2,1,3-Benzoselenadiazole as Mono- and Bidentate N-Donor for Heteroleptic Cu(I) Complexes: Synthesis, Characterization and Photophysical Properties
Abstract
:1. Introduction
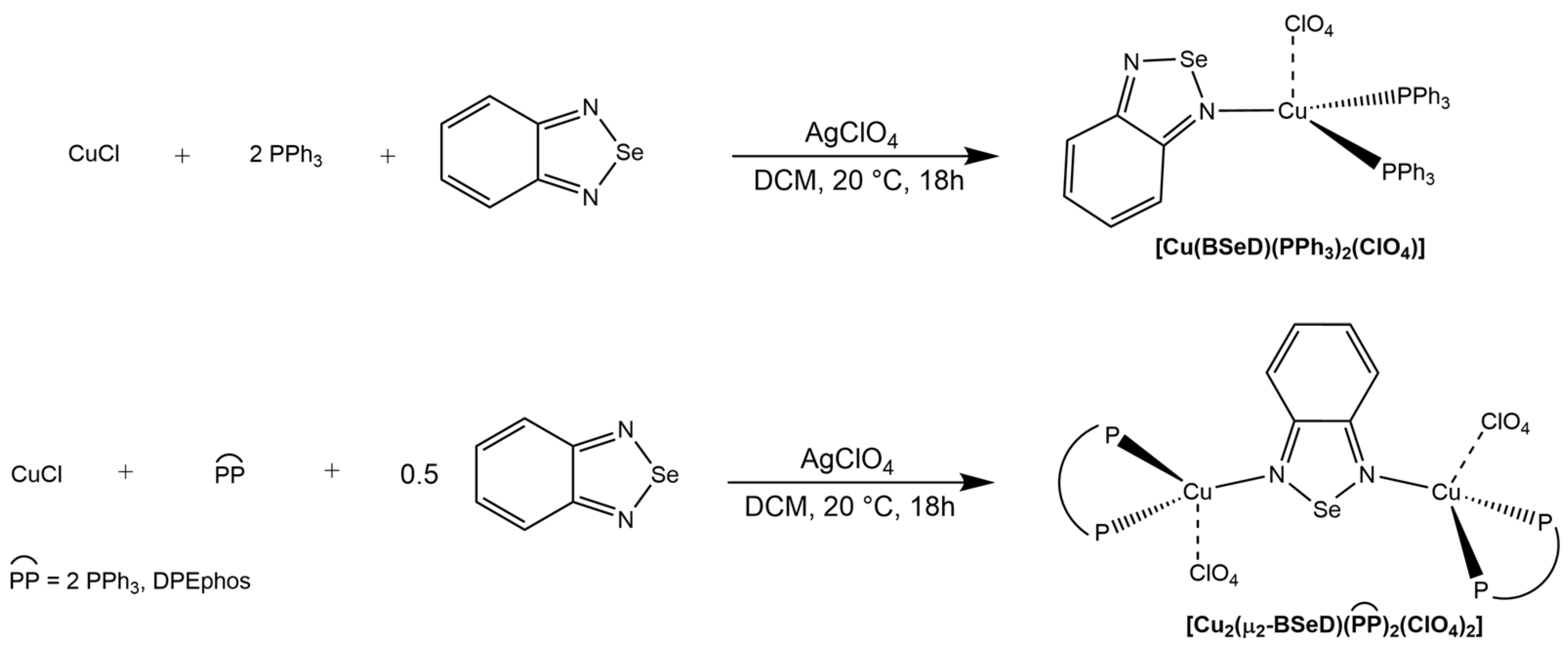
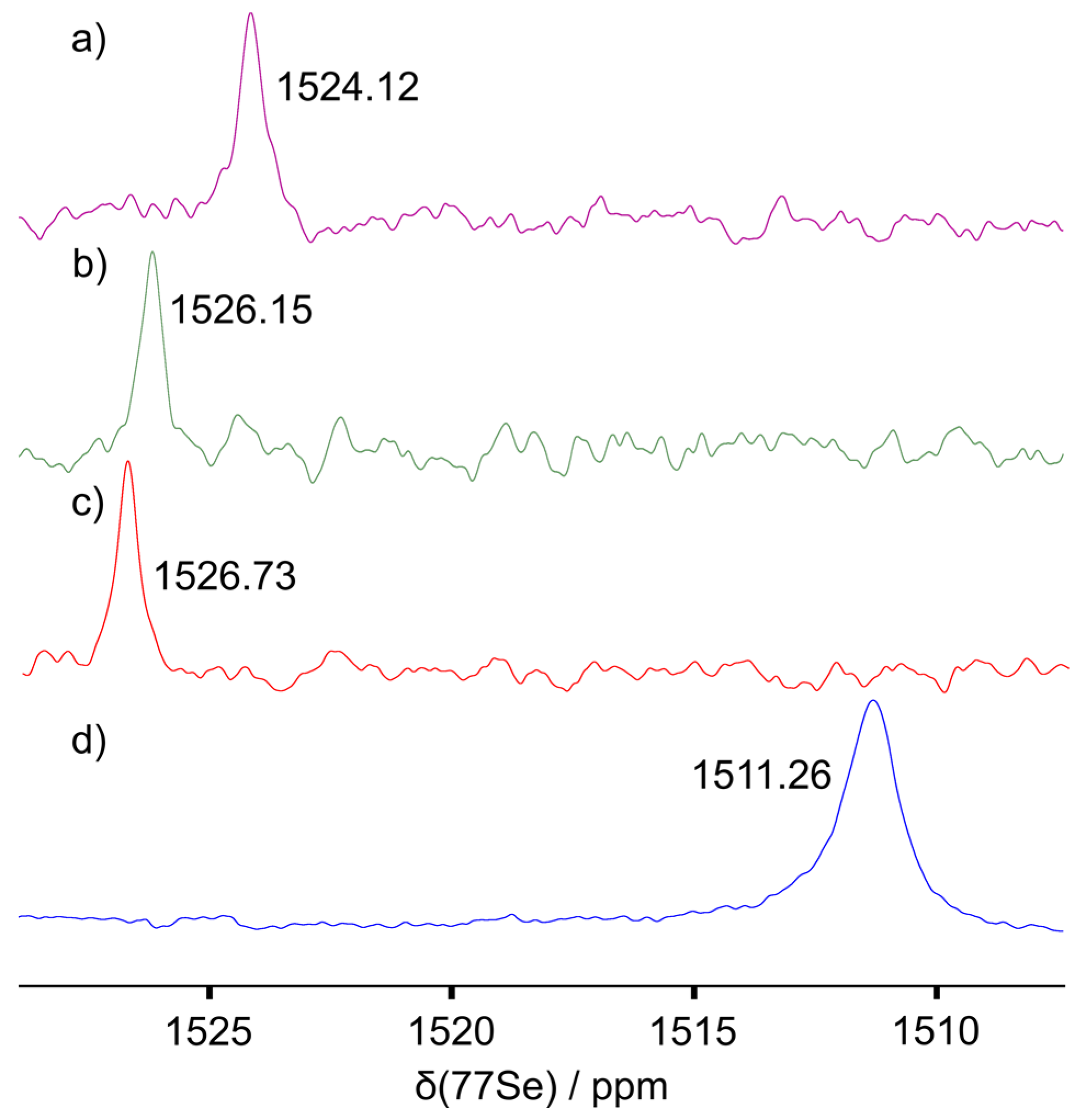
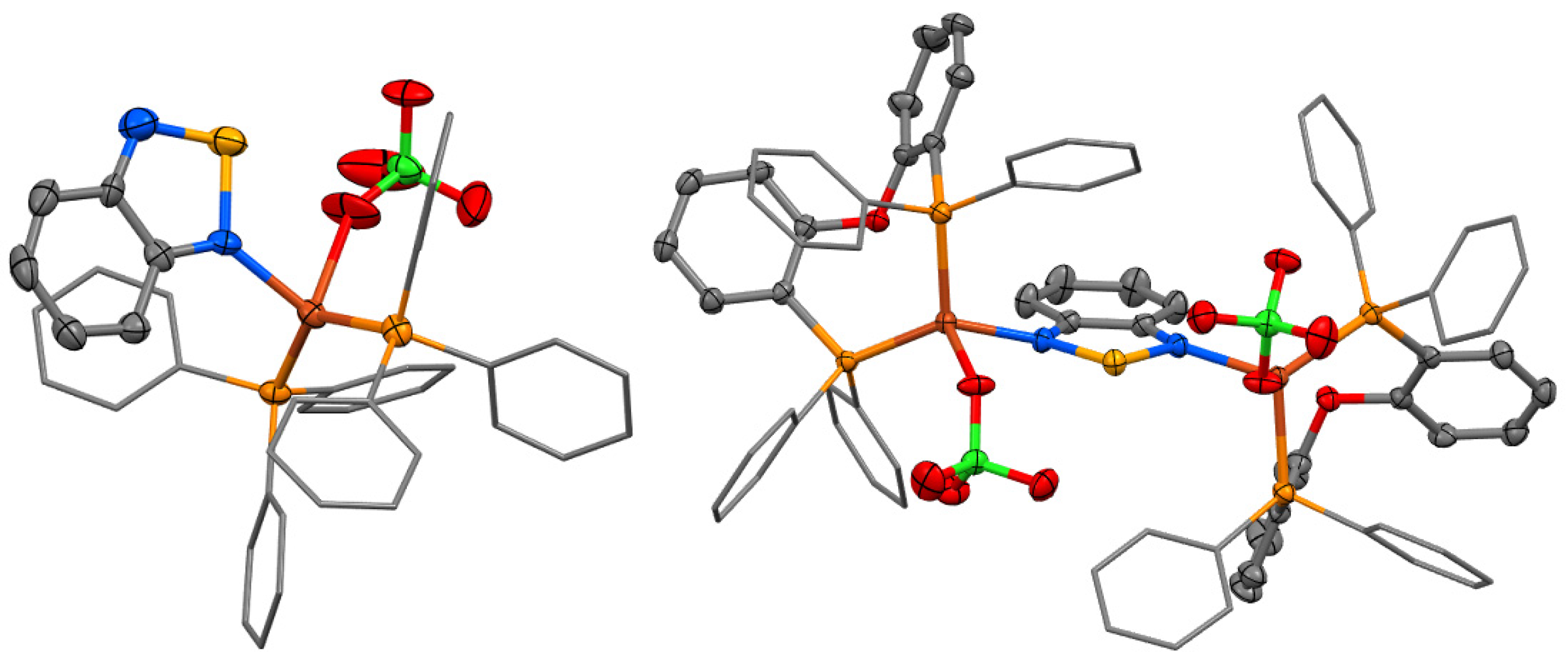
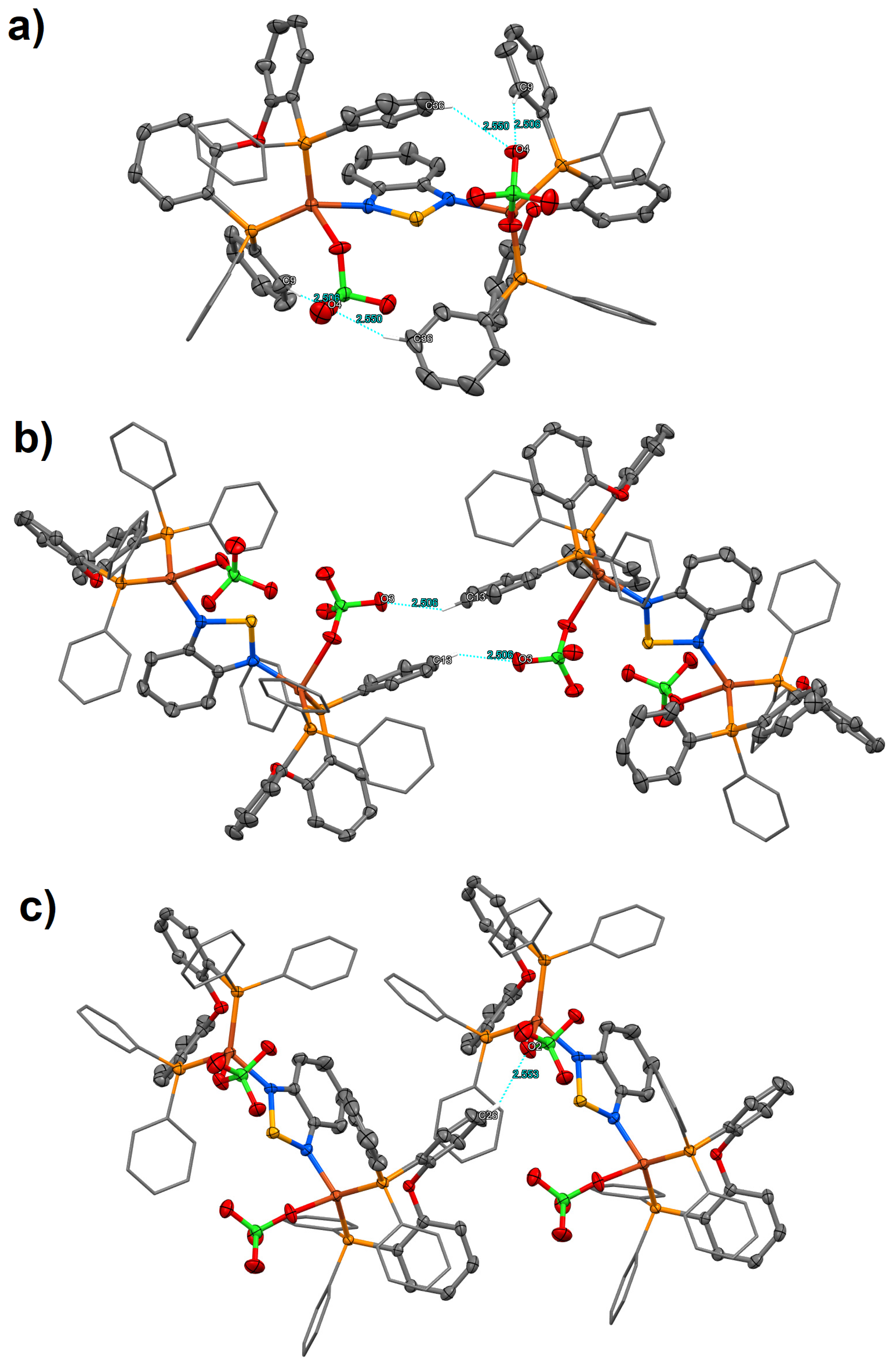
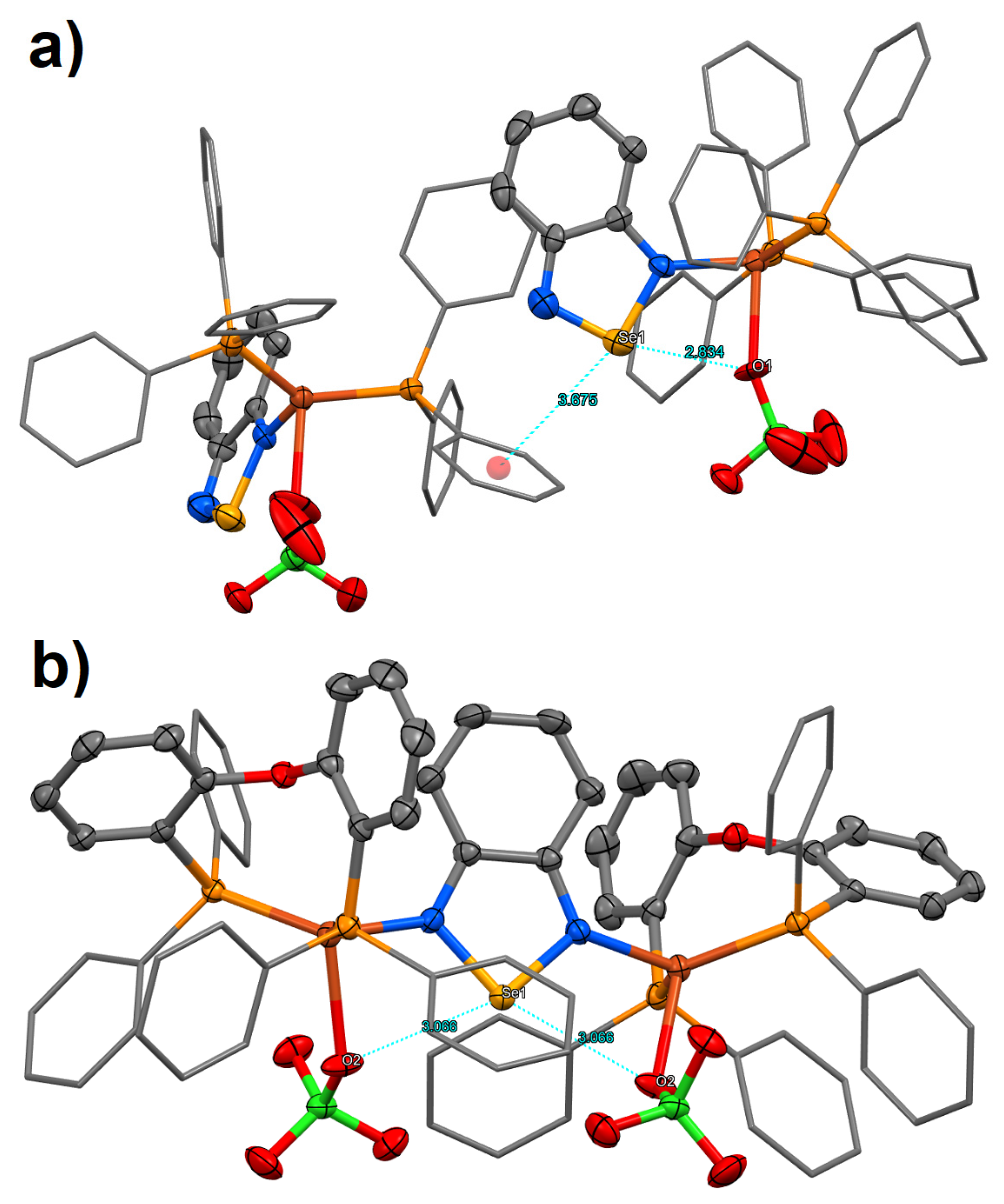
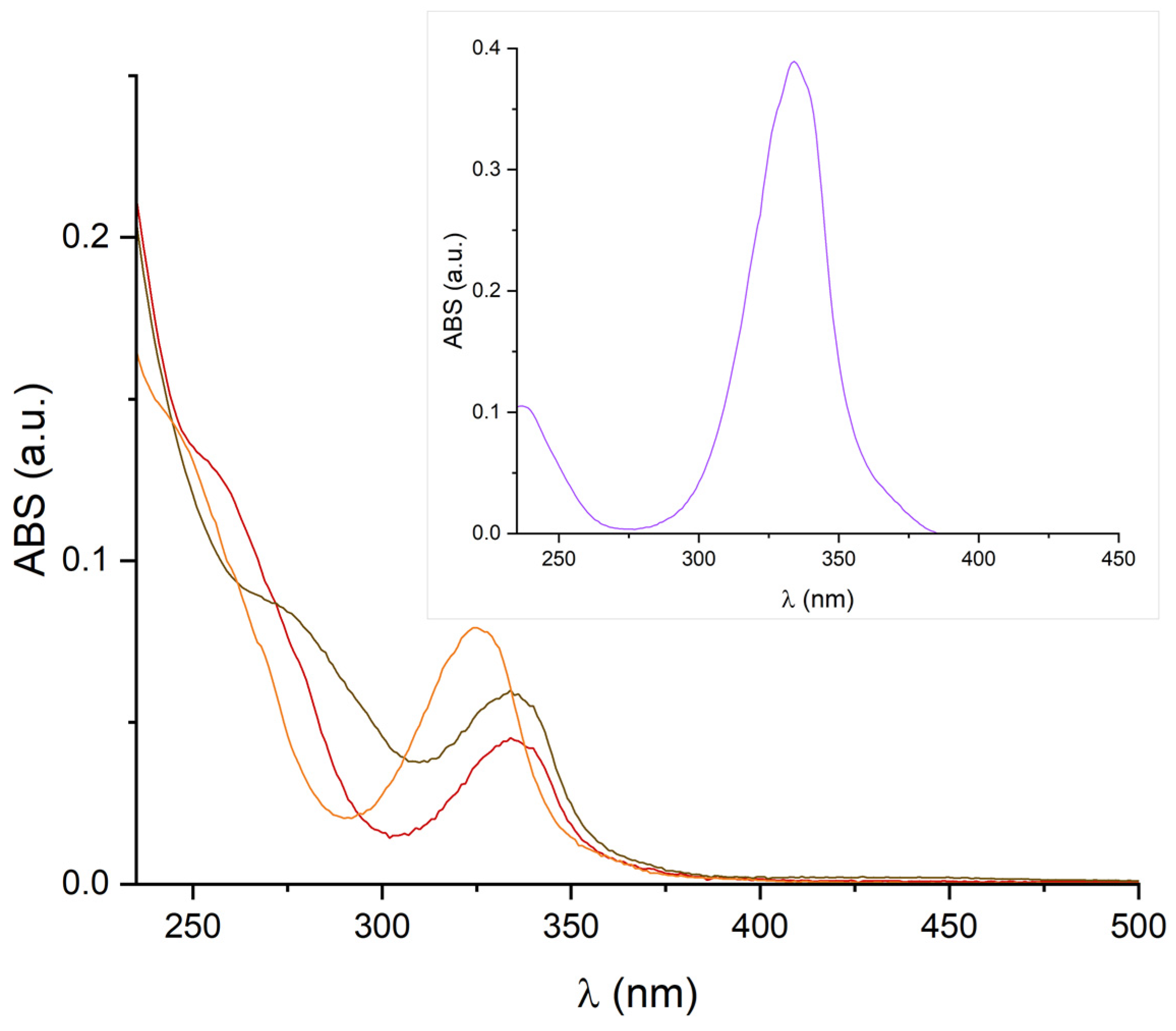
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 2,1,3-Benzoselenadiazole (BSeD)
3.2. Synthesis of the Mononuclear Cu(I) Complex
3.3. Synthesis of the Binuclear Cu(I) Complexes
3.4. Crystal Structure Determination
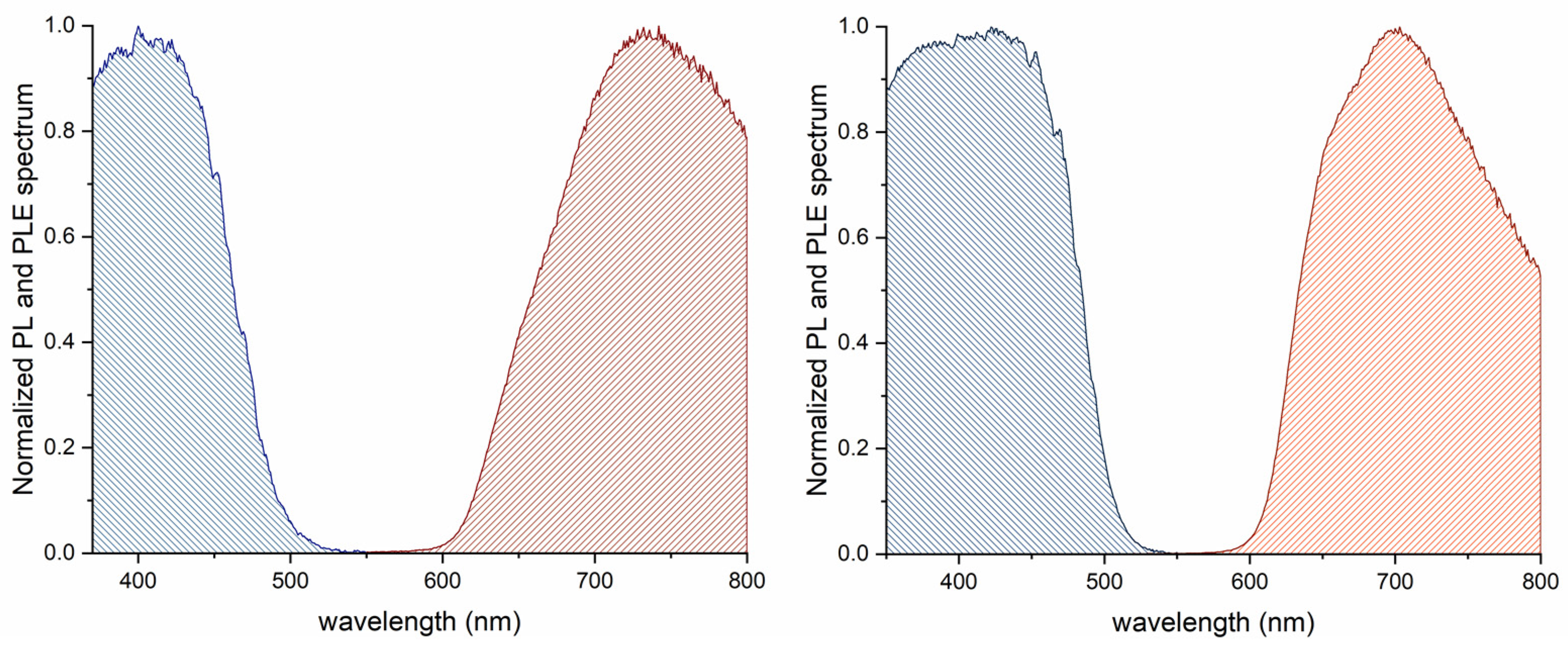
3.5. Photoluminescent Measurements
3.6. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Findlay, N.J.; Breig, B.; Forbes, C.; Inigo, A.R.; Kanibolotsky, A.L.; Skabara, P.J. High brightness solution-processed OLEDs employing linear, small molecule emitters. J. Mater. Chem. C 2016, 4, 3774–3780. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic sensitizers from D–π–A to D–A–π–A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.A.D.; Lapis, A.A.M.; Da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Du, J.; Biewer, M.C.; Stefan, M.C. Benzothiadiazole building units in solution-processable small molecules for organic photovoltaics. J. Mater. Chem. A 2016, 4, 15771–15787. [Google Scholar] [CrossRef]
