Efficient Disposal of Basic Fuchsin Dye from Aqueous Media Using ZrO2/MgMn2O4/Mg(Mg0.333Mn1.333)O4 as a Novel and Facilely Synthesized Nanocomposite
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
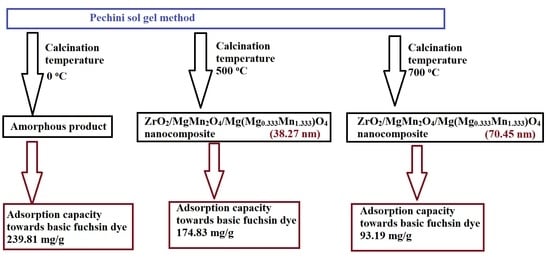
2.2. Synthesis of Zr/Mg/Mn/O Adsorbents
2.3. Characterization of the Synthesized Adsorbents
2.4. Removal of BFD Dye from Aqueous Solutions
3. Results and Discussion
3.1. Characterization of the Fabricated Products
3.1.1. XRD
3.1.2. EDS
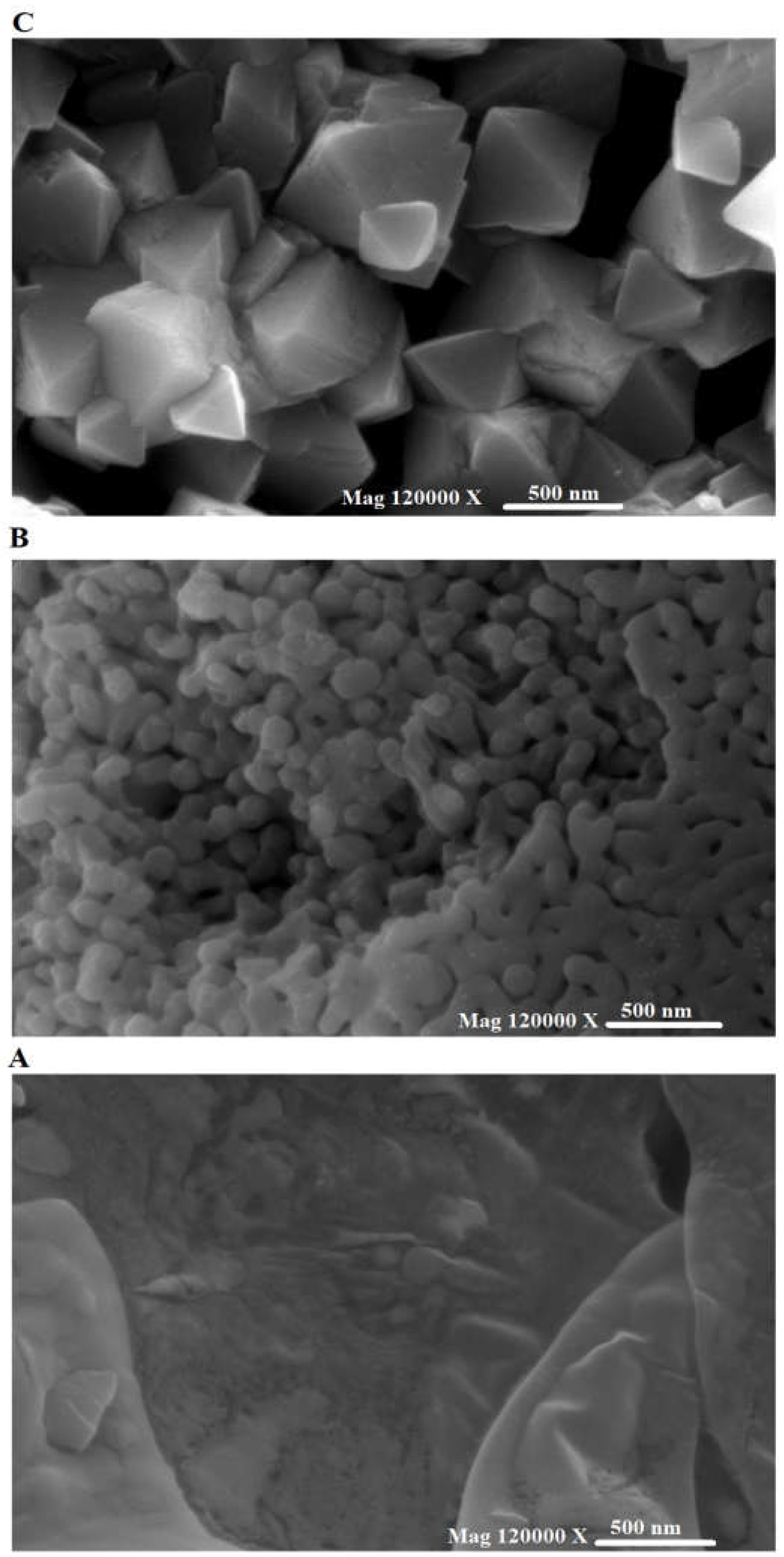
3.1.3. FE-SEM and HR-TEM
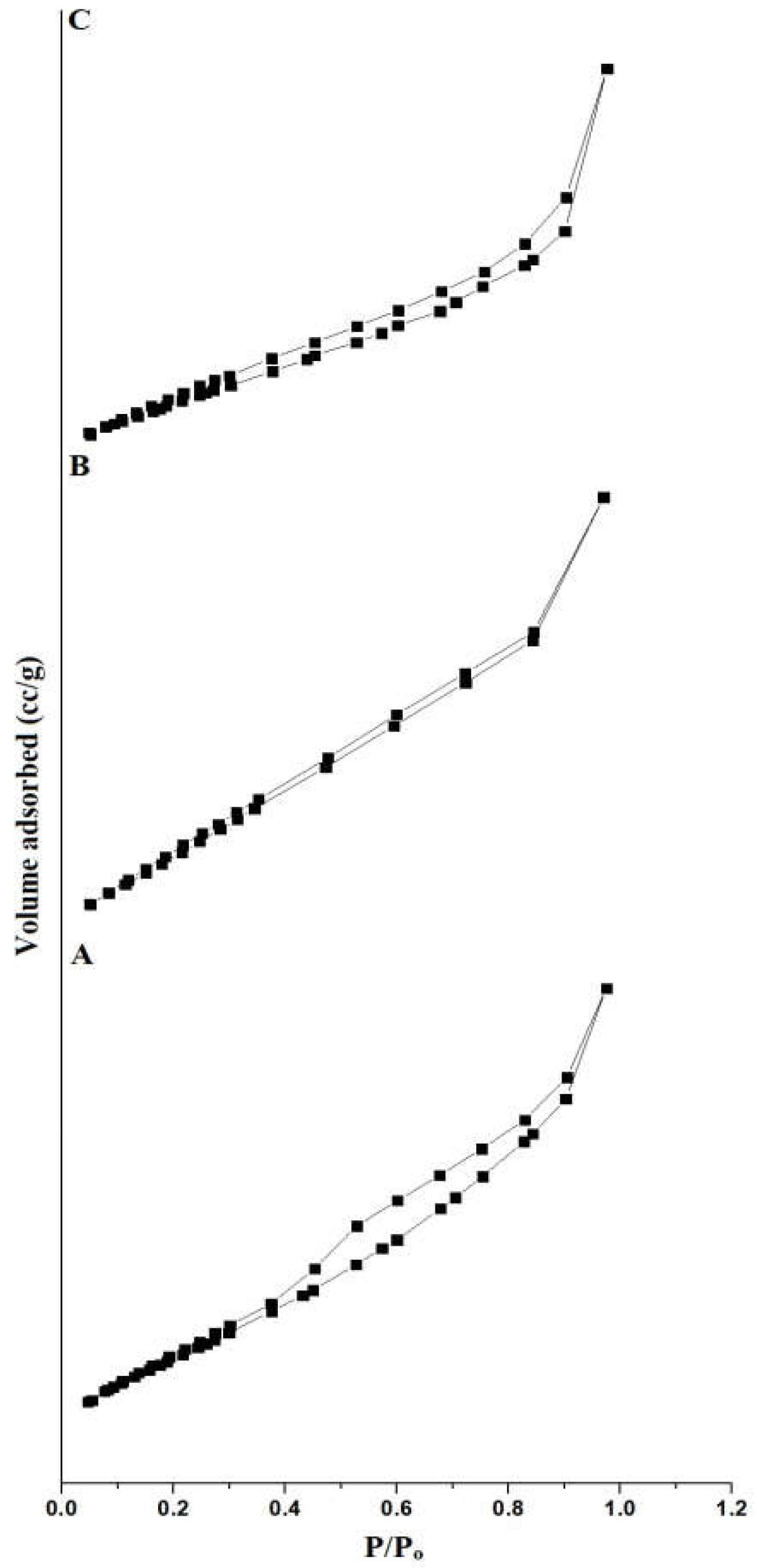
3.1.4. Nitrogen Adsorption/Desorption
3.2. Removal of BFD Dye from Aqueous Media
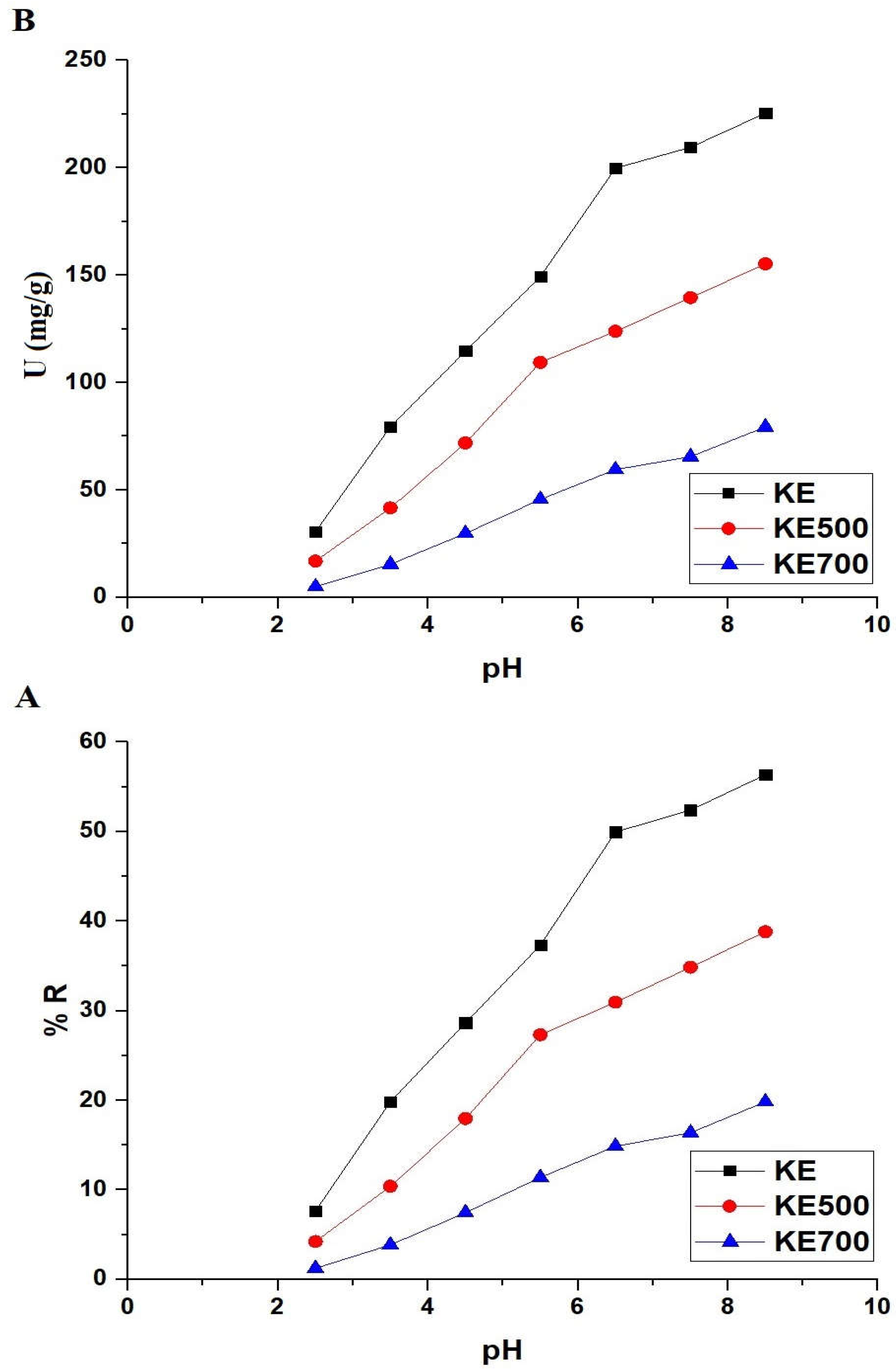
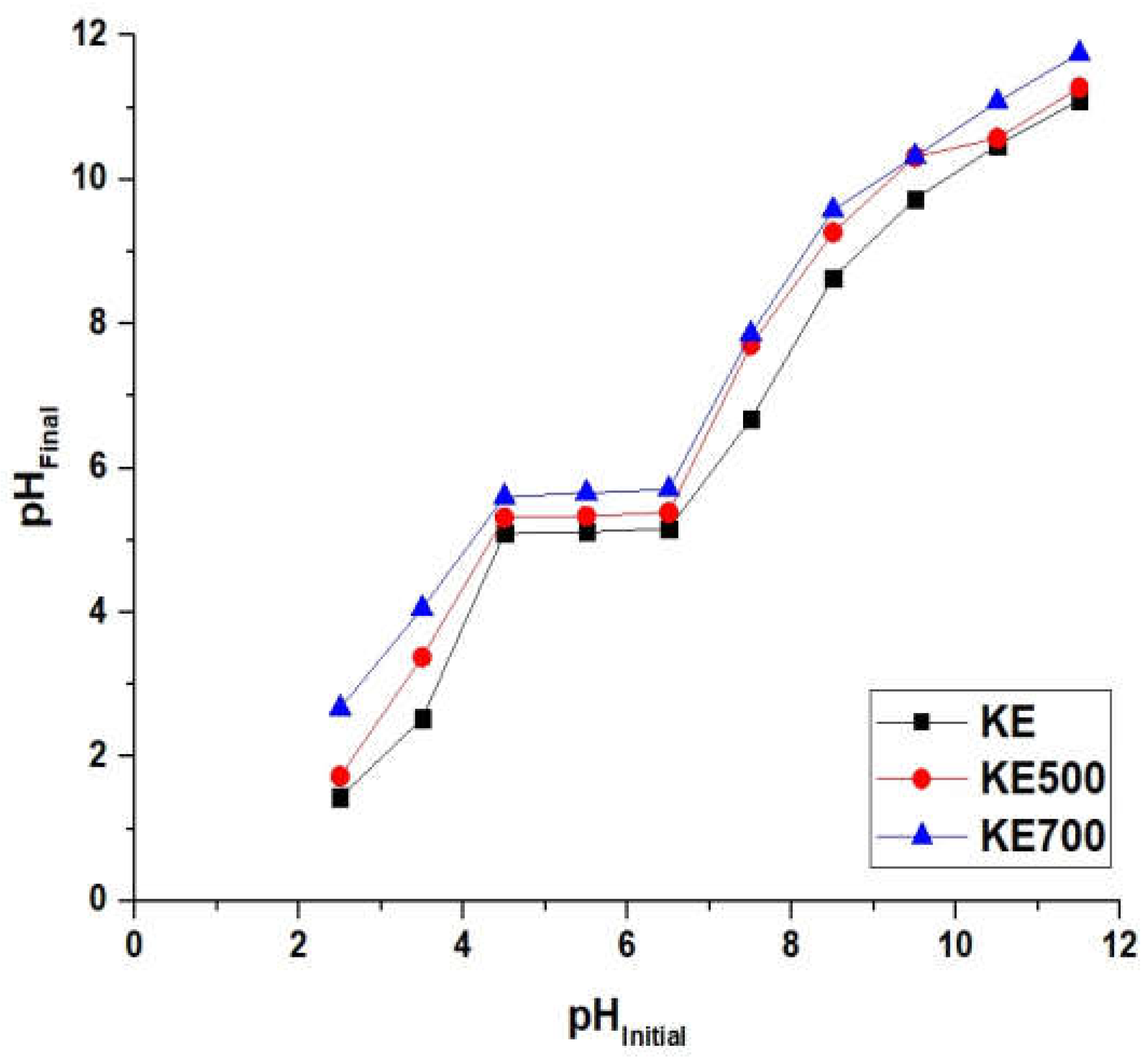
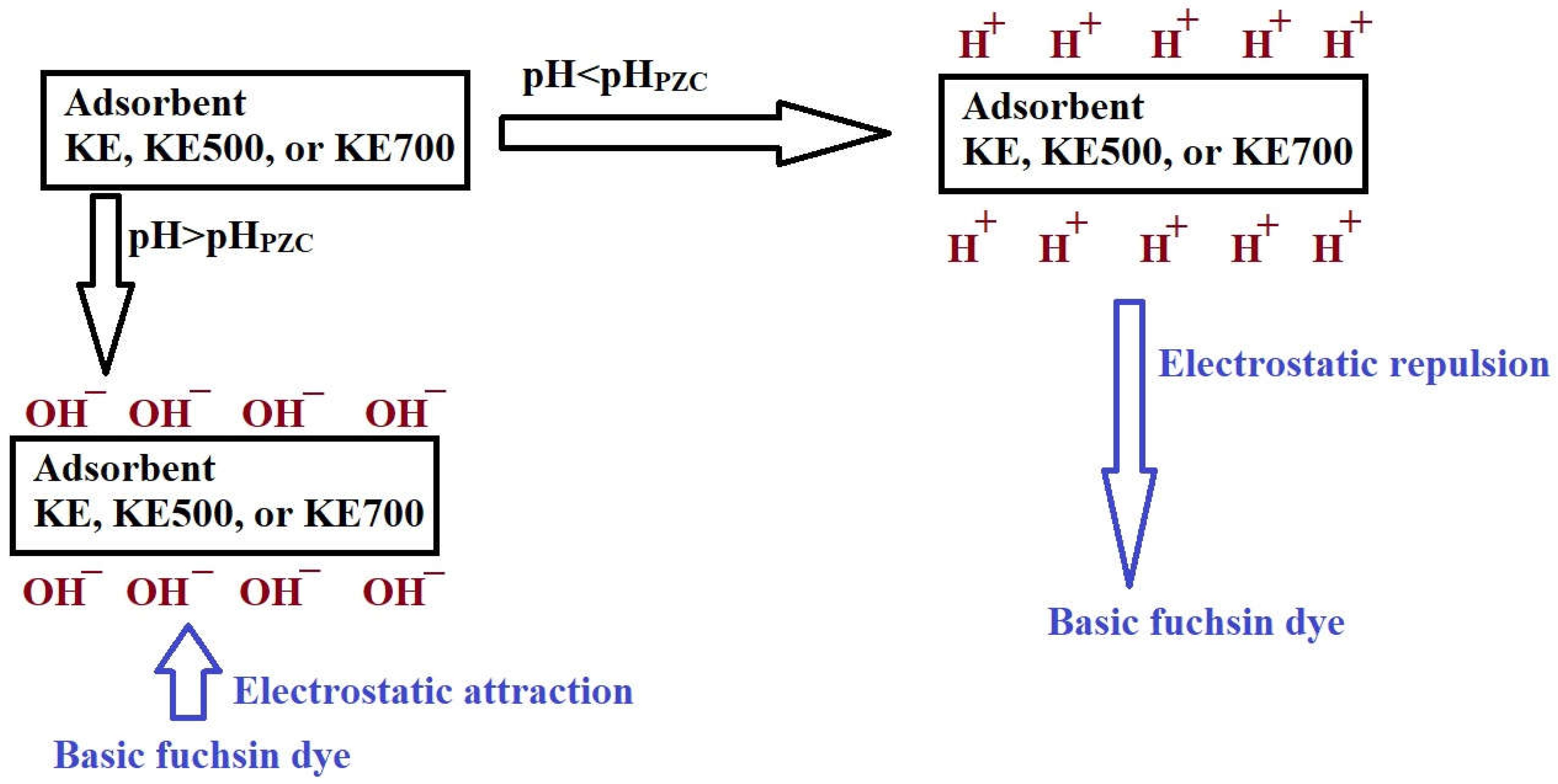
3.2.1. Effect of pH
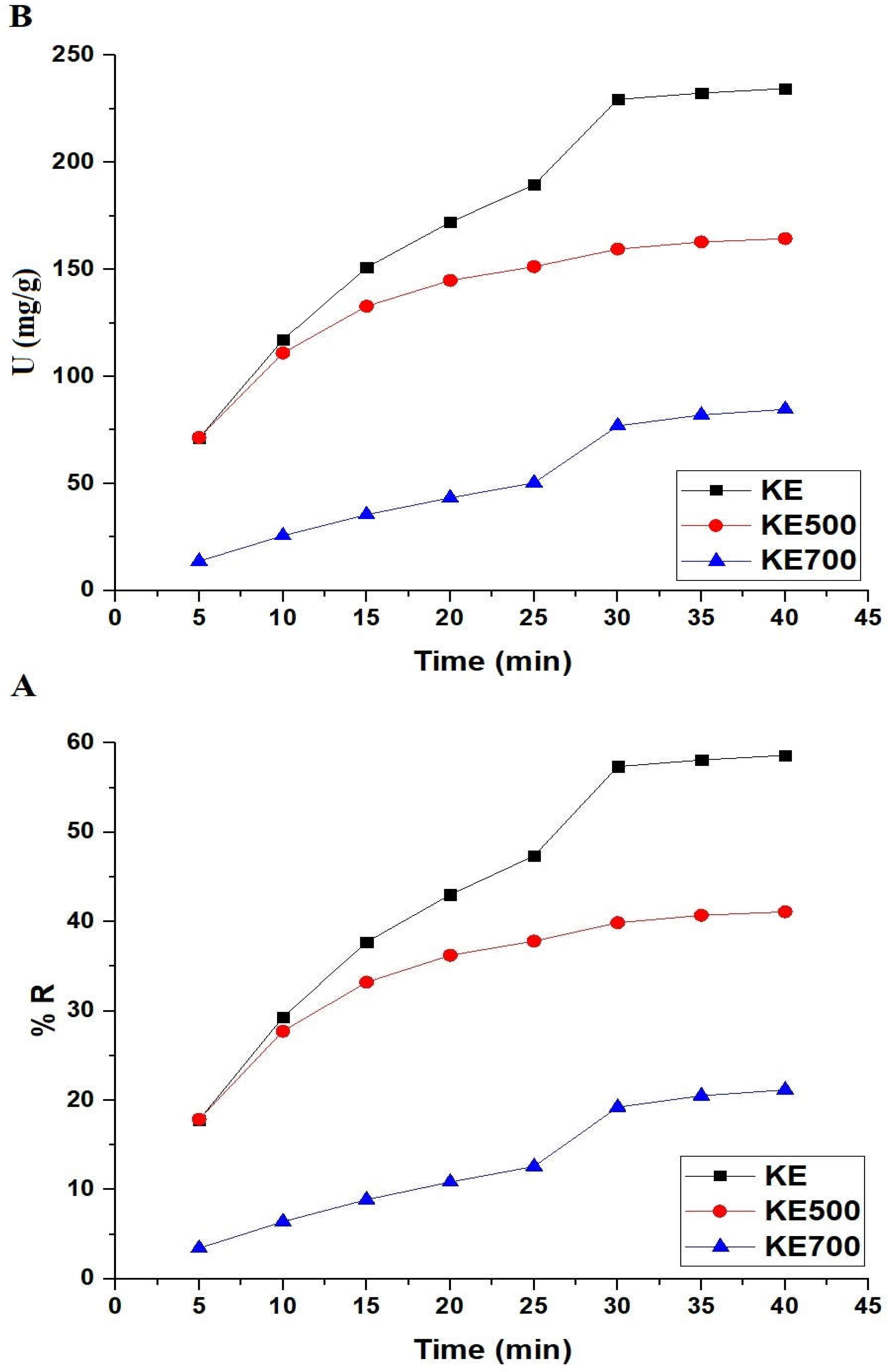
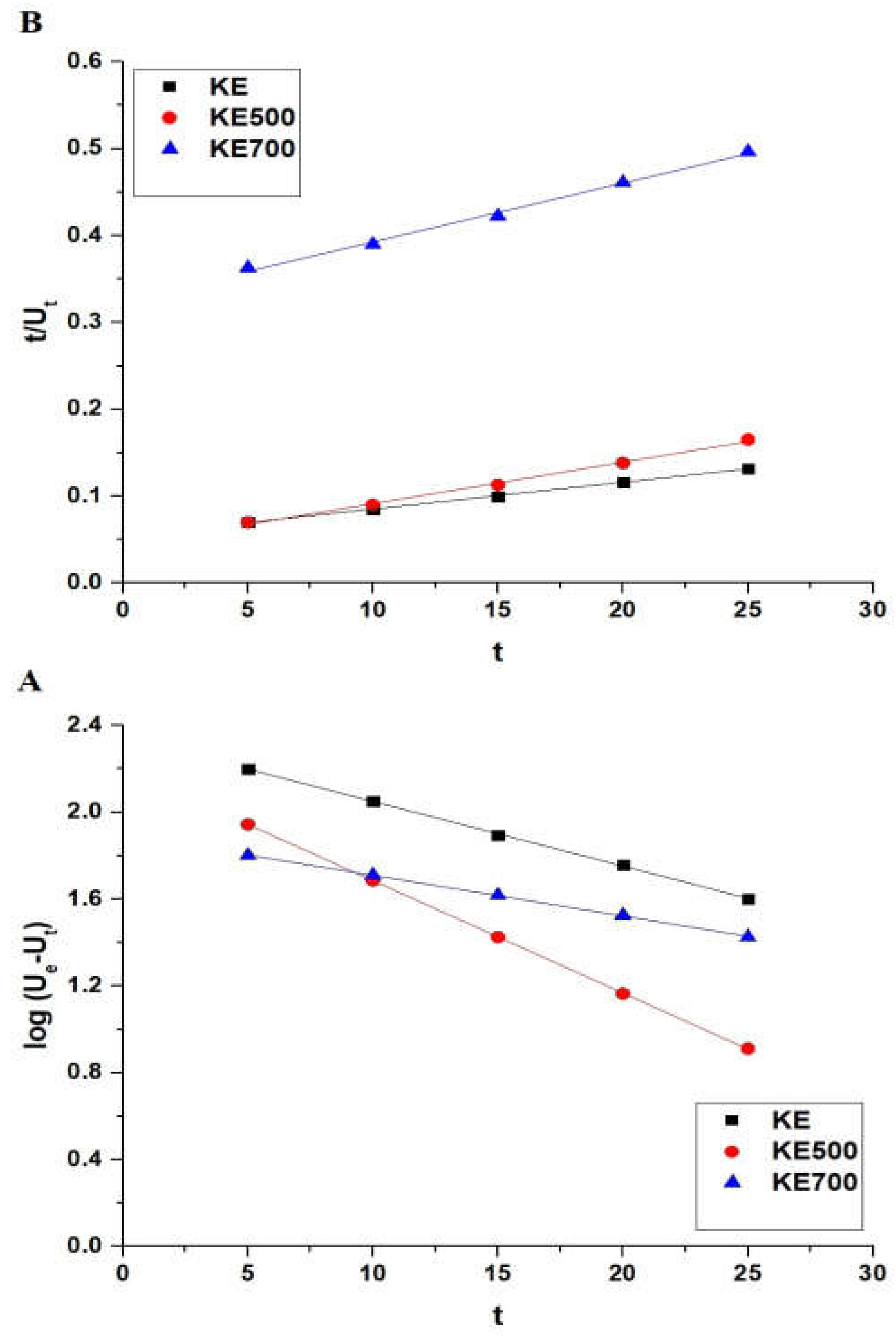
3.2.2. Effect of Interaction Time
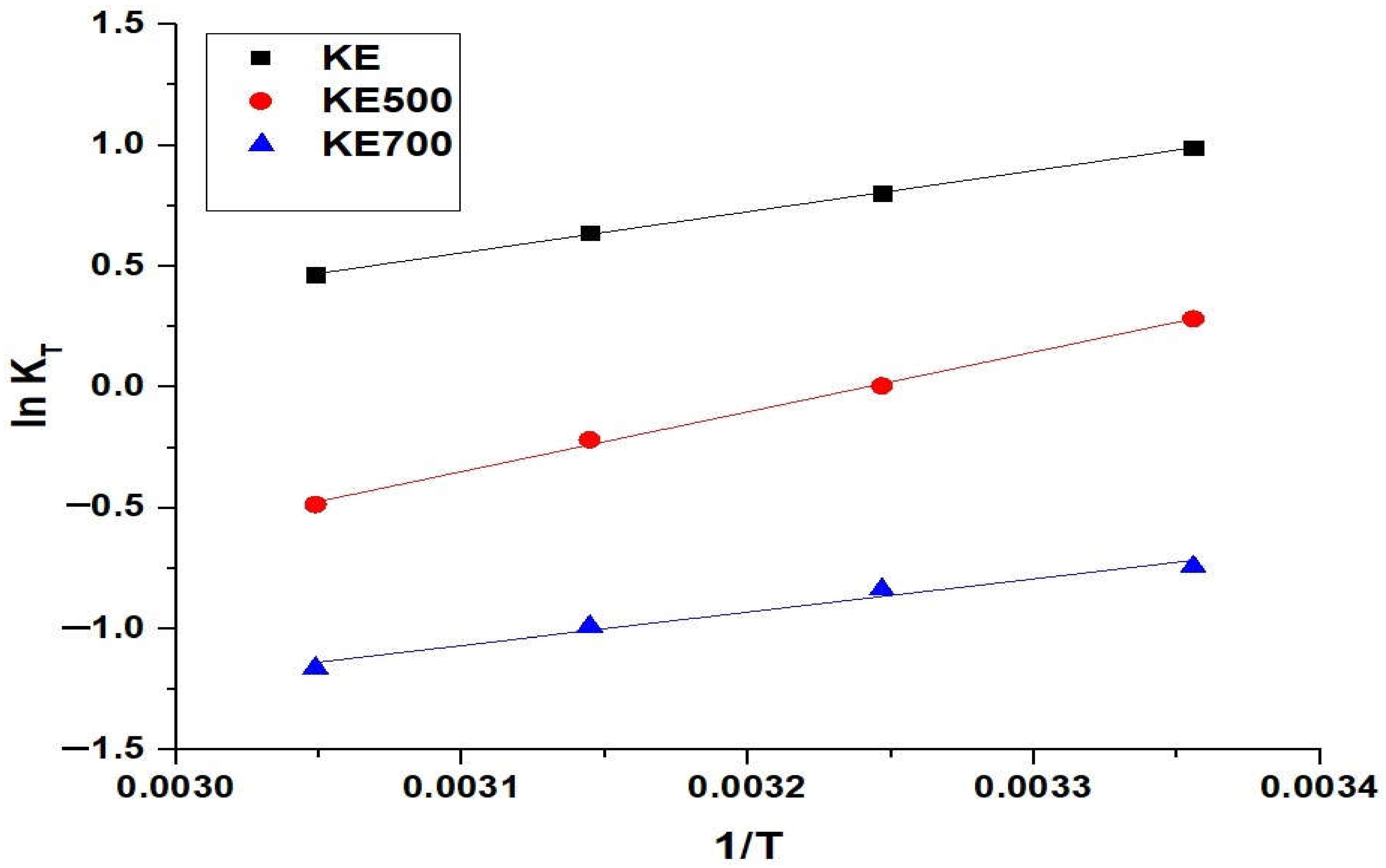
3.2.3. Effect of Interaction Temperature
3.2.4. Effect of BFD Dye Concentration
3.2.5. Impact of Desorption and Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmadian, M.; Jaymand, M. Interpenetrating Polymer Network Hydrogels for Removal of Synthetic Dyes: A Comprehensive Review. Coord. Chem. Rev. 2023, 486, 215152. [Google Scholar] [CrossRef]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; El Messaoudi, N.; Nazir, A. Cellulose-Based Materials and Their Adsorptive Removal Efficiency for Dyes: A Review. Int. J. Biol. Macromol. 2023, 224, 1337–1355. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ihsanullah, I.; Hassan Shah, M.U.; Bhaskar Reddy, A.V.; Aminabhavi, T.M. Recent Advances in the Removal of Dyes from Wastewater Using Low-Cost Adsorbents. J. Environ. Manag. 2022, 321, 115981. [Google Scholar] [CrossRef]
