Abstract
The increasing presence of emerging contaminants (ECs) has attracted considerable attention due to their potential harm to human health and ecosystems. Graphitic carbon nitride (g-C3N4), a semiconductor devoid of metals, stands out due to its distinctive optical properties and strong resistance to chemical degradation, which holds significant promise in the photocatalytic degradation of ECs. However, the inherent limitations of g-C3N4, such as its reduced specific surface area and the swift recombination of photogenerated electron-hole pairs, have prompted extensive research on modification strategies to enhance its photocatalytic performance. Current research on g-C3N4-based materials is often constrained in scope, with most reviews focusing solely on modification strategies or its application in degrading a single category of emerging contaminants (ECs). In this review, a systematic overview of synthesis methods and advanced modification strategies for g-C3N4-based materials is discussed, highlighting their recent advances in the photocatalytic degradation of various ECs using g-C3N4-based materials, which underscores their potential for environmental remediation. Moreover, this article critically examines the current challenges and outlines future research directions, with particular emphasis on integrating artificial intelligence and machine learning to accelerate the development of g-C3N4-based photocatalysts and optimize degradation processes, thereby promoting their efficient application in the photocatalytic degradation of ECs.
1. Introduction
In recent years, the accelerated growth of global industry and the extensive usage of chemicals have resulted in a continuous increase in residual emerging contaminants (ECs) in the environment, which has garnered extensive attention from the international community [1]. The United Nations Environment Programme (UNEP) defines ECs as all pollutants stemming from human activities that are detectable in the environment but not fully regulated by laws, regulations, and standards, thus posing potential risks to human life and the ecological environment, which are recognized as issues demanding urgent global cooperation. These contaminants mainly originate from industrial effluents, domestic waste, agricultural runoff, and hospital discharges, and are generally classified into four groups: persistent organic pollutants (POPs), endocrine disrupting chemicals (EDCs), antibiotics, and microplastics (MPs) [2].
Conventional wastewater treatment methods (such as physical and biological methods) exhibit limitations in effectively removing ECs [3]. As an important component of advanced oxidation processes (AOPs), photocatalytic oxidation technology is widely recognized as an effective strategy for treating organic pollutants [4]. Owing to its advantages such as high solar energy utilization efficiency, no secondary pollution, and strong environmental applicability, this technology has shown broad prospects in the field of organic pollutant degradation, opening up a new approach for the treatment of ECs [5].
In recent years, g-C3N4 has garnered significant attention in photocatalysis due to its abundant availability, non-toxicity, chemical stability, and narrow bandgap (2.7 eV), allowing effective visible light harvesting [6]. Since its photocatalytic characteristics were first reported by Wang et al. in 2009 [7], research on g-C3N4 as a photocatalyst has rapidly expanded. It is extensively used across diverse fields, including water splitting, CO2 reduction, and organic pollutant degradation. This characteristic arises from the sp2 hybridization of carbon and nitrogen atoms in g-C3N4, creating a π-conjugated electronic system [8], and is based on the tri-s-triazine ring as fundamental structural unit and is composed solely of C and N atoms. It demonstrates high thermal stability (up to 600 °C), facile synthesis, and low production cost, making it a practical photocatalyst [9]. Furthermore, g-C3N4 has a suitable band structure (conduction band at −1.3 V and valence band at +1.4 V vs. normal hydrogen electrode (NHE)), which enables photocatalytic activity under visible light and provides strong reducibility.
However, g-C3N4 has intrinsic limitations, including a small specific surface area [10], low visible-light utilization efficiency, and rapid recombination of photogenerated electron–hole pairs [11]. Despite its intrinsic limitations, its tunable structure allows its performance to be improved through strategies including morphological control, elemental doping, defect creation, and heterojunction construction. Modified g-C3N4 demonstrates significantly enhanced photocatalytic degradation efficiency for emerging contaminants, with notable advantages particularly in broadening the spectral response range, strengthening reactive oxygen species (ROS) generation efficiency, and augmenting specific adsorption capacity.
To elucidate recent progress in g-C3N4 synthesis and modification strategies, this review systematically examines the development of high-performance, cost-effective g-C3N4-Based Materials (CNMBs) for the remediation of ECs (Figure 1). This review also evaluates the challenges associated with g-C3N4-based photocatalytic technology and explores its potential in solar-driven applications. Finally, recommendations are provided to improve ECs removal and to maximize its practical efficiency, offering theoretical support for advancing environmental governance.
Figure 1.
Synthesis, modification, and application of carbon nitride-based materials.
2. Synthesis of g-C3N4-Based Materials
g-C3N4 can be readily synthesized through condensation and addition polymerization at various temperatures, employing organic precursors like melamine, thiourea, and urea, which contain carbon, nitrogen, and sulfur. Common approaches for synthesizing g-C3N4 encompass thermal polymerization, hydrothermal synthesis, sol–gel processing, chemical vapor deposition, and microwave-assisted synthesis, providing multiple pathways for material preparation. By adjusting precursors and reaction conditions, these methods enable precise modulation of the structural and functional properties of g-C3N4 to suit specific application requirements. A detailed comparison and summary of these preparation methods are provided in Table 1.
2.1. Thermal Polymerization (TP)
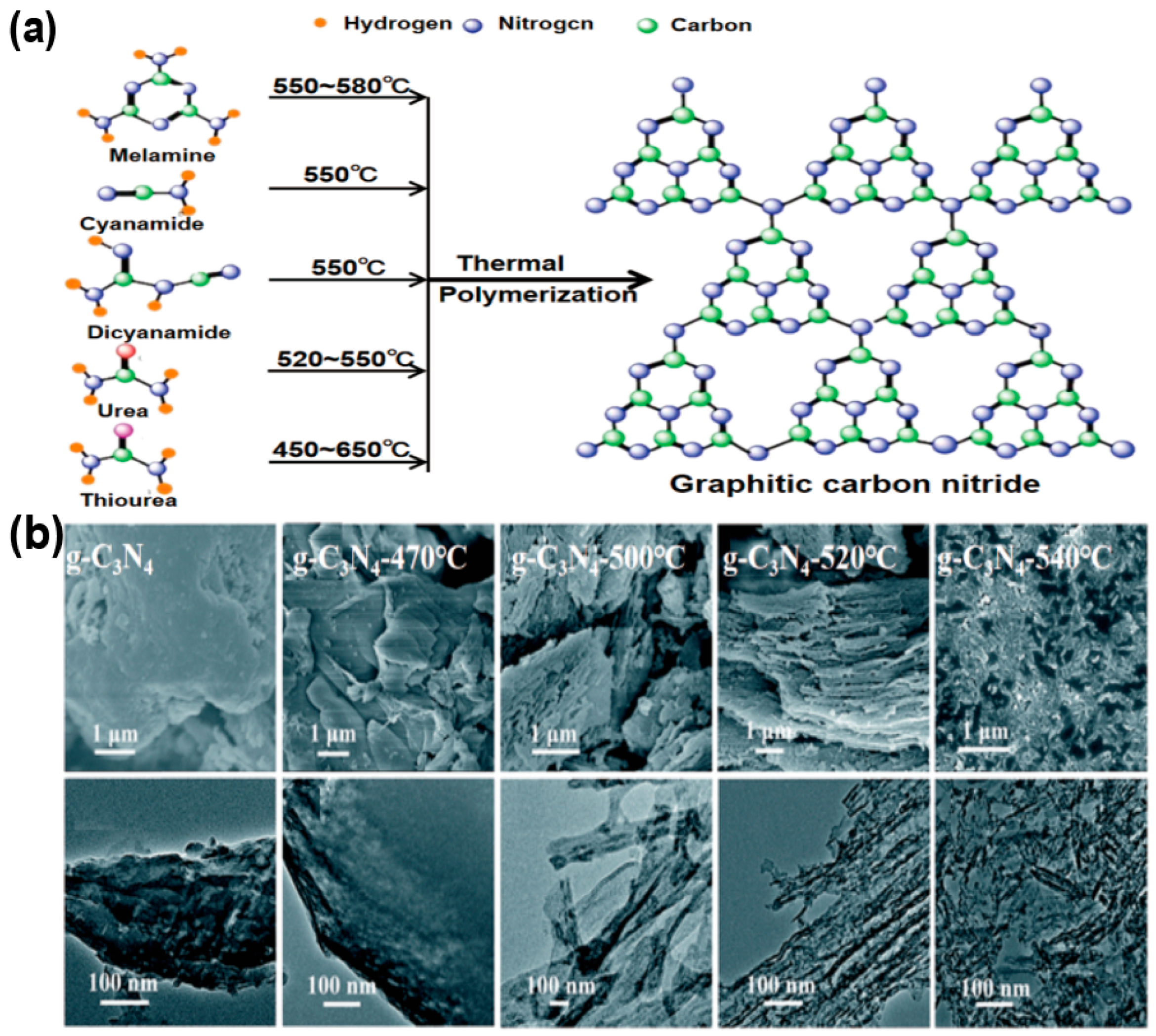
Thermal polymerization is the dominant method for synthesizing g-C3N4, where carbon-, nitrogen-, sulfur-rich organic precursors such as melamine [12], cyanamide [13], dicyandiamide [14], urea [15] and thiourea [16] undergo polycondensation reactions under thermal treatment (Figure 2a). The chemical properties of the precursors directly influence the polymerization pathway, morphology, and porosity of g-C3N4. For instance, melamine tends to form dense layers with small surface area [12], and urea [15] leads to the creation of porous structures that enhance surface area and charge separation, while thiourea [17] and dicyandiamide [18] introduce defects that extend visible light response. Moreover, the polymerization temperature significantly influences the crystallinity, defect formation, and electronic band structure, thereby enabling controlled tuning of g-C3N4 morphology (Figure 2b). Gu et al. obtained bulk-like, lamellar, nanotubular, and nanodot structures of g-C3N4 through temperature control [19], whereas Mo et al. [20] prepared nanosheets and bulk forms using urea as the precursor. Reaction atmosphere (e.g., N2 or Ar) [21] influences doping and defects, inhibiting oxidation, maintaining stoichiometry, and potentially introducing carbon vacancies that modify energy bands, light absorption, and charge separation.
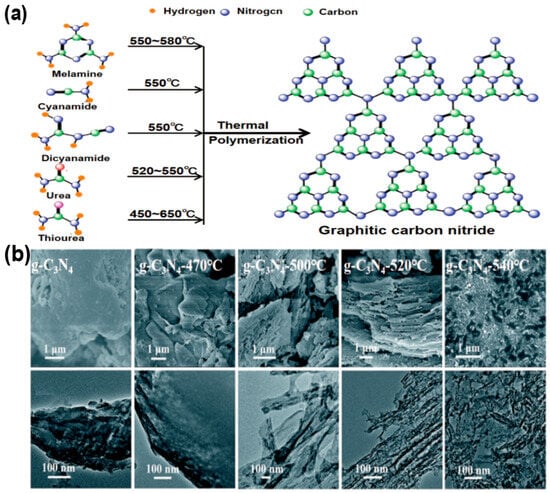
Figure 2.
(a) A schematic diagram visually represents the thermal polymerization process employed to synthesize g-C3N4 at different temperatures (C, N, H atoms denoted with green, blue and orange balls), (It is adapted from Ref. [22]). (b) SEM and TEM images of bulk g-C3N4, g-C3N4-470, g-C3N4-500, g- g-C3N4-520, and g- g-C3N4-540, respectively [19]. (reproduced with permission from Ref. [19], copyright 2015 RSC Advances).
2.2. Hydrothermal Method (HT Method)
Hydrothermal synthesis of g-C3N4 occurs at relatively low temperatures and pressures, allowing precise control over morphology, defects, and surface properties by adjusting solvents, precursors, temperature, and reaction time [23]. Hydrothermal synthesis, aided by solvents or templates, produces nanosheets, quantum dots, and porous structures, and facilitates the incorporation of heteroatoms or composites in the liquid phase. Additionally, the hydrothermal environment also promotes hydroxyl and amino groups, enhancing hydrophilicity and chemical reactivity [23]. Zhang et al. [24] synthesized C-doped g-C3N4 through hydrothermal synthesis, to optimize electron transport and photocatalytic performance. Carbon doping reduced the bandgap from 2.6 eV to 2.0 eV, red-shifting the UV-vis absorption edge to expand visible light response, prolonged photogenerated carrier lifetime, and enhanced separation efficiency and visible-light photocurrent, and thereby boosted the degradation efficiency of 4-nitrophenol
2.3. Sol–Gel Method (Sol–Gel)
The sol–gel method enables precise control over the structural, optical, and morphological characteristics of g-C3N4 during its preparation, which is crucial for enhancing photocatalytic efficiency and achieving sustainable environmental reactions [25]. This method is favored for its high purity, excellent uniformity, low cost, and room-temperature processing capability, making it one of the effective approaches for fabricating g-C3N4 and its nanocomposites. In the sol–gel method, precursors like metal alkoxides and salts experience hydrolysis and polycondensation, forming a sol that transitions into a 3D gel structure. Two fundamental reaction mechanisms, hydrolysis and condensation, enable precise control over M-OH-M and M-O-M (oxygen-based bonding) [26]. Developing g-C3N4 photocatalysts with controllable structural, optical, and morphological properties through this method will enable efficient photocatalysis for sustainable reaction environments. Kailasam et al. [27] synthesized mesoporous carbon nitride with uniform 12 nm spherical pores via this method, achieving 3.2 times higher photocatalytic hydrogen evolution than bulk graphitic carbon nitride. Hollmann et al. [28] produced SG-CN photocatalysts with 20 times higher hydrogen evolution rate than bulk CN, attributed to porous framework, partial crystallographic disorder, and extensive surface area enabling rapid electron transport and proton adsorption.
2.4. Microwave-Assisted Synthesis (MAWA)
Microwave-assisted synthesis (MAWA) for g-C3N4, valued for rapidity and efficiency, uses microwave dielectric heating of precursors to quickly construct composites, producing highly crystalline g-C3N4 in minutes—greatly boosting preparation efficiency versus traditional high-temperature, long-duration methods. Chen et al. [29] applied it to dope phosphorus into g-C3N4 and optimized catalyst dosage via ion exchange, with advantages like instantaneous volumetric heating, faster reactions, and higher precision. Liu et al. [30] developed a microwave-enhanced molten salt technique, using melamine to fabricate isotype heterojunctions from triazine-heptazine derivatives in g-C3N4 which achieved a visible-light photocatalytic hydrogen evolution rate of 1480 μmol g−1 h−1, outperforming er-ms-g-C3N4 by 5 times, er-g-C3N4 by 15 times, and mw-g-C3N4 by 23 times. Integrating microwave heating with molten salt polycondensation efficiently and versatilely enables rapid synthesis of g-C3N4-derived photocatalysts, enhancing productivity and consistency for large-scale photocatalytic applications.
2.5. Chemical Vapor Deposition (CVD)
Chemical vapor deposition (CVD) is highly effective in synthesizing carbon nitride composite photocatalysts. This method involves nitrogen-containing precursors (e.g., NH3, N2), carbon sources (e.g., C2H2, CH3OH), and carrier gases (e.g., Ar, N2). These substances undergo pretreatment (such as high-temperature gas infusion) and then undergo controlled decomposition at high temperatures in a reaction chamber. High temperatures and appropriate reaction times are beneficial for improving the yield and purity of the products. Wang et al. [31] prepared irregular mesoporous g-C3N4 nanotubes (MCN) via vapor deposition using a modified halloysite template. Compared with traditional bulk g-C3N4, these nanotubes have smaller sizes, denser stacking of structural units, and larger specific surface areas. This reduces light-induced electron-hole recombination, enhances photon-capturing ability, and increases the visible light-induced photocatalytic hydrogen production efficiency by 14.3 times.

Table 1.
Comparison and Summary of g-C3N4 Preparation Methods.
Table 1.
Comparison and Summary of g-C3N4 Preparation Methods.
| Preparation Method | Advantages | Disadvantages | Product Morphologies | Application | Ref. |
|---|---|---|---|---|---|
| Thermal polymerization | Cost-efficient equipment, mature scalable process, high-purity product output | severe aggregation, low specific surface area, crystallinity, poor charge transfer efficiency, inadequate morphology control, high energy | Bulk/granular morphologies | photocatalysts, electrodes, catalyst supports | [32] |
| Hydrothermal method | Low-temperature synthesis (<200 °C): well-crystallized products with tunable morphologies | prolonged reaction duration; solvent/impurity residues | Nanosheets, nanorods, hollow spheres | photocatalytic water splitting, CO2 reduction, biosensing apps | [33,34] |
| Sol–gel method | Low-temp synthesis (<200 °C), mesoporous/porous architectures, homogeneous doping, precise structural control, compositional homogeneity | Intricate multi-step process, drying-shrinkage cracking, scalability challenges, high precursor costs | Mesoporous, Porous particles | Composite coatings, thin films fabrication, sensors, anticorrosive apps, flexible electronics | [35] |
| Microwave-assisted synthesis | Rapid reaction kinetics, low energy consumption, well-dispersed products | Elusive process parameter optimization | Nanoparticles, Porous architectures | pollutant degradation, energy storage, high-throughput lab studies | [36] |
| Chemical vapor deposition | High-purity dense films: precise thickness, composition control, large-area fabrication | Costly vacuum equipment, excessive energy use, low yields | Thinfilms, Coatings, Nanoarrays | High-quality thin films fabrication, electronic devices, optical coatings, semiconductor heterojunctions | [37] |
3. Modification Strategies for g-C3N4-Based Materials
Enhancement of g-C3N4 photocatalytic activity requires effective light harvesting, efficient separation and migration of charge carriers, increased surface area, and abundant active sites. Morphology control, elemental doping, defect engineering, and heterostructure formation are key strategies to modulate its electronic and physicochemical properties and improve photocatalytic efficiency.
