Enhancements in Hydrogen Storage Properties of Magnesium Hydride Supported by Carbon Fiber: Effect of C–H Interactions
Abstract
1. Introduction
2. Results and Discussion
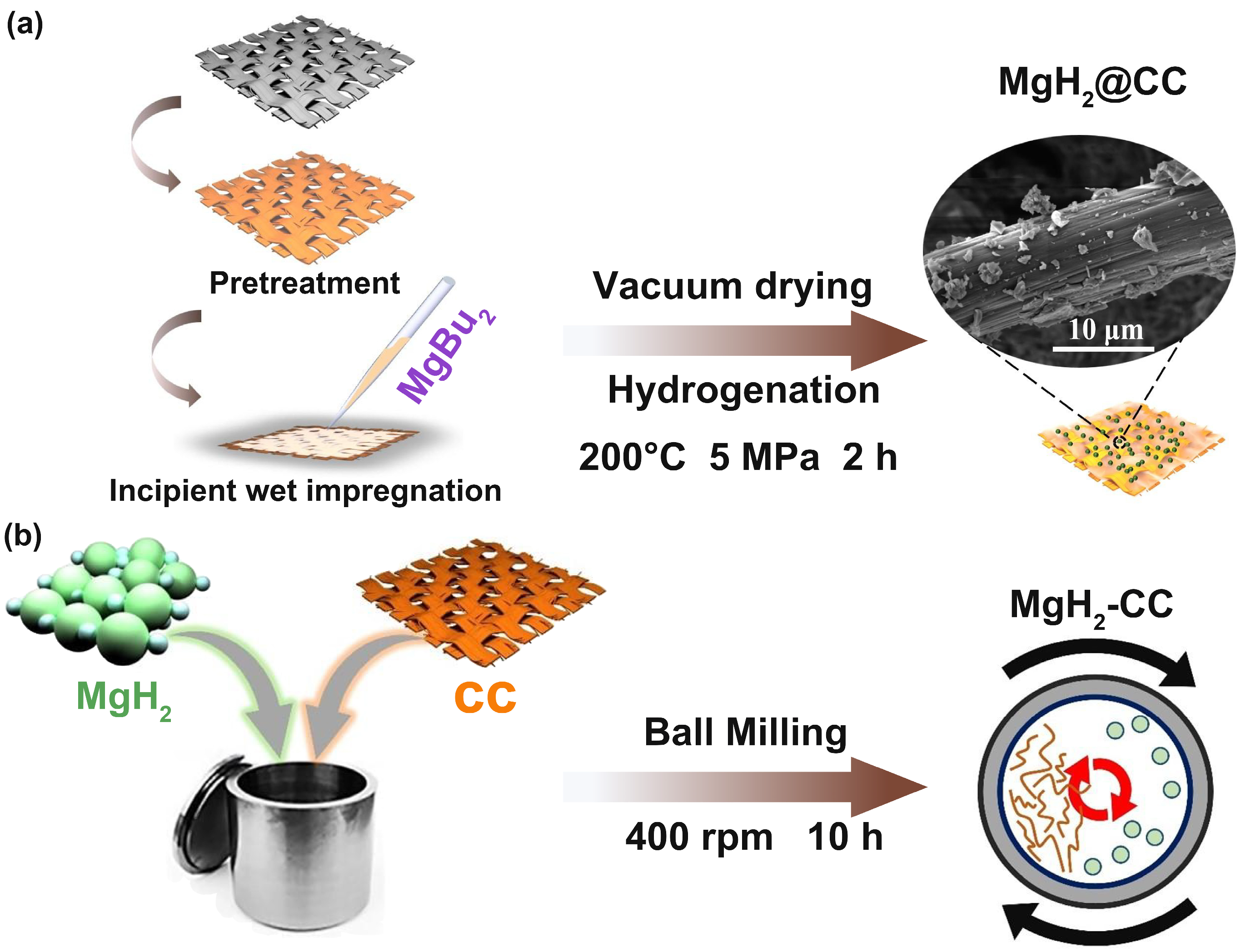
2.1. Structural Features and Microstructures Induced by Different Processes
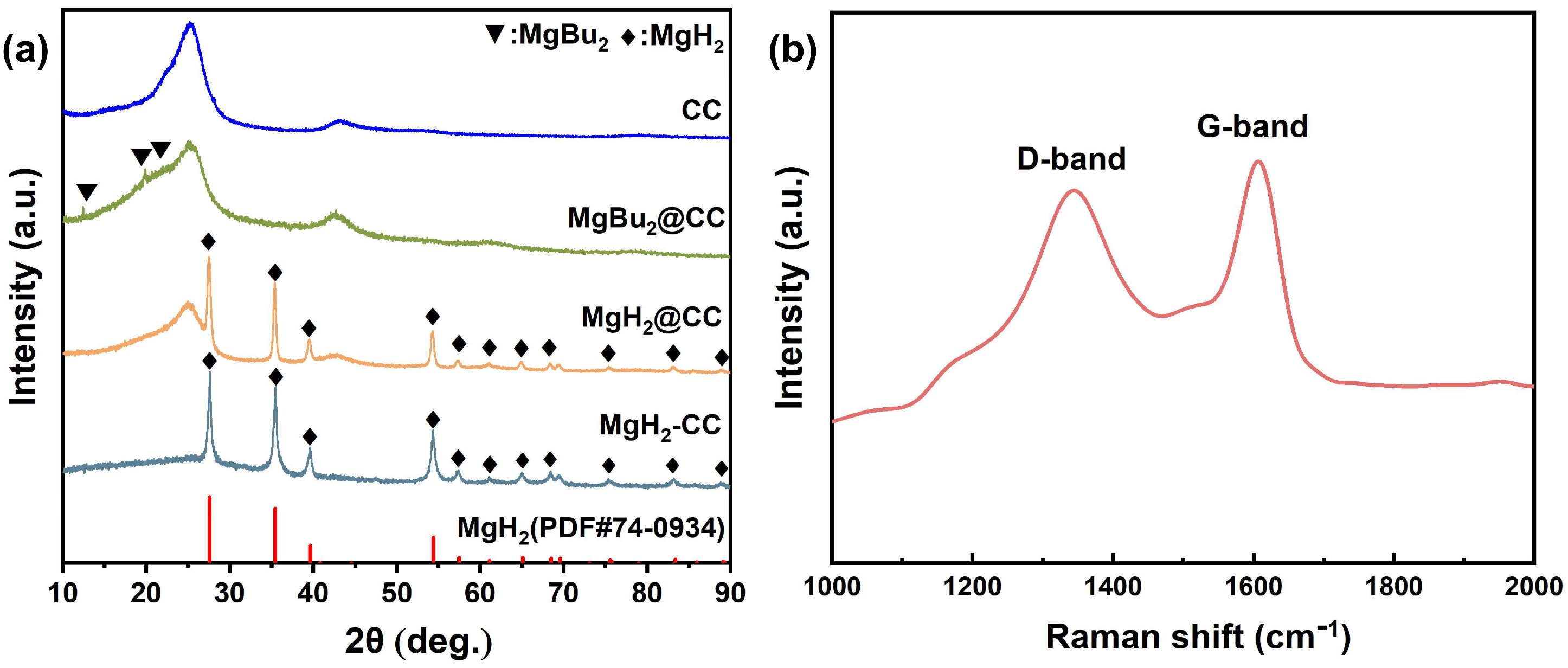
2.1.1. Structural Features of MgH2@CC and MgH2-CC Composites
2.1.2. Distinct Morphologies of MgH2@CC and MgH2-CC Composites
2.2. Improved Hydrogen Storage Performance
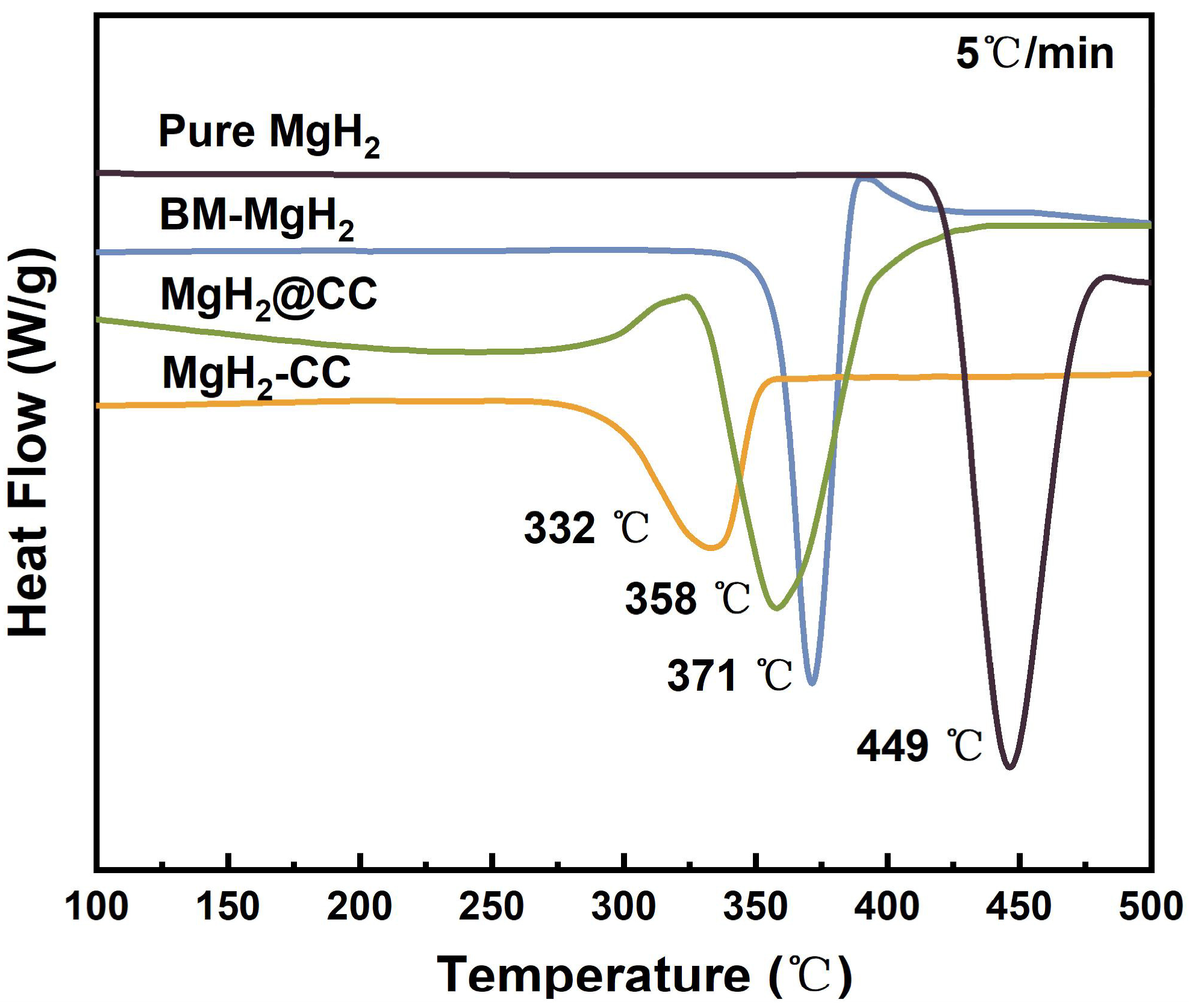
2.2.1. Kinetic Properties of MgH2@CC and MgH2-CC Composites
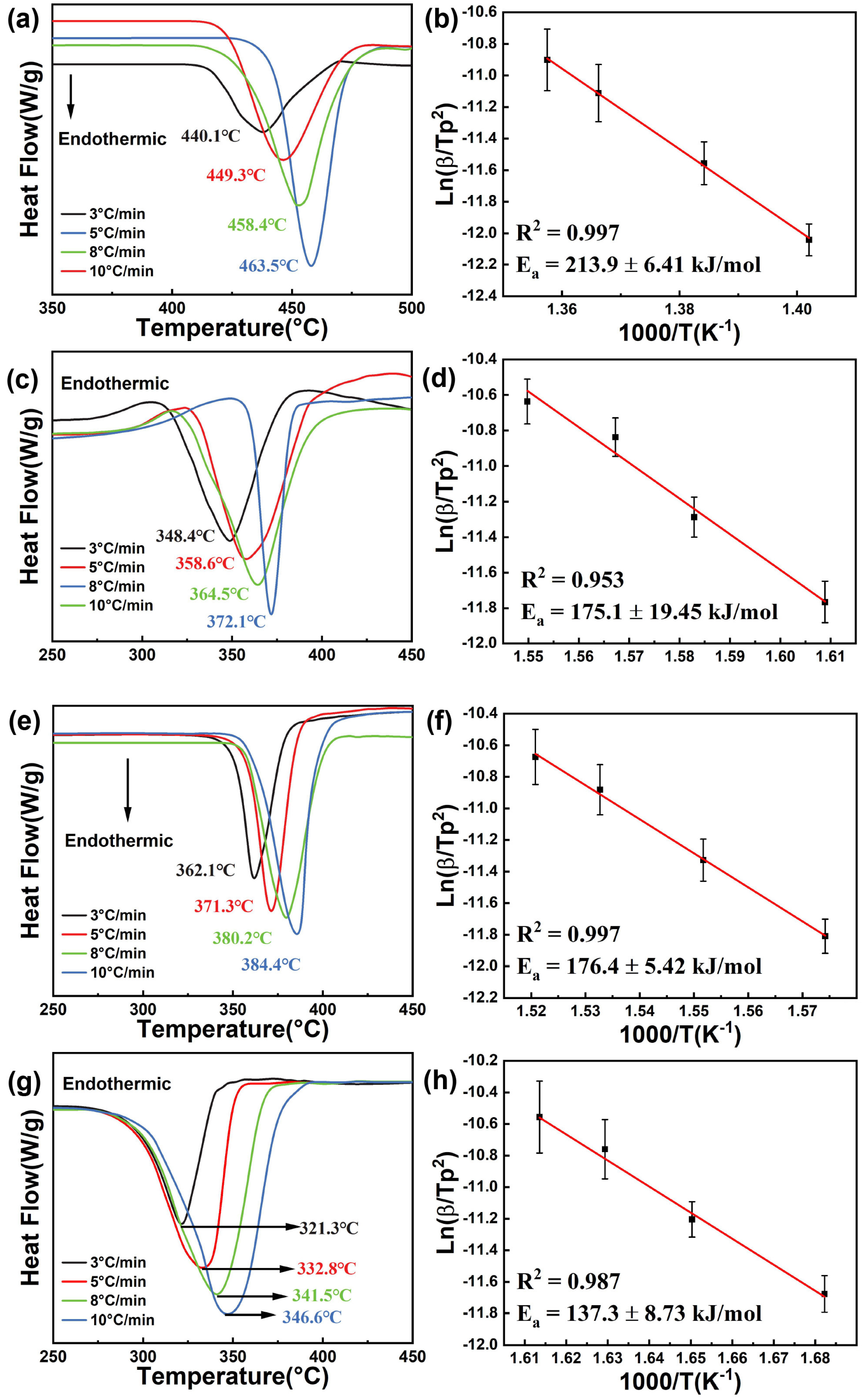
2.2.2. Thermodynamic Properties of MgH2@CC and MgH2-CC Composites
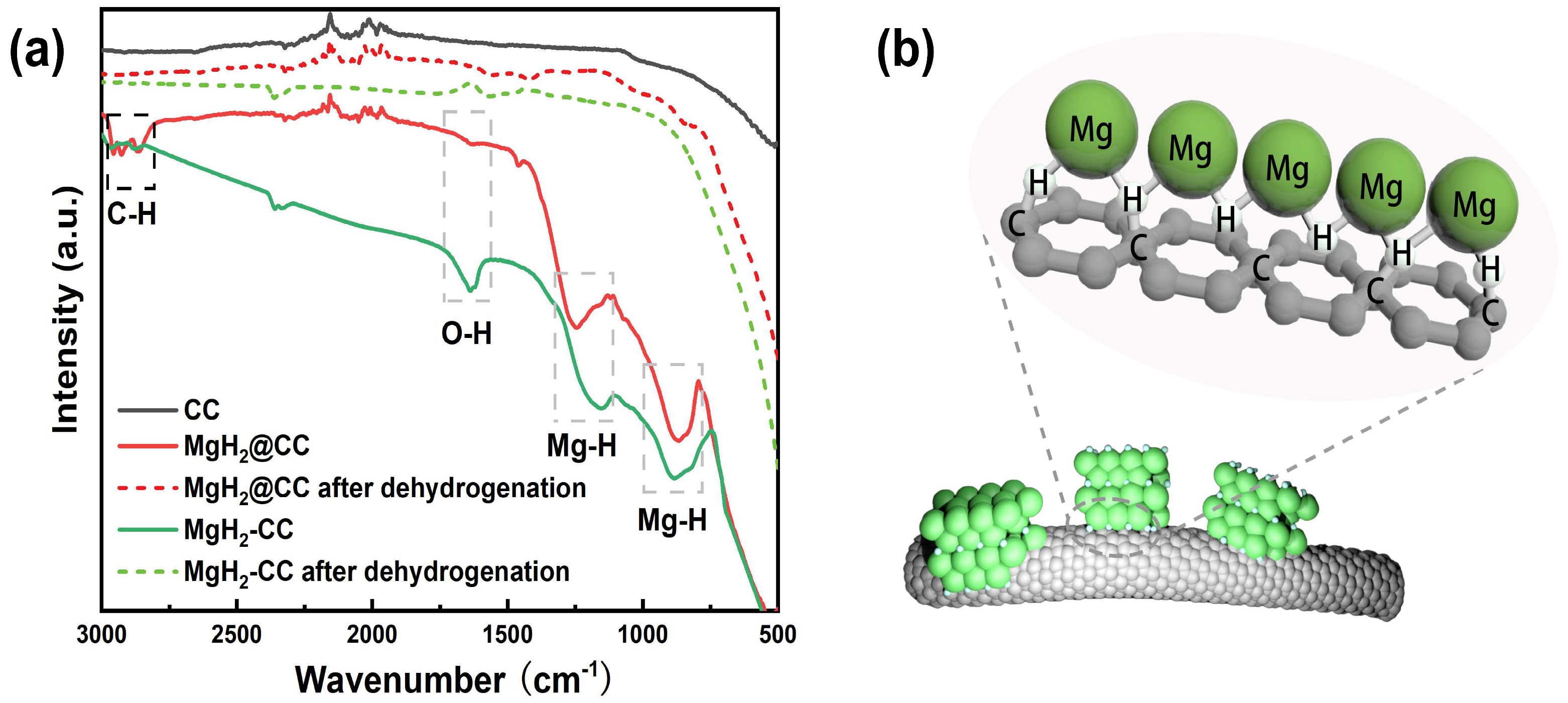
2.3. Mechanism Understanding
3. Experimental
3.1. Material Preparation
3.2. Material Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Li, Z.; Wu, Y.; Guo, X.; Ye, J.; Yuan, B.; Wang, S.; Jiang, L. Recent Advances on the Thermal Destabilization of Mg-Based Hydrogen Storage Materials. RSC Adv. 2019, 9, 408–428. [Google Scholar] [CrossRef] [PubMed]
- Bolarin, J.A.; Zou, R.; Li, Z.; Munyentwali, A.; Zhang, Z.; Cao, H. Recent Path to Ultrafine Mg/MgH2 Synthesis for Sustainable Hydrogen Storage. Int. J. Hydrogen Energy 2024, 52, 251–274. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Zhang, Y.; Wu, Z.; Zhang, Z.; Zhao, F.; Huot, J.; Grobivć Novaković, J.; Novaković, N. Recent Progress on the Development of High Entropy Alloys (HEAs) for Solid Hydrogen Storage: A Review. Int. J. Hydrogen Energy 2022, 47, 11236–11249. [Google Scholar] [CrossRef]
- Baum, Z.J.; Diaz, L.L.; Konovalova, T.; Zhou, Q.A. Materials Research Directions Toward a Green Hydrogen Economy: A Review. ACS Omega 2022, 7, 32908–32935. [Google Scholar] [CrossRef]
- Lu, Y.; Kim, H.; Sakaki, K.; Hayashi, S.; Jimura, K.; Asano, K. Destabilizing the Dehydrogenation Thermodynamics of Magnesium Hydride by Utilizing the Immiscibility of Mn with Mg. Inorg. Chem. 2019, 58, 14600–14607. [Google Scholar] [CrossRef]
