Multistimuli Luminescence and Anthelmintic Activity of Zn(II) Complexes Based on 1H-Benzimidazole-2-yl Hydrazone Ligands
Abstract
1. Introduction
2. Results
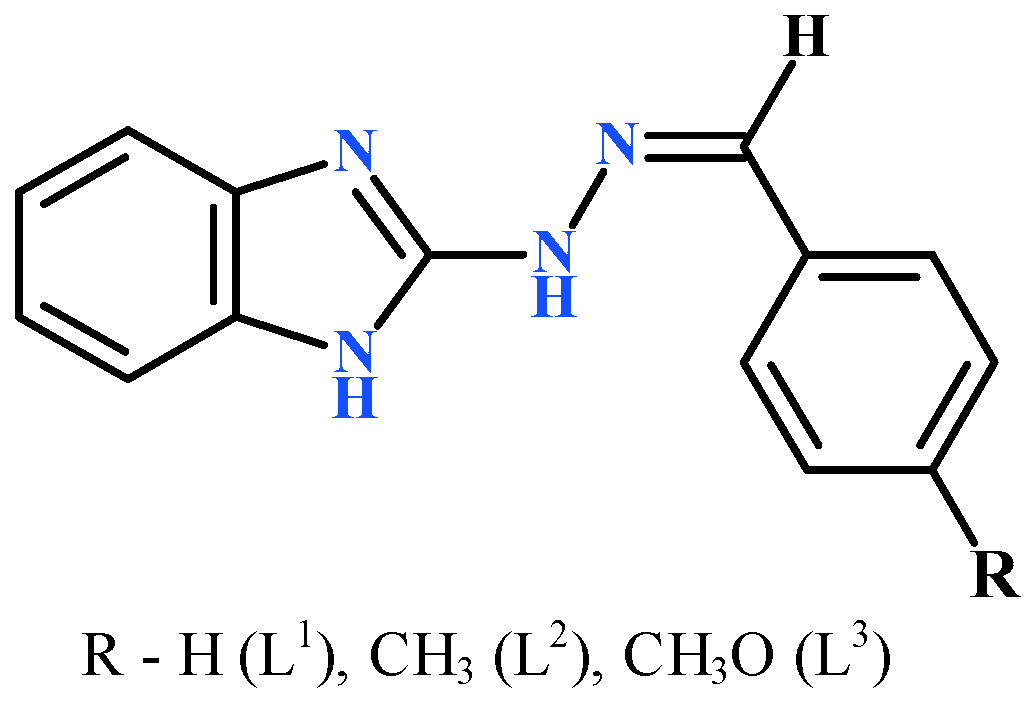
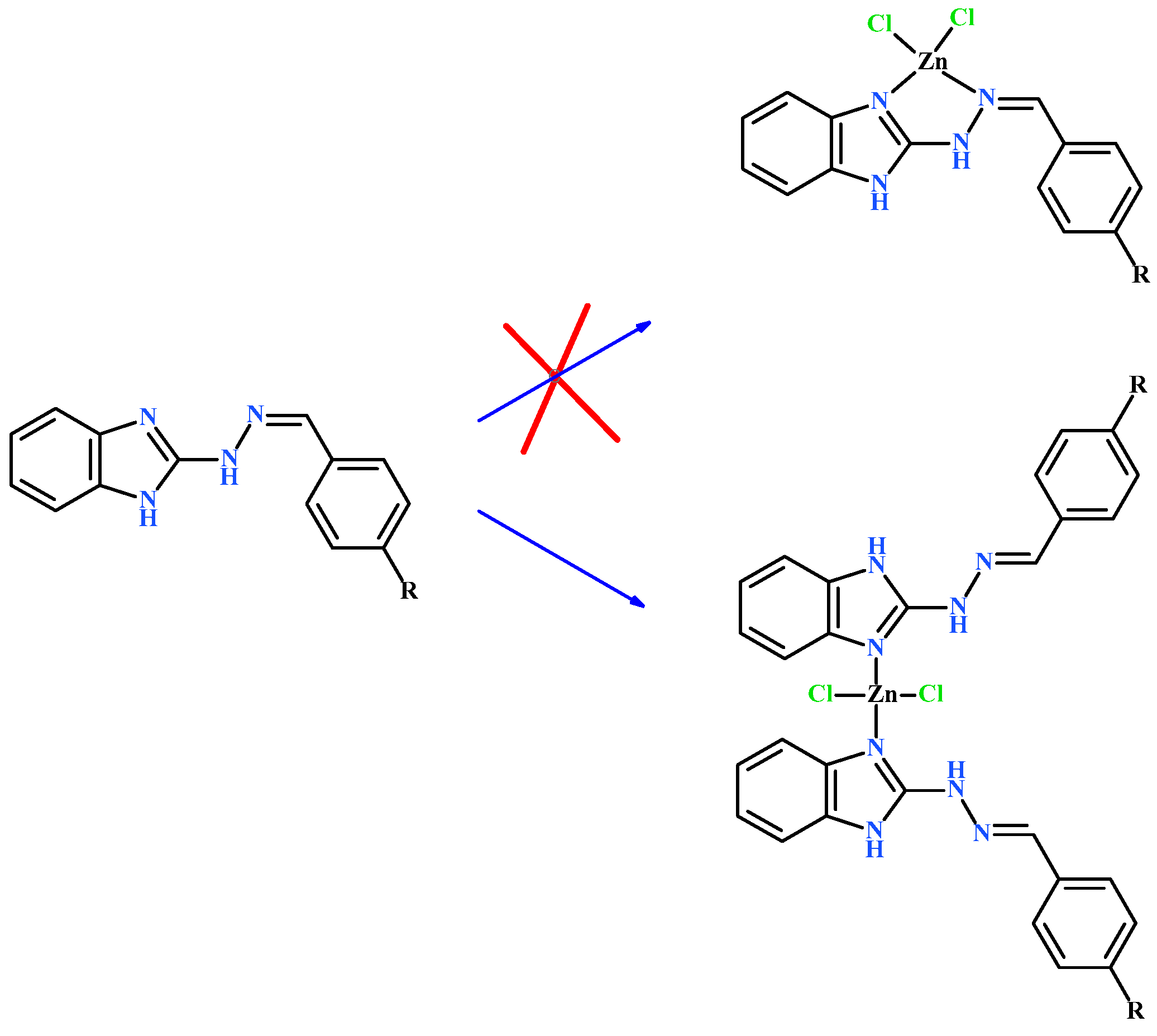
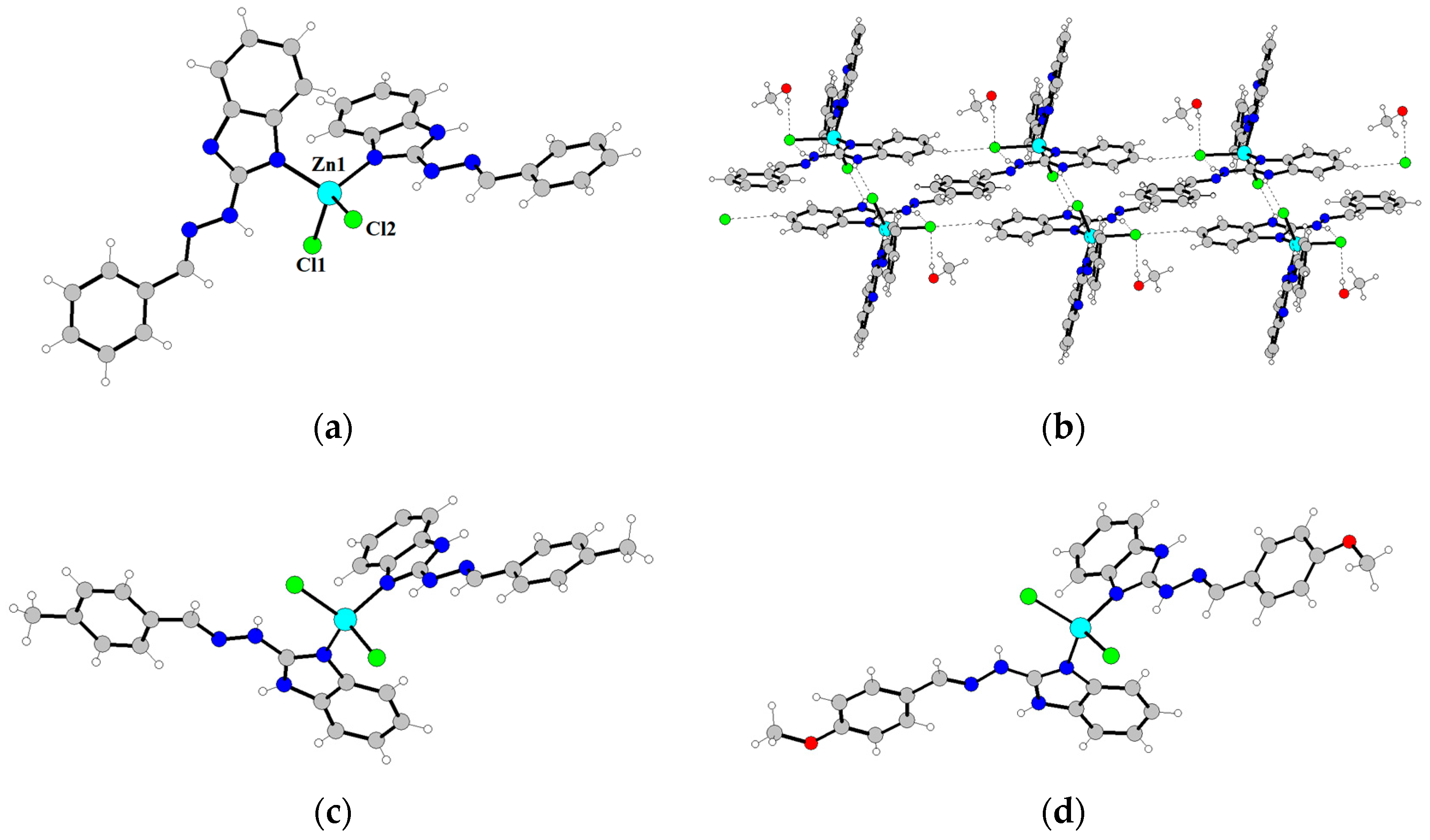
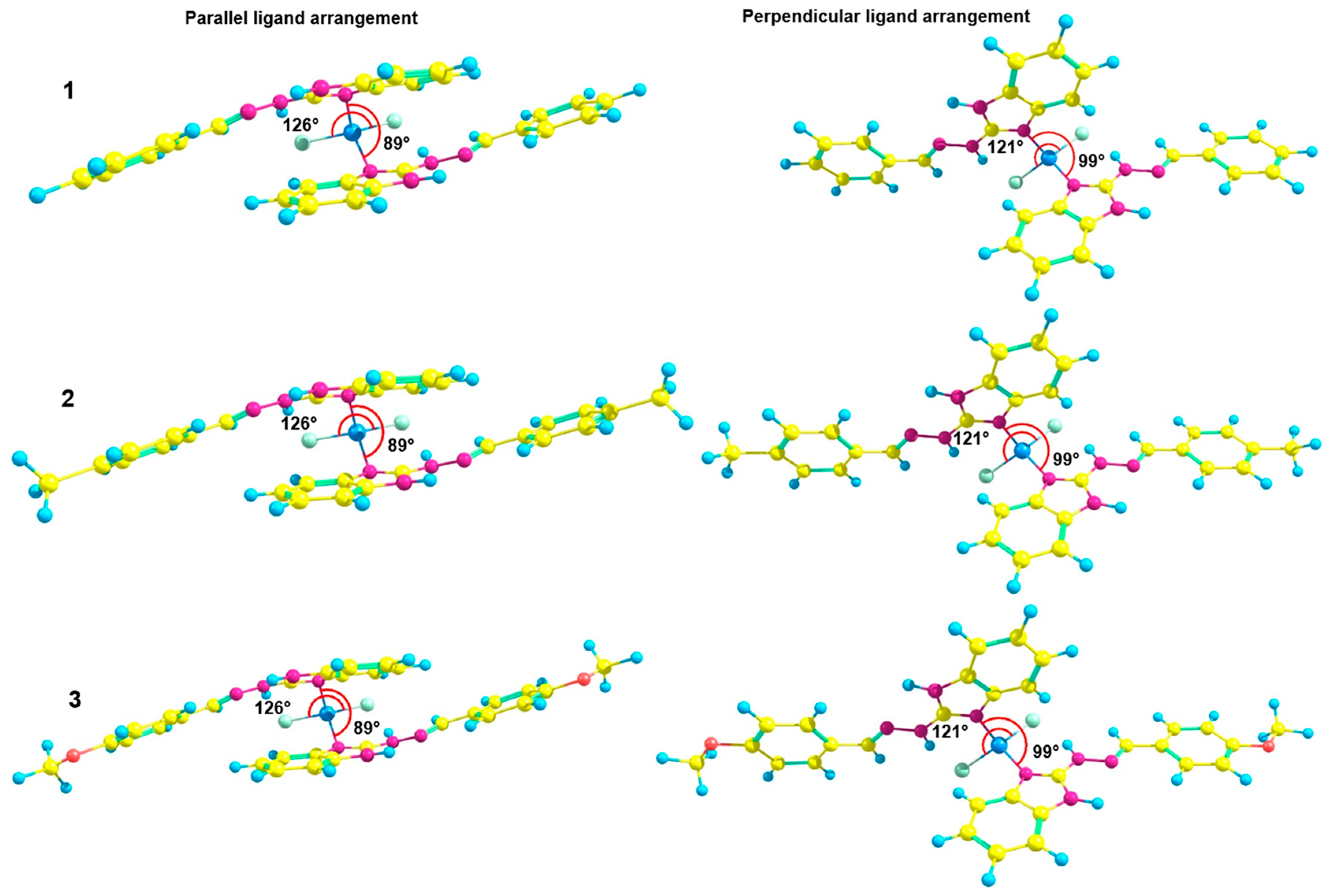
2.1. General Characterization of Complexes
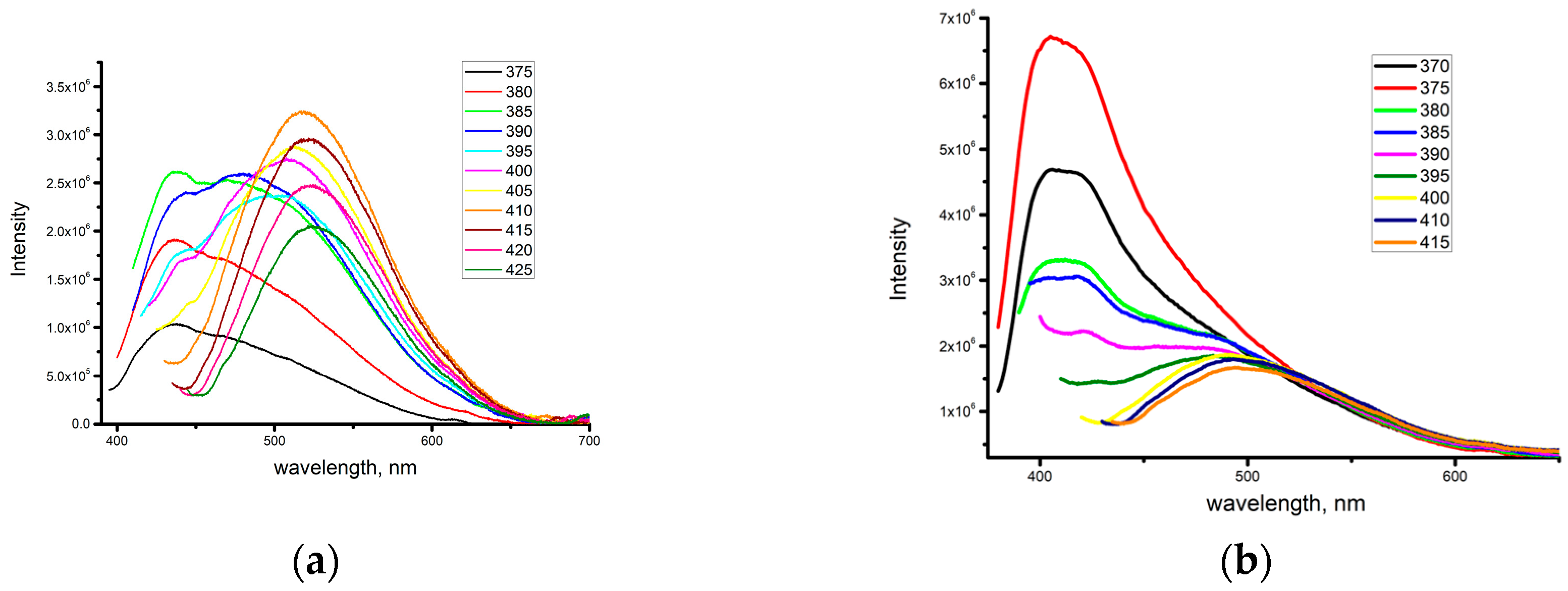
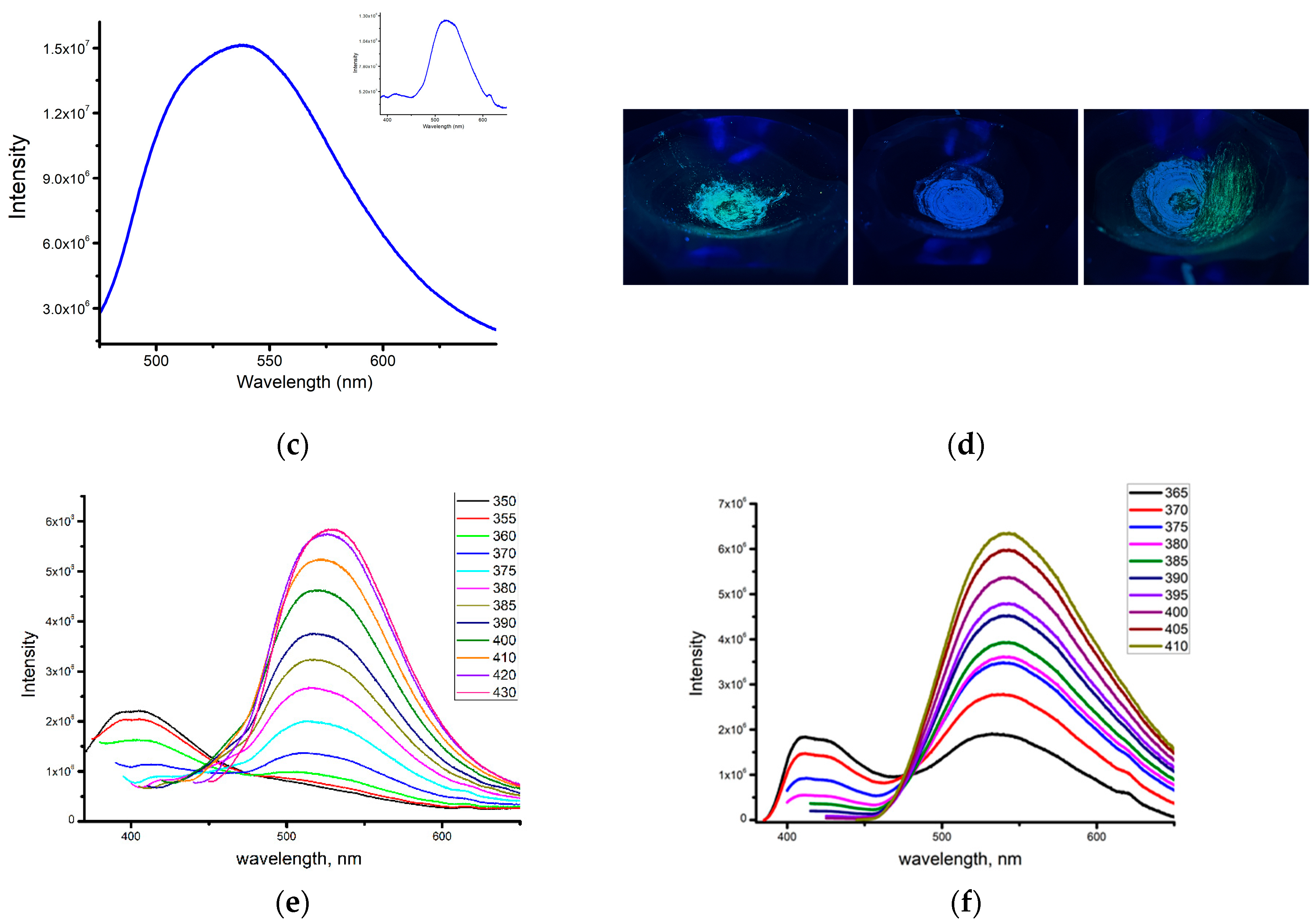
2.2. Photophysical Properties of Zinc Complexes
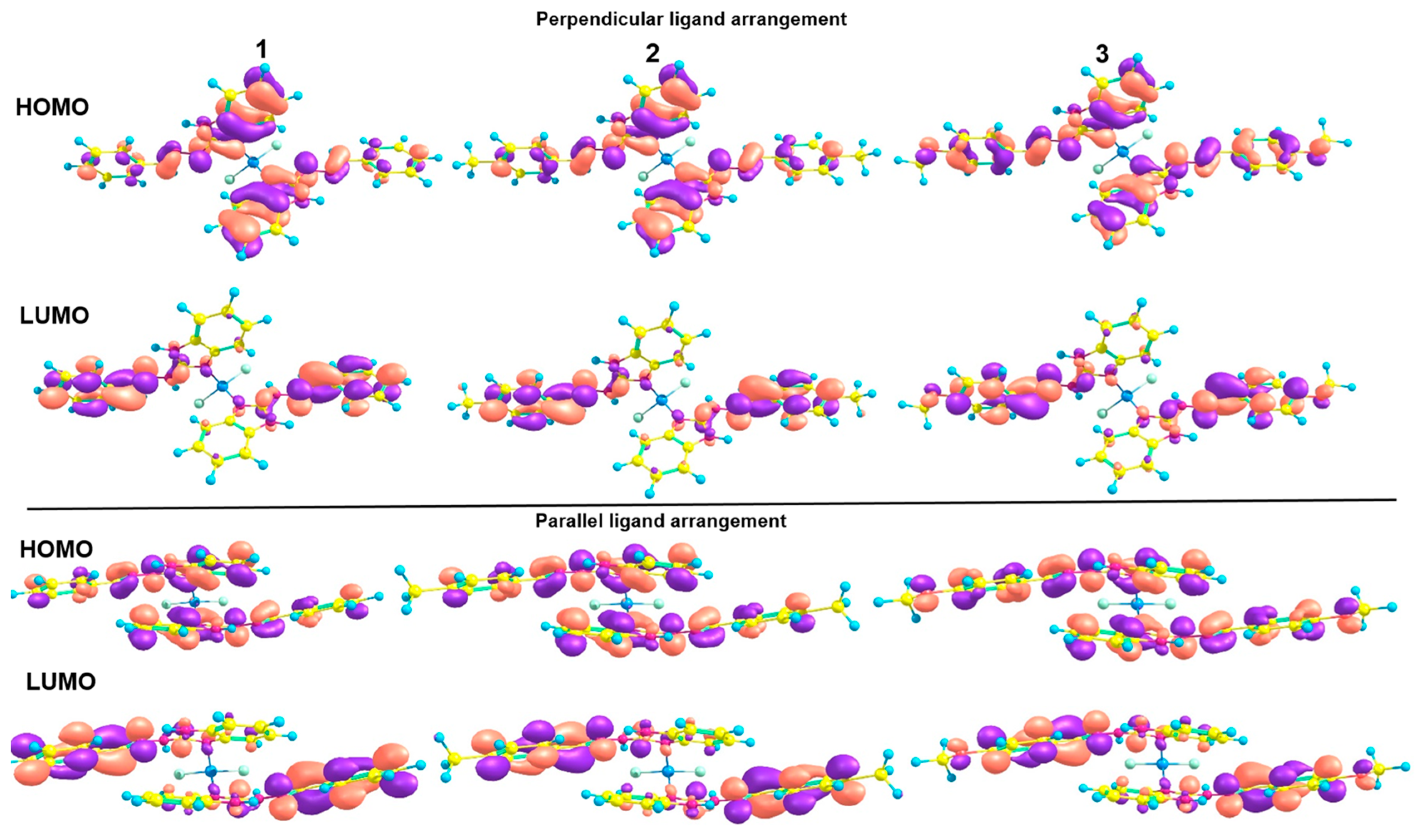
2.3. Theoretical Studies
2.4. Antiparasitic Activities
3. Materials and Methods
3.1. Synthetic Procedures
3.2. Computational Details
3.3. Anthelmintic Activity Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirai, Y. On Sense and Deform: Molecular Luminescence for Mechanoscience. ACS Appl. Opt. Mater. 2024, 2, 1025–1045. [Google Scholar] [CrossRef]
