Zn(II) Three-Dimensional Metal-Organic Frameworks Based on 2,5-Diiodoterephthalate and N,N Linkers: Structures and Features of Sorption Behavior
Abstract
:1. Introduction
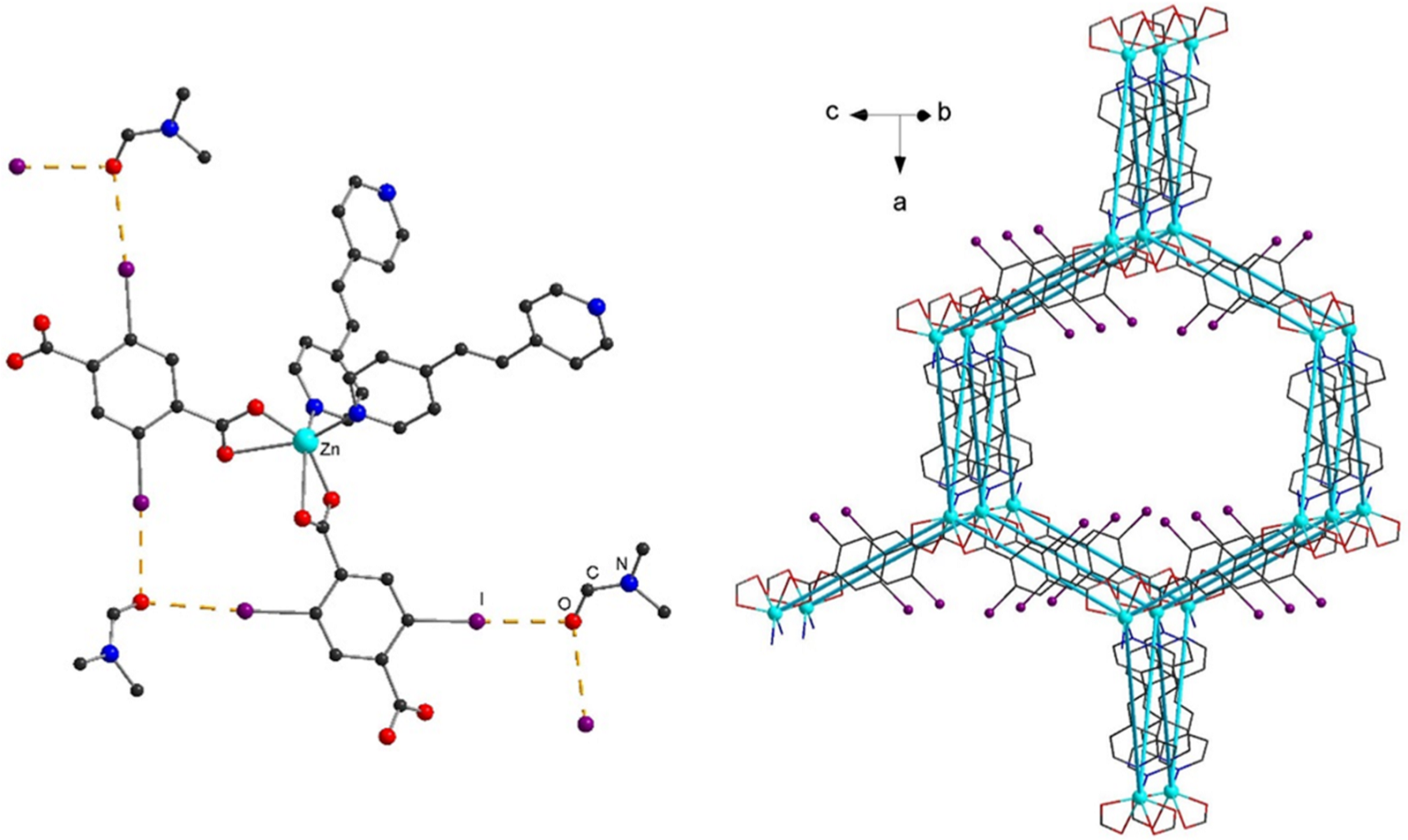
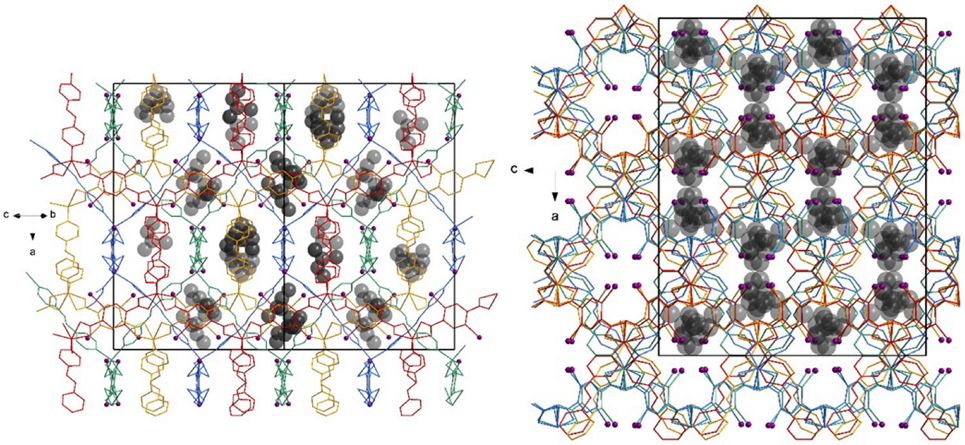
2. Results and Discussion
3. Experimental Part
3.1. Synthesis of 1
3.2. Synthesis of 2
3.3. X-ray Diffractometry
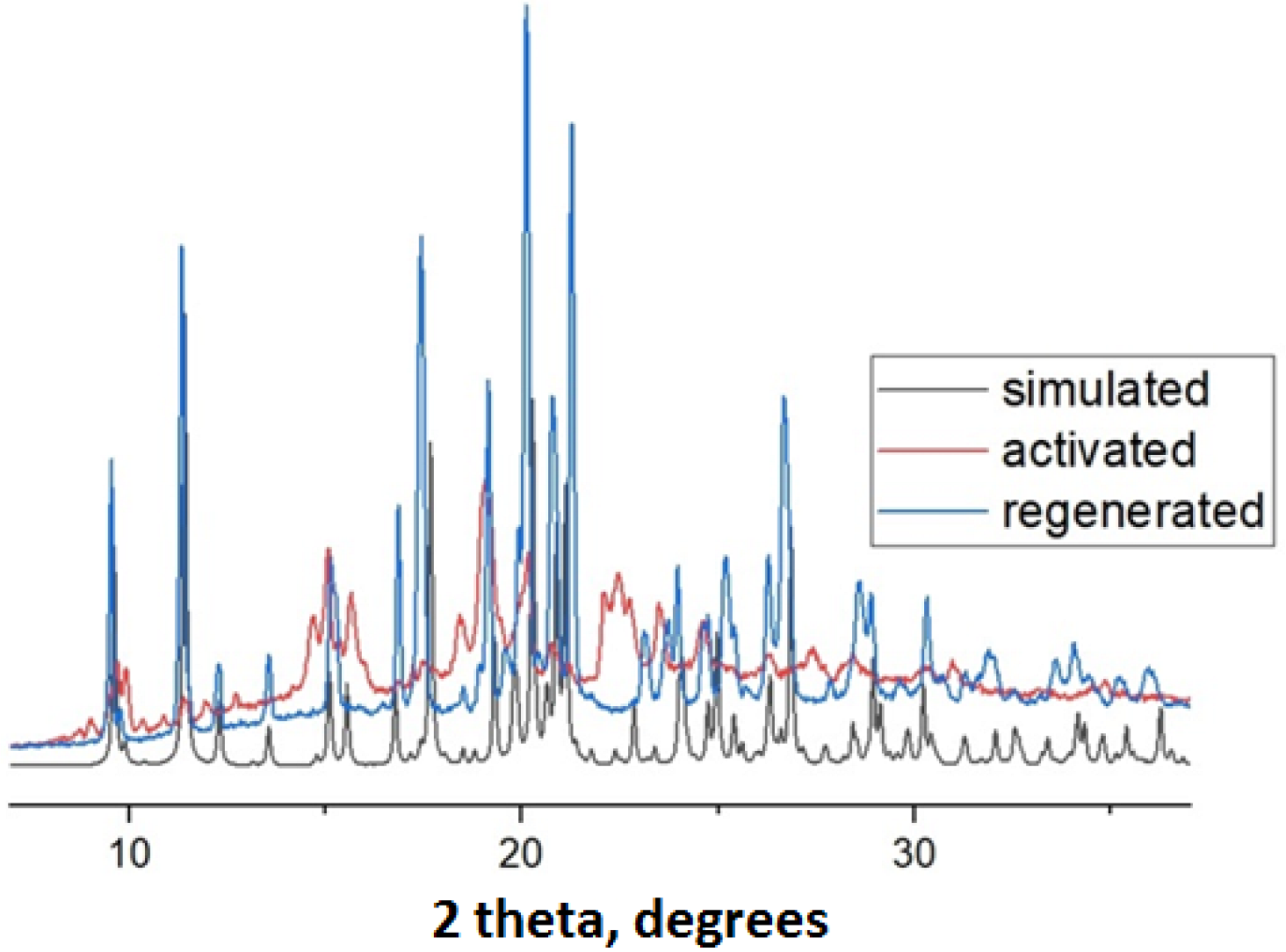
3.4. Powder X-ray Diffraction (PXRD)
3.5. Sorption of Organic Substrates
3.6. Thermogravimetric Analysis (TGA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorbunova, Y.G.; Fedin, V.P.; Blatov, V.A. Metal-organic frameworks as the basis for new-generation functional materials. Russ. Chem. Rev. 2022, 91, RCR5050. [Google Scholar] [CrossRef]
- Rubtsova, I.K.; Melnikov, S.N.; Shmelev, M.A.; Nikolaevskii, S.A.; Yakushev, I.A.; Voronina, J.K.; Barabanova, E.D.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. Facile synthesis and structure elucidation of metal-organic frameworks with {ZnCa} and {Zn2Ca} metal cores. Mendeleev Commun. 2020, 30, 722–724. [Google Scholar] [CrossRef]
- Sidorov, A.A.; Gogoleva, N.V.; Bazhina, E.S.; Nikolaevskii, S.A.; Shmelev, M.A.; Zorina-Tikhonova, E.N.; Starikov, A.G.; Kiskin, M.A.; Eremenko, I.L. Some aspects of the formation and structural features of low nuclearity heterometallic carboxylates. Pure Appl. Chem. 2020, 92, 1093–1110. [Google Scholar] [CrossRef]
- Kovalenko, K.A.; Potapov, A.S.; Fedin, V.P. Micro-and mesoporous metal-organic frameworks for hydrocarbon separation. Russ. Chem. Rev. 2022, 91, RCR5026. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Elsayed, S.M.; Mahmoud, S.E.M.E.; Nabil, G.M.; Salam, M.A. Recent progress of metal organic frameworks-derived composites in adsorptive removal of pharmaceuticals. Polyhedron 2022, 226, 116082. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kovalenko, K.A.; Samsonenko, D.G.; Barsukova, M.O.; Dybtsev, D.N.; Fedin, V.P. Exceptionally effective benzene/cyclohexane separation using a nitro-decorated metal-organic framework. Chem. Commun. 2020, 56, 8241–8244. [Google Scholar] [CrossRef]
- Li, J.; Han, X.; Kang, X.; Chen, Y.; Xu, S.; Smith, G.L.; Tillotson, E.; Cheng, Y.; McCormick McPherson, L.J.; Teat, S.J.; et al. Purification of Propylene and Ethylene by a Robust Metal–Organic Framework Mediated by Host–Guest Interactions. Angew. Chem. Int. Ed. 2021, 60, 15541–15547. [Google Scholar] [CrossRef]
