Synthesis, Spectral Characterization, and Structural Modelling of Di- and Trinuclear Iron(III) Monensinates with Different Bridging Patterns
Abstract
:1. Introduction
- (i)
- glutathione level reduction and decreased activity of glutathione peroxidase, which results in the deposition of harmful lipid-reactive oxygen species (L-ROS) from polyunsaturated fatty acids in the presence of high concentrations of iron ions, thus promoting cell death [34];
- (ii)
- overexpression of transferrin receptor 1 (TFR1) and decrease in ferritin levels, since the upregulation of TFR1 is detected in many malformations such as glioblastoma, leukaemia, breast cancer, ovarian cancer, hepatic cancer, thyroid cancer, and colorectal cancer [35];
- (iii)
- Fenton reaction that strongly depends on the intracellular iron concentration and can be a possible mechanism of ROS generation.
2. Results and Discussion
2.1. General Remarks
2.2. Physicochemical Properties of Complexes 1 and 2
2.2.1. Vibrational and Thermal Analysis
2.2.2. Magnetic Studies
2.2.3. Mössbauer Studies
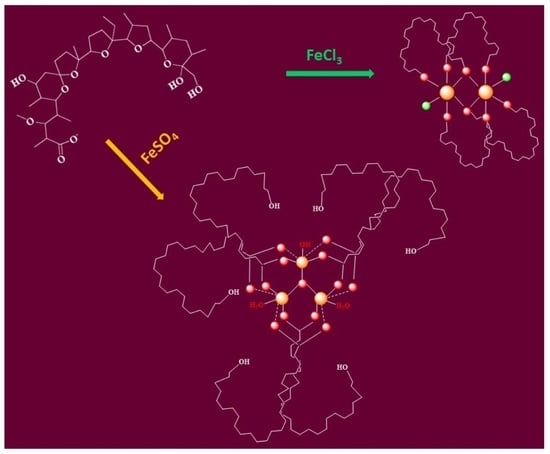
2.2.4. Proposed Structures of Complexes 1–2
- (i)
- two monensinates are bound to each iron(III) ion in a bidentate manner through their terminal carboxylate and hydroxyl functions, and two chloride anions link the metal centres (Figure 6a);
- (ii)
- two bidentate monensinate ligands bridge the iron ions via tail hydroxyl groups and each chloride anion is terminally bound to the metal cation (Figure 6b);
- (iii)
- the presence of a carboxylate linker as a structural motif in 1 is excluded due to the higher Δ-value(s) detected in the IR-spectra of the solid complex.
2.3. Theoretical Studies
2.3.1. Molecular Modelling of 1
2.3.2. Molecular Design of 2
2.4. Antibacterial Activity
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Complexes 1–2
3.3. Computational Protocol
3.4. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crichton, R.R.; Pierre, J.-L. Old iron, young copper: From Mars to Venus. BioMetals 2001, 14, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R. Iron Metabolism: From Molecular Mechanisms to Clinical Consequences, 4th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; ISBN 978-1-118-92561-4. [Google Scholar]
- Ward, R.J.; Crichton, R.R. Ironing Out the Brain. Metal Ions in Life Science; De Gruyter: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Neurodegenerative diseases and therapeutic strategies using iron chelators. J. Trace Elem. Med. Biol. 2015, 31, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 48, 640–656. [Google Scholar] [CrossRef]
- Thich, J.A.; Ou, C.-C.; Powers, D.; Vasilious, B.; Mastropaolo, D.; Potenza, J.A.; Schugar, H.J. Molecular structure and magnetic properties of mu-dihydroxo-bis[2,6-pyridine dicarboxylato aquoiron(III)] and mu-dihydroxo-bis[4-hydroxo-2,6-pyridine dicarboxylato aquoiron(III)] tetrahydrate. J. Am. Chem. Soc. 1976, 98, 1425–1433. [Google Scholar] [CrossRef]
- Ou, C.-C.; Lalancette, R.A.; Potenza, J.A.; Schugar, H.J. Molecular structure and magnetic properties of mu-dihydroxo-bis[4-dimethylamino-2,6-pyridinedicarboxylatoaquo iron(III)] dihydrate, [(CH3)2NC7H2NO4(H2O)FeOH]2.2H2O. J. Am. Chem. Soc. 1978, 100, 2053–2057. [Google Scholar] [CrossRef]
