Polymer-Based Immobilized FePMo12O40@PVP Composite Materials for Photocatalytic RhB Degradation
Abstract
:1. Introduction
2. Results and Discussion
2.1. FT-IR Analysis
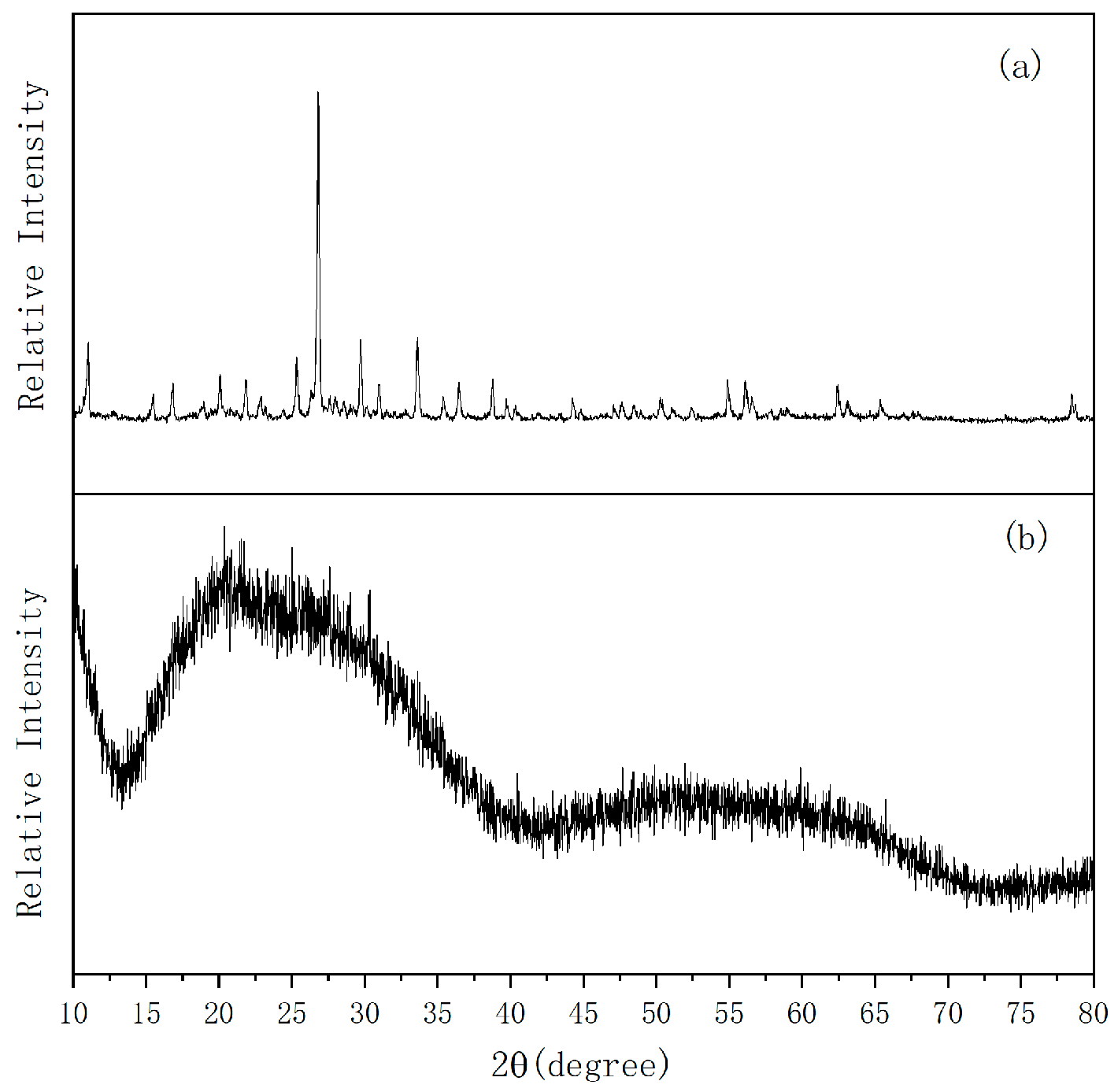
2.2. XRD Analysis
2.3. XPS Analysis
2.4. SEM and TEM
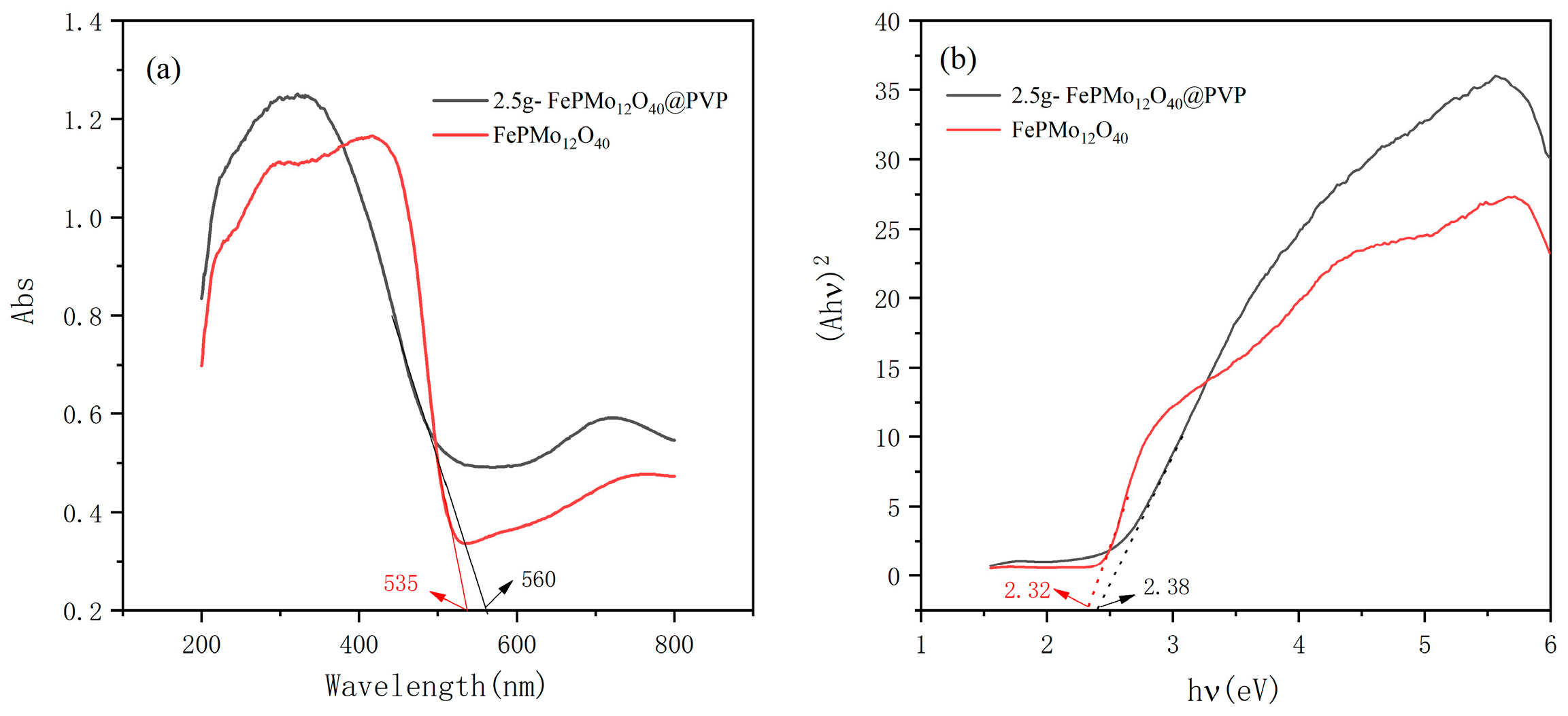
2.5. UV-Vis DRS Analysis
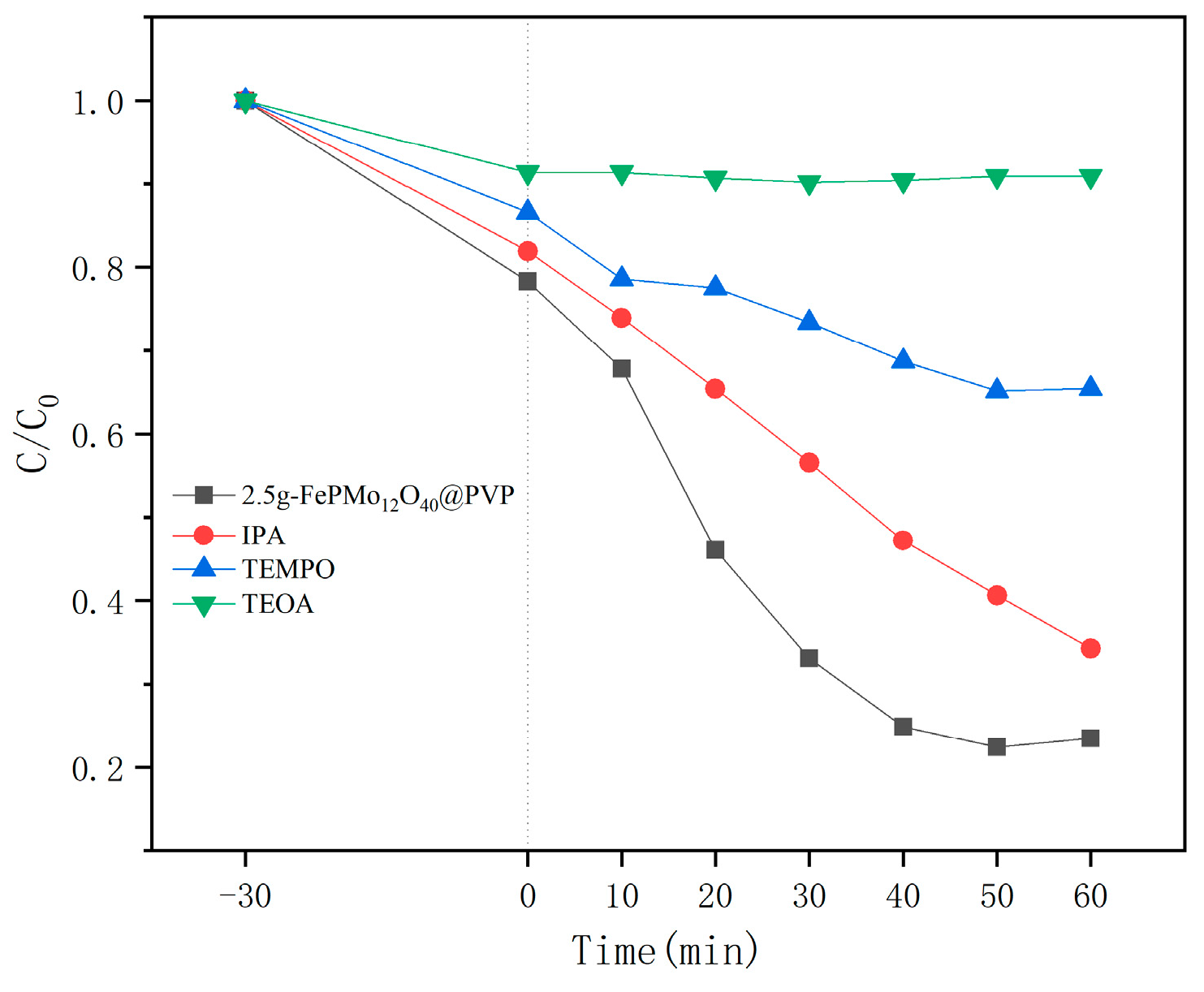
2.6. Photocatalytic Performance Analysis
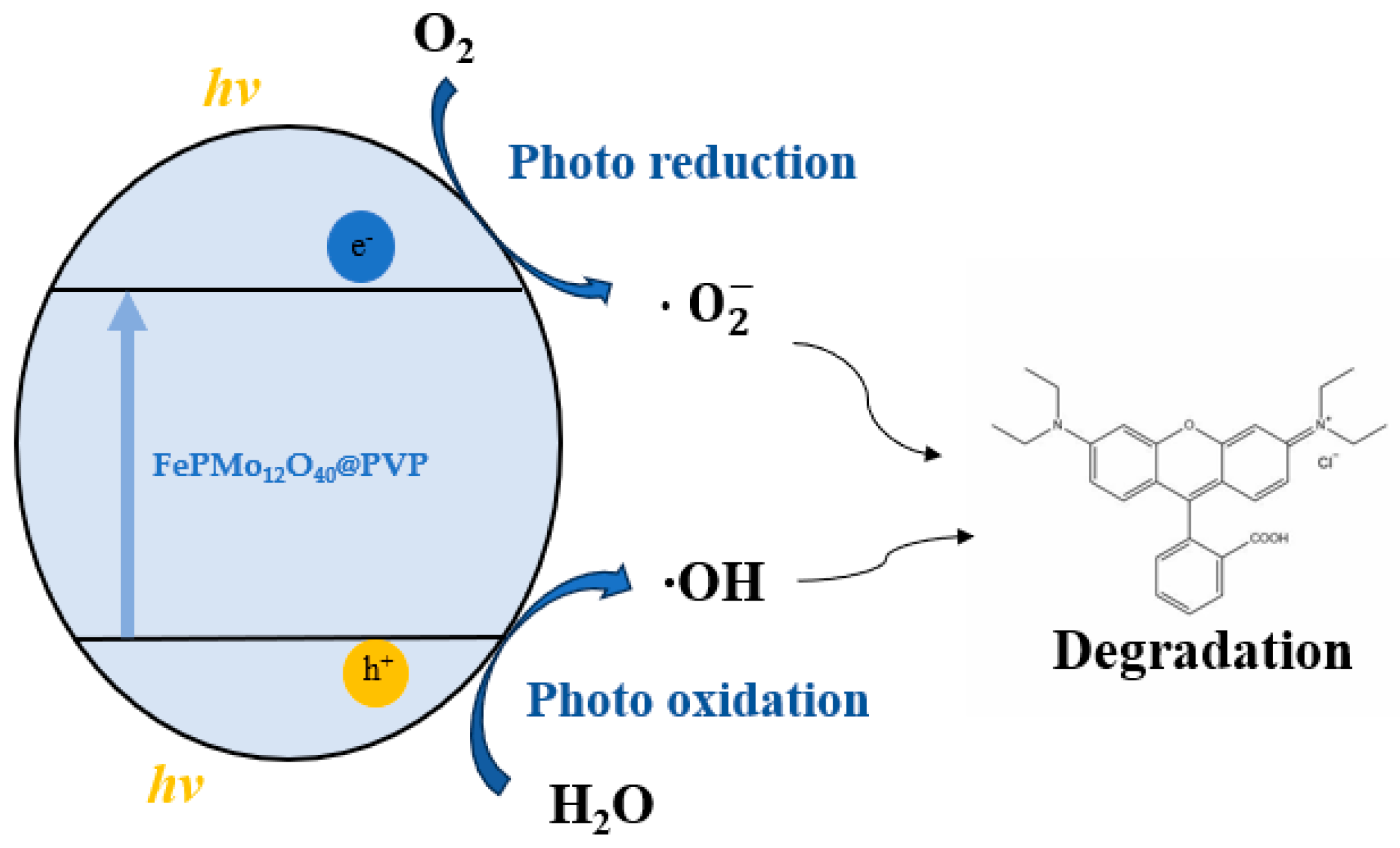
2.7. Possible Mechanism of Photocatalytic Degradation
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Catalyst Preparation
3.3. Photocatalytic Degradation of Rhodamine B Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Liu, Y.; Wang, C.; Yang, L.; Wu, N.; Chen, R. Fabrication of (H2PO4−, Ni2+)—α-Fe2O3/Bi2S3 heterojunction photocatalysts with improved visible-light catalytic activity. Chem. Eng. Sci. 2024, 289, 119874. [Google Scholar] [CrossRef]
- Zaman, F.U.; Kumar, A.; Yasin, G.; Boakye, F.O.; Muhammad, F.; Iqbal, S.; Alotaibi, K.M.; Hou, L.; Yuan, C. Enhanced photocatalytic degradation of organic dyes by carbon quantum dots-ZnFe2O4 composites. J. Alloys Compd. 2024, 983, 173860. [Google Scholar] [CrossRef]
- Wang, W.; Luo, R.; Yin, Y.; Wang, R.; Zhang, D.; Cui, Z.; Bi, S.; Shao, F. Three novel MOFs with various dimensions as efficient Fenton-like photocatalysts for degradation of Rhodamine B. Polyhedron 2024, 252, 116879. [Google Scholar] [CrossRef]
- Ng, K.H.; Lin, Y.; Chen, L. Stably suspended SiO2-supported CdS photocatalyst for a promising organic pollutant degradation in the absence of mechanical stirring. Chemosphere 2024, 350, 141084. [Google Scholar] [CrossRef]
