Abstract
This article focuses on the galvanic replacement synthesis of Ti-Ni and Zr-Ni metal systems with a “core-shell” structure, which are potential precursors for intermetallics. The authors defined effective synthesis parameters and formation features of polymetallic systems characterized by granulometric, phase, and elemental composition. X-ray fluorescence and X-ray phase analysis methods showed that the deposition of nickel on dispersed titanium and zirconium leads to the production of test samples with phase composition representing a mechanical mixture of Ni and Ti, and Ni and Zr. The method of X-ray fluorescence analysis showed that the presence of hydrofluoric acid with a 0.5-1.5 M concentration results in the formation of fixed quantitative ratios of elements in the precipitate, which allows the quantitative composition of dispersed systems “titanium-nickel” and “zirconium-nickel” to be regulated within a relatively wide range. Scanning electron microscopy proved that all synthesized systems are characterized by a highly porous structure that follows the titanium and zirconium particle surface contour and the presence of spherical nanoscale subunits on the formed particle surface.
1. Introduction
Ti-Ni and Zr-Ni metal–powder systems are widely used to produce bulk materials with excellent shape memory properties, hydrogen absorption capacity, superelasticity, corrosion resistance, and biocompatibility [1,2,3,4]. Recent years have seen the problem of finding new materials for the rapid development of additive technologies, including composite metal powders used for molding products [5,6,7]. Traditionally, methods for producing composite metal powders are based on mechanochemistry [8,9,10], chemical reduction [11,12], and electrolytic deposition [13,14,15]. However, these methods are considered material- and energy-intensive [16]. The main disadvantages of mechanochemical synthesis are the duration of the mixing process (40 h) and the contamination of metal powders with fragments of grinding media. Methods of chemical reduction from solutions are characterized by the complexity of controlling the quantitative ratio of metals in powders, the inclusion of components (elements) of a reducing agent in the composition, and the instability of solutions. Electrode position methods require a large amount of electricity from an external source, which limits their application.
The galvanic replacement process, widely used in hydrometallurgy for metal extraction, is simpler, more reliable, and less energy-intensive [17,18,19,20,21,22]. The advantages of the process are a more uniform distribution of the surface layer on a reducing metal and the ability to work without an external source of electrical energy for coating. The difference in electrochemical potentials between a reducing metal and ions of elements with more positive potential values initiates the spontaneous reaction of the more noble metal release on the metal surface in a solution [23]:
nM + mMn+noble = nMm+ + mMnoble
Uniform distribution of powder components can be used to obtain a homogeneous microstructure and highly efficient composite materials for powder metallurgy and additive technologies. For composite materials based on titanium or zirconium, it is more difficult to obtain a multicomponent metal–powder system with a uniform distribution of elements in it due to the significant difference between the density of each component and the melting point [24]. According to the structural orientation concept, the growing precipitate is to reproduce the crystal structure of the matrix to ensure good adhesion to the base metal [25]. Studies show that the sediment distribution uniformity is achieved during the formation of “core-shell” structures in the process of galvanic replacement [26]. The formation of “core-shell” structures reduces the interfacial energy significantly, which ensures the sintering activity and a smooth process of powder material compaction.
The number of articles focused on the galvanic replacement synthesis of powders based on titanium, zirconium, and metals of the iron family elements is very low [27,28,29]. In these studies, titanium and zirconium demonstrate good reducing ability in aqueous solutions in relation to nickel(II) ions. The lack of scientific and practical studies of the kinetics and mechanisms of reactions of the galvanic substitution of titanium or zirconium with metals of the iron family elements is a major barrier to obtaining promising materials with a “core-shell” particle structure [30].
2. Results and Discussion
Due to the high reactivity of titanium and zirconium, their surfaces are always covered with a chemically inert and durable oxide film under normal conditions. This film forms spontaneously in both air and aqueous environments. Depending on the formation mode and rate, the film can be either amorphous or crystalline [25,26,27]. The physicochemical properties of the oxide layer depend on its stoichiometry and defect density. It is important to note that the oxide film at grain boundaries provides fewer protective properties compared to the film on grains where secondary phases are present.
Film stability is critical for many electrochemical reactions and is primarily determined by corrosion potential, pH, and solution temperature [27]. Titanium oxide film consists of layers of complex configuration: a continuous protective barrier layer adjacent to the metal surface (with anatase structure) and a non-continuous outer layer (with rutile structure). In this case, the total thickness of the oxide film formed on bulk titanium is 8–10 nm [28], and on dispersed titanium (with a particle size d50 = 40 μm), it is approximately 18 nm [26,27,28].
It can be seen from Pourbaix diagrams for the Ti-H2O and Zr-H2O systems that the phases are divided into regions of immunity, passivation, and corrosion. As the pH increases, the electrochemical potential decreases, indicating a more stable state for metallic titanium and zirconium under acidic conditions. This stability is maintained through the potential formation of a hydride film of TiH2 and ZrH2. The passivity region exists within the pH range of 2 < pH < 12, indicating a thermodynamically favorable state for the existence of titanium and zirconium oxides in aqueous media.
The polarization diagram method reveals that without the presence of an activating agent, such as hydrofluoric acid in the electrolyte, the cathodic and anodic polarization curves do not intersect. The addition of hydrofluoric acid results in the shifting of the anodic polarization curve towards more negative values, which characterize the oxidation process of titanium. This allows us to determine the stationary potentials that correspond to the equal rates of the anodic and cathodic processes [28,29].
The passive layer on the surface of titanium exists in various stoichiometric compositions, including TiO, Ti2O3, and TiO2 [27]. On the other hand, the passive layer on zirconium’s surface remains thermodynamically stable only in the form of stoichiometric oxide ZrO2. In [27], it is demonstrated that the process of titanium oxidation in 1.00 M NaCl is controlled by mixed diffusion kinetics. Conversely, the electrode process of zirconium oxidation is limited only by the stage of charge transfer [27]. A notable distinction between zirconium and titanium during anodic polarization is the breakdown of the protective oxide film, which is characterized by a sharp increase in current density on the polarization curve. As a result, pittings occur on the surface of zirconium. The higher the concentration of chloride ions in the solution and the lower the potential sweep rate, the greater the number of pittings. Titanium, on the other hand, is less susceptible to repassivation.
The presence of titanium in a passive state is confirmed by the type and characteristics of chronopotentiograms recorded for the corresponding solutions.
Pretreating the working surfaces of titanium and zirconium with a 3% solution of hydrofluoric acid increases the rate of their anodic oxidation. In a hydrofluoric acid medium (0.05 M), a significant shift of the stationary potential for both titanium and zirconium towards more negative values is observed. This shift is accompanied by the appearance of peaks in the oxidation current of metallic titanium.
Analyzing the chronopotentiograms and polarization curves, it can be concluded that the surface oxide layers of titanium are more resistant to aggressive media, such as halide ions, compared to zirconium.
The corrosion potential of compact titanium decreases with the increase in the concentration of metal chloride in the solution, indicating some surface activation.
Titanium and zirconium were used as reducing agents in the process of galvanic replacement of nickel (II) in an aqueous solution. Their standard potential in aqueous solutions is E°(Ti3+/Ti) = −1.21 V [4], E°(Zr4+/Zr) = −1.54 V [31], and their stationary potential ranges are Estac = −0.35 ± 0.1 V [32]. A strong shift in the potential of titanium and zirconium towards more positive values is due to the presence of an oxide film on their surface, the transformation or removal of which allows us to use metallic titanium and zirconium as reducing agents of ions of more electropositive metals [33].
It has been established that the cathodic reduction process of the iron family metals is limited by diffusion, while the anodic process of titanium ionization is limited by an electrochemical reaction. It has been observed that in a series of solutions containing metal ions Co(II)>Ni(II)>Fe(III), there is an increase in the degree of contact precipitation of metals on the surface of bulk titanium [29]. The addition of 0.90 M HF to the reaction mixture containing nickel(II) ions initiates the contact exchange process rapidly. The introduction of 1.50 M HF to the solution results in an induction period seen on the chronopotentiogram, lasting approximately five minutes.
Since galvanic replacement is an electrochemical process that occurs at the interface between heterogeneous phases, it can be described using standard methods of electrochemical reaction kinetics. In particular, corrosion diagrams of coupled cathodic and anodic reactions can be used. The polarization curves allow us to determine compromise potentials that can roughly represent the equality of rates between the anodic and cathodic processes.