- Kato, M.; Yamada, T.; Inagaki, T.; Mori, W.; Sakai, K.; Tsubomura, T.; Sato, M.; Yano, S. Structures and magnetic properties of iron(III) dinuclear complexes with alkoxo and carboxylato bridges. Inorg. Chem. 1995, 34, 2645–2651. [Google Scholar] [CrossRef]
- Norman, R.E.; Yan, S.; Que, L., Jr.; Backes, G.; Ling, J.; Sanders-Loehr, J.; Zhang, J.H.; O’Connor, C.J. (mu-Oxo)(mu-carboxylato) diiron(III) complexes with distinct iron sites. Consequences of the inequivalence and its relevance to dinuclear iron-oxo proteins. J. Am. Chem. Soc. 1990, 112, 1554–1562. [Google Scholar] [CrossRef]
- Trukhan, V.M.; Gritsenko, O.N.; Nordlander, E.; Shteinman, A.A. Design and synthesis of new models for diiron biosites. J. Inorg. Biochem. 2000, 79, 41–46. [Google Scholar] [CrossRef]
- Pardo, E.; Lloret, F.; Carrasco, R.; Muñoz, M.C.; Temporal-Sánchez, T.; Ruiz-García, R. Chemistry and reactivity of dinuclear iron oxamate complexes: Alkane oxidation with hydrogen peroxide catalysed by an oxo-bridged diiron(III) complex with amide and carboxylate ligation. Inorg. Chim. Acta 2004, 357, 2713–2720. [Google Scholar] [CrossRef]
- Romakh, V.B.; Therrien, B.; Labat, G.; Stoekli-Evans, H.; Shul’pin, G.B.; Süss-Fink, G. Dinuclear iron, ruthenium and cobalt complexes containing 1,4-dimethyl-1,4,7-triazacyclononane ligands as well as carboxylato and oxo or hydroxo bridges. Inorg. Chim. Acta 2006, 359, 3297–3051. [Google Scholar] [CrossRef]
- Do, L.H.; Lippard, S.J. Toward functional carboxylate-bridged diiron protein mimics: Achieving structural stability and con-formational flexibility using a macrocyclic ligand framework. J. Am. Chem. Soc. 2011, 133, 10568–105681. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Sakiyama, S.; Nishida, T. Structural investigation of four dinuclear iron(III) complexes relevant to renal injuries. J. Comp. Chem. 2015, 14, 23–29. [Google Scholar] [CrossRef]
- Comba, P.; Daumann, L.; Lefebvre, J.; Linti, G.; Martin, B.; Straub, J.; Zessin, T. Mono- and dinuclear copper(II) and iron(III) complexes of a tetradentate bispidine-diacetate ligand. Aust. J. Chem. 2009, 62, 1238–1245. [Google Scholar] [CrossRef]
- Paredes-García, V.; Latorre, R.O.; Spodine, E. Electronic and magnetic properties of iron(III) dinuclear complexes with carbox-ylate bridges. Polyhedron 2014, 23, 1869–1876. [Google Scholar] [CrossRef]
- Bell, M.; Edwards, A.J.; Hoskins, B.F.; Kachab, E.H.; Robson, R. Synthesis and crystal structure of a complex of a tetranucleating macrocyclic ligand which binds four NiII ions in a square arrangement with a µ4-hydroxo group at the centre. J. Chem. Soc. Chem. Commun. 1987, 24, 1852–1854. [Google Scholar] [CrossRef]
