Effect of Salt Variability on the Low-Temperature Metal-Catalyzed Graphitization of PAN/DMSO Solutions for the Synthesis of Nanostructured Graphitic Carbon
Abstract
:1. Introduction
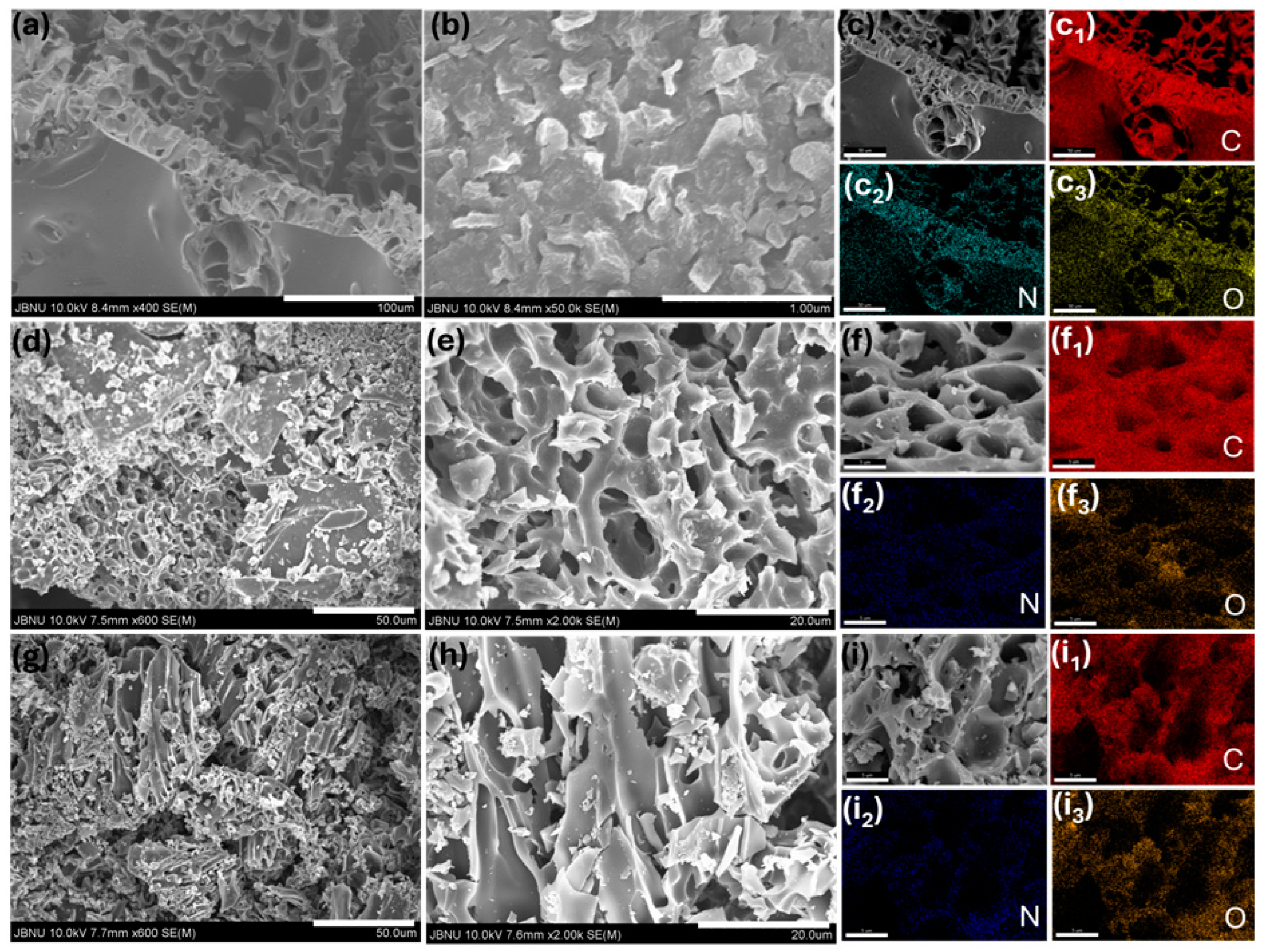
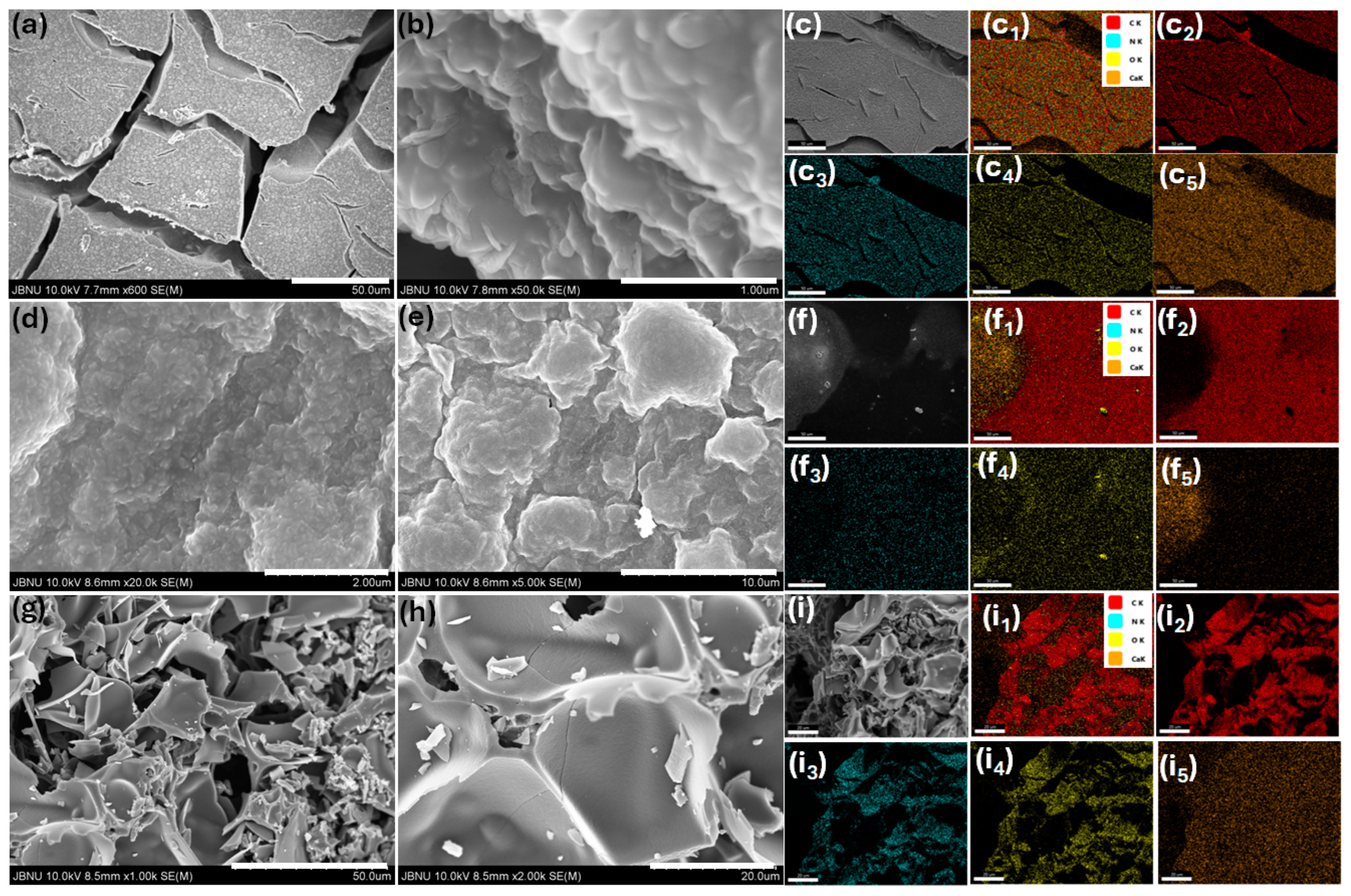
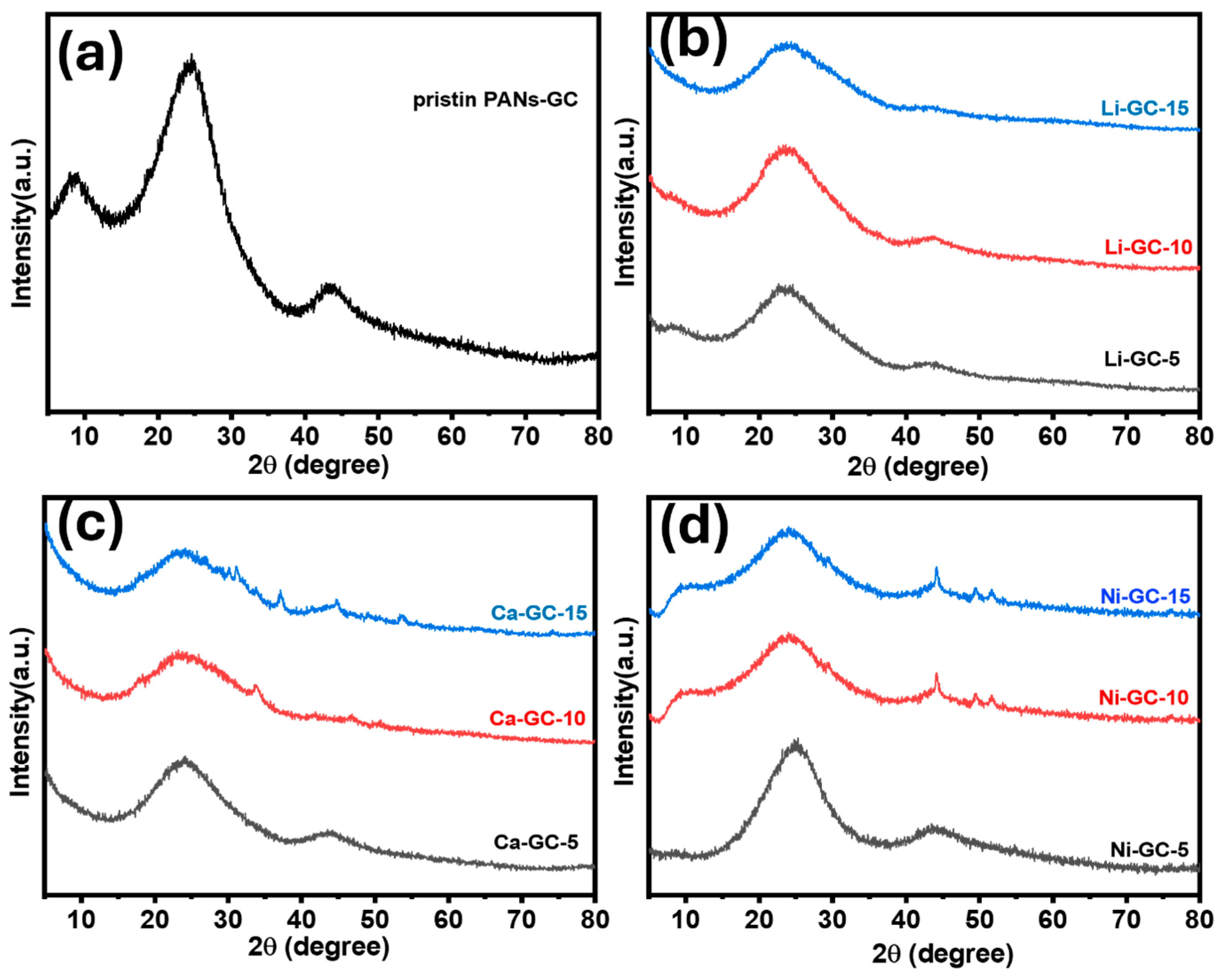
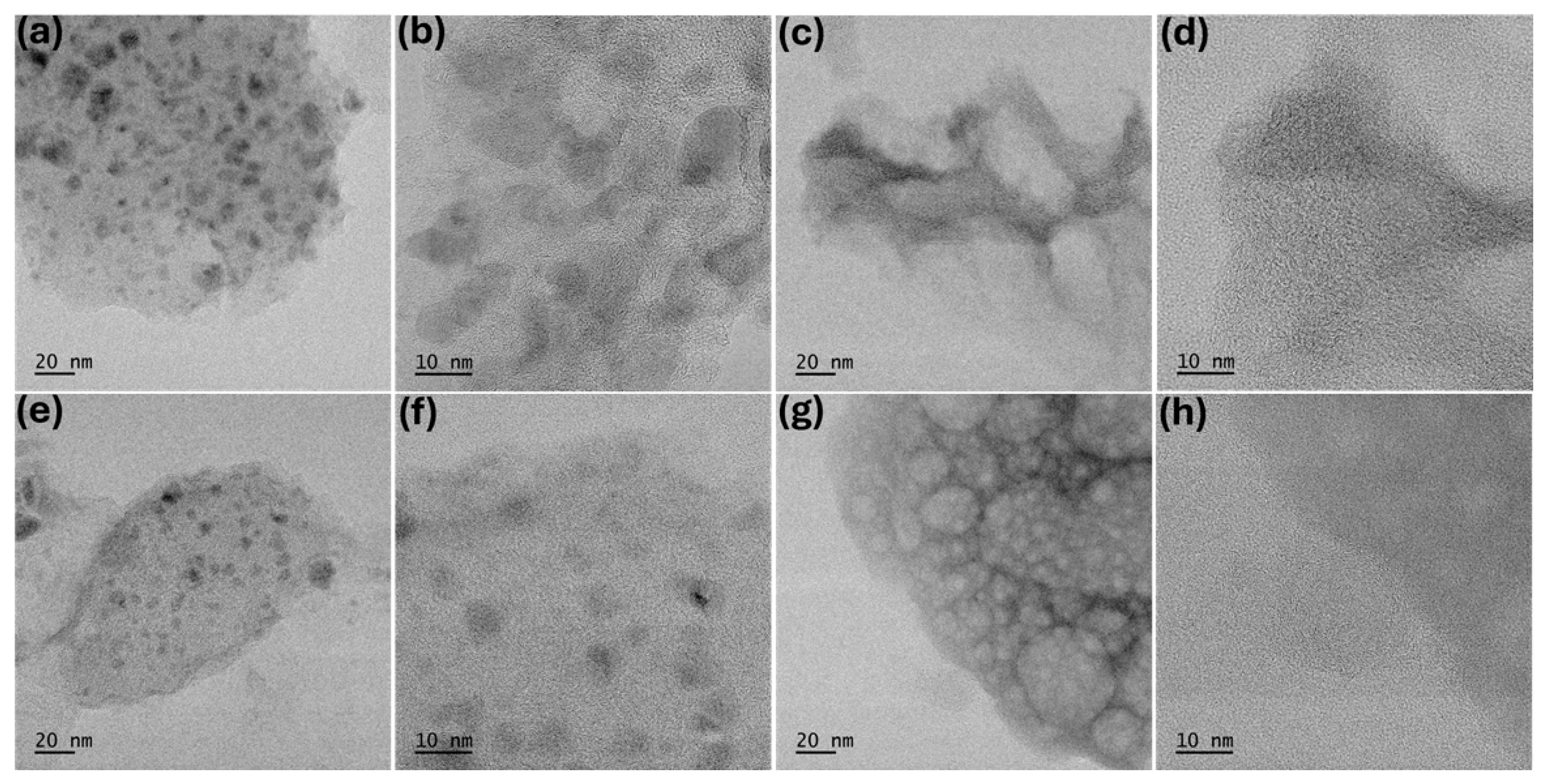
2. Results and Discussion
3. Materials and Methods
3.1. Chemical and Materials
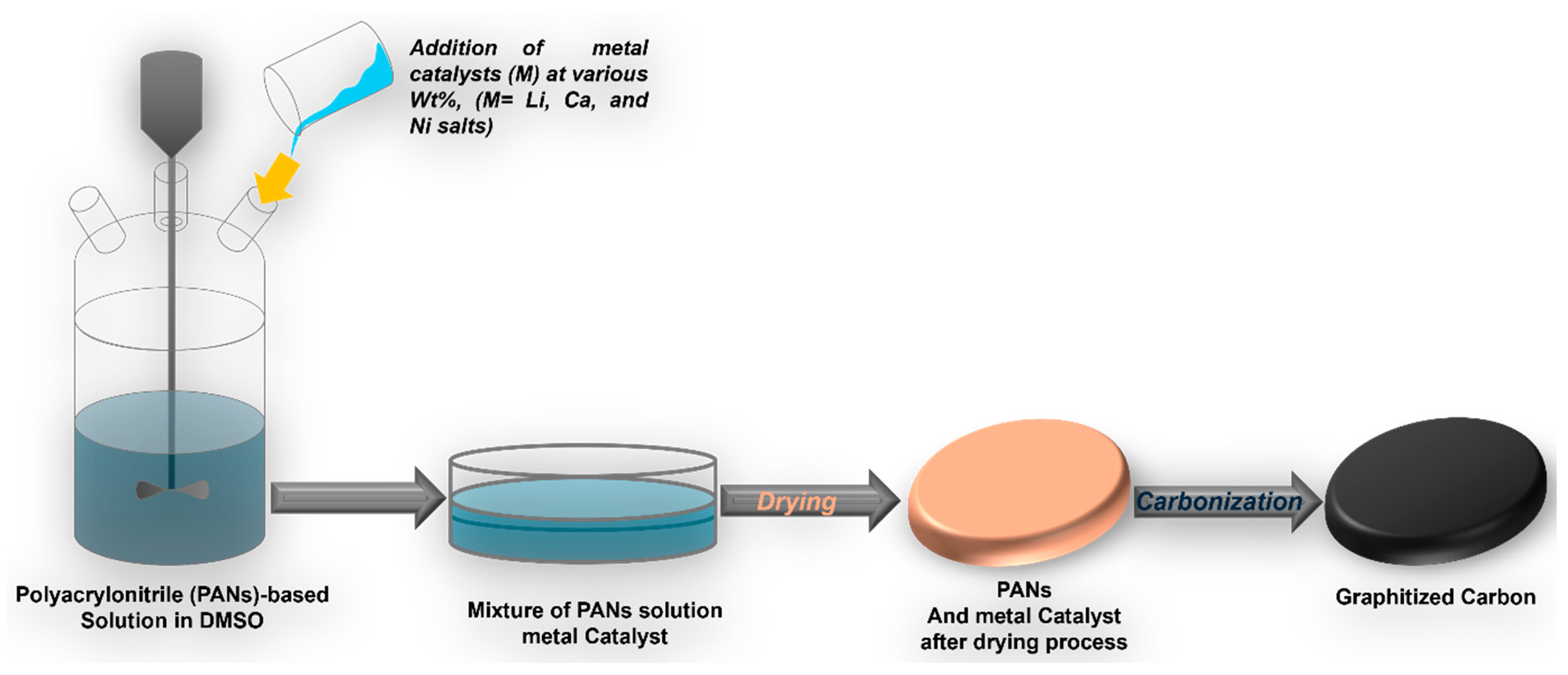
3.2. Preparation of the Nanostructured Graphitic Carbons at Low Temperature
3.3. Material Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hunter, R.D.; Ramírez-Rico, J.; Schnepp, Z. Iron-catalyzed graphitization for the synthesis of nanostructured graphitic carbons. J. Mater. Chem. A 2022, 10, 4489–4516. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; Chen, F.; Sun, Y.; Liu, Y.; Wang, L.; Li, C. String of ZIF-derived hollow beaded nanocage embedded into carbon nanofiber with intensified exposed Co-Nx sites for efficient oxygen catalysis in various fuel cell devices. Chem. Eng. J. 2023, 463, 142498. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Le, N.; Chhetri, K.; Acharya, D.; Pandiyarajan, S.M.S.; Saud, S.; Kim, S.J.; Mok, Y.S. Synergistic Performance Boosts of Dopamine-Derived Carbon Shell Over Bi-metallic Sulfide: A Promising Advancement for High-Performance Lithium-Ion Battery Anodes. Adv. Sci. 2024, 11, 2308160. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, E.; Zanetti, M.; Bracco, P.; Brunella, V.; Luda, M.P.; Costa, L. Crosslinking and carbonization processes in PAN films and nanofibers. Polym. Degrad. Stab. 2016, 123, 178–188. [Google Scholar] [CrossRef]