- Sagara, Y.; Yamane, S.; Mitani, M.; Weder, C.; Kato, T. Mechanoresponsive Luminescent Molecular Assemblies: An Emerging Class of Materials. Adv. Mater. 2016, 28, 1073. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, Z.; Teng, M.; Xu, Z.; Jia, X. Mechanically Induced Multicolor Change of Luminescent Materials. ChemPhysChem 2015, 16, 1811. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Lv, L.; Sun, L.; Hou, Y.; Hou, Z.; Chen, Z. Recent Advances in Mechanochromism of Metal-Organic Compounds. Front. Chem. 2022, 10, 865198. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kato, M. Stimuli-responsive Luminescent Copper(I) Complexes for Intelligent Emissive Devices. Chem. Lett. 2017, 46, 154. [Google Scholar] [CrossRef]
- Jin, M.; Ito, H. Solid-state luminescence of Au(I) complexes with external stimuli-responsive properties. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100478. [Google Scholar] [CrossRef]
- López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E. Luminescent aryl–group eleven metal complexes. Dalton Trans. 2017, 46, 2046. [Google Scholar] [CrossRef]
- Gusev, A.; Kiskin, M.; Braga, E.; Zamnius, E.; Kryukova, M.; Karaush-Karmazin, N.; Baryshnikov, G.; Minaev, B.; Linert, W. Structure and emission properties of dinuclear copper(I) complexes with pyridyltriazole. RSC Adv. 2023, 13, 3899. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, C.A.; Campos-Gaxiola, J.J.; Soto-Rojo, R.; Cruz-Enríquez, A.; Reynoso-Soto, E.A.; Miranda-Soto, V.; García, J.J.; Flores-Álamo, M.; Baldenebro-López, J.; Glossman-Mitnik, D. Synthesis of a New Dinuclear Cu(I) Complex with a Triazine Ligand and Diphenylphosphine Methane: X-ray Structure, Optical Properties, DFT Calculations, and Application in DSSCs. Inorganics 2023, 11, 379. [Google Scholar] [CrossRef]
- Malakhova, J.A.; Berezin, A.S.; Glebov, E.M.; Sannikova, V.A.; Vorob’ev, A.Y.; Pervukhina, N.V.; Naumov, D.Y.; Kolybalov, D.S.; Syrokvashin, M.M.; Vinogradova, K.A. Luminescent polymorphism of mononuclear Cu(I) complexes with pyrazolo [1,5-a][1,10]phenanthrolines. Inorganica Chim. Acta 2023, 555, 121604. [Google Scholar] [CrossRef]
