Reduction of Ferric Chloride in Yeast Growth Media, by Sugars and Aluminum
Abstract
:1. Introduction
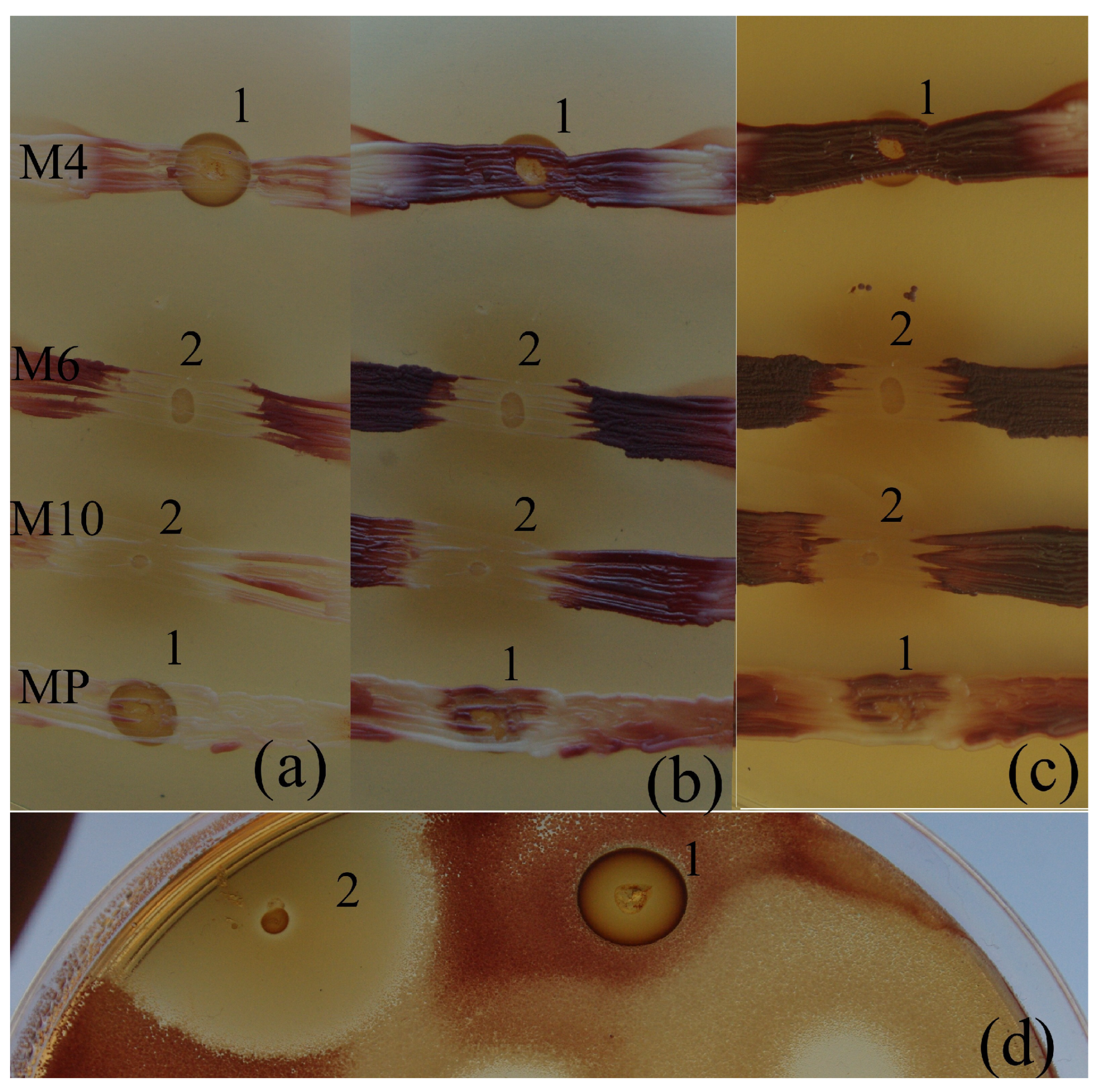
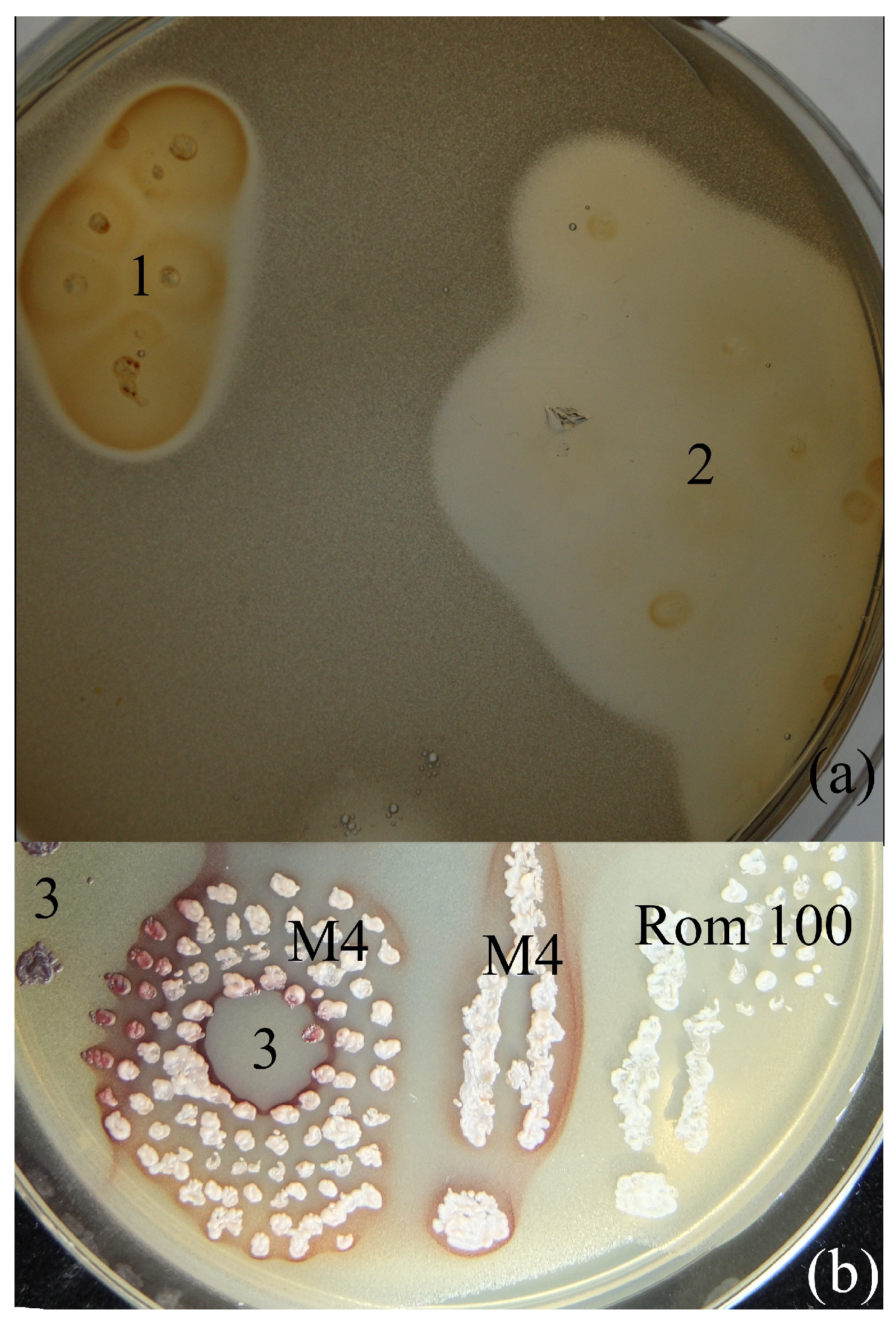
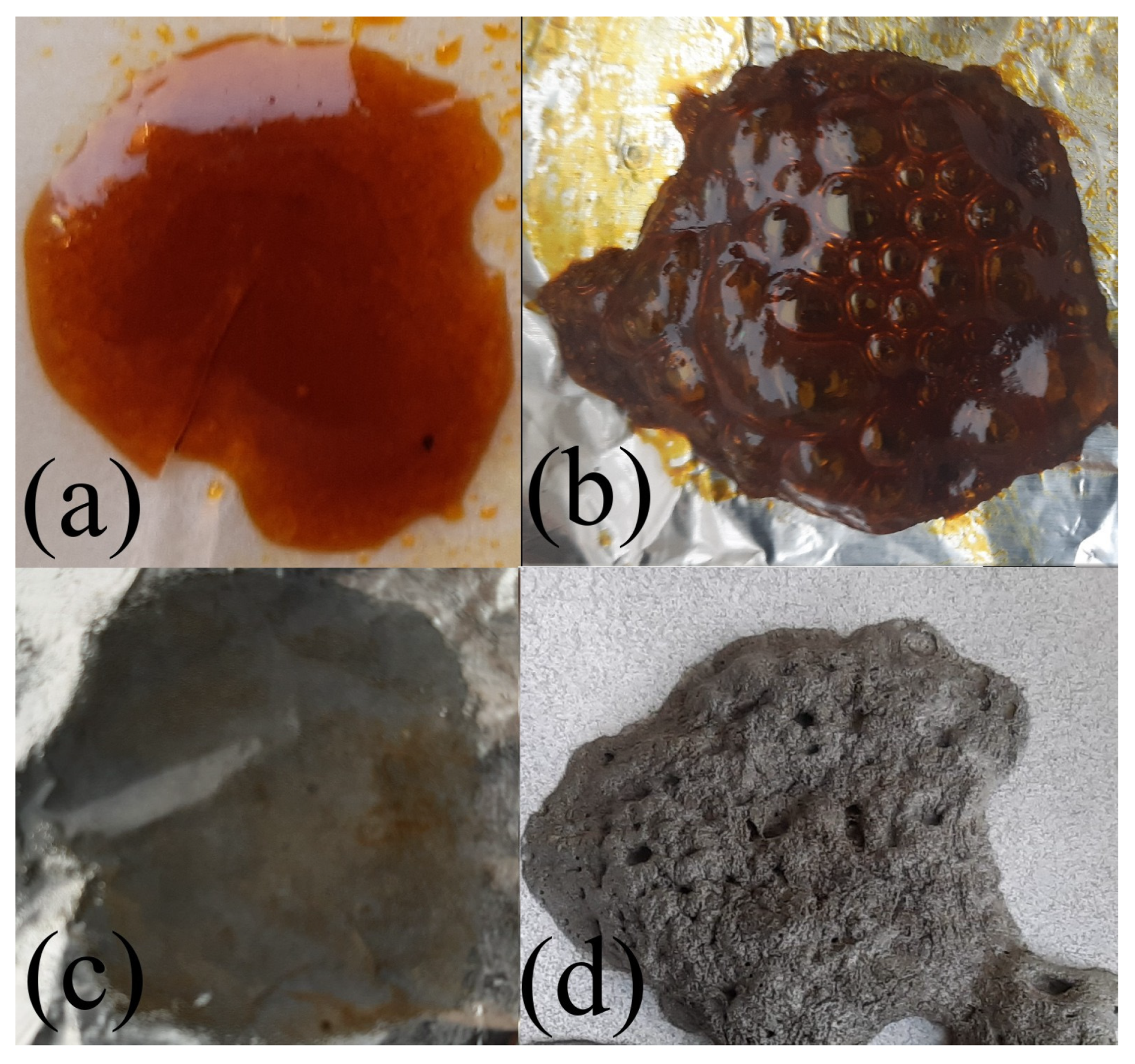
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luidold, S.; Antrekowitsch, H. Hydrogen as a Reducing Agent: State-of-the-Art Science and Technology. JOM 2007, 59, 20–26. [Google Scholar] [CrossRef]
- Xing, Z.Y.; Lu, J.; Ji, X. A brief review of metallothermic reduction reactions for materials preparation. Small Methods 2018, 2, 1800062. [Google Scholar] [CrossRef]
- Mažeika, K.; Šiliauskas, L.; Skridlaite, G.; Matelis, A.; Garjonytė, R.; Paškevicius, A.; Melvydas, V. Features of iron accumulation at high concentration in pulcherrimin-producing Metschnikowia biomass. JBIC J. Biol. Inorg. Chem. 2021, 26, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Melvydas, V.; Staneviciene, R.; Balynaite, A.; Vaiciuniene, J.; Garjonyte, R. Formation of self-organized periodic patterns around yeasts secreting a precursor of red pigment. Microbiol. Res. 2016, 193, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82/83, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. BBA-Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Christides, T.P.; Sharp, P. Sugars increase non-heme iron bioavailability in human epithelial intestinal and liver cells. PLoS ONE 2013, 8, e83031. [Google Scholar] [CrossRef] [PubMed]
- Pulla Rao, C.; Geetha, K.; Raghava, M.S.S. Fe(III) complexes of D-glucose and D-fructose. BioMetals 1994, 7, 25–29. [Google Scholar]