- Qin, Z.-K.; He, L.-Q.; Ding, X.-L.; Si, T.-Z.; Cui, P.; Li, H.-W.; Li, Y.-T. Liquid Channels Built-In Solid Magnesium Hydrides for Boosting Hydrogen Sorption. Inorganics 2023, 11, 216. [Google Scholar] [CrossRef]
- Chen, Y.; Habibullah; Xia, G.; Jin, C.; Wang, Y.; Yan, Y.; Chen, Y.; Gong, X.; Lai, Y.; Wu, C. Hydrogen Storage Properties of Economical Graphene Materials Modified by Non-Precious Metal Nickel and Low-Content Palladium. Inorganics 2023, 11, 251. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Ding, Z. Carbon-Based Materials for Mg-Based Solid-State Hydrogen Storage Strategies. Int. J. Hydrogen Energy 2024, 69, 645–659. [Google Scholar] [CrossRef]
- Floriano, R.; Zepon, G.; Edalati, K.; Fontana, G.L.B.G.; Mohammadi, A.; Ma, Z.; Li, H.-W.; Contieri, R.J. Hydrogen Storage in TiZrNbFeNi High Entropy Alloys, Designed by Thermodynamic Calculations. Int. J. Hydrogen Energy 2020, 45, 33759–33770. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ikeda, Y.; Edalati, P.; Mito, M.; Grabowski, B.; Li, H.-W.; Edalati, K. High-Entropy Hydrides for Fast and Reversible Hydrogen Storage at Room Temperature: Binding-Energy Engineering via First-Principles Calculations and Experiments. Acta Mater. 2022, 236, 118117. [Google Scholar] [CrossRef]
- Cao, W.; Ding, X.; Chen, R.; Zhang, J.; Zhang, Y.; Guo, J.; Fu, H. Dual Tuning of the De-/Hydrogenation Thermodynamics and Kinetics of the Mg–Ni Alloy by Introducing the Ag–H Bond: Enhanced Hydrogen Storage Properties at Moderate Temperatures. J. Mater. Chem. A 2023, 11, 20761–20773. [Google Scholar] [CrossRef]
- Panigrahi, P.K.; Chandu, B.; Motapothula, M.R.; Puvvada, N. Potential Benefits, Challenges and Perspectives of Various Methods and Materials Used for Hydrogen Storage. Energy Fuels 2024, 38, 2630–2653. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Bowman, R.C.; Fang, Z.Z. Roles of Ti-Based Catalysts on Magnesium Hydride and Its Hydrogen Storage Properties. Inorganics 2021, 9, 36. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Lu, J.; Zhang, X. Thermodynamic and Kinetic Destabilization of Magnesium Hydride Using Mg–In Solid Solution Alloys. J. Am. Chem. Soc. 2013, 135, 10982–10985. [Google Scholar] [CrossRef] [PubMed]
- Kefi, C.; Huot, J. Entropy-Enthalpy Compensation in Ti-V-Mn-Cr BCC Alloys Used as Hydrogen Storage Materials. Inorganics 2023, 11, 479. [Google Scholar] [CrossRef]
- Zhang, Q.; Fang, M.; Si, T.; Fang, F.; Sun, D.; Ouyang, L.; Zhu, M. Phase Stability, Structural Transition, and Hydrogen Absorption−Desorption Features of the Polymorphic La4MgNi19 Compound. J. Phys. Chem. C 2010, 114, 11686–11692. [Google Scholar] [CrossRef]
- Zhou, C.; Peng, Y.; Zhang, Q. Growth Kinetics of MgH2 Nanocrystallites Prepared by Ball Milling. J. Mater. Sci. Technol. 2020, 50, 178–183. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Liu, D.D.; Wang, Q.Q.; Fang, F.; Sun, D.L.; Ouyang, L.Z.; Zhu, M. Superior Hydrogen Storage Kinetics of Mg12YNi Alloy with a Long-Period Stacking Ordered Phase. Scr. Mater. 2011, 65, 233–236. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Fang, F.; Sun, D.; Zhang, Q. Hydrogen-Induced Magnesium–Zirconium Interfacial Coupling: Enabling Fast Hydrogen Sorption at Lower Temperatures. J. Mater. Chem. A 2017, 5, 5067–5076. [Google Scholar] [CrossRef]
- Zhang, K.; Chang, Y.; Lei, J.; Chen, J.; Si, T.; Ding, X.; Cui, P.; Li, H.-W.; Zhang, Q.; Li, Y. Synergy of inside Doped metals–Outside Coated Graphene to Enhance Hydrogen Storage in Magnesium-Based Alloys. J. Magnes. Alloys 2024, 12, 2462–2471. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Ares-Fernández, J.-R. Hydrogen in Magnesium: New Perspectives toward Functional Stores. Energy Environ. Sci. 2010, 3, 526. [Google Scholar] [CrossRef]
- Lotoskyy, M.; Denys, R.; Yartys, V.A.; Eriksen, J.; Goh, J.; Nyamsi, S.N.; Sita, C.; Cummings, F. An Outstanding Effect of Graphite in Nano-MgH2–TiH2 on Hydrogen Storage Performance. J. Mater. Chem. A 2018, 6, 10740–10754. [Google Scholar] [CrossRef]