- Kunwar, J.; Acharya, D.; Chhetri, K.; Karki, B.; Deo, B.; Bhattarai, R.M.; Neupane, S.; Adhikari, M.P.; Yadav, A.P. Cobalt oxide decorated 2D MXene: A hybrid nanocomposite electrode for high-performance supercapacitor application. J. Electroanal. Chem. 2023, 950, 117915. [Google Scholar] [CrossRef]
- Li, H.; Wu, A.; Qiu, Z.; Li, J.; Wu, Z.; Ma, Y.; Wang, J.; He, S.; Huang, H. Carbonization of Ni@SiC@C nanoparticles reinforced PAN nanofibers for adjustable impedance matching. Chem. Eng. J. 2023, 476, 146582. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Pan, F.; Guo, H.; Wang, X.; Cheng, J.; Cai, L.; Xiu, Z.; Chen, L.; Batalu, D.; et al. Electrospinning technology on one dimensional microwave absorbers: Fundamentals, current progress, and perspectives. Chem. Eng. J. 2023, 470, 144236. [Google Scholar] [CrossRef]
- Storck, J.L.; Hellert, C.; Brockhagen, B.; Wortmann, M.; Diestelhorst, E.; Frese, N.; Grothe, T.; Ehrmann, A. Metallic Supports Accelerate Carbonization and Improve Morphological Stability of Polyacrylonitrile Nanofibers during Heat Treatment. Materials 2021, 14, 4686. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, J.; Wang, H.-L. Tuning of structural/functional feature of carbon fibers: New insights into the stabilization of polyacrylonitrile. Polymer 2023, 282, 126157. [Google Scholar] [CrossRef]