- Maicas, S.; Mateo, J.J. The Life of Saccharomyces and Non-Saccharomyces Yeasts in Drinking Wine. Microorganisms 2023, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Abeln, F.; Hicks, R.H.; Auta, H.; Moreno-Beltrán, M.; Longanesi, L.; Henk, D.A.; Chuck, C.J. Semi-continuous pilot-scale microbial oil production with Metschnikowia pulcherrima on starch hydrolysate. Biotechnol. Biofuels 2020, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microb. Biot. 2019, 35, 154. [Google Scholar] [CrossRef] [PubMed]
- Melvydas, V.; Svediene, J.; Skridlaite, G.; Vaiciuniene, J.; Garjonyte, R. In vitro inhibition of Saccharomyces cerevisiae growth by Metschnikowia spp. triggered by fast removal of iron via two ways. Braz. J. Microbiol. 2020, 51, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Commenges, A.; Lessard, M.-H.; Coucheney, F.; Labrie, S.; Drider, D. The biopreservative properties of Metschnikowia pulcherrima LMA 2038 and Trichosporon asahii LMA 810 in a model fresh cheese, are presented. Food Biosci. 2024, 58, 103458. [Google Scholar] [CrossRef]
- Kregiel, D.; Czarnecka-Chrebelska, K.H.; Schusterová, H.; Vadkertiová, R.; Nowak, A. The Metschnikowia pulcherrima clade as a model for assessing inhibition of Candida spp. and the toxicity of its metabolite, pulcherrimin. Molecules 2023, 28, 5064. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, E.; James, S.A.; Breierova, E.; Kregiel, H.A.D. Biocontrol capability of local Metschnikowia sp. isolates. Antonie Van Leeuwenhoek J. Microb. 2019, 112, 1425–1445. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M. Metschnikowia pulcherrima and related pulcherrimin-producing yeasts: Fuzzy species boundaries and complex antimicrobial antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, C. The structure of pulcherriminic acid. Can. J. Chem. 1963, 41, 165–172. [Google Scholar] [CrossRef]
