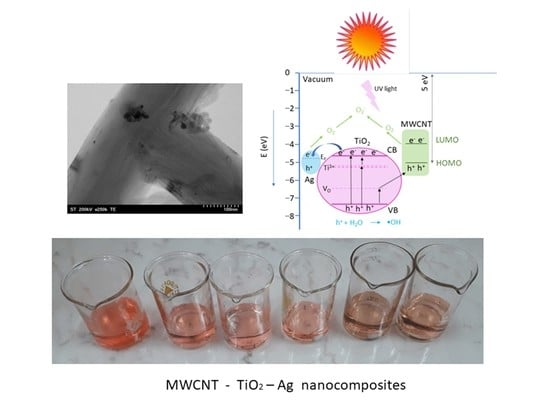
The Influence of Ag+/Ti4+ Ratio on Structural, Optical and Photocatalytic Properties of MWCNT–TiO2–Ag Nanocomposites
Abstract
1. Introduction
2. Results
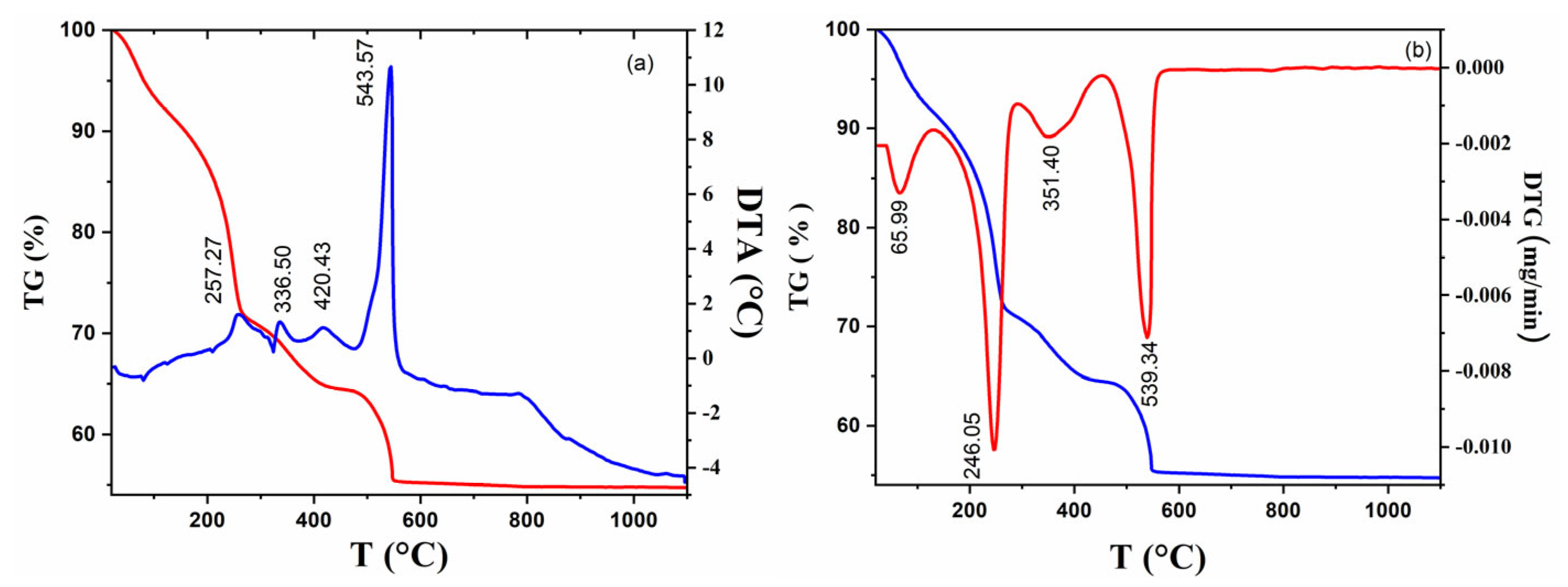
2.1. Investigation of Precursors
2.2. Structural, Morphologic, and Optical Properties
2.3. FT–IR Spectroscopy
2.4. Raman Spectroscopy
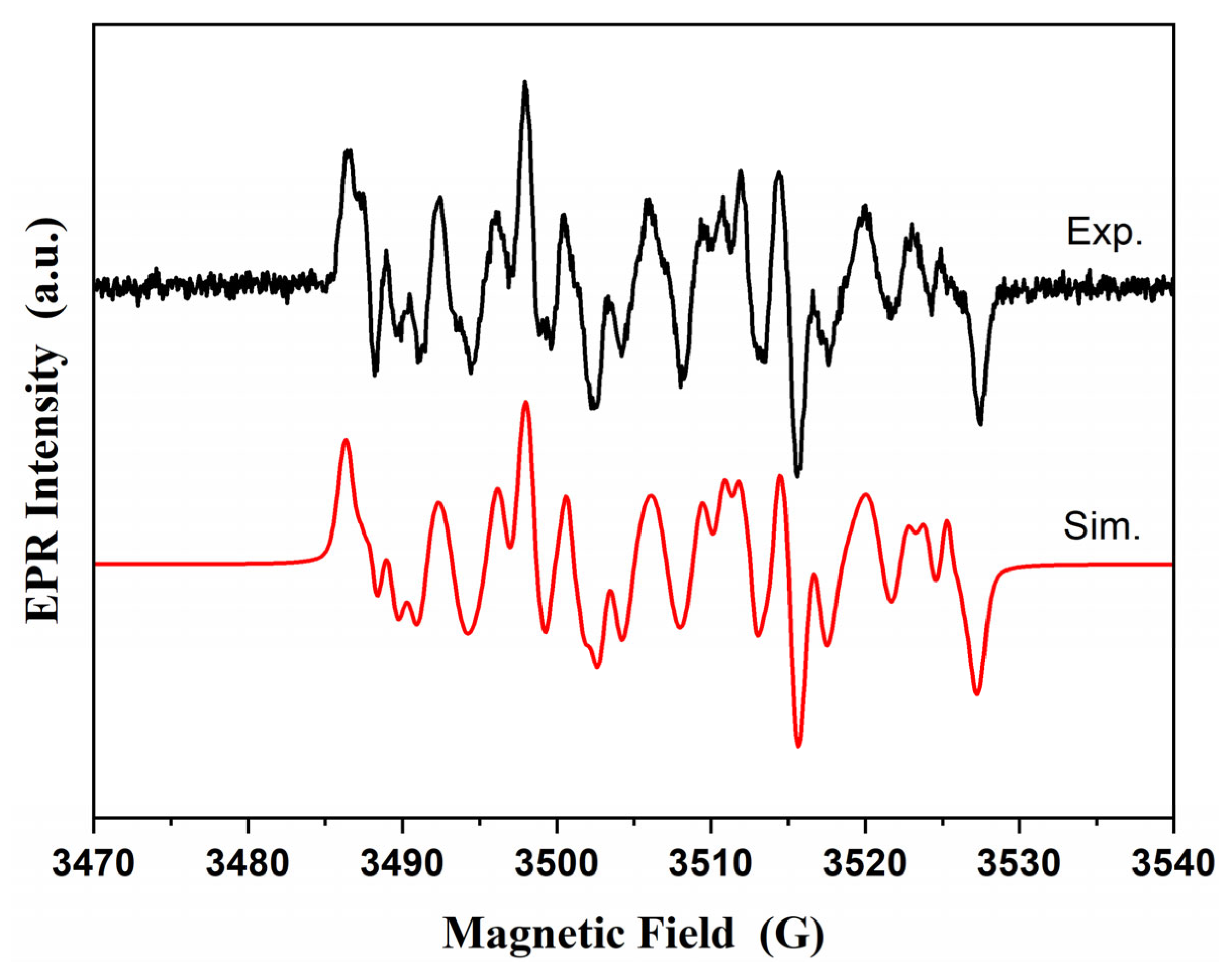
2.5. Electron Spin Resonance Measurements
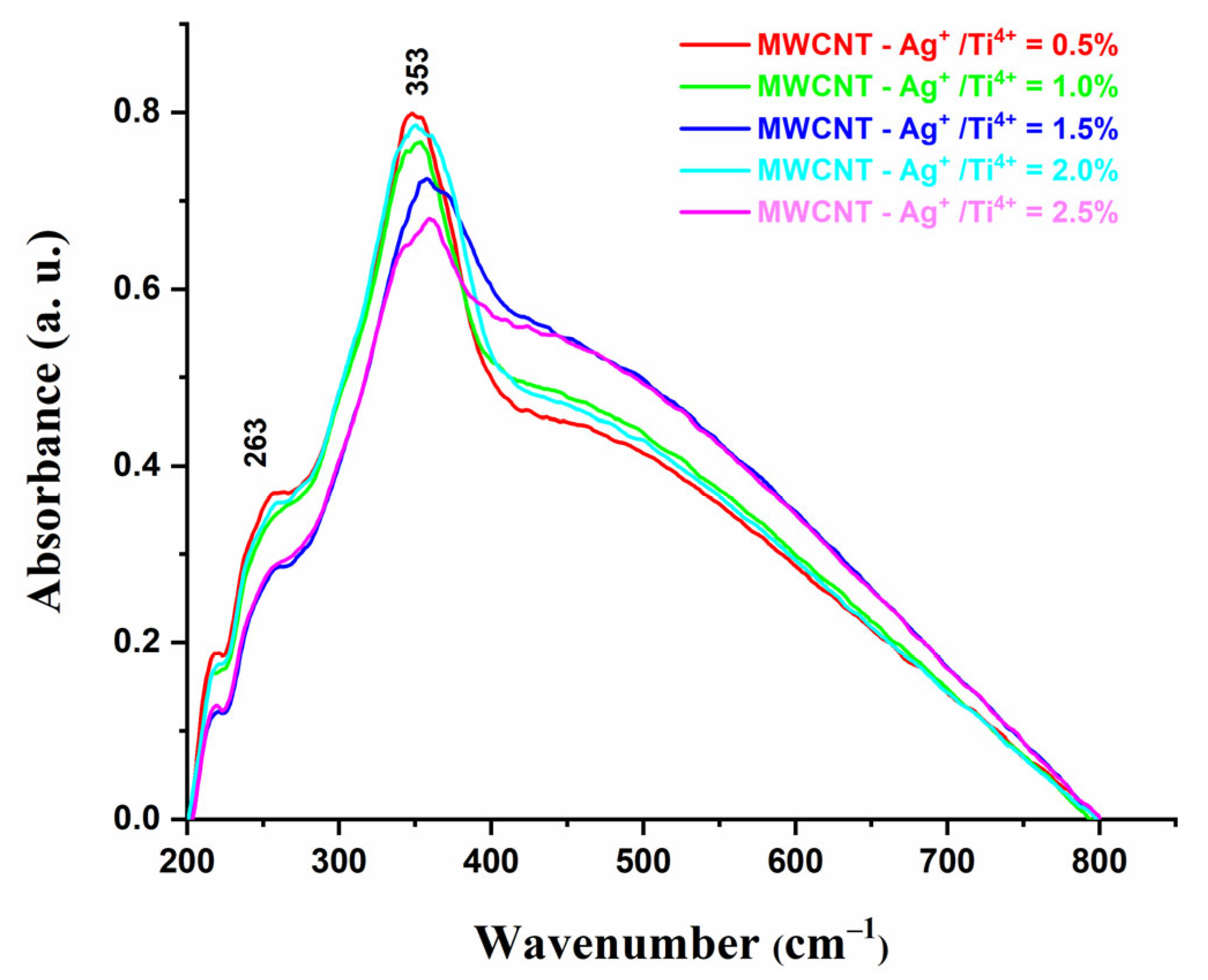
2.6. UV–ViS Spectroscopy
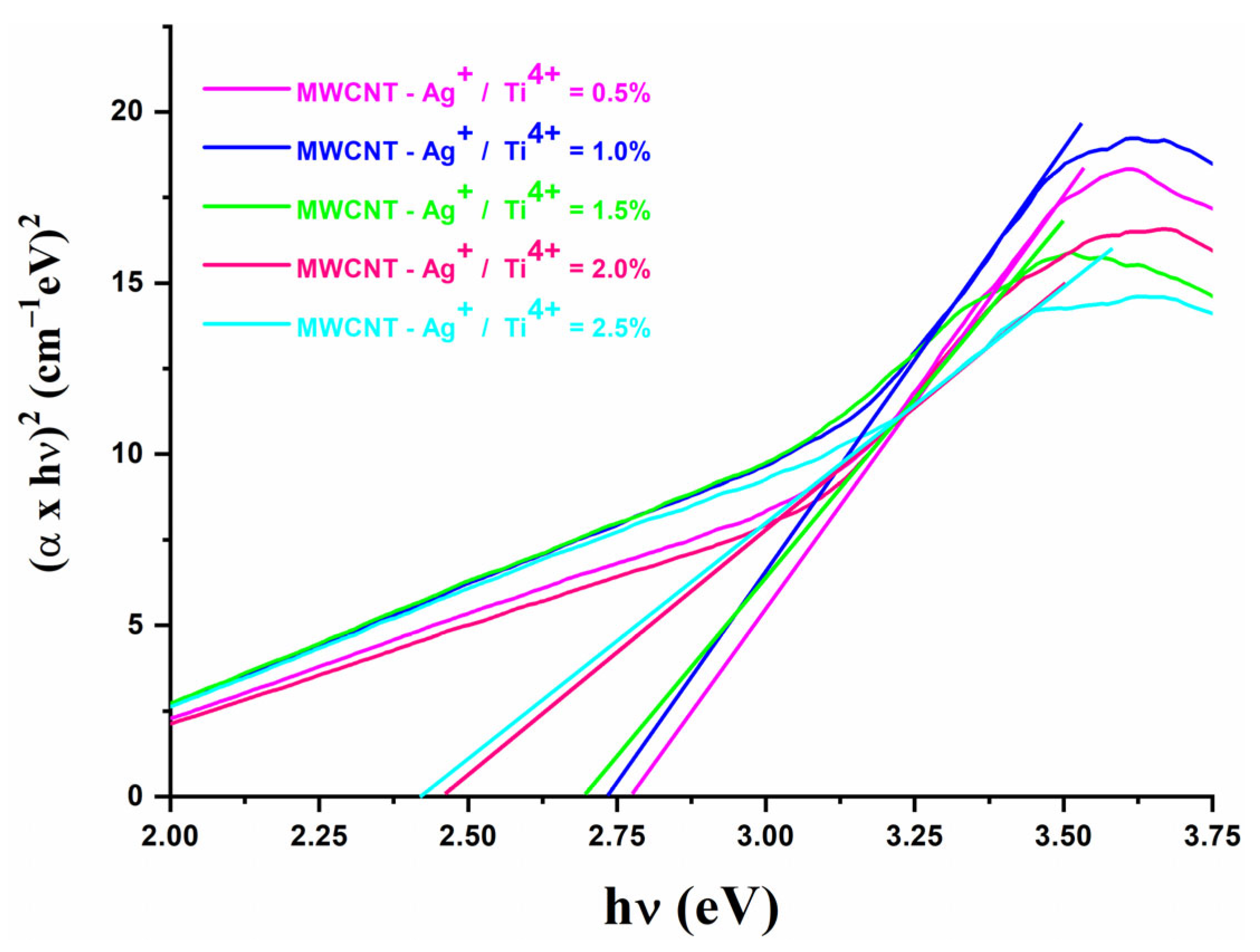
2.7. Optical Band Gap Energy, Eg Determination
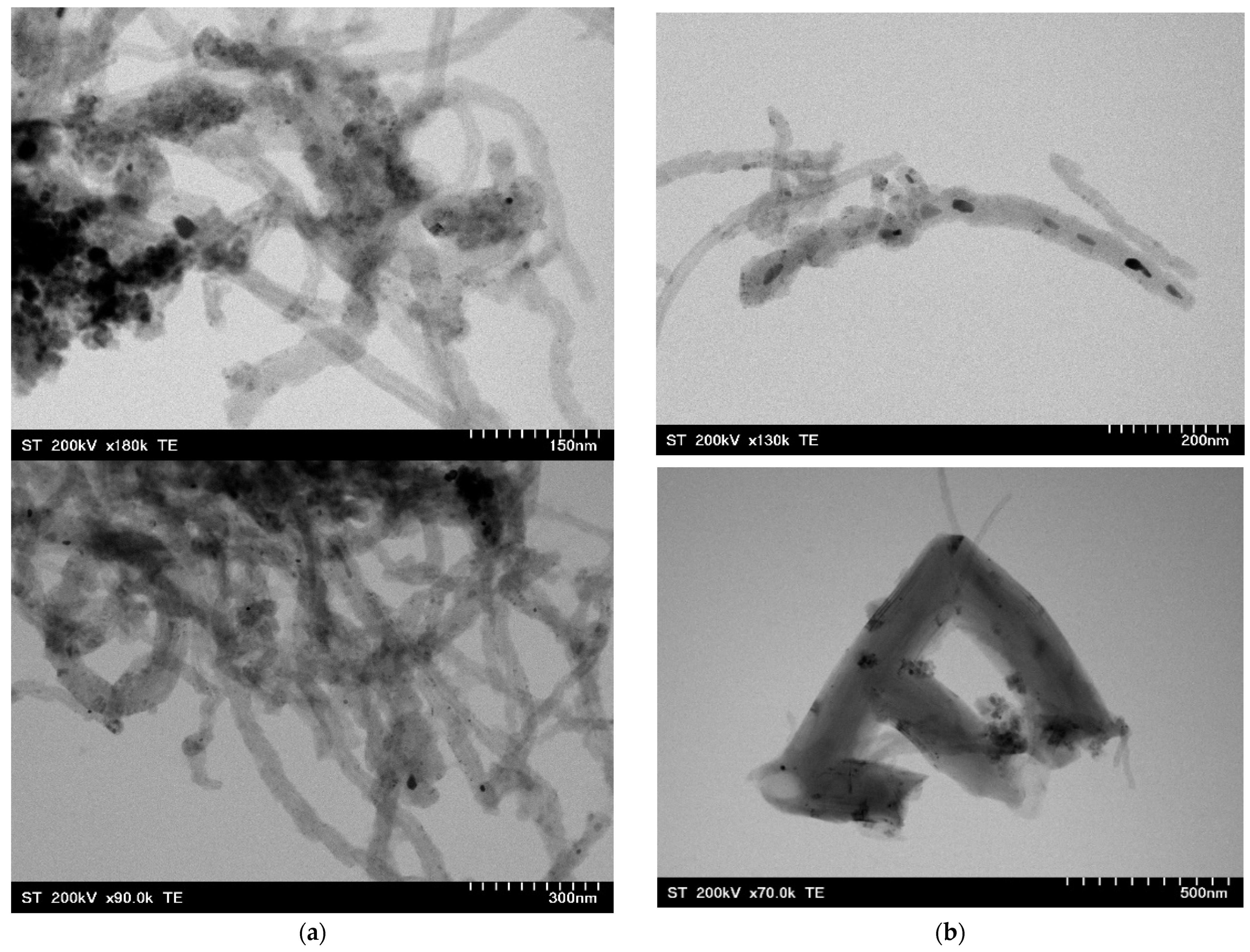
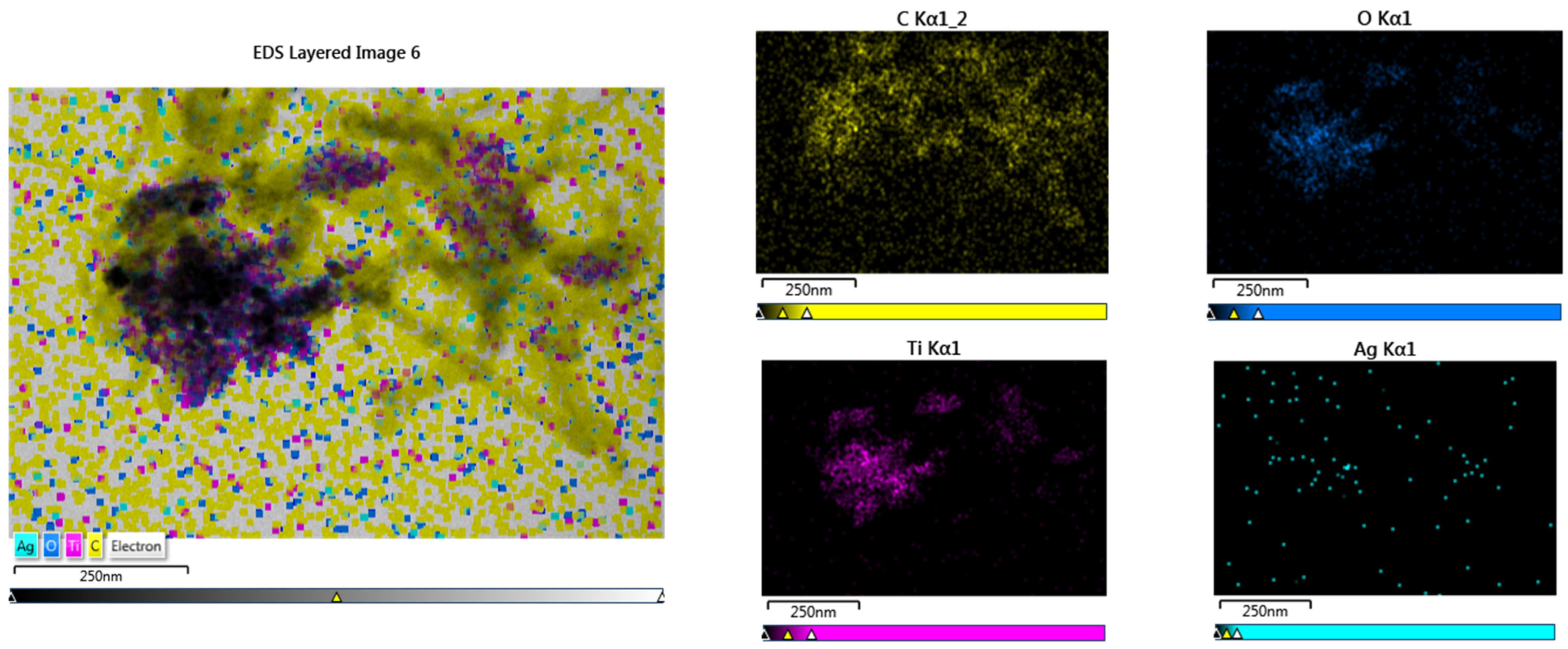
2.8. Morphology of Nanocomposites
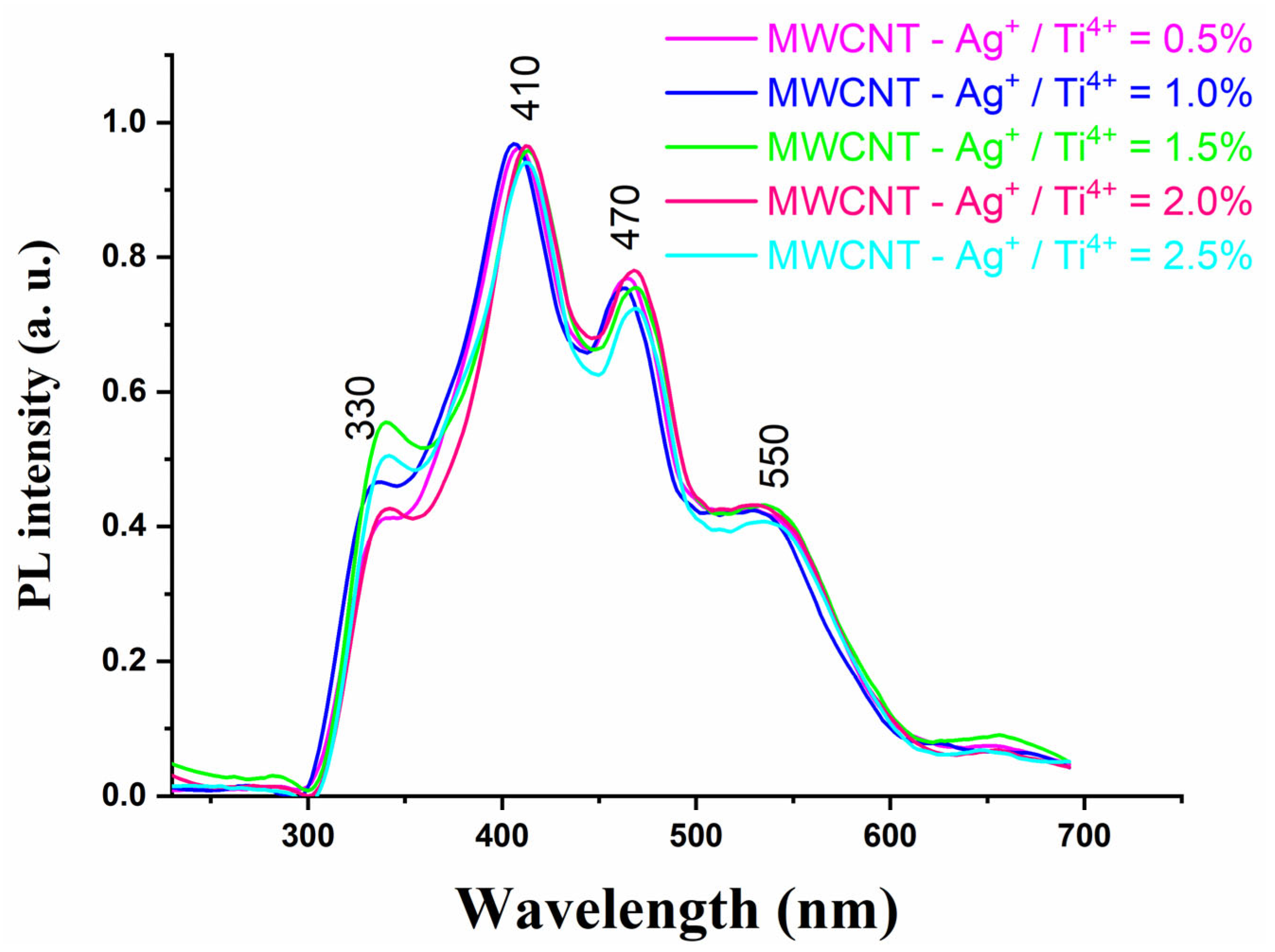
2.9. Photoluminescence Spectroscopy
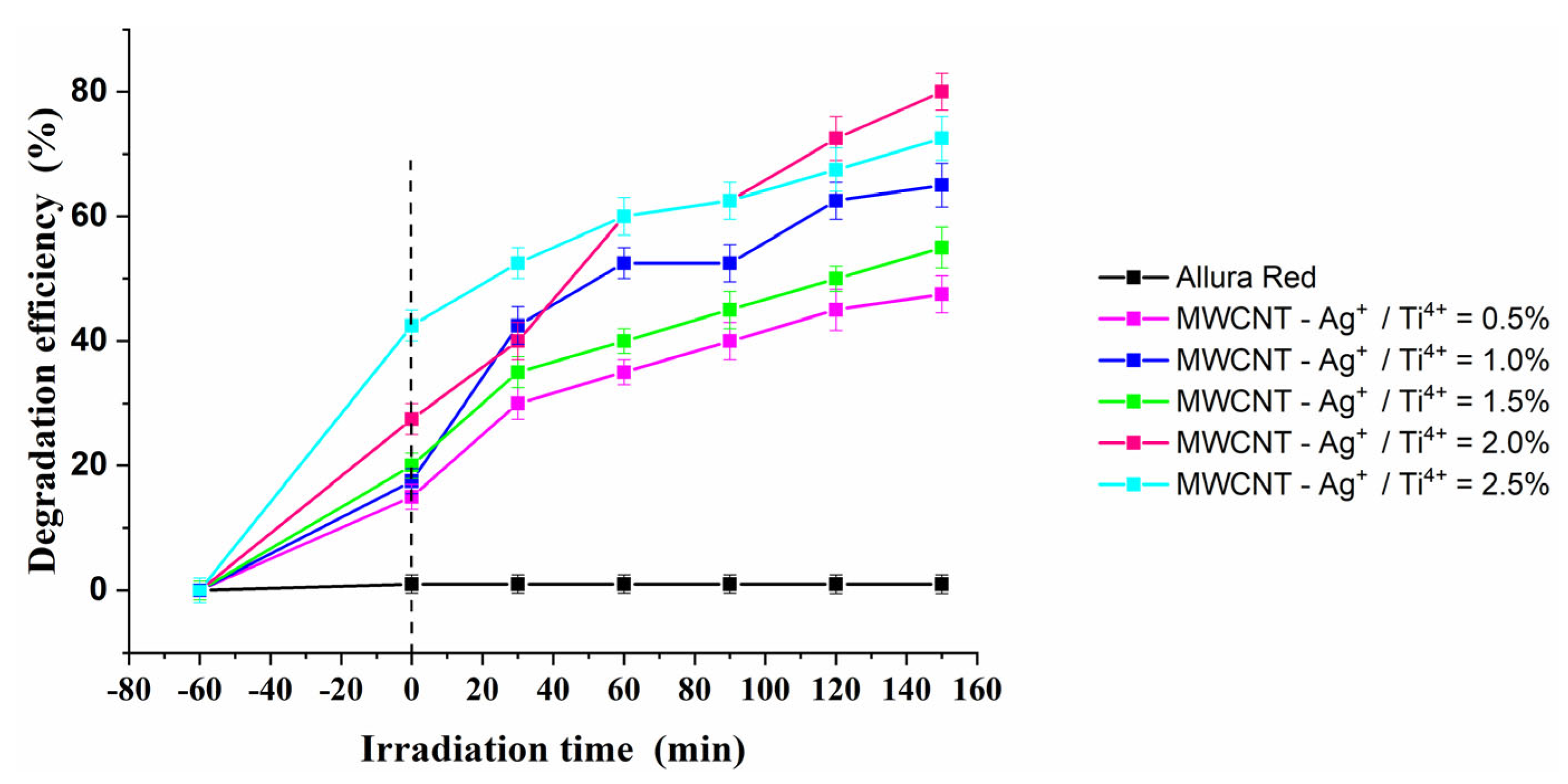
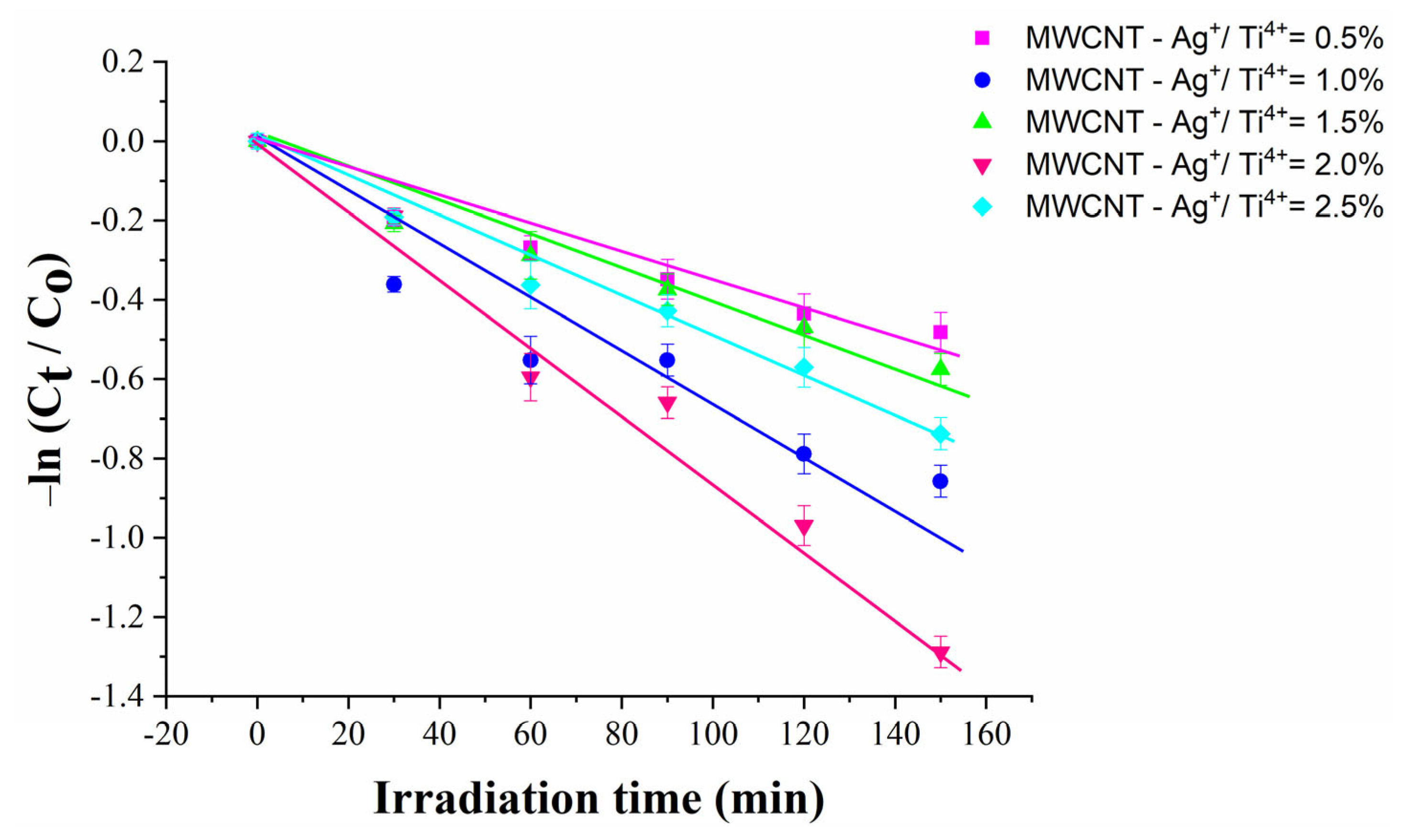
2.10. Photocatalytic Activity
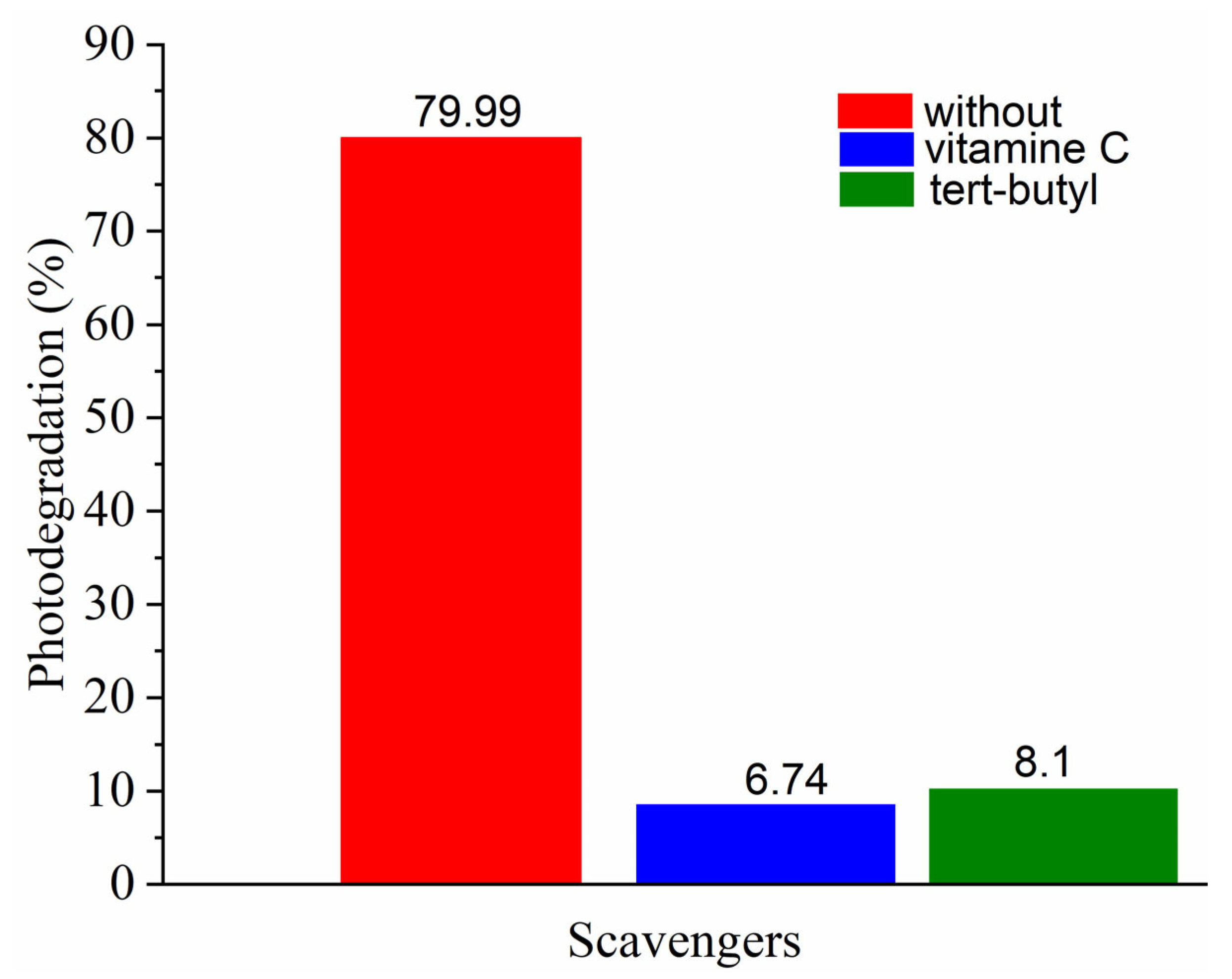
2.10.1. Scavanger Experiments
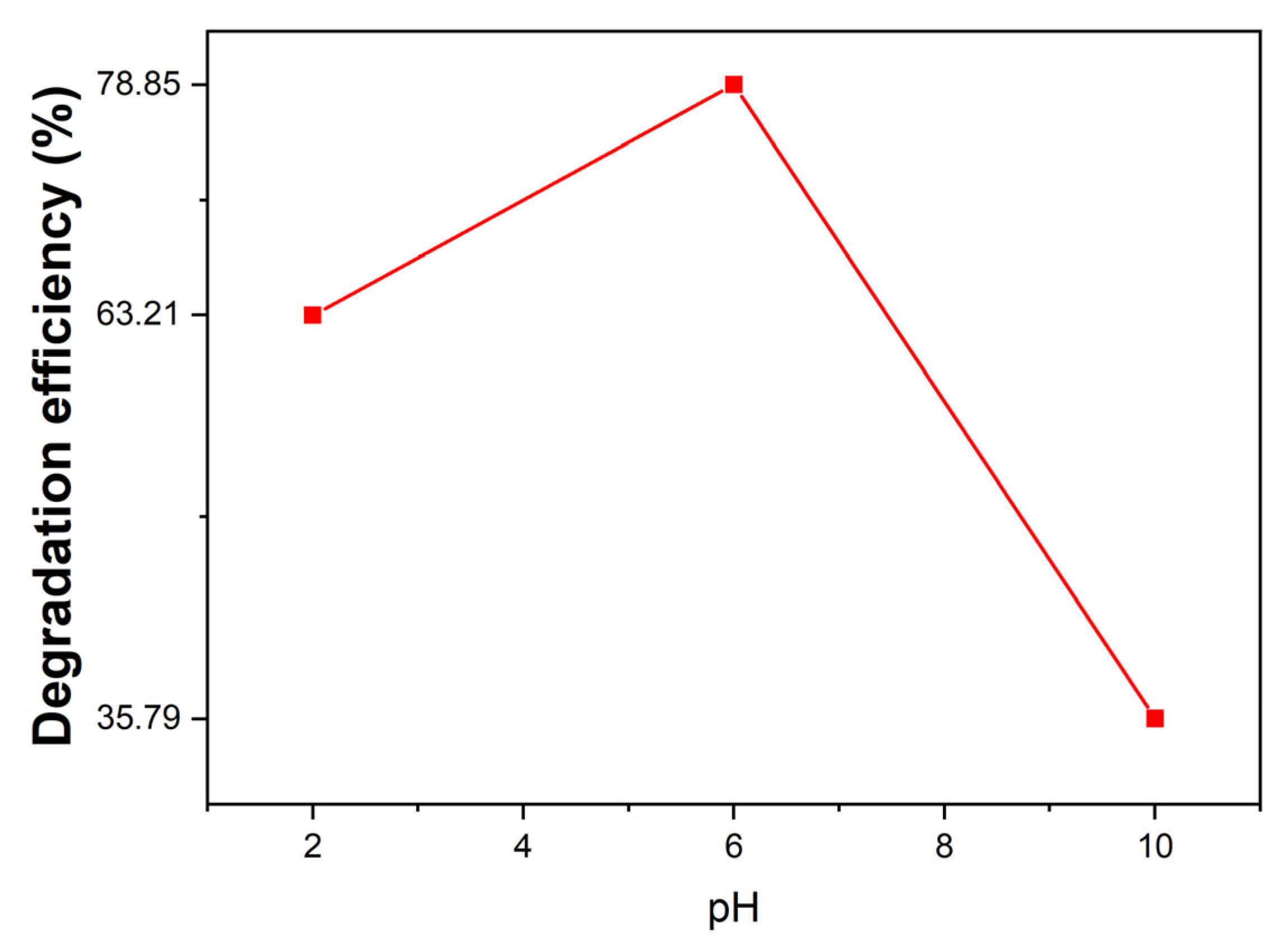
2.10.2. Effect of the Solution pH on Pollutant Eliminations
2.10.3. Photocatalyst Cycling Experiments
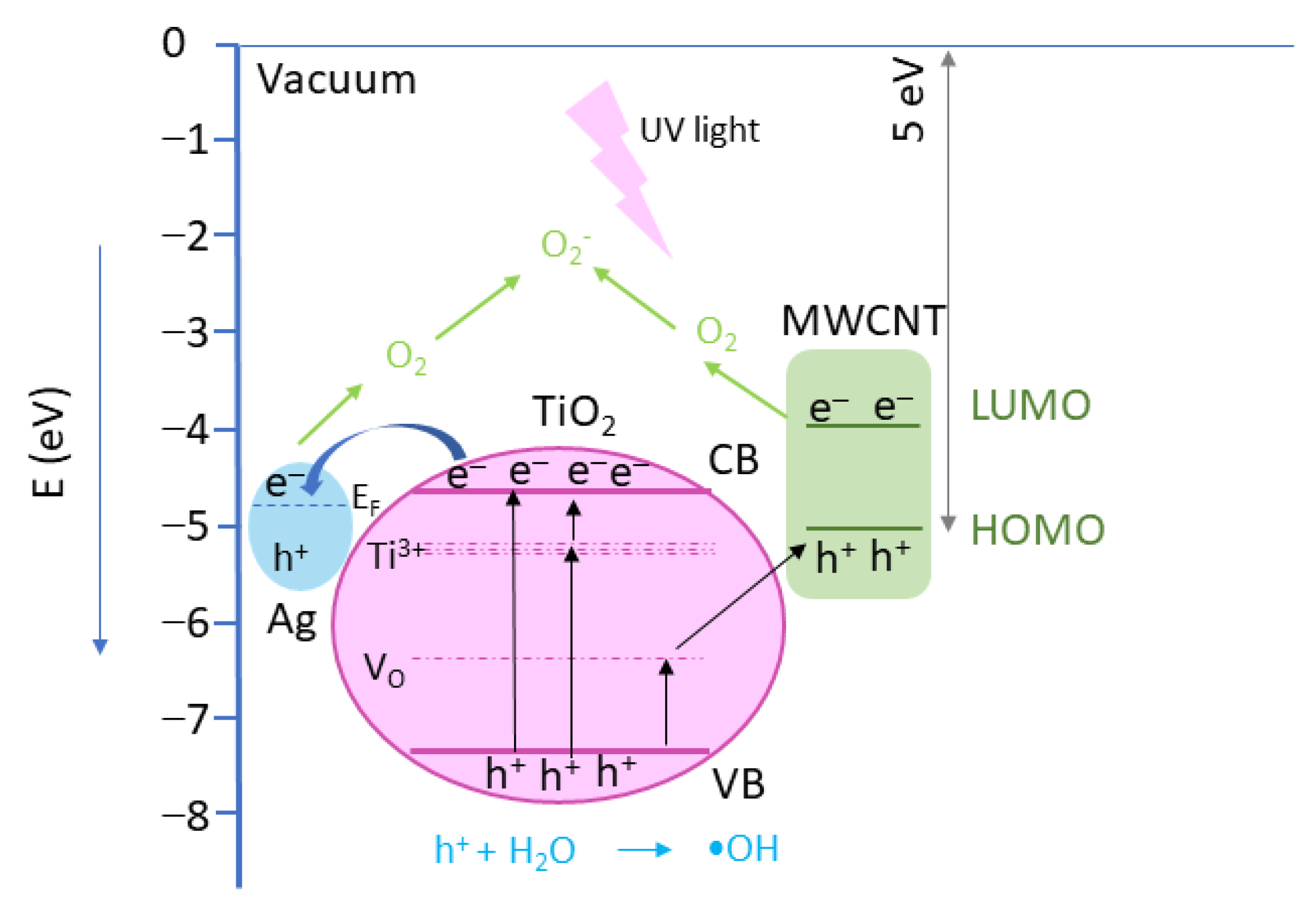
2.10.4. Photocatalytic Mechanism
Reactiv Oxigen Species (ROS) Generation
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.2.1. Functionalization of MWCNTs
3.2.2. Synthesis of TiO2 Modified by Ag
3.3. Decoration of MWCNT with TiO2 Modified by Ag
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, D.; Cai, C. Cerium-based UiO-66 metal-organic framework for synergistic dye adsorption and photodegradation: A discussion of the mechanism. Dye. Pigment. 2021, 185, 108957. [Google Scholar] [CrossRef]
- Xiang, C.; Wang, W.; Liu, S.; Huang, Y.; Li, M.; Wang, D. Humidity-stimulated film actuator with dual-responsive of bending deformation and discoloration. Sens. Actuators B Chem. 2023, 380, 133344. [Google Scholar] [CrossRef]
- Rajakani, V.; Shajan, X.S.; Arulgnanam, A.; Premkumar, P.S. Photovoltaic studies on iodine incorporated titania aerogel nanocomposites. Results Opt. 2023, 10, 100346. [Google Scholar] [CrossRef]
- Mandić, V.; Panžić, I.; Brnardić, I.; Jajčinović, I.; Mičetić, M. Lateral and vertical evolution of the compositional and morphological profiles in nanostructured photocatalytic titania thin films. Appl. Surf. Sci. 2023, 613, 56047. [Google Scholar] [CrossRef]
- Kausar, F.; Varghese, A.; Pinheiro, D.; Sunaja, D.K.R. Recent trends in photocatalytic water splitting using titania based ternary photocatalysts-A review. Int. J. Hydrog. Energy 2022, 47, 22371–22402. [Google Scholar] [CrossRef]
