Synthesis and Characterization of Zn-Salophen Complexes with Different D–A Distances: An Approach to Tuning the Intersystem-Crossing Process
Abstract
:1. Introduction
2. Results and Discussion
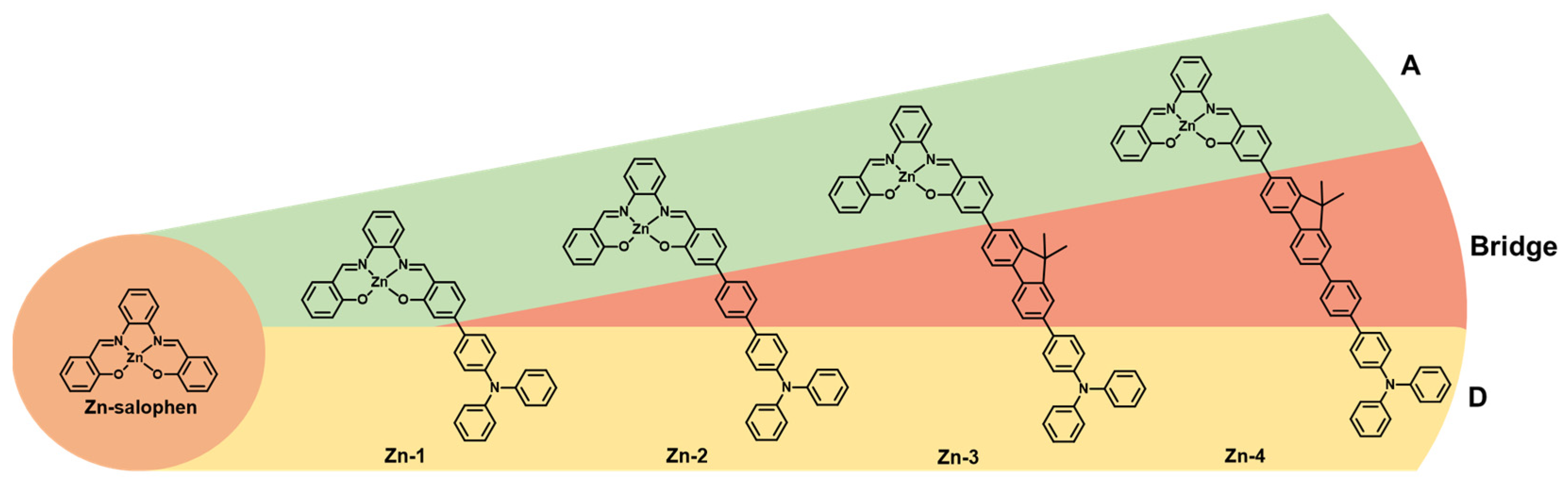
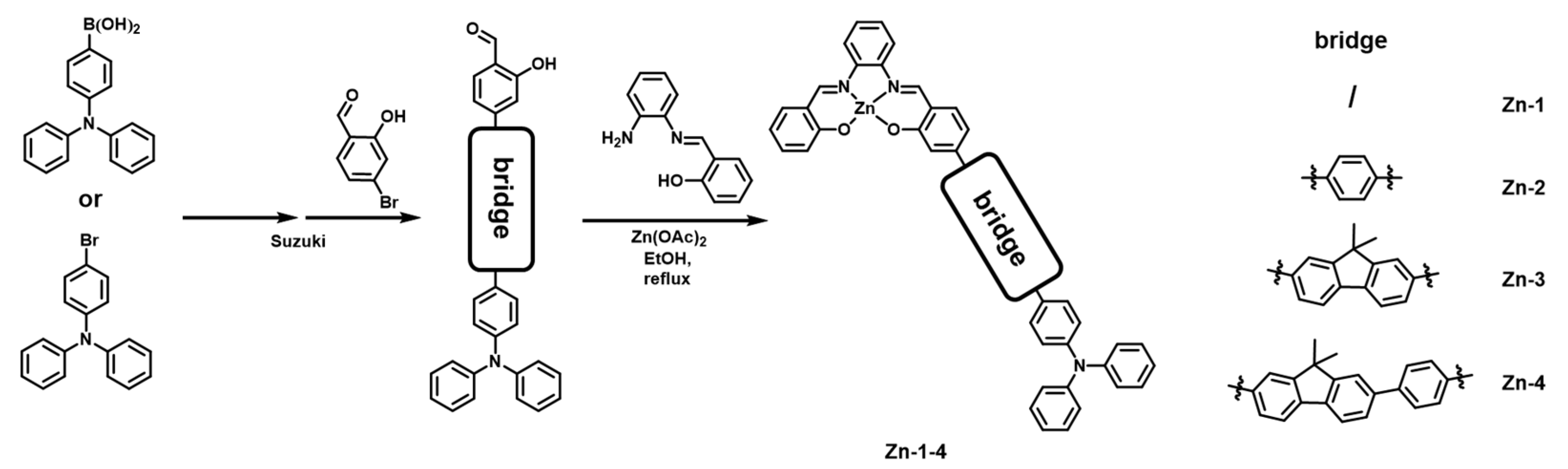
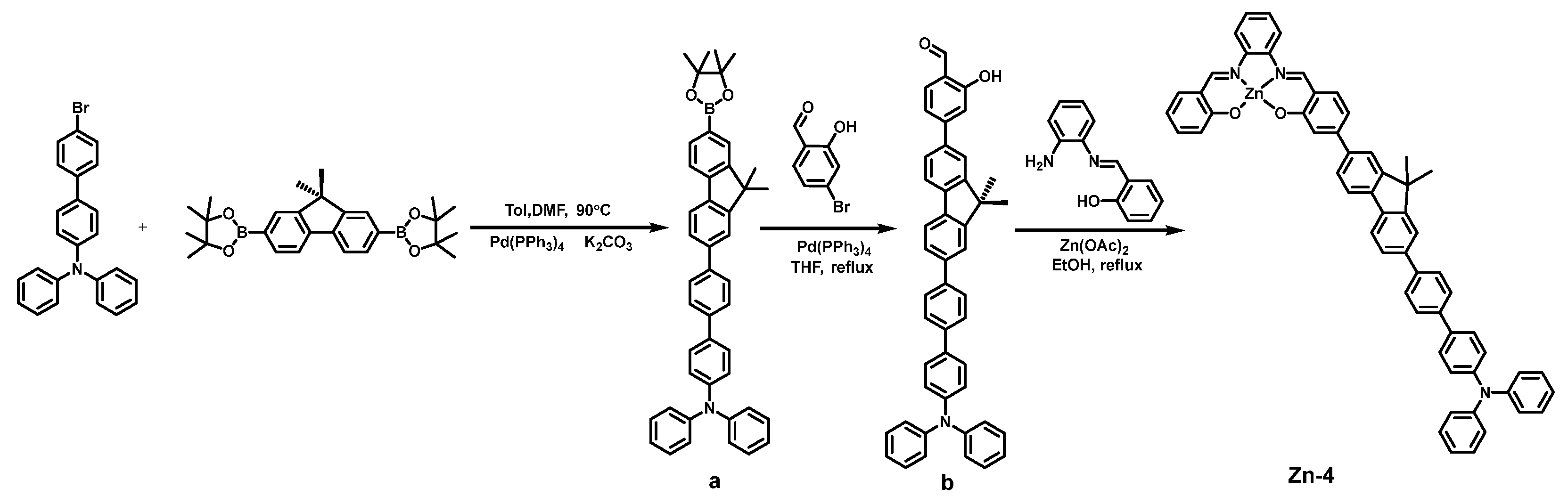
2.1. Synthesis and Characterization
2.2. NMR Spectroscopy
2.3. FTIR Spectroscopy Studies
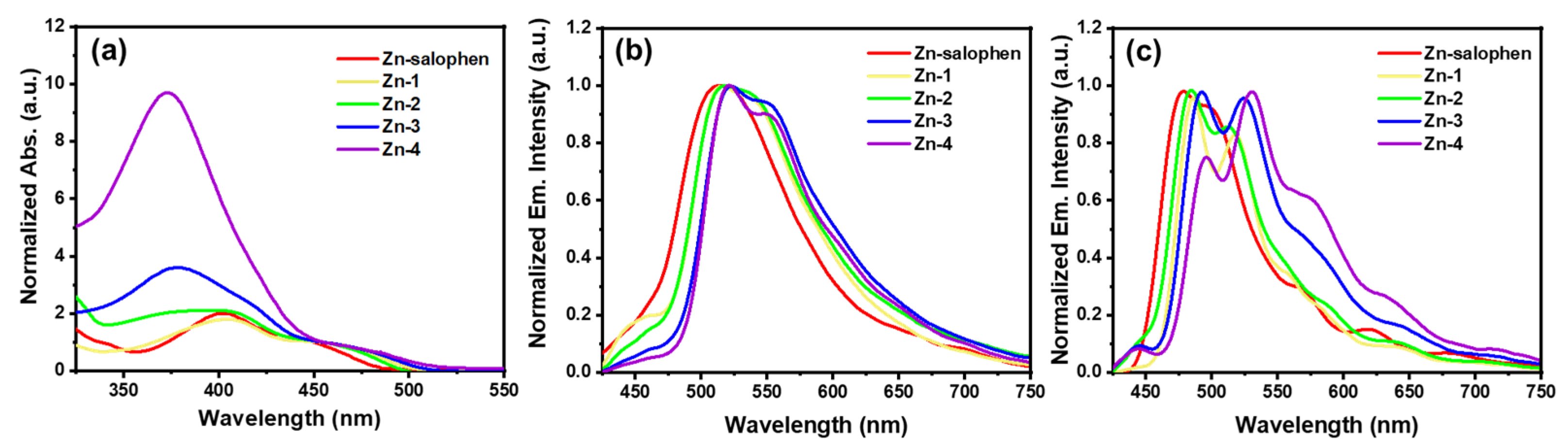
2.4. Absorbance and Emission
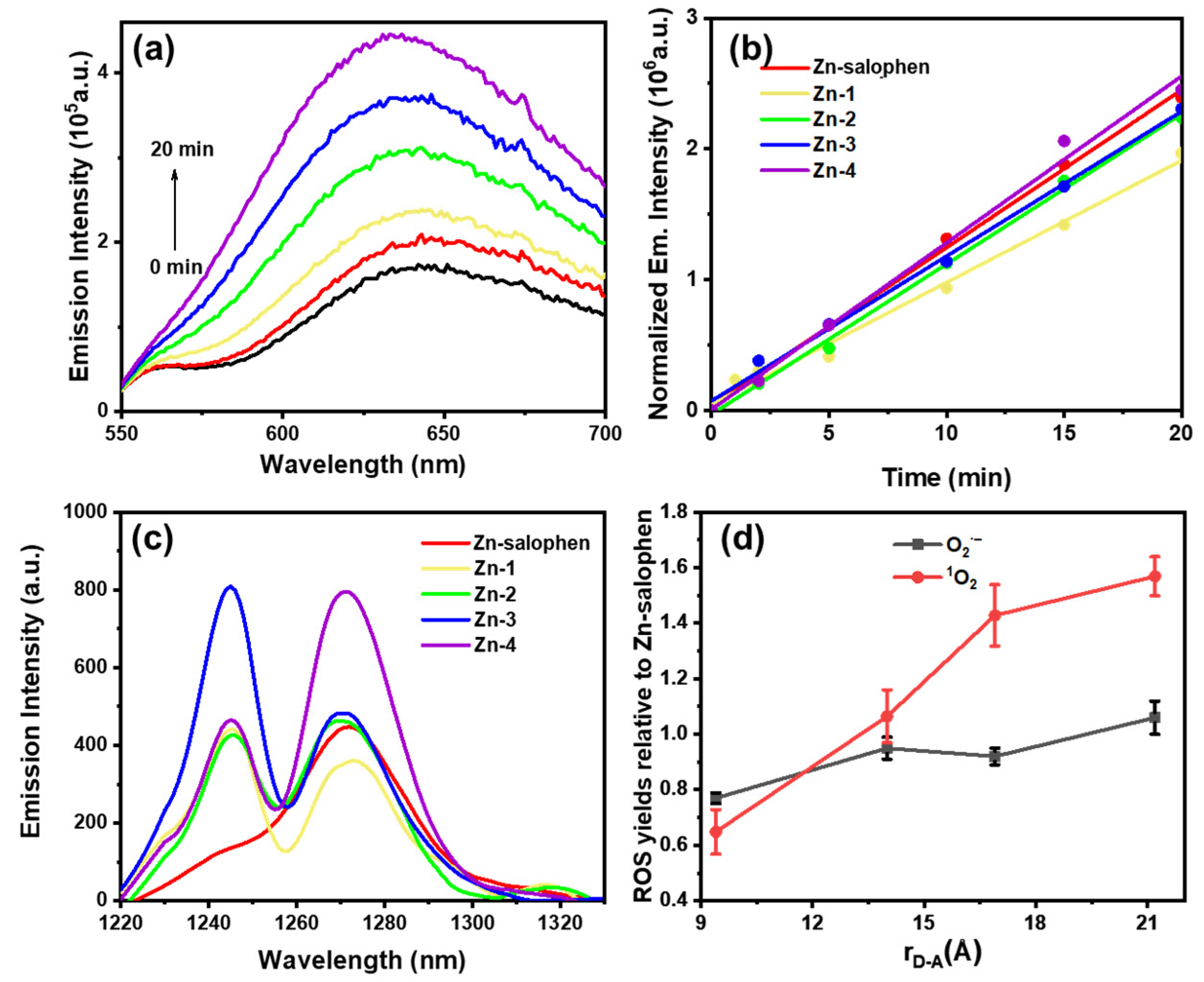
2.5. Air Quenches Emission
2.6. Reactive Oxygen Species (ROS)
3. Materials and Methods
3.1. Synthesis of Zn-1
3.2. Synthesis of Zn-2
3.3. Synthesis of Zn-3
3.4. Synthesis of Zn-4
3.5. Measurement
3.5.1. Measurement of Absorption Spectrum and Extinction Coefficient
3.5.2. Measurement of Excitation and Emission Spectroscopy
3.5.3. Measurement of the Fluorescence Lifetime
- t: decay time
- F(t): fluorescence emission intensity
- τi, Ai: the lifetime and amplitude of the i-th lifespan component
3.5.4. Measurement of the Low Temperature (77 K) Emission Spectroscopy
3.5.5. Freeze Extraction Deoxygenation
3.5.6. Measurement of the ns-TA
3.5.7. Measurement of Superoxide Anion Radical
- ΔI: the variation of emission intensity at 610 nm relative to the initial moment
- t: total illumination time of 405 nm laser
3.5.8. Measurement of Singlet Oxygen
3.5.9. Computations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Langellotti, V.; Melchiorre, M.; Cucciolito, M.E.; Esposito, R.; Grieco, D.; Pinto, G.; Ruffo, F. Biodiesel from Waste Cooking Oil: Highly Efficient Homogeneous Iron (III) Molecular Catalysts. Catalysts 2023, 13, 1496. [Google Scholar] [CrossRef]
