Tetrahydroxidohexaoxidopentaborate(1-) Salts of C6-Linked Substituted Diimidazolium and Dipyrrolidinium Cations: Synthesis, Characterization and XRD Studies
Abstract
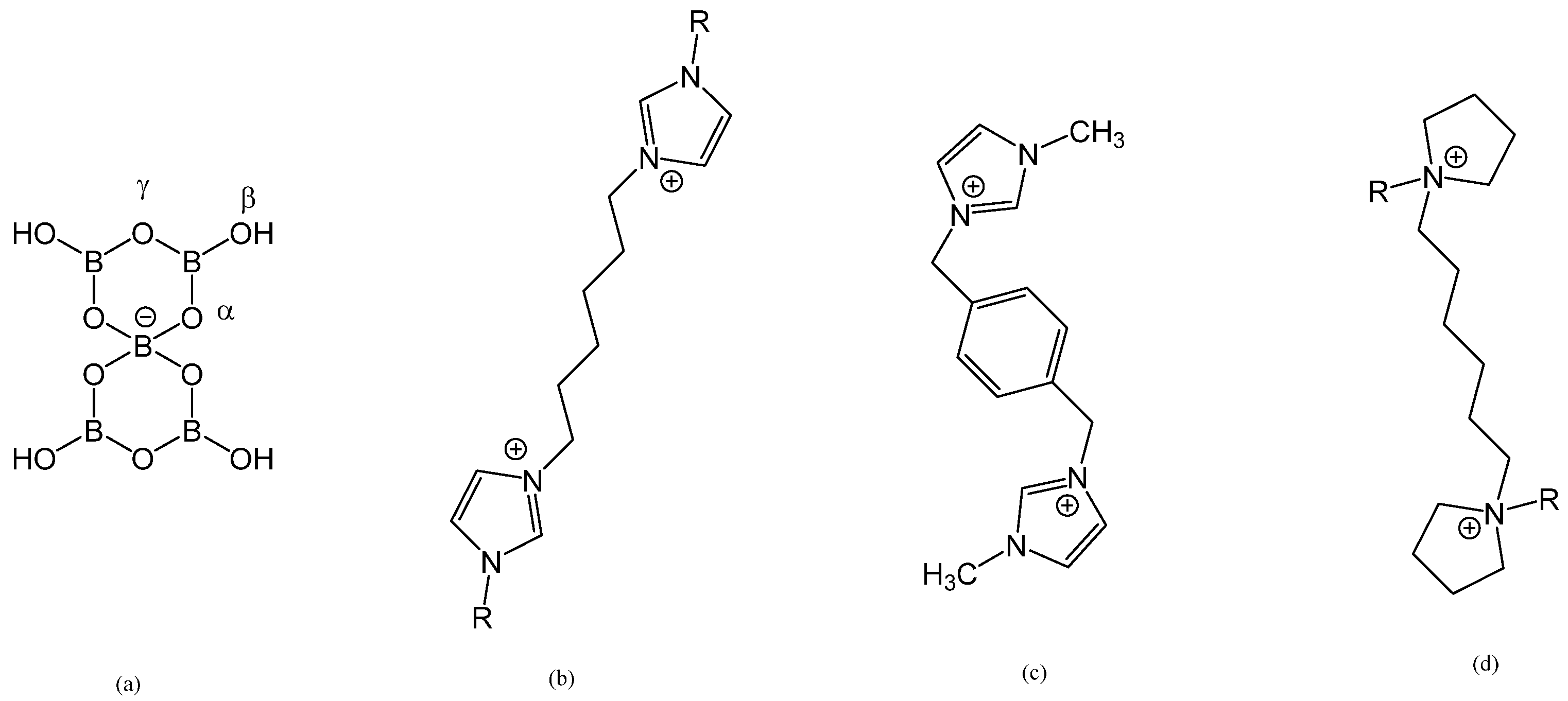
:1. Introduction
2. Results and Discussion
2.1. General and Synthetic Procedures
2.2. Spectroscopic and Thermal Analyses
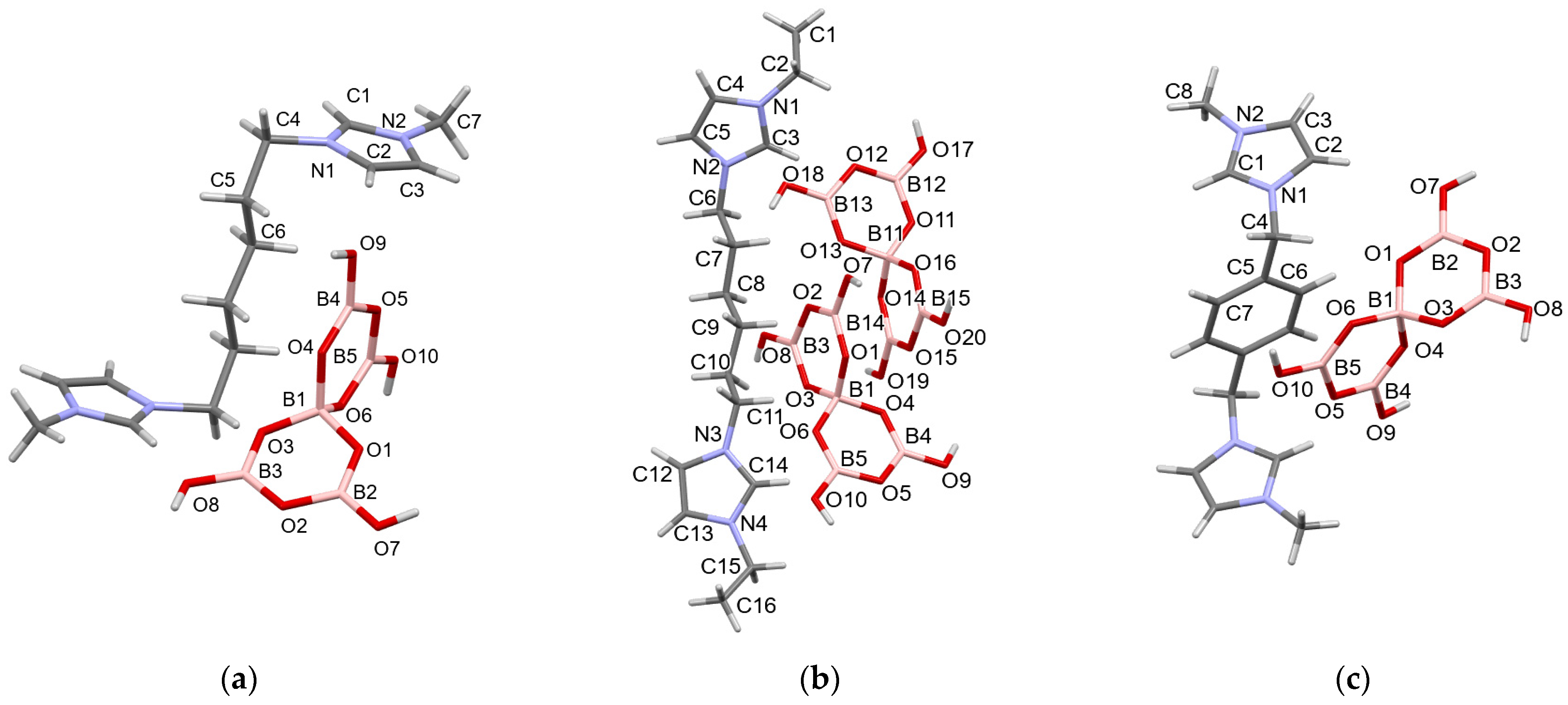
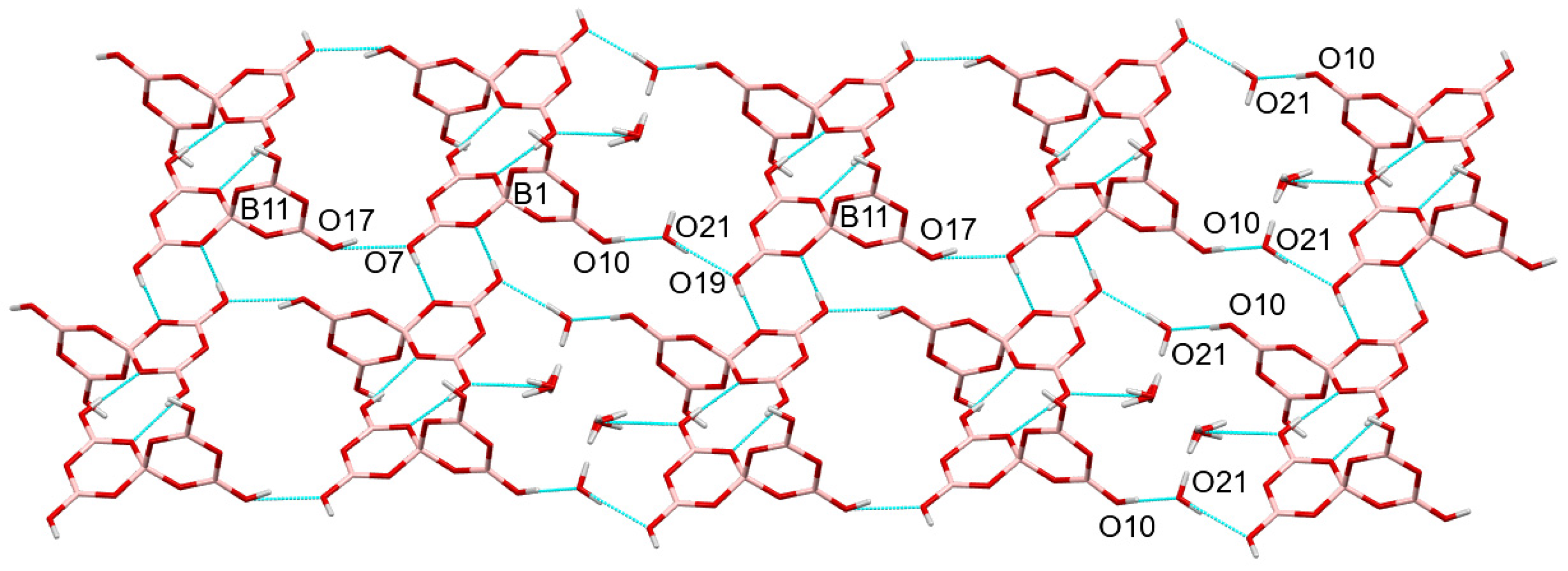
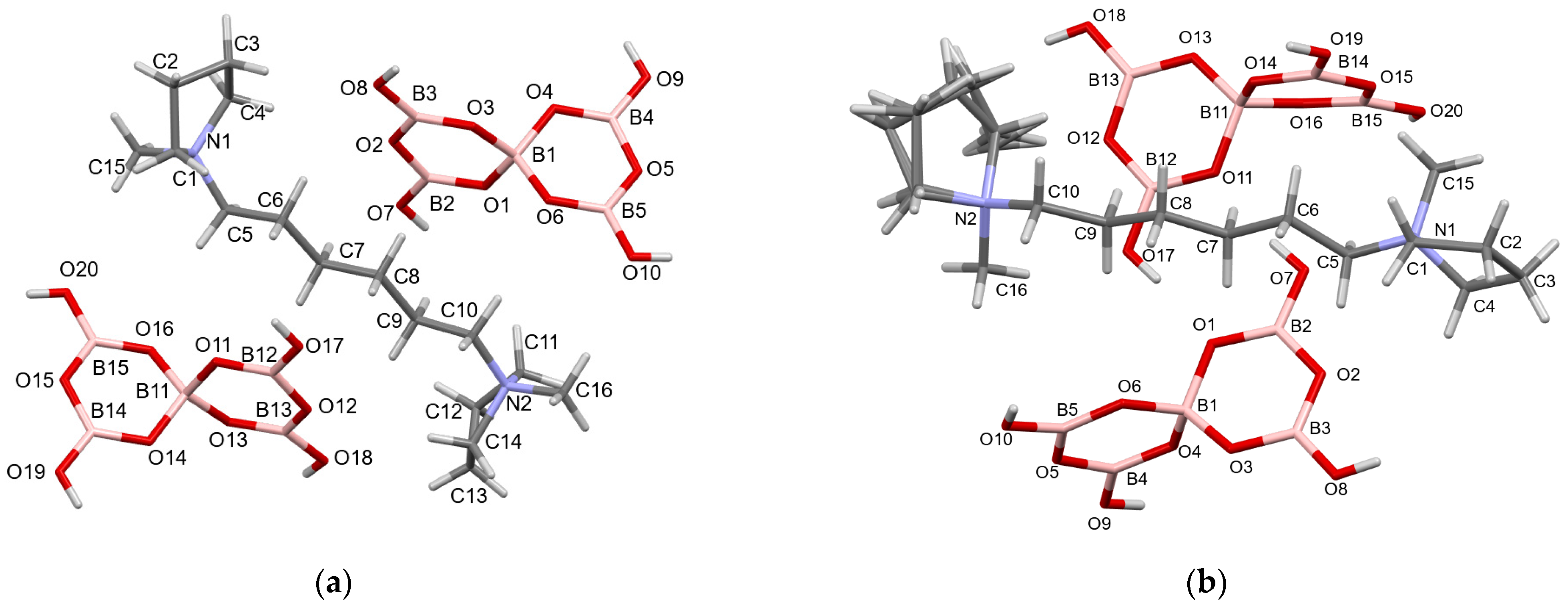
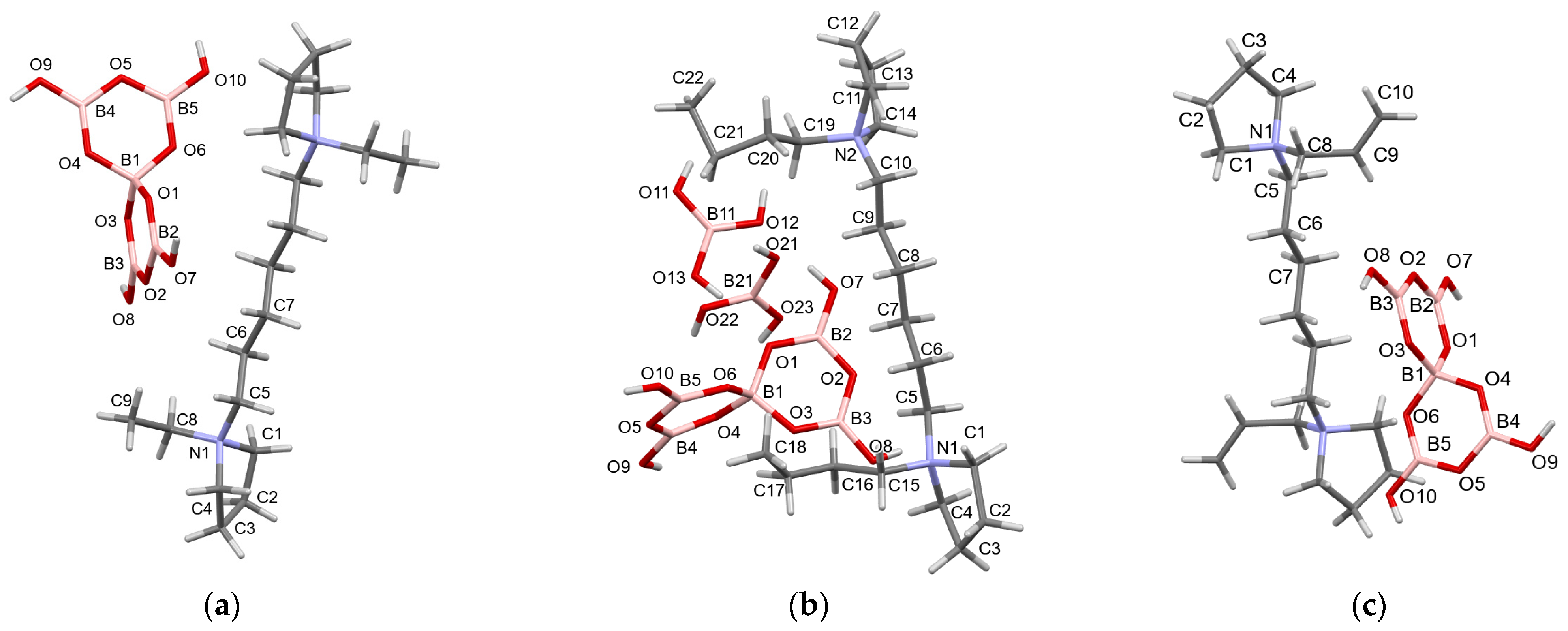
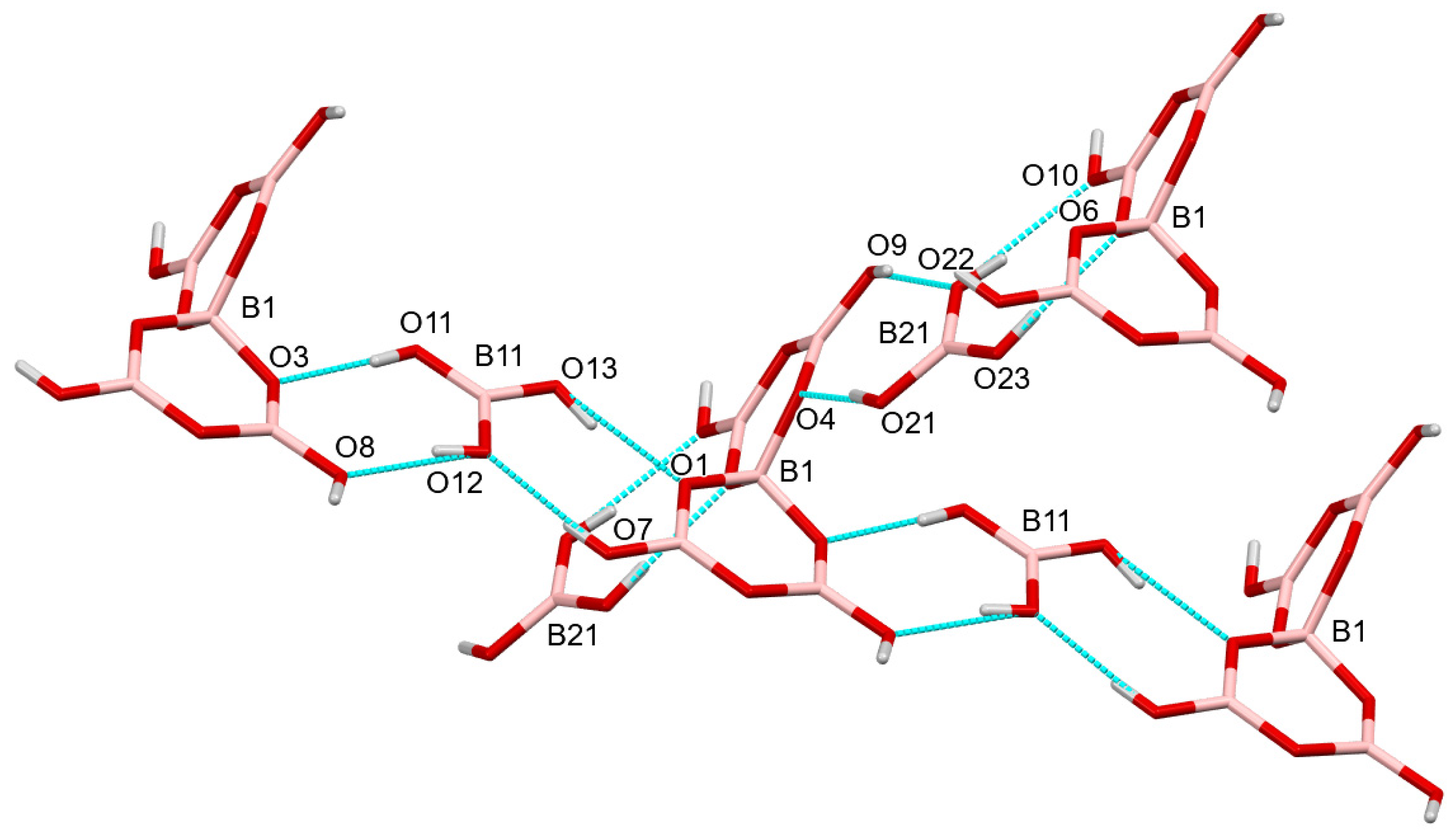
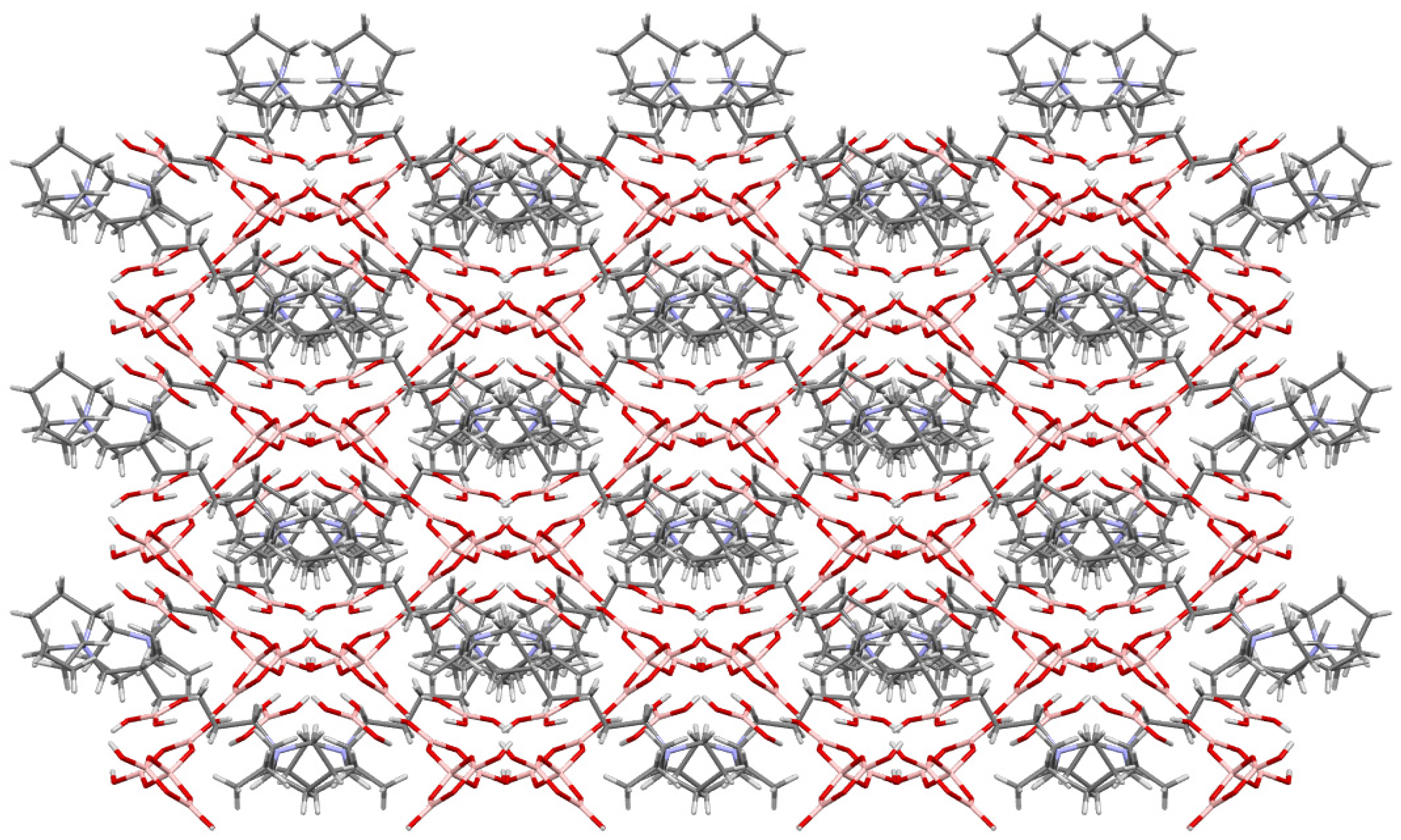
2.3. Crystallographic Studies
3. Experimental
3.1. General
3.2. X-ray Crystallography
3.3. Synthesis of Dication C6-Linked Bispentaborate Salts
3.3.1. Preparation of 1,1′-(1,6-Hexanediyl)bis(3-methyl-1H-imidazol-3-ium)bispentaborate [CH3(C3H3N2)(CH2)6(C3H3N2)CH3][B5O6(OH)4]2 (1)
3.3.2. Preparation of 1,1′-(1,6-Hexanediyl)bis(3-ethyl-1H-imidazol-3-ium)bispentaborate [C2H5(C3H3N2)(CH2)6(C3H3N2)C2H5][B5O6(OH)4]2·3H2O (2)
3.3.3. Preparation of 1,1′-[1,4-Phenylenebis(methylene)]bis(2-methyl-1H-imidazol-3-ium)bispentaborate [CH3(C3H3N2)CH2(C6H4)CH2(C3H3N2)CH3][B5O6(OH)4]2 (3)
3.3.4. Preparation of 1,1′-(1,6-Hexanediyl)bis(1-methylpyrrolidin-1-ium)bispentaborate [CH3(C4H8N)(CH2)6(C4H8N)CH3][B5O6(OH)4]2 (4)
3.3.5. Preparation of 1,1′-(1,6-Hexanediyl)bis(1-ethylpyrrolidin-1-ium)bispentaborate [C2H5(C4H8N)(CH2)6(C4H8N)C2H5][B5O6(OH)4]2 (5)
3.3.6. Preparation of 1,1′-(1,6-Hexanediyl)bis(1-butylpyrrolidin-1-ium) bispentaborate [C4H9(C4H8N)(CH2)6(C4H8N)C4H9][B5O6(OH)4]2·4B(OH)3 (6)
3.3.7. Preparation of 1,1′-(1,6-Hexanediyl)bis(1-allylpyrrolidin-1-ium)bispentaborate [C3H5(C4H8N)(CH2)6(C4H8N)C3H5][B5O6(OH)4]2 (7)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farmer, J.B. Metal borates. Adv. Inorg. Chem. Radiochem. 1982, 25, 187–237. [Google Scholar]
- Heller, G. A survey of structural types of borates and polyborates. Top. Curr. Chem. 1986, 131, 39–98. [Google Scholar]