- Chhetri, K.; Kim, T.; Acharya, D.; Muthurasu, A.; Dahal, B.; Bhattarai, R.M.; Lohani, P.C.; Pathak, I.; Ji, S.; Ko, T.H.; et al. Hollow Carbon Nanofibers with Inside-outside Decoration of Bi-metallic MOF Derived Ni-Fe Phosphides as Electrode Materials for Asymmetric Supercapacitors. Chem. Eng. J. 2022, 450, 138363. [Google Scholar] [CrossRef]
- Li, G.; Guo, H.; Wang, Z.; Yan, G.; Wang, J.; Li, X. A robust in-situ catalytic graphitization combined with salt-template strategy towards fast lithium-ions storage. J. Alloys Compd. 2022, 908, 164717. [Google Scholar] [CrossRef]
- Mennani, M.; Ait Benhamou, A.; Mekkaoui, A.A.; El Bachraoui, F.; El Achaby, M.; Moubarik, A.; Kassab, Z. Probing the evolution in catalytic graphitization of biomass-based materials for enduring energetic applications. J. Mater. Chem. A 2024, 12, 6797–6825. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Cao, F.; Zhang, Y.; Xia, X.; Zhang, L. Employing Ni-Embedded Porous Graphitic Carbon Fibers for High-Efficiency Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2022, 14, 10457–10466. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Gu, J.; Kang, D.; Zhou, F.; Zhang, W.; Wu, Y.; Zhang, D. Highly porous graphitic materials prepared by catalytic graphitization. Carbon 2013, 64, 132–140. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Sun, R.; Yao, L.; Zuo, H.; Ruan, F.; Feng, Q.; Wang, J. Preparation of hierarchical micro-meso porous carbon and carbon nanofiber from polyacrylonitrile/polysulfone polymer via one-step carbonization for supercapacitor electrodes. Electrochim. Acta 2023, 441, 141827. [Google Scholar] [CrossRef]