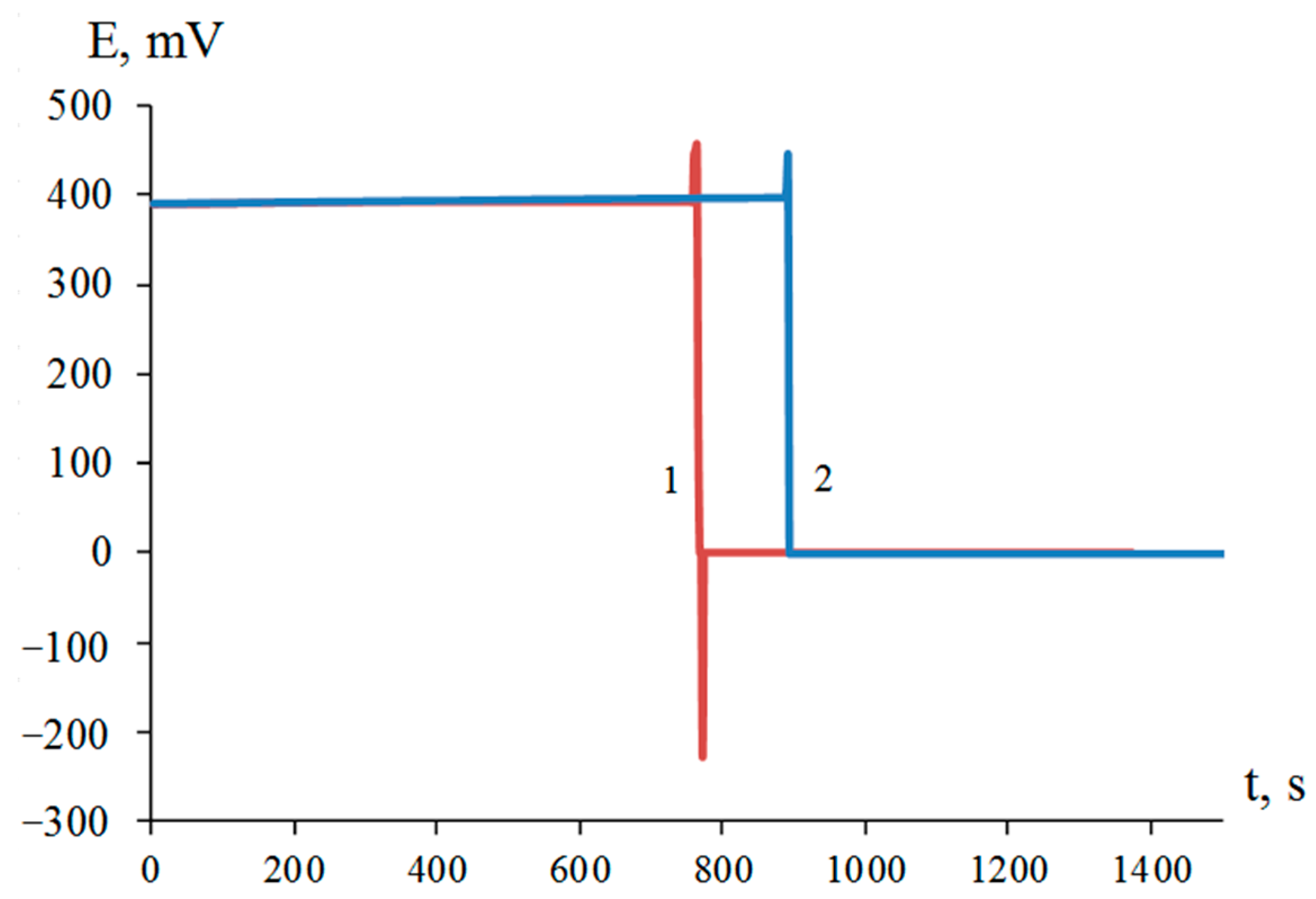
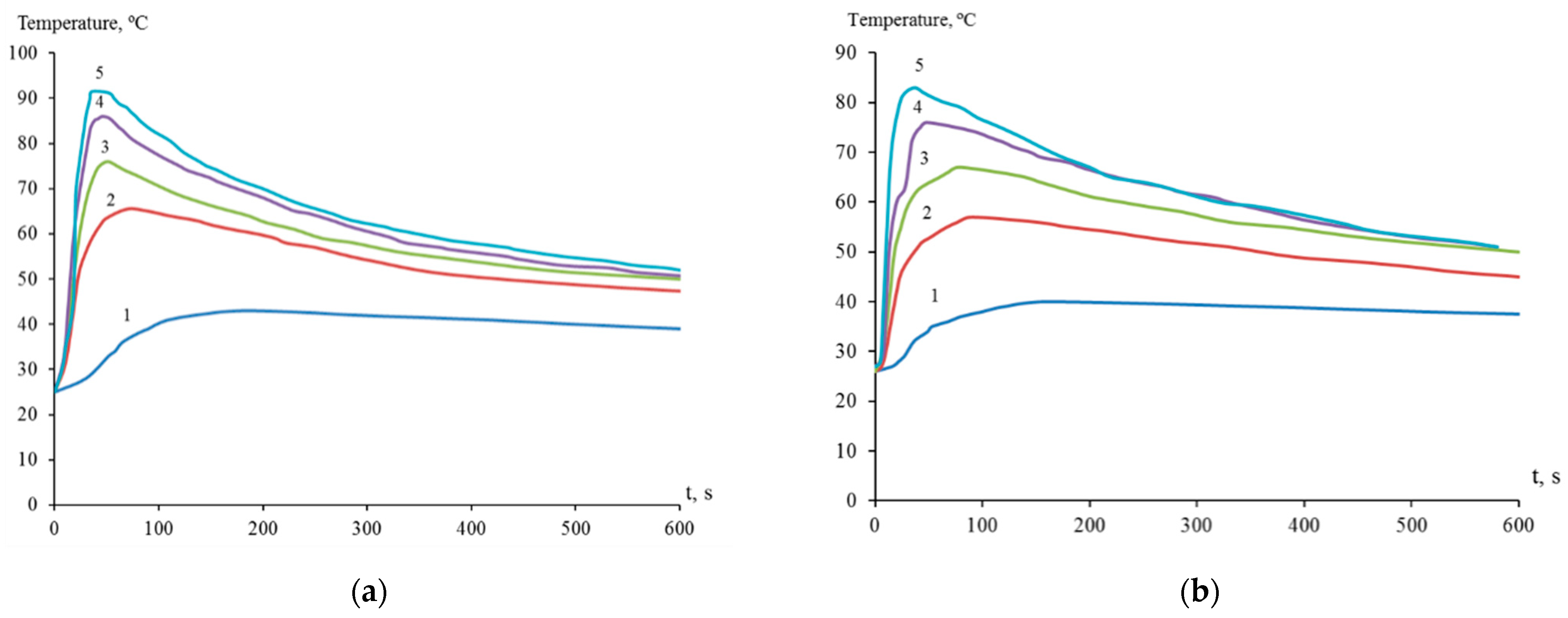
Thus, the interaction of dispersed titanium and zirconium with a solution of nickel(II) chloride was initiated in the presence of hydrofluoric acid, resulting in the release of elemental nickel on the surface of titanium and zirconium microparticles (Figure 1). The process of galvanic replacement in a suspension of dispersed titanium in a solution of 1.00 M NiCl2 with the introduction of 0.45, 0.90, 1.20, 1.50, and 2.00 M HF was accompanied by an increase in temperature to 43, 65, 76, 86, and 91 °C, respectively (Figure 2a). The temperature of the suspension of dispersed zirconium under similar conditions reached 40, 57, 67, 76, and 83 °C, respectively (Figure 2b). Suspension heating was a combined result of redox and other reactions occurring with different thermal effects.
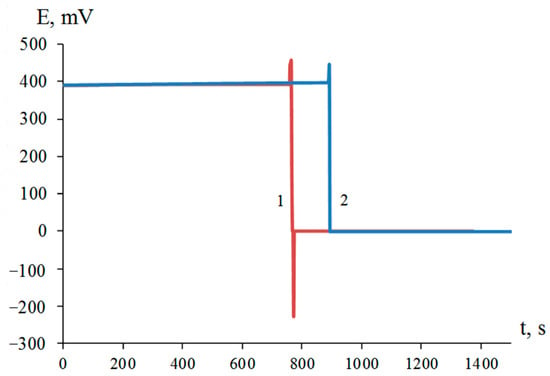
Figure 1.
Redox potential of platinum electrode in suspensions of titanium (1) and zirconium (2) dispersed in 1.00 M NiCl2 solution in the presence of 0.45 M HF.
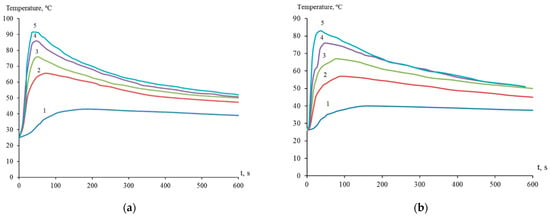
Figure 2.
