Halide Perovskites’ Multifunctional Properties: Coordination Engineering, Coordination Chemistry, Electronic Interactions and Energy Applications beyond Photovoltaics
Abstract
1. Background
2. Halide Perovskites
2.1. HC(NH2)2PbI3 and Its Derivatives
2.2. (CH3NH3)x(HC(NH2)2)1−xPbI3 Perovskite
2.3. (HC(NH2)2)1−xCsxPbI3 Perovskites
2.4. The Difficulty of Replacing Lead Atom by Other Metals
- (a)
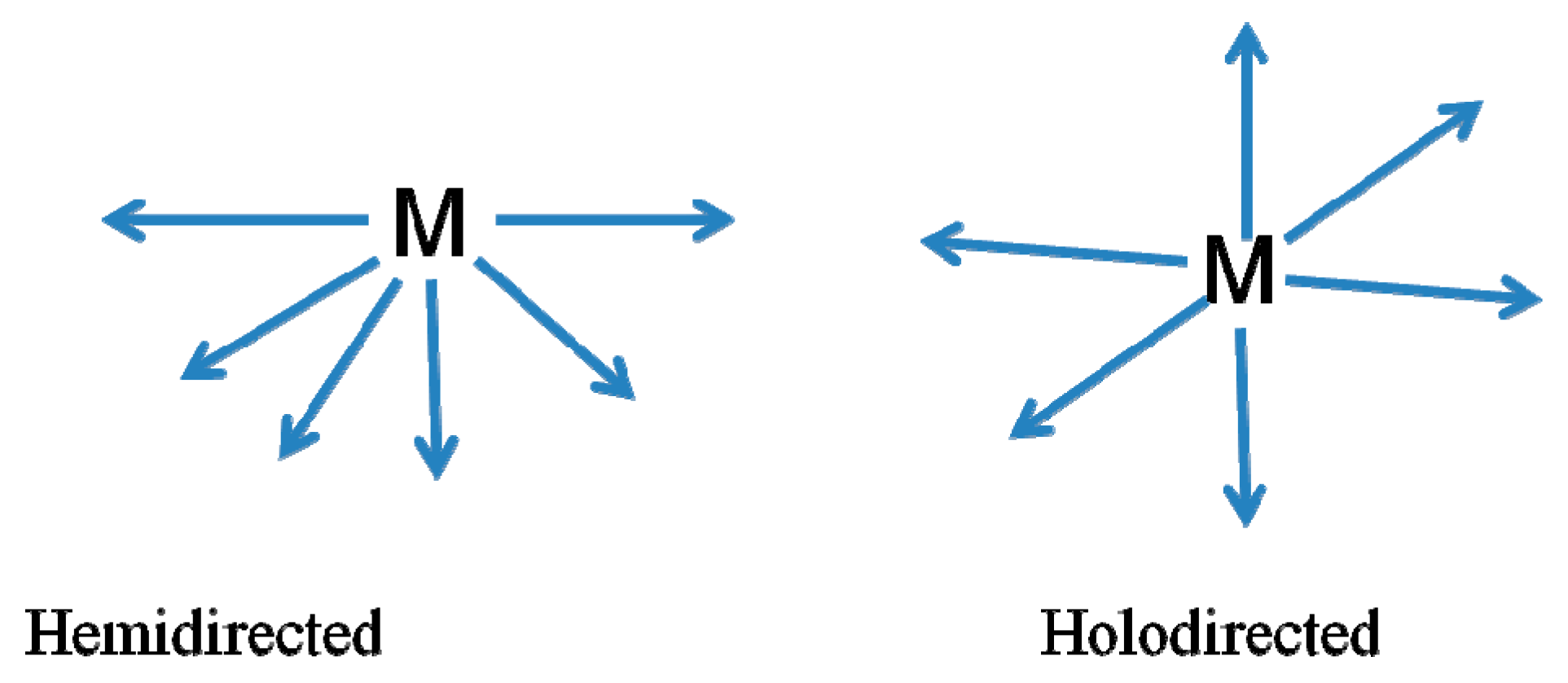
- Ionic radius: This is best outlined by the tolerance factor. Replacing the toxic Pb in perovskite crystals needs an atom of similar size. The excellent performance of Pb-based perovskites is mainly because of high structural symmetry and strong anti-bonding coupling between Pb and I.
- (b)
- High polarizability: The lead (II) atom is considered to be a softer or borderline hard/soft cation that has polarizable outer electrons, large size, low electronegative and should interact most strongly with donor types. Typically, a soft cation will covalently bond with a soft donor atom, which has low electronegativity, highly polarizable low-lying empty orbitals and is easily oxidized, and a hard cation will form an ionic bond with a donor atom, which has high electronegativity, low polarizability, and high energy empty orbitals and is hard to oxidize.
- (c)
- Valence: A B site atom has in a perfect world a 2+ valence. Different configurations are conceivable, and they require remuneration to accomplish charge neutrality. Lead (II) has a stable oxidation state of +2 with a coordination number of 6. All six PbII-X bonds of the halogen ligands, the holo-directed structures in which the ligand atoms are connected to each other, are clearly ionic, but the ionic character of the bonds decreases as the atomic number of the halogen ligand increases and greater transfer of electron density from the ligands to the lead occurs as the electronegativity of the ligand decreases and the bond become covalent bond. If the arrangement is holo-directed geometry, the PbII-ligand bonds are all similar.
- (d)
- Lone pairs: Ideally, the B-site displays a 6s2 lone pair. When considering every one of these elements, of each of the 120+ elements, just lead has this alluring mix of properties.
3. Coordination Engineering of Halide Perovskite Crystals
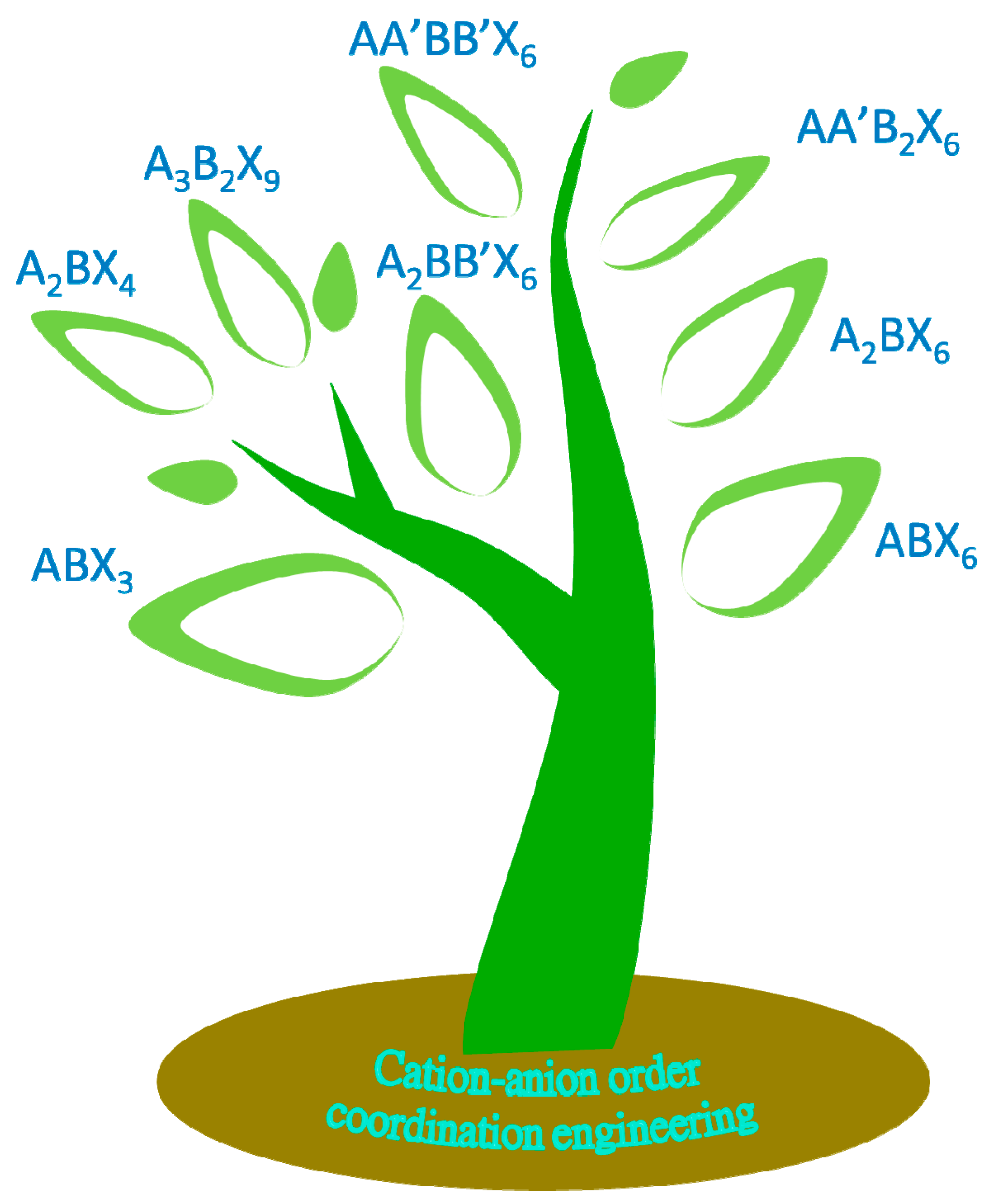
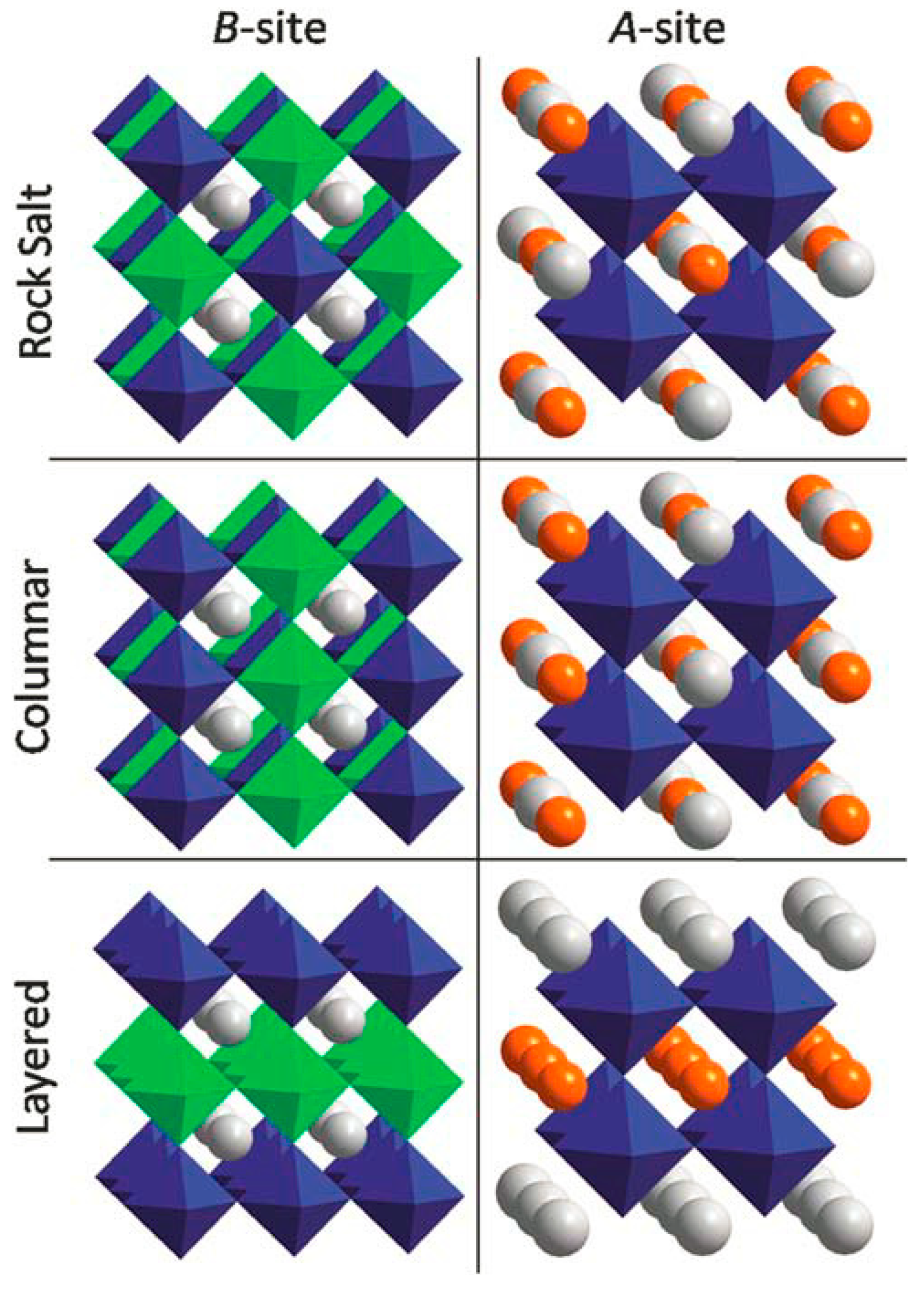
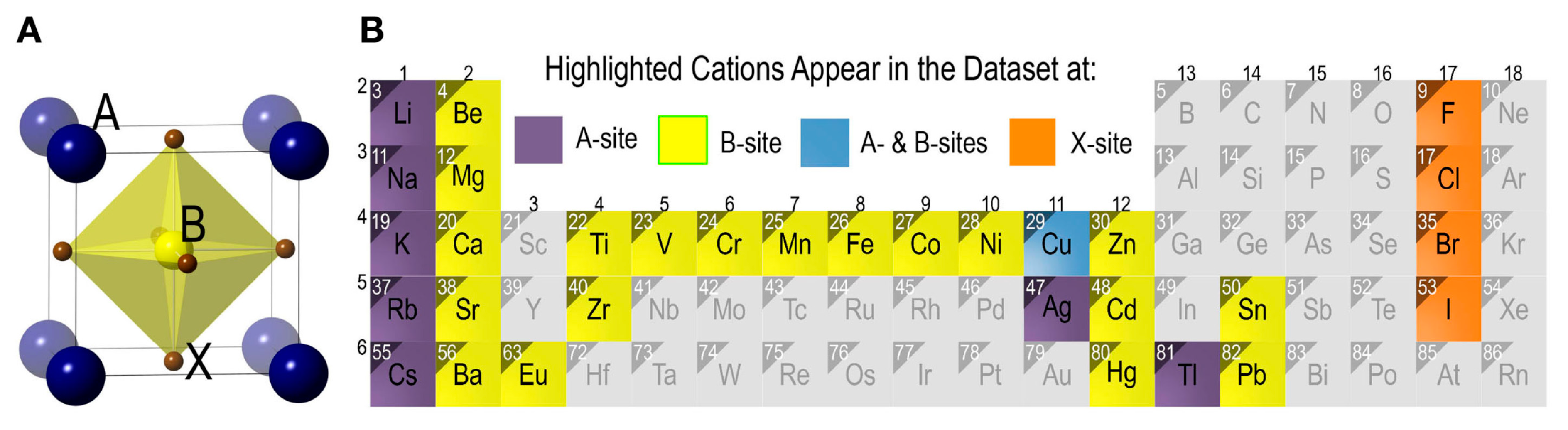
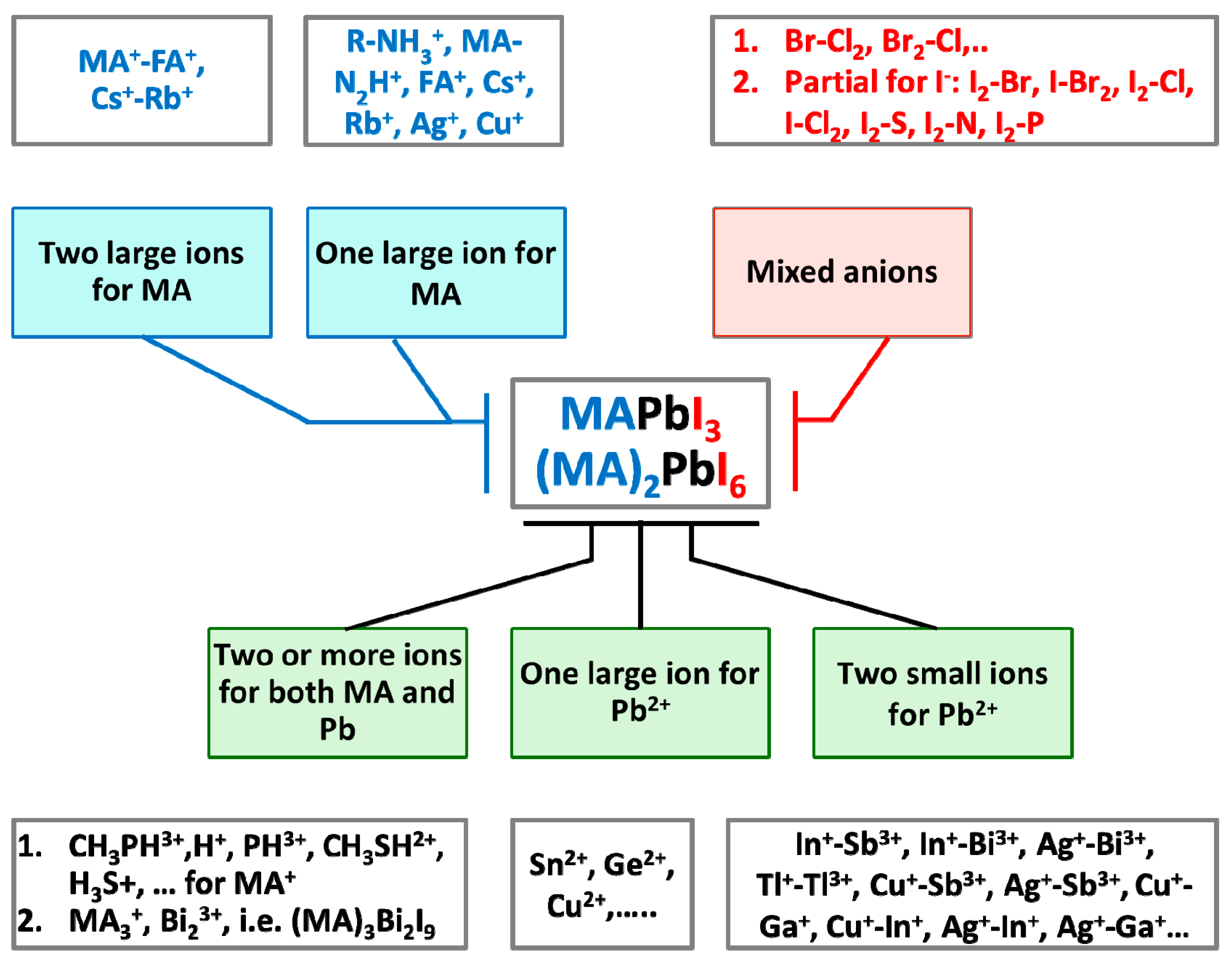
3.1. Cation and Anion Order Engineering of New Halide Perovskite
3.2. ABX3 Perovskite
3.3. ABX6 Perovskite