3.1. Morphology Control
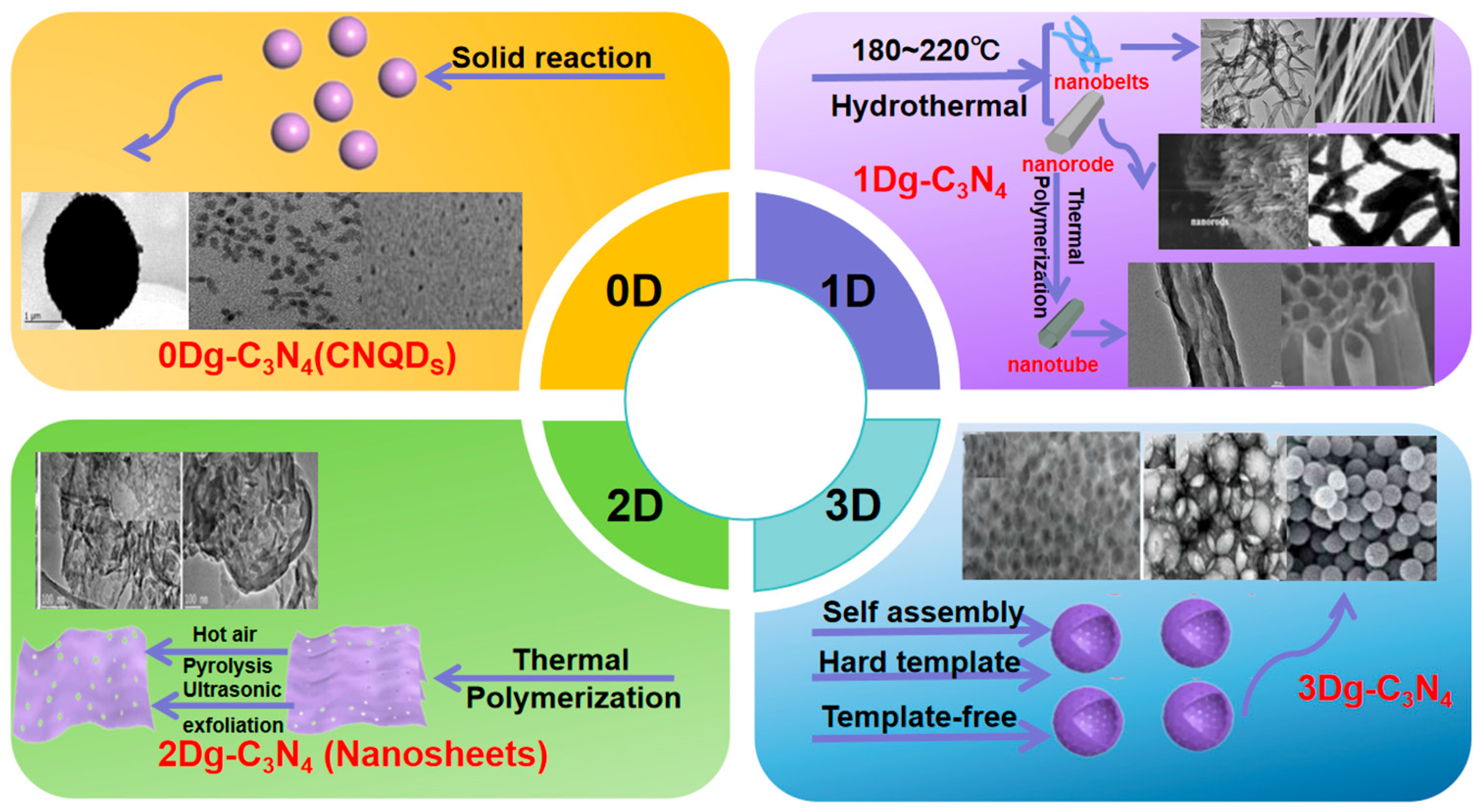
Photocatalytic activity correlates with catalyst dimensions, surface morphology, and structure, enhanced by increasing surface area and accessible active sites. This promotes light reflection/scattering, shortens charge carrier travel distance, and boosts efficiency [38]. Soft and hard templating and exfoliation regulate g-C3N4 morphology, producing 0D–3D structures that aid charge transport and improve properties (Figure 3) [39]. Wang et al. [13] used silica templates for mesoporous g-C3N4 (HCNS), enhancing visible-light hydrogen evolution via optimized band structure and light harvesting. Supramolecular approaches with triazine address hollow structure collapse, yielding larger surface area, better light harvesting, and more reactive sites, with internal reflection boosting charge generation. Dong et al. [40] prepared ultra-thin nanosheets via HCl-assisted exfoliation, where protonation dissociates bulk material and etching induces defects to improve charge separation. Gu et al. [41] created hierarchical nanoporous microspheres via template-free solvothermal treatment and calcination, achieving broader light absorption and better carrier transport to enhance hydrogen production.
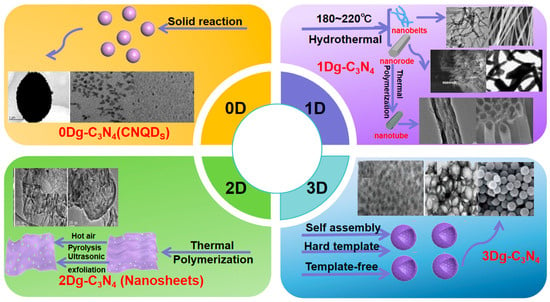
Figure 3.
The applications of g-C3N4-based materials with different dimensions (from 0D to 3D). (Adapted with permission from Ref [42]).
3.2. Elemental Doping
Metal or non-metal doping enhances g-C3N4 photocatalytic performance by modifying electronic and band configurations, boosting light harvesting, and optimizing charge separation. Metal doping narrows the bandgap via donor and acceptor states to broaden light absorption. Cation doping includes hole doping (e.g., Mn2+ coordinating with nitrogen) (Figure 4a) and interlayer doping (e.g., K+ bridging layers) (Figure 4b), which enhance the carrier mobility, charge transport, and visible light capture [43]. Xiong et al. [44] showed K doping narrows the bandgap and improves oxidation strength.
Non-metal doping, differing from metal doping, forms covalent bonds and alters electron distribution via charge polarization, dispersing π-electrons, reducing recombination, and expanding light absorption. As illustrated in Figure 4c, specific dopants such as P [45], O [46], N [47], B [48], S [49] and C [50] along with halogens [51] showed improved activity, particularly in hydrogen generation and pollutant breakdown [52].
Dual or multi-element co-doping extends visible-light absorption and promotes charge separation. Chen et al. [53] synthesized porous g-C3N4 with Rh-P modification, achieving 33 times higher hydrogen yield than unaltered g-C3N4, significantly boosting activity.
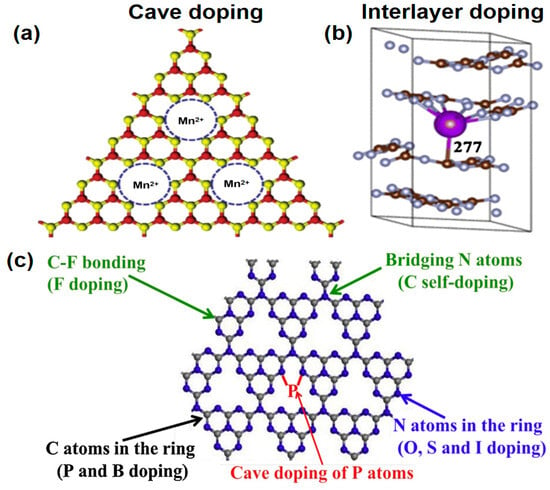
Figure 4.
(a) Potential dopant candidate locations within the monolayer of g-C3N4: Metal ion cave doping, color scheme: C, red; N, yellow. (Adapted with permission from Ref. [54]). (b) Metal ion interlayer doping. (reproduced with permission from Ref. [44], copyright 2016 ACS Publications). (c) Possible substitution sites for non-metal doping. (Adapted with permission from Ref. [55]).
Figure 4.
(a) Potential dopant candidate locations within the monolayer of g-C3N4: Metal ion cave doping, color scheme: C, red; N, yellow. (Adapted with permission from Ref. [54]). (b) Metal ion interlayer doping. (reproduced with permission from Ref. [44], copyright 2016 ACS Publications). (c) Possible substitution sites for non-metal doping. (Adapted with permission from Ref. [55]).
3.3. Defect Engineering
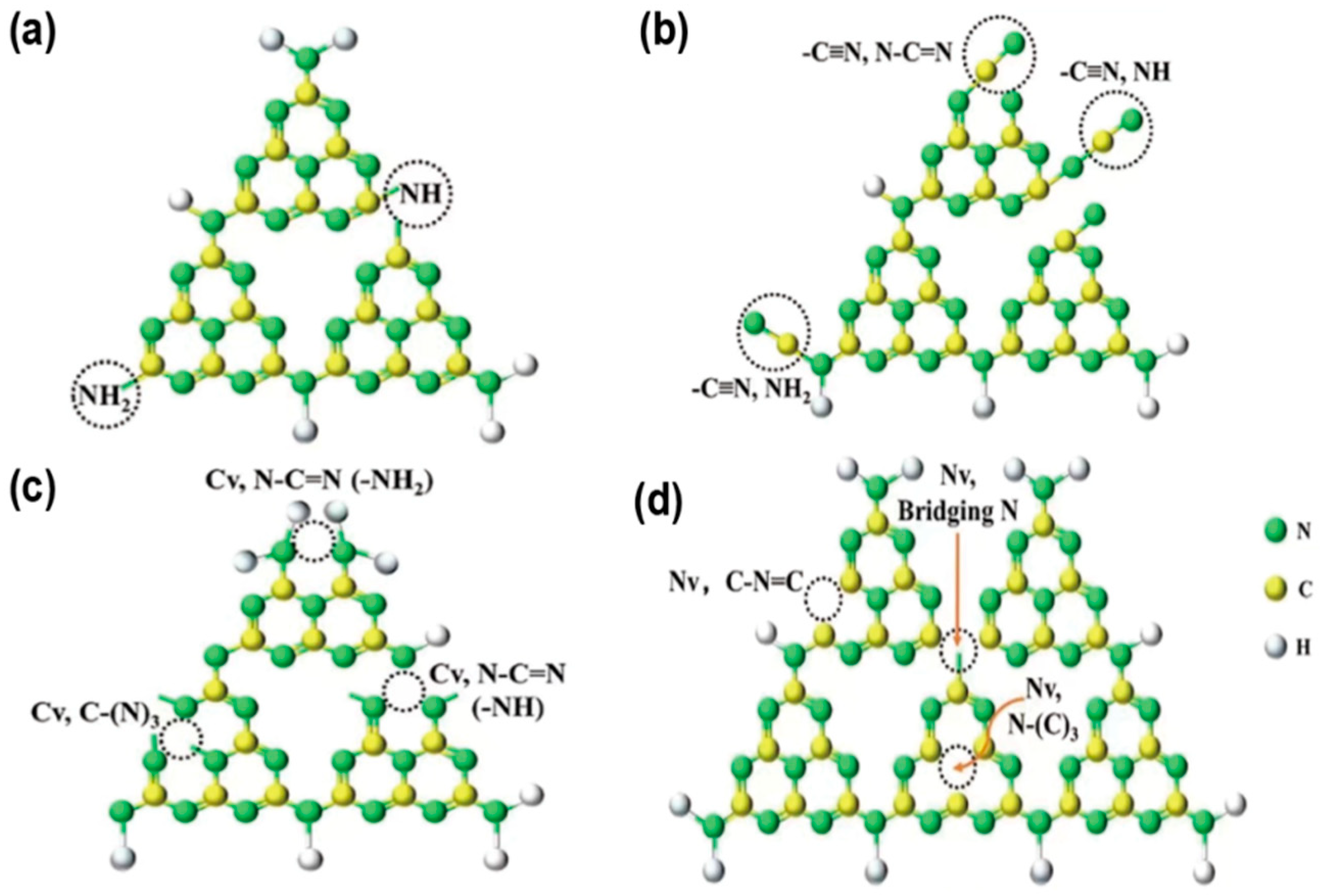
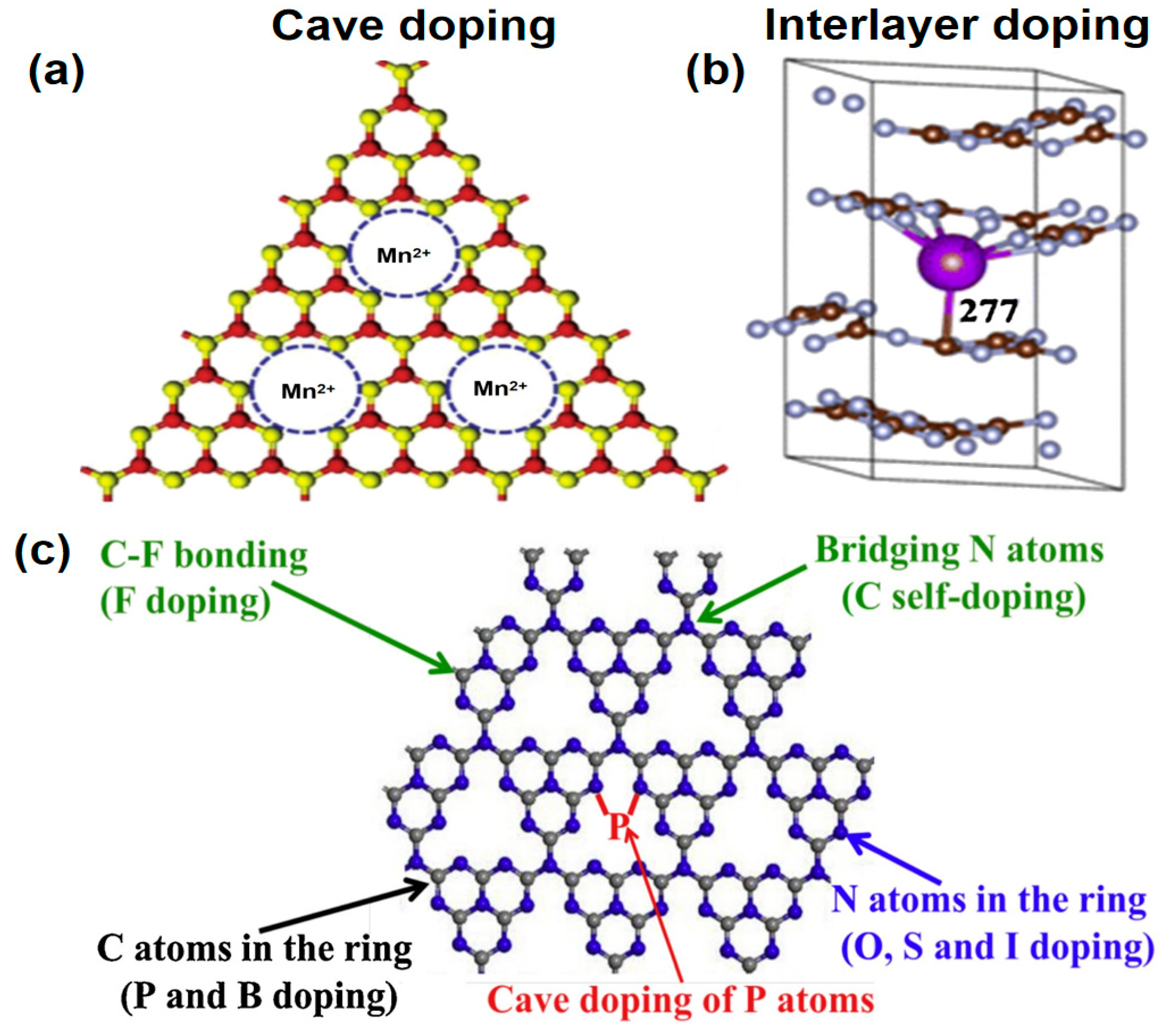
Defect engineering, by introducing specific types of defects, can change the periodicity of the g-C3N4 crystal structure. These defects act as trapping centers, modify the band structure, reduce electron-hole recombination, and enhance charge separation. They create new electronic states in the bandgap, broadening photon harvesting range. Typical defects include amino defects, cyano groups (−C≡N), carbon vacancies (CVs), and nitrogen vacancies (NVs) (Figure 5a–d) [56]. To accurately analyze the content of these defects, X-ray Photoelectron Spectroscopy (XPS) is the most widely used for amino defects and cyano groups. For instance, the presence of cyano groups can be identified by a characteristic peak at approximately 284.8 eV in the C 1 s spectrum, while amino defects correspond to a signal at around 399.8 eV in the N 1 s spectrum [21]. Carbon vacancies (CVs) and nitrogen vacancies (NVs) both exhibit typical paramagnetism due to the presence of unpaired electrons resulting from unsaturated electron orbitals. In this case, Electron Paramagnetic Resonance (EPR) spectroscopy serves as an effective complement to XPS for the quantitative analysis of vacancy defects. Such vacancies produce a characteristic signal in EPR measurements, and the absolute concentration of CVs or NVs can be determined [57].
