Thionitrosyl Complexes of Rhenium and Technetium with PPh3 and Chelating Ligands—Synthesis and Reactivity
Abstract
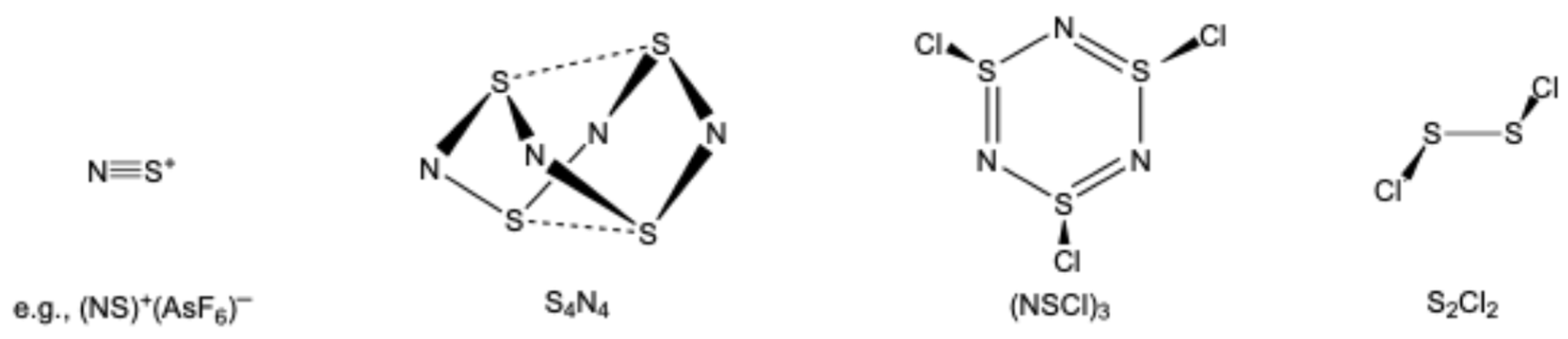
:1. Introduction
2. Results and Discussion
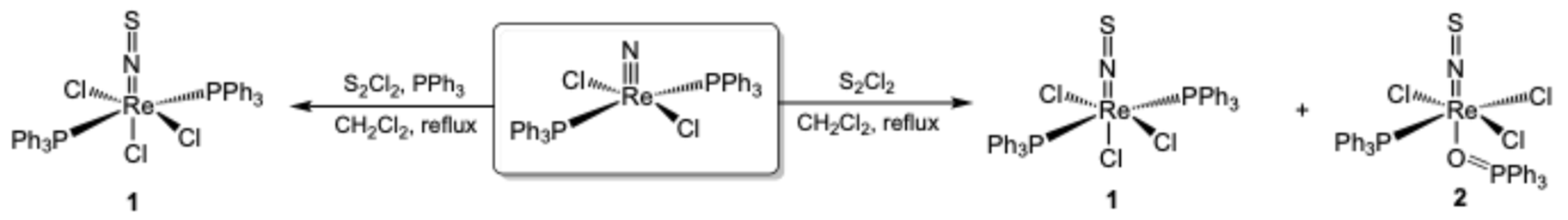
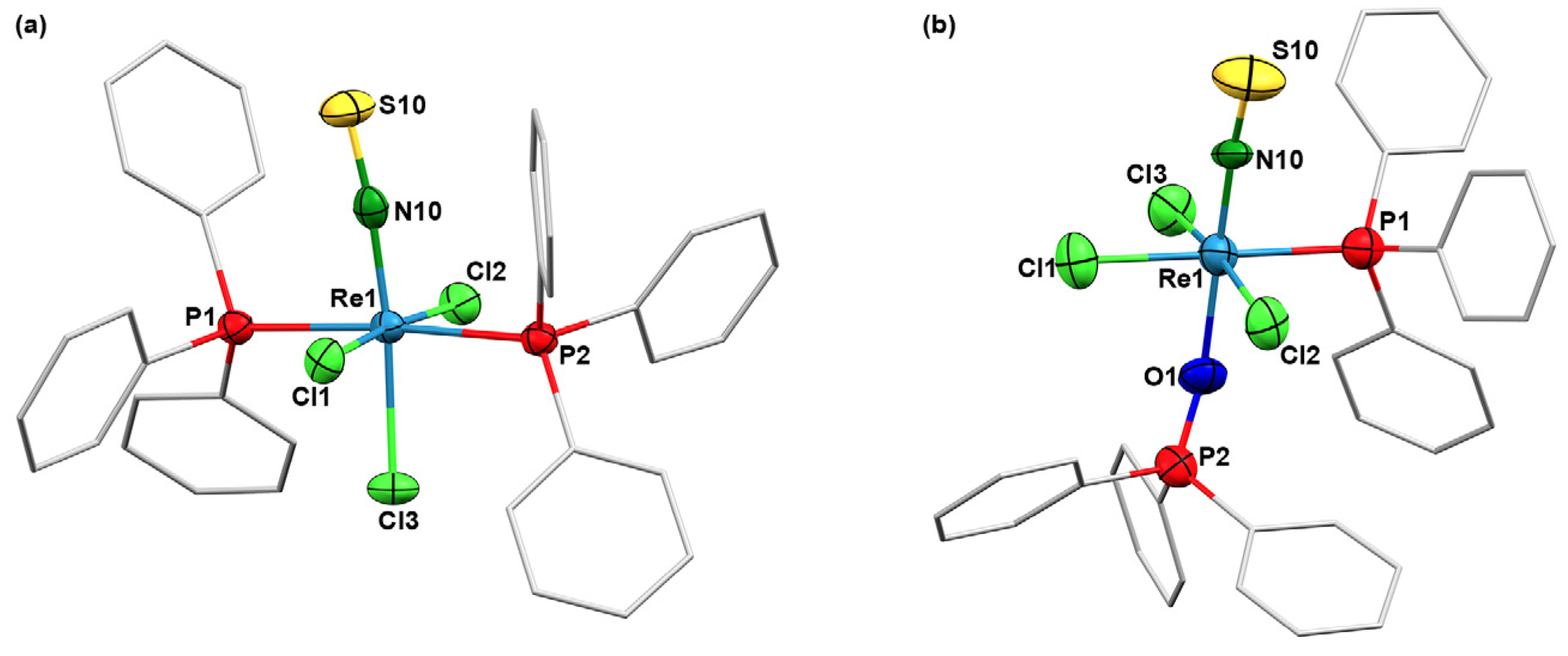
