Calcium Ferrite Nanoparticles: A Simple Synthesis Approach for the Effective Disposal of Congo Red Dye from Aqueous Environments
Abstract
1. Introduction
2. Experimental
2.1. Materials
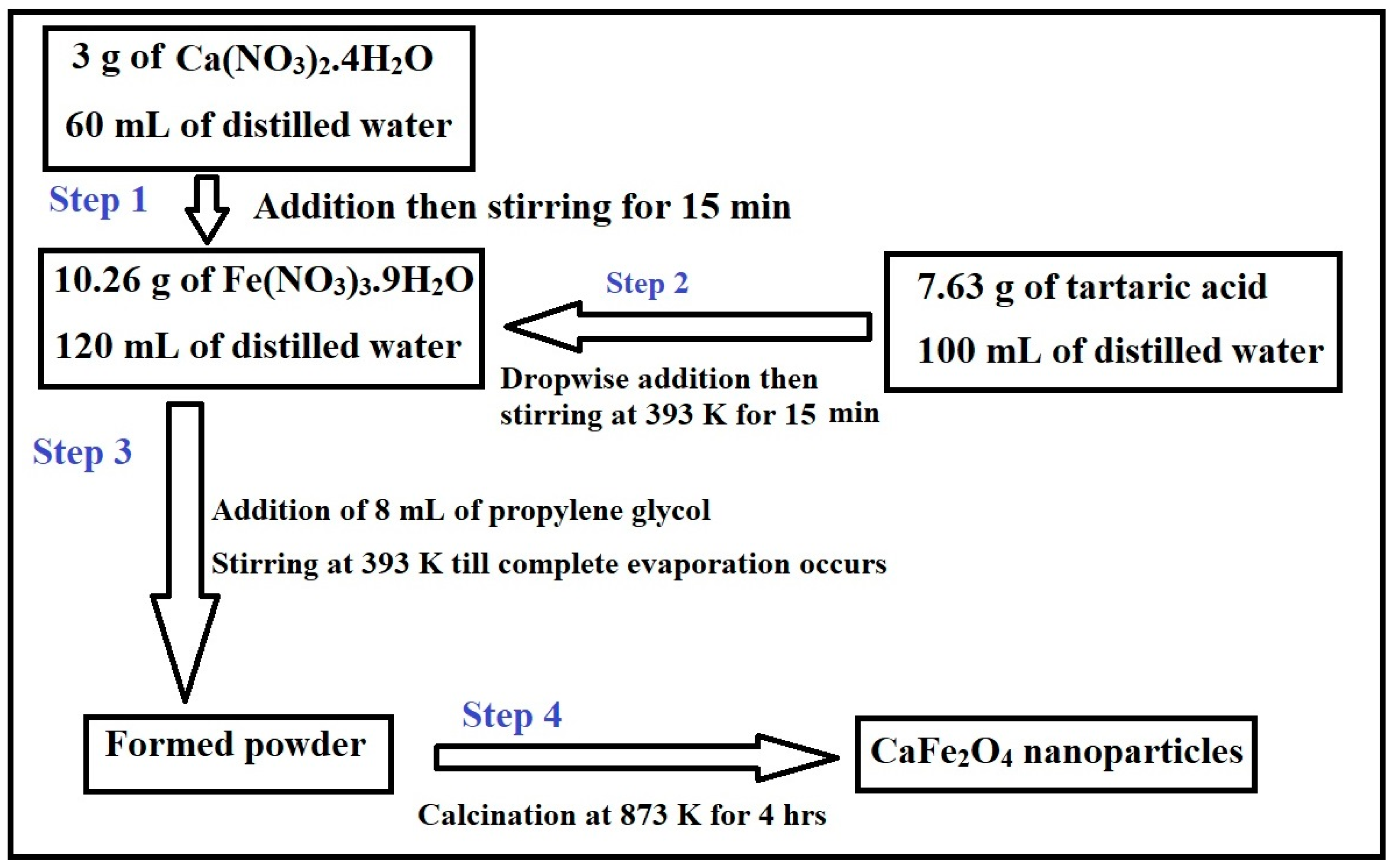
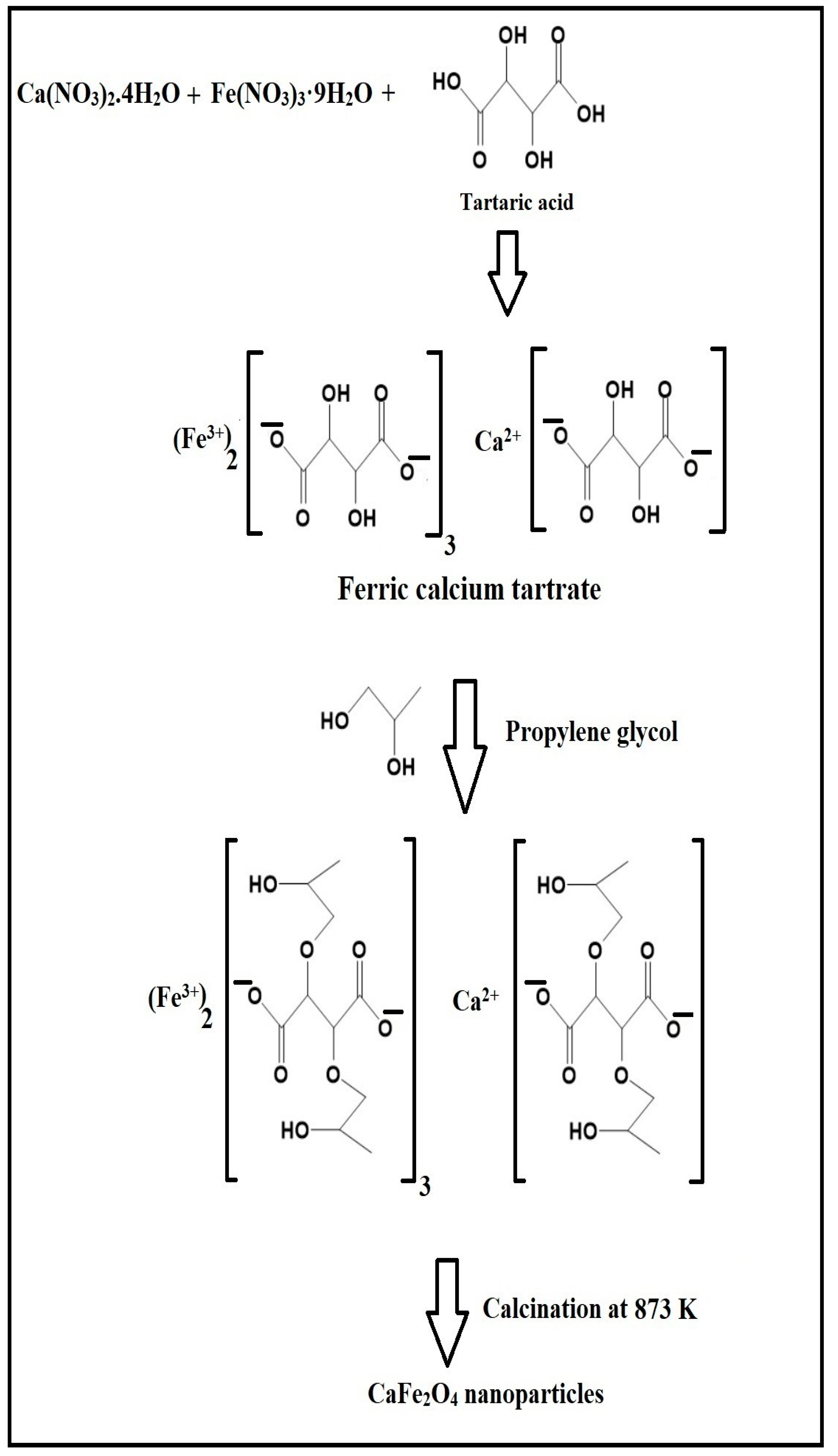
2.2. Synthesis of CaFe2O4 Nanoparticles
2.3. Characterization
2.4. Removal of Congo Red Dye from Aqueous Media
3. Results and Discussion
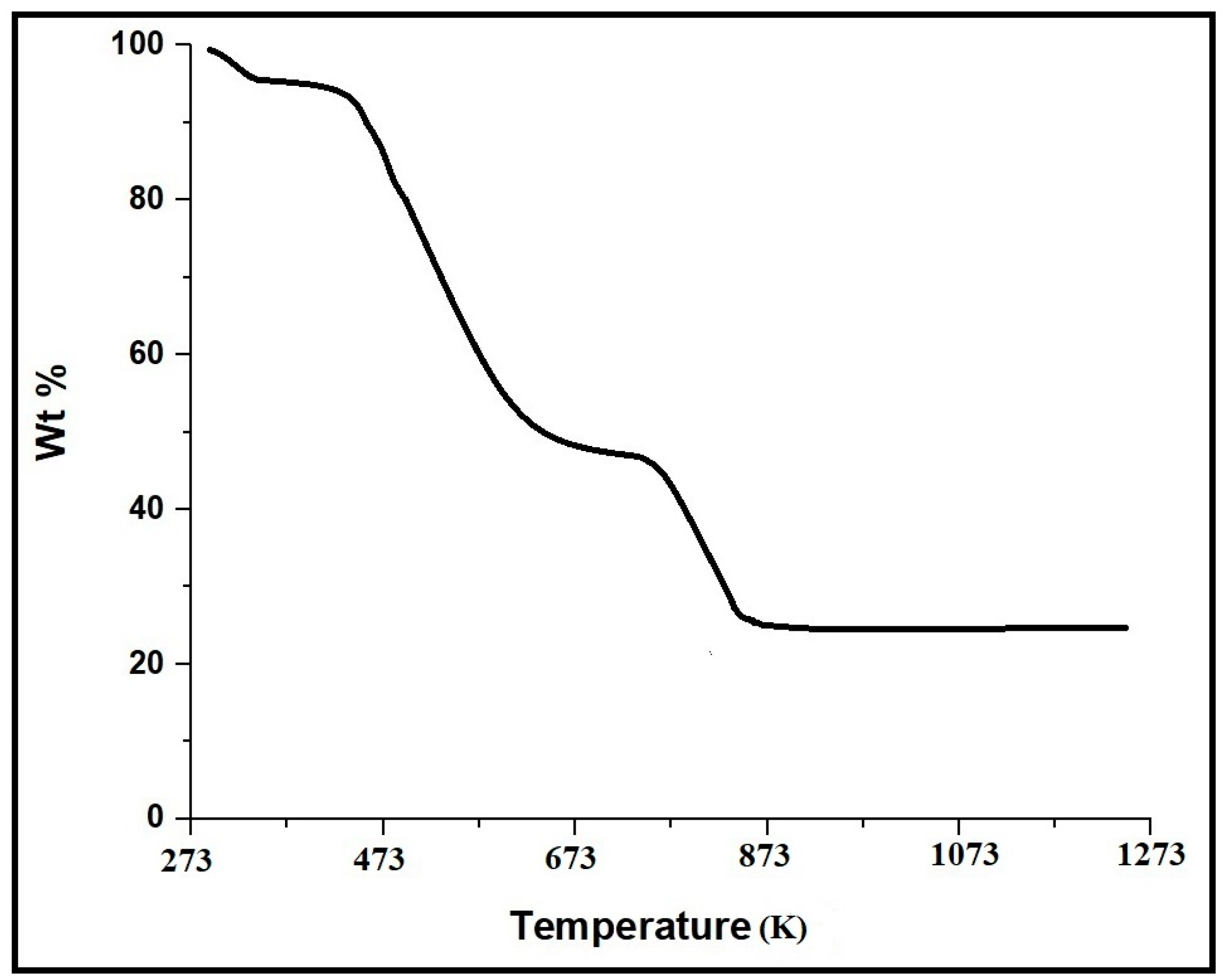
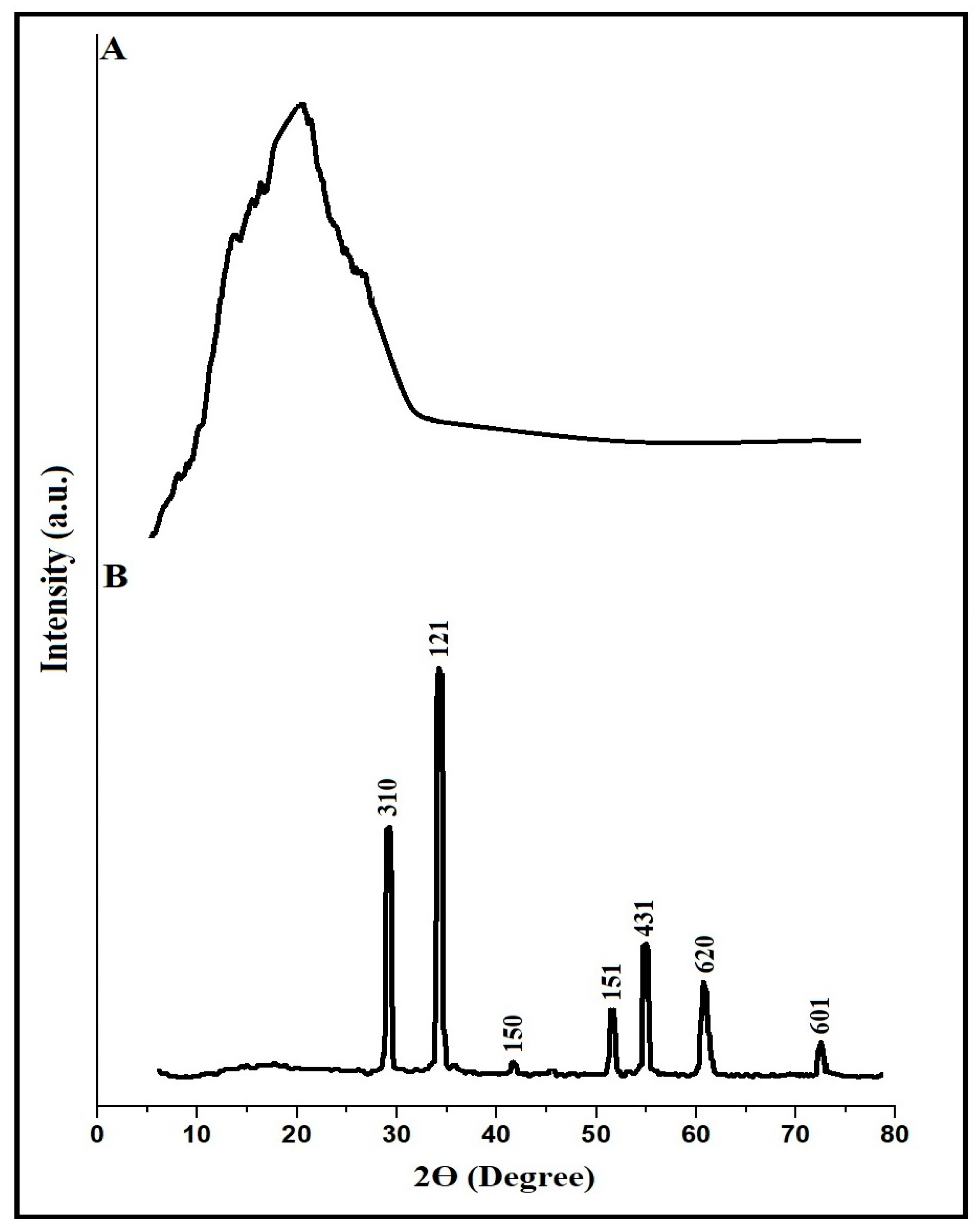
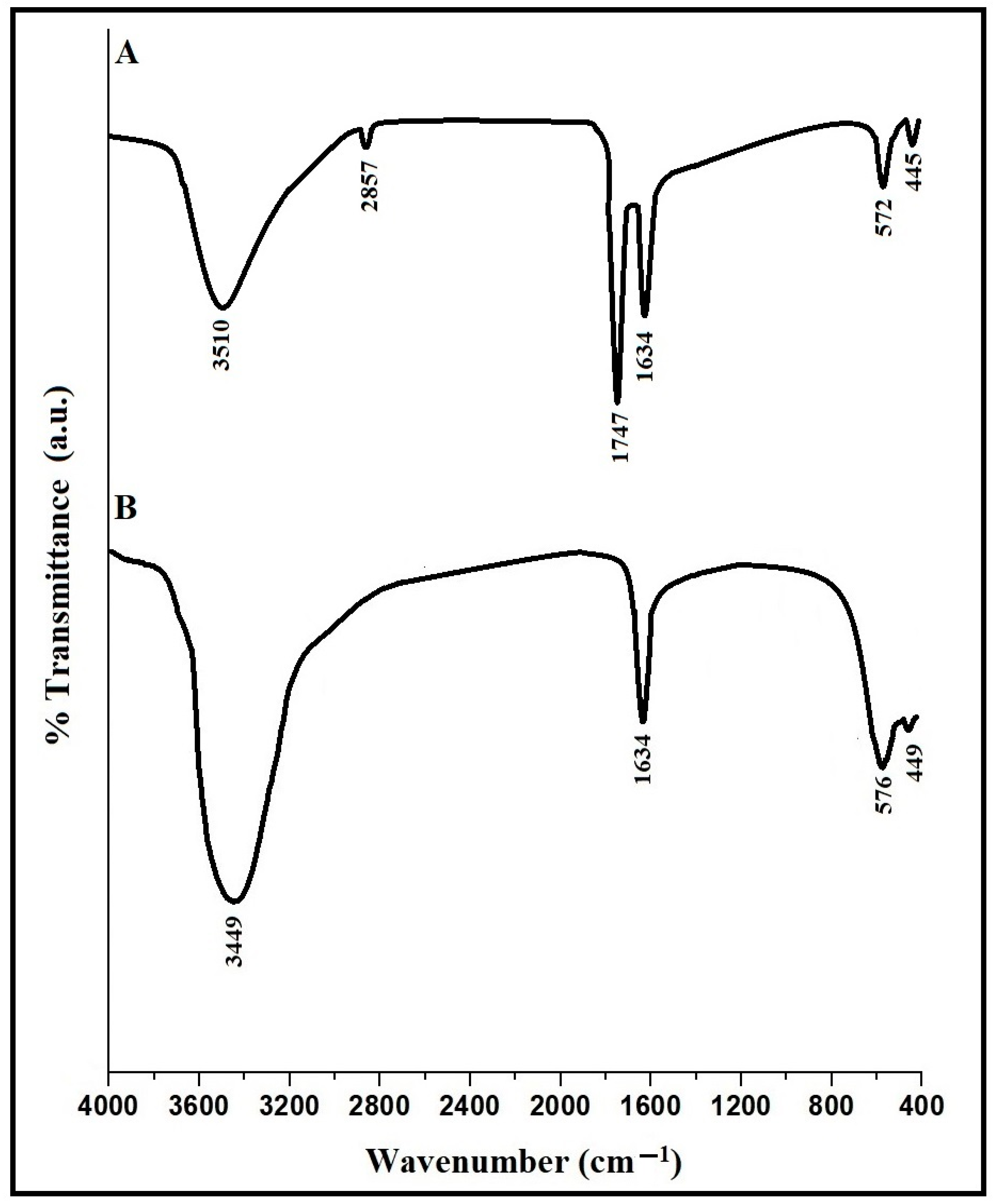
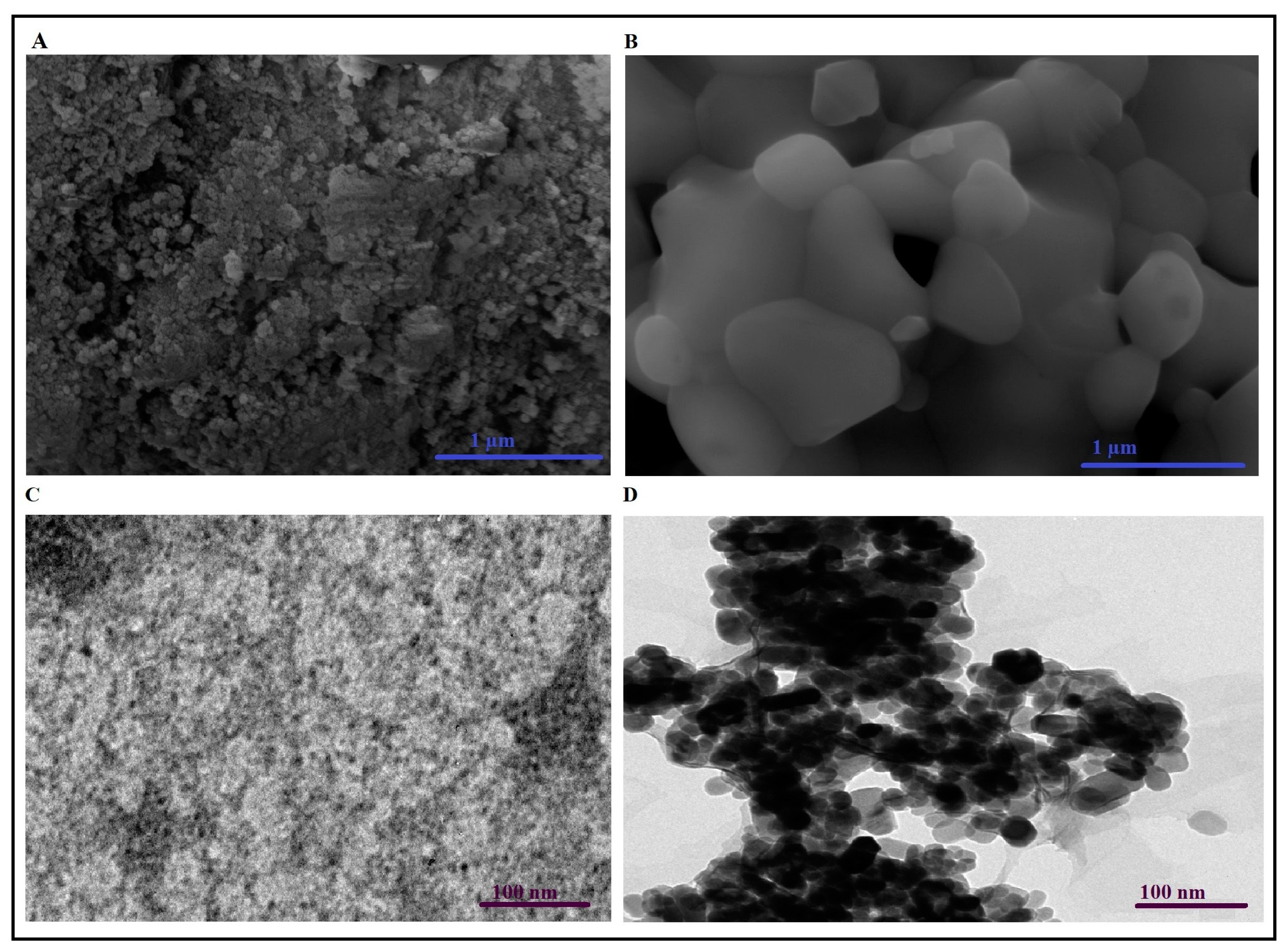
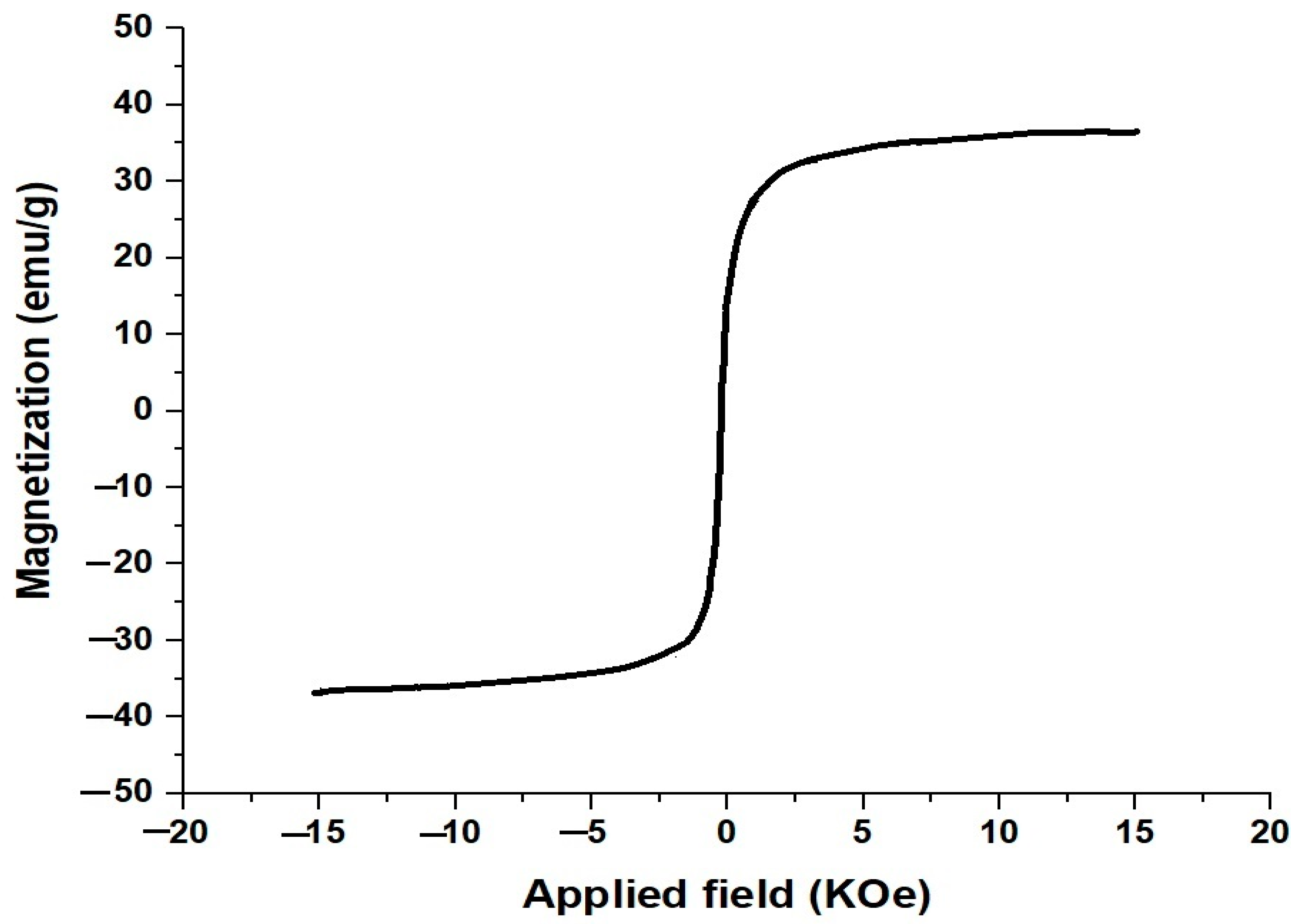
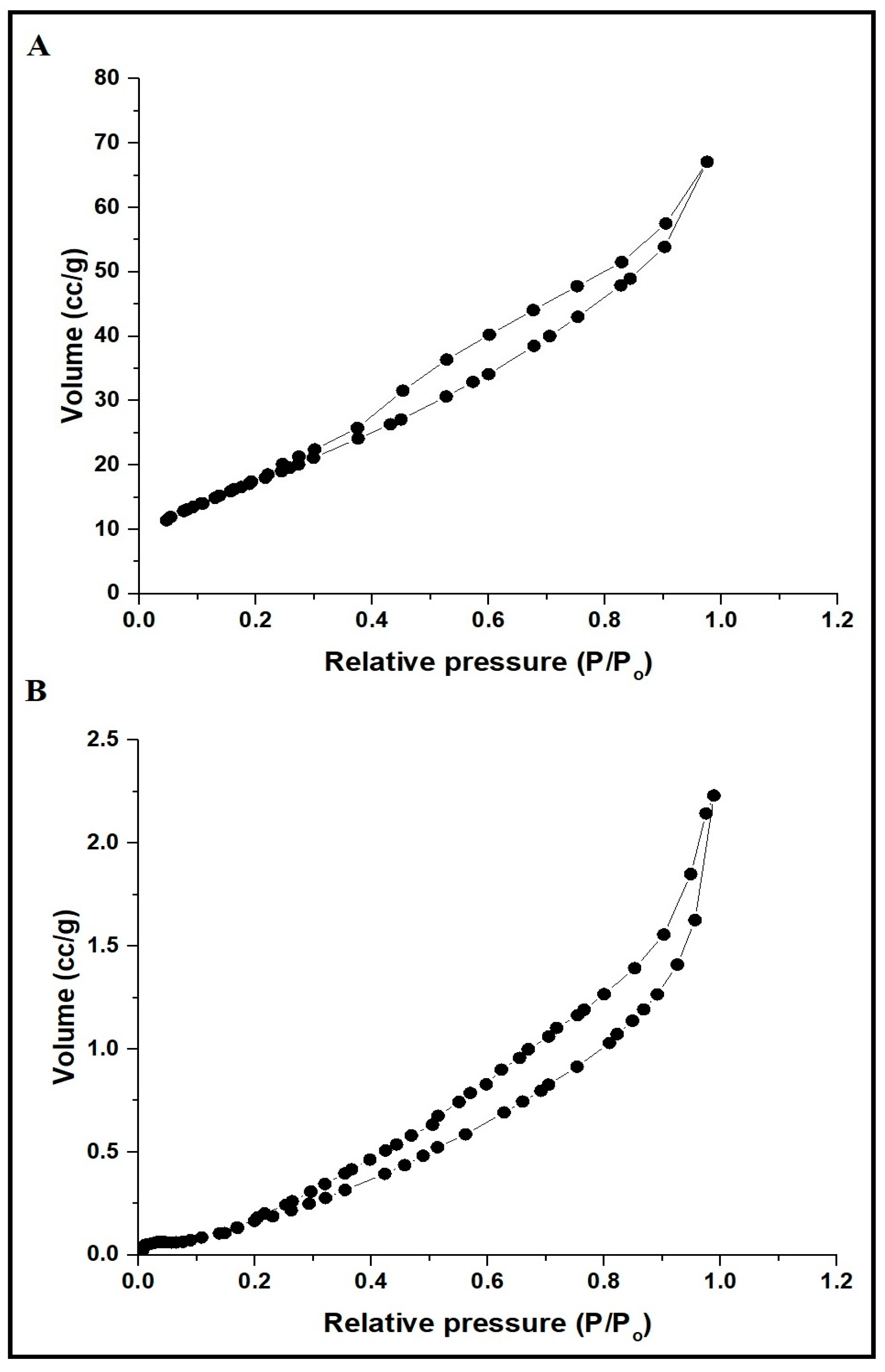
3.1. Synthesis and Characterization of CaFe2O4 Nanoparticles
3.2. Removal of Congo Red Dye from Aqueous Media
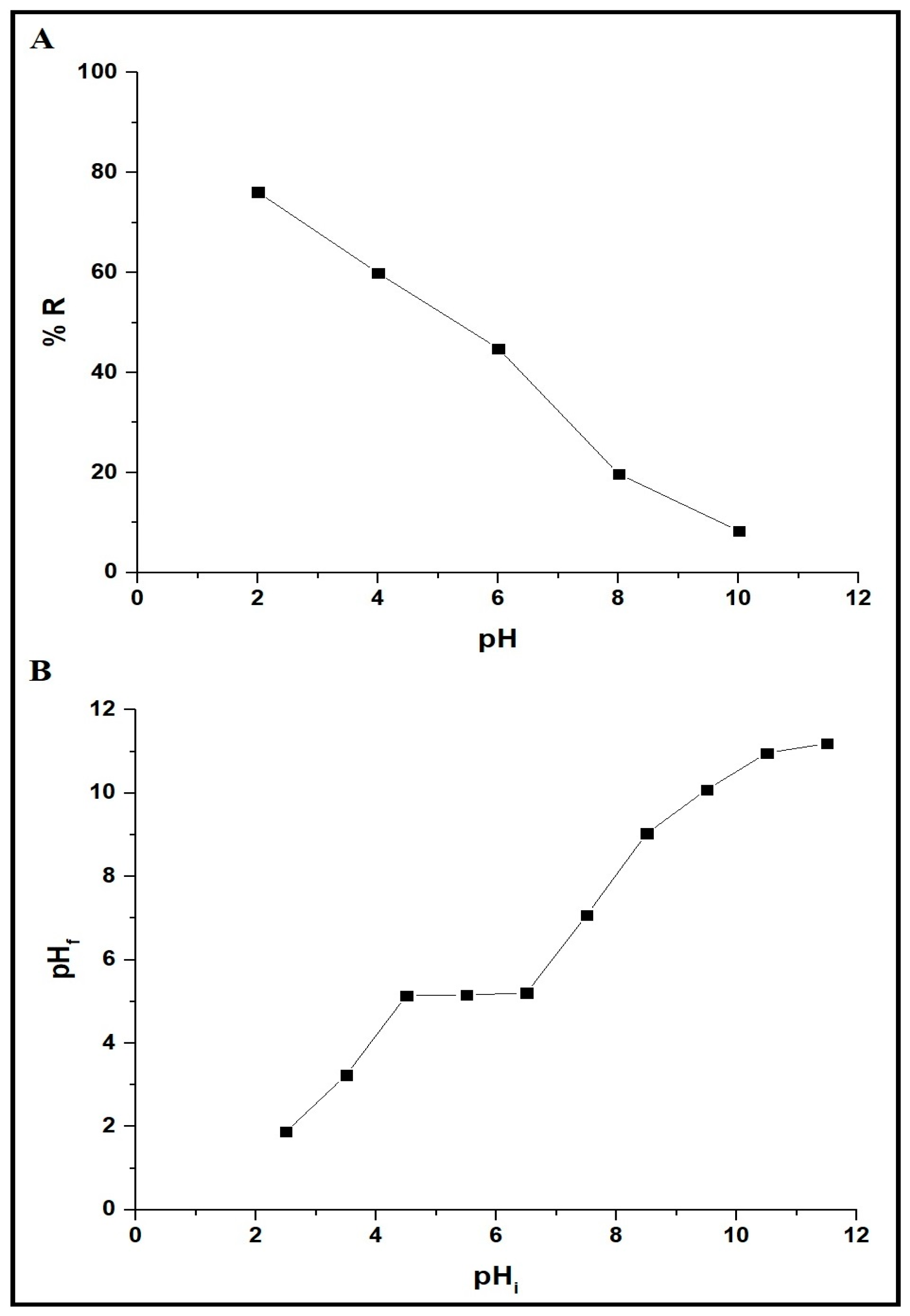
3.2.1. Effect of Dye Solution pH
3.2.2. Effect of Contact Time and Adsorption Kinetics
3.2.3. Effect of Solution Temperature and Thermodynamic Parameters
3.2.4. Effect of Concentration and Adsorption Isotherms
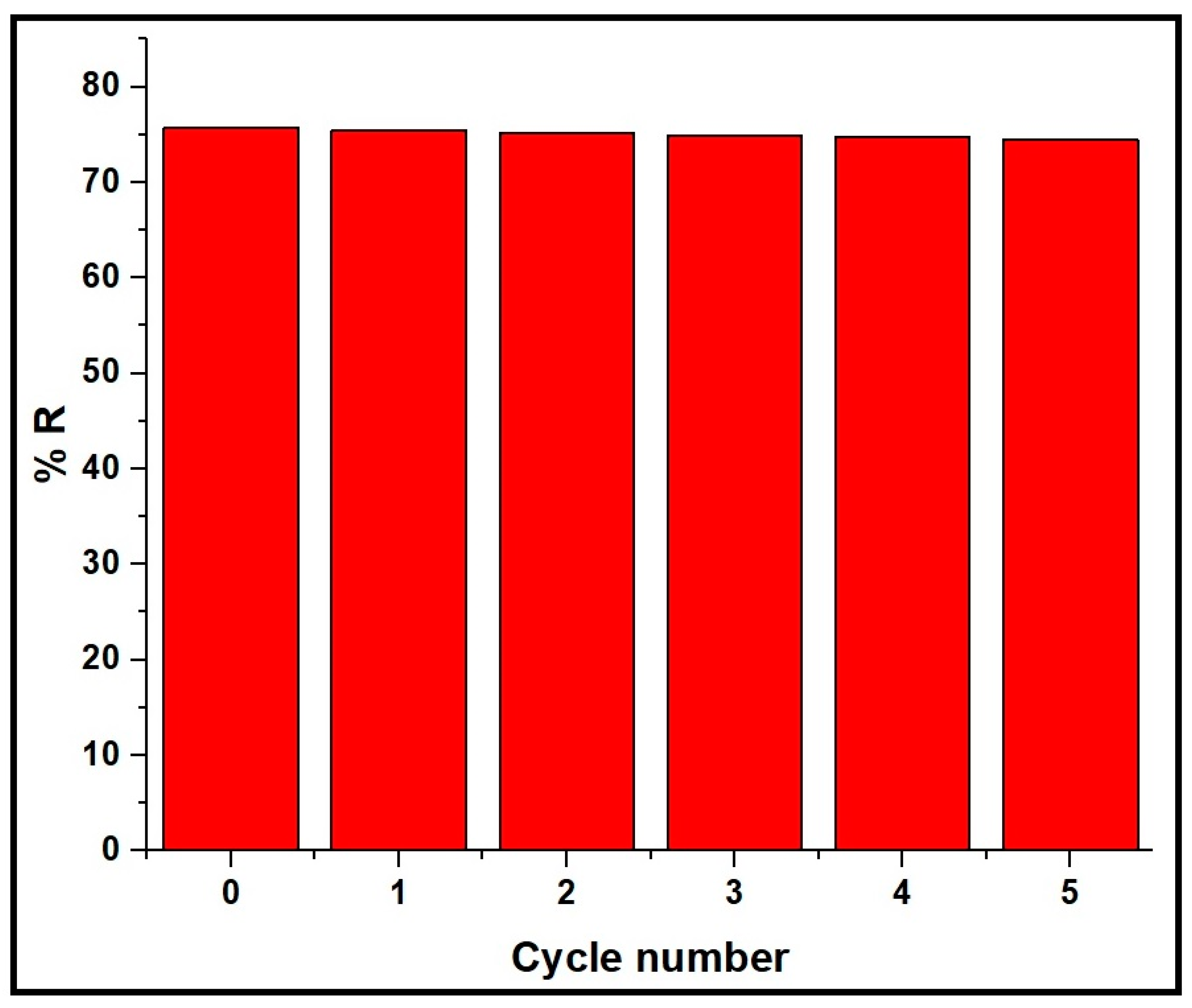
3.2.5. Effect of Regeneration and Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akrami, M.; Danesh, S.; Eftekhari, M. Comparative Study on the Removal of Cationic Dyes Using Different Graphene Oxide Forms. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1785–1797. [Google Scholar] [CrossRef]
- Ghiasi, E.; Malekzadeh, A. Removal of Various Textile Dyes Using LaMn(Fe)O3 and LaFeMn0.5O3 Nanoperovskites; RSM Optimization, Isotherms and Kinetics Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2789–2804. [Google Scholar] [CrossRef]
- Pawariya, V.; De, S.; Dutta, J. Synthesis and Characterization of a New Developed Modified-Chitosan Schiff Base with Improved Antibacterial Properties for the Removal of Bismarck Brown R and Eosin Y Dyes from Wastewater. Carbohydr. Polym. Technol. Appl. 2023, 6, 100352. [Google Scholar] [CrossRef]
