On the Current Status of Ullmann-Type N-Arylation Reactions Promoted by Heterogeneous Catalysts
Abstract
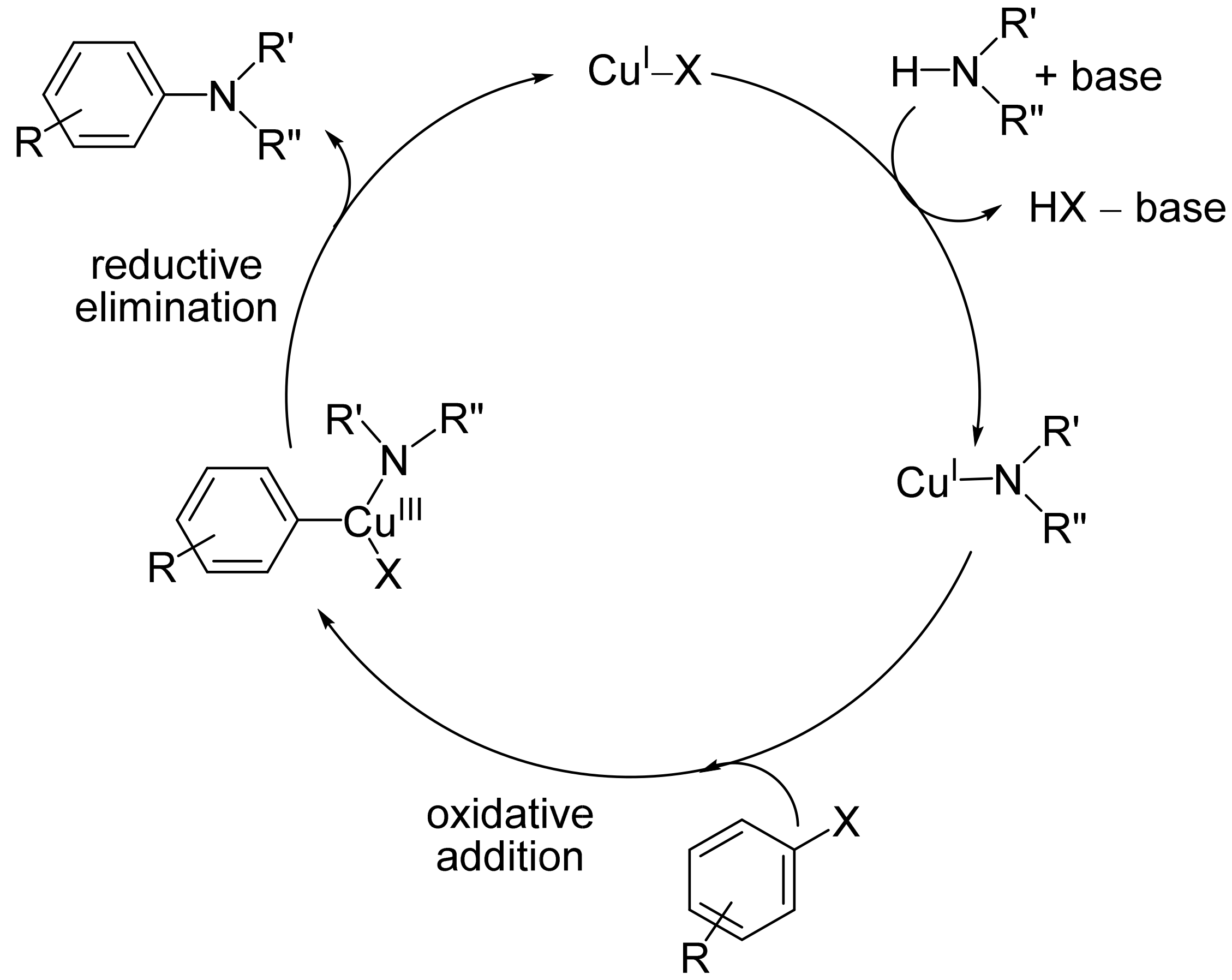
1. Introduction
2. Results
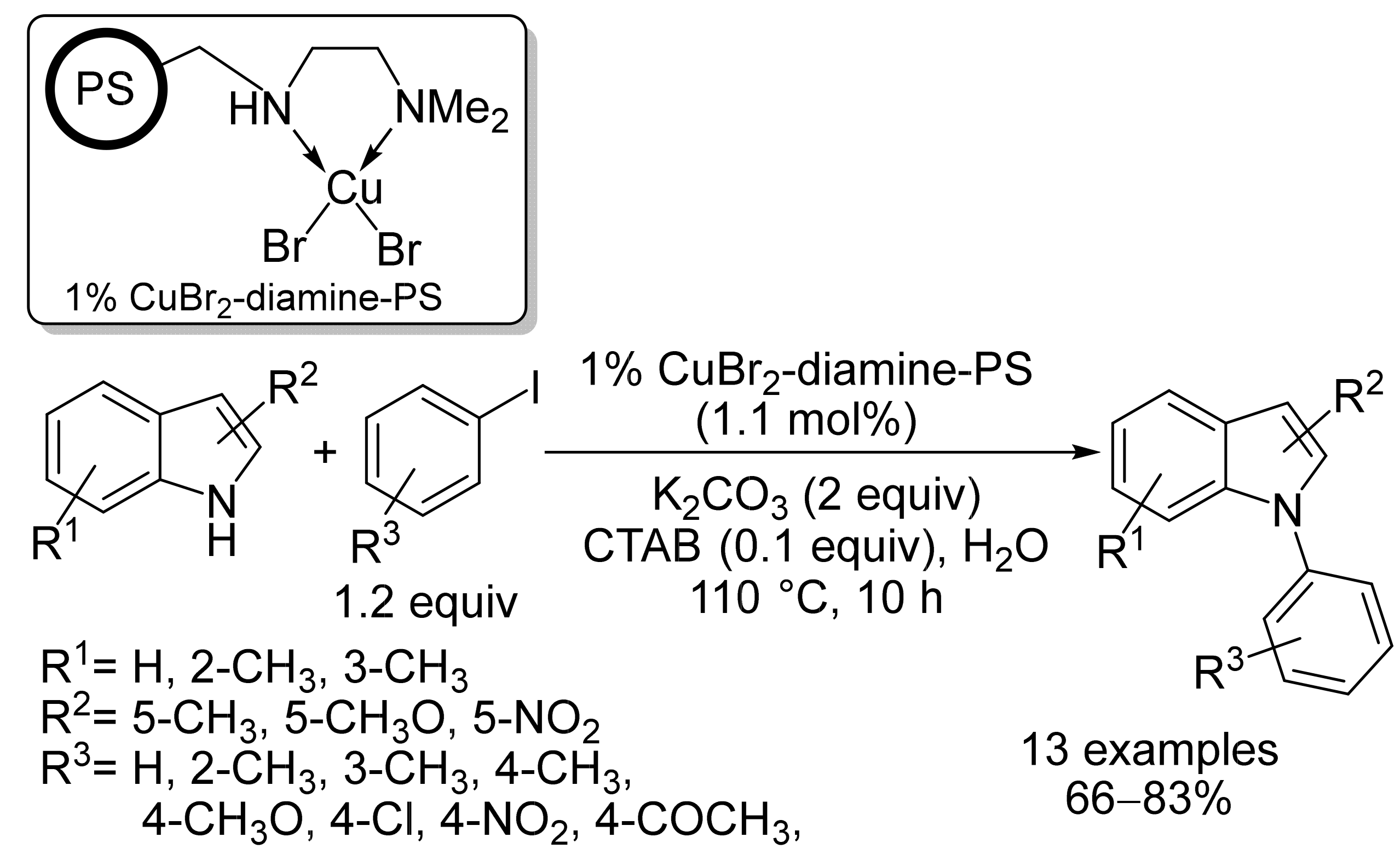
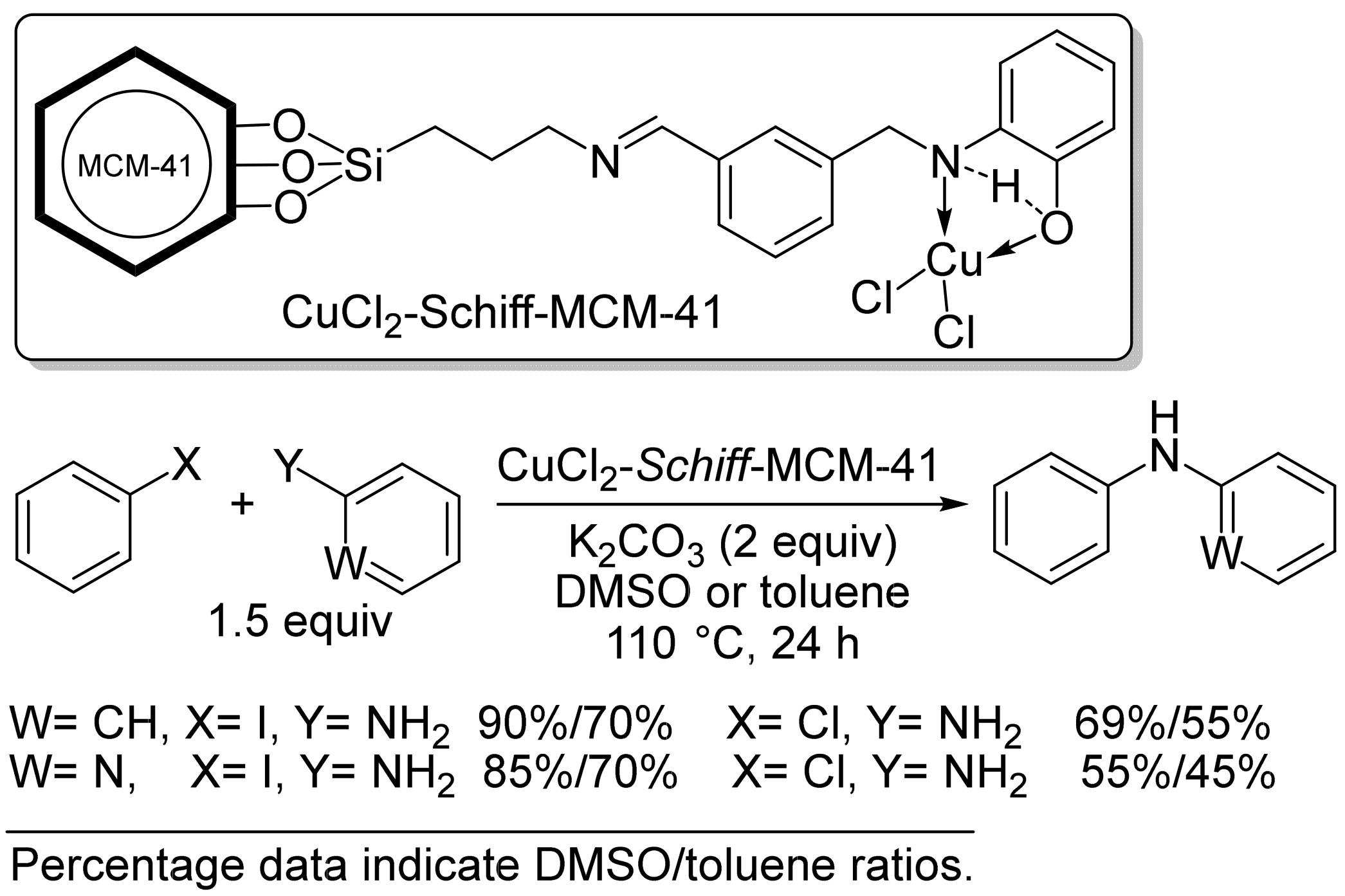
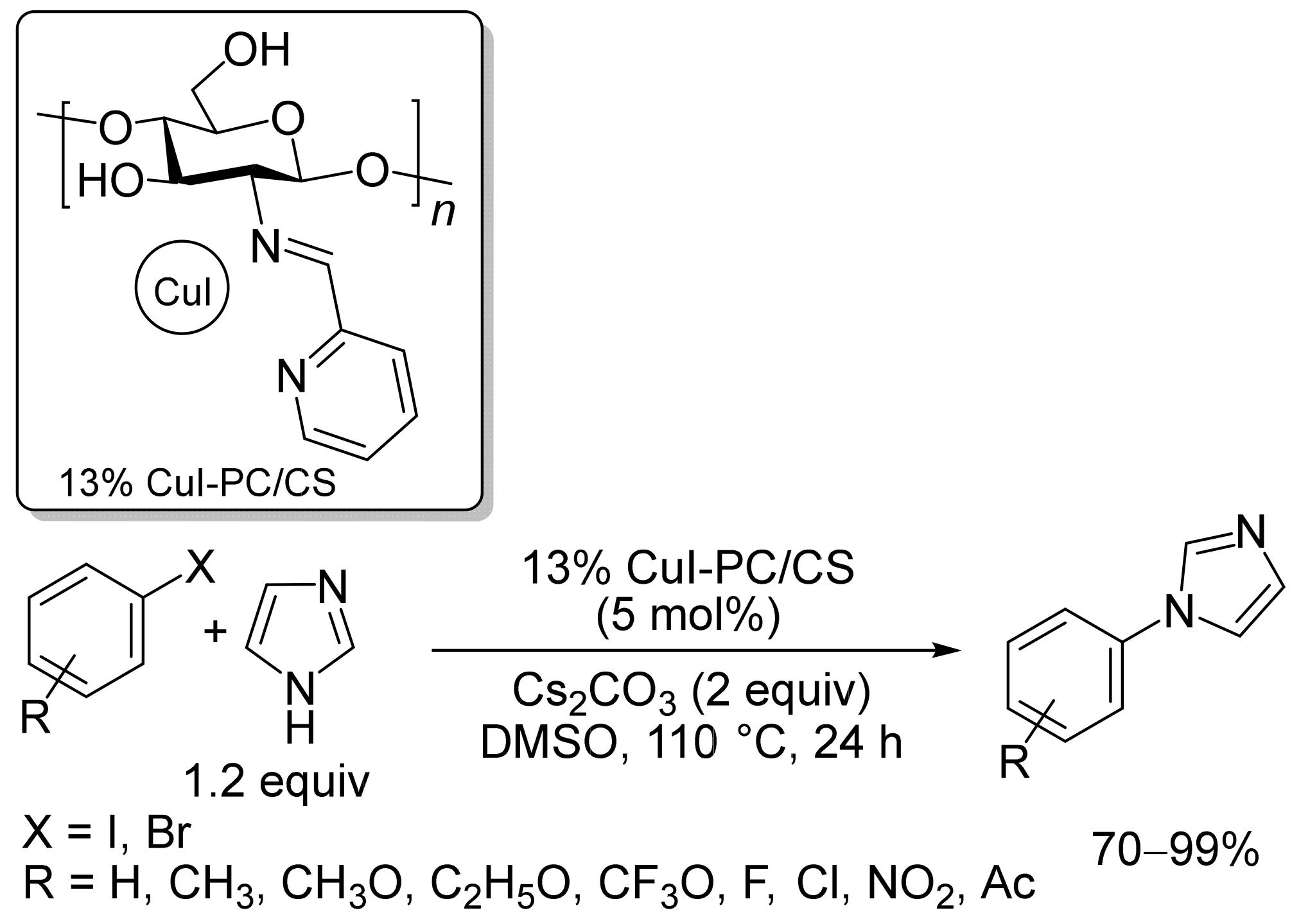
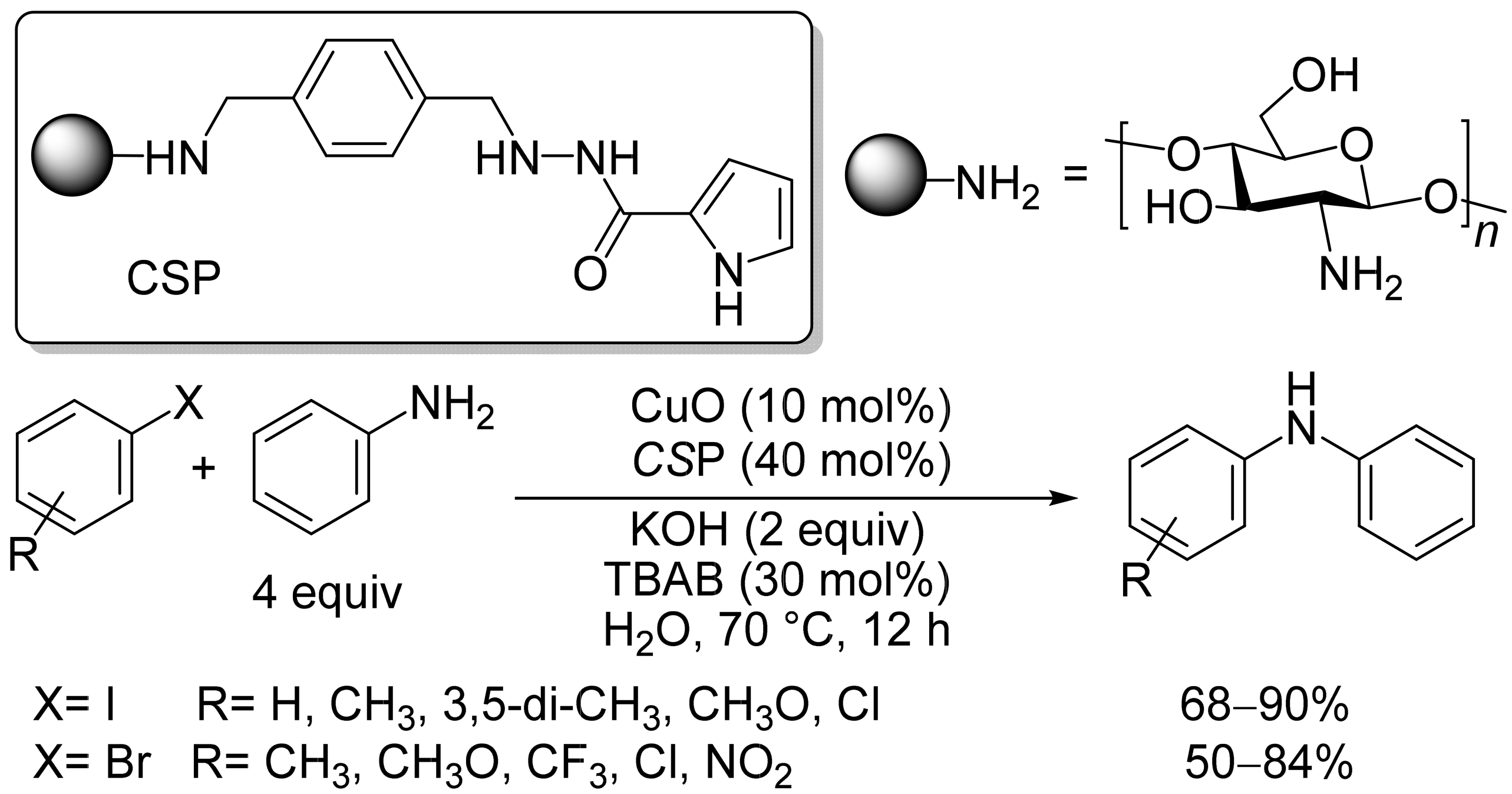
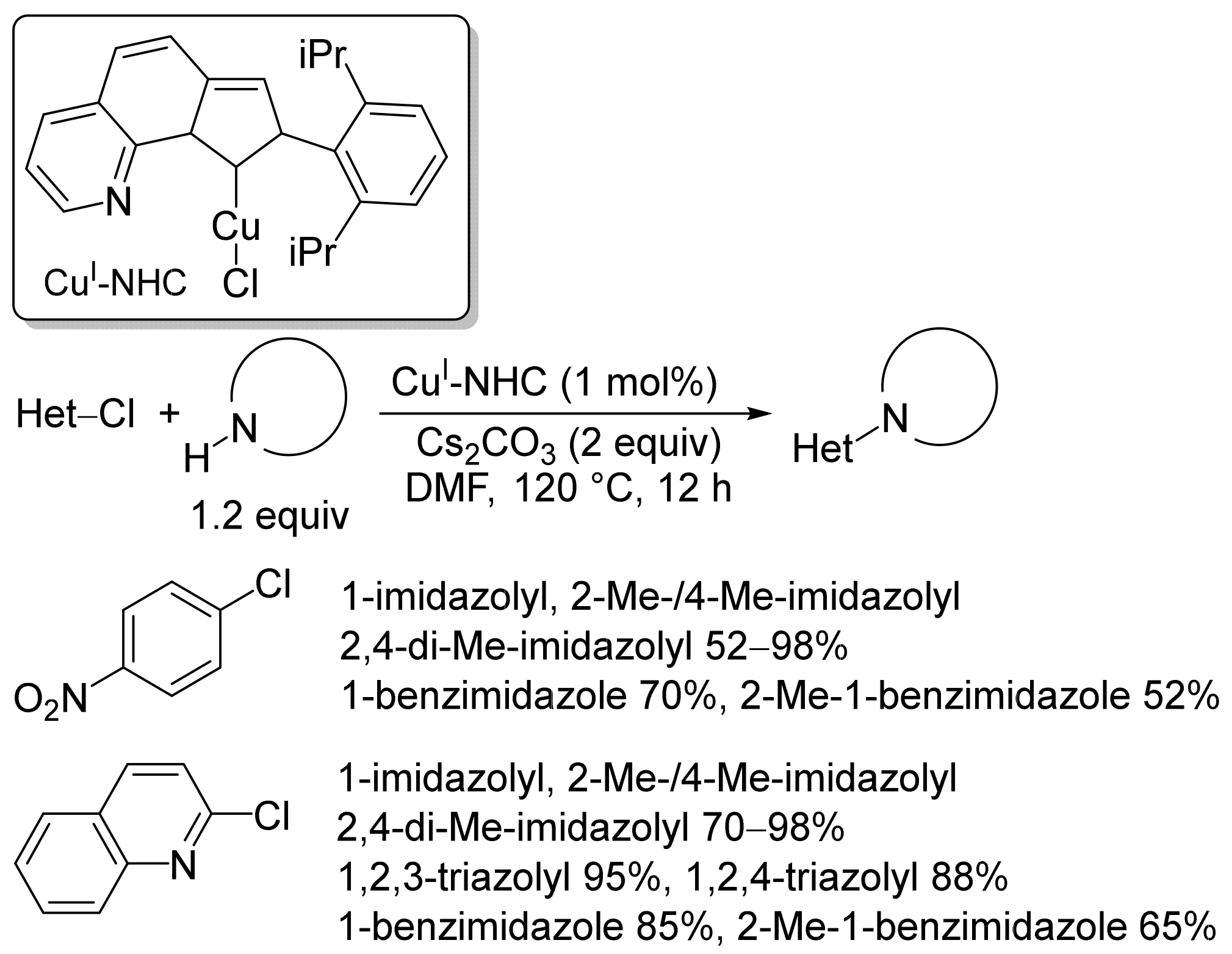
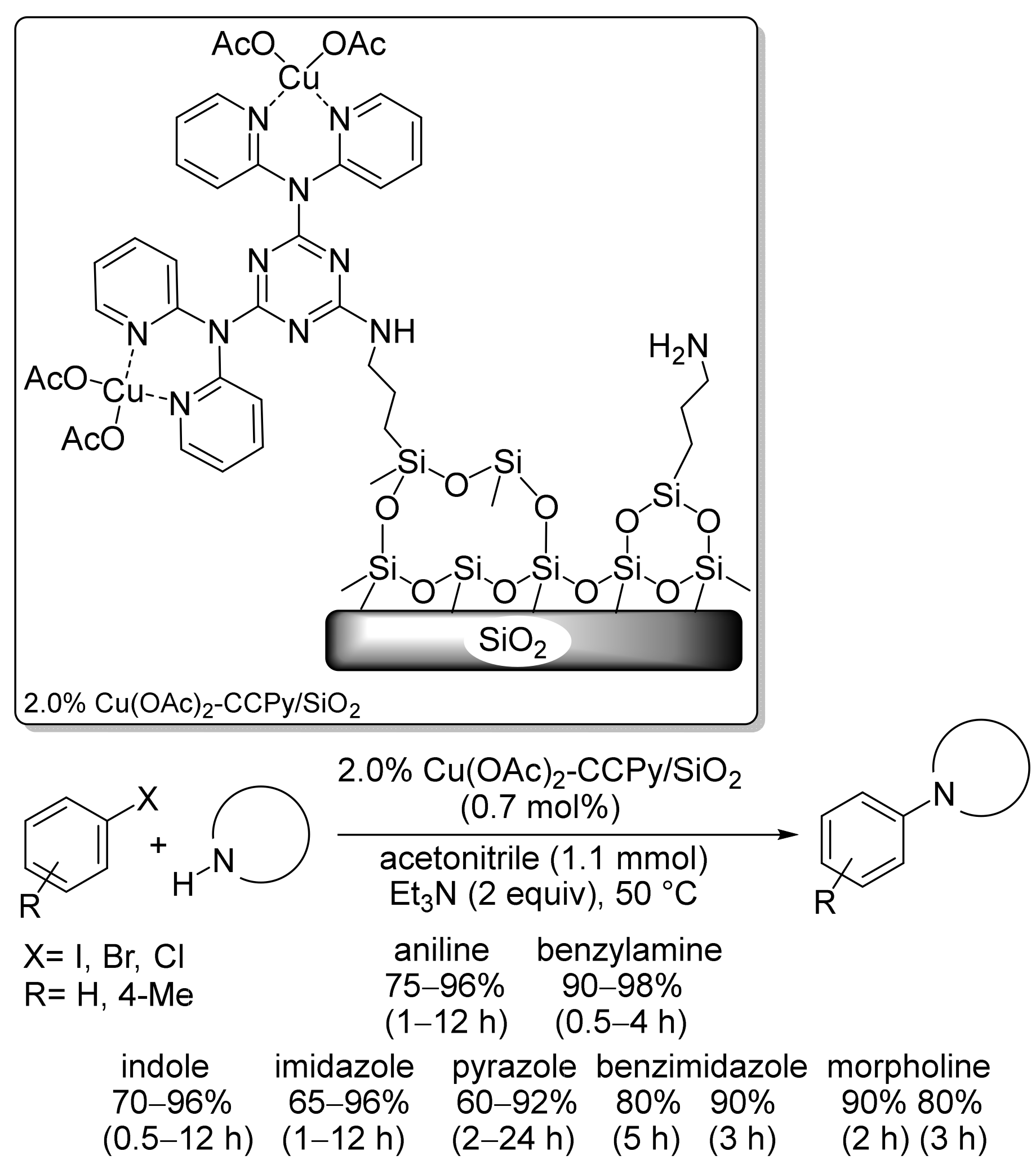
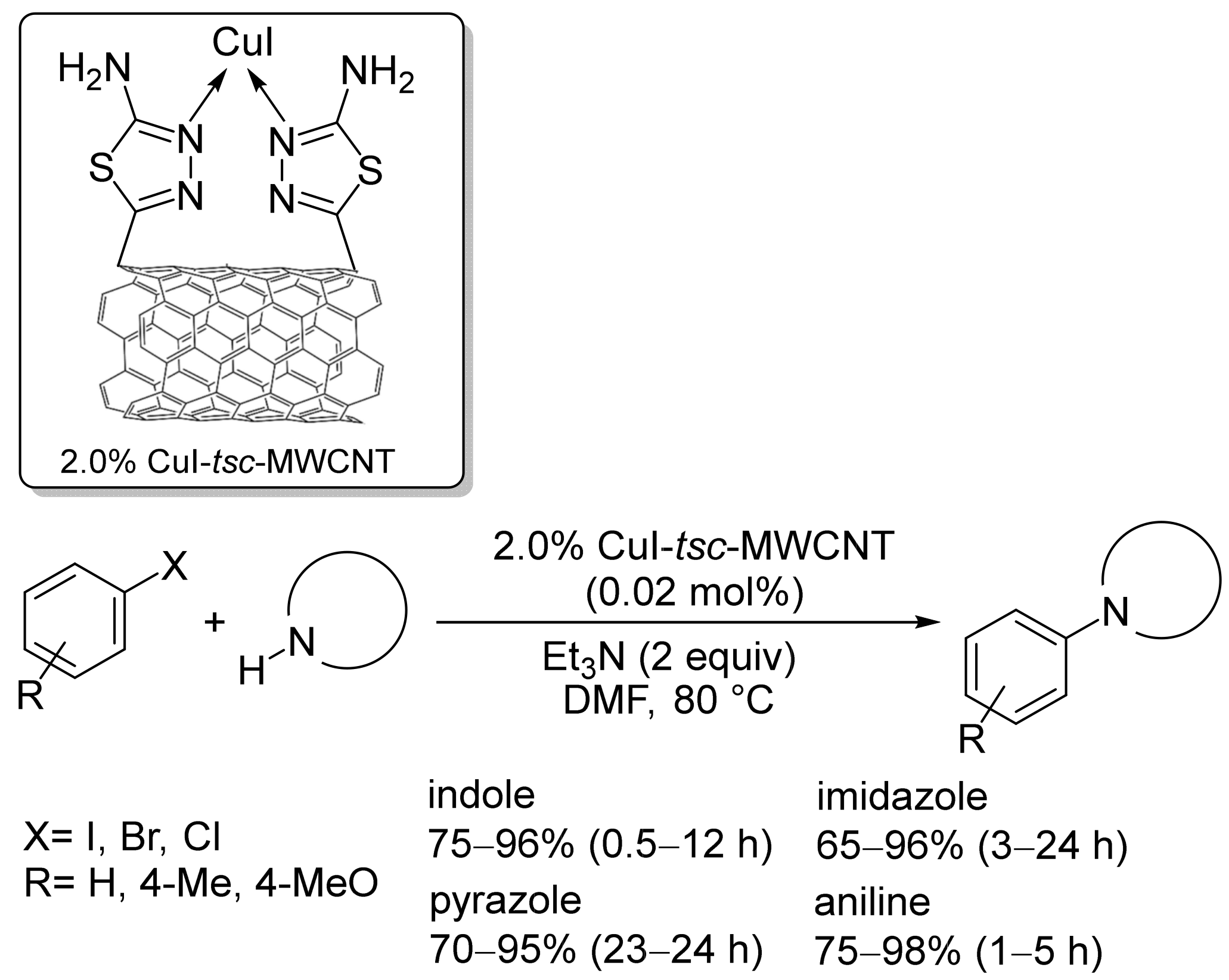
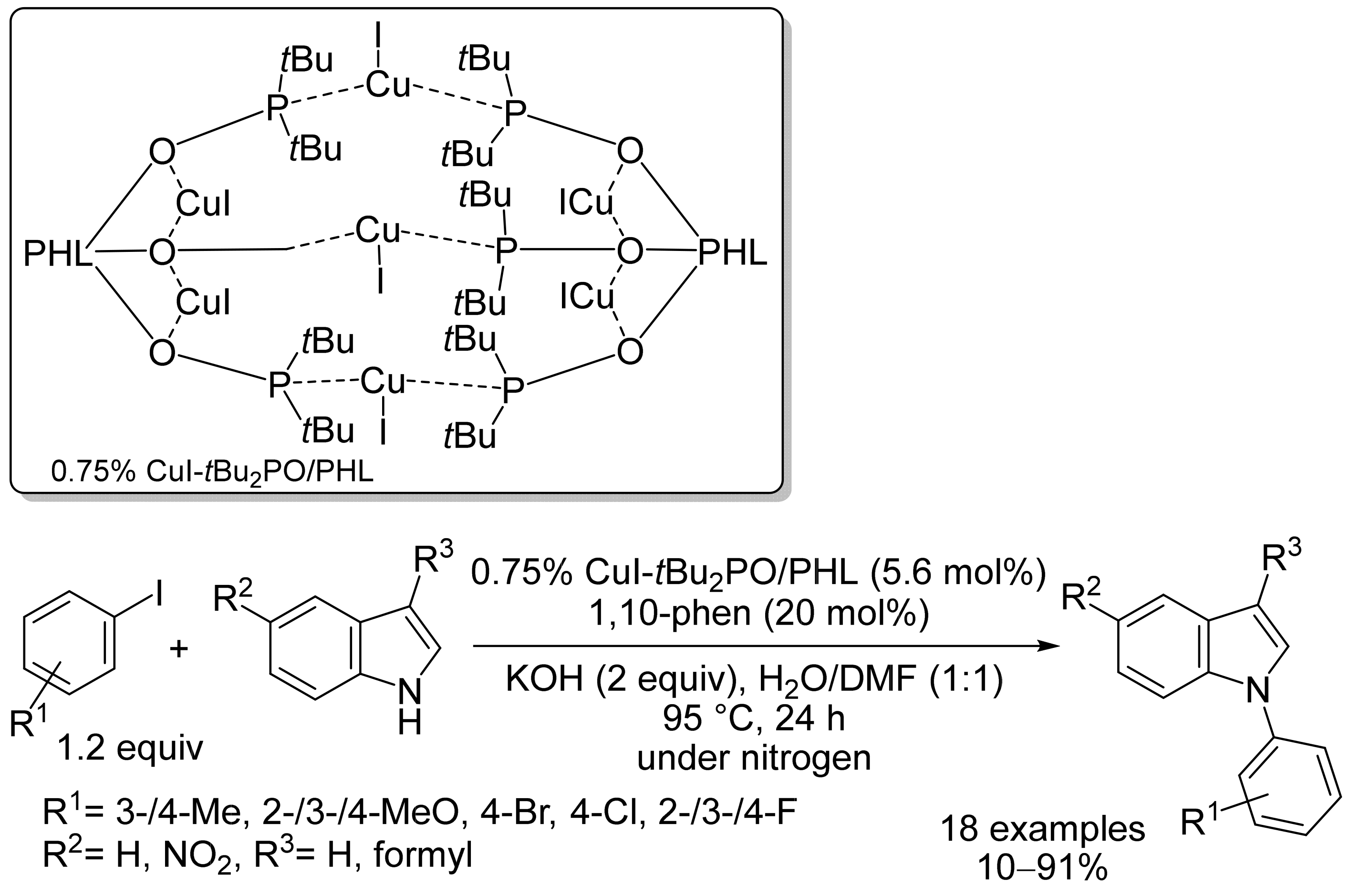
2.1. Copper-Based Catalysts
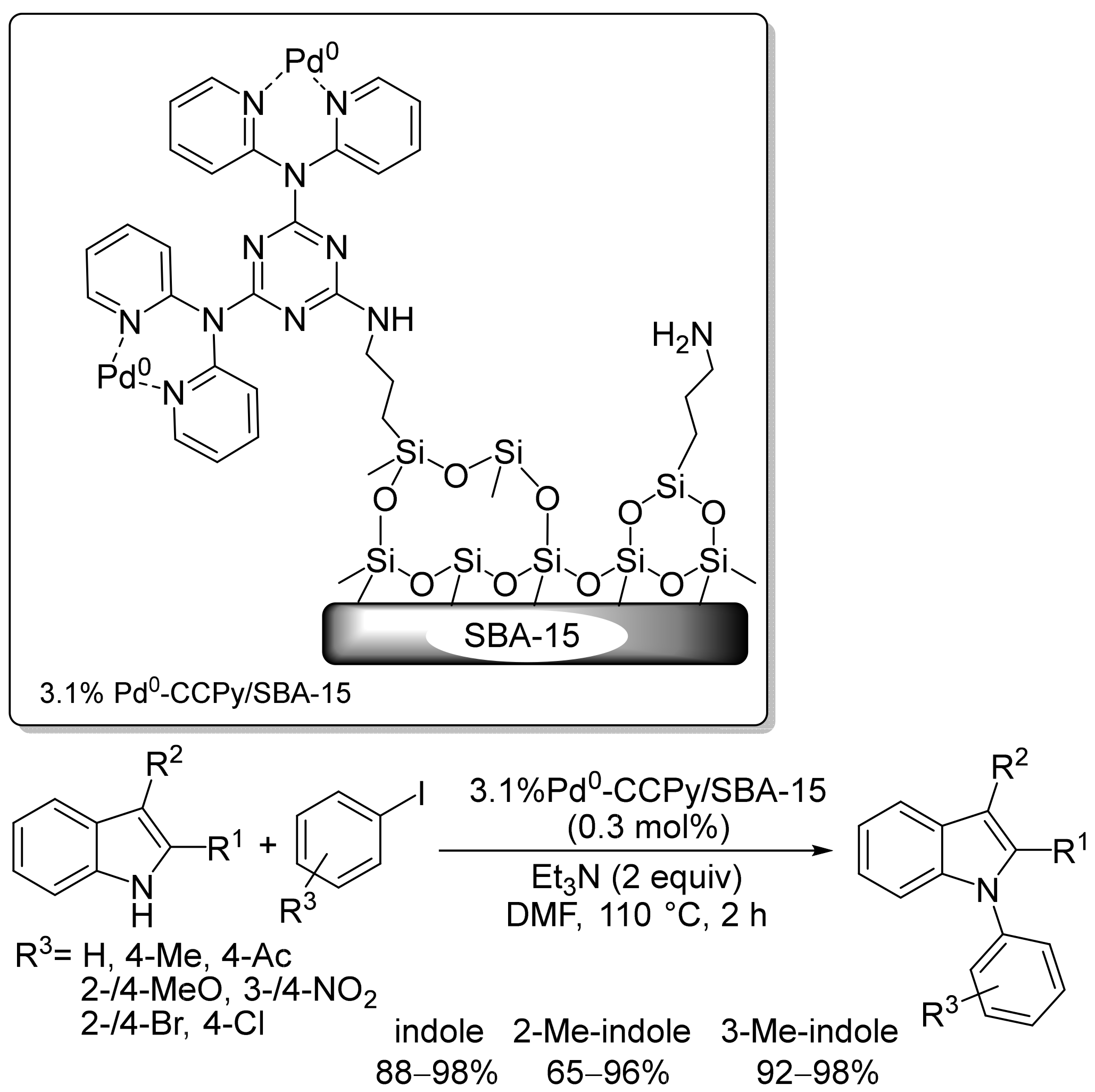
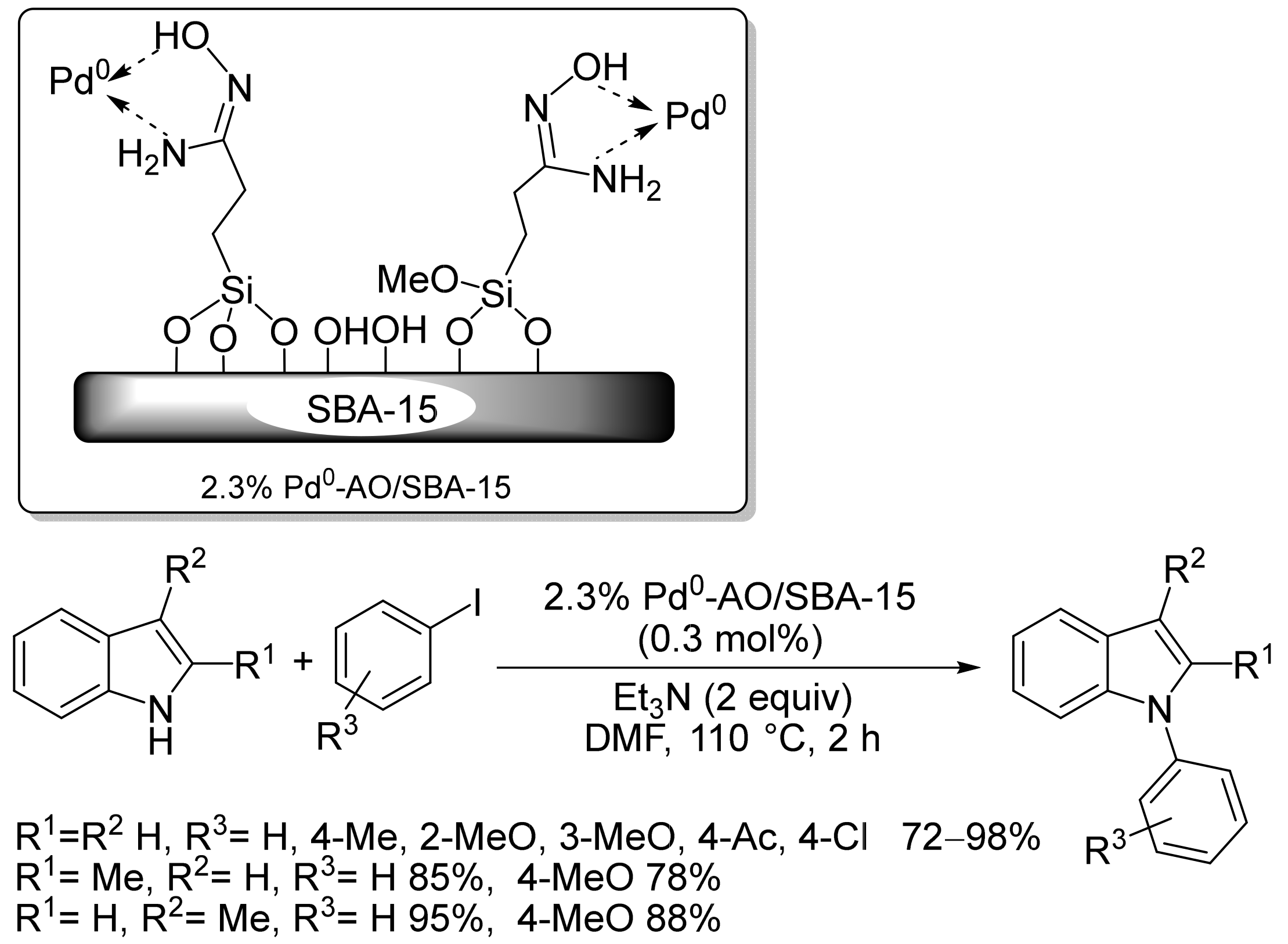
2.2. Pd-Based Catalysts
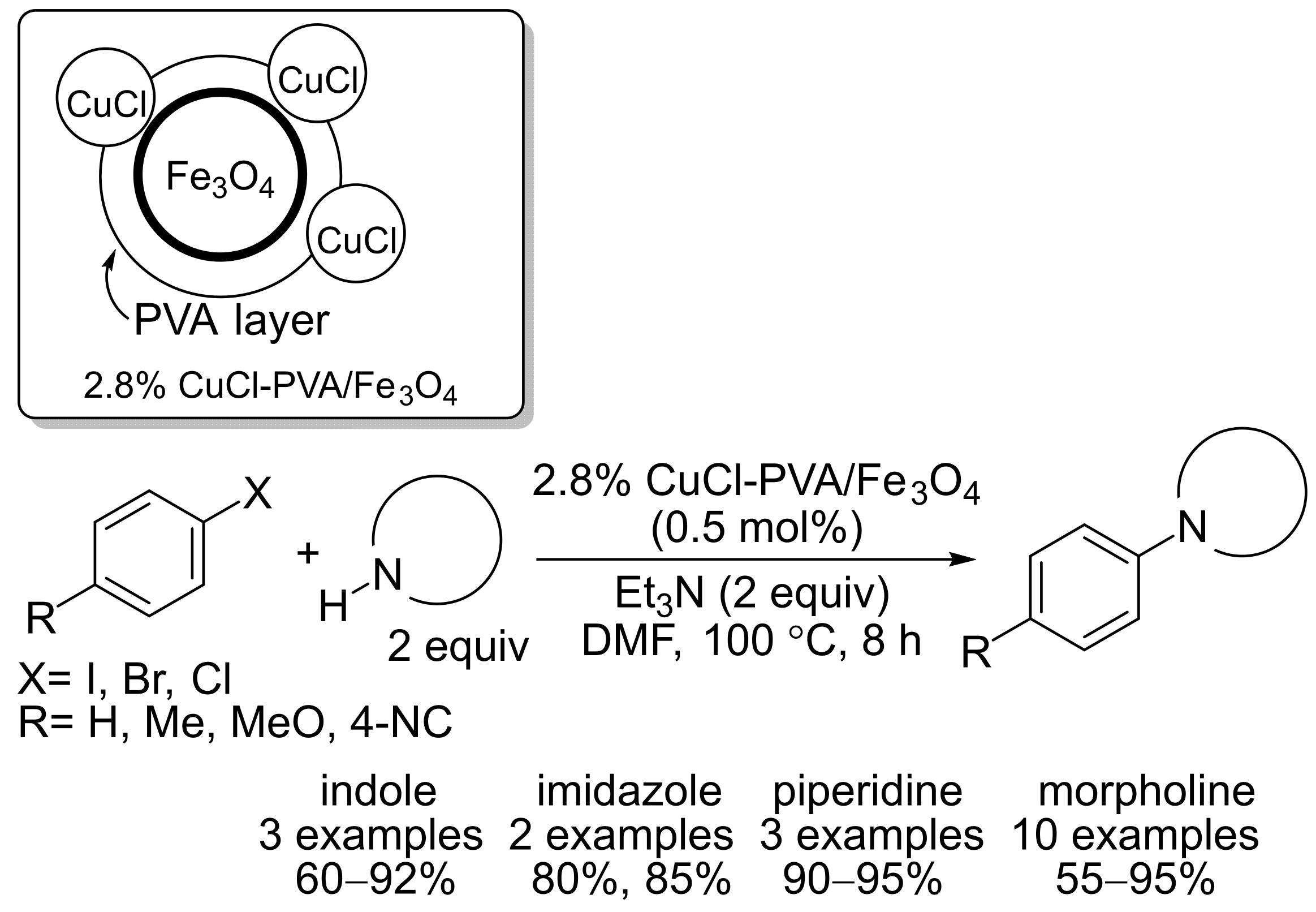
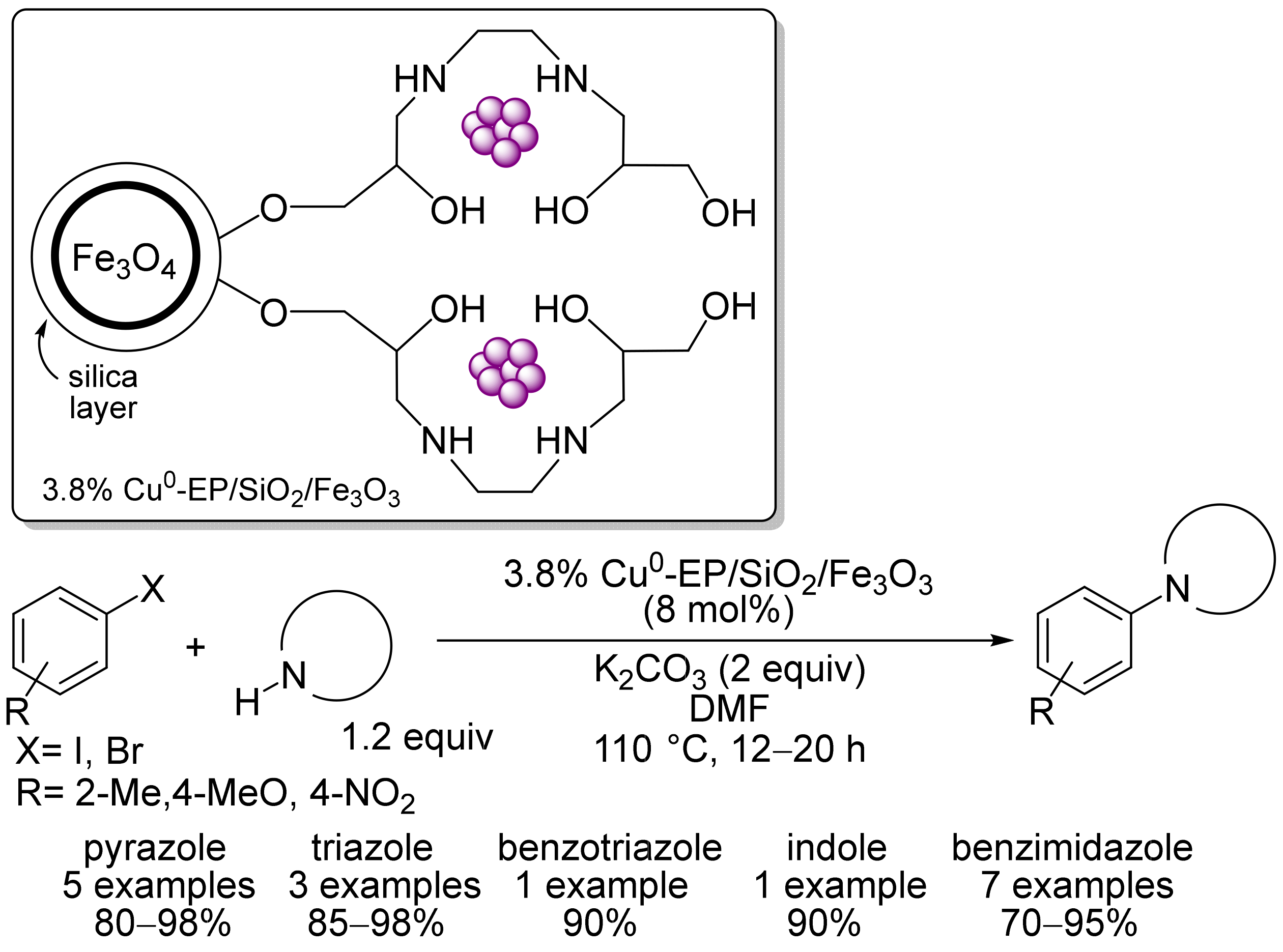
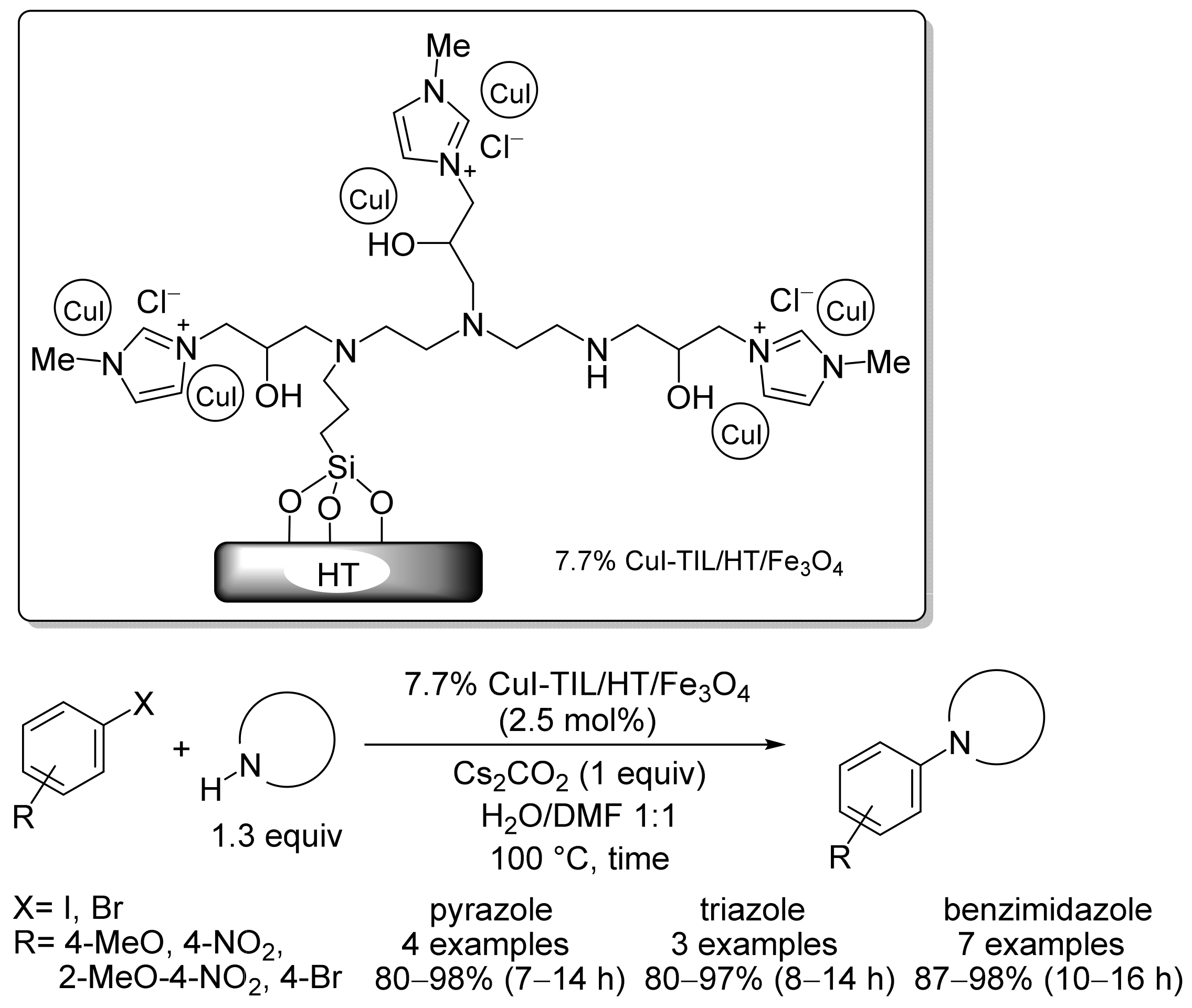
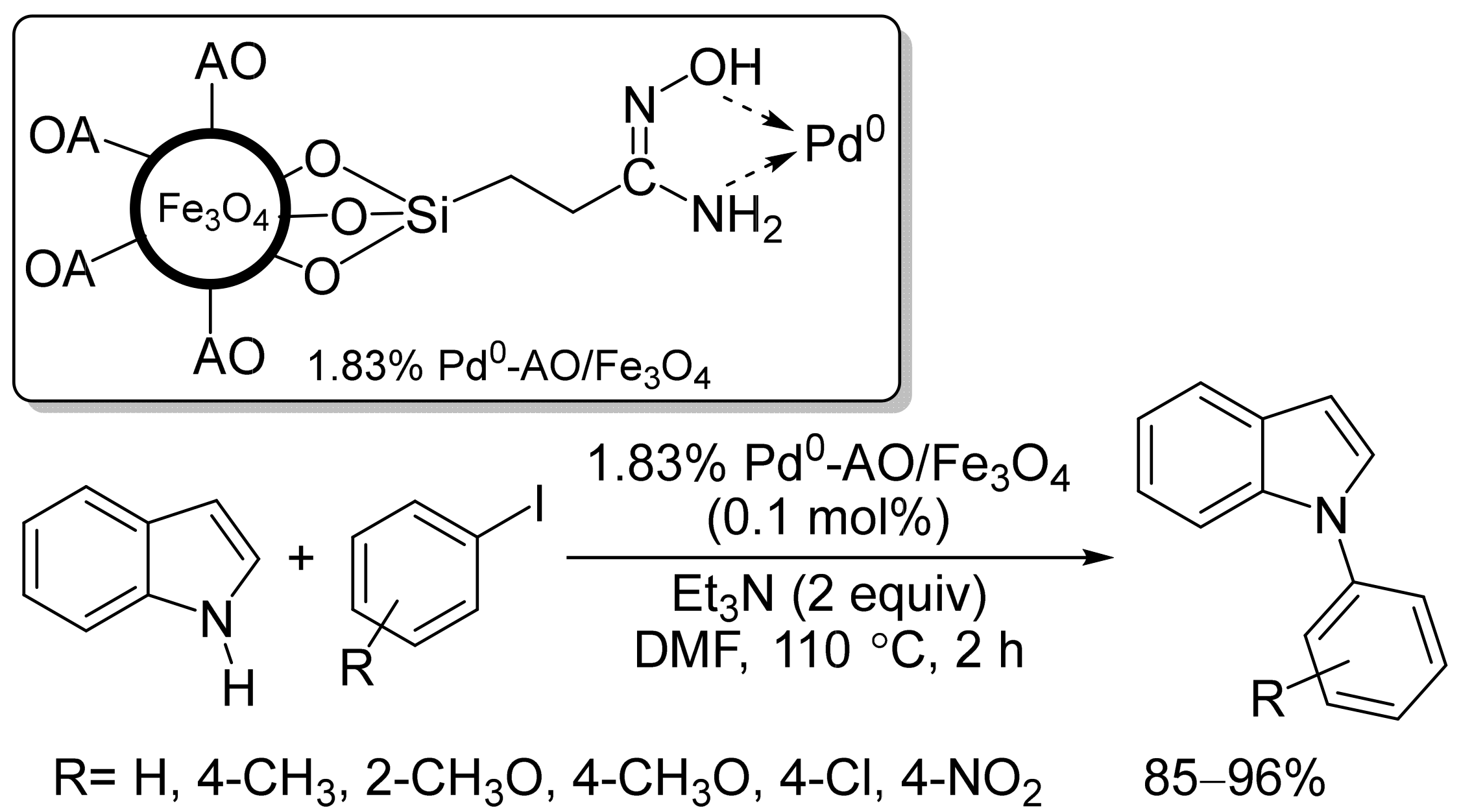
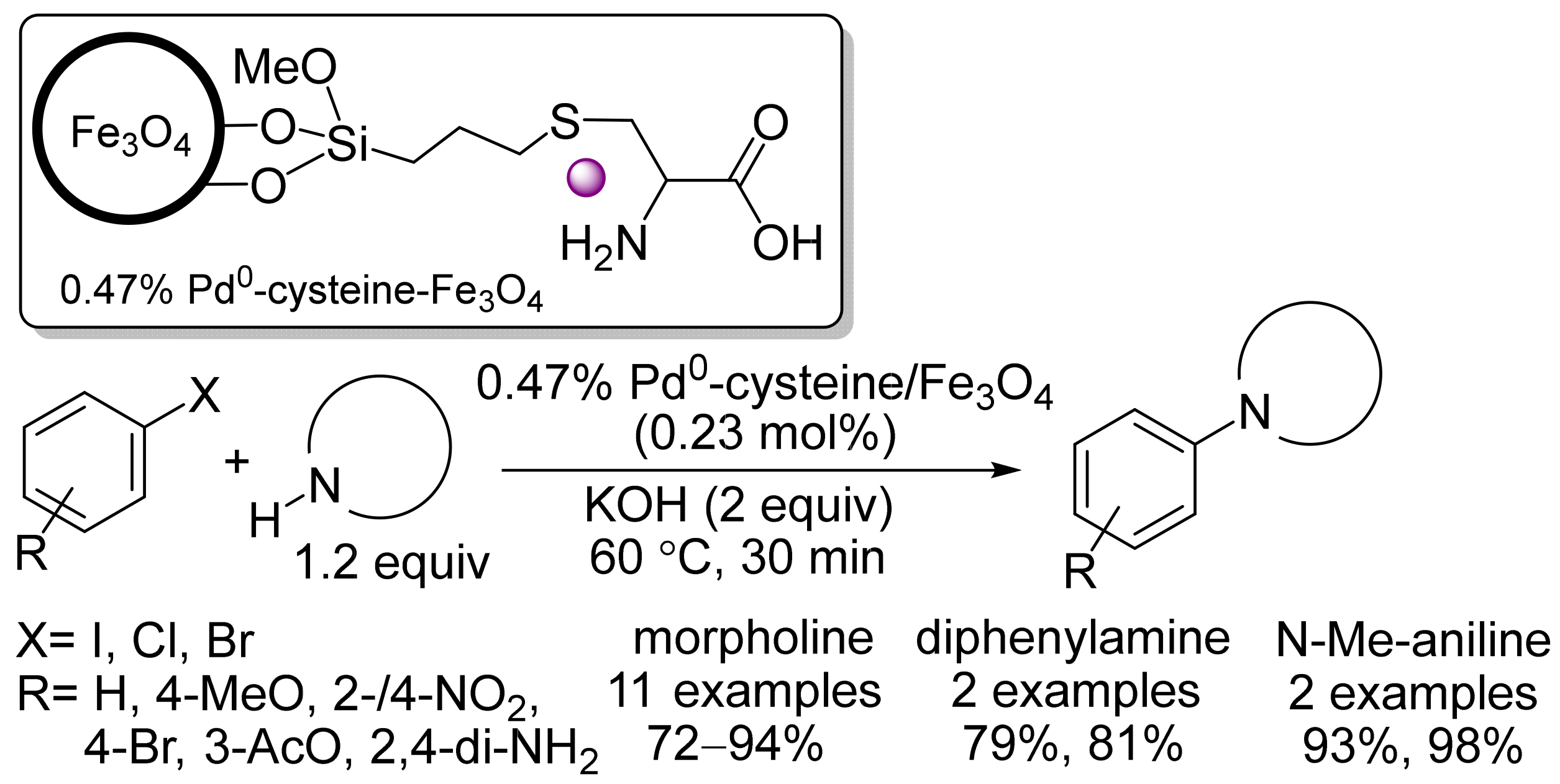
2.3. Magnetic Catalysts
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dai, L.X. C-S coupling with nitro group as leaving group via inorganic salt catalysis. Prog. Chem. 2018, 30, 1257–1297. [Google Scholar]
- Fischer, C.; Koenig, B. Palladium and copper-mediated N-aryl bond formation reactions for the synthesis of biological active compounds. Beilstein J. Org. Chem. 2011, 7, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef] [PubMed]
- Yashwantrao, G.; Saha, S. Sustainable strategies of C-N bond formation via Ullmann coupling employing earth abundant copper catalyst. Tetrahedron 2021, 97, 132406. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, R.; Mandal, M.; Babu, M.; Chakraborty, D. Air-stable palladium(0) phosphine sulfide catalysts for Ullmann-type C–N and C–O coupling reactions. J. Organomet. Chem. 2015, 781, 23–34. [Google Scholar] [CrossRef]
- Kuil, M.; Bekedam, E.K.; Visser, G.M.; van den Hoogenband, A.; Terpstra, J.W.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; van Strijdonck, G.P.F. Mild copper-catalyzed N-arylation of azaheterocycles with aryl halides. Tetrahedron Lett. 2005, 46, 2405–2409. [Google Scholar] [CrossRef]
- Verma, A.K.; Singh, J.; Sankar, V.K.; Chaudhary, R.; Chandra, R. Benzotriazole: An excellent ligand for Cu-catalyzed N-arylation of imidazoles with aryl and heteroaryl halides. Tetrahedron Lett. 2007, 48, 4207–4210. [Google Scholar] [CrossRef]
- Phipps, R.J.; Grimster, N.P.; Gaunt, M.J. Cu(II)-catalyzed direct and site-selective arylation of indoles under miled conditions. J. Am. Chem. Soc. 2008, 130, 8172–8174. [Google Scholar] [CrossRef]