- Topnikova, A.P.; Belokoneva, E.L. The structure and classification of complex borates. Russ. Chem. Rev. 2019, 88, 204–228. [Google Scholar] [CrossRef]
- Christ, C.L.; Clark, J.R. A crystal-chemical classification of borate structures with emphasis on hydrated borates. Phys. Chem. Miner. 1977, 2, 59–87. [Google Scholar] [CrossRef]
- Burns, P.C.; Grice, J.D.; Hawthorne, F.C. Borate minerals I. Polyhedral clusters and fundamental building blocks. Can. Mineral. 1995, 33, 1131–1151. [Google Scholar]
- Burns, P.C. Borate clusters and fundamental building blocks containing four polyhedral: Why few clusters are utilized as fundamental building blocks of structures. Can. Mineral. 1995, 33, 1167–1176. [Google Scholar]
- Grice, J.D.; Burns, P.C.; Hawthorne, F.C. Borate minerals II. A hierarchy of structures based upon the borate fundamental building block. Can. Mineral. 1999, 37, 731–762. [Google Scholar]
- Touboul, M.; Penin, N.; Nowogrocki, G. Borates: A survey of main trends concerning crystal chemistry, polymorphism and dehydration process of alkaline and pseudo-alkaline borates. Solid State Sci. 2003, 5, 1327–1342. [Google Scholar] [CrossRef]
- Belokoneva, E.L. Borate crystal chemistry in terms of extended OD theory: Topology and symmetry analysis. Crystallogr. Rev. 2005, 11, 151–198. [Google Scholar] [CrossRef]
- Schubert, D.M.; Knobler, C.B. Recent studies in polyborate anions. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. B 2009, 50, 71–78. [Google Scholar]
- Schubert, D.M. Borates in industrial use. Struct. Bond. 2003, 105, 1–40. [Google Scholar]
- Schubert, D.M. Boron oxide, boric acid, and borates. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; J. Wiley Sons: New York, NY, USA, 2011; pp. 1–68. [Google Scholar]
- Schubert, D.M. Hydrated zinc borates and their industrial uses. Molecules 2019, 24, 2419. [Google Scholar] [CrossRef]
- Becker, P. Borate materials in nonlinear optics. Adv. Mater. 1998, 10, 979–992. [Google Scholar] [CrossRef]
