Cu-Doped TiO2 Thin Films by Spin Coating: Investigation of Structural and Optical Properties
Abstract
:1. Introduction
2. Results and Discussion
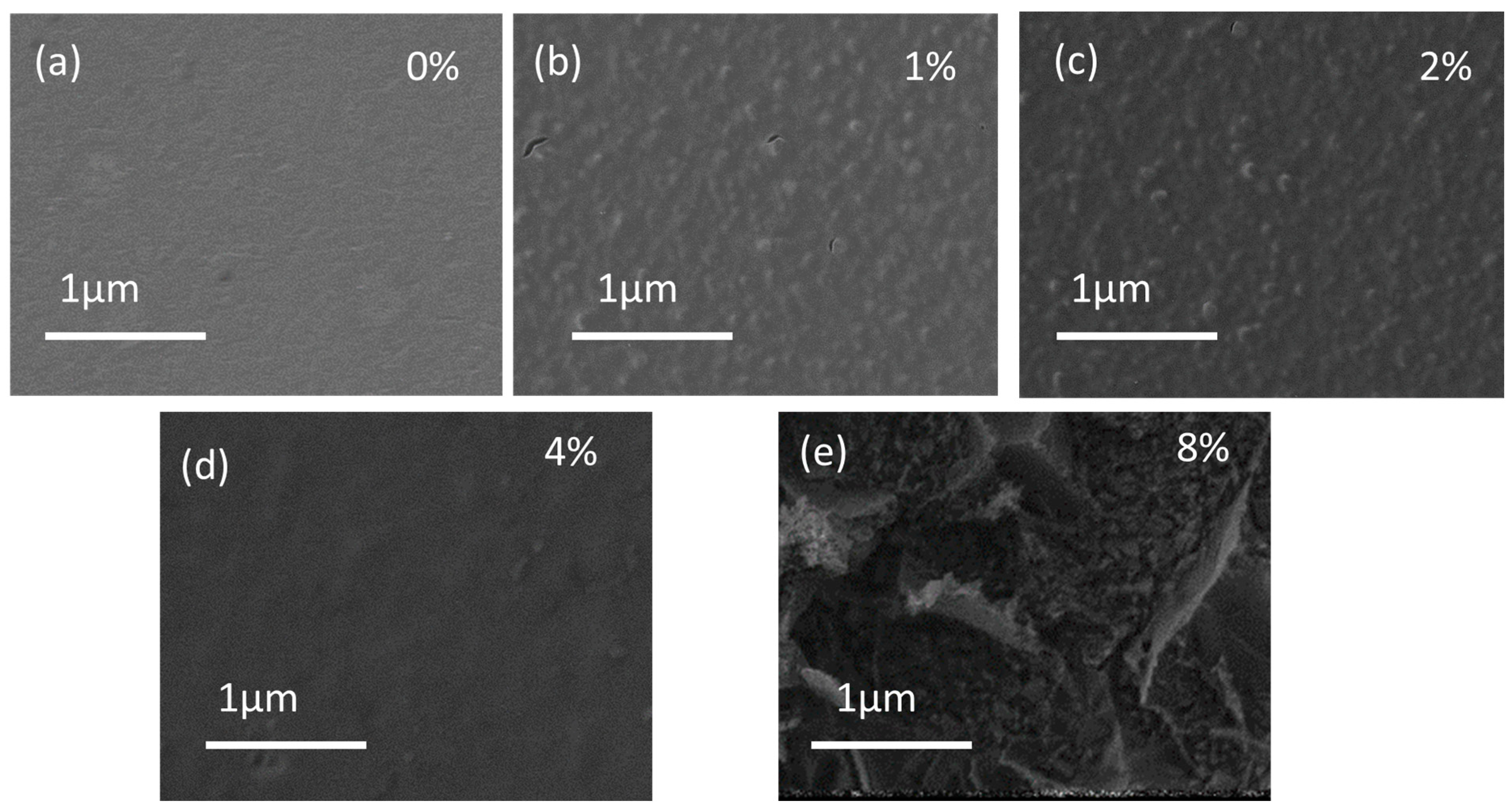
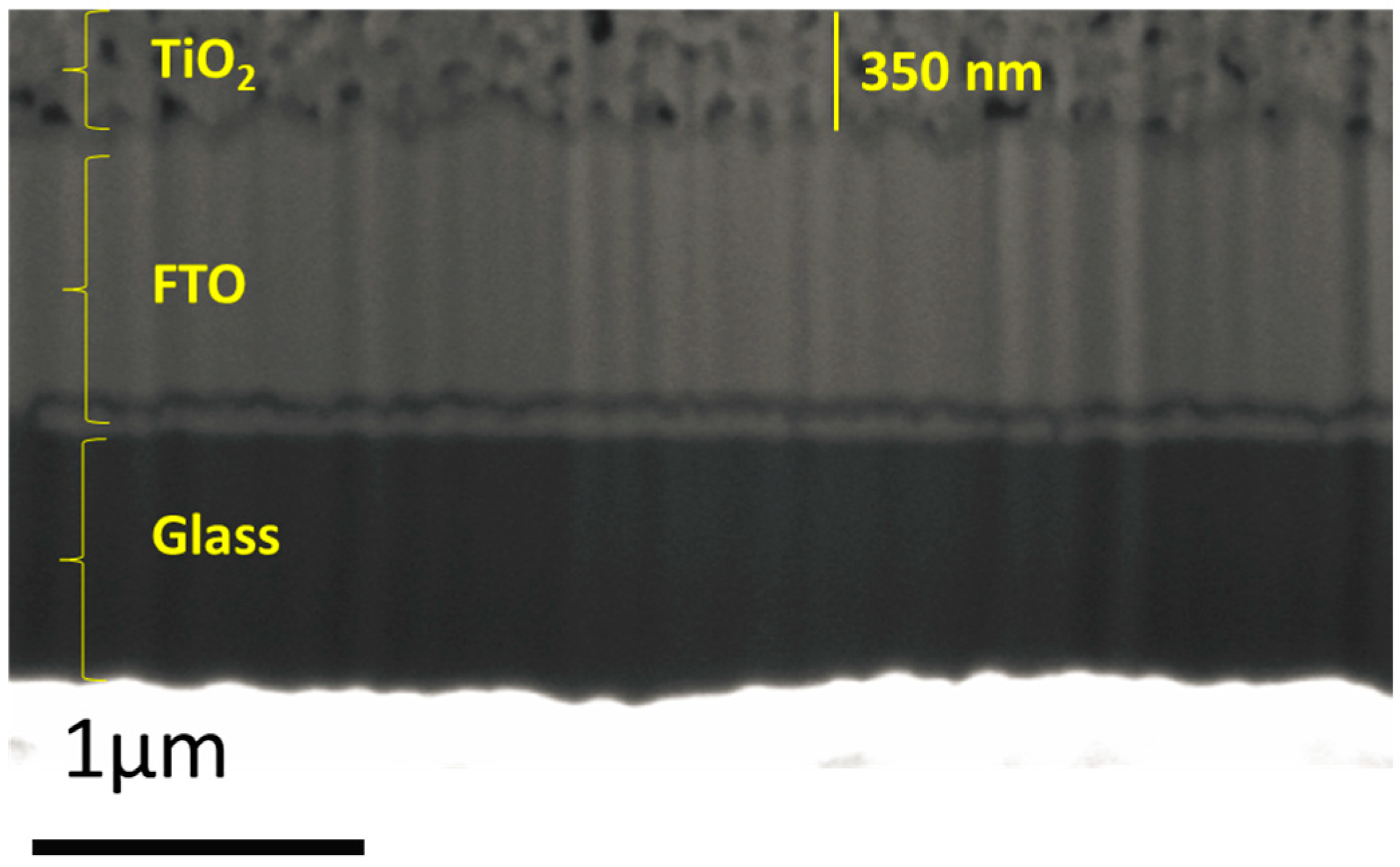
2.1. Morphological Properties
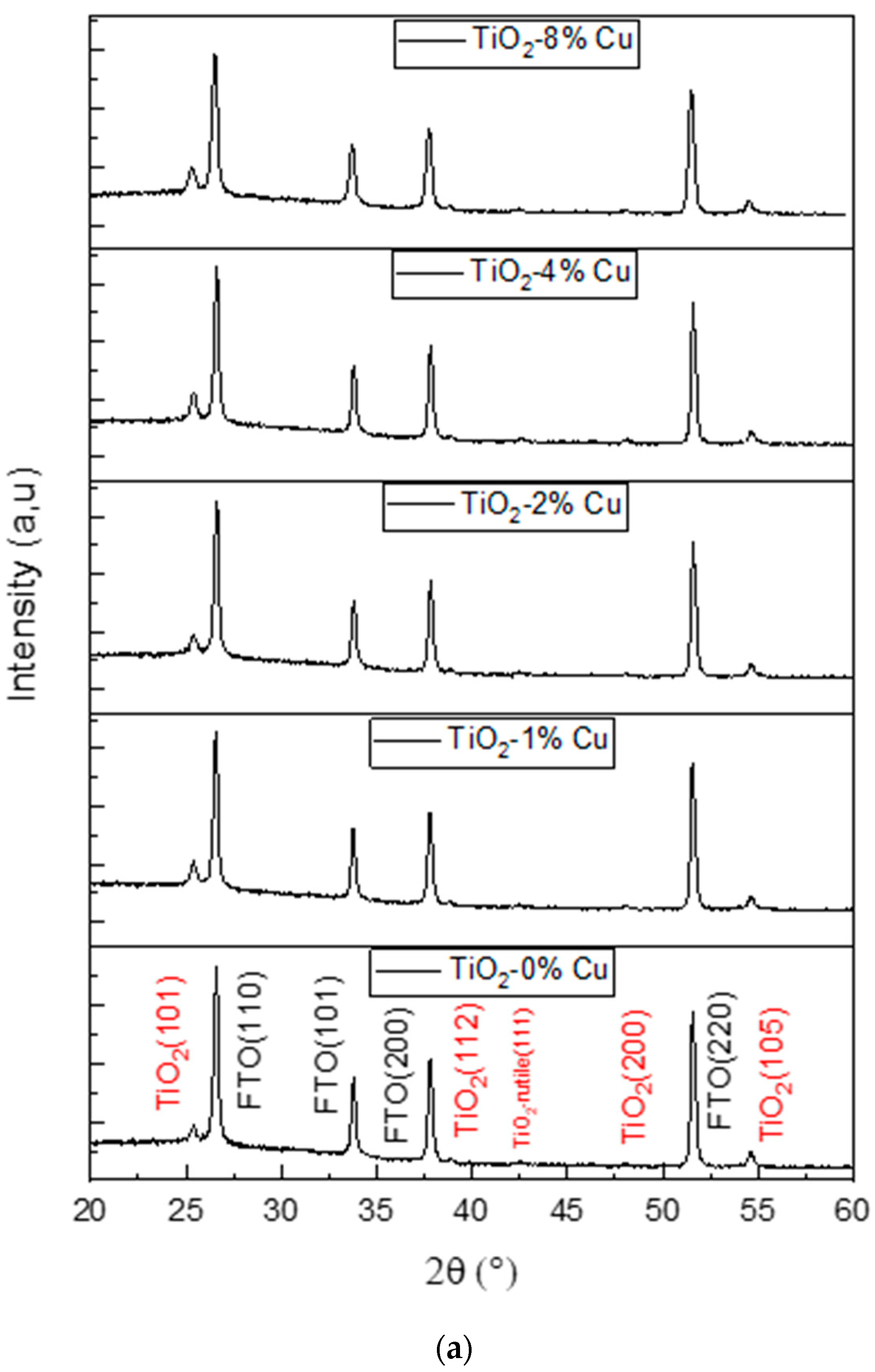
2.2. Structural Properties
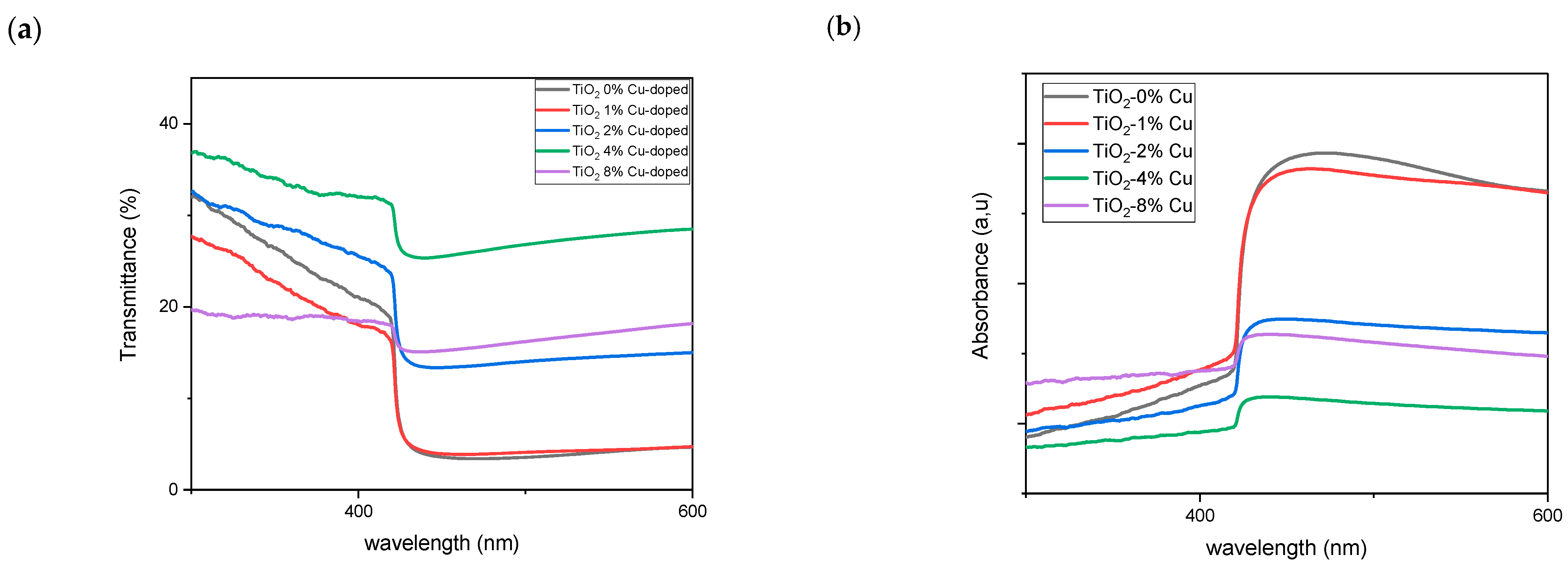
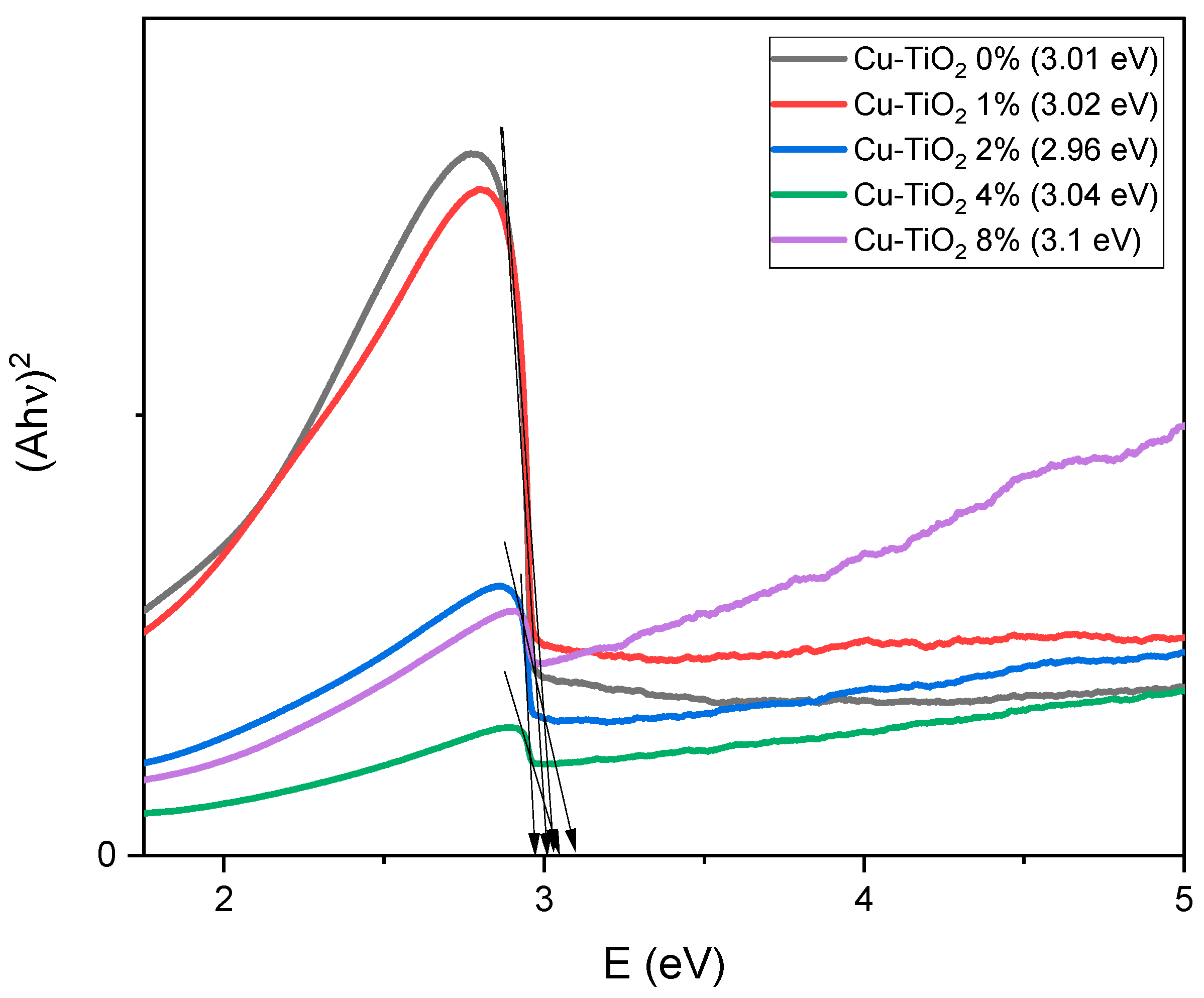
2.3. Optical Properties
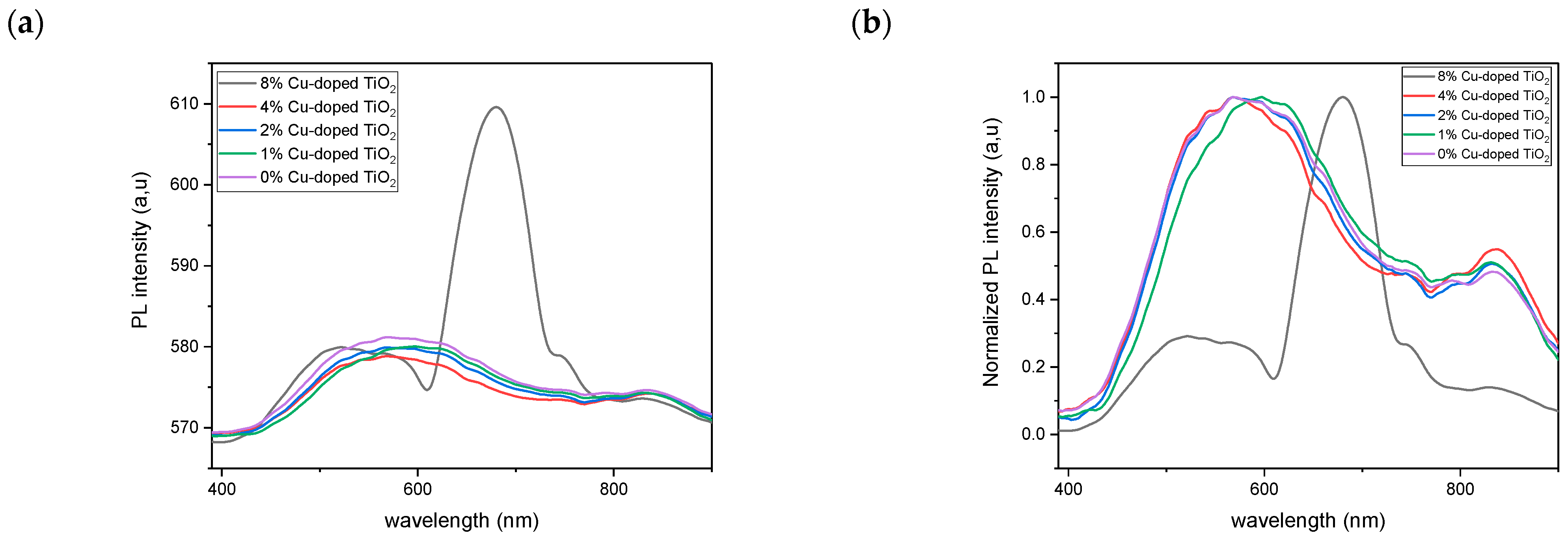
2.4. Photoluminescence
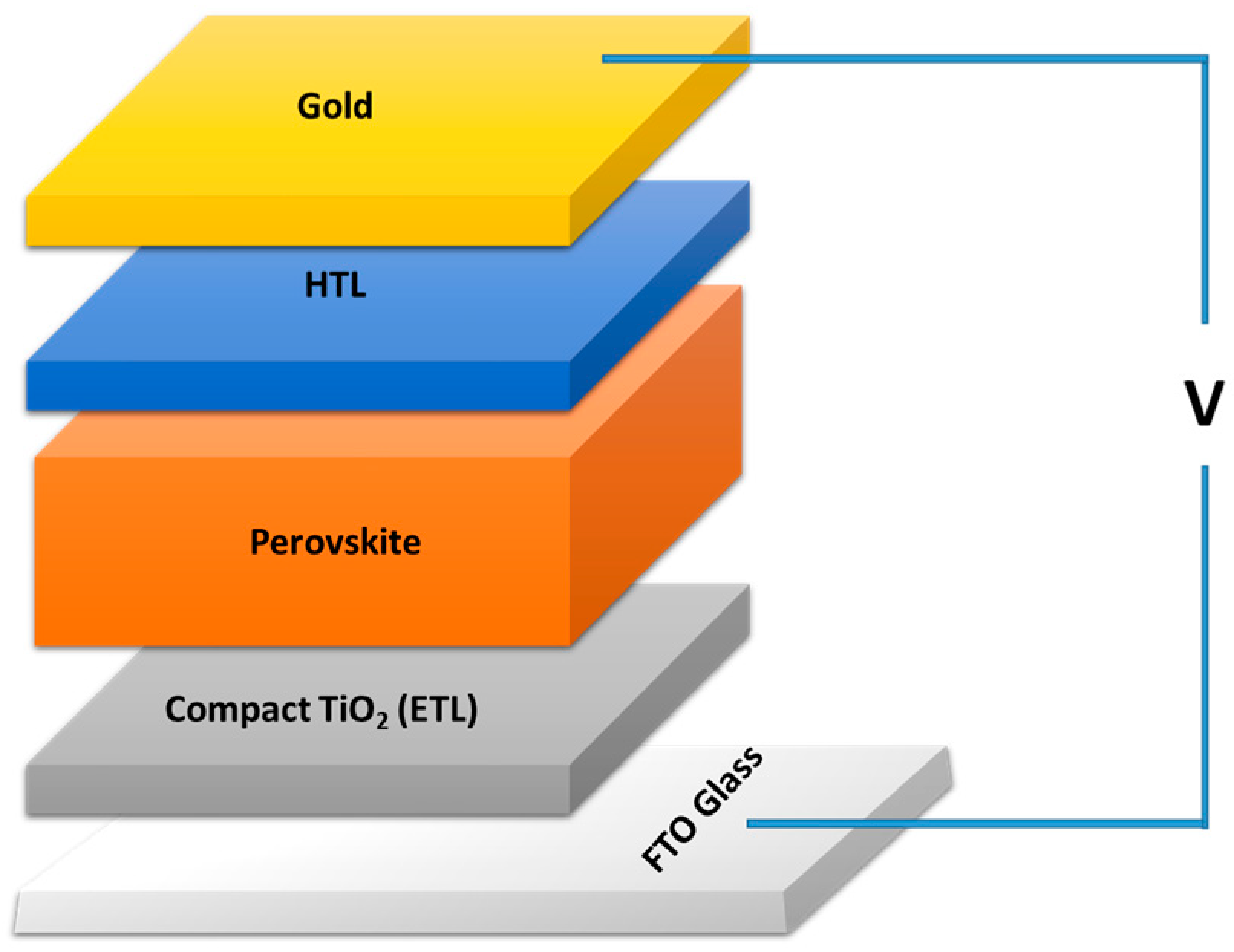
3. Experimental Details Materials and Methods
3.1. Materials
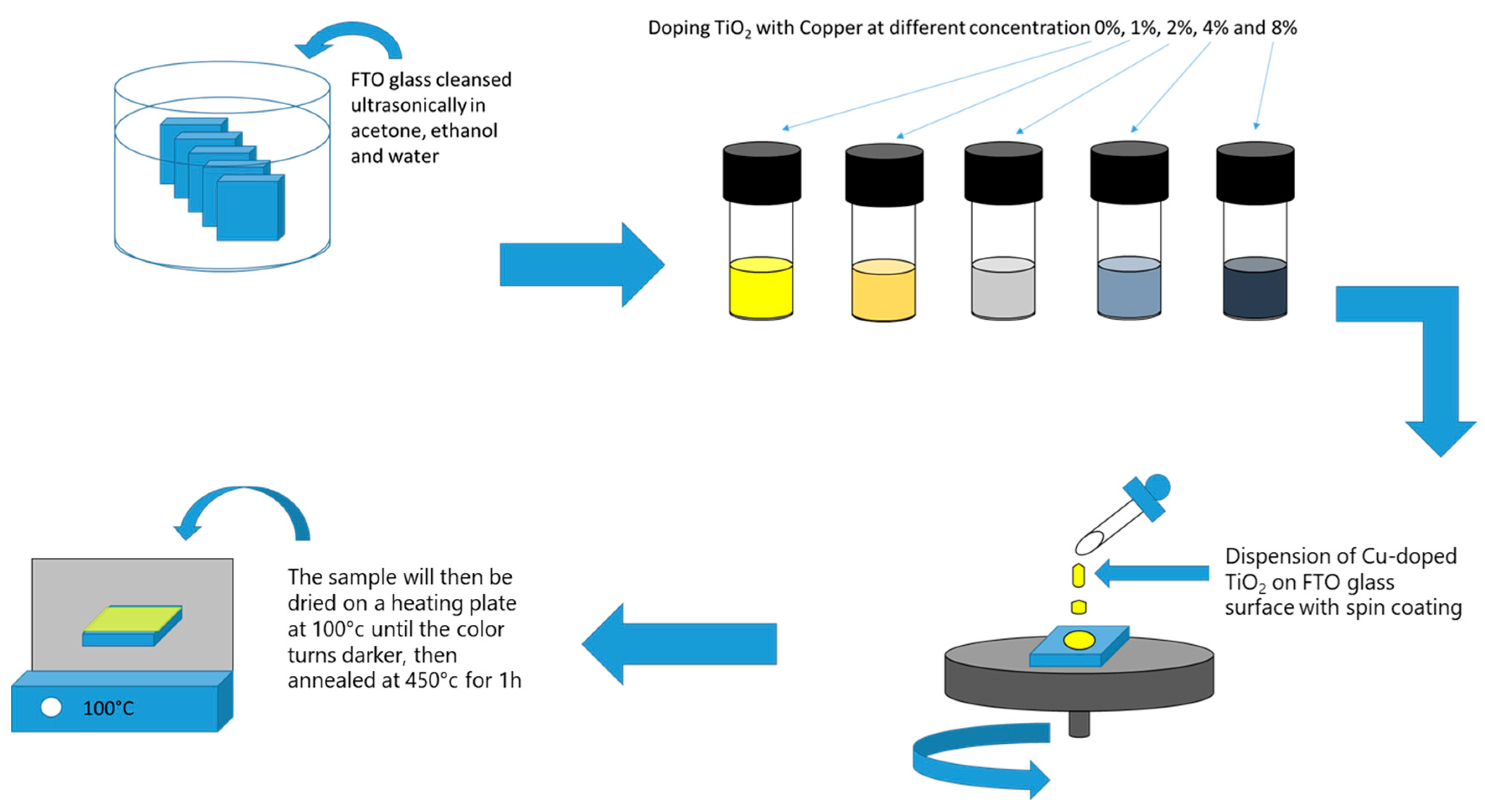
3.2. Pure and Cu-Doped TiO2 Film Elaboration
3.3. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hsu, A.; Rosengarten, C.; Weinfurter, A.; Xie, Y. Renewable Energy and Energy Efficiency in Developing Countries: Contributions to Reducing Global Emissions; United Nations Environment Programme: New York, NY, USA, 2017. [Google Scholar]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (Version 45). Prog. Photovolt. Res. Appl. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Saïdi, H.; Boujmil, M.F.; Durand, B.; Lazzari, J.L.; Bouaïcha, M. Elaboration and characterization of CuInSe2 thin films using one-step electrodeposition method on silicon substrate for photovoltaic application. Mater. Res. Express 2018, 5, 016414. [Google Scholar] [CrossRef]
- Rathore, N.; Panwar, N.L.; Yettou, F.; Gama, A. A comprehensive review of different types of solar photovoltaic cells and their applications. Int. J. Ambient. Energy 2021, 42, 1200–1217. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Y.; Qiu, L.; Dong, G.; Xia, D.; Liu, X.; Li, M.; Fan, R. Polyoxometalate-Based Inorganic-Organic Hybrid [Cu(phen)2]2[(α-Mo8O26)]: A New Additive to Spiro-OMeTAD for Efficient and Stable Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 4224–4233. [Google Scholar] [CrossRef]
