Influence of a One-Pot Approach on a Prepared CuS Macro/Nanostructure from Various Molecular Precursors
Abstract
:1. Introduction
2. Results and Discussions
2.1. NMR Spectra Studies of Copper(II) Complexes
2.2. Electronic Spectra of Copper(II) Complexes
2.3. FTIR Results of Copper(II) Complexes
2.4. TGA Results
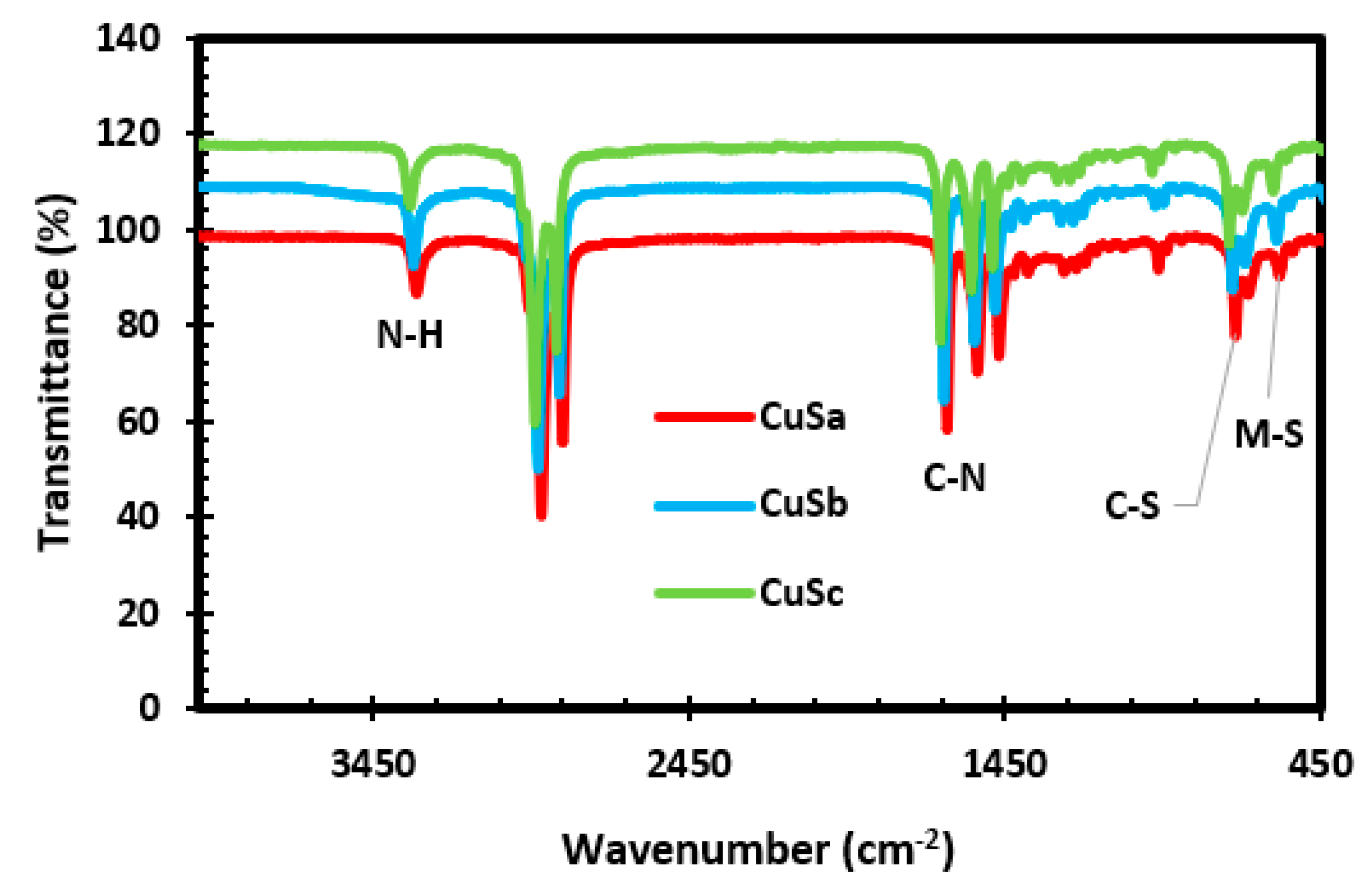
2.5. FTIR Results of CuS Nanoparticles
2.6. XRD Results of CuS Nanoparticles
2.7. UV-Vis of CuS Nanoparticles
2.8. PL Results of CuS Nanoparticles
2.9. HRTEM Results of CuS Nanoparticles
2.10. FESEM and EDS Results of CuS Macro/Nanoparticles
2.11. Cyclic Voltammetry Analysis of CuSa, CuSb, and CuSc Photosensitizers
2.12. Current–Voltage Analysis of CuSa, CuSb, and CuSc Photosensitizers
3. Experimental Section
3.1. Materials
3.2. Ammonium N-Aniline-4-Dithiocarbamate and N-Piperidine-1-Dithiocarbamate Preparation
3.3. Preparation of Copper Complexes: Anil-Piper-Cu(DTC)1, Anil-Cu(DTC)2 and Piper-Cu(DTC)3
3.4. Preparation of CuSa, CuSb, and CuSc Nanoparticles
3.5. Assembling of DSSCs
3.6. Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agoro, M.A.; Mbese, J.Z.; Meyer, E.L.; Onyenankeya, K. Electrochemical signature of CuS photosensitizers thermalized from alkyldithiocarbamato Cu(II) molecular precursors for quantum dots sensitized solar cells. Mater. Lett. 2021, 285, 129191. [Google Scholar] [CrossRef]
- Agoro, M.A.; Meyer, E.L.; Mbese, J.Z.; Manu, K. Electrochemical fingerprint of CuS-hexagonal chemistry from (bis(N-1,4-Phenyl-N-(4-morpholinedithiocarbamato) copper(II) complexes) as photon absorber in quantum-dot/dye-sensitised solar cells. Catalysts 2020, 10, 300. [Google Scholar] [CrossRef] [Green Version]
- Sáez, L.; Molina, J.; Florea, D.I.; Planells, E.M.; Cabeza, M.C.; Quintero, B. Characterization of l-cysteine capped CdTe quantum dots and application to test Cu(II) deficiency in biological samples from critically ill patients. Anal. Chim. Acta 2013, 785, 111–118. [Google Scholar] [CrossRef]
- Du, W.; Liao, L.; Yang, L.; Qin, A.; Liang, A. Aqueous synthesis of functionalized copper sulfide quantum dots as near-infrared luminescent probes for detection of Hg2+, Ag+ and Au3+. Sci. Rep. 2017, 7, 11451. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, M.; Jiang, Y.; Chen, M.; Ding, Y.; Liu, Q. A facile preparation of montmorillonite-supported copper sulfide nanocomposites and their application in the detection of H2O2. Sens. Actuators B Chem. 2017, 239, 28–35. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, B.; Singh, K.; Kumar, R.; Chand, S. Study of precursor-dependent CuS nanostructures: Crystallographic, morphological, optical and photocatalytic activity. Bull. Mater. Sci. 2021, 44, 268. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, Y.; Wang, D.; Chen, C.; Peng, Q.; Li, Y. Ultra-thin Cu2S nanosheets: Effective cocatalysts for photocatalytic hydrogen production. Chem. Commun. 2015, 51, 13305–13308. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Zhou, B.; Law, W.C.; Cartwright, A.N.; Swihart, M.T. Size-controlled synthesis of Cu2-xE (E= S, Se) nanocrystals with strong tunable near-infrared localized surface plasmon resonance and high conductivity in thin films. Adv. Funct. Mater. 2013, 23, 1256–1264. [Google Scholar] [CrossRef]