- Yan, S.; Wei, L.; Gong, Y.; Yang, K. Enhanced Hydrogen Storage Properties of Magnesium Hydride by Multifunctional Carbon-Based Materials: A Review. Int. J. Hydrogen Energy 2024, 55, 521–541. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering Hydrogen Storage Performance of MgH2 by Nanoengineering and Nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Realizing 6.7 Wt% Reversible Storage of Hydrogen at Ambient Temperature with Non-Confined Ultrafine Magnesium Hydrides. Energy Environ. Sci. 2021, 14, 2302–2313. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, S.; Xiao, X.; Chen, M.; Sun, C.; Yao, Z.; Hu, Z.; Chen, L. Novel 1D Carbon Nanotubes Uniformly Wrapped Nanoscale MgH2 for Efficient Hydrogen Storage Cycling Performances with Extreme High Gravimetric and Volumetric Capacities. Nano Energy 2019, 61, 540–549. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Ali, N.; Al-Ajmi, F.; Banyan, M.; Al-Duweesh, A.A. Hydrogen Storage Behavior and Performance of Multiple Cold-Rolled MgH2/Nb2O5 Nanocomposite Powders. Processes 2022, 10, 1017. [Google Scholar] [CrossRef]
- Maafa, I.M.; Zouli, N.; Abutaleb, A.; Yousef, A.; Qudsieh, I.Y.; Matar, S.M.; Adam, A.S.M.; El-Halwany, M.M. In Situ Preparation of 2D Co-B Nanosheets@1D TiO2 Nanofibers as a Catalyst for Hydrogen Production from Sodium Borohydride. Inorganics 2023, 11, 342. [Google Scholar] [CrossRef]
- Mehrabi, M.; Reyhani, A.; Parvin, P.; Mortazavi, S.Z. Surface Structural Alteration of Multi-Walled Carbon Nanotubes Decorated by Nickel Nanoparticles Based on Laser Ablation/Chemical Reduction Methods to Enhance Hydrogen Storage Properties. Int. J. Hydrogen Energy 2019, 44, 3812–3823. [Google Scholar] [CrossRef]