- Campos-Delgado, J.; Mendoza, M.E. Ternary Graphene Oxide and Titania Nanoparticles-Based Nanocomposites for Dye Photocatalytic Degradation: A Review. Materials 2024, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Wang, Y.; Jiang, W.; Liu, J.; Mayyas, M.; Shen, J.; Wei, X. Sulfur quantum dot sensitized anatase TiO2 for enhanced photocatalytic activity. Chem. Eng. Sci. 2024, 288, 119840. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Yu, X.; Yin, Y.; Ru, Y.; Tian, G. In situ construction of Schottky junctions on MOF-derived defective TiO2 oval nanocages for enhanced photocatalytic CO2 reduction. J. Alloys Compd. 2024, 983, 173735. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, J.; Tan, Q.; Li, K.; Li, Q.; Correia Carabineiro, S.A.; Lv, K. Remarkable Enhancement of Photocatalytic Activity of High-Energy TiO2 Nanocrystals for NO Oxidation through Surface Defluorination. Acs Appl. Mater. Interfaces 2024, 16, 11479–11488. [Google Scholar] [CrossRef]
- Manna, R.; Bhattacharya, G.; Sardar, P.; Raj, S.; Jain, A.; Samanta, A.N. Construction of a visible light responsive dual Z-scheme NH2-MIL-101(Fe)/CdS/g-C3N4 ternary heterojunction photocatalyst for efficient CO2 photoreduction to methanol. Chem. Eng. Sci. 2024, 288, 119811. [Google Scholar] [CrossRef]
- Sharma, K.; Sudhaik, A.; Kumar, R.; Nguyen, V.H.; Le, Q.V.; Ahamad, T.; Thakur, S.; Kaya, S.; Nguyen, L.H.; Raizada, P.; et al. Advanced photo-Fenton assisted degradation of tetracycline antibiotics using α-Fe2O3/CdS/SiO2 based S-scheme photocatalyst. J. Water Process Eng. 2024, 59, 105011. [Google Scholar] [CrossRef]
- Yu, P.; Zhuang, R.; Liu, H.; Wang, Z.; Zhang, C.; Wang, Q.; Sun, H.; Huang, W. Recycling alkali lignin-derived biochar with adsorbed cadmium into cost-effective CdS/C photocatalyst for methylene blue removal. Waste Manage. Res. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xu, K.; Wang, Y.; Li, X.; Zhang, X.; Wang, J.; Zhang, Y.; Liu, Q.; Fang, Q. Efficient metal–organic framework-based dual co-catalysts system assist CdS for hydrogen production from photolysis of water. J. Colloid. Interface. Sci. 2024, 661, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Mutahir, S.; Asim Khan, M.; Qunhui, Y.; Mehboob, S.; Bououdina, M.; Mostafa Elkholi, S.; Khan, A.; Abumousa, R.A.; Humayun, M. MOF-derived ZnO/g-C3N4 nanophotocatalyst for efficient degradation of organic pollutant. J. Saudi Chem. Soc. 2024, 28, 101821. [Google Scholar] [CrossRef]