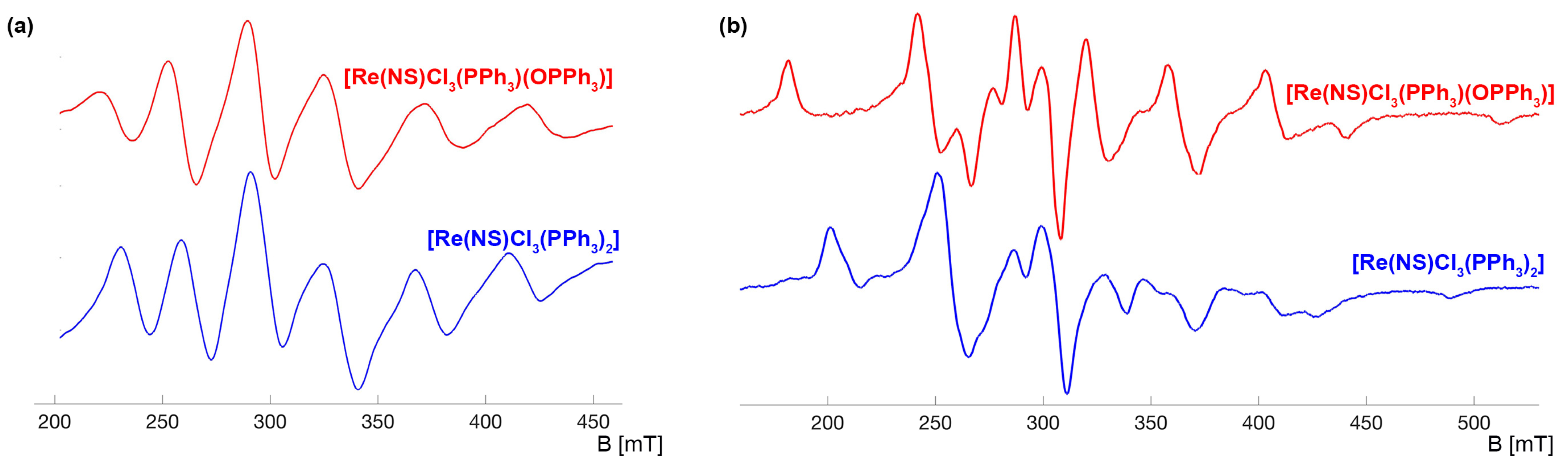
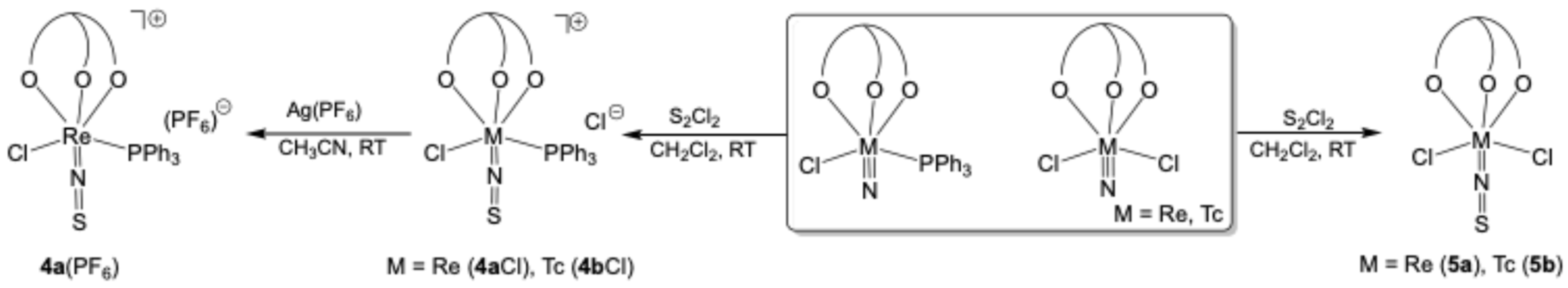
2.1. Triphenylphosphine Complexes
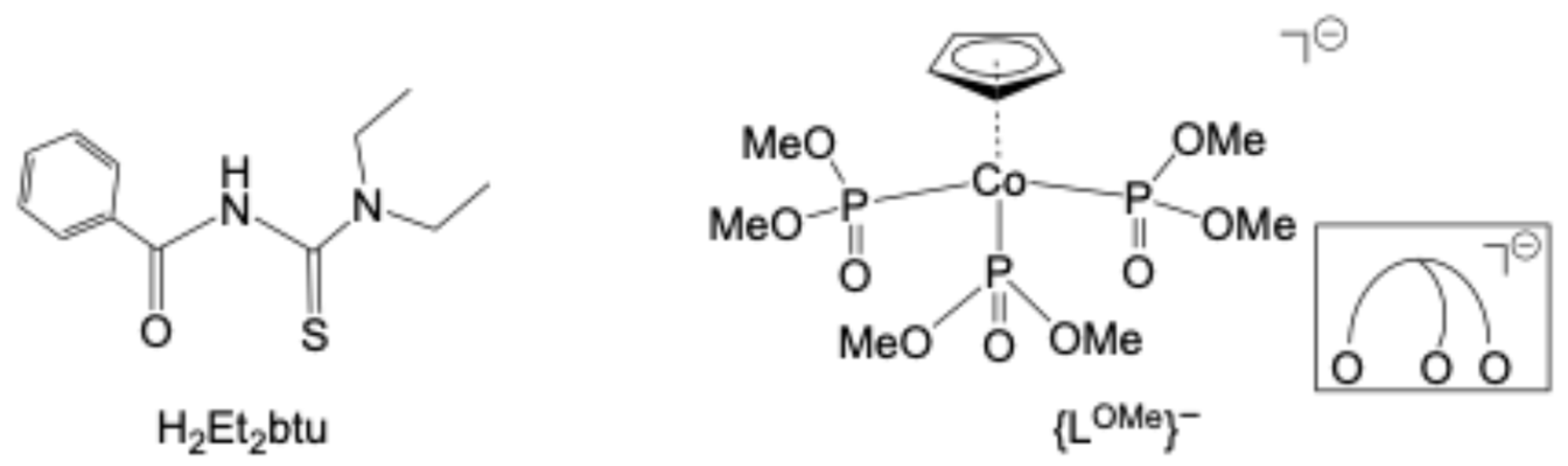
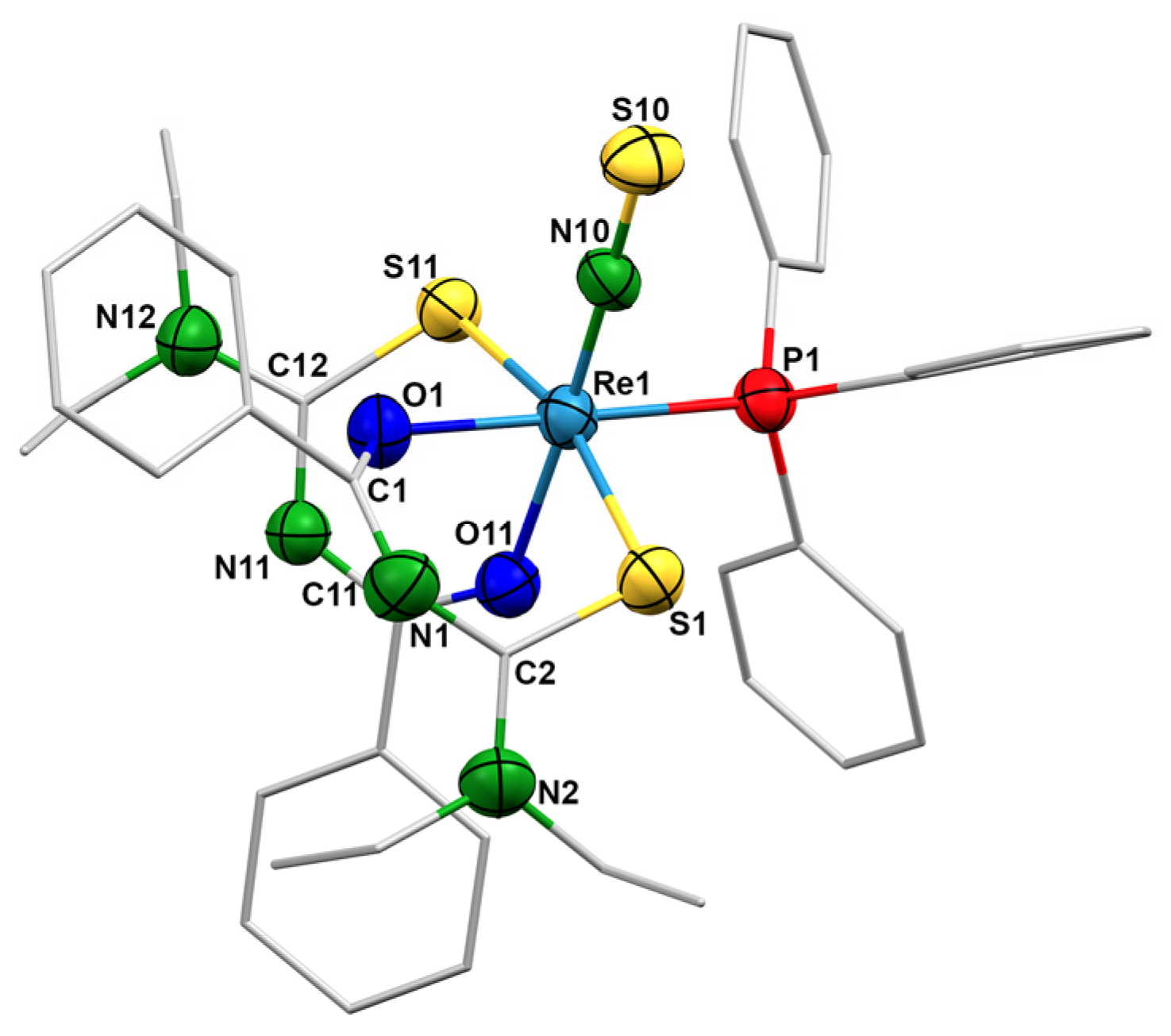
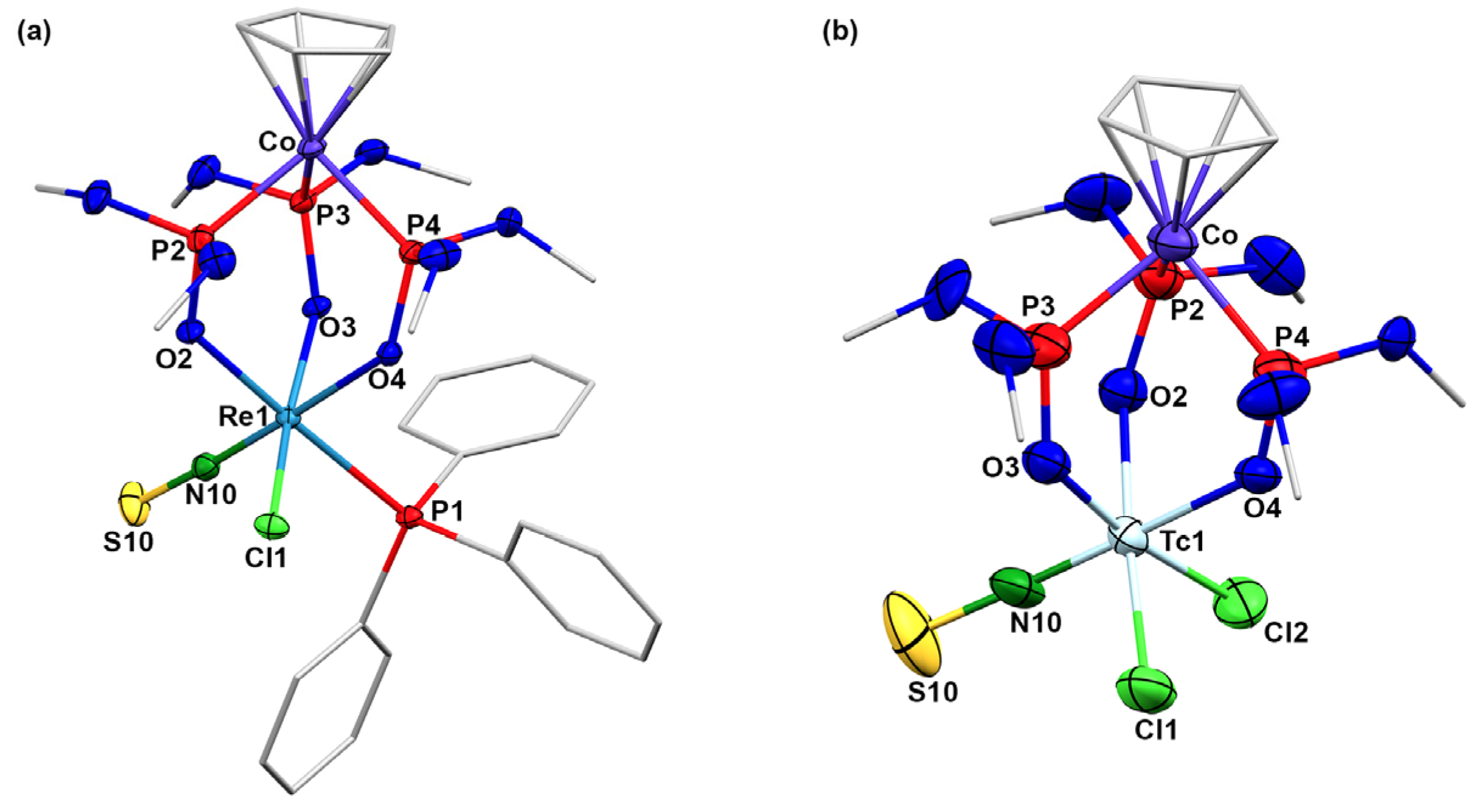
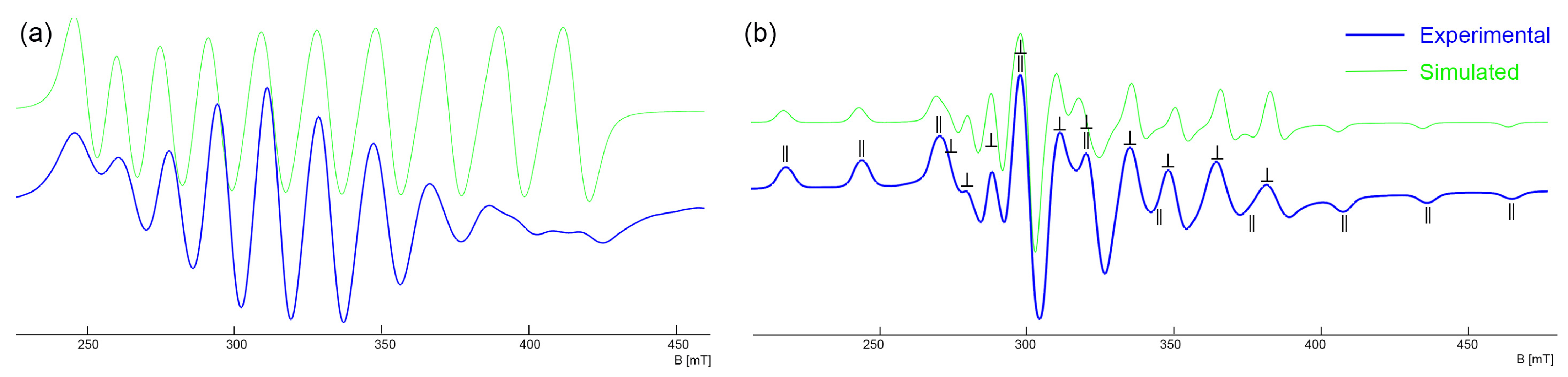
2.2. Complexes with Chelating Ligands
3. Materials and Methods
3.1. Syntheses
3.2. Spectroscopic and Analytical Methods
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Technetium-99m Radiopharmaceuticals: Manufacture of Kits; IAEA Technical Reports Series No. 466; International Atomic Energy Agency: Vienna, Austria, 2008; pp. 126–129.