- Charron-Lamoureux, V.; Haroune, L.; Pomerleau, M.; Hall, L.; Orban, F.; Leroux, J.; Rizzi, A.; Bourassa, J.-S.; Fontaine, N.; d’Astous, É.V.; et al. Pulcherriminic acid modulates iron availability and protects against oxidative stress during microbial interactions. Nat. Commun. 2023, 14, 2536. [Google Scholar] [CrossRef] [PubMed]
- Zhike, W.; Jianting, C.; Cunling, Y. Application of ferric chloride both as oxidant and complexant to enhance the dissolution of metallic copper. Hydrometallurgy 2010, 105, 69–74. [Google Scholar] [CrossRef]
- Cakır, O. Chemical etching of aluminium. J. Mater. Process. Technol. 2008, 199, 337–340. [Google Scholar] [CrossRef]
- Ogura, Y.; Kobayashi, C.; Ooba, Y.; Yahata, N.; Sakamoto, H. Low temperature deposition of metal films by metal chloride reduction chemical vapor deposition. Surf. Coat. Technol. 2006, 200, 3347–3350. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chonga, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- So, R.C.; Carreon-Asok, A.C. Molecular design, synthetic strategies, and applications of cationic polythiophenes. Chem. Rev. 2019, 119, 11442–11509. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, J.; Cai, C.; Wang, S.; Pei, H.; Zhang, J. Corn stover pretreatment by inorganic salts and its effects on hemicellulose and cellulose degradation. Bioresour. Technol. 2009, 100, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, N.; Pan, X.; Wu, S.; Xie, J. Oxidative conversion of glucose to gluconic acid by iron(III) chloride in water under mild conditions. Green Chem. 2016, 18, 2308–2312. [Google Scholar] [CrossRef]