- Wu, Y.; Wadia, C.; Ma, W.; Sadtler, B.; Alivisatos, A.P. Synthesis and photovoltaic application of copper(I) sulfide nanocrystals. Nano Lett. 2008, 8, 2551–2555. [Google Scholar] [CrossRef]
- Tu, X.; Ge, L.; Deng, L.; Zhang, L. Morphology Adjustment and Optimization of CuS as Enzyme Mimics for the High Efficient Colorimetric Determination of Cr (VI) in Water. Nanomaterials 2022, 12, 2087. [Google Scholar] [CrossRef]
- Swaidan, A.; Borthakur, P.; Boruah, P.K.; Das, M.R.; Barras, A.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Szunerits, S.; Boukherroub, R. A facile preparation of CuS-BSA nanocomposite as enzyme mimics: Application for selective and sensitive sensing of Cr (VI) ions. Sens. Actuators B Chem. 2019, 294, 253–262. [Google Scholar] [CrossRef]
- Hu, X.S.; Shen, Y.; Xu, L.H.; Wang, L.M.; Lu, L.S.; Zhang, Y.T. Preparation of flower-like CuS by solvothermal method for photocatalytic, UV protection and EMI shielding applications. Appl. Surf. Sci. 2016, 385, 162–170. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, S.; Liang, Y.; Li, Z.; Cui, Z.; Yang, X.; Inoue, A. Nanoporous CuS with excellent photocatalytic property. Sci. Rep. 2015, 5, 18125. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Han, G.; Li, M. Fabrication of copper sulfide microstructures with the bottle-and thorny rod-shape. Mater. Chem. Phys. 2010, 120, 431–437. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Q.; Cheng, J.H.; Meng, Q.H.; Jiao, Y. Preparation and characteristics of porous CuS microspheres consisted of polycrystalline nanoslices. Powder Technol. 2010, 199, 139–143. [Google Scholar] [CrossRef]
- Nelwamondo, S.M.M.; Moloto, M.J.; Krause, R.W.M.; Moloto, N. Synthesis and characterization of alanine-capped water soluble copper sulphide quantum dots. Mater. Lett. 2012, 75, 161–164. [Google Scholar] [CrossRef]
- Chen, L.; Shang, Y.; Liu, H.; Hu, Y. Synthesis of CuS nanocrystal in cationic gemini surfactant W/O microemulsion. Mater. Des. 2010, 31, 1661–1665. [Google Scholar] [CrossRef]
- Ding, T.Y.; Wang, M.S.; Guo, S.P.; Guo, G.C.; Huang, J.S. CuS nanoflowers prepared by a polyol route and their photocatalytic property. Mater. Lett. 2008, 62, 4529–4531. [Google Scholar] [CrossRef]
- Roy, P.; Mondal, K.; Srivastava, S.K. Synthesis of twinned CuS nanorods by a simple wet chemical method. Cryst. Growth Des. 2008, 8, 1530–1534. [Google Scholar] [CrossRef]
- Saranya, M.; Nirmala Grace, D.A. Hydrothermal synthesis of CuS nanostructures with different morphology. J. Nano Res. 2012, 18, 43–51. [Google Scholar] [CrossRef]
- Saranya, M.; Ramachandran, R.; Samuel, E.J.J.; Jeong, S.K.; Grace, A.N. Enhanced visible light photocatalytic reduction of organic pollutant and electrochemical properties of CuS catalyst. Powder Technol. 2015, 279, 209–220. [Google Scholar] [CrossRef]