- Abram, U.; Alberto, R. Technetium and rhenium—coordination chemistry and nuclear medical applications. J. Braz. Chem. Soc. 2006, 17, 1486–1500. [Google Scholar] [CrossRef]
- Bartholomä, M.D.; Louie, A.S.; Valliant, J.F.; Zubieta, J. Technetium and gallium derived radiopharmaceuticals: Comparing and contrasting the chemistry of two important radiometals for the molecular imaging era. Chem. Rev. 2010, 110, 2903–2920. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Dixit, M. Metallic radionuclides in the development of diagnostic and therapeutic radiopharmaceuticals. Dalton Trans. 2011, 40, 6112–6128. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chakraborty, S. 99mTc-centered one-pot synthesis for preparation of 99mTc radiotracers. Dalton Trans. 2011, 40, 6077–6086. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, J.R.; Pascu, S.I. The Radiopharmaceutical Chemistry of Technetium and Rhenium. In The Chemistry of Molecular Imaging; Long, N., Wong, W.-T., Eds.; John Wiley & Sons: Chichester, UK, 2015; pp. 163–174. [Google Scholar]
- Kuntic, V.; Brboric, J.; Vujic, Z.; Uskokovic-Markovic, S. Radioisotopes Used as Radiotracers in vitro and in vivo Diagnostics: A Review. Asian J. Chem. 2016, 28, 235–411. [Google Scholar] [CrossRef]
- Papagiannopoulou, D. Technetium-99m radiochemistry for pharmaceutical applications. J. Labelled Compd. Radiopharm. 2017, 60, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Duatti, A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2021, 92, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Alberto, R.; Nadeem, Q. 99mTechnetium-Based Imaging Agents and Developments in 99Tc Chemistry. In Metal Ions in Bio-Imaging Techniques; Sigel, A., Freisinger, E., Sigel, K.O., Eds.; De Gruyter: Berlin/Munich, Germany; Boston, MA, USA, 2021; pp. 196–238. [Google Scholar]
- Alberto, R. Role of Pure Technetium Chemistry: Are There Still Links to Applications in Imaging? Inorg. Chem. 2023, 62, 20539–20548. [Google Scholar] [CrossRef] [PubMed]
- World Nuclear Association. Radioisotopes in Medicine. Available online: https://world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine (accessed on 1 July 2024).
- Dash, A.; Knapp, F.F., Jr.; Pillai, M.R.A. 99Mo/99mTc separation: An assessment of technology options. Nucl. Med. Biol. 2013, 40, 167–176. [Google Scholar] [CrossRef]
- Lepareur, N.; Lacœuille, F.; Bouvry, C.; Hindré, F.; Garcion, E.; Chérel, M.; Noiret, N.; Garin, E.; Knapp, F.F. Rhenium-188 Labeled Radiopharmaceuticals: Current Clinical Applications in Oncology and Promising Perspectives. Front. Med. 2019, 6, 132. [Google Scholar] [CrossRef]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S. Radiometals for Combined Imaging and Therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Kronauge, J.F.K.; Mindiola, D.J. The value of Stable Metal-Carbon Bonds in Nuclear Medicine and the Cardiolite Story. Organometallics 2016, 35, 3432–3435. [Google Scholar] [CrossRef]
- Morais, G.R.; Paulo, A.; Santos, I. Organometallic Complexes for SPECT Imaging and/or Radionuclide Therapy. Organometallics 2012, 31, 5693–5714. [Google Scholar] [CrossRef]
- Alberto, R. From oxo to carbonyl and arene complexes; A journey through technetium chemistry. J. Organomet. Chem. 2018, 869, 264–269. [Google Scholar] [CrossRef]
- Benz, M.; Braband, H.; Schmutz, P.; Halter, J.; Alberto, R. From TcVII to TcI; facile syntheses of bis-arene complexes [99(m)Tc(arene)2]+ from pertechnetate. Chem. Sci. 2015, 6, 165–169. [Google Scholar] [CrossRef]
- Meola, G.; Braband, H.; Jordi, S.; Fox, T.; Blacque, O.; Spingler, B.; Alberto, R. Structure and reactivities of rhenium and technetium bis-arene sandwich complexes [M(η6-arene)2]+. Dalton Trans. 2017, 46, 14631–14637. [Google Scholar] [CrossRef]
- Nadeem, Q.; Meola, G.; Braband, H.; Bollinger, R.; Blancque, O.; Hernandez-Valdes, D.; Alberto, R. To Sandwich Technetium: Highly Functionalized Bis-Arene Complexes [99mTc(η6-arene)2]+ Directly from Water and [TcO4−]. Angew. Chem. Int. Ed. Engl. 2020, 59, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, Q.; Battistin, F.; Blancque, O.; Alberto, R. Naphtalene Exchange in [Re(η6-napht)2]+ with Pharmaceutical Leads to Highly Functionalized Sandwich Complexes [M(η6-pharm)2]+ (M = Re/99mTc). Chemistry 2022, 28, e202103566. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Marvelli, L.; Cittanti, C.; Giganti, M.; Martini, P. Technetium-99m Radiopharmaceuticals for Ideal Myocardial Perfusion Imaging: Lost and Found Opportunities. Molecules 2022, 27, 1188. [Google Scholar] [CrossRef]
- Pasqualini, R.; Duatti, A.; Bellande, E.; Comazzi, V.; Brucato, V.; Hoffschir, D.; Fagret, D.; Comet, M. Bis (Dithiocarbamato) Nitrido Technetium-99m Radiopharmaceuticals: A Class of Neutral Myocardial Imaging Agents. J. Nucl. Med. 1994, 35, 334–341. [Google Scholar]
- Boschi, A.; Uccelli, L.; Bolzati, C.; Duatti, A.; Sabba, N.; Moretti, E.; di Domenico, G.; Zavattini, G.; Refosco, F.; Giganti, M. Synthesis and Biologic Evaluation of Monocationic Asymmetric 99mTc-Nitride Heterocomplexes Showing High Heart Uptake and Improved Imaging Properties. J. Nucl. Med. 2003, 44, 806–814. [Google Scholar] [PubMed]
- Salvarese, N.; Carta, D.; Marzano, C.; Gerardi, G.; Melendez-Alafort, L.; Bolzati, C. [99mTc][Tc(N)(DASD)(PNPn)]+ (DASD = 1,4-Dioxa-8-Azaspiro[4,5]Decandithiocarbamate, PNP n = Bisphosphinoamine) for Myocardial Imaging: Synthesis, Pharmacological and Pharmacokinetic Studies. J. Med. Chem. 2018, 61, 11114–11126. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, L.K.; Dose, A.; Biagini, S.C.G.; Blower, P.J. Hydrazinonicotinic acid (HYNIC)—Coordination chemistry and applications in radiopharmaceutical chemistry. Inorg. Chim. Acta 2010, 363, 1059–1069. [Google Scholar] [CrossRef]