Temperature of suspensions of dispersed titanium (a) and zirconium (b) in aqueous 1.00 M NiCl2 with the presence of HF, mol/L: 0.45—1; 0.90—2; 1.20—3; 1.50—4; 2.00—5.
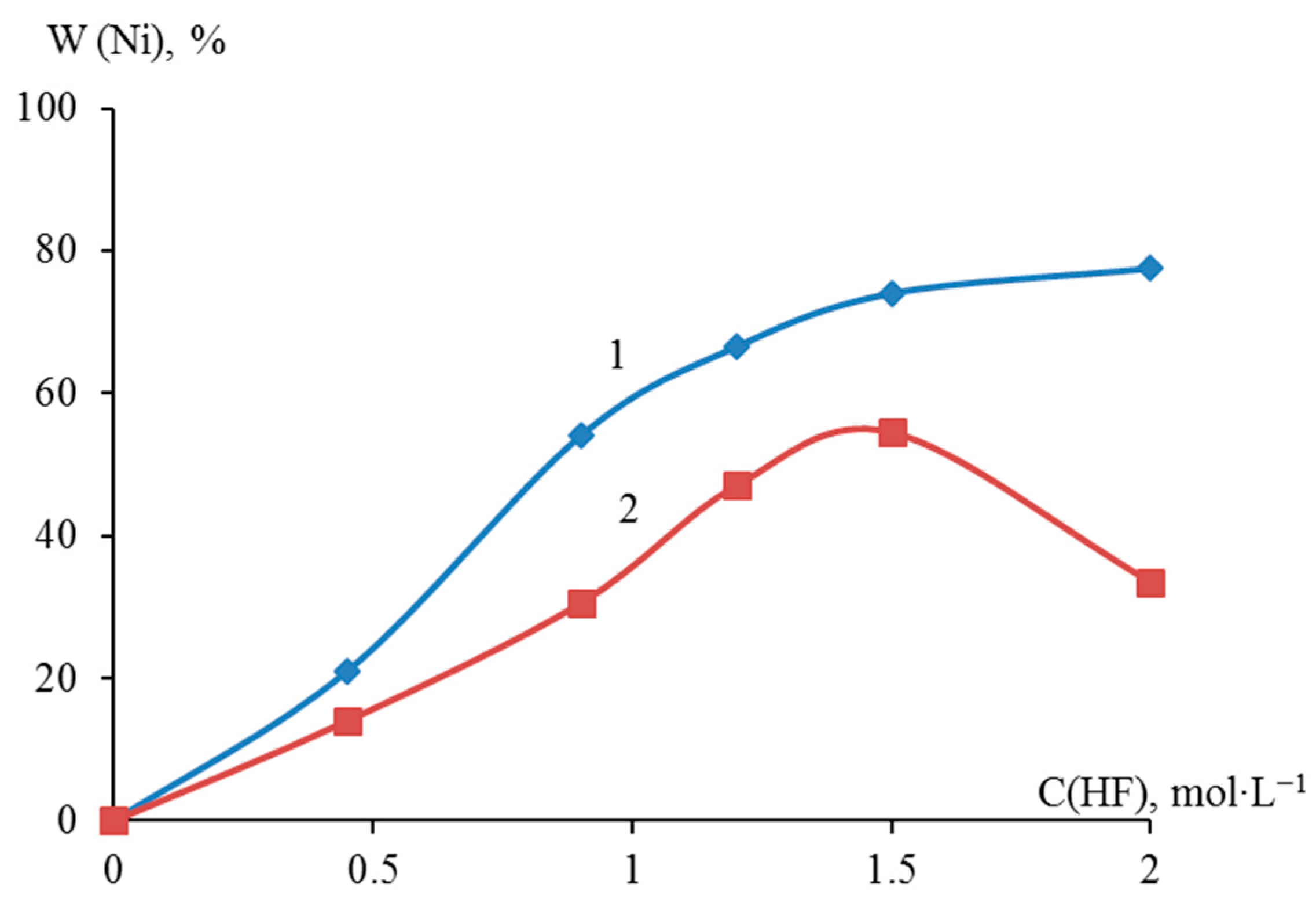
Sampling with titrimetric determination showed that the deposition degree of metallic nickel in the presence of 0.45, 0.90, 1.20, 1.50, and 2.00 M HF on dispersed titanium reached 21.00, 54.00, 66.50, 74.00, and 77.50 wt.%, respectively. The degree of deposition of nickel on dispersed zirconium reached 14.00, 30.50, 47.00, 54.50, and 33.50% wt., respectively. A comparative analysis of the nickel yield on dispersed titanium and zirconium showed that as the concentration of HF in the suspension increased to 1.50 M, the nickel yield increased monotonically. At an HF content of more than 1.50 M, the yield of nickel on zirconium decreased compared to a similar process on titanium (Figure 3).
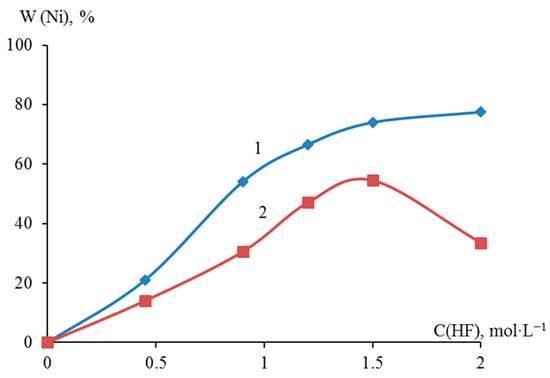
Figure 3.
Dependence of galvanic replacement degree of dispersed titanium (1) and zirconium (2) with metallic nickel on HF concentration after 10 min of the process.
The process of galvanic replacement with the iron family elements can be described using the example of titanium.
According to modern concepts, the process of galvanic replacement of titanium with nickel is associated with the interaction of fluorides and aqua complexes of these metals with the surface of dispersed HF-preactivated titanium.
TiO2 + 6HF → H2[TiF6] + 2H2O,
2Ti + 3[Ni(H2O)6]2+→ 2[Ti(H2O)6]3+ + 3Ni + 6H2O.
When electropositive metals are deposited on the surface of titanium particles, conjugated hydrogen is evolved.
2H3O+ + 2e = H2 + 2H2O.