- Wu, M.C.; Chan, S.H.; Lee, K.M.; Chen, S.H.; Jao, M.H.; Chen, Y.F.; Su, W.F. Enhancing the efficiency of perovskite solar cells using mesoscopic zinc-doped TiO2 as the electron extraction layer through band alignment. Mater. Chem. A 2018, 6, 16920–16931. [Google Scholar] [CrossRef]
- Teimouri, R.; Heydari, Z.; Ghaziani, M.P.; Madani, M.; Abdy, H.; Kolahdouz, M.; Asl-Soleimani, E. Synthesizing Li doped TiO2 electron transport layers for highly efficient planar perovskite solar cell. Superlattices Microstruct. 2020, 145, 106627. [Google Scholar] [CrossRef]
- Mohammad, A.; Mahjabeen, F. Promises and Challenges of Perovskite Solar Cells: A Comprehensive Review. BULLET J. Multidisiplin. Ilmu 2023, 2, 1147–1157. [Google Scholar]
- Ma, T.; Wang, S.; Zhang, Y.; Zhang, K. Yi, The development of all-inorganic CsPbX3 perovskite solar cells. J. Mater. Sci. 2020, 55, 464–479. [Google Scholar] [CrossRef]
- Bouich, A.; Torres, J.C.; Khattak, Y.H.; Baig, F.; Marí-Guaita, J.; Soucase, B.M.; Mendez-Blas, A.; Palacios, P. Bright future by controlling α/δ phase junction of formamidinium lead iodide doped by imidazolium for solar cells: Insight from experimental, DFT calculations and SCAPS simulation. Surf. Interfaces 2023, 40, 103159. [Google Scholar] [CrossRef]
- Ding, M.; Sun, L.; Chen, X.; Luo, T.; Ye, T.; Zhao, C.; Zhang, W.; Chang, H. Airprocessed, large grain perovskite films with low trap density from perovskite crystal engineering for high-performance perovskite solar cells with improved ambient stability. J. Mater. Sci. 2019, 54, 12000–12011. [Google Scholar] [CrossRef]
