In Situ Techniques for Characterization of Layered Double Hydroxide-Based Oxygen Evolution Catalysts
Abstract
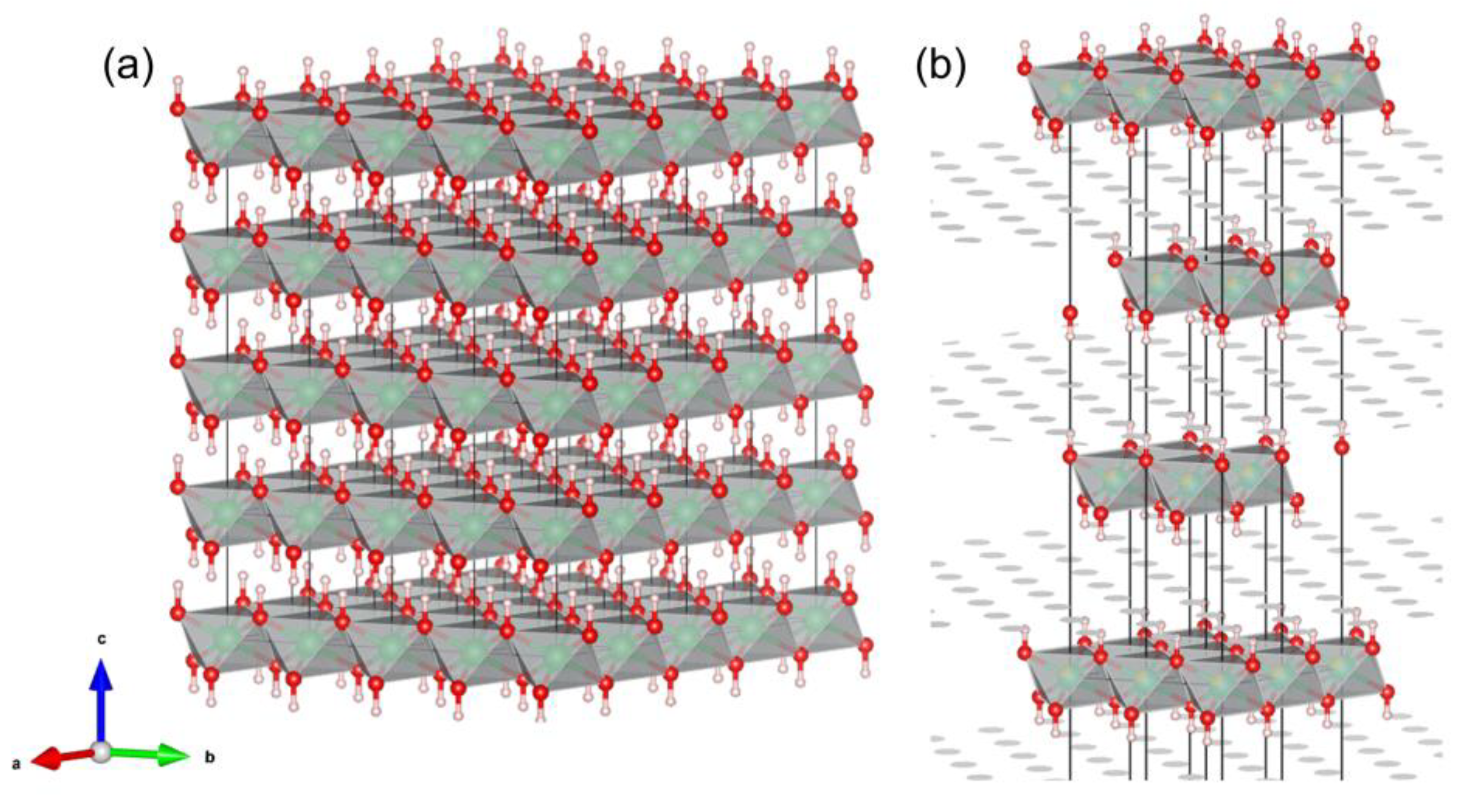
1. Introduction
2. In Situ UV-Vis
| Ni Valence | Form | Wavelength (nm) | Reference |
|---|---|---|---|
| Ni2+ | Ni2+(aq) | 230–250 | [49] |
| Ni2+ | α-Ni(OH)2 | 305 | [49] |
| Ni2+ | β-Ni(OH)2 | 290 | [49] |
| Ni3+ | β-NiOOH | 490–510 and 590–620 | [44,45] |
| Ni3+/4+ | γ-NiOOH | 430 | [44,45] |
| Ni4+ | β/γ-NiOOH | 600–650 | [48] |

3. In Situ Raman Spectroscopy
4. In Situ Infrared Spectroscopy
5. In Situ X-ray Absorption Spectroscopy

6. In Situ Mössbauer Spectroscopy
7. Miscellaneous In Situ Techniques
7.1. Online Application of Mass Spectrometry
7.2. Scanning Electrochemical Microscopy

7.3. Electrochemical Quartz Crystal Microbalance
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stevens, M.B.; Enman, L.J.; Batchellor, A.S.; Cosby, M.R.; Vise, A.E.; Trang, C.D.M.; Boettcher, S.W. Measurement Techniques for the Study of Thin Film Heterogeneous Water Oxidation Electrocatalysts. Chem. Mater. 2017, 29, 120–140. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S. Do the Evaluation Parameters Reflect Intrinsic Activity of Electrocatalysts in Electrochemical Water Splitting? ACS Energy Lett. 2019, 4, 1260–1264. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Appropriate Use of Electrochemical Impedance Spectroscopy in Water Splitting Electrocatalysis. Chemelectrochem 2020, 7, 2297–2308. [Google Scholar] [CrossRef]
- Banares, M.A. Operando methodology: Combination of in situ spectroscopy and simultaneous activity measurements under catalytic reaction conditions. Catal. Today 2005, 100, 71–77. [Google Scholar] [CrossRef]
- Mavrič, A.; Fanetti, M.; Lin, Y.; Valant, M.; Cui, C. Spectroelectrochemical Tracking of Nickel Hydroxide Reveals Its Irreversible Redox States upon Operation at High Current Density. ACS Catal. 2020, 10, 9451–9457. [Google Scholar] [CrossRef]
- Mavric, A.; Cui, C. Advances and Challenges in Industrial-Scale Water Oxidation on Layered Double Hydroxides. ACS Appl. Energy Mater. 2021, 4, 12032–12055. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A 2015, 471, 20140792. [Google Scholar] [CrossRef]
- Oliva, P.; Leonardi, J.; Laurent, J.F.; Delmas, C.; Braconnier, J.J.; Figlarz, M.; Fievet, F.; Guibert, A.d. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J. Power Sources 1982, 8, 229–255. [Google Scholar] [CrossRef]
- Desilvestro, J.; Corrigan, D.A.; Weaver, M.J. Characterization of Redox States of Nickel Hydroxide Film Electrodes by In Situ Surface Raman Spectroscopy. J. Electrochem. Soc. 1988, 135, 885–892. [Google Scholar] [CrossRef]
- Van der Ven, A.; Morgan, D.; Meng, Y.S.; Ceder, G. Phase Stability of Nickel Hydroxides and Oxyhydroxides. J. Electrochem. Soc. 2006, 153, A210–A215. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Zhang, Y.; Rao, G.; Wu, C.; Hu, Y.; Wang, X.; Lu, R.; Li, Y.; Xiong, J. Identification of Key Reversible Intermediates in Self-Reconstructed Nickel-Based Hybrid Electrocatalysts for Oxygen Evolution. Angew. Chem. Int. Ed. 2019, 58, 17458–17464. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Klaus, S.; Cai, Y.; Louie, M.W.; Trotochaud, L.; Bell, A.T. Effects of Fe Electrolyte Impurities on Ni(OH)(2)/NiOOH Structure and Oxygen Evolution Activity. J. Phys. Chem. C 2015, 119, 7243–7254. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, H.; Chen, X.; Liu, H.; Zhao, Y.; Li, H.; Xie, W.; Cheng, F.; Chen, J. Anion insertion enhanced electrodeposition of robust metal hydroxide/oxide electrodes for oxygen evolution. Nat. Commun. 2018, 9, 2373. [Google Scholar] [CrossRef]