- Girish, Y.; Prashantha, K.; Byrappa, K. Recent advances in aggregation-induced emission of mechanochromic luminescent organic materials. Emergent Mater. 2021, 4, 673–724. [Google Scholar] [CrossRef]
- Zheng, H.-W.; Wu, M.; Yang, D.-D.; Liang, Q.-F.; Li, J.-B.; Zheng, X.-J. Multistimuli Responsive Solid-State Emission of a Zinc(II) Complex with Multicolour Switching. Inorg. Chem. 2021, 60, 11609–11615. [Google Scholar] [CrossRef]
- Li, S.; Wu, M.; Kang, Y.; Zheng, H.W.; Zheng, X.J.; Fang, D.C.; Jin, L.P. Grinding-Triggered Single Crystal-to-Single Crystal Transformation of a Zinc(II) Complex: Mechanochromic Luminescence and Aggregation-Induced Emission Properties. Inorg. Chem. 2019, 58, 4626–4633. [Google Scholar] [CrossRef]
- Wang, D.; Shao, T.-F.; Li, S.-M.; Cao, W.; Yao, Q.; Ma, Y. A triphenylamine derivative with multistimuli responsive behavior and high-contrast vapochromism of its Zn(II) complex. Dye. Pigment. 2024, 226, 112114. [Google Scholar] [CrossRef]