- Sankar Sana, S.; Haldhar, R.; Parameswaranpillai, J.; Chavali, M.; Kim, S.C. Silver Nanoparticles-Based Composite for Dye Removal: A Comprehensive Review. Clean. Mater. 2022, 6, 100161. [Google Scholar] [CrossRef]
- Aramesh, N.; Bagheri, A.R.; Bilal, M. Chitosan-Based Hybrid Materials for Adsorptive Removal of Dyes and Underlying Interaction Mechanisms. Int. J. Biol. Macromol. 2021, 183, 399–422. [Google Scholar] [CrossRef]
- Peramune, D.; Manatunga, D.C.; Dassanayake, R.S.; Premalal, V.; Liyanage, R.N.; Gunathilake, C.; Abidi, N. Recent Advances in Biopolymer-Based Advanced Oxidation Processes for Dye Removal Applications: A Review. Environ. Res. 2022, 215, 114242. [Google Scholar] [CrossRef] [PubMed]
- Dadban Shahamat, Y.; Masihpour, M.; Borghei, P.; Hoda Rahmati, S. Removal of Azo Red-60 Dye by Advanced Oxidation Process O3/UV from Textile Wastewaters Using Box-Behnken Design. Inorg. Chem. Commun. 2022, 143, 109785. [Google Scholar] [CrossRef]
- Wu, S.; Shi, W.; Li, K.; Cai, J.; Xu, C.; Gao, L.; Lu, J.; Ding, F. Chitosan-Based Hollow Nanofiber Membranes with Polyvinylpyrrolidone and Polyvinyl Alcohol for Efficient Removal and Filtration of Organic Dyes and Heavy Metals. Int. J. Biol. Macromol. 2023, 239, 124264. [Google Scholar] [CrossRef] [PubMed]
- Aziz, G.M.; Hussein, S.I.; M-Ridha, M.J.; Mohammed, S.J.; Abed, K.M.; Muhamad, M.H.; Hasan, H.A. Activity of Laccase Enzyme Extracted from Malva Parviflora and Its Potential for Degradation of Reactive Dyes in Aqueous Solution. Biocatal. Agric. Biotechnol. 