Hyperthermia and Photocatalytic Performance of Magnetic Polyvinyl Alcohol under External Magnetic Field
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Nanoparticles
2.2. Magnetic Behavior of the Particles
2.3. Photoactivity of the Prepared Nanoparticles
2.4. Hyperthermia Performance
2.5. Hydrogen Production Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of Magnetic Materials
3.2.1. Synthesis of Iron Oxide Nanoparticles (IONPs)
3.2.2. Synthesis of Polymeric Polyvinyl Alcohol–Stabilized Iron Oxide Nanoparticles (PVA@IONPs)
3.3. Hyperthermia Performance of the Fabricated Magnetic Nanoparticles
3.4. Photocatalytic Performance of the Fabricated Nanoparticles
3.5. Characterizations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vera, M.; Mella, C.; Urbano, B.F. Smart polymer nanocomposites: Recent advances and perspectives. J. Chil. Chem. Soc. 2020, 65, 4973–4981. [Google Scholar] [CrossRef]
- Talaat, A.; Suraj, M.V.; Byerly, K.; Wang, A.; Wang, Y.; Lee, J.K.P.; Ohodnicki, R., Jr. Review on soft magnetic metal and inorganic oxide nanocomposites for power applications. J. Alloys Compd. 2021, 870, 159500. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric Nanocomposites for Environmental and Industrial Applications. Int. J. Mol. Sci. 2022, 23, 1023. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fahy, W.P.; Kim, S.; Kim, H.; Zhao, N.; Pilato, L.; Kafi, A.; Bateman, S.; Koo, J.H. Recent developments in polymers/polymer nanocomposites for additive manufacturing. Prog. Mater. Sci. 2020, 111, 100638. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Bhaduri, D.; Sarkar, B.; Rusmin, R.; Hou, D.; Khanam, R.; Sarkar, S.; Biswas, J.K.; Vithanage, M.; Bhatnagar, A. Clay–polymer nanocomposites: Progress and challenges for use in sustainable water treatment. J. Hazard. Mater. 2020, 383, 121125. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kulawik, P.; Kopel, P. The effect of nanofillers on the functional properties of biopolymer-based films: A review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef] [PubMed]
- Jawed, A.; Saxena, V.; Pandey, L.M. Engineered nanomaterials and their surface functionalization for the removal of heavy metals: A review. J. Water Process Eng. 2020, 33, 101009. [Google Scholar] [CrossRef]