- Hong, L.; Liu, X.; Chi, B.; Xia, G.; Wang, H. Diatomic molecule catalysts toward synergistic electrocatalytic carbon dioxide reduction. J. Mater. Chem. A 2023, 11, 6321–6328. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Gupta, K.C.; Kumar Sutar, A.; Lin, C.-C. Polymer-supported Schiff base complexes in oxidation reactions. Coord. Chem. Rev. 2009, 253, 1926–1946. [Google Scholar] [CrossRef]
- Jacobsen, E.N. Asymmetric catalysis of epoxide ring opening reactions. Acc. Chem. Res. 2000, 33, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Groves, J.T. Manganese Catalyzed C-H Halogenation. Acc. Chem. Res. 2015, 48, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.-R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Y.; Ettelaie, R.; Guan, R.; Zhang, M.; Zhang, Y.; Yang, H. Pickering Emulsion-Derived Liquid-Solid Hybrid Catalyst for Bridging Homogeneous and Heterogeneous Catalysis. J. Am. Chem. Soc. 2019, 141, 5220–5230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Yu, X.; Chen, Y.; Tang, X.; Hu, T.; Shi, L.; Pudukudy, M.; Shan, S.; Zhi, Y. Cellulose nanofibers (CNF) supported (Salen) Cr (III) composite as an efficient heterogeneous catalyst for CO2 cycloaddition. Mol. Catal. 2023, 547, 113344. [Google Scholar] [CrossRef]
- Lu, S.; Ge, Q.; Fan, C.; Wang, X.; Chen, Y.; Lin, S.; Pan, Q. Synthesis of sulfur-containing polycarbonate block copolymers via Salen-metal catalyzed copolymerization of CO2, propylene oxide, and phthalic thioanhydride. J. Macromol. Sci. A 2023, 60, 484–491. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.