- Ahmadian, M.; Jaymand, M. Interpenetrating Polymer Network Hydrogels for Removal of Synthetic Dyes: A Comprehensive Review. Coord. Chem. Rev. 2023, 486, 215152. [Google Scholar] [CrossRef]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; El Messaoudi, N.; Nazir, A. Cellulose-Based Materials and Their Adsorptive Removal Efficiency for Dyes: A Review. Int. J. Biol. Macromol. 2023, 224, 1337–1355. [Google Scholar] [CrossRef]
- Micheletti, D.H.; da Silva Andrade, J.G.; Porto, C.E.; Alves, B.H.M.; de Carvalho, F.R.; Sakai, O.A.; Batistela, V.R. A Review of Adsorbents for Removal of Yellow Tartrazine Dye from Water and Wastewater. Bioresour. Technol. Rep. 2023, 24, 101598. [Google Scholar] [CrossRef]
- Chen, D.; Cao, Y.; Chen, N.; Feng, P. Synthesis and Characterization of Cobalt Metal Organic Frameworks Prepared by Ultrasonic Wave-Assisted Ball Milling for Adsorptive Removal of Congo Red Dye from Aqueous Solutions. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1231–1240. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Safari, Z.; Madady, N. Synthesis of Co3O4@SiO2 Core/Shell–Nylon 6 Magnetic Nanocomposite as an Adsorbent for Removal of Congo Red from Wastewater. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3199–3212. [Google Scholar] [CrossRef]
- Liu, M.; Yin, W.; Zhao, T.L.; Yao, Q.Z.; Fu, S.Q.; Zhou, G.T. High-Efficient Removal of Organic Dyes from Model Wastewater Using Mg(OH)2-MnO2 Nanocomposite: Synergistic Effects of Adsorption, Precipitation, and Photodegradation. Sep. Purif. Technol. 2021, 272, 118901. [Google Scholar] [CrossRef]
- Munyai, S.; Tetana, Z.N.; Mathipa, M.M.; Ntsendwana, B.; Hintsho-Mbita, N.C. Green Synthesis of Cadmium Sulphide Nanoparticles for the Photodegradation of Malachite Green Dye, Sulfisoxazole and Removal of Bacteria. Optik 2021, 247, 167851. [Google Scholar] [CrossRef]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials (Bentonite and Opuntia Ficus Indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Mcyotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W.K. Effect of Dye Structure on Color Removal Efficiency by Coagulation. Chem. Eng. J. 2021, 405, 126674. [Google Scholar] [CrossRef]