- Li, N.; Hu, Z.; Li, Y.; Zhou, R.; Lan, G.; Gong, X. WO3-ZnO as an anti-gastric cancer agent and efficient photocatalyst in the synthesis of substituted benzimidazoles. Inorg. Chem. Commun. 2024, 161, 112025. [Google Scholar] [CrossRef]
- Jansanthea, P.; Inyai, N.; Chomkitichai, W.; Ketwaraporn, J.; Ubolsook, P.; Wansao, C.; Wanaek, A.; Wannawek, A.; Kuimalee, S.; Pookmanee, P. Green synthesis of CuO/Fe2O3/ZnO ternary composite photocatalyst using grape extract for enhanced photodegradation of environmental organic pollutant. Chemosphere 2024, 351, 141212. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, M.; Younas, A.; Iqbal, T.; Afsheen, S.; Zubair, M.; Kamran, S.K.S.; Syed, A.; Bahkali, A.H.; Wong, L.S. Synthesis and characterization of ZnO/BiVO4 nanocomposites as heterogeneous photocatalysts for antimicrobial activities and waste water treatment. Mater. Chem. Phys. 2024, 315, 128923. [Google Scholar] [CrossRef]
- Ye, M.; Wang, Y.; Xu, B.; Wang, K.; Zhang, L.; He, Z.; Zhang, P.; Zhang, S.; Jin, L.; Feng, J. Bi5O7I/Bi2O3 heterojunction photocatalyst for pollutant degradation assisted by H2O2. Opt. Mater. 2024, 148, 114918. [Google Scholar] [CrossRef]
- Mazouzi, D.E.; Djani, F.; Soukeur, A.; Bouchal, W.; Manseri, A.; Derkaoui, K.; Martínez-Arias, A.; Ksouri, A.; Şen, F.; Kaci, M.M. Auto-combustion designed BiFeO3/Bi2O3 photocatalyst for improved photodegradation of nitrobenzene under visible light and sunlight irradiation. Surf. Interfaces 2024, 44, 103581. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, A.; Yadav, S.; Kumar, N.; Dahiya, H.; Makgwane, P.R.; Hosseini Bandegharaei, A.; Jindal, J. A facile synthesis of Ag incorporated Bi2O3/CuS nanocomposites as photocatalyst for degradation of environmental contaminants. Inorg. Chem. Commun. 