- Knapp, C.E.; Carmalt, C.J. Solution based CVD of main group materials. Chem. Soc. Rev. 2016, 45, 1036–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, P.B.; McGregor, I.J.; Bourke, S.; Burkitt-Gray, M.; Fairclough, S.; Ma, M.T.; Hogarth, G.; Thanou, M.; Long, N.; Green, M. An atom efficient, single-source precursor route to plasmonic CuS nanocrystals. Nanoscale Adv. 2019, 1, 522–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, S.L.; Bailey, S.G.; Raffaelle, R.P.; Banger, K.K.; Hepp, A.F. Nanocrystalline chalcopyrite materials (CuInS2 and CuInSe2) via low-temperature pyrolysis of molecular single-source precursors. Chem. Mater. 2003, 15, 3142–3147. [Google Scholar] [CrossRef] [Green Version]
- Banger, K.K.; Jin, M.H.C.; Harris, J.D.; Fanwick, P.E.; Hepp, A.F. A new facile route for the preparation of single-source precursors for bulk, thin-film, and nanocrystallite I−III−VI semiconductors. Inorg. Chem. 2003, 42, 7713–7715. [Google Scholar] [CrossRef]
- Andrew, F.P.; Ajibade, P.A. Synthesis, characterization and anticancer studies of bis (1-phenylpiperazine dithiocarbamato) Cu(II), Zn(II) and Pt(II) complexes: Crystal structures of 1-phenylpiperazine dithiocarbamato-S, S′ zinc(II) and Pt(II). J. Mol. Struct. 2018, 1170, 24–29. [Google Scholar] [CrossRef]
- Agoro, M.A.; Meyer, E.L. The formation of SnS nanorods orthorhombic phases grown from different molecular precursors. Results Chem. 2023, 5, 100690. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Duraisamy, S.; Kasi, M.; Kandasamy, S.; Sarkar, R.; Kumarasamy, A. Syntheses, physicochemical characterization, antibacterial studies on potassium morpholine dithiocarbamate nickel (II), copper (II) metal complexes and their ligands. Heliyon 2019, 5, 01687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agoro, M.A.; Meyer, E.L. Proficient One-Step Heat-Up Synthesis of Manganese Sulfide Quantum Dots for Solar Cell Applications. Molecules 2022, 27, 6678. [Google Scholar] [CrossRef]
- Kumar, L.V.; Nath, G.R. Synthesis and Characterization Studies of Cobalt(II), Nickel(II), Copper(II) and Zinc(II) Complexes of Carboxymethyl-N-Methyl-N-Phenyl Dithiocarbamate. Orient. J. Chem. 2018, 34, 3064. [Google Scholar] [CrossRef] [Green Version]
- Gopal, K.V.; Jyothi, P.S.; Raju, P.A.G.; Rameshbabu, K.; Sreeramulu, J. Synthesis and characterization of 2-amino pyridine dithiocarbamate ligand and its Cu(II), Co(II) metal complexes. J. Chem. Pharma Res. 2013, 5, 50–59. [Google Scholar]
- Agoro, M.A.; Meyer, E.L. Roles of TOPO Coordinating Solvent on Prepared Nano-Flower/Star and Nano-Rods Nickel Sulphides for Solar Cells Applications. Nanomaterials 2022, 12, 3409. [Google Scholar] [CrossRef]
- Botha, N.L.; Ajibade, P.A.; Ashafa, A.O. Synthesis, spectroscopic characterization, antifungal and antibacterial studies of copper(II) dithiocarbamate complexes. J. Pharm. Sci. 2018, 10, 2111–2114. [Google Scholar]