3.4. A2BX4 Perovskite
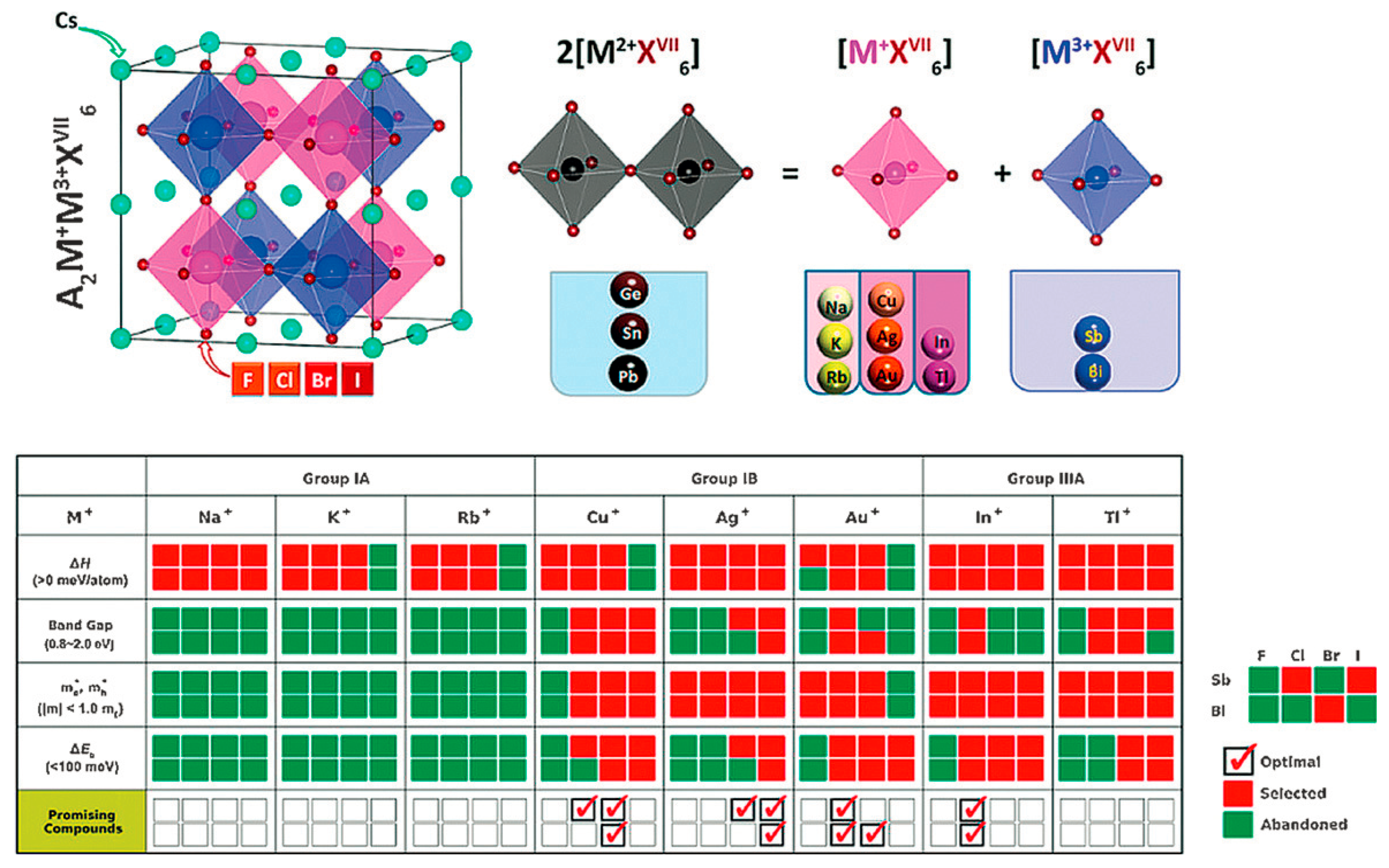
3.5. A2BX6 Perovskite
3.6. A3B2X9 Perovskite-like 3D Framework
3.7. A2BB’X6 Double Perovskites
3.8. AA’B2X6 Double Perovskite
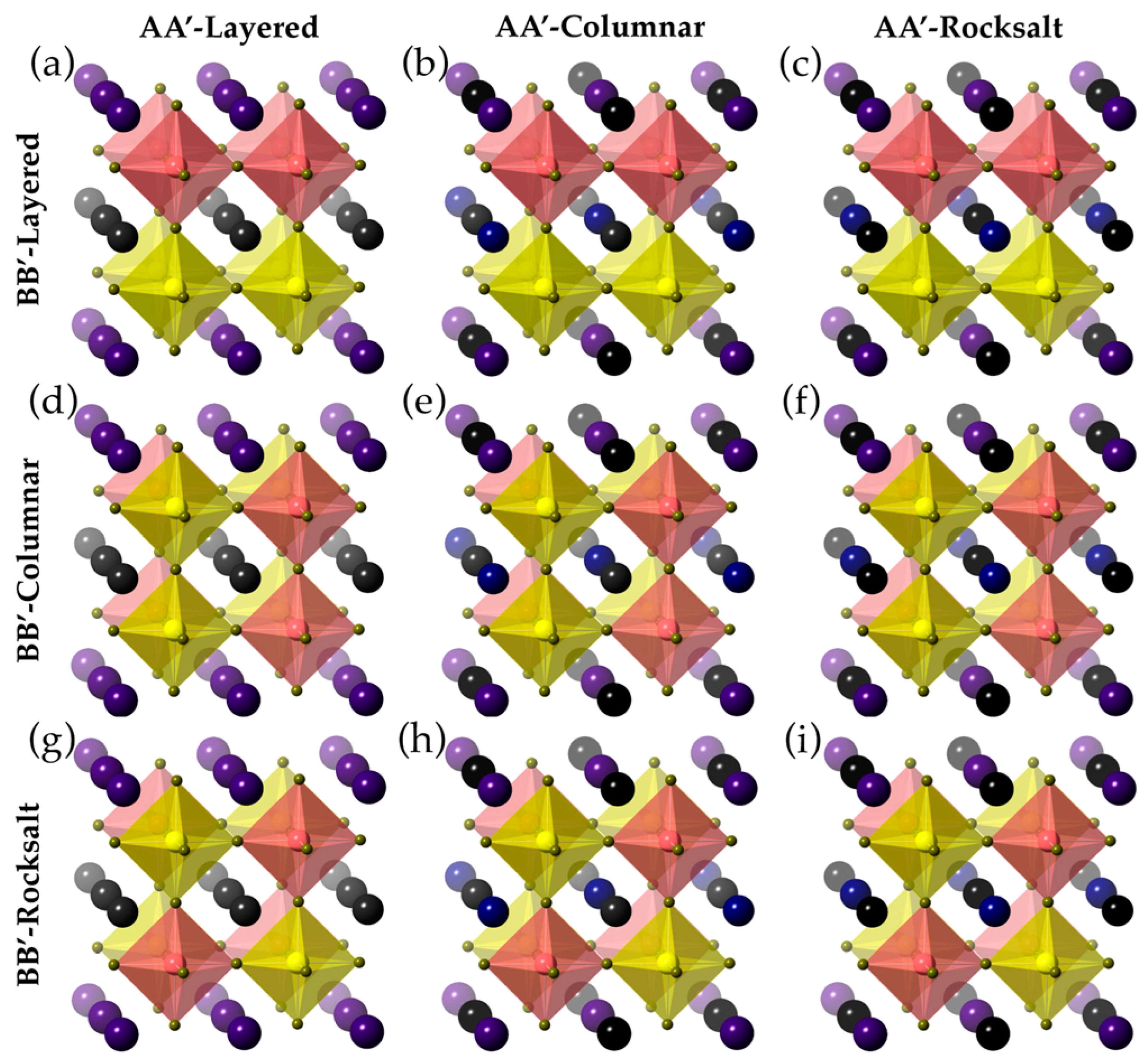
3.9. AA’BB’X6 Double Perovskite
4. Coordination Chemistry of Halide Perovskite Structures
4.1. Coordination Chemistry of Post-Transition-Metal Atoms
4.2. Proposed Ion Exchange and Ion Mixing Chemistry in Perovskites
4.3. Coordination Chemistry of Single-Crystal Complex Formation
4.4. Coordination Chemistry Limits Crystallization of Halide Perovskites
5. Electronic Interaction during Coordination Chemistry
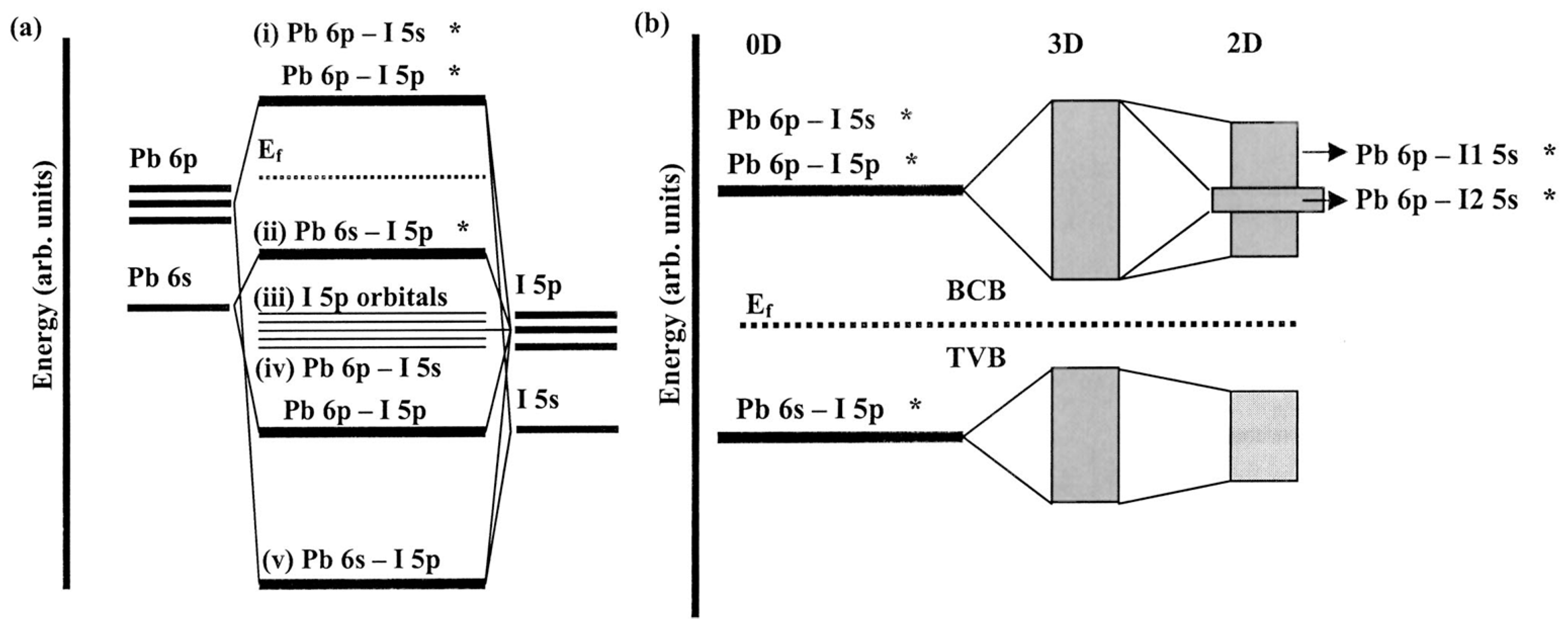
5.1. Bonding Idea in Lead Halide Perovskites
5.2. Complex Bonding Idea in Lead Halide Perovskites
5.3. Electronegativity and Electronic Bandgap Tuning
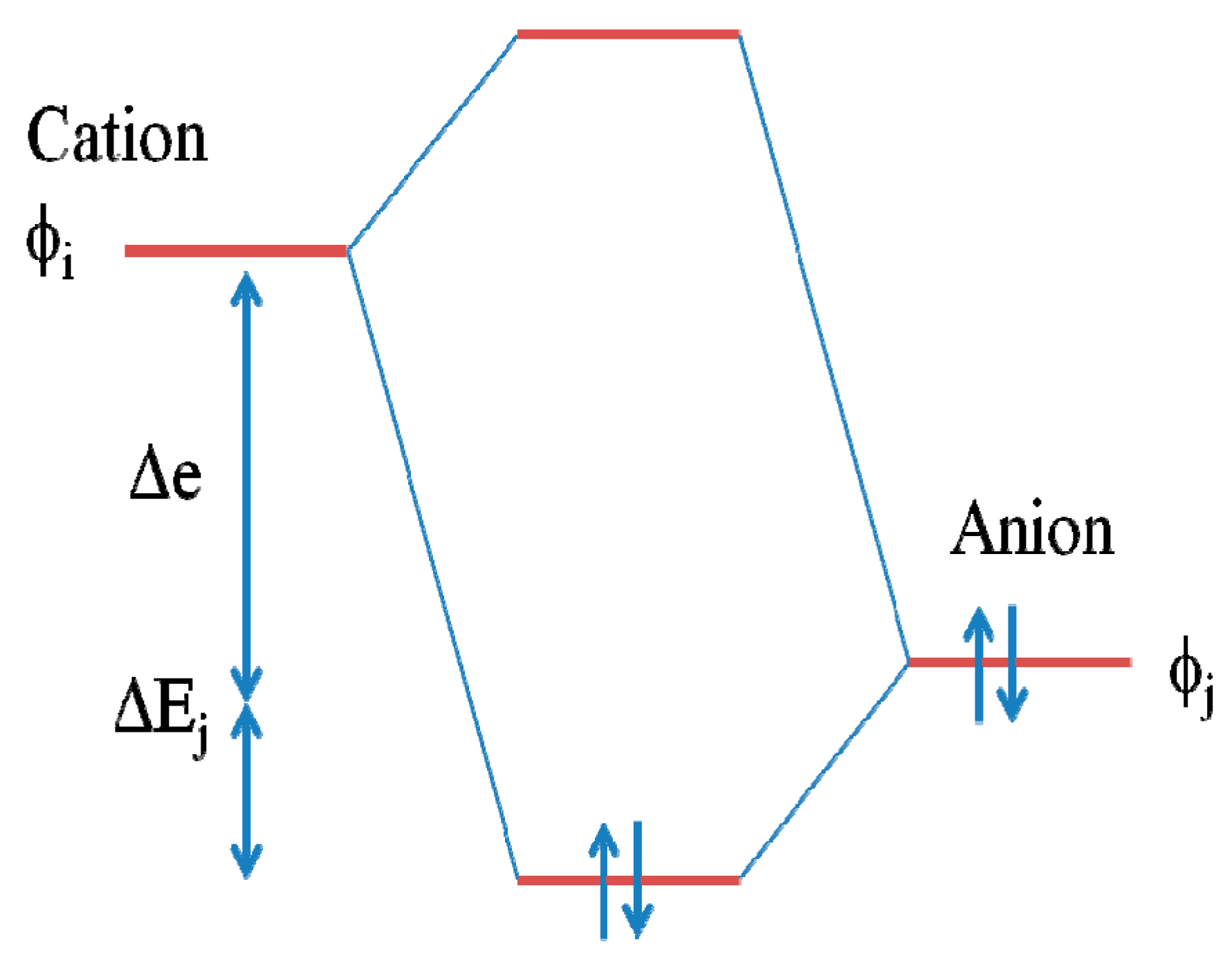
5.4. Cation–Anion Orbital Interaction
6. Properties of Different Halide Perovskite Structures
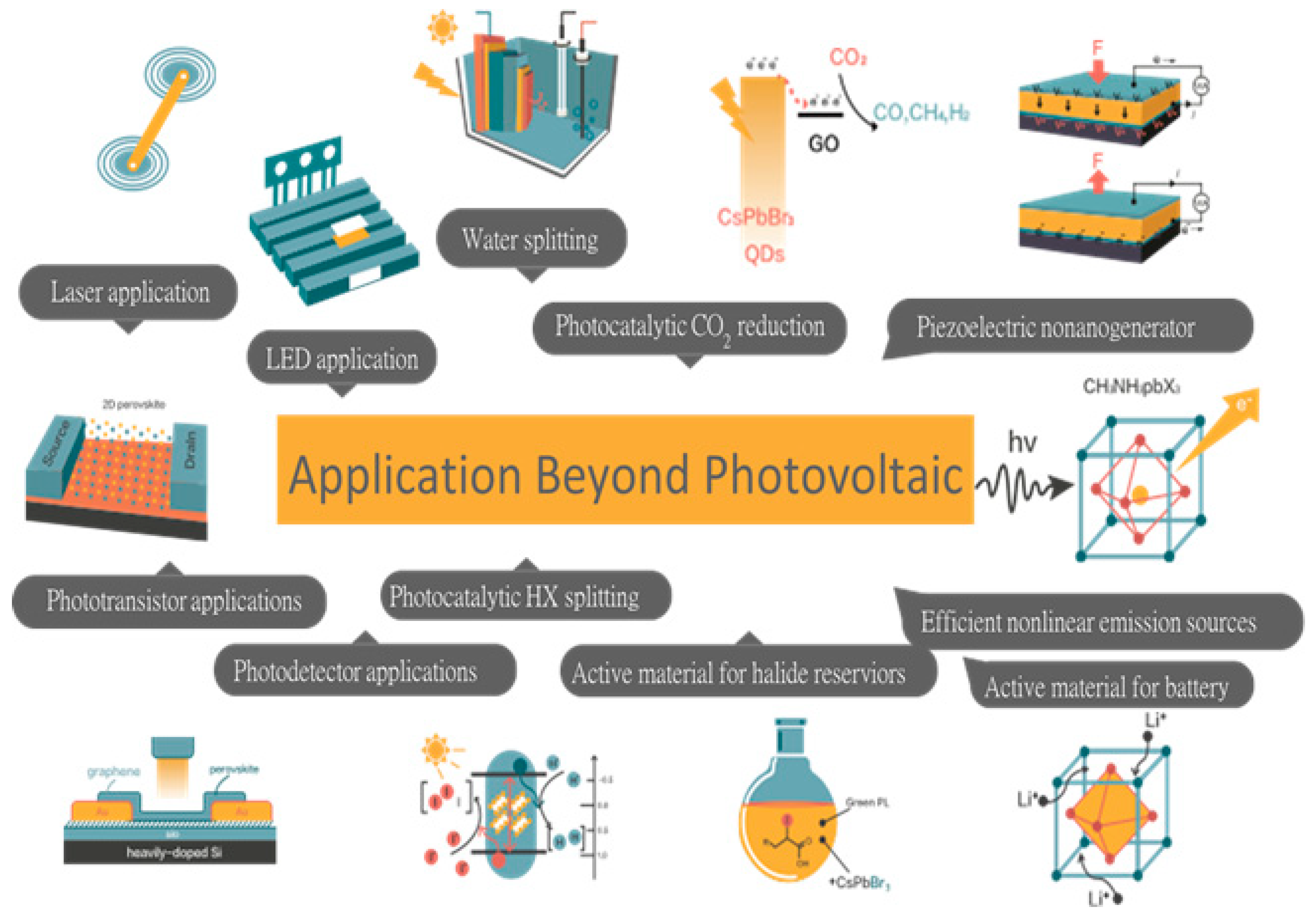
7. Energy Applications of Halide Perovskites beyond Photovoltaic
7.1. MAPbI3 as a Photocatalytic Material for HI Splitting
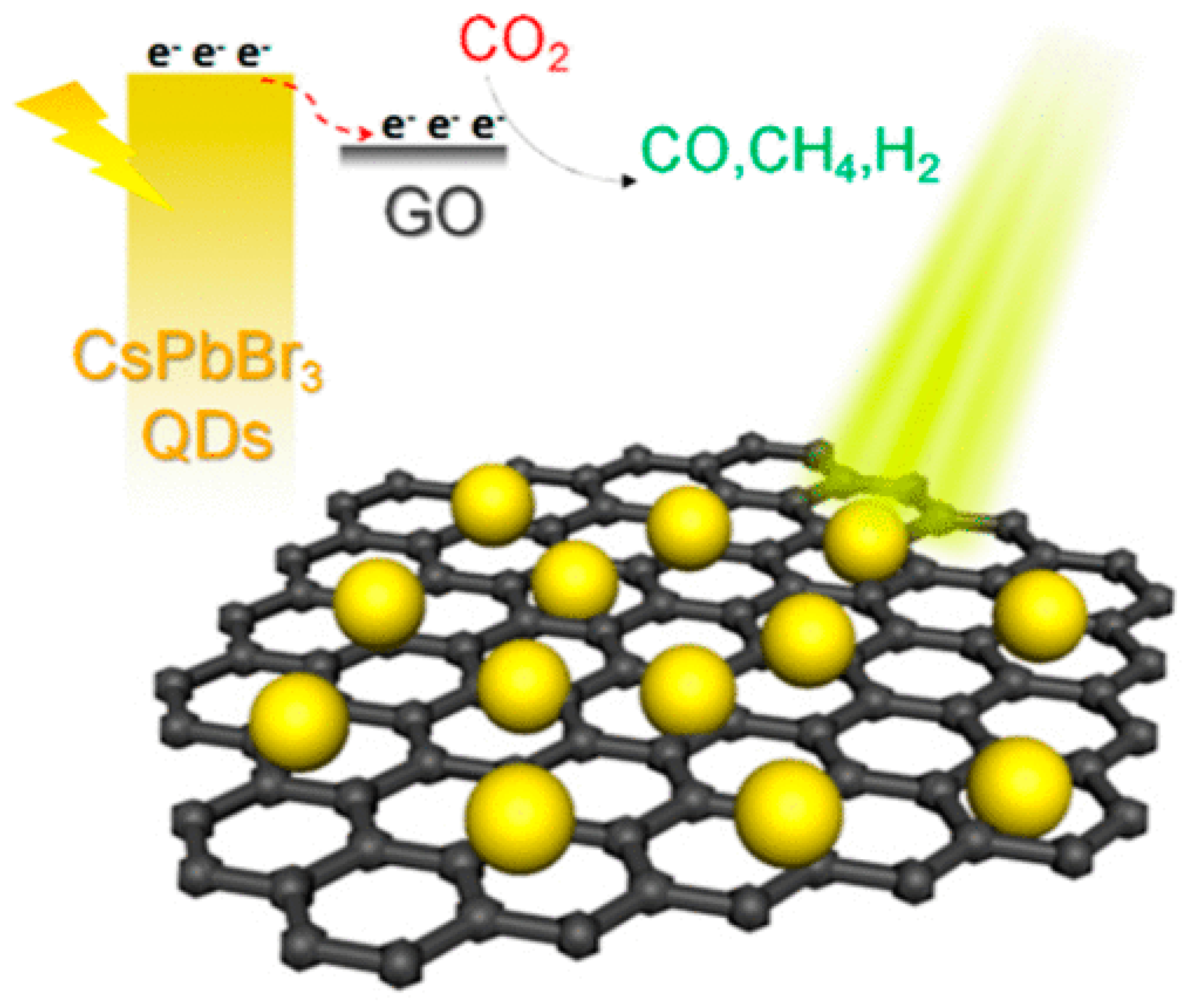
7.2. Perovskite QD-GO Nanocomposite for Photocatalytic Reduction of CO2
7.3. Halide Perovskite as Active Material for Battery
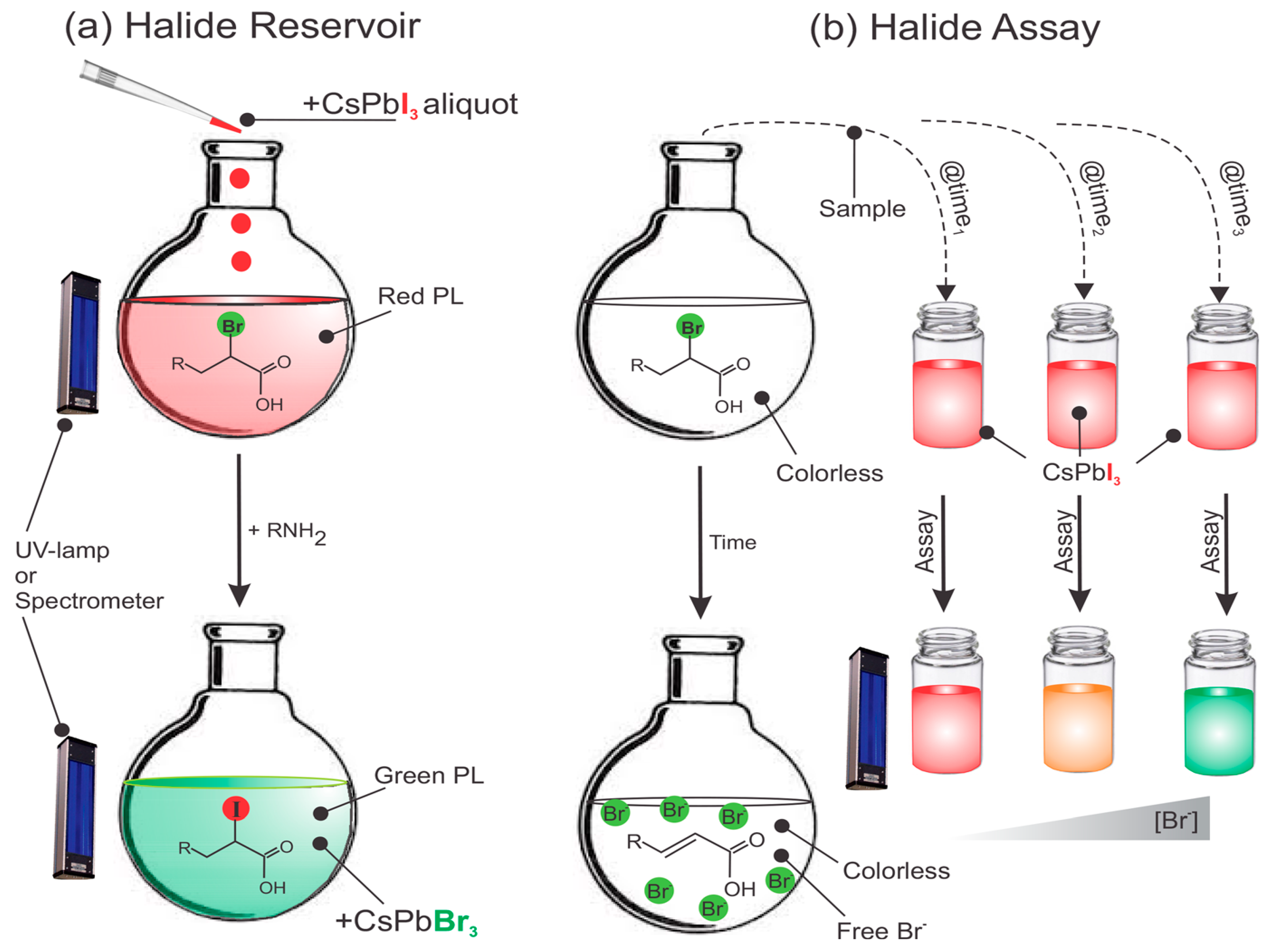
7.4. Halide Reservoir in Catalysis Applications
7.5. Piezoelectric Generators
7.6. What Could Happen in the Future of Halide Perovskites?
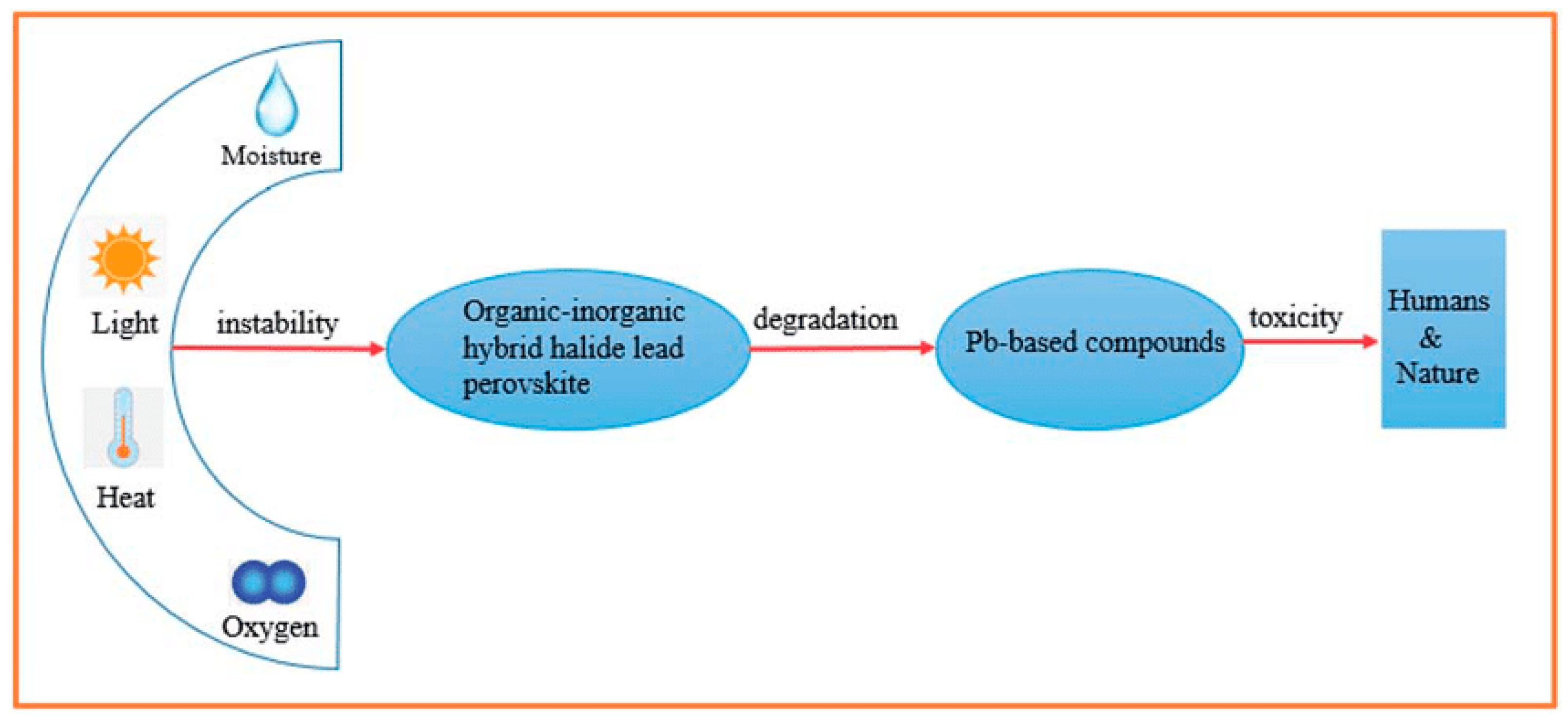
8. Concluding Remarks
- (1)
- Stability improvement as a way for flexible practical applications: Currently, this is the first challenge that blocks practical applications of halide perovskite materials. This limitation is not only for devices, but also the material itself is easily prone to degrade. As a key parameter for any optoelectronic applications, materials’ environmental stability and durability determine the lifespan of the device. Hence, in-depth sympathetic chemistry and engineering of halide perovskites is helpful in enhancing stability in two ways: (a) Enhancing the hydrophobic character of halide perovskites to overcome the solubility and dissolution of these materials. This can be done by increasing the carbon chain in the organic tail to reduce its hydrophilic character or to increase the inorganic character of the halide perovskites by completely replacing CH3NH3+ with water-resistant metal atoms such as Cs, Rb, etc. Using stoichiometric composition engineering of the organic tail with smaller amount of the organic tail could also enhance the hydrophobicity of these materials. (b) The coordination engineering framework of the halide perovskite structure can overcome the stability issues in these materials.
