Synthesis, Characterization, and Cytotoxicity of a Ga(III) Complex with Warfarin
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Analytical and Spectroscopic Measurements
2.3. Tumor Cell Lines
2.4. Cytotoxicity Assay
3. Computational Details
4. Results and Discussion
4.1. Coordination Ability of Warfarin to Ga(III)
4.2. Optimized Geometry Analysis
4.3. Natural Bond Orbital Analysis
4.4. Natural Population Analysis
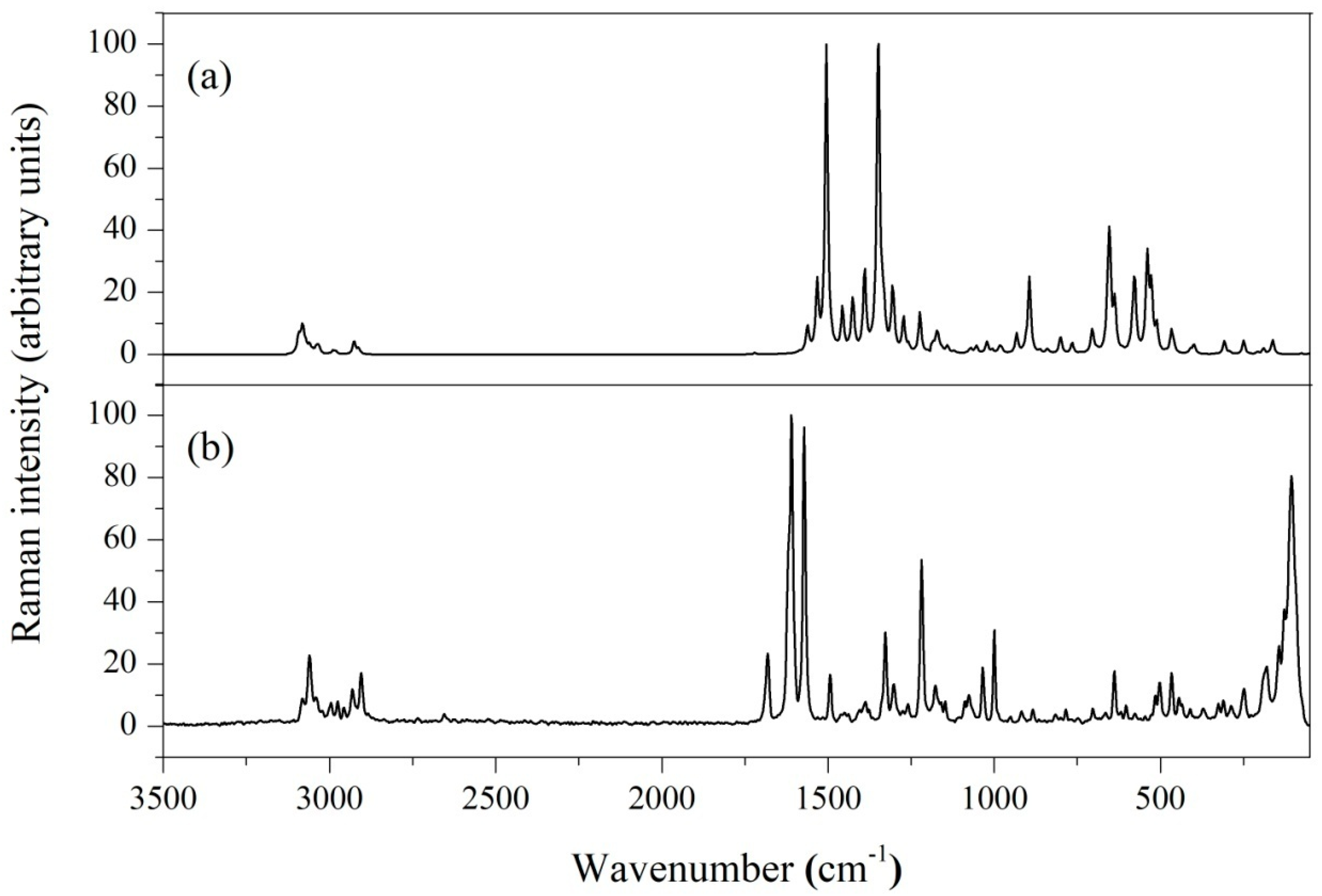
4.5. Vibrational Spectral Analysis
4.5.1. Coumarin Ring Vibrations
4.5.2. Phenyl Ring Vibration
4.5.3. Methyl and Methylene Vibrations
4.5.4. C=O, C-O, and O-H Stretching Vibrations
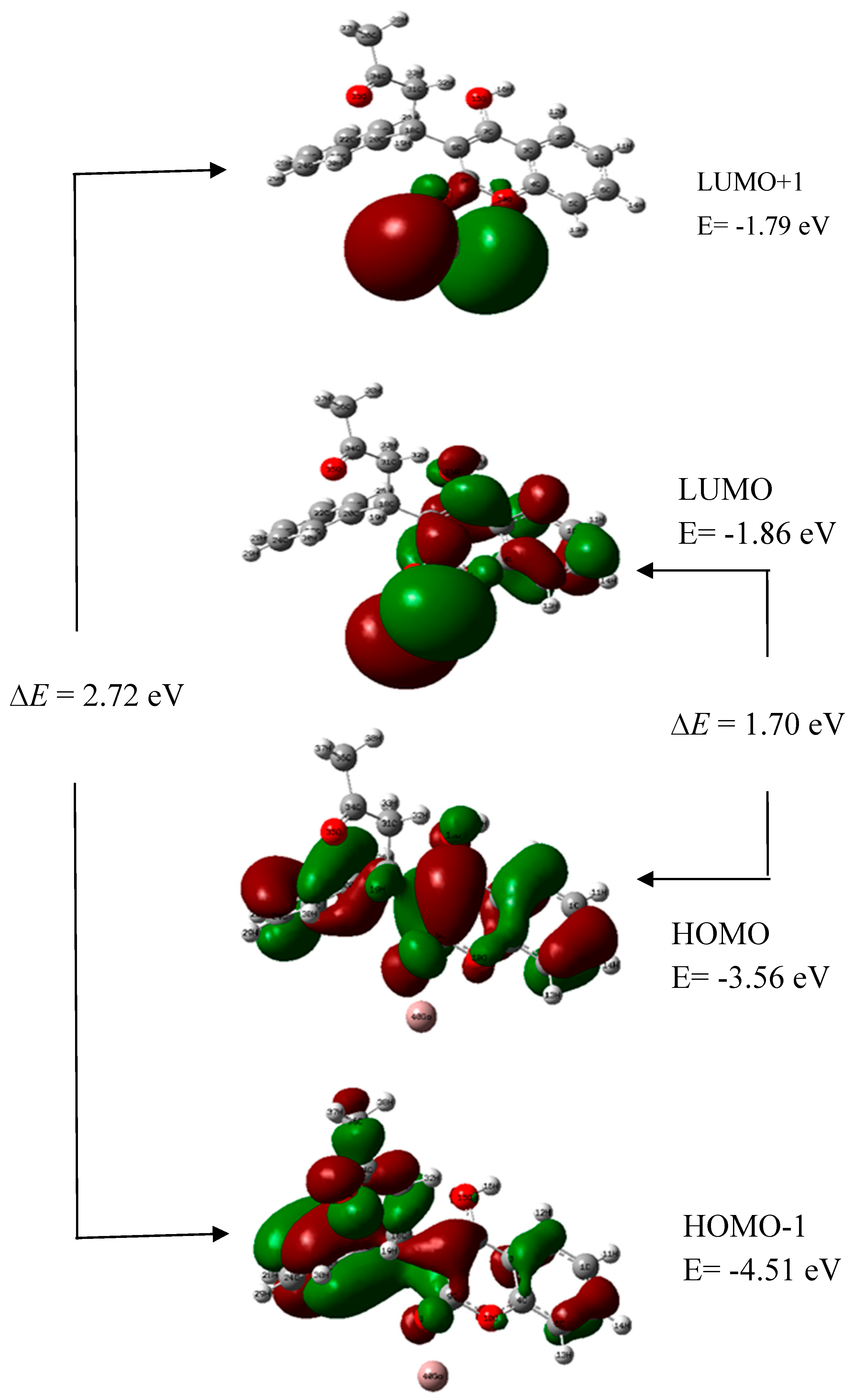
4.6. HOMO and LUMO Energy Analysis
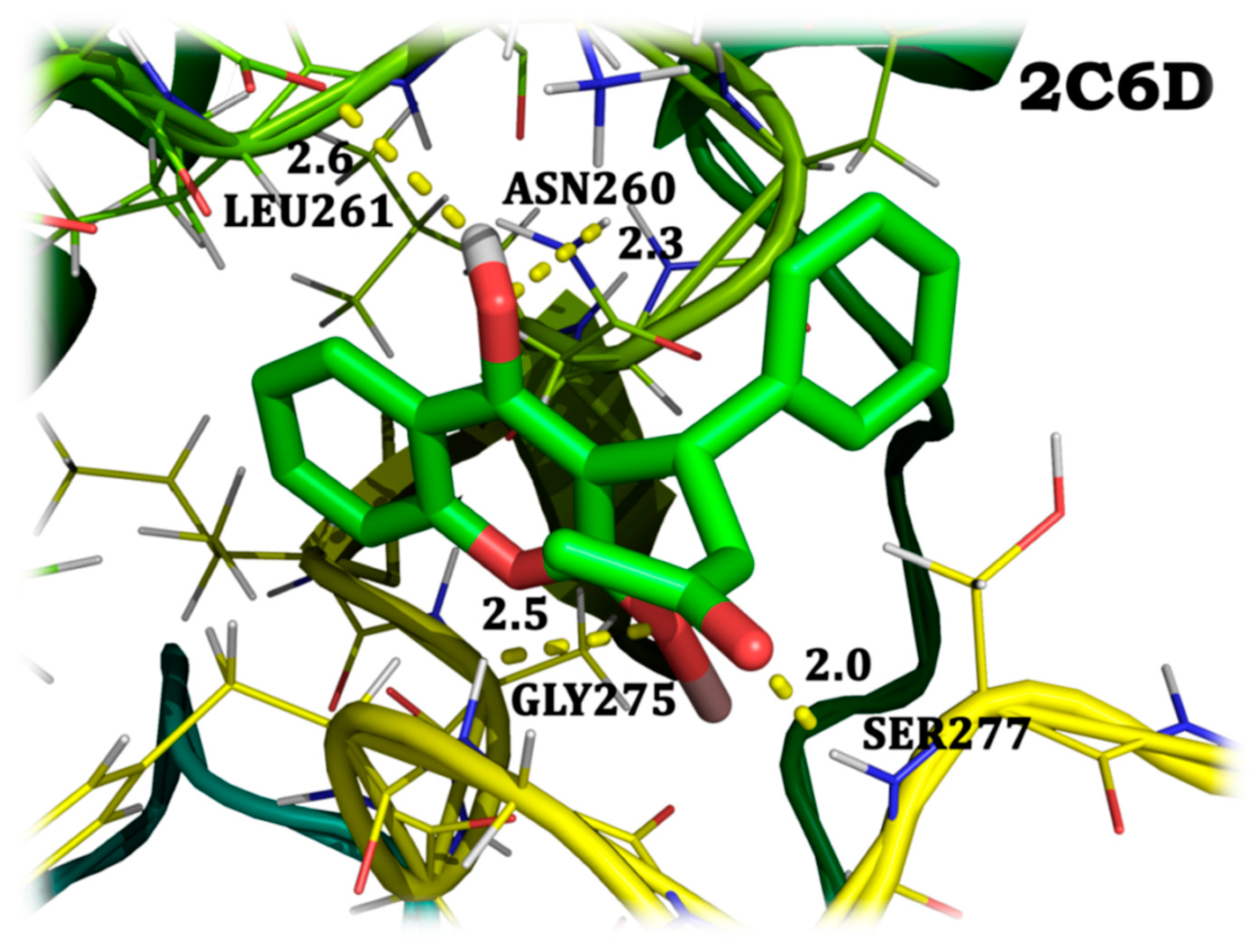
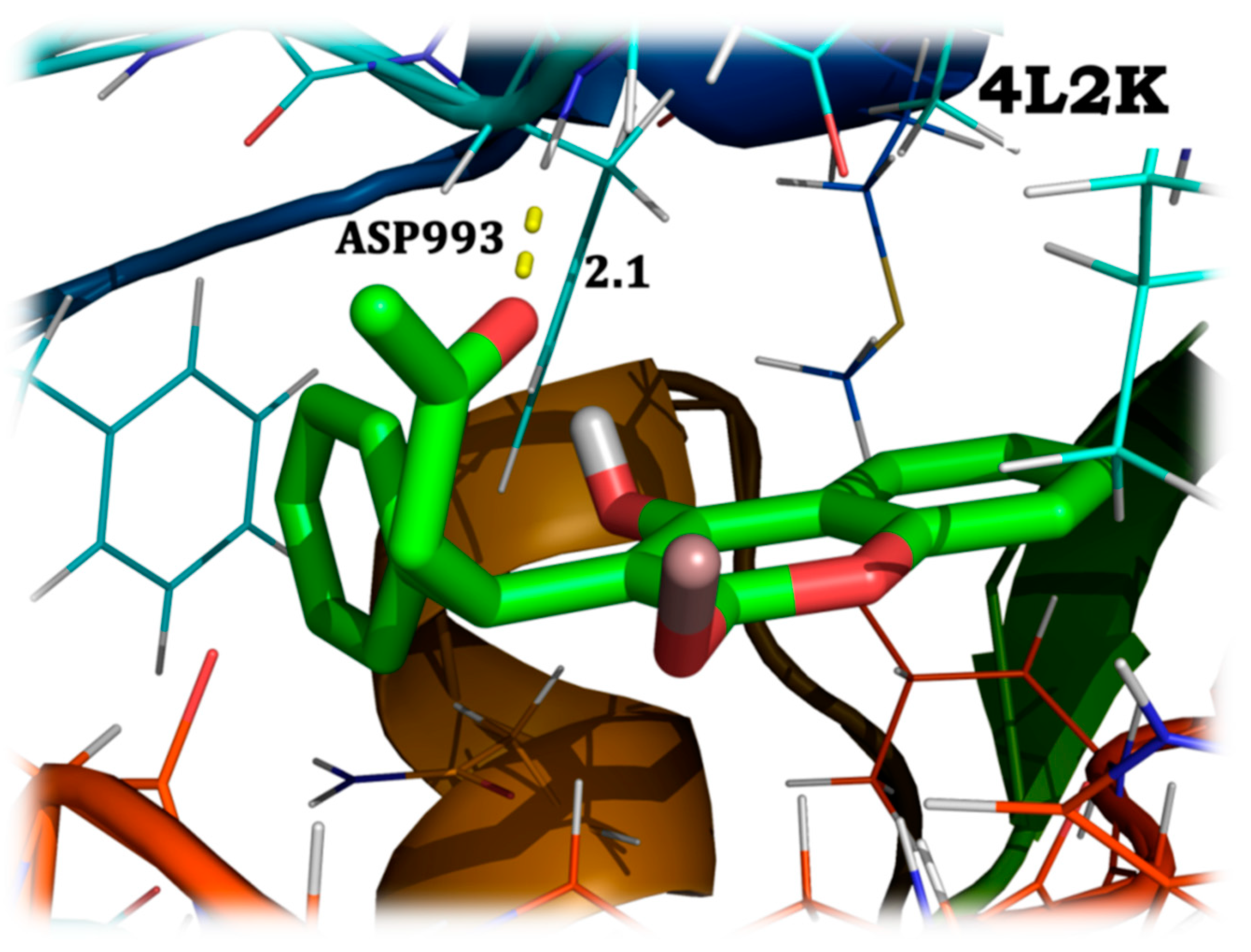
4.7. Molecular Docking Analysis
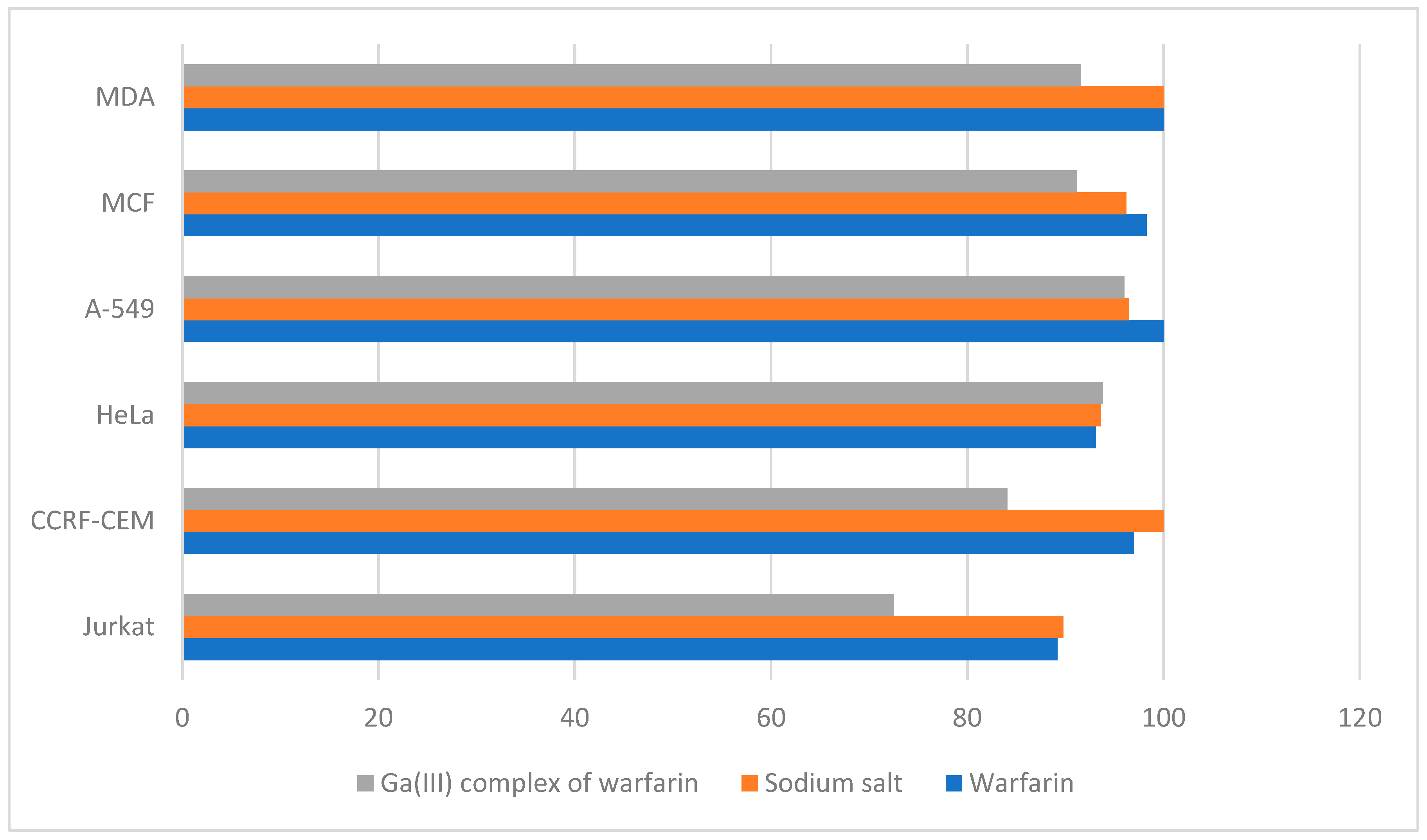
4.8. Pharmacology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kostova, I. Synthetic and Natural Coumarins as Cytotoxic Agents. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Mihaylov, T.; Trendafilova, N.; Georgieva, I.; Kostova, I. Coordination properties of warfarin towards Pr(III) predicted from DFT and FT-IR studies. Chem. Phys. 2010, 374, 37–45. [Google Scholar] [CrossRef]
- Amalanathan, M.; Joe, I.H.; Kostova, I. Density Functional Theory Calculation and Vibrational Spectral Analysis of 4-Hydroxy-3-(3-Oxo-1-Phenylbutyl)-2H-1-Benzopyran-2-One. J. Raman Spectrosc. 2010, 41, 1076–1084. [Google Scholar] [CrossRef]
- Kostova, I.; Amalanathan, M.; Joe, I.H. Molecular First Order Hyperpolarizability and Vibrational Spectral Investigation of Warfarin Sodium. Chem. Phys. 2010, 378, 88–102. [Google Scholar] [CrossRef]
- Mandakmare, A.; Narwade, M. Complexing activity of coumarin dervatives. Orient. J. Chem. 1997, 13, 155–158. [Google Scholar]
- Kostova, I.P.; Manolov, I.; Konstantinov, S.; Karaivanova, M. Synthesis, physicochemical characterisation and cytotoxic screening of new complexes of cerium, lanthanum and neodymium with Warfarin and Coumachlor sodium salts. Eur. J. Med. Chem. 1999, 34, 63–68. [Google Scholar] [CrossRef]
- Kostova, I.; Manolov, I.; Karaivanova, M. Synthesis, Physicochemical Characterization, and Cytotoxic Screening of New Zirconium Complexes with Coumarin Derivatives. Arch. Pharm. 2001, 334, 157–162. [Google Scholar] [CrossRef]
- Kostova, I.; Stefanova, T. Synthesis, Characterization and Cytotoxic/Cytostatic Activity of Sm(III) and Gd(III) Complexes. J. Coord. Chem. 2009, 62, 3187–3197. [Google Scholar] [CrossRef]
- Collery, P.; Keppler, B.; Madoulet, C.; Desoize, B. Gallium in Cancer Treatment. Crit. Rev. Oncol./Hematol. 2002, 42, 283–296. [Google Scholar] [CrossRef]
- Peng, X.X.; Gao, S.; Zhang, J.L. Gallium (III) complexes in cancer chemotherapy. Eur. J. Inorg. Chem. 2022, 6, e202100953. [Google Scholar] [CrossRef]
- Kurtuldu, F.; Mutlu, N.; Boccaccini, A.R.; Galusek, D. Gallium containing bioactive materials: A review of anticancer, antibacterial, and osteogenic properties. Bioact. Mater. 2022, 17, 125–146. [Google Scholar] [CrossRef]
- Pribisko, M.; Palmer, J.; Grubbs, R.H.; Gray, H.B.; Termini, J.; Lim, P. Cellular uptake and anticancer activity of carboxylated gallium corroles. Proc. Natl. Acad. Sci. USA 2016, 113, E2258–E2266. [Google Scholar] [CrossRef]
- Cen, J.H.; Xie, Q.H.; Guo, G.H.; Xu, S.Y.; Liu, Z.Y.; Liao, Y.H.; Liu, H.Y. Construction of 5-Fluorouracil and Gallium Corrole Conjugates for Enhanced Photodynamic Therapy. J. Med. Chem. 2024, 67, 9054–9068. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Parashar, S.; Gómez-Ruiz, S. Anticancer Applications and Recent Investigations of Metallodrugs Based on Gallium, Tin and Titanium. Inorganics 2017, 5, 4. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium-containing anticancer compounds. Future Med. Chem. 2012, 4, 1257–1272. [Google Scholar] [CrossRef]
