Exploring the Frontiers of Cathode Catalysts in Lithium–Carbon Dioxide Batteries: A Mini Review
Abstract
:1. Introduction
2. Overview of Lithium–Carbon Dioxide Batteries
2.1. Mechanism of Lithium–Carbon Dioxide Batteries
2.2. Product Analysis of Lithium–Carbon Dioxide Batteries
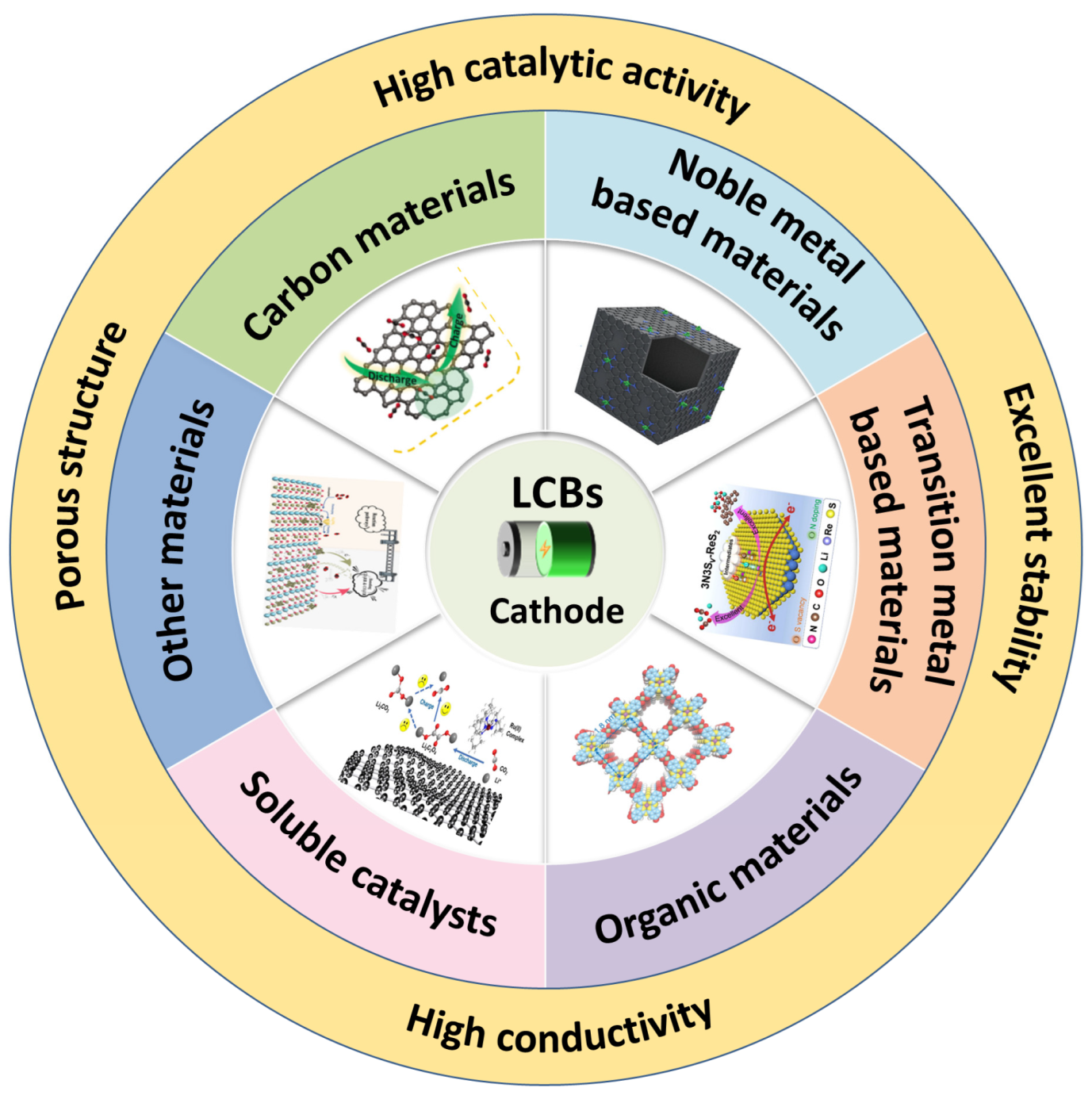
3. Lithium–Carbon Dioxide Battery Catalysts
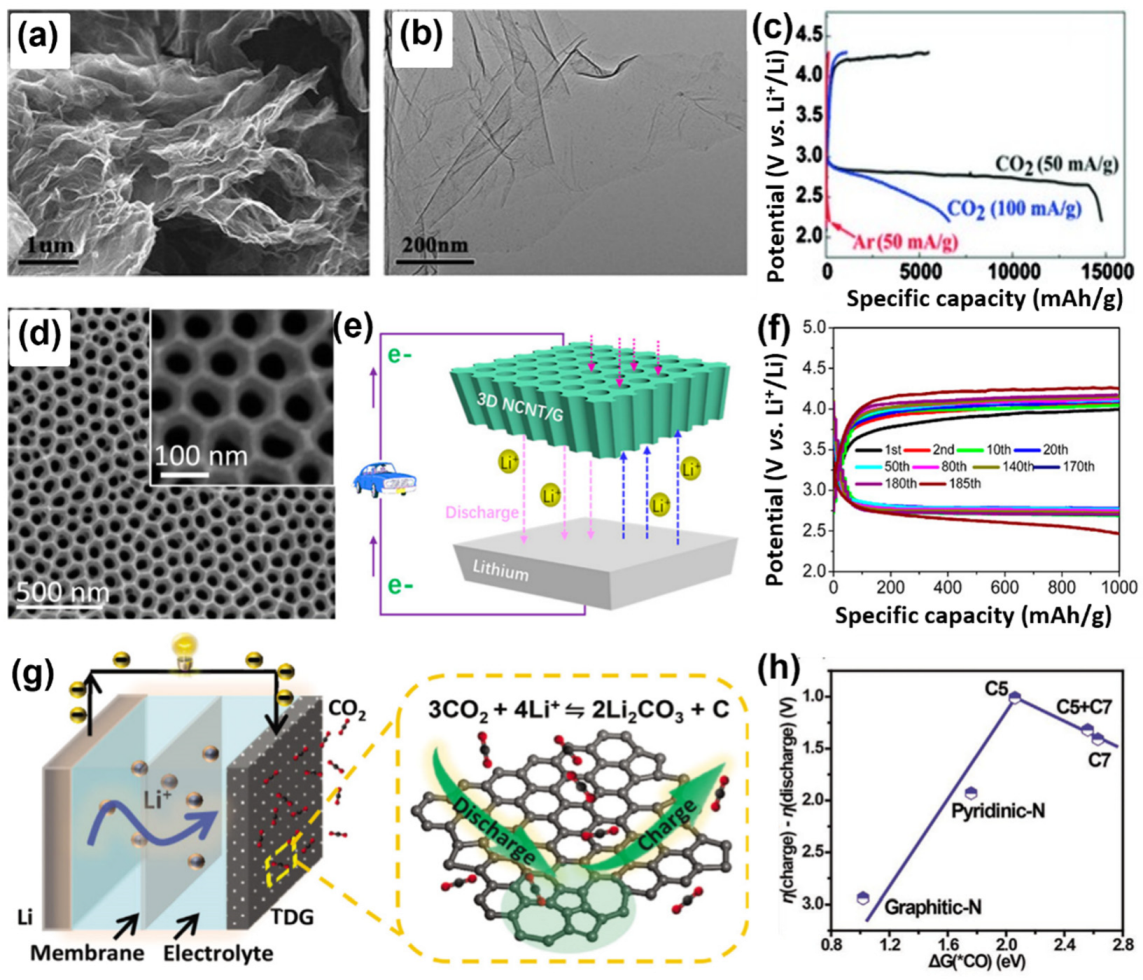
3.1. Carbon-Based Catalysts
3.2. Noble Metal-Based Catalyst
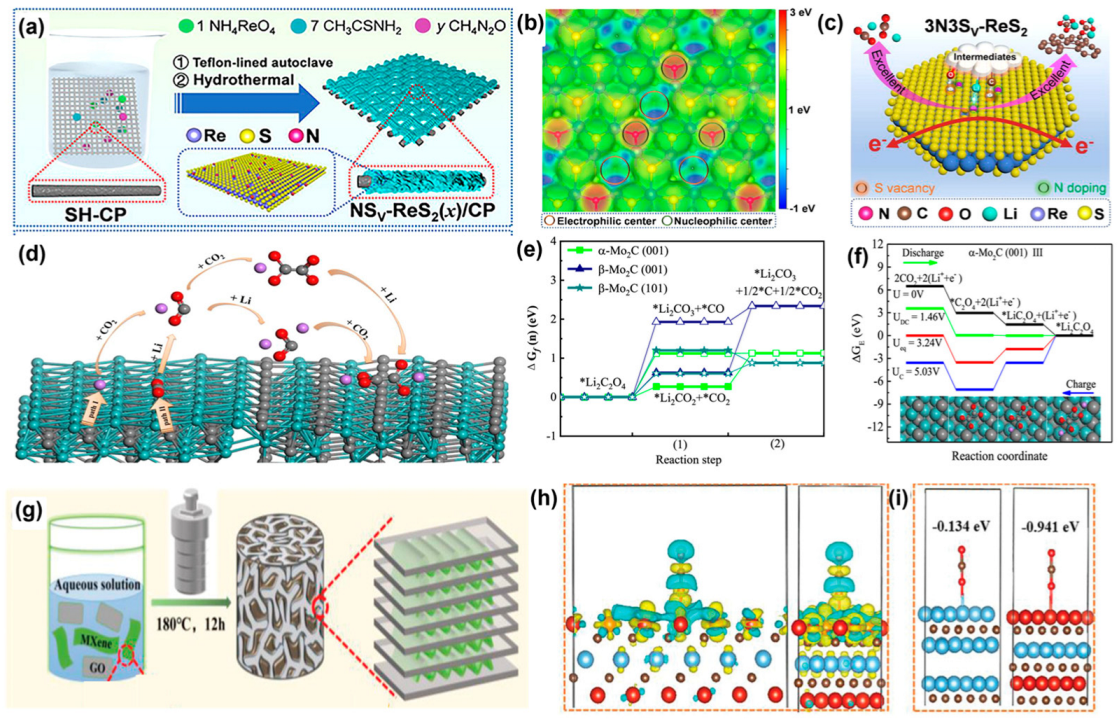
3.3. Transition Metal Compound-Based Catalysts
3.4. Organic Catalysts
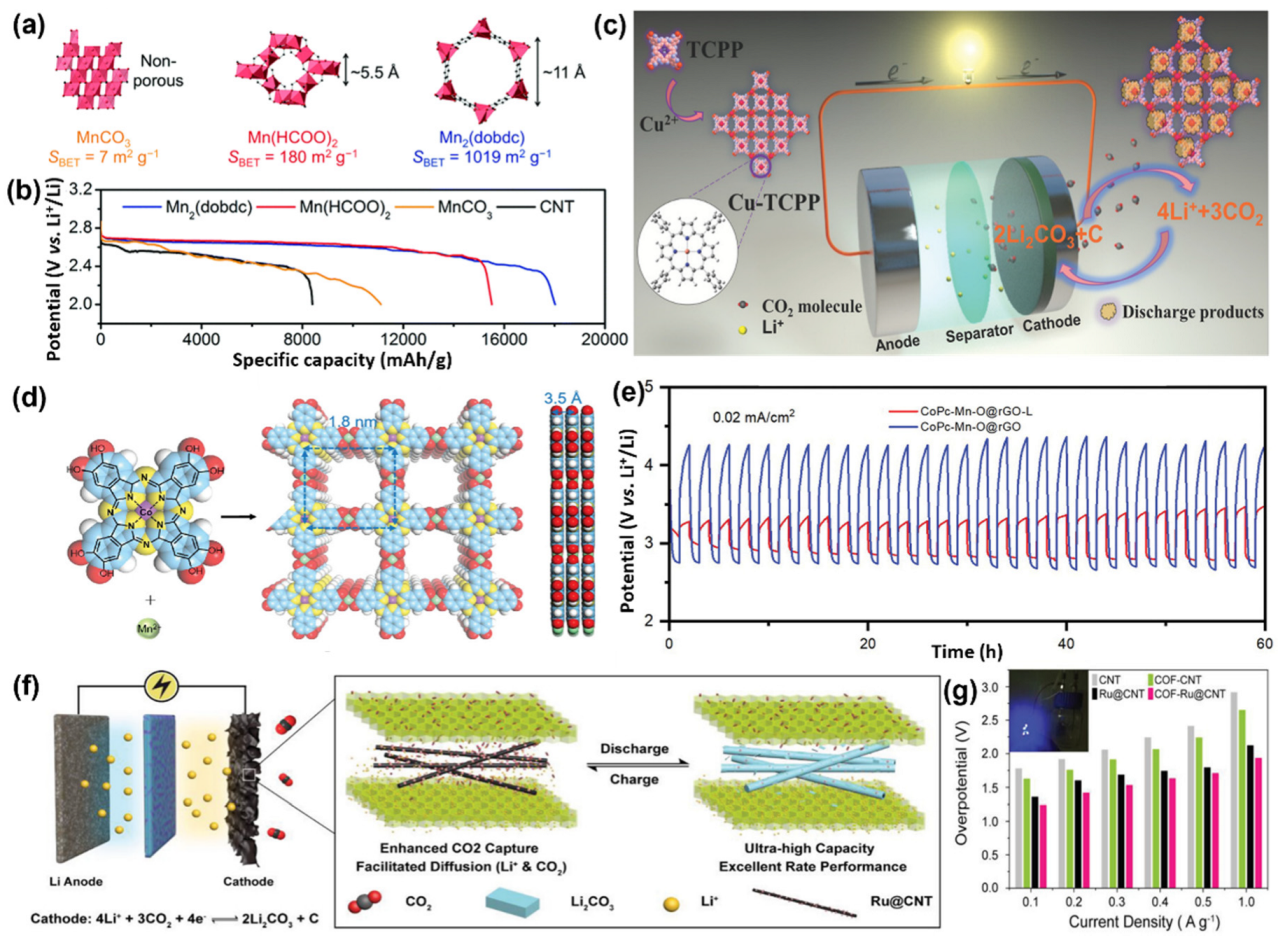
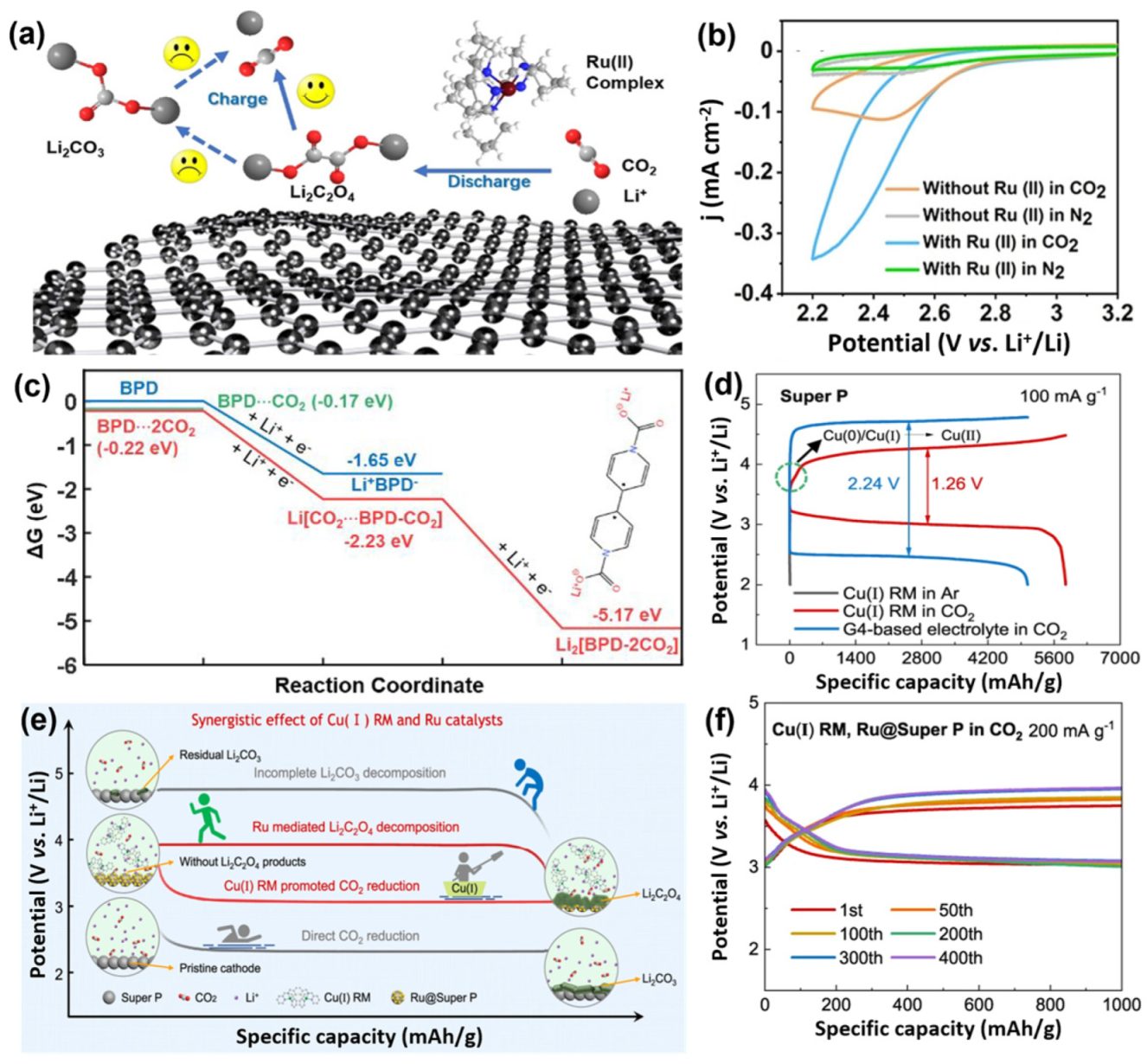
3.5. Soluble Catalysts
3.6. Others

4. Conclusions and Outlook
- (1)
- Unstable intermediates and discharge products have been identified in lithium–carbon dioxide batteries, and successfully detected and characterized using in situ characterization methods. The complexity of the electrochemical reaction mechanisms in Li–CO2 batteries arises from the