- Bouich, A.; Marí-Guaita, J.; Soucase, B.M.; Palacios, P. Bright future by enhancing the stability of methylammonium lead triiodideperovskites thin films through Rb, Cs and Li as dopants. Mater. Res. Bull. 2023, 163, 112213. [Google Scholar] [CrossRef]
- Hossain, M.K.; Samajdar, D.P.; Das, R.C.; Arnab, A.A.; Rahman, M.F.; Rubel, M.H.; Islam, M.R.; Bencherif, H.; Pandey, R.; Madan, J.; et al. Design and simulation of Cs2BiAgI6 double perovskite solar cells with different electron transport layers for efficiency enhancement. Energy Fuels 2023, 37, 3957–3979. [Google Scholar] [CrossRef]
- Mukametkali, T.M.; Ilyassov, B.R.; Aimukhanov, A.K.; Serikov, T.M.; Baltabekov, A.S.; Aldasheva, L.S.; Zeinidenov, A.K. Effect of the TiO2 electron transport layer thickness on charge transfer processes in perovskite solar cells. Phys. B Condens. Matter 2023, 659, 414784. [Google Scholar] [CrossRef]
- Cheng, M.; Zuo, C.; Wu, Y.; Li, Z.; Xu, B.; Hua, Y.; Ding, L. Charge-transport layer engineering in perovskite solar cells. Sci. Bull. 2020, 65, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jones, T.W.; Yang, T.C.; Duffy, N.W.; Li, J.; Zhao, L.; Chi, B.; Wang, X.; Wilson, G.J. Inorganic electron transport materials in perovskite solar cells. Adv. Funct. Mater. 2021, 31, 2008300. [Google Scholar] [CrossRef]
- Li, F.; Shen, Z.; Weng, Y.; Lou, Q.; Chen, C.; Shen, L.; Guo, W.; Li, G. Novel electron transport layer material for perovskite solar cells with over 22% efficiency and long-term stability. Adv. Funct. Mater. 2020, 30, 2004933. [Google Scholar] [CrossRef]
- Valadi, K.; Gharibi, S.; Taheri-Ledari, R.; Akin, S.; Maleki, A.; Shalan, A.E. Metal oxide electron transport materials for perovskite solar cells: A review. Environ. Chem. Lett. 2021, 19, 2185–2207. [Google Scholar] [CrossRef]