- (2)
- Toxicity reduction in mass production of halide perovskites: Toxicity is the second most challenging issue that hinders the commercialization and mass production of halide perovskites. Understanding the chemistry and engineering of halide perovskites is highly relevant to partially or completely avoid toxicity in these materials. This can be completed by (a) complete removal of the lead atom and replacing it with environmentally friendly metal atoms such as Ti, Sb, Bi, etc., or (b) completely replacing the lead atom with at least less toxic metal atoms such as Sn and Ge, which do not affect the environment significantly. (c) If both mechanisms are unsuccessful, mixing metal ions can be the last alternative for optimizing toxicity in lead-based halide perovskites. (d) If all these modifications are unsuccessful, engineering and other perovskite materials with new framework and new stoichiometric composition and structure could be the last alternative to avoid toxicity.
- (3)
- Enhanced semiconducting properties, such as optical and electrical properties, as well as efficiency enhancement. This basic intention of coordination chemistry and coordination engineering of halide perovskite materials is to improve optical and electrical properties and to design new material with better semiconducting properties for better performance.
- (4)
- Another exciting behavior of halide perovskites is their wide range of potential applications owing to enhanced semiconducting properties that may benefit from and require the fundamental concepts of chemistry and engineering in addition to their photophysics properties: A line of inquiry is whether halide perovskites are applicable beyond photovoltaic applications, for instance, (a) many optoelectronic devices such as lasers, LED, photodetectors, transistors and nonlinear emission sources, (b) photocatalytic activities such as efficient water, CO2 and HX splitting, (c) storage devices such as active materials for LIB and Na ion batteries as well as halide reservoirs for catalysis purposes. Moreover, the discovery of new perovskite materials with multifunctional properties and improved semiconducting properties: this point of view may be important to fabricate a new device that fulfills the ‘Triple E’ rule: efficient, economical and environmentally friendly solar cell device.
