In Situ Preparation of 2D Co-B Nanosheets@1D TiO2 Nanofibers as a Catalyst for Hydrogen Production from Sodium Borohydride
Abstract
:1. Introduction
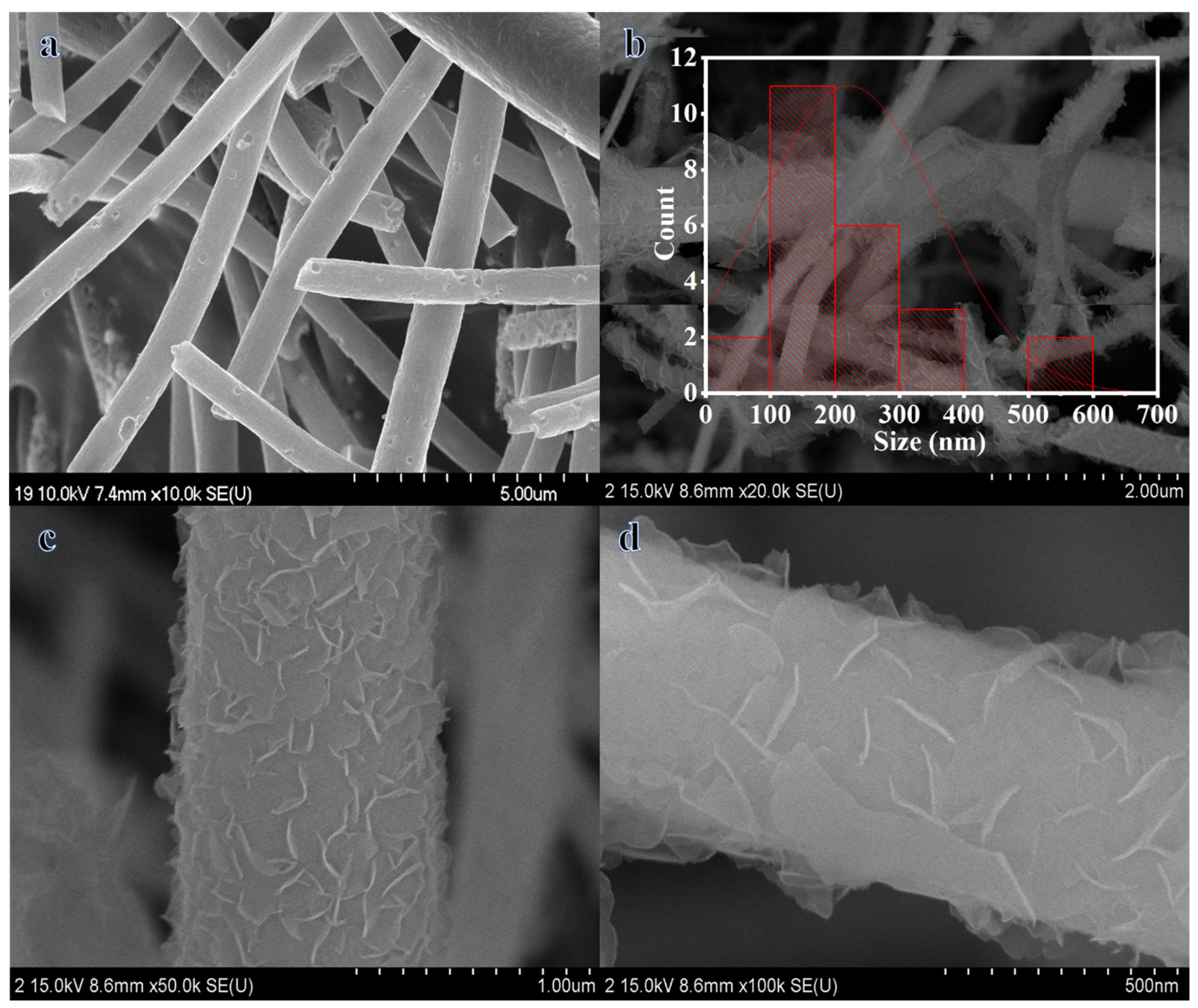
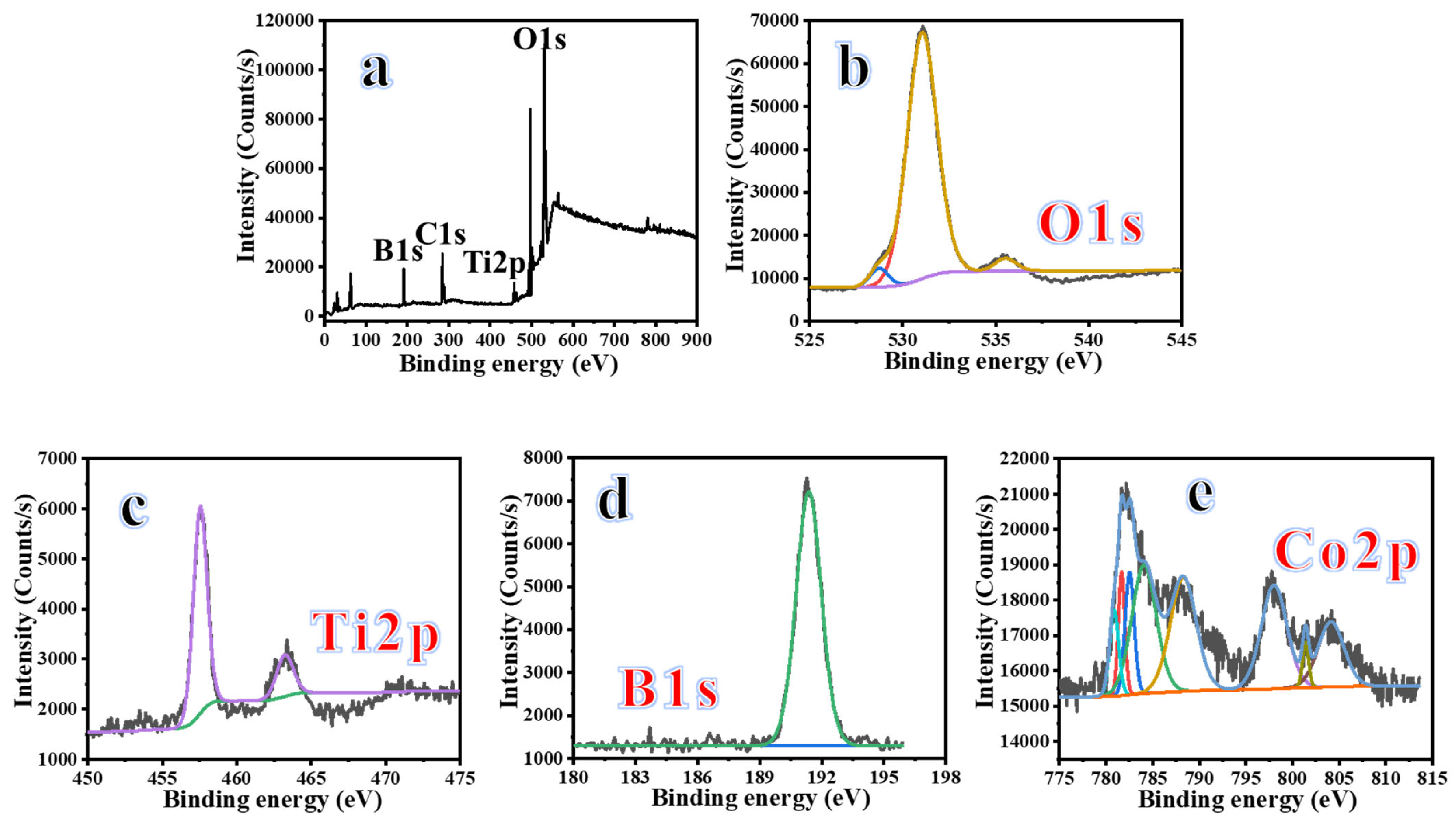
2. Results and Discussion
2.1. Influence of 2D Co-B NS@1D TiO2 NFs Loading
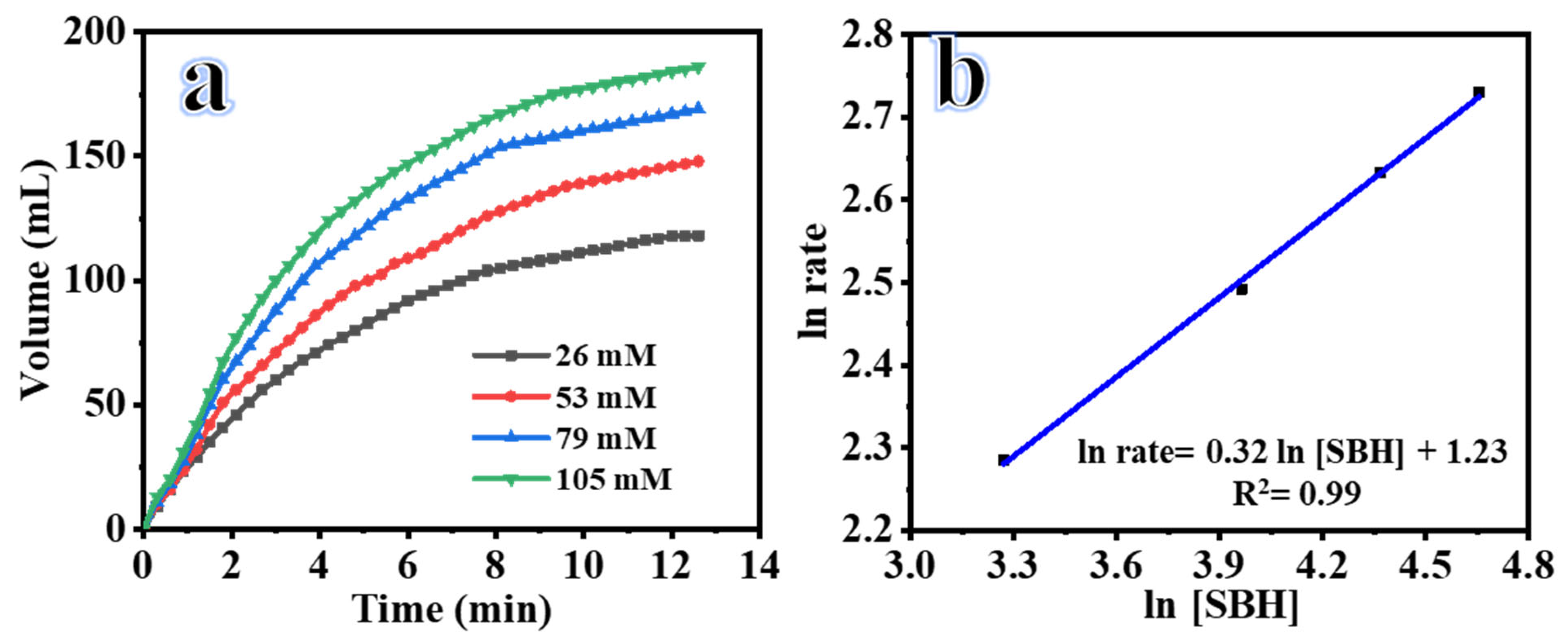
2.2. Influence of SBH Concentration
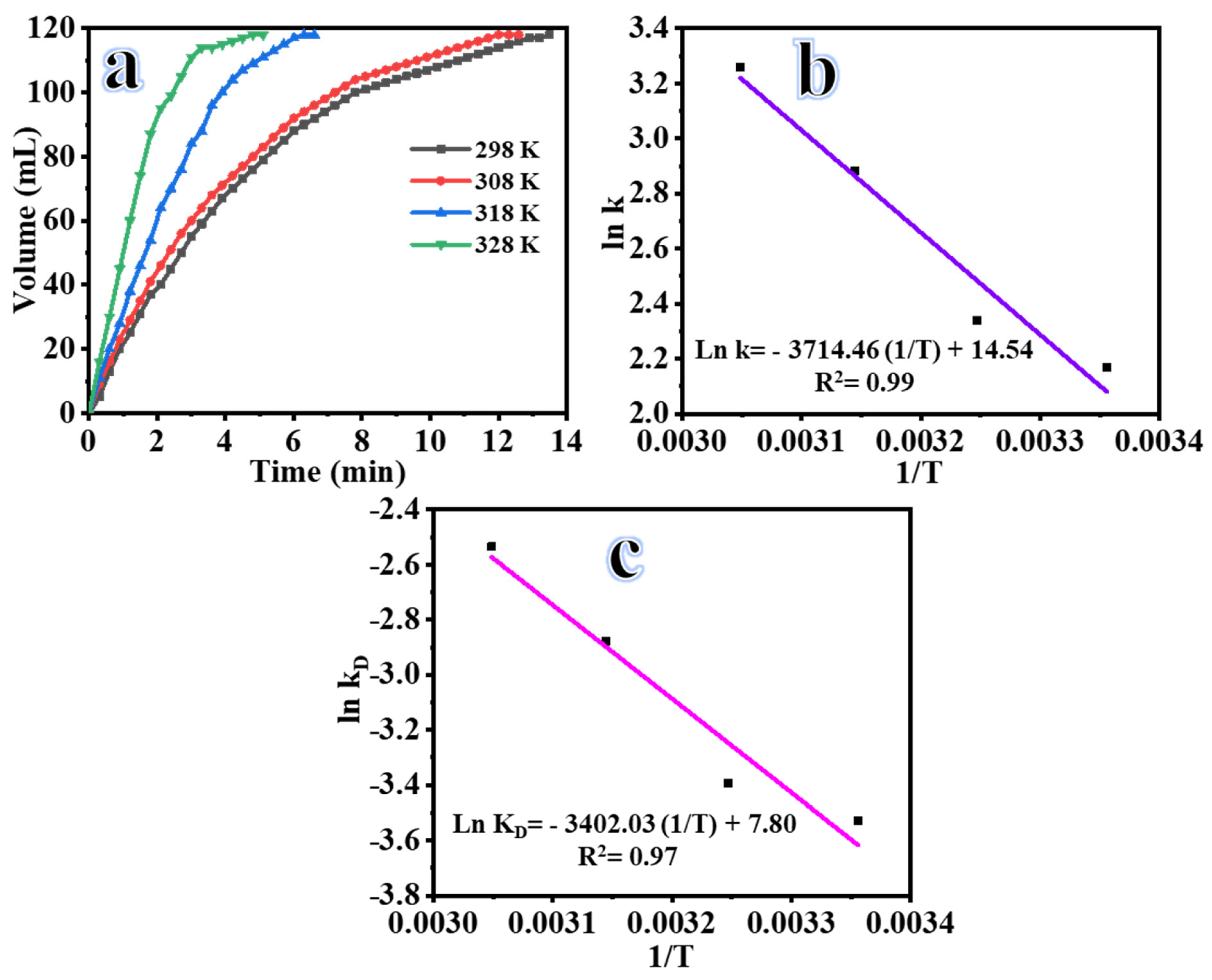
2.3. Influence of Reaction Temperature
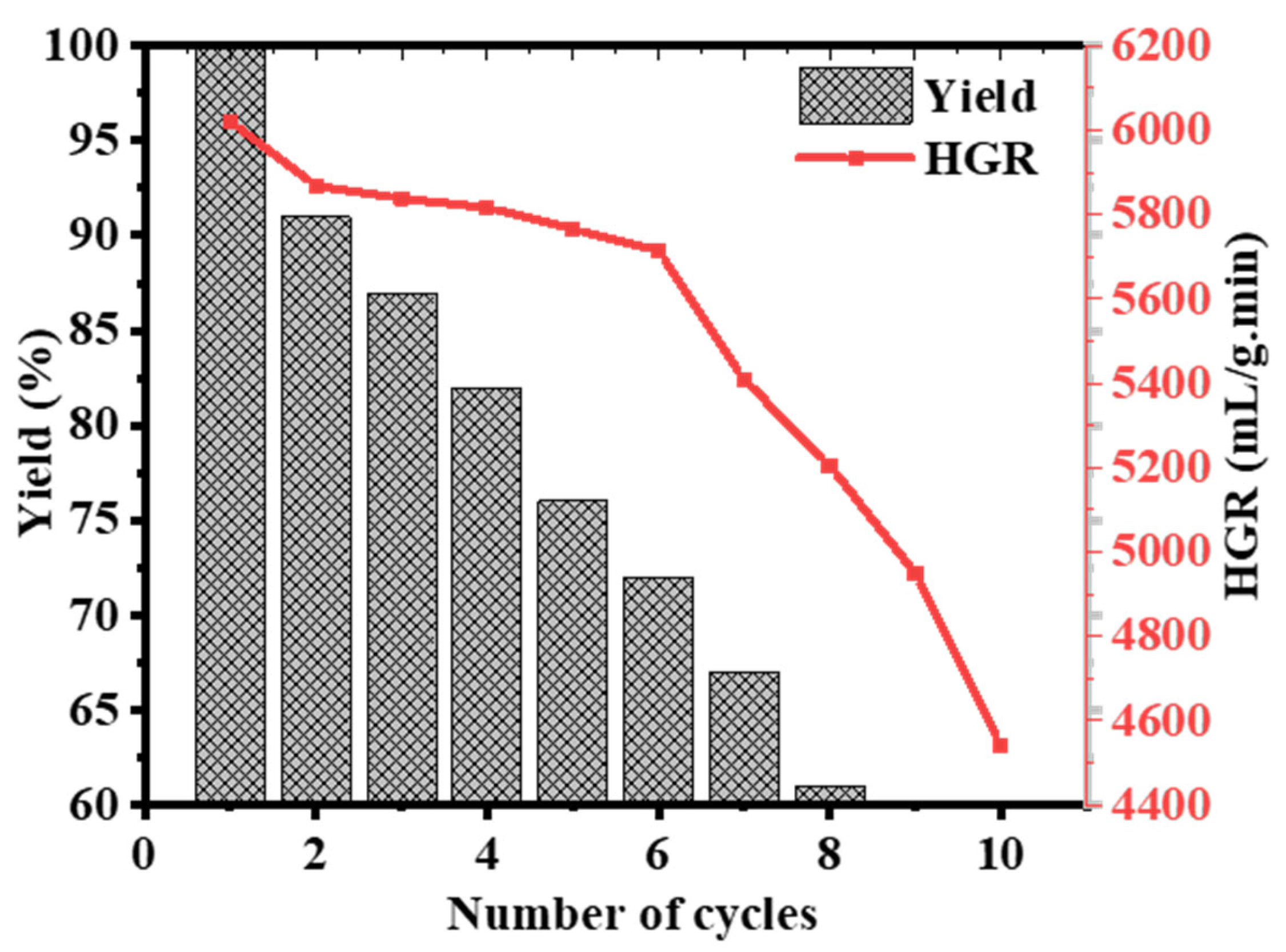
2.4. 2D Co-B NS@1D TiO2 NFs Recyclability
3. Experimental
3.1. Preparation of 2D Co-B NS-Decorated 1D TiO2 NFs
3.2. Characterization
3.3. Dehydrogenation of SBH Using 2D@1D Co-B@TiO2 NFs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Cen, X.; He, J.-F.; Tong, Y. Coupled W–Co2P hybrid nanosheets as a robust bifunctional electrocatalyst for hydrazine-assisted hydrogen production. Chem. Comm. 2023, 59, 5575–5578. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tong, Y. Interface Coupling of Cobalt Hydroxide/Molybdenum Disulfide Heterostructured Nanosheet Arrays for Highly Efficient Hydrazine-Assisted Hydrogen Generation. ACS Sustain. Chem. Eng. 2023, 11, 3219–3227. [Google Scholar] [CrossRef]
- Feng, D.; Liu, X.-Y.; Ye, R.; Huang, W.; Tong, Y. Carbon-encapsulated Co2P/P-modified NiMoO4 hierarchical heterojunction as superior pH-universal electrocatalyst for hydrogen production. J. Colloid Interface Sci. 2023, 634, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Sodium borohydride as a fuel for the future. Renew. Sustain. Energ. Rev. 2011, 15, 3980–4001. [Google Scholar] [CrossRef]