- Zhan, C.-H.; Huang, D.-P.; Wang, Y.; Mao, W.-T.; Wang, X.-J.; Jiang, Z.-G.; Feng, Y.-L. Four anionic Ln-MOFs for remarkable separation of C2H2-CH4/CO2-CH4 and highly sensitive sensing of nitrobenzene. CrystEngComm 2021, 23, 2788–2792. [Google Scholar] [CrossRef]
- Ye, C.-R.; Wang, W.-J.; Chen, W.; Xiao, Y.; Zhang, H.-F.; Dai, B.-L.; Chen, S.-H.; Wu, X.-D.; Li, M.; Huang, X.-C. Harnessing Shape Complementarity for Upgraded Cyclohexane Purification through Adaptive Bottlenecked Pores in an Imidazole-Containing MOF. Angew. Chem. Int. Ed. 2021, 60, 23590–23595. [Google Scholar] [CrossRef]
- Dey, G.; Shadab; Aijaz, A. Metal-Organic Framework Derived Nanostructured Bifunctional Electrocatalysts for Water Splitting. ChemElectroChem 2021, 8, 3782–3803. [Google Scholar] [CrossRef]
- Wang, X.; Zou, Y.; Zhang, Y.; Marchetti, B.; Liu, Y.; Yi, J.; Zhou, X.-D.; Zhang, J. Tin-based metal organic framework catalysts for high-efficiency electrocatalytic CO2 conversion into formate. J. Colloid Interface Sci. 2022, 626, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Verma, K.; Kaushal, S.; Badru, R. A novel 2-D accordion like Al-BPED MOF as reusable and selective catalyst for N-alkylation of amines with dialkylcarbonates. Appl. Organomet. Chem. 2022, 36, e6814. [Google Scholar] [CrossRef]
- Zheng, L.; Gu, Y.; Hua, B.; Fu, J.; Li, F. Hierarchical porous melamine sponge@MIL-101-Fe-NH2 composite as Fenton-like catalyst for efficient and rapid tetracycline hydrochloride removal. Chemosphere 2022, 307, 135728. [Google Scholar] [CrossRef]
- Diyali, N.; Rasaily, S.; Biswas, B. Metal–Organic Framework: An Emergent Catalyst in C–N Cross-Coupling Reactions. Coord. Chem. Rev. 2022, 469, 214667. [Google Scholar] [CrossRef]
- Mulik, N.; Bokade, V. Immobilization of HPW on UiO-66-NH2 MOF as efficient catalyst for synthesis of furfuryl ether and alkyl levulinate as biofuel. Mol. Catal. 2022, 531, 112689. [Google Scholar] [CrossRef]
- Fan, Y.; Guan, H.; Zhang, J.; Wang, W.; Liu, C.; Li, X.; Zhou, J.; Ruan, S. Synthesis and gas sensing properties of β-Fe2O3 derived from Fe/Ga bimetallic organic framework. J. Alloys Compd. 2022, 921, 166193. [Google Scholar] [CrossRef]
- Mullaney, B.R.; Thompson, A.L.; Beer, P.D. An All-Halogen Bonding Rotaxane for Selective Sensing of Halides in Aqueous Media. Angew. Chem. Int. Ed. 2014, 53, 11458–11462. [Google Scholar] [CrossRef]