- Sadia, M.; Naz, R.; Khan, J.; Zahoor, M.; Ullah, R.; Khan, R.; Naz, S.; Almoallim, H.S.; Alharbi, S.A. Metal doped titania nanoparticles as efficient photocatalyst for dyes degradation. J. King Saud Univ. Sci. 2021, 33, 101312. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Mohammed, M.K.A.; Mohammad, M.R. Sol–gel synthesis of Ag-doped titania-coated carbon nanotubes and study their biomedical applications. Chem. Pap. 2020, 74, 197–208. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Carbon Nanostructures Decorated with Titania: Morphological Control and Applications. Appl. Sci. 2021, 11, 6814. [Google Scholar] [CrossRef]
- Rajakani, V.; Shajan, X.S.; Arulgnanam, A.; Premkumar, P.S. Studies on the silver incorporated titania aerogel nanostructure as a photoanode in quasi solid dye- sensitized solar cells. Mater. Today Proc. 2022, 65, 2473–2479. [Google Scholar] [CrossRef]
- Topolski, A. Functionalization of titania nanotubes surface with platinum(II) complexes. Polyhedron 2023, 230, 116218. [Google Scholar] [CrossRef]
- Paszkiewicz, O.; Wang, K.; Rakoczy, R.; Kordas, M.; Leniec, G.; Kowalska, E.; Markowska-Szczupak, A. Antimicrobial properties of pristine and Pt-modified titania P25 in rotating magnetic field conditions. Chem. Eng. Process. Intensif. 2022, 178, 109010. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Sohrabi, L.; Taleshi, F. Effect of carbon nanotubes support on band gap energy of MgO nanoparticles. J. Mater. Sci. Mater. Electron. 2014, 25, 4110–4114. [Google Scholar] [CrossRef]
- Sameera, I.; Bhatia, R.; Prasad, V. Preparation, characterization and electrical conductivity studies of MWCNT/ZnO nanoparticles hybrid. Phys. B Condens. Matter 2010, 405, 1709–1714. [Google Scholar] [CrossRef]
- Younas, M.; Gondal, M.A.; Dastageer, M.A.; Harrabi, K. Efficient and cost-effective dye-sensitized solar cells using MWCNT-TiO2 nanocomposite as photoanode and MWCNT as Pt-free counter electrode. Sol. Energy 2019, 188, 1178–1188. [Google Scholar] [CrossRef]
- Hosny, N.M.; Gomaa, I.; Elmahgary, M.G. Adsorption of polluted dyes from water by transition metal oxides: A review. Appl. Surf. Sci. Adv. 2023, 15, 100395. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.; Yuan, Y.; Wang, S. Spatiotemporal distribution patterns and ecological risk of multi-pesticide residues in the surface water of a typical agriculture area in China. Sci. Total. Environ. 2023, 870, 161872. [Google Scholar] [CrossRef]
