Abstract
The main objective of this work was to analyze the transport by diffusion of salts containing some metal ions (that is, CoCl2, CrCl3, HgCl2, and PbCl2), at 25.0 °C, in two different aqueous solutions with varying pH levels, namely, sodium chloride and hydrochloric chloride, by using Nernst–Hartley model equations. To analyze the applicability of this model, experimental data for two aqueous systems (CoCl2 + NaCl) and (CoCl2 + HCl), were obtained by using the Taylor technique and compared with the predicted values. The Nernst–Hartley model equations were used in this study to estimate the mutual diffusion coefficients of the eight ternary systems, which included (CoCl2 + NaCl), (HgCl2 + NaCl), (PbCl2 + NaCl), (CrCl3 + NaCl), and (CoCl2 + HCl), (HgCl2 + HCl), (PbCl2 + HCl), and (CrCl3 + HCl). By using the limiting equations of Nernst–Hartley coefficients, we were able to understand the composition dependence of the mutual diffusion coefficients of these mixed electrolyte solutions and the electrostatic mechanism for the strongly coupled diffusion of these components.
1. Introduction
1.1. Diffusion of Heavy Metal Ions in Solutions and Its Impact
The study of the diffusion processes of heavy metal ions in solution is important both for practical application in different fields, such as corrosion related to dental restoration [1,2], and for fundamental reasons, helping us to understand the nature of these aqueous electrolyte structures.
It is known that these metals exist naturally in the Earth’s crust, and some of them, at physiological concentrations, are necessary for living things to carry out a variety of biological functions, but larger amounts of these elements can be unhealthy [3]. For example, one of the biggest issues related to heavy metals is their bioaccumulation, since they are neither metabolized nor decomposed. Exposure to these elements can occur through water and food consumption, air intake, or occupational exposure, causing grave problems in health [4]. Among these metals, we highlight in this work four heavy metal ions, that is, cobalt, mercury, lead, and chromium.
To reduce the negative impacts of heavy metals, and to face growing public pressure to reduce relevant health and environmental risks, fundamental chemical studies are required [5], including the study of transport properties. Coupled diffusion techniques involve measurements that are difficult to perform, so the application of mathematical models that are predictable and reproductive is of added value for the scientific community dedicated to this area, obtaining a greater amount of data in a simpler, faster way, eliminating a lot of laboratory time.
1.2. Concepts and Models
1.2.1. Binary Mutual Diffusion
Mutual diffusion is a quantitative measure of a mass transport process in aqueous solution. In the case of the one-dimensional diffusion of a binary system (two components, solute + solvent), these parameters can be described by Fick’s equation (Equation (1)) [6,7,8].
where and represent the binary Fick diffusion coefficient, the flow of solute 1 across a suitable chosen reference plane per area unit and per time unit, in a one-dimensional system, and the gradient in the concentration of the solute. C is the concentration of solute 1 in moles per volume unit at the point considered.
It is very important to understand the differences between two concepts: mutual diffusion (also known as inter-diffusion, collective diffusion, concentration diffusion, or salt diffusion) and self-diffusion (also called intra diffusion, tracer diffusion, single ion diffusion, or ionic diffusion). In mutual diffusion, due to the salt concentration gradient in a binary electrolyte solution, the constraint in maintaining electrical neutrality ensures that positive and negative ions move randomly from the higher- to lower-concentration region at the same speed. In self-diffusion, the electrical neutrality restraint does not apply, and three species (solvents and ions) have independent diffusion coefficients. Furthermore, the activity coefficient in the trace species does not change in a virtually uniform solution, and thus the thermodynamic factor, dlnai/dlnxi (a is the activity and x is the mole faction of component i), which appears in the equations describing mutual diffusion, becomes equal to one.
It is worth highlighting there is no simple relation between mutual and self-diffusion coefficients, except when the solute is at an infinitesimal concentration. For example, in aqueous binary solutions containing symmetrical uni-univalent electrolytes, we have the Nernst–Hartey equation (Equation (2)) [6,7,8].
where D0, , and represent the limiting mutual diffusion coefficient of the salt and the limiting self-diffusion coefficients of the anion and the cation, respectively.
1.2.2. Ternary Mutual Diffusion
The Fick’s laws generalized by Onsager to describe multicomponent mutual diffusion can be represented by Equation (3) [7],
Dik gives the flux Ji of component i caused by a gradient in the concentration of component k (). Considering the ternary system formed by two solutes (components 1 and 2), and water (component 0), in this case, there are three different fluxes, J0, J1, and J2.
However, in the laboratory (volume fixed) frame of reference, diffusion produces no volume flow across any plane, and hence
where V0, V1, and V2 are the partial molar volumes of components 0, 1, and 2.
J0 V0 + J1 V1 + J2 V2 = 0
In the other words, due to the volume fixed constraint (Equation (4)), only two fluxes are independent.
The diffusion flux of solutes 1 and 2 in the direction of their concentration gradient can be determined by Fick’s equations, Equations (5) and (6) [7].
J1 = −D11∇C1 − D12∇C2
J2 = −D21∇C1 − D22∇C2
J1 and J2 represent the molar fluxes of solute 1 and solute 2 driven by the concentration gradients ∇C1 and ∇C2 in the solutions. The main coefficients D11 and D22 allow for the molar fluxes of solute 1 and solute 2, driven by their own concentration gradients. Cross-diffusion coefficients D12 and D21 allow for the coupled flux of each solute, driven by a concentration gradient in the other solute. It is worth noting that D12 ≠ D21, and that these parameters can only be determined experimentally, and cannot be derived from any simple theory [9,10,11,12,13,14,15,16,17,18,19,20,21].