- Mellsop, S.R.; Gardiner, A.; Johannessen, B.; Marshall, A.T. Structure and transformation of oxy-hydroxide films on Ni anodes below and above the oxygen evolution potential in alkaline electrolytes. Electrochim. Acta 2015, 168, 356–364. [Google Scholar] [CrossRef]
- Bode, H.; Dehmelt, K.; Witte, J. Zur Kenntnis der Nickelhydroxidelektrode. II. Über die Oxydationsprodukte von Nickel(II)-hydroxiden. Z. Anorg. Allg. Chem. 1969, 366, 1–21. [Google Scholar] [CrossRef]
- Cornilsen, B.C.; Shan, X.; Loyselle, P.L. Structural comparison of nickel electrodes and precursor phases. J. Power Sources 1990, 29, 453–466. [Google Scholar] [CrossRef]
- Huang, B.; Xu, H.Y.; Jiang, N.N.; Wang, M.H.; Huang, J.R.; Guan, L.H. Tensile-Strained RuO2 Loaded on Antimony-Tin Oxide by Fast Quenching for Proton-Exchange Membrane Water Electrolyzer. Adv. Sci. 2022, 9, 2201654. [Google Scholar] [CrossRef]
- Zhang, H.J.; Gao, Y.X.; Xu, H.Y.; Guan, D.Q.; Hu, Z.W.; Jing, C.; Sha, Y.C.; Gu, Y.X.; Huang, Y.C.; Chang, Y.C.; et al. Combined Corner-Sharing and Edge-Sharing Networks in Hybrid Nanocomposite with Unusual Lattice-Oxygen Activation for Efficient Water Oxidation. Adv. Funct. Mater. 2022, 32, 07618. [Google Scholar] [CrossRef]
- Guan, D.; Sh, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, X.; Li, J.P.; Yang, P.; Cheng, X. Phase and morphology evolution of ultrathin Co(OH)2 nanosheets towards supercapacitor application. Crystengcomm 2017, 19, 5780–5786. [Google Scholar] [CrossRef]
- Darbandi, M.; Shaabani, B.; Alizadeh, A.; Yardani, P.; Shahryari, E.; Hosseini, M.G. Preparation and characterization of hexagonal mesoporous beta-Co(OH)2 nanorings. Micropor. Mesopor. Mat. 2019, 284, 421–426. [Google Scholar] [CrossRef]
- Barde, F.; Palacin, M.R.; Beaudoin, B.; Delahaye-Vidal, A.; Tarascon, J.M. New approaches for synthesizing gamma III-CoOOH by soft chemistry. Chem. Mater. 2004, 16, 299–306. [Google Scholar] [CrossRef]
- Dionigi, F.; Zhu, J.; Zheng, Z.; Merzdorf, T.; Sarodnik, H.; Gliech, M.; Pan, L.; Li, W.X.; Greeley, J.; Strasser, P. Intrinsic Electrocatalytic Activity for Oxygen Evolution of Crystalline 3d-Transition Metal Layered Double Hydroxides. Angew. Chem. Int. Ed. 2021, 60, 14446–14457. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.I.; Koseki, M.; Kumagai, N. Effect of cobalt addition to nickel hydroxide as a positive material for rechargeable alkaline batteries. J. Power Sources 1996, 58, 23–28. [Google Scholar] [CrossRef]
- Delmas, C.; Tessier, C. Stacking faults in the structure of nickel hydroxide: A rationale of its high electrochemical activity. J. Mater. Chem. 1997, 7, 1439–1443. [Google Scholar] [CrossRef]
- Huang, J.C.; Lei, T.; Wei, X.P.; Liu, X.W.; Liu, T.; Cao, D.X.; Yin, J.L.; Wang, G.L. Effect of Al-doped beta-Ni(OH)2 nanosheets on electrochemical behaviors for high performance supercapacitor application. J. Power Sources 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Jayashree, R.S.; Kamath, P.V. Suppression of the alpha -> beta-nickel hydroxide transformation in concentrated alkali: Role of dissolved cations. J. Appl. Electrochem. 2001, 31, 1315–1320. [Google Scholar] [CrossRef]
