MoO3 Solubility and Chemical Durability of V2O5-Bearing Borosilicate Glass
Abstract
1. Introduction
2. Experimental
2.1. Strategy
2.2. Phase Equilibrium Experiments
2.3. Chemical Durability Test
3. Results and Discussion
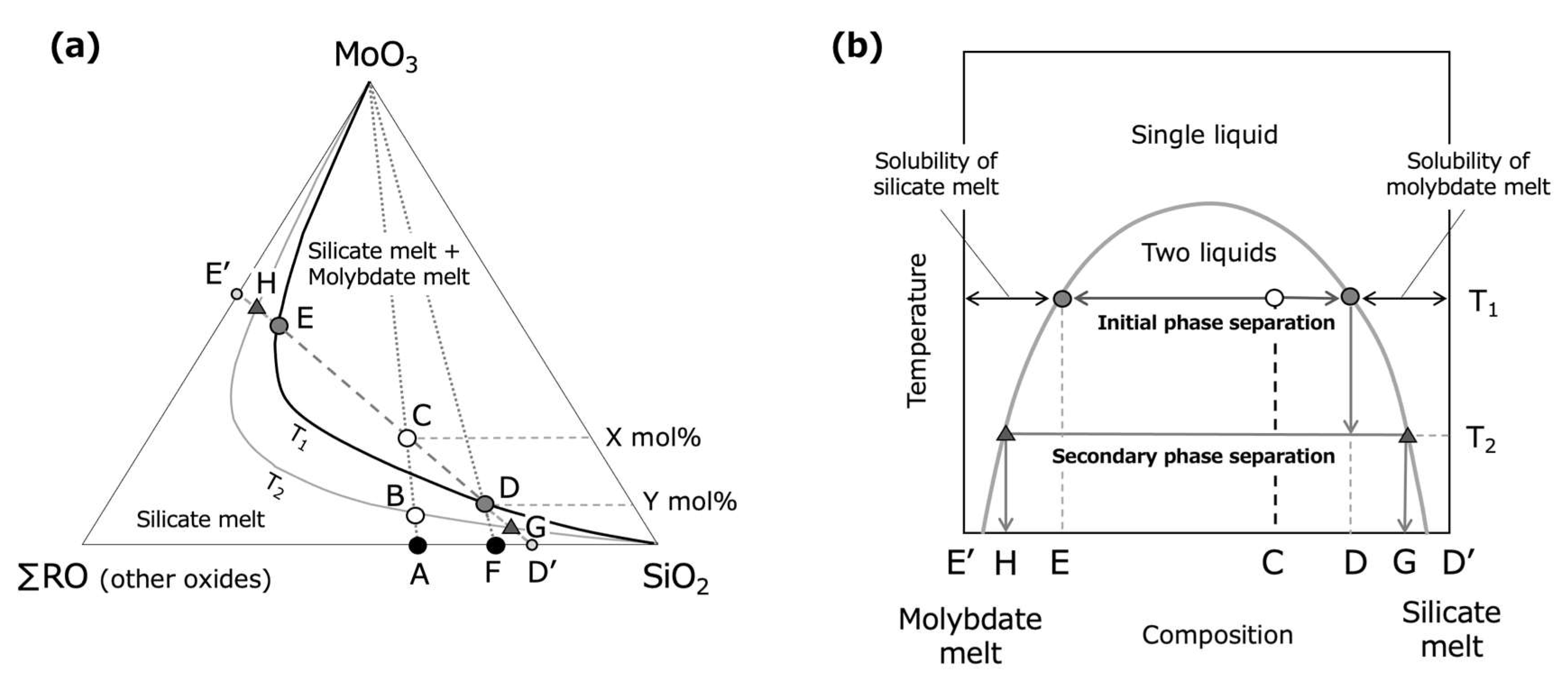
3.1. Phase Separation of Borosilicate Melt and Molybdate Melt
3.2. Effect of V2O5 on MoO3 Solubility in Glass
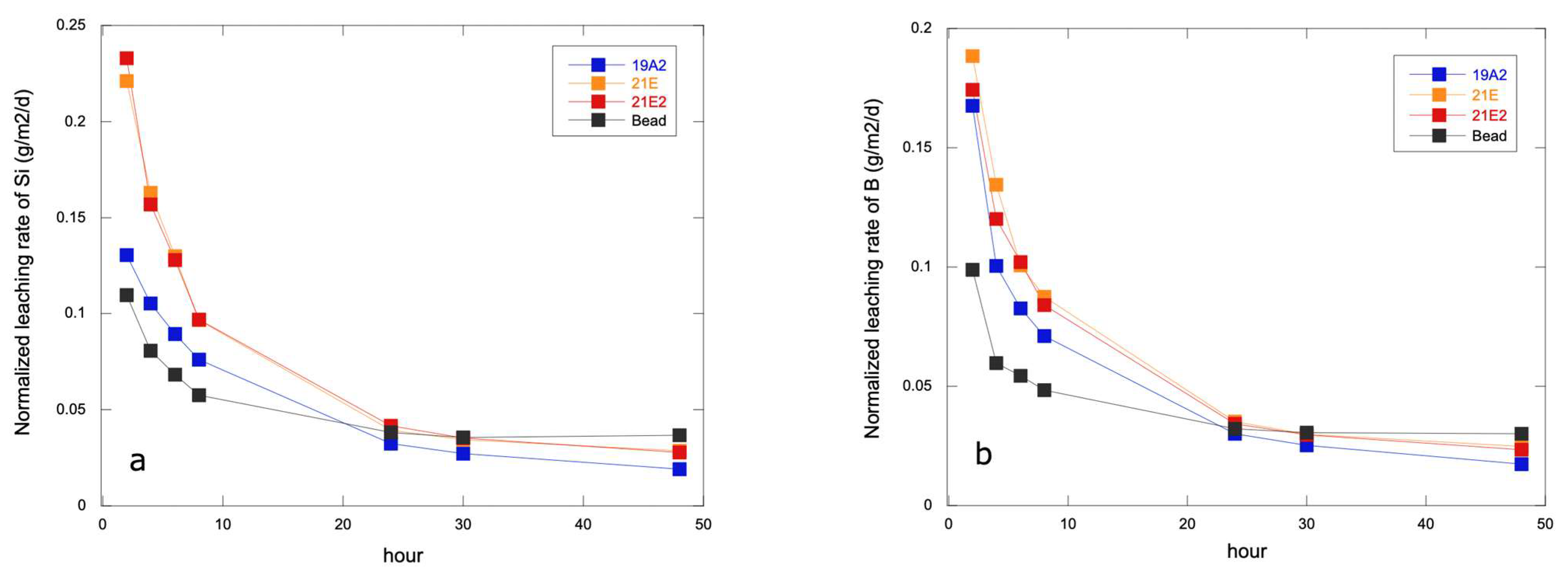
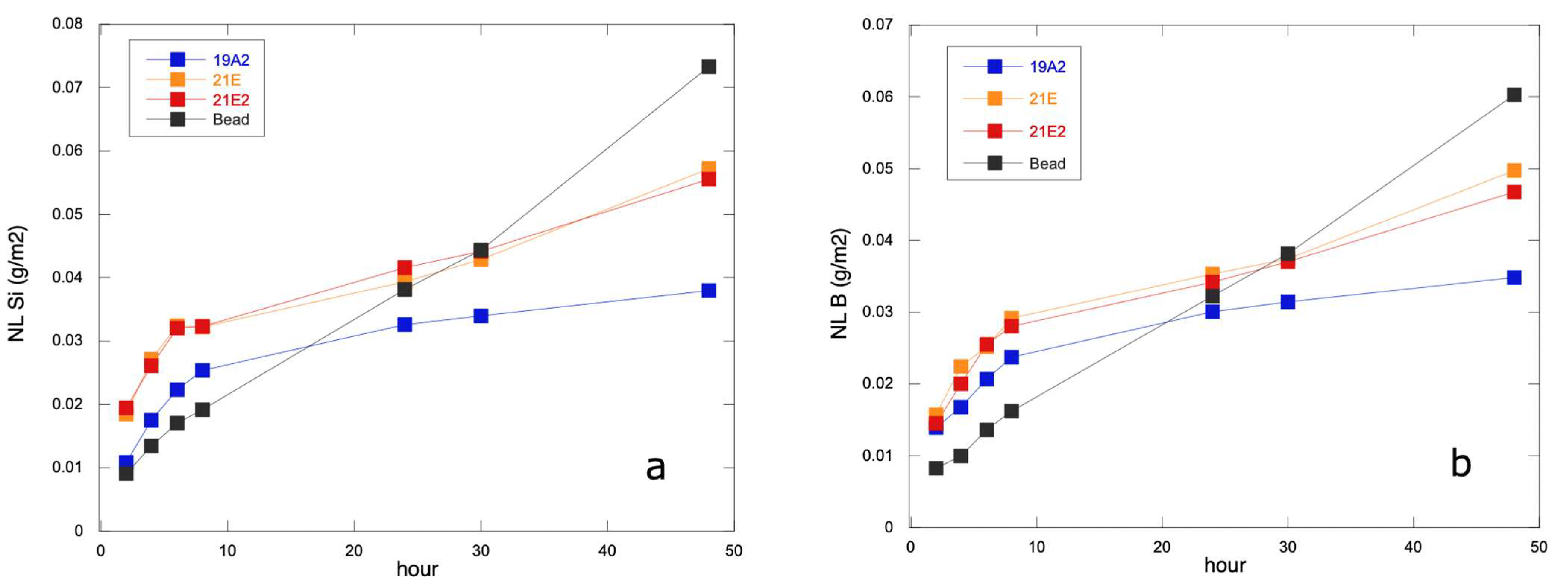
3.3. Chemical Durability Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lutze, W. Radioactive Waste Forms for the Future; Ewing, R.C., Ed.; North-Holland: Amsterdam, The Netherlands, 1988; pp. 335–392. [Google Scholar]