- Retnamma, R.; Novais, A.Q.; Rangel, C.M. Kinetics of hydrolysis of sodium borohydride for hydrogen production in fuel cell applications: A review. Int. J. Hydrogen Energy 2011, 36, 9772–9790. [Google Scholar] [CrossRef]
- Hansu, T.A. A novel and active ruthenium based supported multiwalled carbon nanotube tungsten nanoalloy catalyst for sodium borohydride hydrolysis. Int. J. Hydrogen Energy 2023, 48, 6788–6797. [Google Scholar] [CrossRef]
- Farrag, M.; Ali, G.A.M. Hydrogen generation of single alloy Pd/Pt quantum dots over Co3O4 nanoparticles via the hydrolysis of sodium borohydride at room temperature. Sci. Rep. 2022, 12, 17040. [Google Scholar] [CrossRef]
- Patel, N.; Miotello, A. Progress in Co–B Related Catalyst for Hydrogen Production by Hydrolysis of Boron-Hydrides: A Review and the Perspectives to Substitute Noble Metals. Int. J. Hydrogen Energy 2015, 40, 1429–1464. [Google Scholar] [CrossRef]
- Abutaleb, A.; Maafa, I.M.; Zouli, N.; Yousef, A.; El-Halwany, M.M. Electrospun Co Nanoparticles@PVDF-HFP Nanofibers as Efficient Catalyst for Dehydrogenation of Sodium Borohydride. Polymers 2023, 15, 597. [Google Scholar] [CrossRef]
- Zouli, N.; Maafa, I.M.; Abutaleb, A.; Yousef, A.; El-Halwany, M.M. Membrane Nanofiber-Supported Cobalt–Nickel Nanoparticles as an Effective and Durable Catalyst for H2 Evolution via Sodium Borohydride Hydrolysis. Polymers 2023, 15, 814. [Google Scholar] [CrossRef] [PubMed]