- Onwudiwe, D.C.; Ajibade, P.A. Synthesis, characterization and thermal studies of Zn(II), Cd(II) and Hg(II) complexes of N-methyl-N-phenyldithiocarbamate: The single crystal structure of [(C6H5)(CH3) NCS2] 4Hg2. Int. J. Mol. Sci. 2011, 12, 1964–1978. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Bhattacharya, S. Synthesis and characterization of some triorgano, diorgano, monoorganotin and a triorganolead heteroaromatic dithiocarbamate complexes. J. Organomet. Chem. 2012, 700, 69–77. [Google Scholar] [CrossRef]
- Agoro, M.A.; Meyer, E.L. FeS/FeS2 nanoscale structures synthesized in one step from Fe(ll) dithiocarbamate complexes as a single source precursor. Front. Chem. 2022, 1500, 1035594. [Google Scholar] [CrossRef]
- Tetyana, P.; Mphuthi, N.; Jijana, A.N.; Moloto, N.; Shumbula, P.M.; Skepu, A.; Vilakazi, L.S.; Sikhwivhilu, L. Synthesis, Characterization, and Electrochemical Evaluation of Copper Sulfide Nanoparticles and Their Application for Non-Enzymatic Glucose Detection in Blood Samples. Nanomaterials 2023, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Babar, D.G.; Garje, S.S. Ligand-based stoichiometric tuning in copper sulfide nanostructures and their catalytic ability. Appl. Nanosci. 2019, 9, 353–367. [Google Scholar] [CrossRef]
- Mnqiwu, K.; Xaba, T.; Moloto, M.; Mubiayi, K.; Mofokeng, T. Effect of Concentration on Synthesis of Organic Passivated Cu2-xS Nanoparticles from N-Pyrrolidine Dithiocarbamate Molecular Precursor. Asian J. Chem. 2018, 30, 1978–1982. [Google Scholar] [CrossRef]
- Han, L.; Li, H.; Lei, Y.; Cao, D. Preparation and transparent heat insulating properties of aqueous acrylic–amino–alkyd coatings with CuS nanoplates. J. Mater. Sci. Mater. 2017, 28, 14596–14604. [Google Scholar] [CrossRef]
- Matos, R.; Kuźniarska-Biernacka, I.; Rocha, M.; Belo, J.H.; Araújo, J.P.; Estrada, A.C.; Lopes, J.L.; Shah, T.; Korgel, B.A.; Pereira, C.; et al. Design and photo-Fenton performance of Graphene/CuS/Fe3O4 tertiary nanocomposites for Rhodamine B degradation. Catal. Today 2023, 418, 114132. [Google Scholar] [CrossRef]
- Liang, L.; Peng, S.; Yuan, Z.; Wei, C.; He, Y.; Zheng, J.; Gu, Y.; Chen, H. Biocompatible tumor-targeting nanocomposites based on CuS for tumor imaging and photothermal therapy. RSC Adv. 2018, 8, 6013–6026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Yang, G.; Zhang, Y.; Zhang, S.; Zhang, P. A simple preparation of HDA-CuS nanoparticles and their tribological properties as a water-based lubrication additive. Tribol. Lett. 2019, 67, 1–11. [Google Scholar] [CrossRef]
- Raj, S.I.; Jaiswal, A.; Uddin, I. Ultrasmall aqueous starch-capped CuS quantum dots with tunable localized surface plasmon resonance and composition for the selective and sensitive detection of mercury(II) ions. RSC Adv. 2020, 10, 14050–14059. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Tripathy, E.C. Antibacterial and Photocatalytic Properties of Wet Chemically Fabricated CuS/Graphene nanocomposite. Int. J. Sci. Technol. Res. 2020, 9, 265. [Google Scholar]