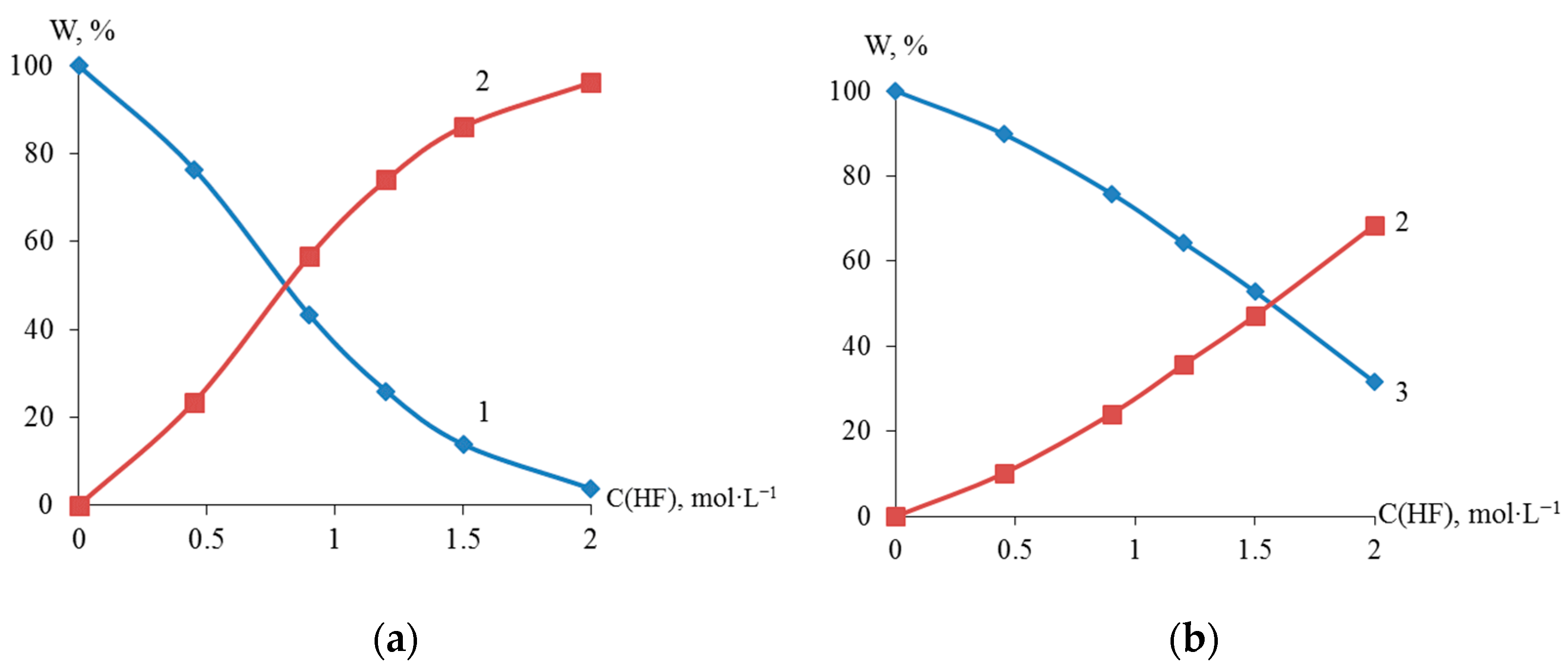
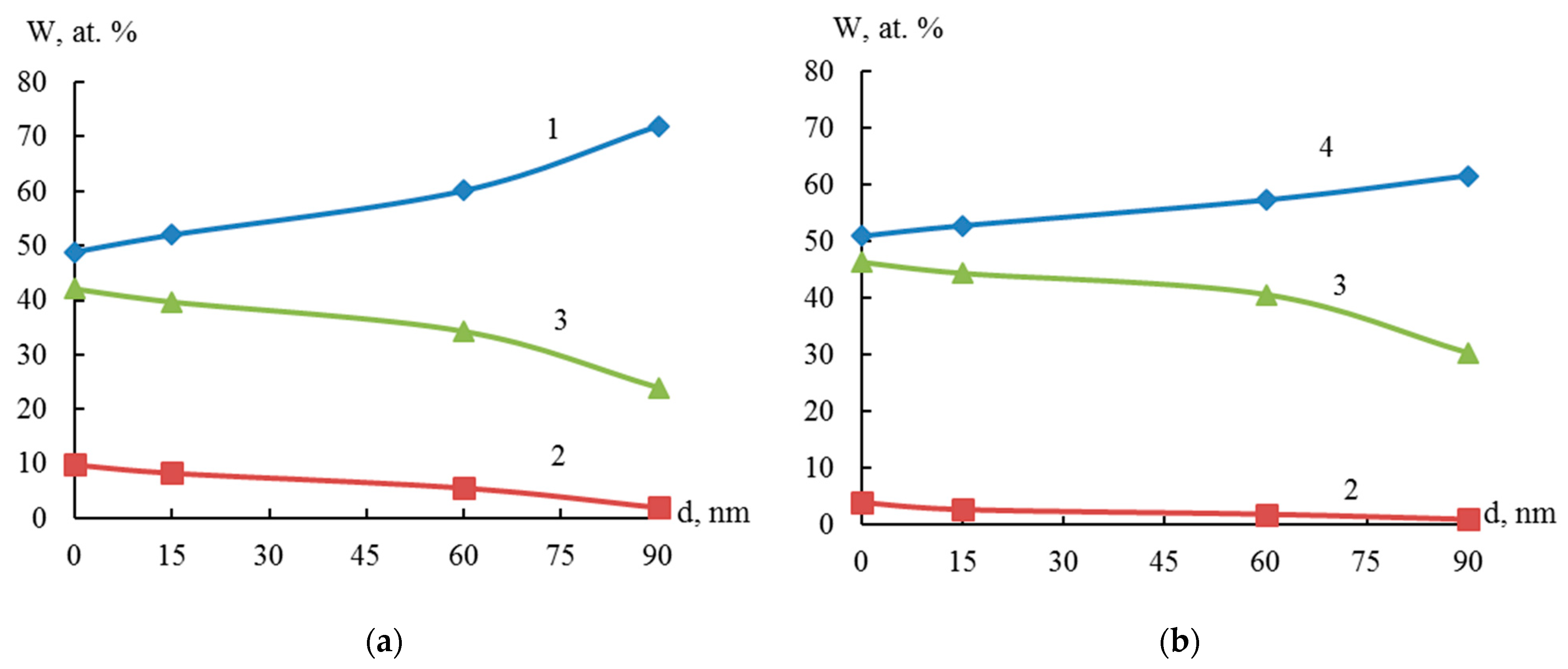
X-ray fluorescence analysis established an S-shaped dependence of the content (wt %) of titanium, zirconium, and nickel in the dispersed Ti-Ni and Zr-Ni systems obtained through galvanic replacement on the concentration of hydrofluoric acid (Figure 4). The data obtained indicate the possibility of regulating the quantitative composition of titanium or zirconium and nickel in the dispersed and bulk Ti-Ni or Zr-Ni systems.
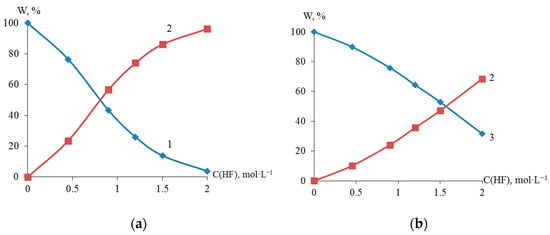
Figure 4.
Elemental composition of Ti (1), Ni (2) and Zr (3) of dispersed systems Ti-Ni (a) and Zr-Ni (b), obtained in solutions with various HF concentrations.
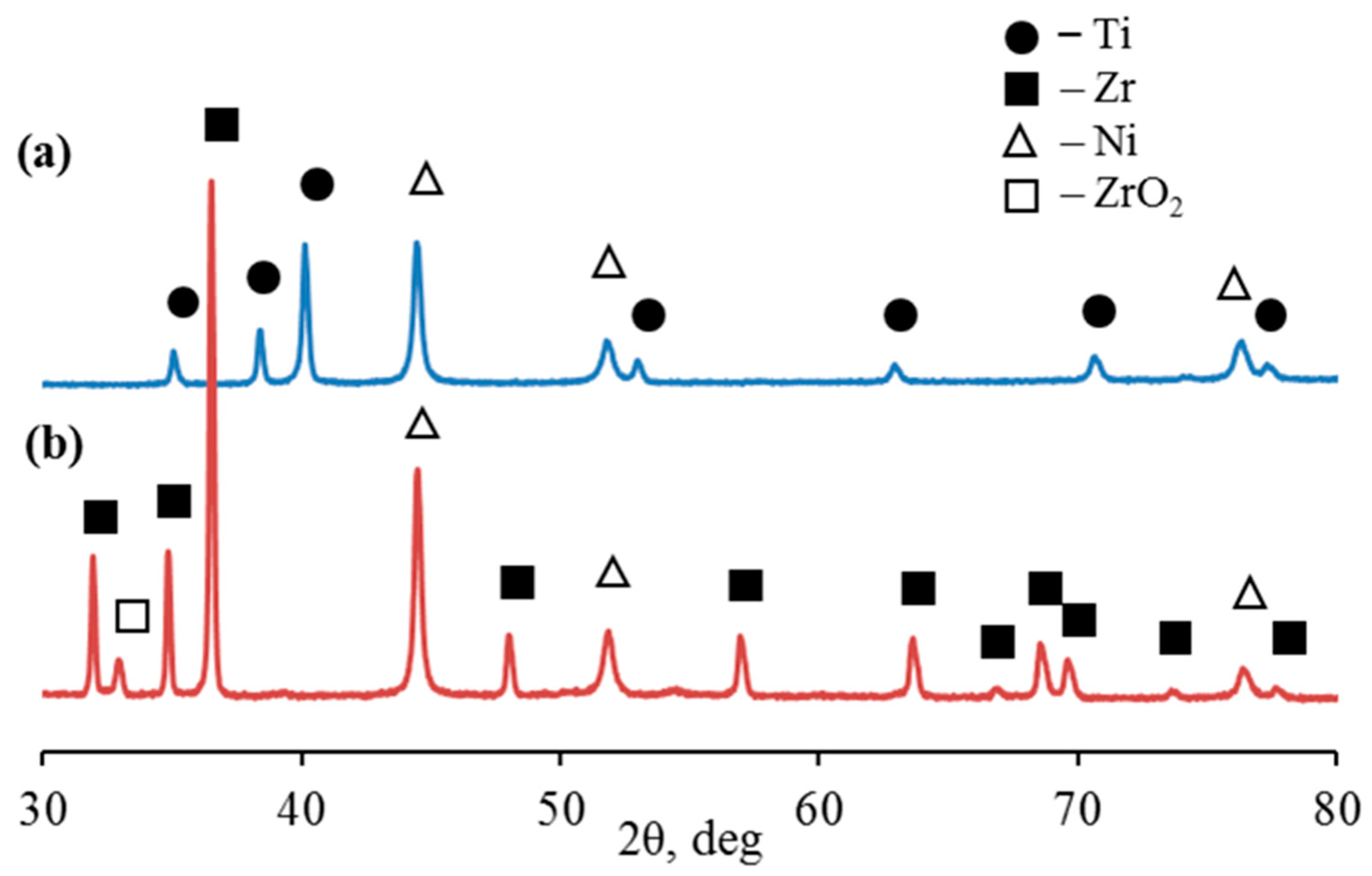
X-ray diffraction showed that in the 2θ scanning range from 30 to 80°, microparticles of the Ti-Ni system are characterized by diffraction peaks corresponding to the hexagonal close-packed lattice of titanium Ti (002), Ti (101), Ti (102), Ti (110), Ti (103), Ti (112), and Ti (201) at angles of diffraction of 35.05°, 38.38°, 40.12°, 52.96°, 62.91°, 70.66°, and 76, 49°, respectively (Figure 5a, Table 1). At the same time, microparticles of the Zr-Ni system are characterized by diffraction peaks corresponding to the hexagonal close-packed lattice of α-zirconium Zr(100), Zr(002), Zr(101), Zr(102), Zr(110), Zr(103), Zr(200), Zr(112), Zr(201), Zr(004), and Zr(202) at angles of diffraction of 31.95°, 34.85°, 36.49°, 47.99°, 56.94 °, 63.58°, 66.79°, 68.52°, 69.56°, 73.58°, and 77.60°, respectively (Figure 5b, Table 1). The diffraction pattern also showed distinct reflections of nickel (β-Ni) with a face-centered cubic lattice (fcc).
