A New Azide-Bridged Polymeric Manganese (III) Schiff Base Complex with an Allylamine-Derived Ligand: Structural Characterization and Activity Spectra
Abstract
:1. Introduction
2. Results and Discussion
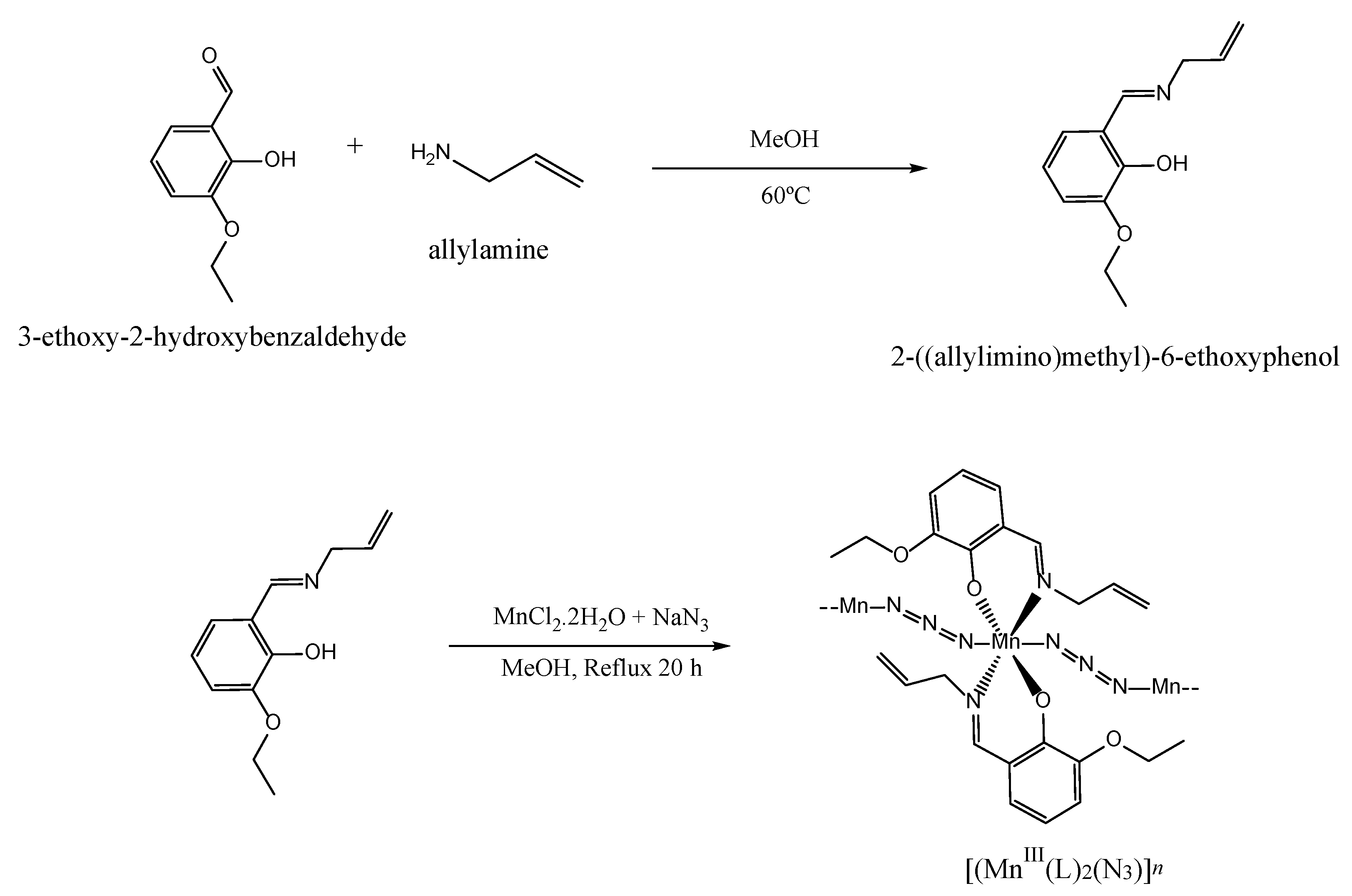
2.1. Synthesis and Spectroscopic Characterization of the SCHIFF Base and Complex
2.2. Electrochemical Studies
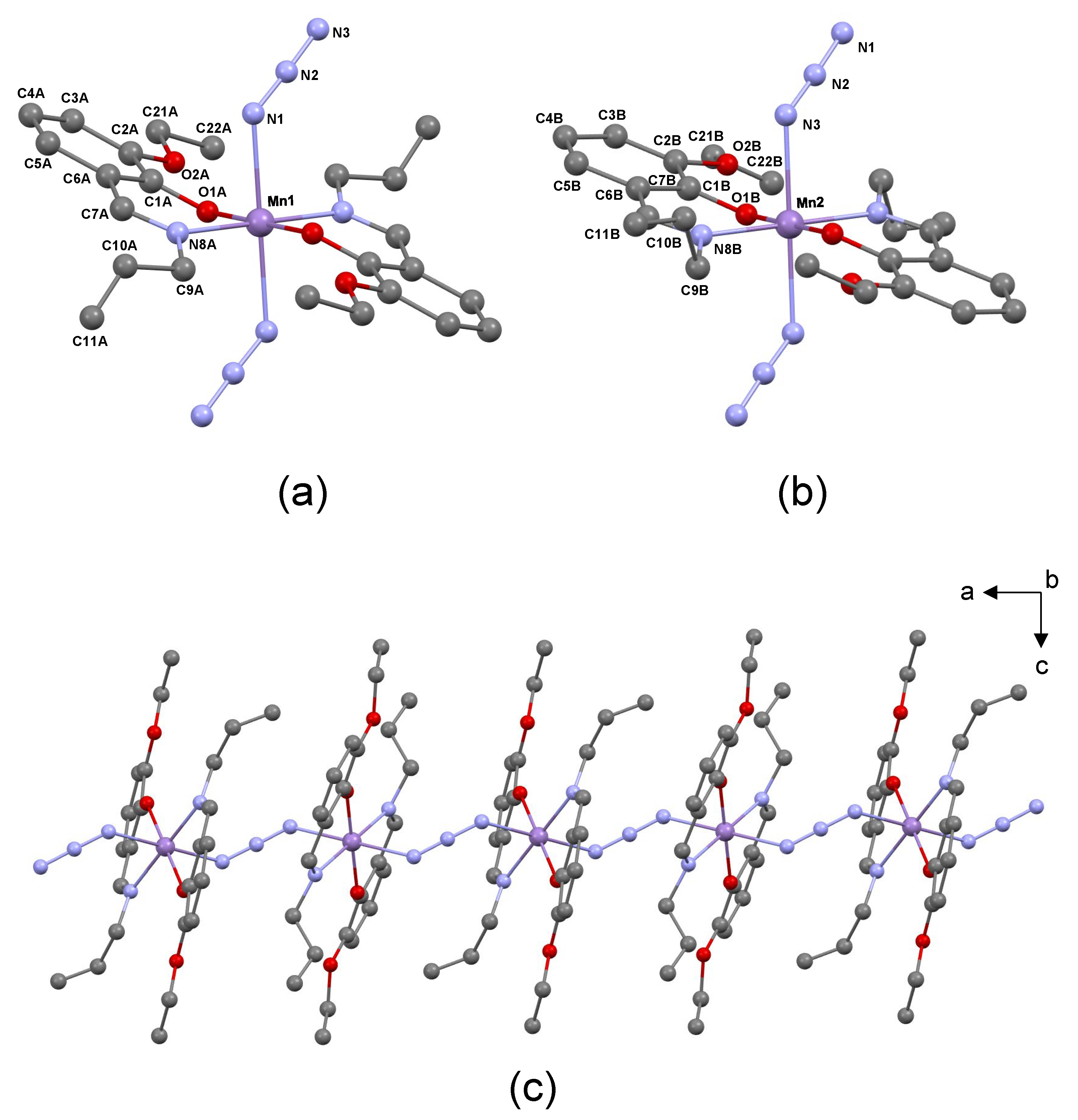
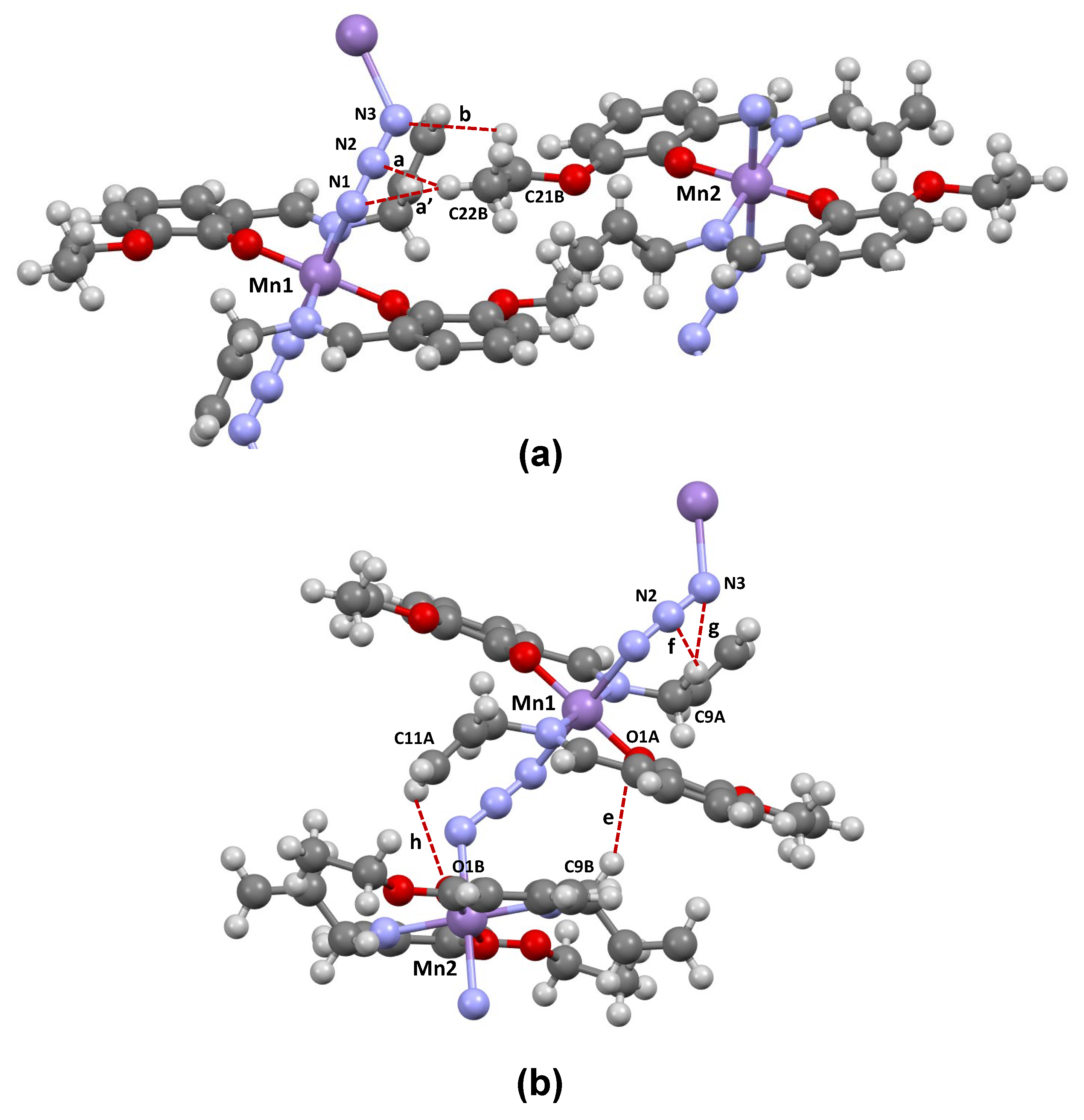
2.3. Crystal Structure
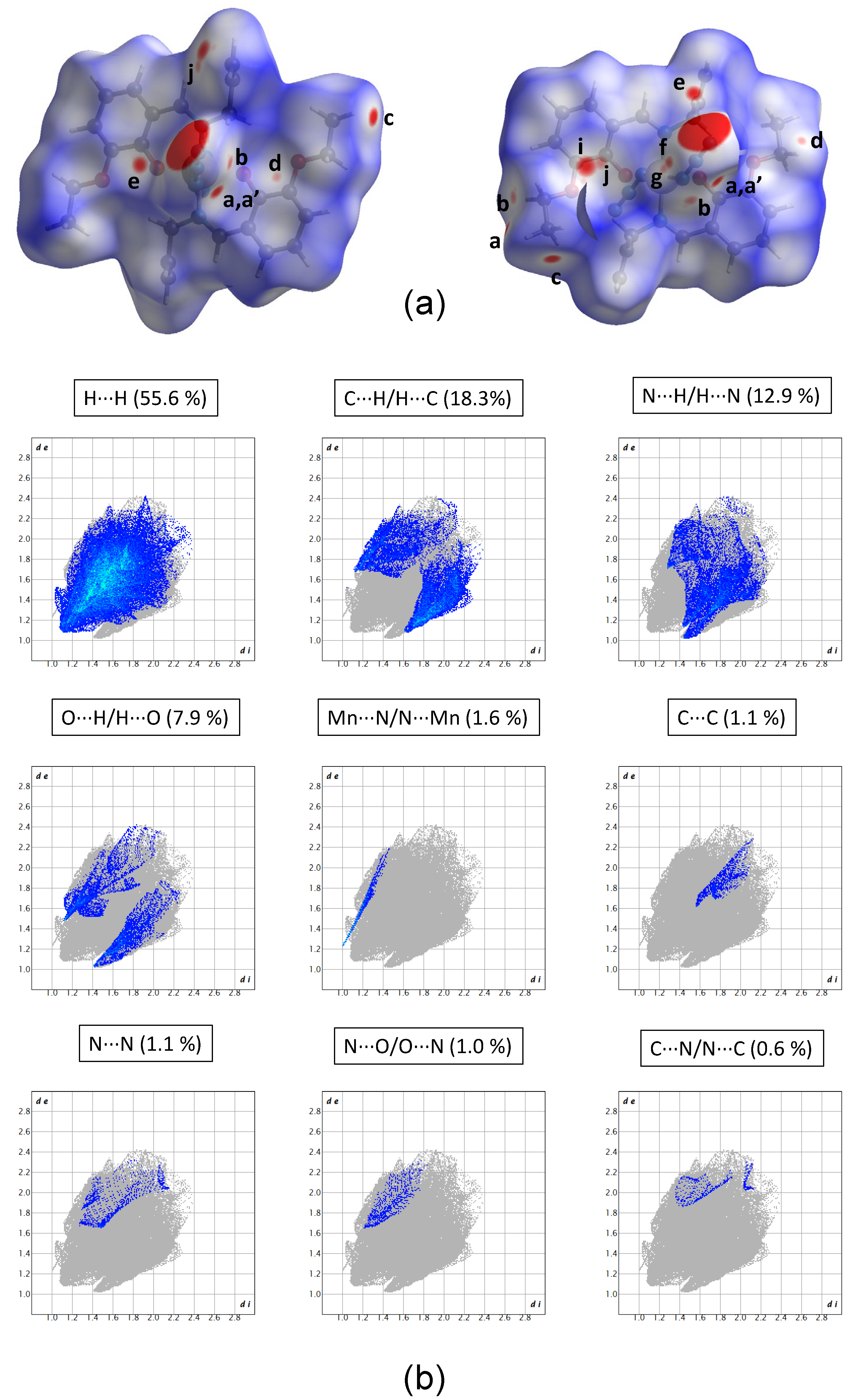
2.4. Hirshfeld Surface Analysis
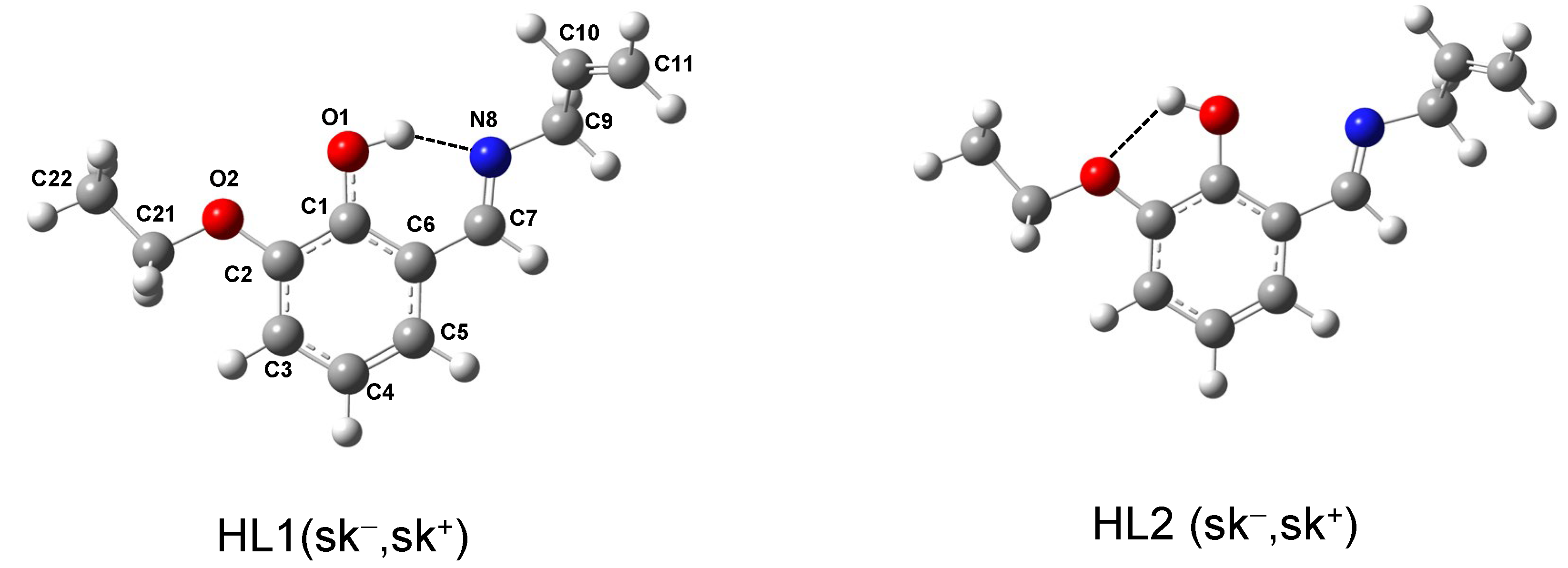
2.5. Conformational Analysis of the Schiff Base Ligand
2.6. In Silico PASS Analysis of Biological Activity
3. Experimental and Computational Methodologies
3.1. Materials and Instrumentation
3.2. Synthesis of the Polymeric Schiff Base Complex [MnIII(L)2(N3)]n
3.3. X-ray Data Collection and Refinement Parameters
3.4. Cyclic Voltammetry
3.5. Hirshfeld Surface Analysis
3.6. Theoretical Calculations
3.7. Prediction of Probable Activity Spectra of Substances (PASS Online Software)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiff, H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef]
- Fabbrizzi, L. Beauty in Chemistry: Making Artistic Molecules with Schiff Bases. J. Org. Chem. 2020, 85, 12212–12226. [Google Scholar] [CrossRef] [PubMed]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases mdash—Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Vigato, P.A.; Tamburini, S. The challenge of cyclic and acyclic schiff bases and related derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar]