- Neto, B.A.; Carvalho, P.H.; Correa, J.R. Benzothiadiazole Derivatives as Fluorescence Imaging Probes: Beyond Classical Scaffolds. Acc. Chem. Res. 2015, 48, 1560–1569. [Google Scholar] [CrossRef]
- Li, C.C.; Cao, J.; Wang, L.; Ji, X.; Xu, Y.; Wang, J.Y. π-Extended BTD Derivatives: Synthesis, Photophysical Properties, and Applications in Biological Systems Imaging for Discriminating Living and Dead Cells. Chem. Asian J. 2023, 18, e202300038. [Google Scholar] [CrossRef]
- Chen, C.-P.; Wu, P.-J.; Liou, S.-Y.; Chan, Y.-H. Ultrabright benzoselenadiazole-based semiconducting polymer dots for specific cellular imaging. RSC Adv. 2013, 3, 17507. [Google Scholar] [CrossRef]
- Patel, H.A.; Bhanvadia, V.J.; Mande, H.M.; Zade, S.S.; Patel, A.L. Benzochalcogendiazole-based conjugated molecules: Investigating the effects of substituents and heteroatom juggling. Org. Biomol. Chem. 2019, 17, 9467–9478. [Google Scholar] [CrossRef]
- Li, H.; Chi, Z.; Zhang, X.; Xu, B.; Liu, S.; Zhang, Y.; Xu, J. New thermally stable aggregation-induced emission enhancement compounds for non-doped red organic light-emitting diodes. Chem. Commun. 2011, 47, 11273. [Google Scholar] [CrossRef]