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Berry, J.; Buonassisi, T.; Egger, D.A.; Hodes, G.; Kronik, L.; Loo, Y.; Lubomirsky, I.; Marder, S.R.; Mastai, Y.; Miller, J.S.; et al. Hybrid Organic-Inorganic Perovskites (HOIPs): Opportunities and Challenges. Adv. Mater. 2015, 27, 5102–5112. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wu, Y.; Chen, H.; Yang, X.; Qiang, Y.; Han, L. Cost-Performance Analysis of Perovskite Solar Modules. Adv. Sci. 2017, 4, 1600269. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Morales Vilches, A.B.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Collavini, S.; Völker, S.F.; Delgado, J.L. Understanding the Outstanding Power Conversion Efficiency of Perovskite-Based Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 9757–9759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Bailie, C.D.; Christoforo, M.G.; Mailoa, J.P.; Bowring, A.R.; Unger, E.L.; Nguyen, W.H.; Burschka, J.; Pellet, N.; Lee, J.Z.; Gratzel, M.; et al. Semi-transparent perovskite solar cells for tandems with silicon and CIGS. Energy Environ. Sci. 2015, 8, 956–963. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Dang, T.-V.; Choi, H.-J.; Park, B.-J.; Eom, J.-H.; Song, H.-A.; Seol, D.; Kim, Y.; Shin, S.-H.; Nah, J.; et al. Piezoelectric properties of CH3NH3PbI3 perovskite thin films and their applications in piezoelectric generators. J. Mater. Chem. A 2016, 4, 756–763. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.-L.; Jiao, W.-B.; Wang, Q.; Wei, M.; Cantone, I.; Lü, J.; Abate, A. Biological impact of lead from halide perovskites reveals the risk of introducing a safe threshold. Nat. Commun. 2020, 11, 310. [Google Scholar] [CrossRef]
- Ren, M.; Qian, X.; Chen, Y.; Wang, T.; Zhao, Y. Potential lead toxicity and leakage issues on lead halide perovskite photovoltaics. J. Hazard. Mater. 2022, 426, 127848. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Burda, C. Coordination engineering toward high performance organic–inorganic hybrid perovskites. Coord. Chem. Rev. 2016, 320–321, 53–65. [Google Scholar] [CrossRef]
- Li, Z.; Johnston, A.; Wei, M.; Saidaminov, M.I.; Martins de Pina, J.; Zheng, X.; Liu, J.; Liu, Y.; Bakr, O.M.; Sargent, E.H. Solvent-Solute Coordination Engineering for Efficient Perovskite Luminescent Solar Concentrators. Joule 2020, 4, 631–643. [Google Scholar] [CrossRef]
- Yan, K.; Long, M.; Zhang, T.; Wei, Z.; Chen, H.; Yang, S.; Xu, J. Hybrid Halide Perovskite Solar Cell Precursors: Colloidal Chemistry and Coordination Engineering behind Device Processing for High Efficiency. J. Am. Chem. Soc. 2015, 137, 4460–4468. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, D.; Yan, H.; Meng, C.; Yang, Y.; Liu, J.; Wang, M.; Xu, P.; Peng, Z.; Chen, J.; et al. A multiple-coordination framework for CsPbI2Br perovskite solar cells. J. Mater. Chem. C 2024, 12, 4112–4122. [Google Scholar] [CrossRef]
- Zuo, S.; Chu, S.; An, P.; Hu, H.; Yin, Z.; Zheng, L.; Zhang, J. Solvent coordination engineering for high-quality hybrid organic-inorganic perovskite films. J. Mater. Sci. 2021, 56, 9903–9913. [Google Scholar] [CrossRef]
- Qin, Y.; Zhong, H.; Intemann, J.; Leng, S.; Cui, M.; Qin, C.; Xiong, M.; Liu, F.; Jen, A.; Yao, K. Coordination Engineering of Single-Crystal Precursor for Phase Control in Ruddlesden-Popper Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1904050. [Google Scholar] [CrossRef]
- Mangrulkar, M.; Stevenson, K.J. The Progress of Additive Engineering for CH3NH3PbI3 Photo-Active Layer in the Context of Perovskite Solar Cells. Crystals 2021, 11, 814. [Google Scholar] [CrossRef]
- Stumpp, M.; Ruess, R.; Müßener, J.; Schlettwein, D. Freezing the polarization of CH3NH3PbI3 and CH3NH3PbI3-xClx perovskite films. Mater. Today Chem. 2017, 4, 97–105. [Google Scholar] [CrossRef]
- Olga, S.; Yudanova, E.S.; Yeryukov, N.A.; Zhivodkov, Y.A.; Shamirzaev, T.; Maximovskiy, E.A.; Gromilov, S.; Troitskaia, I. Perovskite CH3NH3PbI3 Crystals and Films. Synthesis and Characterization. J. Cryst. Growth 2017, 462, 45–49. [Google Scholar] [CrossRef]
- Shao, F.; Qin, P.; Wang, D.; Zhang, G.; Wu, B.; He, J.; Peng, W.; Sum, T.C.; Wang, D.; Huang, F. Enhanced Photovoltaic Performance and Thermal Stability of CH3NH3PbI3 Perovskite through Lattice Symmetrization. ACS Appl. Mater. Interfaces 2019, 11, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Bin Mohd Yusoff, A.R.; Nazeeruddin, M.K. Dimensionality engineering of hybrid halide perovskite light absorbers. Nat. Commun. 2018, 9, 5028. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-J.; Kopyl, S.; Kholkin, A.; Rocha, J. Hybrid organic-inorganic perovskites: Polar properties and applications. Coord. Chem. Rev. 2019, 387, 398–414. [Google Scholar] [CrossRef]
- Mohd Yusoff, A.R.B.; Gao, P.; Nazeeruddin, M.K. Recent progress in organohalide lead perovskites for photovoltaic and optoelectronic applications. Coord. Chem. Rev. 2018, 373, 258–294. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, Z.; Xu, J.; Cao, G. Synergistic combination of semiconductor quantum dots and organic-inorganic halide perovskites for hybrid solar cells. Coord. Chem. Rev. 2018, 374, 279–313. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Abdelhady, A.L.; Murali, B.; Alarousu, E.; Burlakov, V.M.; Peng, W.; Dursun, I.; Wang, L.; He, Y.; Maculan, G.; et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 2015, 6, 7586. [Google Scholar] [CrossRef] [PubMed]
- Govinda, S.; Kore, B.P.; Bokdam, M.; Mahale, P.; Kumar, A.; Pal, S.; Bhattacharyya, B.; Lahnsteiner, J.; Kresse, G.; Franchini, C.; et al. Behavior of Methylammonium Dipoles in MAPbX3 (X = Br and I). J. Phys. Chem. Lett. 2017, 8, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Harikesh, P.C.; Mulmudi, H.K.; Ghosh, B.; Goh, T.W.; Teng, Y.; Krishnamoorthy, T.; Lockrey, M.; Weber, K.; Koh, T.M.; Li, S.; et al. Rb as an Alternative Cation for Templating Inorganic Lead-Free Perovskites for Solution Processed Photovoltaics. Chem. Mater. 2016, 28, 7496–7504. [Google Scholar] [CrossRef]
- Govinda, S.; Kore, B.P.; Swain, D.; Hossain, A.; De, C.; Guru Row, T.N.; Sarma, D.D. Critical Comparison of FAPbX3 and MAPbX3 (X = Br and Cl): How Do They Differ? J. Phys. Chem. C 2018, 122, 13758–13766. [Google Scholar] [CrossRef]
- Debnath, T.; Sarker, D.; Huang, H.; Han, Z.-K.; Dey, A.; Polavarapu, L.; Levchenko, S.V.; Feldmann, J. Coherent vibrational dynamics reveals lattice anharmonicity in organic–inorganic halide perovskite nanocrystals. Nat. Commun. 2021, 12, 2629. [Google Scholar] [CrossRef]
- Saparov, B.; Hong, F.; Sun, J.-P.; Duan, H.-S.; Meng, W.; Cameron, S.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-Film Preparation and Characterization of Cs3Sb2I9: A Lead-Free Layered Perovskite Semiconductor. Chem. Mater. 2015, 27, 5622–5632. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B. Effective Masses and Electronic and Optical Properties of Nontoxic MASnX3 (X = Cl, Br, and I) Perovskite Structures as Solar Cell Absorber: A Theoretical Study Using HSE06. J. Phys. Chem. C 2014, 118, 19655–19660. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.N.; Hwang, B.J. Hybride Halide Perovskites: Coordination Engineering, Coordination Chemistry, Electronic Interactions and Energy Applications beyond Photovoltaic. Preprints 2024, 2024031615. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Tsarev, S.A.; Elshobaki, M.; Luchkin, S.Y.; Zhidkov, I.S.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; Troshin, P.A. Comparative Intrinsic Thermal and Photochemical Stability of Sn(II) Complex Halides as Next-Generation Materials for Lead-Free Perovskite Solar Cells. J. Phys. Chem. C 2019, 123, 26862–26869. [Google Scholar] [CrossRef]
- Ke, W.; Stoumpos, C.C.; Zhu, M.; Mao, L.; Spanopoulos, I.; Liu, J.; Kontsevoi, O.Y.; Chen, M.; Sarma, D.; Zhang, Y.; et al. Enhanced photovoltaic performance and stability with a new type of hollow 3D perovskite {en}FASnI3. Sci. Adv. 2017, 3, e1701293. [Google Scholar] [CrossRef] [PubMed]
- Irshad, Z.; Adnan, M.; Lee, J.K. Simple preparation of highly efficient MAxFA1−xPbI3 perovskite films from an aqueous halide-free lead precursor by all dip-coating approach and application in high-performance perovskite solar cells. J. Mater. Sci. 2022, 57, 1936–1946. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Zhao, C.; Zhang, B.; Liu, X.; Dai, S.; Yao, J. Efficient Planar Heterojunction FA1–xCsxPbI3 Perovskite Solar Cells with Suppressed Carrier Recombination and Enhanced Open Circuit Voltage via Anion-Exchange Process. ACS Appl. Mater. Interfaces 2019, 11, 4597–4606. [Google Scholar] [CrossRef]
- López-Fraguas, E.; Masi, S.; Mora-Seró, I. Optical Characterization of Lead-Free Cs2SnI6 Double Perovskite Fabricated from Degraded and Reconstructed CsSnI3 Films. ACS Appl. Energy Mater. 2019, 2, 8381–8387. [Google Scholar] [CrossRef]
- Yue, S.; McGuire, S.C.; Yan, H.; Chu, Y.S.; Cotlet, M.; Tong, X.; Wong, S.S. Synthesis, Characterization, and Stability Studies of Ge-Based Perovskites of Controllable Mixed Cation Composition, Produced with an Ambient Surfactant-Free Approach. ACS Omega 2019, 4, 18219–18233. [Google Scholar] [CrossRef]
- Liga, S.M.; Konstantatos, G. Colloidal synthesis of lead-free Cs2TiBr6−xIx perovskite nanocrystals. J. Mater. Chem. C 2021, 9, 11098–11103. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Z.; Li, K.; Han, Z.; Wei, S.; Guo, C.; Zhou, S.; Wu, Z.; Guo, W.; Wu, C.-m.L. First-principles insight into the photoelectronic properties of Ge-based perovskites. RSC Adv. 2016, 6, 86976–86981. [Google Scholar] [CrossRef]
- Alnujaim, S.; Bouhemadou, A.; Chegaar, M.; Guechi, A.; Bin-Omran, S.; Khenata, R.; Al-Douri, Y.; Yang, W.; Lu, H. Density functional theory screening of some fundamental physical properties of Cs2InSbCl6 and Cs2InBiCl6 double perovskites. Eur. Phys. J. B 2022, 95, 114. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Frazer, L.; Clark, D.J.; Kim, Y.S.; Rhim, S.H.; Freeman, A.J.; Ketterson, J.B.; Jang, J.I.; Kanatzidis, M.G. Hybrid Germanium Iodide Perovskite Semiconductors: Active Lone Pairs, Structural Distortions, Direct and Indirect Energy Gaps, and Strong Nonlinear Optical Properties. J. Am. Chem. Soc. 2015, 137, 6804–6819. [Google Scholar] [CrossRef]
- Ashfaq, A.; Tahir, S.; Mushtaq, S.; Alqurashi, R.S.; Haneef, M.; Almousa, N.; Rehman, U.u.; Bonilla, R.S. Comparative performance analysis of Cs2TiX6 (X = Br−, Cl−, I−) lead-free perovskite solar cells incorporating single, double and triple layer halides by SCAPS −1D. Mater. Today Commun. 2023, 35, 106016. [Google Scholar] [CrossRef]
- Zhao, X.-G.; Yang, D.; Sun, Y.; Li, T.; Zhang, L.; Yu, L.; Zunger, A. Cu–In Halide Perovskite Solar Absorbers. J. Am. Chem. Soc. 2017, 139, 6718–6725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hao, F.; Li, J.; Zhou, Y.; Wei, Y.; Lin, H. Perovskite solar cells: Must lead be replaced—And can it be done? Sci. Technol. Adv. Mater. 2018, 19, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Hnuna, L.; Pachuau, Z. Electronic, optical and thermoelectric properties of halide double perovskites Rb2AgInX6 (X = Cl, Br, I). Phys. Scr. 2023, 98, 3. [Google Scholar] [CrossRef]
- Wang, M.; Wang, W.; Ma, B.; Shen, W.; Liu, L.; Cao, K.; Chen, S.; Huang, W. Lead-Free Perovskite Materials for Solar Cells. Nano-Micro Lett. 2021, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.R.; Hillman, S.; Haghighirad, A.A.; Snaith, H.J.; Giustino, F. Band Gaps of the Lead-Free Halide Double Perovskites Cs2BiAgCl6 and Cs2BiAgBr6 from Theory and Experiment. J. Phys. Chem. Lett. 2016, 7, 2579–2585. [Google Scholar] [CrossRef]
- Yao, H.; Zhou, F.; Li, Z.; Ci, Z.; Ding, L.; Jin, Z. Strategies for Improving the Stability of Tin-Based Perovskite (ASnX3) Solar Cells. Adv. Sci. 2020, 7, 1903540. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Q.; Lu, Y.; Lu, F.; Mu, X.; Wei, S.-H.; Sui, M. Hydrogenated Cs2AgBiBr6 for significantly improved efficiency of lead-free inorganic double perovskite solar cell. Nat. Commun. 2022, 13, 3397. [Google Scholar] [CrossRef]
- Berhe, T.A.; Ashebir, E.K.; Abay, B.T. Cs2AgBiBr6 and Cs2TiBr6 Perovskite Solar Cells: The Challenges and Research Roadmap for Power Conversion Efficiency Improvement. Preprints 2024, 2024031340. [Google Scholar] [CrossRef]
- Zibouche, N.; Islam, M.S. Structure–Electronic Property Relationships of 2D Ruddlesden–Popper Tin- and Lead-based Iodide Perovskites. ACS Appl. Mater. Interfaces 2020, 12, 15328–15337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cleveland, I.J.; Tran, M.N.; Aydil, E.S. Stability of the Halide Double Perovskite Cs2AgInBr6. J. Phys. Chem. Lett. 2023, 14, 3000–3006. [Google Scholar] [CrossRef]
- Ke, W.; Stoumpos, C.; Kanatzidis, M. “Unleaded” Perovskites: Status Quo and Future Prospects of Tin-Based Perovskite Solar Cells. Adv. Mater. 2018, 31, 1803230. [Google Scholar] [CrossRef]
- Zhao, X.-H.; Tang, Y.-L.; Tang, T.-Y.; Diao, X.-F.; Gao, L.-K.; Xie, Q.; Shi, B.; Yuan, L.; Lu, L.-M. Study on mechanical, electronic and optical properties of Pb-free double halide perovskites In2TiX6 (X = Cl, Br, I) for solar cells based on first-principles. Mater. Today Commun. 2021, 26, 102180. [Google Scholar] [CrossRef]