-Z.; Luo, D.; Zhou, X.-P.; Li, D. A chiral Salen-based Zn (II)-Cd (II) heterometallic metal-organic framework: Synthesis, crystal structure, and optical properties. J. Coord. Chem. 2022, 75, 1670–1678. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Zhou, C.-W.; Wang, B.; Lai, Y.-L.; Lian, Z.-X.; Liu, Y.-T.; Li, Y.Y.; Zhou, X.-P. Chiral two-dimensional metal-organic frameworks based on Zn(salen) ligands: Subcomponent self-assembly and circularly polarised luminescence. CrystEngComm 2023, 25, 484–489. [Google Scholar] [CrossRef]
- Song, F.; Wang, C.; Falkowski, J.M.; Ma, L.; Lin, W. Isoreticular Chiral Metal-Organic Frameworks for Asymmetric Alkene Epoxidation: Tuning Catalytic Activity by Controlling Framework Catenation and Varying Open Channel Sizes. J. Am. Chem. Soc. 2010, 132, 15390–15398. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Guo, J.; Qi, C.; Feng, R.; Yang, B.; Zhou, Y.; Tian, L.; Cui, J. Core-shell structure flame retardant Salen-PZN-Cu@Ni-Mof microspheres enhancing fire safety of epoxy resin through the synergistic effect. J. Polym. Res. 2022, 29, 27. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, W.; He, K.; Wang, L.; Wang, Q.; Liu, J. Half-salen Fe(III) covalently post-modified MIL-125(Ti)-NH2 MOF for effective photocatalytic peroxymonosulfate activation. Appl. Surf. Sci. 2022, 591, 153115. [Google Scholar] [CrossRef]