- Liu, L.; Frohn, M.; Xi, N.; Dominguez, C.; Hungate, R.; Reider, P.J. A soluble base for the copper-catalyzed imidazole N-arylations with aryl halides. J. Org. Chem. 2005, 70, 10135–10138. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. Copper in cross-coupling reactions: The post-Ullmann chemistry. Coord. Chem. Rev. 2004, 248, 2337–2364. [Google Scholar] [CrossRef]
- Ley, S.V.; Thomas, A.W. Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef]
- Hisana, K.N.; Abdulla, C.M.A.; Anilkumar, G. Copper-catalyzed N-arylation of indoles. Curr. Org. Chem. 2022, 26, 857–886. [Google Scholar]
- Oeser, P.; Koudelka, J.; Petrenko, A.; Tobrman, T. Recent progress concerning the N-arylation of indoles. Molecules 2021, 26, 5079. [Google Scholar] [CrossRef]
- Panda, N.; Jena, A.K.; Mohapatra, S.; Rout, S.R. Copper ferrite nanoparticle-mediated N-arylation of heterocycles: A ligand-free reaction. Tetrahedron Lett. 2011, 52, 1924–1927. [Google Scholar] [CrossRef]
- Mastalir, Á.; Molnár, Á. A novel insight into the Ullmann homocoupling reactions performed in heterogeneous catalytic systems. Molecules 2023, 28, 1769. [Google Scholar] [CrossRef]
- Shafir, A.; Lichtor, P.A.; Buchwald, S.L. N-versus O-arylation of aminoalcohols: Orthogonal selectivity in copper-based catalysts. J. Am. Chem. Soc. 2007, 129, 3490–3491. [Google Scholar] [CrossRef]
- Khatri, P.K.; Jain, S.L. Glycerol ingrained copper: An efficient recyclable catalyst for the N-arylation of amines with aryl halides. Tetrahedron Lett. 2013, 54, 2740–2743. [Google Scholar] [CrossRef]
- Danqing, L.; Ming, J.; Li, L.; Mohammadnia, M. Preparation and characterization of Cu supported on 2-(1H-benzol[d]imidazole-2-yl)aniline-functionalized Fe3O4 nanoparticles as a novel magnetic catalyst for Ullmann and Suzuki cross-coupling reactions. Appl. Organomet. Chem. 2020, 34, e5820. [Google Scholar] [CrossRef]
- Kundu, D.; Bhadra, S.; Mukherjee, N.; Sreedhar, B.; Ranu, B.C. Heterogeneous CuII-catalyzed solvent-controlled selective N-arylation of cyclic amides and amines with bromo-iodoarenes. Chem. Eur. J. 2013, 19, 15759–15768. [Google Scholar] [CrossRef]
- Islam, S.M.; Mondal, S.; Mondal, P.; Roy, A.S.; Tuhina, K.; Salam, N.; Mobarak, M. A reusable polymer supported copper catalyst for the C–N and C–O bond cross-coupling reaction of aryl halides as well as arylboronic acids. J. Organomet. Chem. 2012, 696, 4264–4274. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, Y.; Ma, D. Cu-mediated Ullmann-type cross-coupling and industrial applications in route design, process development, and scale-up of pharmaceutical and agrochemical processes. Org. Process Res. Dev. 2022, 26, 1690–1750. [Google Scholar] [CrossRef]