- Zhen, C.; Wu, T.; Chen, R.; Wang, L.; Liu, G.; Cheng, H.M. Strategies for Modifying TiO2 Based Electron Transport Layers to Boost Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 4586–4618. [Google Scholar] [CrossRef]
- Kaewprajak, A.; Kumnorkaew, P.; Lohawet, K.; Duong, B.; Chonsut, T.; Kayunkid, N.; Saranrom, N.; Promarak, V. An unconventional blade coating for low-cost fabrication of PCDTBT: PC70BM polymer and CH3NH3PbIxCl3-x perovskite solar cells. Surf. Interfaces 2021, 23, 100969. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Nishat, S.S.; Hossain, M.J.; Mullick, F.E.; Kabir, A.; Chowdhury, S.; Islam, S.; Hossain, M. Performance analysis of perovskite solar cells using DFT-extracted parameters of metal-doped TiO2 electron transport layer. J. Phys. Chem. C 2021, 125, 13158–13166. [Google Scholar] [CrossRef]
- Che Halin, D.S.; Azhari, A.W.; Mohd Salleh, M.A.; Muhammad Nadzri, N.I.; Vizureanu, P.; Abdullah, M.M.; Wahab, J.A.; Sandu, A.V. Metal-Doped TiO2 Thin Film as an Electron Transfer Layer for Perovskite Solar Cells: A Review. Coatings 2022, 13, 4. [Google Scholar] [CrossRef]
- Chen, S.H.; Ho, C.M.; Chang, Y.H.; Lee, K.M.; Wu, M.C. Efficient perovskite solar cells with low J-V hysteretic behavior based on mesoporous Sn-doped TiO2 electron extraction layer. Chem. Eng. J. 2022, 445, 136761. [Google Scholar] [CrossRef]
- Chen, S.H.; Chan, S.H.; Lin, Y.T.; Wu, M.C. Enhanced power conversion efficiency of perovskite solar cells based on mesoscopic Ag-doped TiO2 electron transport layer. Appl. Surf. Sci. 2019, 469, 18–26. [Google Scholar]
- Dahlan, D.; Saad, S.K.; Berli, A.U.; Bajili, A.; Umar, A.A. Synthesis of two-dimensional nanowall of Cu-Doped TiO2 and its application as photoanode in DSSCs. Phys. E Low-Dimens. Syst. Nanostructures 2017, 91, 185–189. [Google Scholar] [CrossRef]