- Miao, Q.; Hakimifar, A.; Akbar Razavi, S.A.; Abbasi, H.; Tehrani, A.A.; Chen, J.-Q.; Hu, M.-L.; Morsali, A.; Retailleau, P. Multi-functionalization strategy for environmental monitoring: A metal-organic framework for high capacity Mercury(II) removal and exceptionally sensitive detection of nitroaromatics. J. Clean. Prod. 2022, 376, 134301. [Google Scholar] [CrossRef]
- Saedi, Z.; Roushani, M.; Khaleghian-Moghadam, R.; Darabi, A. Selective and sensitive Detection of Cu2+ in aqueous solution based on cation exchange by Metal−Organic framework TMU-16 as a fluorescent sensor. J. Lumin. 2022, 251, 119165. [Google Scholar] [CrossRef]
- Mousavi, S.; Zeinali, S. VOC s detection using resistive gas nanosensor based on MIL-101(Cr) as a metal organic framework. Sensors Actuators A Phys. 2022, 346, 113810. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Davoodian, N.; Taghdisi, S.M.; Abnous, K. Metal organic frameworks as advanced functional materials for aptasensor design. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2022, 276, 121251. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, F.; Zhao, N.; Yuan, R.; Zhu, G. Three novel zinc(ii) metal-organic frameworks based on three tetrazolate ligands: Synthesis, structures and photoluminescence. RSC Adv. 2014, 4, 21535–21540. [Google Scholar] [CrossRef]
- Qu, X.; Yan, B. Zn(ii)/Cd(ii)-based metal-organic frameworks: Crystal structures, Ln(iii)-functionalized luminescence and chemical sensing of dichloroaniline as a pesticide biomarker. J. Mater. Chem. C 2020, 8, 9427–9439. [Google Scholar] [CrossRef]
- Li, X.-L.; Liu, G.-Z.; Xin, L.-Y.; Wang, L.-Y. Three Zn(II) metal-organic frameworks assembled from a versatile tecton 5-ethyl-pyridine-2,3-dicarboxylate and dipyridyl-type coligand. CrystEngComm 2012, 14, 1729–1736. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.-X.; Luo, Y.-H.; Chen, F.-Y.; Jia, C.-Y.; Tan, X.-Q.; Zhang, Y.-Y.; Zhang, D.-E. Co2+ and nitrobenzene sensing using indium-based metal-organic framework. Polyhedron 2022, 224, 116016. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Nabipour, H.; Pahlevani, F.; Zhao, Y.; Hussain, Z.; Hojjati-Najafabadi, A.; Hoang, H.Y.; Pei, R. Recent progress on adsorption of cadmium ions from water systems using metal-organic frameworks (MOFs) as an efficient class of porous materials. Environ. Res. 2022, 214, 114113. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-G.; Bigdeli, F.; Sharifzadeh, Z.; Gholizadeh, S.; Morsali, A. Role of metal-organic framework composites in removal of inorganic toxic contaminants. J. Clean. Prod. 2023, 404, 136709. [Google Scholar] [CrossRef]