2023, 157, 111266. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Liu, B.; Qin, W.; Xie, Y. Facile Synthesis of Nano-Flower β-Bi2O3/TiO2 Heterojunction as Photocatalyst for Degradation RhB. Molecules 2023, 28, 882. [Google Scholar] [CrossRef]
- Hiskia, A.; Mylonas, A.; Papaconstantinou, E. Comparison of the photoredox properties of polyoxometallates and semiconducting particles. Chem. Soc. Rev. 2001, 30, 62–69. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, S.; Wågberg, T.; Zhou, H.; Li, S.; Li, Y.; Tan, Y.; Hu, W.; Ding, Y.; Han, X. Developing Insoluble Polyoxometalate Clusters to Bridge Homogeneous and Heterogeneous Water Oxidation Photocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202303290. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Shi, X.; Wu, L. Polyoxometalate-based frameworks for photocatalysis and photothermal catalysis. Nanoscale 2023, 15, 9242–9255. [Google Scholar] [CrossRef]
- Streb, C.; Kastner, K.; Tucher, J. Polyoxometalates in photocatalysis. Phys. Sci. Rev. 2019, 4, 20170177. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Z.; Yang, S.; Xu, Y. Photodegradation of Dye Pollutants Catalyzed by H3PW12O40/SiO2 Treated with H2O2 under Simulated Solar Light Irradiation. J. Adv. Nano 2017, 2, 146–152. [Google Scholar]
- Guo, R.; Bai, L.; Dong, G.; Chai, D.; Lang, K.; Mou, Z.; Zhao, M. Construction of ZnO/Keggin Polyoxometalate Nano-heterojunction Catalyst for Efficient Removal of Rhodamine B in Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1599–1615. [Google Scholar] [CrossRef]
- Wu, Y.; Bi, L. Research Progress on Catalytic Water Splitting Based on Polyoxometalate/Semiconductor Composites. Catalysts 2021, 11, 524. [Google Scholar] [CrossRef]