- Boll, F.; Crisci, M.; Merola, L.; Lamberti, F.; Smarsly, B.; Gatti, T. Assessing the Effect of Stabilization and Carbonization Temperatures on Electrochemical Performance of Electrospun Carbon Nanofibers from Polyacrylonitrile. Adv. Energy Sustain. Res. 2023, 4, 2300121. [Google Scholar] [CrossRef]
- Mao, Q.; Rajabpour, S.; Talkhoncheh, M.K.; Zhu, J.; Kowalik, M.; van Duin, A.C.T. Cost-effective carbon fiber precursor selections of polyacrylonitrile-derived blend polymers: Carbonization chemistry and structural characterizations. Nanoscale 2022, 14, 6357–6372. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.; Xu, W.; Ravichandran, D.; Jambhulkar, S.; Zhu, Y.; Song, K. Reinforcing carbonized polyacrylonitrile fibers with nanoscale graphitic interface-layers. J. Mater. Sci. Technol. 2021, 95, 78–87. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Saud, S.; Teke, S.; Kim, S.J.; Mok, Y.S. Eco-Friendly Synthesis of Cobalt Molybdenum Hydroxide 3d Nanostructures on Carbon Fabric Coupled with Cherry Flower Waste-Derived Activated Carbon for Quasi-Solid-State Flexible Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 160–175. [Google Scholar] [CrossRef]
