Effect of Impregnation of PEDOT:PSS in Etched Aluminium Electrodes on the Performance of Solid State Electrolytic Capacitors
Abstract
1. Introduction
2. Results and Discussions
2.1. Undoped PEDOT:PSS on Flat and Etched Aluminium Electrodes
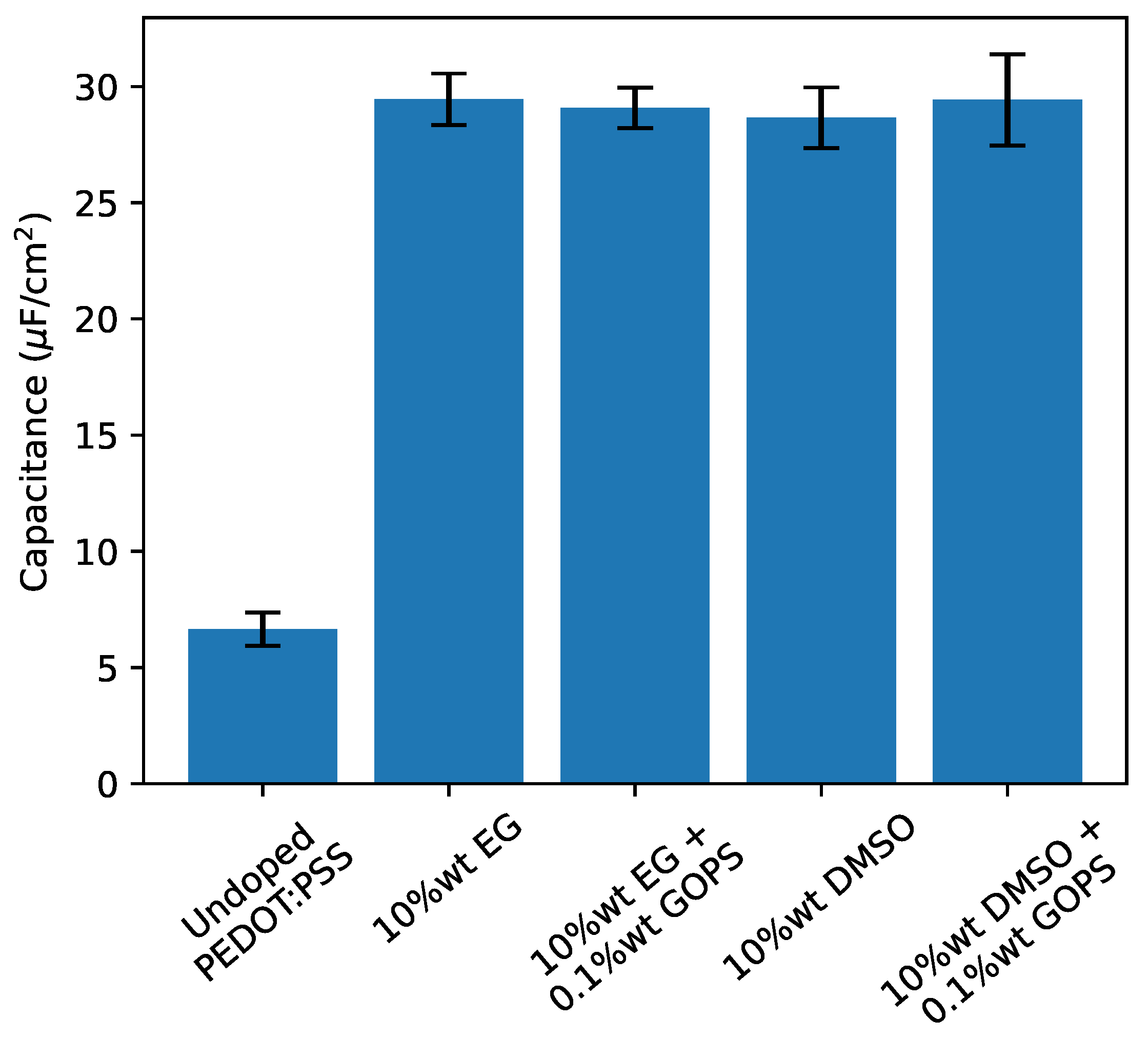
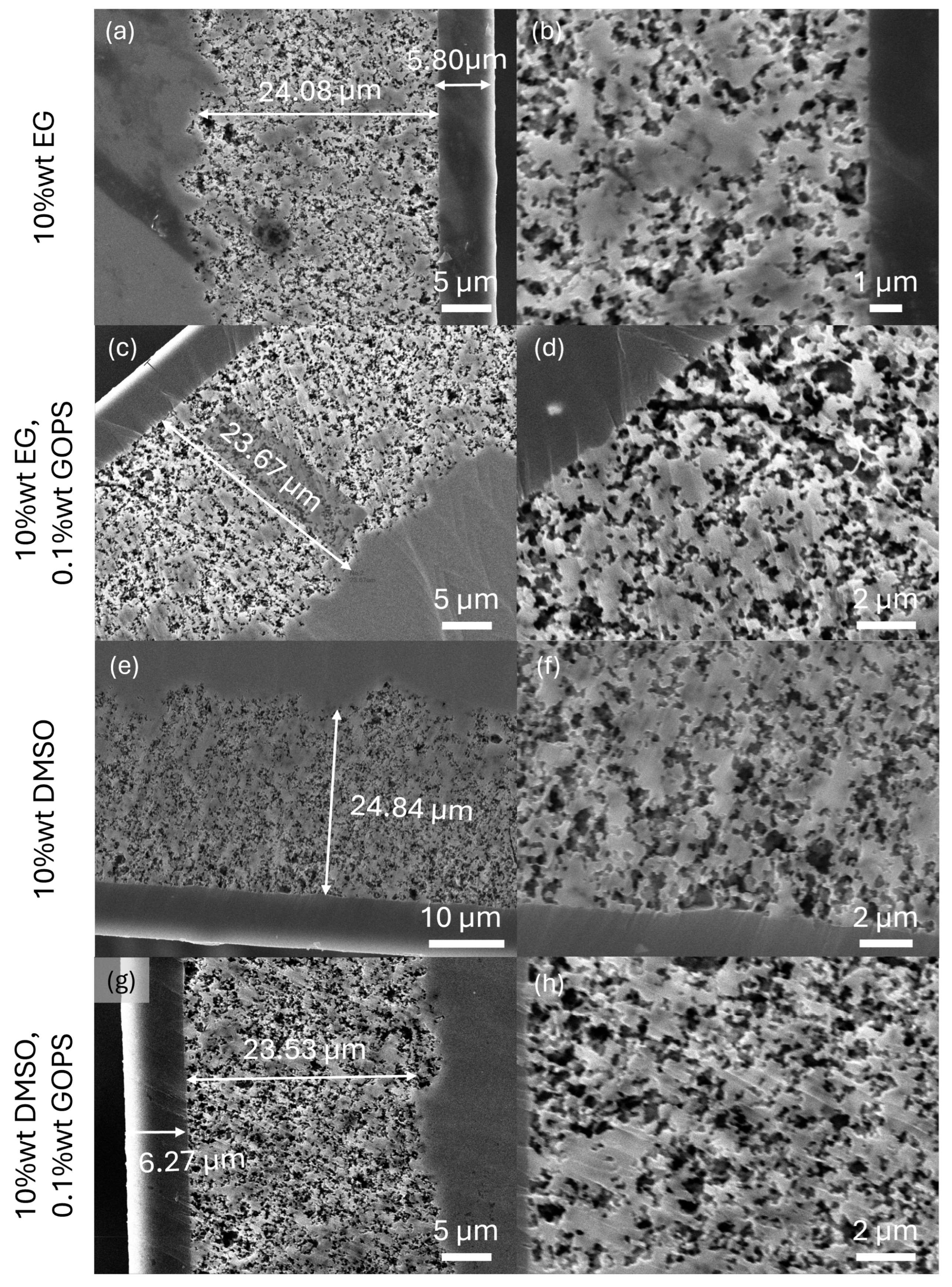
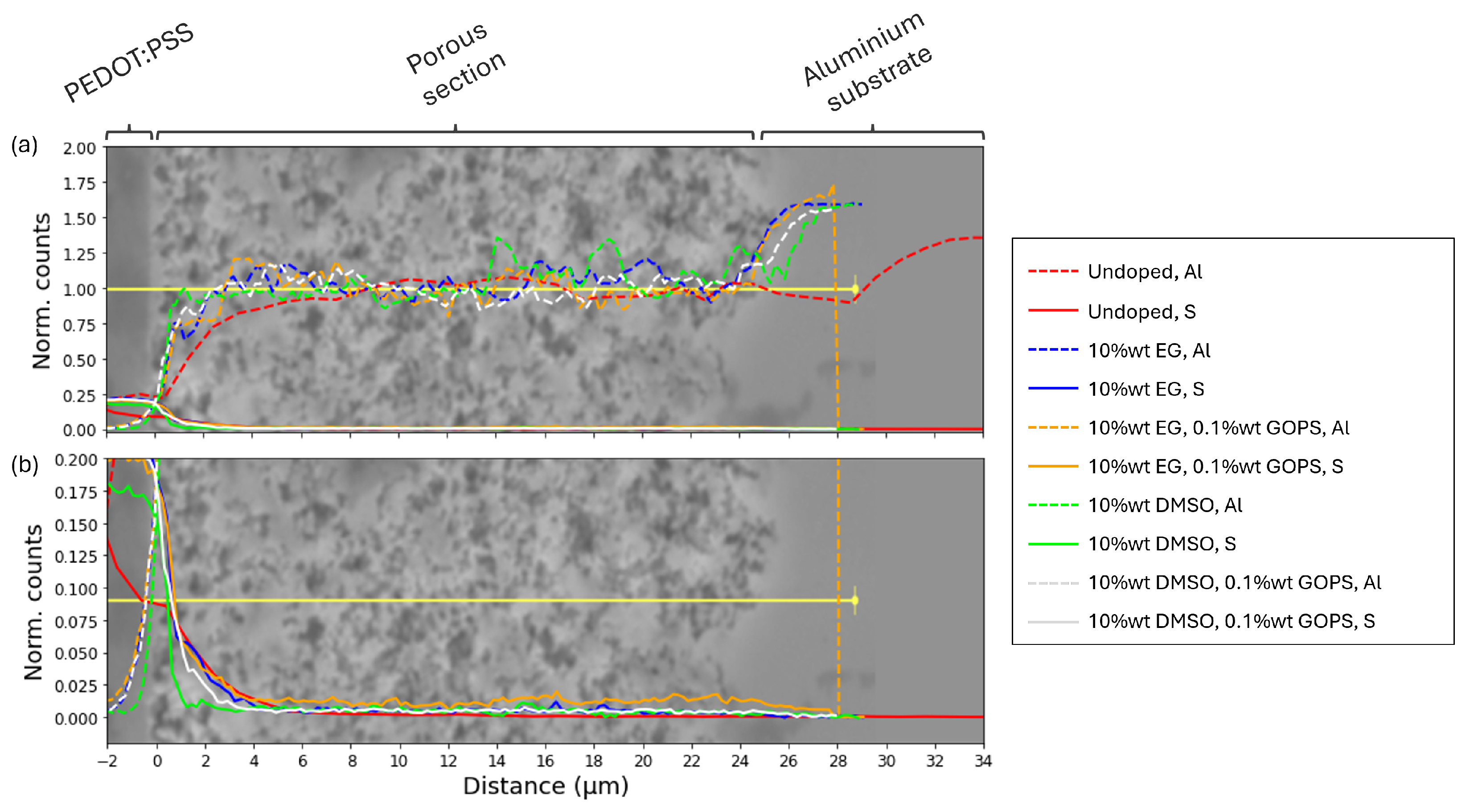
2.2. Influence of Additives on the Behaviour of Polymer Solid Electrolytic Capacitors
3. Materials and Methods
3.1. Materials and Production of Samples
3.2. Characterization Techniques
3.2.1. Electrochemical Characterization
3.2.2. Analysis of the PEDOT:PSS Impregnation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaiser, C.J. The Capacitor Handbook; Springer: Dordrecht, The Netherlands, 1993; p. 129. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatti, T.S. A review on electrochemical double-layer capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Torki, J.; Joubert, C.; Sari, A. Electrolytic capacitor: Properties and operation. J. Energy Storage 2023, 58, 106330. [Google Scholar] [CrossRef]
- Ono, S. Passive Films for Electrolytic Capacitors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 376–395. [Google Scholar] [CrossRef]
- Evangelisti, F.; Stiefel, M.; Guseva, O.; Partovi Nia, R.; Hauert, R.; Hack, E.; Jeurgens, L.P.; Ambrosio, F.; Pasquarello, A.; Schmutz, P.; et al. Electronic and structural characterization of barrier-type amorphous aluminium oxide. Electrochim. Acta 2017, 224, 503–516. [Google Scholar] [CrossRef]
