Abstract
A transparent Er3+,Yb3+:GdMgB5O10 single crystal with dimensions up to 24 × 15 × 12 mm was grown successfully by the high-temperature solution growth on dipped seeds technique from K2Mo3O10-based solvent. The grown crystal was characterized using PXRD, DSC and ATR techniques. Differential scanning calorimetry measurements and SEM analysis of the heat-treated solids revealed Er,Yb:GdMgB5O10 to be an incongruent melting compound with an onset point of 1087 °C. The absorption edge of the Er,Yb:GMBO sample is located in the region of 245 nm, which approximates a value of 4.8 eV. Absorption and emission spectra, and luminescence kinetics, were studied. The energy transfer efficiency from ytterbium to erbium ions was determined. The laser operation in continuous-wave mode was realized and output characteristics were measured. The maximal output power of 0.15 W with a slope efficiency of 11% was obtained at 1568 nm.
1. Introduction
Borate compounds have been extensively studied over the past few decades due to their remarkable structural flexibility and potential applications as laser, nonlinear, scintillation, magnetic and phosphor materials, etc., [1]. The variety of functional properties of borates is based on the diversity of their structural types: boron atoms can be bonded with three or four oxygen atoms, forming planar/non-planar triangular BO3 or tetrahedral BO4 fundamental structural units, respectively. These B-O building groups can be connected to each other by common corners or edges, forming different B-O clusters [2]. Nowadays, rare-earth metal borates are very attractive objects for the research community because of their nonlinear optical and laser applications.
Laser radiation in the 1.5–1.6 µm spectral range is extensively used in range finding, optical locating and telecommunications applications, mainly because of its eye safety, weak absorption in the atmosphere and low dispersion and absorption of quartz fibers. This radiation can be obtained using solid-state lasers based on gain media doped with trivalent erbium ions (transition 4I13/2→4I15/2). However, the main disadvantage of the Er3+ ions is low absorption in the spectral range of InGaAs laser diode emissions (near 1 µm), which limits pumping efficiency. Ytterbium ions are a good sensitizer due to the broad absorption band near 1 µm and the large overlap between Yb3+ emissions and Er3+ absorption, which allows for resonant energy transfer from ytterbium to erbium ions [3].
Recently, the spectroscopic and laser properties of different gain media have been investigated. Among them, phosphate glasses co-doped with Er,Yb ions are the most widely used, since Er,Yb-phosphate glasses are characterized by spectroscopic properties suitable for efficient laser operation (energy transfer from Yb3+ to Er3+ ions of 90% with a long lifetime of the erbium upper laser level 4I13/2 of 7–8 ms and a short lifetime of the 4I11/2 energy level of 2–3 μs) [4]. However, phosphate glass exhibits poor thermo-mechanical properties (a thermal conductivity of 0.85 W × m−1 × K−1) [5], which limits the average output power of Er,Yb:glass lasers in continuous-wave and Q-switched regimes of operation due to the thermal effects.
Crystalline laser hosts are characterized by significantly higher thermal conductivity values than glasses [6,7]. Currently, many crystalline hosts have been investigated for Er,Yb lasers—aluminates, silicates, vanadates, and tungstates [8,9]—but spectroscopic properties of these crystals do not fully meet the requirements for achieving an efficient laser operation. Nowadays, oxoborate crystals are the most important Er,Yb-codoped crystalline laser materials because they possess not only high thermal conductivity but also the necessary spectroscopic properties mentioned above [10,11]. The most efficient laser operation in continuous-wave mode has been demonstrated for huntite-type Er,Yb:RAl3(BO3)4 (R = Y, Gd, Lu) [12,13,14] and pentaborate Er,Yb:RMgB5O10 [15,16,17,18] (R = Y, Gd, La) crystals. The pentaborate crystals demonstrate relatively high thermal conductivity and good spectroscopic properties [16]. The highest output power of 0.61 W and slope efficiency of 23% was realized for Er,Yb:LaMgB5O10 [18]. We have recently exhibited a continuous-wave Er,Yb:YMgB5O10 laser with an output power of 0.2 W at 1570 nm [15]. GdMgB5O10 (GMBO) crystal also proved to be a good choice for both Er,Yb-codoping and single Yb-doping. The maximal output power of 0.22 W with a slope efficiency of 14% at 1569 nm was demonstrated for Er,Yb:GdMgB5O10 [19], while up to 5.35 W and 56% of maximal output power and slope efficiency, respectively, were demonstrated at 1060.8 nm for Yb:GdMgB5O10 [20].
In the present work, a comprehensive characterization including growth technique, thermal stability, IR spectroscopy, bandgap transmission spectra, and spectroscopic and laser properties of as-grown Er,Yb:GdMgB5O10 single crystals was carried out.
2. Results and Discussion
2.1. Growth Technique and Structure Characterization
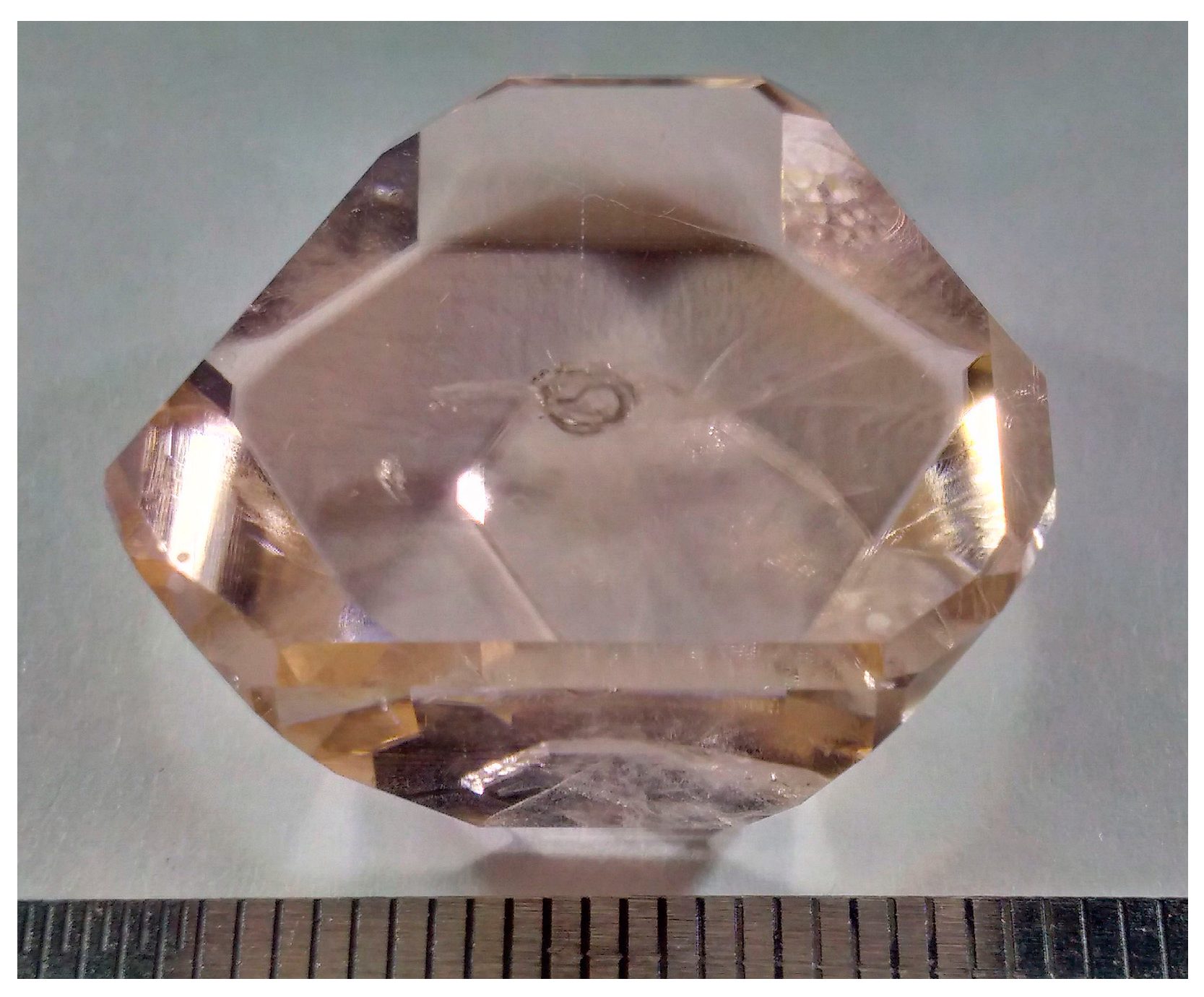
A transparent macrodefect-free Er,Yb-codoped GdMgB5O10 single crystal with a size of 24 × 15 × 12 mm was grown by the high-temperature solution growth on dipped seeds (HT-SGDS) technique (Figure 1). The saturation temperature value obtained was about ∼950 °C.
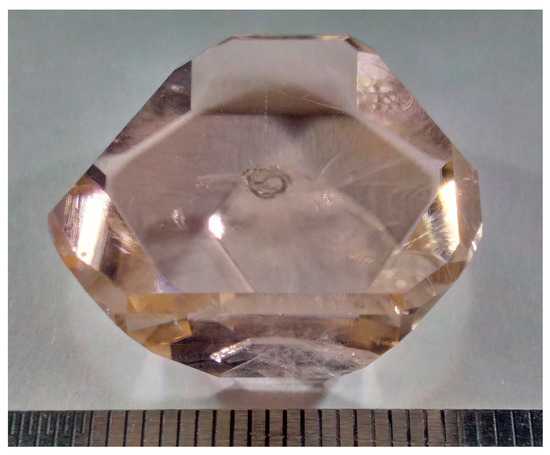
Figure 1.
As-grown Er,Yb:GdMgB5O10 single crystal.
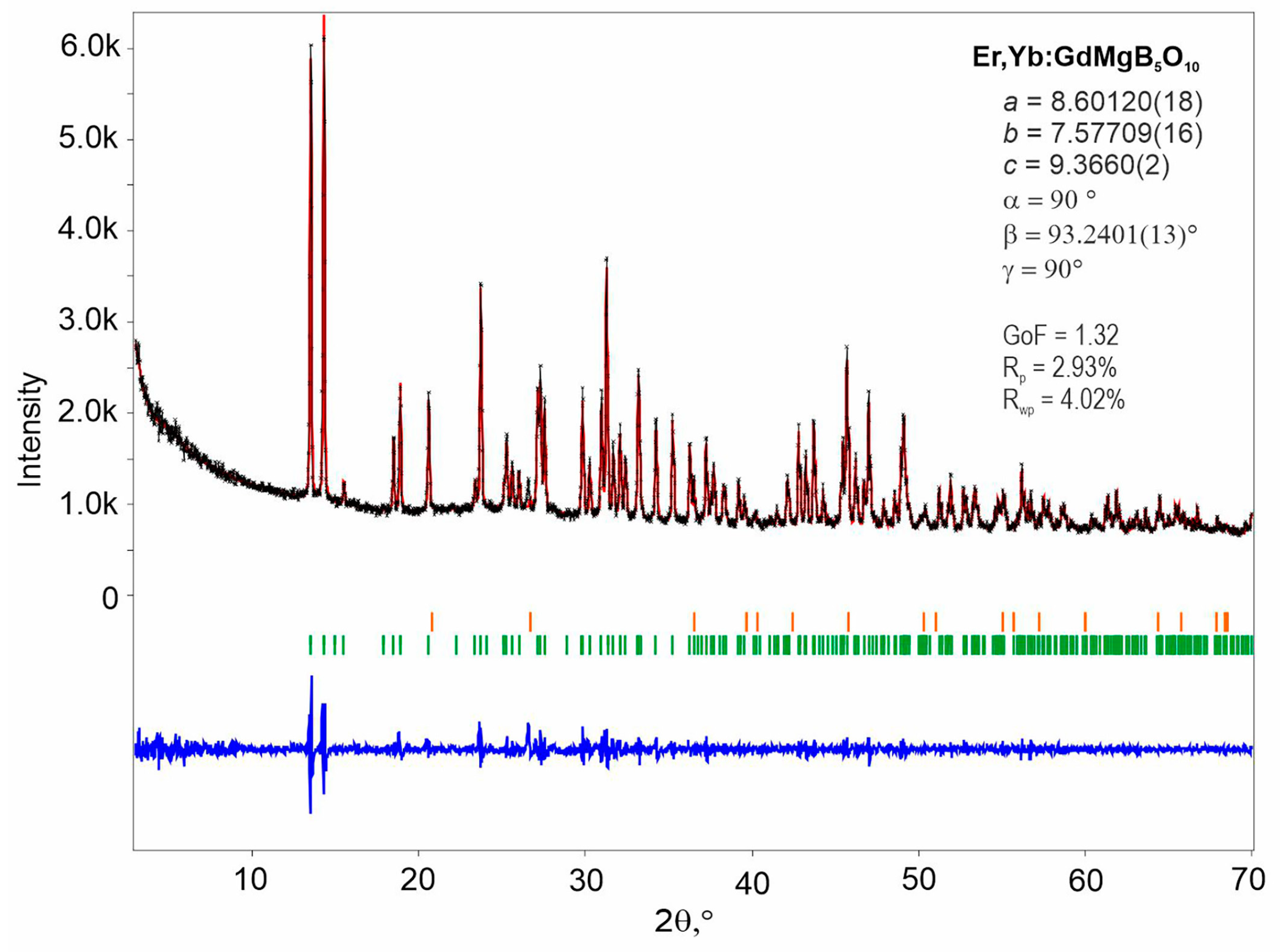
The Le Bail fitting was performed to confirm the structural similarity of Er,Yb:GMBO to rare-earth magnesium pentaborates. To perform the Le Bail method, the crystal symmetry and lattice parameters for GdMgB5O10 (database code: ICSD 157426) were used as input data. Powder X-ray diffraction (PXRD) refinements showed good agreement with the theoretical profile. Nevertheless, pattern matching revealed an impurity phase, SiO2, which appeared after grinding in the agate mortar (Figure 2).