- Kamath, P.V.; Dixit, M.; Indira, L.; Shukla, A.K.; Kumar, V.G.; Munichandraiah, N. Stabilized α-Ni(OH)2 as Electrode Material for Alkaline Secondary Cells. J. Electrochem. Soc. 1994, 141, 2956–2959. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wang, J.M.; Chen, H.; Pan, T.; Zhang, J.Q.; Cao, C.N. Different additives-substituted alpha-nickel hydroxide prepared by urea decomposition. Electrochim. Acta 2004, 50, 91–98. [Google Scholar] [CrossRef]
- Demourgues-Guerlou, L.; Delmas, C. Structure and properties of precipitated nickel-iron hydroxides. J. Power Sources 1993, 45, 281–289. [Google Scholar] [CrossRef]
- Liu, C.J.; Huang, L.H.; Li, Y.W.; Sun, D. Synthesis and electrochemical performance of amorphous nickel hydroxide codoped with Fe3+ and CO32−. Ionics 2010, 16, 215–219. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, X.M.; van Bommel, A.; Rowe, A.W.; Dahn, J.R. Coprecipitation Synthesis of NixMn1-x(OH)(2) Mixed Hydroxides. Chem. Mater. 2010, 22, 1015–1021. [Google Scholar] [CrossRef]
- Luan, C.; Corva, M.; Hagemann, U.; Wang, H.; Heidelmann, M.; Tschulik, K.; Li, T. Atomic-Scale Insights into Morphological, Structural, and Compositional Evolution of CoOOH during Oxygen Evolution Reaction. ACS Catal. 2023, 13, 1400–1411. [Google Scholar] [CrossRef]
- Bott-Neto, J.L.; Rodrigues, M.V.F.; Silva, M.C.; Carneiro-Neto, E.B.; Wosiak, G.; Mauricio, J.C.; Pereira, E.C.; Figueroa, S.J.A.; Fernandez, P.S. Versatile Spectroelectrochemical Cell for In Situ Experiments: Development, Applications, and Electrochemical Behavior. Chemelectrochem 2020, 7, 4306–4313. [Google Scholar] [CrossRef]
- Trzesniewski, B.J.; Diaz-Morales, O.; Vermaas, D.A.; Longo, A.; Bras, W.; Koper, M.T.M.; Smith, W.A. In Situ Observation of Active Oxygen Species in Fe-Containing Ni-Based Oxygen Evolution Catalysts: The Effect of pH on Electrochemical Activity. J. Am. Chem. Soc 2015, 137, 15112–15121. [Google Scholar] [CrossRef]
- Tang, F.; Liu, T.; Jiang, W.L.; Gan, L. Windowless thin layer electrochemical Raman spectroscopy of Ni-Fe oxide electrocatalysts during oxygen evolution reaction. J. Electroanal. Chem. 2020, 871, 114282. [Google Scholar] [CrossRef]
- Nazri, G.; Corrigan, D.A.; Maheswari, S.P. Angle-resolved infrared spectroelectrochemistry. 1. An in situ study of thin-film nickel oxide electrodes. Langmuir 1989, 5, 17–22. [Google Scholar] [CrossRef]
- Drevon, D.; Gorlin, M.; Chernev, P.; Xi, L.F.; Dau, H.; Lange, K.M. Uncovering The Role of Oxygen in Ni-Fe(OxHy) Electrocatalysts using In situ Soft X-ray Absorption Spectroscopy during the Oxygen Evolution Reaction. Sci. Rep. 2019, 9, 1532. [Google Scholar] [CrossRef]
- Chen, J.Y.C.; Dang, L.N.; Liang, H.F.; Bi, W.L.; Gerken, J.B.; Jin, S.; Alp, E.E.; Stahl, S.S. Operando Analysis of NiFe and Fe Oxyhydroxide Electrocatalysts for Water Oxidation: Detection of Fe4+ by Mossbauer Spectroscopy. J. Am. Chem. Soc. 2015, 137, 15090–15093. [Google Scholar] [CrossRef]
- Zaharieva, I.; Gonzalez-Flores, D.; Asfari, B.; Pasquini, C.; Mohammadi, M.R.; Klingan, K.; Zizak, I.; Loos, S.; Chernev, P.; Dau, H. Water oxidation catalysis—Role of redox and structural dynamics in biological photosynthesis and inorganic manganese oxides. Energy Environ. Sci. 2016, 9, 2433–2443. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Pasquini, C.; Loos, S.; Chernev, P.; Klingan, K.; Kubella, P.; Mohammadi, M.R.; Gonzalez-Flores, D.; Dau, H. Spectroscopic identification of active sites for the oxygen evolution reaction on iron-cobalt oxides. Nat. Commun. 2017, 8, 2022. [Google Scholar] [CrossRef] [PubMed]
