Monodentate Ligands in X-Cu(I)-Y Complexes—Structural Aspects
Abstract
1. Introduction
2. Structural Aspects of C-Cu(I)-Y (Y=HL, OL, NL, SL, Si L, BL, PL, Cl, Br, I, CAl, or CSn)
2.1. Structures of C-Cu(I)-Y (Y=HL, OL)
2.2. Structures of C-Cu(I)-Y (Y=NL, SL, SiL)
2.3. Structures of C-Cu(I)-Y (Y=BL, PL)
2.4. Structures of C-Cu(I)-Y (Y=Cl, Br, I, AlL, or SnL)
3. Structural Aspects of X-Cu(I)-Y (X, Y = Variable Combination of Donor Atoms)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-CH3py | 2-methyl pyridine |
| 2-Phpy | 2-phenyl pyridine |
| C2H3O2 | acetate |
| C4H8O | tetrahydrofuran |
| C5H10O2B | (5,5-dimethyl-1,3,2-dioxyborinan-2-yl) |
| C5H3SO2 | (thiophene-2-carboxylate) |
| C6H18Si2P | bis(trimethylsilyl)phosphine |
| C6H23N2BP | bis(diethylamino)phosphanido-borane) |
| C7H8 | toluene |
| C8H10N2B | (1,3-dimethyl-1,3,2-benzodiazaborol-2-yl) |
| C8H11Si | dimethyl(phenyl)silyl |
| C8H4N2 | (1,4-diisocyanobenzene) |
| C8H5N2O | (benzoyliminomethylene amino) |
| C8H7N4O | ((4-methylphenyl)sulfonyl)carbamic azido) |
| C8H7O3 | 4-methoxybenzoate |
| C9H16N2 | (1,3-bis(propan-2-yl)-imidazol-2-ylidene) |
| C11H10F4NO2 | (t-butyl(2,3,5,6-tetrafluorophenyl)carbamatato) |
| C11H20N2 | (1,3-di-t-butyl-imidazol-2-ylidene) |
| C11H21N2 | (4,5-dimethyl-1,3-bis(propan-2-yl)-2,3-dihydro-1H-imidazol-2-ylidene) |
| C11H21N2 | (u-N,N-dipropan-2-ylmethylimidamide)-(4.5-dimethyl-1,3-bis(propan-2-yl)-2,3-dihydro-1H-imidazol-3-ylidene) |
| C12D8N | (perdeutero-9H-carbazol-9-yl)(1,3-bis(2,4,6-trimethylpephonyl)imidazol-2-ylidene) |
| C12H8N | (9H-carbazol-9-yl) |
| C13H19N3O2 | (methyl-2-((bis((dimethylamino)methylidene)amino)benzoate) |
| C14H13O2 | (4-(4-methoxyphenyl)butanoate |
| C16H17PON | (1,1-diphenyl-N-(propan-2-yl)phosphanecarboxamidate) |
| C18H20N2 | (1,3-bis(2-methylphenyl)tetrahydropyrimiden-2-(1H)-ylidene |
| C18HBF15 | hydrido(tris(pentafluorophenyl)borate |
| C19H20N2 | (1,3-bis(2,6-dimethylphenyl)imidazol-2-ylidene) |
| C20H24N2S | (5-(4-(methylsulfanyl)phenyl)-1,3-di-isopropylbezimidazol-2-ylidene) |
| C20H31N | (1-(2,6-di-isopropylphenyl)-3,3′,5,5′-tetramethyl-pyrrolidin-2-ylidene) |
| C20H31N | 4,5-dimethyl-1,3-bis(propan-2-yl)-2,3-dihydro-1H-imidazol-2-ylidene) |
| C21H16N2 | (1,3-bis(3-phenylprop-2-yn-1-yl)2,3-dihydro-1H-imidazol-2-ylidene) |
| C21H24N2 | (1,3-bis(mesityl)imidazol-2-ylidene) |
| C21H24N2S | (1,3-bis(2,4,6-trimethylphenyl)1,3-dihydro-2H-imidazol-2-thione) |
| C21H24N2Se | (1,3-bis(2,6-(2,4,6-trimethylphenyl)1,3-dihydro-2H-imidazole-2-selone) |
| C21H24P | (tris(4-methylyphenylphosphine) |
| C21H26N2 | (1,3-dimesitylimidazolidin-2-ylidene) |
| C21H32N4 | (1-(2-(dimethylamino)ethyl)-3-(N-(2,6-bis(propan-2-yl)phenyl)ethaniminodoyl)-2,3dihydro-1H-imidazil-2-ylidene) |
| C22H21F5N2 | (1-(2,6-di-isopropylphenyl)-3-((pentafluorophenylmethyl)imidazol-2-ylidene) |
| C22H33N | (2-(2,6-di-isopropylphenyl)-1,4,5-trimethyl-2-azabiyclo [2.2.2]octan-3-ylidene |
| C22H35N | (1-(2,6-di-isopropylphenyl)-3,3-diethyl-5,5-dimethylpyrolidin-2-ylidene) |
| C22H36N | ((1-(2,6-di-isopropyl)-4,4-diethyl-2,2-dimethyl-3,4-dihydro-2H-pyrrol-1-ium |
| C22H39N4OP | (1,3-dicyclohexyl-4,6-bis(cycohexylimino)-2-oxo-1,3,5-diazaphophinan-5-yl) |
| C23H35N | (9H-carbazol-9-yl)-2-(2,6-di-isopropylphenyl)-3,3-dimethyl-2-araspirio |
| C24H26N2O2 | (1,3-dimesilyl-5,5-dimethyl-4,6-doxohexahydropyrimidin-2-yl) |
| C24H28N2O2 | (1,3-dimesityl-5,5-dimethyl-4,6-dioxotetrahydropyrimidin-2(1H)-ylidene) |
| C27H30N2 | (1-(2,6-bis(propan-2-yl)phenyl)-3-(1-naphalen-1-yl)ethyl)2,3-dihydro-1H-imidazol-2-yl) |
| C27H36N2 | (1,3-bis(2,6-di-isopropylphenyl)imidazol-2-ylidene) |
| C27H36N2O2 | (2,3-bis(2,6-diisopropylphenyl)amino)acrylate) |
| C27H36N2S | (1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-imidazol-2-thione) |
| C27H37N2 | (1,3-bis(2,6-disopropylphenyl)imidazol-2-ylidene) |
| C27H38N2 | (1,3-bis(2,6-di-isopropylphenyl)-4,5-dihydroimidazol-2-ylidene) |
| C27H39N | (1-(2,6-di-isopropylphenyl)-5,5-dimethyl-2H-spirolpyrolidine-3,2-tricyclo [3.3.1.1.3,7]decan(2-ylidene) |
| C27H40N | (1-(2,6-diisopropylphenyl)-3,3-diethyl-5,5-dimethylpyrolidin-2-ylidene |
| C27H43N | (2-(2,6-bis(propan-2-yl)phenyl)-3,3,9-trimethyl-6-propan-2-yl)-2-azaspiro(4,5)decan-1-ylidene |
| C27H46N2O3Si | (1-(2,6-diisopropylphenyl)-3-(3-(trisopropoxysilyl)propyl)imidazol-2-ylidene) |
| C28H39N2 | (1,3-bis[2,6-bis(propan-2-yl)phenyl)-1,3-diazinan-2-ylidene) |
| C28H40N2 | (1,3-bis[2,6-bis(propan-2-yl)phenyl]tetrahydro-pyrimidin-2(1H)-ylidene) |
| C30H42N4 | (2-(N,N-bis(2,6-diisopropylphenyl))carbammidoyl)-1,3-dimethyl-1H-imidazol-3-iumato) |
| C30H42N6 | (4-((2-azidoethyl)(methyl)amino)-1,3-bis(2,6-di-isopropylphenyl)-2,3-dihydro-1H-imidazol-2-ilydene) |
| C30H49N2Si2Al | ((ethane-1,2-diyl)bis(N-[2,6-bis(propan-2-yl)phenyl))-1,1-dimethylsilanamino))aluminium |
| C30H50N2Si2Al | ((ethane-1,2-diyl)bis(N-[2,6-bis(popan-2-yl)phenyl)1,1-dimethylsilanamino))aluminium) |
| C30H56B11N3 | (2-(3-t-butyl-2-(2,6-di-isopropylphenyl)-1-(1,3-di-isopropyl-4,5-dimethylimidazol-2-ylidene)-2,1-azaborinyl)1,2-dicarbo-closo-dodecaboran-2-yl) |
| C31H32N2 | (2,4-dimesityl-1,2,4,5-tetrahydro-3H-naphtho[1,8-ef] [1,3]diazoein-3-ylidene) |
| C31H38N2 | (1,3-bis(2,6-bis(propan-2-yl)phenyl-2,3-dihydro-1H-benzimidazol-2-ylidene) |
| C32H31N | (2-(2,6-bis(propan-2-yl)phenyl)-3,3-diphenyl-2,3-dihydro-1H-isoindol-1-ylidene) |
| C35H36N2 | (2,3-bis(bis(1-phenylethyl)amino)cycloprop-2-en-1-ylidene) |
| C37H63AlN4Si2 | (ethane-1,2-diyl)bis(N-[2,6-bis(propan-2-yl)phenyl]-1,1-dimethyl-silanamino)-aluminium) |
| C40H47N2Cl | (4-chloro-1,3-bis(2,6-di-isopropylphenyl)-5-methyl(diphenylphosphono)-imidazol-2-ylidene) |
| C40H47N2P | (2-((diphenylphosphanyl)-1,3-bis(2,6-bis(propan-2-yl)phenyl)2,3-dihydro-1H-imidazole) |
| C40H58N4 | (2-N,N-bis(2,6-diisopropylphenyl)(carbamimidoyl)-1,3-dicyclohexyl)-1H-imidazol-3-iumato) |
| C41H38N2O2 | (R,R)-(1,3-bis(8-(4-methocyphenyl)-1,2,3,4-tetrahydronaphthalen-1-yl)benzimidazol-2-ylidene) |
| C45H40N2 | (1,3-bis(2-(diphenylmethyl)-4,6-dimethyl-phenyl)-imidazol-2-ylidene) |
| C45H42N2 | (1,3-bis(2-(diphenylmethyl-4,6-dimethylphenyl)imidazolidin-2-ylidene) |
| C55H44N2 | (7,9-bis(4-(diphenylmethyl)-2,6-dimethylphenyl)-8,9-dihydro-7H-acenaphlo[1,2-d]imidazol-8-ylidene) |
| C60H84AlN4O | (u-N-(cyclohexyl)((cyclohexyl)amino)methanidamido)-(2,7-di-t-butyl-N4,N5-bis(2,6-bis(propan-2-yl)phenyl)-9,9-dimethyl-9H-xanthene-4,5-bis(amido)aluminium) |