- Meng, S.; Li, Y.; Liu, Y.; Zhan, S.; Ma, Q.; Li, Y. Recent advances and mechanisms in magnetic field enhanced photocatalysis: A review. Environ. Surf. Interfaces 2023, 1, 10–23. [Google Scholar] [CrossRef]
- Yang, H.; Wang, F.; Zhang, H.; Guo, L.; Hu, L.; Wang, L.; Xue, D.J.; Xu, X. Solution Synthesis of Layered van der Waals (vdW) Ferromagnetic CrGeTe3 Nanosheets from a Non-vdW Cr2Te3Template. J. Am. Chem. Soc. 2020, 142, 4438–4444. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Xu, T.; Liu, B.; Huang, K.; Qiao, L.; Liu, S.; Cao, J.; Jun, S.C.; Yamauchi, Y.; et al. Atomic-Level Platinum Filling into Ni-Vacancies of Dual-Deficient NiO for Boosting Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2022, 12, 2200434. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Huang, K.; Zheng, X.; Qiao, L.; Liu, S.; Cao, J.; Jun, S.C.; Yamauchi, Y.; Qi, J. Tensile Strain-Mediated Spinel Ferrites Enable Superior Oxygen Evolution Activity. J. Am. Chem. Soc. 2023, 145, 24218–24229. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. 3D printed magnetic polymer composite hydrogels for hyperthermia and magnetic field driven structural manipulation. Prog. Polym. Sci. 2022, 131, 101574. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Design of magnetic hydrogels for hyperthermia and drug delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef]
- Biswas, A.; Patra, A.K.; Sarkar, S.; Das, D.; Chattopadhyay, D.; De, S. Synthesis of highly magnetic iron oxide nanomaterials from waste iron by one-step approach. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124420. [Google Scholar] [CrossRef]
- Maity, D.; Mollick, M.R.; Mondal, D.; Bhowmick, B.; Neogi, S.K.; Banerjee, A.; Chattopadhyay, S.; Bandyopadhyay, S.; Chattopadhyay, D. Synthesis of HPMC stabilized nickel nanoparticles and investigation of their magnetic and catalytic properties. Carbohydr. Polym. 2013, 98, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Wanna, Y.; Chindaduang, A.; Tumcharern, G.; Phromyothin, D.; Porntheerapat, S.; Nukeaw, J.; Hofmann, H.; Pratontep, S. Efficiency of SPIONs functionalized with polyethylene glycol bis (amine) for heavy metal remova. J. Magn. Magn. Mater. 2016, 414, 32–37. [Google Scholar] [CrossRef]