- Ma, S.-Q.; Yu, B.; Yi, X.-H.; Wang, C.-C. Two new Zn-based coordination polymers constructed from a light responsive organic ligand: Efficient clean-up of Cr(VI) and organic pollutants. Polyhedron 2020, 188, 114701. [Google Scholar] [CrossRef]
- Maponya, T.C.; Makgopa, K.; Somo, T.R.; Tshwane, D.M.; Modibane, K.D. Highly adsorptive removal of palladium and platinum ions from wastewater using novel ethylenediamine-glutaraldehyde-grafted metal organic framework. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100805. [Google Scholar] [CrossRef]
- Maponya, T.C.; Modibane, K.D.; Somo, T.R.; Makgopa, K. Selective adsorption of palladium ions from wastewater by ion-imprinted MIL-101(Cr) derived from waste polyethylene terephthalate: Isotherms and kinetics. Sep. Purif. Technol. 2023, 307, 122767. [Google Scholar] [CrossRef]
- Quintero-Álvarez, F.G.; Mendoza-Castillo, D.I.; Rojas-Mayorga, C.K.; García-Hernández, E.; Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A. Mechanism, interfacial interactions and thermodynamics of the monolayer adsorption of trace geogenic pollutants from water using mil metal-organic frameworks: Fluorides and arsenates. J. Mol. Liq. 2023, 380, 121665. [Google Scholar] [CrossRef]
- Colorado-Peralta, R.; María Rivera-Villanueva, J.; Manuel Mora-Hernández, J.; Morales-Morales, D.; Ángel Alfonso-Herrera, L. An overview of the role of supramolecular interactions in gas storage using MOFs. Polyhedron 2022, 224, 115995. [Google Scholar] [CrossRef]
- Torresi, S.; Famulari, A.; Martí-Rujas, J. Kinetically Controlled Fast Crystallization of M12L8Poly-[n]-catenanes Using the 2,4,6-Tris(4-pyridyl)benzene Ligand and ZnCl2in an Aromatic Environment. J. Am. Chem. Soc. 2020, 142, 9537–9543. [Google Scholar] [CrossRef]
- Martí-Rujas, J.; Elli, S.; Famulari, A. Kinetic trapping of 2,4,6-tris(4-pyridyl)benzene and ZnI2 into M12L8 poly-[n]-catenanes using solution and solid-state processes. Sci. Rep. 2023, 13, 5605. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Inomata, Y.; Shimokawa, K.; Fujita, M. A metal-peptide capsule by multiple ring threading. Nat. Commun. 2019, 10, 5687. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Postnikov, P.S.; Suslonov, V.V.; Kissler, T.Y.; Ivanov, D.M.; Yusubov, M.S.