involvement of intricate electrochemical and chemical processes and multiple interfacial reactions. Currently, the correlation between the inherent composition of catalysts and the efficiency of batteries remains inadequately comprehended. Research highlights the necessity for enhanced in situ spectroscopic techniques and distinctive probe technologies to provide real-time and precise qualitative and quantitative analysis. A deeper investigation from kinetic and thermodynamic perspectives is necessary to fully elucidate the electrochemical reaction mechanisms in Li–CO2 batteries. Future research should develop advanced methods such as in situ analysis, isotope calibration, and theoretical calculations to facilitate real-time detection of unstable intermediates and confirm specific reaction pathways involving CO2 electrochemistry.
- (2)
- Future research on solid catalysts should explore more novel materials and elucidate their working mechanisms in CO2 reduction reaction (CO2RR) and CO2 oxidation reaction (CO2ER) kinetics. The impact of soluble catalysts on Li–CO2 battery performance has not received sufficient attention. The design and selection of catalytic materials are crucial for improving the slow kinetics of CO2RR and CO2ER in Li–CO2 batteries. Nevertheless, the precise operational methods of catalysts in electrochemical reactions remain incompletely comprehended, necessitating additional investigation. Enhancing the inherent activity of catalysts through methods such as heteroatom doping, defect control, and strain engineering can improve their activity and conductivity. Additionally, designing electrodes with hierarchical pore structures and high specific surface areas helps increase the utilization efficiency of active sites. Despite some progress in catalysts, the relationship between catalyst structure and battery performance remains unclear and requires further investigation through theoretical calculations and advanced in situ characterization tools. Future research should combine molecular structure simulation, free energy calculations, and electron transfer rates to design efficient catalysts for Li–CO2 batteries.
- (3)
- Research on photoelectric effects and plasmonic interactions is expected to become a focal point in the application of catalytic systems. The photoelectric effect has been widely applied in photocatalytic water splitting and nitrogen fixation, accelerating electrochemical reactions through the formation of photo-generated electrons and holes. In Li–CO2 batteries, photo-generated carriers participate in CO2 electroreduction and redox reactions, improving battery performance. Numerous novel catalyst materials, such as MOFs, show great potential in photo-assisted Li–CO2 batteries. Future research should focus on improving the light transmission of photocatalysts, the effective separation of holes and electrons, and the high photocatalytic activity for CO2 reduction and evolution reactions to advance photo-assisted Li–CO2 battery technology.