- Islam, S.M.; Mondal, P.; Roy, A.S.; Mondal, S.; Hossain, D. Heterogeneous Suzuki and copper-free Sonogashira cross-coupling reactions catalyzed by a reusable palladium(II) complex in water medium. Tetrahedron Lett. 2010, 51, 2067–2070. [Google Scholar] [CrossRef]
- Bakherad, M.; Keivanloo, A.; Bahramian, B.; Jajarmi, S. Suzuki, Heck, and copper-free Sonogashira reactions catalyzed by 4-amino-5-methyl-3-thio-1,2,4-triazole-functionalized polystyrene resin-supported Pd(II) under aerobic conditions in water. J. Organomet. Chem. 2013, 724, 206–212. [Google Scholar] [CrossRef]
- He, Y.; Cai, C. Heterogeneous copper-free Sonogashira coupling reaction catalyzed by a reusable palladium Schiff base complex in water. J. Organomet. Chem. 2011, 696, 2689–2692. [Google Scholar] [CrossRef]
- Kodicherla, B.; Perumgani, P.C.; Mandapati, M.R. A reusable polystyrene-supported copper(II) catalytic system for N-arylation of indoles and Sonogashira coupling reactions in water. Appl. Catal. A 2014, 483, 110–115. [Google Scholar] [CrossRef]
- Khan, D.; Shaily, D.B.S. Synthesis and catalytic application of organo-functionalized MCM-41 catalyst are reviewed. Appl. Organomet. Chem. 2023, 37, e7007. [Google Scholar] [CrossRef]
- Pal, N.; Lee, J.H.; Cho, E.B. Recent trends in morphology-controlled synthesis and application of mesoporous silica nanoparticles. Nanomaterials 2020, 10, 2122. [Google Scholar] [CrossRef]
- Pasricha, S.; Gahlot, P.; Mittal, K.; Rai, D.; Avasthi, N.; Kaur, H.; Rai, S. Functionalized MCM-41: Versatile catalysts for organic transformations. ChemistrySelect 2022, 7, e202103674. [Google Scholar] [CrossRef]
- Mastalir, Á.; Rác, B.; Király, Z.; Molnár, Á. In situ generation of Pd nanoparticles in MCM-41 and catalytic applications in liquid-phase alkyne hydrogenations. J. Mol. Catal. A Chem. 2007, 264, 170–178. [Google Scholar] [CrossRef]
- Mastalir, Á.; Rác, B.; Király, Z.; Tasi, G.; Molnár, Á. Preparation of monodispersed Pt nanoparticles in MCM-41, catalytic applications. Catal. Commun. 2008, 9, 762–768. [Google Scholar] [CrossRef]
- Moorthy, M.; Kannan, B.; Madheswaran, B.; Rangappan, R. Tethering of Cu(II) Schiff base metal complex on mesoporous material MCM-41: Catalyst for Ullmann-type coupling reactions. J. Porous Mater. 2016, 23, 977–986. [Google Scholar] [CrossRef]
