Unusual Metal–organic Multicomponent Ni(II) and Mononuclear Zn(II) Compounds Involving Pyridine dicarboxylates: Supramolecular Assemblies and Theoretical Studies
Abstract
:1. Introduction
2. Results and Discussion
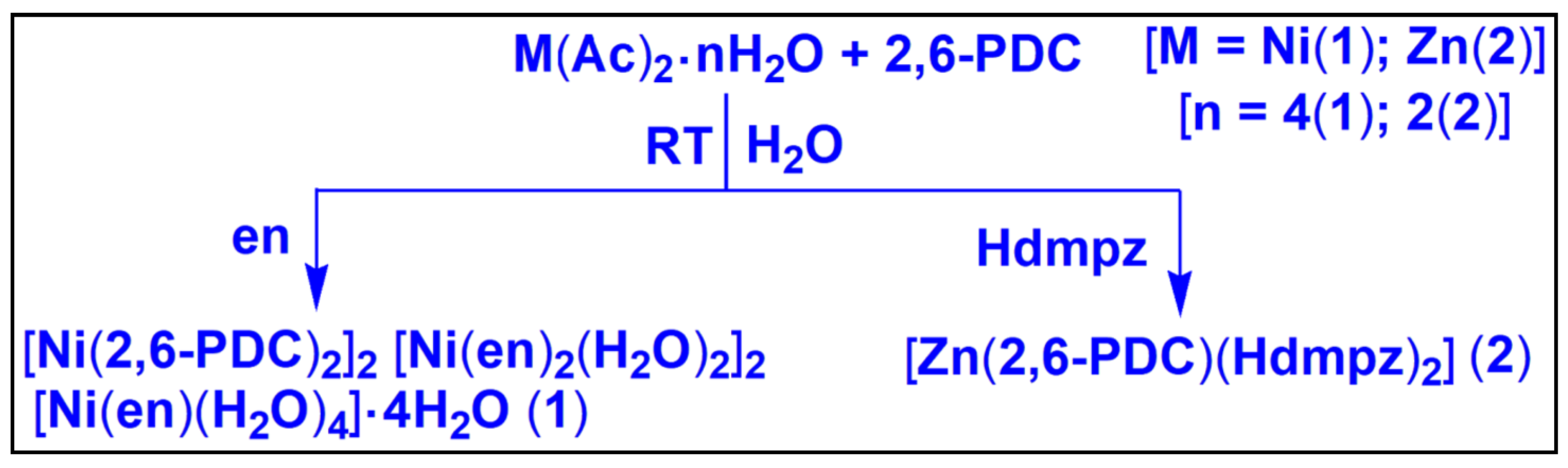
2.1. Syntheses and General Aspects
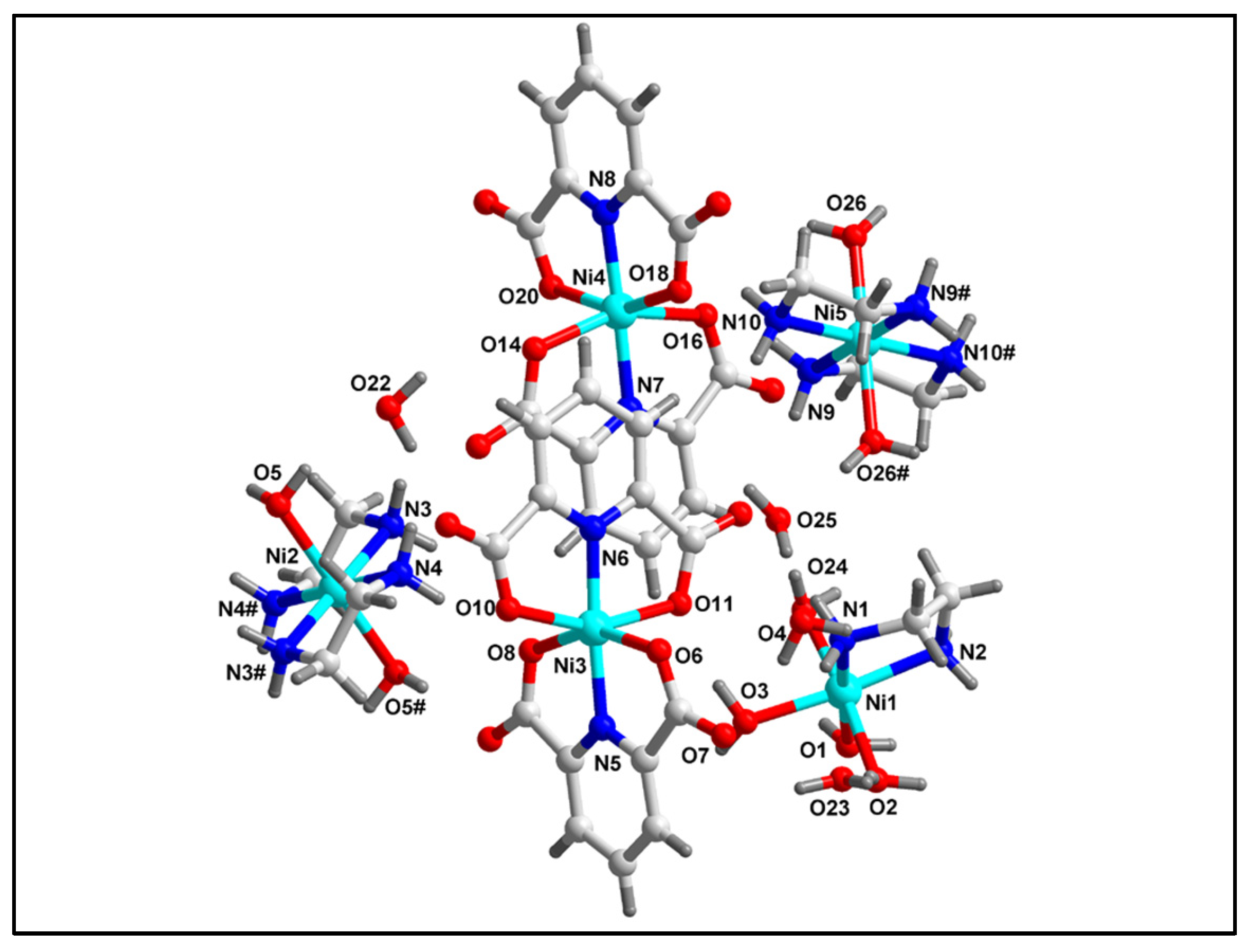
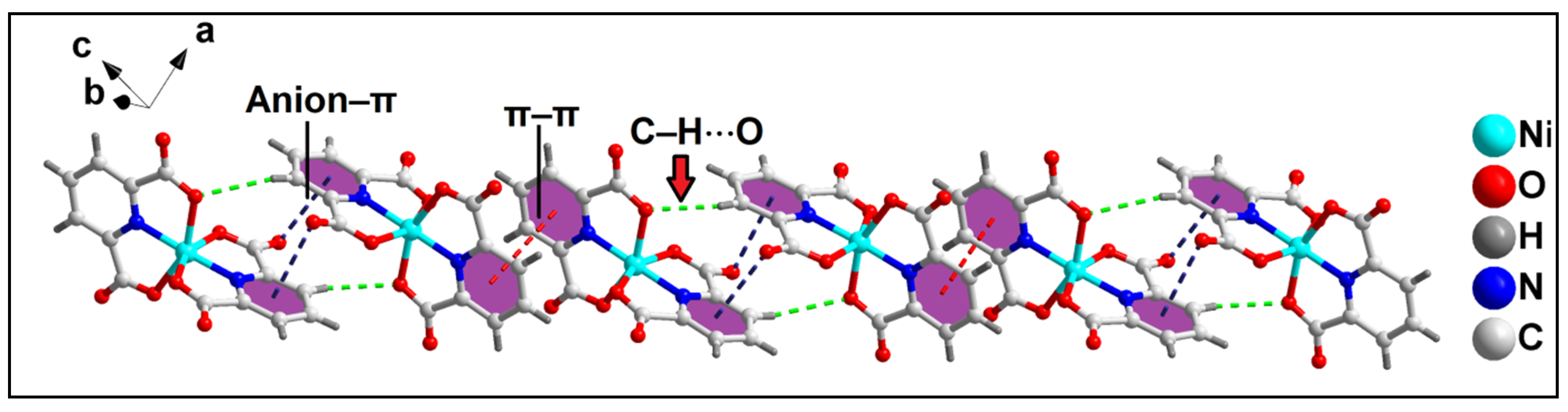
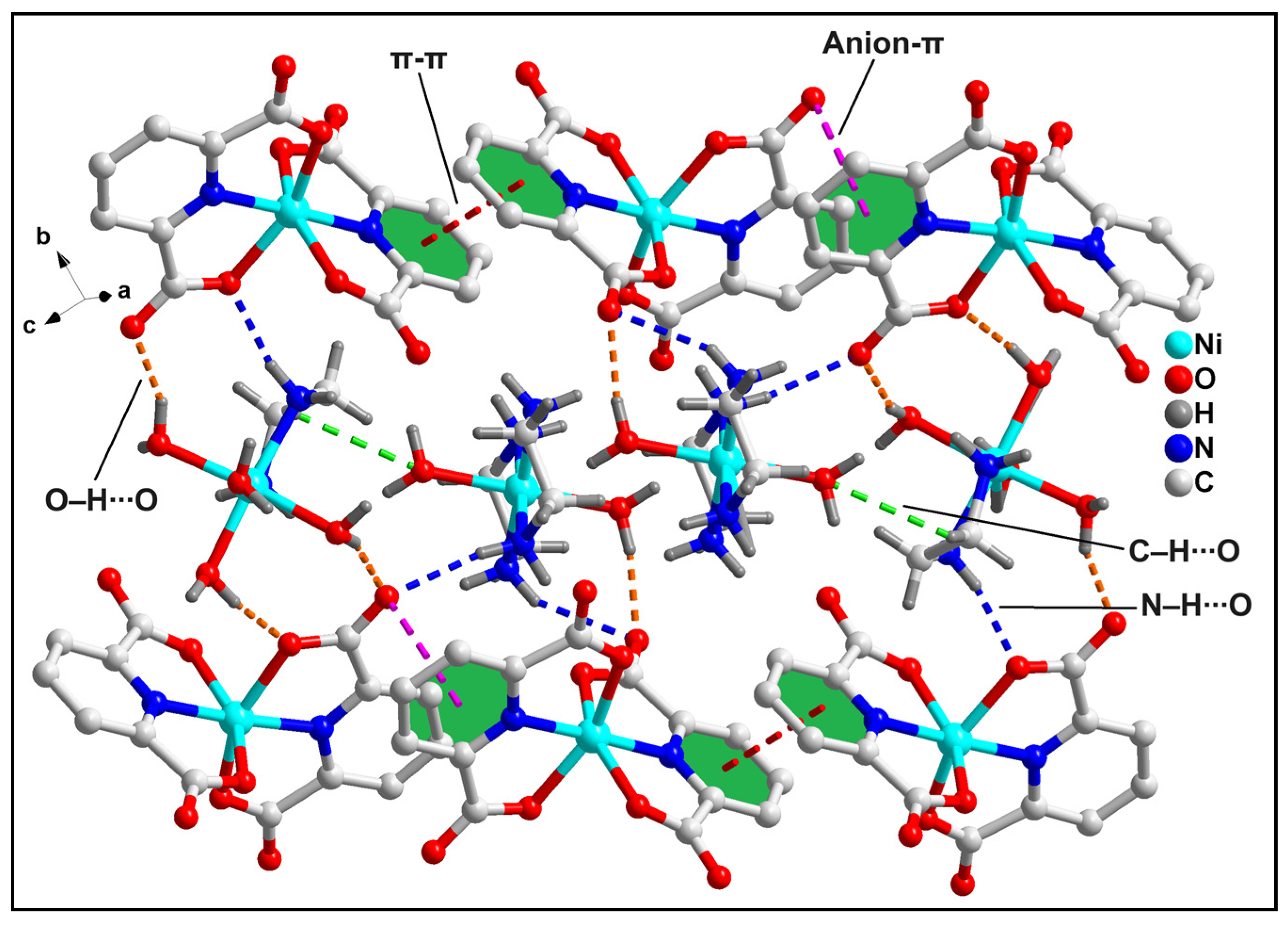
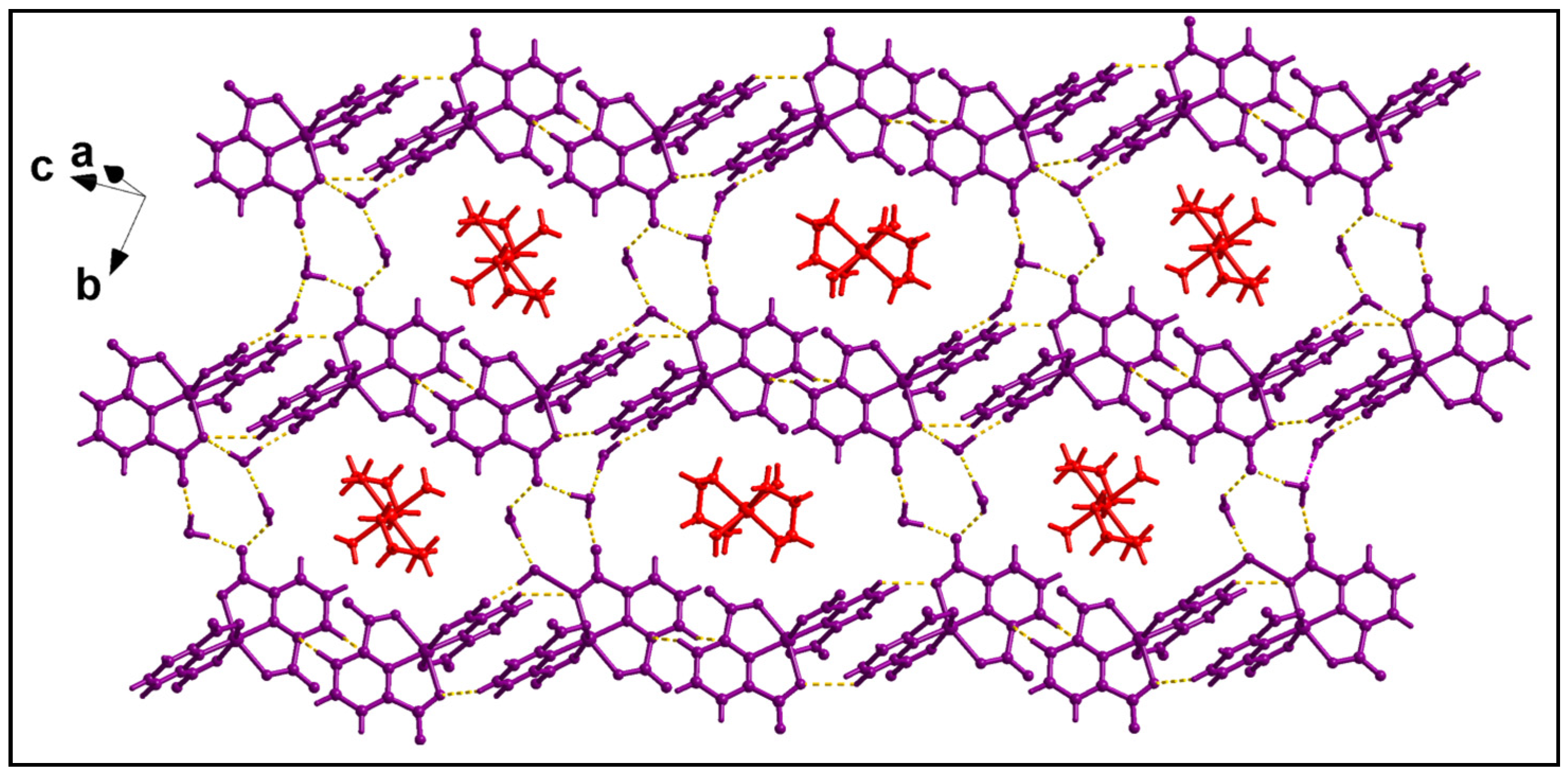
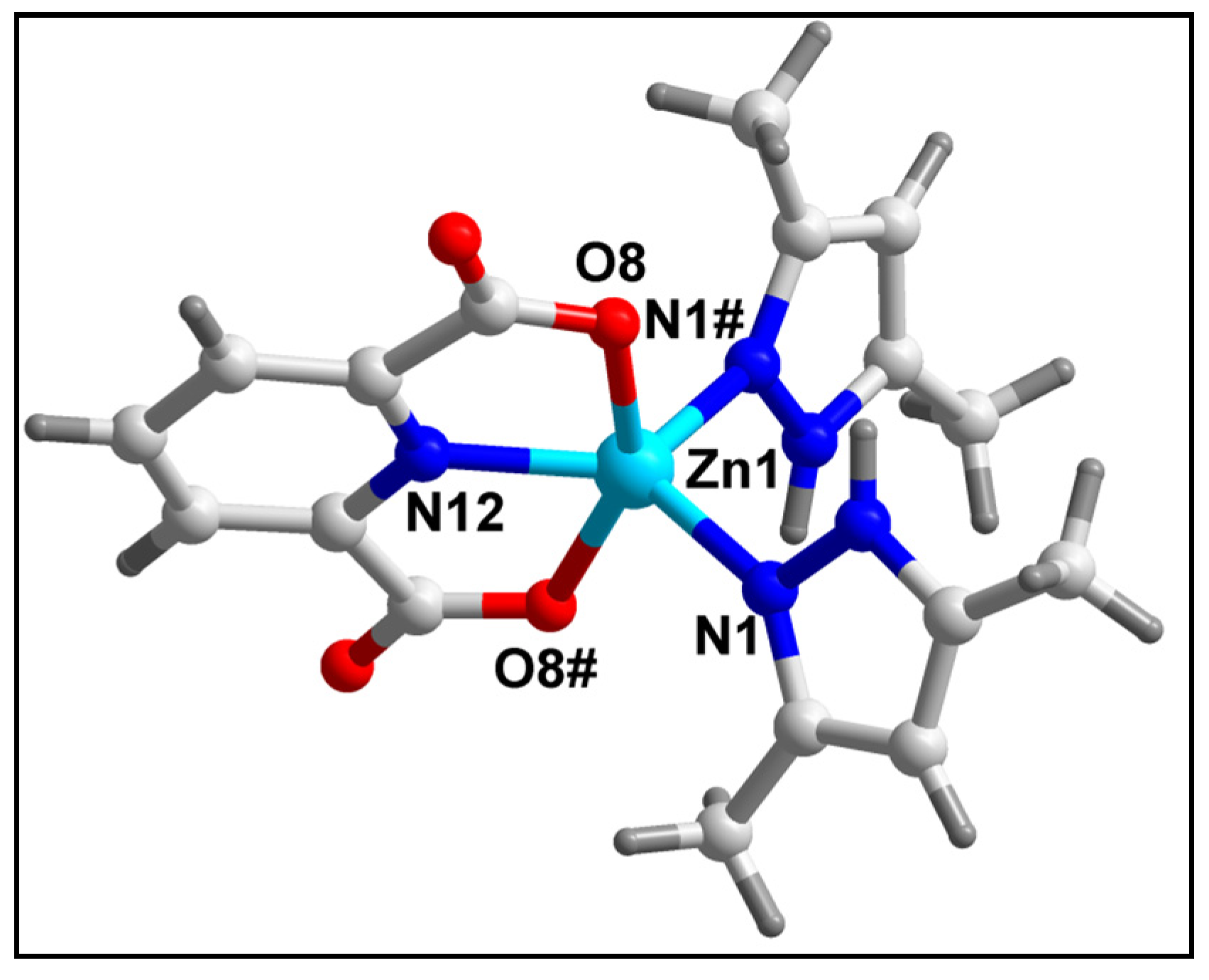
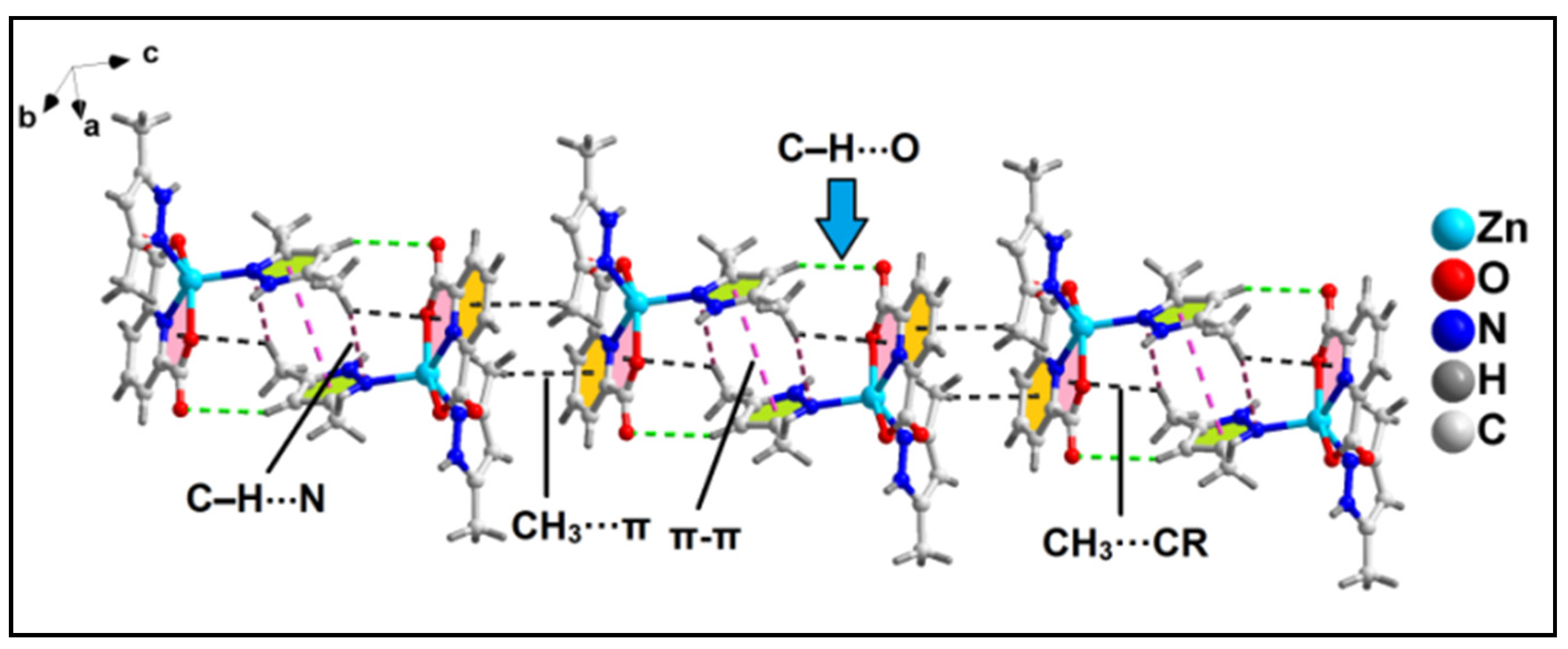
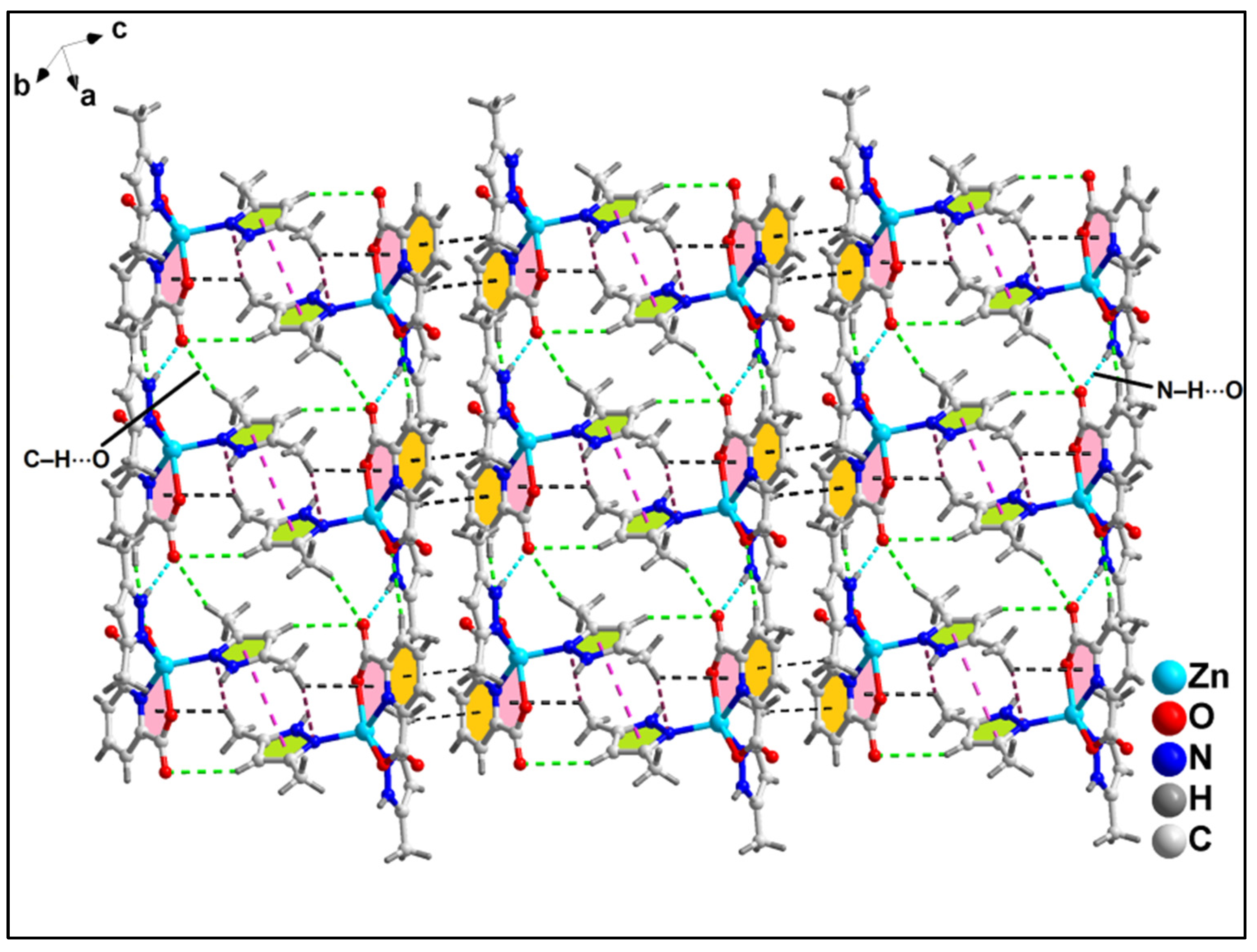
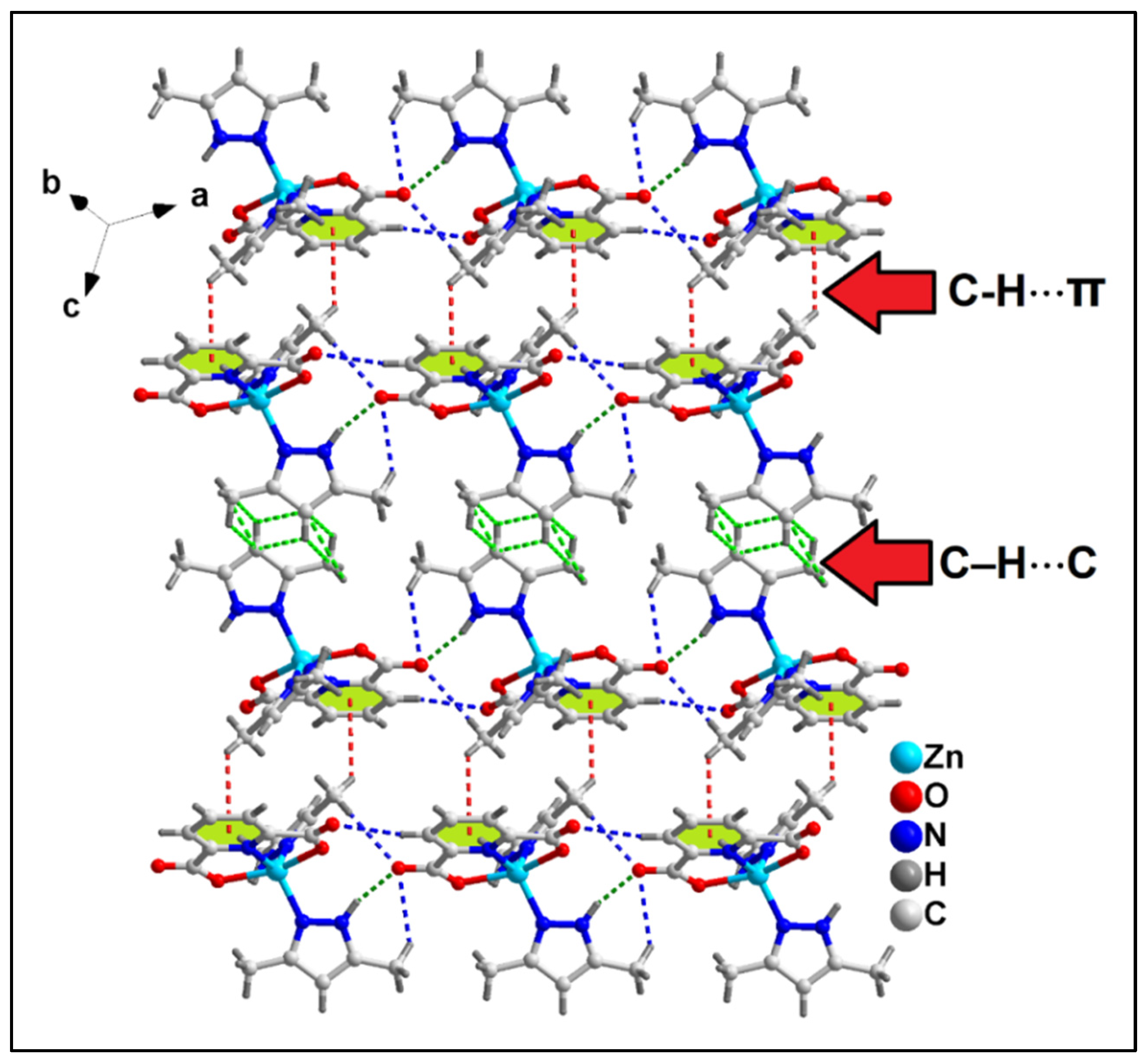
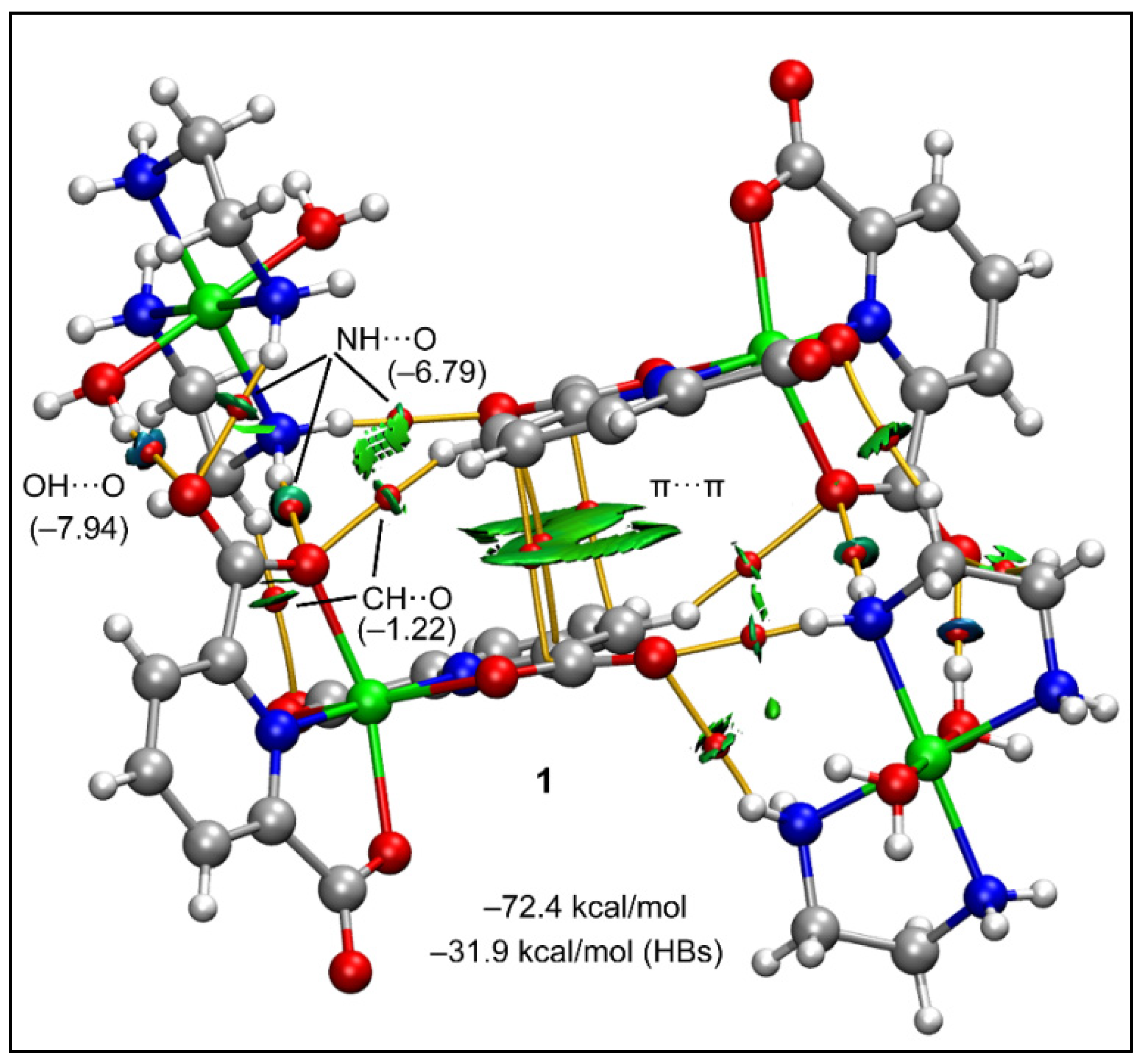
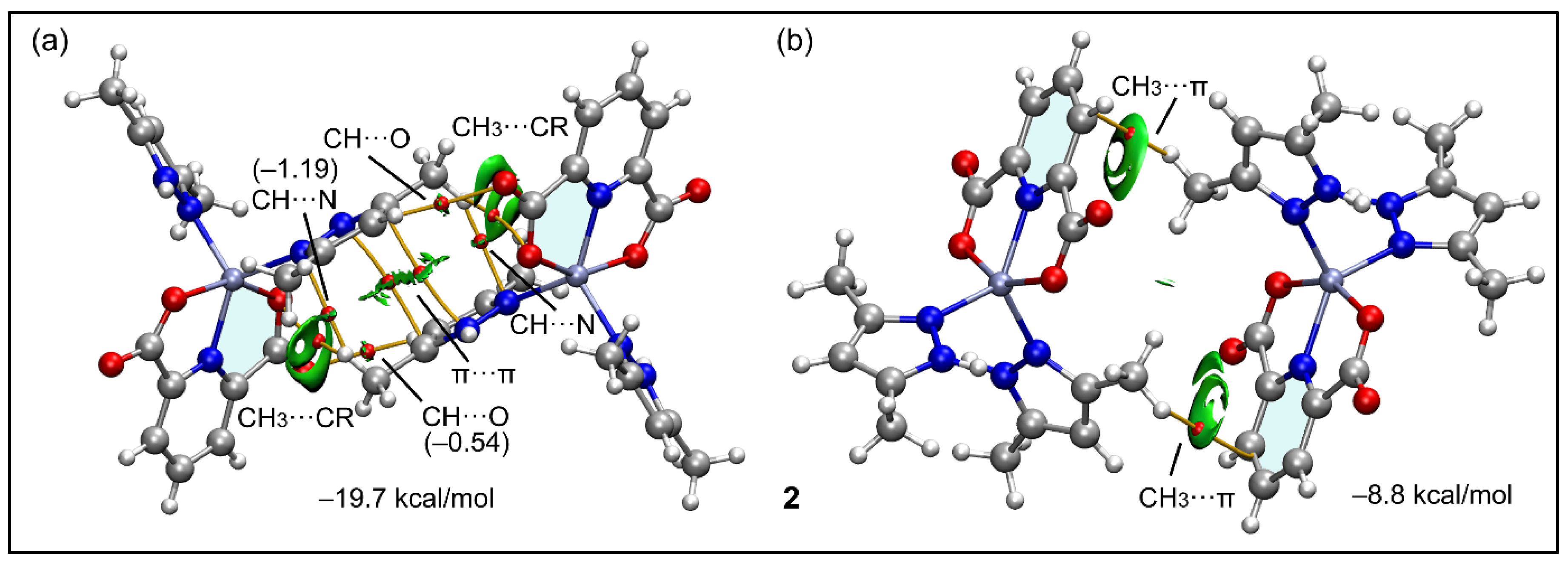
2.2. Crystal Structure Analysis
2.3. Spectral Studies
2.3.1. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.3.2. Electronic Spectroscopy
2.4. Thermogravimetric Study
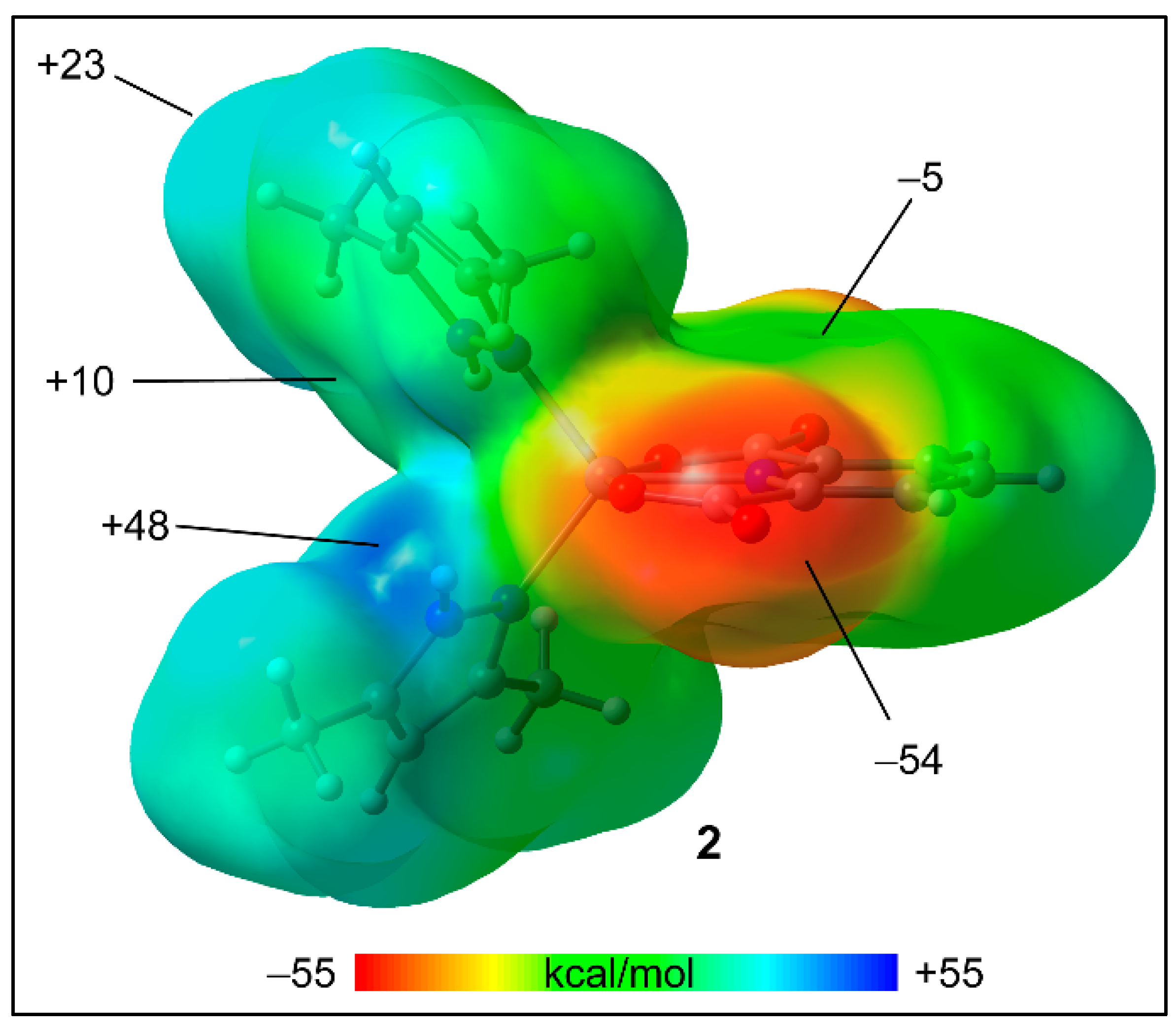
2.5. Theoretical Study
3. Materials and Methods
3.1. Syntheses
3.1.1. Preparation of [Ni(2,6-PDC)2]2[Ni(en)2(H2O)2]2[Ni(en)(H2O)4]·4H2O (1)
3.1.2. Preparation of [Zn(2,6-PDC)(Hdmpz)2] (2)
3.2. Crystallographic Data Collection and Refinement
3.3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Lv, Q.; Yu, R.; Shi, R.; Tan, Z.A. Recent Progress in Organic–Metal Complexes for Organic Photovoltaic Applications. Mater. Chem. Front. 2023, 7, 5063–5103. [Google Scholar] [CrossRef]
- Ding, H.; Deng, X.; Liu, X.; Shang, W.; Du, X.; Wang, S.; Liu, X.; Chen, X.; Liu, H.; Su, H. Exploring Multi-Stimuli-Responsive Pt(II) Complexes: Supramolecular Self-Assembly, Lysosome-Specific Targeted Photodynamic Therapy and Photodegradation of Organic Pollutants. Inorg. Chem. Front. 2024, 11, 1693–1702. [Google Scholar] [CrossRef]
- Dey, N.; Haynes, C.J. Supramolecular Coordination Complexes as Optical Biosensors. ChemPlusChem 2021, 86, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Malenov, D.P.; Zarić, S.D. Stacking Interactions of Aromatic Ligands in Transition Metal Complexes. Coord. Chem. Rev. 2020, 419, 213338. [Google Scholar] [CrossRef]
- Ma, J.F.; Liu, J.F.; Xing, Y.; Jia, H.Q.; Lin, Y.H. Networks with Hexagonal Circuits in Coordination Polymers of Metal Ions (ZnII,CdII) with1,1′-(1,4-Butanediyl)bis(Imidazole). J. Chem. Soc. Dalton Trans. 2000, 14, 2403–2407. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Champness, N.R.; Janiak, C. Recent Advances in Crystal Engineering. CrystEngComm 2010, 12, 22–43. [Google Scholar] [CrossRef]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Bolouri, Z.; Rahmati, Z.; Esmaeilzadeh, A.; Hassanpoor, A.; Bauza, A.; Ballester, P.; Barceló-Oliver, M.; Mague, J.T.; et al. Rationalization of Noncovalent Interactions within Six New MII/8-Aminoquinoline Supramolecular Complexes (MII = Mn, Cu, and Cd): A Combined Experimental and Theoretical DFT Study. Cryst. Growth Des. 2015, 15, 1351–1361. [Google Scholar] [CrossRef]
- Kumar, P.; Banerjee, S.; Radha, A.; Firdoos, T.; Sahoo, S.C.; Pandey, S.K. Role of Non-Covalent Interactions in the Supramolecular Architectures of Mercury (II) Diphenyldithiophosphates: An Experimental and Theoretical Investigation. New J. Chem. 2021, 45, 2249–2263. [Google Scholar] [CrossRef]
- Singh, Y.; Patel, R.N.; Patel, S.K.; Jadeja, R.N.; Patel, A.K.; Patel, N.; Roy, H.; Kumar, P.; Butcher, R.J.; Jasinski, J.P.; et al. Non-Covalent Interactions Governing the Supramolecular Assembly of Copper (II) Complexes with Hydrazone-Type Ligand: Experimental and Quantum Chemical Study. Polyhedron 2021, 200, 115142. [Google Scholar] [CrossRef]
- Talreja, S.; Tiwari, S. Supramolecular Chemistry: Unveiling the Fascinating World of Non-Covalent Interactions and Complex Assemblies. J. Pharm. Pharmacol. Res 2023, 7, 133–139. [Google Scholar] [CrossRef]
- Chauhan, C.; Kumar, S.; Kumar, R.; Saini, A.; Aree, T. Synergistic Effects of Steric Constraints and Non-Covalent Interactions in Copper(II) Chloro-Nitro-Benzoato Complexes: Synthesis, Structural Characterization, Theoretical Investigations, Antimicrobial Studies, and Molecular Docking Analyses. New J. Chem. 2024, 48, 3829–3848. [Google Scholar] [CrossRef]