- Qiu, X.P.; Winnik, F. Preparation and characterization of PVA coated magnetic nanoparticles. Chin. J. Polym. Sci. 2000, 18, 535–539. [Google Scholar]
- Maleki, A.; Rahimi, J.; Hajizadeh, Z.; Niksefat, M. Synthesis and characterization of an acidic nanostructure based on magnetic polyvinyl alcohol as an efficient heterogeneous nanocatalyst for the synthesis of a-aminonitriles. J. Organomet. Chem. 2019, 881, 58–65. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Song, Y.; Han, B.; Hu, X.; Wang, X.; Lin, Y.; Deng, X. Magnetic biodegradable Fe3O4/CS/PVA nanofibrous membranes for bone regeneration. Biomed Mater. 2011, 6, 55008. [Google Scholar] [CrossRef]
- Zélis, P.M.; Muraca, D.; Gonzalez, J.S. Magnetic properties study of iron-oxide nanoparticles/PVA ferrogels with potential biomedical applications. J. Nanopart. Res. 2013, 15, 1613. [Google Scholar] [CrossRef]
- Saghafi, M.; Hosseini, S.A.; Zangeneh, S.; Moghanian, A.H.; Salarvand, V.; Vahedi, S.; Mohajerzadeh, S. Charge storage properties of mixed ternary transition metal ferrites MZnFe oxides (M 14 Al, Mg, Cu, Fe, Ni) prepared by hydrothermal method. SN Appl. Sci. 2019, 1, 1303. [Google Scholar] [CrossRef]
- Ye, Y.; Al-Khaledi, N.; Barker, L.; Darwish, M.S.; El Naggar, A.M.; El-Yahyaoui, A.; Hussein, A.; Hussein, E.S.; Shang, D.; Taha, M.; et al. Uranium resources in China’s phosphate rocks—Identifying low-hanging fruits. IOP Conf. Ser. Earth Environ. Sci. 2019, 227, 052033. [Google Scholar] [CrossRef]
- Motawie, M.; Hanafi, S.A.; Elmelawy, M.S.; Ahmed, S.M.; Mansour, N.A.; Darwish, M.S.; Abulyazied, D.E. Wax co-cracking synergism of high density polyethylene to alternative fuels. Egypt. J. Pet. 2015, 24, 353–361. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Kunz, U.; Peuker, U. Preparation and catalytic use of platinum in magnetic core/shell nanocomposites. J. Appl. Polym. Sci. 2013, 129, 1806–1811. [Google Scholar] [CrossRef]
- Bhagwat, V.R.; Humbe, A.V.; More, S.D.; Jadhav, K.M. Sol-gel auto combustion synthesis and characterizations of cobalt ferrite nanoparticles: Different fuels approach. Mater. Sci. Eng. B 2019, 248, 114388. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; El-Sabbagh, A.; Stibor, I. Hyperthermia properties of magnetic polyethylenimine core/shell nanoparticles: Influence of carrier and magnetic strength. J. Polym. Res. 2015, 22, 239. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Jain, N.; Parekh, K. Investigating the effect of outer layer of magnetic particles on cervical cancer cells HeLa by magnetic fuid hyperthermia. Cancer Nano 2021, 12, 7. [Google Scholar] [CrossRef]