- Li, J.-B.; Zheng, H.-W.; Wu, M.; Liang, Q.-F.; Yang, D.-D.; Zheng, X.-J. Mechanochromic Luminescence of Four Zn(II)/Cd(II) Complexes Based on Same Schiff-base Ligand with Different Coordination Modes. Cryst. Growth Des. 2021, 21, 6937–6946. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, X.; Xu, J.; Xia, L.; Wang, C.; Wu, H. A zinc(II) coordination polymer based on a flexible bis(benzimidazole) ligand: Synthesis, crystal structure and fluorescence study. Z. Naturforsch. 2020, 75, 1005–1009. [Google Scholar] [CrossRef]
- Temerova, D.; Kisel, K.S.; Eskelinen, T.; Melnikov, A.S.; Kinnunen, N.; Hirva, P.; Shakirova, J.R.; Tunik, S.P.; Grachova, E.V.; Koshevoy, I.O. Diversifying the luminescence of phenanthro-diimine ligands in zinc complexes. Inorg. Chem. Front. 2021, 8, 2549. [Google Scholar] [CrossRef]
- Milani, J.L.S.; Oliveira, I.S.; Dos Santos, P.A.; Valdo, A.K.S.M.; Martins, F.T.; Cangussu, D.; Das Chagas, R.P. Chemical fixation of carbon dioxide to cyclic carbonates catalyzed by zinc(II) complex bearing 1,2-disubstituted benzimidazole ligand. Chin. J. Catal. 2018, 39, 245. [Google Scholar] [CrossRef]