- Arshad, M.; Wang, Z.; Nasir, J.A.; Amador, E.; Jin, M.; Li, H.; Chen, Z.; ur Rehman, Z.; Chen, W. Single source precursor synthesized CuS nanoparticles for NIR phototherapy of cancer and photodegradation of organic carcinogen. J. Photochem. Photobiol. B, Biol. 2021, 214, 112084. [Google Scholar] [CrossRef] [PubMed]
- Duran-García, E.I.; Martínez-Santana, J.; Torres-Gómez, N.; Vilchis-Nestor, A.R.; García-Orozco, I. Copper sulfide nanoparticles produced by the reaction of N-alkyldithiocarbamatecopper(II) complexes with sodium borohydride. Mater. Chem. Phys. 2021, 269, 124743. [Google Scholar] [CrossRef]
- Botha, N.L.; Ajibade, P.A. Optical and structural characterization of copper sulphide nanoparticles from copper(II) piperidine dithiocarbamate. Opt. Quantum Electron. 2020, 52, 337. [Google Scholar] [CrossRef]
- Ravele, M.P.; Oyewo, O.A.; Onwudiwe, D.C. Controlled Synthesis of CuS and Cu9S5 and Their Application in the Photocatalytic Mineralization of Tetracycline. Catalysts 2021, 11, 899. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Oluwalana, A.E. Enhanced photocatalytic degradation of ternary dyes by copper sulfide nanoparticles. Nanomaterials 2021, 11, 2000. [Google Scholar] [CrossRef]
- Vinoth, G.; Sakthivel, P.; Abinaya, S.; Kadiresan, M.R. Crystallographic, optical, photoluminescence and electrical properties of CuS quantum dots: Influence of ethylenediamine. Results Opt. 2022, 9, 100283. [Google Scholar] [CrossRef]
- Kumar, S.; Ganguli, A.K. Enhanced photoelectrochemical water splitting and mitigation of organic pollutants under visible light with NaNbO3@ CuS Core-Shell heterostructures. Appl. Surf. Sci. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Srivishnu, K.S.; Prasanthkumar, S.; Giribabu, L. Cu(ii/i) redox couples: Potential alternatives to traditional electrolytes for dye-sensitized solar cells. Mater. Adv. 2021, 2, 1229–1247. [Google Scholar] [CrossRef]
- Coz, E.; Artíñano, B.; Robinson, A.L.; Casuccio, G.S.; Lersch, T.L.; Pandis, S.N. Individual particle morphology and acidity. Aerosol. Sci. Technol. 2008, 42, 224–232. [Google Scholar] [CrossRef]
- Kusior, A.; Jelen, P.; Mazurkow, J.; Nieroda, P.; Radecka, M. Synthesis of anisotropic Cu 2− x S-based nanostructures by thermal oxidation. J. Therm. Anal. Calorim. 2019, 138, 4321–4329. [Google Scholar] [CrossRef] [Green Version]
- Fayemi, O.E.; Pooe, O.G.; Adesanya, F.A.; Ejidike, I.P. Spectroscopy and Cyclic Voltammetry Properties of SPEEK/CuO Nanocomposite at Screen-Printed Gold Electrodes. Nanomaterials 2022, 12, 1825. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M.; Zarghami, Z.; Salavati-Niasari, M. Facile and novel chemical synthesis, characterization, and formation mechanism of copper sulfide (Cu2S, Cu2S/CuS, CuS) nanostructures for increasing the efficiency of solar cells. J. Phy. Chem. C. 2016, 120, 2096–2108. [Google Scholar] [CrossRef]
- Yadav, R.; Waghadkar, Y.; Kociok-Köhn, G.; Kumar, A.; Rane, S.B.; Chauhan, R. Transition metal ferrocenyl dithiocarbamates functionalized dye-sensitized solar cells with hydroxy as an anchoring group. Opt. Mater. 2016, 62, 176–183. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Han, Y.; Chen, E.; Guo, P.; Zhang, W.; An, M.; Pan, Z.; Xu, Q.; Guo, X.; et al. High-performance γ-MnO2 dual-core, pair-hole fiber for ultrafast photonics. Ultrafast Sci. 2023, 3, 0006. [Google Scholar] [CrossRef]