- Rodrigues de Almeida, E.J.; Christofoletti Mazzeo, D.E.; Deroldo Sommaggio, L.R.; Marin-Morales, M.A.; Rodrigues de Andrade, A.; Corso, C.R. Azo Dyes Degradation and Mutagenicity Evaluation with a Combination of Microbiological and Oxidative Discoloration Treatments. Ecotoxicol. Environ. Saf. 2019, 183, 109484. [Google Scholar] [CrossRef] [PubMed]
- Al-Wasidi, A.S.; Khairy, M.; Abdulkhair, B.Y.; Abdelrahman, E.A. Efficient Disposal of Basic Fuchsin Dye from Aqueous Media Using ZrO2/MgMn2O4/Mg(Mg0.333Mn1.333)O4 as a Novel and Facilely Synthesized Nanocomposite. Inorganics 2023, 11, 363. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Algethami, F.K.; Alsalem, H.S.; Binkadem, M.S. Facile Synthesis and Characterization of Novel Nanostructures for the Efficient Disposal of Crystal Violet Dye from Aqueous Media. Inorganics 2023, 11, 339. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Algethami, F.K.; AlSalem, H.S.; Binkadem, M.S.; Khairy, M.; Saad, F.A.; El-Sayyad, G.S.; Alqahtani, Z. Efficient Disposal of Rhodamine 6G and Acid Orange 10 Dyes from Aqueous Media Using ZrO2/CdMn2O4/CdO as Novel and Facilely Synthesized Nanocomposites. Inorganics 2023, 11, 333. [Google Scholar] [CrossRef]
- Salahshoori, I.; Namayandeh Jorabchi, M.; Ghasemi, S.; Mirnezami, S.M.S.; Nobre, M.A.L.; Khonakdar, H.A. Assessing Cationic Dye Adsorption Mechanisms on MIL-53 (Al) Nanostructured MOF Materials Using Quantum Chemical and Molecular Simulations: Toward Environmentally Sustainable Wastewater Treatment. J. Water Process Eng. 2023, 55, 104081. [Google Scholar] [CrossRef]
- Nure, J.F.; Mengistu, A.; Abewaa, M.; Angassa, K.; Moyo, W.; Phiri, Z.; Mafa, P.J.; Kuvarega, A.T.; Nkambule, T.T.I. Adsorption of Black MNN Reactive Dye from Tannery Wastewater Using Activated Carbon of Rumex Abysinicus. J. Taiwan Inst. Chem. Eng. 2023, 151, 105138. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, F.; Wei, W.; Gao, H.; Zhang, K.; Zhang, G.; Xu, Y.; Zhang, P. Efficient and Fast Adsorption of Methylene Blue Dye onto a Nanosheet MFI Zeolite. J. Solid State Chem. 2021, 295, 121917. [Google Scholar] [CrossRef]