- Starosta, W.; Ptasiewicz-Bak, H.; Leciejewicz, J. The crystal and molecular structure of a new calcium(II) complex with pyri-dine-2,6-dicarboxylate, water and nitrate ligands. J. Coord. Chem. 2002, 55, 1147–1153. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Zhang, W.-Z.; Xu, Q. Poly[[mu]2-aqua-[[mu]2-1,1’-(butane-1,4-di yl) diimidazole] bis ([mu]4-naphthalene-1,4-dicarboxyl ato) dimanganese(II)]. Acta Cryst. Sect. E Struct. Rep. Online 2008, 64, m1543. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-Q.; Yao, H.-C.; Yang, R.; Li, Z.-J.; Zhang, H.-Y.; Wu, B.-L.; Hou, H.-W. Synthesis, structure and thermal properties of polynuclear complexes with a new multidentate ligand, N,N′-bis(5-ethyl-1,3,4-thiadiazol-2-yl)-2,6-pyridinedicarboxamide. Polyhedron 2008, 27, 203–209. [Google Scholar] [CrossRef]
- Azadmeher, A.; Amini, M.M.; Hadipour, N.; Khavasi, H.R.; Fun, H.-K.; Chen, C.-J. Synthesis and structural characterization of diorganotin(IV) complexes with 2,6-pyridinedicarboxylic acid. Appl. Organomet. Chem. 2008, 22, 19–24. [Google Scholar] [CrossRef]
- Su, Q.-J.; Li, S.-H.; Wang, L.; Xie, C.-Z.; Ouyang, Y.; Xu, J.-Y. Synthesis, structure and characterization of 1D threefold-bridging copper(II) chain with strong ferromagnetic coupling. Inorg. Chem. Comm. 2010, 13, 1210–1212. [Google Scholar] [CrossRef]
- White, N.G.; Kitchen, J.A.; Joule, J.A.; Brooker, S. Copper-induced N–N bond cleavage results in an octanuclear expanded-core grid-like complex. Chem. Commun. 2012, 48, 6229–6231. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sanudo, E.C.; Hu, J.-Y.; Hu, M.; Fang, S.-M.; Liu, C.-S. Structural diversity and magnetic properties of five Cu(II) complexes with mixed naphthalene-based dicarboxyl tecton and different N-donor co-ligands. Aust. J. Chem. 2013, 66, 963–971. [Google Scholar] [CrossRef]
- Gou, L.; Zhang, H.-X.; Fan, X.-Y.; Li, D.-L. Lithium based coordination polymer as anode for Li-ion battery. Inorg. Chim. Acta 2013, 394, 10–14. [Google Scholar] [CrossRef]
- Long, G.J.; Robinson, W.T.; Tappmeyer, W.P.; Bridges, D.L. The magnetic, electronic, and Mössbauer spectral properties of several trinuclear iron(III) carboxylate complexes. J. Chem. Soc. Dalton Trans. 1973, 6, 573–579. [Google Scholar] [CrossRef]
- Boudalis, A.K.; Sanakis, Y.; Raptopoulou, C.P.; Terzis, A.; Tuchagues, J.-P.; Perlepes, S.P. A trinuclear cluster containing the {Fe3(µ3-O)}7+ core: Structural, magnetic and spectroscopic (IR, Mössbauer, EPR) studies. Polyhedron 2005, 24, 1540–1548. [Google Scholar] [CrossRef]
- Kantouri, M.-L.; Papadopoulos, C.D.; Hatzidimitriou, A.G.; Bakas, T.; Pachini, S. A trinuclear iron(III) complex containing the semi-cubane [Fe3(μ3-O)]7+ core: Structural, spectroscopic, magnetic and electrochemical study. Z. Für Anorg. Und Allg. Chem. 2010, 636, 531–538. [Google Scholar] [CrossRef]
- Balić, T.; Jagličić, Z.; Sadrollah, E.; Litterst, F.J.; Počkaj, M.; Baabe, B.; Kovač-Andrić, E.; Bijelić, J.; Gašo-Sokač, D.; Djerdj, I. Single crystal growth, structural characterization and magnetic properties study of an antiferromagnetic trinuclear iron(III) acetate complex with uncoordinated hexamine. Inorg. Chim. Acta 2021, 520, 120292. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Huang, X.; Retrum, J.; Schmucker, A.; Pink, M.; Stein, B.D.; Dragnea, B. Influence of iron oleate complex structure on iron oxide nanoparticle formation. Chem. Mater. 2007, 19, 3624–3632. [Google Scholar] [CrossRef]