- Abbas, M.M.; Rasheed, M. Investigation of structural, Mechanical, Thermal and Optical Properties of Cu Doped TiO2. Iraqi J. Phys. 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Mane, G.P.; Rane, V.; Tripathi, A.K.; Tyagi, A.K. Selective CO2 photoreduction with Cu-doped TiO2 photocatalyst: Delineating the crucial role of Cu-oxidation state and oxygen vacancies. J. Phys. Chem. C 2021, 125, 1793–1810. [Google Scholar] [CrossRef]
- Dhonde, M.; Sahu, K.; Murty, V.V. Cu-doped TiO2 nanoparticles/graphene composites for efficient dye-sensitized solar cells. Sol. Energy 2021, 220, 418–424. [Google Scholar] [CrossRef]
- Deng, Z.; Li, L.; Ren, Y.; Ma, C.; Liang, J.; Dong, K.; Liu, Q.; Luo, Y.; Li, T.; Tang, B.; et al. Highly efficient two-electron electroreduction of oxygen into hydrogen peroxide over Cu-doped TiO2. Nano Res. 2022, 15, 3880–3885. [Google Scholar] [CrossRef]
- Raguram, T.; Rajni, K.S. Synthesis and characterisation of Cu-Doped TiO2 nanoparticles for DSSC and photocatalytic applications. Int. J. Hydrog. Energy 2022, 47, 4674–4689. [Google Scholar] [CrossRef]
- He, Z.; Zhang, S.; Yin, L.; Hayat, M.D.; Cao, P. Cu–TiO2 nanocomposite coatings prepared from sol-enhanced electrodeposition. Int. J. Mod. Phys. B 2020, 34, 2040038. [Google Scholar] [CrossRef]
- Reda, S.M.; Khairy, M.; Mousa, M.A. Photocatalytic activity of nitrogen and copper doped TiO2 nanoparticles prepared by microwave-assisted sol-gel process. Arab. J. Chem. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Farzaneh, A.; Javidani, M.; Esrafili, M.D.; Mermer, O. Optical and photocatalytic characteristics of Al and Cu doped TiO2: Experimental assessments and DFT calculations. J. Phys. Chem. Solids 2022, 161, 110404. [Google Scholar] [CrossRef]