- Žalneravičius, R.; Paškevičius, A.; Kurtinaitiene, M.; Jagminas, A. Size-dependent antimicrobial properties of the cobalt ferrite nanoparticles. J. Nanopart. Res. 2016, 18, 300. [Google Scholar] [CrossRef]
- Sun, H.-Q.; Lu, X.-M.; Gao, P.-J. The exploration of the antibacterial mechanism of Fe3+ against bacteria. Braz. J. Microb. 2011, 42, 410–414. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Z.; Yao, L.; Zhang, L.; Gou, X.; Mo, H.; Li, H.; Hu, L.; Zhou, X. Ferric chloride induces ferroptosis in Pseudomonas aeruginosa and heals wound infection in a mouse model. Int. J. Antimicrob. Agents 2023, 61, 106794. [Google Scholar] [CrossRef]
- Zhen, W.; Lia, H.; Zhou, W.; Lee, J.; Liu, Z.; An, Z.; Xu, D.; Mo, H.; Hu, L.; Zhou, X. Ferrous sulfate-loaded hydrogel cures Staphylococcus aureus infection via facilitating a ferroptosis-like bacterial cell death in a mouse keratitis model. Biomaterials 2022, 290, 121842. [Google Scholar] [CrossRef]
- Karathia, H.; Vilaprinyo, E.; Sorribas, A.; Alves, R. Saccharomyces cerevisiae as a model organism: A comparative study. PLoS ONE 2011, 6, e16015. [Google Scholar] [CrossRef] [PubMed]
- Duina, A.A.; Miller, M.E.; Keeney, J.B. Budding yeast for budding geneticists: A primer on the Saccharomyces cerevisiae model system. Genetics 2014, 197, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Foley, E.; Moyle, F.J. The reduction of ferric chloride by aluminium in aluminium chloride melts. J. Appl. Chem. Biotechnol. 1972, 22, 867–875. [Google Scholar] [CrossRef]
- Melvydas, V.; Mazeika, K.; Matelis, A.; Paskevicius, A.; Garjonyte, R. Response of pulcherrimin-producing Metschnikowia yeast to solid iron-containing materials: In vitro and in vivo studies. Mycologia, (submitted, under review).