- Singh, J.; Kim, H.; Chi, K.W. Non-Covalent Interaction-Directed Coordination-Driven Self-Assembly of Non-Trivial Supramolecular Topologies. Chem. Rec. 2021, 21, 574–593. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, A.; Brandão, P.; Saha, S.; Mal, D.; Sepay, N. Insight into Non-Covalent Interactions in 1D Gd-Based Coordination Polymer for Solid-State Self-Assembly through a New Supramolecular Synthon. J. Mol. Struct. 2023, 1272, 134204. [Google Scholar] [CrossRef]
- Borovik, A.S. The Use of Non-Covalent Interactions in the Assembly of Metal/Organic Supramolecular Arrays. Comments Inorg. Chem. 2002, 23, 45–78. [Google Scholar] [CrossRef]
- Gou, L.; Wu, Q.R.; Hu, H.M.; Qin, T.; Xue, G.L.; Yang, M.L.; Tang, Z.X. An Investigation of the Positional Isomeric Effect of Terpyridine Derivatives: Self-Assembly of Novel Cadmium Coordination Architectures Driven by N-Donor Covalence and π⋯π Non-Covalent Interactions. Polyhedron 2008, 27, 1517–1526. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Buckingham, A.D.; Del Bene, J.E.; McDowell, S.A.C. The Hydrogen Bond. Chem. Phys. Lett. 2008, 463, 1–10. [Google Scholar] [CrossRef]
- Janiak, C.A. Critical Account on π–π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc., Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Lehn, J.M.; Atwood, J.L.; Davies, J.E.D.; MacNicol, D.D.; Vogtle, F. Comprehensive Supramolecular Chemistry; Pergamon: Oxford, UK, 1996. [Google Scholar]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The σ-Hole Revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef]
- Scheiner, S. Dissection of the Origin of π-Holes and the Noncovalent Bonds in Which They Engage. J. Phys. Chem. A 2021, 125, 6514–6528. [Google Scholar] [CrossRef]
- Kumar, M.; Balaji, P.V. CH···π Interactions in Proteins: Prevalence, Pattern of Occurrence, Residue Propensities, Location, and Contribution to Protein Stability. J. Mol. Model. 2014, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; Wang, M.X. Exploring Anion−π Interactions and Their Applications in Supramolecular Chemistry. Acc. Chem. Res. 2020, 53, 1364–1380. [Google Scholar] [CrossRef]
- Kozelka, J. Lone Pair···π Interactions in Biological Systems: Occurrence, Function, and Physical Origin. Eur. Biophys. J. 2017, 46, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Islam, S.; Seth, S.K. Structural Elucidation and Interpretation of 2D–3D Supramolecular Assemblies Featuring Lone-Pair···π Interaction in Two Cu (II)–PDA Complexes: Experimental and Computational Assessment. J. Mol. Struct. 2024, 1308, 138088. [Google Scholar] [CrossRef]
- Rather, I.A.; Wagay, S.A.; Ali, R. Emergence of Anion-π Interactions: The Land of Opportunity in Supramolecular Chemistry and Beyond. Coord. Chem. Rev. 2020, 415, 213327. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Ou, Q.; Yu, P.; Wang, G.; Duan, Y.; Geng, H.; Peng, Q.; Shuai, Z.; Liao, Y. Molecular Design Strategy for Simultaneously Strong Luminescence and High Mobility: Multichannel CH-π Interaction. J. Phys. Chem. Lett. 2021, 12, 938–946. [Google Scholar] [CrossRef]
- Houser, J.; Kozmon, S.; Mishra, D.; Hammerová, Z.; Wimmerová, M.; Koča, J. The CH–π Interaction in Protein–Carbohydrate Binding: Bioinformatics and In Vitro Quantification. Chem.–Eur. J. 2020, 26, 10769–10780. [Google Scholar] [CrossRef]
- Nagahama, S.; Inoue, K.; Sada, K.; Miyata, M.; Matsumoto, A. Two-Dimensional Hydrogen Bond Networks Supported by CH/π Interaction Leading to a Molecular Packing Appropriate for Topochemical Polymerization of 1,3-Diene Monomers. Cryst. Growth Des. 2003, 3, 247–256. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond: In Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Wheeler, S.E. Local Nature of Substituent Effects in Stacking Interactions. J. Am. Chem. Soc. 2011, 133, 10262–10274. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The Nature and Applications of π–π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523–528. [Google Scholar] [CrossRef]