- Francas, L.; Corby, S.; Selim, S.; Lee, D.; Mesa, C.A.; Godin, R.; Pastor, E.; Stephens, I.E.L.; Choi, K.-S.; Durrant, J.R. Spectroelectrochemical study of water oxidation on nickel and iron oxyhydroxide electrocatalysts. Nat. Commun. 2019, 10, 5208. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Floner, D.; Beden, B.; Lamy, C. In situ investigation of the behaviour of a nickel electrode in alkaline solution by UV-Vis and IR reflectance spectroscopies. Electrochim. Acta 1987, 32, 1631–1636. [Google Scholar] [CrossRef]
- Corrigan, D.A.; Knight, S.L. Electrochemical and Spectroscopic Evidence on the Participation of Quadrivalent Nickel in the Nickel Hydroxide Redox Reaction. J. Electrochem. Soc. 1989, 136, 613–619. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Q.; Zhang, Q.; Xiao, Y.; Zhong, X.; Dong, G.; Delplancke-Ogletree, M.-P.; Terryn, H.; Baert, K.; Reniers, F.; et al. In situ electrochromic efficiency of a nickel oxide thin film: Origin of electrochemical process and electrochromic degradation. J. Mater. Chem. C 2018, 6, 646–653. [Google Scholar] [CrossRef]
- Sahu, D.R.; Wu, T.-J.; Wang, S.-C.; Huang, J.-L. Electrochromic behavior of NiO film prepared by e-beam evaporation. J. Sci.-Adv. Mater. Dev. 2017, 2, 225–232. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, Y.; Wu, W.; Mao, Z.; Xu, H.; Ma, Y. Kinetics of Active Oxide Species Derived from a Metallic Nickel Surface for Efficient Electrocatalytic Water Oxidation. ACS Energy Lett. 2022, 7, 3276–3285. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, M.J.; Li, F.; Xie, J.; Li, Y.; He, J.B. Trace Fe Incorporation into Ni-(oxy)hydroxide Stabilizes Ni3+ Sites for Anodic Oxygen Evolution: A Double Thin-Layer Study. Langmuir 2020, 36, 5126–5133. [Google Scholar] [CrossRef]
- Bendert, R.M.; Corrigan, D.A. Effect of Coprecipitated Metal Ions on the Electrochromic Properties of Nickel Hydroxide. J. Electrochem. Soc. 1989, 136, 1369–1374. [Google Scholar] [CrossRef]
- Gorlin, M.; de Araujo, J.F.; Schmies, H.; Bernsmeier, D.; Dresp, S.; Gliech, M.; Jusys, Z.; Chernev, P.; Kraehnert, R.; Dau, H.; et al. Tracking Catalyst Redox States and Reaction Dynamics in Ni-Fe Oxyhydroxide Oxygen Evolution Reaction Electrocatalysts: The Role of Catalyst Support and Electrolyte pH. J. Am. Chem. Soc. 2017, 139, 2070–2082. [Google Scholar] [CrossRef]
- Loos, S.; Zaharieva, I.; Chernev, P.; Lissner, A.; Dau, H. Electromodified NiFe Alloys as Electrocatalysts for Water Oxidation: Mechanistic Implications of Time-Resolved UV/Vis Tracking of Oxidation State Changes. Chemsuschem 2019, 12, 1966–1976. [Google Scholar] [CrossRef]
- Burke, M.S.; Zou, S.H.; Enman, L.J.; Kellon, J.E.; Gabor, C.A.; Pledger, E.; Boettcher, S.W. Revised Oxygen Evolution Reaction Activity Trends for First-Row Transition-Metal (Oxy)hydroxides in Alkaline Media. J. Phys. Chem. Lett. 2015, 6, 3737–3742. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Pasquini, C.; Loos, S.; Chernev, P.; Klingan, K.; Kubella, P.; Mohammadi, M.R.; Gonzalez-Flores, D.; Dau, H. Geometric distortions in nickel (oxy)hydroxide electrocatalysts by redox inactive iron ions. Energy Environ. Sci. 2018, 11, 2476–2485. [Google Scholar] [CrossRef]