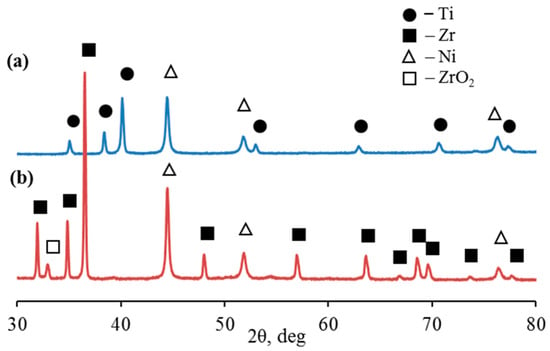
Figure 5.
XRD pattern of Ti-Ni (a) and Zr-Ni (b) systems obtained through galvanic replacement.

Table 1.
Results of X-ray phase analysis of bimetallic systems obtained through galvanic replacement.
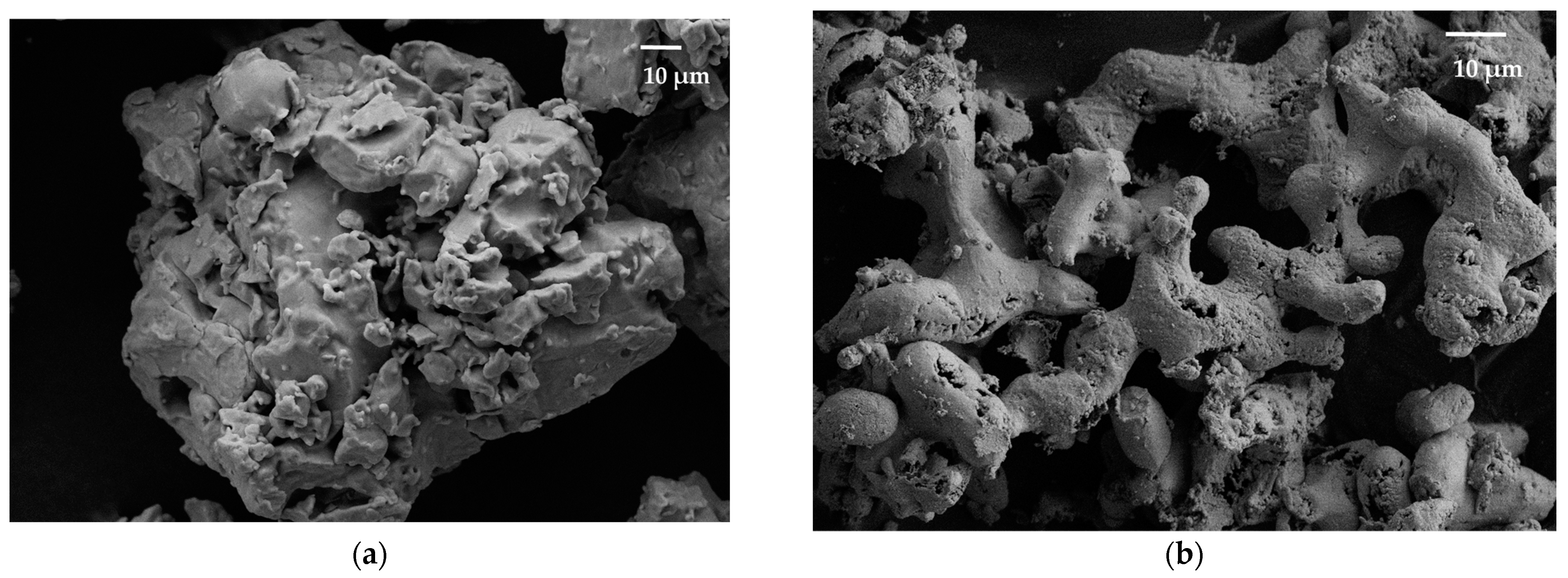
Scanning electron microscopy showed that the formed Ti-Ni particles have the same shapes and geometric dimensions as the original particles (Figure 6). This process is accompanied by the deposition of submicron spherical nickel particles on the surface of titanium microparticles.

Figure 6.
SEM image of titanium (a) and Ti-Ni (b) particles.
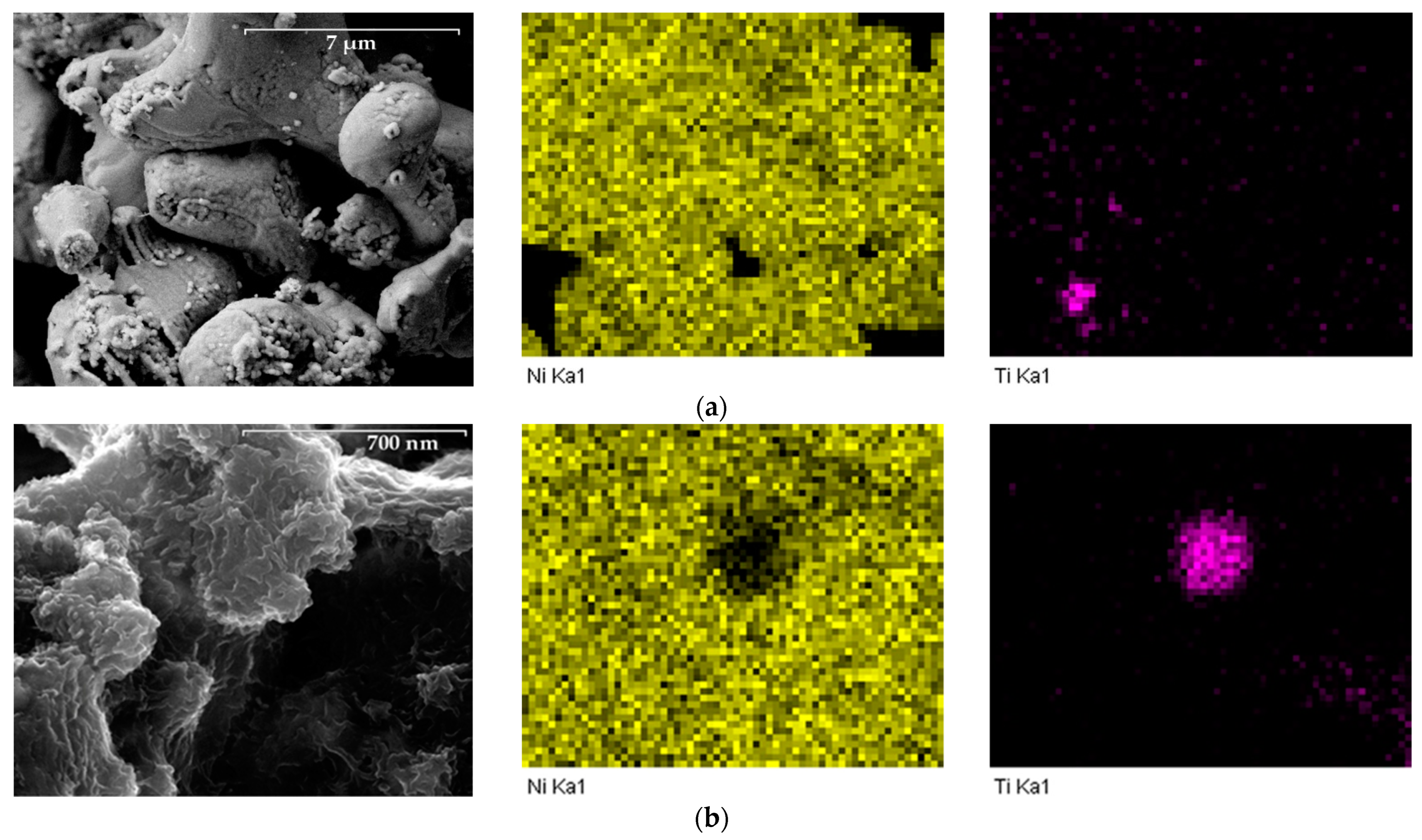
X-ray spectral microanalysis was used to ascertain the distribution of elements on the particles’ surface (Figure 7).