- Golbedaghi, R.; Tabanez, A.M.; Esmaeili, S.; Fausto, R. Biological Applications of Macrocyclic Schiff Base Ligands and Their Metal Complexes: A Survey of the Literature (2005–2019). Appl. Organomet. Chem. 2020, 34, e5884. [Google Scholar] [CrossRef]
- Soroceanu, A.; Bargan, A. Advanced and Biomedical Applications of Schiff-Base Ligands and Their Metal Complexes: A Review. Crystals 2022, 12, 1436. [Google Scholar] [CrossRef]
- Shaygan, S.; Pasdar, H.; Foroughifar, N.; Davallo, M.; Motiee, F. Cobalt (II) Complexes with Schiff Base Ligands Derived from Terephthalaldehyde and ortho-Substituted Anilines: Synthesis, Characterization and Antibacterial Activity. Appl. Sci. 2018, 8, 385. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal Complexes with Schiff Bases: Data Collection and Recent Studies on Biological Activities. Int. J. Mol. Sci. 2022, 23, 14840. [Google Scholar] [CrossRef]
- Naeimi, H.; Sadat Nazifi, Z.; Matin Amininezhad, S.; Amouheidari, M. Synthesis, characterization and in vitro antimicrobial activity of some new Schiff bases and their complexes. J. Antibiot. 2013, 66, 687–689. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Giuzio, F.; Saturnino, C.; Longo, P.; Sinicropi, M.S. Metal Complexes with Schiff Bases as Antimicrobials and Catalysts. Inorganics 2023, 11, 320. [Google Scholar] [CrossRef]
- Amiri Rudbari, H.; Khorshidifard, M.; Askari, B.; Habibi, N.; Bruno, G. New asymmetric Schiff base ligand derived from allylamine and 2,3-dihydroxybenzaldehyde and its molybdenum(VI) complex: Synthesis, characterization, crystal structures, computational studies and antibacterial activity together with synergistic effect against Pseudomonas aeroginosa PTTC 1570. Polyhedron 2015, 100, 180–191. [Google Scholar]