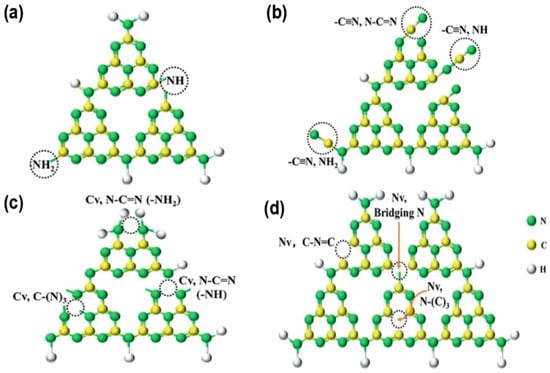
Figure 5.
Typical positions of the (a) amino defects, (b) cyano defects, (c) C vacancies and (d) N vacancies in g-C3N4. (reproduced with permission from Ref. [56], copyright 2021 Elsevier).
Lin et al. [58] synthesized dual vacancy carbon nitride (DACN) with N and O vacancies via thermal polymerization, generating more unpaired electrons in aromatic heterocycles, to reduce electron-hole recombination and enhance visible light absorption. Visible light photocatalysis improves 11.7 times vs. pure g-C3N4. Liao et al. [59] prepared p-n homojunction g-C3N4 with abundant nitrogen vacancies via hydrothermal-calcination, reducing electron-hole recombination and enhancing photon harvesting, boosting visible light photocatalysis 8.7 times vs. conventional g-C3N4, due to the expanded visible response, large surface areas, and p-n homojunction. Yang et al. [60] made nitrogen vacancy-containing CN (NVs-PCN), where nitrogen vacancies regulate electronic structure, promote charge separation, and prolong carrier lifetime, leading to CO2 photocatalytic reduction 279 times that of pristine PCN. Chang et al. [61] prepared AT-CN with carbon and nitrogen vacancies via thermal polymerization, where nitrogen vacancies act as active adsorption sites by extending light absorption, reducing charge recombination, and increasing surface area. Carbon vacancies dissociate C-N bonds in tri-s-triazine by generating unsaturated nitrogen coordination, capturing electrons to reduce recombination, and enhancing hydrophilicity, thus improving visible light photocatalysis.
3.4. Heterostructure Construction
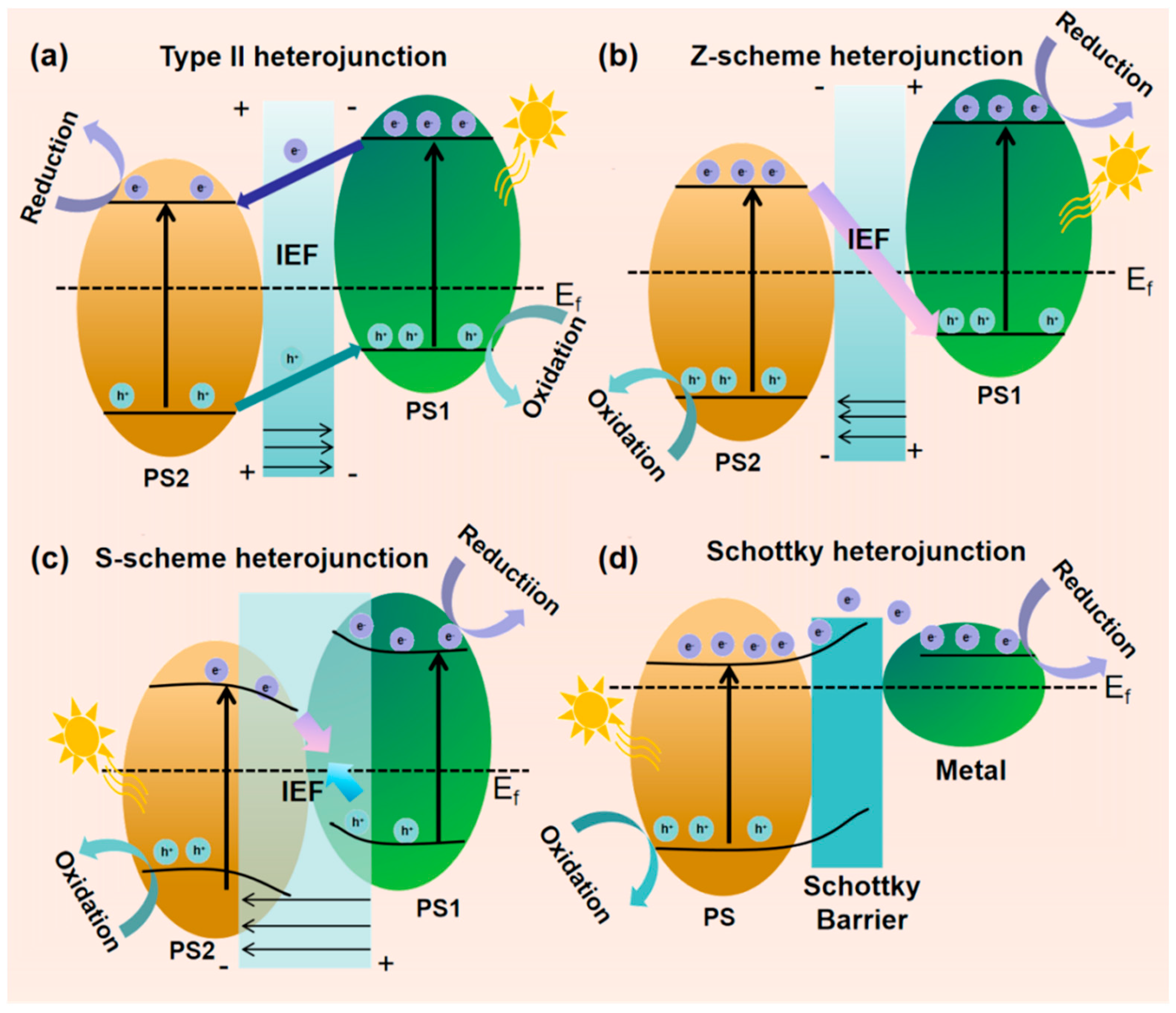
To design high-performance g-C3N4-based composite photocatalysts, materials need a narrow bandgap for visible light harvesting and suitable band positions for redox reactions. Single semiconductors struggled to meet these requirements, while heterojunctions addressed these by leveraging band structure and width differences to promote electron-hole migration and separation at the interface [62], with types including Type-II, Z-scheme, S-scheme, and Schottky heterojunctions [63].
Type II heterojunctions achieve band matching by combining two semiconductors with staggered band structures (designated as PS1 (right) and PS2 (left)) [64]. In this system, the CB position of PS1 is higher than that of PS2, while the VB position of PS2 is deeper than that of PS1, causing electrons to accumulate in the CB of PS2 for reduction reactions, and holes to gather in the VB of PS1 for oxidation reactions (Figure 6a). However, Type II heterojunctions have inherent defects such as difficult coordination of charge transfer impedance and weakening of redox capabilities.
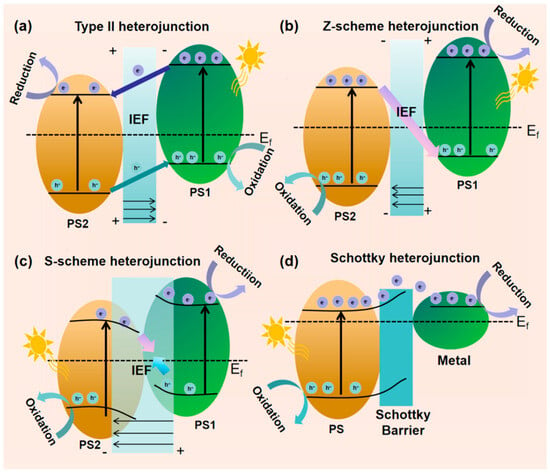
Figure 6.
Processes of charge carrier separation in diverse heterojunctions under light exposure: (a) Type-II; (b) Z-scheme; (c) S-scheme; (d) Schottky heterojunction. (It is adapted from Ref. [63]).
Z-scheme heterojunctions enhance system redox capability by integrating two photocatalytic systems: materials with negative CB potentials (e.g., g-C3N4, Cu2O, etc.) as reduction sites (photosystem I) and those with positive VB potentials (e.g., BiVO4, WO3, etc.) as oxidation sites (photosystem II). They form a unique electron transfer pathway via mediators (Figure 6b), maintaining strong redox capacity of photogenerated electrons and holes, an advantage over Type II heterojunctions. Wisal Muhammad et al. [65] synthesized a 2D/2D NiFe2O4/g-C3N4 Z-scheme heterojunction in the optimized 6NFO/CN, electrons from NFO CB and holes from CN VB are effectively separated via direct Z-scheme transfer, suppressing recombination, significantly enhancing organic pollutant degradation efficiency under visible light, and outperforming pure g-C3N4.
In 2020, Prof. Yu’s team [66] pioneered the S-scheme charge transfer mechanism, derived from Type II heterojunctions and Z-scheme systems. Its core involves staggered band alignment between reducible (PS1) and oxidizing (PS2) photocatalysts (Figure 6c): PS1 band structure (Fermi level, CB, VB) is higher than PS2, driving electron migration to PS2. Contact forms a built-in electric field (IEF) from PS1 to PS2, inducing band bending. Photoexcitation makes PS2 CB electrons transfer to PS1 VB for recombination, with some jumping to PS1 high CB to enhance reduction. Regulated by IEF, band gradient, and interfacial interactions, this retains high-activity carriers, balancing redox driving force and charge separation efficiency. Zhang et al. [67] engineered S-scheme ZIS/CPCN via calcination and hydrothermal methods, using crystalline carbon nitride. It removes quinolones efficiently via internal electric field and low-energy charge recombination, degrading 86% of levofloxacin in 3 h. Chahkandi et al. [68] synthesized HAp@Bi2S3 core–shell structures, loading them onto g-C3N4 to form dual S-scheme HAp@Bi2S3/g-C3N4. This improves charge separation/transfer, extends carrier lifetime, and enhances activity, degrading 98.2% of metformin in 60 min.
g-C3N4 can host noble metal nanoparticles (NPs) to form Schottky junctions, with the surface plasmon resonance (SPR) effect enhancing interfacial localized electric fields [69]. The combination generates a Mott-Schottky barrier due to work function differences, enabling photoexcited electrons to migrate from g-C3N4 CB to the metal Fermi level to reduce electron-hole recombination. Additionally, the photothermal effect enhances light-to-energy conversion and charge carrier utilization (Figure 6d), improving light uptake and photo-generated charge separation to maximize photocatalytic performance.
4. Applications of g-C3N4-Based Materials in the Control of ECs
Persistent organic pollutants (POPs), endocrine-disrupting chemicals (EDCs), antibiotics, and microplastics (MPs) are four major ECs of global concern with significant environmental and ecological risks. g-C3N4-based photocatalysts have demonstrated exceptional potential for their remediation, where photoinduced ROS (•OH, •O2−) mineralize ECs into benign CO2 and H2O through advanced oxidation processes, providing an environmentally sustainable solution. Table 2 summarizes the ROS in the photocatalytic degradation process of g-C3N4-based materials, the corresponding ROS detection methods, and the associated possible pollutant degradation pathways.

Table 2.
Photocatalytic Degradation of g-C3N4-Based Materials: ROS, Detection Methods, and Degradation Pathways.
4.1. POPs Degradation
POPs have garnered widespread international attention due to their environmental persistence, bioaccumulation potential, long-range transport capabilities, and posing threats to human health and ecosystems [75]. POPs include precursors such as dichlorodiphenyltrichloroethane (DDT), polychlorinated biphenyls (PCBs), dioxins, and per-and polyfluoroalkyl substances (PFAS). Owing to their inherent chemical stability, POPs exhibit remarkable resistance to conventional degradation methods, including chemical oxidation and biological treatment. g-C3N4-based photocatalysts can mineralize these pollutants by harnessing the power of light by using electrons and holes to drive oxidation-reduction reactions to effectively break down POPs. This eco-friendly approach is widely regarded as a sustainable solution for eliminating POPs from our surroundings.
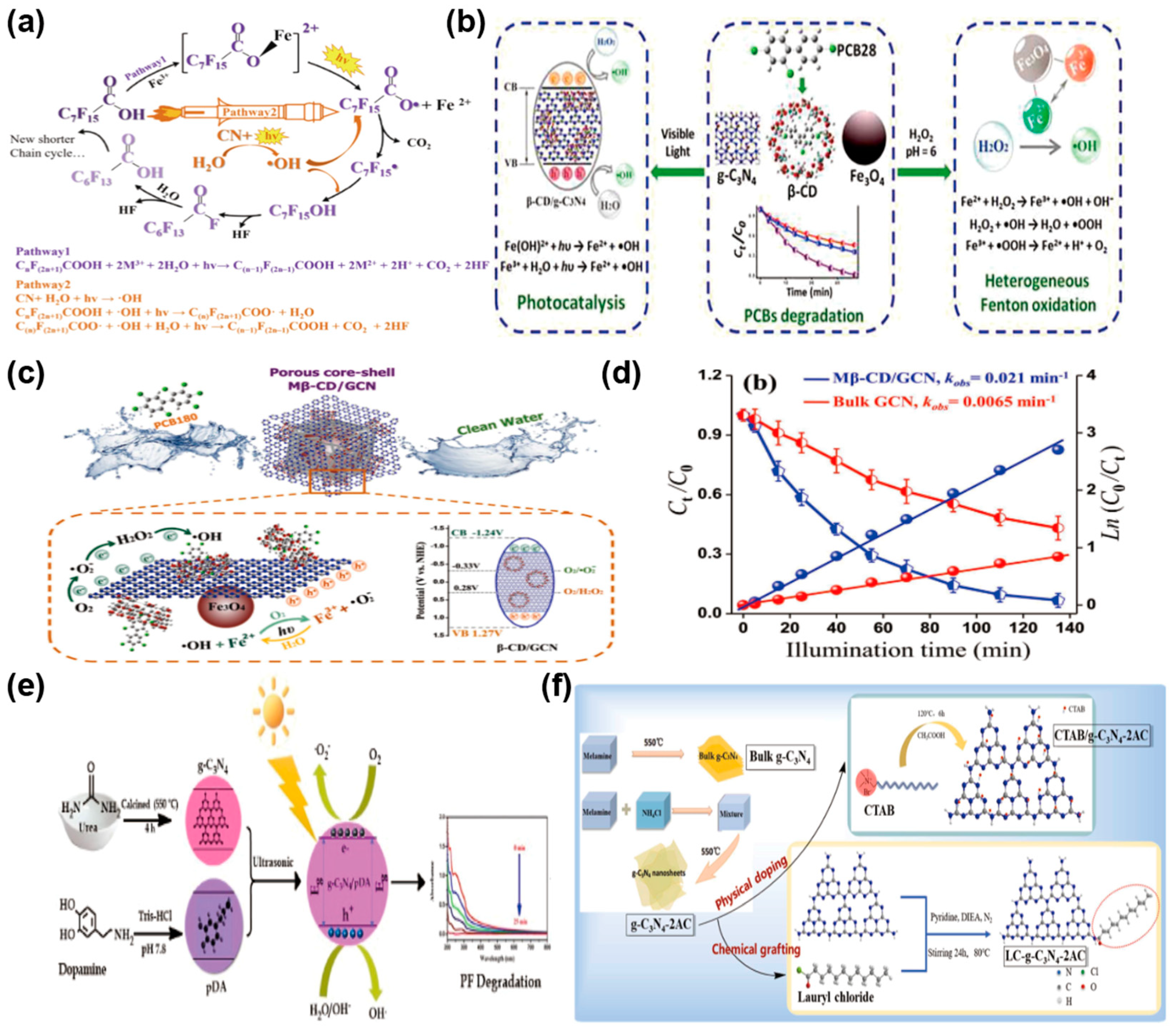
Li et al. [76] achieved efficient degradation of perfluorooctanoic acid (PFOA) under visible light by combining g-C3N4 with Fe3+. This system achieved 80% degradation of PFOA within 20 h under visible light irradiation. Research indicated that PFOA degradation efficiency correlates with the quantity of g-C3N4, and the g-C3N4/Fe3+ system persistently produces strong oxidative •OH radicals to degrade PFOA (Figure 7a), improving photocatalytic efficiency. Wang et al. [77] synthesized a magnetic Fe3O4@β-CD/g-C3N4 nanocomposite photocatalyst for efficient removal of PCBs from wastewater. Fe3O4@β-CD/g-C3N4 managed to degrade six PCBs with efficiencies ranging from 77% to 98% within 55 min and kept its good stability even after six degradation cycles (Figure 7b). Wang et al. [78] developed an innovative photocatalyst with a porous core–shell design, consisting of magnetic β-cyclodextrin integrated with g-C3N4 (Mβ-CD/GCN). This advanced material was specifically tailored for harnessing solar energy to break down PCBs through photocatalytic degradation. Specifically, Fe2+ and Fe3+ in Fe3O4 react with H2O2 and water to generate •OH, while Fe2+ reacts with O2 to produce •O2− and Fe3+. The reversible Fe(III)/Fe(II) redox cycle minimizes iron loss and enhances radical generation. With the synergistic effects of h+, •O2−, and •OH, PCB180 was degraded by 93% in 135 min under light, exhibiting a first-order reaction rate constant 3.23 times higher than that of GCN. PCB180 was ultimately mineralized or transformed into degradation intermediates (Figure 7c,d). Sathish Kumar et al. [79] engineered a nanocomposite by integrating polydopamine (pDA) into g-C3N4 (g-C3N4/pDA) using a hydrothermal synthesis technique. This innovative material was designed to enhance the photocatalytic breakdown of the organophosphorus pesticide profenofos (PF) when exposed to UV light. The pDA establishes a stable bond with the g-C3N4 surface via covalent linkages and π-π interactions. Specifically, the catechol groups in pDA have a strong affinity for the NH2 groups on g-C3N4. By forming strong covalent bonds, a continuous polymer layer is created. π-π interactions with pDA on the g-C3N4 surface significantly reduce electron-hole recombination and enhance the transport of photoexcited charge carriers. This process leads to an impressive photocatalytic degradation rate, reaching 96.4% efficiency for PF in just 25 min (Figure 7e). Su et al. [80] synthesized photocatalytic nanosheets with a high specific surface area through morphology control and developed amphiphilic groups for surface modification, endowing the photocatalyst with Pickering particle functionality (LC-g-C3N4-2AC) (Figure 7f). The engineered photocatalyst firmly anchors itself at the micron-scale interface of the oil-water emulsion, dramatically boosting mass transfer efficiency within the biphasic system. Moreover, the electron-hole recombination in g-C3N4-2AC, which is synthesized by exfoliating bulk g-C3N4, is markedly reduced. This suppression enhances the generation of ROS at the surface reaction sites, thereby improving photodegradation performance, which degrades oily wastewater into various organic acids and aldehydes, eventually oxidizing them into CO2, H2O, and other small molecules. Employing this straightforward PE photocatalytic system, the degradation rate of n-hexane model oily wastewater attained 97.2%. By combining photocatalytic and polyethylene catalytic technologies, researchers developed a stable polyethylene photocatalytic system to degrade organic pollutants in oily wastewater, offering a novel approach for developing efficient g-C3N4-based photocatalysts with potential applications in degrading persistent organic pollutants in aquatic environments. The performance of these various g-C3N4-based photocatalysts for the elimination of POPs is comprehensively summarized in Table 3.