- Anbarasu, M.; Anandan, M.; Chinnasamy, E.; Gopinath, V.; Balamurugan, K. Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim. Acta A 2015, 135, 536–539. [Google Scholar] [CrossRef]
- Sarkar, A.; Biswas, S.K.; Pramanik, P. Design of a new nanostructure comprising mesoporous ZrO2 shell and magnetite core (Fe3O4@mZrO2) and study of its phosphate ion separation efficiency. J. Mater. Chem. 2010, 20, 4417–4424. [Google Scholar] [CrossRef]
- Kmita, A.; Lachowicz, D.; Żukrowski, J.; Gajewska, M.; Szczerba, W.; Kuciakowski, J.; Zapotoczny, S.; Sikora, M. One-step synthe sis of long term stable superparamagnetic colloid of zinc ferrite nanorods in water. Materials 2019, 12, 1048. [Google Scholar] [CrossRef] [PubMed]
- Demerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of poly vinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Vilos, C.; Gutierrez, M.; Escobar, R.; Morales, F.; Denardin, J.; Velásquez, L.; Altbir, D. Superparamagnetic poly (3-hydroxybu tyrate-co-3 hydroxyvalerate) (PHBV) nanoparticles for biomedical applications. Electron. J. Biotechnol. 2013, 16, 5. [Google Scholar] [CrossRef]
- Sethulakshmi, N.; Sooraj, V.; Sajeev, U.S.; Nair, S.S.; Narayanan, T.N.; Joy, L.K.; Joy, P.A.; Ajayan, P.M.; Anantharaman, M.R. Contact potential induced enhancement of magnetization in polyaniline coated nanomagnetic iron oxides by plasma polymerization. Appl. Phys. Lett. 2013, 103, 162414. [Google Scholar] [CrossRef]
- Mol, B.; Beeran, A.E.; Jayaram, P.S.; Prakash, P.; Jayasree, R.S.; Thomas, S.; Chakrapani, B.; Anantharaman, M.R.; Bushiri, M.J. Radio frequency plasma assisted surface modifcation of Fe3O4 nanoparticles using polyaniline/polypyrrole for bioimaging and magnetic hyperthermia applications. J. Mater. Sci. Mater. Med. 2021, 32, 108. [Google Scholar] [CrossRef] [PubMed]