- Rafiee, E.; Pami, N.; Zinatizadeh, A.A.; Eavani, S. A new polyoxometalate-TiO2 nanocomposite for efficient visible photodegradation of dye from wastewater, liquorice and yeast extract: Photoelectrochemical, electrochemical, and physical investigations. J. Photochem. Photobiol. A 2020, 386, 112145. [Google Scholar] [CrossRef]
- Dubey, N.; Rayalu, S.S.; Labhsetwar, N.K.; Naidu, R.R.; Chatti, R.V.; Devotta, S. Photocatalytic properties of zeolite-based materials for the photoreduction of methyl orange. Appl. Catal. A 2006, 303, 152–157. [Google Scholar] [CrossRef]
- Chatti, R.; Rayalu, S.S.; Dubey, N.; Labhsetwar, N.; Devotta, S. Solar-based photoreduction of methyl orange using zeolite supported photocatalytic materials. Sol. Energy Mater. Sol. Cells 2007, 91, 180–190. [Google Scholar] [CrossRef]
- Anandan, S.; Yoon, M. Photocatalytic degradation of methyl orange using heteropolytungstic acid-encapsulated TiSBA-15. Sol. Energy Mater. Sol. Cells 2007, 91, 143–147. [Google Scholar] [CrossRef]
- Fazaeli, R.; Aliyan, H.; Tangestaninejad, S.; Parishani Foroushani, S. Photocatalytic degradation of RhB, MG, MB, Roz.B, 3-BL and CI-50 by PW12-APTES@MCF as nanosized mesoporous photocatalyst. J. Iran. Chem. Soc. 2014, 11, 1687–1701. [Google Scholar] [CrossRef]
- Lei, P.; Chen, C.; Yang, J.; Ma, W.; Zhao, J.; Zang, L. Degradation of Dye Pollutants by Immobilized Polyoxometalate with H2O2 under Visible-Light Irradiation. Environ. Sci. Technol. 2005, 39, 8466–8474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, C.; Cheng, M.; Chen, M.; Chen, S.; Lei, L.; Chen, Y.; Yi, H.; Fu, Y.; Li, L. Polyoxometalate@Metal–Organic Framework Composites as Effective Photocatalysts. ACS Catal. 2021, 11, 13374–13396. [Google Scholar] [CrossRef]
- Buru, C.T.; Farha, O.K. Strategies for Incorporating Catalytically Active Polyoxometalates in Metal–Organic Frameworks for Organic Transformations. ACS Appl. Mater. Interfaces 2020, 12, 5345–5360. [Google Scholar] [CrossRef]