2023, 50, 102671. [Google Scholar] [CrossRef]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials (Bentonite and Opuntia Ficus Indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Hegazey, R.M.; Ismail, S.H.; El-Feky, H.H.; Khedr, A.M.; Khairy, M.; Ammar, A.M. Facile Synthesis and Characterization of β-Cobalt Hydroxide/Hydrohausmannite/Ramsdellitee/Spertiniite and Tenorite/Cobalt Manganese Oxide/Manganese Oxide as Novel Nanocomposites for Efficient Photocatalytic Degradation of Methylene Blue Dye. Arab. J. Chem. 2022, 15, 104372. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Abdelrahman, E.A.; Hassanien, M.M.; Ibrahim, W.A. Application of Mesoporous Silica Nanoparticles Modified with Dibenzoylmethane as a Novel Composite for Efficient Removal of Cd(II), Hg(II), and Cu(II) Ions from Aqueous Media. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2182–2196. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Khalil, M.M.H.; Algethami, F.K.; Khairy, M.; Abou El-Reash, Y.G.; Saad, F.A.; Shah, R.K.; Ammar, A.M. Facile Synthesis of MgO/CuO and MgO/Cu3MgO4 Binary Nanocomposites as Promising Adsorbents for the Disposal of Zn(II) Ions. J. Inorg. Organomet. Polym. Mater. 2023. In Press. [Google Scholar] [CrossRef]
- Bessy, T.C.; Gatasheh, M.K.; Hatamleh, A.A.; Arokiyaraj, S.; Johnson, J.; Bindhu, M.R. Photocatalytic Degradation of Toxic Dyes and Antimicrobial Activities by Cadmium Doped Magnesium Ferrites Synthesized by Combustion Method. J. Inorg. Organomet. Polym. Mater. 2023, in press. [Google Scholar] [CrossRef]
- Badawy, A.A.; Ibrahim, S.M.; Essawy, H.A. Enhancing the Textile Dye Removal from Aqueous Solution Using Cobalt Ferrite Nanoparticles Prepared in Presence of Fulvic Acid. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1798–1813. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Saad, F.A.; Munshi, A.M.; Abdelrahman, E.A. Facile Synthesis and Characterization of Magnesium and Manganese Mixed Oxides for the Efficient Removal of Tartrazine Dye from Aqueous Media. RSC Adv. 2023, 13, 5656–5666. [Google Scholar] [CrossRef]