- Jia, W.-L.; Song, C.; He, L.-Y.; Wang, B.; Gao, F.Z.; Zhang, M.; Ying, G.-G. Antibiotics in soil and water: Occurrence, fate, and risk. Curr. Opin. Environ. Sci. Health 2023, 32, 100437. [Google Scholar] [CrossRef]
- Barboux-Doeuff, S.; Sanchez, C. Synthesis and characterization of titanium oxide-based gels synthesized from acetate modified titanium butoxide precursors. Mater. Res. Bull. 1990, 29, 1–13. [Google Scholar] [CrossRef]
- Kaur, M.; Umar, A.; Mehta, S.K.; Kansal, S.K. Reduced graphene oxide-CdS heterostructure: An efficient fluorescent probe for the sensing of Ag(I) and sunset yellow and a visible-light responsive photocatalyst for the degradation of levofloxacin drug in aqueous phase. Appl. Catal. B Environ. 2019, 245, 143–158. [Google Scholar] [CrossRef]
- Xiang, X.-B.; Yu, Y.; Wen, W.; Wu, J.-M. Control of Selectivity in Heterogeneous Photocatalysis by Tuning TiO2 Morphology for Water Treatment Applications. New J. Chem. 2017, 42, 265–271. [Google Scholar] [CrossRef]
- Salem, M.A.; Abdel-Halim, S.T.; El-Sawy, A.E.-H.M.; Zaki, A.B. Kinetics of degradation of allura red, ponceau 4R and carmosine dyes with potassium ferrioxalate complex in the presence of H2O. Chemosphere 2009, 76, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Ferreira, G.M.D.; Hespanhol, M.D.C.; Rezende, J.D.P.; dos Santos Pires, A.C.; Gurgel, L.; Da Silva, L.H.M. Adsorption of red azo dyes on multi-walled carbon nanotubes and activated carbon: A thermodynamic study. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 531–540. [Google Scholar] [CrossRef]
- Wakrim, A.; Dassaa, A.; Zaroual, Z.; EL Ghachtouli, S.; Jamal Eddine, J.; Azzi, M. Mechanistic study of carmoisine dye degradation in aqueous solution by Fenton process. Mater. Today Proc. 2021, 37, 3847–3853. [Google Scholar] [CrossRef]
- Gui, M.M.; Wong, W.M.P.; Chai, S.-P.; Mohamed, A.R. One-pot synthesis of Ag-MWCNT@TiO2 core–shell nanocomposites for photocatalytic reduction of CO2 with water under visible light irradiation. Chem. Eng. J. 2015, 278, 272–278. [Google Scholar] [CrossRef]
- Chaudhary, D.; Khare, N.; Vankar, V. Ag nanoparticles loaded TiO2/MWCNT ternary nanocomposite: A visible-light-driven photocatalyst with enhanced photocatalytic performance and stability. Ceram. Int. 2016, 42, 15861–15867. [Google Scholar] [CrossRef]
- Honma, M. Evaluation of the in vivo genotoxicity of Allura Red AC (Food Red No. 40). Food Chem. Toxicol. 2015, 84, 270–275. [Google Scholar] [CrossRef]