- Gao, S.; Balan, B.; Yoosaf, K.; Monti, F.; Bandini, E.; Barbieri, A.; Armaroli, N. Highly Efficient Luminescent Solar Concentrators Based on Benzoheterodiazole Dyes with Large Stokes Shifts. Chem. Eur. J. 2020, 26, 11013–11023. [Google Scholar] [CrossRef]
- Ho, P.C.; Wang, J.Z.; Meloni, F.; Vargas-Baca, I. Chalcogen bonding in materials chemistry. Coord. Chem. Rev. 2020, 422, 213464. [Google Scholar] [CrossRef]
- Bryant, J.J.; Lindner, B.D.; Bunz, U.H. Water-soluble bis-triazolyl benzochalcogendiazole cycloadducts as tunable metal ion sensors. J. Org. Chem. 2013, 78, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Girotto, M.; Castro, J.; Bortoluzzi, M. Intense millisecond-long red luminescence from heteroleptic Cu(I) 2,1,3-benzothiadiazole complexes. Dye. Pigm. 2023, 217, 111388. [Google Scholar] [CrossRef]
- Busch, J.M.; Rehak, F.R.; Ferraro, V.; Nieger, M.; Kemell, M.; Fuhr, O.; Klopper, W.; Bräse, S. From Mono- to Polynuclear 2-(Diphenylphosphino)pyridine-Based Cu(I) and Ag(I) Complexes: Synthesis, Structural Characterization, and DFT Calculations. ACS Omega 2024, 9, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Fuhr, O.; Bizzarri, C.; Bräse, S. Substituted Pyrrole-based Schiff Bases: Effect on The Luminescence of Neutral Heteroleptic Cu(I) Complexes. Eur. J. Inorg. Chem. 2024, 27, e202400080. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, K.; Teat, S.J.; Reid, O.G.; Hei, X.; Zhu, K.; Fang, X.; Li, M.; Sojdak, C.A.; Cotlet, M.; et al. Robust and Highly Conductive Water-Stable Copper Iodide-Based Hybrid Single Crystals. Chem. Mater. 2022, 34, 10040–10049. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Khisamov, R.M.; Bashirov, D.A.; Komarov, V.Y.; Molokeev, M.S.; Ryadun, A.A.; Benassi, E.; Konchenko, S.N. Tuning of the Coordination and Emission Properties of 4-Amino-2,1,3-Benzothiadiazole by Introduction of Diphenylphosphine Group. Cryst. Growth Des. 2020, 20, 5796–5807. [Google Scholar] [CrossRef]
- Plebst, S.; Bubrin, M.; Schweinfurth, D.; Záliš, S.; Kaim, W. Metal carbonyl complexes of potentially ambidentate 2,1,3-benzothiadiazole and 2,1,3-benzoselenadiazole acceptors. Z. Naturforsch. B 2017, 72, 839–846. [Google Scholar] [CrossRef]
- Herberhold, M.; Hill, A.F. The coordination chemistry of 2,1,3-benzothiadiazole and 2,1,3-benzoselenadiazole. Complexes of ruthenium, osmium and iridium. J. Organomet. Chem. 1989, 377, 151–156. [Google Scholar] [CrossRef]