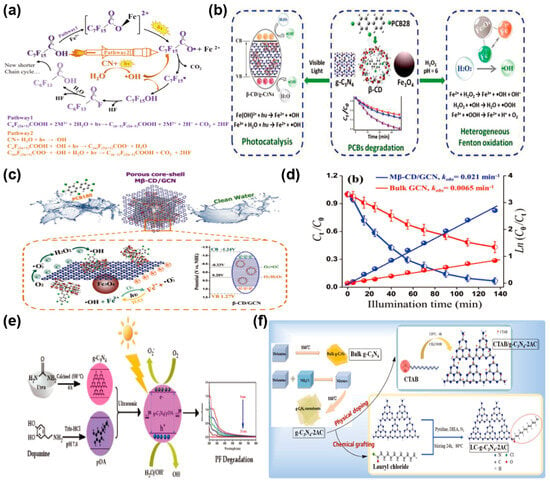
Figure 7.
(a) Schematic of PFOA breakdown in the CN/Fe-Vis process. (reproduced with permission from Ref. [76], copyright 2024 Elsevier). (b) The breakdown process of Fe3O4@β-CD/g-C3N4 for PCB180 degradation. (reproduced with permission from Ref. [77], copyright 2022 Elsevier). (c) Photocatalytic breakdown process of PCB180 using Mβ-CD/GCN composite. (d) Degradation dynamics of PCB180 using dual catalysts, and kinetic rate constant analysis. (reproduced with permission from Ref. [78], copyright 2022 Elsevier). (e) Synthesis process of g-C3N4/pDA nanocomposites and their use in PF photocatalytic degradation. (reproduced with permission from Ref. [79], copyright 2024 Elsevier). (f) Preparation of LC-g-C3N4-2AC photocatalytic materials. (reproduced with permission from Ref. [80], copyright 2023 Elsevier).

Table 3.
Summary of the g-C3N4-based photocatalysts for the removal of POPs.
4.2. EDCs Degradation
EDCs are external chemical agents, which are capable in disrupting the normal operations of the endocrine system in living beings. These chemicals can lead to genetic mutations, cellular alterations, and increase the risk of various cancers, including breast and prostate cancer. These precursors may also result in abnormal reproductive system development, behavioral changes, reduced reproductive capacity, and even species extinction [92]. EDCs mainly consist of synthetic phenolic compounds like BPA, nonylphenol, and octylphenol, commonly found in consumer goods. By the end of 2022, worldwide demand for BPA surged to a staggering of 10.6 million tons [93]. This uptick in both production and usage has exacerbated the contamination of aquatic ecosystems by endocrine-disrupting chemicals, posing a growing environmental threat.
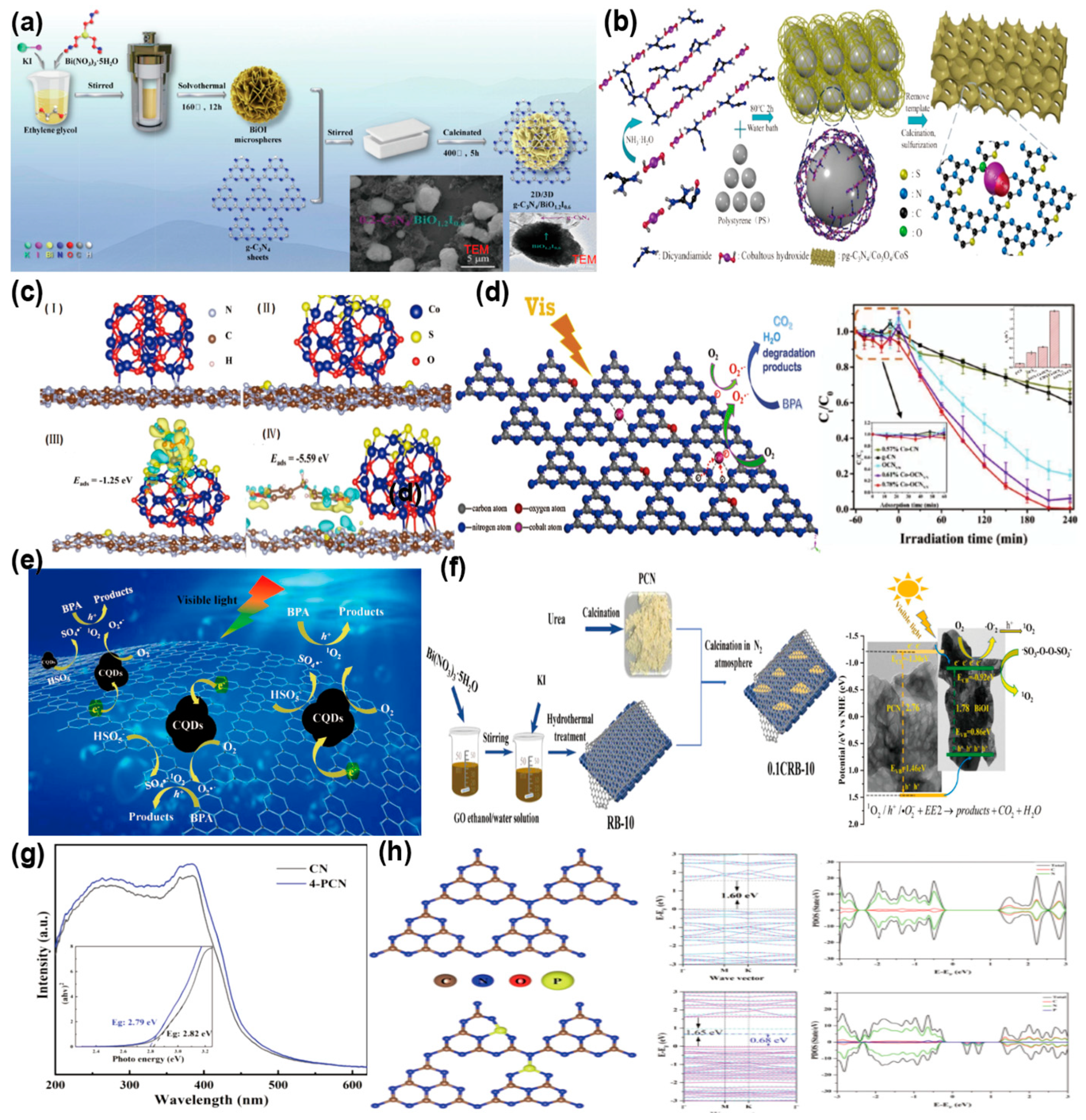
BPA, a prevalent EDC, can be efficiently degraded through g-C3N4 photocatalysis, an eco-friendly and economical approach. Mu et al. [94] effectively fabricated a 2D/3D S-scheme heterostructure (g-C3N4/BiO1.2I0.6) through calcination of g-C3N4 nanosheets and BiOI with a floral morphology. The 2D/3D structure ensures close heterojunction interaction, promoting swift electron transfer and boosting photocatalytic efficiency (Figure 8a). The degradation rate of BPA by 0.2 g-C3N4/BiO1.2I0.6 is 3.2 times higher than that of g-C3N4. Guo et al. [95] engineered the g-C3N4 structure with Co and S (pg-C3N4/Co3O4/CoS) to achieve photocatalytic-PMS oxidation-coupled degradation of Bisphenol F(BPF). The addition of Co markedly improved the activation performance of g-C3N4 and peroxymonosulfate (PMS), while the sulfidation treatment increased the active sites for PMS activation. The incorporation of Co and S expanded the interlayer spacing of g-C3N4 and enhanced its visible-light harvesting efficiency (Figure 8b). DFT calculations (Figure 8c) reveal that electron transfer from carbon nitride to CoS enhances Co3+/Co2+ regeneration. The formation of CoS on Co3O4 shifts the Fermi level towards the CB in g-C3N4, improving its conductivity and facilitating photogenerated charge transfer. The pg-C3N4/Co3O4/CoS system exhibits a degradation rate 4 times higher than the pure PMS system, achieves a 90% TOC removal rate, and retains excellent catalytic activity after 5 cycles. Yu et al. [96] synthesized a cobalt-oxygen co-doped nitrogen-deficient g-C3N4 (Co-O-CNV) charge-transfer complex through thermal polycondensation, employing oxalic acid as a dual-functional precursor serving as both oxygen donor and chelating agent. During copolymerization, cobalt atoms were uniformly distributed and integrated into the OCNVN conjugated framework, establishing “metal-ligand” guest interactions. This structure optimized the optical bandgap of Co-OCNVN and significantly enhanced its light harvesting efficiency ability. The cobalt atoms, serving as separation hubs, greatly enhanced charge carrier separation efficiency. The degradation rate of 0.78% Co-OCNVN was 13 times that of the bulk g-C3N4. And Co-OCNVN could achieve the complete degradation of BPA within 3 h (Figure 8d). Ming et al. [97] hybridized carbon nitride nanosheets with CQDs (PCNC) through thermal polymerization. The Carbon Quantum Dots (CQDs) effectively captured photogenerated electrons, enhancing charge separation and transfer, and activated PMS (PMS/PCNC), resulting in a 95% BPA removal rate (Figure 8e). This study provides an example for developing efficient metal-free photocatalysts for PMS activation and offers a case for removing BPA from wastewater. Luo et al. [98] constructed a porous g-C3N4 sheet/reduced graphene oxide (RGO)/BiOI nanosheet (CRB) catalyst (Figure 8f). RGO, positioned between BiOI nanosheets and porous g-C3N4 nanosheets, acted as an electron shuttle, significantly enhancing carrier separation. Under visible light, CRB activated persulfate (PS). With 0.25 g/L CRB and 0.5 mM PS, 6 mg/L of 17α-ethinylestradiol (EE2) was completely removed within 2 min, with a degradation rate as high as 1.6557 min−1. Xu et al. [99] synthesized an efficient MIL-100(Fe)/ZnFe2O4/porous carbon nitride (MIL/ZC) ternary catalyst. The synergistic interplay between porous carbon nitride (PCN) and complementary catalysts significantly boosted the dissociation of photogenerated electron-hole, thanks to an optimized dual charge transfer mechanism. This advancement led to an impressive 99.84% degradation rate of nonylphenol (NP) when using the 30MIL/ZC2 composite, which showcases its remarkable efficiency in environmental remediation. Bi et al. [100] synthesized phosphorus-doped g-C3N4 (PCN) photocatalysts through thermal polymerization for the degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) under visible light. Phosphorus doping developed impurity bands into g-C3N4, narrowing the bandgap and boosting visible light harvesting efficiency, leading to a PCN degradation rate constant nearly quadruple that of CN (Figure 8g). DFT calculations were used to study the density of states (DOS) and band structures of 4-PCN and CN. The analysis revealed that the doping process preserved the overall trend in the band structure, though the introduction of impurity bands in 4-PCN resulted in a noticeable narrowing of the bandgap. Specifically, the VB edge of CN is dominated by nitrogen orbitals, whereas the CB edge arises from a combined contribution of both nitrogen and carbon orbitals. Phosphorus doping led to interactions between P atoms in 4-PCN and neighboring carbon and nitrogen atoms, giving rise to impurity states. These impurity states formed impurity bands in the band structure, further reducing the bandgap (Figure 8h). Phosphorus doping represents an effective strategy for enhancing the photocatalytic performance of g-C3N4. The performance of these various g-C3N4-based photocatalysts for the elimination of EDCs is comprehensively summarized in Table 4.
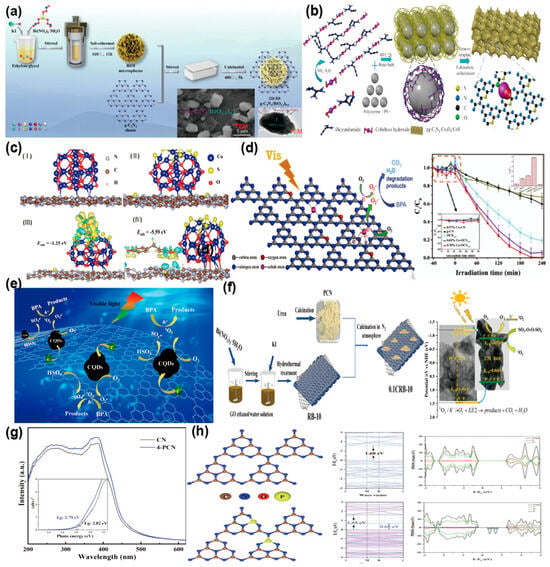
Figure 8.
(a) Schematic of the construction process of 2D/3D g-C3N4/BiO1.2I0.6S-scheme heterojunctions (TEM image of 0.2-C3N4/BiO1.2I0.6 is presented in the illustration). (reproduced with permission from Ref. [94], copyright 2022 Elsevier). (b) Diagram of pg-C3N4/Co3O4/CoS synthesis process. (c) The corresponding geometry structures of (I) pg-C3N4/Co3O4, (II) pg-C3N4/Co3O4/CoS; The charge density variation maps for the adsorption structures of (III) PMS and (IV) BPF. (reproduced with permission from Ref. [95], copyright 2021 Elsevier). (d) Schematic of potential photocatalytic process for Co-OCNVN (Degradation performance of g-CN, OCNVN, x Co-OCNVN, and Co-CN). (The right illustration shows the degradation efficiency of g-CN, OCNVN, x Co-OCNVN, and Co-CN, as well as the corresponding apparent pseudo first order rate constant (k1)). (reproduced with permission from Ref. [96], copyright 2020 Elsevier). (e) Photocatalytic activation and degradation mechanism of PMS/PCNC. (reproduced with permission from Ref. [97], copyright 2021 Elsevier). (f) Preparation route of CRB catalyst and degradation mechanism of EE2 in the 0.1CRB-10/PS/Vis system. (reproduced with permission from Ref. [98], copyright 2024 Elsevier). (g) UV-Vis diffuse reflectance spectra and bandgap energies of CN and 4-PCN. (h) Bandgap Modification through Impurity Band Formation via P Doping. (reproduced with permission from Ref. [100], copyright 2023 Elsevier).

Table 4.
Summary of the g-C3N4 -based photocatalysts for the removal of EDCs.
4.3. Antibiotics Degradation
Antibiotics, as antimicrobial compounds, are extensively utilized in human and veterinary medicine for infection treatment and prevention, as well as growth promotion in aquaculture. Following the discovery of penicillin, multiple antibiotic classes, including sulfonamides, quinolones, tetracyclines, and macrolides, have become pervasive environmental contaminants, detected in surface waters and drinking water systems worldwide [110]. The process of photocatalytic degradation of antibiotics has garnered widespread attention and practical application, thanks to its affordability, remarkable effectiveness, and absence of harmful byproducts, positioning it as a reliable solution for eliminating antibiotics.
