The “Periodic Table” of 1-methylbenzotriazole: Zinc(II) Complexes
Abstract
:1. Introduction
2. Results and Discussion
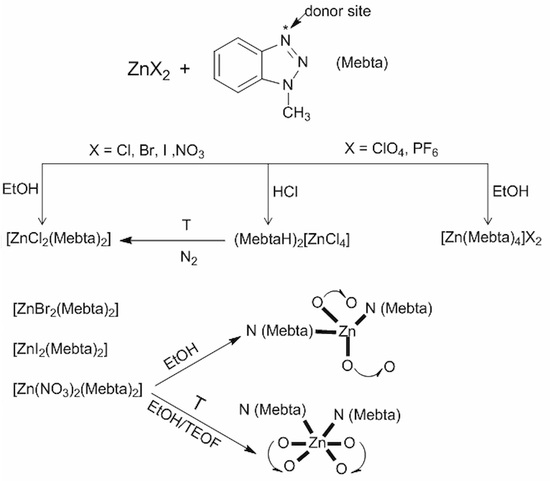
2.1. Synthetic Comments
2.2. Description of Structures
 description of Mebta (Figure 1) might be slightly different due to coordination; this has as a consequence the small decrease of the double bond character between the nitrogen atoms at positions 2 and 3 of the azole ring. The decrease of the double bond character of the triazole ring would be expected as consequence of coordination. The Zn-I bond distances are larger than the Zn-Br ones by ca. 0.2 Å, as expected.
description of Mebta (Figure 1) might be slightly different due to coordination; this has as a consequence the small decrease of the double bond character between the nitrogen atoms at positions 2 and 3 of the azole ring. The decrease of the double bond character of the triazole ring would be expected as consequence of coordination. The Zn-I bond distances are larger than the Zn-Br ones by ca. 0.2 Å, as expected.2.3. Spectroscopic and Physical Characterization in Brief
2.4. Theoretical Calculations
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Syntheses of the Complexes
3.2.1. [ZnCl2(Mebta)2] (1)
3.2.2. (MebtaH)2[ZnCl4] (2)
3.2.3. [ZnBr2(Mebta)2] (3)
3.2.4. [ZnI2(Mebta)2] (4)
3.2.5. tet-[Zn(NO3)2(Mebta)2] (5)
3.2.6. oct-[Zn(NO3)2(Mebta)2] (6)
3.2.7. [Zn(Mebta)4](ClO4)2 (7)
3.2.8. [Zn(Mebta)4](PF6)2 (8)
3.3. Single-Crystal X-ray Crystallography
3.4. Computational Details
4. Concluding Comments and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 1999; pp. 352–353. [Google Scholar]
- Katritzky, A.R.; Rachwall, S.; Hitchings, G.J. Benzotriazole: A Novel Auxiliary. Tetrahedron 1991, 47, 2683–2732. [Google Scholar]
- Hall, C.D.; Panda, S.S. The Benzotriazole Story. Adv. Heter. Chem. 2016, 119, 1–23. [Google Scholar]
- Tomas, F.; Abboud, J.-L.M.; Laynez, J.; Notario, R.; Santos, L.; Nilsson, S.O.; Catalan, J.; Charamunt, R.M.; Elguero, J. Tautomerism and Aromaticity in 1,2,3-Triazoles: The Case of Benzotriazole. J. Am. Chem. Soc. 1989, 111, 7348–7353. [Google Scholar]
- Catalan, J.; Claramunt, R.M.; Elguero, J.; Laynez, J.; Menendez, M.; Anvia, F.; Quian, J.H.; Taagepera, M.; Taft, R.W. Basicity and Acidity of Azoles: The Annelation Effect in Azoles. J. Am. Chem. Soc. 1988, 110, 4105–4111. [Google Scholar]
- Katritzky, A. N-Substituted Benzotriazoles: Properties, Reactivity and Synthetic Utility. Bull. Soc. Chim. Belg. 1992, 101, 409–413. [Google Scholar]
- Patel, P.; Patel, P.; Patel, S. Synthesis, Characterization, Chelating Properties and Biological Activities of Benzotriazole-Salicylic Acid Combined Molecule. Int. J. Pharm. Chem. Sci. 2012, 1, 1799–1804. [Google Scholar]
- Briguglio, I.; Piras, S.; Corona, P.; Gavini, E.; Nieddu, M.; Boatto, G.; Carta, A. Benzotriazole: An overview on its versatile biological behavior. Eur. J. Med. Chem. 2015, 97, 612–648. [Google Scholar]
- Krasevec, I.; Prosen, H. Solid-Phase Extraction of Polar Benzotriazoles as Environmental Pollutants: A Review. Molecules 2018, 23, 2501. [Google Scholar]
- Novak, I.; Abu-Izneid, T.; Kovacs, B.; Klasinc, L. Electronic Structure and Stability of Benzotriazoles. J. Phys. Chem. A 2009, 113, 9751–9756. [Google Scholar]
- Claramunt, R.M.; Sanz, D.; Boyer, G.; Catalan, J.; de Paz, J.L.G.; Elguero, J. Experimental (13C and 15N NMR Spectroscopy) and Theoretical (6-31G) Study of the Protonation of N-Methylazoles and N-Methylbenzazoles. Magn. Reson. Chem. 1993, 31, 791–800. [Google Scholar]