- Chisem, I.C.; Rafelt, J.; Shieh, M.T.; Clark, J.H.; Jachuck, R.; Macquarrie, D.; Ramshaw, C.; Scott, K. Catalytic oxidation of alkyl aromatics using a novel silica supported Schiff base complex. Chem. Commun. 1998, 1949–1950. [Google Scholar] [CrossRef]
- Sudheesh, N.; Sharma, S.K.; Khokhar, M.D.; Shukla, R.S.J. Kinetic investigations on the modified chitosan catalyzed solvent-free synthesis of jasminaldehyde. J. Mol. Catal. A Chem. 2011, 339, 86–91. [Google Scholar] [CrossRef]
- Simsek, M.; Asiyanbi-Hammed, T.T.; Rasaq, N.; Hammed, A.M. Progress in bioactive polysaccharide-derivatives: A review. Food Rev. Int. 2021, 39, 1612–1627. [Google Scholar] [CrossRef]
- Xu, D.; Hein, S.; Wang, K. Chitosan membrane in separation application. Mater. Sci. Technol. 2013, 24, 1076–1087. [Google Scholar] [CrossRef]
- Rafiee, F. Recent advances in the application of chitosan and chitosan derivatives as bio supported catalyst in the cross coupling reactions. Curr. Org. Chem. 2019, 23, 390–408. [Google Scholar] [CrossRef]
- Molnár, Á. The use of chitosan-based metal catalysts in organic transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Shen, C.; Xu, J.; Yu, W.B.; Zhang, P.F. A highly active and easily recoverable chitosan@copper catalyst for the C–S coupling and its application in the synthesis of zolimidine. Green Chem. 2014, 16, 3007–3012. [Google Scholar] [CrossRef]
- Anuradha, K.S.; Pathak, D.D. Synthesis and development of chitosan anchored copper(II) Schiff base complexes as heterogeneous catalysts for N-arylation of amines. Tetrahedron Lett. 2015, 56, 4135–4142. [Google Scholar] [CrossRef]
- Lü, X.M.; Ruan, J.C.; Chen, X.Z.; Qian, C. Cross-linked chitosan bead supported copper complex in water as a green and efficient catalytic protocol for Ullmann reaction. Chin. J. Org. Chem. 2019, 39, 1720–1726. [Google Scholar] [CrossRef]
- Liu, X.; Chang, S.; Chen, X.; Ge, X.; Qian, C. Efficient Ullmann C–X coupling reaction catalyzed by a recoverable functionalized chitosan-supported copper complex. New J. Chem. 2018, 42, 16013–16020. [Google Scholar] [CrossRef]
- Huang, L.; Yu, R.; Zhu, X.; Wan, Y. A recyclable Cu-catalyzed C–N coupling reaction in water and its application to synthesis of imidazo[1,2-a]quinoxaline. Tetrahedron 2013, 69, 8974–8977. [Google Scholar] [CrossRef]