- Thiam, A.; Sirés, I.; Centellas, F.; Cabot, P.L.; Brillas, E. Decolorization and mineralization of Allura Red AC azo dye by solar photoelectro-Fenton: Identification of intermediates. Chemosphere 2015, 136, 1–8. [Google Scholar] [CrossRef]
- Abramsson-Zetterberg, L.; Ilbäck, N.-G. The synthetic food colouring agent Allura Red AC (E129) is not genotoxic in a flow cytometry-based micronucleus assay in vivo. Food Chem. Toxicol. 2013, 59, 86–89. [Google Scholar] [CrossRef]
- Coloma, A.; del Pozo, M.; Martínez-Moro, R.; Blanco, E.; Atienzar, P.; Sánchez, L.; Petit-Domínguez, M.D.; Casero, E.; Quintana, C. MoS2 quantum dots for on-line fluorescence determination of the food additive allura red. Food Chem. 2021, 345, 128628. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.G.; Nagaraj, B.; Rajashekhar, K.E. Synergistic effect of Ag deposition and nitrogen doping in TiO2 for the degradation of phenol under solar irradiation in presence of electron acceptor. Chem. Eng. J. 2012, 181–182, 259–266. [Google Scholar] [CrossRef]
- Bouazza, N.; Ouzzine, M.; Lillo-Ródenas, M.; Eder, D.; Linares-Solano, A. TiO2 nanotubes and CNT–TiO2 hybrid materials for the photocatalytic oxidation of propene at low concentration. Appl. Catal. B Environ. 2009, 92, 377–383. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Ghaedi, M.; Kokhdan, S.N.; Vashaee, D. Remarkably improved electrochemical hydrogen storage by multi-walled carbon nanotubes decorated with nanoporous bimetallic Fe–Ag/TiO2 nanoparticles. Dalton Trans. 2019, 48, 898–907. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Fakhari, M.; Hamidinezhad, H.; Haddadi, H.; Nourani, M.R. TiO2 nanoparticles immobilized on carbon nanotubes for enhanced visible-light photo-induced activity. J. Mater. Res. Technol. 2015, 4, 126–132. [Google Scholar] [CrossRef]
- Yuliati, L.; Kimi, M.; Shamsuddin, M. High activity of Ag-doped Cd0.1Zn0.9S photocatalyst prepared by the hydrothermal method for hydrogen production under visible-light irradiation. Beilstein J. Nanotechnol. 2014, 5, 587–595. [Google Scholar] [CrossRef]
- Aldea, N.; Indrea, E. XRLINE, a program to evaluate the crystallite size of supported metal catalysts by single X-ray profile Fourier analysis. Comput. Phys. Commun. 1990, 60, 155–163. [Google Scholar] [CrossRef]
- Indrea, E.; Barbu, A. Indirect photon interaction in PbS photodetectors. Appl. Surf. Sci. 1996, 106, 498–501. [Google Scholar] [CrossRef]