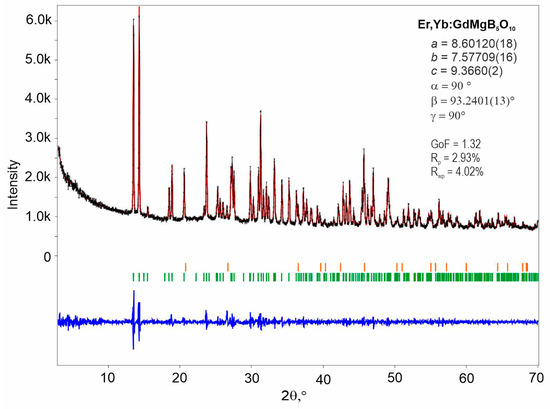
Figure 2.
Diffraction profiles showing observed (black crosses) and calculated (red continuous line) profiles, and the difference curve (blue continuous line) between observed and calculated spectra. Green and orange vertical markers represent Bragg reflections corresponding to the main phase of GdMgB5O10 (ICSD 157426) and the impurity phase of SiO2 (COD 1526860), respectively.
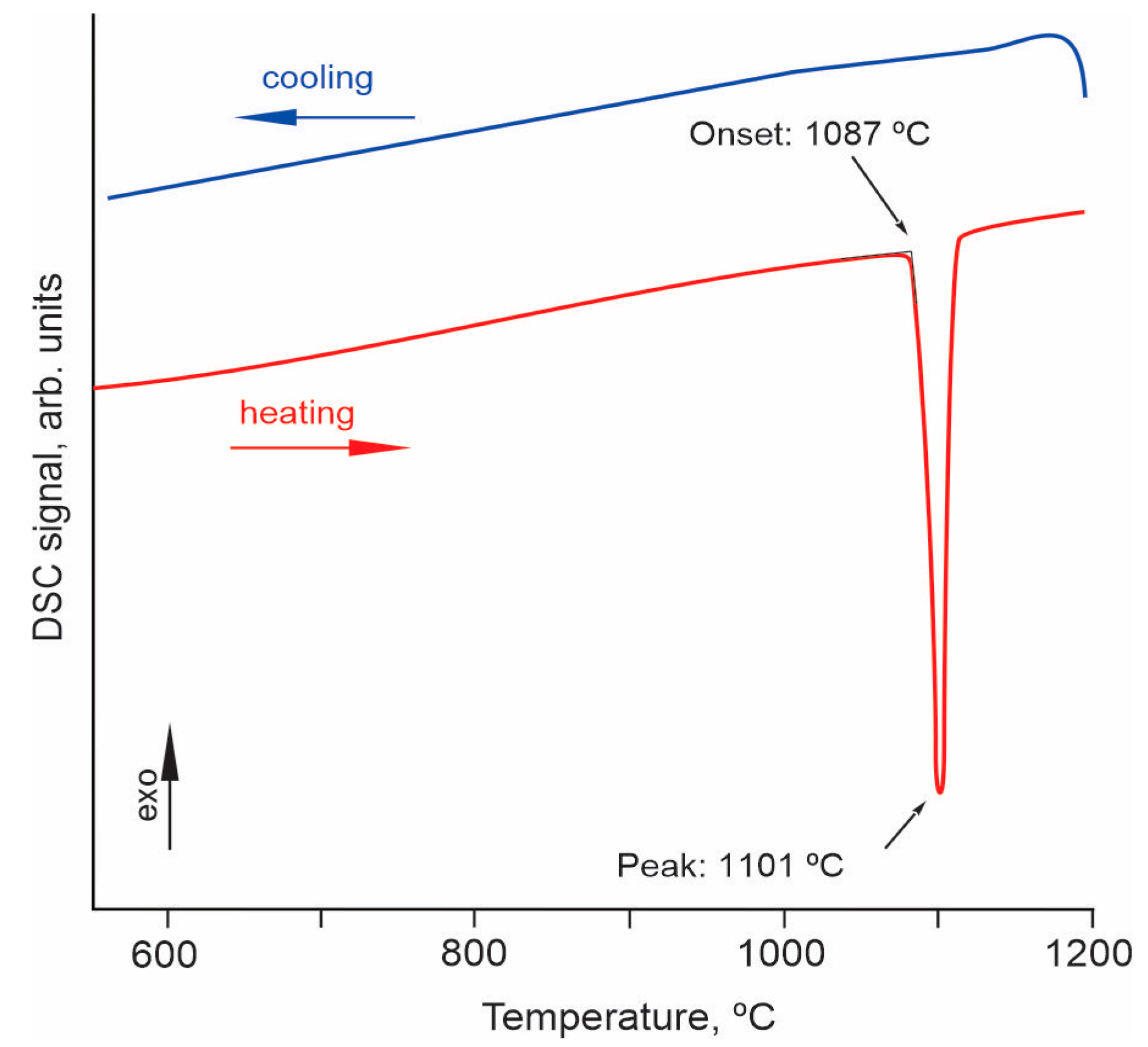
The thermal behavior of the Er,Yb:GMBO compound was determined by differential scanning calorimetry (DSC). A fragment of calorimetric data in the temperature range 600–1200 °C is shown in Figure 3. The heating curve is characterized by a sharp endothermic peak with an onset temperature of ~1087 °C. However, no exothermic peak is observed on the cooling curve, indicating the incongruent melting of the compound under investigation.
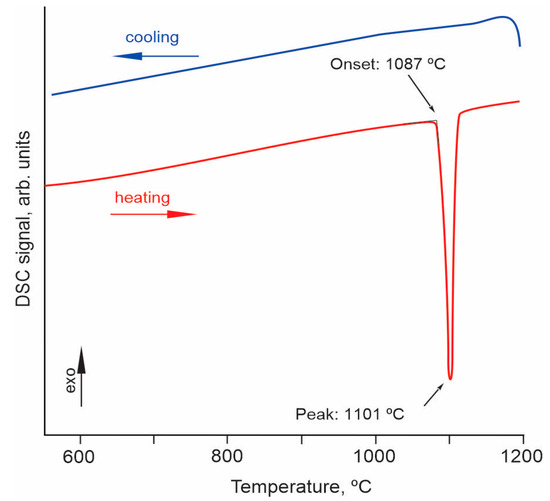
Figure 3.
DSC curves of Er,Yb:GdMgB5O10 crystal in the temperature range of 600–1200 °C.