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. Diaryliodonium as a double s-hole donor: The dichotomy of thiocyanate halogen bonding provides divergent solid state arylation by diaryliodonium cations. Org. Chem. Front. 2020, 7, 2230–2242. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Asadollahi, S.; Dadban Shahamat, Y. Competition between chalcogen bond and halogen bond interactions in YOX4:NH3 (Y = S, Se; X = F, Cl, Br) complexes: An ab initio investigation. Struct. Chem. 2016, 27, 1439–1447. [Google Scholar] [CrossRef]
- Mikherdov, A.S.; Novikov, A.S.; Kinzhalov, M.A.; Boyarskiy, V.P.; Starova, G.L.; Ivanov, A.Y.; Kukushkin, V.Y. Halides Held by Bifurcated Chalcogen–Hydrogen Bonds. Effect of μ (S,N–H) Cl Contacts on Dimerization of Cl(carbene)Pd II Species. Inorg. Chem. 2018, 57, 3420–3433. [Google Scholar] [CrossRef]
- Norouzi, F.; Khavasi, H.R. Iodine decorated-UiO-67 MOF as a fluorescent sensor for the detection of halogenated aromatic hydrocarbons. New J. Chem. 2020, 44, 8937–8943. [Google Scholar] [CrossRef]
- Kalaj, M.; Momeni, M.R.; Bentz, K.C.; Barcus, K.S.; Palomba, J.M.; Paesani, F.; Cohen, S.M. Halogen bonding in UiO-66 frameworks promotes superior chemical warfare agent simulant degradation. Chem. Commun. 2019, 55, 3481–3484. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal-organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Chen, L.; Chen, S.-C.; Zhang, Z.-H.; Sun, F.-A.; Cui, A.-J.; Gao, H.-B.; Hea, M.-Y.; Chen, Q. A Zinc(II) Coordination Polymer with Tetraiodoterephthalate: Synthesis, Crystal Structure, and Luminescence. Z. Naturforsch. B 2012, 67, 843–848. [Google Scholar] [CrossRef]
- Chen, S.-C.; Hu, M.; Zhang, Z.-H.; Sun, F.-A.; Wang, L.; Zhou, W.-Y.; He, M.-Y.; Chen, Q. Syntheses, structures, and properties of zinc(II), cadmium(II), cobalt(II), and manganese(II) coordination polymers with tetraiodoterephthalate. Transit. Met. Chem. 2012, 37, 619–627. [Google Scholar] [CrossRef]
- deKrafft, K.; Xie, Z.; Cao, G.; Tran, S.; Ma, L.; Zhou, O.; Lin, W. Iodinated Nanoscale Coordination Polymers as Potential Contrast Agents for Computed Tomography. Angew. Chem. Int. Ed. 2009, 48, 9901–9904. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of Metal-Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Schubert, U. Clusters with a Zr6O8 core. Coord. Chem. Rev. 2022, 469, 214686. [Google Scholar] [CrossRef]