- Van Berkum, J.G.M.; Vermeulen, A.C.; Delhez, R.; De Keijser, T.H.; Mittemeijer, E.J. Applicabilities of the Warren–Averbach analysis and an alternative analysis for separation of size and strain broadening. J. Appl. Crystallogr. 1994, 27, 345–357. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Thiel, J.; Pakstis, L.; Buzby, S.; Raffi, M.; Ni, C.; Pochan, D.J.; Shah, S.I. Antibacterial Properties of Silver-Doped Titania. Small 2007, 3, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Soler-Illia, G.; Louis, A.; Sanchez, C. Synthesis and Characterization of Mesostructured Titania-Based Materials through Evaporation-Induced Self-Assembly. Chem. Mater. 2002, 14, 750–759. [Google Scholar] [CrossRef]
- Chen, C.S.; Liu, T.G.; Lin, L.W.; Xie, X.D.; Chen, X.H.; Liu, Q.C.; Liang, B.; Yu, W.W.; Qiu, C.Y. Multi-walled carbon nanotube-supported metal-doped ZnO nanoparticles and their photocatalytic property. J. Nano Res. 2013, 15, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Alsharaeh, E.H.; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M.G.; Abu-Salah, K.M.; Dutta, J. Sol-Gel-Assisted Microwave-Derived Synthesis of Anatase Ag/TiO2/GO Nanohybrids toward Efficient Visible Light Phenol Degradation. Catalysts 2017, 7, 133. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef]
- Abbasi, S.; Zebarjad, S.M.; Baghban, S.H.N.; Youssefi, A. Synthesis of TiO2 Nanoparticles and Decorated Multiwalled Carbon Nanotubes with Various Content of Rutile Titania. Synth. React. Inorg. Met. Nano Met. Chem. 2015, 45, 1539–1548. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Misra, S.; Andronenko, S.; Tipikin, D.; Freed, J.; Somani, V.; Prakash, O. Study of paramagnetic defect centers in as-grown and annealed TiO2 anatase and rutile nanoparticles by a variable-temperature X-band and high-frequency (236 GHz) EPR. J. Magn. Magn. Mater. 2016, 401, 495–505. [Google Scholar] [CrossRef]
- Caretti, I.; Keulemans, M.; Verbruggen, S.W.; Lenaerts, S.; Doorslaer, S. Van. Light-Induced Processes in Plasmonic Gold/TiO2 Photocatalysts Studied by Electron Paramagnetic Resonance. Top Catal. 2015, 58, 776–782. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Trykowski, G.; Zaleska-Medynska, A. Noble metal modified TiO2 microspheres: Surface properties and photocatalytic activity under UV–vis and visible light. J. Mol. Catal. A Chem. 2016, 423, 191–206. [Google Scholar] [CrossRef]