- Lutze, L. Glassy and crystalline high-level nuclear waste forms - An attempt at critical evaluation. In Proceedings of the International Symposium on Ceramics in Nuclear Waste Management, Cincinnati, OH, USA, 30 April–2 May 1979; pp. 47–51. [Google Scholar]
- Ojovan, M.I.; Lee, W.E. Glassy Wasteforms for Nuclear Waste Immobilization. Metall. Mater. Trans. A 2011, 42, 837–851. [Google Scholar] [CrossRef]
- Vernaza, E.T.; Bruezière, J.R.M. History of Nuclear Waste Glass in France. Proc. Mater. Sci. 2014, 7, 3–9. [Google Scholar] [CrossRef]
- Thorpe, C.L.; Neeway, J.J.; Pearce, C.I.; Hand, R.J.; Fisher, A.J.; Walling, S.A.; Hyatt, N.C.; Kruger, A.A.; Schweiger, M.; Kosson, D.S.; et al. Forty years of durability assessment of nuclear waste glass by standard methods. Npj Mater. Degrad. 2021, 5, 61. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. New Developments in Glassy Nuclear Waste Forms; Nova Science Publishers: NewYork, NY, USA, 2007. [Google Scholar]
- McCloy, J.S.; Goel, A. Glass-ceramics for nuclear-waste immobilization. MRS Bull. 2017, 42, 233–240. [Google Scholar] [CrossRef]
- Donald, I.W. Waste Immobilization in Glass and Ceramic Based Hosts, Radioactive, Toxic and Hazardous Wastes; Wiley: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. An Introduction to Nuclear Waste Immobilization; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Terai, R.; Eguchi, K.; Yamanaka, H. In Proceedings of the International Symposium on Ceramics in Nuclear Waste Management. Cincinnati, OH, USA, 30 April–2 May 1979, pp. 62–65.
