Phase Formation of Co and Cr Co-Doped Bismuth Niobate with Pyrochlore Structure
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valant, M.; Babu, G.S.; Vrcon, M.; Kolodiazhnyi, T.; Axelsson, A.-K. Pyrochlore Range from Bi2O3-Fe2O3-TeO3 System for LTCC and Photocatalysis and the Crystal Structure of New Bi3(Fe0.56Te0.44)3O11. J. Am. Ceram. Soc. 2011, 95, 644–650. [Google Scholar] [CrossRef]
- Murugesan, S.; Huda, M.N.; Yan, Y.; Al-Jassim, M.M.; Subramanian, V. Band-Engineered Bismuth Titanate Pyrochlores for Visible Light Photocatalysis. J. Phys. Chem. C 2010, 114, 10598–10605. [Google Scholar] [CrossRef]
- Khaw, C.C.; Tan, K.B.; Lee, C.K. High temperature dielectric properties of cubic bismuth zinc tantalate. Ceram. Intern. 2009, 35, 1473–1480. [Google Scholar] [CrossRef]
- Giampaoli, G.; Siritanon, T.; Day, B.; Li, J.; Subramanian, M.A. Temperature in-dependent low loss dielectrics based on quaternary pyrochlore oxides. Prog. Solid State Chem. 2018, 50, 16–23. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1982, 15, 55–143. [Google Scholar] [CrossRef]
- McCauley, R.A. Structural Characteristics of Pyrochlore Formation. J. Appl. Phys. 1980, 51, 290–294. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Sekushin, N.A.; Semenov, V.G.; Fedorova, A.V.; Selyutin, A.A.; Krzhizhanovskaya, M.G.; Lutoev, V.P.; Makeev, B.A.; Kharton, V.V.; Sivkov, D.N.; et al. Dielectric properties, Mössbauer study, ESR spectra of Bi2FeTa2O9.5 with pyrochlore structure. J. Alloys Compd. 2022, 903, 163928. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Nekipelov, S.V.; Kharton, V.V.; Sekushin, N.A. Thermal Expansion, XPS Spectra, and Structural and Electrical Properties of a New Bi2NiTa2O9 Pyrochlore. Inorgan. Chem. 2021, 60, 4924–4934. [Google Scholar] [CrossRef]