- Haque, A.; Alenezi, K.M.; Khan, M.S.; Wong, W.Y.; Raithby, P.R. Non-Covalent Interactions (NCIs) in π-Conjugated Functional Materials: Advances and Perspectives. Chem. Soc. Rev. 2023, 52, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.Y.; Zhen, Q.; Xiao, D.; Tao, G.; Xing, X.; Yu, P.; Xu, C. Engineered Non-Covalent π Interactions as Key Elements for Chiral Recognition. Nat. Commun. 2022, 13, 3276. [Google Scholar] [CrossRef] [PubMed]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-Covalent Intramolecular Interactions through Ligand-Design Promoting Efficient Photoluminescence from Transition Metal Complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Chettri, A.; Subba, A.; Singh, G.P.; Bag, P.P. Pharmaceutical Co-Crystals: A Green Way to Enhance Drug Stability and Solubility for Improved Therapeutic Efficacy. J. Pharm. Pharmacol. 2023, 76, 1–12. [Google Scholar] [CrossRef]
- Hegde, T.A.; Vinitha, G. Chloridocobaltate(II) Metal–Organic Cocrystal Delivering Intermolecular-Charge Transfer-Enhanced Passive Optical Limiting: A Comprehensive Study on Structure–Property Relation. Eur. Phys. J. D 2021, 75, 214. [Google Scholar] [CrossRef]
- Hegde, T.A.; Dutta, A.; Sabari Girisun, T.C.; Vinitha, G. A Novel Organic-Inorganic Ionic Cocrystal—Piperazine-1,4-Diium Tetrachloridocuprate(II) Dihydrate Delivering Efficient Optical Limiting. Chem. Phys. Lett. 2021, 781, 138971–138980. [Google Scholar] [CrossRef]
- Kargar, H.; Behjatmanesh-Ardakani, R.; Torabi, V.; Sarvian, A.; Kazemi, Z.; Chavoshpour-Natanzi, Z.; Mirkhani, V.; Sahraei, A.; Tahir, M.N.; Ashfaq, M. Novel Copper(II) and Zinc(II) Complexes of Halogenated Bidentate N, O-Donor Schiff Base Ligands: Synthesis, Characterization, Crystal Structures, DNA Binding, Molecular Docking, DFT and TD-DFT Computational Studies. Inorg. Chim. Acta 2021, 514, 120004. [Google Scholar] [CrossRef]
- Kargar, H.; Behjatmanesh-Ardakani, R.; Torabi, V.; Kashani, M.; Chavoshpour-Natanzi, Z.; Kazemi, Z.; Mirkhani, V.; Sahraei, A.; Tahir, M.N.; Ashfaq, M.; et al. Synthesis, Characterization, Crystal Structures, DFT, TD-DFT, Molecular Docking and DNA Binding Studies of Novel Copper(II) and Zinc(II) Complexes Bearing Halogenated Bidentate N, O-Donor Schiff Base Ligands. Polyhedron 2021, 195, 114988. [Google Scholar] [CrossRef]
- Ay, B.; Yildiz, E.; Enomoto, M.; Okazawa, A.; Kojima, N. Crystal Structures, Gas Storage and Magnetic Properties of Lanthanide-Organic Frameworks Built Up from Dicarboxylates, [Ln2(2,5-pydc)2(2,5-pipdc)(H2O)2]n (Ln = Ce, Pr, Eu) and (H2pip)n [Ln2(2,6-pydc)4(H2O)2]n (Ln = Ce, Pr, Eu, Sm). Polyhedron 2022, 226, 116110. [Google Scholar] [CrossRef]
- Shahraki, S.; Shiri, F.; Majd, M.H.; Razmara, Z. Comparative Study on the Anticancer Activities and Binding Properties of a Hetero Metal Binuclear Complex [Co(dipic)2Ni(OH2)5]·2H2O (dipic = Dipicolinate) with Two Carrier Proteins. J. Pharm. Biomed. Anal. 2017, 145, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Halim, H.F.; Mohamed, G.G. Synthesis, Spectroscopic, Thermal Analyses, Biological Activity and Molecular Docking Studies on Mixed Ligand Complexes Derived from Schiff Base Ligands and 2, 6-Pyridine Dicarboxylic Acid. Appl. Organomet. Chem. 2018, 32, e4176. [Google Scholar] [CrossRef]
- Murinzi, T.W.; Hosten, E.; Watkins, G.M. Synthesis and Characterization of a Cobalt-2,6-Pyridinedicarboxylate MOF with Potential Application in Electrochemical Sensing. Polyhedron 2017, 137, 188–196. [Google Scholar] [CrossRef]
- Ma, C.; Li, J.; Zhang, R.; Wang, D. Syntheses and Characterization of Triorganotin Complexes: X-ray Crystallographic Study of Triorganotin Pyridinedicarboxylates with Trinuclear, 1D Polymeric Chain and 2D Network Structures. J. Organomet. Chem. 2006, 691, 1713–1721. [Google Scholar] [CrossRef]