- Beckett, M.A. Recent Advances in crystalline hydrated borates with non-metal or transition-metal complex cations. Coord. Chem. Rev. 2016, 323, 2–14. [Google Scholar] [CrossRef]
- Beckett, M.A.; Horton, P.N.; Hursthouse, M.B.; Timmis, J.L. Synthesis and thermal studies of two phosphonium tetrahydroxidohexaoxidooentaborate(1-) salts: Single-crystal XRD characterization of [iPrPPh3][B5O6(OH)4]·3.5H2O and [MePPh3][B5O6(OH)4]·B(OH)3·0.5H2O. Molecules 2023, 28, 6867. [Google Scholar] [CrossRef]
- Xin, S.-S.; Zhou, M.-H.; Beckett, M.A.; Pan, C.-Y. Recent advances in crystalline oxidopolyborate complexes of d-block or p-block metals: Structural aspects, synthesis, and physical properties. Molecules 2021, 26, 3815. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Gavezzotti, A. Supramolecular synthons: Validation and ranking of intermolecular interaction energies. Cryst. Growth Des. 2012, 12, 5873–5877. [Google Scholar] [CrossRef]
- Sola, J.; Lafuente, M.; Atcher, J.; Alfonso, I. Constitutional self-selection from dynamic combinatorial libraries in aqueous solution through supramolecular interactions. Chem. Commun. 2014, 50, 4564–4566. [Google Scholar] [CrossRef]
- Corbett, P.T.; Leclaire, J.; Vial, L.; West, K.R.; Wietor, J.-L.; Sanders, J.K.M.; Otto, S. Dynamic combinatorial chemistry. Chem. Rev. 2006, 106, 3652–3711. [Google Scholar] [CrossRef] [PubMed]
- Beckett, M.A.; Coles, S.J.; Horton, P.N.; Jones, C.L. Polyborate anions partnered with large non-metal cations: Triborate(1-), pentaborate(1-) and heptaborate(2-) salts. Eur. J. Inorg. Chem. 2017, 2017, 4510–4518. [Google Scholar] [CrossRef]
- Beckett, M.A.; Coles, S.J.; Davies, R.A.; Horton, P.N.; Jones, C.L. Pentaborate(1−) salts templated by substituted pyrrolidinium cations: Synthesis, structural characterization, and modelling of solid-state H-bond interactions by DFT calculations. Dalton Trans. 2015, 44, 7032–7040. [Google Scholar] [CrossRef] [PubMed]