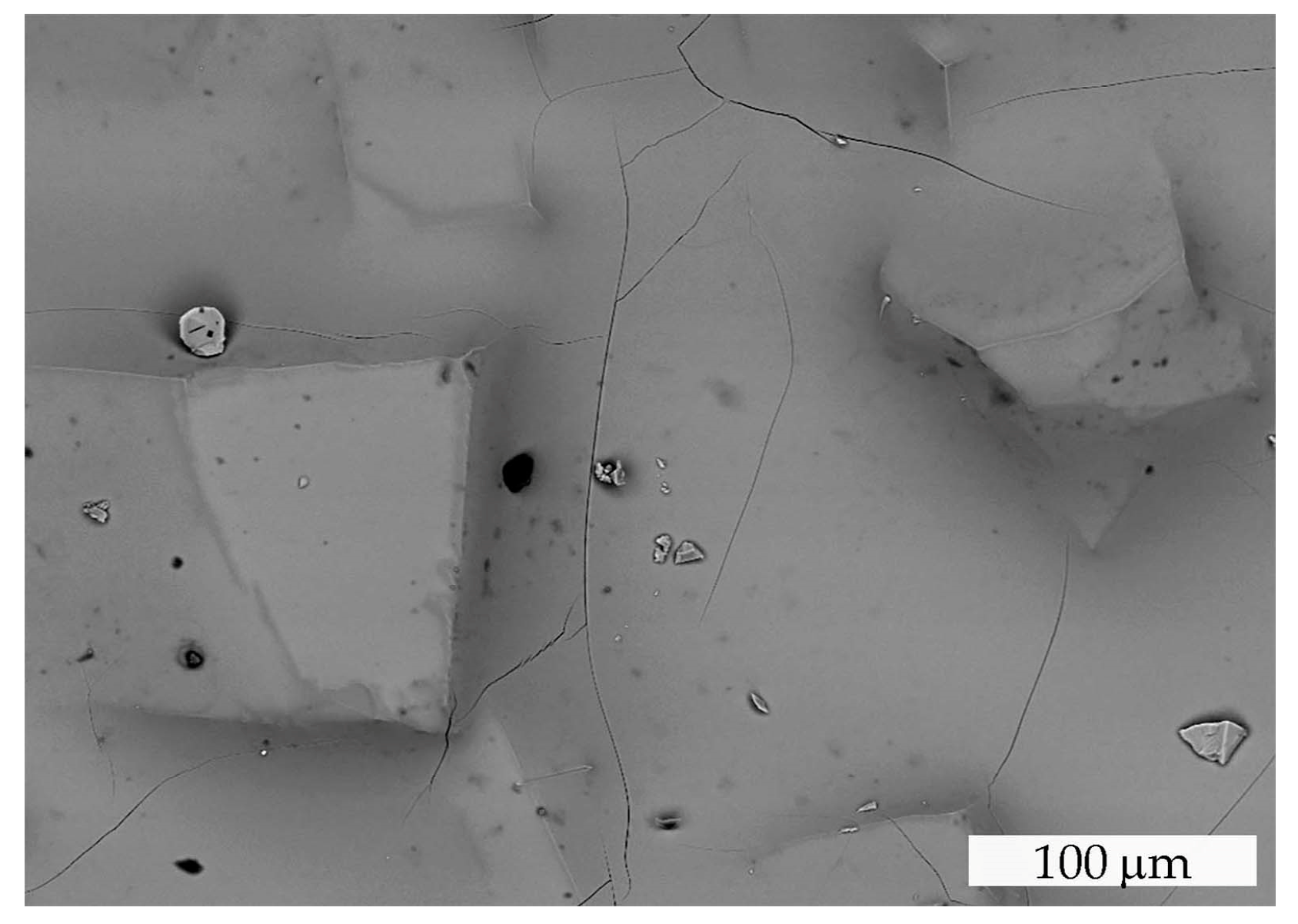
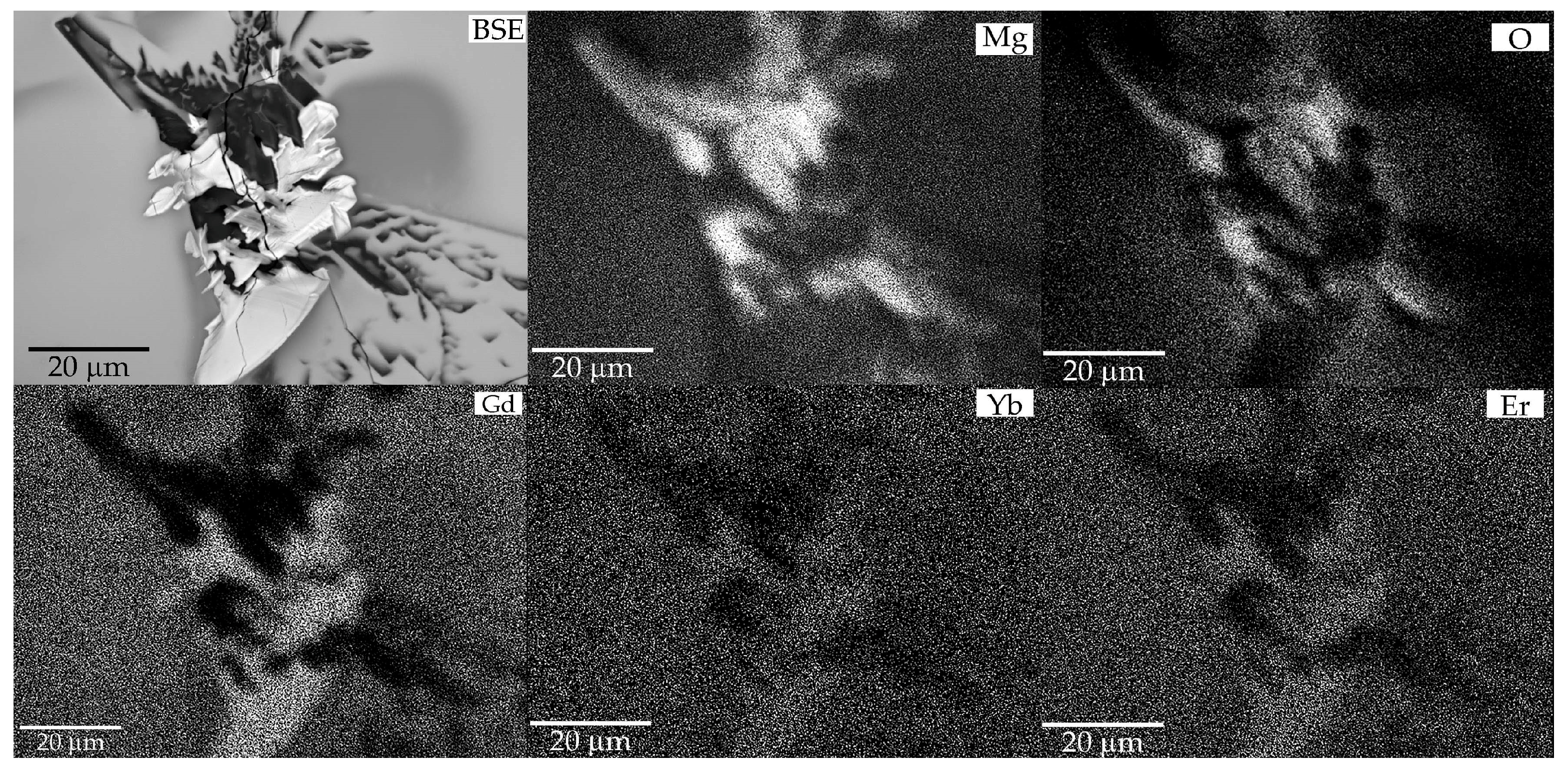
Similar thermal behavior has been previously described for TmMgB5O10 and YMgB5O10, which undergo incongruent melting to form RBO3 and Mg2B2O10 phases [21]. Spontaneous Er,Yb:GMBO crystals from the crystallized (after cooling) molten bath were annealed in an alundum crucible at 1050 °C and characterized using the SEM/EDXS technique. The residue in the crucible after heat treatment was a white, translucent, dense mass containing mostly partially melted pentaborate crystals (Figure 4). SEM/EDXS data revealed the existence of two phases other than Er,Yb:GMBO.
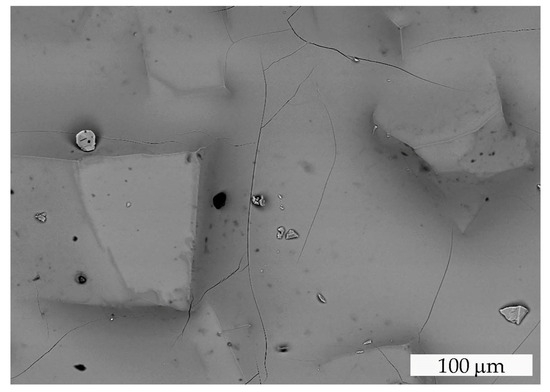
Figure 4.
SEM image of the Er,Yb:GMBO spontaneous crystals heat-treated at 1050 °C .
The distribution patterns of the elements in the X-ray characteristic radiation show that the bright phase formed is enriched in the rare-earth component and the darker one is enriched in magnesium (Figure 5). It is impossible to determine the distribution of boron in the newly formed phases from the SEM data, but it can be assumed that the first phase is represented by rare-earth orthoborate RBO3 (due to the high stability of this phase in a wide temperature range), and the secondby the magnesium phase. Considering previous studies on the thermal properties of Tm and YMg pentaborates, the thermally induced process can be described by the following reaction:
2RMgB5O10→ 2RBO3 + Mg2B2O5 + 3B2O3, where R = Er, Yb, and Gd.
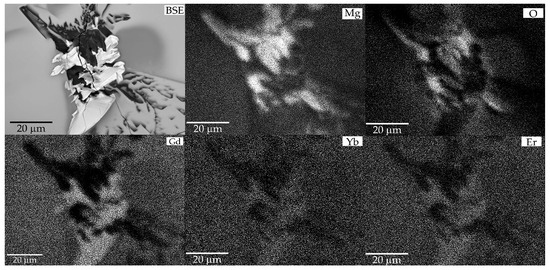
Figure 5.
SEM/EDXS patterns of the decomposition products after annealing Er,Yb:GMBO spontaneous crystals.
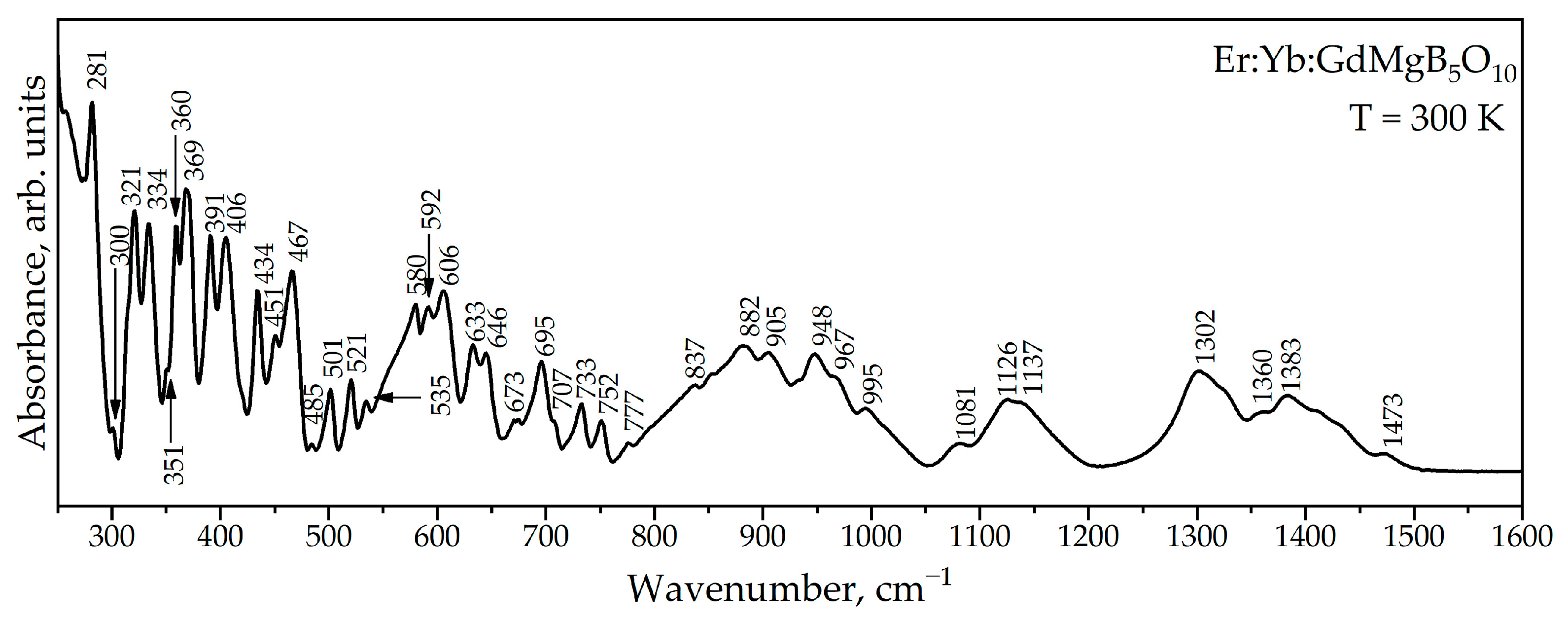
Figure 6 shows the ATR spectrum of Er,Yb:GdMgB5O10. Factor-group analysis for RMgB5O10 compounds was performed in RMgB5O10 compounds [21]. According to it, the GdMgB5O10 compound exhibits 201 optically active modes, of which 99 are IR active. The ATR spectrum contains 40 modes. The smaller number of observed modes is explained by the fact that they cannot be resolved due to the large number of lattice vibrations with close frequencies, and that below 250 cm−1 there is strong absorption, associated with intense modes [21].
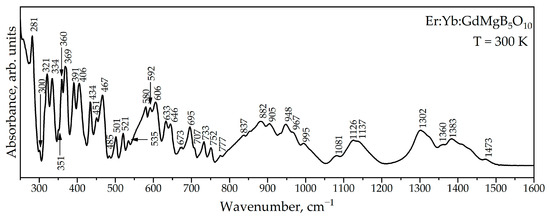
Figure 6.
ATR spectrum of Er:Yb:GdMgB5O10.