- Alwitt, R.S.; Uchi, H.; Beck, T.; Alkire, R. Electrochemical Tunnel Etching of Aluminum. J. Electrochem. Soc. 1984, 131, 13. [Google Scholar] [CrossRef]
- Abruña, H.D.; Kiya, Y.; Henderson, J.C. Batteries and electrochemical capacitors. Phys. Today 2008, 61, 43–47. [Google Scholar] [CrossRef]
- Bauman, J.; Kazerani, M. A comparative study of fuel-cell-battery, fuel-cell-ultracapacitor, and fuel-cell-battery-ultracapacitor vehicles. IEEE Trans. Veh. Technol. 2008, 57, 760–769. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Yu, A.; Chabot, V.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Delivery; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–355. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Akin, M.; Zhou, X. Recent advances in solid-state supercapacitors: From emerging materials to advanced applications. Int. J. Energy Res. 2022, 46, 10389–10452. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, J.; Kim, Y.S. Liquid electrolyte-free cylindrical Al polymer capacitor review: Materials and characteristics. J. Power Sources 2015, 284, 466–480. [Google Scholar] [CrossRef]
- Holze, R. Metal Oxide/Conducting Polymer Hybrids for Application in Supercapacitors. In Metal Oxides in Supercapacitors; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Chapter 9; pp. 219–245. [Google Scholar] [CrossRef]
- Bryan, A.M.; Santino, L.M.; Lu, Y.; Acharya, S.; D’Arcy, J.M. Conducting Polymers for Pseudocapacitive Energy Storage. Chem. Mater. 2016, 28, 5989–5998. [Google Scholar] [CrossRef]