- Catalan, J.; Perez, P.; Elguero, J. Structure of Benzotriazole in the Gas Phase: A UV Experimental Study. J. Org. Chem. 1993, 58, 5276–5277. [Google Scholar]
- Thomas, S.; Venkateswaran, S.; Kapoor, S.; D’Cunha, R.; Mukherjee, T. Surface enhanced Raman scattering of benzotriazole: A molecular orientational study. Spectrochim. Acta A 2004, 60, 25–29. [Google Scholar]
- Sockalingum, D.; Fleischmann, M.; Musiani, M.M. Near-infrared Fourier transform surface-enhanced Raman scattering of azole copper corrosion inhibitors in aqueous chloride media. Spectrochim. Acta A 1991, 47, 1475–1485. [Google Scholar]
- Madsen, H.B. A preliminary note on the use of benzotriazole for stabilizing bronze objects. Stud. Conserv. 1967, 12, 163–167. [Google Scholar]
- Kokalj, A.; Peljhan, S.; Finsgar, M.; Milosev, I. What Determines the Inhibition Effectiveness of ATA, BTAH, and BTAOH Corrosion Inhibitors on Copper? J. Am. Chem. Soc. 2010, 132, 16657–16668. [Google Scholar] [PubMed]
- Kokalj, A. Ab initio modeling of the bonding of benzotriazole corrosion inhibitor to reduced and oxidized copper surfaces. Faraday Discuss. 2015, 180, 415–438. [Google Scholar] [PubMed]
- Malow, M.; Wehrstedt, K.D.; Neuenfeld, S. On the explosive properties of 1H-benzotriazole and 1H-1,2,3-triazole. Tetrahedron Lett. 2007, 48, 1233–1235. [Google Scholar]
- Srinivas, D.; Ghule, V.D.; Tewari, S.P.; Muralidharan, K. Synthesis of Amino, Azido, Nitro, and Nitrogen-Rich Azole-Substituted Derivatives of 1H-Benzotriazole for High-Energy Materials Applications. Chem. Eur. J. 2012, 18, 15031–15037. [Google Scholar]
- Flippen-Anderson, J.L.; Gilardi, R.D.; Pitt, A.M.; Wilson, W.S. Synthesis and Explosive Properties of Benzotriazoles. Aust. J. Chem. 1992, 45, 513–524. [Google Scholar]
- Paterson, M.J.; Robb, M.A.; Blancafort, L.; DeBellis, A.D. Theoretical Study of Benzotriazole UV Photostability: Ultrafast Deactivation through Coupled Proton and Electron Transfer Triggered by a Charge-Transfer State. J. Am. Chem. Soc. 2004, 126, 2912–2922. [Google Scholar]
- Lai, H.-J.; Ying, G.-G.; Ma, Y.-B.; Chen, Z.-F.; Chen, F.; Liu, Y.-S. Field dissipation and plant uptake of benzotriazole ultraviolet stabilizers in biosolid-amended soils. Environ. Sci. Process. Impacts 2014, 16, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yan, Z.; Feng, L.; Zhai, F.; Chen, X.; Xu, Y.; Tang, S.; Huang, C.; Li, L.; Pan, N.; et al. Benzotriazole decorated graphene oxide for efficient removal of U(VI). Environ. Pollut. 2019, 253, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Aromi, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazole and tetrazole. Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Loukopoulos, E.; Kostakis, G.E. Recent advances in the coordination chemistry of benzotriazole-based ligands. Coord. Chem. Rev. 2019, 395, 193–229. [Google Scholar] [CrossRef]
- Biswas, S.; Tonigold, M.; Volkmer, D. Homo- and Heteronuclear Coordination Compounds with Td Symmetry- the Solid State Structures of [MZn4(L)4(L’)6] (M = CoII or ZnII.; L = chloride or acac; L’ = 1,2,3-benzotriazolate). Z. Anorg. Allg. Chem. 2008, 634, 2532–2538. [Google Scholar] [CrossRef]
- Biswas, S.; Tonigold, M.; Speldrich, M.; Kögerler, P.; Volkmer, D. Nonanuclear Coordination Compounds Featuring {M9L12}6+ Cores (M = NiII, CoII, or ZnII.; L = 1,2,3-Benzotriazolate). Eur. J. Inorg. Chem. 3094–3101.