- Lin, M.M.; Kim, D.K. In situ thermolysis of magnetic nanoparticles using non-hydrated iron oleate complex. J. Nanopart. Res. 2012, 14, 688. [Google Scholar] [CrossRef]
- Sánchez, M.; Sabio, L.; Gálvez, N.; Capdevila, M.; Dominguez-Vera, J.M. Iron chemistry at the service of life. IUBMB Life 2017, 69, 382–388. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X. The chemistry and biology of ferroptosis. Cell Chem. Biol. 2020, 27, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Cao, Y.; Cao, W.; Jia, Y.; Lu, N. The application of ferroptosis in diseases. Pharmacol. Res. 2020, 159, 104919. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, L.; Hou, S.; Yuan, Z.; Li, C.; Zhang, W.; Zheng, L.; Li, X. The role of iron in cancer progression. Front. Oncol. 2021, 11, 778492. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhong, M.; Liu, Y.; Xiong, Y.; Gao, Z.; Ma, J.; Zhuang, G.; Hong, X. Application of natural products for inducing ferroptosis in tumor cells. Biotechnol. Appl. Biochem. 2022, 69, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; Al Qudsy, L.H.H.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Hamaï, A.; Hienzsch, A.; Cañeque, T.; Müller, S.; Wicinski, J.; Cabaud, O.; Leroy, C.; David, A.; Acevedo, V.; et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 2017, 9, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Versini, A.; Colombeau, L.; Hienzch, A.; Gaillet, C.; Retailleau, P.; Debieu, S.; Müller, S.; Cañeque, T.; Rodriguez, R. Salinomycin derivatives kill breast cancer stem cells by lysosomal iron targeting. Chem.-Eur. J. 2020, 26, 7416–7424. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Müller, S.; Cañeque, T.; Colombeau, L.; Dusetti, N.; Santofimia-Castaño, P.; Gaillet, G.; Puisieux, A.; Iovanna, J.L.; Rodriguez, R. Iron-sensitive prodrugs that trigger active ferroptosis in drug-tolerant pancreatic cancer cells. J. Am. Chem. Soc. 2022, 144, 11536–11545. [Google Scholar] [CrossRef] [PubMed]
- Petkov, N.; Tadjer, A.; Encheva, E.; Cherkezova-Zheleva, Z.; Paneva, D.; Stoyanova, R.; Kukeva, R.; Dorkov, P.; Pantcheva, I. Experimental and DFT study of monensinate and salinomycinate complexes containing {Fe3(µ3–O)}7+ core. Molecules 2024, 26, 364. [Google Scholar] [CrossRef]
- Pantcheva, I.N.; Ivanova, J.; Zhorova, R.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W.S. Nickel(II) and zinc(II) dim-onensinates: Crystal structure, spectral properties and bactericidal activity. Inorg. Chim. Acta 2010, 363, 1879–1886. [Google Scholar] [CrossRef]
- Ivanova, J.; Pantcheva, I.N.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W.S. Crystal structures and spectral properties of new Cd(II) and Hg(II) complexes of monensic acid with different coordination modes of the ligand. Centr. Eur. J. Chem. 2010, 8, 852–860. [Google Scholar] [CrossRef]
- Pantcheva, I.; Dimitrova, R.; Nedzhib, A.; Dorkov, P.; Stoyanova, R.; Zhecheva, E. Coordination compounds of polyether ionophore Monensin with gadolinium(III) ions. In Nanoscience & Nanotechnology (Sofia); Balabanova, E., Mileva, E., Eds.; Acad. Evgeni Budevski Institute of Electrochemistry and Energy Systems (IEES-BAS), Bulgarian Academy of Sciences (BAS): Sofia, Bulgaria, 2019; Volume 19, pp. 40–46, ISSN 1313-8995 (print), ISSN 2738-8743 (online). [Google Scholar]