- Li, G.; Zhang, K.; Li, C.; Gao, R.; Cheng, Y.; Hou, L.; Wang, Y. Solvent-free method to encapsulate polyoxometalate into metal-organic frameworks as efficient and recyclable photocatalyst for harmful sulfamethazine degrading in water. Appl. Catal. B 2019, 245, 753–759. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.; Yan, J.; Li, L.; Wu, G.; Shi, W.; Yang, G.; Guan, N.; Cheng, P. Polyoxometalate-Based Metal–Organic Frameworks as Visible-Light-Induced Photocatalysts. Inorg. Chem. 2018, 57, 5030–5037. [Google Scholar] [CrossRef]
- Khan, S.U.; Akhtar, M.; Khan, F.U.; Peng, J.; Hussain, A.; Shi, H.; Du, J.; Yan, G.; Li, Y. Polyoxometalates decorated with metal-organic moieties as new molecular photo- and electro-catalysts. J. Coord. Chem. 2018, 71, 2604–2621. [Google Scholar] [CrossRef]
- Xia, L.; Kong, L.; Tan, J.; Wang, Y.; Jin, S.; Wang, C. Advanced photocatalytic performance of PVP-modified BiOBr materials for the removal of 2,4-DCP and mechanism insight. Catal. Sci. Technol. 2022, 12, 6771–6781. [Google Scholar] [CrossRef]
- Senasu, T.; Chankhanittha, T.; Hemavibool, K.; Nanan, S. Solvothermal synthesis of BiOBr photocatalyst with an assistant of PVP for visible-light-driven photocatalytic degradation of fluoroquinolone antibiotics. Catal. Today 2022, 384–386, 209–227. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, M.; Zhang, L.; Bingham, P.A.; Li, W.; Kubuki, S. PVP surfactant-modified flower-like BiOBr with tunable bandgap structure for efficient photocatalytic decontamination of pollutants. Appl. Surf. Sci. 2020, 530, 147233. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Wang, X.; Yang, J.; Dai, Y.; He, Y.; Chen, W.; Zhang, W. Photocatalytic degradation of rhodamin B and diclofenac sodium on hollow hierarchical microspheres of BiOBr modified with sepiolite and polyvinyl pyrrolidone (PVP). Mater. Sci. Eng. B 2019, 244, 12–22. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Wang, T.; Su, C.; Dong, X. Preparation of ultrafine PVP-BiO2−x by mechanochemistry modification for enhanced photocatalytic activity. Surf. Interfaces 2024, 44, 103812. [Google Scholar] [CrossRef]