- Thrane, N.; Trumpy, G. Spin-spin relaxation and Karyagin-Gol’danskii effect in FeCl3 6H2O. Phys. Rev. B 1970, 1, 153–155. [Google Scholar] [CrossRef]
- Stefansson, A. Iron(III) hydrolysis and solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Irto, A.; Cigala, R.M.; De Stefano, C.; Crea, F. Advances in iron(III) hydrolysis studies. Effect of the metal concentration, ionic medium and ionic strength. J. Mol. Liq. 2023, 1, 123361. [Google Scholar] [CrossRef]
- Millero, F.J.; Yao, W.; Aicher, J. The speciation of Fe(II) and Fe(III) in natural waters. Mar. Chem. 1995, 50, 21–39. [Google Scholar] [CrossRef]
- Cotton, S.A. Iron(III) chloride and its coordination chemistry. J. Coord. Chem. 2018, 71, 3415–3443. [Google Scholar] [CrossRef]
- Neilands, J.B. Methodology of siderophores. In Siderophores from Microorganisms and Plants; Clarke, M.J., Goodenough, J.B., Ibers, J.A., Jorgensen, C.K., Mingos, D.M.P., Neilands, J.B., Palmer, G.A., Reinen, D., Sadler, P.J., Weiss, R., Eds.; Springer: Berlin, Germany, 1984; pp. 1–25. [Google Scholar]
- Murphy, J.M.; Powell, B.A.; Brumaghim, J.L. Stability constants of bio-relevant, redox-active metals with amino acids: The challenges of weakly binding ligands. Coord. Chem. Rev. 2020, 412, 212253. [Google Scholar] [CrossRef]