| C69H56N2 | (1,3-bis(2,6-bis(diphenylmethyl)-4-methylphenyl)imidazol-2-ylidene) |
| C69H56N2S | (1,3-bis(2,6-bis(diphenylmethyl)-4-methylphenyl)-1,3-dihydro-2H-imidazol-2-thione |
| C69H56N2Se | (1,3-bis(2,6-bis(diphenylmethyl)-4-methylphenyl)-1,3-dihydro-2H-imidazole-2-selone |
| CHO2 | formate |
| Ph2P | diphenylphophido |
| Ph3Si | triphenylsilyl |
| Ph3SiO | triphenylsilanolate |
| PhCOO | benzoate |
| py | pyridine |
| t-Bu3 P=N | (tri-t-butylphosphanylidene)azanide |
| t-Bu3P | tri-t-bitylphosphine |
References
- Österberg, R. Models for copper-protein interaction based on solution and crystal structure studies. Coord. Chem. Rev. 1974, 12, 309–347. [Google Scholar] [CrossRef]
- Colman, P.M.; Freeman, H.C.; Guss, J.M.; Murata, M.; Norris, B.A.; Ramshaw, J.A.M.; Venkatappa, M.P. X-ray crystal structure analysis of plastocyanin at 2.7 Å resolution. Nature 1978, 272, 319–324. [Google Scholar] [CrossRef]
- Spiro, T.G. Copper Proteins, 1st ed.; Krieger Pub. Co. Publisher: Malabar, FL, USA, 1981; p. 376. [Google Scholar]
- Dockal, E.R.; Diaddario, L.L.; Glick, M.D.; Rorabacher, D.B. Structure of 1,4,8,11-tetrathiacyclotetradecanecopper(I) perchlorate: Comparative geometries of analogous copper(I) and copper(II) complexes. J. Am. Chem. Soc. 1977, 99, 4530–4532. [Google Scholar] [CrossRef]
- Baker, E.N.; Norris, G.E. Copper co-ordination to thioether ligands: Crystal and molecular structures of bis(2,5-dithiahexane)copper(II) bis(tetrafluoroborate) and bis(3,6-dithiaoctane)copper(I) tetrafluoroborates. J. Chem. Soc. Dalton Trans. 1977, 24, 877–882. [Google Scholar] [CrossRef]
- Olmstead, M.M.; Musker, W.K.; Kessler, R.M. Crystal structures and properties of bis(2,5-dithiahexane)copper(I) and copper(II) and mixed-valence complexes. Comparison of tetrahedral and distorted octahedral geometries of thioether-coordinated copper(II). Inorg. Chem. 1981, 20, 151–157. [Google Scholar] [CrossRef]
- Henriksson, H.A.; Sjöberg, B.; Österberg, R. Model structures for a copper(I)–copper(II) redox couple in copper proteins: X-ray powder structure of bis(imidazole)copper(I) perchlorate and crystal structure of bis(imidazole)copper(II) diacetate. J. Chem. Soc. Chem. Commun. 1976, 4, 130–131. [Google Scholar] [CrossRef]
- Ainscough, E.W.; Baker, E.N.; Brodie, A.M.; Larsen, N.G. Copper co-ordination to thioether ligands. Spectroscopic studies of dimeric copper(II) complexes of 2-(3,3-dimethyl-2-thiabutyl)pyridine and the crystal structure of di-µ-bromo-bis{bromo[2-(3,3-dimethyl-2-thiabutyl)pyridine-NS]copper(II)}. J. Chem. Soc. Dalton Trans. 1981, 10, 2054–2058. [Google Scholar] [CrossRef]
- Hathaway, B.J. Copper. Coord. Chem. Rev. 1981, 35, 211–252. [Google Scholar] [CrossRef]
- Hathaway, B.J. Copper. Coord. Chem. Rev. 1982, 41, 423–487. [Google Scholar] [CrossRef]
- Hathaway, B.J. Copper. Coord. Chem. Rev. 1983, 52, 87–169. [Google Scholar] [CrossRef]
- O’Brien, P. Copper. Coord. Chem. Rev. 1984, 58, 169–244. [Google Scholar] [CrossRef]
- Caulton, K.G.; Davies, G.; Holt, E.M. Synthesis, molecular structures, properties and reactions of halo- and carbonyl(amine)copper(I) complexes. Polyhedron 1990, 9, 2319–2351. [Google Scholar] [CrossRef]
- Holloway, C.E.; Melník, M. Copper(I) compounds: Classification and analysis of crystallographic and structural data. Rev. Inorg. Chem. 1995, 15, 147–386. [Google Scholar] [CrossRef]
- Melník, M.; Mikušová, V.; Mikuš, P. The structural aspects of mutually trans-X–Cu(I)–X (X=OL, NL, CL, PL, SL, SeL, Cl or Br) complexes. Inorganics 2024, 12, 245. [Google Scholar] [CrossRef]
- Romero, E.A.; Zhao, T.; Nakano, R.; Hu, X.; Wu, Y.; Jazzar, R.; Bertrand, G. Tandem copper hydride–Lewis pair catalysed reduction of carbon dioxide into formate with dihydrogen. Nat. Catal. 2018, 1, 743–747. [Google Scholar] [CrossRef]
- Hall, J.W.; Unson, D.M.L.; Brunel, P.; Collins, L.R.; Cybulski, M.K.; Mahon, M.F.; Whittlesey, M.K. Copper-NHC-Mediated Semi-Hydrogenation and Hydroboration of Alkynes: Enhanced Catalytic Activity Using Ring-Expanded Carbenes. Organometallics 2018, 37, 3102–3110. [Google Scholar] [CrossRef]
- Jones, C.; Mills, D.P.; Rose, R.P.; Stasch, A.; Woodul, W.D. Synthesis and further reactivity studies of some transition metal gallyl complexes. J. Organomet. Chem. 2010, 695, 2410–2417. [Google Scholar] [CrossRef]
- Mankad, N.P.; Gray, T.G.; Laitar, D.S.; Sadighi, J.P. Synthesis, Structure, and CO2 Reactivity of a Two-Coordinate (Carbene)copper(I) Methyl Complex. Organometallics 2004, 23, 1191–1193. [Google Scholar] [CrossRef]
- Goj, L.A.; Blue, E.D.; Delp, S.A.; Gunnoe, T.B.; Cundari, T.R.; Petersen, J.L. Single-Electron Oxidation of Monomeric Copper(I) Alkyl Complexes: Evidence for Reductive Elimination through Bimolecular Formation of Alkanes. Organometallics 2006, 25, 4097–4104. [Google Scholar] [CrossRef]
- Nolte, C.; Mayer, P.; Straub, B.F. Isolation of a copper(I) triazolide: A “click” intermediate. Angew. Chem. Int. Ed. 2007, 46, 2101–2103. [Google Scholar] [CrossRef]
- McReynolds, K.A.; Lewis, R.S.; Ackerman, L.K.G.; Dubinina, G.G.; Brennessel, W.W.; Vicic, D.A. Decarboxylative Trifluoromethylation of Aryl Halides Using Well-Defined Copper-Trifluoroacetate and –Chlorodifluoroacetate Precursors. J. Fluorine Chem. 2010, 131, 1108–1112. [Google Scholar] [CrossRef]
- Bhattacharyya, K.X.; Akana, J.A.; Laitar, D.S.; Berlin, J.M.; Sadighi, J.P. Carbon−Carbon Bond Formation on Reaction of a Copper(I) Stannyl Complex with Carbon Dioxide. Organometallics 2008, 27, 2682–2684. [Google Scholar] [CrossRef]
- Ohishi, T.; Nishiura, M.; Hou, Z. Carboxylation of Organoboronic Esters Catalyzed by N-Heterocyclic Carbene Copper(I) Complexes. Angew. Chem. Int. Ed. 2008, 47, 5792–5795. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Collins, L.R.; Mahon, M.F.; Djurovich, P.I.; Thompson, M.E.; Whittlesey, M.K. Synthesis and characterization of phosphorescent two-coordinate copper(i) complexes bearing diamidocarbene ligands. Dalton Trans. 2017, 46, 745–752. [Google Scholar] [CrossRef]
- Yang, W.J.; Kang, B.N.; Lee, J.H.; Choi, Y.M.; Kim, C.H.; Yun, J. NHC-copper-thiophene-2-carboxylate complex for the hydroboration of terminal alkynes. Org. Biomol. Chem. 2019, 17, 5249–5252. [Google Scholar] [CrossRef]
- Ohishi, T.; Zhang, L.; Nishiura, M.; Hou, Z. Carboxylation of alkylboranes by N-heterocyclic carbene copper catalysts: Synthesis of carboxylic acids from terminal alkenes and carbon dioxide. Angew. Chem. Int. Ed. 2011, 50, 8114–8117. [Google Scholar] [CrossRef]