- Kurchania, R.; Sawant, S.S.; Ball, R.J. Synthesis and characterization of magnetite/polyvinyl alcohol core-shell composite nanoparticles. J. Am. Ceram. Soc. 2014, 97, 3208–3215. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mater. Sci. Eng. C Biom. Mater. Sens. Syst. 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Badawy, S.M.; Abd, E.-L. Synthesis and characterizations of magnet ite nanocomposite films for radiation shielding. Polym. Compos. 2017, 38, 974–980. [Google Scholar] [CrossRef]
- Piñeiro-Redondo, Y.; Bañobre-López, M.; Pardiñas-Blanco, I.; Goya, G.; López-Quintela, M.A.; Rivas, J. The influence of colloidal parameters on the specific power absorption of PAA-coated magnetite nanoparticles. Nanoscale Res. Lett. 2011, 6, 383. [Google Scholar] [CrossRef]
- Ma, M.; Wu, Y.; Zhou, J.; Sun, Y.; Zhang, Y.; Gu, N. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J. Magn. Magn. Mater. 2004, 268, 33–39. [Google Scholar] [CrossRef]
- Hergt, R.; Hiergeist, R.; Zeisberger, M.; Glockl, G.; Weitschies, W.; Ramirez, L.P.; Hilger, I.; Kaiser, W.A. Enhancement of AC-losses of magnetic nanoparticles for heating applications. J. Magn. Magn. Mater. 2004, 80, 358–368. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—Biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Muller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials develop ment for cancer therapy. J. Phys. Condens. Matter. 2006, 18, S2919. [Google Scholar] [CrossRef]
- Nigam, S.; Barick, K.; Bahadur, D. Development of citrate-stabilized Fe3O4 nanoparticles: Conjugation and release of doxorubicin for therapeutic applications. J. Magn. Magn. Mater. 2011, 323, 237–243. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, Á.V.; Schneider, E.K.; Checa Fernández, B.L.; Iglesias, G.R. Magnetic nanoparticles coated with a thermosensitive polymer with hyperthermia properties. Polymers 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Shete, P.B.; Patil, R.M.; Thorat, N.D.; Prasad, A.; Ningthoujam, R.S.; Ghosh, S.J.; Pawar, S.H. Magnetic chitosan nanocomposite for hyperthermia therapy application: Preparation, characterization and in vitro experiments. Appl. Surf. Sci. 2014, 288, 149–157. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Stibor, I. Preparation and characterization of magnetite–PDMS composites by magnetic 1053 induction heating. Mater. Chem. Phys. 2015, 164, 163–169. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Polyethyleneimine-mediated synthesis of folic acid-targeted iron oxide nanoparticles for in vivo tumor MR imaging. Biomaterials 2013, 34, 8382–8392. [Google Scholar] [CrossRef]