- Biswas, S.; Tonigold, M.; Kelm, H.; Krüger, H.-J.; Volkmer, D. Thermal spin-crossover in the [M3Zn6Cl6L12] (M = Zn, FeII.; L = 5,6-dimethoxy-1,2,3-benzotriazolate) system: Structural, electrochemical, Mössbauer, and UV-Vis spectroscopic studies. Dalton Trans. 2010, 39, 9851–9859. [Google Scholar] [CrossRef]
- Biswas, S.; Tonigold, M.; Speldrich, M.; Kögerler, P.; Weill, M.; Volkmer, D. Syntheses and Magnetostructural Investigations on Kuratowski-Type Homo- and Heteropentanuclear Coordination Compounds [MZn4Cl4(L)6] (MII = Zn, Fe, Co, Ni, or Cu; L = 5,6-Dimethyl-1,2,3-benzotriazolate) Represented by the Nonplanar K3,3 Graph. Inorg. Chem. 2010, 49, 7424–7434. [Google Scholar] [CrossRef]
- Xue, X.; Li, G.-T.; Peng, Y.-H.; Wu, L.; Wu, B.-L. Two photoluminescent pentanuclear homo- and hetero-metal complexes based on benzotriazole bridge. J. Coord. Chem. 2011, 64, 1593–1962. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Grzywa, M.; Tonigold, M.; Sastre, G.; Schüttrigkeit, T.; Leeson, N.S.; Volkmer, D. Photophysical properties of Kuratowski-type coordination compounds [MIIZn4Cl4(Me2bta)6] (MII = Zn or Ru) featuring long-lived excited electronic states. Dalton Trans. 2011, 40, 5926–5938. [Google Scholar] [CrossRef]
- Werner, T.W.; Reschke, S.; Bunzen, H.; Krug von Nidda, H.-A.; Deisenhofer, J.; Loidl, A.; Volkmer, D. [Co5Tp4*(Me2bta)6]: A Highly Symmetrical Pentanuclear Kuratowski Complex Featuring Tris(pyrazolyl)borate and Benzotriazolate Ligands. Inorg. Chem. 2016, 55, 1053–1060. [Google Scholar] [CrossRef]
- Bunzen, H.; Grzywa, M.; Kalytta-Mewes, A.; Volkmer, D. One-pot synthesis of ultrastable pentanuclear alkylzinc complexes. Dalton Trans. 2017, 46, 2613–2625. [Google Scholar] [CrossRef]
- Zhou, G.-J.; Chen, W.-P.; Yu, Y.; Qin, L.; Han, T.; Zheng, Y.-Z. Filling the Missing Lings of M3n Prototype 3d-4f and 4f Cyclic Coordination Cages: Syntheses, Structures and Magnetic Properties of the Ni10Ln5 and the Er3n Wheels. Inorg. Chem. 2017, 56, 12821–12829. [Google Scholar] [CrossRef]
- Tangoulis, V.; Raptopoulou, C.P.; Terzis, A.; Bakalbassis, E.G.; Diamantopoulou, E.; Perlepes, S.P. Polynuclear Nickel(II) Complexes: Preparation, Characterization, Magnetic Properties, and Quantum-Chemical Study of [Ni5(OH)(Rbta)5(acac)4(H2O)4] (R = Benzotriazole and 5,6-Dimethylbenzotriazole). Inorg. Chem. 1998, 37, 3142–3153. [Google Scholar] [CrossRef]
- Müller-Buschbaum, K.; Mokaddem, Y. Rare Earth Benzotriazolates: Coordination Polymers Incorporating Decomposition Products from Ammonia to 1,2-Diaminobenzene in 1/∞ [Ln(Btz)3(BtzH)] (Ln = Ce, Pr), 1/∞ [Ln(Btz)3{Ph(NH2)2}] (Ln = Nd, Tb, Yb), and 1/∞ [Ho2(Btz)6(BtzH)(NH3)]. Eur. J. Inorg. Chem. 2006, 2000–2010. [Google Scholar] [CrossRef]
- Müller-Buschbaum, K.; Mokaddem, Y. MOFs by Transformation of 1D-Coordination Polymers: From 1/∞ [Ln(Btz)3BtzH] to the Homoleptic Rare Earth 3D-Benzotriazolate Frameworks 3/∞ [Ln(Btz)3], Ln = La, Ce. Z. Anorg. Allg. Chem. 2008, 634, 2360–2366. [Google Scholar] [CrossRef]