- Shultz, A.M.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. Post-Synthesis Modification of a Metal-Organic Framework to Form Metallosalen-Containing MOF Materials. J. Am. Chem. Soc. 2011, 133, 13252–13255. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, M.; Dong, W.; Jin, Z.; Tang, J.; Fan, S.; Lu, Y.; Wang, G. Co(II) complexes loaded into metal-organic frameworks as efficient heterogeneous catalysts for aerobic epoxidation of olefins. Catal. Sci. Technol. 2016, 6, 161–168. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, L.; He, C.; Duan, C. Metal-Organic Framework-Encapsulated Anthraquinone for Efficient Photocatalytic Hydrogen Atom Transfer. ACS Appl. Mater. Interfaces 2022, 14, 7980–7989. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, L.; Fu, G.; Li, W.; Lü, X.; He, H.; Wong, W.-Y. Efficient white polymer light-emitting diodes (WPLEDs) based on double emitting layers of a PVK:Eu(III)-complex and Alq(3). J. Mater. Chem. C 2019, 7, 4800–4807. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.S. Quantum chemical analysis of salen-aluminum complexes for organic light emitting diodes. Chem. Phys. Lett. 2013, 585, 143–148. [Google Scholar] [CrossRef]
- Li, X.-X.; Ma, C.-Y.; Du, M.-X.; Dong, W.-K.; Ding, Y.-J. A rare salamo-salophen type “on-off-on” fluorescent probe for relay recognition of Hg2+ and phosphate ions and its applications. J. Mol. Struct. 2024, 1299, 137188. [Google Scholar] [CrossRef]
- Yin, H.; Gao, J.; Chen, X.; Ma, B.; Yang, Z.; Tang, J.; Wang, B.; Chen, T.; Wang, C.; Gao, S.; et al. A Gallium(III) Complex that Engages Protein Disulfide Isomerase A3 (PDIA3) as an Anticancer Target. Angew. Chem. Int. Ed. 2020, 59, 20147–20153. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-X.; Zhang, H.; Zhang, R.; Li, Z.-H.; Yang, Z.-S.; Zhang, J.; Gao, S.; Zhang, J.-L. Gallium Triggers Ferroptosis through a Synergistic Mechanism. Angew. Chem. Int. Ed. 2023, 62, e202307838. [Google Scholar] [CrossRef] [PubMed]