- Yang, B.; Mao, Z.; Zhu, X.; Wan, Y. Functionalised chitosan as a green, recyclable, supported catalyst for the copper-catalysed Ullmann C–N coupling reaction in water. Catal. Commun. 2015, 60, 92–95. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-heterocyclic carbene complexes of copper, nickel and cobalt. Chem. Rev. 2019, 109, 3730–3961. [Google Scholar] [CrossRef]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-heterocyclic carbenes in late transition metal catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Zhang, H.; Zeng, Y.; Liu, G. N-heterocyclic carbene copper(I) complex catalyzed coupling of (hetero)aryl chlorides and nitrogen heterocycles: Highly efficient catalytic system. Chin. J. Chem. 2020, 38, 1252–1256. [Google Scholar] [CrossRef]
- Kriechbaum, M.; List, M.; Berger, R.J.F.; Patzschke, M.; Monkowius, U. Silver and gold complexes with a new 1,10-phenantroline analogue N-heterocyclic carbene: A combined structural, theoretical, and photophysical study. Chem. Eur. J. 2012, 18, 5506–5509. [Google Scholar] [CrossRef]
- Cooper, A.I. Conjugated microporous polymers. Adv. Mater. 2009, 21, 1291–1295. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Zhang, Y.; Luo, X.; Xia, H.; Liu, X.; Mua, Y. Metallosalen-based microporous organic polymers: Synthesis and carbon dioxide uptake. RSC Adv. 2014, 4, 37767–37772. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, K.; Li, T.; Tan, B.; Gu, Y. Functionalized hypercrosslinked polymers with knitted N-heterocyclic carbene-copper complexes as efficient and recyclable catalysts for organic transformations. Catal. Sci. Technol. 2016, 6, 4345–4355. [Google Scholar] [CrossRef]
- Xu, S.; Song, K.; Li, T.; Tan, B. Palladium catalyst coordinated in knitting N-heterocyclic carbene porous polymers for efficient Suzuki-Miyaura coupling reactions. J. Mater. Chem. A 2015, 3, 1272–1278. [Google Scholar] [CrossRef]
- Campisciano, V.; Giacalone, F.; Gruttadauria, M. Supported ionic liquids: A versatile and useful class of materials. Chem. Rec. 2017, 17, 918–938. [Google Scholar] [CrossRef]
- Valdebenito, C.; Pinto, J.; Nazarkovsky, M.; Chacón, G.; Martínez-Ferraté, O.; Wrighton-Araneda, K.; Cortés-Arriagada, D.; Camarada, M.B.; Fernandes, J.A.; Abarca, G. Highly modulated supported triazolium-based ionic liquids: Direct control of the electronic environment of Cu nanoparticles. Nanoscale Adv. 2020, 2, 1325–1332. [Google Scholar] [CrossRef]
- Hemmati, S.; Naderi, A.; Ghadermazi, M.; Veisi, H. SiO2-functionalized melamine-pyridine group-supported Cu(OAc)2 as an efficient heterogeneous and recyclable nanocatalyst for the N-arylation of amines. Comptes Rendus Chim. 2018, 21, 659–668. [Google Scholar] [CrossRef]