- Jonas, F.; Heywang, G.; Schmidtberg, W. Feststoff-Elektrolyte und Diese Enthaltende Elektrolyt-Kondensatoren. European Patent DE3814730A1, 9 November 1989. [Google Scholar]
- Carlberg, J.C. Poly(3,4-ethylenedioxythiophene) as Electrode Material in Electrochemical Capacitors. J. Electrochem. Soc. 1997, 144, L61. [Google Scholar] [CrossRef]
- Chen, H.W.; Li, C. PEDOT: Fundamentals and Its Nanocomposites for Energy Storage. Chin. J. Polym. Sci. 2020, 38, 435–448. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, Q.; Yang, Y.; Tian, D. Abnormal Capacitance Increasing at Elevated Temperature in Tantalum Capacitors with PEDOT:PSS Electrodes. Act. Passiv. Electron. Compon. 2018, 2018, 9864387. [Google Scholar] [CrossRef]
- Zhao, Z.; Richardson, G.F.; Meng, Q.; Zhu, S.; Kuan, H.C.; Ma, J. PEDOT-based composites as electrode materials for supercapacitors. Nanotechnology 2016, 27, 042001. [Google Scholar] [CrossRef] [PubMed]
- Freeman, Y.; Harrell, W.R.; Luzinov, I.; Holman, B.; Lessner, P. Electrical characterization of tantalum capacitors with poly(3,4-ethylenedioxythiophene) counter electrodes. J. Electrochem. Soc. 2009, 156, 4–10. [Google Scholar] [CrossRef]
- Shrivastava, A.; Azarian, M.H.; Pecht, M. Failure of Polymer Aluminum Electrolytic Capacitors Under Elevated Temperature Humidity Environments. IEEE Trans. Compon. Packag. Manuf. Technol. 2017, 7, 745–750. [Google Scholar] [CrossRef]