- Liske, A.; Wallbaum, L.; Holzel, T.; Foller, J.; Gernert, M.; Hupp, B.; Ganter, C.; Marian, C.M.; Steffen, A. Cu-F Interactions between Cationic Linear N-Heterocyclic Carbene Copper(I) Pyridine Complexes and Their Counterions Greatly Enhance Blue Luminescence Efficiency. Inorg. Chem. 2019, 58, 5433–5445. [Google Scholar] [CrossRef]
- Bai, T.; Yang, Y.; Han, C. Isolation and characterization of hydrocarbon soluble NHC copper(I) phosphoranimide complex and catalytic application for alkyne hydroboration reaction. Tetrahedron Lett. 2017, 58, 1523–1527. [Google Scholar] [CrossRef]
- Tzouras, N.V.; Martynova, E.A.; Ma, X.; Scattolin, T.; Hupp, B.; Busen, H.; Saab, M.; Zhang, Z.; Falivene, L.; Pisano, G.; et al. Simple Synthetic Routes to Carbene-M-Amido (M=Cu, Ag, Au) Complexes for Luminescence and Photocatalysis Applications. Chem. A Eur. J. 2021, 27, 11904–11911. [Google Scholar] [CrossRef]
- Downie, T.M.H.; Hall, J.W.; Finn, T.P.C.; Liptrot, D.J.; Lowe, J.P.; Mahon, M.F.; McMullin, C.J.; Whittlesey, M.K. The first ring-expanded NHC–copper(i) phosphides as catalysts in the highly selective hydrophosphination of isocyanates. Chem. Commun. 2020, 56, 13359–13362. [Google Scholar] [CrossRef]
- Hamze, R.; Idris, M.; Ravinson, D.S.M.; Jung, M.C.; Haiges, R.; Djurovich, P.I.; Thompson, M.E. Highly Efficient Deep Blue Luminescence of 2-Coordinate Coinage Metal Complexes Bearing Bulky NHC Benzimidazolyl Carbene. Front. Chem. 2020, 8, 535809. [Google Scholar] [CrossRef] [PubMed]
- Trose, M.; Nahra, F.; Cordes, D.B.; Slawin, A.M.Z.; Cazin, C.S.J. Cu–NHC azide complex: Synthesis and reactivity. Chem. Commun. 2019, 55, 12068–12071. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Zhao, Z.; Li, X.; Yu, X.; Huo, P.; Jin, Q.; Liu, Z.; Brian, Z.; Huang, C. Two-Coordinate Copper(I)/NHC Complexes: Dual Emission Properties and Ultralong Room-Temperature Phosphorescence. Angew. Chem. Int. Ed. 2020, 59, 8210–8217. [Google Scholar] [CrossRef] [PubMed]
- Dodds, C.A.; Kennedy, A.R.; Thompson, R. Taming Copper(I) Cyanate and Selenocyanate with N-Heterocyclic Carbenes. Eur. J. Inorg. Chem. 2019, 2019, 3581–3587. [Google Scholar] [CrossRef]
- Xie, W.; Yoon, J.H.; Chang, S. (NHC)Cu-Catalyzed Mild C–H Amidation of (Hetero)arenes with Deprotectable Carbamates: Scope and Mechanistic Studies. J. Am. Chem. Soc. 2016, 138, 12605–12614. [Google Scholar] [CrossRef]
- Liu, H.Y.; Schwamm, R.J.; Hill, M.S.; Mahon, M.F.; McMullin, C.L.; Rajabi, N.A. Ambiphilic Al-Cu Bonding. Angew. Chem. Int. Ed. 2021, 60, 14390–14393. [Google Scholar] [CrossRef]
- Zhai, Y.; Filatov, A.S.; Hillhouse, G.L.; Hopkins, M.D. Synthesis, structure, and reactions of a copper–sulfido cluster comprised of the parent Cu2S unit: {(NHC)Cu}2(μ-S). Chem. Sci. 2016, 7, 589–595. [Google Scholar] [CrossRef]
- McCarly, B.Y.; Thomas, B.M.; Zeller, M.; Van Hoveln, R. Synthesis of a Copper Silyl Complex by Disilane Activation. Organometallics 2018, 37, 2937–2940. [Google Scholar] [CrossRef]
- Plotzitzka, J.; Kleeberg, C. [(NHC)CuI–ER3] Complexes (ER3 = SiMe2Ph, SiPh3, SnMe3): From Linear, Mononuclear Complexes to Polynuclear Complexes with Ultrashort CuI⋯CuI Distances. Inorg. Chem. 2016, 55, 4813–4823. [Google Scholar] [CrossRef]
- Parvin, N.; Hossain, Y.; George, A.; Parameswaran, P.; Khan, S. N-heterocyclic silylene stabilized monocordinated copper(i)–arene cationic complexes and their application in click chemistry. Chem. Commun. 2020, 56, 273–276. [Google Scholar] [CrossRef]
- Kleeberg, C.; Borner, C. Syntheses, Structures, and Reactivity of NHC Copper(I) Boryl Complexes: A Systematic Study. Organometallics 2018, 37, 4136–4146. [Google Scholar] [CrossRef]
- Drescher, W.; Borner, C.; Kleeberg, C. Stability and decomposition of copper(I) boryl complexes: [(IDipp)Cu–Bneop], [(IDipp*)Cu–Bneop] and copper clusters. New. J. Chem. 2021, 45, 14957–14964. [Google Scholar] [CrossRef]
- Najafabadi, B.K.; Corrigan, J.F. Enhanced thermal stability of Cu–silylphosphido complexes via NHC ligation. Dalton Trans. 2015, 44, 14235–14241. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Ruiz, D.A.; Dahcheh, F.; Bertrand, G.; Suter, R.; Tondreau, A.M.; Grutzmacher, H. Isolation of Au-, Co-η1PCO and Cu-η2PCO complexes, conversion of an Ir–η1PCO complex into a dimetalladiphosphene, and an interaction-free PCO anion. Chem. Sci. 2016, 7, 2335–2341. [Google Scholar] [CrossRef]
- Blum, M.; Kappler, J.; Schlindwein, S.H.; Nieger, M.; Gudat, D. Synthesis, spectroscopic characterisation and transmetalation of lithium and potassium diaminophosphanide-boranes. Dalton Trans. 2018, 47, 112–119. [Google Scholar] [CrossRef]
- McManus, C.; Hicks, J.; Cui, X.; Zhao, L.; Frenking, G.; Goicoechea, J.M.; Aldridge, S. Coinage metal aluminyl complexes: Probing regiochemistry and mechanism in the insertion and reduction of carbon dioxide. Chem. Sci. 2021, 12, 13458–13468. [Google Scholar] [CrossRef]
- Collins, L.R.; Riddlestone, I.M.; Mahon, M.F.; Whittlesey, M.K. A comparison of the stability and reactivity of diamido- and diaminocarbene copper alkoxide and hydride complexes. Chem. Eur. J. 2015, 21, 14075–14084. [Google Scholar] [CrossRef]
- Kuehn, L.; Eichhorn, A.F.; Marder, T.B.; Radius, U. Copper(I) complexes of N-alkyl-substituted N-Heterocyclic carbenes. J. Organomet. Chem. 2019, 881, 25–33. [Google Scholar] [CrossRef]
- Rasale, D.; Patil, K.; Sauter, B.; Geigle, S.; Zhanybekova, S.; Gillingham, D. A new water soluble copper N-heterocyclic carbene complex delivers mild O6G-selective RNA alkylation. Chem. Commun. 2018, 54, 9174–9177. [Google Scholar] [CrossRef]
- Manar, K.K.; Chakrabortty, S.; Porwal, V.K.; Prakash, D.; Thakur, S.K.; Choudhury, A.R.; Singh, S. Two-Coordinate Cu (I) and Au (I) Complexes Supported by BICAAC and CAAC Ligands. ChemistrySelect 2020, 5, 9900–9907. [Google Scholar] [CrossRef]
- Shaw, P.; Kennedy, A.R.; Nelson, D.J. Synthesis and characterisation of an N-heterocyclic carbene with spatially-defined steric impact. Dalton Trans. 2016, 45, 11772–11780. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, G.; Wood, S.H.; Kennedy, A.R.; Nelson, D.J. An N-Heterocyclic Carbene with a Saturated Backbone and Spatially-Defined Steric Impact. Z. Anorg. Allg. Chem. 2019, 645, 105–112. [Google Scholar] [CrossRef]
- Dodds, C.A.; (University of Glasgow, Glasgow, UK); Kennedy, A.R.; (University of Strathclyde, Glasgow, UK). Personal communication, 2018.