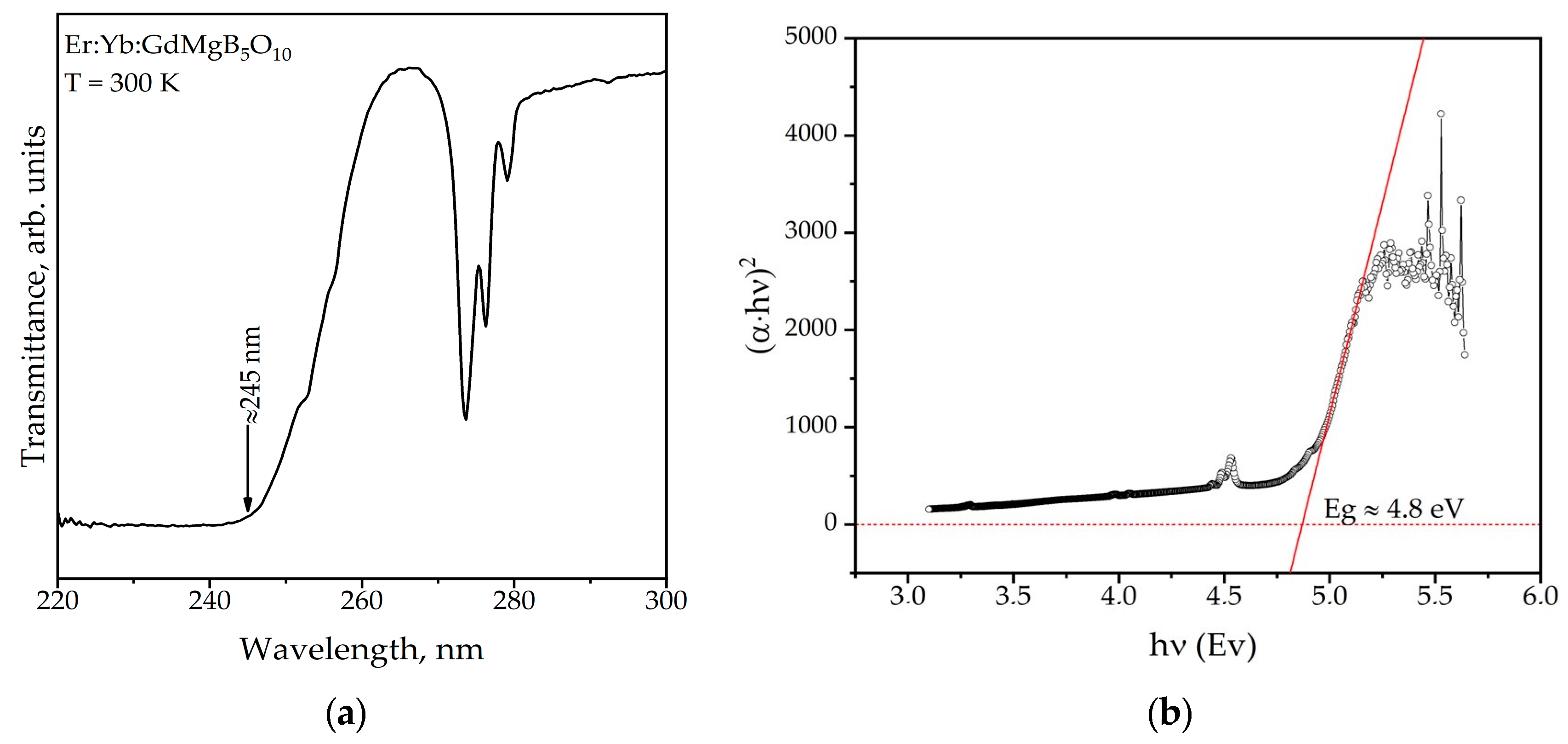
Figure 7a demonstrates the transmission spectrum of Er,Yb:GdMgB5O10 crystal in the range of 220–300 nm. The absorption edge of the Er,Yb:GMBO sample is located in the region of 245 nm, which is about 30 nm larger than for YMgB5O10 [22]. The optical band gap (Eg) obtained from transmission spectra approximates a value of 4.8 eV (Figure 7b) according to methodology from [23]. The formulas for indirect gap semiconductors were used to calculate the band gap.
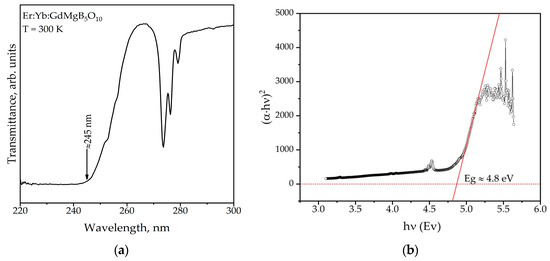
Figure 7.
Transmission spectrum (a) and related Tauc plot (b) of the Er:Yb:GdMgB5O10 crystal.
2.2. Spectroscopy
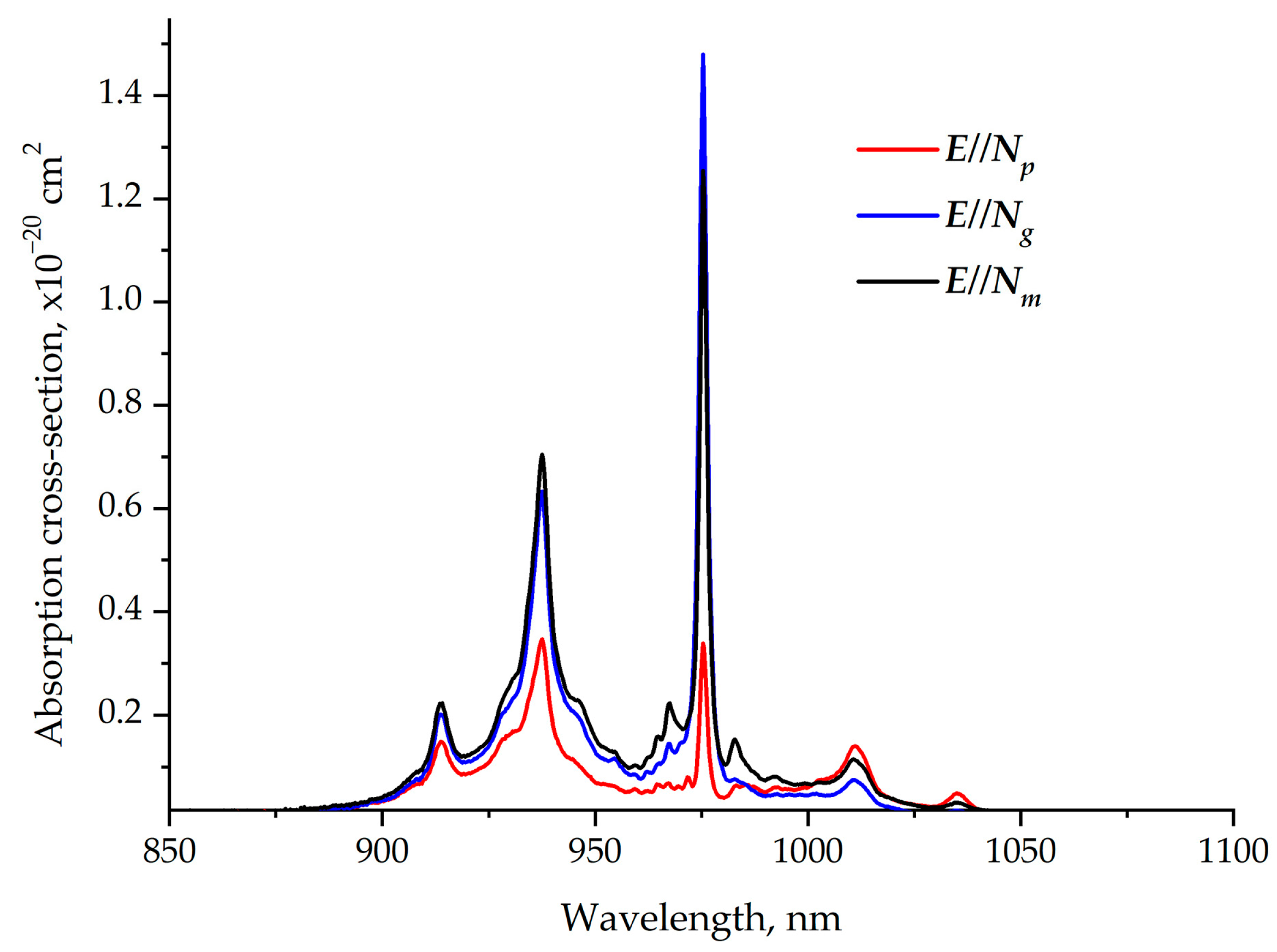
The room-temperature polarized absorption cross-section spectra of the Er,Yb:GMBO crystal in the 850–1100 nm spectral range are shown in Figure 8. The peak absorption cross-sections around 976 nm (the wavelength is close to the emission wavelengths of commercial InGaAs laser diodes) corresponding to the 2F7/2→2F5/2 transitions of Yb3+ ions are about 1.5 × 10−20 cm2 for E//Ngpolarization. Therefore, the polarization of the pump beam that matches the Ng axis of the crystal is preferred.The FWHM near 976 nm is ~2 nm, which imposes additional requirements for the wavelength stabilization of laser diodes.
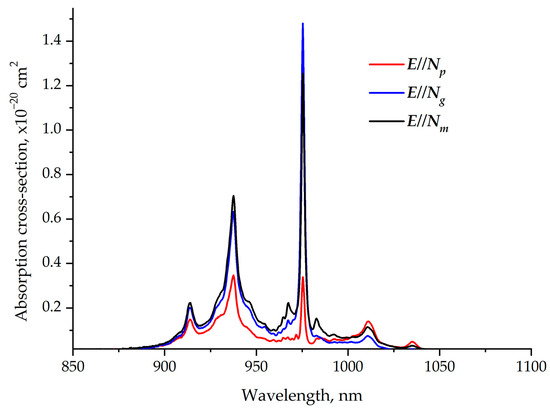
Figure 8.
Polarized room-temperature absorption cross-section spectra of Er,Yb:GMBO crystal near 1 µm.
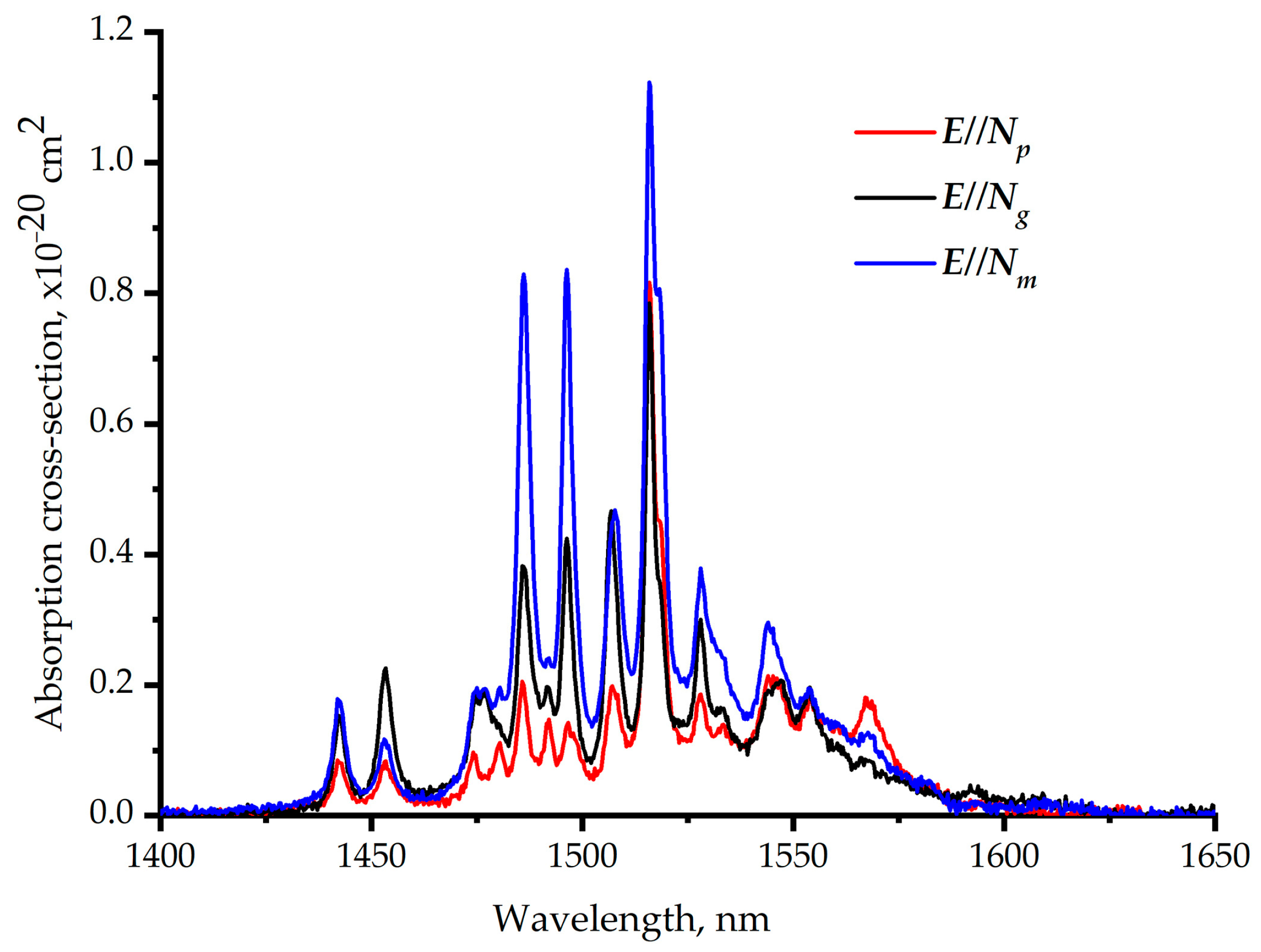
Figure 9 shows the polarized absorption cross-section of the Er,Yb:GMBO crystal at room temperature near 1.5 µm. Structured spectra with several narrow bands related to the 4I15/2→4I13/2transition of Er3+ ions are observed in the 1400–1650 nm spectral range. Multiple peaks in the absorption cross-section spectra correspond to transitions between the stark sublevels of the lower and upper multiplets. One can note the similarity with the data presented for Er,Yb:YMBO in [15].The maximum absorption cross-section does not exceed 1.1 × 10–20 cm2 at the wavelength of 1530 nm.