- Pantcheva, I.; Dimitrova, R.; Ivanova, V.; Nedzhib, A.; Dorkov, P.; Dinev, D.; Spasov, R.; Alexandrova, R. Spectral properties and biological activity of La(III) and Nd(III) monensinates. Open Chem. 2019, 17, 1423–1434. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Muralidhara, R.S.; Kesavulu, C.R.; Rao, J.L.; Anavekar, R.V.; Chakradhar, R.P.S. EPR and optical absorption studies of Fe3+ ions in sodium borophosphate glasses. J. Phys. Chem. Solids 2010, 71, 1651–1655. [Google Scholar] [CrossRef]
- Kakazey, M.; Vlasova, M.; Gonzalez-Rodriguez, G.; Salazar-Hernández, B. Fine structure of EPR spectra of Fe3+ in α-Al2O3 crystal, powders, and textured ceramics. Mat. Sci. Engin. 2002, 90, 114–119. [Google Scholar] [CrossRef]
- Borer, L.; Thalken, L.; Zhang, J.H.; Reiff, W.M. Characterization of a dimeric iron(III) complex of N,N’-ethylenebis(salicylamine). Inorg. Chem. 1983, 22, 3174–3176. [Google Scholar] [CrossRef]
- Vercamer, V.; Lelong, G.; Hijiya, H.; Kondo, Y.; Galoisy, L.; Calas, G. Diluted Fe3+ in silicate glasses: Structural effects of Fe-redox state and matrix composition. An optical absorption and X-band/Q-band EPR study. J. Non-Cryst. Solids 2015, 428, 138–145. [Google Scholar] [CrossRef]
- Murray, K.S. Binuclear oxo-bridged iron(III) complexes. Coord. Chem. Rev. 1974, 12, 1–35. [Google Scholar] [CrossRef]
- Rodriguez, F.; Moreno, M. The influence of water addition on solutions of FeCl3 in isopropanol. Z. Für Naturforschung 1983, 38, 701–702. [Google Scholar] [CrossRef]
- Vertes, A.; Korecz, L.; Burger, K. Mössbauer Spectroscopy; Elsevier: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Reedijk, J.; van Der Kraan, A.M. Mössbauer effect of octahedral iron(II) solvates. Recl. Trav. Chim. Pays-Bas. 1969, 88, 828–832. [Google Scholar] [CrossRef]
- Goldanskii, V.I.; Herber, R.H. Chemical Applications of Mossbauer Spectroscopy; Academic Press: Cambridge, MA, USA, 1968. [Google Scholar]
- Greenwood, N.N.; Gibb, T.C. Mössbauer Spectroscopy; Chapman and Hall: London, UK, 1971. [Google Scholar]
- You, Z.-L.; Zhu, H.-L. A dinuclear Schiff base iron(III) complex with the ligand N,N’-bis(2-oxidophenylmethyleneimino) pro-pane-1,2-diamine. Acta Cryst. Sect. E: Struct. Rep. Online 2004, 60, m1046–m1048. [Google Scholar] [CrossRef]
- Gertenbach, P.G.; Popov, A.I. Solution chemistry of monensin and its alkali metal ion complexes. Potentiometric and spec-troscopic studies. J. Am. Chem. Soc. 1975, 97, 4738–4744. [Google Scholar] [CrossRef]
- Petkov, N.; Pantcheva, I.; Ivanova, A.; Stoyanova, R.; Kukeva, R.; Alexandrova, R.; Abudalleh, A.; Dorkov, P. Novel cerium(IV) coordination compounds of monensin and salinomycin. Molecules 2023, 28, 4676. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; GaussView 5.0.; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comp. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Yang, Y.; Ratner, M.A.; Schatz, G.C. Computational modeling of octahedral iron oxide clusters: Hexaaquairon(III) and its dimers. J. Phys. Chem. C 2013, 117, 21706–21717. [Google Scholar] [CrossRef]