- Chitambar, C.R.; Antholine, W.E. Iron-targeting antitumor activity of gallium compounds and novel insights into triapine®-metal complexes. Antioxid. Redox Signal. 2012, 18, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R.; Seligman, P.A. Effects of different transferrin forms on transferrin receptor expression, iron uptake and cellular proliferation of human leukemic HL60 cells: Mechanisms responsible for the specific cytotoxicity of transferrin-gallium. J. Clin. Investig. 1986, 78, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.J.; Clagett-Carr, K.; Hoth, D.; Leyland-Jones, B. Gallium nitrate: The second metal with clinical activity. Cancer Treat. Rep. 1988, 70, 1311–1319. [Google Scholar]
- Chitambar, C.R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef] [PubMed]
- Lessa, J.A.; Gabrieli, L.P.; Heloisa, B. Gallium complexes as new promising metallodrug candidates. Inorg. Chim. Acta 2012, 393, 53–63. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. IV. A New Dynamical Correlation Functional and Implications for Exact-Exchange Mixing. J. Chem. Phys. 1996, 104, 1040–1046. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, R.M.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.P.; Nakatsuji, H.; Caricato, M.; et al. Gaussian-09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Rauhut, G.; Pulay, P. Transferable Scaling Factors for Density Functional Derived Vibrational Force Fields. J. Phys. Chem. 1995, 99, 3093–3100. [Google Scholar] [CrossRef]
- Pulay, P.; Fogarasi, G.; Pang, F.; Boggs, J.E. Systematic AB Initio Gradient Calculation of Molecular Geometries, Force Constants, and Dipole Moment Derivatives. J. Am. Chem. Soc. 1979, 101, 2550–2560. [Google Scholar] [CrossRef]
- Varsányi, G. Vibrational Spectra of Benzene Derivatives; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Colthup, N. Introduction to Infrared and Raman Spectroscopy; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Tammer, M.G. Sokrates: Infrared and Raman Characteristic Group Frequencies: Tables and Charts. Colloid Polym. Sci. 2004, 283, 235. [Google Scholar] [CrossRef]
- Silverstein, R.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Smith, B. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: New York, NY, USA, 1999. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Heron, N.M.; Anderson, M.; Blowers, D.P.; Breed, J.; Eden, J.M.; Green, S.; Hill, G.B.; Johnson, T.; Jung, F.H.; McMiken, H.H.; et al. SAR and Inhibitor Complex Structure Determination of a Novel Class of Potent and Specific Aurora Kinase Inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 1320–1323. [Google Scholar] [CrossRef]
- Narwal, M.; Koivunen, J.; Haikarainen, T.; Obaji, E.; Legala, O.E.; Venkannagari, H.; Joensuu, P.; Pihlajaniemi, T.; Lehtiö, L. Discovery of Tankyrase Inhibiting Flavones with Increased Potency and Isoenzyme Selectivity. J. Med. Chem. 2013, 56, 7880–7889. [Google Scholar] [CrossRef]
- Jeffrey, G.; Jeffrey, G. An Introduction to Hydrogen Bonding; Oxford University Press: London, UK, 1997. [Google Scholar]
- Avdović, E.H.; Antonijević, M.; Simijonović, D.; Roca, S.; Topić, D.V.; Grozdanić, N.; Marković, Z. Synthesis and Cytotoxicity Evaluation of Novel Coumarin–Palladium (II) Complexes against Human Cancer Cell Lines. Pharmaceuticals 2022, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Avdović, E.H.; Petrović, I.P.; Stevanović, M.J.; Saso, L.; Marković, J.M.D.; Filipović, N.D.; Marković, Z.S. Synthesis and Biological Screening of New 4-Hydroxycoumarin Derivatives and Their Palladium (II) Complexes. Oxidative Med. Cell. Longev. 2021, 2021, 8849568. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Correia, I. Misinterpretations in evaluating interactions of vanadium complexes with proteins and other biological targets. Inorganics 2021, 9, 17. [Google Scholar] [CrossRef]