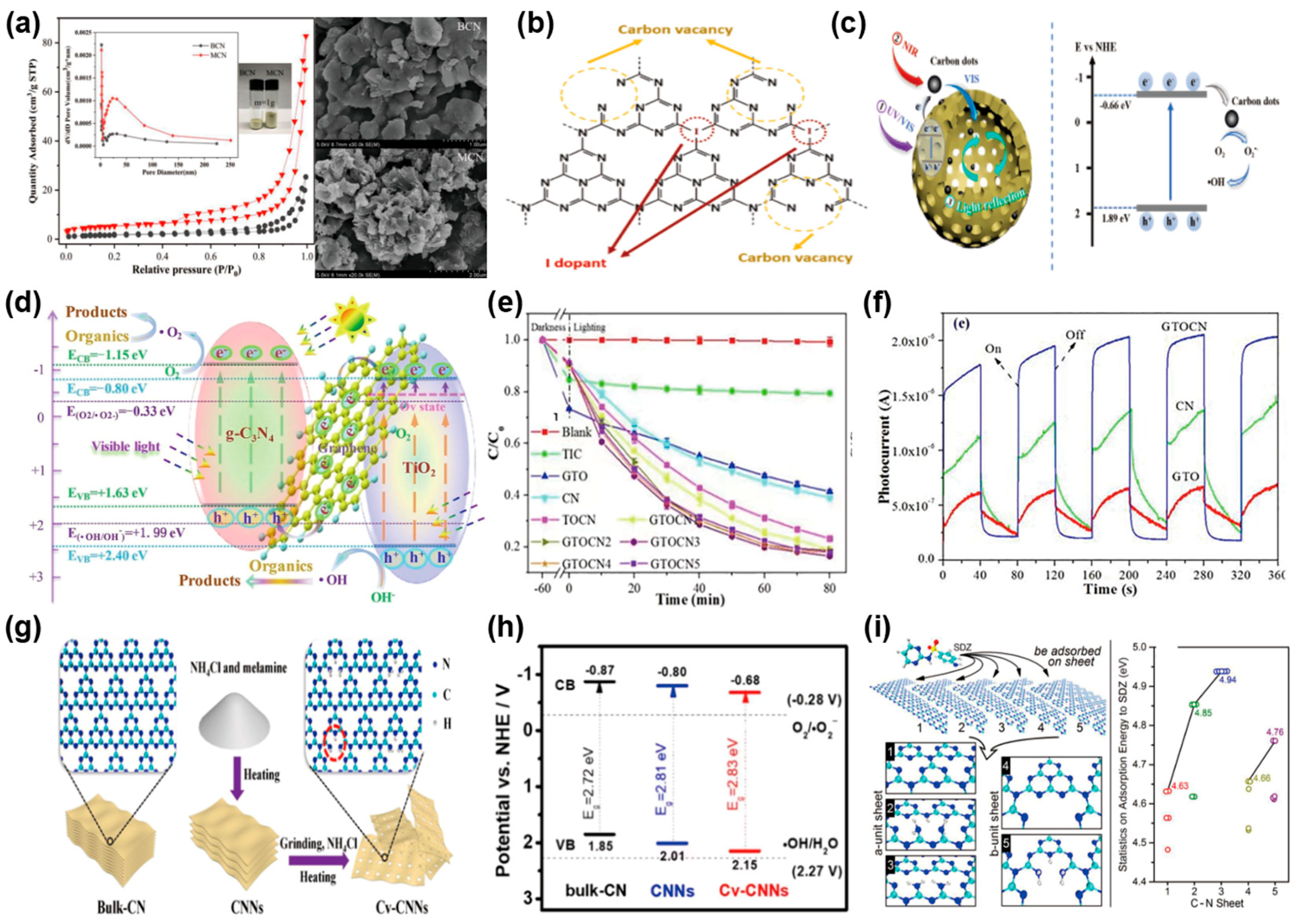
Dou et al. [111] synthesized mesoporous g-C3N4 (MCN) through morphology control, achieving degradation rates of amoxicillin (AMX) and cefotaxime (CFX) under visible light irradiation that were 2.5 and 4 times higher than those of BCN, respectively. The improved photocatalytic activity of MCN is primarily attributed to the formation of a porous structure (Figure 9a). Phoon et al. [112] synthesized I-doped mesoporous g-C3N4 (GCN-I) via thermal polymerization, and found that with the increase in the amount of I dopant, carbon vacancies appeared, leading to a decrease in the C/N ratio. This phenomenon allows more electrons to delocalize in the GCN structure, promoting the generation of more radicals and enhancing photodegradation performance (Figure 9b). Under visible light, GCN-I0.50 achieved a 99.8% removal rate of tetracycline within 180 min. Constructing heterojunctions and doping with carbon quantum dots can significantly enhance the photocatalytic activity of g-C3N4, thereby improving the removal efficiency of organic pollutants. Wu et al. [113] effectively produced a photocatalyst of hollow porous carbon nitride nanospheres modified with carbon quantum dots (HCNS/CDs), which effectively degrades multiple antibiotics under visible and natural light. The hollow nanosphere structure of HCNS/CDs provides abundant reactive sites, and optical harvesting efficiency is enhanced due to internal light reflection. Carbon quantum dots can convert long-wavelength visible and near-infrared light into short-wavelength light, thereby enhancing the photocatalytic degradation efficiency of HCNS/CDs (Figure 9c). Wu et al. [114] created a Z-scheme highly photoactive graphene layer and TiO2/g-C3N4 (GTOCN) composite photocatalyst for the degradation of antibiotics such as tetracycline and ciprofloxacin. In this ternary structure, electrons from the CB of TiO2 are rapidly captured by graphene and transferred to the VB of g-C3N4, promoting efficient charge transport and improving the division of photoinduced electron-hole pairs. GTOCN3 achieved an 83.5% removal rate of tetracycline within 80 min, with a total organic carbon removal rate of 66.3%, demonstrating significant degradation and mineralization effects on antibiotics under visible light (Figure 9d–f). Liu et al. [115] developed ultrathin carbon nitride nanosheets featuring atomic porosity and carbon vacancies (Cv-CNNs) through a two-step thermal method. Carbon vacancies led to the conversion of exposed nitrogen atoms into -NH2 groups, serving as hole stabilizers to extend the excited state lifetime of the photocatalyst and improve the dispersibility of CN sheets in water. Quantum mechanical simulations have shown that the presence of carbon vacancies in CN sheets actually modifies their two-dimensional structure. This adjustment enhances charge separation, narrows the gap for charge to travel to the surface, and cuts down on the likelihood of charge carrier recombination. The CvCNNs exhibited significantly enhanced sulfadiazine (SDZ) adsorption capacity. The introduction of carbon vacancies conversely has modulated the band energy levels in carbon nitride photocatalysts, enhancing their redox potentials and consequently promoting both photocatalytic water splitting for hydrogen evolution and SDZ degradation (Figure 9g–i). Experimental results showed that SDZ on Cv-CNNs was completely degraded within 90 min under visible light irradiation, and the material maintained 95% stability over five cycles. Hassanzadeh et al. [116] developed a Z-scheme g-C3N4/NiFe2O4/Ag nanocomposite by anchoring NiFe2O4/Ag nanoparticles onto g-C3N4. This cutting-edge approach substantially reduced the recombination of electron-hole, resulting in a remarkable improvement in the material’s photocatalytic efficiency and redox capabilities under visible light exposure. The nanocomposite exhibited a 92.1% degradation efficiency for tetracycline (TC) in 120 min. Zhou et al. [117] prepared nitrogen-deficient carbon nitride (FCN-5) through formic acid-aided pyrolysis. The inclusion of formic acid did not just bring about nitrogen defects and boost active sites, but also emitted gases during pyrolysis. This resulted in a porous framework, increased surface area, and reduced electron-hole recombination. As a result, the photoresponse performance was enhanced. In fact, FCN-5 managed to achieve a degradation efficiency of 95.1% for TC within 60 min. The performance of these various g-C3N4-based photocatalysts for the elimination of antibiotics is comprehensively summarized in Table 5.
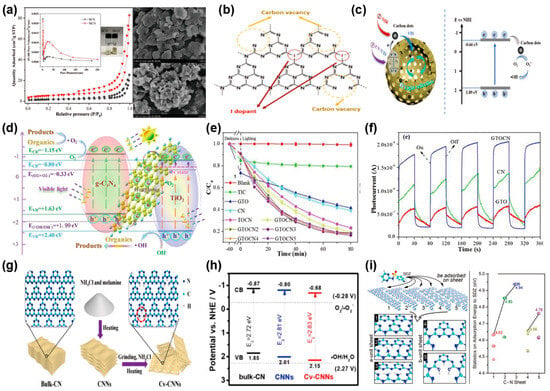
Figure 9.
(a) The N2 adsorption–desorption isotherms and corresponding BJH pore size distribution plots (inset) were analyzed for both BCN and MCN samples. A comparative pore volume analysis (inset) was also conducted at a fixed mass (m = 1 g). Furthermore, the morphological characteristics of BCN and MCN were examined via scanning electron microscopy (SEM). (reproduced with permission from Ref. [111], copyright 2020 Elsevier). (b) The possible structure of I-doped GCN. (reproduced with permission from Ref. [112], copyright 2024 Elsevier). (c) Illustration of the photocatalytic mechanism of HCNS/CD when exposed to broad-spectrum light. (reproduced with permission from Ref. [113], copyright 2020 Elsevier). (d) Visible-light-driven photocatalytic breakdown of organic contaminants using GTOCN3. (e) Photocatalytic degradation performance of GTOCN3 towards TC. (f) Electrochemical impedance spectroscopy of GTOCN3. (reproduced with permission from Ref. [114], copyright 2020 Elsevier). (g) Schematic diagram of the synthesis of Cv-CNNs. (h) Valence and CB potential calculations of CN, CNN, and Cv-CNN. (i) Development of CN layers for SDZ uptake and their corresponding adsorption energies (1–5 denote CNNsa, Cv-CNNsa1, Cv-CNNsa2, CNNsb, and Cv-CNNsb, respectively). (Reproduced with permission from Ref. [115], copyright 2020 Elsevier).

Table 5.
Summary of the g-C3N4-based photocatalysts for the removal of antibiotics.
4.4. MPs Degradation
MPs are plastic particles less than 5 mm in size. They mainly result from the fragmentation of larger plastic debris. Due to their persistence, MPs have emerged as a major environmental pollutant, now infiltrating our water bodies, food sources, air, and even bodily tissues such as blood and lungs. This MP contamination mainly arises from airborne and aquatic microplastics, significantly harming marine ecosystems [128]. Globally, 19–23 million tons of plastic debris are discharged annually. By 2030, this quantity is expected to soar to 53 million tons annually, with around 10 million tons ultimately reaching the oceans. Despite the growing buildup of plastic waste, merely 11% undergoes recycling [129].
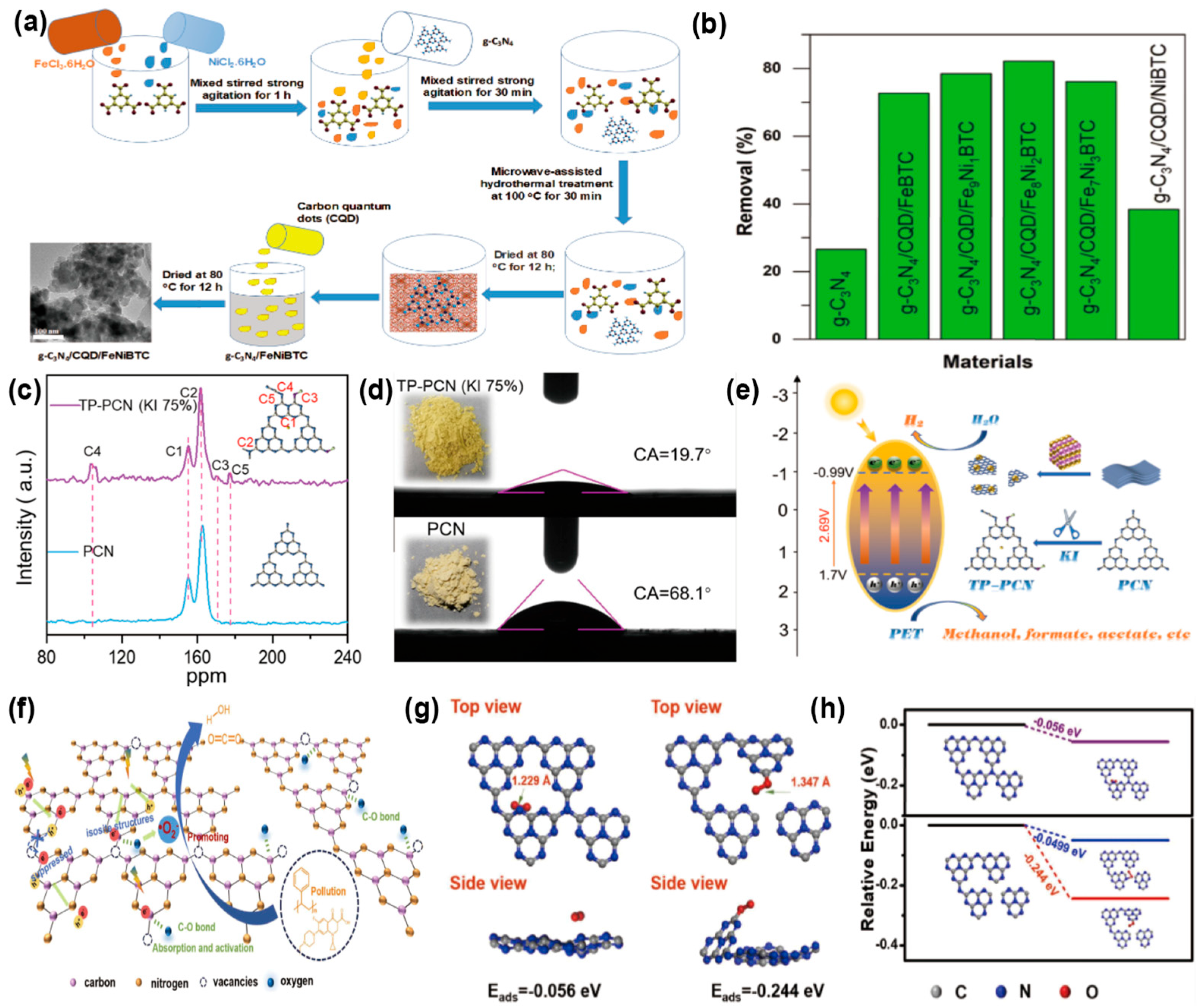
Photocatalysis offers an energy-efficient solution to reduce MPs pollution by converting them into CO2 and H2O. Moreover, MPs act as inexpensive sacrificial precursors. Their polymer chains are degraded and converted into valuable chemicals and fuels. Nguyen et al. [130] employed hydrothermal and microwave methods to integrate g-C3N4 with FeNi-BTC (benzene-1,3,5-tricarboxylic acid) and CQDs, resulting in the formation of (g-C3N4/CQD/FeNi-BTC). The fabricated photocatalytic composite boasted a substantial pore volume of 1.192 cm3 g−1, a high specific surface area of 1090 m2 g−1, a 40–60 nm particle size, and visible-light harvesting efficiency in the range of 2.27–2.52 eV (Figure 10a). With a concentration of 1200 mg/L, the material showed remarkable adsorption efficiency for microplastics like polystyrene (PS) and polyethylene terephthalate (PET), reaching almost 100% adsorption in 45 min. Moreover, after 3 days of visible-light irradiation, the degradation rate of MPs with a concentration of 2500 mg/L reached 82.16% (Figure 10b). Wang et al. [131] developed a Z-scheme heterojunction by integrating WO3 with g-C3N4 (WO3/g-C3N4) through a hydrothermal synthesis process. This novel technique significantly enhanced the separation efficiency of photogenerated electron-hole pairs. Notably, the 30%WO3/g-C3N4 composite demonstrated exceptional performance in this regard. The 30%WO3/g-C3N4 composite increased the hydrogen evolution rate from PET degradation to 14.21 mM. This study opens new pathways for sustainable energy generation. Liu et al. [132] developed −C≡N and C-OH groups into PCN by terminating the polymerization reaction with iodide ions (I−) (Figure 10c), which led to the production of a series of hydrophilic fragmented homogeneous carbon nitride (TP-PCN) (Figure 10d). I− acted as an inhibitor during the exfoliation process by disrupting π-π bonds, reducing the stacking of ultrathin layers in PCN, thereby increasing the specific surface area of TP-PCN and significantly shortening the electron transfer path to the surface. Additionally, it enhanced in-plane electron migration, diminished energy dissipation caused by electron-hole recombination, and effectively promoted PET degradation and hydrogen production (Figure 10e). The photocatalytic conversion of PET to H2 by TP-PCN reached 600.3 μmol g−1 h−1. Liang et al. [127] synthesized porous carbon nitride nanoparticles with N3c vacancies (NCN0.48) through morphology control (Figure 10f). Specifically, the N3c vacancies acted as capture sites for photogenerated charges, effectively reducing carrier recombination. They also improved O2 adsorption and activation via strong C-O interactions, boosting •O2− production (Figure 10g,h). Meanwhile, the large specific surface area and highly porous structure of NCN0.48 provided abundant reactive sites, enhancing pollutant adsorption capacity and facilitating superior photocatalytic performance. Within 150 h, the photocatalytic degradation efficiency of NCN0.48 for PET is 43.1%, much higher than that of CN at 13.6%. The performance of these various g-C3N4-based photocatalysts for the elimination of MPs is comprehensively summarized in Table 6.