- Guan, M.; Chen, D.; Hu, S.; Zhao, H.; You, P.; Meng, S. Theoretical insights into ultrafast dynamics in quantum materials. Ultrafast Sci. 2022, 2022, 9767251. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Gao, Y.; Li, X.; Lu, H.; Wang, Y.; Feng, J.J.; Lu, J.; Ma, K.; Chen, X. Porous nickel oxide micron polyhedral particles for high-performance ultrafast photonics. Opt. Laser Technol. 2022, 146, 107546. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Chen, E.; Liu, H.; Shum, P.P.; Chen, X.H. Hydrazone organics with third-order nonlinear optical effect for femtosecond pulse generation and control in the L-band. Opt. Laser Technol. 2022, 151, 108016. [Google Scholar] [CrossRef]
- Li, X.; An, M.; Li, G.; Han, Y.; Guo, P.; Chen, E.; Hu, J.; Song, Z.; Lu, H.; Lu, J. MOF-Derived Porous Dodecahedron rGO-Co3O4 for Robust Pulse Generation. Adv. Mater. Interfaces 2022, 9, 2101933. [Google Scholar] [CrossRef]
- Yu, J.; Wang, W.; Pan, Z.; Du, J.; Ren, Z.; Xue, W.; Zhong, X. Quantum dot sensitized solar cells with efficiency over 12% based on tetraethyl orthosilicate additive in polysulfide electrolyte. J. Mater. Chem. A 2017, 5, 14124–14133. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, C.; Du, Z.; Pan, D.; Zhong, X. Graphene hydrogel-based counter electrode for high efficiency quantum dot-sensitized solar cells. J. Mater. Chem. A 2017, 5, 1614–1622. [Google Scholar] [CrossRef]
- Jiao, S.; Du, J.; Du, Z.; Long, D.; Jiang, W.; Pan, Z.; Li, Y.; Zhong, X. Nitrogen-doped mesoporous carbons as counter electrodes in quantum dot sensitized solar cells with a conversion efficiency exceeding 12%. J. Phys. Chem. 2017, 8, 559–564. [Google Scholar] [CrossRef]
- Kottayi, R.; Maurya, D.K.; Sittaramane, R.; Angaiah, S. Recent Developments in Metal Chalcogenides based Quantum Dot Sensitized Solar Cells. ES Energy Environ. 2022, 18, 1–40. [Google Scholar] [CrossRef]
- Jun, H.K.; Tung, H.T. A Short Overview on Recent Progress in Semiconductor Quantum Dot-Sensitized Solar Cells. J. Nanomater. 2022, 2022, 1382580. [Google Scholar] [CrossRef]
- Kim, H.J.; Myung-Sik, L.; Gopi, C.V.; Venkata-Haritha, M.; Rao, S.S.; Kim, S.K. Cost-effective and morphology controllable PVP based highly efficient CuS counter electrodes for high-efficiency quantum dot-sensitized solar cells. Dalton Trans. 2015, 44, 11340–11351. [Google Scholar] [CrossRef]
- Punnoose, D.; Kumar, C.S.P.; Rao, S.S.; Varma, C.V.T.; Naresh, B.; Reddy, A.E.; Kundarala, N.; Lee, Y.S.; Kim, M.Y.; Kim, H.J. In situ synthesis of CuS nano platelets on nano wall networks of Ni foam and its application as an efficient counter electrode for quantum dot sensitized solar cells. Org. Electron. 2017, 42, 115–122. [Google Scholar] [CrossRef]