- Zouli, N.; Maafa, I.M.; Abutaleb, A.; Yousef, A.; El-Halwany, M.M. Electrospun NiPd Nanoparticles Supported on Polymer Membrane Nanofibers as an Efficient Catalyst for NaBH4 Dehydrogenation. Polymers 2023, 15, 1083. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; Barakat, N.A.M.; EL-Newehy, M.H.; Ahmed, M.M.; Kim, H.Y. Catalytic Hydrolysis of Ammonia Borane for Hydrogen Generation Using Cu(0) Nanoparticles Supported on TiO2 Nanofibers. Colloid Surf. Physicochem. Eng. Aspect. 2015, 470, 194–201. [Google Scholar] [CrossRef]
- Rakap, M.; Kalu, E.E.; Özkar, S. Polymer-Immobilized Palladium Supported on TiO2 (Pd–PVB–TiO2) as Highly Active and Reusable Catalyst for Hydrogen Generation from the Hydrolysis of Unstirred Ammonia–Borane Solution. Int. J. Hydrogen Energy 2011, 36, 1448–1455. [Google Scholar] [CrossRef]
- Viswanathamurthi, P.; Bhattarai, N.; Kim, C.K.; Kim, H.Y.; Lee, D.R. Ruthenium Doped TiO2 Fibers by Electrospinning. Inorg. Chem. Comm. 2004, 7, 679–682. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Wan, D.; Xue, C.; Zhao, J.; Shao, G. Direct Evidence of 2D/1D Heterojunction Enhancement on Photocatalytic Activity through Assembling MoS2 Nanosheets onto Super-Long TiO2 Nanofibers. Appl. Surf. Sci. 2020, 504, 144361. [Google Scholar] [CrossRef]