- Diop, T.; Ndioléne, A.; Diop, M.B.; Boye, M.S.; van der Lee, A.; Dumitru, F.; Khadir Diop, C.A.; Sidibé, M. Synthesis, Spectral (FT-IR, 1H, 13C) Studies, and Crystal Structure of [(2,6-CO2)2C5H3NSnBu2(H2O)]2•CHCl3. Z. Naturforsch. B 2021, 76, 127–132. [Google Scholar] [CrossRef]
- Dutta, D.; Sharma, P.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Baishya, T.; Bhattacharyya, M.K. Supramolecular Assemblies Involving Unconventional Non-Covalent Contacts in Pyrazole-Based Coordination Compounds of Co(II) and Cu(II) Pyridinedicarboxylates: Antiproliferative Evaluation and Theoretical Studies. Polyhedron 2022, 224, 116025. [Google Scholar] [CrossRef]
- Jin, S.W.; Huang, Y.; Wang, D.Q.; Fang, H.; Wang, T.; Fu, P.; Ding, L. Non-Covalently Bonded 2D–3D Metal–Organic Frameworks from the Reactions of Cd(II) and Zn(II) with 3,5-Dimethylpyrazole and Carboxylate Ligands. Polyhedron 2013, 60, 10–22. [Google Scholar] [CrossRef]
- Jin, S.W.; Lin, Z.H.; Zhou, Y.; Wang, D.Q.; Chen, G.Q.; Ji, Z.Y.; Huang, T.S. Syntheses, Characterization and Crystal Structures of Eight Cd(II) Carboxylates Containing 3,5-Dimethylpyrazole. Polyhedron 2014, 74, 79–92. [Google Scholar] [CrossRef]
- Ding, C.; Xia, M.; Wang, F.; Lei, W.; Ni, Y. The Sensitive Detection and Mechanism of Fe-3,5-Dimethyl Pyrazole Fluorescent Sensor to Diethylenetriamine Pentamethylene Phosphonic Acid: Experimental Study and Quantum Chemical Calculation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 281, 121623. [Google Scholar] [CrossRef]
- Guerrero, M.; Pons, J.; Font-Bardia, M.; Calvet, T.; Ros, J. Synthesis, Characterization, and Crystal Structure of a Novel Two-Dimensional Network Formed by the Reaction of a Pyrazole Ligand with Nickel(II) Ions. Polyhedron 2010, 29, 1083. [Google Scholar] [CrossRef]
- Aljuhani, E.; Aljohani, M.M.; Alsoliemy, A.; Shah, R.; Abumelha, H.M.; Saad, F.A.; Hossan, A.; Al-Ahmed, Z.A.; Alharbi, A.; El-Metwaly, N.M. Synthesis and Characterization of Cu(II)-Pyrazole Complexes for Possible Anticancer Agents; Conformational Studies as well as Compatible In-Silico and In-Vitro Assays. Heliyon 2021, 7, e08485. [Google Scholar] [CrossRef] [PubMed]
- Bouroumane, N.; El Kodadi, M.; Touzani, R.; El Boutaybi, M.; Oussaid, A.; Hammouti, B.; Nandiyanto, A.B.D. New Pyrazole-Based Ligands: Synthesis, Characterization, and Catalytic Activity of Their Copper Complexes. Arab. J. Sci. Eng. 2022, 47, 269–279. [Google Scholar] [CrossRef]
- Santra, A.; Mondal, G.; Acharjya, M.; Bera, P.; Panja, A.; Mandal, T.K.; Mitra, P.; Bera, P. Catechol Oxidase Mimetic Activity of Copper(I) Complexes of 3,5-Dimethyl Pyrazole Derivatives: Coordination Behavior, X-ray Crystallography and Electrochemical Study. Polyhedron 2016, 113, 5–15. [Google Scholar] [CrossRef]
- Devkule, S.S.; More, M.S.; Chavan, S.S. Synthesis, Characterization, Luminescence and Catalytic Properties of Copper(I) Complexes with N-(2-Pyridylmethylene)-1,5-Dimethyl-2-Pyrazole-3-(2H)-one and Triphenylphosphine as Ligands. Inorg. Chim. Acta 2017, 455, 183–189. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Averin, A.A.; Shubina, E.S. Copper(I) Complex with BINAP and 3,5-Dimethylpyrazole: Synthesis and Photoluminescent Properties. Mendeleev Commun. 2019, 29, 570–572. [Google Scholar] [CrossRef]
- Turecka, K.; Chylewska, A.; Rychłowski, M.; Zakrzewska, J.; Waleron, K. Antibacterial Activity of Co(III) Complexes with Diamine Chelate Ligands Against a Broad Spectrum of Bacteria with a DNA Interaction Mechanism. Pharmaceutics 2021, 13, 946. [Google Scholar] [CrossRef] [PubMed]
- Condé, C.A.; De Almeida, M.V.; Da Silva, G.D.S.; Sodré, M.B.P.D.A.; Rodrigues, J.C.F.; Navarro, M. Synthesis, Characterization and Antileishmanial Activity of Copper(II) and Zinc(II) Complexes with Diamine Ligands. Transit. Met. Chem. 2022, 47, 147–156. [Google Scholar] [CrossRef]
