Effect of Doping ZrO2 on Structural and Thermal Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of ZrO2-LSGYN
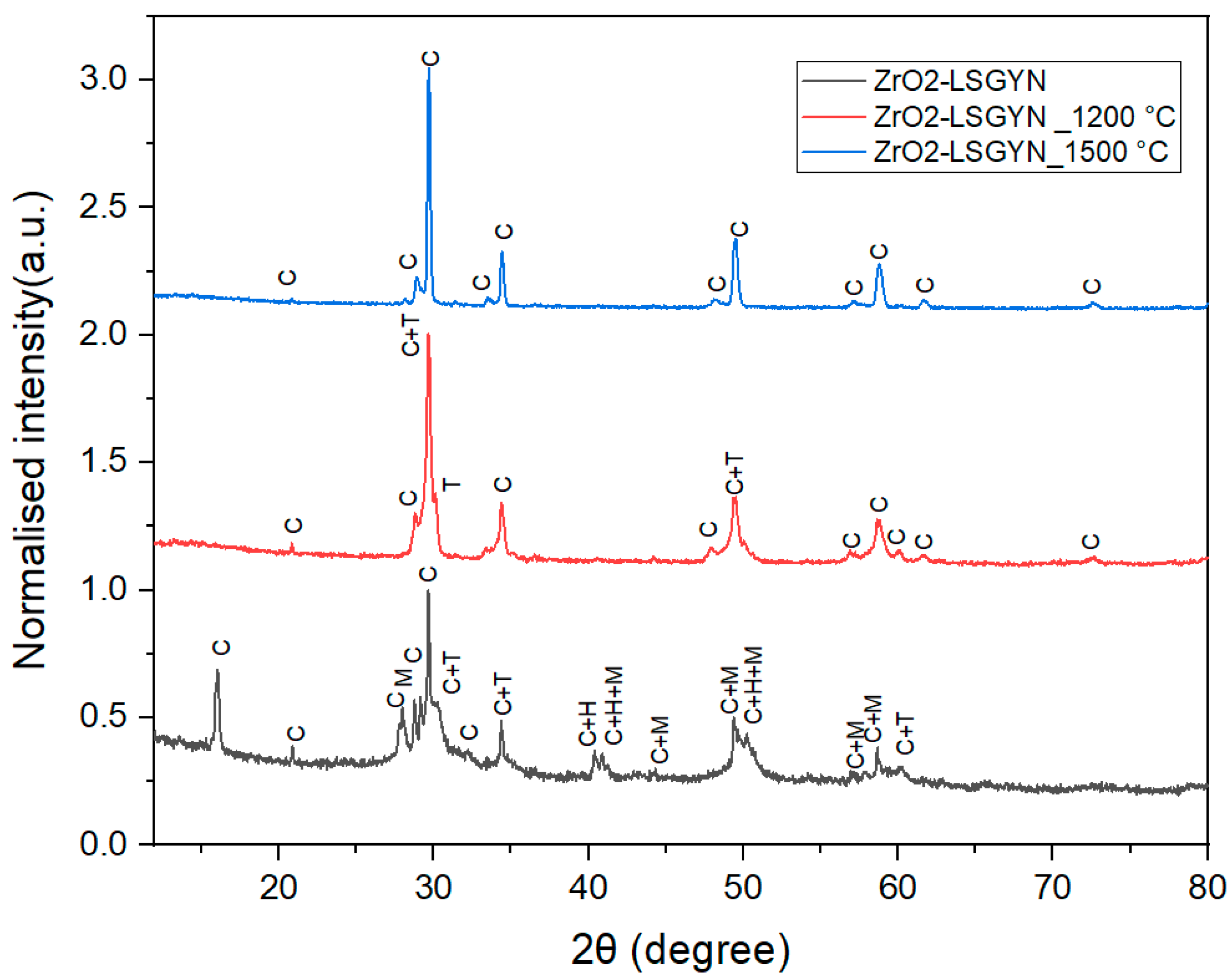
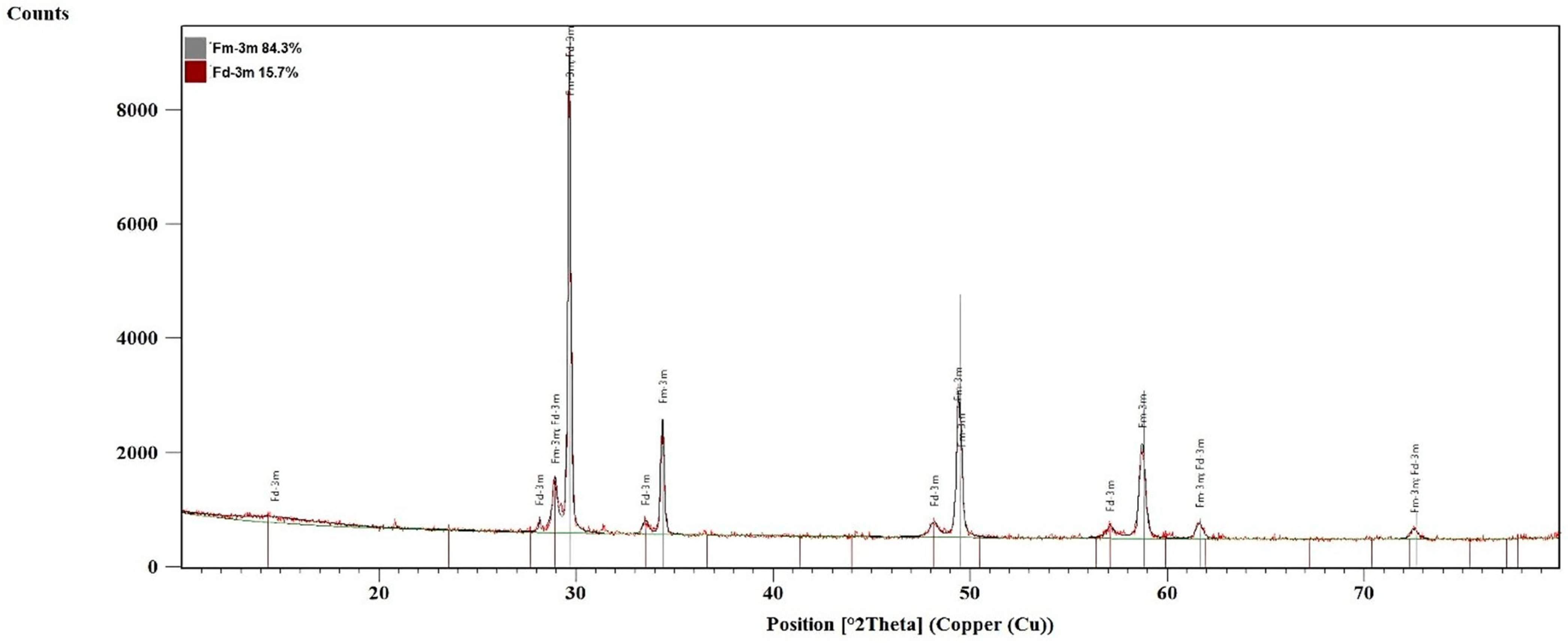
2.2. X-Ray Analysis of ZrO2-LSGYN Samples
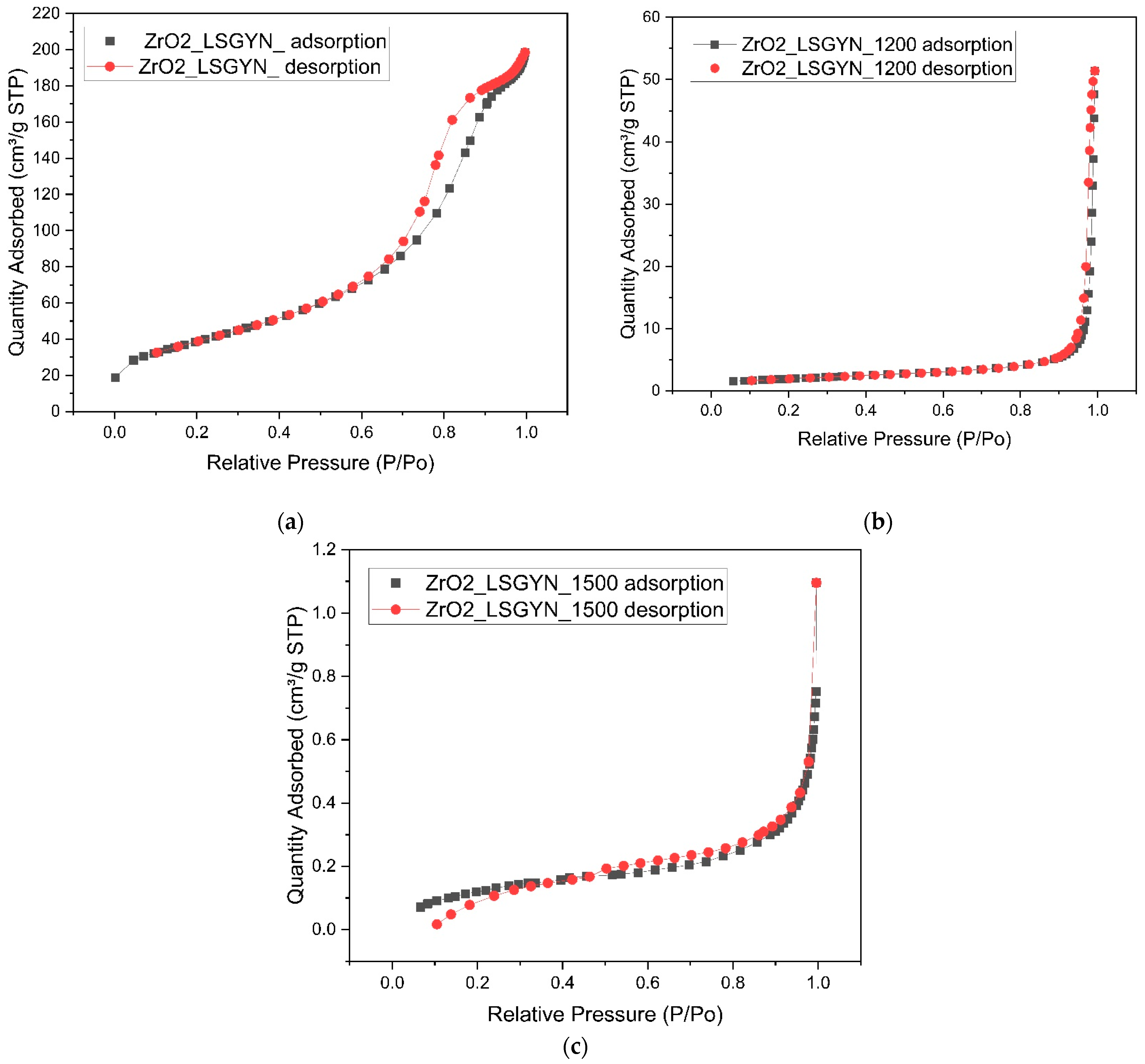
2.3. BET Surface Analysis of ZrO2-LSGYN
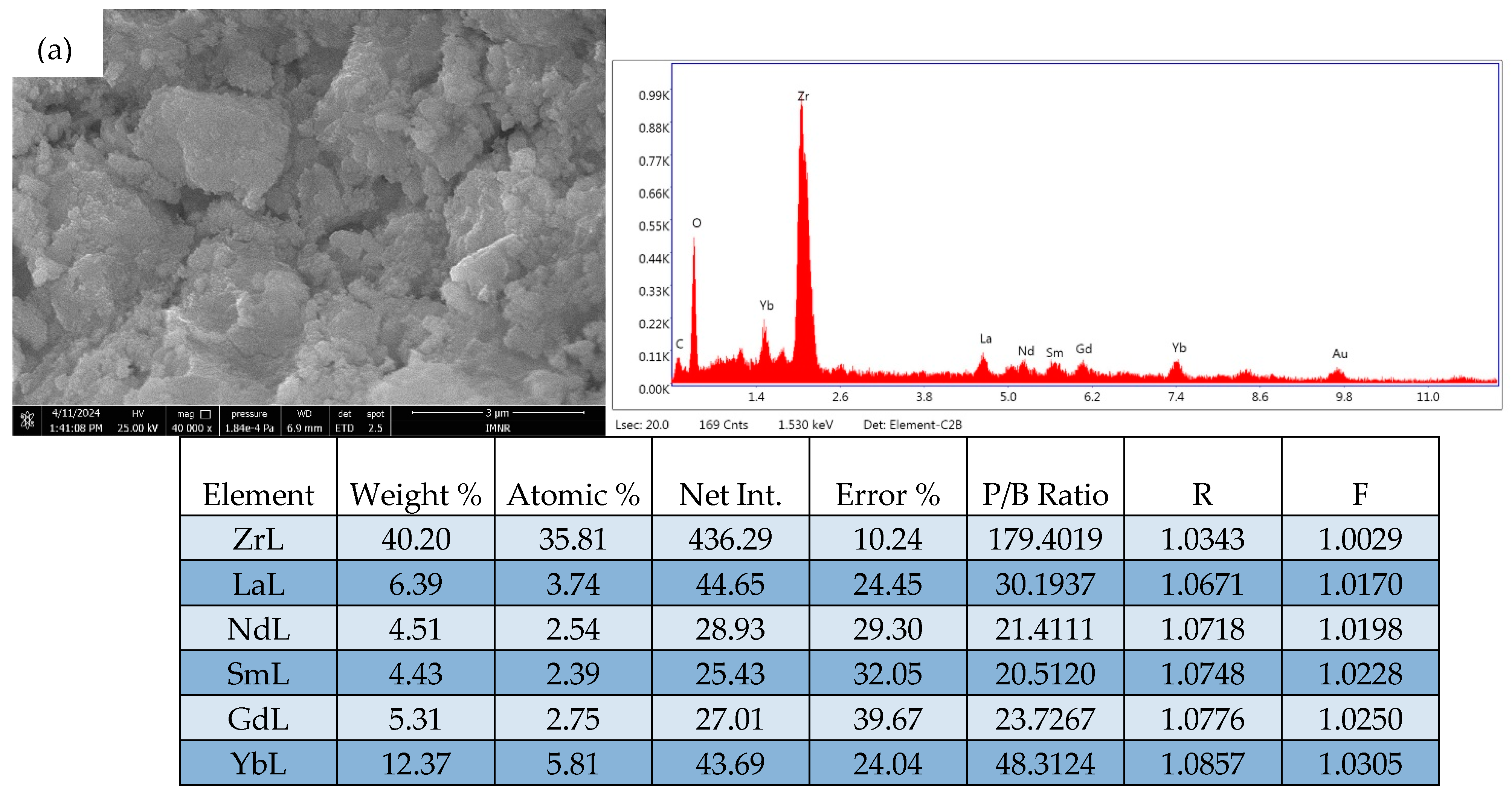
2.4. Scanning Electron Microscopy Analysis of ZrO2-LSGYN
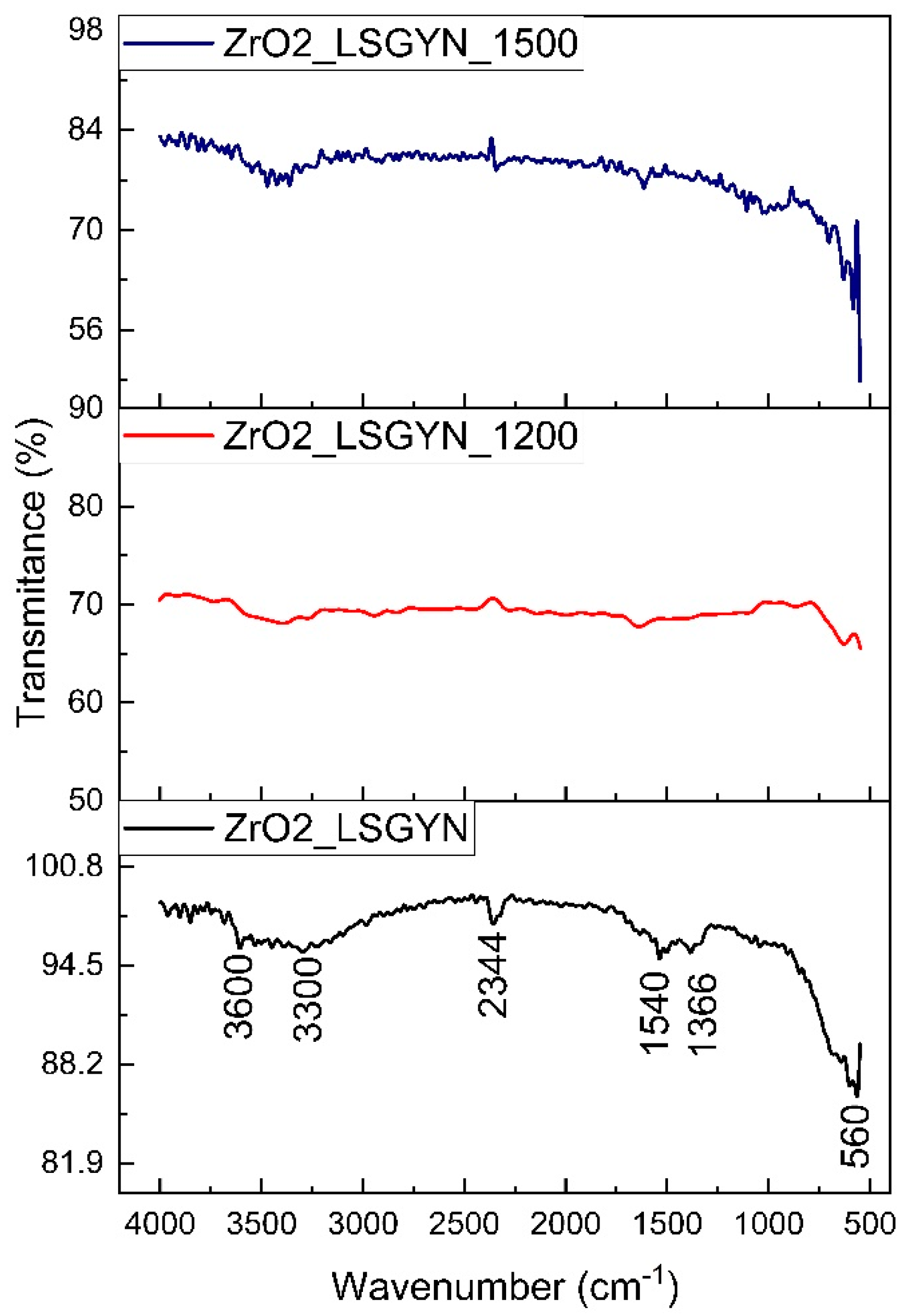
2.5. FT-IR Analysis
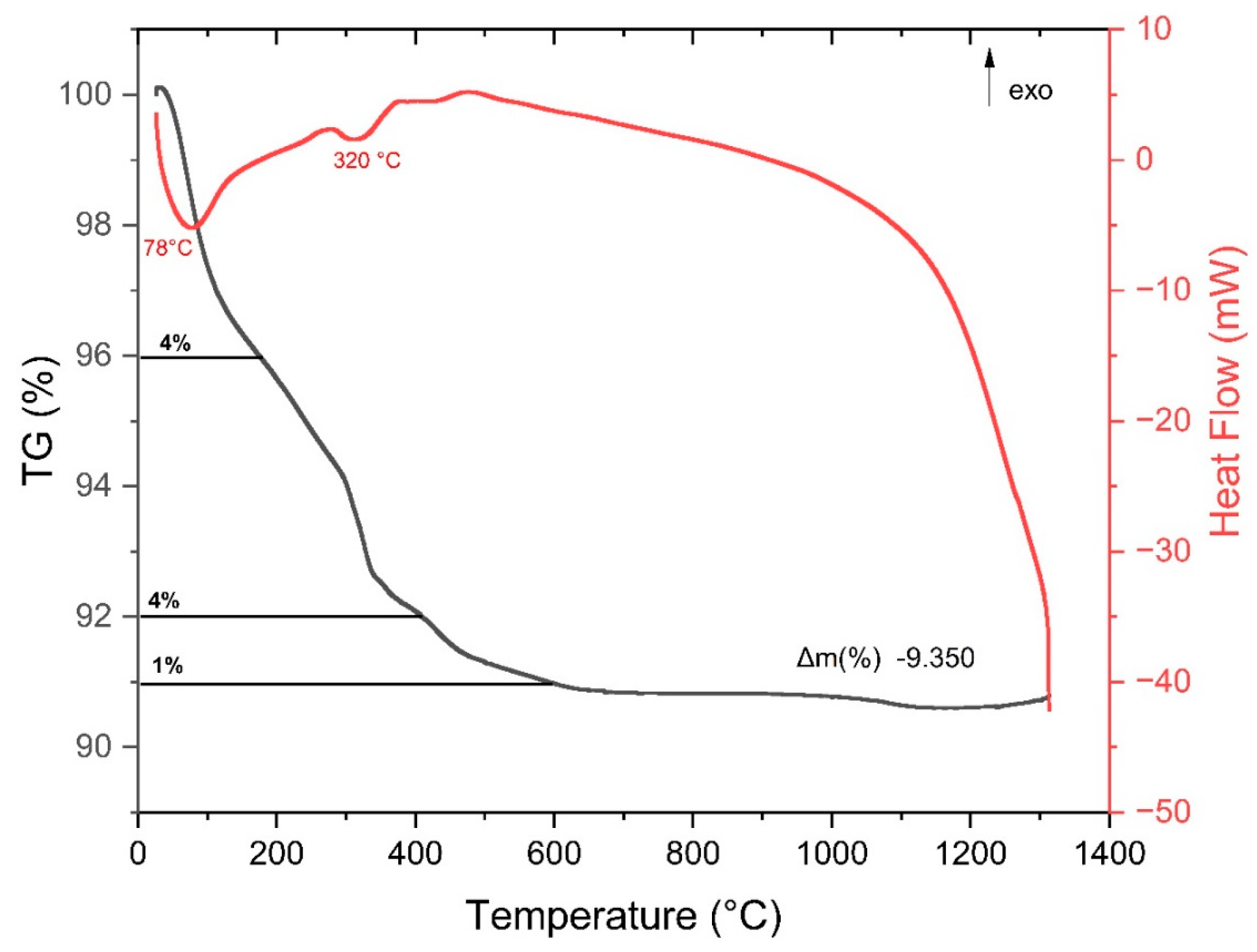
2.6. DSC/TG Analysis of ZrO2-LSGYN
2.7. Thermal Conductivity
| Material | k (W/mK) | α (mm2 /s) | Volumetric Cp (106 J/m3K) | Cp (J/gK) | Ref. |
|---|---|---|---|---|---|
| ZrO2-LSGYN | 0.61 ± 0.006 | 0.34 ± 0.02 | 1.82 ± 0.11 | 0.42 | This work |
| La2Zr2O7 | 1.56 | - | - | - | [44] |
| La1.7(DyNd)0.15(Zr0.8Ce0.2)2O7 | 1.30 | 0.65 | 0.32 | [41] | |
| La1.7Dy0.3Zr2O7 | 1.65 | 0.75 | 0.37 | [41] | |
| La2Zr2O7 | 1.85 | 0.85 | 0.38 | [41] |
3. Materials and Methods
3.1. Doped ZrO2 Hydrothermal Synthesis
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Guo, X.; Jung, Y.G.; Li, L.; Knapp, J. Lanthanum Zirconate Based Thermal Barrier Coatings: A Review. Surf. Coat. Technol. 2017, 323, 18–29. [Google Scholar] [CrossRef]
- Gorelov, V.P. High-Temperature Phase Transitions in ZrO2. Phys. Solid State 2019, 61, 1288–1293. [Google Scholar] [CrossRef]
- Graeve, O.A. Zirconia. In Ceramic and Glass Materials; Springer: New York, NY, USA, 2008. [Google Scholar]
- Tolborg, K.; Walsh, A. Exploring the High-Temperature Stabilization of Cubic Zirconia from Anharmonic Lattice Dynamics. Cryst. Growth Des. 2023, 23, 3314–3319. [Google Scholar] [CrossRef]