- Feng, D.; Ye, R.; Tong, Y.; Ren, X.; Chen, P. Engineering cobalt molybdate nanosheet arrays with phosphorus-modified nickel as heterogeneous electrodes for highly-active energy-saving water splitting. J. Colloid Interface Sci. 2023, 636, 425–434. [Google Scholar] [CrossRef]
- Chen, P.; Li, K.; Ye, Y.; Wu, D.; Tong, Y. Coupled MoO3−x@CoP heterostructure as a pH-universal electrode for hydrogen generation at a high current density. Dalton Trans. 2023, 52, 2262–2271. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kordás, K.; Kiss, J.; Kónya, Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef]
- Kiss, J.; Révész, K.; Solymosi, F. Segregation of boron and its reaction with oxygen on Rh. Appl. Surf. Sci. 1989, 37, 95–110. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Cobalt in NaBH4 hydrolysis. Phys. Chem. Chem. Phys. 2010, 12, 14651–14665. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Guella, G.; Kale, A.; Miotello, A.; Patton, B.; Zanchetta, C.; Mirenghi, L.; Rotolo, P. Thin films of Co–B prepared by pulsed laser deposition as efficient catalysts in hydrogen producing reactions. Appl. Catal. Gen. 2007, 323, 18–24. [Google Scholar] [CrossRef]
- Wang, X.; Liao, J.; Li, H.; Wang, H.; Wang, R.; Pollet, B.G.; Ji, S. Highly active porous Co–B nanoalloy synthesized on liquid-gas interface for hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2018, 43, 17543–17555. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Chen, M.-S.; Chen, Y.-W. Hydrogen Generation by Sodium Borohydride Hydrolysis on Nanosized Co-B Catalysts Supported on TiO2, Al2O3 and CeO2. Int. J. Hydrogen Energy 2012, 37, 4254–4258. [Google Scholar] [CrossRef]