- Sakshi, S.; Dey, S.; Chowdhury, S.; Ray, S. Characterization of a Zeolite-Y-Encapsulated Zn(II)Salmphen Complex with Targeted Anticancer Property. ACS Appl. Mater. Interfaces 2023, 15, 55518–55532. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; Fragala, I.; Ledoux, I.; Marks, T.J. Role of Metal Electronic Properties in Tuning the Second-Order Nonlinear Optical Response of Coordination Complexes. A Combined Experimental and Theoretical Investigation of a Homologous Series of (N,N′-Disalicylidene-1,2-phenylenediaminato)M(II) (M = Co, Ni, Cu) Complexes. J. Am. Chem. Soc. 1995, 117, 9481–9485. [Google Scholar]
- Yang, Z.; Zhang, Z.; Sun, Y.; Lei, Z.; Wang, D.; Ma, H.; Tang, B.Z. Incorporating spin-orbit coupling promoted functional group into an enhanced electron D-A system: A useful designing concept for fabricating efficient photosensitizer and imaging-guided photodynamic therapy. Biomaterials 2021, 275, 120934. [Google Scholar] [CrossRef] [PubMed]
- Filatov, M.A.; Karuthedath, S.; Polestshuk, P.M.; Savoie, H.; Flanagan, K.J.; Sy, C.; Sitte, E.; Telitchko, M.; Laquai, F.; Boyle, R.W.; et al. Generation of triplet excited states via photoinduced electron transfer in meso-anthra-BODIPY: Fluorogenic response toward singlet oxygen in solution and in vitro. J. Am. Chem. Soc. 2017, 139, 6282–6285. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, X.; Chen, K.; Liu, D.; Wang, Z.; Liu, Q.; Zhao, J.; Barbon, A. Charge separation, charge recombination, long-lived charge transfer state formation and intersystem crossing in organic electron donor/acceptor dyads. J. Mater. Chem. C 2019, 7, 12048–12074. [Google Scholar] [CrossRef]