- Deilami, A.B.; Salehi, M.; Amiri, A.; Arab, A. New copper(II) and vanadium(IV) complexes based on allylamine-derived Schiff base ligand; synthesis, crystal structure, electrochemical properties and DFT calculations. J. Mol. Struct. 2019, 1181, 190–196. [Google Scholar] [CrossRef]
- Deilami, A.B.; Salehi, M.; Arab, A.; Amiri, A. Synthesis, crystal structure, electrochemical properties and DFT calculations of three new Zn(II), Ni(II) and Co(III) complexes based on 5-bromo-2-((allylimino)methyl)phenol Schiff-based ligand. Inorg. Chim. Acta 2018, 476, 93–100. [Google Scholar] [CrossRef]
- Kazemi, Z.; Rudbari, H.A.; Sahihi, M.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Mohammadpoor-Baltork, I.; Gharaghani, S. Synthesis, characterization and biological application of four novel metal-Schiff base complexes derived from allylamine and their interactions with human serum albumin: Experimental, molecular docking and ONIOM computational study. J. Photochem. Photobiol. B 2016, 162, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Ramezanipoor, S.; Parvarinezhad, S.; Salehi, M.; Grześkiewicz, A.M.; Kubicki, M. Crystal structures, electrochemical properties and theoretical studies of three New Zn(II), Mn(III) and Co(III) Schiff base complexes derived from 2-hydroxy-1-allyliminomethyl-naphthalen. J. Mol. Struct. 2022, 1257, 132541. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Abe, S. Magnetic assemblies based on Mn(III) salen analogues. Coord. Chem. Rev. 2007, 251, 2622–2664. [Google Scholar] [CrossRef]
- Chakrabarty, P.P.; Saha, S.; Schollmeyer, D.; Boudalis, A.K.; Jana, A.D.; Luneau, D. Azide-bridged manganese(III) one-dimensional chain: Synthesis, structure, and magnetic study. J. Coord. Chem. 2013, 66, 9–17. [Google Scholar] [CrossRef]