- Popa, A.; Stefan, M.; Macavei, S.; Muresan, L.E.; Leostean, C.; Floare-Avram, C.V.; Toloman, D. Photoluminescence and Photocatalytic Properties of MWNTs Decorated with Fe-Doped ZnO Nanoparticles. Materials 2023, 16, 2858. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ichinose, I.; Kunitake, T.; Nakao, A. In Situ Synthesis of Noble Metal Nanoparticles in Ultrathin TiO2−Gel Films by a Combination of Ion-Exchange and Reduction Processes. Langmuir 2002, 18, 10005–10010. [Google Scholar] [CrossRef]
- Siwach, O.P.; Sen, P. Fluorescence properties of Ag nanoparticles in water, methanol and hexane. J. Lumin. 2009, 129, 6–11. [Google Scholar] [CrossRef]
- Chrysicopoulou, P.; Davazoglou, D.; Trapalis, C.; Kordas, G. Optical properties of very thin (<100 nm) sol–gel TiO2 films. Thin Solid Film. 1998, 323, 188–193. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Li, Y.; Ma, M.; Chen, W.; Li, L.; Zen, M. Preparation of Ag-doped TiO2 nanoparticles by a miniemulsion method and their photoactivity in visible light illuminations. Mater. Chem. Phys. 2011, 129, 501–505. [Google Scholar] [CrossRef]
- Chen, X.; Luo, W. Optical Spectroscopy of Rare Earth Ion-Doped TiO2 Nanophosphors. J. Nanosci. Nanotechnol. 2010, 10, 1482–1494. [Google Scholar] [CrossRef]
- Selvam, K.; Swaminathan, M. Cost effective one-pot photocatalytic synthesis of quinaldines from nitroarenes by silver loaded TiO. J. Mol. Catal. A Chem. 2011, 351, 52–61. [Google Scholar] [CrossRef]
- Wen, L.; Liu, B.; Liu, C.; Zhao, X. Preparation, characterization and photocatalytic property of Ag-loaded TiO2 powders using photodeposition method. J. Wuhan Univ. Technol. Sci. Ed. 2009, 24, 258–263. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, J.; Cheng, B.; Liu, S. Fabrication and characterization of Ag–TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Appl. Catal. B Environ. 2005, 60, 211–221. [Google Scholar] [CrossRef]
- Falaras, P.; Arabatzis, I.M.; Stergiopoulos, T.; Bernard, M.C. Enhanced activity of silver modified thin-filmTiO2 photocatalysts. Int. J. Photoenergy 2003, 5, 123–130. [Google Scholar] [CrossRef]
- Sobczynski, A. Photoassisted hydrogen production from a methanol-water mixture on platinized Cr2O3-DOPED TiO. J. Mol. Catal. 1987, 39, 43–53. [Google Scholar] [CrossRef]
- Klein, S.M.; Cohen, G.; Cederbaum, A.I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochemistry 1981, 20, 6006–6012. [Google Scholar] [CrossRef] [PubMed]