- Mariyam, A.; Shahid, M.; Mantasha, I.; Khan, M.S.; Ahmad, M.S. Tetrazole Based Porous Metal Organic Framework (MOF): Topological Analysis and Dye Adsorption Properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1935–1943. [Google Scholar] [CrossRef]
- Ma, T.; Wu, Y.; Liu, N.; Wu, Y. Iron Manganese Oxide Modified Multi-Walled Carbon Nanotube as Efficient Adsorbent for Removal of Organic Dyes: Performance, Kinetics and Mechanism Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4027–4042. [Google Scholar] [CrossRef]
- Danesh, S.M.S.; Faghihian, H.; Shariati, S. Sulfonic Acid Functionalized SBA-3 Silica Mesoporous Magnetite Nanocomposite for Safranin O Dye Removal. Silicon 2019, 11, 1817–1827. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mohamed, A.S. Adsorption Removal of Safranin Dye Contaminants from Water Using Various Types of Natural Zeolite. Silicon 2019, 11, 1635–1647. [Google Scholar] [CrossRef]
- Safa, F.; Alinezhad, Y. Ternary Nanocomposite of SiO2/Fe3O4/Multi-Walled Carbon Nanotubes for Efficient Adsorption of Malachite Green: Response Surface Modeling, Equilibrium Isotherms and Kinetics. Silicon 2020, 12, 1619–1637. [Google Scholar] [CrossRef]
- Ba Mohammed, B.; Hsini, A.; Abdellaoui, Y.; Abou Oualid, H.; Laabd, M.; El Ouardi, M.; Ait Addi, A.; Yamni, K.; Tijani, N. Fe-ZSM-5 Zeolite for Efficient Removal of Basic Fuchsin Dye from Aqueous Solutions: Synthesis, Characterization and Adsorption Process Optimization Using BBD-RSM Modeling. J. Environ. Chem. Eng. 2020, 8, 104419. [Google Scholar] [CrossRef]
- Khan, T.A.; Khan, E.A. Shahjahan Removal of Basic Dyes from Aqueous Solution by Adsorption onto Binary Iron-Manganese Oxide Coated Kaolinite: Non-Linear Isotherm and Kinetics Modeling. Appl. Clay Sci. 2015, 107, 70–77. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Fast Removal of Organic Dyes from Aqueous Solutions by AC/Ferrospinel Composite. Desalination 2010, 262, 134–140. [Google Scholar] [CrossRef]