- Biswas, S.; Grzywa, M.; Nayek, H.P.; Dehnen, S.; Senkovska, I.; Kaskel, S.; Volkmer, D. A cubic coordination framework constructed from benzobistriazolate ligands and zinc ions having selective gas sorption properties. Dalton Trans. 2009, 6487–6495. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Liu, B.-Y.; Wei, G.; Huang, X.-C. Solvent Induced Diverse Dimensional Coordination Assemblies of Cupric Benzotriazole-5-carboxylate: Syntheses, Crystal Structures, and Magnetic Properties. Inorg. Chem. 2011, 50, 11032–11038. [Google Scholar] [CrossRef]
- Li, Z.-H.; Xue, L.-P.; Zhao, B.-T.; Kan, J.; Su, W.-P. 2D lanthanide-organic frameworks constructed from lanthanide acetate skeletons and benzotriazole-5-carboxylic acid connectors: Synthesis, structure, luminescence and magnetic properties. Cryst. Eng. Commun. 2012, 14, 8485–8491. [Google Scholar] [CrossRef]
- Li, Z.-H.; Hong, D.-F.; Xue, L.-P.; Fu, W.-J.; Zhao, B.-T. Two lanthanide-bound 1H-benzotriazole polymers: New potential metal-organic scaffold for solid-phase organic chemistry. Inorg. Chim. Acta 2013, 400, 239–243. [Google Scholar] [CrossRef]
- Schmieder, P.; Denysenko, D.; Grzywa, M.; Baumgärtner, B.; Senkovska, I.; Kaskel, S.; van Wüllen, L.; Volkmer, D. CFA-1: The first chiral metal-organic framework containing Kuratowski-type secondary building units. Dalton Trans. 2013, 42, 10786–10797. [Google Scholar] [CrossRef]
- Bunzen, H.; Grzywa, M.; Hambach, M.; Spirkl, S.; Volkmer, D. From Micro to Nano: A Toolbox for Tuning Crystal Size and Morphology of Benzotriazolate-Based Metal-Organic Frameworks. Cryst. Growth Des. 2016, 16, 3190–3197. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.-B.; Tan, Y.-X.; Wang, F.; Kang, Y.; Zhang, J. Structural Diversity and Photoluminescent Properties of Zinc Benzotriazole-5-carboxylate Coordination Polymers. Inorg. Chem. 2014, 53, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Qin, J.-S.; He, W.-W.; Shao, K.-Z.; Su, Z.-M.; Du, D.-Y.; Li, S.-L.; Lan, Y.-Q. Encapsulation of an iridium complex in a metal–organic framework to give a composite with efficient white light emission. Inorg. Chem. Front. 2017, 4, 547–552. [Google Scholar] [CrossRef]
- Xie, W.; Ning, S.; Zhang, Y.; Tang, Z.; Zhang, S.; Tang, R. A 3D supramolecular network as highly selective and sensitive luminescent sensor for PO43- and Cu2+ ions in aqueous media. Dyes Pigm. 2018, 150, 36–43. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; An, X.; Xu, L.; Yan, J.-H.; Zhang, S.; Xie, W.; Su, Z.-M. Syntheses, structure and properties of an especially stable Cd metal-organic framework driven by benzotriazole-5-carboxylic acid. Inorg. Chem. Commun. 2020, 112, 107726. [Google Scholar] [CrossRef]
- Tangoulis, V.; Raptopoulou, C.P.; Psycharis, V.; Terzis, A.; Skorda, K.; Perlepes, S.P.; Cador, O.; Kahn, O.; Bakalbassis, E.G. Ferromagnetism in an Extended Three-Dimensional, Diamond-like Copper(II) Network: A New Copper(II)/1-hydroxybenzotriazolate Complex Exhibiting Solf-Magnet Properties and two Transitions at 6.4 and 4.4 K. Inorg. Chem. 2000, 39, 2522–2529. [Google Scholar] [CrossRef]