- Chernysheva, M.V.; Bulatova, M.; Ding, X.; Haukka, M. Influence of Substituents in the Aromatic Ring on the Strength of Halogen Bonding in Iodobenzene Derivatives. Cryst. Growth Des. 2020, 20, 7197–7210. [Google Scholar] [CrossRef]
- Christine, T.; Tabey, A.; Cornilleau, T.; Fouquet, E.; Hermange, P. Syntheses of o-iodobenzyl alcohols—BODIPY structures as potential precursors of bimodal tags for positron emission tomography and optical imaging. Tetrahedron 2019, 75, 13076. [Google Scholar] [CrossRef]
- Zaguzin, A.S.; Sukhikh, T.S.; Kolesov, B.A.; Sokolov, M.N.; Fedin, V.P.; Adonin, S.A. Iodinated vs non-iodinated: Comparison of sorption selectivity by [Zn2(bdc)2dabco]n and superstructural 2-iodoterephtalate-based metal-organic framework. Polyhedron 2021, 212, 115587. [Google Scholar] [CrossRef]
- Cherezova, S.V.; Barsukova, M.O.; Samsonenko, D.G.; Fedin, V.P. Crystal structure of dense metal-organic frameworks based on sc(III) and two types of ligands. J. Struct. Chem. 2021, 62, 897–904. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kiskin, M.A.; Samsonenko, D.G.; Ryadun, A.A.; Dybtsev, D.N.; Fedin, V.P. Luminescent detection by coordination polymers derived from a pre-organized heterometallic carboxylic building unit. Polyhedron 2018, 145, 147–153. [Google Scholar] [CrossRef]
- Martí-Rujas, J.; Islam, N.; Hashizume, D.; Izumi, F.; Fujita, M.; Kawano, M. Dramatic structural rearrangements in porous coordination networks. J. Am. Chem. Soc. 2011, 133, 5853–5860. [Google Scholar] [CrossRef]
- Martí-Rujas, J.; Bonafede, S.; Tushi, D.; Cametti, M. Multiple single-crystal-to-single-crystal guest exchange in a dynamic 1D coordination polymer. Chem. Commun. 2015, 51, 12357–12360. [Google Scholar] [CrossRef]
- Perry, R.J.; Wilson, B.D.; Turner, S.R.; Blevins, R.W. Synthesis of Polyimides via the Palladium-Catalyzed Carbonylation of Bis(o-iodo esters) and Diamines. Macromolecules 1995, 28, 3509–3515. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

| No. | Substrates | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| 1 | 1,2-dichloroethane:benzene | 1:1 | 1:1 | 1.2:1 | 2.54:1 |
| 2 | Benzene:chloroform | 1:2.15 | 1:1.5 | 1:1.3 | 1:3.3 |
| 3 | Benzene:cyclohexane | 6:1 | 10.6:1 | 25:1 | 43.8:1 |
| 4 | Benzene:1,2-dibromoethane | 1:1.3 | 1:2 | n/a | 1:8.3 |
| 5 | 1,2-dibromoethane:cyclohexane | 25.7:1 | 8.1:1 | 116:1 | n/a |