- Nitin, N.; LaConte, L.E.W.; Zurkiya, O.; Hu, X.; Bao, G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J. Biol. Inorg. Chem. 2004, 9, 706–712. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating Magnetic Fluid with Alternating Magnetic Field. J. Magn. Magn. Mater. 2002, 252, 370. [Google Scholar] [CrossRef]
- Noh, S.H.; Na, W.; Jang, J.T.; Lee, J.H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Gupta, R. Magnetically mediated release of ciprofloxacin from polyvinyl alcohol based superparamagnetic nanocomposites. J. Mater. Sci. Mater. Med. 2011, 22, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Le Renard, P.E.; Lortz, R.; Senatore, C.; Rapin, J.P.; Buchegger, F.; Petri-Fink, A.; Hofmann, H.; Doelker, E.; Jordan, O. Magnetic and in vitro heating properties of implants formed in situ from injectable formulations and containing superparamagnetic iron oxide nanoparticles (SPIONs) embedded in silica microparticles for magnetically induced local hyperthermia. J. Magn. Magn. Mater. 2011, 323, 1054–1063. [Google Scholar] [CrossRef]
- Owens, F. Ferromagnetic resonance observation of a phase transition in magnetic field-aligned Fe2O3 nanoparticles. J. Magn. Magn. Mater. 2009, 321, 2386–2391. [Google Scholar] [CrossRef]
- Vassallo, M.; Martella, D.; Barrera, G.; Celegato, F.; Coïsson, M.; Ferrero, R.; Olivetti, E.S.; Troia, A.; Sözeri, H.; Parmeggiani, C.; et al. Improvement of Hyperthermia Properties of Iron Oxide Nanoparticles by Surface Coating. ACS Omega 2023, 8, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K.; Lemine, O.M.; Ali, R.; Huwaizi, S.M.; Al-Humaid, S.; AlKushi, A. Evaluating magnetic and thermal effects of various Polymerylated magnetic iron oxide nanoparticles for combined chemo-hyperthermia. New J. Chem. 2022, 46, 5489–5504. [Google Scholar] [CrossRef]