- Bull, J.N.; Maclagan, R.G.A.R.; Fitchett, C.M.; Craighead Tennant, W. A new isomorph of ferrous chloride tetrahydrate: A 57Fe Mossbauer and X-ray crystallography study. J. Phys. Chem. Sol. 2010, 71, 1746–1753. [Google Scholar] [CrossRef]
- Charley, P.J.; Sarkar, B.; Stitt, C.F.; Saltman, P. Chelation of iron by sugars. Biochim. Biophys. Acta 1963, 69, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kótai, L.; Lippart, J.; Gács, I. Deuterium isotope separation in the chemical reaction of aluminium amalgam and water. Eur. Chem. Bull. 2012, 1, 37–38. [Google Scholar] [CrossRef]
- Mažeika, K.; Reklaitis, J.; Nicolenco, A.; Vainoris, M.; Tsyntsaru, N.; Cesiulis, H. Magnetic state instability of disordered electrodeposited nanogranular Fe films. J. Magn. Magn. Mat. 2021, 540, 168433. [Google Scholar] [CrossRef]
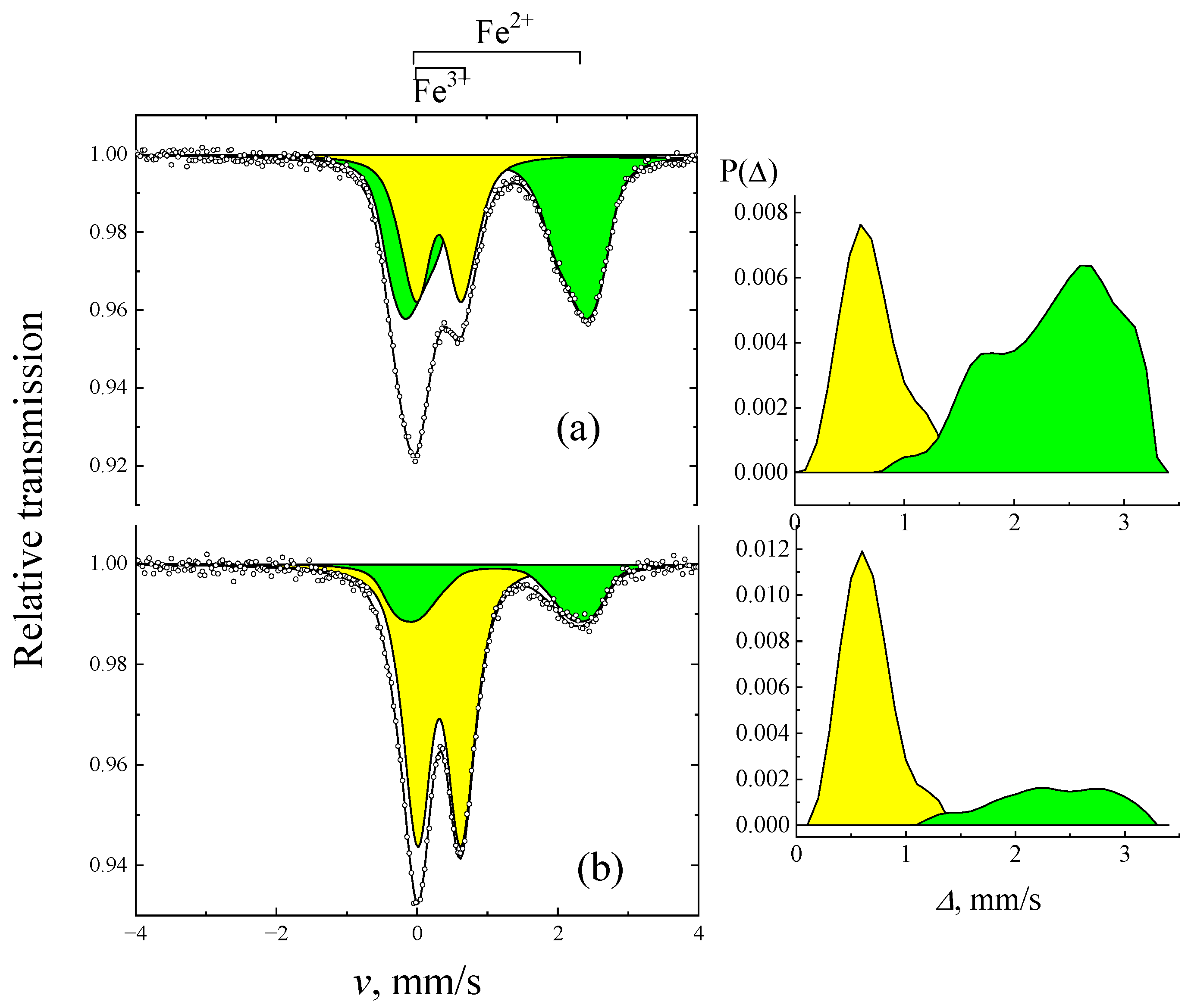
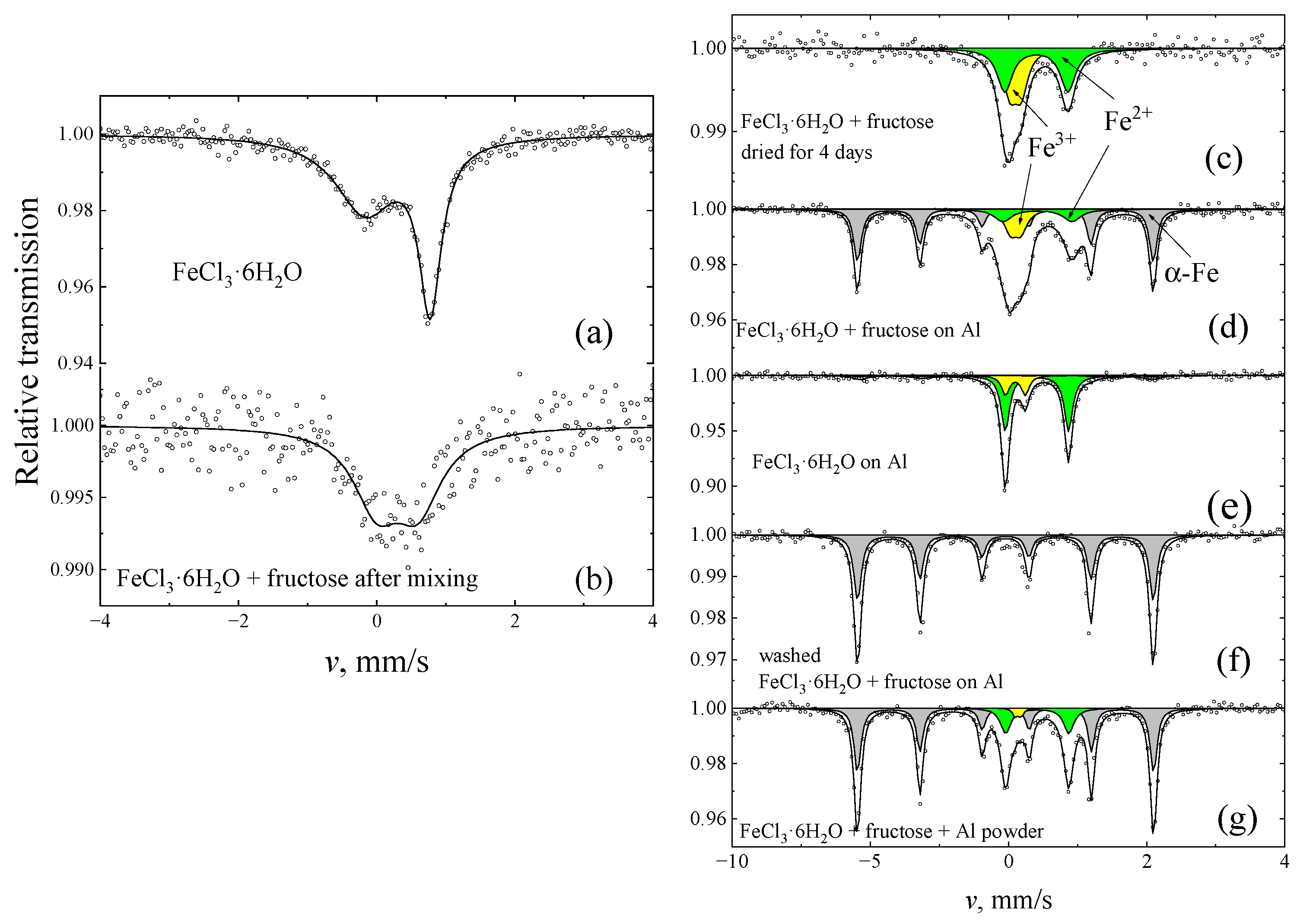
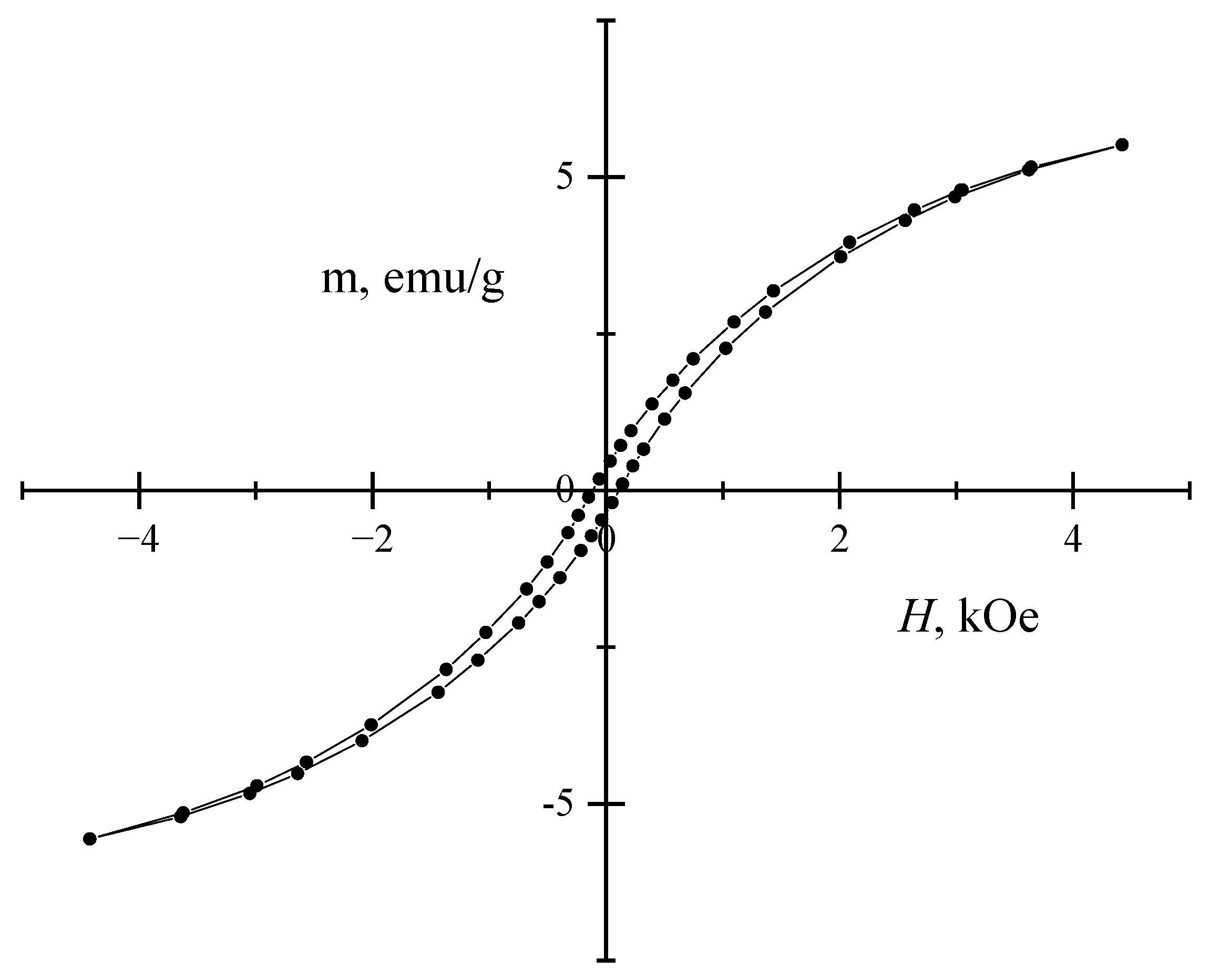
| Sample | I, % | δ, mm/s | Δ, mm/s | |
|---|---|---|---|---|
| FeSO4·7H2O | 35 (34 ± 1) * | 0.427 ± 0.004 | 0.695 (0.66 ± 0.01) | Fe3+ |
| 65 (35 ± 1; 31 ± 1) | 1.245 ± 0.004 | 2.36 (1.97 ± 0.02; 2.75 ± 0.01) | Fe2+ | |
| FeCl3·6H2O | 74 (73 ± 1) | 0.423 ± 0.002 | 0.656 (0.63 ± 0.01) | Fe3+ |
| 26 (27 ± 1) | 1.215 ± 0.010 | 2.33 (2.44 ± 0.02) | Fe2+ |