- Dey, S.; Hollis, T.K. Accessing low-valent titanium CCC-NHC complexes: Toward nitrogen fixation. Inorganics 2021, 9, 15. [Google Scholar] [CrossRef]
- Avdović, E.H.; Milanović, Ž.B.; Živanović, M.N.; Šeklić, D.S.; Radojević, I.D.; Čomić, L.R.; Marković, Z.S. Synthesis, spectroscopic characterization, biological activity, DFT and molecular docking study of novel 4-hydroxycoumarine derivatives and corresponding palladium (II) complexes. Inorganica Chim. Acta 2020, 504, 119465. [Google Scholar] [CrossRef]
- Tacka, K.A.; Dabrowiak, J.C.; Goodisman, J.; Penefsky, H.S.; Souid, A.K. Effects of cisplatin on mitochondrial function in Jurkat cells. Chem. Res. Toxicol. 2004, 17, 1102–1111. [Google Scholar] [CrossRef][Green Version]
- Vascellari, S.; Valletta, E.; Perra, D.; Pinna, E.; Serra, A.; Isaia, F.; Pivetta, T. Cisplatin, glutathione and the third wheel: A copper-(1, 10-phenanthroline) complex modulates cisplatin–GSH interactions from antagonism to synergism in cancer cells resistant to cisplatin. RSC Adv. 2019, 9, 5362–5376. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Zhang, K.J.; Yue, X.T.; Wang, Y.Q.; Yang, Y.; Li, G.C.; Wang, Y.G. Enhancement of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacol. Sin. 2009, 30, 467–477. [Google Scholar] [CrossRef]
- Ray, R.; Al Khashali, H.; Haddad, B.; Wareham, J.; Coleman, K.L.; Alomari, D.; Evans, H.G. Regulation of cisplatin resistance in lung cancer cells by nicotine, BDNF, and a β-adrenergic receptor blocker. Int. J. Mol. Sci. 2022, 23, 12829. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Hassiotou, F.; Blancafort, P.; Filgueira, L. Cisplatin induces differentiation of breast cancer cells. Front. Oncol. 2013, 3, 134. [Google Scholar] [CrossRef] [PubMed]
- Todorov, L.T.; Saso, L.; Benarous, K.; Traykova, M.L.; Linani, A.; Kostova, I. Synthesis, Structure and Impact of 5-Aminoorotic Acid and Its Complexes with Lanthanum(III) and Gallium(III) on the Activity of Xanthine Oxidase. Molecules 2021, 26, 4503. [Google Scholar] [CrossRef] [PubMed]
- Todorov, L.T.; Traykova, M.L.; Saso, L.; Kostova, I. In Vitro Interaction of 5-Aminoorotic Acid and Its Gallium(III) Complex with Superoxide Radical, Generated by Two Model Systems. Int. J. Mol. Sci. 2020, 21, 8862. [Google Scholar] [CrossRef]
- Todorov, L.T.; Kostova, I.; Traykova, M.L. Lanthanum, Gallium and their Impact on Oxidative Stress. Curr. Med. Chem. 2019, 26, 4280–4295. [Google Scholar] [CrossRef] [PubMed]

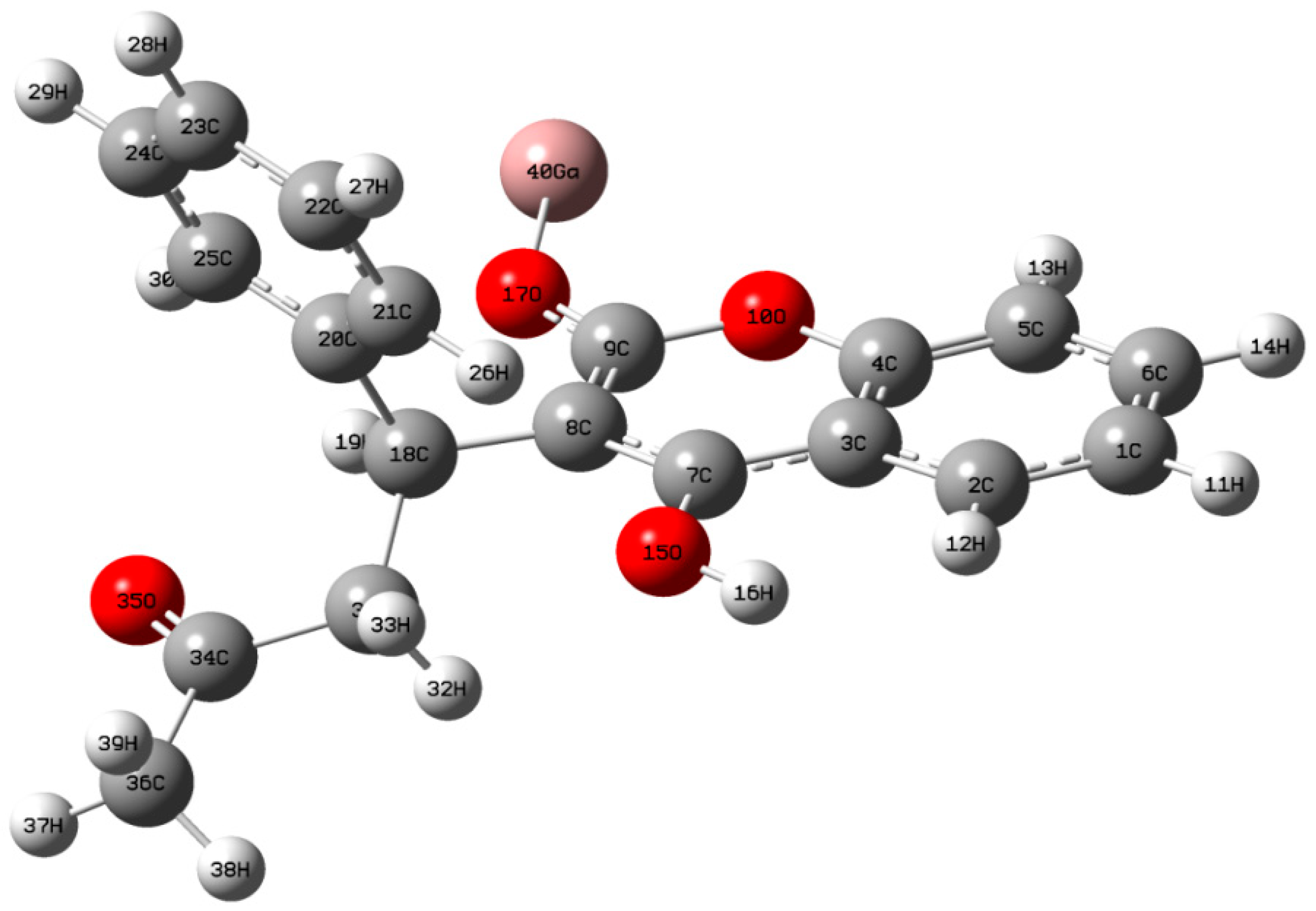
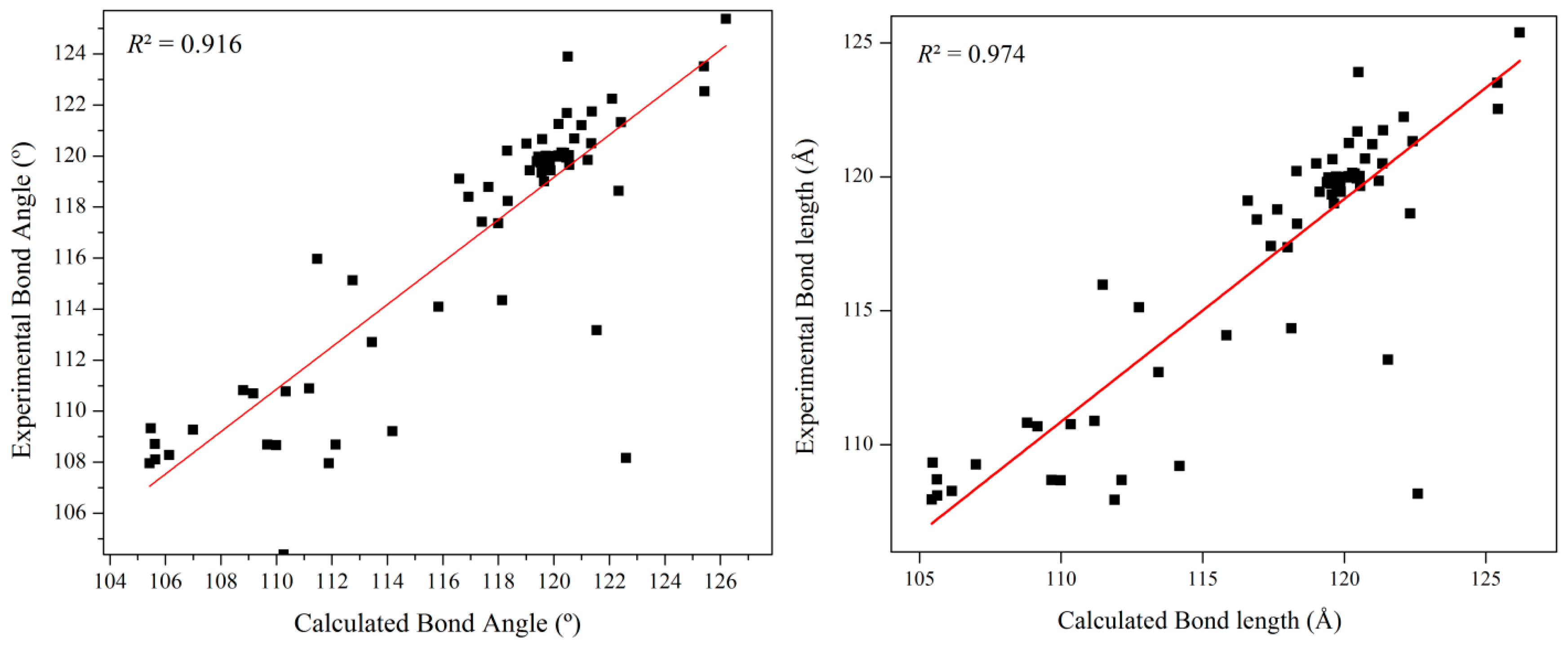
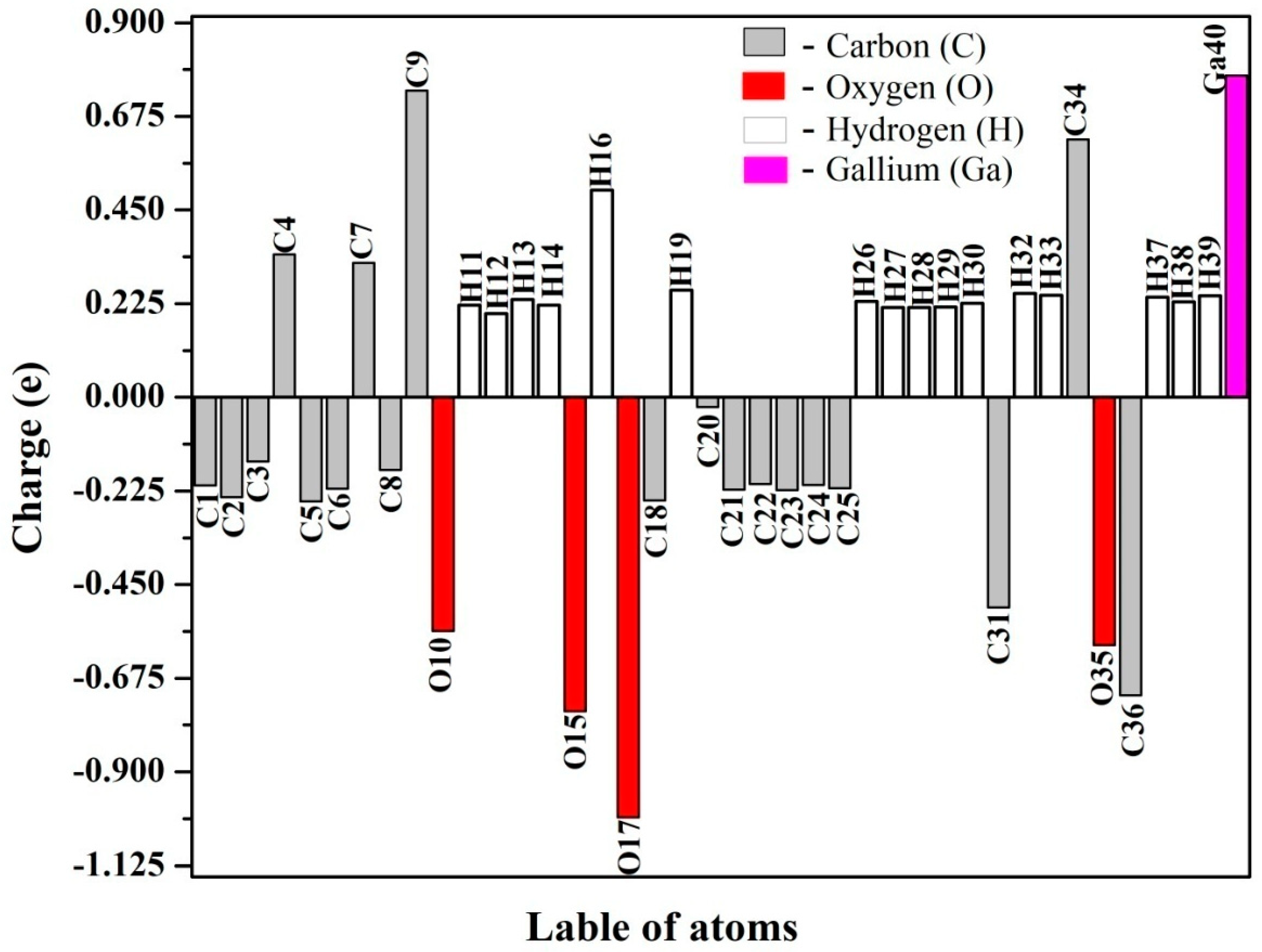



| Bond Length (Å) | Bond Angle | Dihedral Angle (°) | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Calc. (Å) | XRD (Å) | Parameters | Calc. (°) | XRD (°) | Parameters | Calc. (°) | XRD (°) |
| C1-C2 | 1.391 | 1.374 | C2-C1-C6 | 120.54 | 120.03 | C6-C1-C2-C3 | 0.38 | −0.22 |
| C1-C6 | 1.393 | 1.388 | C2-C1-H11 | 119.44 | 119.97 | C6-C1-C2-H12 | −178.76 | 179.64 |
| C1-H11 | 1.084 | 0.951 | C6-C1-H11 | 120.02 | 120.00 | H11-C1-C2-C3 | −179.95 | 179.8 |
| C2-C3 | 1.417 | 1.401 | C1-C2-C3 | 121.22 | 119.85 | H11-C1-C2-H12 | 0.91 | −0.34 |
| C2-H12 | 1.086 | 0.949 | C1-C2-H12 | 118.32 | 120.21 | C2-C1-C6-C5 | 0.25 | −0.86 |
| C3-C4 | 1.416 | 1.382 | C3-C2-H12 | 120.45 | 119.94 | C2-C1-C6-H14 | 179.97 | 179.04 |
| C3-C7 | 1.422 | 1.449 | C2-C3-C4 | 116.59 | 119.11 | H11-C1-C6-C5 | −179.41 | −179.12 |
| C4-C5 | 1.381 | 1.377 | C2-C3-C7 | 125.41 | 123.51 | H11-C1-C6-H14 | 0.31 | −0.98 |
| C4-O10 | 1.381 | 1.378 | C4-C3-C7 | 118.00 | 117.37 | C1-C2-C3-C4 | −0.88 | 1.37 |
| C5-C6 | 1.402 | 1.381 | C3-C4-C5 | 122.42 | 121.32 | C1-C2-C3-C7 | 178.85 | −177.67 |
| C5-H13 | 1.083 | 0.948 | C3-C4-O10 | 120.17 | 121.26 | H12-C2-C3-C4 | 178.24 | −178.49 |
| C6-H14 | 1.083 | 0.953 | C5-C4-O10 | 117.41 | 117.42 | H12-C2-C3-C7 | −2.03 | 2.47 |
| C7-C8 | 1.416 | 1.360 | C4-C5-C6 | 119.64 | 119.01 | C2-C3-C4-C5 | 0.80 | −1.48 |