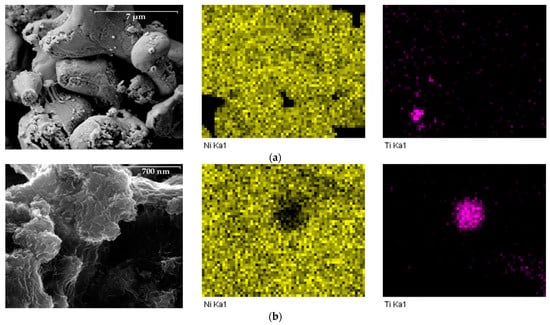
Figure 7.
Results of X-ray spectral microanalysis of titanium particles with deposited nickel layer: (a) 10,000×, (b) 100,000×.
The electron microscopy data indicate a fairly uniform distribution of deposited metals over the particle surfaces. Titanium is found only in the deep pores of the nickel layer. Thus, it can be claimed that the particles of the “core-shell” type were obtained by depositing elemental nickel on the surface of titanium.
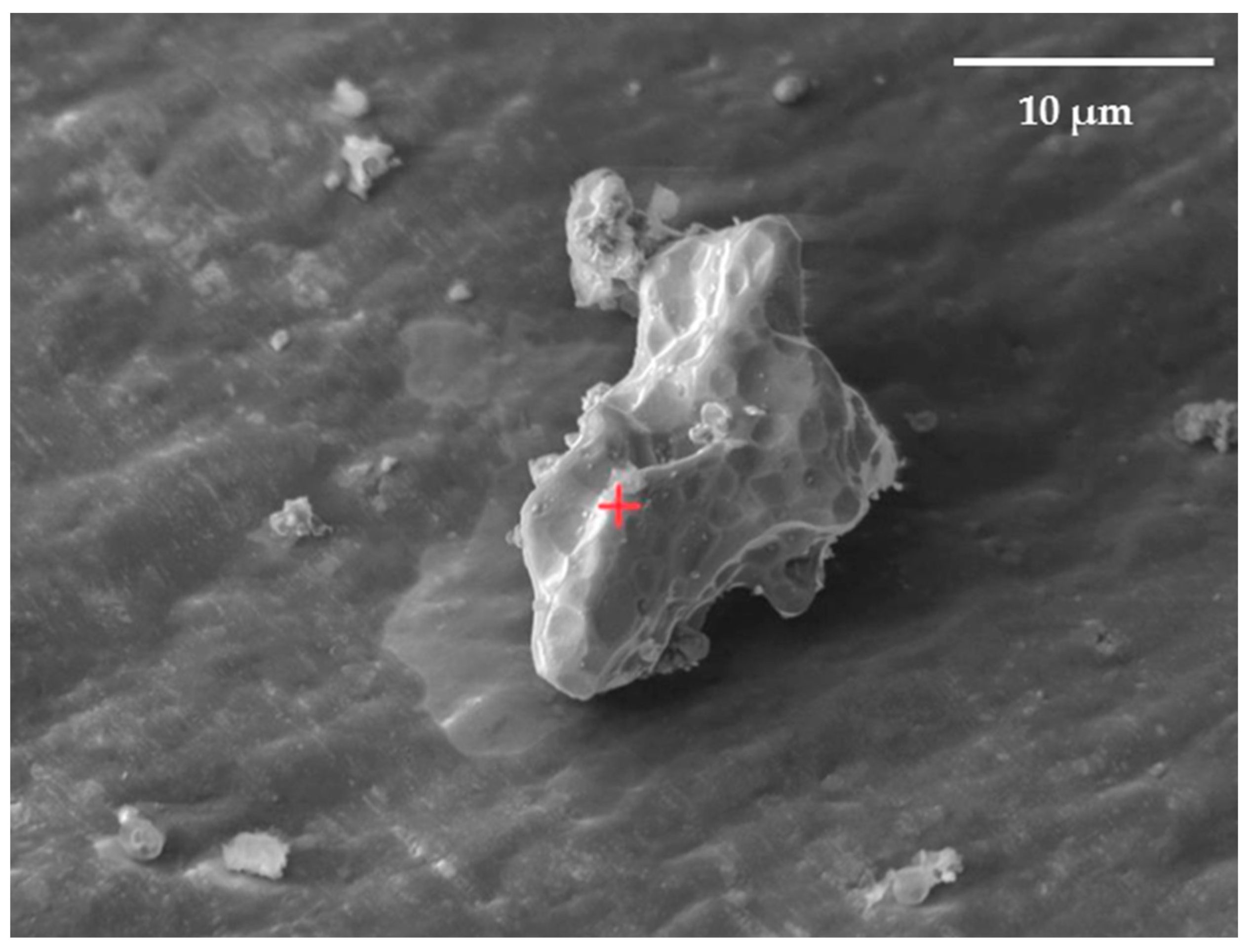
The distribution of elements in the surface layers of the synthesized Ni-Ti and Ni-Zr polymetallic systems was studied using Auger electron spectroscopy. Electron microscopic images were used to select the analyzed areas on the particle surface (Figure 8).
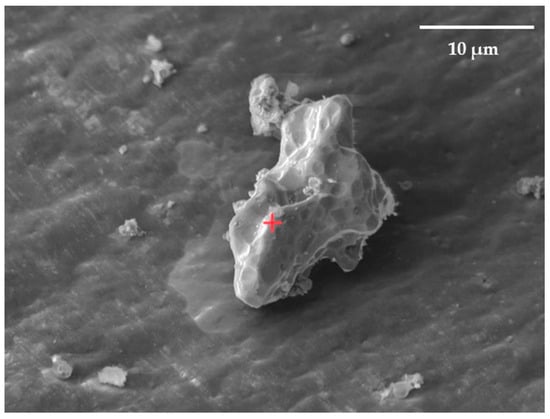
Figure 8.
SEM image of zirconium microparticle surface with deposited nickel. The powder particle is on the aluminum foil. The size of the analysis point is Ø ≤ 0.02 μm (focused beam). The red cross is the designation of the analyzed point.
Figure 9 shows that the amount of nickel decreases and the amount of zirconium increases from the surface to the center of the particle. This indicates that the particle structure of the resulting system can be characterized as a “core-shell” system. The thickness of the nickel film on zirconium is at least 33 nm (the rate of ion etching with Ar+ at 3 kV on the particle surface is approximately 22 to 45 nm/min).
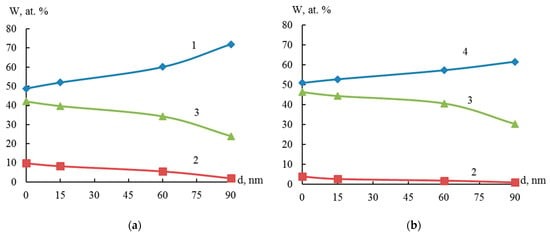
Figure 9.
Element distribution profile according to Auger spectroscopy data: Ti (1), Ni (2), O (3), and Zr (4), according to particle depth of Ti-Zr (a) and Zr-Ni (b) systems.