- Pathak, I.; Muthurasu, A.; Acharya, D.; Chhetri, K.; Dahal, B.; Rosyara, Y.R.; Kim, T.; Ko, T.H.; Kim, H.Y. Electronically modulated bimetallic telluride nanodendrites atop 2D nanosheets using a vanadium dopant enabling a bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2024, 12, 17544–17556. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Kuvarega, A.T.; Onwudiwe, D.C. Graphitic carbon nitride-based catalysts and their applications: A review. Nano-Struct. Nano-Objects 2020, 24, 100577. [Google Scholar] [CrossRef]
- Kandel, D.R.; Poudel, M.B.; Radoor, S.; Chang, S.; Lee, J. Decoration of dandelion-like manganese-doped iron oxide microflowers on plasma-treated biochar for alleviation of heavy metal pollution in water. Chemosphere 2024, 357, 141757. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Yang, Y.; Yang, X.; Wang, G.; Zhu, L.; Zeng, Z. Effective solar-driven evaporators with urchin-like CuO particles and directional polyvinylidene fluoride scaffolds via directional dimethyl sulfoxide crystallization and non-solvent induced phase separation. Sep. Purif. Technol. 2024, 345, 127446. [Google Scholar] [CrossRef]
- An, X.; Shin, D.; Ocon, J.D.; Lee, J.K.; Son, Y.-I.; Lee, J. Electrocatalytic oxygen evolution reaction at a FeNi composite on a carbon nanofiber matrix in alkaline media. Chin. J. Catal. 2014, 35, 891–895. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Duan, L.; Liu, L.; Xiao, X.; Xin, X.; Li, X. Uniform Li-deposition induced by Ni-catalyzing graphitization of natural carbon materials in lithium metal batteries. ChemNanoMat 2023, 9, e202300194. [Google Scholar] [CrossRef]