- Zhao, N.; Peng, J.; Liu, G.; Zhang, Y.; Lei, W.; Yin, Z.; Li, J.; Zhai, M. PVP-capped CdS nanopopcorns with type-II homojunctions for highly efficient visible-light-driven organic pollutant degradation and hydrogen evolution. J. Mater. Chem. A 2018, 6, 18458–18468. [Google Scholar] [CrossRef]
- Keerthana, S.; Yuvakkumar, R.; Ravi, G.; Mustafa, A.E.M.A.; Al-Ghamdi, A.A.; Soliman Elshikh, M.; Velauthapillai, D. PVP influence on Mn–CdS for efficient photocatalytic activity. Chemosphere 2021, 277, 130346. [Google Scholar] [CrossRef]
- Abdolalian, S.; Taghavijeloudar, M. Performance evaluation and optimization of ZnO-PVP nanoparticles for photocatalytic wastewater treatment: Interactions between UV light intensity and nanoparticles dosage. J. Clean. Prod. 2022, 365, 132833. [Google Scholar] [CrossRef]
- Yue, L.; Wang, L.; Shi, F.; Guo, J.; Yang, J.; Lian, J.; Luo, X. Application of response surface methodology to the decolorization by the electrochemical process using FePMo12O40 catalyst. J. Ind. Eng. Chem. 2015, 21, 971–979. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, X.; Wang, J.; Liu, Y.; Liu, Z.; Tang, L.; Shao, B.; Zhang, W.; Gong, S.; Cheng, M.; et al. In-situ self-assembly construction of hollow tubular g-C3N4 isotype heterojunction for enhanced visible-light photocatalysis: Experiments and theories. J. Hazard. Mater. 2021, 401, 123355. [Google Scholar] [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Chang, C.; Hu, C. Graphene oxide-derived carbon-doped SrTiO3 for highly efficient photocatalytic degradation of organic pollutants under visible light irradiation. Chem. Eng. J. 2020, 383, 123116. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Ke, Y. A novel approach of preparing TiO2 films at low temperature and its application in photocatalytic degradation of methyl orange. J. Hazard. Mater. 2010, 177, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Lu, Y.; Xuan, L.; Xia, S.; Feng, W.; Han, X. Preparation and visible-light photochromism of phosphomolybdic acid/polyvinylpyrrolidone hybrid film. Chem. Res. Chin. Univ. 2014, 30, 703–708. [Google Scholar] [CrossRef]
- Jing, X.; Zou, D.; Meng, Q.; Zhang, W.; Zhang, F.; Feng, W.; Han, X. Fabrication and visible-light photochromism of novel hybrid inorganic–organic film based on polyoxometalates and ethyl cellulose. Inorg. Chem. Commun. 2014, 46, 149–154. [Google Scholar] [CrossRef]
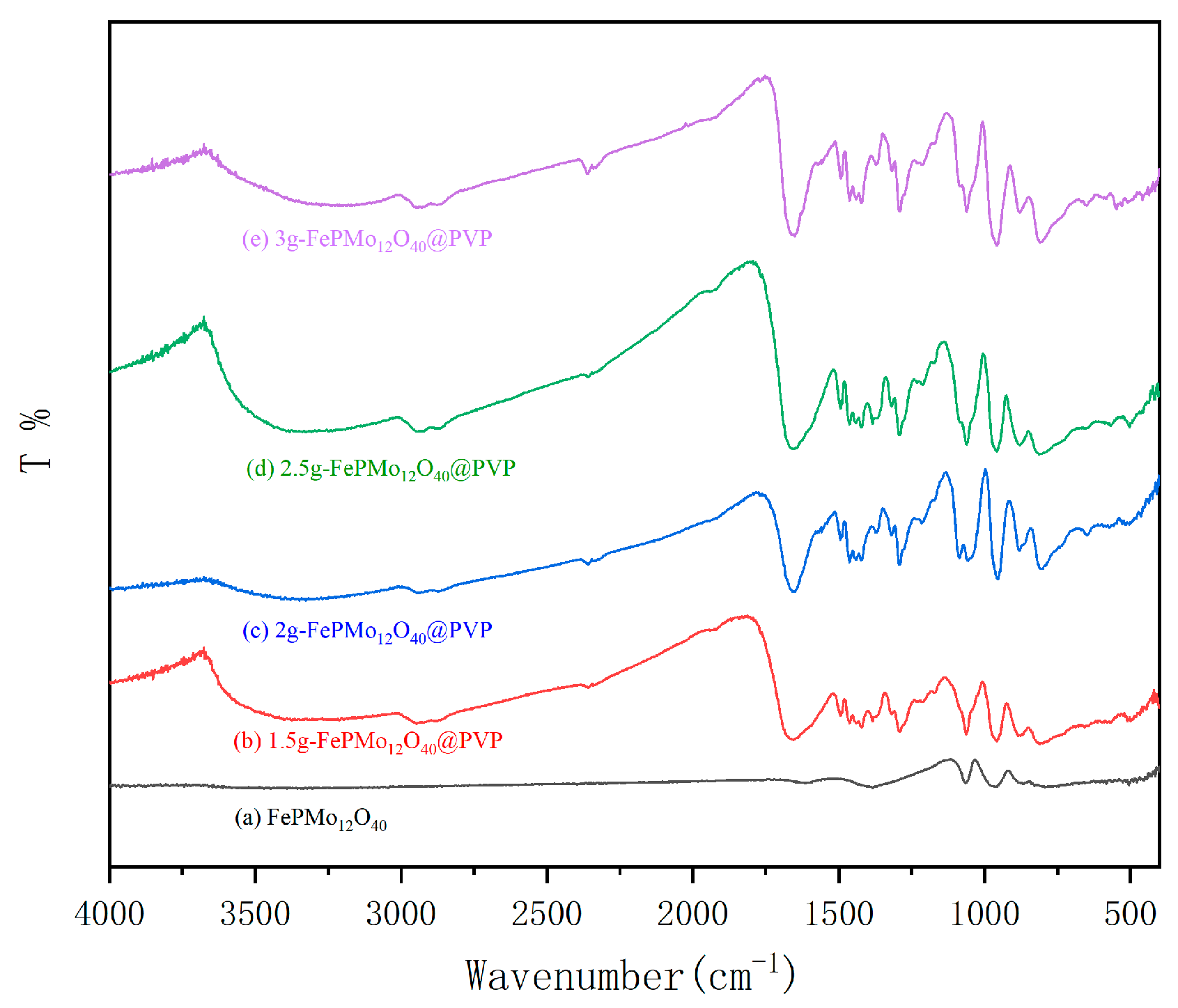




| ν(P-Oa) | ν(M-Od) | ν(M-Ob-M) | (M-OC-M) | |
|---|---|---|---|---|
| FePMo12O40 | 1066 | 962 | 865 | 794 |
| 1.5 g-FePMo12O40@PVP | 1062 | 958 | 883 | 806 |
| 2 g-FePMo12O40@PVP | 1060 | 954 | 881 | 804 |
| 2.5 g-FePMo12O40@PVP | 1062 | 956 | 883 | 811 |
| 3 g-FePMo12O40@PVP | 1060 | 956 | 881 | 809 |
| K (min−1) | R2 | |
|---|---|---|
| 1.5 g-FePMo12O40@PVP | 0.011 | 0.990 |
| 2 g-FePMo12O40@PVP | 0.017 | 0.989 |
| 2.5 g-FePMo12O40@PVP | 0.027 | 0.990 |
| 3 g-FePMo12O40@PVP | 0.013 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Tang, Y.; Ai, L.; Liu, M.; Wang, Y. Polymer-Based Immobilized FePMo12O40@PVP Composite Materials for Photocatalytic RhB Degradation. Inorganics 2024, 12, 144. https://doi.org/10.3390/inorganics12060144
Wang Z, Tang Y, Ai L, Liu M, Wang Y. Polymer-Based Immobilized FePMo12O40@PVP Composite Materials for Photocatalytic RhB Degradation. Inorganics. 2024; 12(6):144. https://doi.org/10.3390/inorganics12060144
Chicago/Turabian StyleWang, Zijing, Yuze Tang, Limei Ai, Minghui Liu, and Yurong Wang. 2024. "Polymer-Based Immobilized FePMo12O40@PVP Composite Materials for Photocatalytic RhB Degradation" Inorganics 12, no. 6: 144. https://doi.org/10.3390/inorganics12060144