- Calabia Gascón, N.; Terryn, H.; Hubin, A. The role of PEDOT:PSS in (super)capacitors: A review. Next Nanotechnol. 2023, 2, 100015. [Google Scholar] [CrossRef]
- Freeman, Y.; Luzinov, I.; Burtovyy, R.; Lessner, P.; Harrell, W.R.; Chinnam, S.; Qazia, J. Capacitance stability in polymer tantalum capacitors with PEDOT counter electrodes. ECS J. Solid State Sci. Technol. 2017, 6, N104–N110. [Google Scholar] [CrossRef]
- Koseki, K.; Sakakura, M. Electrolytic Capacitor Having a Solid Electrolyte Layer and Manufacturing Method Thereof. U.S. Patent US10115529B2, 30 October 2018. [Google Scholar]
- Sato, K.; Sakakura, M.; Koseki, K. Solid Electrolytic Capacitor and Method for Manufacturing Solid Electrolytic Capacitor. European Patent PCT/JP2016/073037, 8 December 2015. [Google Scholar]
- Wakabayashi, T.; Katsunuma, M.; Kudo, K.; Okuzaki, H. PH-Tunable High-Performance PEDOT:PSS Aluminum Solid Electrolytic Capacitors. ACS Appl. Energy Mater. 2018, 1, 2157–2163. [Google Scholar] [CrossRef]
- Calabia Gascón, N.; Revilla, R.I.; Wouters, B.; Terryn, H.; Hubin, A. On the Interaction between PEDOT:PSS Dispersions and Aluminium Electrodes for Solid State Electrolytic Capacitors. Inorganics 2024, 12, 104. [Google Scholar] [CrossRef]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pébère, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric Properties of Materials Showing Constant-Phase-Element (CPE) Impedance Response. J. Electrochem. Soc. 2013, 160, C215–C225. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Scisco, G.P.; Orazem, M.E.; Ziegler, K.J.; Jones, K.S. On the rate capability of supercapacitors characterized by a constant-phase element. J. Power Sources 2021, 516, 230700. [Google Scholar] [CrossRef]