- Goldsmith, Z.K.; Harshan, A.K.; Gerken, J.B.; Voros, M.; Galli, G.; Stahl, S.S.; Hammes-Schiffer, S. Characterization of NiFe oxyhydroxide electrocatalysts by integrated electronic structure calculations and spectroelectrochemistry. Proc. Natl. Acad. Sci. USA 2017, 114, 3050–3055. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An Investigation of Thin-Film Ni-Fe Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef]
- Gao, P.; Gosztola, D.; Leung, L.-W.H.; Weaver, M.J. Surface-enhanced Raman scattering at gold electrodes: Dependence on electrochemical pretreatment conditions and comparisons with silver. J. Electroanal. Chem. Interf. Electrochem. 1987, 233, 211–222. [Google Scholar] [CrossRef]
- Desilvestro, J.; Weaver, M.J. Surface structural changes during oxidation of gold electrodes in aqueous media as detected using surface-enhanced Raman spectroscopy. J. Electroanal. Chem. Interf. Electrochem. 1986, 209, 377–386. [Google Scholar] [CrossRef]
- Yeo, B.S.; Bell, A.T. In Situ Raman Study of Nickel Oxide and Gold-Supported Nickel Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Phys. Chem. C 2012, 116, 8394–8400. [Google Scholar] [CrossRef]
- Kostecki, R.; McLarnon, F. Electrochemical and in situ Raman spectroscopic characterization of nickel hydroxide electrodes. J. Electrochem. Soc. 1997, 144, 485–493. [Google Scholar] [CrossRef]
- Merrill, M.; Worsley, M.; Wittstock, A.; Biener, J.; Stadermann, M. Determination of the “NiOOH” charge and discharge mechanisms at ideal activity. J. Electroanal. Chem. 2014, 717, 177–188. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Ferrus-Suspedra, D.; Koper, M.T.M. The importance of nickel oxyhydroxide deprotonation on its activity towards electrochemical water oxidation. Chem. Sci. 2016, 7, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Hwang, B.J. In situ Raman studies on cathodically deposited nickel hydroxide films and electroless Ni-P electrodes in 1 M KOH solution. Langmuir 1998, 14, 944–950. [Google Scholar] [CrossRef]
- Chen, D.C.; Xiong, X.H.; Zhao, B.T.; Mahmoud, M.A.; El-Sayed, M.A.; Liu, M.L. Probing Structural Evolution and Charge Storage Mechanism of NiO2Hx Electrode Materials using In Operando Resonance Raman Spectroscopy. Adv. Sci. 2016, 3, 1500433. [Google Scholar] [CrossRef] [PubMed]
- Moysiadou, A.; Lee, S.; Hsu, C.-S.; Chen, H.M.; Hu, X. Mechanism of Oxygen Evolution Catalyzed by Cobalt Oxyhydroxide: Cobalt Superoxide Species as a Key Intermediate and Dioxygen Release as a Rate-Determining Step. J. Am. Chem. Soc. 2020, 142, 11901–11914. [Google Scholar] [CrossRef]
- Yeo, B.S.; Bell, A.T. Enhanced Activity of Gold-Supported Cobalt Oxide for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2011, 133, 5587–5593. [Google Scholar] [CrossRef]
- Ye, S.; Wang, J.; Hu, J.; Chen, Z.; Zheng, L.; Fu, Y.; Lei, Y.; Ren, X.; He, C.; Zhang, Q.; et al. Electrochemical Construction of Low-Crystalline CoOOH Nanosheets with Short-Range Ordered Grains to Improve Oxygen Evolution Activity. ACS Catal. 2021, 11, 6104–6112. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Silva, S.V.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Steimecke, M.; Seiffarth, G.; Bron, M. In Situ Characterization of Ni and Ni/Fe Thin Film Electrodes for Oxygen Evolution in Alkaline Media by a Raman-Coupled Scanning Electrochemical Microscope Setup. Anal. Chem. 2017, 89, 10679–10686. [Google Scholar] [CrossRef]