- Anichina, K.; Mavrova, A.; Vuchev, D.; Popova-Daskalova, G.; Bassi, G.; Rossi, A.; Montesi, M.; Panseri, S.; Fratev, F.; Naydenova, E. Benzimidazoles Containing Piperazine Skeleton at C-2 Position as Promising Tubulin Modulators with Anthelmintic and Antineoplastic Activity. Pharmaceuticals 2023, 16, 1518. [Google Scholar] [CrossRef]
- Argirova, M.A.; Georgieva, M.K.; Hristova-Avakumova, N.G.; Vuchev, D.I.; Popova-Daskalova, G.V.; Anichina, K.K.; Yancheva, D.Y. New 1H-benzimidazole-2-yl hydrazones with combined antiparasitic and antioxidant activity. RSC Adv. 2021, 11, 39848–39868. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Braga, E.; Zamnius, E.; Kiskin, M.; Kryukova, M.; Baryshnikova, A.; Minaev, B.; Baryshnikov, G.; Ågren, H.; Linert, W. Structure and excitation-dependent emission of novel zinc complexes with pyridyltriazoles. RSC Adv. 2019, 9, 22143–22152. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Díaz-Tinoco, M.; Romero, J.; Ortiz, J.V.; Reyes, A.; Flores-Moreno, R. A generalized any-particle propagator theory: Prediction of proton affinities and acidity properties with the proton propagator. J. Chem. Phys. 2013, 138, 194108. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
| Gaseous Phase | Methanol | |||
|---|---|---|---|---|
| Gaseous Phase | Methanol | λfl (nm) | λabs (nm) | λfl (nm) |
| 1 | 346 (f = 0.595) | 539 (f = 0.03) | 345 (f = 0.937) | 457 (f = 0.136) |
| 2 | 346 (f = 0.73) | 527 (f = 0.04) | 345 (f = 1.12) | 437 (f = 1.037) |
| 3 | 345 (f = 0.90) | 525 (f = 0.05) | 345 (f = 1.35) | 440 (f = 0.154) |
| Compounds | 24 h | 48 h | ||
|---|---|---|---|---|
| Dicrocoelium Lanceatum | Fasciola Hepatica | Dicrocoelium Lanceatum | Fasciola Hepatica | |
| L1 | 54 | 61 | 78 | 69 |
| 1 | 86 | 94 | 100 | 96 |
| L2 | 48 | 55 | 54 | 57 |
| 2 | 69 | 62 | 84 | 64 |
| L3 | 20 | 9 | 27 | 16 |
| 3 | 51 | 16 | 58 | 35 |
| Albendazole | 29 | 21 | 32 | 27 |
| Triclabendazole | 68 | 47 | 72 | 58 |
| ZnCl2 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusev, A.; Braga, E.; Kaleukh, A.; Baevsky, M.; Kiskin, M.; Linert, W. Multistimuli Luminescence and Anthelmintic Activity of Zn(II) Complexes Based on 1H-Benzimidazole-2-yl Hydrazone Ligands. Inorganics 2024, 12, 256. https://doi.org/10.3390/inorganics12090256
Gusev A, Braga E, Kaleukh A, Baevsky M, Kiskin M, Linert W. Multistimuli Luminescence and Anthelmintic Activity of Zn(II) Complexes Based on 1H-Benzimidazole-2-yl Hydrazone Ligands. Inorganics. 2024; 12(9):256. https://doi.org/10.3390/inorganics12090256
Chicago/Turabian StyleGusev, Alexey, Elena Braga, Alexandr Kaleukh, Michail Baevsky, Mikhail Kiskin, and Wolfgang Linert. 2024. "Multistimuli Luminescence and Anthelmintic Activity of Zn(II) Complexes Based on 1H-Benzimidazole-2-yl Hydrazone Ligands" Inorganics 12, no. 9: 256. https://doi.org/10.3390/inorganics12090256
APA StyleGusev, A., Braga, E., Kaleukh, A., Baevsky, M., Kiskin, M., & Linert, W. (2024). Multistimuli Luminescence and Anthelmintic Activity of Zn(II) Complexes Based on 1H-Benzimidazole-2-yl Hydrazone Ligands. Inorganics, 12(9), 256. https://doi.org/10.3390/inorganics12090256