| C7-O15 | 1.375 | 1.351 | C4-C5-H13 | 119.01 | 120.49 | C2-C3-C4-O10 | −178.91 | 178.43 |
| C8-C9 | 1.379 | 1.443 | C6-C5-H13 | 121.35 | 120.5 | C7-C3-C4-C5 | −178.95 | 177.61 |
| C8-C18 | 1.527 | 1.506 | C1-C6-C5 | 119.58 | 120.66 | C7-C3-C4-O10 | 1.33 | −2.48 |
| C9-O10 | 1.386 | 1.381 | C1-C6-H14 | 120.56 | 119.65 | C2-C3-C7-C8 | 179.41 | 178.63 |
| C9-O17 | 1.300 | 1.214 | C5-C6-H14 | 119.86 | 119.69 | C2-C3-C7-O15 | 0.20 | −0.26 |
| O15-H16 | 0.963 | - | C3-C7-C8 | 121.37 | 121.74 | C4-C3-C7-C8 | −0.87 | −0.42 |
| O17-Ga40 | 1.922 | - | C3-C7-O15 | 120.50 | 123.9 | C4-C3-C7-O15 | 179.93 | −179.31 |
| C18-H19 | 1.090 | 0.949 | C8-C7-O15 | 118.13 | 114.35 | C3-C4-C5-C6 | −0.20 | 0.43 |
| C18-C20 | 1.530 | 1.518 | C7-C8-C9 | 117.64 | 118.78 | C3-C4-C5-H13 | 179.94 | −179.51 |
| C18-C31 | 1.543 | 1.545 | C7-C8-C18 | 125.43 | 122.53 | O10-C4-C5-C6 | 179.52 | −179.49 |
| C20-C21 | 1.401 | 1.388 | C9-C8-C18 | 116.92 | 118.4 | O10-C4-C5-H13 | −0.34 | 0.57 |
| C20-C25 | 1.399 | 1.392 | C8-C9-O10 | 122.33 | 118.63 | C3-C4-O10-C9 | −0.79 | −0.51 |
| C21-C22 | 1.393 | 1.385 | C8-C9-O17 | 126.20 | 125.38 | C5-C4-O10-C9 | 179.48 | 179.41 |
| C21-H26 | 1.083 | 0.949 | O10-C9-O17 | 111.47 | 115.97 | C4-C5-C6-C1 | −0.34 | 0.76 |
| C22-C23 | 1.395 | 1.387 | C4-O10-C9 | 120.47 | 121.69 | C4-C5-C6-H14 | 179.94 | −179.14 |
| C22-H27 | 1.085 | 0.951 | C7-O15-H16 | 109.64 | - | H13-C5-C6-C1 | 179.51 | −179.31 |
| C23-C24 | 1.393 | 1.372 | C9-O17-Ga40 | 131.75 | - | H13-C5-C6-H14 | −0.21 | 0.79 |
| C23-H28 | 1.085 | 0.950 | C8-C18-H19 | 105.43 | 107.96 | C3-C7-C8-C9 | −0.16 | 6 |
| C24-C25 | 1.395 | 1.383 | C8-C18-C20 | 112.75 | 115.13 | C3-C7-C8-C18 | −179.00 | 179.7 |
| C24-H29 | 1.085 | 0.952 | C8-C18-C31 | 114.18 | 109.21 | O15-C7-C8-C9 | 179.07 | −175.22 |
| C25-H30 | 1.084 | 0.951 | H19-C18-C20 | 106.14 | 108.28 | O15-C7-C8-C18 | 0.23 | −1.53 |
| C31-H32 | 1.099 | 0.951 | H19-C18-C31 | 105.63 | 108.11 | C3-C7-O15-H16 | −11.02 | - |
| C31-H33 | 1.091 | 0.950 | C20-C18-C31 | 111.89 | 107.95 | C8-C7-O15-H16 | 169.75 | - |
| C31-C34 | 1.525 | 1.502 | C18-C20-C21 | 122.10 | 122.24 | C7-C8-C9-O10 | 0.76 | −8.8 |
| C34-O35 | 1.213 | 1.290 | C18-C20-C25 | 119.56 | 119.34 | C7-C8-C9-O17 | −178.90 | −172.64 |
| C34-C36 | 1.516 | 1.510 | C21-C20-C25 | 118.34 | 118.24 | C18-C8-C9-O10 | 179.70 | 177.24 |
| C36-H37 | 1.090 | 0.948 | C20-C21-C22 | 120.74 | 120.68 | C18-C8-C9-O17 | 0.04 | −1.32 |
| C36-H38 | 1.094 | 0.951 | C20-C21-H26 | 119.74 | 119.57 | C7-C8-C18-H19 | −158.84 | −106.78 |
| C36-H39 | 1.096 | 0.952 | C22-C21-H26 | 119.52 | 119.74 | C7-C8-C18-C20 | 85.80 | 132.15 |
| C21-C22-C23 | 120.37 | 120.12 | C7-C8-C18-C31 | −43.36 | 10.54 | |||
| C21-C22-H27 | 119.64 | 119.9 | C9-C8-C18-H19 | 22.31 | 66.94 | |||
| C23-C22-H27 | 119.98 | 119.98 | C9-C8-C18-C20 | −93.05 | −54.14 | |||
| C22-C23-C24 | 119.39 | 119.8 | C9-C8-C18-C31 | 137.80 | −175.74 | |||
| C22-C23-H28 | 120.28 | 120.14 | C8-C9-O10-C4 | −0.30 | 6.17 | |||
| C24-C23-H28 | 120.33 | 120.06 | O17-C9-O10-C4 | 179.40 | −175.14 | |||
| C23-C24-C25 | 120.16 | 120.02 | C8-C9-O17-Ga40 | 175.02 | - | |||
| C23-C24-H29 | 120.13 | 119.97 | O10-C9-O17-Ga40 | −4.67 | - | |||
| C25-C24-H29 | 119.71 | 120.01 | C8-C18-C20-C21 | −57.85 | −28.35 | |||
| C20-C25-C24 | 120.99 | 121.21 | C8-C18-C20-C25 | 122.66 | 156.58 | |||
| C20-C25-H30 | 119.13 | 119.44 | H19-C18-C20-C21 | −172.78 | −149.25 | |||
| C24-C25-H30 | 119.88 | 119.44 | H19-C18-C20-C25 | 7.73 | 35.68 | |||
| C18-C31-H32 | 109.99 | 108.67 | C31-C18-C20-C21 | 72.48 | 93.93 | |||
| C18-C31-H33 | 112.14 | 108.69 | C31-C18-C20-C25 | −107.01 | −81.14 | |||
| C18-C31-C34 | 113.44 | 112.71 | C8-C18-C31-H32 | −35.58 | −31.08 | |||
| H32-C31-H33 | 105.47 | 109.33 | C8-C18-C31-H33 | 81.46 | −160.03 | |||
| H32-C31-C34 | 105.62 | 108.71 | C8-C18-C31-C34 | −153.61 | −39.48 | |||
| H33-C31-C34 | 109.67 | 108.69 | H19-C18-C31-H32 | 79.79 | −161.7 | |||