- Veisi, H.; Metghalchi, Y.; Hekmati, M.; Samadzadeh, S. CuI heterogenized on thiosemicarbazide modified multi-walled carbon nanotubes (thiosemicarbazide-MWCNTs –CuI): Novel heterogeneous and reusable nanocatalyst in the C–N Ullmann coupling reactions. Appl. Organomet. Chem. 2017, 31, e3676. [Google Scholar] [CrossRef]
- Zhang, J. Preparation, characterization and application of thiosemicarbazide grafted multiwalled carbon nanotubes for solid-phase extraction of Cd(II), Cu(II) and Pb(II) in environmental samples. J. Environ. Sci. 2013, 25, 2331–2337. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Bidgoli, N.S.S.; Karimkhani, M.M. Valorization of lignin: Antibacterial and catalytic activities of copper complex stabilized on magnetic lignosulfonate for N-formylation of amines under solvent-free conditions. Biomass Conv. Biorefin. 2021. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; An, X.; Seta, F.T.; Li, C.; Zhang, H.; Luo, B.; Hu, Q.; Zhang, R.; Nie, S. Facile preparation of lignosulfonate induced silver nanoparticles for high efficient removal of organic contaminants in wastewater. Ind. Crop. Prod. 2021, 169, 113644. [Google Scholar] [CrossRef]
- Marulasiddeshwara, M.B.; Kumar, P.R. Synthesis of Pd(0) nanocatalyst using lignin in water for the Mizoroki-Heck reaction under solvent-free conditions. Int. J. Biol. Macromol. 2016, 83, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Bharamanagowda, M.M.; Phanchangam, R.K. Fe3O4-lignin @Pd-NPs: A highly efficient, magnetically recoverable and recyclable catalyst for Mizoroki-Heck reaction under solvent-free conditions. Appl. Organomet. Chem. 2020, 34, e5837. [Google Scholar]
- Marulasiddeshwara, M.B.; Kumar, P.R. Phosphine and copper-free Sonogashira coupling reaction catalyzed by lignin supported palladium nanoparticles. Mater. Today Proc. 2018, 5, 20811–20818. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; D’Alessandro, N.; D’Ambrosio, P.; Bressan, M. Palladium nanoparticles, stabilized by lignin, as catalyst for cross-coupling reactions in water. Inorg. Chim. Acta 2013, 399, 12–18. [Google Scholar] [CrossRef]
- Mo, B.; Li, Z.; Peng, J.; Chen, C. Novel lignin-supported copper complex as a highly efficient and recyclable nanocatalyst for Ullmann reaction. Int. J. Biol. Macromol. 2023, 239, 124263. [Google Scholar] [CrossRef]
- Veisi, H.; Heravi, M.R.P.; Hamelian, M. SBA-15-functionalized melamine-pyridine group-supported palladium(0) as an efficient heterogeneous and recyclable nanocatalyst for N-arylation of indoles through Ullmann-type coupling reactions. Appl. Organomet. Chem. 2015, 29, 334–337. [Google Scholar] [CrossRef]