- Ko, H.H.; Lim, J.H.; Kim, H.C.; Hong, C.S. Coexistence of Spin Canting and Metamagnetism in a One-Dimensional Mn(III) Complex Bridged by a Single End-to-End Azide. Inorg. Chem. 2006, 45, 8847–8849. [Google Scholar] [CrossRef]
- Panja, A.; Shaikh, N.; Vojtíšek, P.; Gao, S.; Banerjee, P. Synthesis, crystal structures and magnetic properties of 1D polymeric [MnIII(salen)N3] and [MnIII(salen)Ag(CN)2] complexes. New J. Chem. 2002, 26, 1025–1028. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Li, L.; Liao, D.; Jiang, Z. A one-dimensional azido-bridged manganese(III) complex with bidentate Schiff base: Crystal structure and magnetic properties. J. Solid State Chem. 2007, 180, 2973–2977. [Google Scholar] [CrossRef]
- Qian, K.; Xu, Y.; Wang, Z.; Yang, J. Synthesis, crystal structure, and magnetic properties of an azido-bridged Mn(II) complex [C3H5NH3][Mn(N3)3]. Z. Für Naturforschung B 2017, 72, 409–413. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, W.R.; Ryu, D.W.; Lee, J.W.; Yoon, S.W.; Suh, B.J.; Kim, H.C.; Hong, C.S. End-to-End Azide-Bridged Manganese(III) Chain Compounds: Field-Induced Magnetic Phase Transitions and Variation of TC to 38 K depending on the Side Groups of the Schiff Bases. Inorg. Chem. 2011, 50, 10777–10785. [Google Scholar] [CrossRef]
- Hong, C.S.; Koo, J.-e.; Son, S.-K.; Lee, Y.S.; Kim, Y.-S.; Do, Y. Unusual Ferromagnetic Couplings in Single End-to-End Azide-Bridged Cobalt(II) and Nickel(II) Chain Systems. Chem. Eur. J. 2001, 7, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, M.Y.; Morsali, A. Morphological study and potential applications of nano metal–organic coordination polymers. RSC Adv. 2013, 3, 19191–19218. [Google Scholar] [CrossRef]