| C31-C34-O35 | 122.60 | 108.17 | H19-C18-C31-H33 | −163.18 | −42.81 | |||
| C31-C34-C36 | 115.84 | 114.09 | H19-C18-C31-C34 | −38.25 | 77.75 | |||
| O35-C34-C36 | 121.54 | 113.17 | C20-C18-C31-H32 | −165.16 | −44.77 | |||
| C34-C36-H37 | 110.26 | 104.38 | C20-C18-C31-H33 | −48.13 | 74.12 | |||
| C34-C36-H38 | 111.18 | 110.89 | C20-C18-C31-C34 | 76.80 | −165.33 | |||
| C34-C36-H39 | 108.80 | 110.82 | C18-C20-C21-C22 | −179.76 | −174.61 | |||
| H37-C36-H38 | 110.34 | 110.77 | C18-C20-C21-H26 | 0.46 | 5.46 | |||
| H37-C36-H39 | 109.17 | 110.69 | C25-C20-C21-C22 | −0.26 | 0.52 | |||
| H38-C36-H39 | 106.99 | 109.27 | C25-C20-C21-H26 | 179.95 | −179.42 | |||
| Donor | ED a (i) (e) | Acceptor | ED a (j) (e) | E(2) b kcal mol−1 | E(j)-E(i) c a.u | F(i,j) d a.u. |
|---|---|---|---|---|---|---|
| π (C1-C2) | 0.87 | π*(C3-C7) | 0.32 | 15.93 | 0.26 | 0.09 |
| π (C3-C7) | 0.89 | π*(C8-C9) | 0.18 | 16.38 | 0.29 | 0.09 |
| π (C4-C5) | 0.88 | π*(C3-C7) | 0.32 | 13.28 | 0.27 | 0.09 |
| π(C22-C23) | 0.83 | π*(C20-C21) | 0.17 | 10.39 | 0.28 | 0.07 |
| π(C24-C25) | 0.83 | π*(C20-C21) | 0.17 | 11 | 0.28 | 0.07 |
| π(C24-C25) | 0.83 | π*(C22-C23) | 0.17 | 10.72 | 0.28 | 0.07 |
| LP(O15) | 0.99 | σ*(C3-C7) | 0.02 | 3.05 | 1.09 | 0.07 |
| LP(O15) | 0.98 | π*(C3-C7) | 0.32 | 4.60 | 0.34 | 0.06 |
| LP(O15) | 0.98 | σ*(C21-H26) | 0.01 | 0.32 | 0.87 | 0.02 |
| LP(O17) | 0.96 | σ*(C9-O10) | 0.03 | 1.48 | 0.97 | 0.05 |
| LP(O17) | 0.95 | σ*(C8-C9) | 0.02 | 4.39 | 0.91 | 0.08 |
| LP(O17) | 0.95 | σ*(C9-O10) | 0.03 | 6.35 | 0.62 | 0.08 |
| LP(O35) | 0.95 | σ*(C31-C34) | 0.03 | 8.56 | 0.64 | 0.09 |
| LP(O35) | 0.95 | σ*(C34-C36) | 0.03 | 8.81 | 0.63 | 0.1 |
| σ(C1-C2) | 0.99 | σ*(C3-C7) | 0.32 | 2.39 | 1.19 | 0.07 |
| σ(C1-H11) | 0.99 | σ*(C2-C3) | 0.01 | 2.82 | 1.02 | 0.07 |
| σ(C1-H11) | 0.99 | σ*(C5-C6) | 0.01 | 2.45 | 1.05 | 0.06 |
| σ(C2-C3) | 0.98 | σ*(C4-O10) | 0.02 | 2.40 | 0.95 | 0.06 |
| π(C3-C7) | 0.89 | π*(C4-C5) | 0.17 | 5.17 | 0.30 | 0.05 |
| σ(C5-H13) | 0.99 | σ*(C3-C4) | 0.02 | 3.19 | 1.02 | 0.07 |
| σ(C6-H14) | 0.99 | σ*(C1-C2) | 0.01 | 2.55 | 1.06 | 0.07 |
| π(C8-C9) | 0.95 | π*(C3-C7) | 0.32 | 2.88 | 0.29 | 0.04 |
| σ(C8-C18) | 0.98 | σ*(C9-O10) | 0.03 | 2.83 | 0.86 | 0.06 |
| π(C20-C21) | 0.82 | π*(C22-C23) | 0.17 | 11.31 | 0.27 | 0.07 |
| π(C20-C21) | 0.82 | π*(C24-C25) | 0.17 | 10.50 | 0.28 | 0.07 |
| σ(C21-H26) | 0.99 | σ*(C20-C25) | 0.01 | 2.93 | 1.05 | 0.07 |
| σ(C22-H27) | 0.99 | σ*(C20-C21) | 0.01 | 2.77 | 1.05 | 0.07 |
| σ(C31-H32) | 0.97 | π* (C34-O35) | 0.05 | 4.07 | 0.48 | 0.06 |
| LP (Ga40) | 0.99 | σ*(C9-O17) | 0.02 | 60.32 | 0.66 | 0.05 |
| Mode No | Symbol | Type | Definition |
|---|---|---|---|
| Stretching | |||
| 1–12 | Ri | C-C (ring) | C1-C2, C2-C3, C3-C4, C4-C5, C5-C6, C6-C1, C20-C21, C21-C22, C22-C23, C23-C24, C24-C25, C25-C20 |
| 13–21 | ri | C-H (ring) | C1-H11, C2-H12, C5-H13, C6-H14, C21-H26, C22-H27, C23-H28, C24-H29, C25-H30 |
| 22–27 | ri | C-C | C3-C7, C7-C8, C8-C9, C31-C34, C34-C36, C18-C20 |
| 28–31 | Qi | C-O (ring) | C9-O10, O10-C4, C9-O17, C7-O15 |
| 32 | Qi | C-H | C18-H19 |
| 33–34 | ri | C-H (methylene) | C31-H32, C31-H33 |
| 35 | Qi | O-GA | O17-Ga40 |
| 36 | Qi | C=O | C34-O35 |
| 37–38 | ri | C-C | C8-C18, C31-C18 |
| 39–41 | ri | C-H (methyl) | C36-H38, C36-H37, C36-H39 |
| 42 | Pi | O-H | O15-H16 |
| Bending | |||
| 43–60 | δi | C-C-C (ring) | C6-C1-C2, C1-C2-C3, C2-C3-C4, C3-C4-C5, C4-C5-C6, C5-C6-C1, C7-C3-C4, C3-C4-O10, C4-O10-C9, O10-C9-C8, C9-C8-C7, C8-C7-C3, C25-C20-C21, C20-C21-C22, C21-C22-C23, C22-C23-C24, C23-C24-C25, C24-C25-C20 |
| 61–78 | βi | H-C-C (ring) | H11-C1-C6, H11-C1-C2, H12-C2-C1, H12-C2-C3, H14-C6-C5, H14-C6-C1, H13-C5-C4, H13-C5-C6, H26-C21-C20, H26-C21-C22, H27-C22-C21, H27-C22-C23, H28-C23-C22, H28-C23-C24, H29-C24-C23, H29-C24-C25, H30-C25-C24, H30-C25-C20 |
| 79–82 | βi | O-C-C (ring) | O15-C7-C8, O15-C7-C3, O17-C9-O10, O17-C9-C8 |