- Scisco, G.P.; Orazem, M.E.; Ziegler, K.J.; Jones, K.S. Resistivity of mesopore-confined ionic liquid determined by electrochemical impedance spectroscopy. Electrochim. Acta 2021, 378, 138112. [Google Scholar] [CrossRef]
- Alexander, C.L.; Tribollet, B.; Orazem, M.E. Contribution of Surface Distributions to Constant-Phase-Element (CPE) Behavior: 1. Influence of Roughness. Electrochim. Acta 2015, 173, 416–424. [Google Scholar] [CrossRef]
- Song, H.K.; Hwang, H.Y.; Lee, K.H.; Dao, L.H. Effect of pore size distribution on the frequency dispersion of porous electrodes. Electrochim. Acta 2000, 45, 2241–2257. [Google Scholar] [CrossRef]
- Lee, S.H.; Sohn, J.S.; Kulkarni, S.B.; Patil, U.M.; Jun, S.C.; Kim, J.H. Modified physico-chemical properties and supercapacitive performance via DMSO inducement to PEDOT:PSS active layer. Org. Electron. 2014, 15, 3423–3430. [Google Scholar] [CrossRef]
- Wang, G.F.; Tao, X.M.; Xin, J.H.; Fei, B. Modification of conductive polymer for polymeric anodes of flexible organic light-emitting diodes. Nanoscale Res. Lett. 2009, 4, 613–617. [Google Scholar] [CrossRef]
- Shahrim, N.A.; Ahmad, Z.; Wong Azman, A.; Fachmi Buys, Y.; Sarifuddin, N. Mechanisms for doped PEDOT:PSS electrical conductivity improvement. Mater. Adv. 2021, 2, 7118–7138. [Google Scholar] [CrossRef]