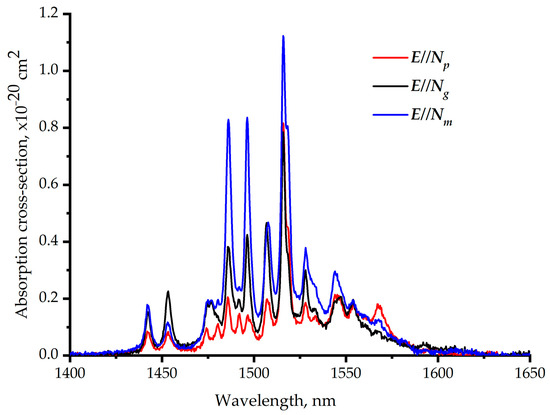
Figure 9.
Polarized room-temperature absorption cross-section spectra of the Er,Yb:GMBO crystal near 1.5 µm.
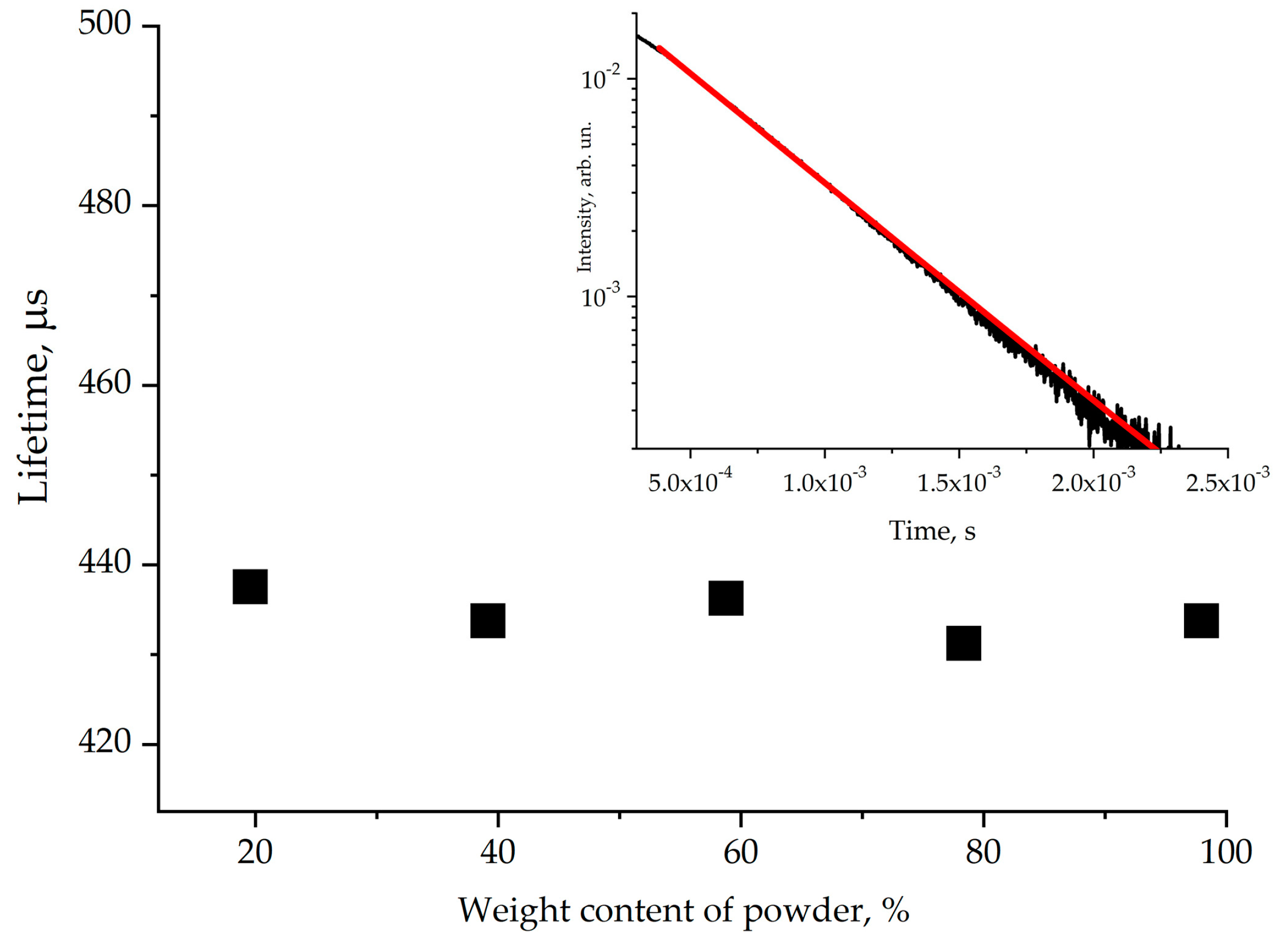
The decay curve of the 1.5 μm emission is shown in Figure 10. It was approximated by a single exponential function, and the 4I13/2 energy level of Er3+ was found to be 430 ± 20 μs. We can note that the influence of reabsorption is not significant for the 4I13/2 erbium energy level measurements. The emission lifetime was calculated using the Judd–Ofelt method and amounted to 6.30 ms (the corresponding data are given in [16]). Thus, the quantum yield of luminescence does not exceed 7%, which is comparable to values demonstrated previously for other oxoborate crystals with high phonon energy [13].
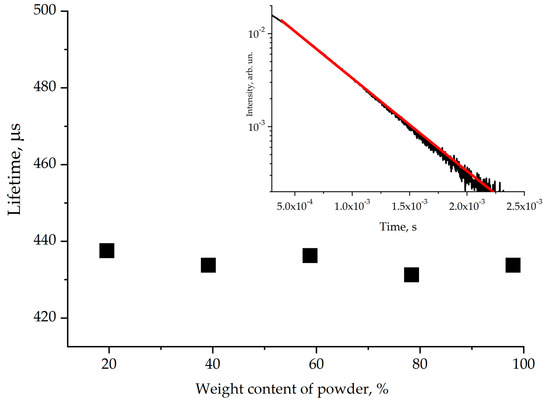
Figure 10.
The lifetime of the erbium 4I13/2 energy level measured using Er,Yb:GMBO crystalline powder in glycerin. The inset shows luminescence decay kinetics near 1.5 µm.
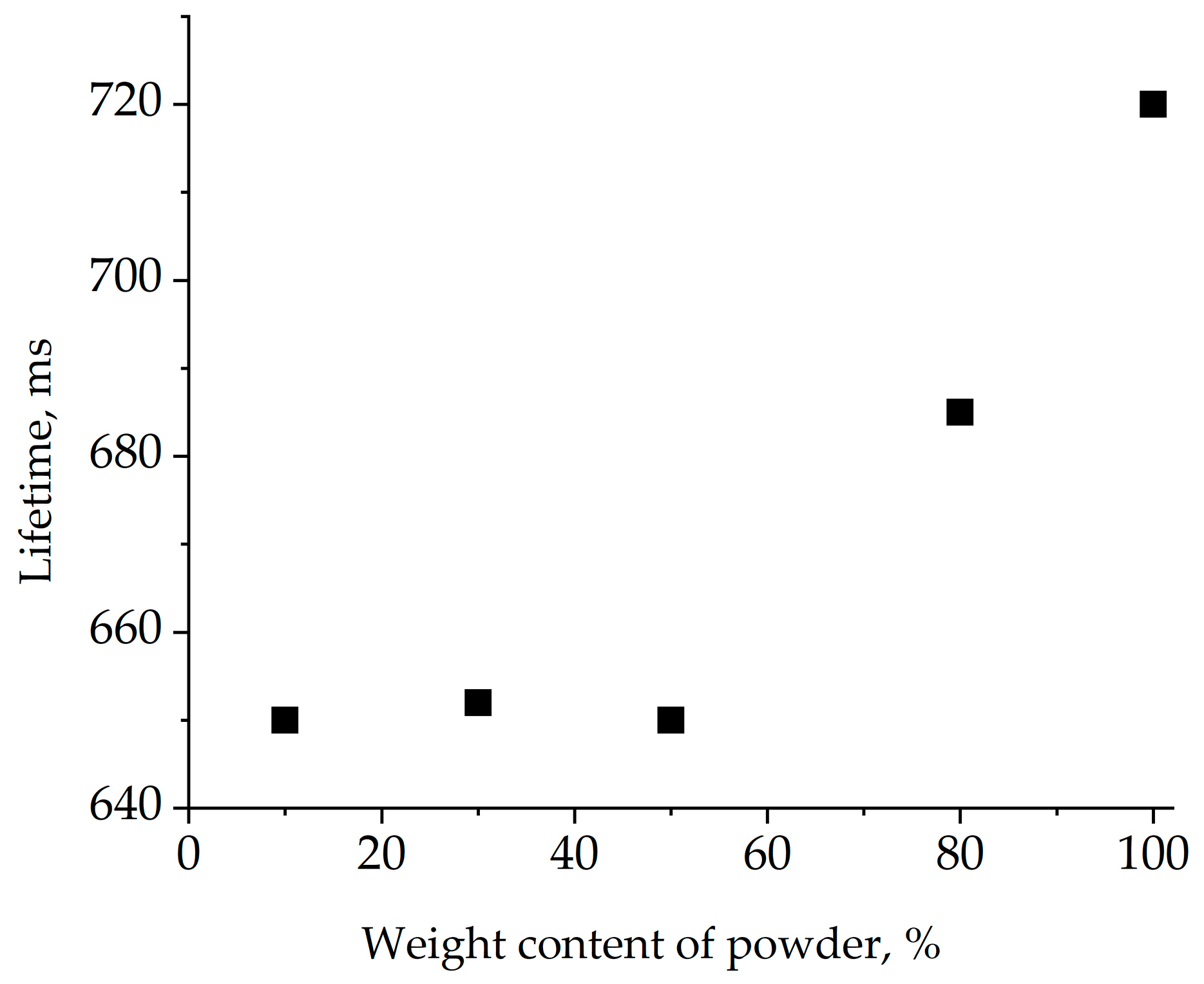
To calculate energy transfer efficiency from ytterbium to erbium according to Equation (1), the lifetime of the 2F5/2energy level of Yb3+ in Yb single-doped crystal and the Er,Yb-codoped one was measured. The dependence of the measured 2F5/2-energy-level lifetime of Yb3+on ytterbium content using Yb:GMBO powder in glycerin is presented in Figure 11.