| 83–86 | βi | H-C-C | C18-C8-C9, C18-C8-C7, C18-C20-C25, C18-C20-C21 |
| 87 | δi | C-O-Ga (ring) | C9-O17-Ga40 |
| 88 | βi | C-O-H (ring) | C7-O15-H16 |
| 89 | αi | C-C-C | C31-C34-C36 |
| 90–91 | βi | C-C-C | O35-C34-C31, O35-C34-C36 |
| 92–94 | αi | H-C-H (methyl) | H37-C36-H39, H38-C36-H37, H38-C36-H39 |
| 95–97 | βi | C-C-H (methyl) | C34-C36-H38, C34-C36-H39, C34-C36-H37 |
| 98 | αi | H-C-H (methylene) | H32-C31-H33 |
| 99 | γi | C-C-C (methylene) | C18-C31-C34 |
| 100 | αi | C-C-C | C8-C18-C31 |
| 101 | γi | H-C-C | H19-C18-C20 |
| 102–109 | βi | H-C-C (methylene) | H32-C31-C34, H33-C31-C34, H32-C31-C18, H33-C31-C18, C8-C18-C20, C31-C18-C20, C8-C18-H19, C31-C18-H19 |
| 110–111 | βi | Butt | C2-C3-C4-O10, C7-C3-C4-C5 |
| Wagging | |||
| 112 | ωi | C-C (ring) | C18-C8-C9-C7 |
| 113–114 | ωi | O-C (ring) | O15-C7-C8-C3, O17-C9-O10-C8 |
| 115–119 | ωi | C-C | H26-C21-C20-C22, H27-C22-C21-C23, H28-C23-C22-C24, H29-C24-C23-C25, H30-C25-C24-C20 |
| 120 | ωi | C18-C20-C25-C21 | |
| 121–124 | ωi | H11-C1-C6-C2, H12-C2-C1-C3, H14-C6-C5-C1, H13-C5-C4-C6 | |
| 125 | ωi | O35-C34-C31-C36 | |
| Torsion | |||
| 126–143 | τi | tC-C (ring) | C6-C1-C2-C3, C1-C2-C3-C4, C2-C3-C4-C5, C3-C4-C5-C6, C4-C5-C6-C1, C5-C6-C1-C2, C7-C3-C4-O10, C3-C4-O10-C9, C4-O10-C9-C8, O10-C9-C8-C7, C9-C8-C7-C3, C8-C7-C3-C4, C25-C20-C21-C22, C20-C21-C22-C23, C21-C22-C23-C24, C22-C23-C24-C25, C23-C24-C25-C20, C24-C25-C20-C21 |
| 144–145 | τi | tC-O | Ga40-O17-C9-O10, Ga40-O17-C9-C8 |
| 146–147 | τi | tC-O | H16-O15-C7-C3, H16-O15-C7-C8 |
| 148–153 | τi | tC-C | C7-C8-C18-C20, C7-C8-C18-C31, C7-C8-C18-H19, C9-C8-C18-C20, C9-C8-C18-C31, C9-C8-C18-H19 |
| 154–159 | τi | tC-C | C8-C18-C20-C21, C8-C18-C20-C25, C31-C18-C20-C21, C31-C18-C20-C25, H19-C18-C20-C21, H19-C18-C20-C25 |
| 160–168 | τi | tC-C | C8-C18-C31-H32, C8-C18-C31-H33, C8-C18-C31-C34, H19-C18-C31-H32, H19-C18-C31-H33, H19-C18-C31-C34, C20-C18-C31-H32, C20-C18-C31-H33, C20-C18-C31-C34 |
| 169–174 | tC-C | C18-C31-C34-O35, C18-C31-C34-C36, H32-C31-C34-O35, H32-C31-C34-C36, H33-C31-C34-C36, H33-C31-C34-O35 | |
| 175–180 | τi | tC1-C | C31-C34-C36-H38, C31-C34-C36-H37, C31-C34-C36-H39, O35-C34-C36-H38, O35-C34-C36-H37, O35-C34-C36-H38 |
| 181–193 | τi | tC-C | O33-C32-C31-C27, O33-C32-C31-C30, O34-C32-C31-C27, O34-C32-C31-C30, O34-C35-C38-H39, O34-C35-C38-H41, O34-C35-C38-H40, H37-C35-C38-H39, H37-C35-C38-H41, H37-C35-C38-H40, H36-C35-C38-H39, H37-C35-C38-H41, H37-C35-C38-H40 |
| Scaled Wavenumber (cm−1) | Experimental Wavenumber (cm−1) | Vibrational Assignment with PED% (≥10) | |
|---|---|---|---|
| IR | Raman | ||
| 3710 | 3278 s | - | ν O15-H16 (100) |
| 3094 | - | - | ν C1-H11 (12), ν C5-H13 (33), ν C6-H14 (54) |
| 3089 | - | - | ν C21-H26 (80), ν C22-H27 (12), |
| 3082 | 3085 w | 3082 w | ν C1-H11 (29), ν C5-H13 (58), ν C6-H14 (12) |
| 3081 | - | - | ν C23-H28 (22), ν C24-H29 (30), ν C25-H30 (36), |
| 3073 | - | - | ν C36-H37 (12), ν C36-H38 (40), ν C36-H39 (44) |
| 3071 | - | - | ν C1-H11 (53), ν C6-H14 (34) |
| 3062 | 3062 w | 3061 w | ν C22-H27 (42), ν C24-H29 (40), ν C25-H30 (14), |
| 3053 | - | - | ν C22-H27 (33), ν C23-H28 (34), ν C24-H29 (27) |
| 3040 | - | 3041 w | ν C36-H39 (62), ν C36-H37 (23) |
| 3033 | 3032 w | - | ν C31-H32 (83), ν C31-H33 (12) |
| 3022 m | - | ||
| 3003 | - | - | ν C18-H19 (51), ν C31-H33 (46) |
| 2991 | 2995 w | 2995 w | ν C18-H19 (48), ν C31-H33 (47) |
| 2982 | 2976 w | 2976 w | ν C36-H38 (53), ν C36-H39 (42) |
| 2957 w | 2956 w | ||
| 2927 | 2928 w | 2931 w | ν C36-H37 (15), ν C36-H38 (32), ν C36-H39 (50) |
| 2914 | - | 2904 w | ν C31-H32 (93) |
| 2882 w | 2885 w | ν C31-H33 (42) | |
| 2852 w | ν C31-H33 (24) | ||
| 1721 | 1757 w | - | ν O35-C34 (89) |
| 1682 s | 1682 m | ν O35-C34 (32) | |
| 1654 s | - | ν O35-C34 (18) | |
| 1587 | - | 1610 s | ν C21-C22 (22), ν C24-C25 (21), |
| 1568 | 1572 m | 1572 s | ν C22-C23 (23), ν C23-C24 (14), ν C20-C25 (14), ν C20-C21 (18) |