- Pyda, W.; Haberko, K.; Zurek, Z. Zirconia Stabilized Earth Oxides with a Mixture of the Rare. J. Eur. Ceram. Soc. 1992, 10, 453–459. [Google Scholar] [CrossRef]
- Vassen, R.; Cao, X.; Tietz, F.; Basu, D.; Stöver, D. Zirconates as New Materials for Thermal Barrier Coatings. J. Am. Ceram. Soc. 2000, 83, 2023–2028. [Google Scholar] [CrossRef]
- Liu, D.; Shi, B.; Geng, L.; Wang, Y.; Xu, B.; Chen, Y. High-Entropy Rare-Earth Zirconate Ceramics with Low Thermal Conductivity for Advanced Thermal-Barrier Coatings. J. Adv. Ceram. 2022, 11, 961–973. [Google Scholar] [CrossRef]
- Xu, L.; Wang, H.; Su, L.; Lu, D.; Peng, K.; Gao, H. A New Class of High-Entropy Fluorite Oxides with Tunable Expansion Coefficients, Low Thermal Conductivity and Exceptional Sintering Resistance. J. Eur. Ceram. Soc. 2021, 41, 6670–6676. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, H.; Li, G.; Geng, Y.; Xiao, Y.; Guo, H.; Peng, P. CMAS Corrosion Resistant of La3+-Yb3+-Ce4+ Co-Doped ZrO2 Based TBCs: Experimental and Theoretical Research. Ceram. Int. 2023, 49, 19402–19411. [Google Scholar] [CrossRef]
- Colbea, C.; Avram, D.; Cojocaru, B.; Negrea, R.; Ghica, C.; Kessler, V.G.; Seisenbaeva, G.A.; Parvulescu, V.; Tiseanu, C. Full Tetragonal Phase Stabilization in ZrO2 Nanoparticles Using Wet Impregnation: Interplay of Host Structure, Dopant Concentration and Sensitivity of Characterization Technique. Nanomaterials 2018, 8, 988. [Google Scholar] [CrossRef]