- Cowley, A.R.; Hector, A.L.; Hill, A.F.; White, A.J.P.; Williams, D.J.; Wilton-Ely, J.D.E.T. Synthesis and Reactions of Five-Coordinate Mono- and Binuclear Thiocarbonyl−Alkenyl and Thioacyl Complexes of Ruthenium (II). Organometallics 2007, 26, 6114–6125. [Google Scholar] [CrossRef]
- Tan, C.K.; Wang, J.; Leng, J.D.; Zheng, L.L.; Tong, M.L. The Use of 2,1,3-Benzoselenadiazole as an Auxiliary Ligand for the Construction of New 2D Silver(I)/Benzene- or Cyclohexane-1,3,5-tricarboxylate Honeycomb Networks. Eur. J. Inorg. Chem. 2008, 2008, 771–778. [Google Scholar] [CrossRef]
- Milios, C.J.; Ioannou, P.V.; Raptopoulou, C.P.; Papaefstathiou, G.S. Crystal engineering with 2,1,3-benzoselenadiazole and mercury (II) chloride. Polyhedron 2009, 28, 3199–3202. [Google Scholar] [CrossRef]
- Mitra, R.; Sridevi, V.S.; Somasundaram, K.; Samuelson, A.G. Photophysical and Biological Studies with Organometallic Ruthenium Complexes of Selenodiazole Ligands. Proc. Natl. Acad. Sci. India A 2016, 86, 511–520. [Google Scholar] [CrossRef]