- Abrams, M.J.; Juweid, M.; tenKate, C.I.; Schwartz, D.A.; Hauser, M.M.; Gaul, F.E.; Fuccello, A.J.; Rubin, R.H.; Strauss, H.W.; Fischman, A.J. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazine nicotinamide derivative for imaging focal sites of infection in rats. J. Nucl. Med. 1990, 31, 2022–2028. [Google Scholar] [PubMed]
- Jiang, Y.; Tian, Y.; Feng, B.; Zhao, T.; Yu, X.; Zhao, Q. A novel molecular imaging probe [99mTc]Tc-HYNIC-FAPI targeting cancer-associated fibroblasts. Sci. Rep. 2023, 13, 3700. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.T.; Newman, J.L.; Nowotnik, D.P.; Thornback, J.R. Synthesis and biological studies of the [99mTc]tetrachloronitrosyltechnetium(II) anion—An alternative low valent technetium starting material. Int. J. Rad. Appl. Instrum. Nucl. Med. Biol. 1987, 14, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Machura, B. Structural and spectroscopic properties of rhenium nitrosyl complexes. Coord. Chem. Rev. 2005, 249, 2277–2307. [Google Scholar] [CrossRef]
- Dilworth, J.R. Rhenium chemistry—Then and Now. Coord. Chem. Rev. 2021, 436, 213822. [Google Scholar] [CrossRef]
- Mahmood, A.; Akgun, Z.; Peng, Y.; Mueller, P.; Jiang, Y.; Berke, H.; Jones, A.G.; Nicholson, T. The synthesis and characterization of rhenium nitrosyl complexes. The X-ray crystal structures of [ReBr2(NO)(NCMe)3], [Re(NO)(N5)](BPh4)2] and [ReBr2(NO)(NCMe){py-CH2-NHCH2CH2-N(CH2-py)2}]. Inorg. Chim. Acta 2013, 405, 455–460. [Google Scholar] [CrossRef]
- Abram, U.; Ortner, K.; Hübener, R.; Voigt, A.; Caballho, R.; Vazquez-Lopez, E. Darstellung, Strukturen und EPR-Spektren der Rhenium(II)-Nitrosylkomplexe [Re(NO)Cl2(PPh3)(OPPh3)(OReO3)], [Re(NO)Cl2(OPPh3)2(OReO3)] und [Re(NO)Cl2(PPh3)3][ReO4]. Z. Anorg. Allg. Chem. 1998, 624, 1662–1668. [Google Scholar] [CrossRef]
- Agbossou, F.; O’Connor, E.J.; Garner, C.M.; Quiros Mendez, N.; Fernandez, J.M.; Patton, A.T.; Ramsden, J.A.; Gladysz, J.A. Cyclopentadienyl Rhenium Complexes. Inorg. Synth. 1992, 29, 211–225. [Google Scholar]
- Seidel, S.N.; Prommesberger, M.; Eichenseher, S.; Meyer, O.; Hampel, F.; Gladysz, J.A. Syntheses and structural analyses of chiral rhenium containing amines of the formula (η5-C5H5)Re(NO)(PPh3)((CH2)nNRR′) (n = 0, 1). Inorg. Chim. Acta 2010, 363, 533–548. [Google Scholar] [CrossRef]
- Bernasconi, C.F.; Bhattacharya, S.; Wenzel, P.J.; Olmstead, M.M. Kinetic and Thermodynamic Acidity of [Cp(NO)(PPh3)Re(2,5-dimethyl-3-thienyl)carbene]+. Transition State Imbalance and Intrinsic Barriers. Organometallics 2006, 25, 4322–4330. [Google Scholar] [CrossRef]
- Dilsky, S.; Schenk, W.A. Diastereomeric Halfsandwich Rhenium Complexes Containing Hemilabile Phosphane Ligands. Eur. J. Inorg. Chem. 2004, 2004, 4859–4870. [Google Scholar] [CrossRef]
- Nicholson, T.; Chun, E.; Mahmood, A.; Mueller, P.; Davison, A.; Jones, A.G. Synthesis, spectroscopy and structural analysis of Technetium and Rhenium nitrosyl complexes. Commun. Inorg. Chem. 2015, 3, 31–39. [Google Scholar]
- Blanchard, S.S.; Nicholson, T.; Davison, A.; Davis, W.; Jones, A.G. The synthesis, characterization and substitution reactions of the mixed technetium(I) nitrosyl complex trans-trans-[(NO)(NCCH3)Cl2(PPh3)2Tc]. Inorg. Chim. Acta 1996, 244, 121–130. [Google Scholar] [CrossRef]
- Balasekaran, S.M.; Hagenbach, A.; Drees, M.; Abram, U. [TcII(NO)(trifluoroacetate)4F]2−—Synthesis and reactions. Dalton Trans. 2017, 46, 13544–13552. [Google Scholar] [CrossRef] [PubMed]
- Linder, K.E.; Davison, A.; Dewan, J.C.; Costello, C.E.; Melaknia, S. Nitrosyl complexes of technetium: Synthesis and characterization of [TcI(NO)(CNCMe3)5](PF6)2 and Tc(NO)Br2(CNCMe3)3 and the crystal structure of Tc(NO)Br2(CNCMe3)3. Inorg. Chem. 1986, 25, 2085–2089. [Google Scholar] [CrossRef]
- Ackermann, J.; Noufele, C.N.; Hagenbach, A.; Abram, U. Nitrosyltechnetium(I) Complexes with 2-(Diphenylphosphanyl)aniline. Z. Anorg. Allg. Chem. 2019, 645, 8–13. [Google Scholar] [CrossRef]
- Ackermann, J.; Hagenbach, A.; Abram, U. {Tc(NO)(Cp)(PPh3)}+—A novel technetium(I) core. Chem. Commun. 2016, 52, 10285–10288. [Google Scholar] [CrossRef]
- Ackermann, J.; Abdulkader, A.; Scholtysik, C.; Jungfer, M.R.; Hagenbach, A.; Abram, U. [TcI(NO)X(Cp)(PPh3)] Complexes (X− = I−, I3−, SCN−, CF3SO3−, or CF3COO−) and Their Reactions. Organometallics 2019, 38, 4471–4478. [Google Scholar] [CrossRef]
- Abdulkader, A.; Hagenbach, A.; Abram, U. [Tc(NO)Cl(Cp)(PPh3)]—A Technetium(I) Compound with an Unexpected Synthetic Potential. Eur. J. Inorg. Chem. 2021, 2021, 3812–3818. [Google Scholar]
- Schibli, R.; Marti, N.; Maurer, P.; Spingler, B.; Lehaire, M.-L.; Gramlich, V.; Barnes, C.L. Syntheses and Characterization of Dicarbonyl–Nitrosyl Complexes of Technetium(I) and Rhenium(I) in Aqueous Media: Spectroscopic, Structural, and DFT Analyses. Inorg. Chem. 2005, 44, 683–690. [Google Scholar] [CrossRef]
- Brown, D.S.; Newman, J.L.; Thornback, J.R.; Davison, A. Structure of the tetra-n-butylammoium salt of tetrachloro(methanol)nitrosyltechnetium(II) anion. Acta Cryst. 1987, C43, 1692–1694. [Google Scholar]
- Brown, D.S.; Newman, J.L.; Thornback, J.R.; Pearlstein, R.M.; Davison, A.; Lawson, A. The synthesis and characterisation of the trichloronitrosyl(acetylacetonato)technetium(II) anion, a novel technetium(II) complex. Inorg. Chim. Acta 1988, 150, 193–196. [Google Scholar] [CrossRef]
- Nicholson, T.; Hirsch-Kuchma, M.; Freiberg, E.; Davison, A.; Jones, A.G. The reaction chemistry of a technetium(I) nitrosyl complex with potentially chelating organohydrazines: The X-ray crystal structure of [TcCl2(NO)(HNNC5H4N)(PPh3)]. Inorg. Chim. Acta 1998, 279, 206–209. [Google Scholar]