- Doud, E.A.; Inkpen, M.S.; Lovat, G.; Montes, E.; Paley, D.W.; Steigerwald, M.L.; Vazquez, H.; Venkataraman, L.; Roy, X. In Situ Formation of N-Heterocyclic Carbene-Bound Single-Molecule Junctions. J. Am. Chem. Soc. 2018, 140, 8944–8949. [Google Scholar] [CrossRef] [PubMed]
- Romanov, A.S.; Becker, C.R.; James, C.E.; Di, D.; Credgington, D.; Linnolahti, M.; Bochmann, M. Copper and Gold Cyclic (Alkyl)(amino)carbene Complexes with Sub-Microsecond Photoemissions: Structure and Substituent Effects on Redox and Luminescent Properties. Chem. A Eur. J. 2017, 23, 4625–4637. [Google Scholar] [CrossRef]
- Ren, X.; Wesolek, M.; Braunstein, P. Cu(i), Ag(i), Ni(ii), Cr(iii) and Ir(i) complexes with tritopic NimineCNHCNamine pincer ligands and catalytic ethylene oligomerization. Dalton Trans. 2019, 48, 12895–12909. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Xie, Z. Reversible Photothermal Isomerization of Carborane-Fused Azaborole to Borirane: Synthesis and Reactivity of Carbene-Stabilized Carborane-Fused Borirane. Angew. Chem. Int. Ed. 2017, 56, 9198–9201. [Google Scholar] [CrossRef]
- Deng, M.; Mukthar, N.F.M.; Schley, N.D.; Ung, G. Yellow Circularly Polarized Luminescence from C1 -Symmetrical Copper(I) Complexes. Angew. Chem. Int. Ed. 2020, 59, 1228–1231. [Google Scholar] [CrossRef]
- Romanov, A.S.; Di, D.; Yang, L.; Fernandez-Cestau, J.; Becker, C.R.; James, C.E.; Zhu, B.; Linolahti, M.; Credgington, D.; Bochmann, M. Highly photoluminescent copper carbene complexes based on prompt rather than delayed fluorescence. Chem. Commun. 2016, 52, 6379–6382. [Google Scholar] [CrossRef]
- Liu, C.; Shen, H.Q.; Chen, M.W.; Zhou, Y.G. C2-Symmetric Hindered “Sandwich” Chiral N-Heterocyclic Carbene Precursors and Their Transition Metal Complexes: Expedient Syntheses, Structural Authentication, and Catalytic Properties. Organometallics 2018, 37, 3756–3769. [Google Scholar] [CrossRef]
- Tarrieu, R.; Dumas, A.; Thongpaen, J.; Vives, T.; Roisnel, T.; Dorcet, V.; Crevisy, C.; Basle, O.; Mauduit, M. Readily accessible unsymmetrical unsaturated 2, 6-diisopropylphenyl N-heterocyclic carbene ligands. Applications in enantioselective catalysis. J. Org. Chem. 2017, 82, 1880–1887. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Y.; Liu, F.S.; Lin, H.; Zhou, Y. Copper(I)–NHCs complexes: Synthesis, characterization and their inhibition against the biofilm formation of Streptococcus mutans. Polyhedron 2021, 197, 115033. [Google Scholar] [CrossRef]
- Pape, F.; Brechmann, L.T.; Teichert, J.F. Catalytic Generation and Chemoselective Transfer of Nucleophilic Hydrides from Dihydrogen. Chem. Eur. J. 2019, 25, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Lorkowski, J.; Krahfuss, M.; Kubicki, M.; Radius, U.; Pietraszuk, C. Intramolecular Ring-Expansion Reaction (RER) and Intermolecular Coordination of In Situ Generated Cyclic (Amino)(aryl)carbenes (cAArCs). Chem. Eur. J. 2019, 25, 11365–11374. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, C.; Bestgen, S.; Gamer, M.T.; Kuhn, M.; Lebedkin, S.; Weigend, F.; Kappes, M.M.; Roesky, P.W. Coinage Metal Complexes of Bis-Alkynyl-Functionalized N-Heterocyclic Carbenes: Reactivity, Photophysical Properties, and Quantum Chemical Investigations. Chem. Eur. J. 2017, 23, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Schwedtmann, K.; Schoemaker, R.; Hennersdorf, F.; Bauza, A.; Fontera, A.; Weiss, R.; Weigand, J.J. Cationic 5-phosphonio-substituted N-heterocyclic carbenes. Dalton Trans. 2016, 45, 11384–11396. [Google Scholar] [CrossRef]
- Delgado-Rebollo, M.; Garcia-Morales, C.; Maya, C.; Prieto, A.; Echavarren, A.M.; Perez, P.J. Coinage metal complexes bearing fluorinated N-Heterocyclic carbene ligands. J. Organomet. Chem. 2019, 898, 120856. [Google Scholar] [CrossRef]
- Chesnokov, G.A.; Dzhevakov, P.B.; Rybakov, V.B.; Nechaev, M.S.; (M. V. Lomonosov Moscow State University, Moscow, Russia); Topchiy, M.A.; Gribanov, P.S.; Asachenko, A.F.; (Russian Academy of Sciences, Moscow, Russia); Khrustalev, V.N.; (Peoples’ Friendship University of Russia, Moscow, Russia). Personal communication, 2016.
- Gernert, M.; Balles-Wof, L.; Kerner, F.; Muller, U.; Schmiedel, A.; Holzapfel, M.; Marian, C.M.; Pflaum, J.; Lambert, C.; Steffen, A. Cyclic (Amino)(aryl)carbenes Enter the Field of Chromophore Ligands: Expanded π System Leads to Unusually Deep Red Emitting CuI Compounds. J. Am. Chem. Soc. 2020, 142, 8897–8909. [Google Scholar] [CrossRef]
- Garces, K.; Fernandez-Alvarez, F.J.; Garcia-Orduna, P.; Lahoz, F.J.; Perez-Torrente, J.J.; Oro, L.A. Grafting of Copper(I)–NHC Species on MCM-41: Homogeneous versus Heterogeneous Catalysis. ChemCatChem 2015, 7, 2501–2507. [Google Scholar] [CrossRef]
- Hayakawa, T.; Sakata, T.; (Josai University, Saitama, Japan). Personal communication, 2017.
- Jones, P.G.; Holschumacher, D.; Hrib, C.G.; Tamm, M.; (Technische Universität Carolo-Wilhelmina, Braunschweig, Germany). Personal communication, 2019.