- Yan, Z.; Huang, D.; Lai, A.; Chu, Y.; Zheng, F.; Cai, Y.; Pan, Q.; Wang, H.; Huang, Y.; Li, Q. Nickel catalyzed graphitized carbon coated LiFe1-xNixPO4 composites as cathode material for high-performance lithium-ion batteries. Electrochim. Acta 2020, 353, 136565. [Google Scholar] [CrossRef]
- Nazarov, E.E.; Tyablikov, O.A.; Nikitina, V.A.; Antipov, E.V.; Fedotov, S.S. Polyacrylonitrile-Derived Carbon Nanocoating for Long-Life High-Power Phosphate Electrodes. Appl. Nano 2023, 4, 25–37. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, G.; Liu, J.; Xiang, Y.; Guo, S. Interaction between thermal stabilization temperature program, oxidation reaction, and mechanical properties of polyacrylonitrile (PAN) based carbon fibers. Diam. Relat. Mater. 2024, 141, 110588. [Google Scholar] [CrossRef]
- Made Joni, I.; Vanitha, M.; Camellia, P.; Balasubramanian, N. Augmentation of graphite purity from mineral resources and enhancing % graphitization using microwave irradiation: XRD and Raman studies. Diam. Relat. Mater. 2018, 88, 129–136. [Google Scholar] [CrossRef]
- Nguyen, L.; Bhattarai, R.M.; Teke, S.; Chhetri, K.; Acharya, D.; Sasikumar, R.; Mok, Y.S. Unlocking enhanced electrochemical performance through oxygen–nitrogen dual functionalization of iron–nickel–sulfide for efficient energy storage systems. J. Mater. Chem. A 2024, 12, 17369–17381. [Google Scholar] [CrossRef]