- Manaktala, H.K. An Assessment of Borosilicate Glass as a High-level Waste Form; Nuclear Waste Regulatory Analyses: San Antonio, TX, USA, 1992; CNW 92-017. [Google Scholar]
- Donald, I.W.; Metcalfe, B.L.; Taylor, R.N.J. The immobilization of high level radioactive wastes using ceramics and glasses. J. Mater. Sci. 1997, 32, 5851–5887. [Google Scholar] [CrossRef]
- Goel, A.; McCloy, J.S.; Pokorny, R.; Kruger, A.A. Challenges with vitrification of Hanford High-Level Waste (HLW) to borosilicate glass—An overview. J. Non-Cryst. Solids 2019, 4, 100033. [Google Scholar] [CrossRef]
- Mazurin, O.V.; Porai-Koshits, E.A. Phase Separation in Glass; North-Holland: Amsterdam, The Netherlands, 1984. [Google Scholar] [CrossRef]
- Lee, W.E.; Ojovan, M.I.; Stennett, M.C.; Hyatt, N.C. Immobilisation of radioactive waste in glasses, glass composite materials and ceramics. Adv. Appl. Ceram. 2006, 105, 3–12. [Google Scholar] [CrossRef]
- Nicoleau, E.; Schuller, S.; Angeli, F.; Charpentier, T.; Jollivet, P.; Alexandre, L.G.; Fournier, M.; Mesbah, A.; Vasconcelos, F. Phase separation and crystallization effects on the structure and durability of molybdenum borosilicate glass. J. Non-Cryst. Solids 2015, 427, 120–133. [Google Scholar] [CrossRef]
- Kroeker, S.; Schuller, S.; Wren, J.E.C.; Greer, B.J.; Mesbah, A. 133Cs and 23Na MAS NMR spectroscopy of molybdate crystallization in model nuclear glasses. J. Am. Ceram. Soc. 2016, 99, 1557–1564. [Google Scholar] [CrossRef]
- Yamane, M.; Nakao, Y. Phase separation in the glass containing high level radioactive waste. Yogyo-Kyokai-Shi 1979, 87, 328–332. [Google Scholar] [CrossRef]
- Caurant, D.; Majérus, O.; Fadel, E.; Lenoir, M.; Gervais, C.; Pinet, O. Effect of molybdenum on the Sstructure and on the crystallization of SiO2–Na2O–CaO–B2O3 Glasses. J. Am. Ceram. Soc. 2007, 90, 774–783. [Google Scholar] [CrossRef]
- Magnin, M.; Schuller, S.; Caurant, D.; Majérus, O.; Ligny, D.D.; Mercier, C. Effect of compositional changes on the structure and crystallization tendency of a borosilicate glass containing MoO3. Ceram. Trans. Ser. 2009, 13, 59–67. [Google Scholar]
- Chouard, N.; Caurant, D.; Majérus, O.; Dussossoy, J.L.; Ledieu, A.; Peuget, S.; Hadjean, R.B.; Ramos, J.P.P. Effect of neodymium oxide on the solubility of MoO3 in an aluminoborosilicate glass. J. Non-Cryst. Solids 2011, 357, 2752–2762. [Google Scholar] [CrossRef]
- Taurines, T.; Boizot, B. Microstructure of powellite-rich glass-ceramics: A model system for high level waste immobilization. J. Am. Ceram. Soc. 2012, 95, 1105–1111. [Google Scholar] [CrossRef]