- Islamoglu, T.; Goswami, S.; Li, Z.; Howarth, A.J.; Farha, O.K.; Hupp, J.T. Postsynthetic Tuning of Metal–Organic Frameworks for Targeted Applications. Acc. Chem. Res. 2017, 50, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Aung, T.; Guo, N.; Weichselbaum, R.; Lin, W. Nanoscale Metal–Organic Frameworks for Therapeutic, Imaging, and Sensing Applications. Adv. Mater. 2018, 30, 1707634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.J.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef]
- Keypour, H.; Salehzadeh, S.; Pritchard, R.G.; Parish, R.V. Synthesis and Crystal Structure Determination of Some Asymmetrical and Symmetrical CR-Type Macrocyclic Schiff Base Complexes, with a Single Pendant Coordinating 2-Aminoethyl Arm. Inorg. Chem. 2000, 39, 5787–5790. [Google Scholar] [CrossRef]
- Suydam, F.H. The C=N Stretching Frequency in Azomethines. Anal. Chem. 1963, 35, 193–195. [Google Scholar] [CrossRef]
- Verma, A.L.; Venkateswarlu, P. Vibrational spectra and rotational isomerism of allyl amine. J. Mol. Spectrosc. 1971, 39, 227–241. [Google Scholar] [CrossRef]
- İspir, E.; Kurtoğlu, M.; Purtaş, F.; Serin, S. Synthesis and Antimicrobial Activity of New Schiff Bases Having the –SiOR Group (R = CH3 or CH2CH3), and their Transition Metal Complexes. Transit. Met. Chem. 2005, 30, 1042–1047. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Frantz, E.B. Manganese(III) Schiff Base Complexes: Chemistry Relevant to the Copolymerization of Epoxides and Carbon Dioxide. Inorg. Chem. 2007, 46, 5967–5978. [Google Scholar] [CrossRef] [PubMed]