- Duyar, C.; Pendar, A.; Bektaş, N.; Zorlu, Y.; Davarcı, D. 2D and 3D Ag(I) Coordination Polymers with a 2-Methylimidazole Substituted Cyclotriphosphazene Ligand: Structures and Dye Adsorption Properties. Polyhedron 2023, 244, 116589. [Google Scholar] [CrossRef]
- Kanwal, A.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Basic Dye Adsorption onto Clay/MnFe2O4 Composite: A Mechanistic Study. Water Environ. Res. 2017, 89, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Adeogun, A.I. Removal of Methylene Blue Dye from Aqueous Solution Using Activated Charcoal Modified Manganese Ferrite (AC-MnFe2O4): Kinetics, Isotherms, and Thermodynamics Studies. Part. Sci. Technol. 2020, 38, 756–767. [Google Scholar] [CrossRef]
- Hassan, M.R.; Aly, M.I. Magnetically Synthesized MnFe2O4 Nanoparticles as an Effective Adsorbent for Lead Ions Removal from an Aqueous Solution. Aqua Water Infrastruct. Ecosyst. Soc. 2021, 70, 901–920. [Google Scholar] [CrossRef]
- Algarni, T.S.; Al-Mohaimeed, A.M.; Al-Odayni, A.B.; Abduh, N.A.Y. Activated Carbon/ZnFe2O4 Nanocomposite Adsorbent for Efficient Removal of Crystal Violet Cationic Dye from Aqueous Solutions. Nanomaterials 2022, 12, 3224. [Google Scholar] [CrossRef]
- Vergis, B.R.; Kottam, N.; Hari Krishna, R.; Nagabhushana, B.M. Removal of Evans Blue Dye from Aqueous Solution Using Magnetic Spinel ZnFe2O4 Nanomaterial: Adsorption Isotherms and Kinetics. Nano-Struct. Nano-Objects 2019, 18, 100290. [Google Scholar] [CrossRef]
- Gupta, A.; Viltres, H.; Gupta, N.K. Sono-Adsorption of Organic Dyes onto CoFe2O4/Graphene Oxide Nanocomposite. Surf. Interfaces 2020, 20, 100563. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Li, F.; Khaimanov, S.; Tsidaeva, N.; Lahoubi, M. PEG-Assisted Hydrothermal Synthesis of CoFe2O4 Nanoparticles with Enhanced Selective Adsorption Properties for Different Dyes. Appl. Surf. Sci. 2016, 389, 1003–1011. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Mohamed, A.A. Synthesis and Characterization of Magnetically Separable and Recyclable Crumbled MgFe2O4/Reduced Graphene Oxide Nanoparticles for Removal of Methylene Blue Dye from Aqueous Solutions. J. Phys. Chem. Solids 2021, 149, 109760. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Algethami, F.K.; Saad, F.A.; Abdelrahman, E.A. Remarkable High Adsorption of Methylene Blue Dye from Aqueous Solutions Using Facilely Synthesized MgFe2O4 Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2035–2045. [Google Scholar] [CrossRef]
- Kazemi, J.; Javanbakht, V. Alginate Beads Impregnated with Magnetic Chitosan@Zeolite Nanocomposite for Cationic Methylene Blue Dye Removal from Aqueous Solution. Int. J. Biol. Macromol. 2020, 154, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.K.; Pandey, L.M.; Uppaluri, R.V.S. Synthesized Carboxymethyl-Chitosan Variant Composites for Cyclic Adsorption-Desorption Based Removal of Fe, Pb, and Cu. Chemosphere 2023, 340, 139780. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; El-Aziz, M.E.A.; Fayez, A.E.S.; Kamel, A.H.; Youssef, A.M. Synthesis and Characterization of Bio-Nanocomposite Based on Chitosan and CaCO3 Nanoparticles for Heavy Metals Removal. Int. J. Biol. Macromol. 2024, 255, 128007. [Google Scholar] [CrossRef]
- Kenawy, I.M.M.; Abou El-Reash, Y.G.; Hassanien, M.M.; Alnagar, N.R.; Mortada, W.I. Use of Microwave Irradiation for Modification of Mesoporous Silica Nanoparticles by Thioglycolic Acid for Removal of Cadmium and Mercury. Microporous Mesoporous Mater. 2018, 258, 217–227. [Google Scholar] [CrossRef]
- Bingül, Z. Determination of Affecting Parameters on Removal of Methylene Blue Dyestuff from Aqueous Solutions Using Natural Clay: Isotherm, Kinetic, and Thermodynamic Studies. J. Mol. Struct. 2022, 1250, 131729. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Khalil, M.M.H.; Algethami, F.K.; Khairy, M.; Abou El-Reash, Y.G.; Saad, F.A.; Shah, R.K.; Ammar, A.M. Facile Synthesis of MgO/CuO and MgO/Cu3MgO4 Binary Nanocomposites as Promising Adsorbents for the Disposal of Zn(II) Ions. J. Inorg. Organomet. Polym. Mater. 2023, 34, 266–281. [Google Scholar] [CrossRef]