- Hinojosa-Reyes, M.; Camposeco-Solis, R.; Ruiz, F. H2Ti3O7 Titanate Nanotubes for Highly Effective Adsorption of Basic Fuchsin Dye for Water Purification. Microporous Mesoporous Mater. 2019, 276, 183–191. [Google Scholar] [CrossRef]
- Apostol, I.; Anghel, N.; Doroftei, F.; Bele, A.; Spiridon, I. Xanthan or Esterified Xanthan/Cobalt Ferrite-Lignin Hybrid Materials for Methyl Blue and Basic Fuchsine Dyes Removal: Equilibrium, Kinetic and Thermodynamic Studies. Mater. Today Chem. 2023, 27, 101299. [Google Scholar] [CrossRef]
- Bichave, M.S.; Kature, A.Y.; Koranne, S.V.; Shinde, R.S.; Gongle, A.S.; Choudhari, V.P.; Topare, N.S.; Raut-Jadhav, S.; Bokil, S.A. Nano-Metal Oxides-Activated Carbons for Dyes Removal: A Review. Mater. Today Proc. 2022, 77, 19–30. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Khan, M.; Malik, A.; Khraisheh, M.; Hijazi, D.; Mohamed, S.; Alsorour, S.; Eltayeb, R.; Al Mahmoud, F.; Alahmad, J. Development of Novel Nano-γ-Al2O3 Adsorbent from Waste Aluminum Foil for the Removal of Boron and Bromide from Aqueous Solution. J. Water Process Eng. 2022, 50, 103312. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Rezaee, A. Tetracycline Removal Using Microbial Cellulose@nano-Fe3O4 by Adsorption and Heterogeneous Fenton-like Processes. J. Mol. Liq. 2022, 366, 120199. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, W.; Tan, F.; Luo, F.; Chen, J.; Qiao, X. Investigation on the Efficiency and Mechanism of Cd(II) and Pb(II) Removal from Aqueous Solutions Using MgO Nanoparticles. J. Hazard. Mater. 2015, 299, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, M.A.; Jasim, L.S.; Ranjeh, M.; Masjedi-Arani, M.; Salavati-Niasari, M. Improved Pechini Sol-Gel Fabrication of Li2B4O7/NiO/Ni3(BO3)2 Nanocomposites to Advanced Photocatalytic Performance. Arab. J. Chem. 2022, 15, 103768. [Google Scholar] [CrossRef]