- Jusoh, F.A.; Tan, K.B.; Zainal, Z.; Chen, S.K.; Khaw, C.C.; Lee, O.J.; Balachandran, R.; Ananda Murthy, H.C. Novel pyrochlore-structured bismuth iron antimonates: Structural, impedance and electrochemical studies. Results Phys. 2021, 27, 104542. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Vanderah, T.A.; Pazos, I.M.; Levin, I.; Roth, R.S.; Nino, J.C.; Provenzano, V.; Schenck, P.K. Phase formation, crystal chemistry, and properties in the system Bi2O3–Fe2O3–Nb2O5. J. Sol. St. Chem. 2006, 179, 3900–3910. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Ellert, O.G.; Maksimov, Y.V.; Volodin, V.D.; Efimov, N.N.; Novotortsev, V.M. Subsolidus phase equilibria and magnetic characterization of the pyrochlore in the Bi2O3–Fe2O3–Sb2Ox system. J. Alloys Compd. 2013, 579, 311–314. [Google Scholar] [CrossRef]
- Vanderah, T.A.; Lufaso, M.W.; Adler, A.U.; Levin, I.; Nino, J.C.; Provenzano, V.; Schenck, P.K. Subsolidus phase equilibria and properties in the system Bi2O3:Mn2O3±x:Nb2O5. J. Sol. St. Chem. 2006, 179, 3467–3477. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Nekipelov, S.V.; Sivkov, D.V.; Sivkov, V.N.; Lebedev, A.M.; Chumakov, R.G.; Makeev, B.A.; Kharton, V.V.; et al. Spectroscopic characterization of cobalt doped bismuth tantalate pyrochlore. Sol. St. Sci. 2022, 125, 106820. [Google Scholar] [CrossRef]
- Greedan, J.E. Frustrated rare earth magnetism: Spin glasses, spin liquids and spin ices in pyrochlore oxides. J. Alloys Comp. 2006, 408–412, 444–455. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, L.; Tan, Y.; Zeng, S.; Xia, Y.; Wang, Y.; Zhang, H. Synthesis and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc. 2020, 40, 1639–1643. [Google Scholar] [CrossRef]