- (4)
- From the perspective of future development of flexible Li–CO2 battery catalysts, research should focus on developing flexible cathode materials that can maintain high efficiency and stability under bending and deformation conditions. To achieve this, new material morphologies need to be explored, and their working mechanisms in CO2 reduction reaction (CO2RR) and CO2 oxidation reaction (CO2ER) should be elucidated. The impact of electrolytes and solvents on battery performance also needs in-depth study, especially when applying certain RMs or additives. Future research directions should focus on improving the performance of flexible cathode materials, reducing preparation costs, and developing environmentally friendly catalysts to promote the further development and commercialization of flexible Li–CO2 battery technology. By addressing these key issues, flexible Li–CO2 batteries are expected to become an ideal energy storage solution for future wearable devices.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Liu, Y.; Wang, J.; Tan, Y.; Liang, Z.; Zhou, G. Toward Circular Energy: Exploring Direct Regeneration for Lithium-Ion Battery Sustainability. Adv. Mater. 2024, 36, 2403818. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Zhang, Y.; Zhu, B.; Wei, G.; Wang, Q.; Qu, J. Reducing the Environmental Impact of Lithium-Ion Battery Recycling through Co-Processing of NCM and LFP. Process Saf. Environ. Prot. 2024, 187, 810–819. [Google Scholar] [CrossRef]
- Mao, J.; Ye, C.; Zhang, S.; Xie, F.; Zeng, R.; Davey, K.; Guo, Z.; Qiao, S. Toward Practical Lithium-Ion Battery Recycling: Adding Value, Tackling Circularity and Recycling-Oriented Design. Energy Environ. Sci. 2022, 15, 2732–2752. [Google Scholar] [CrossRef]
- Chigbu, B.I. Advancing Sustainable Development through Circular Economy and Skill Development in EV Lithium-Ion Battery Recycling: A Comprehensive Review. Front. Sustain. 2024, 5, 1409498. [Google Scholar] [CrossRef]
- Abu, S.M.; Hannan, M.A.; Hossain Lipu, M.S.; Mannan, M.; Ker, P.J.; Hossain, M.J.; Mahlia, T.M.I. State of the Art of Lithium-Ion Battery Material Potentials: An Analytical Evaluations, Issues and Future Research Directions. J. Clean. Prod. 2023, 394, 136246. [Google Scholar] [CrossRef]
- Yang, X.; Wen, H.; Lin, Y.; Zhang, H.; Liu, Y.; Fu, J.; Liu, Q.; Jiang, G. Emerging Research Needs for Characterizing the Risks of Global Lithium Pollution under Carbon Neutrality Strategies. Environ. Sci. Technol. 2023, 57, 5103–5106. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hua, W.; Huang, X.; Stenzel, D.; Wang, J.; Ding, Z.; Cui, Y.; Wang, Q.; Ehrenberg, H.; Breitung, B.; et al. Synergy of Cations in High Entropy Oxide Lithium Ion Battery Anode. Nat. Commun. 2023, 14, 1487. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Lee, Y.; Choi, J.; Lee, M.; Chung, K.Y.; Jung, H.; Jeong, S.; Kim, H. In Situ Mesopore Formation in SiOx Nanoparticles by Chemically Reinforced Heterointerface and Use of Chemical Prelithiation for Highly Reversible Lithium-Ion Battery Anode. Small 2023, 19, 2206238. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Gu, H.; Chen, Q.; Tang, X.; Zhou, Y.; Gao, F.; Han, X.; Guo, Y.; Bhagat, R.; Zheng, Y. Investigating Greenhouse Gas Emissions and Environmental Impacts from the Production of Lithium-Ion Batteries in China. J. Clean. Prod. 2022, 372, 133756. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Wang, L.; Chen, F.; Shao, J.; Hu, X. Efficient Nitrogen Doped Porous Carbonaceous CO2 Adsorbents Based on Lotus Leaf. J. Environ. Sci. 2021, 103, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation Technologies for CO2 Utilisation: Current Status, Challenges and Future Prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Valluri, S.; Claremboux, V.; Kawatra, S. Opportunities and Challenges in CO2 Utilization. J. Environ. Sci. 2022, 113, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Manthiram, A. Recent Advances in Lithium-Carbon Dioxide Batteries. Small Struct. 2020, 1, 2000027. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Xia, X.; Cai, W.; Chen, Q.; Chen, M. Metal-CO2 Electrochemistry: From CO2 Recycling to Energy Storage. Adv. Energy Mater. 2021, 11, 2100667. [Google Scholar] [CrossRef]