A positive Dab cross-coefficient (a ≠ b) shows the co-current coupled transport of solute a from regions of higher to lower concentrations of solute b. A negative Dab coefficient shows the counter-current coupled transport of solute a from regions of lower to higher concentrations of solute b.
1.2.3. Limiting Nernst–Hartley Equations
For very diluted solutions, Dab coefficients can be estimated by using the limiting Nernst–Hartley equations (in references [9,10,11,12,13,14,15,16,17,18,19,20], which show some examples of these estimations for some systems). Under these conditions, the solutions become ideal, and the thermodynamic factors are equal to one.
We conducted research on the diffusion behaviour (Dab) of four chemical aqueous systems involving four heavy metal ions, Co2+, Hg2+, Pb2+, and Cr3+, in different media (NaCl and HCl). As the limiting Nernst–Hartley equation is similar when applied to these systems, and they have been well-described in the literature [10,11,12], we will only present these equations for the limiting ternary mutual diffusion coefficients of the aqueous systems CoCl2 (component 1) + NaCl (component 2) and CoCl2 (component 1) + HCl (component 2) as examples.
The mutual diffusion in dilute aqueous solutions containing cobalt chloride and sodium chloride causes fluxes of three different solute species (Na+, Co2+, and Cl− ions). Bearing in mind the zero-electric current,
we can consider two independent diffusion fluxes, that is, J1 and J2, as representing the indices 1 and 2 the components CoCl2 and NaCl. On the other hand, each flux of the solute component, used in the Fick equations, can always be expressed as the sum of the fluxes of the i-containing solute species, js,
, DNa, and are the limiting diffusion coefficients of the Co2+, Na+, and Cl− ions, respectively, and and represent the fraction of the total current carried by the Co2+ and Na+ ions, respectively. The ionic diffusion coefficients used in the Nernst–Hartley equations can be calculated from limiting ionic conductivities (see Table 1).

Table 1.
The limiting ionic conductivities (λion) [8] and diffusion coefficients (Dion) a for different ions at 25.0 °C.
Relative to the aqueous system CoCl2 (C1) + HCl (C2) solutions, we have also three different solute species (Co2+, H+, and Cl− ions) and two independent diffusion fluxes.
The Nernst–Hartley Diko coefficients for this aqueous system are calculated from Equations (8) and (14)–(16).
DCl, DH, DCo, tCo, and tH represent the limiting diffusion coefficients of the Cl−, H+, and Co2+ ions, and the fraction of the total current carried by the Co2+ and H+ ions (Equation (17)), respectively.
The values for these parameters are indicated in Table 1.
2. Results and Discussion
2.1. Predicted Data from Nernst–Hartley Equations and Comparison with New Experimental Data and Their Analysis for Two Aqueous Systems: (CoCl2 Plus NaCl) and (CoCl2 Plus HCl)
Table 2 and Table 3 summarize the predicted and experimental values of the Dab diffusion coefficients for solutions of different compositions for two aqueous systems {(CoCl2 plus NaCl) and (CoCl2 plus HCl)}. Regarding the last values, they were obtained from at least six independent measurements, all carried out in the present work. The main diffusion coefficients D11 and D22 were generally reproducible within ±(0.020 × 10−9 m2 s−1, and the cross-coefficients were in general reproducible within about ±(0.040 × 10−9 m2 s−1).

Table 2.
Experimental and predicted ternary diffusion coefficients, D11, D12, D21, and D22, by Nernst–Hartley equations [9,12] for aqueous CoCl2 (C1) + NaCl (C2) + solutions at 25.0 °C.

Table 3.
Predicted ternary diffusion coefficients, D11, D12, D21, and D22, by Nernst–Hartley equations [9,12], for aqueous CoCl2 (C1) + HCl (C2) + solutions at 25.0 °C.
An analysis of Table 2 and Table 3 shows that there is a reasonable agreement between the experimental and predicted data for diluted solutions. The deviations between experimental and predicted main diffusion coefficients are generally less than 5%.
Nonideal solution behaviour and electrophoretic terms not included in the Nernst limiting Dab estimations can explain the highest deviations observed.
It is important to highlight that there is a similar behaviour observed in the variation of these parameters Dab (experimental and theoretical) as a function of their composition. Furthermore, it should be noted that although the predicted Dab values are the same for equal CoCl2 solute fractions, X1, but calculated with different concentrations of each component, the same does not apply to the experimental data.