- Whyman, G.; Kalashnikov, A.; Zinigrad, M. On the Dependence of the Ionic Conductivity on Dopant Concentration in the Cubic Zirconium Oxide Doped with Oxides of Trivalent Metals. Solid State Ion. 2018, 316, 34–37. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xiong, X. Phase Stability and Thermal Conductivity of La2O3, Y2O3 stabilized ZrO2 ceramic for Thermal Barrier Coating Application. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2014; Volume 1033–1034, pp. 907–911. [Google Scholar]
- Sasikumar, K.; Bharathikannan, R.; Raja, M.; Mohanbabu, B. Fabrication and Characterization of Rare Earth (Ce, Gd, and Y) Doped ZrO2 Based Metal-Insulator-Semiconductor (MIS) Type Schottky Barrier Diodes. Superlattices Microstruct. 2020, 139, 106424. [Google Scholar] [CrossRef]
- Loong, C.-K.; Thiyagarajan, P.; Richardson, J.W.; Ozawa, M.; Suzuki, S. Microstructural Evolution of Zirconia Nanoparticles Caused by Rare-Earth Modification and Heat Treatment. J. Catal. 1997, 171, 498–505. [Google Scholar] [CrossRef]
- Park, M.-s.; Kim, B.; Kim, T.; Hong, E.; Lee, H. Phase Formation and Lattice Distortion of Lu2O3-Doped ZrO2 in Comparison with Y2O3-Doped ZrO2. Int. J. Appl. Ceram. Technol. 2020, 17, 1224–1230. [Google Scholar] [CrossRef]
- Jeong, J.; Han, Y.; Sohn, H. Effect of La Doping on Dielectric Constant and Tetragonality of ZrO2 Thin Films Deposited by Atomic Layer Deposition. J. Alloys Compd. 2022, 927, 166961. [Google Scholar] [CrossRef]
- Madhusudhana, H.C.; Shobhadevi, S.N.; Nagabhushana, B.M.; Krishna, R.H.; Murugendrappa, M.V.; Nagabhushana, H. Structural Characterization and Dielectric Studies of Gd Doped ZrO2 Nano Crystals Synthesized by Solution Combustion Method. Mater. Today Proc. 2018, 5, 21195–21204. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Salavati-Niasari, M. Facile Route to Synthesize Zirconium Dioxide (ZrO2) Nanostructures: Structural, Optical and Photocatalytic Studies. J. Mol. Liq. 2016, 216, 545–551. [Google Scholar] [CrossRef]