- Dönmez, F.; Ayas, N. Synthesis of Ni/TiO2 Catalyst by Sol-Gel Method for Hydrogen Production from Sodium Borohydride. Int. J. Hydrogen Energy 2021, 46, 29314–29322. [Google Scholar] [CrossRef]
- Xu, J.; Du, X.; Wei, Q.; Huang, Y. Efficient Hydrolysis of Sodium Borohydride by Co-B Supported on Nitrogen-doped Carbon. Chem. 2020, 5, 6683–6690. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Hydroxyapatite-supported cobalt(0) nanoclusters as efficient and cost-effective catalyst for hydrogen generation from the hydrolysis of both sodium borohydride and ammonia-borane. Catal. Today 2012, 183, 17–25. [Google Scholar] [CrossRef]
- Jeong, S.U.; Kim, R.K.; Cho, E.A.; Kim, H.-J.; Nam, S.-W.; Oh, I.-H.; Hong, S.-A.; Kim, S.H. A Study on Hydrogen Generation from NaBH4 Solution Using the High-Performance Co-B Catalyst. J. Power Source 2005, 144, 129–134. [Google Scholar] [CrossRef]
- Vernekar, A.A.; Bugde, S.T.; Tilve, S. Sustainable Hydrogen Production by Catalytic Hydrolysis of Alkaline Sodium Borohydride Solution Using Recyclable Co–Co2B and Ni–Ni3B Nanocomposites. Int. J. Hydrogen Energy 2012, 37, 327–334. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Bai, S.; Qi, K.; Cao, Z.; Zhang, K.; Wu, S.; Wang, D. Catalytic hydrolysis of sodium borohydride via nanostructured cobalt–boron catalysts. Int. J. Hydrogen Energy 2016, 41, 276–284. [Google Scholar] [CrossRef]