- Sysoeva, A.A.; Novikov, A.S.; Suslonov, V.V.; Bolotin, D.S.; Il’in, M.V. 2,1,3-Benzoselenadiazole-containing zinc (II) halide complexes: Chalcogen bonding in the solid state and catalytic activity in the Schiff condensation. Inorg. Chim. Acta 2024, 561, 121867. [Google Scholar] [CrossRef]
- Parsons, S. Determination of absolute configuration using X-ray diffraction. Tetrahedron: Asymmetry 2017, 28, 1304–1313. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 21, 2832. [Google Scholar] [CrossRef] [PubMed]
- Bera, J.K.; Nethaji, M.; Samuelson, A.G. Synthesis and Structures of Oxyanion Encapsulated Copper(I)−dppm Complexes (dppm = Bis(diphenylphosphino)methane). Inorg. Chem. 1999, 38, 1725. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Han, X.; Li, C.; Xu, Y.; Shen, K.; Shi, X.; Wu, H. Cu(I) complexes regulated by N-heterocyclic ligands: Syntheses, structures, fluorescence and electrochemical properties. Spectrochim. Acta A 2018, 203, 408. [Google Scholar] [CrossRef]
- Chou, C.-C.; Su, C.-C.; Yeh, A. Mononuclear and Dinuclear Copper(I) Complexes of Bis(3,5-dimethylpyrazol-1-yl) methane: Synthesis, Structure, and Reactivity. Inorg. Chem. 2005, 44, 6122–6128. [Google Scholar] [CrossRef]