- Chouard, N.; Caurant, D.; Majérus, O.; Guezi-Hasni, N.; Dussossoy, J.L.; Baddour-Hadjean, R.; Pereira-Ramos, J.P. Thermal stability of SiO2-B2O3-Al2O3-Na2O-CaO glasses with high Nd2O3 and MoO3 concentrations. J. Alloys Compd. 2016, 671, 84–99. [Google Scholar] [CrossRef]
- Brehault, A.; Patil, D.; Kamat, H.; Youngman, R.E.; Thirion, L.M.; Mauro, J.C.; Corkhill, C.L.; McCloy, J.S.; Goel, A. Compositional Dependence of Solubility/Retention of Molybdenum Oxides in Aluminoborosilicate-Based Model Nuclear Waste Glasses. J. Phys. Chem. B 2018, 122, 1714–1729. [Google Scholar] [CrossRef]
- Sugawara, T.; Ohira, T.; Oowak, K.; Kanehira, N. Thermodynamic optimization of phase separation of molybdenum phase in high-level waste glass. In Proceedings of the ICG Annual Meeting, Yokohama, Japan, 23–28 September 2018. ICGY037. [Google Scholar]
- Lian, Q.; Zhang, X.; Ji, H.; Yu, P.; Guo, X.; Wan, W.; Liu, H.; Zheng, K.; Zhu, Y.; Wang, H.; et al. Effect of V2O5 on crystallization tendency and chemical durability of Mo-bearing aluminoborosilicate glass. Mater. Res. Express 2020, 7, 045201. [Google Scholar] [CrossRef]
- Zhou, J.; Liao, Q.; Wang, F.; Wang, Y.; Zhu, H.; Zhu, Y. Effect of Na2O and CaO on the solubility and crystallization of Mo in borosilicate glasses. J. Non-Cryst. Solids 2021, 557, 120623. [Google Scholar] [CrossRef]
- Sugawara, T.; Ohira, T.; Oowak, K.; Tsukada, T. International Year of Glass 2022; Future prospects of glass science in the reprocessing process: (3) The reaction behavior of Molybdenum in a glass melter: Recent progress. In Proceedings of the Atomic Energy Society of Japan, Annual Meeting, Online, 16–18 March 2022. 3I_PL03. [Google Scholar]
- Sugawara, T.; Ohira, T.; Sekine, A.; Adachi, M.; Sato, H. Crystallization of molybdenum oxide phase from simulated high-level waste glass under slow cooling. J. Ceram. Soc. Jpn. 2020, 130, 933–942. [Google Scholar] [CrossRef]
- Manara, D.; Grandjean, A.; Pinet, O.; Dussossoy, J.L.; Neuville, D.R. Sulfur behavior in silicate glasses and melts: Implications for sulfate incorporation in nuclear waste glasses as a function of alkali cation and V2O5 content. J. Non-Cryst. Solids 2007, 353, 12–23. [Google Scholar] [CrossRef]
- Stemprok, M.; Voldan, J. Homogenous silicate glasses in systems Na2O-SiO2-WO3 and Na2O-SiO2-MoO3. Ceramics-Silikaty 1974, 18, 19–30. [Google Scholar]
- Uruga, K.; Tsukada, T.; Usami, T. Generation mechanism and prevention method of secondary molybdate phase during vitrification of PUREX wastes in liquid-fed ceramic melter. J. Nucl. Sci. Technol. 2020, 57, 433–443. [Google Scholar] [CrossRef]