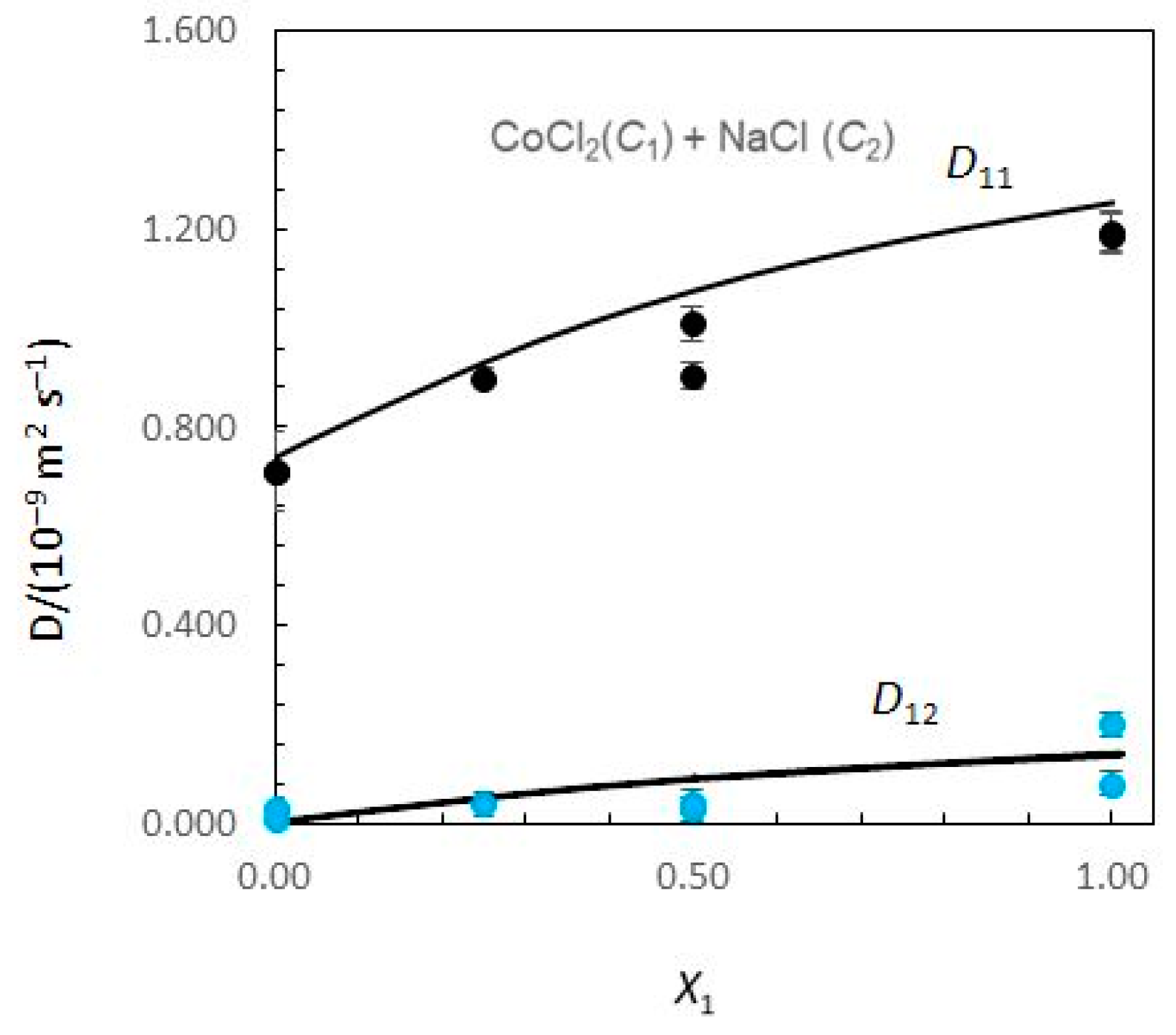
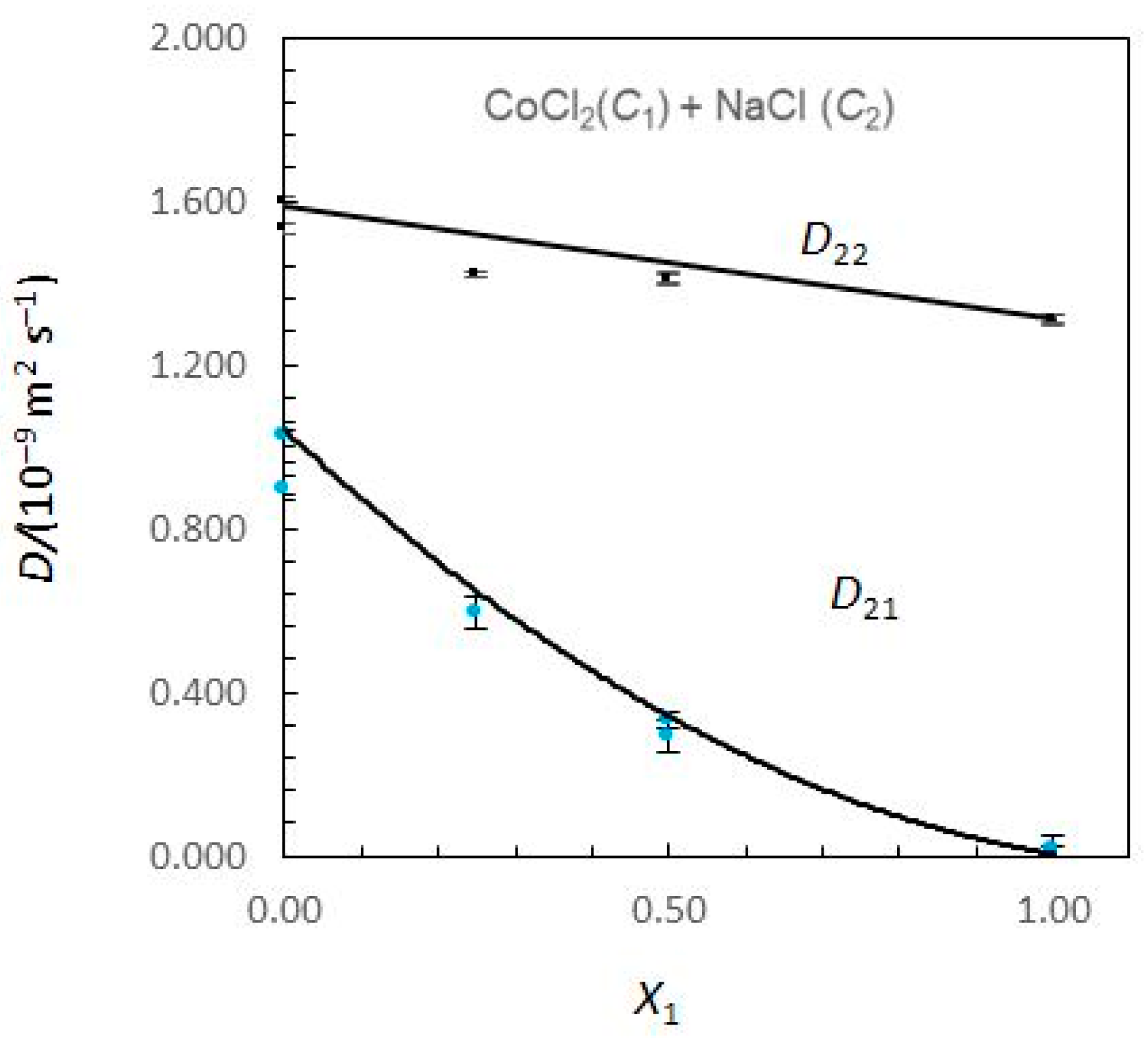
Figure 1 and Figure 2 show the limiting Nernst–Hartley Dik coefficients for aqueous CoCl2 (C1) + NaCl (C2) solutions plotted against the solute fraction of CoCl2.
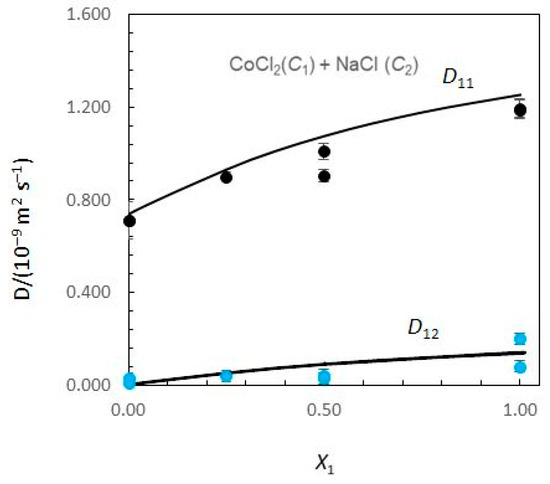
Figure 1.
Ternary mutual diffusion coefficients D11 and D12 values (Equations (8)–(13)) for aqueous CoCl2 (C1) + NaCl (C2) solutions at 25.0 °C plotted against the solute fraction of CoCl2. (X1 = C1/(C1 + C2)). Measured Dik values: symbols. Predicted Dik values: solid curves.
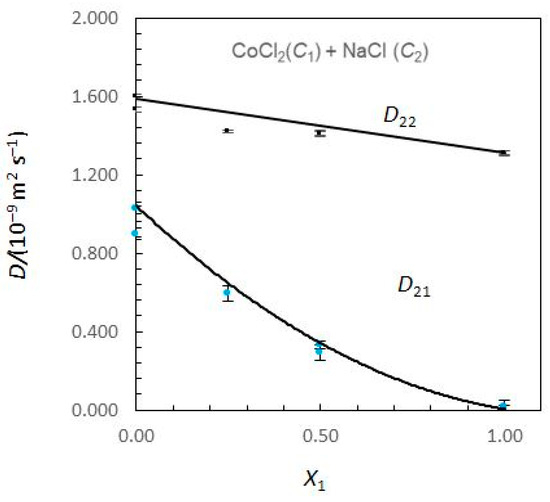
Figure 2.
Ternary mutual diffusion coefficients D22 and D21 values (Equations (8)–(13)) for aqueous CoCl2 (C1) + NaCl (C2) solutions at 25.0 °C plotted against the solute fraction of CoCl2. (X1 = C1/(C1 + C2)). Measured Dik values: symbols. Predicted Dik values: solid curves.
Considering Figure 1, the Nernst–Hartley D11 values for composition limits X1 = 0 and X1 = 1 are DCo (the tracer diffusion coefficient of Co2+ ions in supporting NaCl solutions) and (the binary mutual diffusion coefficient of aqueous CoCl2), respectively. Equivalently, the limiting D22 values for X1 = 0 and X1 = 1 are and DNa. As the solute fraction of CoCl2 increases from 0 to 1, D12 changes from 0 to and D21 changes from to 0. In these intervals of concentrations, a significant variation of D12 and D21 is observed. While D12 are negative, D21 are positive. At X1 = 1, D21 is zero, because sodium chloride concentration gradients cannot drive coupled flows of cobalt chloride in solutions. Similarly, D12 → 0 as X1 → 0.
A possible justification for the behaviour of coupled diffusion can be made on the basis of the electrostatic mechanism. The diffusion coefficient of the Cl− ions is larger than that of the Co2+ ions (Table 1). Consequently, a CoCl2 concentration gradient produces an electric field that slows down the Cl− ions and speeds up the Co2+ ions to maintain electroneutrality along the diffusion path. If NaCl is present in the solution, the electric field generated by a CoCl2 concentration gradient drives a coupled flow of Na+ ions in the same direction as the flux of Co2+, helping to explain the positive values estimated for cross-coefficient D21. Similarly, DNa < DCl and D12 > 0. However, it can also be worth noticing the higher values obtained for D21 when compared with those obtained for D12.