- Ghrib, T.; Rached, A.; Algrafy, E.; Al-nauim, I.A.; Albalawi, H.; Ashiq, M.G.B.; Haq, B.U.; Mahmood, Q. A new lead free double perovskites K2Ti(Cl/Br)6; a promising materials for optoelectronic and transport properties; probed by DFT. Mater. Chem. Phys. 2021, 264, 124435. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Sun, Q.; Dong, J.; Lu, Y.; Zhang, B.; Jie, W. Lead free halide perovskite Cs3Bi2I9 bulk crystals grown by a low temperature solution method. CrystEngComm 2018, 20, 4935–4941. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Morrison, C.A.; Wu, W.; Han, H.; Robertson, N. Lead-free pseudo-three-dimensional organic–inorganic iodobismuthates for photovoltaic applications. Sustain. Energy Fuels 2017, 1, 308–316. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Brock, P. Structure and Optical Properties of Several Organic−Inorganic Hybrids Containing Corner-Sharing Chains of Bismuth Iodide Octahedra. Inorg. Chem. 2001, 40, 2096–2104. [Google Scholar] [CrossRef]
- Alsalamah, I.M.; Shaari, A.; Alsaif, N.A.M.; Yamusa, S.A.; Lakshminarayana, G.; Rekik, N. Exploring the structural properties and the optoelectronic features of RbPbX3 (X = Cl, F) perovskite crystals for solar cells solicitations: Showcasing the DFT predictions. Chem. Phys. 2023, 573, 111978. [Google Scholar] [CrossRef]
- Ju, D.; Jiang, X.; Xiao, H.; Chen, X.; Hu, X.; Tao, X. Narrow band gap and high mobility of lead-free perovskite single crystal Sn-doped MA3Sb2I9. J. Mater. Chem. A 2018, 6, 20753–20759. [Google Scholar] [CrossRef]
- Amgar, D.; Binyamin, T.; Uvarov, V.; Etgar, L. Near ultra-violet to mid-visible band gap tuning of mixed cation RbxCs1−xPbX3 (X = Cl or Br) perovskite nanoparticles. Nanoscale 2018, 10, 6060–6068. [Google Scholar] [CrossRef] [PubMed]
- Jayan, K.D. Enhancement of efficiency of (FA)2BiCuI6 based perovskite solar cells with inorganic transport layers. Opt. Mater. 2021, 122, 111671. [Google Scholar] [CrossRef]
- Kshirsagar, B.; Jaykhedkar, N.; Jain, K.; Kishor, S.; Shah, V.; Ramaniah, L.M.; Tiwari, S. Green CsSnX3 (X = Cl, Br, I)-Derived Quantum Dots for Photovoltaic Applications: First-Principles Investigations. J. Phys. Chem. C 2021, 125, 2592–2606. [Google Scholar] [CrossRef]
- El-Mellouhi, F.; Bentria, E.T.; Marzouk, A.; Rashkeev, S.N.; Kais, S.; Alharbi, F.H. Hydrogen bonding: A mechanism for tuning electronic and optical properties of hybrid organic–inorganic frameworks. npj Comput. Mater. 2016, 2, 16035. [Google Scholar] [CrossRef]
- Farshad Akhtarianfar, S.; Shojaei, S.; Khameneh Asl, S. Organic cation rotation in HC(NH2)2PbI3 perovskite solar cells: DFT & DOE approach. Sol. Energy 2021, 220, 70–79. [Google Scholar] [CrossRef]
- Chen, R.; Liu, C.; Chen, Y.; Ye, C.; Chen, S.; Cheng, J.; Cao, S.; Wang, S.; Cui, A.; Hu, Z.; et al. Ferroelectric CsGeI3 Single Crystals with a Perovskite Structure Grown from Aqueous Solution. J. Phys. Chem. C 2023, 127, 635–641. [Google Scholar] [CrossRef]
- Jung, M.-H. Formation of cubic perovskite alloy containing the ammonium cation of 2D perovskite for high performance solar cells with improved stability. RSC Adv. 2021, 11, 32590–32603. [Google Scholar] [CrossRef]
- Mahmood, Q.; Nazir, G.; Bouzgarrou, S.; Aljameel, A.I.; Rehman, A.; Albalawi, H.; Ul Haq, B.; Ghrib, T.; Mera, A. Study of new lead-free double perovskites halides Tl2TiX6 (X = Cl, Br, I) for solar cells and renewable energy devices. J. Solid State Chem. 2022, 308, 122887. [Google Scholar] [CrossRef]
- Ishikawa, R.; Ueno, K.; Shirai, H. Improved efficiency of methylammonium-free perovskite thin film solar cells by fluorinated ammonium iodide treatment. Org. Electron. 2019, 78, 105596. [Google Scholar] [CrossRef]
- Berhe, T.A.; Tsai, M.-C.; Su, W.-N.; Hwang, B. CuPbX3 and AgPbX3 Inorganic Perovskites for Solar cell Applications. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Zheng, H.; Dai, J.; Duan, J.; Chen, F.; Zhu, G.; Wang, F.; Xu, C. Temperature-dependent photoluminescence properties of mixed-cation methylammonium–formamidium lead iodide [HC(NH2)2]x[CH3NH3]1−xPbI3 perovskite nanostructures. J. Mater. Chem. C 2017, 5, 12057–12061. [Google Scholar] [CrossRef]
- Robert, F. Service. Perovskite solar cells gear up to go commercial. Science 2016, 354, 1214–1215. [Google Scholar] [CrossRef]
- Koh, T.M.; Fu, K.; Fang, Y.; Chen, S.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G.; Boix, P.P.; Baikie, T. Formamidinium-Containing Metal-Halide: An Alternative Material for Near-IR Absorption Perovskite Solar Cells. J. Phys. Chem. C 2014, 118, 16458–16462. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Pang, S.; Hu, H.; Zhang, J.; Lv, S.; Yu, Y.; Wei, F.; Qin, T.; Xu, H.; Liu, Z.; Cui, G. NH2CH=NH2PbI3: An Alternative Organolead Iodide Perovskite Sensitizer for Mesoscopic Solar Cells. Chem. Mater. 2014, 26, 1485–1491. [Google Scholar] [CrossRef]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Mixed-organic-cation perovskite photovoltaics for enhanced solar-light harvesting. Angew. Chem. 2014, 53, 3151–3157. [Google Scholar] [CrossRef]
- Lee, J.W.; Seol, D.J.; Cho, A.N.; Park, N.G. High-efficiency perovskite solar cells based on the black polymorph of HC(NH2)2 PbI3. Adv. Mater. 2014, 26, 4991–4998. [Google Scholar] [CrossRef]
- Hu, M.; Liu, L.; Mei, A.; Yang, Y.; Liu, T.; Han, H. Efficient hole-conductor-free, fully printable mesoscopic perovskite solar cells with a broad light harvester NH2CH=NH2PbI3. J. Mater. Chem. A 2014, 2, 17115–17121. [Google Scholar] [CrossRef]
- Binek, A.; Hanusch, F.C.; Docampo, P.; Bein, T. Stabilization of the Trigonal High-Temperature Phase of Formamidinium Lead Iodide. J. Phys. Chem. Lett. 2015, 6, 1249–1253. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.H.; Kim, H.S.; Seo, S.W.; Cho, S.M.; Park, N.G. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.; Park, N.G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Barnes, P.W.; Woodward, P.M. Structure prediction of ordered and disordered multiple octahedral cation perovskites using SPuDS. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J.; Kennedy, B.J.; Woodward, P.M. Ordered double perovskites—A group-theoretical analysis. Acta Crystallogr. Sect. B Struct. Sci. 2003, 59, 463–471. [Google Scholar] [CrossRef]
- Iwanaga, D.; Inaguma, Y.; Itoh, M. Structure and magnetic properties of Sr2NiAO6 (A. = W, Te). Mater. Res. Bull. 2000, 35, 449–457. [Google Scholar] [CrossRef]
- Chakraverty, S.; Ohtomo, A.; Okuyama, D.; Saito, M.; Okude, M.; Kumai, R.; Arima, T.; Tokura, Y.; Tsukimoto, S.; Ikuhara, Y.; et al. Ferrimagnetism and spontaneous ordering of transition metals in double perovskite La2CrFeO6 films. Phys. Rev. B 2011, 84, 064436. [Google Scholar] [CrossRef]
- Battle, P.D.; Gibb, T.C.; Jones, C.W.; Studer, F. Spin-glass behavior in Sr2FeRuO6 and BaLaNiRuO6: A comparison with antiferromagnetic BaLaZnRuO6. J. Solid State Chem. 1989, 78, 281–293. [Google Scholar] [CrossRef]
- Anderson, M.T.; Greenwood, K.B.; Taylor, G.A.; Poeppelmeier, K.R. B-cation arrangements in double perovskites. Prog. Solid State Chem. 1993, 22, 197–233. [Google Scholar] [CrossRef]
- Barnes, P.W.; Lufaso, M.W.; Woodward, P.M. Structure Determination of A2M3+TaO6 and A2M3+NbO6 Ordered Perovskites: Octahedr vskites: Octahedral Tilting and Pseudosymmetr al Tilting and Pseudosymmetry. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Woodward, P.; Hoffmann, R.D.; Sleight, A.W. Order-disorder in A2M3+M5+O6 perovskites. J. Mater. Res. 1994, 9, 2118–2127. [Google Scholar] [CrossRef]
- Davies, P.K.; Wu, H.; Borisevich, A.Y.; Molodetsky, I.E.; Farber, L. Crystal Chemistry of Complex Perovskites: New Cation-Ordered Dielectric Oxides. Annu. Rev. Mater. Res 2008, 38, 369–401. [Google Scholar] [CrossRef]
- King, G.; Woodward, P.M. Cation ordering in perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar] [CrossRef]
- Selivanov, N.; Samsonova, A.; Kevorkyants, R.; Krauklis, I.; Stroganov, B.; Triantafyllou Rundell, M.; Bahnemann, D.; Stoumpos, C.; Emeline, A.; Kapitonov, Y. Hybrid Organic–Inorganic Halide Post-Perovskite 3-Cyanopyridinium Lead Tribromide for Optoelectronic Applications. Adv. Funct. Mater. 2021, 31, 2102338. [Google Scholar] [CrossRef]
- Alaei, A.; Circelli, A.; Yuan, Y.; Yang, Y.; Lee, S. Polymorphism in Metal Halide Perovskites. Mater. Adv. 2020, 2, 47–63. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Tang, J.; Xiao, Z. Material exploration via designing spatial arrangement of octahedral units: A case study of lead halide perovskites. Front. Optoelectron. 2021, 14, 252–259. [Google Scholar] [CrossRef]
- Muller, O.; Roy, R. The Major Ternary Structural Families; Springer: New York, NY, USA, 1974. [Google Scholar]
- Bisquert, J.; Fabregat-Santiago, F.; Mora-Seró, I.; Garcia-Belmonte, G.; Barea, E.M.; Palomares, E. A review of recent results on electrochemical determination of the density of electronic states of nanostructured metal-oxide semiconductors and organic hole conductors. Inorganica Chim. Acta 2008, 361, 684–698. [Google Scholar] [CrossRef]
- Pilania, G.; Balachandran, P.V.; Kim, C.; Lookman, T. Finding New Perovskite Halides via Machine Learning. Front. Mater. 2016, 3, 19. [Google Scholar] [CrossRef]
- Adonin, S.A.; Frolova, L.A.; Sokolov, M.N.; Shilov, G.V.; Korchagin, D.V.; Fedin, V.P.; Aldoshin, S.M.; Stevenson, K.J.; Troshin, P.A. Antimony (V) Complex Halides: Lead-Free Perovskite-Like Materials for Hybrid Solar Cells. Adv. Energy Mater. 2017, 8, 1701140. [Google Scholar] [CrossRef]
- Lawton, S.L.; Jacobson, R.A. Crystal Structure Studies of Some Unusual Antimony Bromide Salts1. J. Am. Chem. Soc. 1966, 88, 616–618. [Google Scholar] [CrossRef]
- Lawton, S.L.; Jacobson, R.A. Crystal structure of quinuclidinium dodecarbromoantimon (III) antimonate (V)-2-dibromine, (C7H13NH)4Sb(III)Sb(V)Br12.2Br2. Inorg. Chem. 1971, 10, 709–712. [Google Scholar] [CrossRef]
- Lawton, S.L.; Jacobson, R.A. Crystal structure of di-. alpha.-picolinium nonabromoantimonate (V). Inorg. Chem. 1968, 7, 2124–2134. [Google Scholar] [CrossRef]
- Lawton, S.L.; Jacobson, R.A. The Crystal Structure of Ammonium Hexabromoantimonate, (NH4)4SbIIISbVBr12. Inorg. Chem. 1966, 5, 743–749. [Google Scholar] [CrossRef]
- Lawton, S.L.; Jacobson, R.A. The Crystal Structure of Quinuclidinium Dodecabromoantimon(III)antimon(V)ate-2-Dibromine (C7H13NH)4SbIIISbVBr12 · 2Br2. Inorg. Chem. 1971, 10, 2813–2816. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, J. Layered Organic-Inorganic Hybrid Perovskites: Structure, Optical Properties, Film Preparation, Patterning and Templating Engineering. CrystEngComm. 2010, 12, 2646–2662. [Google Scholar] [CrossRef]
- Dohner, E.R.; Hoke, E.T.; Karunadasa, H.I. Self-Assembly of Broadband White-Light Emitters. J. Am. Chem. Soc. 2014, 136, 1718–1721. [Google Scholar] [CrossRef]
- Dohner, E.R.; Jaffe, A.; Bradshaw, L.R.; Karunadasa, H.I. Intrinsic White-Light Emission from Layered Hybrid Perovskites. J. Am. Chem. Soc. 2014, 136, 13154–13157. [Google Scholar] [CrossRef]
- Lee, B.; Stoumpos, C.C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P. Air-Stable Molecular Semiconducting Iodosalts for Solar Cell Applications: Cs2SnI6 as a Hole Conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. Int. Ed. 2014, 126, 11232–11235. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.E.; Kurchin, R.C.; Hoye, R.L.; Poindexter, J.R.; Wilson, M.W.; Sulekar, S.; Lenahan, F.; Yen, P.X.; Stevanovic, V.; Nino, J.C.; et al. Investigation of bismuth triiodide (BiI3) for photovoltaic applications. J. Phys. Chem. Lett. 2015, 6, 4297–4302. [Google Scholar] [CrossRef] [PubMed]
- Hahn, N.T.; Rettie, A.J.; Beal, S.K.; Fullon, R.R.; Mullins, C.B. n-BiSI thin films: Selenium doping and solar cell behavior. J. Phys. Chem. C 2012, 116, 24878–24886. [Google Scholar] [CrossRef]
- Sfaelou, S.; Raptis, D.; Dracopoulos, V.; Lianos, P. BiOI solar cells. RSC Adv. 2015, 5, 95813–95816. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X = Br, Cl): New visible light absorbing, lead-free halide perovskite semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, Z.; Jain, A.; Voznyy, O.; Kim, G.H.; Liu, M.; Quan, L.N.; García de Arquer, F.P.; Comin, R.; Fan, J.Z.; et al. Pure Cubic-Phase Hybrid Iodobismuthates AgBi2I7 for Thin-Film Photovoltaics. Angew. Chem. Int. Ed. 2016, 55, 9586–9590. [Google Scholar] [CrossRef] [PubMed]
- Hebig, C.; Kühn, I.; Flohre, J.; Kirchartz, T. Optoelectronic Properties of (CH3NH3)3Sb2I9 Thin Films for Photovoltaic Applications. ACS Energy Lett. 2016, 1, 309–314. [Google Scholar] [CrossRef]
- Huang, X.; Huang, S.; Biswas, P.; Mishra, R. Band gap insensitivity to large chemical pressures in ternary bismuth iodides for photovoltaic applications. J. Phys. Chem. C. 2016, 120, 28924–28932. [Google Scholar] [CrossRef]
- Lyu, M.; Yun, J.H.; Cai, M.; Jiao, Y.; Bernhardt, P.V.; Zhang, M.; Wang, Q.; Du, A.; Wang, H.; Liu, G.; et al. Organic-inorganic bismuth (III)-based material: A lead-free, air-stable and solution-processable light-absorber beyond organolead perovskites. Nano Res. 2016, 9, 692–702. [Google Scholar] [CrossRef]
- Zhao, X.G.; Yang, J.H.; Fu, Y.; Yang, D.; Xu, Q.; Yu, L.; Wei, S.H.; Zhang, L. Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J. Am. Chem. Soc. 2017, 139, 2630–2638. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef]
- Xiao, Z.; Du, K.Z.; Meng, W.; Wang, J.; Mitzi, D.B.; Yan, Y. Intrinsic instability of Cs2In(I)M(III)X6 (M = Bi, Sb; X = halogen) double perovskites: A combined density functional theory and experimental study. J. Am. Chem. Soc. 2017, 139, 6054–6057. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, W.; Wang, J.; Mitzi, D.B.; Yan, Y. Searching for promising new perovskite-based photovoltaic absorbers: The importance of electronic dimensionality. Mater. Horiz. 2017, 4, 206–216. [Google Scholar] [CrossRef]
- Amirbekova, G.; Abdykadyrov, B.; Takibaev, N.Z. Ferroelectricity of CsRbPb2I6 Superlattice; Physics Department, al-Farabi Kazakh National University: Almaty, Kazakhstan, 2017. [Google Scholar]
- Abdykadyrov, B. ANM Abstracts. 2015. Available online: https://pps.kaznu.kz/ru/Main/FileShow2/40200/114/3/3417/2015// (accessed on 22 July 2015).