- Shojaei, B.; Miri, R.; Bazyari, A.; Thompson, L.T. Asphaltene Adsorption on MgO, CaO, SiO2, and Al2O3 Nanoparticles Synthesized via the Pechini-Type Sol−Gel Method. Fuel 2022, 321, 124136. [Google Scholar] [CrossRef]
- Ranjeh, M.; Beshkar, F.; Amiri, O.; Salavati-Niasari, M.; Moayedi, H. Pechini Sol-Gel Synthesis of Cu2O/Li3BO3 and CuO/Li3BO3 Nanocomposites for Visible Light-Driven Photocatalytic Degradation of Dye Pollutant. J. Alloys Compd. 2020, 815, 152451. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Highly Stable and Selective Ethanol Sensor Based on α-Fe2O3 Nanoparticles Prepared by Pechini Sol-Gel Method. Ceram. Int. 2016, 42, 6136–6144. [Google Scholar] [CrossRef]
- Alhaji, A.; Razavi, R.S.; Ghasemi, A.; Loghman-Estarki, M.R. Modification of Pechini Sol–Gel Process for the Synthesis of MgO-Y2O3 Composite Nanopowder Using Sucrose-Mediated Technique. Ceram. Int. 2017, 43, 2541–2548. [Google Scholar] [CrossRef]
- Krishna Kumar, A.S.; Warchol, J.; Matusik, J.; Tseng, W.L.; Rajesh, N.; Bajda, T. Heavy Metal and Organic Dye Removal via a Hybrid Porous Hexagonal Boron Nitride-Based Magnetic Aerogel. Npj Clean Water 2022, 5, 24. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Santhana Krishna Kumar, A.; Rajesh, V.; Rajesh, N. Enhanced Adsorption of Hexavalent Chromium Arising out of an Admirable Interaction between a Synthetic Polymer and an Ionic Liquid. Chem. Eng. J. 2013, 222, 454–463. [Google Scholar] [CrossRef]