- Rajeshwari, K.; Anantha Lakshmi, P.V.; Archana, J.; Sumakanth, M. Ternary Cobalt(II), Nickel(II), and Copper(II) Complexes Containing Metformin and Ethylenediamine: Synthesis, Characterization, Thermal, In Vitro DNA Binding, In Silico Molecular Docking, and In Vivo Antihyperglycemic Studies. Appl. Organomet. Chem. 2021, 35, e6100. [Google Scholar] [CrossRef]
- Boudiombo, J.S.B.; Su, H.; Ravenscroft, N.; Bourne, S.A.; Nassimbeni, L.R. Selective Enclathration of Xylenols: Synergistic Effects of Mixed Hosts. CrystEngComm 2020, 22, 7389–7398. [Google Scholar] [CrossRef]
- Morohashi, N.; Hattori, T. Selective Guest Inclusion by Crystals of Calixarenes: Potential for Application as Separation Materials. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 261–277. [Google Scholar] [CrossRef]
- Colorado-Peralta, R.; Rivera-Villanueva, J.M.; Mora-Hernández, J.M.; Morales-Morales, D.; Alfonso-Herrera, L.Á. An Overview of the Role of Supramolecular Interactions in Gas Storage Using MOFs. Polyhedron 2022, 224, 115995. [Google Scholar] [CrossRef]
- Das, R.K.; Aijaz, A.; Sharma, M.K.; Lama, P.; Bharadwaj, P.K. Direct Crystallographic Observation of Catalytic Reactions Inside the Pores of a Flexible Coordination Polymer. Chem.-Eur. J. 2012, 18, 6866–6872. [Google Scholar] [CrossRef] [PubMed]
- Baishya, T.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Bhattacharyya, M.K. Enclathration of Mn(II)(H2O)6 Guests and Unusual Cu⋯O Bonding Contacts in Supramolecular Assemblies of Mn(II) Co-Crystal Hydrate and Cu(II) Pyridinedicarboxylate: Antiproliferative Evaluation and Theoretical Studies. Polyhedron 2023, 230, 116243. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Banik, S.; Baishya, T.; Sharma, P.; Dutta, K.K.; Gomila, R.M.; Barcelo-Oliver, M.; Frontera, A. Fascinating Inclusion of Metal–Organic Complex Moieties in Dinuclear Mn(II) and Zn(II) Compounds Involving Pyridinedicarboxylates and Phenanthroline: Experimental and Theoretical Studies. Polyhedron 2024, 254, 116947. [Google Scholar] [CrossRef]
- Fouad, R.; Shaaban, I.A.; Ali, T.E.; Assiri, M.A.; Shenouda, S.S. Co(II), Ni(II), Cu(II), and Cd(II)-Thiocarbonohydrazone Complexes: Spectroscopic, DFT, Thermal, and Electrical Conductivity Studies. RSC Adv. 2021, 11, 37726. [Google Scholar] [CrossRef]
- Lv, Y.; Qi, Y.; Sun, L.; Luo, F.; Che, Y.; Zheng, J. Construction of Metal-Organic Frameworks with the Pyridine-3,5-Dicarboxylate Anion and Bis(imidazole)Ligands: Synthesis, Structure, and Thermostability Studies. Inorg. Chem. 2010, 49, 5592. [Google Scholar] [CrossRef]
- Das, A.; Choudhury, S.R.; Estarellas, C.; Dey, B.; Frontera, A.; Hemming, J.; Helliwell, M.; Gamez, P.; Mukhopadhyay, S. Supramolecular Assemblies Involving Anion–π and Lone Pair–π Interactions: Experimental Observation and Theoretical Analysis. CrystEngComm 2011, 13, 4519. [Google Scholar] [CrossRef]
- Basak, T.; Frontera, A.; Chattopadhyay, S. Synthesis and Characterization of a Mononuclear Zinc(II) Schiff Base Complex: On the Importance of C–H⋯π Interactions. RSC Adv. 2021, 11, 30148–30155. [Google Scholar] [CrossRef]
- Adhav, V.A.; Saikrishnan, K. The Realm of Unconventional Noncovalent Interactions in Proteins: Their Significance in Structure and Function. ACS Omega 2023, 8, 22268–22284. [Google Scholar] [CrossRef]
- Kokina, T.E.; Glinskaya, L.A.; Tkachev, A.V.; Plyusnin, V.F.; Tsoy, Y.V.; Bagryanskaya, I.Y.; Vasilyev, E.S.; Piryazev, D.A.; Sheludyakova, L.A.; Larionov, S.V. Chiral Zinc(II) and Cadmium(II) Complexes with a Dihydrophenanthroline Ligand Bearing (–)-α-Pinene Fragments: Synthesis, Crystal Structures and Photophysical Properties. Polyhedron 2016, 117, 437. [Google Scholar] [CrossRef]
- Das, S.; Bharadwaj, P.K. Self-Assembly of a Luminescent Zinc(II) Complex: A Supramolecular Host–Guest Fluorescence Signaling System for Selective Nitrobenzene Inclusion. Inorg. Chem. 2006, 45, 5257. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, T.S.; Bauzá, A.; Dutta, D.; Mishra, S.; Frontera, A.; Ray, D. Carboxylate Coordination Assisted Aggregation for Quasi-Tetrahedral and Partial-Dicubane [Cu4] Coordination Clusters. Chem. Select. 2016, 1, 64. [Google Scholar] [CrossRef]