- Uberuaga, B.P. Cation ordering and effect of biaxial strain in double perovskite CsRbCaZnCl6. J. Appl. Phys. 2015, 117, 114103. [Google Scholar]
- Stamplecoskie, K.G.; Manser, J.S.; Kamat, P.V. Dual Nature of the Excited State in Organic−Inorganic Lead Halide Perovskites. Energy Environ. Sci. 2015, 8, 208–215. [Google Scholar] [CrossRef]
- Manser, J.S.; Saidaminov, M.I.; Christians, J.A.; Bakr, O.M.; Kamat, P.V. Making and Breaking of Lead Halide Perovskites. Acc. Chem. Res. 2016, 49, 330–338. [Google Scholar] [CrossRef]
- Hancock, R.D.; Shaikjee, M.S.; Dobson, S.M.; Boeyens, J.C. The Stereochemical activity or non-activity of the ‘Inert’ pair of electrons on lead(II) in relation to its complex stability and structural properties. Some considerations in ligand design. Inorg. Chim. Acta 1988, 154, 229–238. [Google Scholar] [CrossRef]
- Pyykko, P. Relativistic effects in structural chemistry. Chem. Rev. 1988, 88, 563–594. [Google Scholar] [CrossRef]
- Schwerdtfeger, P.; Heath, G.A.; Dolg, M.; Bennett, M.A. Low valencies and periodic trends in heavy element chemistry. A theoretical study of relativistic effects and electron correlation effects in Group 13 and Period 6 hydrides and halides. J. Am. Chem. Soc. 1992, 114, 7518–7527. [Google Scholar] [CrossRef]
- Andrés, A.; Bencini, A.; Carachalios, A.; Bianchi, A.; Dapporto, P.; García-España, E.; Paoletti, P.; Paoli, P. Interaction of lead(II) with highly-dentate linear and cyclic polyamines. J. Chem. Soc. Dalton Trans. 1993, 3507–3513. [Google Scholar] [CrossRef]
- Parr, J. Some recent coordination chemistry of lead(ll). Polyhedron 1997, 16, 551–566. [Google Scholar] [CrossRef]
- Chung, J.-H.; Min, B.-K.; Kim, Y.K.; Kim, K.-H.; Kwon, T.-Y. From discrete to infinite 3D coordination polymer: Sonochemical syntheses and structural characterization of a new nano flower lead (II) coordination compound. J. Mol. Struct. 2014, 1076, 698–703. [Google Scholar] [CrossRef]
- Platas-Iglesias, C.; Esteban-Gómez, D.; Enriquez-Perez, T.; Avecilla, F.; A de Blas Rodriguez-Blas, T. Lead(II) Thiocyanate Complexes with Bibracchial Lariat Ethers: An X-ray and DFT Study. Inorg. Chem. 2005, 44, 2224–2233. [Google Scholar] [CrossRef]
- Nugent, J.W.; Lee, H.; Reibenspies, J.H.; Hancock, R.D. Spectroscopic, structural, and thermodynamic aspects of the stereochemically active lone pair on lead(II): Structure of the lead(II) dota complex. Polyhedron 2015, 91, 120–127. [Google Scholar] [CrossRef]
- Shimoni-Livny, L.; Glusker, J.P.; Bock, C.W. Lone Pair Functionality in Divalent Lead Compounds. Inorg. Chem. 1998, 37, 1853–1867. [Google Scholar]
- Walsh, A.; Watson, G.W. The origin of the stereochemically active Pb(II) lone pair: DFT calculations on PbO and PbS. J. Solid State Chem. 2005, 178, 1422–1428. [Google Scholar] [CrossRef]
- Hoffman, J.B.; Schleper, A.L.; Kamat, P.V. Transformation of sintered CsPbBr3 nanocrystals to cubic CsPbI3 and gradient CsPbBrxI3−x through halide exchange. J. Am. Chem. Soc. 2016, 138, 8603–8611. [Google Scholar] [CrossRef]
- Li, G.; Ho, J.Y.-L.; Wong, M.; Kwok, H.S. Reversible anion exchange reaction in solid halide perovskites and its implication in photovoltaics. J. Phys. Chem. C 2015, 119, 26883–26888. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef]
- Markov, I.V. Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth and Epitary; World Scientific: Singapore, 1995; ISBN 9810215312. ISBN 9810221770. [Google Scholar]
- Yang, S.; Zheng, Y.C.; Hou, Y.; Chen, X.; Chen, Y.; Wang, Y.; Zhao, H.; Yang, H.G. Formation Mechanism of Freestanding CH3NH3PbI3 Functional Crystals: In Situ Transformation vs Dissolution–Crystallization. Chem. Mater. 2014, 26, 6705–6710. [Google Scholar] [CrossRef]
- Horváth, O.; Mikó, I. Spectra, equilibrium and photoredox chemistry of tri- and tetraiodoplumbate (II) complexes in acetonitrile. J. Photochem. Photobiol. A 1998, 114, 95–101. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.N.; Cheng, J.H.; Lin, M.H.; Ibrahim, K.B.; Kahsay, A.W.; Lin Li, C.; Tripathi, A.M.; Tang, M.T.; Hwang, B.J. Scalable Synthesis of Micron Size Crystals of CH3NH3PbI3 at Room Temperature in Acetonitrile via Rapid Reactive Crystallization. ChemistrySelect 2020, 5, 3266–3271. [Google Scholar] [CrossRef]
- van Gorkom, B.T.; van der Pol, T.P.A.; Datta, K.; Wienk, M.M.; Janssen, R.A.J. Revealing defective interfaces in perovskite solar cells from highly sensitive sub-bandgap photocurrent spectroscopy using optical cavities. Nat. Commun. 2022, 13, 349. [Google Scholar] [CrossRef]
- Zeiske, S.; Sandberg, O.J.; Zarrabi, N.; Wolff, C.M.; Raoufi, M.; Peña-Camargo, F.; Gutierrez-Partida, E.; Meredith, P.; Stolterfoht, M.; Armin, A. Static Disorder in Lead Halide Perovskites. J. Phys. Chem. Lett. 2022, 13, 7280–7285. [Google Scholar] [CrossRef]
- Yan, J.; Li, H.; Aldamasy, M.H.; Frasca, C.; Abate, A.; Zhao, K.; Hu, Y. Advances in the Synthesis of Halide Perovskite Single Crystals for Optoelectronic Applications. Chem. Mater. 2023, 35, 2683–2712. [Google Scholar] [CrossRef]
- Fateev, S.A.; Petrov, A.A.; Ordinartsev, A.A.; Grishko, A.Y.; Goodilin, E.A.; Tarasov, A.B. Universal Strategy of 3D and 2D Hybrid Perovskites Single Crystal Growth via In Situ Solvent Conversion. Chem. Mater. 2020, 32, 9805–9812. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, B.; Yang, H.; Boschloo, G.; Johansson, E.M.J. Solvent Engineering of Perovskite Crystallization for High Band Gap FAPbBr3 Perovskite Solar Cells Prepared in Ambient Condition. ACS Appl. Energy Mater. 2023, 6, 7102–7108. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, S. Composition Engineering of Perovskite Single Crystals for High-Performance Optoelectronics. Adv. Funct. Mater. 2023, 33, 2210335. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Kovalenko, A.; Forés, S.M.; Aranda, C.; Guerrero, A. Coordination Chemistry Dictates the Structural Defects in Lead Halide Perovskites. ChemPhysChem 2016, 17, 2795–2798. [Google Scholar] [CrossRef]
- Brivio, F.; Butler, K.T.; Walsh, A.; van Schilfgaarde, M. Relativistic Quasiparticle Self-Consistent Electronic Structure of Hybrid Halide Perovskite Photovoltaic Absorbers. Phys. Rev. B: Condens. Matter Mater. Phys. 2014, 89, 155204. [Google Scholar] [CrossRef]
- Kepenekian, M.; Robles, R.; Katan, C.; Sapori, D.; Pedesseau, L.; Even, J. Rashba and Dresselhaus Effects in Hybrid Organic−Inorganic Perovskites: From Basics to Devices. ACS Nano 2015, 9, 11557–11567. [Google Scholar] [CrossRef]
- Kim, M.; Im, J.; Freeman, A.J.; Ihm, J.; Jin, H. Switchable S = 1/2 and J = 1/2 Rashba Bands in Ferroelectric Halide Perovskites. Proc. Natl. Acad. Sci. USA 2014, 111, 6900–6904. [Google Scholar] [CrossRef]
- Even, J.P. Guide to Symmetry Properties of the Reference Cubic Structure of 3D All-Inorganic and Hybrid Perovskites. J. Phys. Chem. Lett. 2015, 6, 2238–2242. [Google Scholar] [CrossRef]
- Amat, A.; Mosconi, E.; Ronca, E.; Quarti, C.; Umari, P.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. Cation-Induced Band-Gap Tuning in Organohalide Perovskites: Interplay of Spin-Orbit Coupling and Octahedra Tilting. Nano Lett. 2014, 14, 3608–3616. [Google Scholar] [CrossRef]
- Stroppa, A.; Di Sante, D.; Barone, P.; Bokdam, M.; Kresse, G.; Franchini, C.; Whangbo, M.-H.; Picozzi, S. Tunable Ferroelectric Polarization and Its Interplay with Spin−orbit Coupling in Tin Iodide Perovskites. Nat. Commun. 2014, 5, 5900. [Google Scholar] [CrossRef]
- Koutselas, I.B.; Ducasse, L.; Papavassiliou, G.C. Electronic Properties of Three- and Low-Dimensional Semiconducting Materials with Pb Halide and Sn Halide Units. J. Phys. Condens. Matter. 1996, 8, 1217–1227. [Google Scholar] [CrossRef]
- Chiarella, F.; Zappettini, A.; Licci, F.; Borriello, I.; Cantele, G.; Ninno, D.; Cassinese, A.; Vaglio, R. Combined Experimental and Theoretical Investigation of Optical, Structural, and Electronic Properties of CH3NH3SnX3 Thin Films (X = Cl, Br). Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 045129. [Google Scholar]
- Umebayashi, T.; Asai, K.; Kondo, T.; Nakao, A. Electronic Structures of Lead Iodide Based Low-Dimensional Crystals. Phys. Rev. B: Condens. Matter Mater. Phys. 2003, 67, 155405. [Google Scholar] [CrossRef]
- Brivio, F.; Walker, A.B.; Walsh, A. Structural and Electronic Properties of Hybrid Perovskites for High-Efficiency Thin-Film Photovoltaics from First-Principles. APL Mater. 2013, 1, 042111. [Google Scholar] [CrossRef]
- Chang, Y.H.; Park, C.H.; Matsuishi, K. First-Principles Study of the Structural and the Electronic Properties of the Lead-Halide-Based Inorganic-Organic Perovskites (CH3NH3)PbX3 and CsPbX3 (X = Cl, Br, I). J. Korean Phys. Soc. 2004, 44, 889–893. [Google Scholar]
- Borriello, I.; Cantele, G.; Ninno, D. Ab Initio Investigation of Hybrid Organic-Inorganic Perovskites Based on Tin Halides. Phys. Rev. B: Condens. Matter Mater. Phys. 2008, 77, 235214. [Google Scholar] [CrossRef]
- Vincent, B.R.; Robertson, K.N.; Cameron, T.S.; Knop, O. Alkylammonium Lead Halides. Part 1. Isolated PbI64− Ions in (CH3NH3)4PbI6·2H2O. Can. J. Chem. 1987, 65, 1042–1046. [Google Scholar] [CrossRef]
- García-Martín, S.; Urones-Garrote, E.; Knapp, M.C.; King, G.; Woodward, P.M. Structural complexity in AA′MM′O6 Perovskites. A Transmission Electron Microscopy Study. Mater. Res. Soc. Symp. Proc. 2009, 1148, 1148-PP15-05. [Google Scholar]
- Kobayashi, K.-I.; Kimura, T.; Sawada, H.; Terakura, K.; Tokura, Y. Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature 1998, 395, 677–680. [Google Scholar] [CrossRef]
- Ubic, R.; Subodh, G.; Sebastian, M.T.; Gout, D.; Proffen, T. Effective size of vacancies in the Sr1-3x/2CexTiO3 superstructure. Ceram. Trans. 2009, 204, 177–185. [Google Scholar]
- Brown, I.D. Recent Developments in the Methods and Applications of the Bond Valence Model. Chem. Rev. 2009, 109, 6858–6919. [Google Scholar]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef]
- Dittrich, T.; Awino, C.; Prajongtat, P.; Rech, B.; Lux-Steiner, M.C. Temperature Dependence of the Band Gap of CH3NH3PbI3 Stabilized with PMMA: A Modulated Surface Photovoltage Study. J. Phys. Chem. C 2015, 119, 23968–23972. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond; Cornell University Press: Ithaca, NY, USA, 1960. [Google Scholar]
- Hao, F.; Stoumpos, C.C.; Chang, R.P.; Kanatzidis, M.G. Anomalous Band Gap Behavior in Mixed Sn and Pb Perovskites Enables Broadening of Absorption Spectrum in Solar Cells. J. Am. Chem. Soc. 2014, 136, 8094–8099. [Google Scholar] [CrossRef]
- Albright, T.A.; Burdett, J.K.; Whangbo, M.H. Orbital Interactions in Chemistry, 2nd ed.; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Sabba, D.; Mulmudi, H.K.; Prabhakar, R.R.; Krishnamoorthy, T.; Baikie, T.; Boix, P.P.; Mhaisalkar, S.; Mathews, N. Impact of Anionic Br– Substitution on Open Circuit Voltage in Lead Free Perovskite (CsSnI3−xBrx) Solar Cells. J. Phys. Chem. C 2015, 119, 1763–1767. [Google Scholar] [CrossRef]
- Goyal, A.; McKechnie, S.; Pashov, D.; Tumas, W.; van Schilfgaarde, M.; Stevanović, V. Origin of Pronounced Nonlinear Band Gap Behavior in Lead–Tin Hybrid Perovskite Alloys. Chem. Mater. 2018, 30, 3920–3928. [Google Scholar] [CrossRef]
- Chung, I.; Song, J.-H.; Im, J.; Androulakis, J.; Malliakas, C.D.; Li, H.; Freeman, A.J.; Kenney, J.T.; Kanatzidis, M.G. CsSnI3: Semiconductor or Metal? High Electrical Conductivity and Strong Near-Infrared Photoluminescence from a Single Material. High Hole Mobility and Phase-Transitions. J. Am. Chem. Soc. 2012, 134, 8579–8587. [Google Scholar] [CrossRef]
- Bernal, C.; Yang, K. First-principles hybrid functional study of the organic-inorganic perovskites CH3NH3SnBr3 and CH3NH3SnI3. J. Phys. Chem. C 2014, 118, 24383–24388. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Lambrecht, W.R.L. Electronic band structure, phonons, and exciton binding energies of halide perovskites CsSnCl3, CsSnBr3, and CsSnI3. Phys. Rev. B 2013, 88, 165203. [Google Scholar] [CrossRef]
- Iftikhar, F.J.; Wali, Q.; Yang, S.; Iqbal, Y.; Jose, R.; Munir, S.; Gondal, I.A.; Khan, M.E. Structural and optoelectronic properties of hybrid halide perovskites for solar cells. Org. Electron. 2021, 91, 106077. [Google Scholar] [CrossRef]
- Kim, T.; Park, B. Ambipolar Charge Carrier Transport Properties at the S-Benzyl-l-cysteine-Induced 2D/3D Halide Perovskite Interface. Chem. Mater. 2024, 36, 675–681. [Google Scholar] [CrossRef]
- Giorgi, G.; Yamashita, K. Organic–inorganic halide perovskites: An ambipolar class of materials with enhanced photovoltaic performances. J. Mater. Chem. A 2015, 3, 8981–8991. [Google Scholar] [CrossRef]
- He, C.; Liu, X. The rise of halide perovskite semiconductors. Light Sci. Appl. 2023, 12, 15. [Google Scholar] [CrossRef]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, L.; Ghimire, S.; Subrahmanyam, C.; Miyasaka, T.; Biju, V. Synthesis, optoelectronic properties and applications of halide perovskites. Chem. Soc. Rev. 2020, 49, 2869–2885. [Google Scholar] [CrossRef]
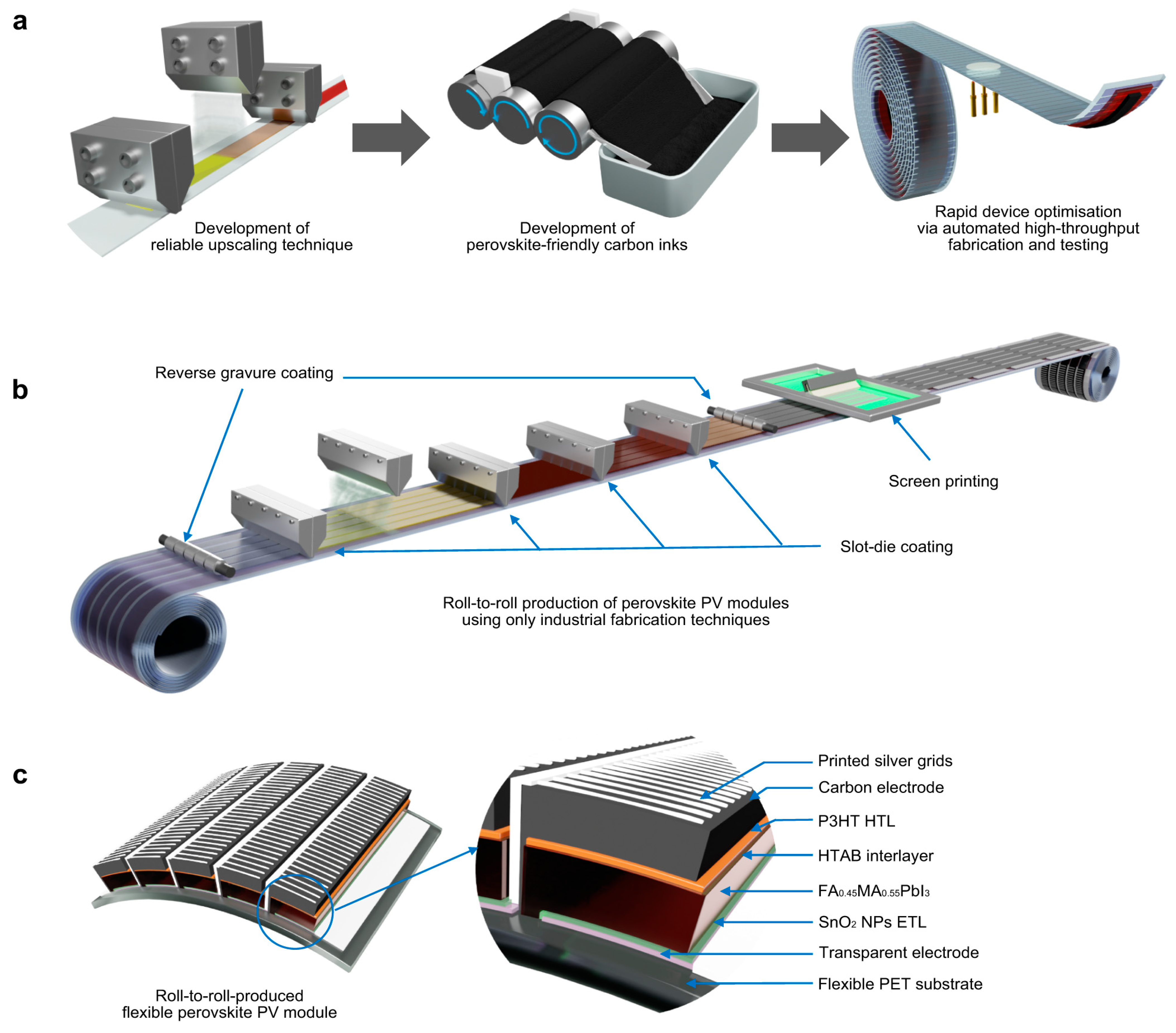