- Lövenich, W. PEDOT-properties and applications. Polym. Sci. Ser. C 2014, 56, 135–143. [Google Scholar] [CrossRef]
- D’Angelo, P.; Tarabella, G.; Romeo, A.; Marasso, S.L.; Verna, A.; Cocuzza, M.; Peruzzi, C.; Vurro, D.; Iannotta, S. PEDOT:PSS Morphostructure and ion-to-electron transduction and amplification mechanisms in organic electrochemical transistors. Materials 2018, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.M.; Rivnay, J.; Malliaras, G.G. Understanding volumetric capacitance in conducting polymers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1433–1436. [Google Scholar] [CrossRef]
- Savva, A.; Wustoni, S.; Inal, S. Ionic-to-electronic coupling efficiency in PEDOT:PSS films operated in aqueous electrolytes. J. Mater. Chem. C 2018, 6, 12023–12030. [Google Scholar] [CrossRef]
- Stavrinidou, E.; Leleux, P.; Rajaona, H.; Khodagholy, D.; Rivnay, J.; Lindau, M.; Sanaur, S.; Malliaras, G.G. Direct measurement of ion mobility in a conducting polymer. Adv. Mater. 2013, 25, 4488–4493. [Google Scholar] [CrossRef]
- Håkansson, A.; Han, S.; Wang, S.; Lu, J.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X.; Fabiano, S. Effect of (3-glycidyloxypropyl)trimethoxysilane (GOPS) on the electrical properties of PEDOT:PSS films. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 814–820. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Ouyang, J.; Xu, Q.; Chu, C.W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4- ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Naitoh, Y.; Ishida, T. Morphological change and mobility enhancement in PEDOT:PSS by adding co-solvents. Adv. Mater. 2013, 25, 2831–2836. [Google Scholar] [CrossRef]