- Sahu, K.; Dhonde, M.; Murty, V.V. Microwave-assisted hydrothermal synthesis of Cu-doped TiO2 nanoparticles for efficient dye-sensitized solar cell with improved open-circuit voltage. Int. J. Energy Res. 2021, 45, 5423–5432. [Google Scholar] [CrossRef]
- Jasima, F.H.; Shakirb, H.R.; Chiada, S.S.; Habubic, N.F.; Mosad, Z.S.; Kadhime, Y.H.; Jadanf, M. Characterizations of sprayed TiO2 and Cu doped TiO2 thin films prepared by spray pyrolysis method. Dig. J. Nanomater. Biostructures (DJNB) 2023, 18, 1385–1393. [Google Scholar] [CrossRef]
- Lukong, V.T.; Ukoba, K.; Jen, T.C. Review of self-cleaning TiO2 thin films deposited with spin coating. Int. J. Adv. Manuf. Technol. 2022, 122, 3525–3546. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Gong, Z.; Shen, Z.; Ye, Y.; Yang, B.; Qiu, Y.; Ye, B.; Xu, L.; Guo, T.; et al. Weak light-stimulated synaptic hybrid phototransistors based on islandlike perovskite films prepared by spin coating. ACS Appl. Mater. Interfaces 2021, 13, 13362–13371. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chang, Y.H.; Lin, T.H.; Chan, S.H.; Lee, K.M.; Hsu, K.H.; Hsu, J.F.; Wu, M.C. Boosting the power conversion efficiency of perovskite solar cells based on Sn doped TiO2 electron extraction layer via modification the TiO2 phase junction. Sol. Energy 2020, 205, 390–398. [Google Scholar] [CrossRef]
- Hajjaji, M.A.; Missaoui, K.; Trabelsi, K.; Bouzaza, A.; Bessais, B.; Hajjaji, A.; Assadi, A.A. Electrodeposited Platinum Nanoparticles on Highly Ordered Titanium Dioxide Nanotubes for Photocatalytic Application: Enhancement of Photocatalytic Degradation of Amido Black Dye. Catal. Lett. 2023, 154, 1242–1254. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Bark, C.W. Synthesis of Cobalt-Doped TiO2 Based on Metal-Organic Frameworks as an Effective Electron Transport Material in Perovskite Solar Cells. ACS Omega 2020, 5, 2280–2286. [Google Scholar] [CrossRef] [PubMed]