- Weerasinghe, H.C.; Macadam, N.; Kim, J.-E.; Sutherland, L.J.; Angmo, D.; Ng, L.W.T.; Scully, A.D.; Glenn, F.; Chantler, R.; Chang, N.L.; et al. The first demonstration of entirely roll-to-roll fabricated perovskite solar cell modules under ambient room conditions. Nat. Commun. 2024, 15, 1656. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-S.; Hwang, K.; Heo, Y.-J.; Kim, J.-E.; Vak, D.; Kim, D.-Y. Progress in Scalable Coating and Roll-to-Roll Compatible Printing Processes of Perovskite Solar Cells toward Realization of Commercialization. Adv. Opt. Mater. 2018, 6, 1701182. [Google Scholar] [CrossRef]
- Gong, C.; Tong, S.; Huang, K.; Li, H.; Huang, H.; Zhang, J.; Yang, J. Flexible Planar Heterojunction Perovskite Solar Cells Fabricated via Sequential Roll-to-Roll Microgravure Printing and Slot-Die Coating Deposition. Sol. RRL 2020, 4, 1900204. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Yang, T.-Y.; Suhonen, R.; Välimäki, M.; Maaninen, T.; Kemppainen, A.; Jeon, N.J.; Seo, J. Gravure-Printed Flexible Perovskite Solar Cells: Toward Roll-to-Roll Manufacturing. Adv. Sci. 2019, 6, 1802094. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, X.; Lei, H.; Ke, W.; Chen, C.; Chen, Z.; Yang, G.; Fang, G. Highly Efficient and Stable Planar Perovskite Solar Cells With Large-Scale Manufacture of E-Beam Evaporated SnO2 Toward Commercialization. Sol. RRL 2017, 1, 1700118. [Google Scholar] [CrossRef]
- Li, Z.; Li, P.; Chen, G.; Cheng, Y.; Pi, X.; Yu, X.; Yang, D.; Han, L.; Zhang, Y.; Song, Y. Ink Engineering of Inkjet Printing Perovskite. ACS Appl. Mater. Interfaces 2020, 12, 39082–39091. [Google Scholar] [CrossRef]
- Verma, A.; Martineau, D.; Abdolhosseinzadeh, S.; Heier, J.; Nüesch, F. Inkjet printed mesoscopic perovskite solar cells with custom design capability. Mater. Adv. 2020, 1, 153–160. [Google Scholar] [CrossRef]
- Shan, C.; Wang, Z.; Wang, Z.; Wang, T.; Luo, D.; Wang, K.; Sun, X.W.; Kyaw, A.K.K. Screen printing strategy for fabricating flexible crystallized perovskite nanocomposite patterns with high photoluminescence. Flex. Print. Electron. 2022, 7, 015010. [Google Scholar] [CrossRef]
- Vescio, G.; Sanchez-Diaz, J.; Frieiro, J.L.; Sánchez, R.S.; Hernández, S.; Cirera, A.; Mora-Seró, I.; Garrido, B. 2D PEA2SnI4 Inkjet-Printed Halide Perovskite LEDs on Rigid and Flexible Substrates. ACS Energy Lett. 2022, 7, 3653–3655. [Google Scholar] [CrossRef]
- Richmond, D.; McCormick, M.; Ekanayaka, T.K.; Teeter, J.D.; Swanson, B.L.; Benker, N.; Hao, G.; Sikich, S.; Enders, A.; Sinitskii, A.; et al. Inkjet Printing All Inorganic Halide Perovskite Inks for Photovoltaic Applications. J. Vis. Exp. 2019, e58760. [Google Scholar] [CrossRef]
- Chen, C.; Ran, C.; Yao, Q.; Wang, J.; Guo, C.; Gu, L.; Han, H.; Wang, X.; Chao, L.; Xia, Y.; et al. Screen-Printing Technology for Scale Manufacturing of Perovskite Solar Cells. Adv. Sci. 2023, 10, 2303992. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Kanatzidis, M.G. The Renaissance of Halide Perovskites and Their Evolution as Emerging Semiconductors. Acc. Chem. Res. 2015, 48, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Laska, M.; Krzemińska, Z.; Kluczyk-Korch, K.; Schaadt, D.; Popko, E.; Jacak, W.A.; Jacak, J.E. Metallization of solar cells, exciton channel of plasmon photovoltaic effect in perovskite cells. Nano Energy 2020, 75, 104751. [Google Scholar] [CrossRef]
- Jacak, J.E.; Jacak, W.A. Routes for Metallization of Perovskite Solar Cells. Materials 2022, 15, 2254. [Google Scholar] [CrossRef]
- Ren, M.; Fang, L.; Zhang, Y.; Eickemeyer, F.T.; Yuan, Y.; Zakeeruddin, S.M.; Grätzel, M.; Wang, P. Durable Perovskite Solar Cells with 24.5% Average Efficiency: The Role of Rigid Conjugated Core in Molecular Semiconductors. Adv. Mater. 2024, e2403403. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Lim, S.; Yantara, N.; Liu, X.; Sabba, D.; Graetzel, M.; Mhaisalkar, S.; Sum, T. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 2014, 13, 476–480. [Google Scholar] [CrossRef]
- Tan, Z.-K.M.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Chin, X.Y.; Cortecchia, D.; Yin, J.; Bruno, A.; Soci, C. Lead iodide perovskite light-emitting field-effect transistor. Nat. Commun. 2015, 6, 7383. [Google Scholar] [CrossRef]
- Huskinson, B.; Rugolo, J.; Mondal, S.K.; Aziz, M.J. A high power density, high e ciency hydrogen chlorine regenerative fuel cell with a low precious metal content catalyst. Energy Environ. Sci. 2012, 5, 8690–8698. [Google Scholar] [CrossRef]
- Taylor, G.R.; Butler, M. A comparison of the virucidal properties of chlorine, chlorine dioxide, bromine chloride and iodine. J. Hyg. 1982, 89, 321–328. [Google Scholar] [CrossRef]
- Yeo, R.S.; Chin, D.-T. A hydrogen bromine cell for energy storage applications. J. Electrochem. Soc. 1980, 127, 549–555. [Google Scholar] [CrossRef]
- Baglio, J.A.; Calabrese, G.S.; Kamieniecki, E.; Kershaw, R.; Kubiak, C.P.; Ricco, A.J.; Wold, A.; Wrighton, M.S.; Zoski, G.D. Characterization of n-type semiconducting tungsten disulfide photoanodes in aqueous and nonaqueous electrolyte solutions photo-oxidation of halides with high e ciency. J. Electrochem. Soc. 1982, 129, 1461–1472. [Google Scholar] [CrossRef]
- Singh, N.; Mubeen, S.; Lee, J.; Metiu, H.; Moskovits, M.; McFarland, E.W. Stable electrocatalysts for autonomous photoelectrolysis of hydrobromic acid using single-junction solar cells. Energy Environ. Sci. 2014, 7, 978–981. [Google Scholar] [CrossRef]
- Powers, D.C.; Hwang, S.J.; Zheng, S.-L.; Nocera, D.G. Halide-bridged binuclear HX-splitting catalysts. Inorg. Chem. 2014, 53, 9122–9128. [Google Scholar] [CrossRef]
- McKone, J.R.; Potash, R.A.; DiSalvo, F.J.; Abruña, H.D. Unassisted HI photoelectrolysis using n-WSe2 solar absorbers. Phys. Chem. Chem. Phys. 2015, 17, 13984–13991. [Google Scholar] [CrossRef]
- Ardo, S.; Park, S.H.; Warren, E.L.; Lewis, N.S. Unassisted solar-driven photoelectrosynthetic HI splitting using membrane-embedded Si microwire arrays. Energy Environ. Sci. 2015, 8, 1484–1492. [Google Scholar] [CrossRef]
- Park, S.; Chang, W.J.; Lee, C.W.; Park, S.; Ahn, H.Y.; Nam, K.T. Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution. Nat. Energy 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Kazim, S.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. Perovskite as Light Harvester: A Game Changer in Photovoltaics. Angew. Chem. Int. Ed. 2014, 53, 2812–2824. [Google Scholar] [CrossRef]
- He, M.; Zhang, D.; Wang, M.; Lin, C.; Lin, Z. High efficiency perovskite solar cells: From complex nanostructure to planar heterojunction. J. Mater. Chem. A 2014, 2, 5994–6003. [Google Scholar] [CrossRef]
- He, M.; Pang, X.; Liu, X.; Jiang, B.; He, Y.; Snaith, H.; Lin, Z. Monodisperse Dual-Functional Upconversion Nanoparticles Enabled Near-Infrared Organolead Halide Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 4280–4284. [Google Scholar] [CrossRef]
- Kim, Y.; Yassitepe, E.; Voznyy, O.; Comin, R.; Walters, G.; Gong, X.; Kanjanaboos, P.; Nogueira, A.F.; Sargent, E.H. Efficient Luminescence from Perovskite Quantum Dot Solids. ACS Appl. Mater. Interfaces 2015, 7, 25007–25013. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; Gonzaález-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Peárez-Prieto, J.J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yang, M.Z.; Chen, B.X.; Wang, X.D.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Vicente, N.; Garcia-Belmonte, G. Methylammonium Lead Bromide Perovskite Battery Anodes Reversibly Host High Li-ion Concentrations. J. Phys. Chem. Lett. 2017, 8, 1371–1374. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Dai, L. Efficiently photo-charging lithium-ion battery by perovskite solar cell. Nat. Comm. 2015, 6, 8103. [Google Scholar] [CrossRef]
- Wong, A.B.; Lai, M.; Eaton, S.W.; Yu, Y.; Lin, E.; Dou, L.; Fu, A.; Yang, P. Growth and Anion Exchange Conversion of CH3NH3PbX3 Nanorod Arrays for Light-Emitting Diodes. Nano Lett. 2015, 15, 5519–5524. [Google Scholar] [CrossRef]
- Pellet, N.; Teuscher, J.; Maier, J.; Grätzel, M. Transforming Hybrid Organic Inorganic Perovskites by Rapid Halide Exchange. Chem. Mater. 2015, 27, 2181–2188. [Google Scholar] [CrossRef]
- Jang, D.M.; Park, K.; Kim, D.H.; Park, J.; Shojaei, F.; Kang, H.S.; Ahn, J.-P.; Lee, J.W.; Song, J.K. Reversible Halide Exchange Reaction of Organometal Trihalide Perovskite Colloidal Nanocrystals for Full-Range Band Gap Tuning. Nano Lett. 2015, 15, 5191–5199. [Google Scholar] [CrossRef] [PubMed]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Ryan, K.L.; Pathade, L.; Cruz, K.J.; Zang, H.; Cotlet, M.; Maye, M.M. Using Perovskite Nanoparticles as Halide Reservoirs in Catalysis and as Spectrochemical Probes of Ions in Solution. ACS Nano 2016, 10, 5864–5872. [Google Scholar] [CrossRef]
- Starks, C.M.; Liotta, C. Phase Transfer Catalysis: Principles and Techniques; Academic Press: New York, NY, USA, 1978; pp. 13–56. [Google Scholar]
- Gribble, G.W. The Diversity of Naturally Occurring Organobromine Compounds. Chem. Soc. Rev. 1999, 28, 335–346. [Google Scholar] [CrossRef]
- De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Van Driessche, I.; Kovalenko, M.V.; Hens, Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Liu, H.; Zhang, X.; Xiao, J.; Kishor, R.; Sun, H.; Zhu, B.; Chen, G.; Gao, F.; Feng, X.; et al. Flexible Piezoelectric Nanocomposite Generators Based on Formamidinium Lead Halide Perovskite Nanoparticles. Adv. Funct. Mater. 2016, 26, 7708–7716. [Google Scholar] [CrossRef]
- Siegler, T.D.; Dawson, A.; Lobaccaro, P.; Ung, D.; Beck, M.E.; Nilsen, G.; Tinker, L.L. The Path to Perovskite Commercialization: A Perspective from the United States Solar Energy Technologies Office. ACS Energy Lett. 2022, 7, 1728–1734. [Google Scholar] [CrossRef]
- Qiu, L.; Ono, L.K.; Qi, Y. Advances and challenges to the commercialization of organic–inorganic halide perovskite solar cell technology. Mater. Today Energy 2018, 7, 169–189. [Google Scholar] [CrossRef]
- Soleimanioun, N.; Rani, M.; Singh, B.; Saini, G.S.S.; Tripathi, S.K. Potential replacement to lead: Alkali metal potassium and transition metal zinc in organo-metal halide perovskite materials. J. Alloys Compd. 2021, 861, 158207. [Google Scholar] [CrossRef]
- Murugan, S.; Lee, E.C. Recent Advances in the Synthesis and Application of Vacancy-Ordered Halide Double Perovskite Materials for Solar Cells: A Promising Alternative to Lead-Based Perovskites. Materials 2023, 16, 5275. [Google Scholar] [CrossRef]
- Chauhan, V.; Tripathi, D.; Singh, P.; Sharma, A.; Khanna, M.K.; Kumar, R.; Bhatnagar, R.; Kumar, T. Prospects for lead free perovskite for photovoltaic applications and biological impacts: Challenges and opportunities. Inorg. Chem. Commun. 2023, 157, 111421. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, X.; Chen, X.; Jiang, X.; Wang, H.; Zhang, G. Lead detoxification of edible fungi Auricularia auricula and Pleurotus ostreatus: The purification of the chelation substances and their effects on rats. Front. Nutr. 2023, 10, 1162110. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.; Meki, A.; Abd El-Mottaleb, N. Protective effect of green tea on lead-induced oxidative damage in rat’s blood and brain tissue homogenates. J. Physiol. Biochem. 2010, 66, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Narbad, A.; Chen, W. Dietary Strategies for the Treatment of Cadmium and Lead Toxicity. Nutrients 2015, 7, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.E. Chelation: Harnessing and enhancing heavy metal detoxification—A review. Sci. World J. 2013, 2013, 219840. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, Toxicity, and Detoxification in Plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Jang, T.-W.; Chae, H.-J.; Choi, W.-J.; Ha, M.-N.; Ye, B.-J.; Kim, B.-G.; Jeon, M.-J.; Kim, S.-Y.; Hong, Y.-S. Evaluation and management of lead exposure. Ann. Occup. Environ. Med. 2015, 27, 30. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ladi, N.; Shai, X.; Li, H.; Shen, Y.; Wang, M. Will the organic-inorganic hybrid halide lead perovskites be eliminated from the optoelectronic application? Nanoscale Adv. 2019, 1, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Jiang, J.; Yan, C.; Lin, Y.; Mortazavi, M.; Kaul, A.B.; Jiang, Q. Progress and Application of Halide Perovskite Materials for Solar Cells and Light Emitting Devices. Nanomaterials 2024, 14, 391. [Google Scholar] [CrossRef]
- Chen, H. Two-Step Sequential Deposition of Organometal Halide Perovskite for Photovoltaic Application. Adv. Funct. Mater. 2017, 27, 1605654. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Stranks, S.D.; You, F. Life cycle energy use and environmental implications of high-performance perovskite tandem solar cells. Sci. Adv. 2020, 6, eabb0055. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Bhattarai, S.; Sharma, K.; Madan, J.; Al-Mousoi, A.K.; Mohammed, M.K.A.; Hossain, M.K. Halide Composition Engineered a Non-Toxic Perovskite–Silicon Tandem Solar Cell with 30.7% Conversion Efficiency. ACS Appl. Electron. Mater. 2023, 5, 5303–5315. [Google Scholar] [CrossRef]