- Marquez, A.; Avila, E.; Urbaneja, C.; Alvarez, E.; Palma, P.; Campora, J. Copper(I) Complexes of Zwitterionic Imidazolium-2-Amidinates, a Promising Class of Electroneutral, Amidinate-Type Ligands. Inorg. Chem. 2015, 54, 11007–11017. [Google Scholar] [CrossRef]
- Florke, U.; (Universität Paderborn, Paderborn, Germany); Nagel, C.; (Julius-Maximilians-Universität Würzburg, Würzburg, Germany); Henkel, G.; (Westfälische Wilhelms-Universität Münster, Münster, Germany). Personal communication, 2017.
- Srinivas, K.; Prabusankar, G. Role of C, S, Se and P donor ligands in copper(i) mediated C–N and C–Si bond formation reactions. RSC Adv. 2018, 8, 32269–32282. [Google Scholar] [CrossRef]
- Rheingold, A.L.; (University of California, San Diego, CA, USA); Rabinovich, D.; (The University of North Carolina at Charlotte, Charlotte, NC, USA); Golen, J.A.; (University of Massachusetts Dartmouth, North Dartmouth, MA, USA). Personal communication, 2020.
- Parvin, N.; Pal, S.; Khan, S.; Das, S.; Pati, S.K.; Roesky, H.W. Unique approach to copper(I) silylene chalcogenone complexes. Inorg. Chem. 2017, 56, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Lui, M.W.; Shynkaruk, O.; Oakley, M.S.; Sinelnikov, R.; McDonald, R.; Ferguson, M.J.; Meldrum, A.; Klobukowski, M.; Rivard, E. Engaging dual donor sites within an N-heterocyclic olefin phosphine ligand. Dalton Trans. 2017, 46, 5946–5954. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Putz, H.; Berndt, M. DIAMOND; Crystal Impact GbR: Bonn, Germany, 1999. [Google Scholar]
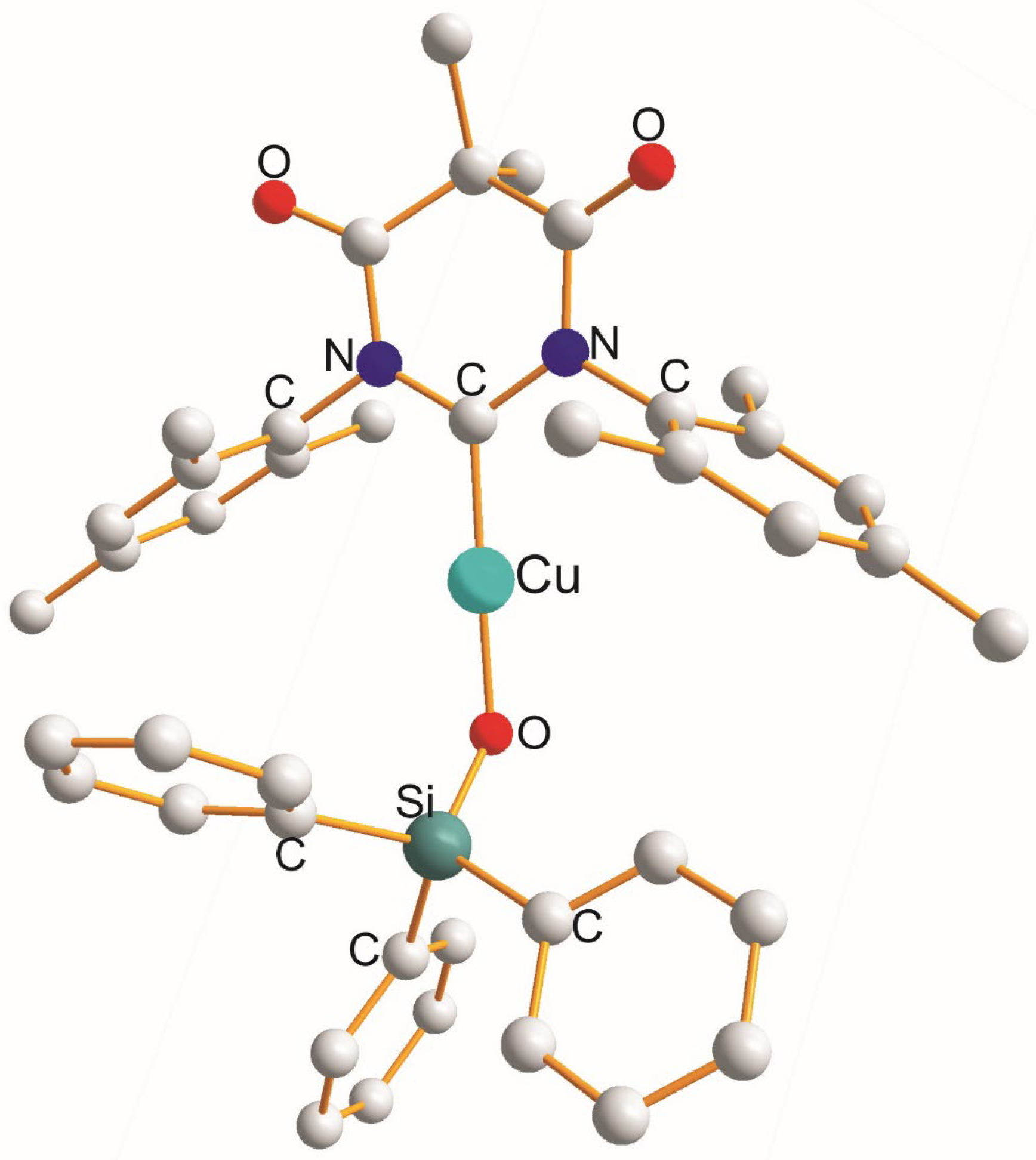
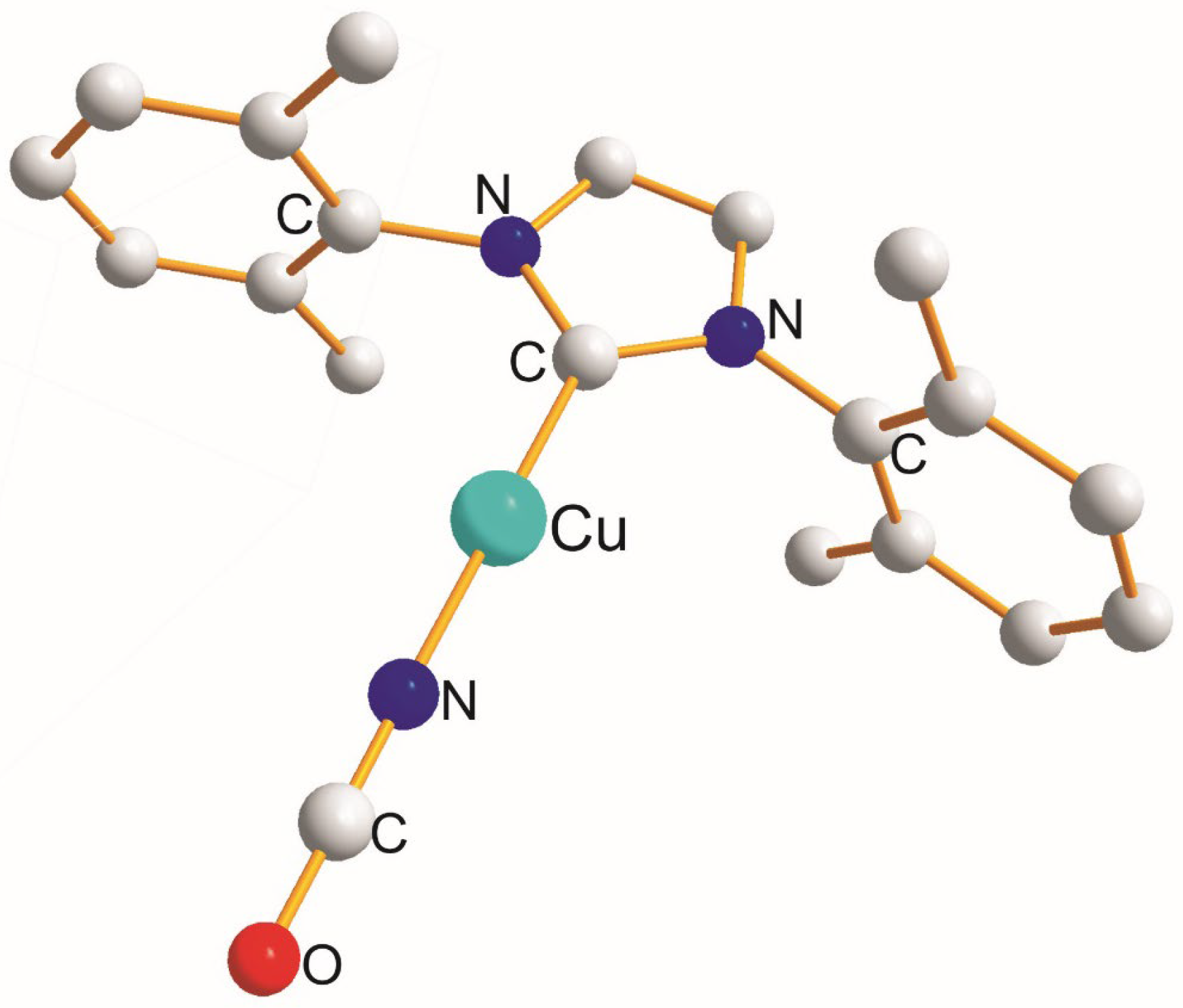


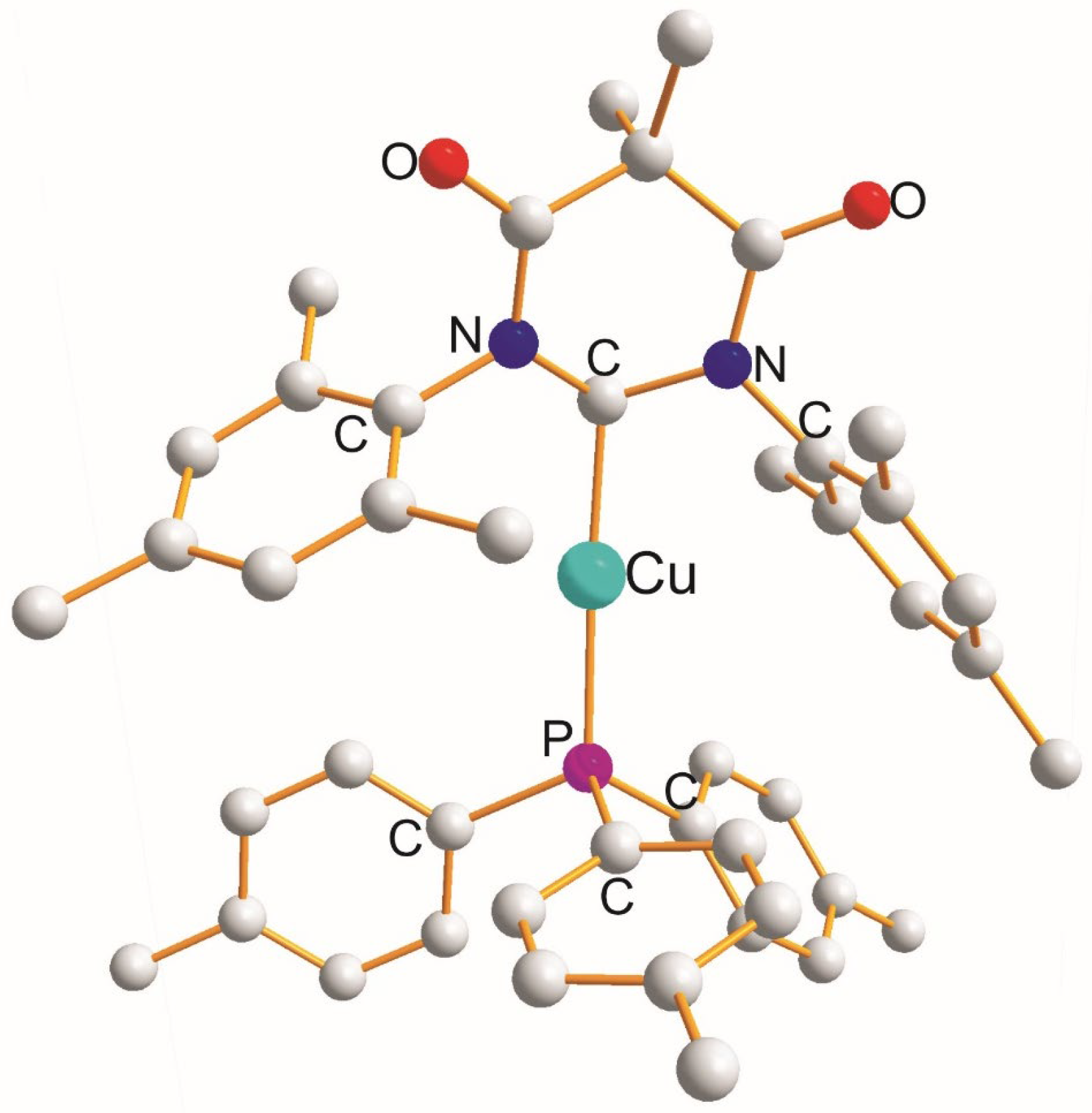

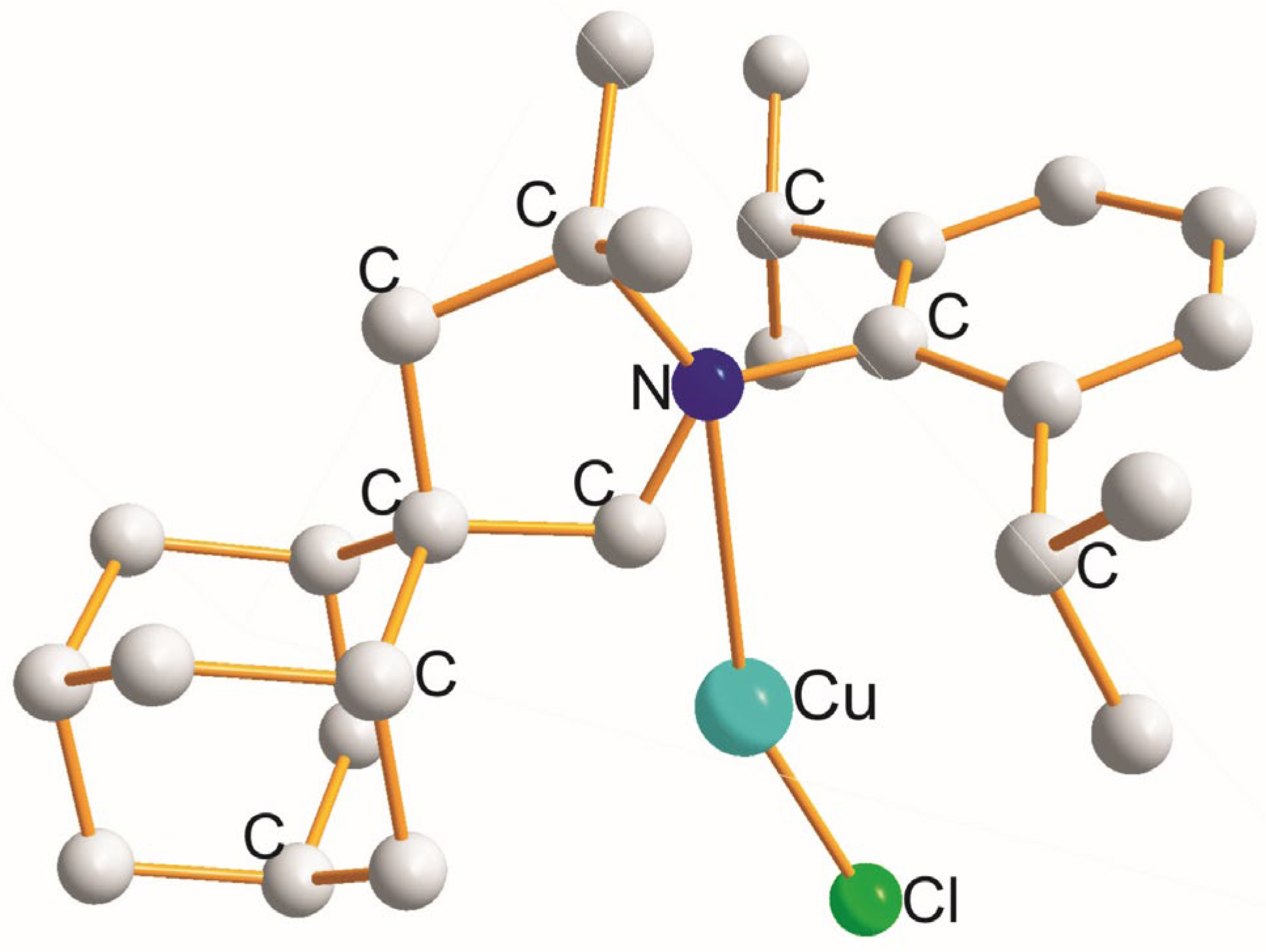
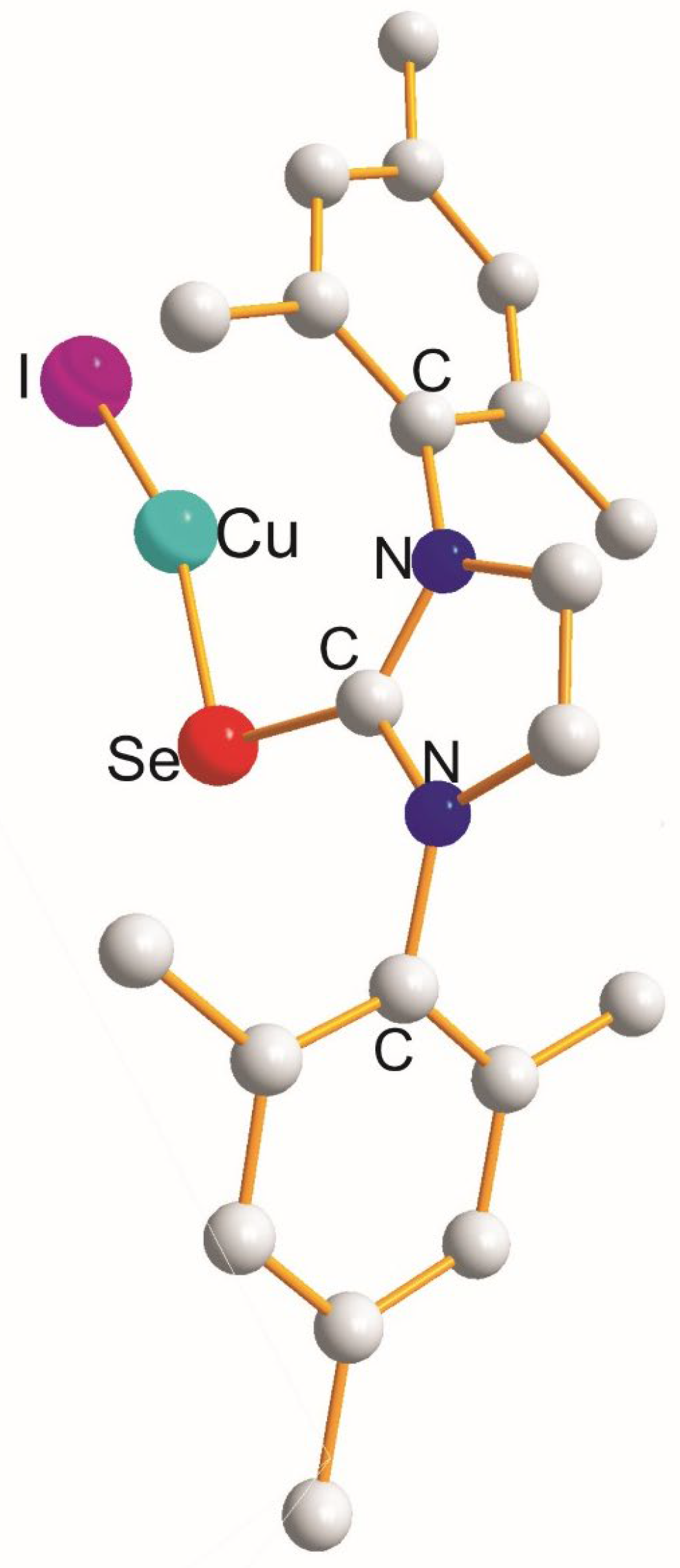
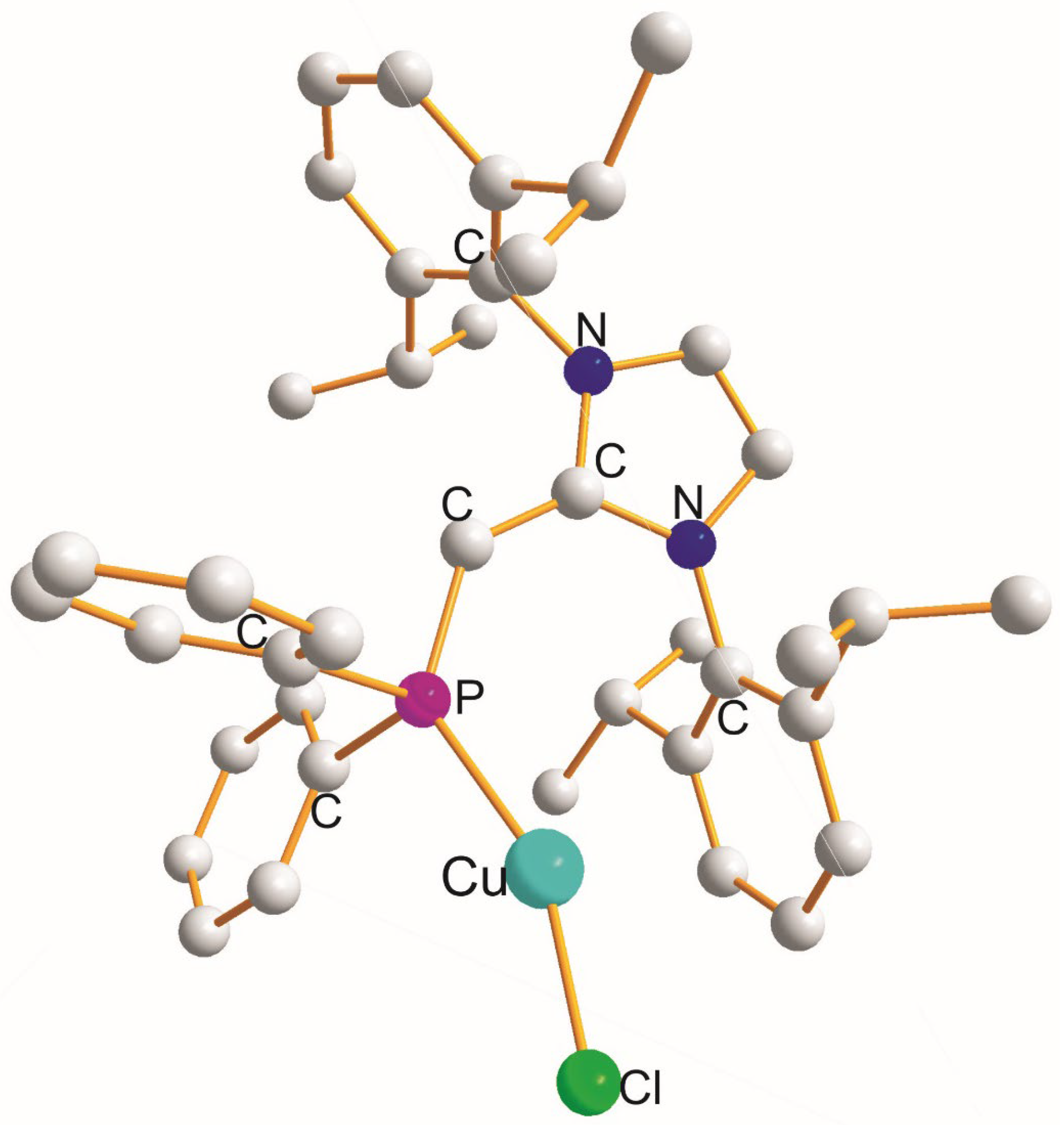
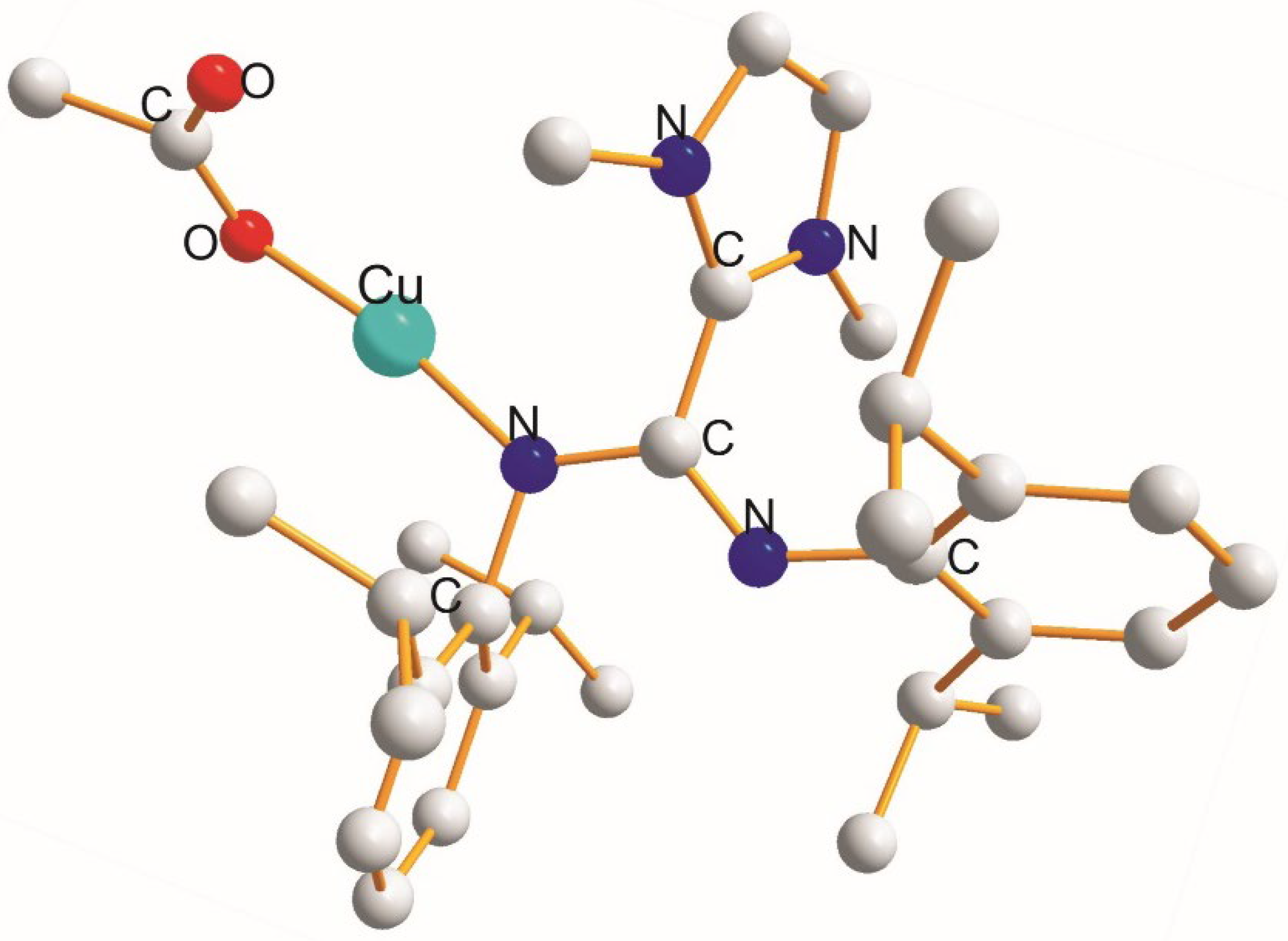