- Lee, S.; Bai, L.C.; Hu, X.L. Deciphering Iron-Dependent Activity in Oxygen Evolution Catalyzed by Nickel-Iron Layered Double Hydroxide. Angew. Chem. Int. Ed. 2020, 59, 8072–8077. [Google Scholar] [CrossRef]
- Lee, S.; Banjac, K.; Lingenfelder, M.; Hu, X. Oxygen Isotope Labeling Experiments Reveal Different Reaction Sites for the Oxygen Evolution Reaction on Nickel and Nickel Iron Oxides. Angew. Chem. Int. Ed. 2019, 58, 10295–10299. [Google Scholar] [CrossRef]
- Bo, X.; Li, Y.B.; Chen, X.J.; Zhao, C. Operando Raman Spectroscopy Reveals Cr-Induced-Phase Reconstruction of NiFe and CoFe Oxyhydroxides for Enhanced Electrocatalytic Water Oxidation. Chem. Mater. 2020, 32, 4303–4311. [Google Scholar] [CrossRef]
- Sagui, N.A.; Strom, P.; Edvinsson, T.; Pehlivan, I.B. Nickel Site Modification by High-Valence Doping: Effect of TantalumImpurities on the Alkaline Water Electro-Oxidation by NiO Probedby Operando Raman Spectroscopy. ACS Catal. 2022, 12, 6506–6516. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Poirier, S.; Bock, C.; MacDougall, B.R. Applications of in Situ Raman Spectroscopy for Identifying Nickel Hydroxide Materials and Surface Layers during Chemical Aging. ACS Appl. Mater. Inter. 2014, 6, 3141–3149. [Google Scholar] [CrossRef]
- Qiu, Z.; Tai, C.-W.; Niklasson, G.A.; Edvinsson, T. Direct observation of active catalyst surface phases and the effect of dynamic self-optimization in NiFe-layered double hydroxides for alkaline water splitting. Energy Environ. Sci. 2019, 12, 572–581. [Google Scholar] [CrossRef]
- Desilvestro, J.; Corrigan, D.A.; Weaver, M.J. Spectroelectrochemistry of thin nickel hydroxide films on gold using surface-enhanced Raman spectroscopy. J. Phys. Chem. 1986, 90, 6408–6411. [Google Scholar] [CrossRef]
- Yu, P.C.; Lampert, C.M. In-situ spectroscopic studies of electrochromic hydrated nickel-oxide films. Sol. Energy Mater. 1989, 19, 1–16. [Google Scholar] [CrossRef]
- Zhang, M.; Frei, H. Water Oxidation Mechanisms of Metal Oxide Catalysts by Vibrational Spectroscopy of Transient Intermediates. Annu. Rev. Phys. Chem. 2017, 68, 209–231. [Google Scholar] [CrossRef]
- Mansour, A.N.; Melendres, C.A. Analysis of X-ray absorption spectra of some nickel oxycompounds using theoretical standards. J. Phys. Chem. A 1998, 102, 65–81. [Google Scholar] [CrossRef]
- Goerlin, M.; Chernev, P.; de Araujo, J.F.; Reier, T.; Dresp, S.; Paul, B.; Kraehnert, R.; Dau, H.; Strasser, P. Oxygen Evolution Reaction Dynamics, Faradaic Charge Efficiency, and the Active Metal Redox States of Ni-Fe Oxide Water Splitting Electrocatalysts. J. Am. Chem. Soc. 2016, 138, 5603–5614. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.J.; Sokaras, D.; Weng, T.C.; Alonso-Mori, R.; et al. Identification of Highly Active Fe Sites in (Ni,Fe)OOH for Electrocatalytic Water Splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef]
- Gonzalez-Flores, D.; Klingan, K.; Chernev, P.; Loos, S.; Mohammadi, M.R.; Pasquini, C.; Kubella, P.; Zaharieva, I.; Smith, R.D.L.; Dau, H. Nickel-iron catalysts for electrochemical water oxidation—Redox synergism investigated by in situ X-ray spectroscopy with millisecond time resolution. Sustain. Energy Fuels 2018, 2, 1986–1994. [Google Scholar] [CrossRef]