- Mehrabi, M.; Parvin, P.; Reyhani, A.; Mortazavi, S.Z. Hybrid Laser Ablation and Chemical Reduction to Synthesize Ni/Pd Nanoparticles Decorated Multi-Wall Carbon Nanotubes for Effective Enhancement of Hydrogen Storage. Int. J. Hydrogen Energy 2018, 43, 12211–12221. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Sun, L.-X.; Xu, F.; Luo, Y.-M.; Xia, Y.-P.; Wei, S.; Zhang, C.-C.; Cheng, R.-G.; Ye, C.-F.; Liu, M.-Y.; et al. Modulated Noble Metal/2D MOF Heterostructures for Improved Hydrogen Storage of MgH2. Rare Met. 2024, 43, 1672–1685. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Ma, T.; Li, K.; Ye, F.; Wang, X.; Jiao, L.; Yuan, H.; Wang, Y. Facile Synthesis of Small MgH2 Nanoparticles Confined in Different Carbon Materials for Hydrogen Storage. J. Alloys Compd. 2020, 825, 153953. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, J.; Zeng, X.; Wu, X.; Tian, H.; Ding, W.; Wang, J.; Walter, A. Study on Hydrogen Storage Properties of Mg Nanoparticles Confined in Carbon Aerogels. Int. J. Hydrogen Energy 2013, 38, 5302–5308. [Google Scholar] [CrossRef]
- Satawara, A.M.; Shaikh, G.A.; Gupta, S.K.; Andriotis, A.N.; Menon, M.; Gajjar, P.N. An Ab-Initio Analysis of the Hydrogen Storage Behaviour of V Doped Si2BN Nanotube. Int. J. Hydrogen Energy 2024, 52, 1560–1567. [Google Scholar] [CrossRef]
- Abutaleb, A. Synthesis of Copper/Sulfur Co-Doped TiO2-Carbon Nanofibers as Catalysts for H2 Production via NaBH4 Hydrolysis. Inorganics 2023, 11, 352. [Google Scholar] [CrossRef]
- Zeng, L.; Qing, P.; Cai, F.; Huang, X.; Liu, H.; Lan, Z.; Guo, J. Enhanced Hydrogen Storage Properties of MgH2 Using a Ni and TiO2 Co-Doped Reduced Graphene Oxide Nanocomposite as a Catalyst. Front. Chem. 2020, 8, 207. [Google Scholar] [CrossRef]
- Boateng, E.; Thiruppathi, A.R.; Hung, C.-K.; Chow, D.; Sridhar, D.; Chen, A. Functionalization of Graphene-Based Nanomaterials for Energy and Hydrogen Storage. Electrochim. Acta 2023, 452, 142340. [Google Scholar] [CrossRef]
- Mahamiya, V.; Shukla, A.; Chakraborty, B. Scandium Decorated C24 Fullerene as High Capacity Reversible Hydrogen Storage Material: Insights from Density Functional Theory Simulations. Appl. Surf. Sci. 2022, 573, 151389. [Google Scholar] [CrossRef]