- Beckett, M.A.; Coles, S.J.; Horton, P.N.; Rixon, T.A. Structural (XRD) characterization and an analysis of H-bonding motifs in some tetrahydroxidohexaoxidopentaborate(1-) salts of N-substituted guandinium cations. Molecules 2023, 28, 3273. [Google Scholar] [CrossRef]
- Beckett, M.A.; Brellocks, B.; Chizhevsky, I.T.; Damhus, T.; Hellwich, K.-H.; Kennedy, J.D.; Laitinen, R.; Powell, W.H.; Rabinovich, D.; Vinas, C.; et al. Nomenclature for boranes and related species (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 355–381. [Google Scholar] [CrossRef]
- Rixon, T.A. Stabilised Silicate and Borate Solutions for Foliar Agricultural Sprays. Ph.D. Thesis, Bangor University, Bangor, UK, 2019. [Google Scholar]
- Beckett, M.A.; Coles, S.J.; Horton, P.N.; Rixon, T.A. Synthesis and XRD study of an C2-linked bis(quaternary ammonium) pentaborate: [Me3NCH2CH2NMe3][B5O6(OH)4]2. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 952–955. [Google Scholar] [CrossRef]
- Beckett, M.A.; Meena, B.I.; Rixon, T.A.; Coles, S.J.; Horton, P.N. Pentaborate(1-) salts and a tetraborate(2-) salt derived from C2- or C3-linked bis(alkylammonium) dications: Synthesis, characterization and structural (XRD) studies. Molecules 2020, 25, 53. [Google Scholar] [CrossRef] [PubMed]
- Beckett, M.A.; Horton, P.N.; Hursthouse, M.B.; Knox, D.A.; Timmis, J.L. Structural (XRD) and thermal (DSC, TGA) and BET analysis of materials derived from non-metal cation pentaborate salts. Dalton Trans. 2010, 39, 3944–3951. [Google Scholar] [CrossRef]
- Jones, C.L.; Beckett, M.A.; Coles, S.J.; Davies, R.A.; Horton, P.N. Synthesis and characterization of templated pentaborate(1-) salts: X-ray structure of [(2-HOCH2CH2)C4H7NMeH][B5O6(OH)4]·0.3H2O. Phosphorus Sulfur Silicon 2016, 191, 628–630. [Google Scholar] [CrossRef]
- Beckett, M.A.; Horton, P.N.; Hursthouse, M.B.; Timmis, J.L. Synthesis and X-ray structural studies of pentaborate(1-) salts containing substituted imidazolium cations. Polyhedron 2014, 77, 96–102. [Google Scholar] [CrossRef]