- Radha, F.H.S.; Shwan, D.M.S.; Kaufhold, S. Adsorption Study and Removal of Basic Fuchsin Dye from Medical Laboratory Wastewater Using Local Natural Clay. Adsorpt. Sci. Technol. 2023, 2023, 9398167. [Google Scholar] [CrossRef]
- Labiod, K.; Hazourli, S.; Bendaia, M.; Tlili, M.; Aitbara, A.; Graine, R.; Meradi, H. Removal of Azo Dye Carmoisine by Adsorption Process on Diatomite. Adsorpt. Sci. Technol. 2022, 2022, 1–17. [Google Scholar] [CrossRef]
- Abdullah, E.A.; Abdullah, A.H.; Zainal, Z.; Ban, T.K.; Material, A. Bismuth Basic Nitrate as a Novel Adsorbent for Azo Dye Removal. J. Chem. 2012, 9, 1885–1896. [Google Scholar] [CrossRef]

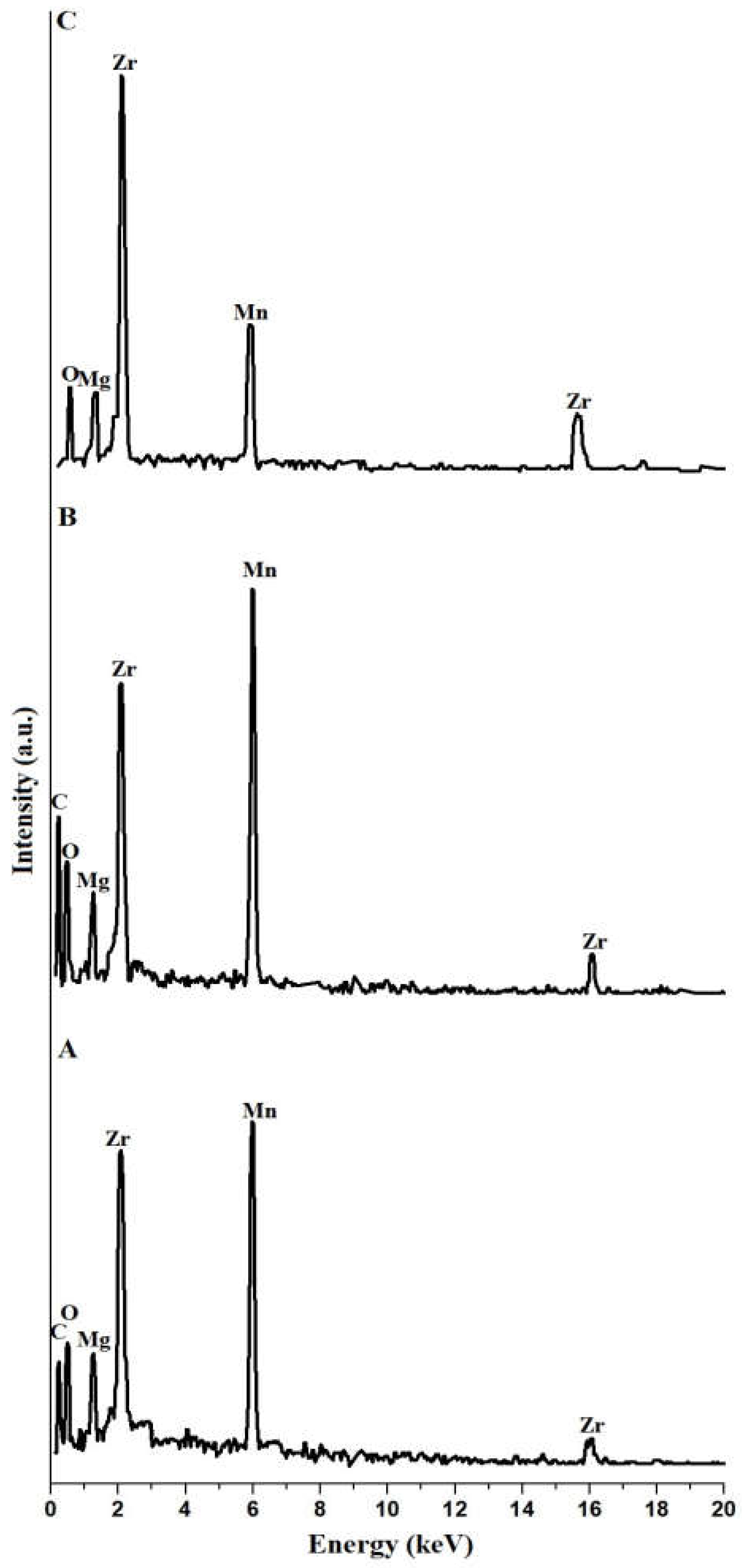






| Samples | % C | % O | % Mg | % Mn | % Zr |
|---|---|---|---|---|---|
| KE | 36.25 | 28.47 | 4.70 | 11.78 | 18.80 |
| KE500 | 33.96 | 23.84 | 5.98 | 13.77 | 22.45 |
| KE700 | ---- | 18.22 | 9.28 | 14.14 | 58.36 |
| Sample | BET Surface Area (m2/g) | Total Pore Volume (cc/g) | Average Pore Size (nm) |
|---|---|---|---|
| KE | 67.85 | 0.1077 | 3.17 |
| KE500 | 20.15 | 0.0265 | 2.63 |
| KE700 | 13.60 | 0.0305 | 4.49 |
| Adsorbent | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| k1 (1/min) | Ue (mg/g) | R2 | k2 (g/mg.min) | Ue (mg/g) | R2 | |
| KE | 0.0685 | 222.36 | 0.9996 | 0.00018 | 323.62 | 0.9989 |
| KE500 | 0.1192 | 159.33 | 0.9999 | 0.00052 | 209.64 | 0.9956 |
| KE700 | 0.0431 | 78.79 | 0.9994 | 0.00014 | 147.71 | 0.9945 |
| Adsorbent | ∆H° (kJ/mol) | ∆S° (kJ/mol Kelvin) | ∆G° (kJ/mol) | |||
|---|---|---|---|---|---|---|
| 298 | 308 | 318 | 328 | |||
| KE | −14.20 | 0.0394 | −25.94 | −26.34 | −26.73 | −27.12 |
| KE500 | −20.66 | 0.0669 | −40.62 | −41.29 | −41.96 | −42.63 |
| KE700 | −11.47 | 0.0445 | −24.73 | −25.17 | −25.62 | −26.06 |
| Langmuir Isotherm | Freundlich Isotherm | |||||
|---|---|---|---|---|---|---|
| Adsorbent | Umax (mg/g) | k3 (L/mg) | R2 | Umax (mg/g) | k4 (mg/g) (L/mg)1/n | R2 |