- EL-Barbary, A.A.; Shabi, A.H. Stone-Wales Defective C60 Fullerene for Hydrogen Storage. Int. J. Hydrogen Energy 2024, 71, 155–164. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.-H. MXenes: Emerging 2D Materials for Hydrogen Storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Huang, T.; Huang, X.; Hu, C.; Wang, J.; Liu, H.; Xu, H.; Sun, F.; Ma, Z.; Zou, J.; Ding, W. MOF-Derived Ni Nanoparticles Dispersed on Monolayer MXene as Catalyst for Improved Hydrogen Storage Kinetics of MgH2. Chem. Eng. J. 2021, 421, 127851. [Google Scholar] [CrossRef]
- Su, Y.; Yang, M.; Wang, X.; Tian, F.; Jia, H.; Li, L. Synthesis of Ni Nanoparticles Embedded Porous Mo2C Nanospheres for Enhancing the Electrochemical Hydrogen Storage Properties of Co2B Material. J. Alloys Compd. 2023, 954, 170143. [Google Scholar] [CrossRef]
- Tseng, F.-G.; Bhalothia, D.; Lo, K.-H.; Syu, C.-H.; Chen, Y.-C.; Sihag, A.; Wang, C.-W.; Chen, H.-Y.T.; Chen, T.-Y. Glucose-Based Highly-Porous Activated Carbon Nanospheres (g-ACNSs) for High Capacity Hydrogen Storage. Energy Adv. 2024, 3, 1283–1292. [Google Scholar] [CrossRef]
- Alsabawi, K.; Webb, T.A.; Gray, E.; Webb, C.J. The Effect of C60 Additive on Magnesium Hydride for Hydrogen Storage. Int. J. Hydrogen Energy 2015, 40, 10508–10515. [Google Scholar] [CrossRef]
- Lakhnik, A.; Kirian, I.; Rud, A. Comparative Analysis of Hydrogen Uptake and Release Kinetics in MgH2/C Composites Synthesized Using Varied Surface Areas Graphite Powders. Appl. Phys. A 2024, 130, 283. [Google Scholar] [CrossRef]
- Guo, S.; Yu, Z.; Li, Y.; Fu, Y.; Zhang, Z.; Han, S. Preparation of Mg-Mg2Ni/C Composite and Its Excellent Hydrogen Storage Properties. J. Alloys Compd. 2024, 976, 173035. [Google Scholar] [CrossRef]