- Motoc, A.M.; Valsan, S.; Slobozeanu, A.E.; Corban, M.; Valerini, D.; Prakasam, M.; Botan, M.; Dragut, V.; Vasile, B.S.; Surdu, A.V.; et al. Design, Fabrication, and Characterization of New Materials Based on Zirconia Doped with Mixed Rare Earth Oxides: Review and First Experimental Results. Metals 2020, 10, 746. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Wang, Z.; Wang, X. Continuous Hydrothermal Flow Synthesis and Characterization of ZrO2 Nanoparticles Doped with CeO2 in Supercritical Water. Nanomaterials 2022, 12, 668. [Google Scholar] [CrossRef]
- Costa, C.Z.; Sousa-Aguiar, E.F.; Couto, M.A.P.G.; Filho, J.F.S.d.C. Hydrothermal Treatment of Vegetable Oils and Fats Aiming at Yielding Hydrocarbons: A Review. Catalysts 2020, 10, 843. [Google Scholar] [CrossRef]
- Koch, C.C. Nanostructured Materials: Processing, Properties and Potential Applications; Noyes Pub.: Hartford, WI, USA; William Andrew Pub.: Hartford, WI, USA, 2002; ISBN 0815514514. [Google Scholar]
- Yang, G.; Park, S.J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef]
- ASTM E1479-24; Standard Practice for Describing and Specifying Inductively Coupled Plasma Atomic Emission Spectrometers. ASTM International: West Conshohocken, PA, USA, 2024.
- Terki, R.; Bertrand, G.; Aourag, H.; Coddet, C. Structural and Electronic Properties of Zirconia Phases: A FP-LAPW Investigations. Mater. Sci. Semicond. Process. 2006, 9, 1006–1013. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Pandey, M.; Singh, M.; Wasnik, K.; Gupta, S.; Patra, S.; Gupta, P.S.; Pareek, D.; Chaitanya, N.S.N.; Maity, S.; Reddy, A.B.M.; et al. Targeted and Enhanced Antimicrobial Inhibition of Mesoporous ZnO-Ag2O/Ag, ZnO-CuO, and ZnO-SnO2 Composite Nanoparticles. ACS Omega 2021, 6, 31615–31631. [Google Scholar] [CrossRef]