- Han, W.-H.; Dang, L.-L.; Zhang, W.-J. Crystal structure of imidazolium tetrahydroxohexaoxopentaborate, [C3H5N2][B5O6(OH)4]. Z. Kristallogr. NCS 2007, 222, 403–404. [Google Scholar] [CrossRef]
- Yang, H.-X.; Zhang, W.-J.; Liu, X.-L.; Liu, Z.-K. 2-Methimidazolium tetrahydroxohexaoxopentaborate. Acta Cryst. 2006, E62, o4877–o4879. [Google Scholar]
- Parker, T.G.; Pubbi, D.; Beehler, A.; Albrecht-Schmitt, T.E. 1-Ethyl-3-methyl-1H-imidazol-3-ium spiropentaborate. Acta Cryst. 2014, E70, o171–o172. [Google Scholar] [CrossRef] [PubMed]
- Visi, M.Z.; Knobler, C.B.; Owen, J.J.; Khan, M.I.; Schubert, D.M. Structures of self-assembled nonmetal borates derived from α,ω-diaminoalkanes. Cryst. Growth Des. 2006, 6, 538–545. [Google Scholar] [CrossRef]
- Schubert, D.M.; Visi, M.Z.; Knobler, C.B. Guanidinium and imidazolium borates containing the first examples of an isolated nonaborate oxoanion: [B9O12(OH)6](3−). Inorg. Chem. 2000, 39, 2250–2251. [Google Scholar] [CrossRef]
- Schubert, D.M.; Smith, R.A.; Visi, M.Z. Studies of crystalline non-metal borates. Glass Technol. 2003, 44, 63–70. [Google Scholar]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic chemistry. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Salentine, G. High-field 11B NMR of alkali borates. Aqueous polyborate equilibria. Inorg. Chem. 1983, 22, 3920–3924. [Google Scholar] [CrossRef]
- Anderson, J.L.; Eyring, E.M.; Whittaker, M.P. Temperature jump rate studies of polyborate formation in aqueous boric acid. J. Phys. Chem. 1964, 68, 1128–1132. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Lan, Y.; Wang, D.; Zhang, L.; Tang, X.; Yang, S.; Luo, Z.; Tian, G. Speciation of borate in aqueous solution studied experimentally by potentiometry and Raman spectroscopy and computationally by DFT calculations. New J. Chem. 2023, 47, 8499–8506. [Google Scholar] [CrossRef]
- Durka, K.; Jarzembska, K.N.; Kaminski, R.; Lulinski, S.; Serwatowski, J.; Wozniak, K. Structure and energetic landscapes of fluorinated 1,4-phenylboronic acids. Cryst. Growth Des. 2012, 12, 3720–3734. [Google Scholar] [CrossRef]