- Laurita, G.; Hickox-Young, D.; Husremovic, S.; Li, J.; Sleight, A.W.; Macaluso, R.; Rondinelli, J.M.; Subramanian, M.A. Covalency-driven Structural Evolution in the Polar Pyrochlore Series Cd2Nb2O7–xSx. Chem. Mater. 2019, 31, 7626–7637. [Google Scholar] [CrossRef]
- Kamba, S.; Porokhonskyy, V.; Pashkin, A.; Bovtun, V.; Petzelt, J.; Nino, J.C.; Trolier-McKinstry, S.; Lanagan, M.T.; Randall, C.A. Anomalous broad dielectric relaxation in Bi1.5Zn1.0Nb1.5O7 pyrochlore. Phys. Rev. B 2002, 66, 054106. [Google Scholar] [CrossRef]
- Valant, M. Dielectric Relaxations in Bi2O3-Nb2O5-NiO Cubic Pyrochlores. J. Am. Ceram. Soc. 2009, 92, 955–958. [Google Scholar] [CrossRef]
- Ismunandar; Kamiyama, T.; Oikawa, K.; Hoshikawa, A.; Kennedy, B.J.; Kubota, Y.; Kato, K. Static bismuth disorder in Bi2−x(CrTa)O7−y. Mater. Res. Bull. 2004, 39, 553–560. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Reveguk, A.A.; Sivkov, D.V.; Nekipelov, S.V. Thermal expansion, crystal structure, XPS and NEXAFS spectra of Fe-doped bismuth tantalate pyrochlore. Ceram. Intern. 2022, 48, 14849–14855. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Sekushin, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Reveguk, A.A.; Nekipelov, S.V.; Sivkov, D.V.; Lutoev, V.P.; Makeev, B.A.; Kharton, V.V.; et al. Cr-doped bismuth tantalate pyrochlore: Electrical and thermal properties, crystal structure and ESR, NEXAFS, XPS spectroscopy. Mater. Res. Bull. 2023, 158, 112067. [Google Scholar] [CrossRef]
- Jusoh, F.A.; Tan, K.B.; Zainal, Z.; Chen, S.K.; Khaw, C.C.; Lee, O.J. Investigation of structural and dielectric properties of subsolidus bismuth iron niobate pyrochlores. J. Asian Ceram. Soc. 2020, 8, 957–969. [Google Scholar] [CrossRef]