- Sepehrfar, S.; Salehi, M.; Parvarinezhad, S.; Grześkiewicz, A.M.; Kubicki, M. New Cu(II), Mn(II) and Mn(III) Schiff base complexes cause noncovalent interactions: X-ray crystallography survey, Hirshfeld surface analysis and molecular simulation investigation against SARS-CoV-2. J. Mol. Struct. 2023, 1278, 134857. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, F.; Zhang, W.; Wang, Z.-M.; Gao, S. Azide-Bridged One-Dimensional MnIII Polymers: Effects of Side Group of Schiff Base Ligands on Structure and Magnetism. Inorg. Chem. 2007, 46, 11235–11242. [Google Scholar] [CrossRef]
- Freitag, R.; Conradie, J. Understanding the Jahn–Teller Effect in Octahedral Transition-Metal Complexes: A Molecular Orbital View of the Mn(β-diketonato)3 Complex. J. Chem. Educ. 2013, 90, 1692–1696. [Google Scholar] [CrossRef]
- Bikas, R.; Shahmoradi, E.; Reinoso, S.; Emami, M.; Lezama, L.; Sanchiz, J.; Noshiranzadeh, N. The effect of the orientation of the Jahn–Teller distortion on the magnetic interactions of trinuclear mixed-valence Mn(II)/Mn(III) complexes. Dalton Trans. 2019, 48, 13799–13812. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.W.; Ryu, D.W.; Yoon, S.W.; Suh, B.J.; Kim, H.C.; Hong, C.S. One-Dimensional End-To-End Azide-Bridged MnIII Complexes Incorporating Alkali Metal Ions: Slow Magnetic Relaxations and Metamagnetism. Chem. Eur. J. 2011, 17, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford Univiversity Press: Oxford, UK, 2006. [Google Scholar]
- Rigaku Oxford Diffraction. CrysAlisPro, Version. 1.171.36.20; Rigaku Oxford Diffraction: Yarnton, UK, 2015. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic-Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Boucher, L.J.; Day, V.W. Manganese Schiff base complexes. 5. Synthesis and spectroscopy of some anion complexes of N,N′-ethylenebis(acetylacetone iminato)manganese(III). Inorg. Chem. 1977, 16, 1360–1367. [Google Scholar] [CrossRef]