| 6 | 1,2-dibromoethane: hexane | 103:1 | 94:1 | 176:1 | n/a |
| 7 | Bromobenzene:toluene | 0.9:1 | 1.1:1 | 1.8:1 | n/a |
| 1·DMF | 2·DMF | |
|---|---|---|
| Empirical Formula | C23H21I2N3O5Zn | C23H19I2N3O5Zn |
| Crystal System | Orthorombic | Orthorombic |
| Formula weight | 738.60 | 736.58 |
| Temperature/K | 150 (2) | 150 (2) |
| Space group | Fdd2 | Fdd2 |
| a/Å | 28.7667 (10) | 28.6666 (11) |
| b/Å | 31.0967 (11) | 31.2803 (15) |
| c/Å | 22.7773 (8) | 22.6871 (11) |
| α/° | 90 | 90 |
| β/° | 90 | 90 |
| γ/° | 90 | 90 |
| Volume/Å3 | 20,375.4 (12) | 20,343.5 (16) |
| Z | 32 | 32 |
| ρcalcg/cm3 | 1.926 | 1.924 |
| μ/mm−1 | 3.427 | 3.432 |
| F (000) | 11,392.0 | 11,328.0 |
| Crystal size/mm3 | 0.07 × 0.04 × 0.04 | 0.06 × 0.04 × 0.04 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 2.63 to 51.37 | 3.854 to 51.364 |
| Index ranges | −35 ≤ h ≤ 35, −37 ≤ k ≤ 35, −27 ≤ l ≤ 27 | −34 ≤ h ≤ 34, −38 ≤ k ≤ 38, −27 ≤ l ≤ 27 |
| Reflections collected | 53,782 | 48,728 |
| Independent reflections | 9670 (Rint = 0.0825, Rsigma = 0.0641) | 9651 (Rint = 0.0606, Rsigma = 0.0486) |
| Data/restraints/parameters | 9670/153/593 | 9651/56/604 |
| Goodness-of-fit on F2 | 1.031 | 1.047 |
| Final R indexes (I > =2σ (I)) | R1 = 0.0615, wR2 = 0.1406 | R1 = 0.0449, wR2 = 0.0951 |
| Final R indexes (all data) | R1 = 0.0862, wR2 = 0.1565 | R1 = 0.0543, wR2 = 0.0999 |
| Largest diff. peak/hole / e Å−3 | 1.85/−1.23 | 1.67/−0.97 |
| Flack parameter | 0.50 (5) | 0.45 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaguzin, A.S.; Sukhikh, T.; Sokolov, M.N.; Fedin, V.P.; Adonin, S.A. Zn(II) Three-Dimensional Metal-Organic Frameworks Based on 2,5-Diiodoterephthalate and N,N Linkers: Structures and Features of Sorption Behavior. Inorganics 2023, 11, 192. https://doi.org/10.3390/inorganics11050192
Zaguzin AS, Sukhikh T, Sokolov MN, Fedin VP, Adonin SA. Zn(II) Three-Dimensional Metal-Organic Frameworks Based on 2,5-Diiodoterephthalate and N,N Linkers: Structures and Features of Sorption Behavior. Inorganics. 2023; 11(5):192. https://doi.org/10.3390/inorganics11050192
Chicago/Turabian StyleZaguzin, Alexander S., Taisiya Sukhikh, Maxim N. Sokolov, Vladimir P. Fedin, and Sergey A. Adonin. 2023. "Zn(II) Three-Dimensional Metal-Organic Frameworks Based on 2,5-Diiodoterephthalate and N,N Linkers: Structures and Features of Sorption Behavior" Inorganics 11, no. 5: 192. https://doi.org/10.3390/inorganics11050192