- Bensouici, F.; Bououdina, M.; Dakhel, A.A.; Tala-Ighil, R.; Tounane, M.; Iratni, A.; Souier, T.; Liu, S.; Cai, W.J. Optical, structural and photocatalysis properties of Cu-doped TiO2 thin films. J. Appl. Sci. 2017, 395, 110–116. [Google Scholar]
- Sassi, S.; Trabelsi, K.; El Jery, A.; Abidi, M.; Hajjaji, A.; Khezami, L.; Karrech, A.; Gaidi, M.; Soucase, B.M.; Bessais, B. Synergistic effect of CuxOy-NPs/TiO2-NTs heterostructure on the photodegradation of amido black staining. Optik 2023, 272, 170234. [Google Scholar] [CrossRef]
- Trabelsi, K.; Jemai, S.; El Jery, A.; Sassi, S.; Guesmi, A.; Khezami, L.; Hajjaji, A.; Gaidi, M.; Bessais, B. Ag-NPs coating influence on TiO2-NTs photocatalytic performances on Amido Black staining. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Sławek, A.; Starowicz, Z.; Lipiński, M. The influence of the thickness of compact TiO2 electron transport layer on the performance of planar CH3NH3PbI3 perovskite solar cells. Materials 2021, 14, 3295. [Google Scholar] [CrossRef]
- Bouich, A.; Torres, J.C.; Chfii, H.; Marí-Guaita, J.; Khattak, Y.H.; Baig, F.; Soucase, B.M.; Palacios, P. Delafossite as hole transport layer a new pathway for efficient perovskite-based solar cells: Insight from experimental, DFT and numerical analysis. Sol. Energy 2023, 250, 18–32. [Google Scholar] [CrossRef]
- Nair, S.S.; Mathews, M.; Anantharaman, M.R. Anantharaman. Evidence for blueshift by weak exciton confinement and tuning of bandgap in superparamagnetic nanocomposites. Chem. Phys. Lett. 2005, 406, 398–403. [Google Scholar] [CrossRef]