- Manohar, A.; Krishnamoorthi, C. Structural, Optical, Dielectric and Magnetic Properties of CaFe2O4 Nanocrystals Prepared by Solvothermal Reflux Method. J. Alloys Compd. 2017, 722, 818–827. [Google Scholar] [CrossRef]
- Josun, J.; Sharma, P.; Kumar Garg, V. Optical and Structural Behavior of Hydrothermally Synthesized ZnO Nanoparticles at Various Temperatures with NaOH Molar Ratios. Results Opt. 2024, 14, 100601. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Al-Farraj, E.S. Facile Synthesis and Characterizations of Mixed Metal Oxide Nanoparticles for the Efficient Photocatalytic Degradation of Rhodamine B and Congo Red Dyes. Nanomaterials 2022, 12, 3992. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Hegazey, R.M.; Ismail, S.H.; El-Feky, H.H.; Khedr, A.M.; Khairy, M.; Ammar, A.M. Facile Synthesis and Characterization of β-Cobalt Hydroxide/Hydrohausmannite/Ramsdellitee/Spertiniite and Tenorite/Cobalt Manganese Oxide/Manganese Oxide as Novel Nanocomposites for Efficient Photocatalytic Degradation of Methylene Blue Dye. Arab. J. Chem. 2022, 15, 104372. [Google Scholar] [CrossRef]
- Jeyaseelan, C.; Kaur, M.; Sen, M. Activated Carbon Modified Chitosan Beads: An Effective Method for Removal of Congo Red Dye. Mater. Today Proc. 2023. In Press. [Google Scholar] [CrossRef]
- Gao, H.J.; Wang, S.F.; Fang, L.M.; Sun, G.A.; Chen, X.P.; Tang, S.N.; Yang, H.; Sun, G.Z.; Li, D.F. Nanostructured Spinel-Type M(M=Mg,Co,Zn)Cr2O4 Oxides: Novel Adsorbents for Aqueous Congo Red Removal. Mater. Today Chem. 2021, 22, 100593. [Google Scholar] [CrossRef]
- Priyan, V.V.; Kumar, N.; Narayanasamy, S. Toxicological Assessment and Adsorptive Removal of Lead (Pb) and Congo Red (CR) from Water by Synthesized Iron Oxide/Activated Carbon (Fe3O4/AC) Nanocomposite. Chemosphere 2022, 294, 133758. [Google Scholar] [CrossRef]