- Dimitropoulos, A.; Stamou, C.; Perlepes, S.P.; Lada, Z.G.; Petsalakis, I.D.; Marinakis, S. A study of 1-methylbenzotriazole (MEBTA) using quantum mechanical calculations and vibrational, electronic, and nuclear magnetic resonances spectroscopies. J. Eng. Sci. Technol. Rev. 2023, in press. [Google Scholar]
- Plakatouras, J.C.; Perlepes, S.P.; Mentzafos, D.; Terzis, A.; Bakas, T.; Papaefthymiou, V. Coordination Chemistry of Corrosion Inhibitors of the Benzotriazole Type: Preparation and Characterization of Cobalt(II) Complexes with 1-methylbenzotriazole (Mebta) and the Crystal Structures of [CoCl2(Mebta)2], trans-[Co(NCS)2(Mebta)4], trans-[Co(NCS)2(MeOH)2(Mebta)2] and cis-[Co(NO3)2(Mebta)2]. Polyhedron 1992, 11, 2657–2672. [Google Scholar]
- Plakatouras, J.C.; Bakas, T.; Huffman, C.J.; Huffman, J.C.; Papaefthymiou, V.; Perlepes, S.P. Two Different Terminal Bonding Modes in [Fe2O(NO3)4(C7H7N3)4]. J. Chem. Soc. Dalton Trans. 1994, 2737–2738. [Google Scholar] [CrossRef]
- Skorda, K.; Stamatatos, T.C.; Vafiadis, A.P.; Lithoxoidou, A.T.; Terzis, A.; Perlepes, S.P.; Mrozinski, J.; Raptopoulou, C.P.; Plakatouras, J.C.; Bakalbassis, E.G. Copper(II) chloride/1-methylbenzotriazole chemistry: Influence of various synthetic parameters on the product identity, structural and magnetic characterization, and quantum-chemical studies. Inorg. Chim. Acta 2005, 358, 565–582. [Google Scholar] [CrossRef]
- Lazari, G.; Grammatikopoulos, S.; Perlepes, S.P.; Stamatatos, T.C. Combining benzotriazoles and azides in copper(II) chemistry: Synthesis, structural and spectroscopic characterization of a 1-D corrugated tape [Cu(N3)2(1-Mebta)]n coordination polymer (1-Mebta = 1-methylbenzotriazole). J. Coord. Chem. 2021, 74, 1823–1833. [Google Scholar] [CrossRef]
- Jones, L.F.; O’Dea, L.; Offermann, D.A.; Jensen, P.; Moubaraki, B.; Murray, K.S. Benzotriazole based 1-D, 2-D and 3-D metal dicyanamide and tricyanomethanide coordination networks. Polyhedron 2006, 25, 360–372. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Sun, N.; Liu, Y. Gold-Catalyzed Cadiot-Chodkiewicz-type Cross-Coupling of Terminal Alkynes with Alkynyl Hypervalent Iodine Reagents: Highly Selective Synthesis of Unsymmetrical 1,3-Diynes. Angew. Chem. Int. Ed. 2017, 56, 6994–6998. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Bekiari, V.; Tzimopoulos, D.I.; Raptopoulou, C.P.; Psycharis, V.; Tsipis, A.; Perlepes, S.P. Reactivity of Coordinated 2-Pyridyl Oximes: Synthesis, Structure, Spectroscopic Characterization and Theoretical Studies of Dichlorodi{(2-Pyridyl)Furoxane}Zinc(II) Obtained from the Reaction between Zinc(II) Nitrate and Pyridine-2-Chloroxime. Inorganics 2020, 8, 47. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Kuzmierkiewicz, W.; Greenhill, I.V. An improved method for the N-alkylation of benzotriazole and 1,2,4-triazole. Recl. Trav. Chim. Pays-Bas 1991, 110, 369–373. [Google Scholar] [CrossRef]