- Saikumari, N.; Dev, S.M.; Dev, S.A. Effect of Calcination Temperature on the Properties and Applications of Bio Extract Mediated Titania Nano Particles. Sci. Rep. 2021, 11, 1734. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Gupta, T.K.; Bechtold, J.H.; Cadoff, L.H.; Rossing, B.R. Stabilization of Tetragonal Phase in Polycrystalline Zirconia. J. Mater. Sci. 1977, 12, 2421–2426. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.A.; Matijevi’c, E.M. Preparation and Characterization of Nanosized Zirconium (Hydrous) Oxide Particles. J. Mater. Res. 1997, 12, 3286–3292. [Google Scholar] [CrossRef]
- Guo, G.Y.; Chen, Y.L.; Ying, W.J. Thermal, Spectroscopic and X-ray Diffractional Analyses of Zirconium Hydroxides Precipitated at Low PH Values. Mater. Chem. Phys. 2004, 84, 308–314. [Google Scholar] [CrossRef]
- Dwivedi, R.; Maurya, A.; Verma, A.; Prasad, R.; Bartwal, K.S. Microwave Assisted Sol-Gel Synthesis of Tetragonal Zirconia Nanoparticles. J. Alloys Compd. 2011, 509, 6848–6851. [Google Scholar] [CrossRef]
- Rashad, M.M.; Baioumy, H.M. Effect of Thermal Treatment on the Crystal Structure and Morphology of Zirconia Nanopowders Produced by Three Different Routes. J. Mater. Process. Technol. 2008, 195, 178–185. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Nataraj, S.K.; Wari, M.N.; Inamdar, S.R. Structural and Optical Properties of Zirconium Oxide (ZrO2) Nanoparticles: Effect of Calcination Temperature. Nano Express 2020, 1, 010022. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Xu, Y.; Gao, Y.; Chen, Z.; Wang, J. A Facile One-Pot Hydrothermal Process to Synthesize Sulfonated Mesoporous ZrO2. J. Porous Mater. 2016, 23, 489–495. [Google Scholar] [CrossRef]