- Fujisawa, K.; Noguchi, Y.; Miyashita, Y.; Okamoto, K.; Lehnert, N. Mononuclear and Binuclear Copper(I) Complexes Ligated by Bis(3,5-diisopropyl-1-pyrazolyl) methane: Insight into the Fundamental Coordination Chemistry of Three-Coordinate Copper(I) Complexes with a Neutral Coligand. Inorg. Chem. 2007, 46, 10607–10623. [Google Scholar] [CrossRef]
- Bianchi, R.; Gervasio, G.; Marabello, D. Experimental Electron Density Analysis of Mn2(CO)10: Metal-Metal and Metal-Ligand Bond Characterization. Inorg. Chem. 2000, 39, 2360–2366. [Google Scholar] [CrossRef] [PubMed]
- Lepetit, C.; Fau, P.; Fajerwerg, K.; Kahn, M.L.; Silvi, B. Topological analysis of the metal-metal bond: A tutorial review. Coord. Chem. Rev. 2017, 345, 150–181. [Google Scholar] [CrossRef]
- Yang, X.; Chin, R.M.; Hall, M.B. Protonating metal-metal bonds: Changing the metal-metal interaction from bonding, to nonbonding, and to antibonding. Polyhedron 2022, 212, 115585. [Google Scholar] [CrossRef]
- Beaudelot, J.; Oger, S.; Perusko, S.; Phan, T.A.; Teunens, T.; Moucheron, C.; Evano, G. Photoactive Copper Complexes: Properties and Applications. Chem. Rev. 2022, 122, 16365–16609. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Bräse, S. Chemotion Repository. Available online: https://doi.org/10.14272/collection/VF_2024-03-05 (accessed on 22 July 2024).