- Chen, B.; Wang, D.; Zhang, B.; Zhong, X.; Liu, Y.; Sheng, J.; Zhang, Q.; Zou, X.; Zhou, G.; Cheng, H.-M. Engineering the Active Sites of Graphene Catalyst: From CO2 Activation to Activate Li-CO2 Batteries. ACS Nano 2021, 15, 9841–9850. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wang, K.; Li, N.; Su, D.; Wong, W.-T.; Huang, B.; Guo, S. Ultrathin RuRh Alloy Nanosheets Enable High-Performance Lithium-CO2 Battery. Matter 2020, 2, 1494–1508. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Dong, L.; Manjunatha, R.; Zuo, Y.; Deng, S.-Q.; Tan, M.; Yan, W.; Zhang, J.; Wilkinson, D.P. A Review of Lithium-O2/CO2 and Lithium-CO2 Batteries: Advanced Electrodes/Materials/Electrolytes and Functional Mechanisms. Nano Energy 2022, 95, 106964. [Google Scholar] [CrossRef]
- Xu, S.; Chen, C.; Kuang, Y.; Song, J.; Gan, W.; Liu, B.; Hitz, E.M.; Connell, J.W.; Lin, Y.; Hu, L. Flexible Lithium-CO2 Battery with Ultrahigh Capacity and Stable Cycling. Energy Environ. Sci. 2018, 11, 3231–3237. [Google Scholar] [CrossRef]
- Song, L.; Hu, C.; Xiao, Y.; He, J.; Lin, Y.; Connell, J.W.; Dai, L. An Ultra-Long Life, High-Performance, Flexible Li-CO2 Battery Based on Multifunctional Carbon Electrocatalysts. Nano Energy 2020, 71, 104595. [Google Scholar] [CrossRef]
- Chen, K.; Huang, G.; Ma, J.; Wang, J.; Yang, D.; Yang, X.; Yu, Y.; Zhang, X. The Stabilization Effect of CO2 in Lithium-Oxygen/CO2 Batteries. Angew. Chem.-Int. Ed. 2020, 59, 16661–16667. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Ren, S.; Mu, X.; Liu, Y.; Zhang, X.; He, P.; Zhou, H. Towards a Stable Li-CO2 Battery: The Effects of CO2 to the Li Metal Anode. Energy Storage Mater. 2020, 26, 443–447. [Google Scholar] [CrossRef]
- Ahmadiparidari, A.; Warburton, R.E.; Majidi, L.; Asadi, M.; Chamaani, A.; Jokisaari, J.R.; Rastegar, S.; Hemmat, Z.; Sayahpour, B.; Assary, R.S.; et al. A Long-Cycle-Life Lithium-CO2 Battery with Carbon Neutrality. Adv. Mater. 2019, 31, 1902518. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Du, F.; Hu, C.; Ding, Y.; Wang, Z.L.; Roy, A.; Dai, L. High-Performance Li-CO2 Batteries from Free-Standing, Binder-Free, Bifunctional Three-Dimensional Carbon Catalysts. ACS Energy Lett. 2020, 5, 916–921. [Google Scholar] [CrossRef]
- Hu, Z.; Xie, Y.; Yu, D.; Liu, Q.; Zhou, L.; Zhang, K.; Li, P.; Hu, F.; Li, L.; Chou, S.; et al. Hierarchical Ti3C2Tx MXene/Carbon Nanotubes for Low Overpotential and Long-Life Li-CO2 Batteries. ACS Nano 2021, 15, 8407–8417. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Das, S.K.; Archer, L.A. The Li-CO2 Battery: A Novel Method for CO2 Capture and Utilization. RSC Adv. 2013, 3, 6656. [Google Scholar] [CrossRef]
- Lim, H.-K.; Lim, H.-D.; Park, K.-Y.; Seo, D.-H.; Gwon, H.; Hong, J.; Goddard, W.A.; Kim, H.; Kang, K. Toward a Lithium-”Air” Battery: The Effect of CO2 on the Chemistry of a Lithium-Oxygen Cell. J. Am. Chem. Soc. 2013, 135, 9733–9742. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, J.; Liu, L.; Liu, Y.; Chou, S.; Shi, D.; Liu, H.; Wu, Y.; Zhang, W.; Chen, J. Mo2C/CNT: An Efficient Catalyst for Rechargeable Li-CO2 Batteries. Adv. Funct. Mater. 2017, 27, 1700564. [Google Scholar] [CrossRef]
- Yang, C.; Guo, K.; Yuan, D.; Cheng, J.; Wang, B. Unraveling Reaction Mechanisms of Mo2C as Cathode Catalyst in a Li-CO2 Battery. J. Am. Chem. Soc. 2020, 142, 6983–6990. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Qin, J.; Sari, H.M.K.; Li, D.; Li, X.; Sun, X. Recent Progress and Prospects of Li-CO2 Batteries: Mechanisms, Catalysts and Electrolytes. Energy Storage Mater. 2021, 34, 148–170. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, J.; Zhao, J.; Li, Q.; Zhang, P.; Hao, C.; Liu, X.; Yang, S.; Liu, Y. Synergistic Effect of Bifunctional Catalytic Sites and Defect Engineering for High-Performance Li-CO2 Batteries. Energy Storage Mater. 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Chen, B.; Wang, D.; Tan, J.; Liu, Y.; Jiao, M.; Liu, B.; Zhao, N.; Zou, X.; Zhou, G.; Cheng, H.-M. Designing Electrophilic and Nucleophilic Dual Centers in the ReS2 Plane toward Efficient Bifunctional Catalysts for Li-CO2 Batteries. J. Am. Chem. Soc. 2022, 144, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Gong, L.; Long, Y.; Talapaneni, S.N.; Zhang, L.; Xiao, Y.; Liu, D.; Hu, C.; Dai, L. Topological Defect-Rich Carbon as a Metal-Free Cathode Catalyst for High-Performance Li-CO2 Batteries. Adv. Energy Mater. 2021, 11, 2101390. [Google Scholar] [CrossRef]