- Boratto, M.H. Semiconducting and Insulating Oxides Applied to Electronic Devices. Ph.D. Thesis, Incheon National University, Incheon, Republic of Korea, 2018. [Google Scholar] [CrossRef]
- Shi, Q.; Yuan, W.; Chao, X.; Zhu, Z. Phase Stability, Thermal Conductivity and Crystal Growth Behavior of RE2O3 (RE = La,Yb,Ce,Gd) Co-Doped Y2O3 Stabilized ZrO2 Powder. J. Solgel. Sci. Technol. 2017, 84, 341–348. [Google Scholar] [CrossRef]
- Sun, L.; Guo, H.; Peng, H.; Gong, S.; Xu, H. Influence of Partial Substitution of Sc2O3 with Gd2O3 on the Phase Stability and Thermal Conductivity of Sc2O3-Doped ZrO2. Ceram. Int. 2013, 39, 3447–3451. [Google Scholar] [CrossRef]
- ZHOU, H.; YI, D. Effect of Rare Earth Doping on Thermo-Physical Properties of Lanthanum Zirconate Ceramic for Thermal Barrier Coatings. J. Rare Earths 2008, 26, 770–774. [Google Scholar] [CrossRef]
- Piticescu, R.R.; Slobozeanu, A.E.; Valsan, S.N.; Ciobota, C.F.; Ghita, A.N.; Motoc, A.M.; Chiriac, S.; Prakasam, M. Hydrothermal Synthesis of Nanocrystalline ZrO2-8Y2O3-XLn2O3 Powders (Ln = La, Gd, Nd, Sm): Crystalline Structure, Thermal and Dielectric Properties. Materials 2021, 14, 7432. [Google Scholar] [CrossRef]
- Available online: https://www.morgantechnicalceramics.com/en-Gb/Materials/Zirconia-ZrO2/ (accessed on 20 September 2024).
- Pasupuleti, K.T.; Ghosh, S.; Ramaswamy, P.; Narayana Murty, S.V.S. Zirconia Based Pyrochlore Thermal Barrier Coatings. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 9 December 2019; Volume 577. [Google Scholar]