- Freyhardt, C.C.; Wiebcke, M.; Felsche, J.; Englehardt, G. N(nPr)4[B5O6(OH)4][B(OH)3]2 and N(nBu)4[B5O6(OH)4][B(OH)3]2: Clathrates with a diamondoid arrangement of hydrogen bonded pentaborate anions. J. Incl. Phenom. Mol. Recogn. Chem. 1994, 18, 161–175. [Google Scholar] [CrossRef]
- Beckett, M.A.; Horton, P.N.; Hursthouse, M.B.; Timmis, J.L.; Varma, K.S. Templated heptaborate and pentaborate salts of cyclo-alkylammonium cations: Structural and thermal properties. Dalton Trans. 2012, 41, 4396–4403. [Google Scholar] [CrossRef]
- Beckett, M.A.; Horton, P.N.; Coles, S.J.; Kose, D.A.; Kreuziger, A.-M. Structural and thermal studies of non-metal cation pentaborate salts with cations derived from 1,5-diazobicyclo[4.3.0]non-5-ene, 1,8-diazobicyclo[5.4.0]undec-7-ene and 1,8-bis(dimethylamino)naphthalene. Polyhedron 2012, 38, 157–161. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, D.; Li, G.; Zhang, Y. Synthesis, crystal structure, and variable-temperature-luminescent property of the organically templated pentaborate [C10N2H9][B5O6(OH)4].H3BO3·H2O. Z. Anorg. Allg. Chem. 2013, 639, 722–727. [Google Scholar] [CrossRef]
- Rajkumar, T.; Ranga Rao, G. Synthesis and characterization of hybrid molecular material prepared by ionic liquid and silicotungstic acid. Mater. Chem. Phys. 2008, 112, 853–857. [Google Scholar] [CrossRef]
- Li, J.; Xia, S.; Gao, S. FT-IR and Raman spectroscopic study of hydrated borates. Spectrochim. Acta 1995, 51A, 519–532. [Google Scholar]
- Babushkina, O.B. Phase Behaviour and FTIR Spectra of Ionic Liquids: The Mixtures of 1-Butyl-1-methylpyrrolidinium Chloride and TaCl5. Z. Naturforsch. 2008, 63A, 66–72. [Google Scholar] [CrossRef]