- Schuller, S.; Gosse, S.; Rogez, J. Glass, crystallization and phase separation: An insight into glass enriched in MoO3. In Proceedings of the Joint ICTP-IAEA Workshop on Fundamentals of Vitrification and Vitreous Materials for Nuclear Waste Immobilization (smr 3159), Trieste, Italy, 6–10 November 2017; cea-02400187. [Google Scholar]
- Buekers, J.; Mertens, J.; Smolders, E. Toxicity of the molybdate anion in soil is partially explained by effects of the accompanying cation or by soil pH. Environ. Toxicol. Chem. 2010, 29, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Ferkl, P.; Hrma, P.; Kruger, A.A. Augmented Adam-Gibbs model for glass melt viscosity and configuration entropy as functions of temperature and composition. J. Non. Cryst. Solids 2022, 595, 121832. [Google Scholar] [CrossRef]
- Suzuki, M.; Umesaki, N.; Tanaka, T.; Ohkubo, T.; Kakihara, T.; Hashimoto, T.; Kawashima, H. Structural behaviour of vanadium ions in alkali borosilicate glass for nuclear waste storage. Phys. Chem. Glasses B 2018, 59, 181–192. [Google Scholar] [CrossRef]
- Sun, K.H. Fundamental condition of glass formation. J. Am. Chem. Soc. 1947, 30, 277–281. [Google Scholar] [CrossRef]
- Grambow, B. Corrosion of glass. In Uhlig’s Corrosion Handbook, 2nd ed.; Revie, R.W., Ed.; Wiley: Chichester, UK, 2000; pp. 411–437. [Google Scholar]
- Barkatt, A.; Macedo, P.B.; Gibson, B.C.; Montrose, C.J. Modelling of waste performance and system release. Mater. Res. Soc. Symp. Proc. 1984, 44, 3–13. [Google Scholar] [CrossRef]
- Delage, F.; Ghaleb, D.; Dussossoy, J.L. A mechanistic model for understanding nuclear waste glass dissolution. J. Nucl. Mater. 1992, 190, 191–197. [Google Scholar] [CrossRef]
- Xing, S.B.; Buechele, A.C.; Pegg, I.L. Effect of surface layers on the dissolution of nuclear waste glasses. Mater. Res. Soc. Symp. Proc. 1994, 333, 541–548. [Google Scholar] [CrossRef]
- Gin, S.; Ribet, I.; Couillard, M. Role and properties of the gel formed during nuclear glass alteration: Importance of gel formation conditions. J. Nucl. Mater. 2001, 298, 1–10. [Google Scholar] [CrossRef]
- Cailleteau, C.; Angeli, F.; Devreux, F.; Gin, S.; Jestin, J.; Jollivet, P.; Spalla, O. Insight into Silicate-Glass Corrosion Mechanisms. Nat. Mater. 2008, 7, 978–983. [Google Scholar] [CrossRef]


| Sample | SiO2 | B2O3 | Al2O3 | ZnO | CaO | Na2O | Li2O | V2O5 | MoO3 |
|---|---|---|---|---|---|---|---|---|---|
| 19A2 | 39.8 | 15.4 | 2.4 | 1.8 | 5.3 | 16.0 | 6.2 | 0.0 | 13.0 |
| 21E | 38.2 | 14.8 | 2.3 | 1.7 | 5.3 | 16.0 | 6.2 | 2.4 | 13.0 |
| 21E2 | 34.6 | 18.4 | 2.3 | 1.7 | 5.3 | 16.0 | 6.2 | 2.4 | 13.0 |
| Sample | SiO2 | B2O3 | Al2O3 | ZnO | CaO | Na2O | Li2O | V2O5 | MoO3 | NF | NM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lian et al. [26] | Vx-M2 | 58.8 | 13.9 | 2.9 | 0.0 | 11.2 | 11.3 | 0.0 | 0.0–3.0 | 2.0 | 75.5 | 24.5 |