- Rondeau, R.E.; Rosenberg, H.M.; Dunbar, D.J. Nuclear Magnetic Resonance Analysis of 1- and 2-Methylbenzotriazole. J. Mol. Spectr. 1969, 29, 305–311. [Google Scholar] [CrossRef]
- Begtrup, M.; Elguero, J.; Faure, R.; Camos, P.; Estopá, C.; Ilavsky, D.; Fruchier, A.; Marzin, C.; de Mendoza, J. Effect of N-Substituents on the 13C NMR Parameters of Azoles. Magn. Reson. Chem. 1988, 26, 134–151. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986; pp. 130–141+147–156+254–257+324–331+464. [Google Scholar]
- Kleywegt, G.J.; Wiesmeijer, W.G.R.; Van Driel, G.; Driessen, W.L.; Reedijk, J.; Noordik, J.H. Unidentate versus symmetrically and unsymmetrically bidentate nitrate co-ordination in pyrazole–containing chelates. The crystal and molecular structures of (nitrato-O)[tris(3,5-dimethylpyrazol-1-ylmethyl)amine]copper(II) nitrate, (nitrato-O,O’)[tris(3,5- dimethylpyrazol-1-ylmethyl)amine]nickel(II) nitrate, and (nitrato-O)(nitrato-O,O’)[tris(3,5- dimethylpyrazol-1-ylmethyl)amine]cadmium(II). J. Chem. Soc. Dalton Trans. 1985, 2177–2184. [Google Scholar]
- Kitos, A.A.; Efthymiou, C.G.; Manos, M.J.; Tasiopoulos, A.J.; Nastopoulos, V.; Escuer, A.; Perlepes, S.P. Interesting copper(II)-assisted transformations of 2-acetylpyridine and 2-benzoylpyridine. Dalton Trans. 2016, 45, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Fujimoto, H. A theoretical account for stereoselective e2 reactions. Tetrahedron Lett. 1965, 4303–4307. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital. Chem. Rev. 1988, 88, 899–926. [Google Scholar]
- Weinhold, F. Natural Bond Orbital Methods. In The Encyclopedia of Computational Chemistry; Schleyer, P.v.R., Allinger, N.L., Clark, T., Casteiger, J., Kollman, P.A., Schaefer, H.F., III, Schreiner, P.R., Eds.; Wiley: Chichester, UK, 1998; p. 1792. [Google Scholar]
- Glendening, E.D.; Bandenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Weinhold, F. NBO 5.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2001. [Google Scholar]
- CrystalClear; Rigaku: The Woodlands, TX, USA; MSC Inc.: Melville, NY, USA, 2005.
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Crystal Impact. Diamond, Crystal and Molecular Structure Visualization; Version 3.1; Crystal Impact: Bonn, Germany, 2018. [Google Scholar]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 Energy Evaluation by Direct Methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Saebo, S.; Almlof, J. Avoiding the integral storage bottleneck in LCAO calculations of electron correlation. Chem. Phys. Lett. 1989, 154, 83–89. [Google Scholar] [CrossRef]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. A direct MP2 gradient method. Chem. Phys. Lett. 1990, 166, 275–280. [Google Scholar] [CrossRef]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. Semi-direct algorithms for the MP2 energy and gradient. Chem. Phys. Lett. 1990, 166, 281–289. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Head-Gordon, T. Analytic MP2 frequencies without fifth-order storage. Theory and application to bifurcated hydrogen bonds in the water hexamer. Chem. Phys. Lett. 1994, 220, 122–128. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2994–3094. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Becke, D. Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. J. Chem. Phys. 1997, 107, 8554–8560. [Google Scholar] [CrossRef]