- Orhan, O.; Çolak, A.T.; Emen, F.M.; Kismali, G.; Meral, O.; Sel, T.; Çilgi, G.K.; Taş, M. Syntheses of Crystal Structures and In Vitro Cytotoxic Activities of New Copper(II) Complexes of Pyridine-2,6-Dicarboxylate. J. Coord. Chem. 2015, 68, 4003. [Google Scholar] [CrossRef]
- Manna, S.C.; Mistri, S.; Jana, A.D. A Rare Supramolecular Assembly Involving Ion Pairs of Coordination Complexes with a Host–Guest Relationship: Synthesis, Crystal Structure, Photoluminescence and Thermal Study. CrystEngComm 2012, 14, 7415. [Google Scholar] [CrossRef]
- Debnath, P.; Singh, K.S.; Singh, K.K.; Singh, S.S.; Sieroń, L.; Maniukiewicz, W. Di-Butyltin(IV) Complexes with Azo-Carboxylates: Synthesis, Characterization, Crystal Structures and Their Anti-Diabetic Assay. New J. Chem. 2020, 44, 5862. [Google Scholar] [CrossRef]
- Hashemi, M.; Mohandes, F.; Ahmadian-Fard-Fini, S.; Sobhani, A.; Shabani-Armaki, N.; Salavati-Niasari, M. Solvent-Free Preparation of Copper Ferrite Microspheres Composed of Nanorods Using a New Coordination Compound as Precursor. J. Mater. Sci. Mater. Electron. 2017, 28, 11682. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1986; p. 203. [Google Scholar]
- Li, J.; Xing, Y.H.; Zhao, H.Y.; Li, Z.P.; Wang, C.G.; Zeng, X.Q.; Ge, M.F.; Niu, S.Y. Constructions of a Set of Hydrogen-Bonded Supramolecules from Reactions of Transition Metals with 3,5-Dimethylpyrazole and Different Dicarboxylate Ligands. Inorg. Chim. Acta 2009, 362, 2788. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, W.; Hu, K.; Jin, S.; Sun, L.; Liu, B.; Wang, D. Synthesis and Structural Characterizations of NineNon-Covalent-Bonded Zn2+and Cd2+ Supramolecules Based on 3,5-Dimethylpyrazole and Carboxylates. Polyhedron 2019, 159, 408. [Google Scholar] [CrossRef]
- Titi, A.; Shiga, T.; Oshio, H.; Touzani, R.; Hammouti, B.; Mouslim, M.; Warad, I. Synthesis of Novel [Cl2Co4L6] Cluster Using 1-Hydroxymethyl-3,5-Dimethylpyrazole (LH) Ligand: Crystal Structure, Spectral, Thermal, Hirschfeld Surface Analysis and Catalytic Oxidation Evaluation. J. Mol. Struct. 2020, 1199, 126995. [Google Scholar] [CrossRef]
- Direm, A.; Tursun, M.; Parlak, C.; Benali-Cherif, N. Trans-Dichlorotetrakis(1H-Pyrazole-κN2) Copper(II): Synthesis, Crystal Structure, Hydrogen Bonding Graph-Sets, Vibrational and DFT Studies. J. Mol. Struct. 2015, 1093, 208. [Google Scholar] [CrossRef]
- Mautner, F.A.; Scherzer, M.; Berger, C.; Fischer, R.C.; Vicente, R.; Massoud, S.S. Synthesis and Characterization of Five New Thiocyanato- and Cyanato-Metal(II) Complexes with 4-Azidopyridine as Co-Ligand. Polyhedron 2015, 85, 20–26. [Google Scholar] [CrossRef]
- Figgis, B.N.; Hitchman, M.A. Ligand Field Theory and Its Applications; Wiley-VCH: New York, NY, USA, 2000; p. 209. [Google Scholar]
- Büyükkıdan, N.; Yenikaya, C.; İlkimen, H.; Karahan, C.; Darcan, C.; Korkmaz, T.; Süzen, Y. Synthesis, Characterization, and Biological Activities of Metal(II) Dipicolinate Complexes Derived from Pyridine-2,6-Dicarboxylic Acid and 2-(Piperazin-1-yl)ethanol. J. Mol. Struct. 2015, 1101, 139. [Google Scholar] [CrossRef]
- Borah, M.J.; Bhubon Singh, R.K.; Sinha, U.B.; Swu, T.; Borah, P.J. Synthesis and Crystal Structure Determination of DimericCo(II) and Ni(II) with Pyridine-2,6-dicarboxylicAcid. J. Chem. Crystallogr. 2012, 42, 67. [Google Scholar] [CrossRef]
- Araújo, E.L.; Barbosa, H.F.G.; Dockal, E.R.; Cavalheiro, E.T.G. Synthesis, Characterization and Biological Activity of Cu(II), Ni(II) and Zn(II) Complexes of Biopolymeric Schiff Bases of Salicylaldehydes and Chitosan. Int. J. Biol. Macromol. 2017, 95, 168. [Google Scholar] [CrossRef]
- Ekennia, A.C.; Onwudiwe, D.C.; Osowole, A.A.; Olasunkanmi, L.O.; Ebenso, E.E. Synthesis, Biological, and Quantum Chemical Studies of Zn(II) and Ni(II) Mixed-Ligand Complexes Derived from N,N-Disubstituted Dithiocarbamate and Benzoic Acid. J. Chem. 2016, 55, 5129010. [Google Scholar] [CrossRef]