- Abbott, D.F.; Fabbri, E.; Borlaf, M.; Bozza, F.; Schaublin, R.; Nachtegaal, M.; Graule, T.; Schmidt, T.J. Operando X-ray absorption investigations into the role of Fe in the electrochemical stability and oxygen evolution activity of Ni1−xFexOy nanoparticles. J. Mater. Chem. A 2018, 6, 24534–24549. [Google Scholar] [CrossRef]
- Trzesniowski, H.; Deka, N.; van der Heijden, O.; Golnak, R.; Xiao, J.; Koper, M.T.M.; Seidel, R.; Mom, R.V. Reversible and Irreversible Cation Intercalation in NiFeOX Oxygen Evolution Catalysts in Alkaline Media. J. Phys. Chem. Lett. 2023, 14, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kuai, C.; Zhang, Y.; Wu, D.; Sokaras, D.; Mu, L.; Spence, S.; Nordlund, D.; Lin, F.; Du, X.-W. Fully Oxidized Ni-Fe Layered Double Hydroxide with 100% Exposed Active Sites for Catalyzing Oxygen Evolution Reaction. ACS Catal. 2019, 9, 6027–6032. [Google Scholar] [CrossRef]
- Yu, H.M.; Yang, X.; Xiao, X.; Chen, M.; Zhang, Q.H.; Huang, L.; Wu, J.B.; Li, T.Q.; Chen, S.M.; Song, L.; et al. Atmospheric-Pressure Synthesis of 2D Nitrogen-Rich Tungsten Nitride. Adv. Mater. 2018, 30, 05655. [Google Scholar] [CrossRef]
- Kuai, C.; Xu, Z.; Xi, C.; Hu, A.; Yang, Z.; Zhang, Y.; Sun, C.-J.; Li, L.; Sokaras, D.; Dong, C.; et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts. Nat. Catal. 2020, 3, 743–753. [Google Scholar] [CrossRef]
- Enman, L.J.; Stevens, M.B.; Dahan, M.H.; Nellist, M.R.; Toroker, M.C.; Boettcher, S.W. Operando X-Ray Absorption Spectroscopy Shows Iron Oxidation Is Concurrent with Oxygen Evolution in Cobalt-Iron (Oxy)hydroxide Electrocatalysts. Angew. Chem. Int. Ed. 2018, 57, 12840–12844. [Google Scholar] [CrossRef]
- Pasquini, C.; Zaharieva, I.; Gonzalez-Flores, D.; Chernev, P.; Mohammadi, M.R.; Guidoni, L.; Smith, R.D.L.; Dau, H. H/D Isotope Effects Reveal Factors Controlling Catalytic Activity in Co-Based Oxides for Water Oxidation. J. Am. Chem. Soc. 2019, 141, 2938–2948. [Google Scholar] [CrossRef]
- Risch, M.; Klingan, K.; Heidkamp, J.; Ehrenberg, D.; Chernev, P.; Zaharieva, I.; Dau, H. Nickel-oxido structure of a water-oxidizing catalyst film. Chem. Commun. 2011, 47, 11912–11914. [Google Scholar] [CrossRef]
- Risch, M.; Khare, V.; Zaharieva, I.; Gerencser, L.; Chernev, P.; Dau, H. Cobalt-Oxo Core of a Water-Oxidizing Catalyst Film. J. Am. Chem. Soc. 2009, 131, 6936. [Google Scholar] [CrossRef]
- Niemantsverdriet, J.W.; Delgass, W.N. In situ Mossbauer spectroscopy in catalysis. Top. Catal. 1999, 8, 133–140. [Google Scholar] [CrossRef]
- Kasian, O.; Geiger, S.; Mayrhofer, K.J.J.; Cherevko, S. Electrochemical On-line ICP-MS in Electrocatalysis Research. Chem. Rec. 2019, 19, 2130–2142. [Google Scholar] [CrossRef]
- Kasian, O.; Geiger, S.; Li, T.; Grote, J.P.; Schweinar, K.; Zhang, S.Y.; Scheu, C.; Raabe, D.; Cherevko, S.; Gault, B.; et al. Degradation of iridium oxides via oxygen evolution from the lattice: Correlating atomic scale structure with reaction mechanisms. Energy Environ. Sci. 2019, 12, 3548–3555. [Google Scholar] [CrossRef]
- Kasian, O.; Geiger, S.; Stock, P.; Polymeros, G.; Breitbach, B.; Savan, A.; Ludwig, A.; Cherevko, S.; Mayrhofer, K.J.J. On the Origin of the Improved Ruthenium Stability in RuO2-IrO2 Mixed Oxides. J. Electrochem. Soc. 2016, 163, F3099–F3104. [Google Scholar] [CrossRef]