- Emery, Q.; Remec, M.; Paramasivam, G.; Janke, S.; Dagar, J.; Ulbrich, C.; Schlatmann, R.; Stannowski, B.; Unger, E.; Khenkin, M. Encapsulation and Outdoor Testing of Perovskite Solar Cells: Comparing Industrially Relevant Process with a Simplified Lab Procedure. ACS Appl. Mater. Interfaces 2022, 14, 5159–5167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ahmad, I.; Leung, T.; Lin, J.; Chen, W.; Liu, F.; Ng, A.M.C.; Zhang, Y.; Djurišić, A.B. Encapsulation and Stability Testing of Perovskite Solar Cells for Real Life Applications. ACS Mater. Au 2022, 2, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.K.; Gurusamy Thangavelu, S.A.; Venkataraj, S.; Krishnamoorthy, A. Materials, methods and strategies for encapsulation of perovskite solar cells: From past to present. Renew. Sustain. Energy Rev. 2021, 151, 111608. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, R.; Gao, J.; Wang, S.; Zhu, J.; Xiong, W.; Yuan, N.; Ding, J. A Facile Approach for the Encapsulation of Perovskite Solar Cells. Energies 2023, 16, 598. [Google Scholar] [CrossRef]
- Zhong, Q.; Cao, M.; Zhang, Q. Encapsulation of lead halide perovskite nanocrystals (NCs) at the single-particle level: Strategies and properties. Nanoscale 2021, 13, 19341–19351. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Yun, J.-H.; Chen, P.; Hao, M.; Wang, L. Addressing Toxicity of Lead: Progress and Applications of Low-Toxic Metal Halide Perovskites and Their Derivatives. Adv. Energy Mater. 2017, 7, 1602512. [Google Scholar] [CrossRef]
- Slavney, A.H.; Smaha, R.W.; Smith, I.C.; Jaffe, A.; Umeyama, D.; Karunadasa, H.I. Chemical Approaches to Addressing the Instability and Toxicity of Lead–Halide Perovskite Absorbers. Inorg. Chem. 2017, 56, 46–55. [Google Scholar] [CrossRef]
- Hu, H.; Dong, B.; Zhang, W. Low-toxic metal halide perovskites: Opportunities and future challenges. J. Mater. Chem. A 2017, 5, 11436–11449. [Google Scholar] [CrossRef]
- Rony, J.K.; Islam, M.; Saiduzzaman, M.; Hossain, K.M.; Alam, S.; Biswas, A.; Mia, M.H.; Ahmad, S.; Mitro, S.K. TlBX3 (B = Ge, Sn; X = Cl, Br, I): Promising non-toxic metal halide perovskites for scalable and affordable optoelectronics. J. Mater. Res. Technol. 2024, 29, 897–909. [Google Scholar] [CrossRef]
- Magliano, E.; Mariani, P.; Agresti, A.; Pescetelli, S.; Matteocci, F.; Taheri, B.; Cricenti, A.; Luce, M.; Di Carlo, A. Semitransparent Perovskite Solar Cells with Ultrathin Protective Buffer Layers. ACS Appl. Energy Mater. 2023, 6, 10340–10353. [Google Scholar] [CrossRef] [PubMed]
- Fangnon, A.; Dvorak, M.; Havu, V.; Todorović, M.; Li, J.; Rinke, P. Protective Coating Interfaces for Perovskite Solar Cell Materials: A First-Principles Study. ACS Appl. Mater. Interfaces 2022, 14, 12758–12765. [Google Scholar] [CrossRef] [PubMed]
- Koushik, D.; Verhees, W.J.H.; Kuang, Y.; Veenstra, S.; Zhang, D.; Verheijen, M.A.; Creatore, M.; Schropp, R.E.I. High-efficiency humidity-stable planar perovskite solar cells based on atomic layer architecture. Energy Environ. Sci. 2017, 10, 91–100. [Google Scholar] [CrossRef]
- Azad, S.S.; Keshavarzi, R.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Mohammadpoor-Baltork, I. Stability enhancement of perovskite solar cells using multifunctional inorganic materials with UV protective, self cleaning, and high wear resistance properties. Sci. Rep. 2024, 14, 6466. [Google Scholar] [CrossRef]
- Hosseinian Ahangharnejhad, R.; Song, Z.; Mariam, T.; Gardner, J.J.; Liyanage, G.K.; Almutawah, Z.S.; Anwar, B.M.M.; Junda, M.; Podraza, N.J.; Phillips, A.B.; et al. Protecting Perovskite Solar Cells against Moisture-Induced Degradation with Sputtered Inorganic Barrier Layers. ACS Appl. Energy Mater. 2021, 4, 7571–7578. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Mei, Y.; Dong, C.; Gangadharan, D.T.; Liu, K.; Wang, Z.; Qu, S.; Saidaminov, M.I.; Zhang, W.; et al. Blade-Coated Carbon Electrode Perovskite Solar Cells to Exceed 20% Efficiency Through Protective Buffer Layers. Adv. Funct. Mater. 2023, 33, 2301920. [Google Scholar] [CrossRef]
- Wang, Y.; Ran, H.; Zhao, Y.; Lu, Y.; Chen, X.; Tang, Y. Highly Stable Perovskite Solar Cells with Protective Layers Obtained via Interfacial Modification. Sol. RRL 2024, 8, 2301075. [Google Scholar] [CrossRef]
- Chu, Q.-Q.; Sun, Z.; Wang, D.; Cheng, B.; Wang, H.; Wong, C.-P.; Fang, B. Encapsulation: The path to commercialization of stable perovskite solar cells. Matter 2023, 6, 3838–3863. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, C.; Dai, J.; Zhang, Y.; Mao, R.; Chen, S.; Huang, J.; Zhu, J. Toward the Commercialization of Perovskite Solar Modules. Adv. Mater. 2024, 36, 2307357. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, T.; Xue, J.; Tong, J.; Zhu, K.; Yang, Y. Prospects for metal halide perovskite-based tandem solar cells. Nat. Photonics 2021, 15, 411–425. [Google Scholar] [CrossRef]
- Bi, H.; Liu, J.; Zhang, Z.; Wang, L.; Beresneviciute, R.; Tavgeniene, D.; Kapil, G.; Ding, C.; Baranwal, A.K.; Sahamir, S.R.; et al. All-Perovskite Tandem Solar Cells Approach 26.5% Efficiency by Employing Wide Bandgap Lead Perovskite Solar Cells with New Monomolecular Hole Transport Layer. ACS Energy Lett. 2023, 8, 3852–3859. [Google Scholar] [CrossRef]
- Leijtens, T.; Bush, K.A.; Prasanna, R.; McGehee, M.D. Opportunities and challenges for tandem solar cells using metal halide perovskite semiconductors. Nat. Energy 2018, 3, 828–838. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Y.; Lu, Q.; Tang, B.; Li, J.; Gao, H.; Gao, Y.; Li, H.; Ding, C.; Wen, J.; et al. All-perovskite tandem solar cells with 3D/3D bilayer perovskite heterojunction. Nature 2023, 620, 994–1000. [Google Scholar] [CrossRef]
- Boukortt, N.E.; Triolo, C.; Santangelo, S.; Patanè, S. All-Perovskite Tandem Solar Cells: From Certified 25% and Beyond. Energies 2023, 16, 3519. [Google Scholar] [CrossRef]
- Chen, B.; Yu, Z.; Onno, A.; Yu, Z.; Chen, S.; Wang, J.; Holman, Z.C.; Huang, J. Bifacial all-perovskite tandem solar cells. Sci. Adv. 2022, 8, eadd0377. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Wang, X.; Lin, R.; Luo, X.; Liu, Z.; Zhou, K.; Xiong, S.; Bao, Q.; Chen, G.; et al. Flexible all-perovskite tandem solar cells approaching 25% efficiency with molecule-bridged hole-selective contact. Nat. Energy 2022, 7, 708–717. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Z.; Yan, Y.; Liu, S.; Yang, D. Perovskite—A Perfect Top Cell for Tandem Devices to Break the S–Q Limit. Adv. Sci. 2019, 6, 1801704. [Google Scholar] [CrossRef]
- Yuan, J.; Hazarika, A.; Zhao, Q.; Ling, X.; Moot, T.; Ma, W.; Luther, J.M. Metal Halide Perovskites in Quantum Dot Solar Cells: Progress and Prospects. Joule 2020, 4, 1160–1185. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Li, H.; Wu, Y.; Lin, C.-H.; Guan, X.; Hu, L.; Kim, J.; Zhu, X.; Zeng, H.; Wu, T. Inorganic Halide Perovskite Quantum Dots: A Versatile Nanomaterial Platform for Electronic Applications. Nano-Micro Lett. 2022, 15, 16. [Google Scholar] [CrossRef]
- Zhao, Q.; Hazarika, A.; Chen, X.; Harvey, S.P.; Larson, B.W.; Teeter, G.R.; Liu, J.; Song, T.; Xiao, C.; Shaw, L.; et al. High efficiency perovskite quantum dot solar cells with charge separating heterostructure. Nat. Commun. 2019, 10, 2842. [Google Scholar] [CrossRef]
- Ling, X.; Yuan, J.; Ma, W. The Rise of Colloidal Lead Halide Perovskite Quantum Dot Solar Cells. Acc. Mater. Res. 2022, 3, 866–878. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Q.; Huang, S.; Zheng, J.; Guan, X.; Patterson, R.; Kim, J.; Shi, L.; Lin, C.-H.; Lei, Q.; et al. Flexible and efficient perovskite quantum dot solar cells via hybrid interfacial architecture. Nat. Commun. 2021, 12, 466. [Google Scholar] [CrossRef]
- Chen, J.; Jia, D.; Johansson, E.M.J.; Hagfeldt, A.; Zhang, X. Emerging perovskite quantum dot solar cells: Feasible approaches to boost performance. Energy Environ. Sci. 2021, 14, 224–261. [Google Scholar] [CrossRef]
- ur Rehman, A.; Van Kerschaver, E.P.; Aydin, E.; Raja, W.; Allen, T.G.; De Wolf, S. Electrode metallization for scaled perovskite/silicon tandem solar cells: Challenges and opportunities. Prog. Photovolt. Res. Appl. 2023, 31, 429–442. [Google Scholar] [CrossRef]
- De Rose, A.; Erath, D.; Nikitina, V.; Schube, J.; Güldali, D.; Minat, Ä.; Rößler, T.; Richter, A.; Kirner, S.; Kraft, A.; et al. Low-temperature metallization & interconnection for silicon heterojunction and perovskite silicon tandem solar cells. Sol. Energy Mater. Sol. Cells 2023, 261, 112515. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Magomedov, A.; Roß, M.; Jošt, M.; Talaikis, M.; Chistiakova, G.; Bertram, T.; Márquez, J.A.; Köhnen, E.; Kasparavičius, E.; et al. Conformal monolayer contacts with lossless interfaces for perovskite single junction and monolithic tandem solar cells. Energy Environ. Sci. 2019, 12, 3356–3369. [Google Scholar] [CrossRef]
- Jošt, M.; Kegelmann, L.; Korte, L.; Albrecht, S. Monolithic Perovskite Tandem Solar Cells: A Review of the Present Status and Advanced Characterization Methods Toward 30% Efficiency. Adv. Energy Mater. 2020, 10, 1904102. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Wang, X.; Sun, Y.; Zhao, Z.; Li, Y.; Zhou, H.; Chen, Q. Cost Analysis of Perovskite Tandem Photovoltaics. Joule 2018, 2, 1559–1572. [Google Scholar] [CrossRef]
- Albrecht, S.; Rech, B. Perovskite solar cells: On top of commercial photovoltaics. Nature Energy 2017, 2, 16196. [Google Scholar] [CrossRef]
- Wu, P.; Wen, J.; Wang, Y.; Liu, Z.; Lin, R.; Li, H.; Luo, H.; Tan, H. Efficient and Thermally Stable All-Perovskite Tandem Solar Cells Using All-FA Narrow-Bandgap Perovskite and Metal-oxide-based Tunnel Junction. Adv. Energy Mater. 2022, 12, 2202948. [Google Scholar] [CrossRef]
- Liu, J.; Aydin, E.; Yin, J.; De Bastiani, M.; Isikgor, F.H.; Rehman, A.U.; Yengel, E.; Ugur, E.; Harrison, G.T.; Wang, M.; et al. 28.2%-efficient, outdoor-stable perovskite/silicon tandem solar cell. Joule 2021, 5, 3169–3186. [Google Scholar] [CrossRef]
- Liu, J.; De Bastiani, M.; Aydin, E.; Harrison, G.T.; Gao, Y.; Pradhan, R.R.; Eswaran, M.K.; Mandal, M.; Yan, W.; Seitkhan, A.; et al. Efficient and stable perovskite-silicon tandem solar cells through contact displacement by MgFx. Science 2022, 377, 302–306. [Google Scholar] [CrossRef]
- Wu, P.; Thrithamarassery Gangadharan, D.; Saidaminov, M.I.; Tan, H. A Roadmap for Efficient and Stable All-Perovskite Tandem Solar Cells from a Chemistry Perspective. ACS Cent. Sci. 2023, 9, 14–26. [Google Scholar] [CrossRef]
- Boyd, C.C.; Xu, J.; Bush, K.A.; Raiford, J.A.; Cheacharoen, R.; McGehee, M.D. Highly Efficient and Stable Perovskite-Silicon Tandem Solar Cells. In Proceedings of the OSA Advanced Photonics Congress (AP) 2019 (IPR, Networks, NOMA, SPPCom, PVLED), Burlingame, CA, USA, 29 July–2 August 2019; p. PM4C.1. [Google Scholar]

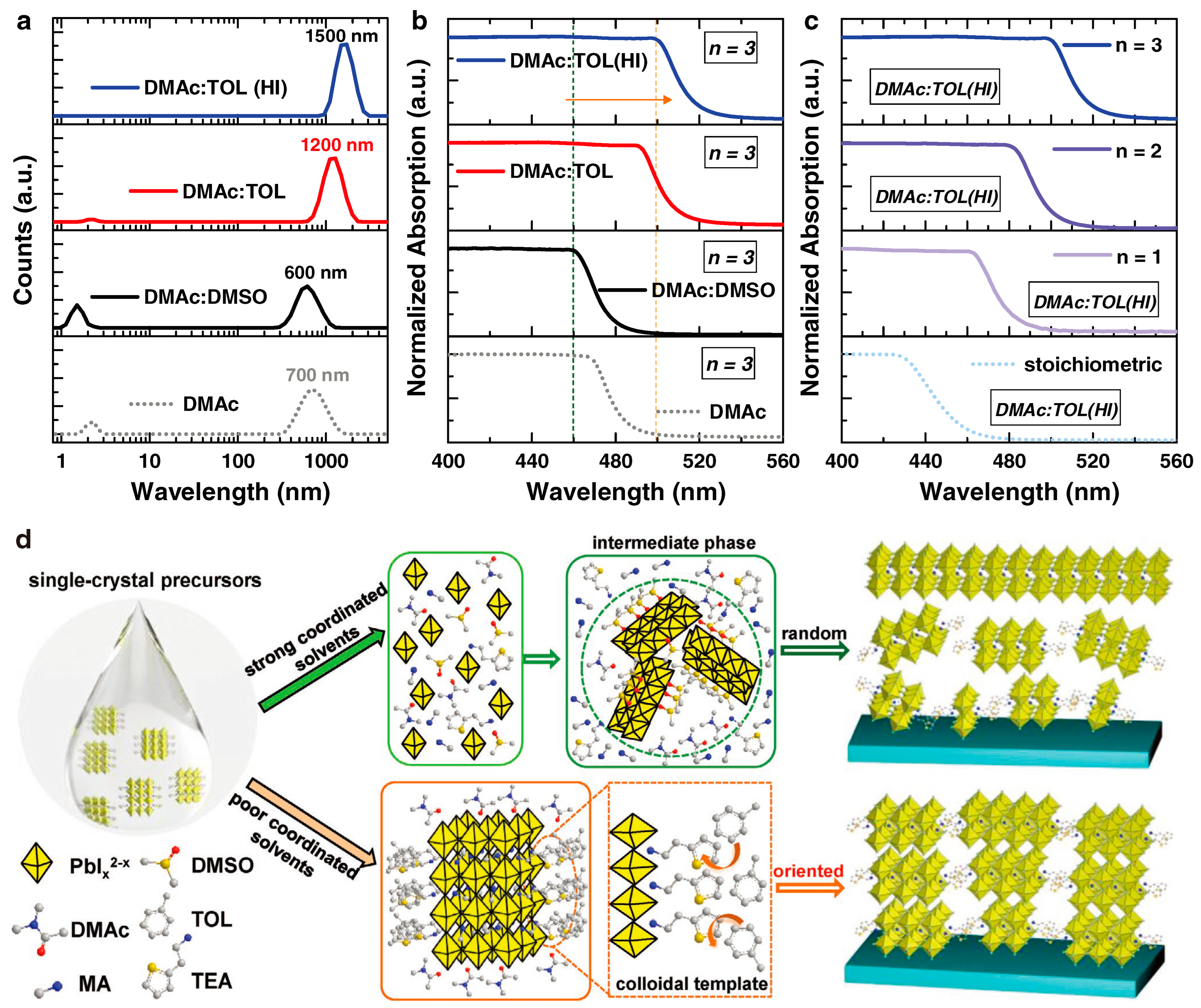





| No. | Hybrid Organic–Inorganic Halide Perovskite | Inorganic Halide Perovskites |
|---|---|---|
| 1 | MAPbX3 [26,27] | Rb3Sb2I9 [28] |
| 2 | FAPbX3 [29,30] | Cs3Sb2I9 [31] |
| 3 | MASnX3 [32] | Rb6Pb5Br16 [33] |
| 4 | FASnX3 [34,35] | Rb6Pb5I16 [33] |
| 5 | MAxFA1−xPbI3 [36] | CsRbPb2I6 |
| 6 | FA1−xCsxPbI3 [37] | Cs2SnI6 [38] |
| 7 | MA1–xFAxGeI3 [39] | Cs2TiIxBr6−x [40] |
| 8 | MAGeX3 [41] | Cs2InBiCl6 [42] |
| 9 | FAGeI3 [43] | Cs2InSbCl6 [42] |
| 10 | C(NH2)3GeI3 [43] | Cs2TiX6 [44] |
| 11 | MAPbxSn1-xBr3 | Rb2CuInCl6 [45] |
| 12 | MAPb1−xInxI3Clx [46] | Rb2AgInBr6 [47] |
| 13 | FA0.8Cs0.2SnI3 [48] | Cs2BiAgCl6 [49] |
| 14 | (PEA)2(FA)8Sn9I28 [50] | Cs2AgBiBr6 [51,52] |
| 15 | (BA)2(MA)3Sn4I13 [53] | Cs2AgInBr6 [54] |
| 16 | (FA)x(MA)1−xSnX3 [55] | In2TiX6 [56] |
| 17 | (CH3)3NHGeI3 [43] | K2TiX6 [57] |
| 18 | CH3C(NH2)2GeI3 [43] | Cs3Bi2I9 [58] |
| 19 | C5H6NBiI4 [59] | CsPbX3 [60] |
| 20 | (H3NC6H12NH3)BiI5 [61] | RbPbX3 [62] |
| 21 | MA3Sb2I9 [63] | Cs1−xRbxPbX3 [64] |
| 22 | (FA)2BiCuI6 [65] | CsSnX3 [66] |
| 23 | (NH4)3Sb2I9 [48] | H3SPbX3 [67] |
| 24 | HC(NH2)2PbI3 [68] | CsGeI3 [69] |
| 25 | (CH3NH3)1−x(HC(NH2)2)xPbI3 [70] | Tl2TiX6 [71] |
| 26 | (HC(NH2)2)0.9Cs0.1PbI3 [72] | CuPbX3 [73] |
| 27 | [HC(NH2)2]x[CH3NH3]1−xPbI3 [74] | AgPbX3 [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berhe, T.A.; Su, W.-N.; Hwang, B.J. Halide Perovskites’ Multifunctional Properties: Coordination Engineering, Coordination Chemistry, Electronic Interactions and Energy Applications beyond Photovoltaics. Inorganics 2024, 12, 182. https://doi.org/10.3390/inorganics12070182
Berhe TA, Su W-N, Hwang BJ. Halide Perovskites’ Multifunctional Properties: Coordination Engineering, Coordination Chemistry, Electronic Interactions and Energy Applications beyond Photovoltaics. Inorganics. 2024; 12(7):182. https://doi.org/10.3390/inorganics12070182
Chicago/Turabian StyleBerhe, Taame Abraha, Wei-Nien Su, and Bing Joe Hwang. 2024. "Halide Perovskites’ Multifunctional Properties: Coordination Engineering, Coordination Chemistry, Electronic Interactions and Energy Applications beyond Photovoltaics" Inorganics 12, no. 7: 182. https://doi.org/10.3390/inorganics12070182
APA StyleBerhe, T. A., Su, W.-N., & Hwang, B. J. (2024). Halide Perovskites’ Multifunctional Properties: Coordination Engineering, Coordination Chemistry, Electronic Interactions and Energy Applications beyond Photovoltaics. Inorganics, 12(7), 182. https://doi.org/10.3390/inorganics12070182