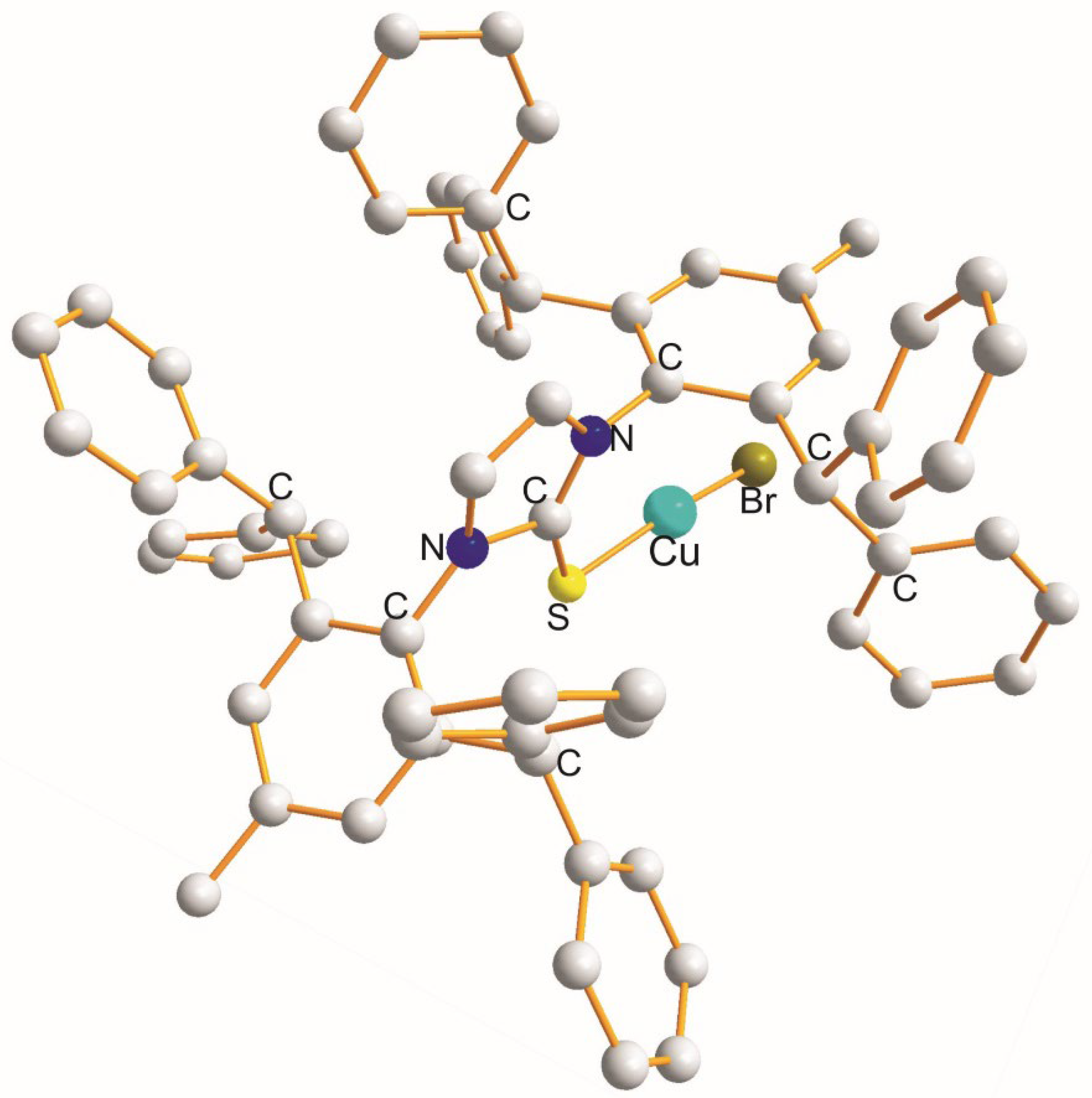
| (X) a Trans to (Y) b [Å] | (X) Trans to (X) < [Å] | (X) a Trans to (Y) b [Å] |
|---|---|---|
| 1.832 (C) < 1.848 (N)< | (O), 1.849, (O) | |
| 1.852 (O) < 1.855 (Cl) < 1.864 (C)< | (N), 1.886, (N) | |
| 2.095 (N) < 2.100 (C)< | (Cl), 2.104, (Cl)< | 2.108 (S) < 2.120 (P) |
| 2.104 (C) < 2.134 (Br)< | (S), 2.137, (S)< | 2.139 (Cl) < 2.142 (I) |
| 2.172 (Cl) < 2.202 (I) < 2.219 (C)< | (P), 2.236, (P) | |
| 2.248 (Br) < 2.252 (I)< | (Se), 2.260, (Se) | |
| 1.867 (S) < 1.869 (O) < 1.875 (N)< | (C), 1.900, (C)< | 1.915 (I) < 1.917 (P) < 1.925 (Sn)< |
| 1.879 (H) < 1.883 (Cl) < 1.892 (Br) | <1.935 (Si) < 1.939 (B) < 1.958 (Al) |
| Organometalic Compounds C-Cu(I)-Y | Coordination Compounds X-Cu(I)-Y |
|---|---|
| 178.9°(Y=I) > 177.8°(S) > 177.0°(H) > 175.9°(Al) > 175.8°(Cl) > 175.7°(N) > 175.1°(O) > 174.8°(Br) > 174.1°(P) > 173.7°(Si) > 173.5°(B) > 163.4°(Sn) | 170.6°(X=N; Y=Cl) > 166.2°(S, Br) > 165.8°(S, Cl) > 164.2°(Se, Br) > 162.2°(N, O) > 160.7°(S, I) > 159.6°(Se, I) > 156.8°(P, Cl) > 144.5°(P, I) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melník, M.; Mikušová, V.; Mikuš, P. Monodentate Ligands in X-Cu(I)-Y Complexes—Structural Aspects. Inorganics 2024, 12, 279. https://doi.org/10.3390/inorganics12110279
Melník M, Mikušová V, Mikuš P. Monodentate Ligands in X-Cu(I)-Y Complexes—Structural Aspects. Inorganics. 2024; 12(11):279. https://doi.org/10.3390/inorganics12110279
Chicago/Turabian StyleMelník, Milan, Veronika Mikušová, and Peter Mikuš. 2024. "Monodentate Ligands in X-Cu(I)-Y Complexes—Structural Aspects" Inorganics 12, no. 11: 279. https://doi.org/10.3390/inorganics12110279
APA StyleMelník, M., Mikušová, V., & Mikuš, P. (2024). Monodentate Ligands in X-Cu(I)-Y Complexes—Structural Aspects. Inorganics, 12(11), 279. https://doi.org/10.3390/inorganics12110279