- Muir, S.S.; Chen, Z.; Wood, B.J.; Wang, L.; Lu, G.Q.; Yao, X. New electroless plating method for preparation of highly active Co–B catalysts for NaBH4 hydrolysis. Int. J. Hydrogen Energy 2014, 39, 414–425. [Google Scholar] [CrossRef]
- Bekiroğullari, M.; Kaya, M.; Saka, C. Highly efficient Co-B catalysts with Chlorella Vulgaris microalgal strain modified using hydrochloric acid as a new support material for hydrogen production from methanolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 7262–7275. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Q.; Wu, Q.; Guo, S.; Zhang, Z.; Sun, Z.; Liu, B.; Wang, Z.; Zhao, B.; Ding, W. CoB supported on Ag-activated TiO2 as a highly active catalyst for hydrolysis of alkaline NaBH4 solution. Energy 2015, 90, 464–474. [Google Scholar] [CrossRef]
- Shi, L.; Chen, Z.; Jian, Z.; Guo, F.; Gao, C. Carbon nanotubes-promoted Co–B catalysts for rapid hydrogen generation via NaBH4 hydrolysis. Int. J. Hydrogen Energy 2019, 44, 19868–19877. [Google Scholar] [CrossRef]
- Shi, L.; Xie, W.; Jian, Z.; Liao, X.; Wang, Y. Graphene modified Co–B catalysts for rapid hydrogen production from NaBH4 hydrolysis. Int. J. Hydrogen Energy 2019, 44, 17954–17962. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Intrazeolite Co-Balt(0) Nanoclusters as Low-Cost and Reusable Catalyst for Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Appl. Catal. B Environ. 2009, 91, 21–29. [Google Scholar] [CrossRef]