- ASTM E1479-16; Standard Practice for Describing and Specifying Inductively Coupled Plasma Atomic Emission Spectrometers. ASTM International: West Conshohocken, PA, USA, 2016.


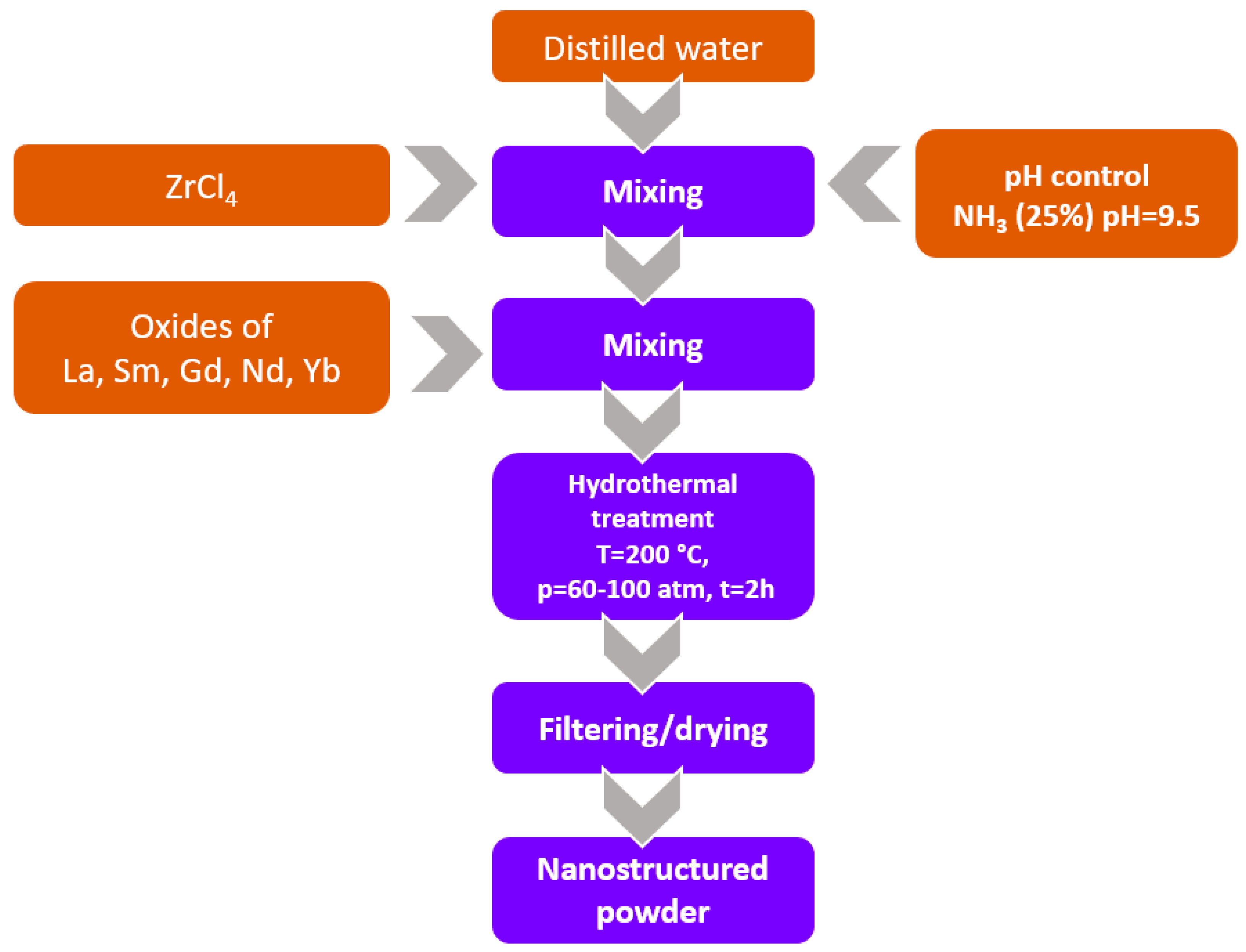
| Hydrothermal Process | |
|---|---|
| Advantages | Disadvantages |
| It is possible to control the size, shape, and morphology of the materials. | The method can be complex, requiring careful control of various parameters. |
| Low environmental impact | The autoclaves can be expensive. |
| Versatility (metal oxide, doped oxides, organic/inorganic structures) | Hydrothermal reactions can sometimes take a long time, impacting efficiency. |
| High-quality powders | The reaction takes place in a closed system, making it impossible to see firsthand. |
| Large-scale production | Working conditions can lead to reactor corrosion. |
| Refs. [21,22] | Refs. [21,23] |
| Chemical Analysis wt.% | |||||
|---|---|---|---|---|---|
| Zr | La | Sm | Gd | Yb | Nd |
| 41.8 | 7.44 | 6.60 | 7.85 | 8.74 | 6.32 |
| ZrO2-LSGYN | ZrO2-LSGYN_1200 | ZrO2-LSGYN_1500 | ||||
|---|---|---|---|---|---|---|
| ICDD PDF4+ | 04-014-2971 | 04-010-3278 | 04-007-2358 | 04-005-9865 | 00-024-1164 | 04-005-9865 |
| Symmetry | P42/nmc | P21/c | I3-3m | Fm-3m | P42/nmc | Fm-3m |
| a [Å] | 3.63293 | 5.12066 | 4.43861 | 5.21287 | 3.60927 | 5.2088 |
| b [Å] | 3.63293 | 5.26207 | 4.43861 | 5.21287 | 3.60927 | 5.2088 |
| c [Å] | 5.22511 | 5.43056 | 4.43861 | 5.21287 | 5.19192 | 5.2088 |
| alpha [°] | 90 | 90 | 90 | 90 | 90 | 90 |
| beta [°] | 90 | 99.68192 | 90 | 90 | 90 | 90 |