- Kim, H.J.; Ko, B.; Gopi, C.V.; Venkata-Haritha, M.; Lee, Y.S. Facile synthesis of morphology dependent CuS nanoparticle thin film as a highly efficient counter electrode for quantum dot-sensitized solar cells. J. Electroanal. Chem. 2017, 791, 95–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Wang, Q.; Zheng, W. One-step synthesized CuS and MWCNTs composite as a highly efficient counter electrode for quantum dot sensitized solar cells. Mater. Des. 2018, 160, 870–875. [Google Scholar] [CrossRef]
- Palve, B.M.; Kadam, V.S.; Jagtap, C.V.; Jadkar, S.R.; Pathan, H.M. A simple chemical route to synthesis the CuSe and CuS counter electrodes for titanium oxide based quantum dot solar cells. J. Mater. Sci. Mater. Electron. 2017, 28, 14394–14401. [Google Scholar] [CrossRef]
- Punnoose, D.; Rao, S.S.; Kim, S.K.; Kim, H.J. Exploring the effect of manganese in lead sulfide quantum dot sensitized solar cell to enhance the photovoltaic performance. RSC Adv. 2015, 5, 33136–33145. [Google Scholar] [CrossRef]
- González-Pedro, V.; Sima, C.; Marzari, G.; Boix, P.P.; Giménez, S.; Shen, Q.; Dittrich, T.; Mora-Seró, I. High performance PbS Quantum Dot Sensitized Solar Cells exceeding 4% efficiency: The role of metal precursors in the electron injection and charge separation. Phys. Chem. Chem. Phys. 2013, 15, 13835–13843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthalif, M.P.A.; Lee, Y.S.; Sunesh, C.D.; Kim, H.J.; Choe, Y. Enhanced photovoltaic performance of quantum dot-sensitized solar cells with a progressive reduction of recombination using Cu-doped CdS quantum dots. Appl. Surf. Sci. 2017, 396, 582–589. [Google Scholar] [CrossRef]
- Dou, Y.; Zhou, R.; Wan, L.; Niu, H.; Zhou, J.; Xu, J.; Cao, G. Nearly monodisperse PbS quantum dots for highly efficient solar cells: An in situ seeded ion exchange approach. Chem. Commun. 2018, 54, 12598–12601. [Google Scholar] [CrossRef]
- Ahmed, R.; Zhao, L.; Mozer, A.J.; Will, G.; Bell, J.; Wang, H. Enhanced electron lifetime of CdSe/CdS quantum dot (QD) sensitized solar cells using ZnSe core–shell structure with efficient regeneration of quantum dots. J. Phys. Chem. C. 2015, 119, 2297–2307. [Google Scholar] [CrossRef]
- Gao, B.; Shen, C.; Yuan, S.; Zhang, B.; Zhang, M.; Yang, Y.; Chen, G. Influence of nanocrystal size on the quantum dots sensitized solar cells’ performance with low temperature synthesized CdSe quantum dots. J. Alloy. Compd. 2014, 612, 323–329. [Google Scholar] [CrossRef]
- Gao, B.; Shen, C.; Zhang, B.; Zhang, M.; Yuan, S.; Yang, Y.; Chen, G. Green synthesis of highly efficient CdSe quantum dots for quantum-dots-sensitized solar cells. J. Appl. Phys. 2014, 115, 193104. [Google Scholar] [CrossRef]
- Quan, L.; Li, W.; Zhu, L.; Geng, H.; Chang, X.; Liu, H. A new in-situ preparation method to FeS counter electrode for quantum dots-sensitized solar cells. J. Power Sources 2014, 272, 546–553. [Google Scholar] [CrossRef]
- Li, Y.; Yin, J.; Chu, C.; Sui, N.; Shi, S.; Wei, J.; Di, F.; Guo, J.; Wang, C.; Xu, W.; et al. Earth-abundant Fe1− x S@ S-doped graphene oxide nano–micro composites as high-performance cathode catalysts for green solar energy utilization: Fast interfacial electron exchange. RSC Adv. 2018, 8, 4340–4347. [Google Scholar] [CrossRef] [Green Version]