| This study | 19A2 | 54.7 | 12.1 | 3.3 | 2.4 | 5.4 | 14.0 | 6.1 | 0.0 | 2.0 | 72.5 | 27.5 |
| 21E | 52.2 | 10.2 | 3.2 | 2.3 | 5.1 | 13.9 | 9.0 | 2.1 | 2.0 | 70.0 | 30.0 | |
| 21E2 | 49.0 | 13.2 | 3.3 | 2.3 | 5.7 | 15.5 | 6.5 | 2.5 | 2.0 | 70.3 | 29.7 | |
| Bead | 51.3 | 14.4 | 3.2 | 2.3 | 3.8 | 10.6 | 8.2 | 0.0 | 2.0 | 71.2 | 24.5 | |
| Waste components in Bead | K2O | MnO2 | Fe2O3 | CoO | NiO | ZrO2 | BaO | La2O3 | Ce2O3 | Pr2O3 | Gd2O3 | |
| 0.13 | 0.29 | 0.37 | 0.16 | 0.52 | 1.20 | 0.25 | 0.62 | 0.35 | 0.08 | 0.30 | ||
| Sample | Temp./°C | SiO2 | B2O3 | Al2O3 | ZnO | CaO | Na2O | Li2O | V2O5 | MoO3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lian et al. [24] Vx-M2 | 1300 | 58.8 | 13.9 | 2.9 | 0.0 | 11.2 | 11.3 | 0.0 | 0.0–3.0 | 2.0 | ||
| This study P | Glass | 19A2 | 1200 | 53.5 | 11.8 | 3.2 | 2.3 | 5.3 | 13.7 | 5.9 | 0.0 | 4.3 |
| 21E | 50.3 | 9.8 | 3.1 | 2.2 | 4.9 | 13.4 | 8.7 | 2.1 | 5.6 | |||
| 21E2 | 45.7 | 12.3 | 3.1 | 2.2 | 5.3 | 14.5 | 6.1 | 2.3 | 8.6 | |||
| 19A2 | 1000 | 53.9 | 14.3 | 3.2 | 2.6 | 4.7 | 13.1 | 5.6 | 0.0 | 2.6 | ||
| 21E2 | 48.5 | 15.7 | 3.2 | 2.3 | 4.4 | 13.7 | 5.6 | 2.1 | 4.5 | |||
| Precipitate | 19A2 | 1200 | 6.4 | 1.9 | 0.1 | 0.9 | 6.3 | 30.6 | 9.4 | 0.0 | 44.5 | |
| 21E | 9.3 | 5.0 | 0.3 | 1.1 | 4.2 | 26.6 | 14.5 | 3.5 | 35.4 | |||
| 21E2 | 6.5 | 2.2 | 0.3 | 1.6 | 7.5 | 28.9 | 9.5 | 6.3 | 37.3 | |||
| 19A2 | 1000 | 1.4 | 1.0 | 0.0 | 0.6 | 7.8 | 31.6 | 9.2 | 0.0 | 48.4 | ||
| 21E2 | 6.4 | 2.2 | 0.3 | 1.6 | 7.4 | 28.7 | 10.2 | 6.2 | 37.0 | |||
| Sample | Temp./°C | Silicate Phase | Molybdata Phase | B2O3-Loss | Residual |
|---|---|---|---|---|---|
| 19A2 | 1200 | 64.3 | 29.3 | 6.4 | 0.55 |
| 21E | 63.6 | 30.3 | 6.1 | 1.63 | |
| 21E2 | 69.0 | 22.7 | 8.4 | 1.15 | |
| 19A2 | 1000 | 64.4 | 31.0 | 4.6 | 0.76 |
| 21E2 | 60.2 | 33.2 | 6.6 | 1.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagata, M.; Sugawara, T. MoO3 Solubility and Chemical Durability of V2O5-Bearing Borosilicate Glass. Inorganics 2023, 11, 311. https://doi.org/10.3390/inorganics11070311
Nagata M, Sugawara T. MoO3 Solubility and Chemical Durability of V2O5-Bearing Borosilicate Glass. Inorganics. 2023; 11(7):311. https://doi.org/10.3390/inorganics11070311
Chicago/Turabian StyleNagata, Minako, and Toru Sugawara. 2023. "MoO3 Solubility and Chemical Durability of V2O5-Bearing Borosilicate Glass" Inorganics 11, no. 7: 311. https://doi.org/10.3390/inorganics11070311
APA StyleNagata, M., & Sugawara, T. (2023). MoO3 Solubility and Chemical Durability of V2O5-Bearing Borosilicate Glass. Inorganics, 11(7), 311. https://doi.org/10.3390/inorganics11070311