Table 3 shows the effects of coupled diffusion between these CoCl2 and HCl components. In this case, as the diffusion coefficient of the H+ ions is much larger than that of the Cl− ions (Table 1); a HCl concentration gradient produces an electric field which slows down the Co2+ ions and speeds up the Cl− ions to maintain electroneutrality. Consequently, D12 < 0.
It can be concluded that strongly coupled diffusion occurs between cobalt chloride and hydrochloric acid, while sodium chloride gradients have a weaker influence on cobalt chloride diffusion. Furthermore, these predicted values are usually acceptable at highly diluted solutions, as the differences between them and experimental values are equal to, or less than, experimental uncertainty. In conclusion, when there is no experimental diffusion data, we can use the Nernst–Hartley equations to comprehend the composition dependence of the mutual diffusion coefficients of ternary systems, despite the limitations of this approach.
2.2. Predicted Ternary Diffusion Coefficients from Nernst Equations for Different Aqueous Systems
Taking all these considerations into account, Nernst–Hartley values were calculated for six aqueous systems for different compositions (Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9).

Table 4.
Predicted ternary diffusion coefficients, D11, D12, D21, and D22, for aqueous HgCl2 (C1) + NaCl (C2) + solutions at 25.00 °C by Nernst–Hartley equations [9,12].

Table 5.
Predicted ternary diffusion coefficients, D11, D12, D21, and D22, for aqueous PbCl2 (C1) + NaCl (C2) + solutions at 25.0 °C by Nernst–Hartley equations [9,12].

Table 6.
Predicted ternary diffusion coefficients, D11, D12, D21, and D22, for aqueous CrCl3 (C1) + NaCl (C2) + solutions at 25.00 °C by Nernst–Hartley equations [9,12].

Table 7.
Predicted ternary diffusion coefficients, D11, D12, D21, and D22, for aqueous HgCl2 (C1) + HCl (C2) + solutions at 25.0 °C by Nernst–Hartley equations [9,12].

Table 8.
Predicted ternary diffusion coefficients, D11, D12, D21, and D22, for aqueous PbCl2 (C1) + HCl (C2) + solutions at 25.0 °C by Nernst–Hartley equations [9,12].

Table 9.
Predicted ternary diffusion coefficients, D11, D12, D21, and D22, for aqueous CrCl3 (C1) + HCl (C2) + solutions at 25.00 °C by Nernst–Hartley equations [9,12].
From the analysis of Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9, it can be inferred that for these solutions of these mixed electrolytes, the tendency of limiting ternary coefficients with the composition is similar. For example, in four aqueous systems {(CoCl2 + HCl), (CrCl3 + HCl), (HgCl2 + HCl), and (PbCl2 + HCL)}, the D22 values are considerably larger than the D11 values and increase dramatically with the solute 1 fraction, defined as X1 = C1/(C1 + C2)). Also, the cross-coefficient D12 becomes very large and negative with the increasing solute 1 fraction.
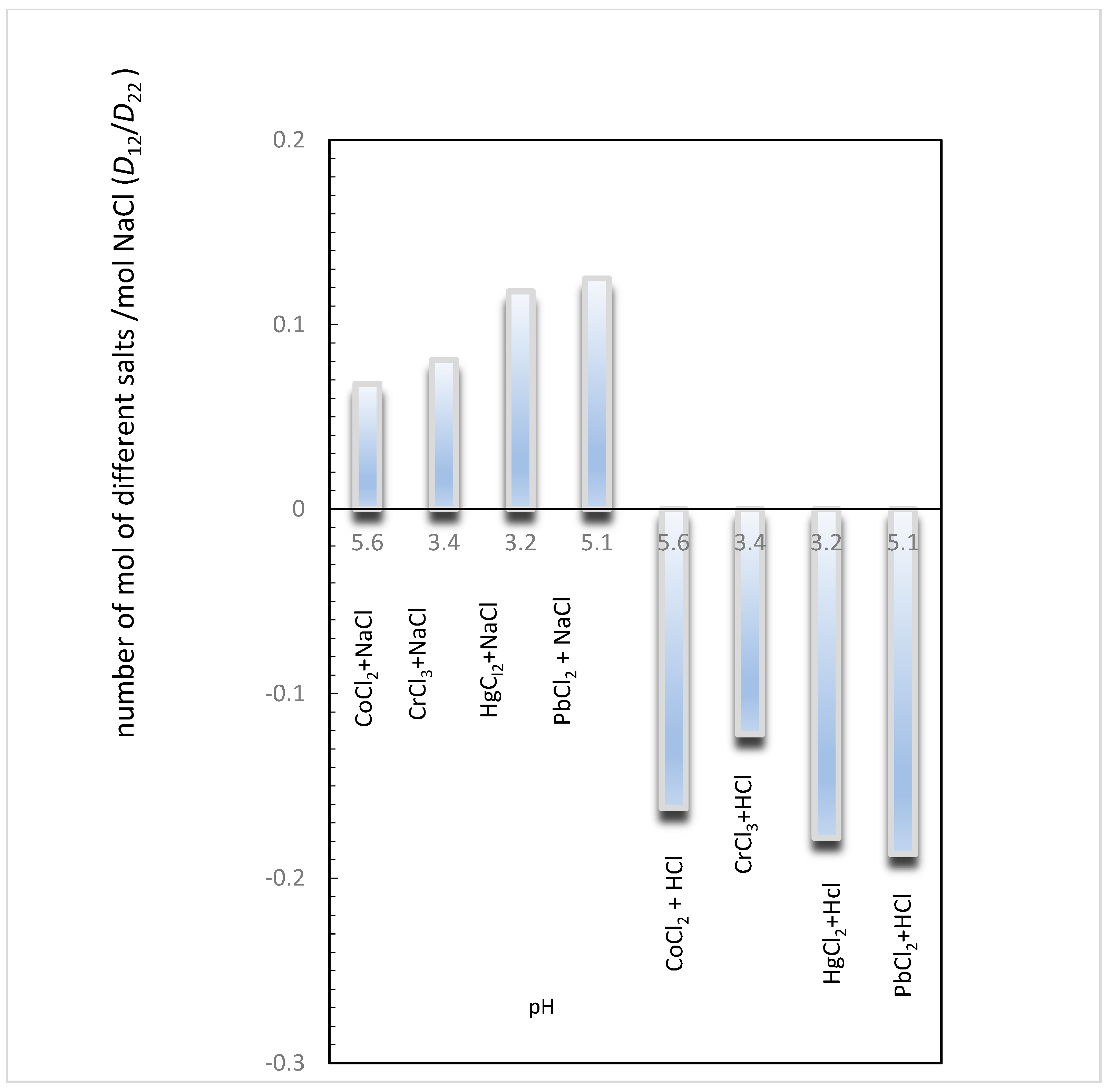