- De Vries, N.; Cook, J.; Davison, A.; Nicholson, T.; Jones, A.G. Synthesis and characterization of a technetium(III) nitrosyl compound: Tc(NO)(Cl)(SC10H13)3. Inorg. Chem. 1990, 29, 1062–1064. [Google Scholar] [CrossRef]
- Nicholson, T.; Mahmood, A.; Limpa-Amara, N.; Salvarese, N.; Takase, M.K.; Müller, P.; Akgun, Z.; Jones, A.G. Reactions of the tridentate and tetradentate amine ligands di-(2-picolyl)(N-ethyl)amine and 2,5-bis-(2-pyridylmethyl)-2,5 diazohexane with technetium nitrosyl complexes. Inorg. Chim. Acta 2011, 373, 301–305. [Google Scholar] [CrossRef]
- Roca Jungfer, M.; Ernst, M.J.; Hagenbach, A.; Abram, U. [{TcI(NO)(LOMe)(PPh3)Cl}2Ag](PF6) and [TcII(NO)(LOMe)(PPh3)Cl](PF6): Two Unusual Technetium Complexes with a “Kläui-type” Ligand. Z. Anorg. Allg. Chem. 2022, 648, e202100316. [Google Scholar]
- Nicholson, T.L.; Mahmood, A.; Muller, P.; Davison, A.; Storm-Blanchard, S.; Jones, A.G. The synthesis and structural characterization of the technetium nitrosyl complexes [TcCl(NO)(SC5H4N)(PPh3)2] and [Tc(NO)(SC5H4N)2(PPh3)]. Inorg. Chim. Acta 2011, 365, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Balasekaran, S.M.; Spandl, J.; Hagenbach, A.; Köhler, K.; Drees, M.; Abram, U. Fluoridonitrosyl Complexes of Technetium(I) and Technetium(II). Synthesis, Characterization, Reactions, and DFT Calculations. Inorg. Chem. 2014, 53, 5117–5128. [Google Scholar] [CrossRef]
- Nicholson, T.; Hirsch-Kuchma, M.; Shellenbarger-Jones, A.; Davison, A.; Jones, A.G. The synthesis and characterization of a technetium nitrosyl complex with cis-{2-pyridyl,diphenylphosphine} coligands. The X-ray crystal structure of [TcCl2(NO)(pyPPh2-P,N) (pyPPh2-P)]. Inorg. Chim. Acta 1998, 267, 319–322. [Google Scholar] [CrossRef]
- Grunwald, A.C.; Scholtysik, C.; Hagenbach, A.; Abram, U. One Ligand, One Metal, Seven Oxidation States: Stable Technetium Complexes with the “Kläui Ligand”. Inorg. Chem. 2020, 59, 9396–9405. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.L.; Mahmood, A.; Refosco, F.; Tisato, F.; Müller, P.; Jones, A.G. The synthesis and X-ray structural characterization of mer and fac isomers of the technetium(I) nitrosyl complex [TcCl2(NO)(PNPpr)]. Inorg. Chim. Acta 2009, 362, 3637–3640. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.; Müller, P.; Davison, A.; Jones, A.G. The synthesis and characterization of a cationic technetium nitrosyl complex: The X-ray crystal structure of [TcCl(NO)(DPPE)2](PF6) × CH2Cl2. Inorg. Chim. Acta 2006, 359, 1296–1298. [Google Scholar] [CrossRef]
- Pandey, K.K. Coordination Chemistry of Thionitrosyl (NS), Thiazate (NSO–), Disulfidothionitrate (S,N–), Sulfur Monoxide (SO), and Disulfur Monoxide (S,O) Ligands. Progr. Inorg. Chem. 1992, 40, 445–502. [Google Scholar]
- Døsing, A. The electronic structure and photochemistry of transition metal thionitrosyl complexes. Coord. Chem. Rev. 2016, 306, 544–557. [Google Scholar] [CrossRef]
- Anhaus, J.; Siddiqi, Z.A.; Roesky, H.W. Reaction of Tetrasulfurtetranitride with Rhenium(VII)-chloronitride. The Crystal Structure of [Ph4As+]2[Cl4Re(NS)(NSCl)2−] × CH2Cl2. Z. Naturforsch. 1985, 40b, 740–744. [Google Scholar] [CrossRef]
- Dirican, D.; Pfister, N.; Wozniak, M.; Braun, T. Reactivity of Binary and Ternary Sulfur Halides towards Transition-Metal Compounds. Chem. Eur. J. 2020, 31, 6945–6963. [Google Scholar] [CrossRef]
- Dietrich, A.; Neumüller, B.; Dehnicke, K. (PPh4)2[(SN)ReCl3(μ-N)(μ-NSN)ReCl3(THF)]—Ein Nitrido-Thionitrosyl-Dinitridosulfato-Komplex des Rheniums. Z. Anorg. Allg. Chem. 2000, 626, 1268–1270. [Google Scholar] [CrossRef]
- Reinel, M.; Höcher, T.; Abram, U.; Kirmse, R. Ein Beitrag zu Rhenium(II)-, Osmium(II)- und Technetium(II)-Thionitrosylkomplexe vom Typ [M(NS)Cl4py]: Darstellung, Strukturen und EPR-Spektren. Z. Anorg. Allg. Chem. 2003, 629, 853–861. [Google Scholar] [CrossRef]
- Voigt, A.; Abram, U.; Kirmse, R. Darstellung, Strukturen und EPR-Spektren der Rhenium(II)-Thionitrosylkomplexe trans-[Re(NS)Cl3(MePh2P)2] und trans-[Re(NS)Br3(Me2PhP)2]. Z. Anorg. Allg. Chem. 1999, 625, 1658–1663. [Google Scholar] [CrossRef]
- Hauck, H.-G.; Willing, W.; Müller, U.; Dehnicke, K. [ReCl2(NS)(NSCl)(Pyridin)2], ein Thionitrosyl-chlorthionitrenkomplex des Rheniums. Z. Anorg. Allg. Chem. 1986, 534, 77–84. [Google Scholar] [CrossRef]
- Hübener, R.; Abram, U.; Strähle, J. Isothiocyanato complexes of rhenium II. Synthesis, characterization and structures of ReN(NCS)2(Me2PhP)3 and Re(NS)(NCS)2(Me2PhP)3. Inorg. Chim. Acta 1994, 216, 223–228. [Google Scholar] [CrossRef]
- Ritter, S.; Abram, U. Gemischtligand-Komplexe des Rheniums. VI. Darstellung und Strukturen der Rhenium Thionitrosyl-Komplexe mer-[Re(NS)Cl2(Me2PhP)3] × CH2Cl2 und trans-[Re(NS)Cl3(Me2PhP)2]. Z. Anorg. Allg. Chem. 1994, 620, 1223–1228. [Google Scholar]
- Ritter, S.; Abram, U. Gemischtligandkomplexe des Rheniums. IX. Reaktionen am Nitridoliganden von [ReN(Me2PhP)(Et2dtc)2]. Synthese, Charakterisierung und Kristallstrukturen von [Re(NBCl3)(Me2PhP)(Et2dtc)2], [Re(NGaCl3)(Me2PhP)(Et2dtc)2] und [Re(NS)Cl(Me2PhP)2(Et2dtc)]. Z. Anorg. Allg. Chem. 1995, 622, 965–973. [Google Scholar] [CrossRef]
- Ruf, C.; Behrens, U.; Lork, E.; Mews, R. Reactions of halides with trans-[Re(CO)4(MeCN)(NS)][AsF6]2: Syntheses and structure of trans-[Re(CO)4(Cl)(NS)][AsF6] and [(OC)5ReNS–NS–N[Re(CO)5]S{N–SNRe(CO)5}–CH2–CH2][AsF6]2, an unusual trinuclear bis(thiazyl)rhenium complex. Chem. Commun. 1996, 939–940. [Google Scholar] [CrossRef]
- Baldas, J.; Bonnyman, J.; Mackay, M.F.; Williams, G.A. Structural studies of technetium complexes. V. The preparation and crystal structure of Dichlorobis(diethyldithiocarbamato)thionitrosyltechnetium(III). Austr. J. Chem. 1984, 37, 751–759. [Google Scholar] [CrossRef]