- Alyani, S.J.; Pirbazari, A.E.; Khalilsaraei, F.E.; Kolur, N.A.; Gilani, N. Growing Co-doped TiO2 nanosheets on reduced graphene oxide for efficient photocatalytic removal of tetracycline antibiotic from aqueous solution and modeling the process by artificial neural network. J. Alloys Compd. 2019, 799, 169–182. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Daza, M.C.; Martínez, F.; Páez-Mozo, E.A.; Guedes, C.; Di Mauro, E. Visible light superoxide radical anion generation by tetra(4-carboxyphenyl)porphyrin/TiO2: EPR characterization. J. Photochem. Photobiol. A Chem. 2010, 215, 172–178. [Google Scholar] [CrossRef]
- Schneider, J.T.; Firak, D.S.; Ribeiro, R.R.; Peralta–Zamora, P. Use of scanenger agents in heterogenous photocatalysis: Truts, half –truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; Cruz, A.; Dunlop, P.S.M.; Anthony Byrne, J.; Dionnysiou, D.D. Use of selected scavangers for the determination of NF—TiO2 reactive oxygen specoes during the degradation of Microcystin—LR under visible light irradiation. J. Mol. A Chem. 2016, 425, 183–189. [Google Scholar] [CrossRef]
- Rengaraj, S.; Li, X.Z. Photocatalytic reduction of nitrate over Fe-modified TiO2. Chemosphere 2007, 66, 930–938. [Google Scholar] [CrossRef]
- Mahboob, I.; Shafiq, I.; Shafique, S.; Akhter, P.; Amjad, U.-E.; Hussain, M.; Park, Y.-K. Effect of active species scavengers in photocatalytic desulfurization of hydrocracker diesel using mesoporous Ag3VO. Chem. Eng. J. 2021, 441, 136063. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Nanan, S. Visible-light-driven photocatalytic degradation of ofloxacin (OFL) antibiotic and Rhodamine B (RhB) dye by solvothermally grown ZnO/Bi2MoO6 heterojunction. J. Colloid Interface Sci. 2020, 582, 412–427. [Google Scholar] [CrossRef]
- Wannakan, K.; Khansamrit, K.; Senasu, T.; Nanan, S. Ultrasound-Assisted Synthesis of a ZnO/BiVO4 S-Scheme Heterojunction Photocatalyst for Degradation of the Reactive Red 141 Dye and Oxytetracycline Antibiotic. ACS Omega 2023, 8, 4835–4852. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.; Pana, O.; Stefan, M.; Toloman, D.; Stan, M.; Leostean, C.; Suciu, R.C.; Vlad, G.; Ulinici, S.; Baisan, G.; et al. Interplay between ferromagnetism and photocatalytic activity generated by Fe3+ ions in iron doped ZnO nanoparticles grown on MWCNTs. Phys. E Low-Dimens. Syst. Nanostructures 2020, 129, 114581–114590. [Google Scholar] [CrossRef]
- Corby, S.; Francàs, L.; Kafizas, A.; Durrant, J.R. Determining the role of oxygen vacancies in the photoelectrocatalytic performance of WO3 for water oxidation. Chem. Sci. 2020, 11, 2907–2914. [Google Scholar] [CrossRef]