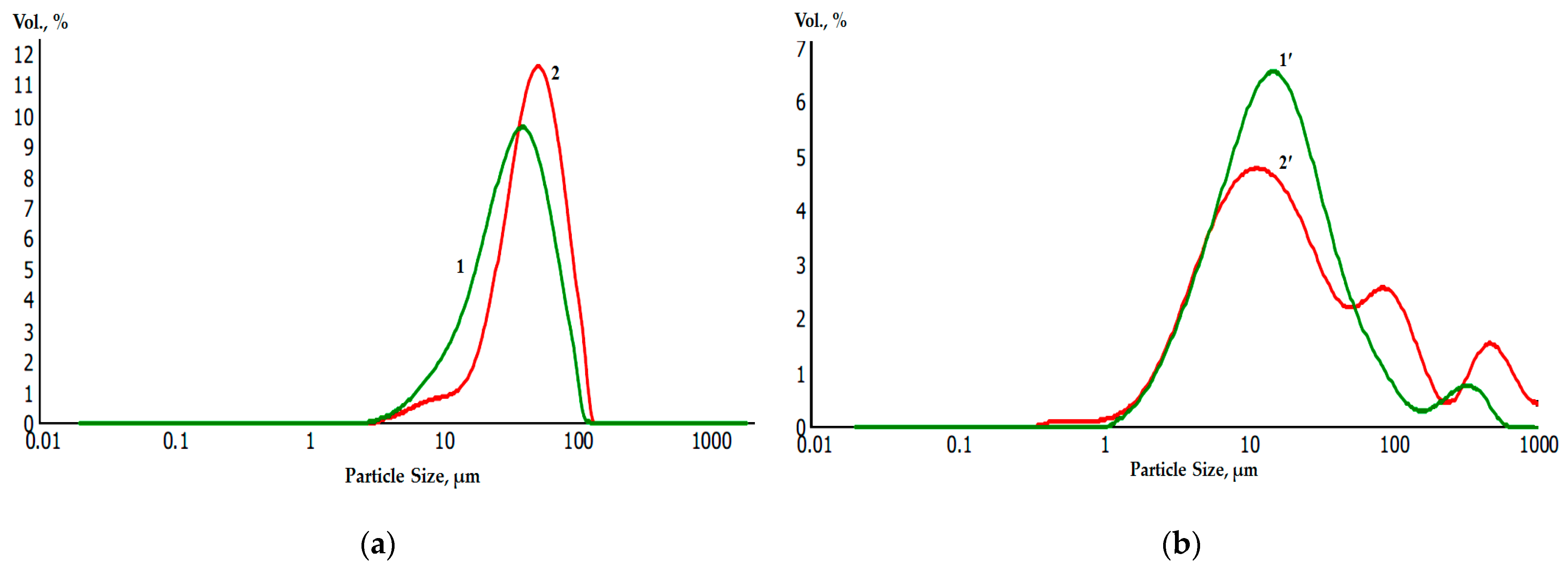
Laser diffraction analysis of the granulometric composition of powders before (a) and after (b) galvanic replacement showed a slight increase in the average diameter of the metal powder particles. At the same time, the average diameter of the Ti and Ti-Ni particles was 30 μm and 42 μm, respectively, while for Zr and Zr-Ni particles, it was 10 μm and 15 μm, respectively (Figure 10).
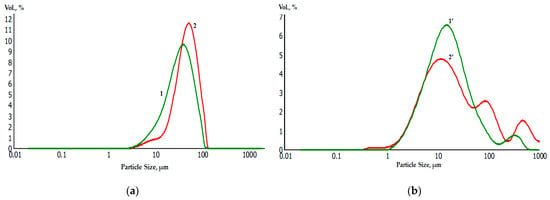
Figure 10.
Granulometric composition of initial and nickel-coated titanium and zirconium microparticles before (Ti—1; Zr—1’) and after (Ti—2; Zr—2’) galvanic replacement.
The synthesized polymetallic systems Ti-Ni and Zr-Ni can be studied in detail to establish phase transitions at high temperatures. The results of these studies can be used to develop new technological methods for obtaining powders of metallic layered systems such as “titanium-nickel“ and “zirconium-nickel”.
3. Materials and Methods
Commercially available dispersed forms of titanium and zirconium (American Elements Co, Los Angeles, CA, USA) were used for the galvanic replacement synthesis of the dispersed bimetallic systems Ti-Ni and Zr-Ni. Table A1 presents the chemical composition of dispersed titanium and zirconium.
To remove or transform the oxide film from the surface of titanium and zirconium and initiate the process of galvanic replacement in aqueous solutions, various concentrations of high-purity hydrofluoric acid solution were used.
To better understand the redox process occurring on dispersed titanium and zirconium, chronopotentiograms were recorded in a three-electrode cell using a P-2X potentiostat (ElectroChemical Instruments, Perm, Russia). A thin-layer platinum laboratory electrode was used as an indicator electrode, and a saturated silver chloride electrode with a Luggin capillary was used as a reference electrode. All values of electrode potentials presented in this study were relative to the reference saturated silver chloride electrode. In parallel, the temperature of the reaction mixture was measured using a contact thermometer.
To synthesize the dispersed bimetallic Ti-Ni and Zr-Ni systems, precise amounts of dispersed titanium (1.88 mol = 60 g/L), zirconium (1.32 mol = 60 g/L), and hydrofluoric acid were introduced into aqueous solutions containing 1.00 M NiCl2, accounting for stoichiometric excess. The metal deposits were thoroughly washed with distilled water and acetone before being subjected to drying at 80 °C for 3 hours in a drying cabinet.
The extent of galvanic replacement of metallic nickel on dispersed titanium and zirconium was determined by sampling the reaction mixture and subsequently quantitatively analyzing nickel(II) ions through complexometric titration.
The elemental composition of the dispersed systems was determined through X-ray fluorescence analysis. The analysis procedure was carried out using an S1 TITAN 500 portable X-ray fluorescence spectrometer (Bruker, Billerica, MA, USA).
The structural phase analysis of the dispersed systems was carried out using a Rigaku Smart Lab multi-functional X-ray diffractometer (Rigaku Corporation, Tokyo, Japan). The investigated dispersed systems were placed in a cuvette made of fused quartz.
In step-by–step scanning mode (step: 2θ = 0.05°, exposure time at the point: 2 s, shooting interval: 2θ = 30–80°), the surface of the test powder was irradiated with X-ray (30 kV, 10 mA). Calculations were performed using Powder cell and X powder software Ver. 2.8.4.0. The full-profile analysis of diffractograms was conducted by fitting the theoretical reflection intensity values to the experimental data and performing calculations using corundum coefficients in the PDXL-2 software package. In all cases, the hardware broadening of diffraction peaks was considered, relative to a standard sample of silicon powder that was free from microstresses and dimensional widenings.
The surface morphology of dispersed samples containing titanium, zirconium, and nickel was studied using an AURIGA CrossBeam electron microscope (Carl Zeiss) equipped with a combined X-ray spectral microanalyzer. Additionally, a JEOL JSM-7100F scanning electron microscope with secondary electron detectors (SEIs) was used.
The elemental composition of the near-surface region of the dispersed samples was investigated using scanning Auger electron spectroscopy using a JAMP-9500F Auger microprobe with an energy resolution of ΔE/E = 0.05% (JEOL Ltd., Tokyo, Japan). The dispersed samples under study were affixed to a conductive carbon tape (cat. № G3939, Agar Scientific, Stansted, UK). To analyze the local distribution of elements within the sample volume, the surface was periodically treated with Ar+ ions with an energy of 3 keV and a beam diameter of approximately 0.02 μm within time intervals of up to 90 seconds (the rate of ion etching with Ar+ 3 kV is approximately from 22 to 45 nm/min according to Zr). The analyzed regions were selected based on images obtained using a scanning electron microscope.
The granulometric composition of the dispersed systems was studied using laser diffraction on the Mastersizer 2000 by Malvern. The test samples, placed in a flow cell, were illuminated with a laser beam (a 630 nm He-Ne gas laser). The average size of the microparticles of the studied samples and their quantitative granulometric composition were determined based on the radiation intensity using the Malvern program, applying the Fraunhofer theory.
4. Conclusions
Dispersed systems consisting of titanium, zirconium, and nickel (Ti-Ni, Zr-Ni) were synthesized through galvanic replacement. The important synthesis parameters, including the quantitative ratios of metal ions and hydrofluoric acid, the process duration (not exceeding 10 minutes), and the formation characteristics of polymetallic systems with adjustable granulometrics, phase, and elemental compositions, were described.
X-ray phase analysis revealed that the deposition of nickel on dispersed titanium and zirconium in aqueous solutions, in the presence of hydrofluoric acid, results in the phase composition of binary systems existing as a mechanical mixture of nickel and titanium, as well as nickel and zirconium, in the specified ratios.
The Auger electron spectroscopy method confirmed that the dispersed Ti-Ni and Zr-Ni systems obtained through galvanic replacement consist of microparticles with a core–shell structure.
Scanning electron microscopy revealed that the synthesized systems exhibit a highly porous structure that conforms the contour of the titanium and zirconium particle surfaces, accompanied by the presence of nanoscale round-shaped subunits on the surfaces of the formed particle.
Author Contributions
This work is the collaborative development of all the authors. Conceptualization, A.F.D. and L.E.K.; methodology, A.F.D. and E.V.P.; software, E.V.P.; validation, A.F.D. and E.V.P.; formal analysis, L.E.K.; investigation, A.F.D., L.E.K. and E.V.P.; resources, A.F.D.; data curation, A.F.D. and L.E.K.; writing—original draft preparation, A.F.D. and L.E.K.; writing—review and editing, A.F.D.; supervision, A.F.D. and E.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation, grant number 075-01508-23-00.