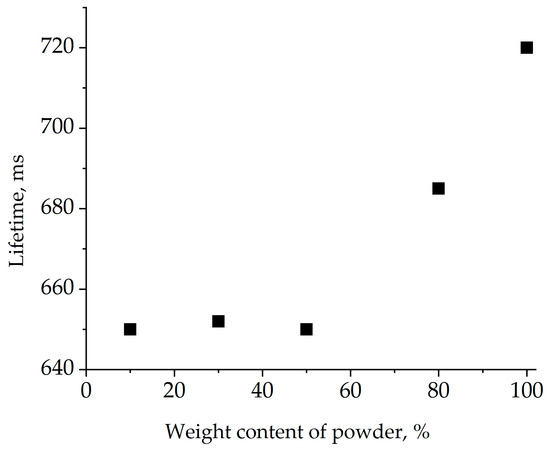
Figure 11.
Lifetime of the 2F5/2 Yb3+ energy level measured using Yb:GMBO crystalline powder in glycerin.
The measured lifetime decreased with decreasing powder content in the suspension. Starting from a certain powder content, the lifetime did not change despite further dilution, suggesting a negligible influence of reabsorption. The lifetime of the Yb3+2F5/2 energy level in the Yb(1 at.%):GMBO crystal was 650 ± 30 μs. The measurement of the Yb3+2F5/2-energy-level lifetime of Er,Yb:GMBO was similar to above and the 2F5/2-energy-level lifetime of Er,Yb:GMBO crystal was determined to be 60 ± 5 μs. Thus, the calculated energy transfer efficiency in the Er,Yb:GMBO crystal doped with 2 at.% Er3+ and 11 at.% Yb3+ was close to 90%. These results show that the almost all of the absorbed energy may be efficiently transferred from the 2F5/2 energy level of the Yb3+ion to the 4I11/2energy level of the Er3+ ion by a non-radiative resonant energy transfer process. It is also worth noting that the energy transfer efficiency in Er,Yb:GMBO crystals is similar to that of Er,Yb:RAl3(BO3)4 and Er,Yb:YMgB5O10 [15].
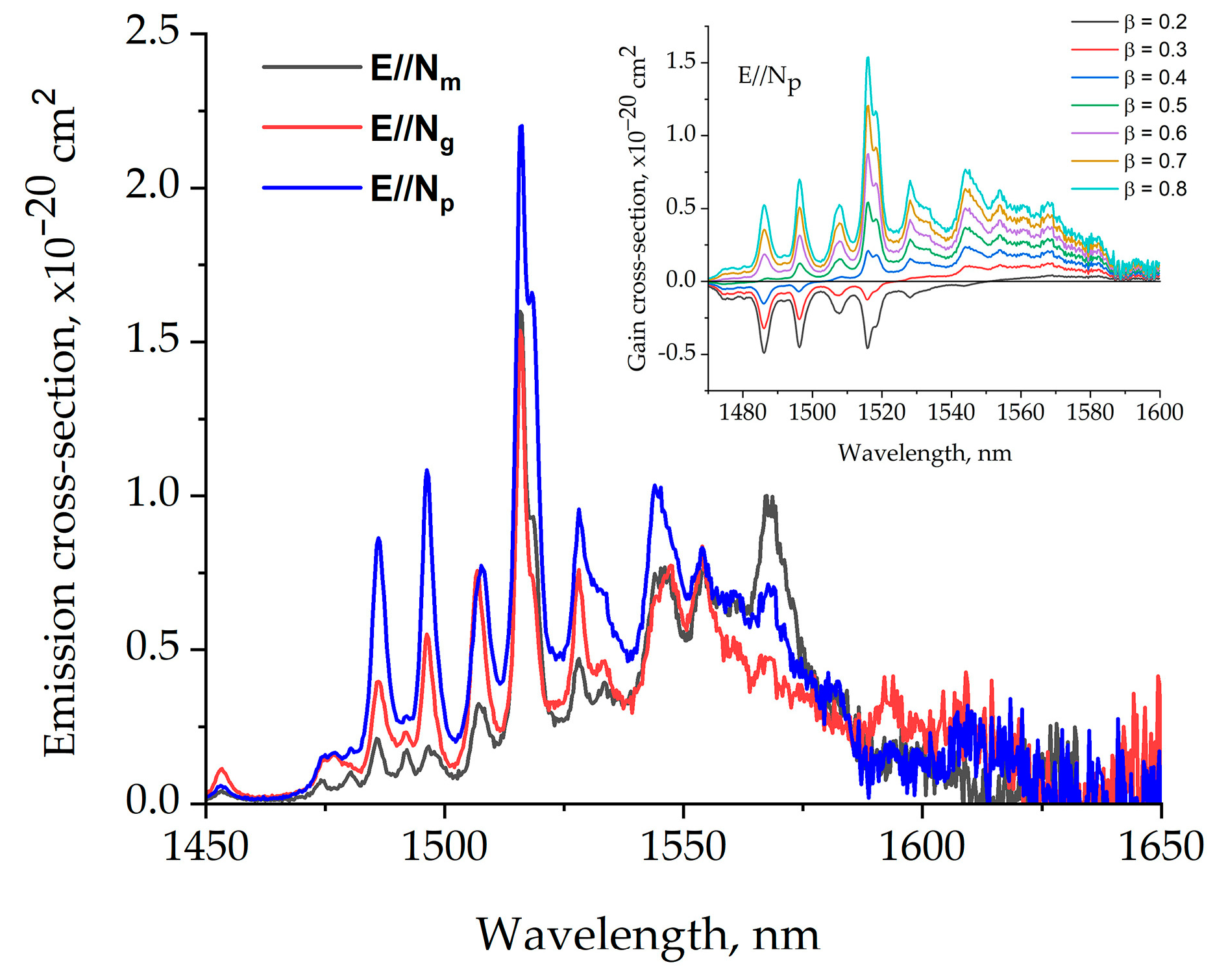
The stimulated emission cross-section spectra of Er,Yb:GMBO crystal in the 1400–1650 nm range are presented in Figure 12. The emission cross-section is 0.7 × 10–20 cm2at a laser wavelength of 1568 nm for the polarization of E//Np. The inset shows the gain cross-section spectra of the Er,Yb:GMBO crystal for different inversion parameters β ranging from 0.2 to 0.8 in the 1480–1600 nm spectral range for E//Np polarization.
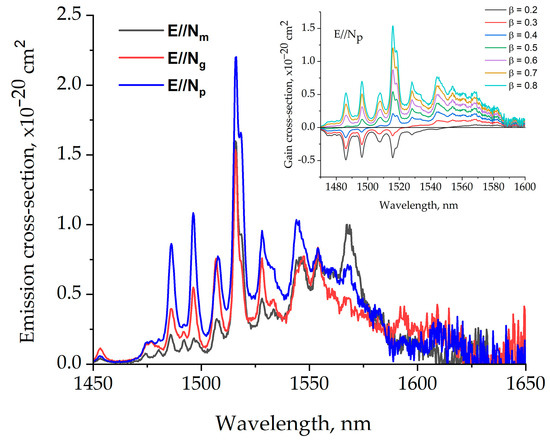
Figure 12.
The stimulated emission cross-section spectra of the Er,Yb:GMBO crystal. The inset shows the gain cross-section spectra of the Er,Yb:GMBO crystal.
2.3. Laser Operation
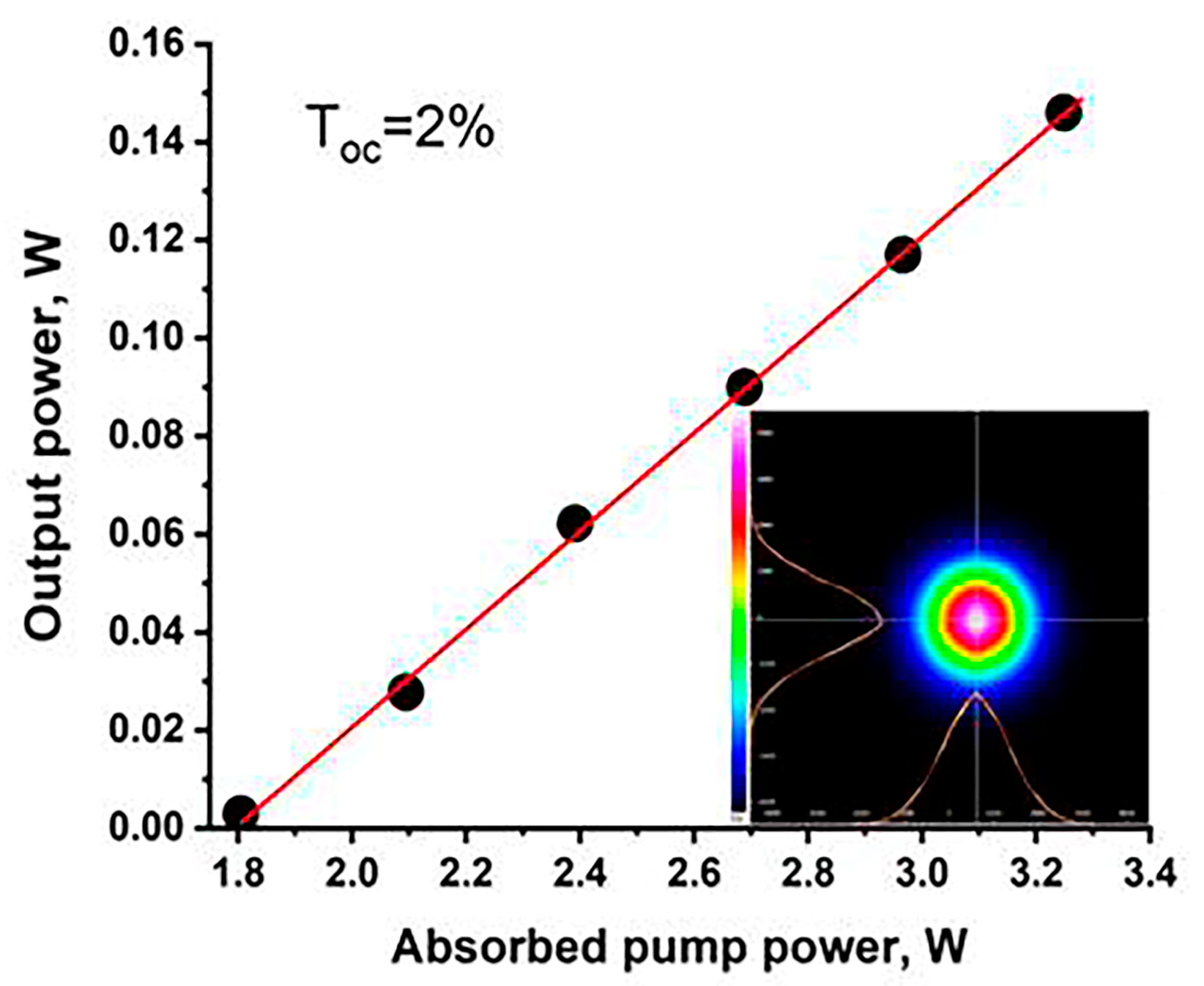
The continuous-wave Er,Yb:GMBO laser operation was realized for output couplers (OCs) with transmissions of 1%, 2% and 5%. The maximal output power and slope efficiency was obtained for OCs with a transmission of 2% at the laser wavelength. The input–output characteristics of a continuous-wave Er,Yb:GMBO laser with a 2% OC is presented in Figure 13. The maximum output power of 0.15 W at 1568 nm with a slope efficiency of 11% was achieved at an absorbed pump power of 3.3 W. Degradation of the output characteristics was observed with increasing incident power, which may be due to the influence of thermal lensing. The laser threshold was approximately 1.8 W of absorbed pump power. The laser radiation was linearly polarized (E//Np). The spatial profile of the output beam was TEM00 mode with M2 < 1.2 during all laser operations (inset in Figure 13).