- Chai, D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, W.T. Empirical correction to density functional theory for van der Waals interactions. J. Chem. Phys. 2002, 116, 515–524. [Google Scholar] [CrossRef]
- Minenkov, Y.; Singstad, A.; Occhipinti, O.; Jensen, V. The accuracy of DFT-optimized geometries of functional transition metal compounds: A validation study of catalysts for olefin metathesis and other reactions in the homogeneous phase. Dalton Trans. 2012, 41, 5526–5541. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Weigend, F.; Ahlirchs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Weigend, F.; Furche, F.; Ahlrichs, R. Gaussian basis sets of quadruple zeta valence quality for atoms H–Kr. J. Chem. Phys. 2003, 119, 12753–12762. [Google Scholar] [CrossRef]











| Bond Lengths (Å) and Angles (°) a | 3 | 4 |
|---|---|---|
| Zn-X1 | 2.367(1) | 2.554(1) |
| Zn-X2 | 2.353(7) | 2.567(1) |
| Zn-N3 | 2.034(3) | 2.050(4) |
| Zn-N13 | 2.070(4) | 2.042(4) |
| N1-N2 | 1.335(4) | 1.334(6) |
| N2-N3 | 1.321(4) | 1.315(7) |
| N11-N12 | 1.329(5) | 1.333(7) |
| N12-N13 | 1.323(4) | 1.321(7) |
| C9-N1 | 1.362(5) | 1.362(8) |
| C8-N3 | 1.384(5) | 1.367(7) |
| C10-N1 | 1.466(5) | 1.461(7) |
| X1-Zn-X2 | 116.4(3) | 115.4(1) |
| X1-Zn-N3 | 108.9(1) | 112.3(1) |
| X1-Zn-N13 | 104.6(1) | 108.0(1) |
| X2-Zn-N3 | 114.4(1) | 104.0(1) |
| X2-Zn-N13 | 107.2(1) | 110.2(1) |
| N3-Zn-N13 | 104.2(1) | 106.7(2) |
| N1-N2-N3 | 107.9(3) | 107.6(4) |
| Complex 5 | |||
|---|---|---|---|
| Distances (Å) | Angles (°) | ||
| Zn-O1 | 2.016(2) | O1-Zn-O4 | 87.9(1) |
| Zn∙∙∙O2 | 2.588(5) | O1-Zn-N3 | 115.7(1) |
| Zn-O4 | 1.992(2) | O1-Zn-N13 | 108.3(1) |
| Zn∙∙∙O6 | 2.722(6) | O4-Zn-N3 | 117.4(1) |
| Zn-N3 | 1.996(2) | O4-Zn-N13 | 105.0(1) |
| Zn-N13 | 1.995(2) | N3-Zn-N13 | 118.2(1) |
| N4-O1 | 1.290(3) | O2∙∙∙Zn-O1 | 53.8(1) |
| N4-O2 | 1.228(3) | O2∙∙∙Zn-O4 | 141.0(1) |
| N4-O3 | 1.214(3) | O2∙∙∙Zn-N3 | 88.9(1) |
| N5-O4 | 1.291(3) | O2∙∙∙Zn-N13 | 83.9(1) |
| N5-O5 | 1.214(3) | O2∙∙∙Zn∙∙∙O6 | 164.3(1) |
| N5-O6 | 1.229(3) | O6∙∙∙Zn-O1 | 139.5(1) |
| N1-N2 | 1.336(3) | O6∙∙∙Zn-O4 | 51.7(1) |
| N2-N3 | 1.319(3) | O6∙∙∙Zn-N3 | 89.9(1) |
| N11-N12 | 1.332(3) | O6∙∙∙Zn-N13 | 82.8(1) |
| N12-N13 | 1.313(3) | ||
| Complex 6 | |||
| Distances (Å) | Angles (°) a | ||
| Zn-N3 | 2.040(2) | N3-Zn-O1 | 105.9(1) |
| Zn-O1 | 2.061(2) | N3-Zn-O1′ | 97.6(1) |
| Zn-O2 | 2.388(2) | N3-Zn-O2 | 92.5(1) |
| N1-N2 | 1.325(3) | N3-Zn-O2′ | 153.7(1) |
| N2-N3 | 1.323(3) | N3-Zn-N3′ | 101.7(1) |
| N4-O1 | 1.275(3) | O1-Zn-O1′ | 142.5(1) |
| N4-O2 | 1.244(3) | O1-Zn-O2 | 56.9(1) |
| N4-O3 | 1.220(3) | O1-Zn-O2′ | 93.9(1) |
| O2-Zn-O2′ | 84.0(1) |
| Bond Lengths (Å) | Bond Angles (°) | ||
|---|---|---|---|
| Zn-N3 | 1.990(2) | N3-Zn-N13 | 111.0(1) |
| Zn-N13 | 1.987(2) | N3-Zn-N23 | 109.8(1) |