| Sample | I, % | Γ, mm/s | δ, mm/s | Δ, mm/s | B, T | |
|---|---|---|---|---|---|---|
| FeCl3·6H2O | 100 | 1.07 ± 0.05 * | 0.41 ± 0.01 | 0.94 ± 0.01 | Fe3+ | |
| FeClFP0d (1:1) | 100 | 0.83 ± 0.05 | 0.40 ± 0.03 | 0.60 ± 0.06 | Fe3+ | |
| FeClFP1–2d (1:1) | 74 ± 2 | 0.55 ± 0.02 | 0.37 ± 0.13 | 0.40 ± 0.01 | Fe3+ | |
| 26 ± 2 | 0.91 ± 0.09 | 1.14 ± 0.04 | 2.39 ± 0.07 | Fe2+ | ||
| FeClFP1d (2:1) | 32 ± 1 | 0.50 ± 0.04 | 0.36 ± 0.01 | 0.41 ± 0.02 | Fe3+ | |
| 68 ± 1 | 0.28 ± 0.01 | 1.17 ± 0.01 | 2.35 ± 0.01 | Fe2+ | ||
| FeClFP4d (1:1) | 41 ± 2 | 0.57 ± 0.09 | 0.37 ** | 0.40 ± 0.04 | Fe3+ | |
| 59 ± 3 | 0.67 ± 0.09 | 1.11 ± 0.02 | 2.28 ± 0.04 | Fe2+ | ||
| FeClFAl1d (1:1) | 24 ± 1 | 0.53 ± 0.04 | 0.37 ** | 0.37 ± 0.02 | Fe3+ | |
| 29 ± 1 | 0.76 ± 0.04 | 1.16 ± 0.01 | 2.49 ± 0.03 | Fe2+ | ||
| 47 ± 1 | 0.33 ± 0.01 | 0.00 ± 0.01 | 0.00 ± 0.01 | 33.17 ± 0.02 | Fe0 | |
| After washing FeClFAl1d | 100 | 0.33 ± 0.02 | 0.00 ± 0.01 | −0.01 ± 0.01 | 33.23 ± 0.03 | Fe0 |
| FeCl3·6H2O on Al | 20 ± 1 | 0.33 ± 0.03 | 0.36 ± 0.05 | 0.71 ± 0.11 | Fe3+ | |
| 70 ± 2 | 0.36 ± 0.01 | 1.14 ± 0.02 | 2.28 ± 0.04 | Fe2+ | ||
| 10 ± 2 | 0.34 ± 0.02 | 0.00 ± 0.06 | 0.00 ** | 33.7 ± 0.4 | Fe0 | |
| FeClFAlp1d | 2 ± 1 | 0.20 ± 0.09 | 0.46 ± 0.04 | 0.22 ± 0.05 | Fe3+ | |