Data Availability Statement
Not applicable.
Acknowledgments
This study was conducted using the equipment at the Center for Collective Use “Nanomaterials and Nanotechnology” at Kazan National Research Technological University.
Conflicts of Interest
The authors declare no conflict of interests.
Appendix A

Table A1.
Chemical composition of dispersed titanium and zirconium.
Table A1.
Chemical composition of dispersed titanium and zirconium.
| Metal | Content, (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ti | Zr | N | C | H | Fe, Ni | Si | Ca | Cl | O | |
| Ti 20 ÷ 40 μm | 98.80 | - | 0.08 | 0.05 | 0.35 | 0.40 | 0.10 | 0.05 | 0.004 | 0.16 |
| Zr 5 ÷ 15 μm | - | 99.81 | 0.01 | 0.03 | 0.30 | 0.05 | 0.10 | 0.05 | 0.001 | 0.25 |
- element missing
References
- Lu, N.H.; Chen, C.H. Improving the functional stability of TiNi-based shape memory alloy by multi-principal element design. Mater. Sci. Eng. A 2023, 872, 144999. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Regmi, R.; Lawes, G.; Salley, S.; Ng, K. Gaseous phase hydrogen storage and electrochemical properties of Zr8Ni21, Zr7Ni10, Zr9Ni11, and ZrNi metal hydride alloys. Int. J. Hydrogen Energy 2012, 37, 16042–16055. [Google Scholar] [CrossRef]
- Hu, L.F.; Li, J.; Tao, Y.F.; Lv, Y.H. Corrosion behaviors of TiNi/Ti2Ni matrix coatings in the environment rich in Cl ions. Surf. Coat. Technol. 2017, 311, 295–306. [Google Scholar] [CrossRef]
- Patel, S.K.; Behera, B.; Swain, B.; Roshan, R.; Sahoo, D.; Behera, A. A review on NiTi alloys for biomedical applications and their biocompatibility. Mater. Today Proc. 2020, 33, 5548–5551. [Google Scholar] [CrossRef]
- Wagner, M.A.; Ocana-Pujol, J.L.; Hadian, A.; Clemens, F.; Spolenak, R. Filament extrusion-based additive manufacturing of NiTi shape memory alloys. Mater. Des. 2023, 225, 111418. [Google Scholar] [CrossRef]
- Wang, C.; Tan, X.P.; Du, Z.; Chandra, S.; Sun, Z.; Lim, C.; Tor, S.; Wong, C. Additive manufacturing of NiTi shape memory alloys using pre-mixed powders. J. Mater. Process. Technol. 2019, 271, 152–161. [Google Scholar] [CrossRef]
- Konovalov, S.; Osintsev, K.; Golubeva, A.; Smelov, V.; Ivanov, Y.; Chen, X.; Komissarova, I. Surface modification of Ti-based alloy by selective laser melting of Ni-based superalloy powder. J. Mater. Res. Technol. 2020, 9, 8796–8807. [Google Scholar] [CrossRef]
- Tsuzuki, T. Mechanochemical synthesis of nanoparticles. Encycl. Nanomater. 2023, 1, 39–47. [Google Scholar] [CrossRef]
- Taha, M.A.; Youness, R.A.; Zawrah, M.F. Review on nanocomposites fabricated by mechanical alloying. Int. J. Miner. Metall. Mater. 2019, 26, 1047–1058. [Google Scholar] [CrossRef]
- Huot, J.; Ravnsbæk, D.B.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T. Mechanochemical synthesis of hydrogen storage materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, C.; Li, Y.; Hu, T. Synthesis of core-shell Ti@Ni-P spherical powder by Ni electroless plating. Micron 2021, 143, 103027. [Google Scholar] [CrossRef]
- Lu, L.; Sevonkaev, I.; Kumar, A.; Goia, D.V. Strategies for tailoring the properties of chemically precipitated metal powders. Powder Technol. 2014, 261, 87–97. [Google Scholar] [CrossRef]
- Deng, N.; Li, J.; Liang, S.; Sun, H.; Guo, Y. Research on process control of intermittent electrodeposition for preparation of core-shell powder. Powder Technol. 2022, 407, 117632. [Google Scholar] [CrossRef]
- Sure, J.; Vishnu, D.S.M. Carsten Schwandt. Single-step electrochemical synthesis of nano-crystalline CoCrFeNi high-entropy alloy powder. Electrochem. Commun. 2022, 143, 107392. [Google Scholar] [CrossRef]
- Djokić, S.S.; Nikolić, N.D.; Živković, P.M.; Popov, K.; Djokić, N. Electrodeposition and Electroless Deposition of Metallic Powders: A Comparison. ECS Trans. 2011, 33, 7–31. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Venkateshalu, S.; Jeong, S.; Tomboc, G.M.; Jo, J.; Park, J.; Lee, K. Galvanic replacement reaction to prepare catalytic materials. Bull. Korean Chem. Soc. 2023, 44, 4–22. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.Y.; Zhang, Q.; Liu, H. Overview of recent developments of resource recovery from wastewater via electrochemistry-based technologies. Sci. Total Environ. 2021, 757, 143901. [Google Scholar] [CrossRef]
- Chen, A.N.; McClain, S.M.; House, S.D.; Tomboc, G.M.; Jo, J.; Park, J.; Lee, K. Mechanistic Study of Galvanic Replacement of Chemically Heterogeneous Templates. Chem. Mater. 2019, 31, 1344–1351. [Google Scholar] [CrossRef]
- Clay, M.; Cui, Q.; Sha, Y.; Chen, J.; Rondinone, A.J.; Wu, Z.; Chen, J.; Gu, Z. Galvanic synthesis of bi-modal porous metal nanostructures using aluminum nanoparticle templates. Mater. Lett. 2012, 88, 143–147. [Google Scholar] [CrossRef]
- Cobley, C.M.; Xia, Y. Engineering the properties of metal nanostructures via galvanic replacement reactions. Mater. Sci. Eng. R 2010, 70, 44–62. [Google Scholar] [CrossRef]
- Carraro, C.; Maboudian, R.; Magagnin, L. Metallization and nanostructuring of semiconductor surfaces by galvanic displacement processes. Surf. Sci. Rep. 2007, 62, 499–525. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Goebl, J.; Yin, Y. Self-templated synthesis of hollow nanostructures. Nanotoday 2009, 4, 494–507. [Google Scholar] [CrossRef]
- Parvizi, S.; Hashemi, S.M.; Asgarinia, F.; Nematollahi, M.; Elahinia, M. Effective parameters on the final properties of NiTi-based alloys manufactured by powder metallurgy methods: A review. Prog. Mater Sci. 2021, 117, 100739. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Sun, Y.; Liu, H.; Jiang, C.; Ji, V.; Li, W. Influences of Al and Ti particles on microstructure, internal stress and property of Ni composite coatings. J. Alloys Compd. 2019, 793, 314–325. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Kalugin, L.E.; Dresvyannikov, A.F. Kinetics of contact precipitation of metallic nickel on the surface of dispersed titanium. Butl. Commun. 2022, 70, 68–75. [Google Scholar]
- Dresvyannikov, A.F.; Kolpakov, M.E.; Ermolaeva, E.A. Formation of a heterogeneous mixture of metals during the reduction of Ni(II) ions on titanium in aqueous solutions. Russ. J. Phys. Chem. A 2022, 96, 309–314. [Google Scholar] [CrossRef]
- Dresvyannikov, A.F.; Kalugin, L.E.; Mironov, M.M.; Shaekhov, M.F. Influence of Plazma High Frequency Discharge on the Physical and Chemical Properties of Ti-Fe-Ni Dispersed System Obtained by Electrochemical Method. Phys. Chem. Mater. Treat. 2022, 4, 15–22. [Google Scholar] [CrossRef]
- Bogdanov, S.; Sychov, M. Core-Shell Powders with Titanium Coating. In Proceedings of the Recent Advances in Technology Research and Education; Springer: Cham, Switzerland, 2017; Volume 660, pp. 81–86. [Google Scholar] [CrossRef]
- Mogoda, A.S. Electrochemical behaviour of zirconium and the anodic oxide film in aqueous solutions containing chloride ions. Thin Solid Films 1999, 357, 202–207. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.D. Corrosion of Titanium: Part 1: Aggressive Environments and Main Forms of Degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, e291–e302. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-L.; Lu, S.-Y.; Shao, Y.; Yao, K.F. Segregating the homogeneous passive film and understanding the passivation mechanism ofTi-based metallic glasses. Corros. Sci. 2021, 178, 109078. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).