- Kaden, L.; Lorenz, B.; Kirmse, R.; Stach, J.; Behm, H.; Beurskens, P.T.; Abram, U. Synthesis, characterization and x-ray molecular and crystal structure of Tc(NS)Cl3(Me2PhP)(Me2PhPO)-a first example of mixed phosphine/phosphine oxide coordination. Inorg. Chim. Acta 1990, 169, 43–48. [Google Scholar] [CrossRef]
- Baldas, J.; Colmanet, S.F.; Williams, G.A. Preparation and Structure of Dibromobis(N,N-diethyldithiocarbamato)-thionitrosyltechnetium(III). Austr. J. Chem. 1991, 44, 1125–1132. [Google Scholar] [CrossRef]
- Lu, J.; Clarke, M.J. Modulation of Tc–NX (X = O or S) bonds by π-acceptor ligands. J. Chem. Soc. Dalton Trans. 1992, 1243–1248. [Google Scholar] [CrossRef]
- Abram, U.; Schulz Lang, E.; Abram, S.; Wegmann, J.; Dilworth, J.R.; Kirmse, R.; Woolins, J.D. Technetium(V) and rhenium(V) nitrido complexes with bis(diphenyl-thiophosphoryl)amide, N(SPPh2)2−. J. Chem. Soc. Dalton Trans. 1997, 623–630. [Google Scholar] [CrossRef]
- Hiller, W.; Hübener, R.; Lorenz, B.; Kaden, L.; Findeisen, M.; Stach, J.; Abram, U. Structural and spectroscopic studies on mer-dichlorotris(dimethylphenylphosphine)(thionitrosyl)technetium(I), mer-[Tc(NS)Cl2(Me2PhP)3]. Inorg. Chim. Acta 1991, 181, 161–165. [Google Scholar] [CrossRef]
- Lu, J.; Clarke, M.J. Sulfur atom transfer with reduction of a [TcVI≡N]3+ core to a [TcI-N≡S]2+ core. Crystal structure of mer-dichlorotris(4-picoline)(thionitrosyl)technetium. Inorg. Chem. 1990, 29, 4123–4125. [Google Scholar] [CrossRef]
- Abram, U.; Hübener, R.; Wollert, R.; Kirmse, R.; Hiller, W. Synthesis, characterization and reactions of [Tc(NS)X4]− complexes (X = Cl, Br, NCS). Inorg. Chim. Acta 1993, 206, 9–14. [Google Scholar] [CrossRef]
- Dressler, K. Ultraviolett- und Schumannspektren der neutralen und ionisierten Moleküle PO, PS, NS, P2. Helv. Phys. Acta 1955, 28, 563–590. [Google Scholar]
- O’Hare, P.A.G. Dissociation Energies, Enthalpies of Formation, Ionization Potentials, and Dipole Moments of NS and NS+. J. Chem. Phys. 1970, 52, 2992–2996. [Google Scholar] [CrossRef]
- Mews, R. The Thionitrosyl Cation NS+ as a Synthetic Reagent. Angew. Chem. Int. Ed. Engl. 1976, 15, 691–692. [Google Scholar] [CrossRef]
- Clegg, W.; Glemser, O.; Harms, K.; Hartmann, G.; Mews, R.; Noltemeyer, M.; Sheldrick, G.M. Crystal structures of thionitrosyl hexafluoroantimonate(V) and thionitrosyl undecafluorodiantimonate(V) at 293 K and of thionitrosyl undecafluorodiantimonate(V) at 121.5 K: The effect of thermal motion on the apparent NS bond length. Acta Cryst. 1981, 37b, 548–552. [Google Scholar] [CrossRef]
- Kaden, L.; Lorenz, B.; Kirmse, R.; Stach, J.; Abram, U. Darstellung und Charakterisierung von Thionitrosylkomplexen des Technetiums(I) und –(II). Z. Chem. 1985, 25, 29–30. [Google Scholar] [CrossRef]
- Abram, U.; Kirmse, R.; Köhler, K.; Lorenz, B.; Kaden, L. Tc(NX)Y3(Me2PhP)2 Complexes (X = O or S; Y = Cl or Br). Preparation, Characterization and EPR Studies. Inorg. Chim. Acta 1987, 129, 15–20. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Abram, U. Rhenium and Technetium Complexes with N,N-Dialkyl-N’-benzoylthioureas. Inorg. Chem. 2007, 46, 5310–5319. [Google Scholar] [PubMed]
- Hayes, T.R.; Powell, A.S.; Benny, P.D. Synthesis and stability of 2 + 1 complexes of N,N-diethylbenzoylthiourea with [MI(CO)3]+ (M = Re, 99mTc). J. Coord. Chem. 2015, 68, 3432–3448. [Google Scholar] [CrossRef]
- Abram, U.; Abram, S.; Alberto, R.; Schibli, R. Ligand exchange reactions starting from [Re(CO)3Br3]2−. Synthesis, characterization and structures of rhenium(I) tricarbonyl complexes with thiourea and thiourea derivatives. Inorg. Chim. Acta 1996, 248, 193–202. [Google Scholar] [CrossRef]
- Borges, A.P.; Possato, B.; Hagenbach, A.; Machado, A.E.H.; Deflon, V.M.; Abram, U.; Maia, P.I.S. Re(V) complexes containing the phenylimido (NPh2-) core and SNS-thiosemicarbazide ligands. Inorg. Chim. Acta 2021, 516, 120110. [Google Scholar] [CrossRef]
- Mukiza, J.; Gerber, T.I.A.; Hosten, E.C.; Betz, R. Crystal structure of fac-κO,S-(Z)-1,1-diethyl-3-(hydroxido(phenyl)methylene) thiourea-(κS’(Z)-1,1-diethyl-3-(hydroxido(phenyl)methylene)-thiourea)-tricarbonyl rhenium(I), C27H31N4O5ReS2. Z. Kristallogr. New Cryst. Struct. 2015, 230, 50–52. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Abram, U. Rhenium and technetium complexes with tridentate S,N,O ligands derived from benzoylhydrazine. Polyhedron 2009, 28, 3945–3952. [Google Scholar] [CrossRef]
- Salsi, F.; Portapilla, G.B.; Simon, S.; Roca Jungfer, M.; Hagenbach, A.; de Albuquerque, S.; Abram, U. Effect of Fluorination on the Structure and Anti-Trypanosoma cruzy Activity of Oxorhenium(V) Complexes with S,N,S-Tridentate Thiosemicarbazones and Benzoylthioureas. Synthesis and Structures of Technetium(V) Analogues. Inorg. Chem. 2019, 58, 10129–10138. [Google Scholar] [CrossRef]
- Roca Jungfer, M.; Elsholz, L.; Abram, U. Technetium Hydrides Revisited: Syntheses, Structures, and Reactions of [TcH3(PPh3)4] and [TcH(CO)3(PPh3)2]. Organometallics 2021, 40, 3095–3112. [Google Scholar] [CrossRef]
- Kläui, W. The Coordination Chemistry and Organometallic Chemistry of Tridentate Oxygen Ligands with π-Donor Properties. Angew. Chem. Int. Ed. Engl. 1990, 29, 627–637. [Google Scholar] [CrossRef]
- Leung, W.-H.; Zhang, Q.-F.; Yi, X.-Y. Recent developments in the coordination and organometallic chemistry of Kläui oxygen tripodal ligands. Coord. Chem. Rev. 2007, 251, 2266–2279. [Google Scholar] [CrossRef]
- Kramer, D.J.; Davison, A.; Jones, A.G. Structural models for [M(CO)3(H2O)3]+ (M = Tc, Re): Fully aqueous synthesis of technetium and rhenium tricarbonyl complexes of tripodal oxygen donor ligands. Inorg. Chim. Acta 2001, 312, 215–220. [Google Scholar] [CrossRef]
- Leung, W.-H.; Chan, E.Y.Y.; Lai, T.C.Y.; Wong, W.-T. Synthesis and reactivity of nitrido-rhenium and -osmium complexes with an oxygen tripod ligand. J. Chem. Soc. Dalton Trans. 2000, 51–56. [Google Scholar] [CrossRef]