- Wang, G.-M.; Sun, Y.-Q.; Yang, G.-Y. Syntheses and crystal structures of two new organically templated borates. J. Solid. State Chem. 2004, 177, 4648–4654. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Zhong, L.-J.; Lu, J.; Li, D.-G.; Zhoa, F.-H.; Yang, H.-M. Synthesis, structure, and luminescent property of the templated borate [C9H14N]·[B5O6(OH)4]. Z. Anorg. Allg. Chem. 2014, 640, 352–356. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Tian, S.; Liao, F.; Lin, J. Synthesis and structure of [C2H10N2][B5O8(OH)]: A nonmetal pentaborate with nonlinear optical properties. Cryst. Growth Des. 2007, 7, 1246–1250. [Google Scholar] [CrossRef]
- Liu, H.-X.; Liang, Y.-X.; Jiang, X. Synthesis, crystal structure and NLO property of a nonmetal pentaborate [C6H13N2][B5O6(OH)4]. J. Solid State Chem. 2008, 181, 3243–3247. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Wen, T.; Zhang, H.; Lin, X.; Wang, Y.; Liao, F.; Lin, J. Synthesis, crystal structure and visible light emission of a new inorganic-organic hybrid pentaborate, [C6H12N][B5O6(OH)4]. J. Mol. Struct. 2013, 1048, 1–5. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Hopfl, H. The tetrahedral character of the boron atom newly defined—A useful tool to evaluate the N→B bond. J. Organomet. Chem. 1999, 581, 129–149. [Google Scholar] [CrossRef]
- Beckett, M.A.; Brassington, D.S.; Owen, P.; Hursthouse, M.B.; Light, M.E.; Malik, K.M.A.; Varma, K.S. p-Bonding in B-O ring species: Lewis acidity of Me3B3O3, synthesis of amine Me3B3O3 adducts, and the crystal and molecular structure of Me3B3O3·NH2iBu·MeB(OH)2. J. Organonmet. Chem. 1999, 585, 7–11. [Google Scholar] [CrossRef]
- Vlahakis, J.Z.; Mitu, S.; Roman, G.; Rodriguez, E.P.; Crandall, I.C.; Szarek, W.A. The anti-malarial activity of imidazolium salts. Biorgan. Med. Chem. 2011, 19, 6525–6542. [Google Scholar] [CrossRef] [PubMed]
- Libman, D.D.; Pain, D.L.; Slac, R. Some bisquaternary salts. J. Chem. Soc. 1952, 2305–2307. [Google Scholar] [CrossRef]
- Cabildo, P.; Sanz, D.; Claramunt, R.M.; Bourne, S.A.; Alkorta, I.; Elguero, J. Synthesis and Structural Studies of Some [14]Paracyclo-bis-(1,2)pyrazolium- and (1,3)imidazolium-phanes. Tetrahedron 1999, 55, 2327–2340. [Google Scholar] [CrossRef]