- Carson, R.M.; Ellis, B.L.; Long, F.; Persaud, S.Y. Structural Evolution of a Mg–C Composite over 1000 H2 Storage Cycles. Int. J. Hydrogen Energy 2024, 51, 676–687. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.; Liang, T.; Zhou, Y.; Guan, P.; Zhou, L.; Hu, L.; Wan, T.; Chu, D. Structural Optimization and Electrocatalytic Hydrogen Production Performance of Carbon-Based Composites: A Mini-Review. Carbon Trends 2024, 15, 100363. [Google Scholar] [CrossRef]
- Qiao, Z.; Bian, K.; Ding, C.; Zhao, Y. Recent Progress of Carbon-Fiber-Based Electrode Materials for Energy Storage. Diam. Relat. Mater. 2023, 138, 110208. [Google Scholar] [CrossRef]
- Fang, B.; Li, L.; Guo, J.; Qin, Y.; Wei, Y.; Zhang, J.; He, C.; Chen, Y. Development of a Novel Co-Doped Carbon Fiber from Textile Waste as Catalyst for the Highly Efficient Degradation of Organic Pollutants: The Key Role of C–O–Co Bond. Chem. Eng. J. 2024, 498, 155494. [Google Scholar] [CrossRef]
- Mishra, M.K.; Ghalsasi, P.; Deo, M.N.; Bhatt, H.; Poswal, H.K.; Ghosh, S.; Ganguly, S. In Situ High Pressure Study of an Elastic Crystal by FTIR Spectroscopy. CrystEngComm 2017, 19, 7083–7087. [Google Scholar] [CrossRef]
- Amaral, M.M.; Yukuhiro, V.Y.; Vicentini, R.; Peterlevitz, A.C.; Da Silva, L.M.; Fernandez, P.; Zanin, H. Direct Observation of the CO2 Formation and C–H Consumption of Carbon Electrode in an Aqueous Neutral Electrolyte Supercapacitor by in-Situ FTIR and Raman. J. Energy Chem. 2022, 71, 488–496. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, T.; Zhou, S.; Liu, P.; Yu, H.; Han, Z. Confinement of Mg Nanoparticles by Bituminous Coal and Associated Synergistic Hydrogen Storage Effect. J. Mater. Eng Perform 2020, 29, 760–768. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Zhang, Q. Enhanced Hydrogen Desorption Properties of MgH2 by Highly Dispersed Ni: The Role of in-Situ Hydrogenolysis of Nickelocene in Ball Milling Process. J. Alloys Compd. 2022, 900, 163547. [Google Scholar] [CrossRef]