The values of the ternary diffusion coefficient ratios D12/D22 and D21/D11 (Table 10 and Table 11) permit us to give the number of moles of one component transported per mole of the other component.


From Table 10, the higher positive value obtained for D12/D22 in the CrCl3/NaCl system, when compared with the others, stands out; therefore, we can say that one mole of diffusing NaCl co-transports up to 2.9 mol of CrCl3.
Relative to the effects of added hydrochloric acid on the diffusion of aqueous CoCl2, CrCl3, HgCl2, and PbCl2, the D12/D22 values are lower and similar between them (that is, one mole of diffusing HCl counter-transports up to 0.1 of MCl2 (M = Co2+, Hg2+, Pb2+) and 0.2 mol of CrCl3.
From the positive D21/D11 values (Table 11), it can be concluded that a mole of diffusing of MCl2 (M = Co2+, Hg2+, Pb2+) and CrCl3 favour the transport of NaCl (and HCl) in the same direction. However, the added HCl is more relevant, considering the highest values for this ratio (achieving the maximum value for system CrCl3 plus HCl). In this case, a mole of diffusing CrCl3 co-transports at most 6 mol of HCl).
Based on these observations, it can be inferred that there is coupled diffusion in such systems that disrupts the thermodynamic equilibrium. For instance, when cobalt–chromium dental alloys are present in the oral cavity and exposed to low pH, it can create favourable conditions for the production of flows of chromium ions into the human body.
Figure 3 illustrates the coupled diffusion occurring in these aqueous systems, revealing the number of moles of salts containing metallic ions (Co2+, Hg2+, Pb2+, and Cr3+) transported by each mole of NaCl and HCl at different pHs. That is, the values D12/D22 are plotted for each system at different pHs (Table 10).
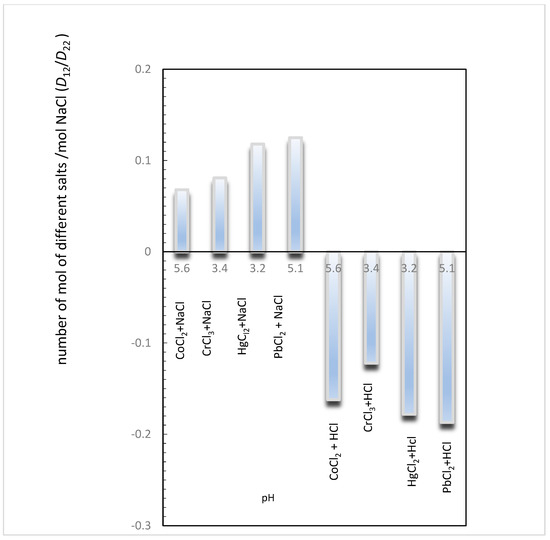
Figure 3.
Estimation of the number of moles of salts (i.e., CoCl2, CrCl3, HgCl2, and PbCl2) transported for each mole of NaCl (or HCl), for different values of pH, by the calculation of D12/D22 ratios.
From Figure 3, we can observe that the D12/D22 values are close, independent of the pH level.
However, the direction of flux of these ions depends strongly on the component added to these systems. While NaCl concentration gradients drive co-current fluxes of Co2+, Hg2+, Pb2+, and Cr3+ in solutions, HCl concentration gradients drive counter-current fluxes of these ions in solutions.
3. Materials and Methods
3.1. Materials
Table 12 shows the reagents used as received in the present work: cobalt chloride, sodium chloride, and hydrogen chloride. All chemicals were used without further purification.

Table 12.
Sample description.
All solutions were prepared using ultrapure water (Millipore, Darmstadt, Germany, Milli-Q Advantage A10, specific resistance = 1.82 × 105 Ω m, at 25.0 °C). The weighing was carried out using a Radwag AS 220C2 balance (Radwag, Radom, Poland), with an accuracy of ±0.0001 g.