- Nisticò, R.; Cesano, F.; Garello, F. Magnetic Materials and Systems: Domain Structure Visualization and Other Characterization Techniques for the Application in the Materials Science and Biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kang, M. Hydrogen production from methanol–water decomposition in a liquid photosystem using the anatase structure of Cu loaded TiO2. Int. J. Hydrog. Energy 2007, 32, 3841–3848. [Google Scholar] [CrossRef]
- Chen, J.; Ollis, D.F.; Rulken, W.H.; Bruning, H. Photocatalyzed oxidation of alcohols and organochlorides in the presence of nativeTiO2 and metallized TiO2 suspensions. Part (II): Photocatalytic mechanisms. Water Res. 1999, 33, 669–676. [Google Scholar] [CrossRef]
- Darwish, M.S.; El Naggar, A.M.; Morshedy, A.S.; Haneklaus, N. Increased production of hydrogen with in situ CO2 capture through the process of water splitting using magnetic core/shell structures as novel photocatalysts. Environ. Sci. Pollut. Res. 2021, 28, 3566–3578. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Wang, Y.H.; Chau, Y.F.C.; Chiang, H.P.; Wu, J.C.S. Magnetic Field-Enhancing Photocatalytic Reaction in Micro Optofluidic Chip Reactor. Nanoscale Res. Lett. 2019, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Peng, R.; Yang, Y.; Zhao, X.; Cui, C.; Su, X.; Qin, W.; Dai, Y.; Ma, Y.; Liu, H.; et al. Electron Spin Polarization-Enhanced Photoinduced Charge Separation in Ferromagnetic ZnFe2O4. ACS Energy Lett. 2021, 6, 2129–2137. [Google Scholar] [CrossRef]
- Li, J.; Pei, Q.; Wang, R.Y.; Zhou, Y.; Zhang, Z.M.; Cao, Q.Q.; Wang, D.H.; Mi, W.B.; Du, Y.W. Enhanced photocatalytic performance through magnetic field boosting carrier transport. ACS Nano 2018, 12, 3351–3359. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, W.; Pan, J.; Zhang, Y.; Wang, F.; Wang, L.; Zhao, Q. External field assisted hydrogen evolution reaction. Nano Res. 2023, 16, 8638–8654. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen production using Fe2O3-based core shell nano particles with ZnS and CdS. Int. J. Hydrog. Energy 2014, 39, 1613–1622. [Google Scholar] [CrossRef]
- Madhumitha, A.; Preethi, V.; Kanmani, S. Photocatalytic hydrogen production using TiO2 coated iron-oxide core shell particles. Int. J. Hydrog. Energy 2018, 43, 3946–3956. [Google Scholar] [CrossRef]