- Wang, N.; Mao, M.; Mao, G.; Yin, J.; He, R.; Zhou, H.; Li, N.; Liu, Q.; Zhi, K. Investigation on carbide slag catalytic effect of Mongolian bituminous coal steam gasification process. Chemosphere 2021, 264, 128500. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tu, J.; Lei, H.; Zhu, H. The effect of graphitization degree of carbonaceous material on the electrochemical performance for aluminum-ion batteries. RSC Adv. 2019, 9, 38990–38997. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.M.; Chhetri, K.; Le, N.; Acharya, D.; Saud, S.; Nguyen, M.C.H.P.L.; Kim, S.J.; Mok, Y.S. Oxygen functionalization-assisted anionic exchange toward unique construction of flower-like transition metal chalcogenide embedded carbon fabric for ultra-long life flexible energy storage and conversion. Carbon Energy 2024, 6, e392. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Natarajan, S.; Saud, S.; Kim, S.J.; Mok, Y.S. Activated carbon derived from cherry flower biowaste with a self-doped heteroatom and large specific surface area for supercapacitor and sodium-ion battery applications. Chemosphere 2022, 303, 135290. [Google Scholar] [CrossRef] [PubMed]
- Kandel, M.R.; Pan, U.N.; Dhakal, P.P.; Ghising, R.B.; Sidra, S.; Kim, D.H.; Kim, N.H.; Lee, J.H. Manganese-Doped Bimetallic (Co,Ni)2P Integrated CoP in N,S Co−Doped Carbon: Unveiling a Compatible Hybrid Electrocatalyst for Overall Water Splitting. Small 2024, 20, 2307241. [Google Scholar] [CrossRef]
- Gautam, K.P.; Acharya, D.; Bhatta, I.; Subedi, V.; Das, M.; Neupane, S.; Kunwar, J.; Chhetri, K.; Yadav, A.P. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics 2022, 10, 86. [Google Scholar] [CrossRef]

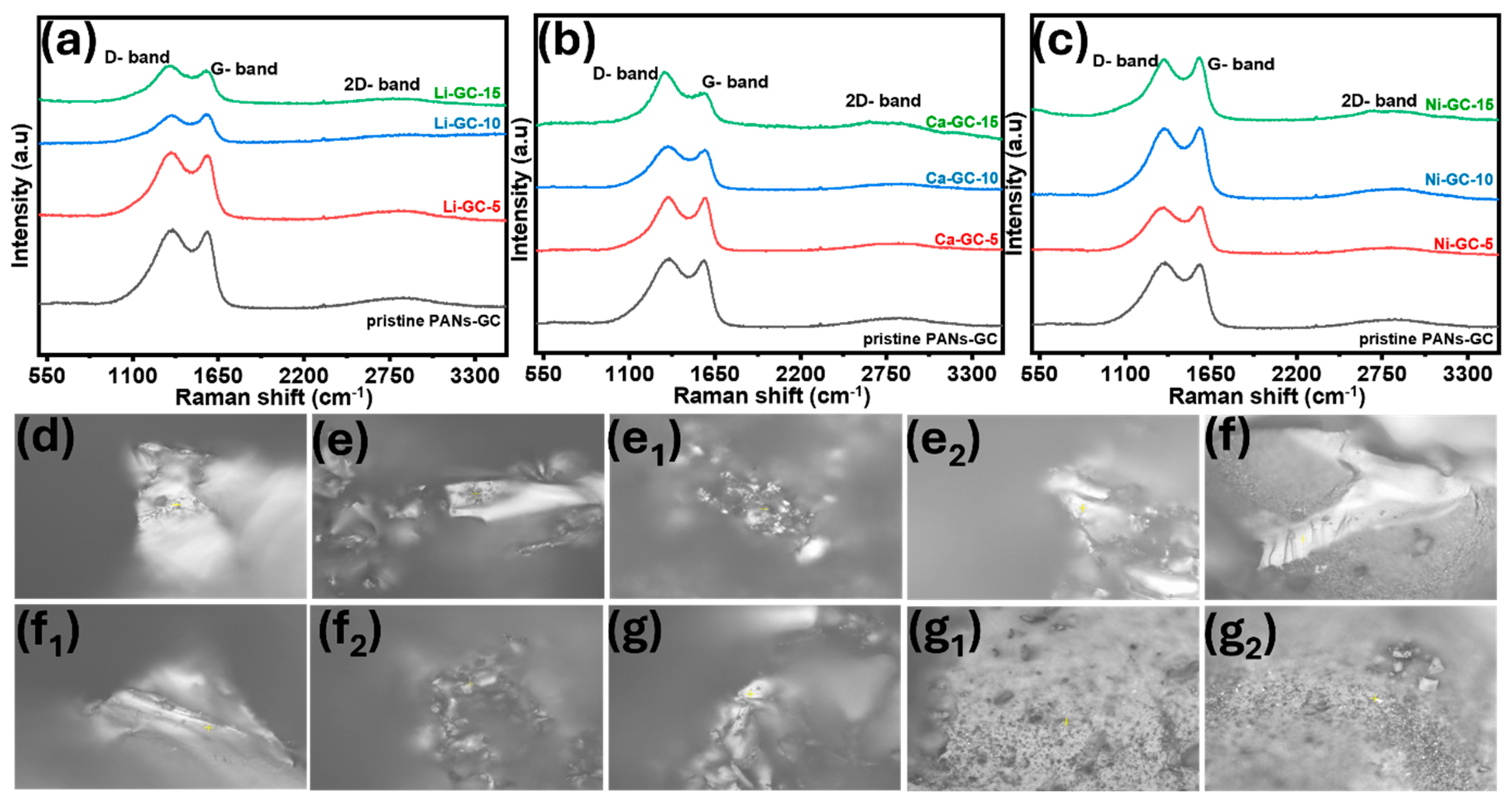