| 1562 | - | - | ν C4-C5 (24), ν C1-C6 (12), ν C1-C2 (18), ν C3-C4 (17) |
| 1533 | - | 1531 w | ν C5-C6 (28), δ C2-C1-C6 (12), |
| 1505 | - | - | ν C8-C9 (34) |
| 1475 | - | - | δ H26-C21-C20 (15), δ H27-C22-C21 (17), δ H29-C24-C25 (16), δ H30-C25-C20 (13) |
| - | - | ||
| 1457 | 1453 m | - | δ H12-C2-C3 (12), δ H13-C5-C6 (13) |
| 1437 | - | - | ν C21-C22 (10), ν C24-C25 (11), δ H28-C23-C24 (23) |
| 1428 | - | - | δ H37-C36-H38 (73), τ C36-H37-C34-H38 (12), |
| 1426 | - | - | δ H11-C1-C6 (31), δ H14-C6-C1 (16), |
| 1416 | - | - | δ H37-C36-C34 (15), δ H38-C36-H39 (47), τ C36-H38-C34-H39 (27), |
| 1406 | - | 1404 w | ν C9-O17 (10), |
| 1390 | 1384 m | 1389 w | ν C7-C8 (26), δ H16-O15-C7 (11), δ H19-C18-C8 (16), |
| 1350 | 1378 m | 1377 m | δ H33-C31-C34 (24), OUT C18-C8-C20-H19 (38) |
| 1349 | - | - | δ H19-C18-C8 (15), |
| 1338 | 1340 w | - | δ H37-C36-C34 (12), δ H38-C36-H39 (26), OUT C36-H38-C34-H39 (16), |
| 1332 | - | δ H38-C36-H39 (13), ν O15-C7 (11), | |
| 1310 | 1307 w | 1302 w | δ H26-C21-C20 (21), δ H30-C25-C20 (19), |
| 1305 | - | - | ν C4-C5 (13), ν C1-C6 (17), ν C1-C2 (11), ν C3-C4 (20) |
| - | - | ||
| 1273 | 1278 w | 1277 w | δ H33-C31-C34 (10), ν O35-C34 (11), δ C8-C9-O10 (10) |
| 1262 | 1262 w | 1260 w | δ C18-C8-C20-H19 (12), δ H32-C31-C18(28), |
| - | |||
| 1257 | 1245 w | 1242 w | ν C20-C25 (11), δ H12-C2-C3 (10), δ H13-C5-C6 (17), δ H19-C18-C8 (17) |
| - | |||
| 1224 | 1219 w | 1219 w | δ H12-C2-C3 (14), ν C7-C8 (16); ν O17-C9 (21) |
| 1199 | 1192 w | - | ν C7-C8 (32), δ H16-O15-C7 (27), |
| 1184 | - | - | δ H32-C31-C18(11), ν O10-C4 (21), ν C7-C3 (19) |
| 1173 | 1178 w | 1176 w | δ H37-C36-H38 (16) |
| 1170 | - | - | δ H26-C21-C20 (19), δ H27-C22-C21 (16), δ H29-C24-C25 (13), δ H30-C25-C20 (17); ν C1-C2 (25) |
| 1168 | 1164 w | 1159 w | δ H29-C24-C25 (19), δ H28-ν C23-C24 (27), |
| 1158 | - | - | δ H33-C31-C34 (10), ν C18-C20 (24) |
| 1142 | - | - | δ H27-C22-C21 (11 δ H11-C1-C6 (24), δ H14-C6-C1 (10) |
| 1141 | - | - | ν C23-C22 (18); δ H11-C1-C6 (10), |
| 1139 | - | - | δ H37-C36-C34 (17), ν C34-C36 (14), ν C31-C34 (10), δ C36-C34-O35 (12) |
| 1121 | 1104 w | - | δ H13-C5-C6 (20), δ H14-C6-C1 (13), ν O15-C7 (12) |
| 1089 m | 1089 w | ||
| 1076 | 1078 m | 1076 w | ν C9-O17 (11), ν C7-O15 (17), δ H14-C6-C1 (12) |
| 1070 | - | - | ν C21-C22 (14), ν C24-C25 (15), δ H28-C23-C24 (10) |
| 1053 | - | - | ν O15-C7 (10), C18-C31 (21), |
| 1022 | - | - | ν C1-C6 (24), ν C5-C6 (11), δ H13-C5-C6 (12), |
| 1020 | 1030 w | 1036 w | ν C22-C23 (19), ν C23-C24 (21) |
| 1006 | 1006 w | - | OUT C36-H37-C34-H38 (23), δ H32-C31-C18(12) |
| 985 | 997 w | 999 w | C20-C21 (11), δ C22-C23-C24 (26), δ C21-C20-C25 (24), |
| 979 | - | - | ν O10-C9 (23), |
| 970 | - | - | τ H26-C21-C22-H27 (51), τ H28-C23-C24-H29 (15) |
| 958 | 952 w | 951 w | τ H26-C21-C22-H27 (26), τ H29-C24-C25-H30 (54) |
| 932 | - | - | τ C36-H38-C34-H39(11), ν C34-C36 (20), ν C18-C31 (11) |
| 925 | 919 w | 918 w | δ H37-C36-C34 (10); τH11-C1-C6-H14 (74), τ C6-C1-C 5-H14 (10); |
| 905 | 909 w | - | τ H29-C24-C25-H30 (23), τ H26-C21-C20-C18 (20), |
| 895 | 899 w | - | τ H28-C23-C24-H29 (15) |
| 876 | 885 w | 883 w | δ H14-C5-C34 (24); τ H13-C5-C6-H14 (78) |
| 862 | - | - | τ C36-H37-C34-H38 (13), τ C31-C 18-C34-H32 (10) |
| 842 | - | - | δ O10-C9-O17 (11), δ C2-C1-C6 (24) |
| 832 | - | - | τ H26-C21-C20-C18 (39), τ H28-C23-C24-H29 (49) |
| 801 | 814 w | 816 w | νC31-C34 (34), ν C34-C36(10) |
| 785 | 784 w | 785 w | τ C6-C1-C5-H14 (30), τ H12-C2-C3-C7(52) |
| 766 | 765 m | - | τ H28-C23-C24-C25(16), τ C20-C25-C24-C23 (13) |
| 740 | 755 m | 748 w | ν C18-C20 (14), τ C31-C18-C34-H33(10), δ C22-C23-C24 (15) |
| 708 | - | - | τ C6-C1-C5-H14 (41), τ H12-C2-C3-C7 (12) |
| 705 | 701 m | 704 w | τ H28-C23-C24-C25 (10), δ C1-C6-C5(10), δ C8-C9-O10 (11) |
| 695 | - | - | τ H12-C2-C3-C7 (12), τ C3-C5-O10-C4(47) |