- Paladini, M.; Arzac, G.M.; Godinho, V.; Hufschmidt, D.; de Haro, M.C.J.; Beltrán, A.M.; Fernández, A. The Role of Co-Balt Hydroxide in Deactivation of Thin Film Co-Based Catalysts for Sodium Borohydride Hydrolysis. Appl. Catal. B Environ. 2017, 210, 342–351. [Google Scholar] [CrossRef]
- Peng, C.; Li, T.; Zou, Y.; Xiang, C.; Xu, F.; Zhang, J.; Sun, L. Bacterial Cellulose Derived Carbon as a Support for Catalytically Active Co–B Alloy for Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2021, 46, 666–675. [Google Scholar] [CrossRef]


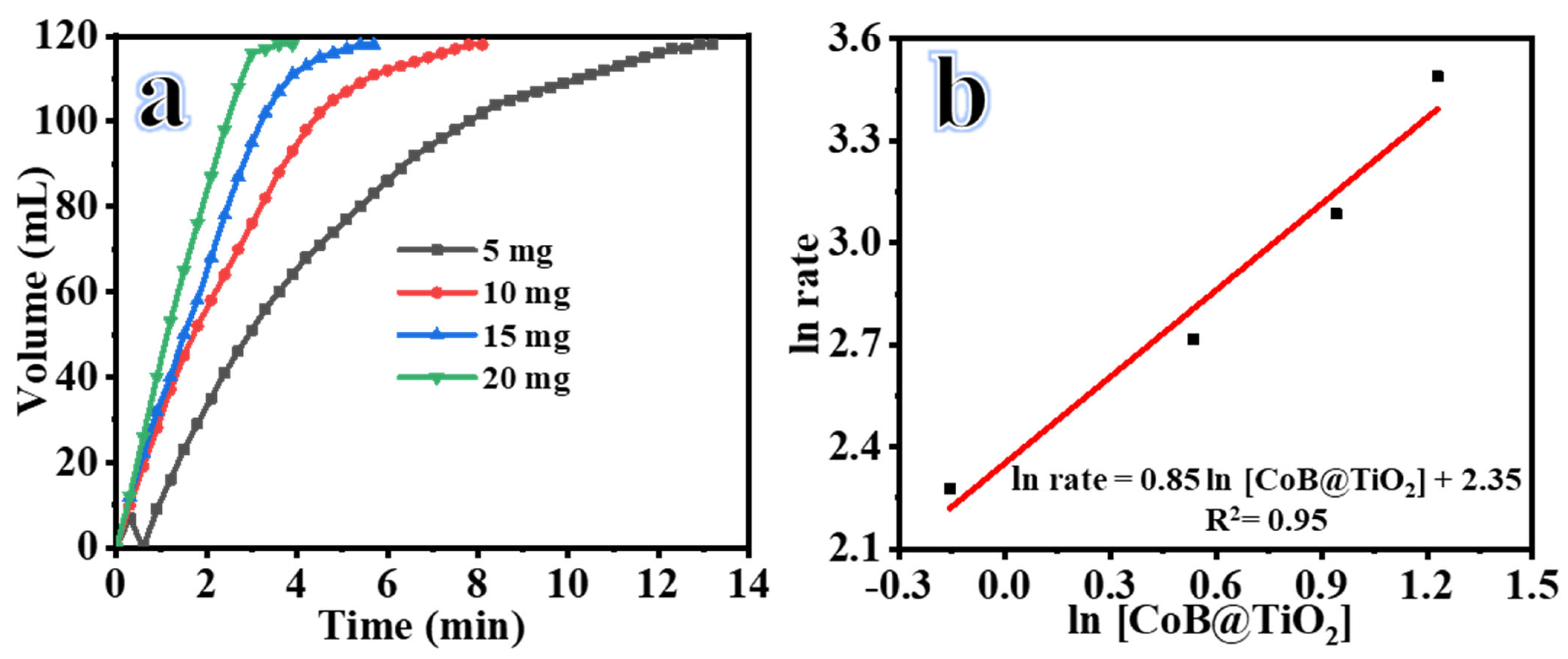
| Catalyst Loading (mg) | ||||
|---|---|---|---|---|
| 5 | 10 | 15 | 20 | |
| Volume, mL | 118 | 118 | 118 | 118 |
| Yield, % | 98.3 | 98.3 | 98.3 | 98.3 |
| Reaction time, min | 12 | 7.5 | 4.8 | 3.3 |
| Reaction Rate, mL min−1 | 9.8 | 15.1 | 21.9 | 32.8 |
| Catalyst Loading (mg) | ||||
|---|---|---|---|---|
| 298 | 308 | 318 | 328 | |
| Volume, mL | 118 | 118 | 118 | 118 |
| Yield, % | 98.3 | 98.3 | 98.3 | 98.3 |
| Reaction time, min | 13.2 | 12 | 6 | 4.5 |
| Reaction Rate, mL min−1 | 8.94 | 9.8 | 19.7 | 26.2 |
| Catalyst | Conditions | Ea, kJ mol−1 | Ref. |
|---|---|---|---|
| Co@ HAP | 819 mg cat, 150 mM SBH, 10 wt% NaOH | 53 | [27] |
| Co-B | 20 mg cat, 20 wt% SBH, 5 wt% NaOH, 20 °C | 64.87 | [28] |
| Co-Co2B | 10 mg cat, 0.019 g SBH, 0.025 M NaOH, 30 °C | 35.245 | [29] |
| Co-B/Cu | 4 × 4 cm2 cat, 1 wt% SBH, 5 wt% NaBH4, 25 °C | 43.3 | [30] |