- Ko, Y.J.; Han, M.H.; Kim, H.; Kim, J.Y.; Lee, W.H.; Kim, J.; Kwak, J.Y.; Kim, C.H.; Park, T.E.; Yu, S.H.; et al. Unraveling Ni-Fe 2D nanostructure with enhanced oxygen evolution via in situ and operando spectroscopies. Chem Catal. 2022, 2, 2312–2327. [Google Scholar] [CrossRef]
- Oliver-Tolentino, M.A.; Vazquez-Samperio, J.; Manzo-Robledo, A.; Gonzalez-Huerta, R.D.; Flores-Moreno, J.L.; Ramirez-Rosales, D.; Guzman-Vargas, A. An Approach to Understanding the Electrocatalytic Activity Enhancement by Superexchange Interaction toward OER in Alkaline Media of Ni-Fe LDH. J. Phys. Chem. C 2014, 118, 22432–22438. [Google Scholar] [CrossRef]
- Dionigi, F.; Zeng, Z.H.; Sinev, I.; Merzdorf, T.; Deshpande, S.; Lopez, M.B.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.X.; et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef]
- Roy, C.; Sebok, B.; Scott, S.B.; Fiordaliso, E.M.; Sorensen, J.E.; Bodin, A.; Trimarco, D.B.; Damsgaard, C.D.; Vesborg, P.C.K.; Hansen, O.; et al. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 2018, 1, 820–829. [Google Scholar] [CrossRef]
- De Araujo, J.F.; Dionigi, F.; Merzdorf, T.; Oh, H.S.; Strasser, P. Evidence of Mars-Van-Krevelen Mechanism in the Electrochemical Oxygen Evolution on Ni-Based Catalysts. Angew. Chem. Int. Ed. 2021, 60, 14981–14988. [Google Scholar] [CrossRef]
- Bard, A.J.; Fan, F.R.F.; Kwak, J.; Lev, O. Scanning Electrochemical Microscopy—Introduction and Principles. Anal. Chem. 1989, 61, 132–138. [Google Scholar] [CrossRef]
- Kai, T.H.; Zoski, C.G.; Bard, A.J. Scanning electrochemical microscopy at the nanometer level. Chem. Commun. 2018, 54, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Bard, A.J. Surface Interrogation Scanning Electrochemical Microscopy of Ni1−xFexOOH (0 < x < 0.27) Oxygen Evolving Catalyst: Kinetics of the “fast” Iron Sites. J. Am. Chem. Soc. 2016, 138, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lopez, J.; Alpuche-Aviles, M.A.; Bard, A.J. Interrogation of Surfaces for the Quantification of Adsorbed Species on Electrodes: Oxygen on Gold and Platinum in Neutral Media. J. Am. Chem. Soc. 2008, 130, 16985–16995. [Google Scholar] [CrossRef]
- Ahn, H.S.; Bard, A.J. Surface Interrogation of CoPi Water Oxidation Catalyst by Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2015, 137, 612–615. [Google Scholar] [CrossRef]
- Wehrens-Dijksma, M.; Notten, P.H.L. Electrochemical Quartz Microbalance characterization of Ni(OH)2-based thin film electrodes. Electrochim. Acta 2006, 51, 3609–3621. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrič, A.; Valant, M. In Situ Techniques for Characterization of Layered Double Hydroxide-Based Oxygen Evolution Catalysts. Inorganics 2023, 11, 296. https://doi.org/10.3390/inorganics11070296
Mavrič A, Valant M. In Situ Techniques for Characterization of Layered Double Hydroxide-Based Oxygen Evolution Catalysts. Inorganics. 2023; 11(7):296. https://doi.org/10.3390/inorganics11070296
Chicago/Turabian StyleMavrič, Andraž, and Matjaž Valant. 2023. "In Situ Techniques for Characterization of Layered Double Hydroxide-Based Oxygen Evolution Catalysts" Inorganics 11, no. 7: 296. https://doi.org/10.3390/inorganics11070296
APA StyleMavrič, A., & Valant, M. (2023). In Situ Techniques for Characterization of Layered Double Hydroxide-Based Oxygen Evolution Catalysts. Inorganics, 11(7), 296. https://doi.org/10.3390/inorganics11070296