- Ferreira, G.A.; Nunes, C.M.; Lopes Jesus, A.J.; Fausto, R. The meta and para OH Substitution Effect on C-Phenyl-Nitrilimine Bond-Shift Isomers. Eur. J. Org. Chem. 2023, 26, e202300310. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; de Lucena Júnior, J.R.; Fausto, R.; Reva, I. Infrared Spectra and Phototransformations of meta-Fluorophenol Isolated in Argon and Nitrogen Matrices. Molecules 2022, 27, 8248. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, M.K.; Brauer, B.; Martin, J.M.L. Frequency and Zero-Point Vibrational Energy Scale Factors for Double-Hybrid Density Functionals (and Other Selected Methods): Can Anharmonic Force Fields Be Avoided? J. Phys. Chem. A 2015, 119, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Parasuraman, S. Prediction of activity spectra for substances. J. Pharmacol. Pharmacother. 2011, 2, 52–53. [Google Scholar]
| Mn1 | Mn2 | ||
|---|---|---|---|
| Bond length/Å | |||
| Mn1–N8A | 2.035 | Mn2–N8B | 2.044 |
| Mn1–O1A | 1.885 | Mn2–O1B | 1.866 |
| Mn1–N1 | 2.272 | Mn2–N3 | 2.256 |
| Bond angle/deg. | |||
| O1A–Mn1–N8A | 89.2–90.8 | O1B-Mn2–N8B | 89.6–90.4 |
| O1A–Mn1–N1 | 89.7–90.3 | O1B–Mn2–N3 | 88.6–91.3 |
| N8A–Mn1–N1 | 87.5–92.6 | N8B–Mn2–N3 | 88.1–91.9 |
| O1A–Mn1–O1A | 180.0 | O1B–Mn2–O1B | 180.0 |
| N8A–Mn1–N8A | 180.0 | N8B–Mn2–N8B | 180.0 |
| N1–Mn1–N1 | 180.0 | N3–Mn2–N3 | 180.0 |
| Interaction | Label | H⋯A/Å | C⋯A/Å | C–H⋯A/° |
|---|---|---|---|---|
| Interchain | ||||