- Veisi, H.; Hamelian, M.; Hemmati, S. Palladium anchored to SBA-15 functionalized with melamine-pyridine groups as a novel and efficient heterogeneous nanocatalyst for Suzuki-Miyaura coupling reactions. J. Mol. Catal. A 2014, 395, 25–33. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Hemmati, S.; Hamelian, M.; Veisi, H. An efficient, mild and selective Ullmann-type N-arylation of indoles catalysed Pd immobilized on amidoxime-functionalized mesoporous SBA-15 as heterogeneous and recyclable nanocatalyst. Appl. Organomet. Chem. 2015, 29, 195–199. [Google Scholar] [CrossRef]
- Pirhayati, M.; Veisi, H.; Kakanejadifard, A. Palladium stabilized by 3,4-dihydroxypyridine-functionalized magnetic Fe3O4 nanoparticles as a reusable and efficient heterogeneous catalyst for Suzuki reactions. RSC Adv. 2016, 6, 27252–27259. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. Fast-growing field of magnetically recyclable nanocatalysts. Chem. Rev. 2014, 114, 6949–6985. [Google Scholar] [CrossRef]
- Karimi, B.; Mansouri, F.; Vali, H. A highly water-dispersible /magnetically separable palladium catalyst based on a Fe3O4@SiO2 anchored TEG-imidazolium ionic liquid for the Suzuki-Miyaura coupling reaction in water. Green Chem. 2014, 16, 2587–2596. [Google Scholar] [CrossRef]
- Hemmati, S.; Kamangar, S.A.; Yousefi, M.; Salehi, M.H.; Hekmati, M. Cu(I)-anchored polyvinyl alcohol coated-magnetic nanoparticles as heterogeneous nanocatalyst in Ullmann-type C–N coupling reactions. Appl. Organomet. Chem. 2020, 34, e5611. [Google Scholar] [CrossRef]
- Veisi, H.; Taheri, S.; Hemmati, S. Preparation of polydopamine sdulfamic acid-functionalized magnetic Fe3O4 nanoparticles with a core/shell nanostructure as heterogeneous and recyclable nanocatalysts for the acetylation of alcohols, phenols, amines and thiols under solvent-free conditions. Green Chem. 2016, 18, 6337–6348. [Google Scholar] [CrossRef]
- Rajabzadeh, M.; Eshghi, H.; Khalifeh, R.; Bakavoli, M. Magnetically recoverable copper nanorods and their catalytic activity in Ullmann cross-coupling reactions. Appl. Organomet. Chem. 2017, 31, e3647. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interf. Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Rajabzadeh, M.; Eshghi, H.; Khalifeh, R.; Bakavoli, M. Generation of Cu nanoparticles on novel designed Fe3O4@SiO2/EP.EN.EG as reusable nanocatalyst for the reduction of nitro compounds. RSC Adv. 2016, 6, 19331–19340. [Google Scholar] [CrossRef]