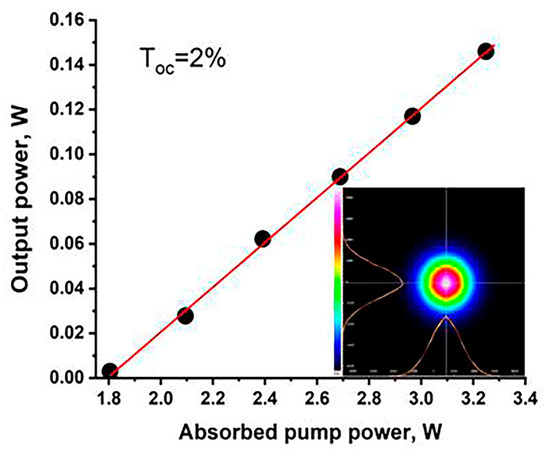
Figure 13.
The input–output characteristics of Er,Yb:GMBO laser; the inset shows the spatial profile of the output beam.
3. Materials and Methods
(Er,Yb):GdMgB5O10 bulk crystal was grown by the HT-SGDS technique from K2Mo3O10 solvent in the temperature range 870–830°C. Previously, experiments on GMBO spontaneous crystallization were carried out to estimate acceptable fluxed melt composition, taking into account the peculiarities of phase formation in other RMBO-K2Mo3O10 systems [24]. Based on the results obtained, a complex system of the composition 20 wt.% Er,Yb:GMBO–80 wt.% K2Mo3O10 was applied in growth experiments. The R2O3/MgO/B2O3 (where R = Yb, Er, Gd) ratio was set according to the pentaborate formula (1:2:5 in molar fractions). Er2O3 (99.96%), Yb2O3 (99.96%), Gd2O3 (99.96%), MgO (A.C.S. grade, by Aldrich), and B2O3 (A.C.S. grade, by Alfa Aesar) were used as crystal-forming agents, which were weighed according to the composition of Er0.02Yb0.11Gd0.87MgB5O10. The solvent, K2Mo3O10, was prepared from a mixture of K2MoO4 (A.C.S. grade, Chimkraft, Kaliningrad, Russia) and MoO3 (A.C.S. grade, Aldrich), compounded in a molar ratio of 1:2.
The starting chemicals were carefull y ground, mixed, and in the 250 mL platinum crucible placed into the furnace at a position that could be adjusted for an optimum temperature gradient. Then, the initial load was heated to a maximum temperature of 100–150 °C above the expected saturation temperature (Tsat). After solution homogenization for 24 h, the system was cooled to 5–10 °C above the expected Tsat. The saturation point was then precisely confirmed by dipping a trial GMBO seed in the solution and observing the growth/dissolutionprocesses of the crystal faces at different temperatures. Er,Yb:GBMO bulk crystal was obtained using a well-faceted spontaneous crystal. A GBMO seed was dipped into the solution and overheated by 2–3 °C above the fluxed melt saturation temperature to slightly dissolve the outer surface and ensure defect-free nucleation. Subsequently, the temperature was lowered to the Tsat in 1 h. During crystal growth, supersaturation was maintained by cooling down from 0.5 to 2 °C per day. At the end, the crystal was extracted and cooled down to room temperature for several days to prevent cracking due to the thermal shock. All experiments were carried out in a vertical tube furnace, equipped with a CrNi alloy resistive heater and a Proterm-100 precision temperature controller connected with a set of Pt-Rh/Pt thermocouples. The temperature in the furnace working zone was kept with a stability of ±0.1°C.
PXRD studies were performed on a Rigaku MiniFlex-600 powder diffractometer (Rigaku Corp., Tokyo, Japan). PXRD data sets were collected in continuous mode at room temperature (CuKα radiation) in the range of 2θ = 3–70°, and a scan speed of 5° per minute. The PXRD data were analyzed using the model-biased Le Bail fitting with Yana2006 software [25]. Phases were identified using the Match! software package, version 3.8.1.143 [26], the Crystallographic Open Database (COD) and the ICSD inorganic crystal database [27].
The composition and homogeneity of the Er,Yb:GdMB crystal were studied using the analytical scanning electron microscope (SEM) Leo 1420 VP equipped with the energy dispersive X-ray spectrometer (EDXS) INCA 350. Qualitative analysis was performed on the as-grown face. The sample was fixed on a conductive carbon adhesive tape and covered with a thin layer of carbon to prevent charge accumulation on the crystal during interactions with an electron beam. The Er3+ and Yb3+ distribution coefficient (Kd) was defined as Kd = Ccryst/Cs, where Ccryst is the Er3+ and Yb3+ content in the crystal and Cs is the nominal concentration of rare-earth oxides in the initial load.
DSC analysis was performed on a STA 449 F5 Jupiter® (Netzsch, Selb, Germany) to investigate the thermal behavior of the Er,Yb:GMBO crystal. DSC measurements were performed in the temperature range of 50–1200 °C at a heating rate of 20 °C/min under argon gas flow. PtRh20 crucibles with a volume of 85 μL were used for the experiments. The melting point was determined based on the onset temperature of the melting process, with an estimated uncertainty of ±5 °C. To further investigate the thermal behavior, crystals obtained were heat-treated in the range of 1050 °C. The products after annealing were analyzed using the SEM/EDXS method to identify any changes in the phase composition.
Spontaneous Er:Yb:GdMgB5O10 crystals were ground into powder in a corundum mortar to record attenuated total reflection (ATR) spectra. The measurements were carried out on a Fourier spectrometer Bruker IFS 125HR at room temperature in the spectral range 50–2000 cm−1 with a resolution of 2 cm−1. In this case, a Mylar beamsplitter and a DTGS receiver were used in the far-infrared(IR) range (50–700 cm−1). A KBr beamsplitter and a DLaTGS receiver were used in the mid-IR range (400–2000 cm−1). In both cases, the source was a Globar.
Transmission spectra in the ultraviolet (UV) range were recorded on an Ocean Insight OCEAN-HDX-UV-VIS spectrometer at room temperature in the spectral range 200–800 nm with a resolution of 0.73 nm. A deuterium lamp was used as a source, and a CCD detector served as a receiver. The measurements used a fragment of a small crystal.
Er,Yb:GMBO is a monoclinic crystal, it is optically biaxial and its optical properties are described along the three main optical axes of the Ng, Nm, and Np indicatrices [28]. For spectroscopic investigations in polarized light, plates oriented along Ng, Nm, and Np indicatrices were cut from the Er,Yb:GMBO crystal. The polarized absorption spectra were measured at room temperature by using spectrophotometer Agilent Cary 5000. The absorption cross-section spectra were calculated according to (1):
where is the absorption coefficient, is the concentration of ytterbium for near-1 µm spectra or erbium for near-1.5 µm spectra.
For lifetime measurements, a parametric oscillator based on β-Ba2B2O4 crystal pumped by a 355 nm Nd:YAG laser (third harmonic) was used as an excitation source. Luminescence of the sample was collected from its surface irradiated with an excitation beam, passed through an MDR-12 monochromator, and recorded by an InGaAs photodiode with a preamplifier coupled to a 500 MHz digital oscilloscope.
The energy transfer efficiency was calculated according to (2) [29]:
where is the ytterbium 2F5/2-level lifetime in Er,Yb-codoped crystal, and is the ytterbium 2F5/2-level lifetime in Yb single-doped crystal. For Yb3+ lifetime measurements, luminescence kinetics were registered by using immersed Er,Yb:GMBO fine powder that enables minimizingreabsorption influence [30].
The calculation of the stimulated emission spectra in the range near 1.5 µm (transition 4I13/2→4I15/2) was performed by using the integral reciprocity method according to (3) [31] and radiative lifetime of the 4I13/2energy level obtained from the Judd–Ofelt theory in [17].
where σem(λ) is the stimulated emission cross-section; α, γ denote the polarization of light; h is Planck’s constant; c is the speed of light in a vacuum; k is the Boltzmann constant; T is the temperature of the environment; n is the refractive index of the crystal; τrad is the radiation lifetime of the 4I13/2 energy level of erbium ions; and σabs(λ) is the absorption cross-section.
The gain cross-section spectra g(λ) were calculated for different inversion parameters β by using the following Equation (3):
where β = Nex/Ntot is the ratio of the population of excited Er3+ ion manifolds to the total erbium ion concentration.
g(λ) = β σem(λ) − (1 − β)σabs(λ)
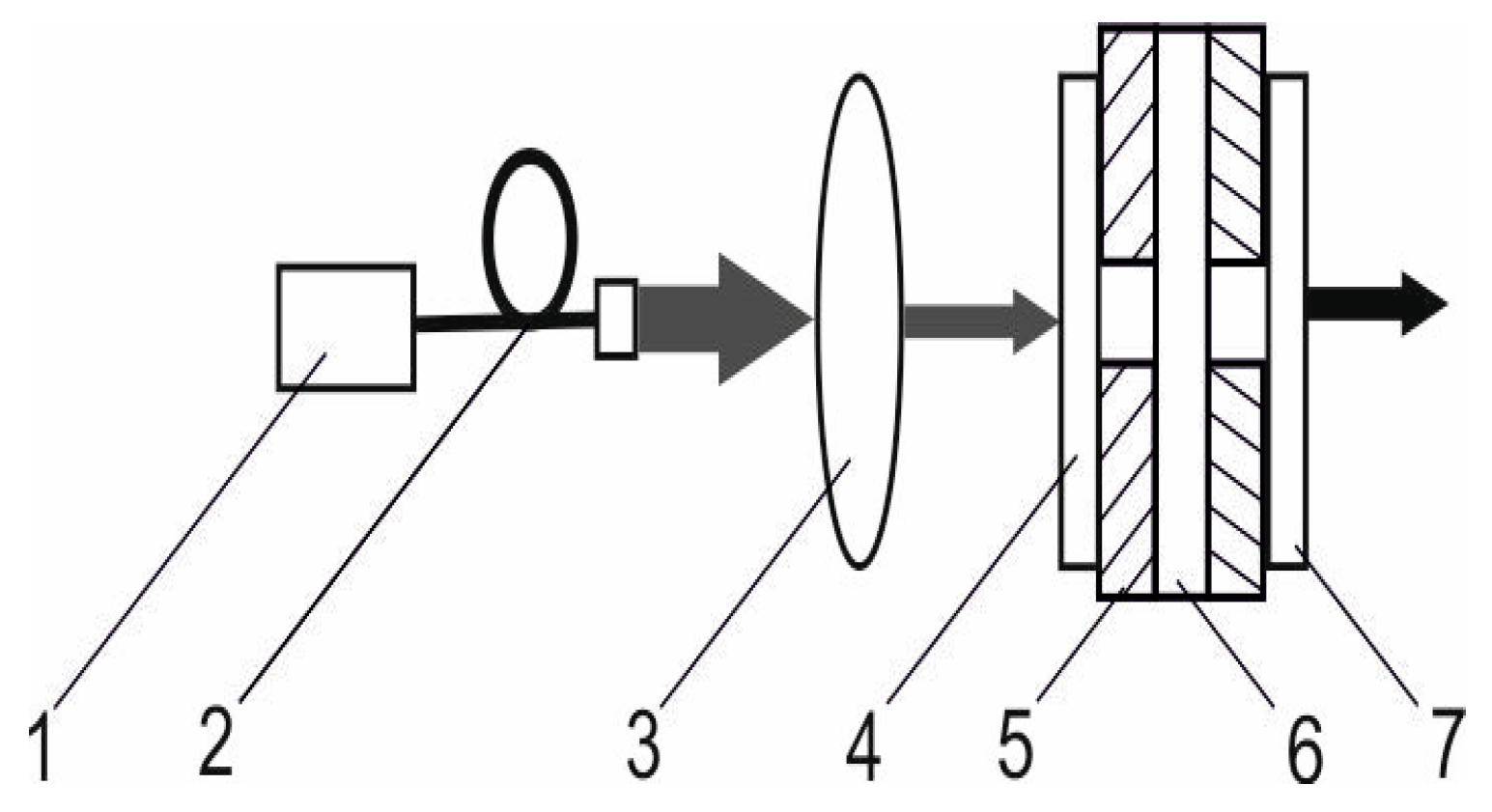
The experimental setup provided in Figure 14 was used to study the laser properties in acontinuous-wave mode of operation. A plane–plane Nm-cut Er,Yb:GMBO crystal with a length of 1.5 mm was used as a gain medium. It was coated with anti-reflection coatings at the pump and laser wavelengths and mounted on an aluminum heat sink kept at 20 °C. A continuous-wave fiber-coupled laser diode (Ø 105 μm, NA = 0.22) emitting at a wavelength of 976 nm was used for the longitudinal pumping of the gain medium. The plano–plano cavity with a geometrical cavity length of 4 mm was applied. A single-lens focusing system focused the pump beam into a 120µm spot inside the laser crystal. Three output couplers with different transmission coefficients at laser wavelengths were used in the laser experiments.