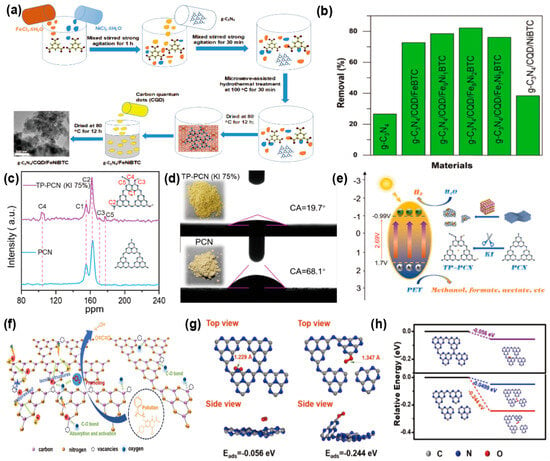
Figure 10.
(a) Microwave hydrothermal synthesis of g-C3N4/CQD/FeNi-BTC. (b) Microplastic elimination performance of g-C3N4/CQD/Fe-BTC versus g-C3N4/CQD/FeNi-BTC. (reproduced with permission from Ref. [130], copyright 2024 Elsevier). (c) Solid-state13C NMR spectra of PCN and TP-PCN (KI 75%). (d) Contact angle test visuals for the synthesized samples in digital format. (e) Schematic illustration of the reaction mechanism for microplastic degradation and hydrogen evolution by hydrophilic TP-PCN photocatalyst. (reproduced with permission from Ref. [132], copyright 2023 Elsevier). (f) Photocatalytic mechanism of microplastic degradation by NCN0.48 under light irradiation. (g) Optimized configurations of O2 adsorption on g-C3N4 surfaces and carbon atoms in N3c-deficient g-C3N4. (h) Gibbs free energy profile for O2 adsorption on g-C3N4 surfaces and g-C3N4 with N3c vacancies. (Reproduced with permission from Ref. [127], copyright 2024 Elsevier).

Table 6.
Summary of the g-C3N4-based photocatalysts for the removal of MPs.
4.5. Application of Carbon Nitride-Based Materials in Degrading ECs in Actual Water Bodies
Currently, extensive research has been conducted on g-C3N4-based materials in the field of photocatalytic degradation of emerging contaminants in real wastewater, and these materials have demonstrated remarkable application potential. Endowed with excellent chemical stability, visible-light response capability, and environmental benignity, g-C3N4-based materials enable efficient degradation of typical ECs present in real wastewater. Bi et al. [100] investigated the photocatalytic performance of 4-PCN for 2,4-D degradation using actual water samples from the rivers and reservoirs of Harbin. Results showed its degradation efficiency was lower than in ultrapure water, attributed to inhibition by dissolved organic matter (DOM) and ions (e.g., Cl−, HCO3−) [136]. Nevertheless, 4-PCN still removed 60~70% of 2,4-D. After 20 min of irradiation, about 45% total organic carbon (TOC) reduction indicated the mineralization of 2,4-D. Additionally, five recycling tests revealed no significant activity loss, and XRD, FTIR, TEM, SEM and XRD analyses confirmed unchanged crystal structure and functional groups of 4-PCN, verifying its good reusability and stability. Considering the complexity of real water matrices, where antibiotics may coexist with inorganic ions and other substances, the influence of common aqueous anions (Cl−, HCO3−, NO3−, SO42−, HPO42−) on the photodegradation of OFX was investigated. Feng et al. [126] prepared nitrogen-deficient, oxygen-doped porous g-C3N4 with a wider bandgap (denoted as RCN-2h), which achieved 100% degradation of ofloxacin (OFX) within 15 min. The results revealed that Cl−, NO3−, and SO42− had negligible effects, whereas HCO3− and HPO42− exhibited inhibitory effects. This inhibition was attributed to the Coulombic repulsion under alkaline conditions between these anions and OFX, as well as their ability to capture photogenerated holes (h+), leading to the formation of weakly oxidative species. The degradation rate constant of RCN-2h was 15.4 times higher than that of bulk g-C3N4 (BCN).
Zhang et al. [137] successfully prepared a Z-scheme heterojunction (5% Ag2V4O11/TCN) composed of silver vanadate quantum dots and tubular carbon nitride. This catalyst achieved a photocatalytic degradation efficiency of 86.25% for TC within 120 min. Four cyclic experiments showed that the degradation efficiency of TC remained at 80% after four cycles, confirming the strong stability of 5% Ag2V4O11/TCN. Furthermore, the study employed liquid chromatography-mass spectrometry (LC-MS) to analyze the intermediates generated during the degradation of TC and investigated their toxic effects as well as their interactions with free radicals. The degradation pathways of TC involved reactions such as hydroxylation, demethylation, and deamidation, yielding various intermediates. These intermediates were further converted into small molecular products through ring-opening and bond-cleavage processes, ultimately mineralizing into CO2 and H2O, accompanied by a significant reduction in toxicity. Quenching experiments and ESR results demonstrated that free radicals (·OH, ·O2−, h+, and 1O2) played crucial roles in the degradation process, with·O2− contributing the most significantly. Highly reactive free radicals attacked TC molecules and their intermediates, thereby promoting the cleavage of C–C, C–N, and C–O bonds as well as structural ring-opening, which accelerated the decomposition and detoxification of toxic intermediates.
In summary, g-C3N4-based materials exhibit notable potential for photocatalytic degradation of ECs in real wastewater. Though their efficiency is inhibited by DOM and ions in real water, they still achieve effective EC removal. Recycling tests and XRD/FTIR confirm their stability, while TOC and LC-MS verify extensive EC mineralization, thereby reducing the risk of hazardous byproducts. Mechanistic studies, including proposed degradation pathways and identification of intermediate products via techniques such as LC-MS, further deepen the understanding of reaction processes and confirm the environmental compatibility of the degradation products. and the degradation performance, cyclic stability, and potential metal leaching risks of g-C3N4-based catalysts for ECs are summarized in Table 7.

Table 7.
Summary of the degradation performance, cyclic stability, and metal leaching risks of g-C3N4-based catalysts for ECs.
5. Conclusions and Perspectives
g-C3N4-based materials show promise in environmental applications but have limited pristine performance. Research thus focuses on high-performance g-C3N4 via key modifications (morphology control, defect engineering, doping, and heterojunction construction) to expand light response and boost carrier separation. For doping: Metal doping needs to tackle active species agglomeration and enhance stability; non-metal doping requires precise content control; co-doping faces challenges of unclear synergies and complex structural regulation. In heterojunction construction: Despite their performance benefits, the structure-activity relationship between fine structures and catalytic activity remains unclear. Core issues include interface charge transfer, energy band matching, and long-term stability. Notably, the structural evolution of g-C3N4 during modification merits attention.
g-C3N4-based materials have been applied in the removal of various ECs. Modifications are typically necessary to enhance their activity and stability to meet the degradation requirements for different types of ECs. AI and ML play critical roles in advancing this process by analyzing large datasets of modification parameters and forecasting EC degradation performance, which can rapidly establish correlations to guide the rational design of high-efficiency modified g-C3N4, avoiding the inefficiency of traditional trial-and-error. However, systematic studies on the correlation between modification methods and specific ECs remain scarce. Furthermore, given that one of the primary applications of g-C3N4 is the remediation of contaminated water environments, the potential environmental impacts of modified g-C3N4 also warrant careful consideration. AI and ML can further support the optimization of g-C3N4-based water treatment processes, they can integrate data on photocatalyst stability and ecological toxicity with reaction conditions to predict both degradation efficiency and secondary pollution risks, promoting the translation of g-C3N4-based technologies from lab-scale to practical applications.
In summary, g-C3N4-based materials exhibit promising prospects for application in the degradation of emerging pollutants. To facilitate their practical implementation, the following recommendations are proposed for future study.
- (i).
- Presently, the charge transfer mechanisms in g-C3N4-based materials are not fully understood, and further studies are required to explore this process to improve their photocatalytic efficiency. Combining DFT calculations and experimental characterization methods to verify and optimize the charge transfer process in g-C3N4-based materials will provide a theoretical foundation for developing more efficient photocatalysts and precisely controlling photocatalytic reaction pathways.
- (ii).
- A thorough investigation into the thermodynamics and kinetics of surface reactions in g-C3N4-based materials is essential for refining material design, boosting charge separation efficiency, and accelerating photocatalytic reactions. AI-assisted analysis, particularly ML-driven kinetic modeling, has emerged as a powerful tool to unravel microscopic mechanisms. It can also reduce trial-and-error costs by summarizing experimental experience: studies have shown that ML algorithms predict surface reaction barriers and charge separation efficiencies by correlating experimental thermodynamic data with structural descriptors of g-C3N4-based materials, which not only deepens mechanism understanding but also enhances the selectivity of pollutant degradation by optimizing active species generation.
- (iii).
- Recent research indicates that g-C3N4-based photocatalytic materials exhibit low efficiency in the removal of POPs and may generate intermediate products with higher toxicity. Therefore, particular heed must be paid to the mineralization degree of emerging pollutants and the toxicity risks of intermediate products during the photocatalytic degradation by g-C3N4-based materials. Additionally, for g-C3N4 composites modified with metals, the potential leaching of metal ions should be monitored, as it helps further reduce the environmental risk of the final degradation system.
- (iv).
- While g-C3N4-based photocatalysts demonstrate remarkable pollutant degradation performance under laboratory conditions, their applications remain restricted to small-scale experiments. Moreover, the inherent powder morphology of g-C3N4 materials complicates recovery processes, thereby limiting their practical engineering applications. Consequently, future research should prioritize enhancing the stability and reusability of g-C3N4-based materials to facilitate their transition from laboratory research to scaled-up environmental engineering applications.
- (v).
- Currently, g-C3N4-based photocatalytic materials primarily rely on photoexcitation to generate strongly oxidative radicals for organic pollutant degradation, whose activity ceases without illumination. To address this limitation, integrating g-C3N4 with piezoelectric materials enables persistent radical generation via the piezoelectric effect even in dark conditions for emerging contaminant degradation. Simultaneously, the built-in electric field from piezoelectric coupling facilitates photogenerated charge separation and reduces recombination, thereby enhancing photocatalytic efficiency.
Author Contributions
Conceptualization, D.X. and D.L.; methodology, D.X. and D.L.; software, H.C., D.L. and X.C.; validation, S.H., F.C. and D.X.; formal analysis, D.X., Z.L., Z.H., Z.C., J.H., W.H., X.T., Y.W. and Y.F.; investigation, H.C. and S.H.; resources, H.C. and S.H.; data curation, D.X. and D.L.; writing—original draft preparation, D.X. and D.L.; writing—review and editing, D.L. and S.H.; supervision, D.L., H.C. and S.H.; project administration, H.C.; funding acquisition, H.C., D.L. and X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Guangdong Provincial Program for Young Innovative Talents in General Higher Education Institutions (2024KQNCX169), the National Natural Science Foundation of China (42477220), and the Foshan City Self-Funded Science and Technology Innovation Project Incubation Program (2420001003624).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Author Chen Feng is employed by Foshan Water Group. The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. Affiliated companies did not participate in the study design, data collection, analysis, interpretation, writing of this manuscript, or the decision to submit it for publication.
References
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive review of emerging contaminants: Detection technologies, environmental impact, and management strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef]
- Field, J.A.; Johnson, C.A.; Rose, J.B. What is “emerging”? Environ. Sci. Technol. 2006, 40, 7105. [Google Scholar] [CrossRef]
- Javed, H.; Lyu, C.; Sun, R.; Zhang, D.; Alvarez, P.J.J. Discerning the inefficacy of hydroxyl radicals during perfluorooctanoic acid degradation. Chemosphere 2020, 247, 125883. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Ren, Y.-M.; Liu, Y.-L.; Xiao, J.-Y.; Lin, L.-Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Feng, M.; Chen, X.; Cui, Y.; Zhao, D.; Sun, J. Fabrication of potassium ion decorated 1D/2D g-C3N4/g-C3N4 homojunction enabled by dual-ions synergistic strategy for enhanced photocatalytic activity towards degradation of organic pollutants. Appl. Surf. Sci. 2022, 575, 151695. [Google Scholar]
- Joy, J.; George, E.; Poornima Vijayan, P.; Anas, S.; Thomas, S. An overview of synthesis, morphology, and versatile applications of nanostructured graphitic carbon nitride (g-C3N4). J. Ind. Eng. Chem. 2024, 133, 74–89. [Google Scholar]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar]
- Maeda, K.; Wang, X.; Nishihara, Y.; Lu, D.; Antonietti, M.; Domen, K. Photocatalytic Activities of Graphitic Carbon Nitride Powder for Water Reduction and Oxidation under Visible Light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Bekyarova, E.B.; Niyogi, S.; Sarkar, S.; Tian, X.; Chen, M.; Moser, M.L.; Ayub, K.; Mitchell, R.H.; Haddon, R.C. Stereochemical effect of covalent chemistry on the electronic structure and properties of the carbon allotropes and graphene surfaces. Synth. Met. 2015, 210, 80–84. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [PubMed]
- Wang, X.; Maeda, K.; Chen, X.; Takanabe, K.; Domen, K.; Hou, Y.; Fu, X.; Antonietti, M. Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light. J. Am. Chem. Soc. 2009, 131, 1680–1681. [Google Scholar] [CrossRef]
- Bai, X.; Yan, S.; Wang, J.; Wang, L.; Jiang, W.; Wu, S.; Sun, C. A Simple and Efficient Strategy for Chemically Tailored g-C3N4 Material. J. Mater. Chem. A 2014, 2. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.L.; Chai, S.-P.; Yong, S.-T. Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene-g-C3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem. Commun. 2015, 5. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Wu, L.; Fu, M.; Wu, Z. Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1332–1335. [Google Scholar] [CrossRef]
- Kesavan, G.; Sorescu, D.; Zeng, Z.; Askari, F.; He, Y.; Rosi, N.; Star, A. Optimizing Dicyandiamide Pretreatment Conditions for Enhanced Structure and Electronic Properties of Polymeric Graphitic Carbon Nitride. J. Mater. Chem. C 2023, 11, 14865–14875. [Google Scholar] [CrossRef]
- Gu, Q.; Gao, Z.; Zhao, H.-J.; Lou, Z.; Liao, Y.; Xue, C. Temperature-controlled morphology evolution of graphitic carbon nitride nanostructures and their photocatalytic activities under visible light. RSC Adv. 2015, 5, 49317–49325. [Google Scholar] [CrossRef]