| 691 | - | - | τ H28-C23-C24-C25(47), τ C20-C21-C22-C23 (22) |
| 657 | 666 w | 665 w | τ C7-C8-C9-O10(22), τ O17-C8-O10-C9(26) |
| 653 | 650 w | - | δ C2-C1-C6 (20) |
| 637 | 639 w | 638 w | δ O10-C9-O17 (30), δ C8-C7-O15(11), δ C3-C4-C5 (10) |
| 614 | 618 w | 619 w | δ C23-C24-C25 (75) |
| 584 | 604 w | 603 w | ν C34-C36(16), δ C36-C34-O35 (27) |
| 577 | 576 w | 577 w | δ C22-C23-C24(12), δ C21-C20-C25 (12) |
| 545 | 547 w | 546 w | τ C2-C1-C6-C5 (46), τ O17-C8-O10-C9(11), τ C3-C5-O10-C4(11) |
| 540 | - | - | δ C36-C34-O35 (18) |
| 527 | 526 w | - | τ C22-C23-C24-C25(12), |
| 512 | 515 w | 515 w | τ C18-C21-C25-C20(16) |
| 484 | 504 w | 503 w | τ C2-C1-C6-C5 (13), τ C4-O10-C9-C8(16), τ C2-C3-C7-O15(29) |
| 466 | 468 w | 467 w | δ C3-C7-C8 (28), ν Ga40-O17 (10), τ O35-C31-C36-C34(11) |
| 459 | 446 w | 443 w | τ O35-C31-C36-C34 (33) |
| 433 | 434 w | 436 w | δ C31-C34-C36 (11), τ C22-C23-C24-C25(15) |
| 410 | 409 w | 411 w | τ C1-C2-C3-C4 (20), τ O17-C8-O10-C9(11) |
| 401 | - | - | τ C20-C21-C22-C23(45), τ C20-C25-C24-C23(46) |
| 399 | - | 372 w | νO10-C4(10) |
| 333 | - | 325 w | τ C1-C2-C3-C4 (33), τ C18-C8-C9-O10(10), τ C2-C3-C7-O15(14) |
| 308 | - | 310 w | δ C5-C4-O10 (15), δ C8-C7-O15(35) |
| 291 | - | 287 w | δ C31-C34-C36 (26), δ C20-C18-C31 (19), τ C22-C23-C24-C25(11) |
| 252 | - | - | τ C6-C5-C4-O10 (61) |
| 250 | - | 249 w | δ C5-C4-O10 (24), ν Ga40-O17 (17) |
| 225 | - | - | δ C18-C20-C25 (38), τ C22-C23-C24-C25(13) |
| 207 | - | - | |
| 190 | - | - | δ C18-C31-C34 (20), ν C8-C18 (16) |
| 172 | - | 179 w | δ C18-C20-C25 (13), τ C7-C8-C9-O10(12) |
| 163 | - | - | δ C9-C8-C18 (14), δ C31-C34-C36 (10), δ C18-C31-C34 (12), νGa40-O17 (14) |
| 150 | - | 143 w | τ C5-C4-O10-C9 (19), τ C4-O10-C9-C8(10), τ C18-C8-C9-O10(11), τ C7-C8-C9-O10(15) |
| 122 | - | - | τ H37-C36-C34-O35 (80) |
| 102 | - | - | τ C5-C4-O10-C9 (22), τ C8-C9-O17-Ga40 (12) |
| 99 | - | - | τ C4-O10-C9-C8(14), τC18-C8-C9-O10(14), τ C2-C3-C7-O15(13), τ C7-C8-C9-O10(13) |
| 75 | - | - | δ C9-C8-C18 (27), δC18-C31-C34 (14) |
| 55 | - | - | τ C31-C8-C20-C18(26) |
| 50 | - | - | δ C9-O17-Ga40 (15), τ C18-C31-C34-C36(10), τC20-C18-C31-C34(59) |
| 38 | - | - | δ C9-O17-Ga40 (14), τ C18-C31-C34-C36(46), |
| 36 | - | - | δ C9-O17-Ga40 (17), |
| 32 | - | - | τ C8-C18-C20-C25(57) |
| 28 | - | - | τ C8-C18-C20-C25(14) |
| 21 | - | - | τ C9-C8-C18-C20 (53), τ C8-C9-O17-Ga40 (20) |
| Ligand | Target Proteins (PDB ID) | Estimated Inhibition Constant (Ki) (μM) | Binding Energy (kcal mol−1) |
|---|---|---|---|
| Warfarin gallium | 2C6D | 68.62 µM | –5.68 |
| 2C6E | 152.15 µM | –5.21 | |
| 4L2K | 4.09 µM | –7.35 |
| Proteins [PDB ID] | Bounded Residues | No. of Hydrogen Bonds | Bond Distance (Å) | H-Bond Energy (kcal mol−1) |
|---|---|---|---|---|
| 2C6D | protein:A:LEU261:CD1 protein:A:ASN260:HD2 protein:A:GLY275:HN protein:A:SER277:HN | 4 | 2.6 2.3 2.5 2.0 | –4.96 –3.76 –3.99 –1.93 |
| 2C6E | protein:A:GLY1132:O | 1 | 2.0 | –3.58 |
| 4L2K | protein:A:ASP993:HN | 1 | 2.1 | –3.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joe, H.; Atanasova, V.; Mojžiš, J.; Kostova, I. Synthesis, Characterization, and Cytotoxicity of a Ga(III) Complex with Warfarin. Inorganics 2024, 12, 177. https://doi.org/10.3390/inorganics12070177
Joe H, Atanasova V, Mojžiš J, Kostova I. Synthesis, Characterization, and Cytotoxicity of a Ga(III) Complex with Warfarin. Inorganics. 2024; 12(7):177. https://doi.org/10.3390/inorganics12070177
Chicago/Turabian StyleJoe, Hubert, Venceslava Atanasova, Jan Mojžiš, and Irena Kostova. 2024. "Synthesis, Characterization, and Cytotoxicity of a Ga(III) Complex with Warfarin" Inorganics 12, no. 7: 177. https://doi.org/10.3390/inorganics12070177
APA StyleJoe, H., Atanasova, V., Mojžiš, J., & Kostova, I. (2024). Synthesis, Characterization, and Cytotoxicity of a Ga(III) Complex with Warfarin. Inorganics, 12(7), 177. https://doi.org/10.3390/inorganics12070177