| Zn-N23 | 1.981(2) | N3-Zn-N33 | 108.6(1) |
| Zn-N33 | 2.007(2) | N13-Zn-N23 | 110.3(1) |
| N1-N2 | 1.325(3) | N13-Zn-N33 | 108.0(1) |
| N2-N3 | 1.320(3) | N23-Zn-N33 | 109.1(1) |
| Compound | νas(NNN) | νs(NNN) | νas(ZnX)t | νs(ZnX)t | ν(ZnO)t |
|---|---|---|---|---|---|
| Mebta | 1197s | 1110m | |||
| (1180w) | (1105s) | ||||
| 1 | 1218s | 1136m | 324s | 300s | |
| (1226m) | (1140s) | (325m) | (300s) | ||
| 2 | 1222s | 1138m | |||
| b | b | ||||
| 3 | 1218s | 1135m | 243s c | 219s | |
| (1224m) | (1140s) | (246m c) | (220s) | ||
| 4 | 1219s | 1132m | 208s | 189s | |
| (1233w, 1224m) | (1136s) | (208m) | (182s) | ||
| 5 | 1220s | 1139m | 348s | 300m | |
| b | b | b | b | ||
| 6 | 1220s | 1137m | 326w, 292sh, 274s d, 255sh | ||
| (1228m) | (1141m) | (326m, 270s d, 260m) | |||
| 7 | 1218m | 1144s | |||
| b | b | ||||
| 8 | 1226m | 1170w | |||
| b | b |
| Compound | ν(ZnCl)t(T2)/ ν(ZnN)(T2) | νas(ZnN) | νs(ZnN) | ν(ZnN) |
| Mebta | ||||
| 1 | 274m | 247m | ||
| (270m) | (249s) | |||
| 2 | 293s | |||
| b | ||||
| 3 | 275s | 243s c | ||
| (271m) | (246m c) | |||
| 4 | 263m | 248m | ||
| (263m) | (246m) | |||
| 5 | 291s | 248m | ||
| b | b | |||
| 6 | 263m | 274s d, 242m | ||
| b | (270s d, 248m) | |||
| 7 | 288s | |||
| b | ||||
| 8 | 287s | |||
| b |
| Complex | Zn-Donor Atom | Bond Length (Å) | Mulliken Pop Anal | Wiberg Bond Indices |
|---|---|---|---|---|
| 5 | Zn-N3 | 2.04 | 0.2233 | 0.2705 |
| Zn-N13 | 2.06 | 0.1871 | 0.2588 | |
| Zn-O1 | 1.99 | 0.2065 | 0.2794 | |
| Zn-O4 | 2.00 | 0.1770 | 0.2663 | |
| Zn-O2 | 2.51 | 0.0723 | 0.1021 | |
| Zn-O6 | 2.66 | 0.0504 | 0.0723 | |
| 6 | Zn-N3 | 2.15 | 0.1568 | 0.2261 |
| Zn-N3′ | 2.15 | 0.1568 | 0.2261 | |
| Zn-O1 | 2.08 | 0.1535 | 0.2250 | |
| Zn-O1′ | 2.08 | 0.1535 | 0.2250 | |
| Zn-O2 | 2.19 | 0.1336 | 0.1911 | |
| Zn-O2′ | 2.19 | 0.1336 | 0.1911 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamou, C.; Barouni, E.; Plakatouras, J.C.; Sigalas, M.M.; Raptopoulou, C.P.; Psycharis, V.; Bakalbassis, E.G.; Perlepes, S.P. The “Periodic Table” of 1-methylbenzotriazole: Zinc(II) Complexes. Inorganics 2023, 11, 356. https://doi.org/10.3390/inorganics11090356
Stamou C, Barouni E, Plakatouras JC, Sigalas MM, Raptopoulou CP, Psycharis V, Bakalbassis EG, Perlepes SP. The “Periodic Table” of 1-methylbenzotriazole: Zinc(II) Complexes. Inorganics. 2023; 11(9):356. https://doi.org/10.3390/inorganics11090356
Chicago/Turabian StyleStamou, Christina, Eleftheria Barouni, John C. Plakatouras, Michael M. Sigalas, Catherine P. Raptopoulou, Vassilis Psycharis, Evangelos G. Bakalbassis, and Spyros P. Perlepes. 2023. "The “Periodic Table” of 1-methylbenzotriazole: Zinc(II) Complexes" Inorganics 11, no. 9: 356. https://doi.org/10.3390/inorganics11090356
APA StyleStamou, C., Barouni, E., Plakatouras, J. C., Sigalas, M. M., Raptopoulou, C. P., Psycharis, V., Bakalbassis, E. G., & Perlepes, S. P. (2023). The “Periodic Table” of 1-methylbenzotriazole: Zinc(II) Complexes. Inorganics, 11(9), 356. https://doi.org/10.3390/inorganics11090356