| Co-B/Ni | 1 × 1 cm2 cat, 15 wt% SBH, 5 wt% NaOH, 30 °C | 94.5 | [31] |
| Co-B/C. Vulgaris | 100 mg cat, 0.66 M SBH, 1% NaOH, 25 °C | 25.22 | [32] |
| CoB/Ag-TiO2 | 10 mg cat, 1 wt% SBH, 5 wt% NaOH, 25 °C | 44 | [33] |
| CoB-MWCNT | 100 mg cat, 5 wt% SBH, 5 wt% NaOH, 25 °C | 23.5 | [34] |
| Co-B/GO | 50 mg cat, 5 wt% SBH, 5 wt% NaOH, 30 °C | 26.2 | [35] |
| 2D Co-B NS@1D TiO2 NFs | 5 mg cat, 26 mM SBH, 25 °C, 1000 rpm | 30.87 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maafa, I.M.; Zouli, N.; Abutaleb, A.; Yousef, A.; Qudsieh, I.Y.; Matar, S.M.; Adam, A.S.M.; El-Halwany, M.M. In Situ Preparation of 2D Co-B Nanosheets@1D TiO2 Nanofibers as a Catalyst for Hydrogen Production from Sodium Borohydride. Inorganics 2023, 11, 342. https://doi.org/10.3390/inorganics11080342
Maafa IM, Zouli N, Abutaleb A, Yousef A, Qudsieh IY, Matar SM, Adam ASM, El-Halwany MM. In Situ Preparation of 2D Co-B Nanosheets@1D TiO2 Nanofibers as a Catalyst for Hydrogen Production from Sodium Borohydride. Inorganics. 2023; 11(8):342. https://doi.org/10.3390/inorganics11080342
Chicago/Turabian StyleMaafa, Ibrahim M., Nasser Zouli, Ahmed Abutaleb, Ayman Yousef, Isam Y. Qudsieh, Saleh M. Matar, Abdel Samed M. Adam, and M. M. El-Halwany. 2023. "In Situ Preparation of 2D Co-B Nanosheets@1D TiO2 Nanofibers as a Catalyst for Hydrogen Production from Sodium Borohydride" Inorganics 11, no. 8: 342. https://doi.org/10.3390/inorganics11080342