3.2. pH Measurements
The pH measurements of solutions were carried out with a Radiometer pH meter PHM 240 (Radiometer, Apeldoorn, The Netherlands) with an Ingold U457-K7pH conjugated electrode (Mettler-Toledo International, Columbus, OH, USA); the pH was measured in fresh solutions, and the electrode was calibrated immediately before each experimental set of solutions using IUPAC-recommended pH 4, 7, and 10 buffers. From pH meter calibration, a zero pH of 6.00 ± 0.03 and sensitivity higher than 98.7% were obtained. All solutions were freshly prepared at 298.15 K and degassed by sonication for about 60 min before each experiment.
3.3. Taylor Dispersion Method
The Taylor dispersion technique for measuring diffusion coefficients is well described in the literature [7,10,12,14,20], and hence its details are not included in this manuscript. However, we sum up some relevant points regarding the method and the equipment. Dispersion profiles were generated by injecting, at the start of each run, using a 6-port Teflon injection valve (Rheodyne, model 5020; Sigma-RBI, Burlington, MA, USA), 0.063 mL of solution into a laminar carrier stream of slightly different compositions at the entrance to a Teflon capillary dispersion tube, of length 3048.0 (±0.1) cm, and with an internal radius, r, of 0.03220 ± (0.00003) cm. This tube and the injection valve were kept at 25.00 (±0.01) °C in an air thermostat. The broadened distribution of the disperse samples was monitored at the tube outlet using a differential refractometer (Waters model 2410; Waters, Milford, MA, USA). The refractometer output voltages, V(t), were measured at 5 s intervals using a digital voltmeter (Agilent 34401 A; Agilent Technologies, Santa Clara, CA, USA).
The dispersion profiles for these ternary solutions {e.g., CoCl2 plus sodium chloride} were analyzed by fitting the following equation (Equation (18)) to obtain the values of the different Dab coefficients.
D1 and D2 represent the eigenvalues of the matrix of the ternary Dab coefficients; V0, V1, and Vmax are the baseline voltage, the baseline slope, and the peak height, respectively; r is the inner radius of the dispersion tube (whose values are indicated above); tR is the mean sample retention time; and W1 and (1 − W1) are normalized pre-exponential factors.
4. Conclusions
Based on the predicted ternary diffusion coefficients for the aqueous ternary systems studied (that is, {(CoCl2 + NaCl), (HgCl2 + NaCl), (PbCl2 + NaCl), and (CrCl3 + NaCl), and (CoCl2 + HCl), (HgCl2 + HCl), (PbCl2 + HCl), and (CrCl3 + HCl)}), it can be concluded that the presence of NaCl and HCl affects the diffusion behaviour of these ions (i.e., Co2+, Hg2+, Pb2+, and Cr3+).
There is a coupled diffusion of these salts and NaCl and HCl, as indicated by cross-diffusion coefficients different to zero, that suggests that there is interaction between the solutes. This behaviour of these coefficients was interpreted based on the electrostatic mechanism between these components. Of course, due to the limitations of the Nernst–Hartley equations, it is not possible to accurately find the diffusion behaviour of these systems, however desirable that would be. In other words, although the accurate prediction of Dik coefficients is not possible, being valid only for very diluted solutions, by taking the appropriate precautions, each researcher can use these values, depending on the necessities of its application to a given real problem. In conclusion, Nernst-Hartley model equations are useful, bearing in mind that from their estimated values we can obtain a better understanding of the transport and structure of those systems.
Author Contributions
Conceptualization, M.M.R., A.C.F.R., S.I.G.F. and M.A.E.; Methodology, M.M.R., F.R.L.F., S.I.G.F. and A.C.F.R.; Software, M.M.R., A.C., F.R.L.F., S.I.G.F. and A.C.F.R.; Validation, M.M.R., M.A.E., F.R.L.F., S.I.G.F. and A.C.F.R.; Formal Analysis, M.M.R., F.R.L.F., S.I.G.F. and A.C.F.R.; Investigation, M.M.R., A.C., F.R.L.F., S.I.G.F. and A.C.F.R.; Resources, A.C., F.R.L.F., S.I.G.F. and A.C.F.R.; Data Curation, M.A.E., F.R.L.F., S.I.G.F. and A.C.F.R.; Writing—Original Draft Preparation, M.M.R., A.C.F.R. and S.I.G.F.; Writing—Review and Editing, M.M.R., M.A.E., S.I.G.F. and A.C.F.R.; Visualization, F.R.L.F., S.I.G.F. and A.C.F.R.; Supervision, A.C.F.R. and S.I.G.F.; Project Administration, A.C.F.R., S.I.G.F. and M.A.E.; Funding Acquisition, S.I.G.F. and A.C.F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coimbra Chemistry Centre, which is supported by the Fundação para a Ciência e a Tecnologia (FCT), Portuguese Agency for Scientific Research, through the project UID/QUI/UI0313/2013 and the COMPETE Programme (Operational Programme for Competitiveness).
Data Availability Statement
The data obtained are presented in the article.