| gamma [°] | 90 | 90 | 90 | 90 | 90 | 90 |
| Volume [Å3] | 68.96 | 144.2437 | 87.44632 | 141.6545 | 67.63442 | 141.3234 |
| Crystal system | Tetragonal | Monoclinic | Cubic | Cubic | Tetragonal | Cubic |
| Rwp | 6.3733 | 4.8630 | 5.9777 | |||
| Analysis Type | Elements wt.% | |||||
|---|---|---|---|---|---|---|
| Zr | La | Sm | Gd | Yb | Nd | |
| Chemical | 41.8 | 7.44 | 6.60 | 7.85 | 8.74 | 6.32 |
| EDS | 40.2 | 6.39 | 4.43 | 5.31 | 13.37 | 4.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petriceanu, M.; Ioniță, F.G.; Piticescu, R.R.; Nicoară, A.I.; Matei, A.C.; Ioța, M.A.; Tudor, I.A.; Caramarin, Ș.; Ciobota, C.F. Effect of Doping ZrO2 on Structural and Thermal Properties. Inorganics 2024, 12, 290. https://doi.org/10.3390/inorganics12110290
Petriceanu M, Ioniță FG, Piticescu RR, Nicoară AI, Matei AC, Ioța MA, Tudor IA, Caramarin Ș, Ciobota CF. Effect of Doping ZrO2 on Structural and Thermal Properties. Inorganics. 2024; 12(11):290. https://doi.org/10.3390/inorganics12110290
Chicago/Turabian StylePetriceanu, Mirela, Florentina Gabriela Ioniță, Radu Robert Piticescu, Adrian Ionuț Nicoară, Alexandru Cristian Matei, Miruna Adriana Ioța, Ioan Albert Tudor, Ștefania Caramarin, and Cristina Florentina Ciobota. 2024. "Effect of Doping ZrO2 on Structural and Thermal Properties" Inorganics 12, no. 11: 290. https://doi.org/10.3390/inorganics12110290
APA StylePetriceanu, M., Ioniță, F. G., Piticescu, R. R., Nicoară, A. I., Matei, A. C., Ioța, M. A., Tudor, I. A., Caramarin, Ș., & Ciobota, C. F. (2024). Effect of Doping ZrO2 on Structural and Thermal Properties. Inorganics, 12(11), 290. https://doi.org/10.3390/inorganics12110290