- Rajabzadeh, M.; Najdi, N.; Zarei, Z.; Khalifeh, R. CuI immobilized on tricationic ionic liquid anchored on functionalized magnetic hydrotalcite (Fe3O4/HT-TIL-CuI) as a novel, magnetic and efficient nanocatalyst for Ullmann-type C–N coupling reaction. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2696–2711. [Google Scholar] [CrossRef]
- Zarei, Z.; Akhlaghinia, B. ZnII doped and immobilized on functionalized magnetic hydrotalcite (Fe3O4/HT-SMTU-ZnII): A novel, green and magnetically recyclable bifunctional nanocatalyst for the one-pot multi-component synthesis of acridinediones under solvent-free conditions. New J. Chem. 2017, 41, 15485–15500. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Hemmati, S.; Hekmati, M. Pd immobilized on modified magnetic Fe3O4 nanoparticles: Magnetically recoverable and reusable Pd nanocatalyst for Suzuki-Miyaura coupling reactions and Ullmann-type N-arylation of indoles. J. Chem. Sci. 2016, 128, 1157–1162. [Google Scholar] [CrossRef]
- Veisi, H.; Gholami, J.; Ueda, H.; Mohammadi, P.; Noroozi, M. Magnetically palladium catalyst stabilized by diaminoglyoxime-functionalized magnetic Fe3O4 nanoparticles as active and reusable catalyst for Suzuki coupling reactions. J. Mol. Catal. A Chem. 2015, 396, 216–223. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Khorsandi, Z.; Metkazini, S.F.M. Palladium nanoparticles supported on cysteine-functionalized MNPs as robust recyclable catalysts for fast O- and N-arylation reactions in green media. J. Organomet. Chem. 2019, 899, 120793. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastalir, Á.; Molnár, Á. On the Current Status of Ullmann-Type N-Arylation Reactions Promoted by Heterogeneous Catalysts. Inorganics 2023, 11, 276. https://doi.org/10.3390/inorganics11070276
Mastalir Á, Molnár Á. On the Current Status of Ullmann-Type N-Arylation Reactions Promoted by Heterogeneous Catalysts. Inorganics. 2023; 11(7):276. https://doi.org/10.3390/inorganics11070276
Chicago/Turabian StyleMastalir, Ágnes, and Árpád Molnár. 2023. "On the Current Status of Ullmann-Type N-Arylation Reactions Promoted by Heterogeneous Catalysts" Inorganics 11, no. 7: 276. https://doi.org/10.3390/inorganics11070276
APA StyleMastalir, Á., & Molnár, Á. (2023). On the Current Status of Ullmann-Type N-Arylation Reactions Promoted by Heterogeneous Catalysts. Inorganics, 11(7), 276. https://doi.org/10.3390/inorganics11070276