| KE | 239.81 | 0.3064 | 0.9999 | 236.49 | 196.81 | 0.9979 |
| KE500 | 174.83 | 0.0917 | 0.9996 | 165.81 | 110.75 | 0.9944 |
| KE700 | 93.19 | 0.0311 | 0.9966 | 79.84 | 36.53 | 0.9495 |
| Adsorbent | Maximum Uptake Capability (mg/g) | Ref |
|---|---|---|
| Iron-manganese-oxide-coated kaolinite | 10.36 | [23] |
| Activated carbon/ferrospinel composite | 101.00 | [24] |
| Hydrogen titanate nanotubes | 183.20 | [25] |
| Xanthan/ferrite/lignin hybrid | 33.33 | [26] |
| KE | 239.81 | This study |
| KE500 | 174.83 | This study |
| KE700 | 93.19 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wasidi, A.S.; Khairy, M.; Abdulkhair, B.Y.; Abdelrahman, E.A. Efficient Disposal of Basic Fuchsin Dye from Aqueous Media Using ZrO2/MgMn2O4/Mg(Mg0.333Mn1.333)O4 as a Novel and Facilely Synthesized Nanocomposite. Inorganics 2023, 11, 363. https://doi.org/10.3390/inorganics11090363
Al-Wasidi AS, Khairy M, Abdulkhair BY, Abdelrahman EA. Efficient Disposal of Basic Fuchsin Dye from Aqueous Media Using ZrO2/MgMn2O4/Mg(Mg0.333Mn1.333)O4 as a Novel and Facilely Synthesized Nanocomposite. Inorganics. 2023; 11(9):363. https://doi.org/10.3390/inorganics11090363
Chicago/Turabian StyleAl-Wasidi, Asma S., Mohamed Khairy, Babiker Y. Abdulkhair, and Ehab A. Abdelrahman. 2023. "Efficient Disposal of Basic Fuchsin Dye from Aqueous Media Using ZrO2/MgMn2O4/Mg(Mg0.333Mn1.333)O4 as a Novel and Facilely Synthesized Nanocomposite" Inorganics 11, no. 9: 363. https://doi.org/10.3390/inorganics11090363
APA StyleAl-Wasidi, A. S., Khairy, M., Abdulkhair, B. Y., & Abdelrahman, E. A. (2023). Efficient Disposal of Basic Fuchsin Dye from Aqueous Media Using ZrO2/MgMn2O4/Mg(Mg0.333Mn1.333)O4 as a Novel and Facilely Synthesized Nanocomposite. Inorganics, 11(9), 363. https://doi.org/10.3390/inorganics11090363