- Salamone, M.; Martin, T.; Milan, M.; Costas, M.; Bietti, M. Electronic and Torsional Effects on Hydrogen Atom Transfer from Aliphatic C–H Bonds: A Kinetic Evaluation via Reaction with the Cumyloxyl Radical. J. Org. Chem. 2017, 82, 13542–13549. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D.; Fagnoni, M. Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C–H Bonds Elaboration. Chem. Rev. 2022, 122, 1875–1924. [Google Scholar] [CrossRef]
- Fan, L.; Long, J.; Gu, Q.; Huang, H.; Lin, H.; Wang, X. Single-Site Nickel-Grafted Anatase TiO2 for Hydrogen Production: Toward Understanding the Nature of Visible-Light Photocatalysis. J. Catal. 2014, 320, 147–159. [Google Scholar] [CrossRef]

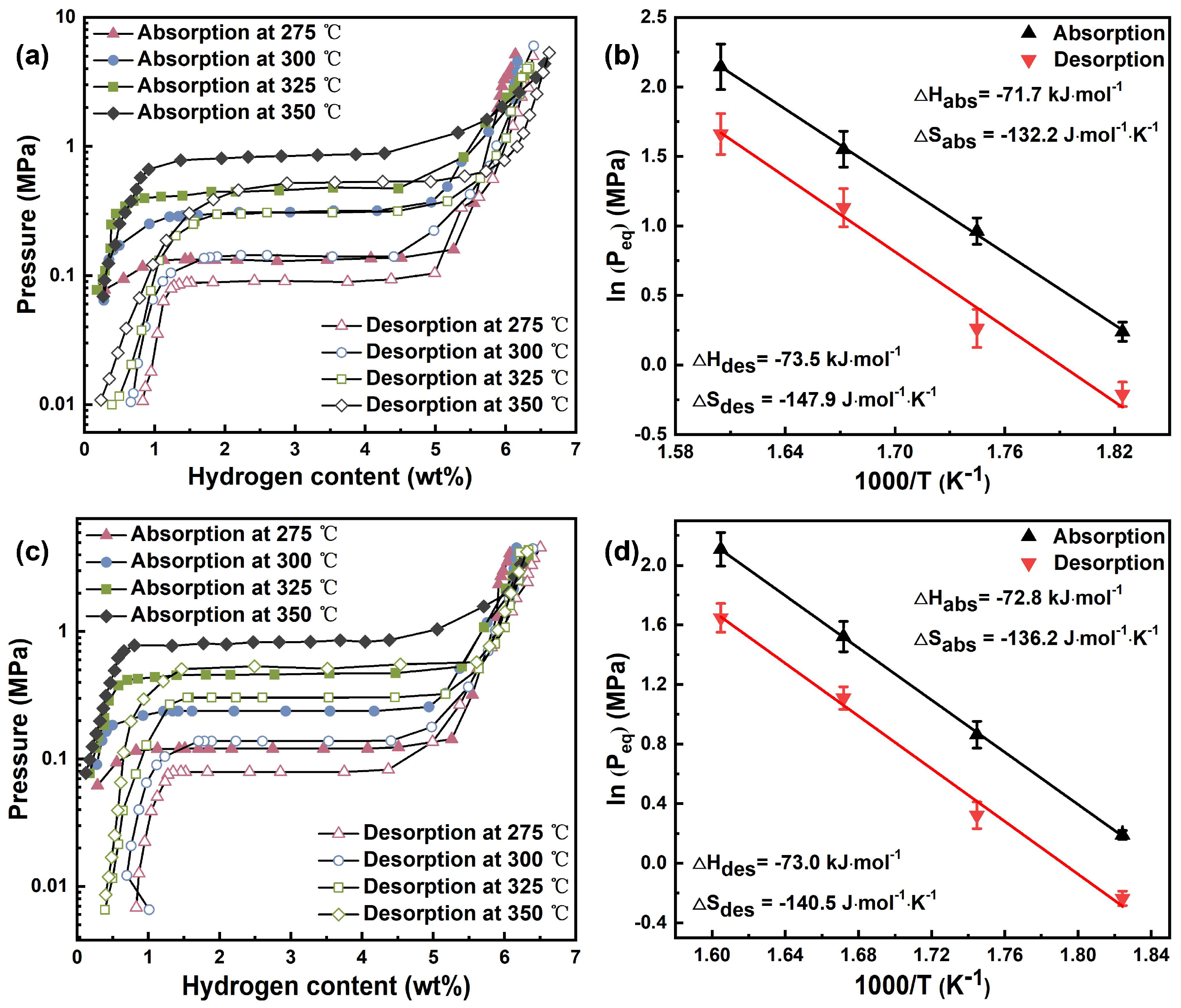
| Temperature (°C) | Absorption Plateau (MPa) | Desorption Plateau (MPa) | ||
|---|---|---|---|---|
| MgH2@CC | MgH2-CC | MgH2@CC | MgH2-CC | |
| 350 | 0.853 | 0.823 | 0.526 | |
| 325 | 0.472 | 0.458 | 0.310 | 0.303 |
| 300 | 0.262 | 0.237 | 0.130 | 0.138 |
| 275 | 0.127 | 0.121 | 0.081 | 0.079 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Jia, X.; Qin, Z.; Ding, X.; Li, Y. Enhancements in Hydrogen Storage Properties of Magnesium Hydride Supported by Carbon Fiber: Effect of C–H Interactions. Inorganics 2024, 12, 273. https://doi.org/10.3390/inorganics12110273
Yang Q, Jia X, Qin Z, Ding X, Li Y. Enhancements in Hydrogen Storage Properties of Magnesium Hydride Supported by Carbon Fiber: Effect of C–H Interactions. Inorganics. 2024; 12(11):273. https://doi.org/10.3390/inorganics12110273
Chicago/Turabian StyleYang, Quan, Xiansong Jia, Zhikang Qin, Xiaoli Ding, and Yongtao Li. 2024. "Enhancements in Hydrogen Storage Properties of Magnesium Hydride Supported by Carbon Fiber: Effect of C–H Interactions" Inorganics 12, no. 11: 273. https://doi.org/10.3390/inorganics12110273
APA StyleYang, Q., Jia, X., Qin, Z., Ding, X., & Li, Y. (2024). Enhancements in Hydrogen Storage Properties of Magnesium Hydride Supported by Carbon Fiber: Effect of C–H Interactions. Inorganics, 12(11), 273. https://doi.org/10.3390/inorganics12110273