- Jusoh, F.A.; Tan, K.B.; Zainal, Z.; Chen, S.K.; Khaw, C.C.; Lee, O.J. Novel pyrochlores in the Bi2O3-Fe2O3-Ta2O5 (BFT) ternary system: Synthesis, structural and electrical properties. J. Mater. Res. Technol. 2020, 9, 11022–11034. [Google Scholar] [CrossRef]
- Chon, M.P.; Tan, K.B.; Khaw, C.C.; Zainal, Z.; Taufiq-Yap, Y.H.; Chen, S.K.; Tan, P.Y. Subsolidus phase equilibria and electrical properties of pyrochlores in the Bi2O3–CuO–Ta2O5 ternary system. J. Alloys Compd. 2016, 675, 116–127. [Google Scholar] [CrossRef]
- Tan, P.Y.; Tan, K.B.; Khaw, C.C.; Zainal, Z.; Chen, S.K.; Chon, M.P. Phase equilibria and dielectric properties of Bi3+(5/2)xMg2−xNb3−(3/2)xO14−x cubic pyrochlores. Ceram. Intern. 2014, 40, 4237–4246. [Google Scholar] [CrossRef]
- Tan, P.Y.; Tan, K.B.; Khaw, C.; Zainal, Z.; Chen, S.K.; Chon, M.P. Structural and electrical properties of bismuth magnesium tantalate pyrochlores. Ceram. Intern. 2012, 38, 5401–5409. [Google Scholar] [CrossRef]
- Youn, H.-J.; Sogabe, T.; Randall, C.A.; Shrout, T.R.; Lanagan, M.T. Phase Relations and Dielectric Properties in the Bi2O3-ZnO-Ta2O5 System. J. Am. Ceram. Soc. 2001, 84, 2557–2562. [Google Scholar] [CrossRef]
- Khaw, C.C.; Tan, K.B.; Lee, C.K.; West, A.R. Phase equilibria and electrical properties of pyrochlore and zirconolite phases in the Bi2O3–ZnO–Ta2O5 system. J. Eur. Ceram. Soc. 2012, 32, 671–680. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G. Thermal expansion of bismuth magnesium tantalate and niobate pyrochlores. Ceram. Int. 2021, 47, 30099–30105. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Sekushin, N.A.; Kharton, V.V.; Makeev, B.A.; Belyy, V.A.; Korolev, R.I. Dielectric performance of pyrochlore-type Bi2MgNb2-xTaxO9 ceramics: The effects of tantalum doping. Ceram. Intern. 