- Van Ingelgem, Y.; Tourwé, E.; Blajiev, O.; Pintelon, R.; Hubin, A. Advantages of odd random phase multisine electrochemical impedance measurements. Electroanalysis 2009, 21, 730–739. [Google Scholar] [CrossRef]
- Van Gheem, E.; Pintelon, R.; Vereecken, J.; Schoukens, J.; Hubin, A.; Verboven, P.; Blajiev, O. Electrochemical impedance spectroscopy in the presence of non-linear distortions and non-stationary behaviour Part I: Theory and validation. Electrochim. Acta 2004, 49, 4753–4762. [Google Scholar] [CrossRef]
- Newville, M.; Stensitzki, T.; Allen, D.B.; Ingargiola, A. LMFIT: Non-Linear Least-Square Minimization and Curve-Fitting for Python, version 0.8.0; Zenodo: Geneva, Switzerland, 2014. [CrossRef]
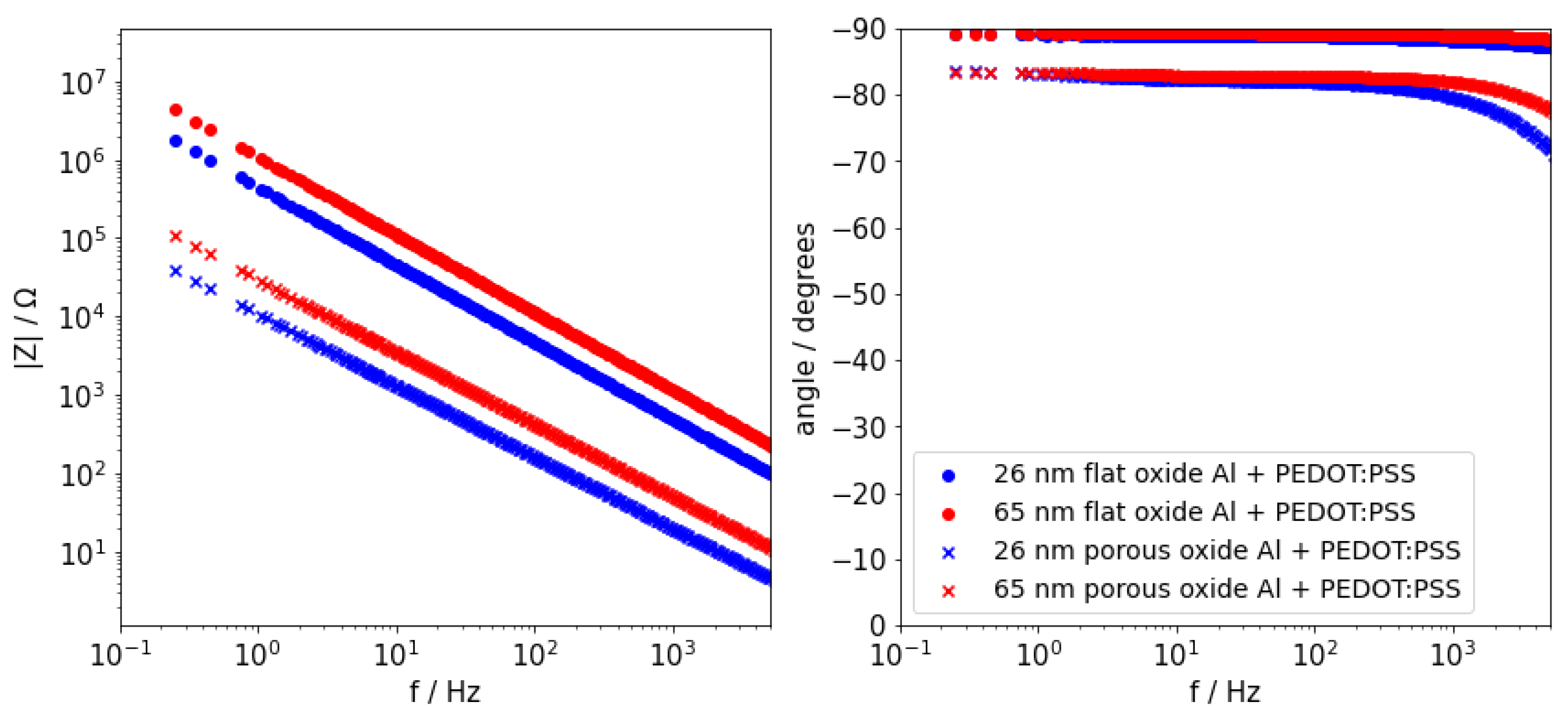



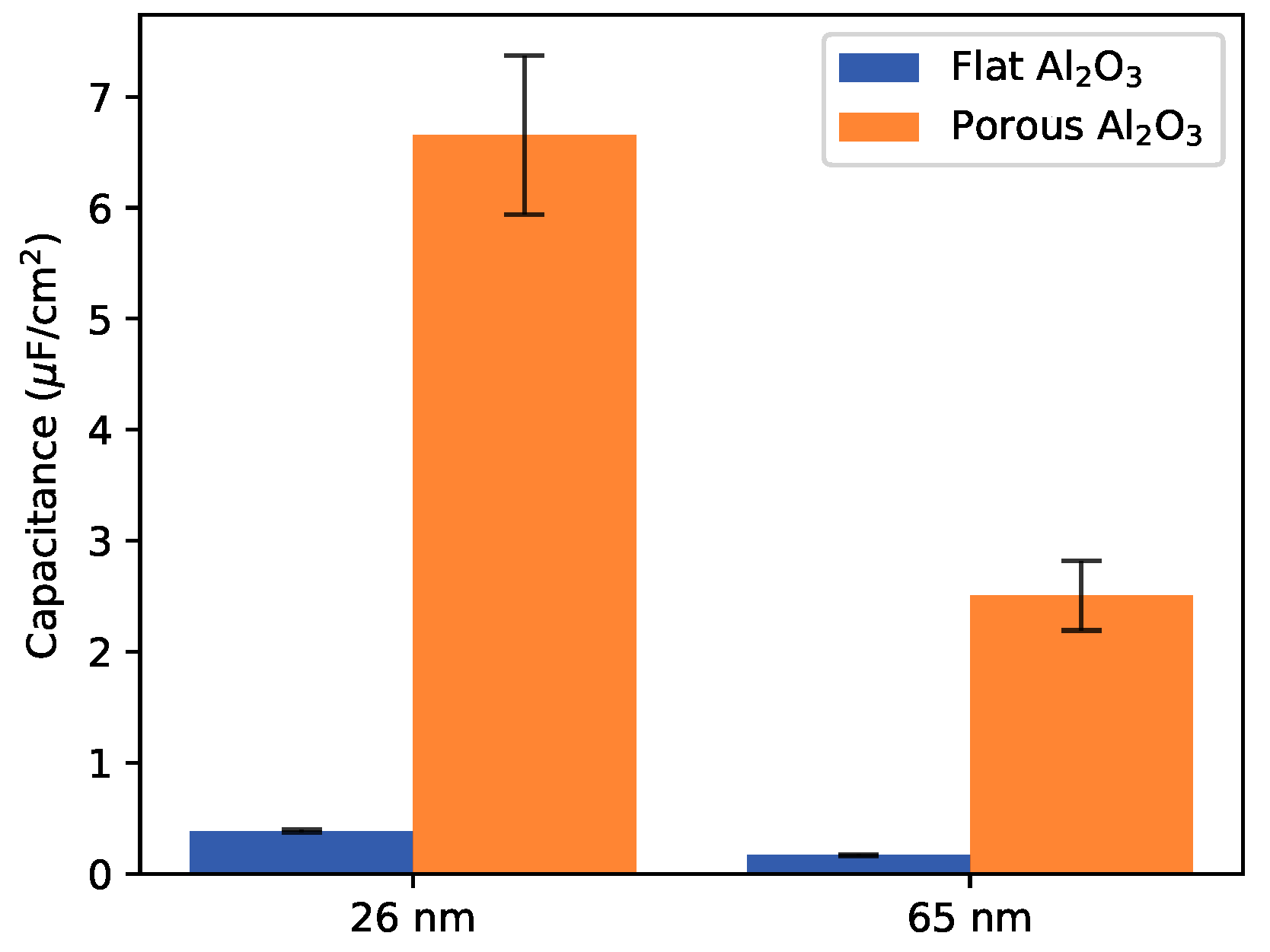

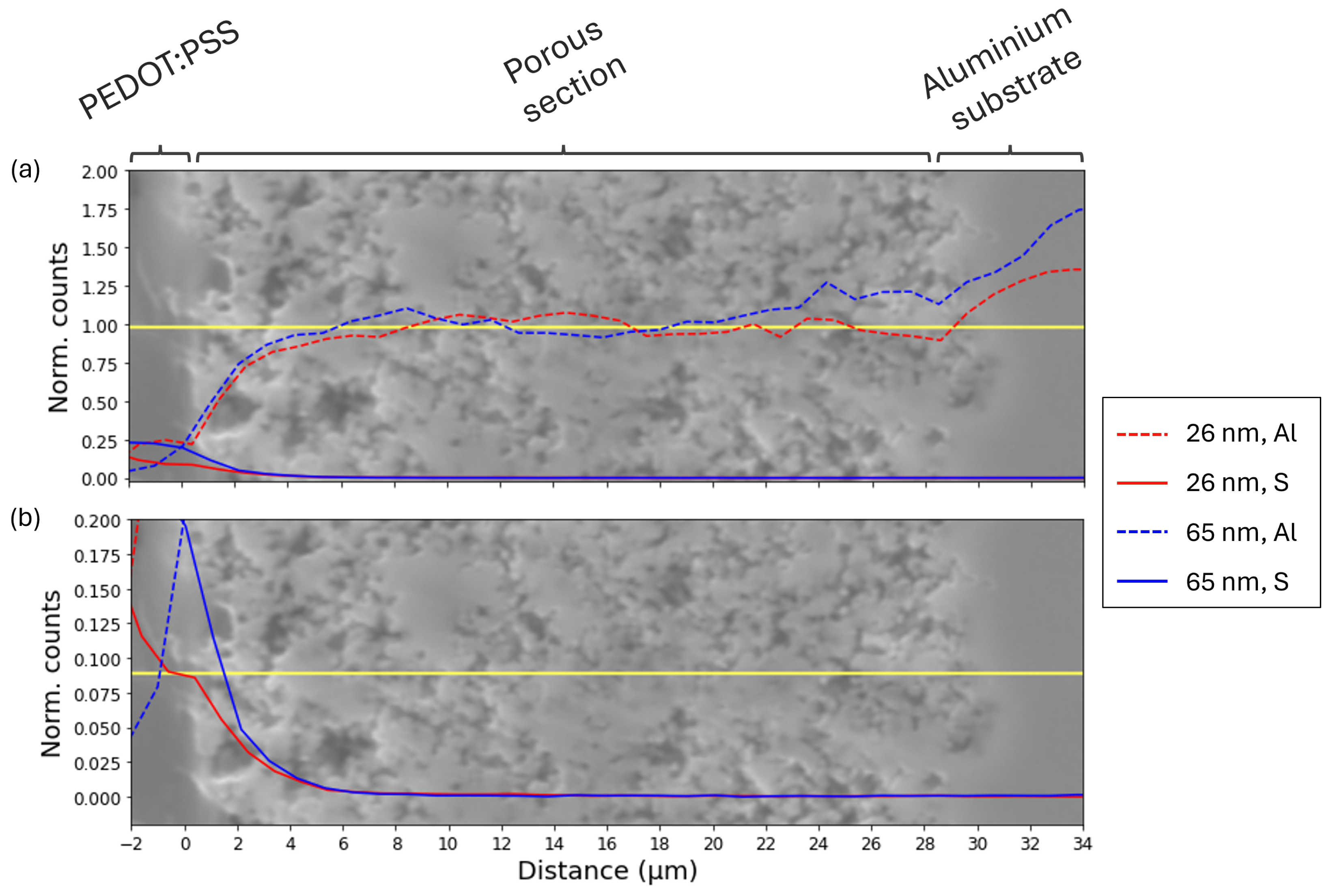
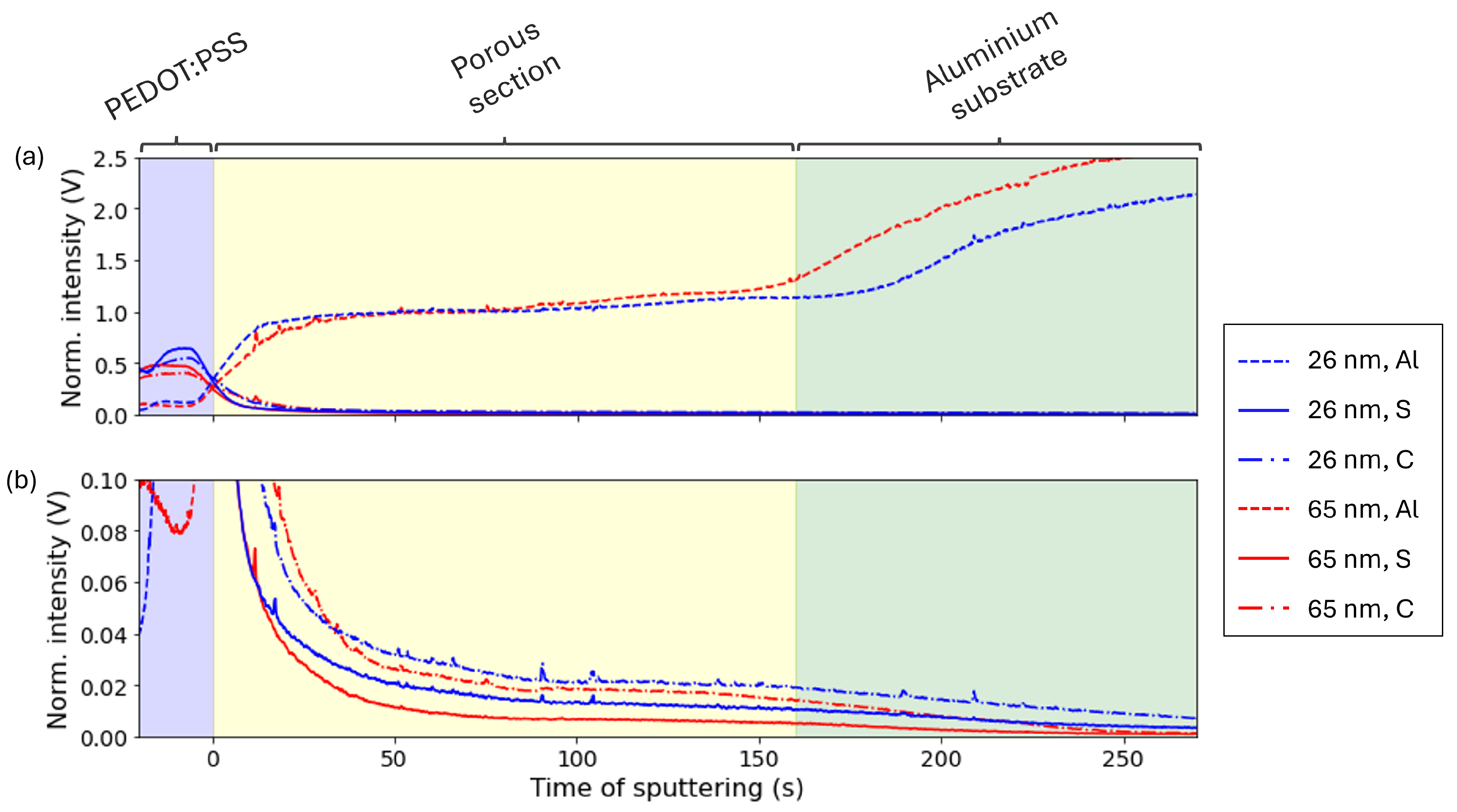

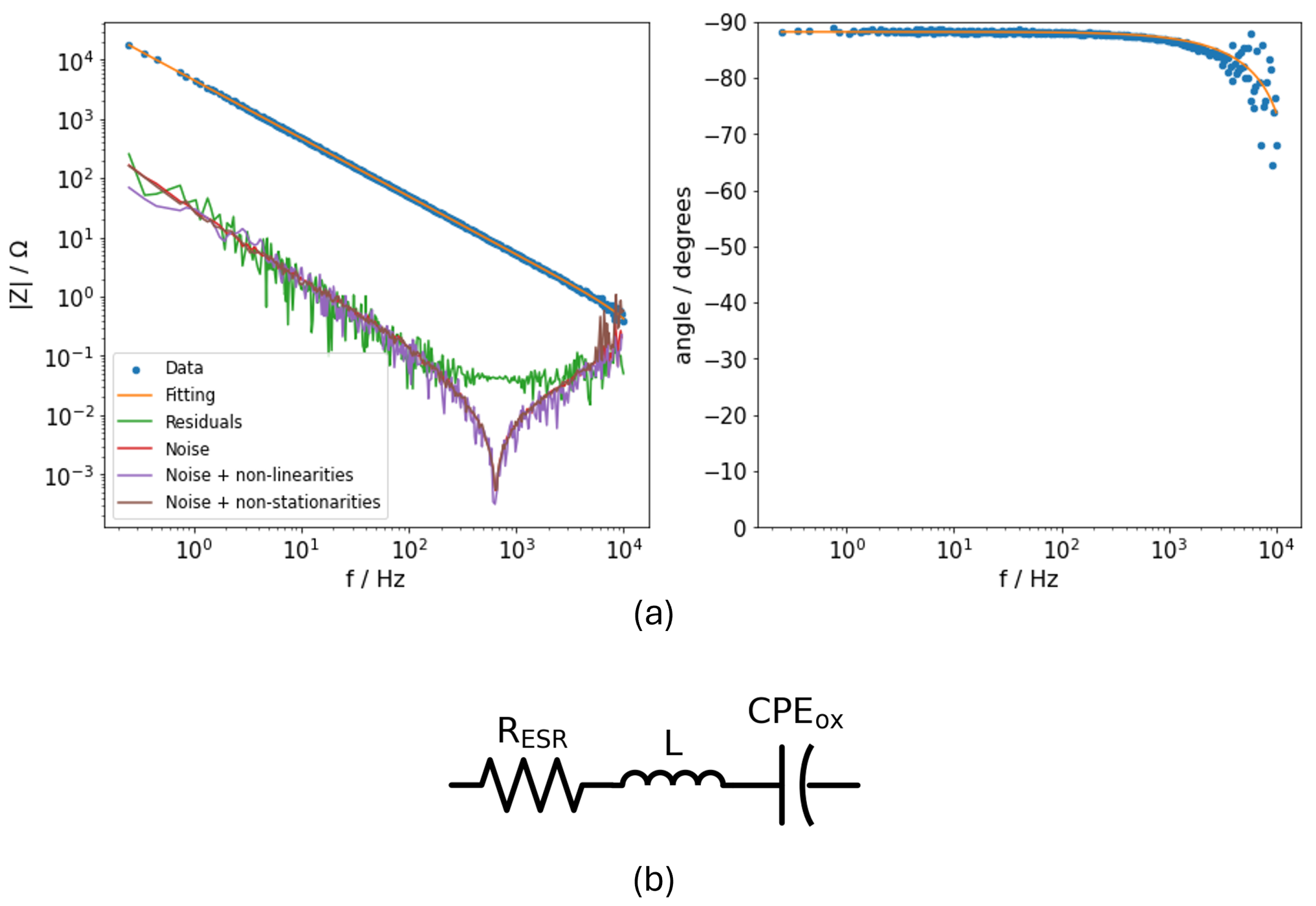


| PEDOT:PSS/Al2O3/Flat Al | PEDOT:PSS/Al2O3/Flat Al | |
|---|---|---|
| = 26 nm | = 65 nm | |
| () | ± | ± |
| () | ± | ± |
| ± | ± | |
| () | ± | ± |
| () | ± | ± |
| ± | ± |
| PEDOT:PSS/ Al2O3/Porous Al | PEDOT:PSS/ Al2O3/Porous Al | |
|---|---|---|
| = 26 nm | = 65 nm | |
| () | ± | ± |
| L (H) | ± | ± |
| () | ± | ± |
| ± | ± | |
| () | ± | ± |
| () | ± | ± |
| ± | ± |
| EG | DMSO | GOPS | |