- Bordbar, M.; Tabatabaee, M.; Alizadeh-Nouqi, M.; Mehri-Lighvan, Z.; Khavasi, H.R.; YeganehFaal, A.; Fallahian, F.; Dolati, M. Synthesis, Characterization, Cytotoxic Activity and DNA-Binding Studies of Cobalt(II) Mixed-Ligand Complex Containing Pyridine-2,6-Dicarboxylate Ion and 2-Aminopyrimidine. J. Iranian Chem. Soc. 2016, 13, 1125–1132. [Google Scholar] [CrossRef]
- Chakravorty, S.; Platts, J.A.; Das, B.K. Novel C–H⋯C Contacts Involving 3,5-Dimethylpyrazole Ligands in a TetracoordinateCo(II) Complex. Dalton Trans. 2011, 40, 11605. [Google Scholar] [CrossRef]
- Ghosh, M.; Majee, A.; Nethaji, M.; Chattopadhyay, T. Syntheses and Characterization of trans-[NiL2(NCS)2][L = 2-(Aminomethyl) Pyridine], trans-[NiL2′(NSC)2][L′ = 2-(2-Aminoethyl) Pyridine] and trans-[NiL2″(NSC)2][L″ = 2-(2-Methylaminoethyl) Pyridine] Complexes: X-Ray Single Crystal Structure of trans-[NiL2′(NSC)2][L′ = 2-(2-Aminoethyl) Pyridine]. Inorganica Chim. Acta 2009, 362, 2052–2055. [Google Scholar]
- Bhattacharyya, M.K.; Gogoi, A.; Chetry, S.; Dutta, D.; Verma, A.K.; Sarma, B.; Franconetti, A.; Frontera, A. Antiproliferative Evaluation and Supramolecular Association in Mn(II) and Zn(II) Bipyridine Complexes: Combined Experimental and Theoretical Studies. J. Inorg. Biochem. 2019, 200, 110803. [Google Scholar] [CrossRef]
- Osypiuk, D.; Cristóvão, B.; Bartyzel, A. New Coordination Compounds of Cu(II) with Schiff Base Ligands—Crystal Structure, Thermal, and Spectral Investigations. Crystals 2020, 10, 1004. [Google Scholar] [CrossRef]
- El-Sonbati, A.Z.; Diab, M.A.; Morgan, S.M.; Abou-Dobara, M.I.; El-Ghettany, A.A. Synthesis, Characterization, Theoretical and Molecular Docking Studies of Mixed-Ligand Complexes of Cu(II), Ni(II), Co(II), Mn(II), Cr(III), UO2(II) and Cd(II). J. Mol. Struct. 2020, 1200, 127065. [Google Scholar] [CrossRef]
- Yang, L.; Wu, B.; Zhang, T.; Liu, Z.; Zhang, J. Preparation, Crystal Structure, Thermal Decomposition, and Explosive Properties of [Cd(en)(N3)2]n. Propellants Explos. Pyrotech. 2010, 35, 521–528. [Google Scholar] [CrossRef]
- Etaiw, S.E.H.; El-Bendary, M.M.; Abdelazim, H. Synthesis, Characterization, and Biological Activity of Cd(II) and Mn(II) Coordination Polymers Based on Pyridine-2,6-Dicarboxylic Acid. Russ. J. Coord. Chem. 2017, 43, 320–330. [Google Scholar] [CrossRef]
- da Silva, P.B.; Terra, P.H.; Frem, R.C.; Netto, A.V.; Mauro, A.E. Synthesis, Characterization, and Investigation of the Thermal Behavior of Cu(II) Pyrazolyl Complexes. J. Therm. Anal. Calorim. 2011, 106, 495–499. [Google Scholar] [CrossRef]
- Liu, M.S.; Yu, Q.Y.; Cai, Y.P.; Su, C.Y.; Lin, X.M.; Zhou, X.X.; Cai, J.W. One-, Two-, and Three-Dimensional Lanthanide Complexes Constructed from Pyridine-2,6-Dicarboxylic Acid and Oxalic Acid Ligands. Cryst. Growth Des. 2008, 8, 4083–4091. [Google Scholar] [CrossRef]
- Alkaya, Z.A.; Abdusalamov, J.; İlkimen, H.; Gulbandilar, A.; Sari, M.; Cevik, S. Synthesis, Characterization, and Antimicrobial Activity of V(IV) Complexes Containing 2,6-Pyridinedicarboxylate(DPA) Ligand. J. Mol. Struct. 2024, 1314, 138623. [Google Scholar] [CrossRef]
- SADABS, Version 2.05; Bruker AXS: Madison, WI, USA, 1999.
- Sheldrick, G.M. TWINABS, TWINABS 2012/1; Bruker: Madison, WI, USA, 2012.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. Diamond 3.1f; Crystal Impact GbR: Bonn, Germany, 2008. [Google Scholar]
- Ahlrichs, R.; Bar, M.; Hacer, M.; Horn, H.; Kömel, C. Electronic Structure Calculations on Workstation Computers: The Program System TURBOMOLE. Chem. Phys. Lett. 1989, 162, 165. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods Without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H–Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules. Chem. Rev. 1991, 91, 893. [Google Scholar] [CrossRef]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Non-Covalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170. [Google Scholar] [CrossRef]