2021, 47, 19424–19433. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Sekushin, N.A.; Kharton, V.V.; Koroleva, A.V.; Nekipelov, S.V.; Sivkov, D.V.; Sivkov, V.N.; Makeev, B.A.; Lebedev, A.M.; et al. Novel Ni-Doped Bismuth–Magnesium Tantalate Pyrochlores: Structural and Electrical Properties, Thermal Expansion, X-ray Photoelectron Spectroscopy, and Near-Edge X-ray Absorption Fine Structure Spectra. ACS Omega 2021, 6, 23262–23273. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Makeev, B.A.; Korolev, R.I.; Sivkov, D.N.; Shpynova, A.D. A study of phase evolution of Fe-doped bismuth tantalate pyrochlore. Ceram. Intern. 2022, 48, 31965–31969. [Google Scholar] [CrossRef]
- Nino, J.C.; Lanagan, M.T.; Randall, C.A. Phase formation and reactions in the Bi2O3–ZnO–Nb2O5–Ag pyrochlore system. J. Mater. Res. 2001, 16, 1460–1464. [Google Scholar] [CrossRef]
- Rylchenko, E.P.; Makeev, B.A.; Sivkov, D.V.; Korolev, R.I.; Zhuk, N.A. Features of phase formation of pyrochlore-type Bi2Cr1/6Mn1/6Fe1/6Co1/6Ni1/6Cu1/6Ta2O9+Δ. Lett. Mater. 2022, 12, 486–492. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Makeev, B.A.; Krzhizhanovskaya, M.G.; Korolev, R.I. Effect of magnesium and zinc on phase formation of pyrochlore-type Bi2Mg(Zn)1−xMxTa2O9.5−Δ (M-Cr, Fe) ceramics. Ceram. Intern. 2023, 49, 5496–5509. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Kovalenko, S.Y.; Korolev, R.I.; Makeev, B.A.; Krzhizhanovskaya, M.G.; Sivkov, D.V.; Nekipelov, S.V.; Sivkov, V.N.; Yermolina, M.V. Features of Phase Formation of Pyrochlore-type Ceramics Bi2Mg(Zn)1−xNixTa2O9. ACS Omega 2023, 8, 11351–11363. [Google Scholar] [CrossRef]