Acknowledgments
The authors would like to thank “The Coimbra Chemistry Centre” and CIROS for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Trindade, A.C.V.; Fangaia, S.I.G.; Nicolau, P.M.G.; Messias, A.; Ribeiro, A.C.F.; Silva, D.S.A.; Valente, A.J.M.; Rodrigo, M.M.; Esteso, M.A. Transport Properties of Carbohydrates: Towards the Minimization Toxicological Risks of Cobalt and Chromium Ions. Processes 2023, 11, 1701. [Google Scholar] [CrossRef]
- Fangaia, S.I.G.; Messias, A.; Guerra, F.A.D.R.A.; Ribeiro, A.C.F.; Valente, A.J.M.; Nicolau, P.M.G. Evaluation of the Tribocorrosion Behavior of Ti-6Al-4V Biomedical Alloy in Simulated Oral Environments. Processes 2024, 12, 1283. [Google Scholar] [CrossRef]
- Devi, V.N.M. Sources and Toxicological Effects of Some Heavy Metals—A Mini Review. J. Toxicol. Stud. 2024, 2, 404. [Google Scholar] [CrossRef]
- Khomdram, S.; Soni, H.; Kumar, S.; Sharma, S. Heavy Metal Toxicity in Human Beings. E3S Web Conf. 2024, 509, 03015. [Google Scholar] [CrossRef]
- Koller, M.; Saleh, H.M. Introductory Chapter: Introducing Heavy Metals. In Heavy Metals; InTech: Nappanee, IN, USA, 2018. [Google Scholar] [CrossRef]
- Asfour, A.A. Diffusion: Mass Transfer in Fluid Systems By E.L. Cussler, Cambridge University Press, 1984, 525pp. AIChE J. 1985, 31, 523. [Google Scholar] [CrossRef]
- Tyrrell, H.J.V.; Harris, K.R. Diffusion in Liquids: A Theoretical and Experimental Study; Butterworths: London, UK, 1984. [Google Scholar]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions, 2nd ed.; Dover Publications, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Leaist, D.G.; Hao, L. Diffusion in Buffered Protein Solutions: Combined Nernst–Planck and Multicomponent Fick Equations. J. Chem. Soc. Faraday Trans. 1993, 89, 2775–2782. [Google Scholar] [CrossRef]
- Rodrigo, M.M.; Valente, A.J.M.; Esteso, M.A.; Cabral, A.M.T.D.P.V.; Ribeiro, A.C.F. Ternary Diffusion in Aqueous Sodium Salicylate + Sodium Dodecyl Sulfate Solutions. J. Chem. Thermodyn. 2022, 174, 106859. [Google Scholar] [CrossRef]
- Rodrigo, M.M.; Cabral, A.M.T.D.P.V.; Nicolau, P.M.G.; Ribeiro, A.C.F.; Valente, A.J.M.; Fangaia, S.I.G.; Esteso, M.A. Effect of Potassium Dihydrogen Citrate on the Diffusion Behaviour of Citric Acid. J. Chem. Thermodyn. 2023, 179, 106996. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Gomes, J.C.S.; Santos, C.I.A.V.; Lobo, V.M.M.; Esteso, M.A.; Leaist, D.G. Ternary Mutual Diffusion Coefficients of Aqueous NiCl2 + NaCl and NiCl2 + HCl Solutions at 298.15 K. J. Chem. Eng. Data 2011, 56, 4696–4699. [Google Scholar] [CrossRef]
- Onsager, L.; Fuoss, R.M. Irreversible Processes in Electrolytes. Diffusion, Conductance and Viscous Flow in Arbitrary Mixtures of Strong Electrolytes. J. Phys. Chem. 1932, 36, 2689–2778. [Google Scholar] [CrossRef]
- Callendar, R.; Leaist, D.G. Diffusion Coefficients for Binary, Ternary, and Polydisperse Solutions from Peak-Width Analysis of Taylor Dispersion Profiles. J. Solut. Chem. 2006, 35, 353–379. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Barros, M.C.F.; Verissimo, L.M.P.; Esteso, M.A.; Leaist, D.G. Coupled Mutual Diffusion in Aqueous Sodium (Salicylate + sodium Chloride) Solutions at 25 °C. J. Chem. Thermodyn. 2019, 138, 282–287. [Google Scholar] [CrossRef]
- Leaist, D.G. Relating Multicomponent Mutual Diffusion and Intradiffusion for Associating Solutes. Application to Coupled Diffusion in Water-in-Oil Microemulsions. Phys. Chem. Chem. Phys. 2002, 4, 4732–4739. [Google Scholar] [CrossRef]
- Leaist, D.G. Fick Equations for the Diffusion of Electrolytes in Ternary Liquid Junctions. J. Chem. Soc. Faraday Trans. 1992, 88, 2897. [Google Scholar] [CrossRef]
- Belova, I.V.; Allnatt, A.R.; Murch, G.E. Interdiffusion in Strongly Ionic Insulating Compounds: The Nernst-Planck Equation. Philos. Mag. 2007, 87, 4169–4180. [Google Scholar] [CrossRef]
- Cussler, E.L. Multicomponent Diffusion: Chemical Engineering Monographs 3; Elsevier Scientific Press: New York, NY, USA, 1976. [Google Scholar]
- Deng, Z.; Leaist, D.G. Ternary Mutual Diffusion Coefficients of MgCl2 + MgSO4 + H2O and Na2SO4 + MgSO4 + H2O from Taylor Dispersion Profiles. Can. J. Chem. 1991, 69, 1548. [Google Scholar] [CrossRef]
- John, G.; Albright, J.G.; Mathew, R.; Miller, D.G.; Rard, J.A. Isothermal diffusion coefficients for sodium chloride-magnesium chloride-water at 25.degree.C. 1. Solute concentration ratio of 3:1. J. Phys. Chem. 1989, 93, 2176. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).