- Zhou, W.; Umezawa, N.; Ma, R.; Sakai, N.; Ebina, Y.; Sano, K.; Liu, M.; Ishida, Y.; Aida, T.; Sasaki, T. Spontaneous Direct Band Gap, High Hole Mobility, and Huge Exciton Energy in Atomic-Thin TiO2 Nanosheet. Chem. Mater. 2018, 30, 6449–6457. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]

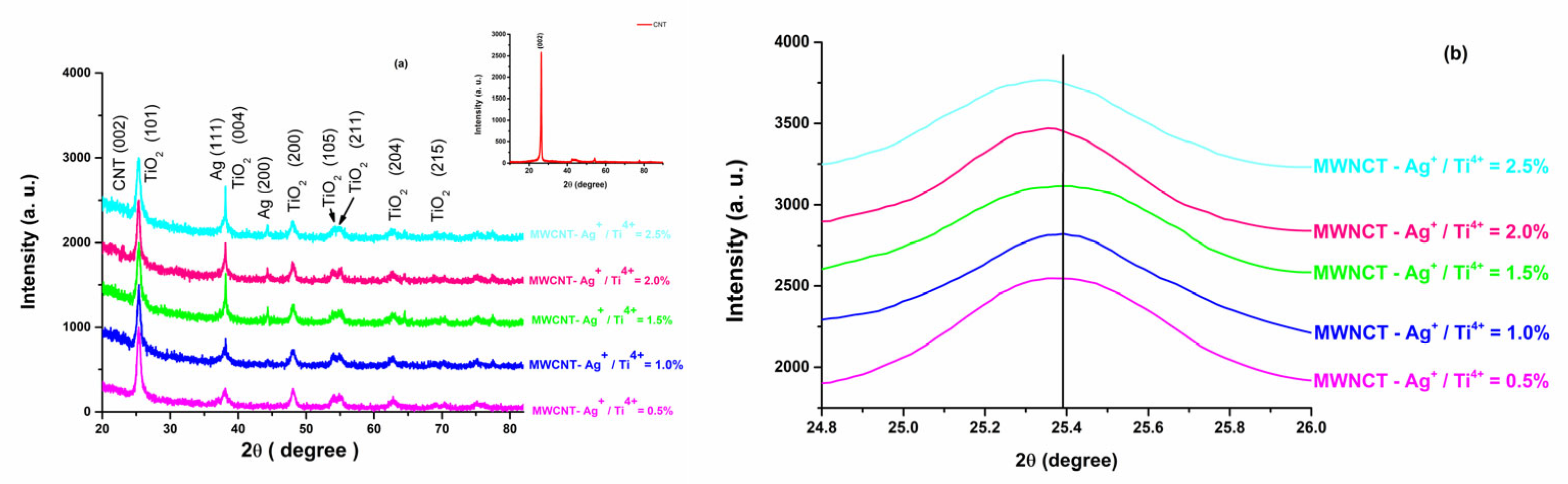




| Sample | Unit Cell Parameter | Cell Volume [Å3] | Effective Crystalline Mean Size, | Microstrains Averaged along the Real Space, | |
|---|---|---|---|---|---|
| a [Å] | c [Å] | ||||
| MWCNT-Ag+/Ti4+ = 0.5% | 3.7717 | 9.4603 | 134.576 | 14.21 | 11.3 |
| MWCNT-Ag+/Ti4+ = 1.0% | 3.7824 | 9.4821 | 135.656 | 14.08 | 12.44 |
| MWCNT-Ag+/Ti4+ = 1.5% | 3.7892 | 9.4888 | 136.240 | 13.86 | 13.86 |
| MWCNT-Ag+/Ti4+ = 2.0% | 3.7920 | 9.4842 | 136.375 | 13.35 | 14.2 |
| MWCNT-Ag+/Ti4+ = 2.5% | 3.7951 | 9.5046 | 136.892 | 13.18 | 15.6 |
| Sample | Eg—Direct Transition |
|---|---|
| MWCNT-Ag+/Ti4+ = 0.5% | 2.77 |
| MWCNT-Ag+/Ti4+ = 1.0% | 2.73 |
| MWCNT-Ag+/Ti4+ = 1.5% | 2.69 |
| MWCNT-Ag+/Ti4+ = 2.0% | 2.46 |
| MWCNT-Ag+/Ti4+ = 2.5% | 2.41 |
| Sample | Photodegradation Rate (%) | R2 | |
|---|---|---|---|
| MWCNT-Ag+/Ti4+ = 0.5% | 47.5 | 3.06 | 0.95097 |
| MWCNT-Ag+/Ti4+ = 1.0% | 65 | 5.21 | 0.95263 |
| MWCNT-Ag+/Ti4+ = 1.5% | 55 | 3.57 | 0.97206 |
| MWCNT-Ag+/Ti4+ = 2.0% | 80 | 8.42 | 0.98006 |
| MWCNT-Ag+/Ti4+ = 2.5% | 72.5 | 4.66 | 0.98538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suciu, R.-C.; Zagrai, M.; Popa, A.; Toloman, D.; Berghian-Grosan, C.; Tudoran, C.; Stefan, M. The Influence of Ag+/Ti4+ Ratio on Structural, Optical and Photocatalytic Properties of MWCNT–TiO2–Ag Nanocomposites. Inorganics 2023, 11, 249. https://doi.org/10.3390/inorganics11060249
Suciu R-C, Zagrai M, Popa A, Toloman D, Berghian-Grosan C, Tudoran C, Stefan M. The Influence of Ag+/Ti4+ Ratio on Structural, Optical and Photocatalytic Properties of MWCNT–TiO2–Ag Nanocomposites. Inorganics. 2023; 11(6):249. https://doi.org/10.3390/inorganics11060249
Chicago/Turabian StyleSuciu, Ramona-Crina, Mioara Zagrai, Adriana Popa, Dana Toloman, Camelia Berghian-Grosan, Cristian Tudoran, and Maria Stefan. 2023. "The Influence of Ag+/Ti4+ Ratio on Structural, Optical and Photocatalytic Properties of MWCNT–TiO2–Ag Nanocomposites" Inorganics 11, no. 6: 249. https://doi.org/10.3390/inorganics11060249
APA StyleSuciu, R.-C., Zagrai, M., Popa, A., Toloman, D., Berghian-Grosan, C., Tudoran, C., & Stefan, M. (2023). The Influence of Ag+/Ti4+ Ratio on Structural, Optical and Photocatalytic Properties of MWCNT–TiO2–Ag Nanocomposites. Inorganics, 11(6), 249. https://doi.org/10.3390/inorganics11060249