- Agoro, M.A.; Meyer, E.L.; Mbese, J.Z.; Fuku, X.; Ahia, C.C. Aliphatic mixed ligands Sn(II) complexes as photon absorbers in quantum dots sensitized solar cell. J. Solid State Chem. 2022, 308, 122890. [Google Scholar] [CrossRef]











| Samples | 2-Theta | Miller’s Index (Lattice Plane) | FWHM | Average Size (nm) from XRD | TEM Particle Size (nm) | Atomic Percentage from EDS | Band Gap (eV) | |
|---|---|---|---|---|---|---|---|---|
| Cu | S | |||||||
| CuSa | 26.2° | (101) | 0.19 | 34.88 | 54.30–167.73 | 5.31 | 1.15 | 2.70 |
| 38.6° | (105) | 0.23 | ||||||
| 52.3° | (108) | 0.26 | ||||||
| CuSb | 27.3° | (101) | 0.19 | 57.25 | 63.02–73.99 | 0.71 | 0.4 | 2.05 |
| 38.5° | (105) | 0.29 | ||||||
| 52.3° | (108) | 0.25 | ||||||
| CuSc | 26.34° | (101) | 0.21 | 25.08 | 105.49–119.22 | 6.3 | 0.35 | 2.87 |
| 38.78° | (105) | 0.39 | ||||||
| 48.96° | (110) | 0.31 | ||||||
| Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitizers | Ep,a (V) | Ep,c (V) | ∆Ep = Ep,a – Ep,c (V) | E° = ½(Ep,a – Ep,c) (V) | ip,a/ip,c | JSC (mA/cm2) | VOC (V) | FF | η (%) |
| CuSa 0 hr | 0.17 | 0.39 | 0.22 | 0.11 | 1.85 | 11 | 0.60 | 0.68 | 4.48 |
| 3 hr | 10 | 0.60 | 0.67 | 4.02 | |||||
| CuSb 0 hr | 0.15 | 0.39 | 0.24 | 0.12 | 0.47 | 10 | 0.61 | 0.76 | 4.63 |
| 3 hr | 10 | 0.60 | 0.67 | 4.02 | |||||
| CuSc 0 hr | 0.17 | 0.39 | 0.22 | 0.11 | 0.44 | 10 | 0.63 | 0.64 | 4.03 |
| 3 hr | 10 | 0.58 | 0.67 | 3.88 | |||||
| Sensitizers | JSC (mA/cm2) | VOC (V) | FF | η (%) | Ref. |
|---|---|---|---|---|---|
| CuS | 12.36 | 0.591 | 0.435 | 3.18 | [68] |
| CuS | 17.8 | 0.61 | 0.54 | 4.93 | [69] |
| CuS2h | 15.52 | 0.612 | 0.452 | 4.29 | [70] |
| CuS/MWCNTs | 18.680 | 0.618 | 0.455 | 5.254 | [71] |
| CuS | 0.71 | 0.58 | 48 | 1.38 | [72] |
| Mn-PbS | 17.34 | 0.53 | 0.48 | 4.25 | [73] |
| Pb(Ac)–Cd(Ac) | 22.28 | 0.416 | 0.453 | 4.20 | [74] |
| CdCuS3 | 9.40 | 0.637 | 0.501 | 3.00 | [75] |
| PbS QD | 25.01 | 0.56 | 67.3 | 9.43 | [76] |
| CdSe/CdS | 13.4 | 580 | 0.51 | 3.99 | [77] |
| CdSe | 10.58 | 0.6 | 0.564 | 3.7 | [78] |
| CdSe | 11.86 | 549.31 | 52 | 3.39 | [79] |
| SnS | 17 | 0.72 | 0.67 | 8.20 | [80] |
| FeS | 20.40 | 0.42 | 0.40 | 3.34 | [81] |
| Fe1−xS | 14.08 | 0.72 | 0.72 | 7.23 | [82] |
| CuSb 0 hr | 10 | 0.61 | 0.76 | 4.63 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agoro, M.A.; Meyer, E.L. Influence of a One-Pot Approach on a Prepared CuS Macro/Nanostructure from Various Molecular Precursors. Inorganics 2023, 11, 266. https://doi.org/10.3390/inorganics11070266
Agoro MA, Meyer EL. Influence of a One-Pot Approach on a Prepared CuS Macro/Nanostructure from Various Molecular Precursors. Inorganics. 2023; 11(7):266. https://doi.org/10.3390/inorganics11070266
Chicago/Turabian StyleAgoro, Mojeed A., and Edson L. Meyer. 2023. "Influence of a One-Pot Approach on a Prepared CuS Macro/Nanostructure from Various Molecular Precursors" Inorganics 11, no. 7: 266. https://doi.org/10.3390/inorganics11070266