| Samples | Peak Position (D-Band) | Peak Position (G-Band) | ID/IG Ratio |
|---|---|---|---|
| Pristine GC | 1361.83 cm−1 | 1570.84 cm−1 | 1.026 |
| Li-GC-5 | 1348.01 cm−1 | 1575.33 cm−1 | 1.016 |
| Li-GC-10 | 1352.62 cm−1 | 1565.35 cm−1 | 0.994 |
| Li-GC-15 | 1343.4 cm−1 | 1570.84 cm−1 | 1.017 |
| Ca-GC-5 | 1352.62 cm−1 | 1584.29 cm−1 | 1.007 |
| Ca-GC-10 | 1348.01 cm−1 | 1588.77 cm−1 | 1.017 |
| Ca-GC15 | 1324.94 cm−1 | 1588.77 cm−1 | 1.079 |
| Ni-GC-5 | 1348.01 cm−1 | 1575.33cm−1 | 1.003 |
| Ni-GC-10 | 1352.62 cm−1 | 1566.35 cm−1 | 0.994 |
| Ni-GC-15 | 1352.62 cm−1 | 1570.84 cm−1 | 0.979 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Kim, B.-S.; Ko, T.H.; Kim, H.Y. Effect of Salt Variability on the Low-Temperature Metal-Catalyzed Graphitization of PAN/DMSO Solutions for the Synthesis of Nanostructured Graphitic Carbon. Inorganics 2024, 12, 212. https://doi.org/10.3390/inorganics12080212
Kim T, Kim B-S, Ko TH, Kim HY. Effect of Salt Variability on the Low-Temperature Metal-Catalyzed Graphitization of PAN/DMSO Solutions for the Synthesis of Nanostructured Graphitic Carbon. Inorganics. 2024; 12(8):212. https://doi.org/10.3390/inorganics12080212
Chicago/Turabian StyleKim, Taewoo, Byoung-Sukh Kim, Tae Hoon Ko, and Hak Yong Kim. 2024. "Effect of Salt Variability on the Low-Temperature Metal-Catalyzed Graphitization of PAN/DMSO Solutions for the Synthesis of Nanostructured Graphitic Carbon" Inorganics 12, no. 8: 212. https://doi.org/10.3390/inorganics12080212