- Kobialka, S.; Müller-Tautges, C.; Schmidt, M.T.S.; Schnakenburg, G.; Hollóczki, O.; Kirchner, B.; Engeser, M. Stretch out or fold-back? Conformations of Dinuclear Gold(I) N-Heterocyclic Carbene Macrocycles. Inorg. Chem. 2015, 54, 6100–6111. [Google Scholar] [CrossRef]
- Rigaku. CrysAlisPro Software System; Rigaku Oxford Diffraction; Rigaku Corporation: Cedar Park, TX, USA, 2019. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. ShelXT-intergrated space-group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with ShelXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dulayymi, A.R.; Beckett, M.A.; Braganca, R.; Coles, S.J.; Horton, P.N.; Rixon, T.A. Tetrahydroxidohexaoxidopentaborate(1-) Salts of C6-Linked Substituted Diimidazolium and Dipyrrolidinium Cations: Synthesis, Characterization and XRD Studies. Inorganics 2024, 12, 220. https://doi.org/10.3390/inorganics12080220
Al-Dulayymi AR, Beckett MA, Braganca R, Coles SJ, Horton PN, Rixon TA. Tetrahydroxidohexaoxidopentaborate(1-) Salts of C6-Linked Substituted Diimidazolium and Dipyrrolidinium Cations: Synthesis, Characterization and XRD Studies. Inorganics. 2024; 12(8):220. https://doi.org/10.3390/inorganics12080220
Chicago/Turabian StyleAl-Dulayymi, Ahmad R., Michael A. Beckett, Radek Braganca, Simon J. Coles, Peter N. Horton, and Thomas A. Rixon. 2024. "Tetrahydroxidohexaoxidopentaborate(1-) Salts of C6-Linked Substituted Diimidazolium and Dipyrrolidinium Cations: Synthesis, Characterization and XRD Studies" Inorganics 12, no. 8: 220. https://doi.org/10.3390/inorganics12080220
APA StyleAl-Dulayymi, A. R., Beckett, M. A., Braganca, R., Coles, S. J., Horton, P. N., & Rixon, T. A. (2024). Tetrahydroxidohexaoxidopentaborate(1-) Salts of C6-Linked Substituted Diimidazolium and Dipyrrolidinium Cations: Synthesis, Characterization and XRD Studies. Inorganics, 12(8), 220. https://doi.org/10.3390/inorganics12080220