| (1:1:1) | 30 ± 1 | 0.45 ± 0.01 | 1.15 ± 0.01 | 2.28 ± 0.01 | Fe2+ | |
| 68 ± 1 | 0.30 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 33.2 ± 0.01 | Fe0 | |
| Washed | 19 ± 2 | 0.6 ± 0.2 | 0.29 ± 0.05 | 0.69 ± 0.09 | Fe3+ | |
| FeClFAlp1d | 81 ± 2 | 0.35 ± 0.01 | 0.00 ± 0.01 | 0.00 ± 0.01 | 33.18 ± 0.04 | Fe0 |
| FeSFP6d (1:1) | 88 ± 1 | 0.33 ± 0.01 | 0.42 ± 0.01 | 0.15 ± 0.01 | Fe3+ | |
| 12 ± 1 | 0.78 ± 0.09 | 1.24 ± 0.06 | 2.57 ± 0.08 | Fe2+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mažeika, K.; Melvydas, V.; Čepukoit, D. Reduction of Ferric Chloride in Yeast Growth Media, by Sugars and Aluminum. Inorganics 2024, 12, 137. https://doi.org/10.3390/inorganics12050137
Mažeika K, Melvydas V, Čepukoit D. Reduction of Ferric Chloride in Yeast Growth Media, by Sugars and Aluminum. Inorganics. 2024; 12(5):137. https://doi.org/10.3390/inorganics12050137
Chicago/Turabian StyleMažeika, Kęstutis, Vytautas Melvydas, and Dovilė Čepukoit. 2024. "Reduction of Ferric Chloride in Yeast Growth Media, by Sugars and Aluminum" Inorganics 12, no. 5: 137. https://doi.org/10.3390/inorganics12050137
APA StyleMažeika, K., Melvydas, V., & Čepukoit, D. (2024). Reduction of Ferric Chloride in Yeast Growth Media, by Sugars and Aluminum. Inorganics, 12(5), 137. https://doi.org/10.3390/inorganics12050137