| D–H⋯A | d(D–H) | d(D⋯A) | d(H⋯A) | (DHA) |
|---|---|---|---|---|
| Compound 1 | ||||
| C14–H14⋯O20 | 0.95 | 3.58 | 2.73 | 150.8 |
| C21–H21⋯O6 | 0.95 | 3.27 | 2.51 | 136.4 |
| N2–H2A′⋯O14 | 0.91 | 2.86 | 1.97 | 167.4 |
| O2–H2A⋯O15 | 0.88 | 2.67 | 1.87 | 151.5 |
| O3–H3A⋯O11 | 0.87 | 2.75 | 1.90 | 163.6 |
| O4–H4A⋯O12 | 0.88 | 2.65 | 1.83 | 155.3 |
| N9–H9A⋯O12 | 0.91 | 3.16 | 2.26 | 170.1 |
| N10–H10A⋯O19 | 0.91 | 3.19 | 2.36 | 153.3 |
| C1–H1D⋯O26 | 0.99 | 3.65 | 3.00 | 123.4 |
| O26–H26A⋯O19 | 0.87 | 2.77 | 1.93 | 163.2 |
| O25–H25B⋯O17 | 0.87 | 2.69 | 1.84 | 167.6 |
| O25–H25A⋯O24 | 0.87 | 2.78 | 1.92 | 177.6 |
| O24–H24B⋯O21 | 0.87 | 2.74 | 1.89 | 162.4 |
| N9–H9B⋯O18 | 0.91 | 3.05 | 2.16 | 169.1 |
| C34–H34B⋯O17 | 0.99 | 3.58 | 2.72 | 145.6 |
| O19–H19B⋯O26 | 0.87 | 2.77 | 1.93 | 163.3 |
| C22–H22⋯O6 | 0.95 | 3.36 | 2.93 | 108.9 |
| O23–H23A⋯O22 | 0.87 | 2.76 | 1.89 | 170.6 |
| O23–H23B⋯O7 | 0.87 | 2.74 | 2.04 | 136.4 |
| O24–H24A⋯O7 | 0.87 | 2.83 | 1.98 | 167.2 |
| O22–H22A⋯O13 | 0.87 | 2.68 | 1.82 | 171.1 |
| O22–H22B⋯O20 | 0.87 | 2.70 | 1.83 | 177.0 |
| Compound 2 | ||||
| C5–H5⋯O10 | 0.95 | 3.63 | 2.84 | 142.2 |
| C4–H4A⋯N1 | 0.98 | 3.54 | 2.64 | 153.0 |
| C7–H7A⋯O10 | 0.97 | 3.51 | 2.63 | 149.9 |
| N2–H2⋯O10 | 0.88 | 2.79 | 1.96 | 156.6 |
| Parameters | 1 | 2 |
|---|---|---|
| Formula | C34H56N10Ni4O26 | C17H19N5O4Zn |
| Formula weight | 1255.72 | 422.74 |
| Temp, [K] | 100(2) | 100(2) |
| Crystal system | Triclinic | Monoclinic |
| Space group | C2/c | |
| a, [Å] | 8.9375(7) | 13.9543(16) |
| b, [Å] | 12.8596(10) | 9.4508(11) |
| c, [Å] | 21.1687(17) | 13.968(2) |
| α, [°] | 85.416(3) | 90 |
| β, [°] | 82.686(2) | 111.638(2) |
| γ, [°] | 89.928(3) | 90 |
| V [Å3] | 2405.4(3) | 1712.3(4) |
| Z | 2 | 4 |
| Absorption coefficient (mm−1) | 2.641 | 2.315 |
| F(0 0 0) | 1300 | 872 |
| D (calcd), [Mg/m3] | 1.734 | 1.640 |
| Index ranges | −10 ≤ h ≤ 10, −15 ≤ k ≤ 15, −0 ≤ l ≤ 25 | −16 ≤ h ≤ 16, −11 ≤ k ≤ 11, −16 ≤ l ≤ 16 |
| Crystal size, [mm3] | 0.31 × 0.28 × 0.15 | 0.21 × 0.16 × 0.13 |
| θ range, [°] | 6.10 to 68.28 | 3.40 to 68.17 |
| Independent Reflections | 8443 | 1491 |
| Reflections collected | 8519 | 1489 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 8519/0/692 | 1491/0/126 |
| Goodness-of-fit on F2 | 1.085 | 1.175 |
| Final R indices [I >2σ(I)] | R1 = 0.0559, wR2 = 0.1573 | R1 = 0.0419, wR2 = 0.1139 |
| R indices (all data) | R1 = 0.0563, wR2 = 0.1577 | R1 = 0.0419, wR2 = 0.1139 |
| Largest hole and peak [e·Å−3] | 1.27 and −0.77 | 0.48 and −0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, K.K.; Sharma, P.; Banik, S.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Bhattacharyya, M.K. Unusual Metal–organic Multicomponent Ni(II) and Mononuclear Zn(II) Compounds Involving Pyridine dicarboxylates: Supramolecular Assemblies and Theoretical Studies. Inorganics 2024, 12, 267. https://doi.org/10.3390/inorganics12100267
Dutta KK, Sharma P, Banik S, Gomila RM, Frontera A, Barcelo-Oliver M, Bhattacharyya MK. Unusual Metal–organic Multicomponent Ni(II) and Mononuclear Zn(II) Compounds Involving Pyridine dicarboxylates: Supramolecular Assemblies and Theoretical Studies. Inorganics. 2024; 12(10):267. https://doi.org/10.3390/inorganics12100267
Chicago/Turabian StyleDutta, Kamal K., Pranay Sharma, Subham Banik, Rosa M. Gomila, Antonio Frontera, Miquel Barcelo-Oliver, and Manjit K. Bhattacharyya. 2024. "Unusual Metal–organic Multicomponent Ni(II) and Mononuclear Zn(II) Compounds Involving Pyridine dicarboxylates: Supramolecular Assemblies and Theoretical Studies" Inorganics 12, no. 10: 267. https://doi.org/10.3390/inorganics12100267
APA StyleDutta, K. K., Sharma, P., Banik, S., Gomila, R. M., Frontera, A., Barcelo-Oliver, M., & Bhattacharyya, M. K. (2024). Unusual Metal–organic Multicomponent Ni(II) and Mononuclear Zn(II) Compounds Involving Pyridine dicarboxylates: Supramolecular Assemblies and Theoretical Studies. Inorganics, 12(10), 267. https://doi.org/10.3390/inorganics12100267