- Mo, Z.; Xu, H.; Chen, Z.; She, X.; Song, Y.; Lian, J.; Zhu, X.; Yan, P.; Lei, Y.; Yuan, S.; et al. Construction of MnO2/Monolayer g-C3N4 with Mn vacancies for Z-scheme overall water splitting. Appl. Catal. B Environ. 2019, 241, 452–460. [Google Scholar]
- Kong, Y.; Li, D.; Zhang, C.; Han, W.; Xue, Y.; Zhang, W.; Sun, H.; Wang, S.; Duan, X. Synergistic silver doping and N vacancy promoting photocatalytic performances of carbon nitride for pollutant oxidation and hydrogen production. Chem. Eng. J. 2024, 479, 147676. [Google Scholar]
- Singh, R.; Chauhan, M.; Garg, P.; Sharma, B.; Attri, P.; Sharma, R.K.; Sharma, D.; Chaudhary, G.R. A critical review on visible light active graphitic carbon nitride (g-CN) based photocatalyst for environment remediation application: A sustainable approach. J. Clean. Prod. 2023, 427, 138855. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar]
- Zhang, P.; Li, X.; Shao, C.; Liu, Y. Hydrothermal synthesis of carbon-rich graphitic carbon nitride nanosheets for photoredox catalysis. J. Mater. Chem. A 2015, 3, 3281–3284. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F. Characterization and biological properties of TiO2/PCL hybrid layers prepared via sol–gel dip coating for surface modification of titanium implants. J. Non-Cryst. Solids 2015, 415, 9–15. [Google Scholar]
- Ouyang, H.; Tu, X.; Fu, Z.; Wang, W.; Fu, S.; Zhu, C.; Du, D.; Lin, Y. Colorimetric and Chemiluminescent Dual-Readout Immunochromatographic Assay for Detection of Pesticide Residues Utilizing g-C3N4/BiFeO3 Nanocomposites with Peroxidase-Like Activity. Biosens. Bioelectron. 2018, 106, 43–49. [Google Scholar]
- Kailasam, K.; Epping, J.; Thomas, A.; Losse, S.; Junge, H. Mesoporous carbon nitride–silica composites by a combined sol–gel/thermal condensation approach and their application as photocatalysts. Energy Environ. Sci. 2011, 4, 4668–4674. [Google Scholar]
- Hollmann, D.; Karnahl, M.; Tschierlei, S.; Kailasam, K.; Schneider, M.; Radnik, J.; Grabow, K.; Bentrup, U.; Junge, H.; Beller, M.; et al. Structure-Activity Relationships in Bulk Polymeric and Sol-Gel-Derived Carbon Nitrides during Photocatalytic Hydrogen Production. Chem. Mater. 2014, 26, 1727–1733. [Google Scholar]
- Chen, P.; Chen, L.; Ge, S.; Zhang, W.; Wu, M.; Xing, P.; Rotamond, T.B.; Lin, H.; Wu, Y.; He, Y. Microwave heating preparation of phosphorus doped g-C3N4 and its enhanced performance for photocatalytic H2 evolution in the help of Ag3PO4 nanoparticles. Int. J. Hydrog. Energy 2020, 45, 14354–14367. [Google Scholar]
- Liu, H.; Chen, D.; Wang, Z.; Jing, H.; Zhang, R. Microwave-assisted molten-salt rapid synthesis of isotype triazine-/heptazine based g-C3N4 heterojunctions with highly enhanced photocatalytic hydrogen evolution performance. Appl. Catal. B-Environ. 2017, 203, 300–313. [Google Scholar]
- Wang, W.; Shu, Z.; Zhou, J.; Li, T.; Duan, P.; Zhao, Z.; Tan, Y.; Xie, C.; Cui, S. Halloysite-derived mesoporous g-C3N4 nanotubes for improved visible-light photocatalytic hydrogen evolution. Appl. Clay Sci. 2018, 158, 143–149. [Google Scholar]
- Zheng, Y.; Ren, J.; Zhang, N.; Li, J. Preparation of carbon nitride from different precursors through pyrolysis: Correlating the photocatalytic activity to the crystallinity and disorder. J. Environ. Chem. Eng. 2021, 9, 106410. [Google Scholar] [CrossRef]
- Lin, F.; Zhou, S.; Wang, G.; Wang, J.; Gao, T.; Su, Y.; Wong, C.-P. Electrostatic self-assembly combined with microwave hydrothermal strategy: Construction of 1D/1D carbon nanofibers/crystalline g-C3N4 heterojunction for boosting photocatalytic hydrogen production. Nano Energy 2022, 99, 107432. [Google Scholar]
- Yan, L.; Zhong, S.; Xiao, C.; Chen, Y. Optimization of hydrothermal pretreatment to prepare g-C3N4 with enhanced catalyst yield and photocatalytic activity for aqueous contaminant removal. Mater. Today Sustain. 2023, 22, 100374. [Google Scholar]
- Adler, C.; Krivtsov, I.; Mitoraj, D.; Dos Santos-Gómez, L.; García-Granda, S.; Neumann, C.; Kund, J.; Kranz, C.; Mizaikoff, B.; Turchanin, A.; et al. Sol-Gel Processing of Water-Soluble Carbon Nitride Enables High-Performance Photoanodes*. ChemSusChem 2021, 14, 2170–2179. [Google Scholar] [PubMed]
- Plubphon, N.; Thongtem, S.; Phuruangrat, A.; Randorn, C.; Kaowphong, S.; Narksitipan, S.; Thongtem, T. Direct microwave heating synthesis and characterization of highly efficient g-C3N4 photocatalyst. Inorg. Chem. Commun. 2022, 139, 109386. [Google Scholar]
- Zhou, Y.; Jin, P.; Zhou, Y.; Zhu, Y. High-performance symmetric supercapacitors based on carbon nanotube/graphite nanofiber nanocomposites. Sci. Rep. 2018, 8, 9005. [Google Scholar]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar]
- Pan, Z.; Ding, W.; Chen, H.; Ji, H. A review on g-C3N4 decorated with silver for photocatalytic energy conversion. Chin. Chem. Lett. 2024, 35, 108567. [Google Scholar]
- Dong, C.; Ma, Z.; Runtian, Q.; Guo, X.; Li, C.; Wang, R.; Shi, Y.; Dai, B.; Jia, X. Morphology and Defects Regulation of Carbon Nitride by Hydrochloric Acid to Boost Visible Light Absorption and Photocatalytic Activity. Appl. Catal. B Environ. 2017, 217, 629–636. [Google Scholar]
- Gu, Q.; Liao, Y.; Yin, L.; Long, J.; Wang, X.; Xue, C. Template-free synthesis of porous graphitic carbon nitride microspheres for enhanced photocatalytic hydrogen generation with high stability. Appl. Catal. B Environ. 2015, 165, 503–510. [Google Scholar]
- Wang, S.; Zhang, J.; Li, B.; Sun, H.; Wang, S.; Duan, X. Morphology-dependent photocatalysis of graphitic carbon nitride for sustainable remediation of aqueous pollutants: A mini review. J. Environ. Chem. Eng. 2022, 10, 107438. [Google Scholar] [CrossRef]
- Gao, H.; Yan, S.; Wang, J.; Huang, Y.; Wang, P.; Li, Z.; Zou, Z. Towards efficient solar hydrogen production by intercalated carbon nitride photocatalyst. Phys. Chem. Chem. Phys. 2013, 15, 18077–18084. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Cen, W.; Zhang, Y.; Dong, F. Bridging the g-C3N4 Interlayers for Enhanced Photocatalysis. ACS Catal. 2016, 6, 2462–2472. [Google Scholar]
- Li, D.; Wen, C.; Huang, J.; Zhong, J.; Chen, P.; Liu, H.; Wang, Z.; Liu, Y.; Lv, W.; Liu, G. High-efficiency ultrathin porous phosphorus-doped graphitic carbon nitride nanosheet photocatalyst for energy production and environmental remediation. Appl. Catal. B Environ. Energy 2022, 307, 121099. [Google Scholar]
- Zhang, B.; Li, X.; Zhao, Y.; Song, H.; Wang, H. Facile synthesis of oxygen doped mesoporous graphitic carbon nitride with high photocatalytic degradation efficiency under simulated solar irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123736. [Google Scholar]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Yu, H.; Mo, D.; Wang, H.; Xiao, Z.; Zhou, C. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation. J. Colloid Interface Sci. 2019, 536, 17–29. [Google Scholar]
- Qureshi, W.A.; Ali, R.N.; Haider, S.N.-U.-Z.; Ahmad, N.; Khan, M.U.; Wang, L.; Cheng, C.; Rao, S.; Ali, A.; Liu, Q.Q.; et al. Construction of B doped g-C3N4/Ni-MOF-74 porous heterojunction with boosted carrier separation for photocatalytic H2 generation. Mater. Today Sustain. 2024, 25, 100679. [Google Scholar]
- Li, Y.; Wang, S.; Chang, W.; Zhang, L.; Wu, Z.; Song, S.; Xing, Y. Preparation and enhanced photocatalytic performance of sulfur doped terminal-methylated g-C 3 N 4 nanosheets with extended visible-light response. J. Mater. Chem. A 2019, 7, 20640–20648. [Google Scholar]
- Ge, F.; Xu, Y.; Zhou, Y.; Tian, D.; Huang, S.; Xie, M.; Xu, H.; Li, H. Surface amorphous carbon doping of carbon nitride for efficient acceleration of electron transfer to boost photocatalytic activities. Appl. Surf. Sci. 2020, 507, 145145. [Google Scholar] [CrossRef]
- Dat, T.D.; Minh, D.T.C.; An, H.; Hai, N.D.; Nam, N.T.H.; Lam, N.H.; Hieu, N.H. Synthesis of selenium and fluorine co-doped graphitic carbon nitride for photodegradation of toxic organic pollutants and hydrogen peroxide photoproduction. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100920. [Google Scholar]
- Liu, Q.; Cui, K.; Cui, M.; Sun, S.; Li, H. Sulfur and chloride co-doped g-C3N4 in photocatalytic peroxymonosulfate activation for enhanced acetaminophen removal: Combination of experiments and DFT calculations. J. Environ. Chem. Eng. 2024, 12, 112857. [Google Scholar]
- Chen, Z.; Bu, Y.; Wang, L.; Wang, X.; Ao, J.-P. Single-sites Rh-phosphide modified carbon nitride photocatalyst for boosting hydrogen evolution under visible light. Appl. Catal. B Environ. 2020, 274, 119117. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar]
- Hu, C.; Hung, W.-Z.; Wang, M.-S.; Lu, P.-J. Phosphorus and sulfur codoped g-C3N4 as an efficient metal-free photocatalyst. Carbon 2018, 127, 374–383. [Google Scholar]
- Tan, J.; Tian, N.; Li, Z.; Li, J.; Yao, X.; Vakili, M.; Lu, Y.; Zhang, T. Intrinsic defect engineering in graphitic carbon nitride for photocatalytic environmental purification: A review to fill existing knowledge gaps. Chem. Eng. J. 2021, 421, 127729. [Google Scholar] [CrossRef]
- Chen, J.; Gao, W.; Lu, Y.; Ye, F.; Huang, S.; Peng, Y.; Yang, X.; Cai, Y.; Qu, J.; Hu, J. Anti-defect Engineering of Crystalline g-C3 N4 Nanostructures for Efficient Photocatalytic In Situ H2O2 Production. ACS Appl. Nano Mater. 2023, 6, 3927–3935. [Google Scholar]
- Lin, Y.; Yang, Y.; Guo, W.; Wang, L.; Zhang, R.; Liu, Y.; Zhai, Y. Preparation of double-vacancy modified carbon nitride to greatly improve the activity of photocatalytic hydrogen generation. Appl. Surf. Sci. 2021, 560, 150029. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, G.; Wang, J.; Wang, K.; Yan, S.; Su, Y. Nitrogen vacancy induced in situ g-C3N4 p-n homojunction for boosting visible light-driven hydrogen evolution. J. Colloid Interface Sci. 2021, 587, 110–120. [Google Scholar]
- Yang, Z.; Zhang, Y.; Zhang, H.; Zhao, J.; Shi, H.; Zhang, M.; Yang, H.; Zheng, Z.; Yang, P. Nitrogen vacancies in polymeric carbon nitrides promote CO2 photoreduction. J. Catal. 2022, 409, 12–23. [Google Scholar] [CrossRef]
- Chang, L.-L.; Hu, C.; Wang, C.-Y.; Chen, W.-L.; Yamada, K.; Wu, A.-Y.; Yoshida, M.; Chien, S.-C.; Tung, K.-L. Synergistic effects of carbon and nitrogen vacancies in carbon nitride for photocatalytic H2 production and tetracycline oxidation. Sep. Purif. Technol. 2025, 354, 129346. [Google Scholar]
- Li, D.; Liu, Y.; Wen, C.; Huang, J.; Li, R.; Liu, H.; Zhong, J.; Chen, P.; Lv, W.; Liu, G. Construction of dual transfer channels in graphitic carbon nitride photocatalyst for high-efficiency environmental pollution remediation: Enhanced exciton dissociation and carrier migration. J. Hazard. Mater. 2022, 436, 129171. [Google Scholar] [CrossRef]
- Deng, A.; Sun, Y.; Gao, Z.; Yang, S.; Liu, Y.; He, H.; Zhang, J.; Liu, S.; Sun, H.; Wang, S. Internal electric field in carbon nitride-based heterojunctions for photocatalysis. Nano Energy 2023, 108, 108228. [Google Scholar] [CrossRef]
- Wen, X.; Shen, C.; Fei, Z.; Fang, D.; Liu, Z.; Dai, J.; Niu, C. Recent developments on AgI based heterojunction photocatalytic systems in photocatalytic application. Chem. Eng. J. 2020, 383, 123083. [Google Scholar] [CrossRef]
- Muhammad, W.; Ali, W.; Khan, M.A.; Ali, F.; Zada, A.; Ansar, M.Z.; Yap, P.-S. Construction of visible-light-driven 2D/2D NiFe2O4/g-C3N4 Z-scheme heterojunction photocatalyst for effective degradation of organic pollutants and CO2 reduction. J. Environ. Chem. Eng. 2024, 12, 113409. [Google Scholar]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar]
- Zhang, C.; Lin, L.; Zhou, M.; Wang, Y.; Xu, S.; Chen, X.; Li, Z. Dual functional S-scheme ZnIn2S4/crystalline polymeric carbon nitride (ZIS/CPCN) heterojunction for efficient photocatalytic hydrogen evolution and degradation of levofloxacin. Chem. Eng. J. 2024, 495, 153563. [Google Scholar] [CrossRef]
- Chahkandi, M.; Zargazi, M.; Ghiasabadi, K.B.; Chung, J.S.; Yazdi, M.K.; Saeb, M.R.; Baghayeri, M. Graphitic carbon nitride nanosheets decorated with HAp@Bi2S3 core–shell nanorods: Dual S-scheme 1D/2D heterojunction for environmental and hydrogen production solutions. Chem. Eng. J. 2024, 499, 155886. [Google Scholar]
- Li, X.; Wang, X.; Antonietti, M. Mesoporous g-C3N4 nanorods as multifunctional supports of ultrafine metal nanoparticles: Hydrogen generation from water and reduction of nitrophenol with tandem catalysis in one step. Chem. Sci. 2012, 3, 2170–2174. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.; Zeng, G.; Huang, J.; Lai, C.; Zhou, C.; Wang, W.; Guo, H.; Xue, W.; et al. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B Environ. 2019, 245, 87–99. [Google Scholar]
- Fan, F.; Yu, J.; Jiang, J.; Xiu, H.; Wang, Y.; Cui, Y.; Wang, Y. Iron-doped and nitrogen-deficient tubular carbon nitride enables synergistic photocatalysis-self-Fenton degradation of tetracycline. J. Environ. Chem. Eng. 2025, 13, 117562. [Google Scholar] [CrossRef]
- Li, L.; Zeng, H.; Zhong, L.; Wu, H.; Zheng, J.; Zhou, Z.; Liu, J.; Tang, R.; Deng, Y.; Huang, Y. Tailoring a donor-acceptor structure in oxygen and cyano group co-modified carbon nitride for enhanced photocatalytic imidacloprid degradation. Chem. Eng. J. 2025, 518, 164671. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhan, X.; Hong, B.; Xia, Y.; Ding, Y.; Cai, T.; Yin, K.; Wang, X.; Yang, L.; Luo, S. Surface atom rearrangement on carbon nitride for enhanced photocatalysis degradation of antibiotics under visible light. Chem. Eng. J. 2023, 452, 139434. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, P.; Che, H.; Ao, Y. Co-doping and N defect synergistically boosting 2e− oxygen reduction reaction on carbon nitride for full-day degradation of nitenpyram. J. Colloid Interface Sci. 2025, 690, 137319. [Google Scholar] [CrossRef]
- Bierbaumer, S.; Nattermann, M.; Schulz, L.; Zschoche, R.; Erb, T.J.; Winkler, C.K.; Tinzl, M.; Glueck, S.M. Enzymatic Conversion of CO2: From Natural to Artificial Utilization. Chem. Rev. 2023, 123, 5702–5754. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Song, Y.; Cao, L.; Dou, Y.; Yu, J.; Zhang, Y.; He, J.; Dai, W.; Yao, C.; et al. Enhanced and accelerated degradation of PFOA using visible light—Applying semiconductor carbon nitride as an accelerator. J. Environ. Chem. Eng. 2024, 12, 111653. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Zhang, X.; Wang, S.; Xia, Z.; Zeng, G.; Ding, J.; Ren, N. Construction of Fe3O4@β-CD/g-C3N4 nanocomposite catalyst for degradation of PCBs in wastewater through photodegradation and heterogeneous Fenton oxidation. Chem. Eng. J. 2022, 429, 132445. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Kong, L.; Wang, Y.; Zhang, S.; Zhang, X.; Ding, J.; Ren, N. Solar light photocatalytic transformation of heptachlorobiphenyl (PCB 180) using g-C3N4 based magnetic porous photocatalyst. J. Hazard. Mater. 2022, 427, 128105. [Google Scholar] [CrossRef]
- Sathish Kumar, P.; Shobana, B.; Prakash, P. Light harvesting enhancement through band structure engineering in graphite carbon nitride/polydopamine nanocomposite photocatalyst: Addressing persistent organophosphorus pesticide pollution in water systems. Chemosphere 2024, 354, 141708. [Google Scholar] [CrossRef]
- Su, B.; Ma, X.; Ran, L.; Wu, S.; Hou, B. Improving the photodegradation efficiency of POPs in oily sewage: Design and synthesis of G-C3N4-based photocatalysts incorporating a pickering emulsion catalytic strategy. J. Environ. Chem. Eng. 2023, 11, 111297. [Google Scholar] [CrossRef]