- Yang, S.; Qiao, Y.; He, P.; Liu, Y.; Cheng, Z.; Zhu, J.; Zhou, H. A Reversible Lithium-CO2 Battery with Ru Nanoparticles as a Cathode Catalyst. Energy Environ. Sci. 2017, 10, 972–978. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Chen, Y.; Bao, J.; Zhou, X.; Xie, Z.; Wei, J.; Zhou, Z. The First Introduction of Graphene to Rechargeable Li-CO2 Batteries. Angew. Chem.-Int. Ed. 2015, 54, 6550–6553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Zhang, Z.; Chen, Y.; Xie, Z.; Wei, J.; Zhou, Z. Rechargeable Li-CO2 Batteries with Carbon Nanotubes as Air Cathodes. Chem. Commun. 2015, 51, 14636–14639. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, L.; Yang, Y.; Li, N.; Chen, Y.; Yin, X.; Niu, J.; Wang, J.; Ding, S. N, O-Diatomic Dopants Activate Catalytic Activity of 3D Self-Standing Graphene Carbon Aerogel for Long-Cycle and High-Efficiency Li-CO2 Batteries. Chem. Eng. J. 2023, 465, 142787. [Google Scholar] [CrossRef]
- Huang, N.-Y.; He, H.; Liu, S.; Zhu, H.-L.; Li, Y.-J.; Xu, J.; Huang, J.-R.; Wang, X.; Liao, P.-Q.; Chen, X.-M. Electrostatic Attraction-Driven Assembly of a Metal-Organic Framework with a Photosensitizer Boosts Photocatalytic CO2 Reduction to CO. J. Am. Chem. Soc. 2021, 143, 17424–17430. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Sun, K.; Liu, S.; Cheong, W.-C.; Chen, Z.; Zhang, C.; Pan, Y.; Cheng, Y.; Zhuang, Z.; Wei, X.; et al. Atomically Dispersed Ni-Ru-P Interface Sites for High-Efficiency pH-Universal Electrocatalysis of Hydrogen Evolution. Nano Energy 2021, 80, 105467. [Google Scholar] [CrossRef]
- Li, F.; Tang, D.; Jian, Z.; Liu, D.; Golberg, D.; Yamada, A.; Zhou, H. Li-O2 Battery Based on Highly Efficient Sb-Doped Tin Oxide Supported Ru Nanoparticles. Adv. Mater. 2014, 26, 4659–4664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-F.; Lu, Y.-Q.; Wu, Y.-J.; Yin, Z.-W.; Li, J.-T.; Zhou, Y.; Hong, Y.-H.; Li, Y.-Y.; Huang, L.; Sun, S.-G. High-Performance Rechargeable Li-CO2/O2 Battery with Ru/N-Doped CNT Catalyst. Chem. Eng. J. 2019, 363, 224–233. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Zhang, J.-Y.; Sheng, T.; Lu, Y.-Q.; Yin, Z.-W.; Li, Y.-Y.; Peng, X.-X.; Zhou, Y.; Li, J.-T.; Wu, Y.-J.; et al. Synergetic Effect of Ru and NiO in the Electrocatalytic Decomposition of Li2CO3 to Enhance the Performance of a Li-CO2/O2 Battery. ACS Catal. 2020, 10, 1640–1651. [Google Scholar] [CrossRef]
- Wu, G.; Li, X.; Zhang, Z.; Dong, P.; Xu, M.; Peng, H.; Zeng, X.; Zhang, Y.; Liao, S. Design of Ultralong-Life Li-CO2 Batteries with IrO2 Nanoparticles Highly Dispersed on Nitrogen-Doped Carbon Nanotubes. J. Mater. Chem. A 2020, 8, 3763–3770. [Google Scholar] [CrossRef]
- Kang, Z.; Wu, C.; Dong, L.; Liu, W.; Mou, J.; Zhang, J.; Chang, Z.; Jiang, B.; Wang, G.; Kang, F.; et al. 3D Porous Copper Skeleton Supported Zinc Anode toward High Capacity and Long Cycle Life Zinc Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 3364–3371. [Google Scholar] [CrossRef]
- Chen, C.-J.; Yang, J.-J.; Chen, C.-H.; Wei, D.-H.; Hu, S.-F.; Liu, R.-S. Improvement of Lithium Anode Deterioration for Ameliorating Cyclabilities of Non-Aqueous Li-CO2 Batteries. Nanoscale 2020, 12, 8385–8396. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ding, J.; Wang, H.; Yang, X.; Zheng, X.; Huang, Z.; Song, W.; Ding, J.; Han, X.; Hu, W. Boosting Energy Efficiency and Stability of Li-CO2 Batteries via Synergy between Ru Atom Clusters and Single-Atom Ru-N4 Sites in the Electrocatalyst Cathode. Adv. Mater. 2022, 34, 2200559. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, F.; Han, X.; Zhang, L.; Hou, Q.; Gong, L.; Wang, J.; Xia, Z.; Hao, J.; Xie, K. Intrinsic Descriptor Guided Noble Metal Cathode Design for Li-CO2 Battery. Adv. Mater. 2023, 35, 2302325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, B.; Yang, X.; Zhao, C.; Dong, P.; Li, X.; Zhang, Y.; Doyle-Davis, K.; Zeng, X.; Zhang, Y.; et al. Dual Catalytic Sites of Alloying Effect Bloom CO2 Catalytic Conversion for Highly Stable Li-CO2 Battery. Adv. Funct. Mater. 2023, 33, 2213931. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, Y.; Deng, Q.; Dai, Q.; Fang, Z.; Fu, X.; Yan, W.; Wu, L.; Zhou, Y. Toward an Understanding of Bimetallic MXene Solid-Solution in Binder-Free Electrocatalyst Cathode for Advanced Li-CO2 Batteries. Adv. Funct. Mater. 2023, 33, 2210037. [Google Scholar] [CrossRef]