- Akselrud, L.G.; Grin, Y.N.; Zavalij, P.Y. CSD-universal program package for single crystal or powder structure data treatment. In Proceedings of the 12th European Crystallographic Meeting, Moscow, Russia, 20–29 August 1989; p. 155. [Google Scholar]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Sekushin, N.A.; Sivkov, D.V.; Abdurakhmanov, I.E. Crystal structure, dielectric and thermal properties of cobalt doped bismuth tantalate pyrochlore. J. Mater. Res. Technol. 2023, 22, 1791–1799. [Google Scholar] [CrossRef]
- Da Cruz, J.A.; Volnistem, E.A.; Ferreira, R.F.; Freitas, D.B.; Sales, A.J.M.; Costa, L.C.; Graça, M.P.F. Structural characterization of Brazilian niobium pentoxide and treatment to obtain the single phase (H-Nb2O5). Therm. Sci. Eng. Progr. 2021, 25, 101015. [Google Scholar] [CrossRef]
- Zahid, A.H.; Han, Q. A review on the preparation, microstructure, and photocatalytic performance of Bi2O3 in polymorphs. Nanoscale 2021, 13, 17687–17724. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Ramanan, A.; Rao, C.N.R.; Jefferson, D.A.; Smith, D.J. A homologous series of recurrent intergrowth structures of the type Bi4Am+n−2Bm+nO3(m+n)+6 formed by oxides of the aurivillius family. Sol. St. Chem. 1984, 55, 101–105. [Google Scholar] [CrossRef]
- Grins, J.; Esmaeilzadeh, S.; Hull, S. Structure and Ionic Conductivity of Bi6Cr2O15, a New Structure Type Containing (Bi12O14)8n+n Columns and CrO42− Tetrahedra. J. Sol. St. Chem. 2002, 163, 144–150. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, J.B.; Lianga, J.K.; Luo, J.; Ji, L.N.; Zhang, J.Y.; Rao, G.H. Phase diagram of the Bi2O3–Cr2O3 system. Mater. Chem. Phys. 2008, 112, 239–243. [Google Scholar] [CrossRef]