- Bharathi, D.; Lee, J.; Albeshr, M.F.; Fahad Alrefaei, A.; Le, T.T.; Mathimani, T. Enhanced photocatalytic degradation of polycyclic aromatic hydrocarbon by graphitic carbonitride-nickel (g–C3N4–Ni) nanocomposite. Chemosphere 2023, 345, 140464. [Google Scholar]
- Karuppusamy, I.; Surendiran, M.; Al-Humaid, L.A.; Aldawsari, M. Understanding the effective breakdown of PAH s in water through the use of g–C3N4–Ag–Cu–Ni nanocomposites. Chemosphere 2023, 344, 140125. [Google Scholar]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Gu, X.; Mei, J.; Lai, J.; Lv, S.; Yang, J.; Cui, S.; Chen, S. Synthesis of Z-Scheme heterojunction ZnNb2O6/g-C3N4 nanocomposite as a high efficient photo-catalyst for the degradation of 2,4-DCP under simulated sunlight. Mater. Res. Bull. 2020, 130, 110939. [Google Scholar] [CrossRef]
- Sim, L.C.; Tai, J.Y.; Leong, K.H.; Saravanan, P.; Tan, S.T.; Chong, W.C.; Aziz, A.A. Metal free and sunlight driven g-C3N4 based photocatalyst using carbon quantum dots from Arabian dates: Green strategy for photodegradation of 2,4-dichlorophenol and selective detection of Fe3+. Diam. Relat. Mater. 2021, 120, 108679. [Google Scholar] [CrossRef]
- Gak, J.C.D.; Mokhtar Mohamed, M.; Ibrahim, I.; Matsushita, Y.; Mawgood, A.A. Efficient sonocatalytic degradation of pyrene using a novel and magnetically recoverable graphitic carbon nitride—Cobalt ferrite—Graphene oxide (g-C3N4/CoFe2O4@GO) nanocomposite with an enhanced Z-scheme electron transfer pathway. Ceram. Int. 2024, 50, 34431–34441. [Google Scholar] [CrossRef]
- Wang, S.; He, F.; Zhao, X.; Zhang, J.; Ao, Z.; Wu, H.; Yin, Y.; Shi, L.; Xu, X.; Zhao, C.; et al. Phosphorous doped carbon nitride nanobelts for photodegradation of emerging contaminants and hydrogen evolution. Appl. Catal. B Environ. 2019, 257, 117931. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, Y.; Yao, J.; Kong, D.; Chu, M.; Zhang, G.; Wang, Y. Enhancing dechlorination and inhibiting H2 evolution: Surface N-hydroxymethylation of Pd-loaded carbon nitride for photocatalytic elimination of polychlorinated aromatics. J. Catal. 2024, 438, 115732. [Google Scholar]
- Liu, X.; Zong, H.; Tan, X.; Wang, X.; Qiu, J.; Kong, F.; Zhang, J.; Fang, S. Facile synthesis of modified carbon nitride with enhanced activity for photocatalytic degradation of atrazine. J. Environ. Chem. Eng. 2021, 9, 105807. [Google Scholar] [CrossRef]
- Mohamed, R.M. Synthesis and characterization of AgCl@graphitic carbon nitride hybrid materials for the photocatalytic degradation of atrazine. Ceram. Int. 2015, 41, 1197–1204. [Google Scholar]
- Padhiari, S.; Tripathy, M.; Hota, G. Nitrogen-Doped Reduced Graphene Oxide Covalently Coupled with Graphitic Carbon Nitride/Sulfur-Doped Graphitic Carbon Nitride Heterojunction Nanocatalysts for Photoreduction and Degradation of 4-Nitrophenol. ACS Appl. Nano Mater. 2021, 4, 7145–7161. [Google Scholar] [CrossRef]
- Vandenberg, L.N. Endocrine disrupting chemicals: Strategies to protect present and future generations. Expert Rev Endocrinol Metab 2021, 16, 135–146. [Google Scholar] [CrossRef]
- Hercog, K.; Maisanaba, S.; Filipič, M.; Sollner-Dolenc, M.; Kač, L.; Žegura, B. Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci. Total Environ. 2019, 687, 267–276. [Google Scholar] [CrossRef]
- Mu, F.; Dai, B.; Wu, Y.; Yang, G.; Li, S.; Zhang, L.; Xu, J.; Liu, Y.; Zhao, W. 2D/3D S-scheme heterojunction of carbon nitride/iodine-deficient bismuth oxyiodide for photocatalytic hydrogen production and bisphenol A degradation. J. Colloid Interface Sci. 2022, 612, 722–736. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Y.; Chen, C.; He, J.; Zhu, M.; Chen, J.; Zhang, C. A multi-structural carbon nitride co-modified by Co, S to dramatically enhance mineralization of Bisphenol f in the photocatalysis-PMS oxidation coupling system. Chem. Eng. J. 2021, 422, 130035. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, S.; Gu, J.; Liu, R.; Wang, Z.; Chen, H.; Jiang, F. Visible-light photocatalytic degradation of bisphenol A using cobalt-to-oxygen doped graphitic carbon nitride with nitrogen vacancies via metal-to-ligand charge transfer. J. Hazard. Mater. 2020, 384, 121247. [Google Scholar] [CrossRef]
- Ming, H.; Wei, D.; Yang, Y.; Chen, B.; Yang, C.; Zhang, J.; Hou, Y. Photocatalytic activation of peroxymonosulfate by carbon quantum dots functionalized carbon nitride for efficient degradation of bisphenol A under visible-light irradiation. Chem. Eng. J. 2021, 424, 130296. [Google Scholar] [CrossRef]
- Luo, L.; Cha, C.; Meng, D.; Zou, W.; Xia, L.; Jiang, F.; Ma, X.; Dai, J. Photocatalytic activation of persulfate for rapid degradation of 17α-ethinylestradiol over quasi-full-visible-responsive Pg-C3N4/RGO/BiOI. J. Environ. Chem. Eng. 2024, 12, 113489. [Google Scholar] [CrossRef]
- Xu, K.; Jiao, L.; Wang, C.; Bu, Y.; Tang, Y.; Qiu, L.; Zhang, Q.; Wang, L. Nonylphenol photodegradation by novel ternary MIL-100(Fe)/ZnFe2O4/PCN composite under visible light irradiation via double charge transfer process. J. Environ. Sci. 2022, 111, 93–103. [Google Scholar] [CrossRef]
- Bi, L.; Fu, G.; Cai, D.; Shen, J.; Zhang, J.; Kang, J.; Cheng, Y.; Yan, P.; Zhou, Y.; Chen, Z. Electron rich P doped g-C3N4 for photodegradation of 2,4-dichlorophenoxyacetic acid under visible light by improving oxygen adsorption: Performance and catalytic mechanism. Sep. Purif. Technol. 2023, 306, 122562. [Google Scholar]
- Liu, X.; Yan, L.; Hu, X.; Feng, H.; Guo, B.; Ha, X.; Xu, H. Facile synthesis of B and P doped g-C3N4 for enhanced synergetic activity between photocatalytic water splitting and BPA degradation. Int. J. Hydrogn Energy 2023, 48, 13181–13188. [Google Scholar] [CrossRef]
- Lu, D.; Loh, J.W.J.; Lau, H.X.Y.; Lin, X.H.; Li, S.F.Y. Hydroxyl-rich carbon nitride microspheres with carbon doping for visible-light driven photocatalytic degradation of endocrine disrupting chemicals. Mater. Today Sustain. 2023, 22, 100347. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Gu, P.; Ma, R.; Wen, T.; Zhao, G.; Li, L.; Ai, Y.; Hu, C.; Wang, X. Enhanced photodegradation of toxic organic pollutants using dual-oxygen-doped porous g-C3N4: Mechanism exploration from both experimental and DFT studies. Appl. Catal. B Environ. 2019, 248, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Shao, L.; Du, J.; Gao, Q.; Fu, S.; Li, L.; Hu, M.; Zhao, F.; Zhou, J. Promoting charge separation in dual defect mediated Z-scheme MoS2/g-C3N4 photocatalysts for enhanced photocatalytic degradation activity: Synergistic effect insight. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124668. [Google Scholar] [CrossRef]
- Katsumata, H.; Higashi, F.; Kobayashi, Y.; Tateishi, I.; Furukawa, M.; Kaneco, S. Dual-defect-modified graphitic carbon nitride with boosted photocatalytic activity under visible light. Sci. Rep. 2019, 9, 14873. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, L.; Wang, X.; Shi, H. Fabrication of AgCl/Ag3PO4/graphitic carbon nitride heterojunctions for enhanced visible light photocatalytic decomposition of methylene blue, methylparaben and E. coli. RSC Adv. 2021, 11, 6383–6394. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.; Sampaio, M.J.; Dražić, G.; Faria, J.L.; Silva, C.G. Efficient removal of parabens from real water matrices by a metal-free carbon nitride photocatalyst. Sci. Total Environ. 2020, 716, 135346. [Google Scholar] [CrossRef]
- Fernandes, E.; Drosopoulou, S.; Mazierski, P.; Miodyńska, M.; Gołaszewska, D.; Zaleska-Medynska, A.; Martins, R.C.; Gomes, J. Carbon nitride photoactivation evaluation and degradation of a mixture of parabens by ozone assistance. J. Water Process Eng. 2022, 49, 103018. [Google Scholar] [CrossRef]
- Shittu, F.B.; Iqbal, A.; Ahmad, M.N.; Yusop, M.R.; Ibrahim, M.N.M.; Sabar, S.; Wilson, L.D.; Yanto, D.H.Y. Insight into the photodegradation mechanism of bisphenol-A by oxygen doped mesoporous carbon nitride under visible light irradiation and DFT calculations††Electronic supplementary information (ESI) available: Effect of reaction parameters and kinetics on the photodegradation of BPA. RSC Adv. 2022, 12, 10409–10423. [Google Scholar]
- Sun, Y.; Luo, H.; Iboleon, R.; Wang, Z. Fate of antibiotic resistance genes and class 1 integrons during sludge treatment using pilot-scale anaerobic digestion with thermal hydrolysis pretreatment. Bioresour. Technol. 2022, 364, 128043. [Google Scholar] [CrossRef]
- Dou, M.; Wang, J.; Gao, B.; Xu, C.; Yang, F. Photocatalytic difference of amoxicillin and cefotaxime under visible light by mesoporous g-C3N4: Mechanism, degradation pathway and DFT calculation. Chem. Eng. J. 2020, 383, 123134. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Chen, C.-Y.; Boonyuen, S.; Tang, W.K.; Juan, J.C. Mesoporous I-doped-g-C3N4 with C vacancies as superior visible-light-driven photocatalyst for removal of tetracycline antibiotic. J. Alloys Compd. 2024, 1009, 176772. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Jin, X.; Zheng, X.; Wang, Y.; Wei, D.; Zhang, Q.; Feng, Y.; Xie, Z.; Chen, P.; et al. Highly active metal-free carbon dots/g-C3N4 hollow porous nanospheres for solar-light-driven PPCPs remediation: Mechanism insights, kinetics and effects of natural water matrices. Water Res. 2020, 172, 115492. [Google Scholar]
- Wu, Z.; Liang, Y.; Yuan, X.; Zou, D.; Fang, J.; Jiang, L.; Zhang, J.; Yang, H.; Xiao, Z. MXene Ti3C2 derived Z–scheme photocatalyst of graphene layers anchored TiO2/g–C3N4 for visible light photocatalytic degradation of refractory organic pollutants. Chem. Eng. J. 2020, 394, 124921. [Google Scholar]
- Liu, M.; Zhang, D.; Han, J.; Liu, C.; Ding, Y.; Wang, Z.; Wang, A. Adsorption enhanced photocatalytic degradation sulfadiazine antibiotic using porous carbon nitride nanosheets with carbon vacancies. Chem. Eng. J. 2020, 382, 123017. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A. Synthesis of NiFe2O4/Ag nanoparticles immobilized on mesoporous g-C3N4 sheets and application for degradation of antibiotics. J. Photochem. Photobiol. A Chem. 2021, 418, 113398. [Google Scholar]
- Zhou, Y.; Jiang, D.; Wang, Z.; Yi, L.; Sun, J.; Liu, D.; Yu, X.; Chen, Y. Bandgap engineering of carbon nitride by formic acid assisted thermal treatment for photocatalytic degradation of tetracycline hydrochloride. Chem. Eng. J. 2024, 485, 149830. [Google Scholar] [CrossRef]
- Razavi-Esfali, M.; Mahvelati-Shamsabadi, T.; Fattahimoghaddam, H.; Lee, B.-K. Highly efficient photocatalytic degradation of organic pollutants by mesoporous graphitic carbon nitride bonded with cyano groups. Chem. Eng. J. 2021, 419, 129503. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, X.; Li, R.; Xie, Y.; Tang, Q.; Ren, B. Hydrothermal synthesis of 2D/2D BiOCl/g-C3N4 Z-scheme: For TC degradation and antimicrobial activity evaluation. Opt. Mater. 2020, 108, 110170. [Google Scholar]
- Saravanakumar, K.; Park, C.M. Rational design of a novel LaFeO3/g-C3N4/BiFeO3 double Z-scheme structure: Photocatalytic performance for antibiotic degradation and mechanistic insight. Chem. Eng. J. 2021, 423, 130076. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, G.; Huang, D.; Zhang, C.; He, R.; Zhou, C.; Wang, W.; Xiong, W.; Song, B.; Huan, Y.; et al. In Situ Grown Single-Atom Cobalt on Polymeric Carbon Nitride with Bidentate Ligand for Efficient Photocatalytic Degradation of Refractory Antibiotics. Small 2020, 16, 2001634. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Ma, C.; Guan, Q.; Lu, Z.; Huo, P.; Yan, Y. Melamine modified P25 with heating method and enhanced the photocatalytic activity on degradation of ciprofloxacin. Appl. Surf. Sci. 2015, 329, 17–22. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Sufian, S. Hybrid 2D/3D g-C3N4/BiVO4 photocatalyst decorated with RGO for boosted photoelectrocatalytic hydrogen production from natural lake water and photocatalytic degradation of antibiotics. J. Mol. Liq. 2020, 314, 113530. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kim, D.G.; Ko, S.O. Changes in the catalytic activity of oxygen-doped graphitic carbon nitride for the repeated degradation of oxytetracycline. Chemosphere 2022, 307, 135870. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; He, H.; Lu, M.; Pei, Y.; Peng, H.; Zhou, W.; Guo, C.; Wang, J. Serine mesoporous organosilicon modified carbon nitride (g-C3N4@Ser-PMO) for degradation of ofloxacin. Microporous Mesoporous Mater. 2024, 373, 113133. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, J.; Yu, F.; Su, Q.; Wang, H.; Wang, L.; Guo, Y.; Xie, H. Broad-bandgap porous graphitic carbon nitride with nitrogen vacancies and oxygen doping for efficient visible-light photocatalytic degradation of antibiotics. Environ. Pollut. 2023, 335, 122268. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Shan, R.; Gu, J.; Wang, S.; Cheng, L.; Yuan, H.; Chen, Y. Unraveling the impact of three coordinate nitrogen (N3c) vacancies in porous carbon nitride nanobelt for boosted photocatalytic degradation of microplastics and antibiotics. Appl. Catal. B Environ. Energy 2024, 358, 124402. [Google Scholar] [CrossRef]
- Haward, M. Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nat. Commun. 2018, 9, 667. [Google Scholar] [CrossRef]
- Kaur, R.; Marwaha, A.; Chhabra, V.; Kaushal, K.; Kim, K.-H.; Tripathi, S.K. Facile synthesis of a Cu-based metal-organic framework from plastic waste and its application as a sensor for acetone. J. Clean. Prod. 2020, 263, 121492. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Doan, H.V.; Le Hoang Tan, D.; Lam, T.D. Advanced g-C3N4 and bimetallic FeNi-BTC integration with carbon quantum dots for removal of microplastics and antibiotics in aqueous environments. J. Environ. Chem. Eng. 2024, 12, 112965. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Z.; Jiang, J.; Li, R.; Xiong, J. Preparation of heterojunction C3N4/WO3 photocatalyst for degradation of microplastics in water. Chemosphere 2023, 337, 139206. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Wan, S.; Li, S.; Ou, M.; Song, F.; Fan, X.; Zhong, Q. Tuning the surface hydrophilicity of a C3N4 nanosheet for efficient photocatalytic H2 evolution coupled with microplastic degradation. Int. J. Hydrog. Energy 2023, 48, 27599–27610. [Google Scholar] [CrossRef]
- Jia, R.; Li, D.; Wang, P.; Li, T.; Chen, J.; Zhang, L.; Zhao, Q.; Cheng, X. Constructing Z-scheme MoO3-x/g-C3N4 heterojunction for highly efficient photocatalytic degradation of microplastic polyaniline. Appl. Surf. Sci. 2025, 715, 164506. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, H.; Li, X.; Li, F.; Tang, H.; She, X. Modulating reactive oxygen species in O, S co-doped C3N4 to enhance photocatalytic degradation of microplastics. Acta Phys. -Chim. Sin. 2025, 41, 100052. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, C.; Hu, J.; Zou, X.; Qi, Y.; Ge, Z.; Zhang, S.; Wang, H.; Wang, Y. Amphiphilic CoP/CN heterojunction for photocatalytic microplastics degradation synergistic hydrogen generation. Chem. Eng. Sci. 2025, 307, 121336. [Google Scholar] [CrossRef]
- Yi, X.-H.; Ma, S.-Q.; Du, X.-D.; Zhao, C.; Fu, H.; Wang, P.; Wang, C.-C. The facile fabrication of 2D/3D Z-scheme g-C3N4/UiO-66 heterojunction with enhanced photocatalytic Cr(VI) reduction performance under white light. Chem. Eng. J. 2019, 375, 121944. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Xue, Z.; Bian, Z.; Lin, X.; Hong, Y. Tubular carbon nitride decorated with Ag2V4O11 quantum dots: A binary heterostructure photocatalyst for tetracycline degradation. J. Phys. Chem. Solids 2025, 208, 113120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).