- Enders, M.; Görling, B.; Braun, A.B.; Seltenreich, J.E.; Reichenbach, L.F.; Rissanen, K.; Nieger, M.; Luy, B.; Schepers, U.; Bräse, S. Cytotoxicity and NMR Studies of Platinum Complexes with Cyclooctadiene Ligands. Organometallics 2014, 33, 4027–4034. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Bühl, M.; Kabrede, H. Geometries of Transition-Metal Complexes from Density-Functional Theory. J. Chem. Theory Comput. 2006, 2, 1282–1290. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Spicher, S.; Caldeweyher, E.; Hansen, A.; Grimme, S. Benchmarking London dispersion corrected density functional theory for noncovalent ion–π interactions. Phys. Chem. Chem. Phys. 2021, 23, 11635–11648. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, C.A. Time-Dependent Density Functional Theory; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- De Souza, B.; Farias, G.; Neese, F.; Izsák, R. Predicting Phosphorescence Rates of Light Organic Molecules Using Time-Dependent Density Functional Theory and the Path Integral Approach to Dynamics. J. Chem. Theory Comput. 2019, 15, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. An sp-hybridized all-carboatomic ring, cyclo [18] carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 2020, 165, 461–467. [Google Scholar] [CrossRef]

| Empirical Formula | C42H34ClCuN2O4P2Se | C78H60Cu2N2O10P4SeCl2 |
|---|---|---|
| Formula weight | 870.60 | 1586.10 |
| Temperature/K | 180 | |
| Crystal system | Monoclinic | |
| Space group | Cc | C2/c |
| a/Å | 12.5156 (8) | 15.8430 (5) |
| b/Å | 19.0888 (15) | 18.1763 (5) |
| c/Å | 16.3818 (10) | 24.8971 (9) |
| β/° | 95.746 (5) | 99.158 (3) |
| Volume/Å3 | 3894.1 (5) | 7078.2 (4) |
| Z | 4 | 4 |
| ρcalc g/cm3 | 1.485 | 1.488 |
| μ/mm−1 | 1.691 | 1.343 |
| F(000) | 1768.0 | 3232.0 |
| Crystal size/mm3 | 0.18 × 0.04 × 0.03 | 0.28 × 0.2 × 0.16 |
| Radiation | MoKα (λ = 0.71073) | |
| 2Θ range for data collection/° | 3.906 to 58.554 | 3.314 to 69.314 |
| Index ranges | −16 ≤ h ≤ 16, −25 ≤ k ≤ 24, −21 ≤ l ≤ 21 | −16 ≤ h ≤ 24, −27 ≤ k ≤ 28, −38 ≤ l ≤ 39 |
| Reflections collected | 23,862 | 44,786 |
| Independent reflections | 8024 [Rint = 0.0468, Rsigma = 0.1472] | 14,221 [Rint = 0.0462, Rsigma = 0.1071] |
| Indep. reflections with I ≥ 2σ (I) | 4400 | 7406 |
| Data/restraints/parameters | 8024/2/478 | 14221/0/447 |
| Goodness-of-fit on F2 | 0.774 | 0.838 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0362, wR2 = 0.0625 | R1 = 0.0349, wR2 = 0.0630 |
| Final R indexes [all data] | R1 = 0.0876, wR2 = 0.0678 | R1 = 0.0947, wR2 = 0.0694 |
| Largest diff. peak/hole/e Å−3 | 0.40/−0.58 | 0.46/−0.47 |
| Flack parameter | −0.007 (7) | – |
| CCDC number | 2353339 | 2353340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, V.; Hoffmann, F.; Fuhr, O.; Luy, B.; Bräse, S. 2,1,3-Benzoselenadiazole as Mono- and Bidentate N-Donor for Heteroleptic Cu(I) Complexes: Synthesis, Characterization and Photophysical Properties. Inorganics 2024, 12, 201. https://doi.org/10.3390/inorganics12080201
Ferraro V, Hoffmann F, Fuhr O, Luy B, Bräse S. 2,1,3-Benzoselenadiazole as Mono- and Bidentate N-Donor for Heteroleptic Cu(I) Complexes: Synthesis, Characterization and Photophysical Properties. Inorganics. 2024; 12(8):201. https://doi.org/10.3390/inorganics12080201
Chicago/Turabian StyleFerraro, Valentina, Fabian Hoffmann, Olaf Fuhr, Burkhard Luy, and Stefan Bräse. 2024. "2,1,3-Benzoselenadiazole as Mono- and Bidentate N-Donor for Heteroleptic Cu(I) Complexes: Synthesis, Characterization and Photophysical Properties" Inorganics 12, no. 8: 201. https://doi.org/10.3390/inorganics12080201
APA StyleFerraro, V., Hoffmann, F., Fuhr, O., Luy, B., & Bräse, S. (2024). 2,1,3-Benzoselenadiazole as Mono- and Bidentate N-Donor for Heteroleptic Cu(I) Complexes: Synthesis, Characterization and Photophysical Properties. Inorganics, 12(8), 201. https://doi.org/10.3390/inorganics12080201