- Struzik, M.; Liu, X.; Abrahams, I.; Krok, F.; Malys, M.; Dygas, J.R. Defect structure and electrical conductivity in the pseudo-binary system Bi3TaO7–Bi3NbO7. Sol. St. Ion. 2012, 218, 25–30. [Google Scholar] [CrossRef]
- Castro, A.; Aguado, E.; Rojo, J.M.; Herrero, P.; Enjalbert, R.; Galy, J. The New Oxygen-Deficient Fluorite Bi3NbO7: Synthesis, Electrical Behavior and Structural Approach. Mater. Res. Bull. 1998, 33, 31–41. [Google Scholar] [CrossRef]
- Roth, R.S.; Waring, J.L. Synthesis and stability of bismutotantalite, stibiotantalite and chemically similar ABO4 compounds. Am. Mineral. 1963, 48, 1348–1356. [Google Scholar]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Belyy, V.A.; Makeev, B.A. High-Temperature Crystal Chemistry of α-, β-, and γ-BiNbO4 Polymorphs. Inorg. Chem. 2019, 58, 1518–1526. [Google Scholar] [CrossRef]
- Guragain, D.; Zequine, C.; Gupta, R.K.; Mishra, S.R. Facile Synthesis of Bio-Template Tubular MCo2O4 (M = Cr, Mn, Ni) Microstructure and Its Electrochemical Performance in Aqueous Electrolyte. Processes 2020, 8, 343. [Google Scholar] [CrossRef] [Green Version]
- Piir, I.V.; Prikhodko, D.A.; Ignatchenko, S.V.; Schukariov, A.V. Preparation and structural investigations of the mixed bismuth niobates, containing transition metals. Sol. St. Ion. 1997, 101–103, 1141–1146. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Selyutin, A.A.; Sekushin, N.A.; Nekipelov, S.V.; Sivkov, D.V.; Kharton, V.V. Cr and Mg codoped bismuth tantalate pyrochlores: Thermal expansion and stability, crystal structure, electrical and optical properties, NEXAFS and XPS study. J. Sol. St. Chem. 2023, 323, 124074. [Google Scholar] [CrossRef]
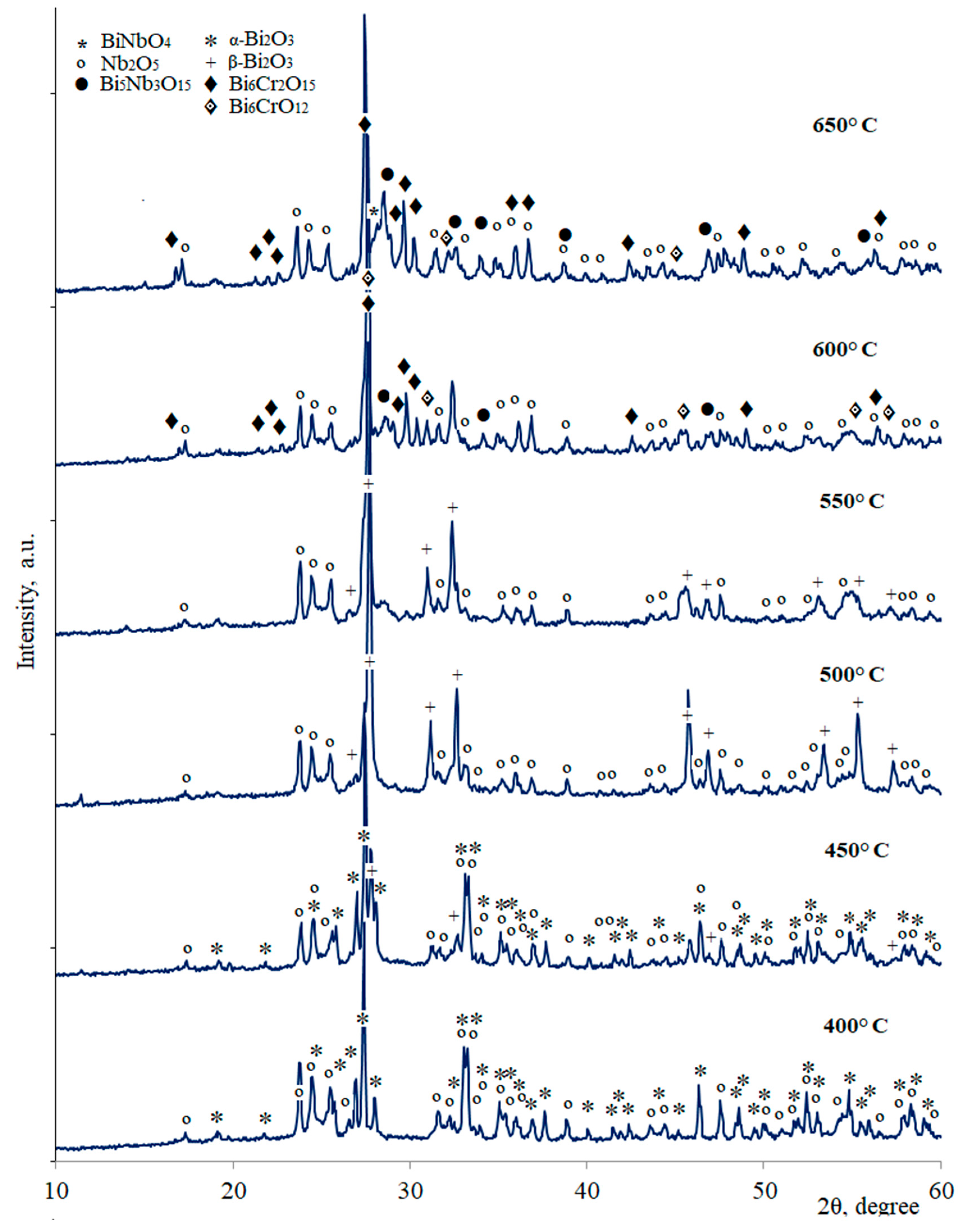
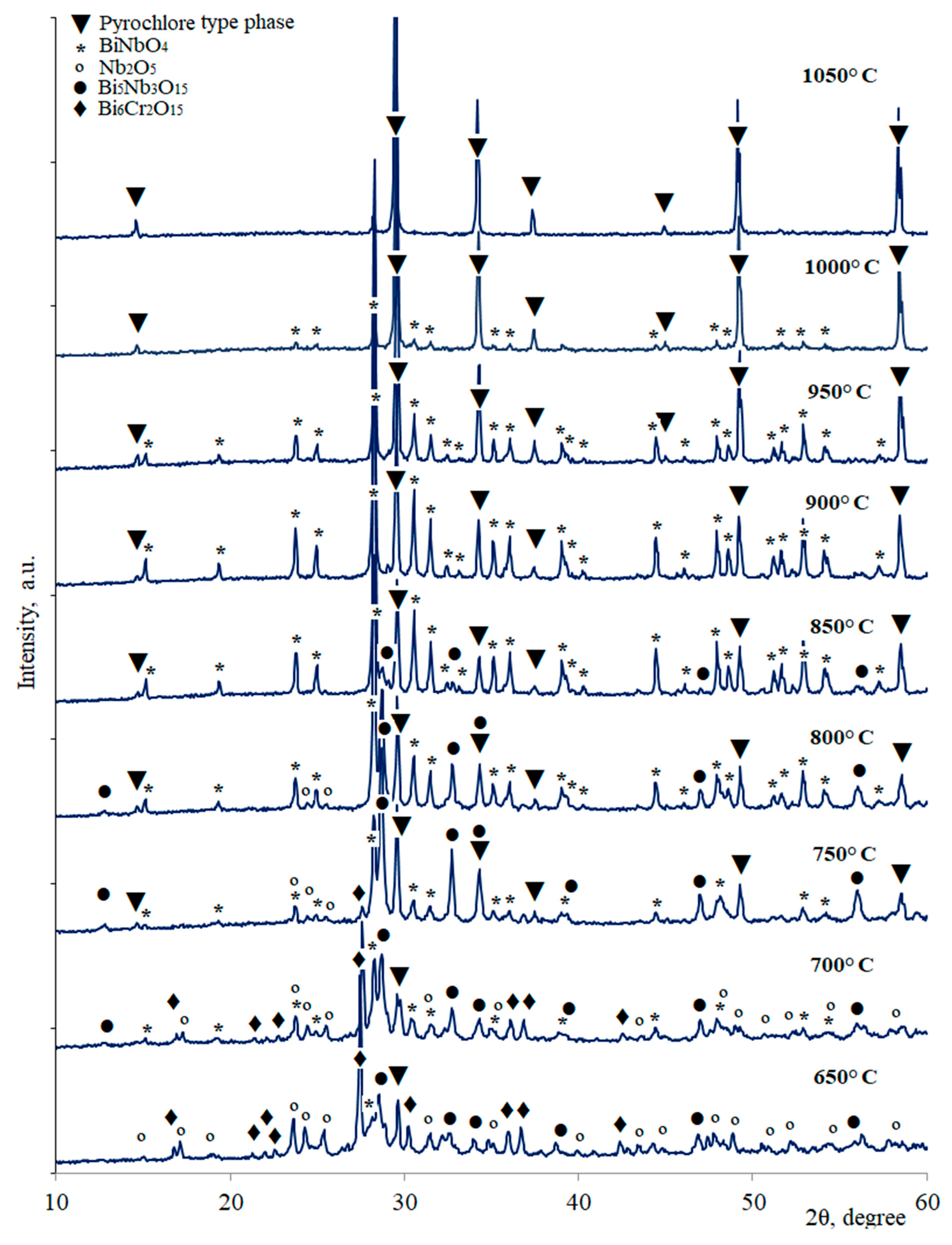
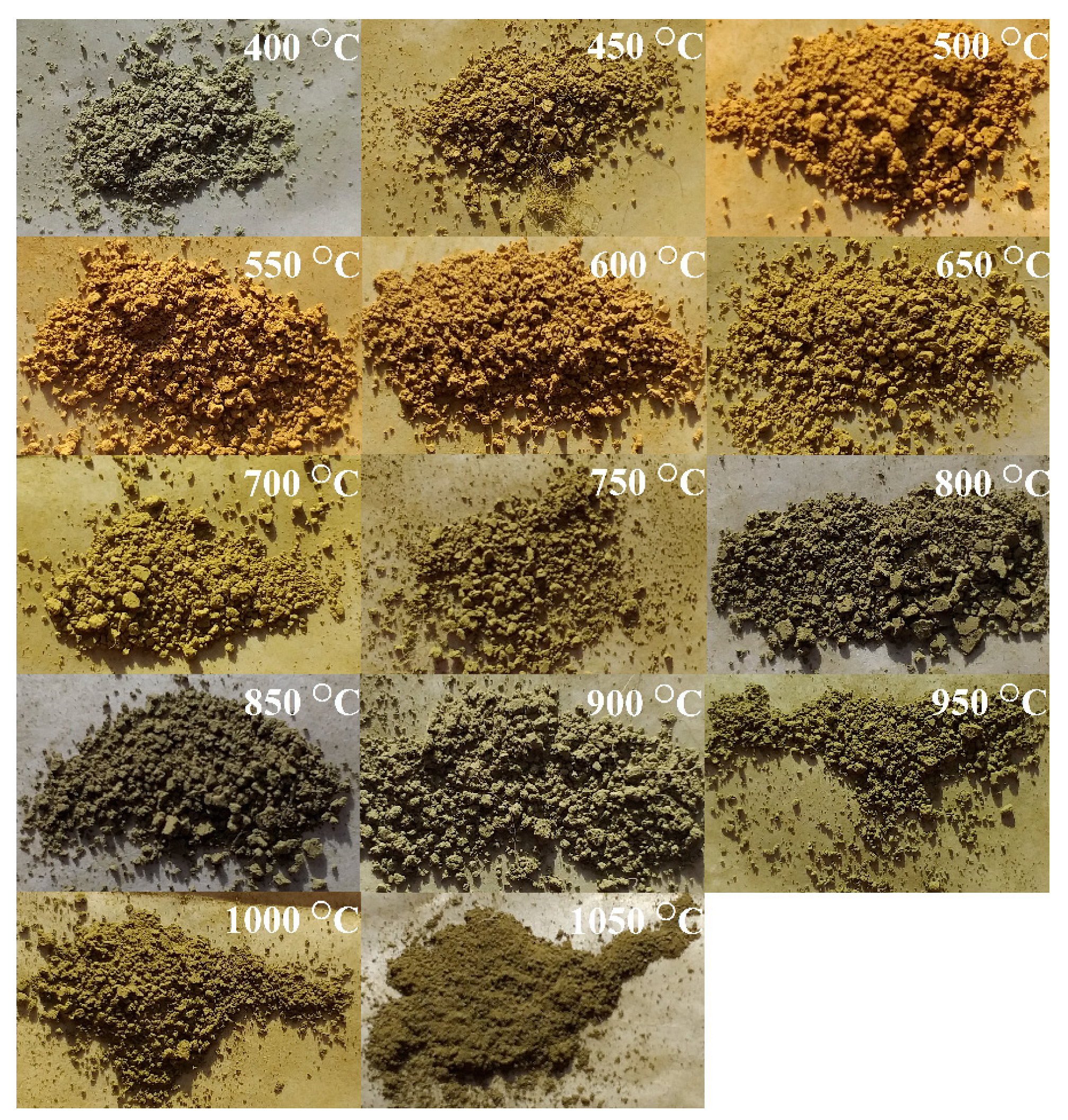

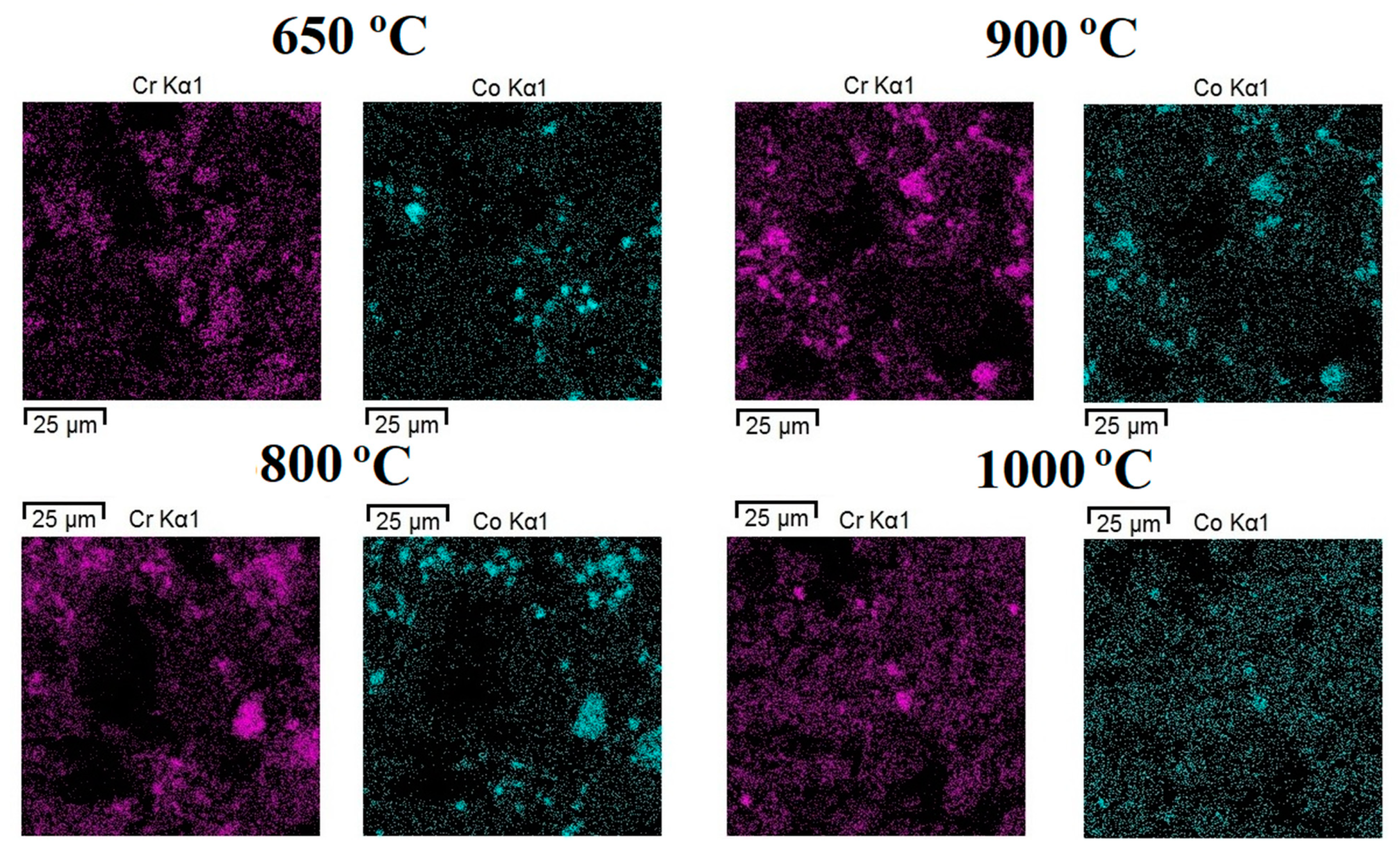