|---|---|---|---|
| PEDOT:PSS 1 | - | - | - |
| PEDOT:PSS 2 | 10%wt | - | - |
| PEDOT:PSS 3 | 10%wt | - | 0.1%wt |
| PEDOT:PSS 4 | - | 10%wt | - |
| PEDOT:PSS 5 | - | 10%wt | 0.1%wt |
| L | ||||
|---|---|---|---|---|
| () | (H) | ( cm2) | - | |
| 10%wt EG | ± | ± | ± | ± |
| 10%wt EG, 0.1%wt GOPS | ± | ± | ± | ± |
| 10%wt DMSO | ± | ± | ± | ± |
| 10%wt DMSO, 0.1%wt GOPS | ± | ± | ± | ± |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabia Gascón, N.; Wouters, B.; Terryn, H.; Hubin, A. Effect of Impregnation of PEDOT:PSS in Etched Aluminium Electrodes on the Performance of Solid State Electrolytic Capacitors. Inorganics 2024, 12, 185. https://doi.org/10.3390/inorganics12070185
Calabia Gascón N, Wouters B, Terryn H, Hubin A. Effect of Impregnation of PEDOT:PSS in Etched Aluminium Electrodes on the Performance of Solid State Electrolytic Capacitors. Inorganics. 2024; 12(7):185. https://doi.org/10.3390/inorganics12070185
Chicago/Turabian StyleCalabia Gascón, Néstor, Benny Wouters, Herman Terryn, and Annick Hubin. 2024. "Effect of Impregnation of PEDOT:PSS in Etched Aluminium Electrodes on the Performance of Solid State Electrolytic Capacitors" Inorganics 12, no. 7: 185. https://doi.org/10.3390/inorganics12070185
APA StyleCalabia Gascón, N., Wouters, B., Terryn, H., & Hubin, A. (2024). Effect of Impregnation of PEDOT:PSS in Etched Aluminium Electrodes on the Performance of Solid State Electrolytic Capacitors. Inorganics, 12(7), 185. https://doi.org/10.3390/inorganics12070185