- Tian, F.-Y.; Hou, D.; Tang, F.; Deng, M.X.-Q.; Zhang, Q.; Wu, T.; Li, D.-S. Novel Zn0.8Cd0.2S@g-C3N4 core–shell heterojunctions with a twin structure for enhanced visible-light-driven photocatalytic hydrogen generation. J. Mater. Chem. A 2018, 6, 17086–17094. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lee, Z.; Wei, M.D.; Chang, C.-C.; Chu, K.-W. Photocatalytic hydrogen production by magnetically separable Fe3O4@ZnS and NiCo2O4@ZnS core-shell nanoparticles. Int. J. Hydrog. Energy 2015, 40, 11436–11443. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Ababtain, A.S.; Alanazi, H.S.; Al-Nafaei, W.S.; Hasan, I. Synthesis of Zn3V2O8/rGO Nanocomposite for Photocatalytic Hydrogen Production. Inorganics 2023, 11, 93. [Google Scholar] [CrossRef]
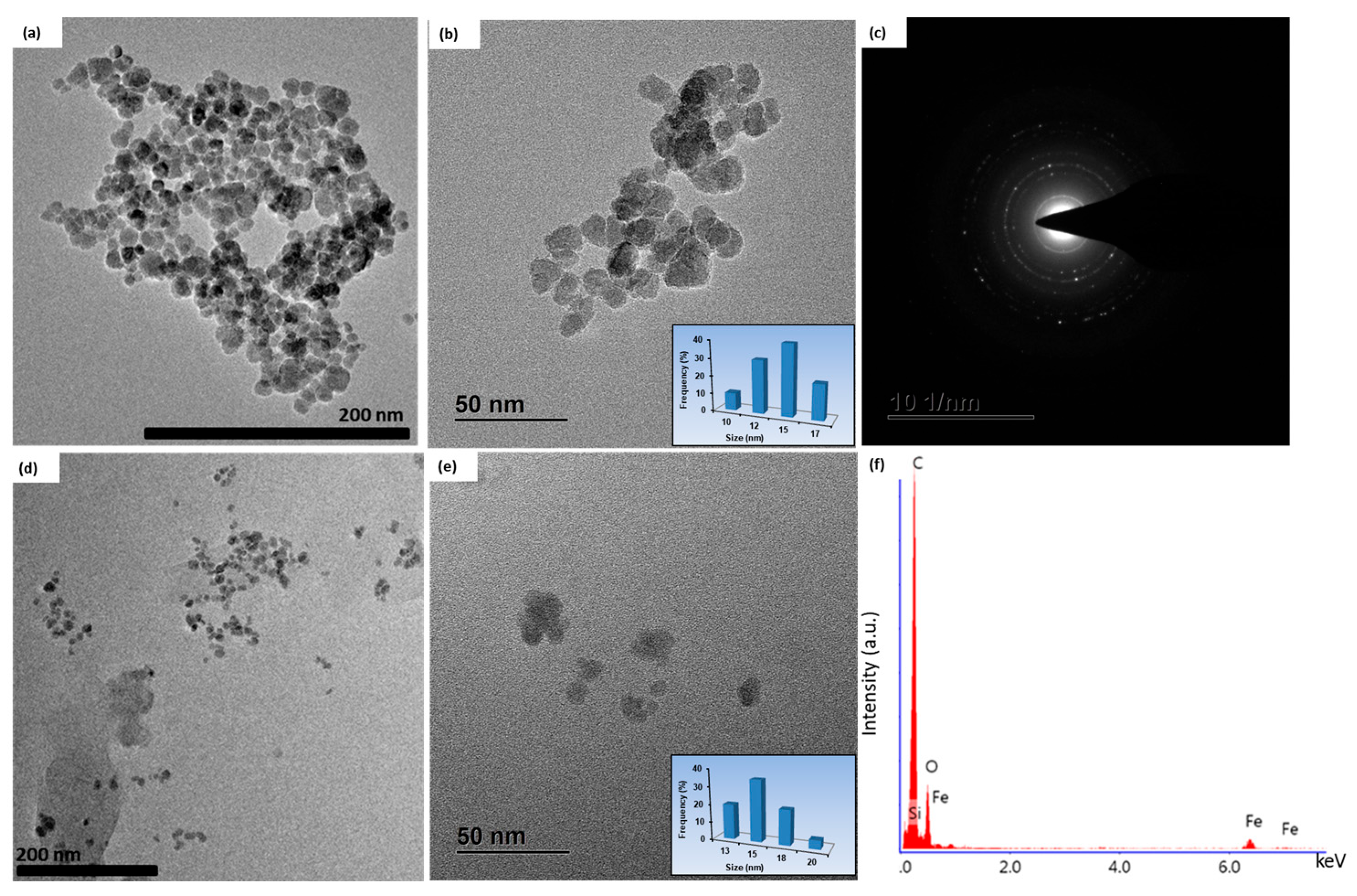
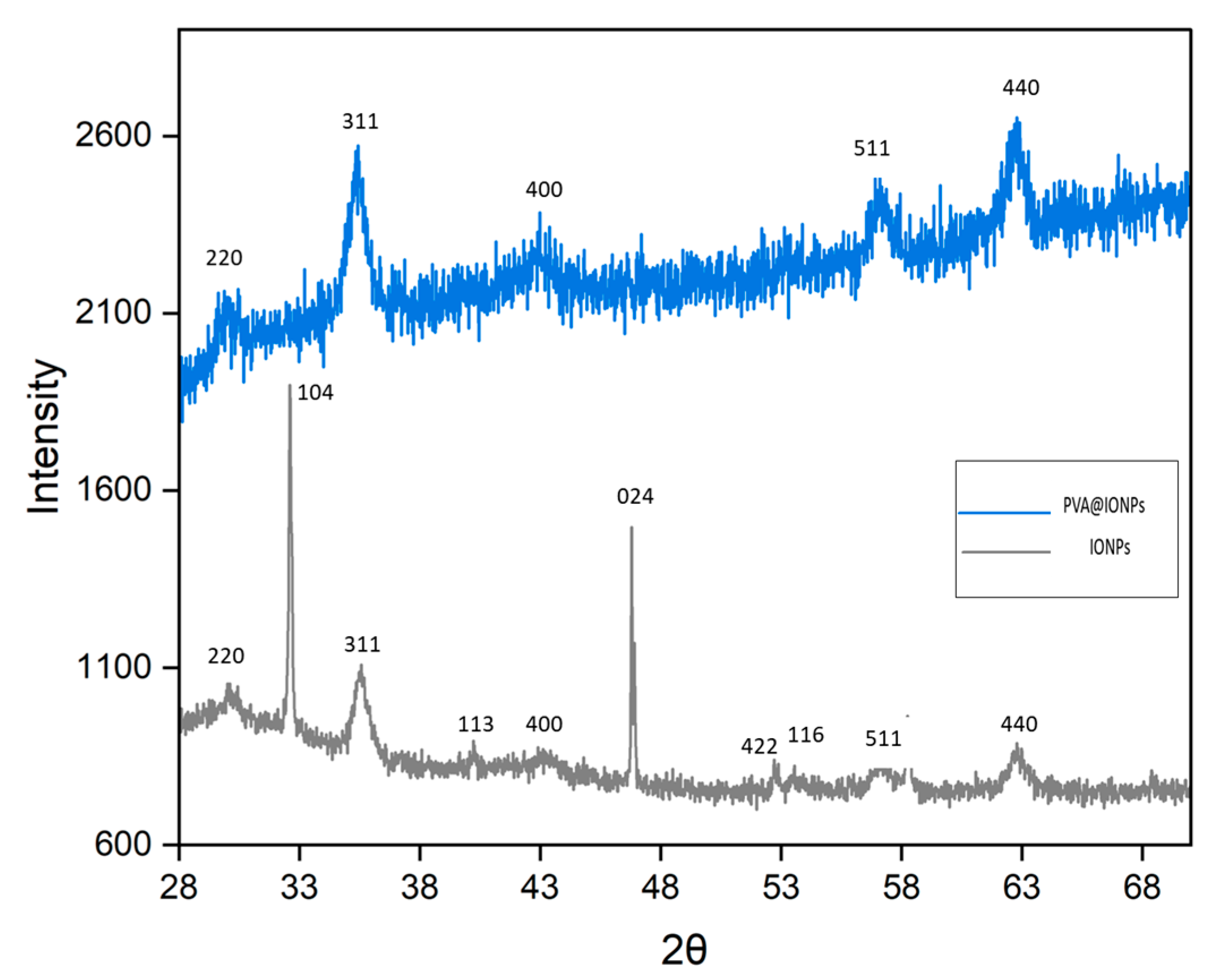

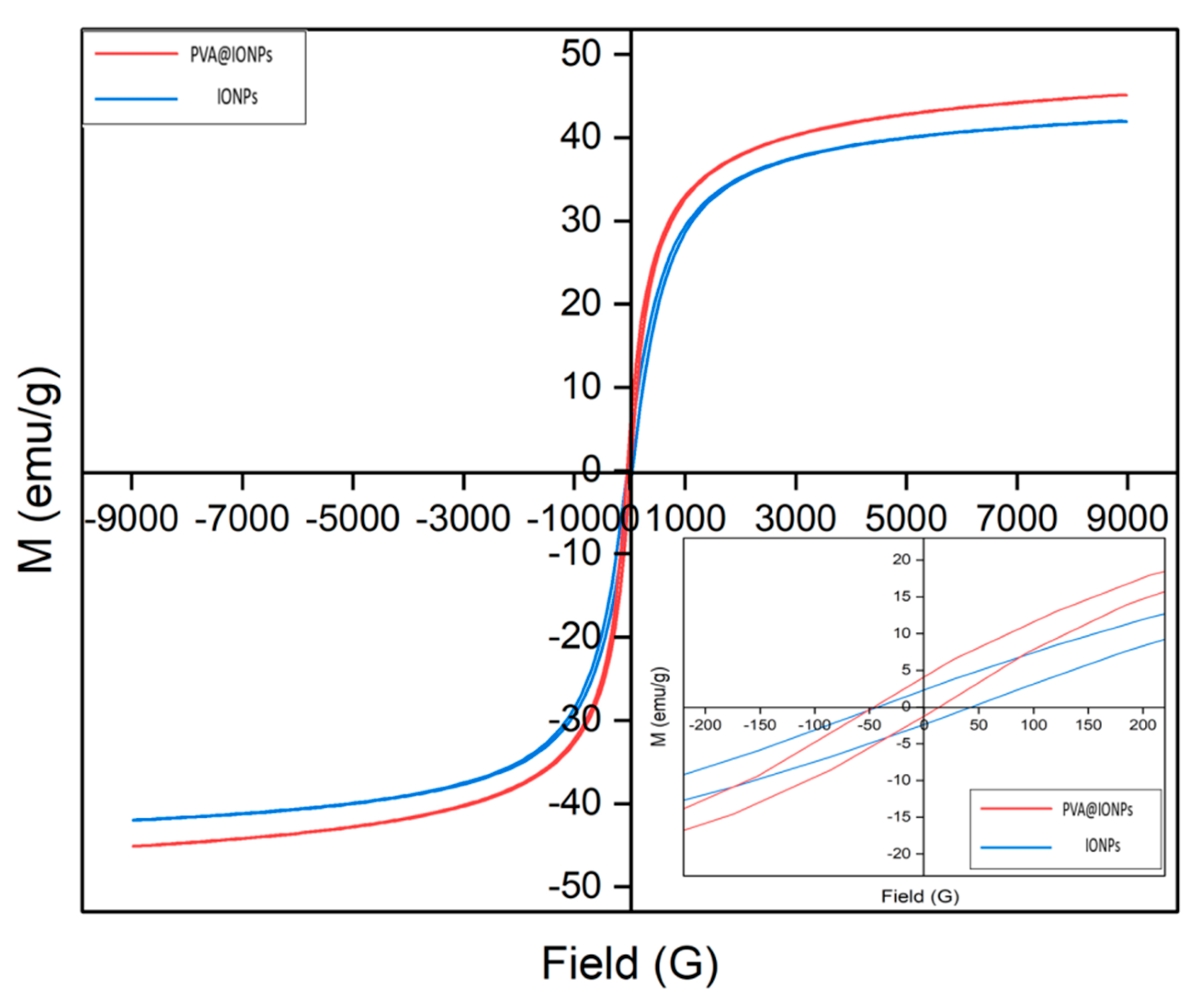

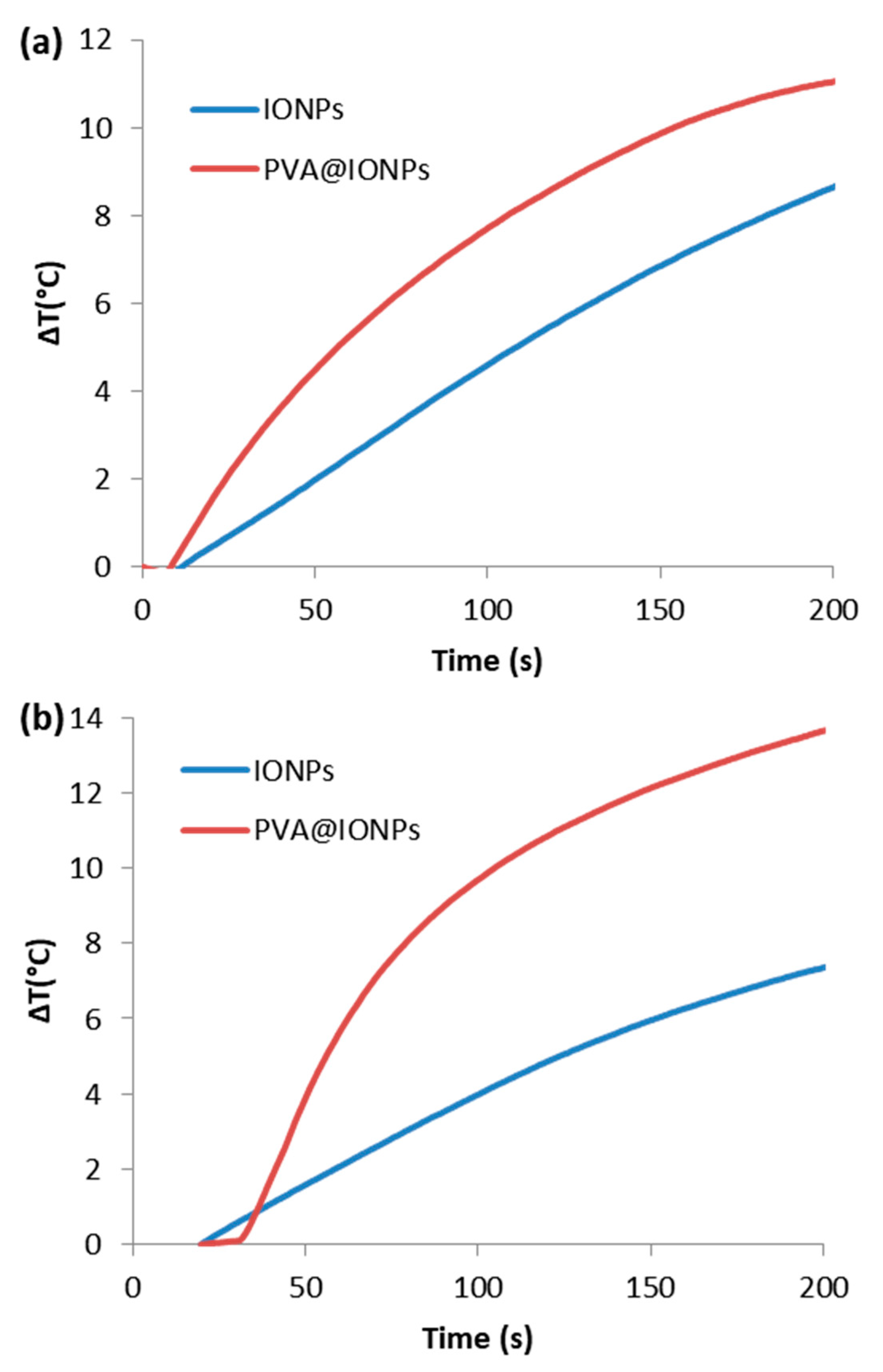

| Magnetic Particles | Coating Layer | Size (nm) | Ms (emu g−1) | SAR (W g−1) | Ref. |
|---|---|---|---|---|---|
| Fe3O4 | PEG | 19 | 80 | 2452 | [43] |
| Fe3O4 | Silica | 10 | 3 | 20 | [54] |
| Fe3O4 | Silica | 14.8 | 2.5 | 20 | [55] |
| Fe3O4 | - | 7.5 | 10 | 15.5 | [40] |
| Fe3O4 | Chitosan | 15.1 ± 5.0 | 49.96 | 118.85 | [46] |
| Fe3O4 | Citrate | 10 | 76 | 171 | [56] |
| Fe3O4 | Dextran | 5–15 | 49 | 36 | [57] |
| Fe3O4 | - | 10–12 | 41.98 | 55.6 | Our study |
| Fe3O4 | PVA | 10–12 | 45.08 | 65.9 | Our study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khowdiary, M.M.; Alsnani, H.; Darwish, M.S.A. Hyperthermia and Photocatalytic Performance of Magnetic Polyvinyl Alcohol under External Magnetic Field. Inorganics 2024, 12, 47. https://doi.org/10.3390/inorganics12020047
Khowdiary MM, Alsnani H, Darwish MSA. Hyperthermia and Photocatalytic Performance of Magnetic Polyvinyl Alcohol under External Magnetic Field. Inorganics. 2024; 12(2):47. https://doi.org/10.3390/inorganics12020047
Chicago/Turabian StyleKhowdiary, Manal M., Hind Alsnani, and Mohamed S. A. Darwish. 2024. "Hyperthermia and Photocatalytic Performance of Magnetic Polyvinyl Alcohol under External Magnetic Field" Inorganics 12, no. 2: 47. https://doi.org/10.3390/inorganics12020047
APA StyleKhowdiary, M. M., Alsnani, H., & Darwish, M. S. A. (2024). Hyperthermia and Photocatalytic Performance of Magnetic Polyvinyl Alcohol under External Magnetic Field. Inorganics, 12(2), 47. https://doi.org/10.3390/inorganics12020047