- Zhao, H.; Fang, C.; Gao, J.; Liu, C. Spin-state energies of heme-related models from spin-flip TDDFT calculations. Phys. Chem. Chem. Phys. 2016, 18, 29486–29494. [Google Scholar] [CrossRef]
- Schaefer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian-basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schaefer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian-basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Neese, F. Prediction and interpretation of the 57Fe isomer shift in Mössbauer spectra by density functional theory. Inorg. Chim. Acta 2002, 337, 181–192. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [PubMed]









| Complex | Component | δ, mm/s | Δ, mm/s | Γ, mm/s | A, % | M, % |
|---|---|---|---|---|---|---|
| 1 | Db1 | 0.43 (0.60) | 0.75 (0.75) | 0.45 (0.45) | 47 (48) | 0.49 (1.48) |
| Db2 | 0.41 (0.61) | 0.74 (0.79) | 0.57 (0.52) | 45 (47) | 0.37 (1.29) | |
| Db3 | 0.21 (0.40) | 0.82 (0.75) | 0.40 (0.40) | 8 (5) | 0.09 (0.19) | |
| 2 | Db1 | 0.37 (0.57) | 0.63 (0.62) | 0.32 (0.31) | 66 (64) | 6.96 (8.41) |
| Db2 | 0.39 (0.58) | 0.96 (1.00) | 0.42 (0.38) | 34 (36) | 2.99 (3.91) |
| Complex | Band Position, cm−1 | ||
|---|---|---|---|
| Exp. | Calc. | SF | |
| 1A | − | 1613 | − |
| 1B (sub-band 1) | 1594 | 1647 | 0.97 |
| 1B (sub-band 2) | 1554 | 1578 | 0.98 |
| 1C (sub-band 1) | 1594 | 1626 | 0.98 |
| 1C (sub-band 2) | 1554 | 1604 | 0.97 |
| 1D (sub-band 1) | 1594 | 1655 | 0.96 |
| 1D (sub-band 2) | 1554 | 1607 | 0.97 |
| 2 | 1527 | 1649 | 0.93 |
| Multiplicity | ΔH, kcal/mol, 293 K | g-Factor, 0 K |
|---|---|---|
| HS | 0.91 | 2.005 |
| AFMSx | 0.00 | 2.004 |
| AFMSx-ooa * | 122.71 | 2.087 |
| Bacteria Compound | MW, g/mol | BC | BS | ||
|---|---|---|---|---|---|
| µg/mL | µM | µg/mL | µM | ||
| MonH × H2O | 688.90 | 3.91 | 5.67 | 15.63 | 22.69 |
| Complex 1 | 2862.08 | 1.95 | 0.68 | 15.63 | 5.46 |
| Complex 2 | 4363.90 | 1.95 | 0.47 | 15.63 | 3.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkov, N.; Tadjer, A.; Simova, S.; Cherkezova-Zheleva, Z.; Paneva, D.; Stoyanova, R.; Kukeva, R.; Dorkov, P.; Pantcheva, I. Synthesis, Spectral Characterization, and Structural Modelling of Di- and Trinuclear Iron(III) Monensinates with Different Bridging Patterns. Inorganics 2024, 12, 114. https://doi.org/10.3390/inorganics12040114
Petkov N, Tadjer A, Simova S, Cherkezova-Zheleva Z, Paneva D, Stoyanova R, Kukeva R, Dorkov P, Pantcheva I. Synthesis, Spectral Characterization, and Structural Modelling of Di- and Trinuclear Iron(III) Monensinates with Different Bridging Patterns. Inorganics. 2024; 12(4):114. https://doi.org/10.3390/inorganics12040114
Chicago/Turabian StylePetkov, Nikolay, Alia Tadjer, Svetlana Simova, Zara Cherkezova-Zheleva, Daniela Paneva, Radostina Stoyanova, Rositsa Kukeva, Petar Dorkov, and Ivayla Pantcheva. 2024. "Synthesis, Spectral Characterization, and Structural Modelling of Di- and Trinuclear Iron(III) Monensinates with Different Bridging Patterns" Inorganics 12, no. 4: 114. https://doi.org/10.3390/inorganics12040114