- Kc, C.B.; Lim, G.N.; Nesterov, V.N.; Karr, P.A.; D’Souza, F. Phenothiazine–BODIPY–fullerene triads as photosynthetic reaction center models: Substitution and solvent polarity effects on photoinduced charge separation and recombination. Chem. Eur. J. 2014, 20, 17100–17112. [Google Scholar] [CrossRef]
- Liao, J.-M.; Chin, Y.-K.; Wu, Y.-T.; Chou, H.-H. Effect of regio-specific arylamine substitution on novel π-extended zinc salophen complexes: Density functional and time-dependent density functional study on DSSC applications. RSC Adv. 2023, 13, 2501–2513. [Google Scholar] [CrossRef]
- Wiederrecht, G.P.; Svec, W.A.; Wasielewski, M.R.; Galili, T.; Levanon, H. Novel mechanism for triplet state formation in short distance covalently linked radical ion pairs. J. Am. Chem. Soc. 2000, 122, 9715–9722. [Google Scholar] [CrossRef]
- Dance, Z.E.X.; Mi, Q.X.; McCamant, D.W.; Ahrens, M.J.; Ratner, M.A.; Wasielewski, M.R. Time-resolved EPR studies of photogenerated radical ion pairs separated by p-phenylene oligomers and of triplet states resulting from charge recombination. J. Phys. Chem. B 2006, 110, 25163–25173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-L.; Yang, M.; Shen, X.; Chen, L.; Liao, L.-F. Study on spectral properties of Salophen-fluorescein and its uranyl complex. Appl. Chem. Ind. 2013, 42, 549–551. [Google Scholar]