- Deotale, A.J.; Nandedkar, R.V. Correlation between particle size, strain and band gap of iron oxide nanoparticles. Mater. Today Proc. 2016, 3, 2069–2076. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Kalarikkal, N.; Sivakumar, J.; Reddy, M.R.; Sayanna, R. Structural, optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts. Ceram. Int. 2019, 45, 25060–25068. [Google Scholar] [CrossRef]
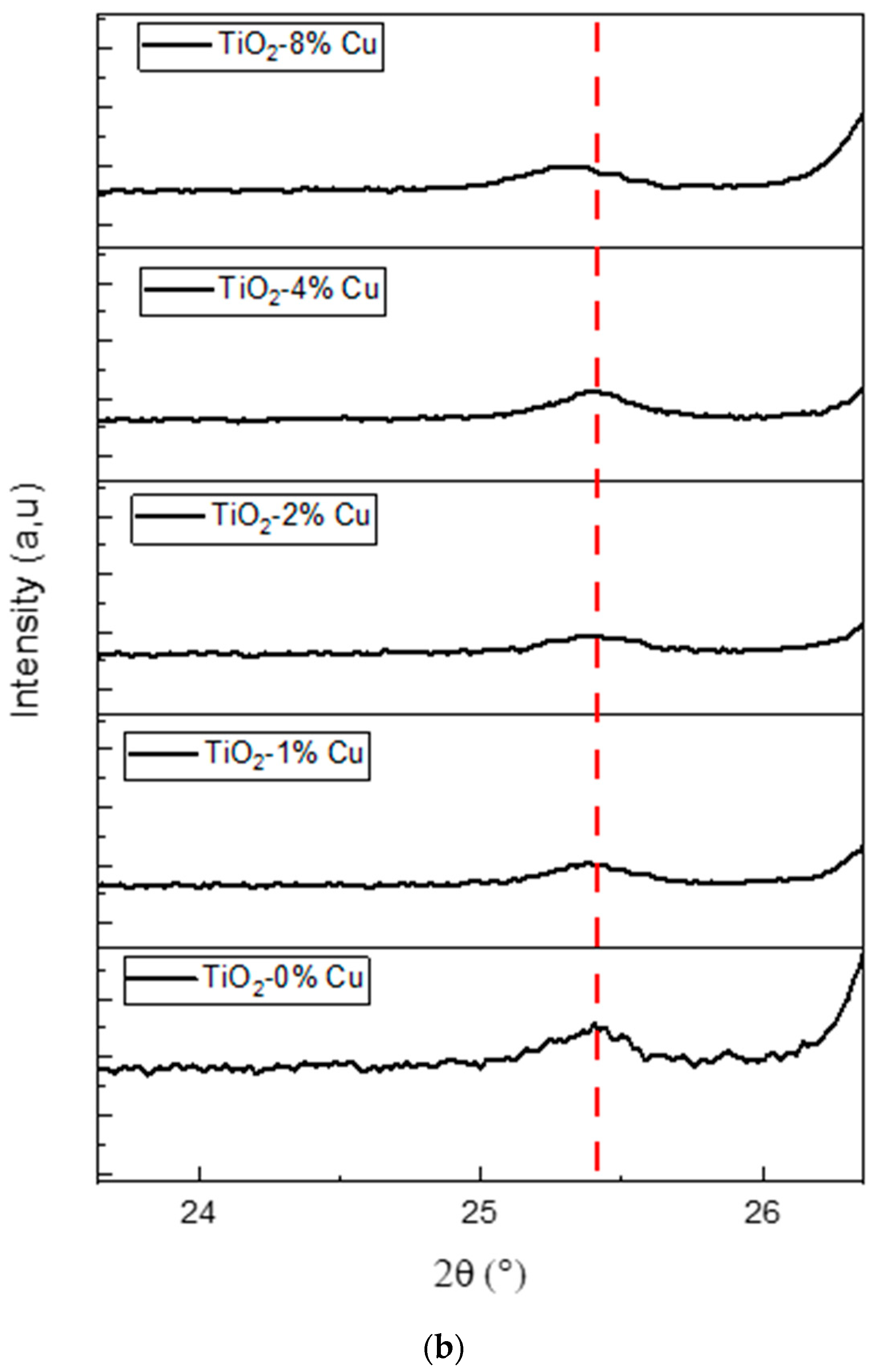
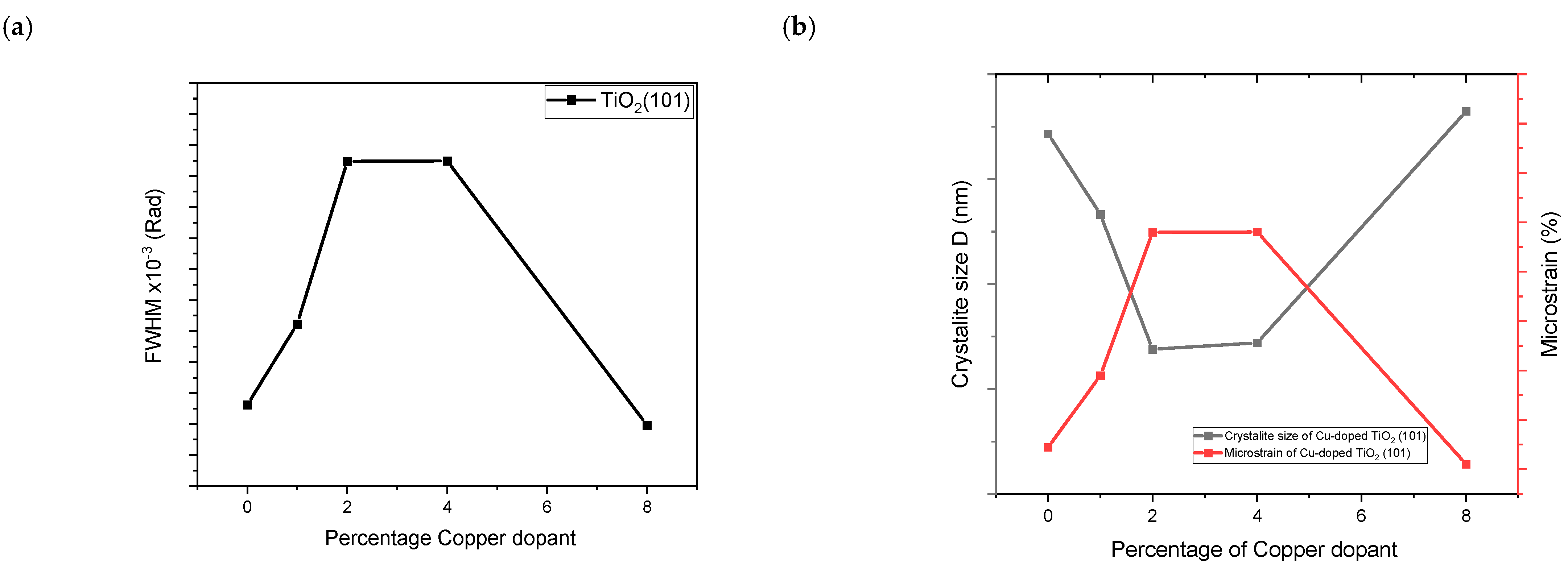
| Cu-Doped TiO2 (101) | 2ϴ (deg) | d(101) (Å) | FWHM (rad) | D (nm) | 1/D (nm−1) | Ԑ (%) × 10−3 |
|---|---|---|---|---|---|---|
| 0% Cu | 25.389 | 3.506934 | 0.002962 | 47.16 | 0.0212 | 3.289 |
| 1% Cu | 25.388 | 3.515507 | 0.003223 | 43.33 | 0.023 | 3.579 |
| 2% Cu | 25.397 | 3.472665 | 0.003748 | 36.9 | 0.0271 | 4.16 |
| 4% Cu | 25.40 | 3.513734 | 0.003749 | 37.2 | 0.0268 | 4.161 |
| 8% Cu | 25.32 | 3.508971 | 0.002896 | 48.23 | 0.0207 | 3.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassi, S.; Bouich, A.; Hajjaji, A.; Khezami, L.; Bessais, B.; Soucase, B.M. Cu-Doped TiO2 Thin Films by Spin Coating: Investigation of Structural and Optical Properties. Inorganics 2024, 12, 188. https://doi.org/10.3390/inorganics12070188
Sassi S, Bouich A, Hajjaji A, Khezami L, Bessais B, Soucase BM. Cu-Doped TiO2 Thin Films by Spin Coating: Investigation of Structural and Optical Properties. Inorganics. 2024; 12(7):188. https://doi.org/10.3390/inorganics12070188
Chicago/Turabian StyleSassi, Syrine, Amal Bouich, Anouar Hajjaji, Lotfi Khezami, Brahim Bessais, and Bernabé Mari Soucase. 2024. "Cu-Doped TiO2 Thin Films by Spin Coating: Investigation of Structural and Optical Properties" Inorganics 12, no. 7: 188. https://doi.org/10.3390/inorganics12070188