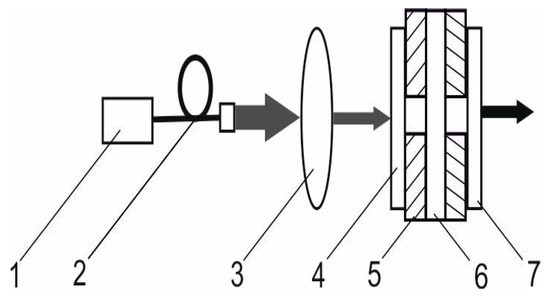
Figure 14.
The experimental setup of a continuous-wave diode-pumped Er,Yb:GMBO laser: 1—laser diode; 2—fiber; 3—focusing system; 4—input mirror; 5—copper heat sink; 6—active element; and 7—output coupler.
4. Conclusions
Er3+,Yb3+:GdMgB5O10 single crystals with dimensions up to 24 × 15 × 12 mm3were successfully grown using an HT-SGDS route from a K2Mo3O10-based flux system. Detailed study of the growth technique and characterization of Er,Yb:GdMgB5O10 single crystalswere carried out. The spectroscopic properties of as-grown crystals were presented. It was shown that Er,Yb:GMBO crystals are characterized by spectroscopic properties to achieve laser operations. A CW diode-pumped Er,Yb:GMBO laser with an output power of about 150 mW and a slope efficiency of 11% was realized at 1568 nm.
Author Contributions
Software, K.N.G., E.V.K. and E.A.V.; validation, K.N.G., V.E.K., V.V.M., E.A.V., E.V.K., D.D.M., N.N.K., E.I.M. and V.L.K.; investigation, K.N.G., V.E.K., V.V.M., E.A.V., E.V.K., D.D.M., N.N.K., E.I.M. and V.L.K.; resources, K.N.G., V.E.K., E.A.V., E.V.K., V.V.M. and N.N.K.; writing—original draft preparation, K.N.G. and E.A.V.; writing—review and editing, K.N.G. and E.A.V.; visualization, E.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out within the framework of the state budget theme AAAA-A16-116033010121-7 of M.V. Lomonosov MSU. ATR and transmission spectra were obtained with the support of the Ministry of Science and Higher Education of the Russian Federation under the program FFUU-2024-004. This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mutailipu, M.; Poeppelmeier, K.R.; Pan, S. Borates: A Rich Source for Optical Materials. Chem. Rev. 2020, 121, 1130–1202. [Google Scholar] [CrossRef]
- Leonyuk, N.I.; Maltsev, V.V.; Volkova, E.A. Crystal Chemistry of High-Temperature Borates. Molecules 2020, 25, 2450. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, S.; Meneses-Nava, M.A.; Barbosa-Garcia, O.; Diaz-Torres, L.A.; Santoyo, M.A.; Mosino, J.F. Energy back transfer, migration and energy transfer (Yb-to-Er and Er-to-Yb) processes in Yb,Er:YAG. J. Lumin. 2003, 102–103, 694–698. [Google Scholar] [CrossRef]
- Karlsson, G.; Laurell, F.; Tellefsen, J.; Denker, B.; Galagan, B.; Osiko, V.; Sverchkov, S. Development and characterization of Yb-Er laser glass for high average power laser diode pumping. Appl. Phys. B 2002, 75, 41–46. [Google Scholar] [CrossRef]
- Taccheo, S.; Sorbello, G.; Laporta, P.; Karlsson, G.; Laurell, F. 230-mW diode-pumped single-frequency Er,Yb laser at 1.5 μm. IEEE Phot. Techn. Let. 2001, 13, 19–21. [Google Scholar] [CrossRef]
- Zagumennyi, A.I.; Lutts, G.B.; Popov, P.A.; Sirota, N.N.; Shcherbakov, I.A. The Thermal conductivity of YAG and YSAG laser crystals. Laser Phys. 1993, 3, 1064–1065. [Google Scholar]
- Krankel, C.; Uvarova, A.; Guguschev, C.; Kalusniak, S.; Hülshoff, L.; Tanaka, H.; Klimm, D. Rare-earth doped mixed sesquioxides for ultrafast lasers. Opt. Mat. Exp. 2022, 12, 1074–1091. [Google Scholar] [CrossRef]
- Schweizer, T.; Jensen, T.; Heumann, E.; Huber, G. Spectroscopic properties and diode-pumped 1.6 μm laser performance in Yb-codoped Er:Y3Al5O12 and Er:Y2SiO5. Opt. Commun. 1995, 118, 557–561. [Google Scholar] [CrossRef]
- Bjurshagen, S.; Brynolfsson, P.; Pasiskevicius, V.; Parreu, I.; Pujol, M.C.; Peña, A.; Aguiló, M.; Díaz, F. Crystal growth, spectroscopic characterization, and eye-safe laser operation of erbium- and ytterbium-codopedKLu(WO4)2. Appl. Opt. 2008, 47, 656–665. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Huang, J.; Gong, X.; Luo, Z.; Huang, Y. Spectroscopic and laser properties of Er3+,Yb3+:LuAl3(BO3)4 crystal at 1.5-1.6 μm. Opt. Express 2010, 18, 13700–13707. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Y.; Gong, X.; Lin, Y.; Luo, Z.; Huang, Y. Spectral and laser properties of Er,Yb:Sr3Lu2(BO3)4 crystal at 1.5–1.6 μm. Opt. Express 2013, 3, 1885–1892. [Google Scholar] [CrossRef]
- Gorbachenya, K.N.; Kisel, V.E.; Yasukevich, A.S.; Deineka, R.V.; Lipinskas, T.; Galinis, A.; Miksys, D.; Maltsev, V.V.; Leonyuk, N.I.; Kuleshov, N.V. Monolithic 1.5 µm Er,Yb:GdAl3(BO3)4 eye-safe laser. Opt. Mat. 2019, 88, 60–66. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, T.; Jiang, Z.; Huang, G.; Huang, Y.; Li, B.; Lin, Z.; Chen, Y.; Huang, Y.; Lin, Y.; et al. 4.55 W continue-wave dual-end pumping of Er:Yb:YAl3(BO3)4 microchip laser at 1.5 μm. Appl. Phys. Lett. 2023, 123, 171101. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Huang, J.; Gong, X.; Luo, Z.; Huang, Y. Fabrication and diode-pumped 1.55 μm continuous-wave laser performance of a diffusion-bonded Er:Yb:YAl3(BO3)4/YAl3(BO3)4 composite crystal. Opt. Express 2017, 25, 17128–17133. [Google Scholar] [CrossRef]
- Gorbachenya, K.N.; Kisel, V.E.; Deineka, R.V.; Yasukevich, A.S.; Kuleshov, N.V.; Maltsev, V.V.; Mitina, D.D.; Volkova, E.A.; Leonyuk, N.I. Continuous-wave laser on Er,Yb-codopedpentaborate crystal. Devices Methods Meas. 2019, 10, 301–307. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, F.; Sun, S.; Lin, Z.; Zhang, L. Thermal, spectral and laser properties of Er3+,Yb3+:GdMgB₅O10: A new crystal for 1.5 μm lasers. Materials 2018, 11, 25. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, S.; Yuan, F.; Zhang, L.; Lin, Z. Spectroscopic properties and continuous-wave laser operation of Er3+,Yb3+:LaMgB5O10 crystal. J. Alloy Compd. 2017, 695, 215–220. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, Q.; Huang, Y.; Lin, Y.; Huang, J.; Gong, X.; Luo, Z.; Lin, Z.; Huang, Y. Efficient continuous-wave diode-pumped Er3+:Yb3+:LaMgB5O10 laser with sapphire cooling at 1.57 μm. Opt. Express 2017, 25, 19320–19325. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Lin, Z.; Huang, Y. Passively Q-switched Er,Yb:GdMgB5O10 pulse laser at 1567 nm. OSA Contin. 2019, 2, 3598–3603. [Google Scholar] [CrossRef]
- Huang, Y.; Lou, F.; Sun, S.; Yuan, F.; Zhang, L.; Lin, Z.; You, Z. Spectroscopy and laser performance of Yb3+:GdMgB5O10 crystal. J. Lumines 2017, 188, 7–11. [Google Scholar] [CrossRef]
- Gorbachenya, K.N.; Yasukevich, A.S.; Lazarchuk, A.I.; Kisel, V.E.; Kuleshov, N.V.; Volkova, E.A.; Maltsev, V.V.; Koporulina, E.V.; Yapaskurt, V.O.; Kuzmin, N.N.; et al. Growth and Spectroscopy of Yb:YMgB5O10 Crystal. Crystals 2022, 12, 986. [Google Scholar] [CrossRef]
- Sun, S.; Wei, Q.; Li, B.; Shi, X.; Yuan, F.; Lou, F.; Zhang, L.; Lin, Z.; Zhong, D.; Huang, Y.; et al. The YMgB5O10 crystal preparation and attractive multi-wavelength emission characteristics of doping Nd3+ ions. J. Mater. Chem. C 2021, 9, 1945–1957. [Google Scholar] [CrossRef]
- Wang l Liu, S.; Gou, H.; Chen, Y.; Yue, G.H.; Peng, D.L.; Hihara, T.; Sumiyama, K. Preparation and characterization of the ZnO:Al/Fe65Co35/ZnO:Al multifunctional films. Appl. Phys. A Mater. Sci. Process. 2011, 106, 717–723. [Google Scholar] [CrossRef]
- Maltsev, V.V.; Mitina, D.D.; Belokoneva, E.L.; Volkova, E.A.; Koporulina, E.V.; Jiliaeva, A.I. Synthesis and flux-growth of rare-earth magnesium pentaborate crystals RMgB5O10 (R = Y, Gd, La, Tm and Yb). J. Cryst. Growth 2022, 587, 126628. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Match!—Phase Analysis Using Powder Diffraction, Crystal Impact; GbR: Bonn, Germany. Available online: https://www.crystalimpact.de/match (accessed on 16 July 2024).
- Inorganic Crystal Structure Data Base—ICSD; Fachinformations Zentrum (FIZ) Karlsruhe: Karlsruhe, Germany, 2021; Available online: https://www.crystallography.net/cod/ (accessed on 16 July 2024).
- Zhang, J.; Tao, X.; Cai, G.; Jin, Z. Phase relation, structure, and properties of borate MgYB5O10 in MgO–Y2O3–B2O3 system. Powder Diffr. 2017, 32, 97–106. [Google Scholar] [CrossRef]
- Burns, P.; Dawes, J.; Dekker, P.; Pipper, J.; Jiang, H.; Wang, J. Optimization of Er,Yb:YCOB for cw laser operation. IEEE J. Quantum Electron. 2004, 40, 1575–1582. [Google Scholar] [CrossRef]
- Sumida, D.S.; Fan, T.Y. Effect of radiation trapping on fluorescence lifetime and emission cross section measurements in solid-state laser media. Opt. Lett. 1994, 19, 1343–1345. [Google Scholar] [CrossRef]
- Yasyukevich, A.S.; Shcherbitskii, V.G.; Kisel, V.E.; Mandrik, A.V.; Kuleshov, N.V. Integral method of reciprocity in the spectroscopy of laser crystals with impurity centers. J. Appl. Spectr. 2004, 71, 202–208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).