- So, Y.-M.; Chiu, W.-H.; Cheung, W.-M.; Ng, H.-Y.; Lee, H.K.; Sung, H.H.-Y.; Williams, I.D.; Leung, W.H. Heterobimetallic rhenium nitrido complexes containing the Kläui tripodal ligand [Co(η5-C5H5){P(O)(OEt)2}3]−. Dalton Trans. 2015, 44, 5479–5487. [Google Scholar] [CrossRef]
- Banberry, H.J.; Hussain, W.; Evans, I.G.; Hamor, T.A.; Jones, C.J.; McCleverty, J.A.; Schulte, H.-J.; Engles, B.; Kläui, W. The syntheses of high oxidation state metal complexes containing the tripodal ligand [(η5-C5H5)Co{P(OMe)2(O)}3]− and the X-ray crystal structure of [(η5-C5H5)Co{P(OMe)2(O)}3 ReO3]. Polyhedron 1990, 9, 2549–2551. [Google Scholar] [CrossRef]
- Dyckhoff, B.; Schulte, H.-J.; Englert, U.; Spaniol, T.P.; Kläui, W.; Schubiger, P.A. Rhenium-Komplexe von Sauerstoffchelatliganden: Ein Weg zu neuen Radiopharmaka? Z. Anorg. Allg. Chem. 1992, 614, 131–141. [Google Scholar] [CrossRef]
- Abram, U.; Abram, S. Synthese und Charakterisierung neuartiger Technetiumkomplexe mit 1,1-disubstituierten Benzoylthioharnstoffen. Z. Chem. 1983, 23, 228. [Google Scholar] [CrossRef]
- Sullivan, B.P.; Brewer, J.C.; Gray, H.B.; Linebarrier, D.; Mayer, J.M. Nitrido and Oxo Complexes of Rhenium(V). Inorg. Synth. 1992, 29, 146–150. [Google Scholar]
- Kaden, L.; Lorenz, B.; Schmidt, K.; Sprinz, H.; Wahren, M. Nitridokomplexe des Technetium(V). Isotopenpraxis 1981, 17, 174–175. [Google Scholar]
- Baldas, J.; Boas, J.F.; Bonnyman, J.; Williams, G.A. Studies of technetium complexes. Part 6. The preparation, characterisation, and electron spin resonance spectra of salts of tetrachloro- and tetrabromonitridotechnetate(VI): Crystal structure of tetraphenylarsonium tetrachloronitridotechnetate(VI). J. Chem. Soc. Dalton Trans. 1984, 11, 2395–2400. [Google Scholar] [CrossRef]
- Abram, U.; Braun, M.; Abram, S.; Kirmse, R.; Voigt, A. [NBu4][ReNCl4]: Facile synthesis, structure, electron paramagnetic resonance spectroscopy and reactions. J. Chem. Soc. Dalton Trans. 1998, 2, 231–238. [Google Scholar] [CrossRef]
- Grunwald, A.C. Metal Complexes with Tripodal and Chelating Thiourea Ligands towards Nuclear Medical Imaging. Doctoral Thesis, Freie Universität Berlin, Berlin, Germany, 2020. Available online: https://refubium.fu-berlin.de/handle/fub188/30387 (accessed on 1 July 2024).
- Nowak, D. Thionitrosylkomplexe des Rheniums und Technetiums. Doctoral Thesis, Freie Universität Berlin, Berlin, Germany, 2022. Available online: https://refubium.fu-berlin.de/handle/fub188/36899 (accessed on 1 July 2024).
- Kleinpeter, E.; Beyer, L. 1H-NMR-Untersuchung der behinderten Rotation um die C-N-Bindung in 1,1’-Diäthyl-3-benzoylharnstoff-Derivaten. J. Prakt. Chem. 1975, 317, 938–942. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Res. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- MATLAB Version: 9.13.0 (R2022b); The MathWorks Inc.: Natick, MA, USA, 2022.
- Sheldrick, G. SADABS, vers. 2014/5; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Coppens, P. The Evaluation of Absorption and Extinction in Single-Crystal Structure Analysis. In Crystallographic Computing; Muksgaard: Copenhagen, Denmark, 1979. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Diamond—Crystal and Molecular Structure Visualization; Vers. 4.6.8.; Crystal Impact: Bonn, Germany, 2022. [Google Scholar]

| Re1–N10 | N10–S10 | Re1–Cl1 | Re1–Cl2 | Re1–Cl3 | Re1–P1 | Re1–P2/O1 | O1–P2 | Re1–N10–S10 | Re1–O1–P2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.795(5) | 1.518(5) | 2.353(1) | 2.340(1) | 2.416(1) | 2.540(2) | 2.555(2) | - | 174.4(3) | - |
| 2 (1) | 1.77(1) 1.77(1) | 1.53(1) 1.52(1) | 2.368(4) 2.357(4) | 2.255(3) 2.332(3) | 2.338(3) 2.366(3) | 2.506(4) 2.584(4) | 2.100(8) 2.099(8) | 1.509(9) 1.497(9) | 167.2(7) 178.4(6) | 155.7(6) 164.5(5) |
| Re1–N10 | N10–S10 | Re1–P1 | Re1–S1 | Re1–O1 | Re1–S11 | Re1–O11 | O1–C1 | C1–N1 | N1–C2 |
|---|---|---|---|---|---|---|---|---|---|
| 1.749(4) | 1.571(4) | 2.362(1) | 2.401(1) | 2.101(3) | 2.439(1) | 2.103(4) | 1.296(6) | 1.301(7) | 1.353(7) |
| C2–N2 | C2–S1 | O11–C11 | C11–N11 | N11–C12 | C12–N12 | C12–S11 | Re1–N10–S10 | ||
| 1.319(7) | 1.755(6) | 1.266(6) | 1.329(6) | 1.338(7) | 1.325(7) | 1.758(6) | 176.5(3) | ||
| M1–N10 | N10–S10 | M1–Cl1 | M1–Cl2 | M1–P1 | M1–O2 | M1–O3 | M1–O4 | M1–N10–S10 | |
|---|---|---|---|---|---|---|---|---|---|
| 4a | 1.752(5) | 1.554(5) | 2.337(1) | - | 2.475(1) | 2.071(3) | 2.032(3) | 2.083(3) | 175.4(3) |
| 5b (1) | 1.732(7) 1.75(1) | 1.540(7) 1.54(1) | 2.339(3) 2.330(3) | 2.343(2) 2.344(3) | - | 2.060(6) 2.068(5) | 2.047(6) 2.057(6) | 2.095(5) 2.088(5) | 171.7(6) 172.0(8) |
| g0 | a0M | g‖ | g⊥ | A‖M | A⊥M | |
|---|---|---|---|---|---|---|
| [Re(NS)Cl(PPh3)(LOMe)]Cl (4aCl) | 1.995 | 420 | 1.842 | 1.986 | 644 | 330 |
| [Tc(NS)Cl(PPh3)(LOMe)]Cl (4bCl) | 2.007 | 168 | 1.966 | 2.023 | 252 | 112 |
| [Re(NS)Cl2(LOMe)] (5a) | 1.990 | 455 | 1.945 | 1.760 | 787 | 398 |
| [Tc(NS)Cl2(LOMe)] (5b) | 1.996 | 177 | 1.943 | 2.021 | 285 | 129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, D.; Hagenbach, A.; Sawallisch, T.E.; Abram, U. Thionitrosyl Complexes of Rhenium and Technetium with PPh3 and Chelating Ligands—Synthesis and Reactivity. Inorganics 2024, 12, 210. https://doi.org/10.3390/inorganics12080210
Nowak D, Hagenbach A, Sawallisch TE, Abram U. Thionitrosyl Complexes of Rhenium and Technetium with PPh3 and Chelating Ligands—Synthesis and Reactivity. Inorganics. 2024; 12(8):210. https://doi.org/10.3390/inorganics12080210
Chicago/Turabian StyleNowak, Domenik, Adelheid Hagenbach, Till Erik Sawallisch, and Ulrich Abram. 2024. "Thionitrosyl Complexes of Rhenium and Technetium with PPh3 and Chelating Ligands—Synthesis and Reactivity" Inorganics 12, no. 8: 210. https://doi.org/10.3390/inorganics12080210