- Buck, J.T.; Wilson, R.W.; Mani, T. Intramolecular long-range charge-transfer emission in donor-bridge-acceptor systems. J. Phys. Chem. Lett. 2019, 10, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Y.; Pan, G.; Han, W.; Wang, B.; Cui, L.; Ma, H.; An, Z.; Xie, Z.; Xu, B.; et al. Exploiting radical-pair intersystem crossing for maximizing singlet oxygen quantum yields in pure organic fluorescent photosensitizers. Chem. Sci. 2020, 11, 10921–10927. [Google Scholar] [CrossRef]
- Lee, J.; Song, I.; Warkad, S.D.; Yeom, G.S.; Shinde, P.B.; Song, K.; Nimse, S.B. Synthesis and evaluation of 2-aryl-1H-benzo[d]imidazole derivatives as potential microtubule targeting agents. Drug Dev. Res. 2022, 83, 769–782. [Google Scholar] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab-initio calculation of vibrational absorption and circular-dichroism spectra using density-functional force-fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations—Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]


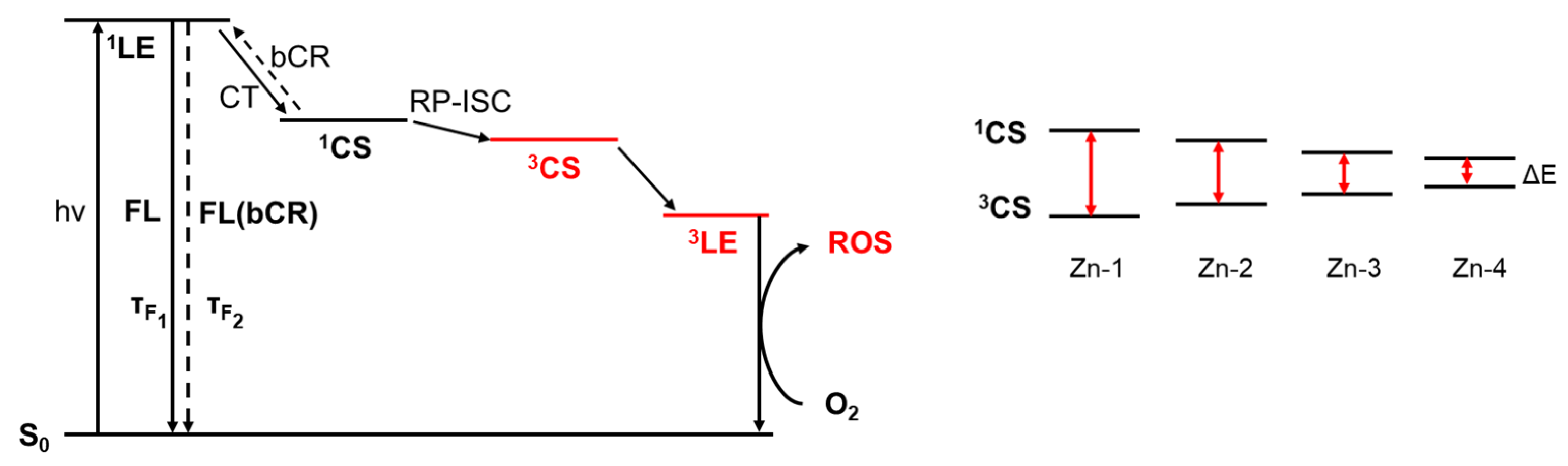



| Compounds | ν(O-H) | ν(C=O) | ν(C=N) | ν(C=C) | ν(C-O) | ν(C-H) |
|---|---|---|---|---|---|---|
| salophen [33] | 3500 | 1606 | 1566, 1497 | 1263 | 1406 | |
| 3b | 3450 | 1660 | 1585, 1487 | 1273 | 1377 | |
| Zn-1 | 1610 | 1579, 1498 | 1298 | 1385 | ||
| Zn-2 | 1610 | 1579, 1491 | 1281 | 1385 | ||
| Zn-3 | 1616 | 1589, 1487 | 1282 | 1385 | ||
| Zn-4 | 1618 | 1589, 1491 | 1290 | 1367 |
| Compounds | λ (nm) [a] | τ (ns) (Rel%) [b] | λ (nm) | τ (ns) (Rel%) |
|---|---|---|---|---|
| Zn-salophen | 520 | 0.21 (35.78%) | 650 | 0.22 (28.37%) |
| 2.56 (64.22%) | 2.45 (51.75%) | |||
| 10.40 (19.87%) | ||||
| Zn-1 | 540 | 0.33 (52.98%) | 680 | 0.27 (20.16%) |
| 2.84 (47.02%) | 2.31 (40.50%) | |||