| C22B–H⋯N1/N2 | a/a’ | 2.742/2.562 | 3.631/3.345 | 150.9/136.8 |
| C21B–H⋯N3 | b | 2.634 | 3.167 | 114.0 |
| C22A–H⋯C22B | c | 2.697 | 3.607 | 154.6 |
| C21B–H⋯C2A | d | 2.789 | 3.574 | 136.6 |
| Intrachain | ||||
| C9B–H⋯O1A | e | 2.549 | 3.499 | 160.9 |
| C9A–H⋯N2/N3 | f/g | 2.603/2.668 | 3.347/3.573 | 131.9/152.1 |
| C11A–H⋯O1B | h | 2.699 | 3.359 | 127.1 |
| C11A⋯C2B | i | 3.219 | ||
| C11A⋯C1B | j | 3.320 |
| Activity | Pa | Pi |
|---|---|---|
| Antiviral (Rhinovirus) | 0.444 | 0.053 |
| Antiviral (Influenza) | 0.350 | 0.064 |
| Antiviral (Picornavirus) | 0.352 | 0.156 |
| Antiviral | 0.211 | 0.086 |
| Antitussive | 0.199 | 0.087 |
| Antiviral (CMV) | 0.224 | 0.122 |
| Antiviral (Influenza A) | 0.227 | 0.145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talebi, A.; Salehi, M.; Jesus, A.J.L.; Kubicki, M.; Fausto, R.; Golbedaghi, R. A New Azide-Bridged Polymeric Manganese (III) Schiff Base Complex with an Allylamine-Derived Ligand: Structural Characterization and Activity Spectra. Inorganics 2024, 12, 234. https://doi.org/10.3390/inorganics12090234
Talebi A, Salehi M, Jesus AJL, Kubicki M, Fausto R, Golbedaghi R. A New Azide-Bridged Polymeric Manganese (III) Schiff Base Complex with an Allylamine-Derived Ligand: Structural Characterization and Activity Spectra. Inorganics. 2024; 12(9):234. https://doi.org/10.3390/inorganics12090234
Chicago/Turabian StyleTalebi, Aynaz, Mehdi Salehi, A. J. Lopes Jesus, Maciej Kubicki, Rui Fausto, and Reza Golbedaghi. 2024. "A New Azide-Bridged Polymeric Manganese (III) Schiff Base Complex with an Allylamine-Derived Ligand: Structural Characterization and Activity Spectra" Inorganics 12, no. 9: 234. https://doi.org/10.3390/inorganics12090234