- Singh, N.; Shah, K.; Pramanik, B.K. Synthesis and Application of Manganese-Doped Zinc Oxide as a Potential Adsorbent for Removal of Congo Red Dye from Wastewater. Environ. Res. 2023, 233, 116484. [Google Scholar] [CrossRef]
- Koohi, P.; Rahbar-kelishami, A.; Shayesteh, H. Efficient Removal of Congo Red Dye Using Fe3O4/NiO Nanocomposite: Synthesis and Characterization. Environ. Technol. Innov. 2021, 23, 101559. [Google Scholar] [CrossRef]
- Nodehi, R.; Shayesteh, H.; Kelishami, A.R. Enhanced Adsorption of Congo Red Using Cationic Surfactant Functionalized Zeolite Particles. Microchem. J. 2020, 153, 104281. [Google Scholar] [CrossRef]
- Ramírez-Ortega, A.A.; Medina-Llamas, M.; da Silva, R.J.; García-Elías, J.; de Lira-Gómez, P.; Medina-Llamas, J.C.; Chávez-Guajardo, A.E. Synthesis of a Maghemite-Polypyrrole Nanocomposite for the Removal of Congo Red Dye from Aqueous Solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100597. [Google Scholar] [CrossRef]
- Shahnaz, T.; Padmanaban, V.C.; Narayanasamy, S. Surface Modification of Nanocellulose Using Polypyrrole for the Adsorptive Removal of Congo Red Dye and Chromium in Binary Mixture. Int. J. Biol. Macromol. 2020, 151, 322–332. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Myslin, M.; Mironyuk, I.; Kosobucki, P.; Scigalski, P.; Kotsyubynsky, V. Removal of Congo Red Dye, Polar and Non-Polar Compounds from Aqueous Solution Using Magnesium Aluminate Nanoparticles. Mater. Today Proc. 2019, 35, 518–522. [Google Scholar] [CrossRef]




| Experimental Qexp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| Qe (mg/g) | kFirst (1/min) | R2 | Qe (mg/g) | kSecond (g/mg·min) | R2 | |
| 303 ± 3.5 | 157.17 ± 17.48 | 0.0275 ± 0.0031 | 0.9524 | 307.69 ± 0.26 | 0.00039 ± 7.61×10-7 | 0.9999 |
| △H° (KJ/mol) | △S° (KJ/mol K) | △G° (KJ/mol) | |||
|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | ||
| −56.30 ± 5.29 | 0.1745 ± 0.017 | −108.31 ± 10.35 | −110.05 ± 10.52 | −111.79 ± 10.68 | −113.54 ± 10.85 |
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| Qmax (mg/g) | kL (L/mg) | R2 | Qmax (mg/g) | kF (mg/g)(L/mg)1/n | R2 |