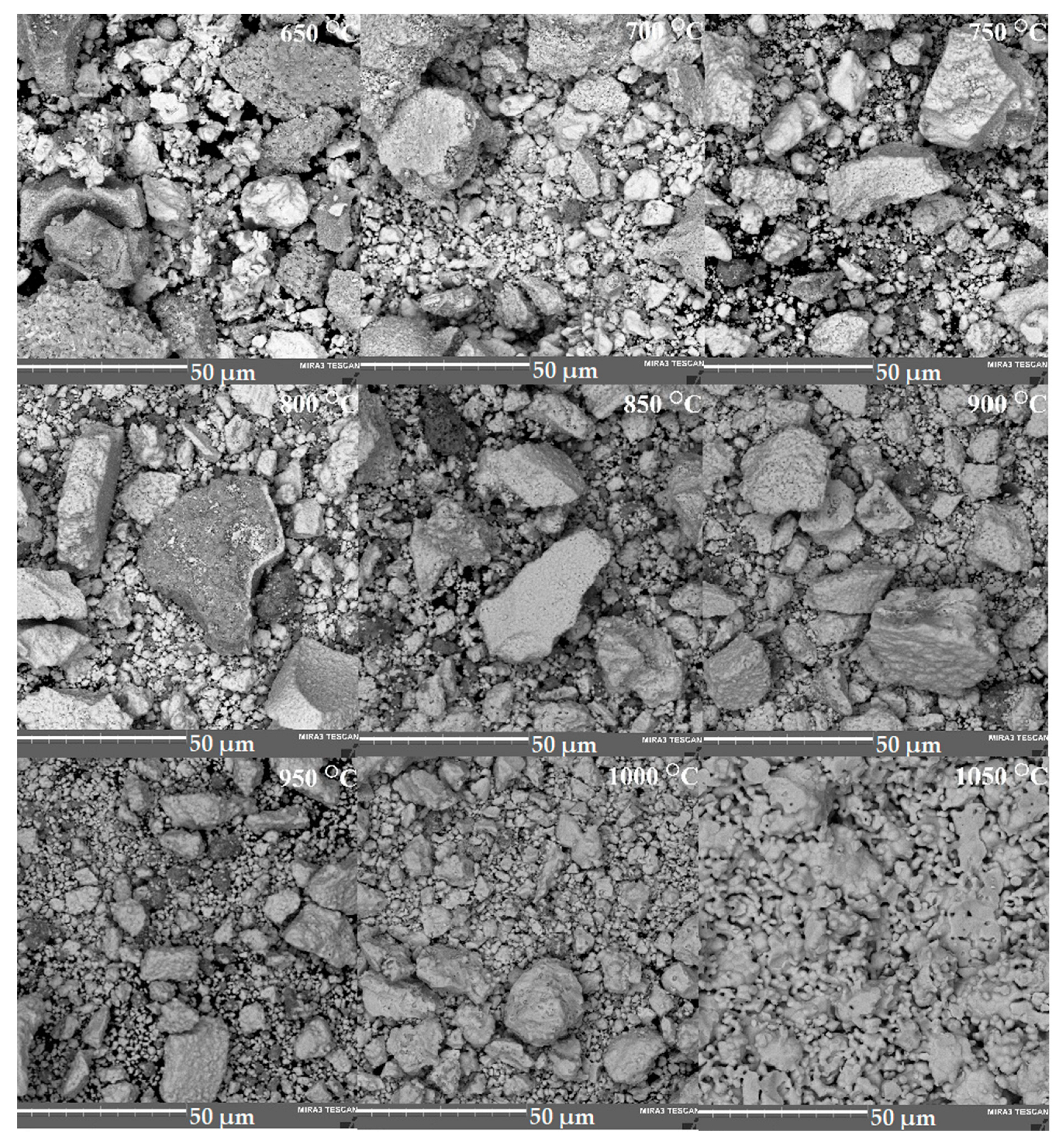

| Annealing Temperature, °C | Phase Composition |
|---|---|
| 400 | Nb2O5, α-Bi2O3 |
| 450 | Nb2O5, α-Bi2O3, β-Bi2O3 |
| 500–550 | Nb2O5, β-Bi2O3 |
| 600 | Nb2O5, Bi5Nb3O15(traces), Bi6CrO12, Bi6Cr2O15 |
| 650 | Nb2O5, Bi5Nb3O15, Bi6Cr2O15, BiNbO4(traces), pyrochlore (traces) |
| 700 | Nb2O5, Bi5Nb3O15, Bi6Cr2O15 (traces), BiNbO4, pyrochlore |
| 750 | Nb2O5, Bi5Nb3O15, BiNbO4, pyrochlore |
| 800 | Nb2O5 (traces), Bi5Nb3O15, BiNbO4, pyrochlore |
| 850 | Pyrochlore, Bi5Nb3O15(traces), BiNbO4 |
| 900–1000 | Pyrochlore, BiNbO4 |
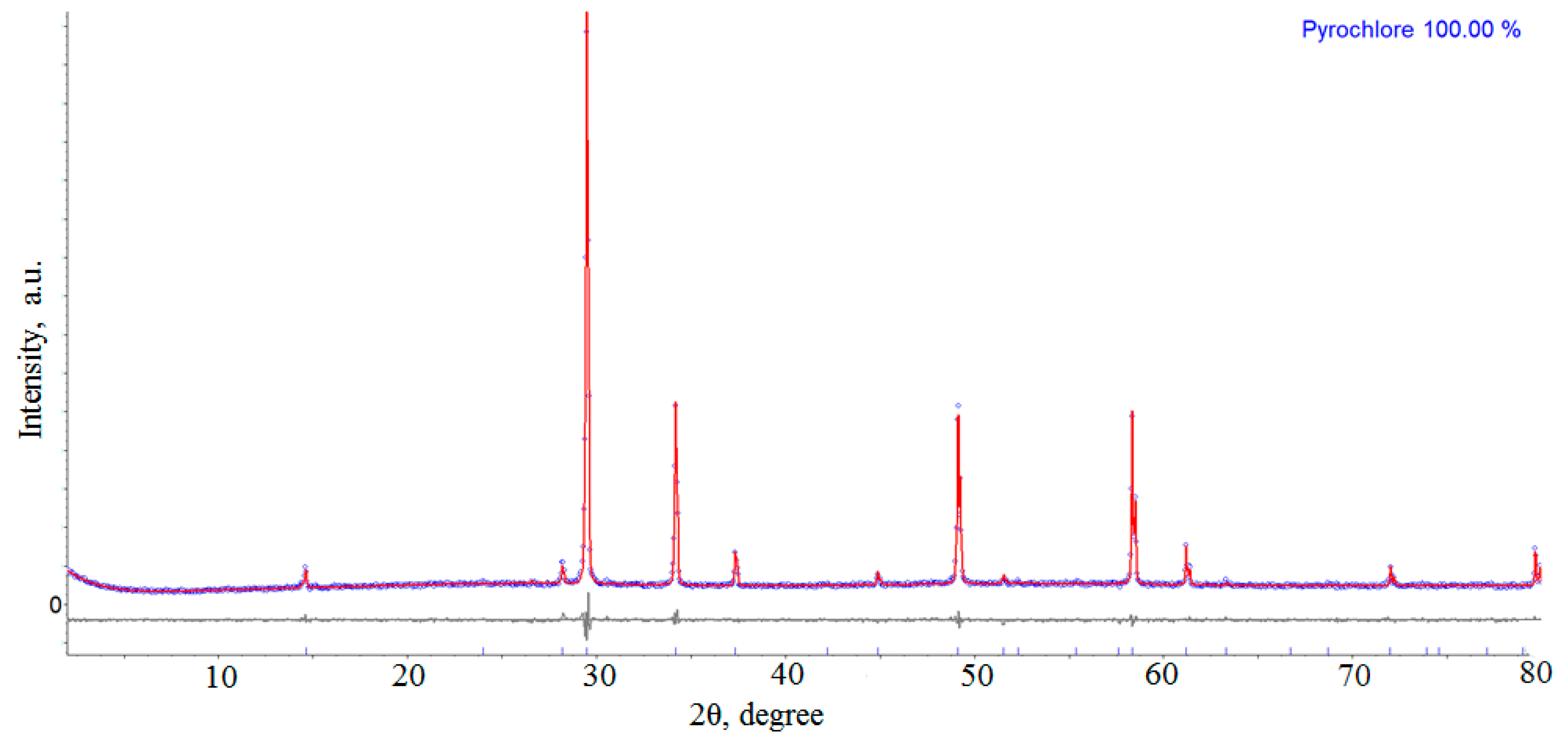
| 1050 | Pyrochlore |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuk, N.A.; Badanina, K.A.; Korolev, R.I.; Makeev, B.A.; Krzhizhanovskaya, M.G.; Kharton, V.V. Phase Formation of Co and Cr Co-Doped Bismuth Niobate with Pyrochlore Structure. Inorganics 2023, 11, 288. https://doi.org/10.3390/inorganics11070288
Zhuk NA, Badanina KA, Korolev RI, Makeev BA, Krzhizhanovskaya MG, Kharton VV. Phase Formation of Co and Cr Co-Doped Bismuth Niobate with Pyrochlore Structure. Inorganics. 2023; 11(7):288. https://doi.org/10.3390/inorganics11070288
Chicago/Turabian StyleZhuk, Nadezhda A., Ksenia A. Badanina, Roman I. Korolev, Boris A. Makeev, Maria G. Krzhizhanovskaya, and Vladislav V. Kharton. 2023. "Phase Formation of Co and Cr Co-Doped Bismuth Niobate with Pyrochlore Structure" Inorganics 11, no. 7: 288. https://doi.org/10.3390/inorganics11070288
APA StyleZhuk, N. A., Badanina, K. A., Korolev, R. I., Makeev, B. A., Krzhizhanovskaya, M. G., & Kharton, V. V. (2023). Phase Formation of Co and Cr Co-Doped Bismuth Niobate with Pyrochlore Structure. Inorganics, 11(7), 288. https://doi.org/10.3390/inorganics11070288