| 14.97 (39.34%) | ||||
| Zn-2 | 540 | 0.24 (56.09%) | 680 | 0.27 (45.12%) |
| 2.23 (43.91%) | 2.48 (42.69%) | |||
| 23.54 (12.19%) | ||||
| Zn-3 | 550 | 0.26 (23.40%) | 680 | 0.31 (17.75%) |
| 2.43 (76.60%) | 2.44 (75.50%) | |||
| 11.85 (6.75%) | ||||
| Zn-4 | 555 | 0.39 (3.13%) | 680 | 0.41 (3.45%) |
| 2.45 (96.87%) | 2.44 (89.28%) | |||
| 13.62 (7.27%) |
| Compound | kt=0 (min–1) [a] | The Ratio of O2·– Yield Relative to Zn-Salophen [b] | 1O2 Yield [c] | The Ratio of 1O2 Yield Relative to Zn-Salophen [d] |
|---|---|---|---|---|
| Zn-salophen | 120,478 | 1.00 | 0.019 | 1.00 |
| Zn-1 | 93,312 | 0.77 | 0.012 | 0.63 |
| Zn-2 | 114,845 | 0.95 | 0.020 | 1.05 |
| Zn-3 | 110,643 | 0.92 | 0.027 | 1.42 |
| Zn-4 | 127,420 | 1.06 | 0.030 | 1.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-H.; Tang, Z.-Y.; Zhang, J.; Zhang, J.-L. Synthesis and Characterization of Zn-Salophen Complexes with Different D–A Distances: An Approach to Tuning the Intersystem-Crossing Process. Inorganics 2024, 12, 108. https://doi.org/10.3390/inorganics12040108
Li Z-H, Tang Z-Y, Zhang J, Zhang J-L. Synthesis and Characterization of Zn-Salophen Complexes with Different D–A Distances: An Approach to Tuning the Intersystem-Crossing Process. Inorganics. 2024; 12(4):108. https://doi.org/10.3390/inorganics12040108
Chicago/Turabian StyleLi, Ze-Hao, Zi-Yi Tang, Jing Zhang, and Jun-Long Zhang. 2024. "Synthesis and Characterization of Zn-Salophen Complexes with Different D–A Distances: An Approach to Tuning the Intersystem-Crossing Process" Inorganics 12, no. 4: 108. https://doi.org/10.3390/inorganics12040108