| 318.55 ± 6.17 | 0.3203 ± 0.09 | 0.9985 | 574.76 ± 409.95 | 107.11 ± 29.20 | 0.6619 |
| Adsorbent | Maximum Disposal Capacity (mg/g) | Ref. |
|---|---|---|
| Activated carbon/chitosan composite | 5.99 | [40] |
| ZnCr2O4 nanoparticles | 44.04 | [41] |
| Fe3O4/activated carbon composite | 122.22 | [42] |
| Mn/ZnO composite | 232.50 | [43] |
| Fe3O4/NiO composite | 89.90 | [44] |
| Cationic surfactant-modified clinoptilolite | 200.00 | [45] |
| Maghemite/polypyrrole composite | 269.50 | [46] |
| Nanocellulose/polypyrrole composite | 298.98 | [47] |
| MgAl2O4 nanoparticles | 24.50 | [48] |
| CaFe2O4 nanoparticles | 318.55 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Kadhi, N.S.; Al-Senani, G.M.; Algethami, F.K.; Shah, R.K.; Saad, F.A.; Munshi, A.M.; Rehman, K.u.; Khezami, L.; Abdelrahman, E.A. Calcium Ferrite Nanoparticles: A Simple Synthesis Approach for the Effective Disposal of Congo Red Dye from Aqueous Environments. Inorganics 2024, 12, 69. https://doi.org/10.3390/inorganics12030069
Al-Kadhi NS, Al-Senani GM, Algethami FK, Shah RK, Saad FA, Munshi AM, Rehman Ku, Khezami L, Abdelrahman EA. Calcium Ferrite Nanoparticles: A Simple Synthesis Approach for the Effective Disposal of Congo Red Dye from Aqueous Environments. Inorganics. 2024; 12(3):69. https://doi.org/10.3390/inorganics12030069
Chicago/Turabian StyleAl-Kadhi, Nada S., Ghadah M. Al-Senani, Faisal K. Algethami, Reem K. Shah, Fawaz A. Saad, Alaa M. Munshi, Khalil ur Rehman, Lotfi Khezami, and Ehab A. Abdelrahman. 2024. "Calcium Ferrite Nanoparticles: A Simple Synthesis Approach for the Effective Disposal of Congo Red Dye from Aqueous Environments" Inorganics 12, no. 3: 69. https://doi.org/10.3390/inorganics12030069
APA StyleAl-Kadhi, N. S., Al-Senani, G. M., Algethami, F. K., Shah, R. K., Saad, F. A., Munshi, A. M., Rehman, K. u., Khezami, L., & Abdelrahman, E. A. (2024). Calcium Ferrite Nanoparticles: A Simple Synthesis Approach for the Effective Disposal of Congo Red Dye from Aqueous Environments. Inorganics, 12(3), 69. https://doi.org/10.3390/inorganics12030069