- Lamagni, P.; Miola, M.; Catalano, J.; Hvid, M.S.; Mamakhel, M.A.H.; Christensen, M.; Madsen, M.R.; Jeppesen, H.S.; Hu, X.; Daasbjerg, K.; et al. Restructuring Metal-Organic Frameworks to Nanoscale Bismuth Electrocatalysts for Highly Active and Selective CO2 Reduction to Formate. Adv. Funct. Mater. 2020, 30, 1910408. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, 1703663. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-Pore Apertures in a Series of Metal-Organic Frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, Y.; Zhou, J.; Liu, Y.; Wang, J.; Gao, X.; Han, Y.; Qi, P.; Wang, B. Carbon Dioxide in the Cage: Manganese Metal-Organic Frameworks for High Performance CO2 Electrodes in Li-CO2 Batteries. Energy Environ. Sci. 2018, 11, 1318–1325. [Google Scholar] [CrossRef]
- Xu, Y.; Gong, H.; Ren, H.; Fan, X.; Li, P.; Zhang, T.; Chang, K.; Wang, T.; He, J. Highly Efficient Cu-Porphyrin-Based Metal-Organic Framework Nanosheet as Cathode for High-Rate Li-CO2 Battery. Small 2022, 18, 2203917. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Chen, Y.; Dong, L.; Liu, M.; Shi, J.; Li, S.; Lan, Y. Phthalocyanine Based Metal-Organic Framework Ultrathin Nanosheet for Efficient Photocathode toward Light-Assisted Li-CO2 Battery. Adv. Funct. Mater. 2022, 32, 2210259. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Jiang, D. Covalent Organic Frameworks: A Materials Platform for Structural and Functional Designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The Atom, the Molecule, and the Covalent Organic Framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef] [PubMed]
- Kandambeth, S.; Dey, K.; Banerjee, R. Covalent Organic Frameworks: Chemistry beyond the Structure. J. Am. Chem. Soc. 2019, 141, 1807–1822. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Chen, Z.; Xu, H.; Yu, W.; Liu, C.; Wang, X.; Zhang, K.; Xie, K.; Loh, K.P. Covalent-Organic-Framework-Based Li-CO2 Batteries. Adv. Mater. 2019, 31, 1905879. [Google Scholar] [CrossRef]
- Bharti, A.; Achutharao, G.; Bhattacharyya, A.J. Efficient Rechargeable Li-CO2 Battery with a Liquid Electrolyte-Soluble CuCl2 Electrocatalyst. ACS Appl. Mater. Interfaces 2023, 15, 53342–53350. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, W.; Cai, Z.; Cheng, J.; Kuang, H.; Dong, B.; Wang, Y.; Wang, K.; Chen, J. Enhanced Electrochemical Performance of Aprotic Li-CO2 Batteries with a Ruthenium-Complex-Based Mobile Catalyst. Angew. Chem.-Int. Ed. 2021, 60, 16404–16408. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Z.; Peng, Z. Tailoring Li-CO2 Electrochemistry Based on 4,4′-Bipyridine Redox Cycle. ACS Energy Lett. 2023, 8, 3430–3436. [Google Scholar] [CrossRef]
- Sun, X.; Mu, X.; Zheng, W.; Wang, L.; Yang, S.; Sheng, C.; Pan, H.; Li, W.; Li, C.-H.; He, P.; et al. Binuclear Cu Complex Catalysis Enabling Li-CO2 Battery with a High Discharge Voltage above 3.0V. Nat. Commun. 2023, 14, 536. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Li, R.; Wang, Z.; Yu, F.; Chi, B.; Pu, J. Synergistic Effect of Cu-La0.96Sr0.04Cu0.3Mn0.7O3−δ Heterostructure and Oxygen Vacancy Engineering for High-Performance Li-CO2 Batteries. Electrochim. Acta 2021, 395, 139209. [Google Scholar] [CrossRef]
- Jaradat, A.; Zhang, C.; Shashikant Sutar, S.; Shan, N.; Wang, S.; Singh, S.K.; Yang, T.; Kumar, K.; Sharma, K.; Namvar, S.; et al. A High-Rate Li-CO2 Battery Enabled by 2D Medium-Entropy Catalyst. Adv. Funct. Mater. 2023, 33, 2300814. [Google Scholar] [CrossRef]
- Chen, J.; Zou, K.; Ding, P.; Deng, J.; Zha, C.; Hu, Y.; Zhao, X.; Wu, J.; Fan, J.; Li, Y. Conjugated Cobalt Polyphthalocyanine as the Elastic and Reprocessable Catalyst for Flexible Li-CO2 Batteries. Adv. Mater. 2019, 31, 1805484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, H.; Zhang, Z.; Wang, X.; Jiang, Z.; Tang, Y.; Zhang, J.; Emley, B.; Zhang, Y.; Zhou, H.; et al. Dispersed Nickel Phthalocyanine Molecules on Carbon Nanotubes as Cathode Catalysts for Li-CO2 Batteries. Small 2023, 19, 2302768. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Yan, X.; Meng, X.; Li, P.; Wang, Q.; Zhang, Y.; Yan, S.; Luo, S. Exploring the Frontiers of Cathode Catalysts in Lithium–Carbon Dioxide Batteries: A Mini Review. Inorganics 2024, 12, 222. https://doi.org/10.3390/inorganics12080222
Guo J, Yan X, Meng X, Li P, Wang Q, Zhang Y, Yan S, Luo S. Exploring the Frontiers of Cathode Catalysts in Lithium–Carbon Dioxide Batteries: A Mini Review. Inorganics. 2024; 12(8):222. https://doi.org/10.3390/inorganics12080222
Chicago/Turabian StyleGuo, Jing, Xin Yan, Xue Meng, Pengwei Li, Qin Wang, Yahui Zhang, Shenxue Yan, and Shaohua Luo. 2024. "Exploring the Frontiers of Cathode Catalysts in Lithium–Carbon Dioxide Batteries: A Mini Review" Inorganics 12, no. 8: 222. https://doi.org/10.3390/inorganics12080222






