Studies Toward Persilylation of π-Cyclopentadienyl Complexes of Fe and Ru. Molecular Structures of [Fe(C5H5){C5(SiMe2H)5}], [Fe(C5H5){C5Br3(SiMe3)2}] and [Fe(C5H5){C5Br2(SiMe3)3}]
Abstract
1. Introduction
2. Results
2.1. Synthesis
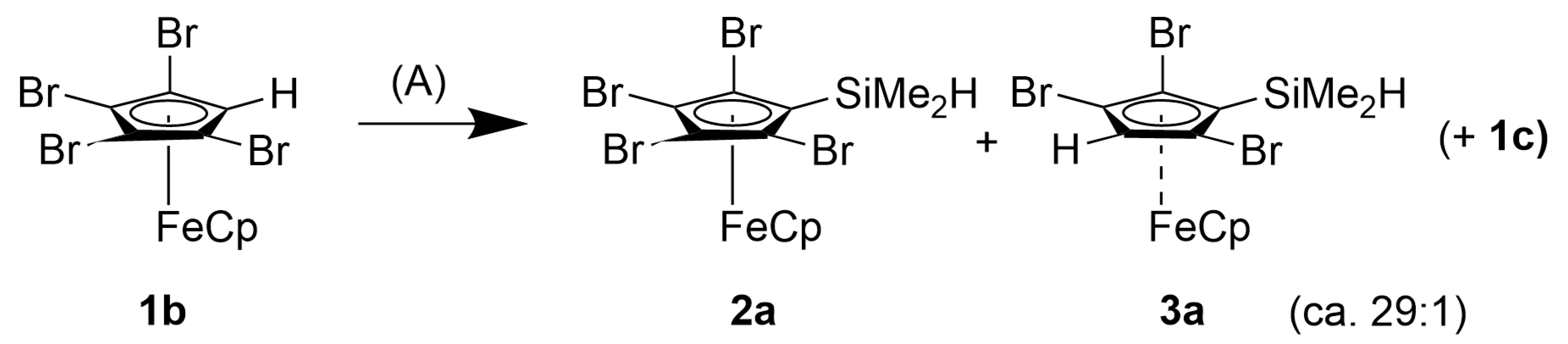
2.1.1. Dimethylsilylferrocenes
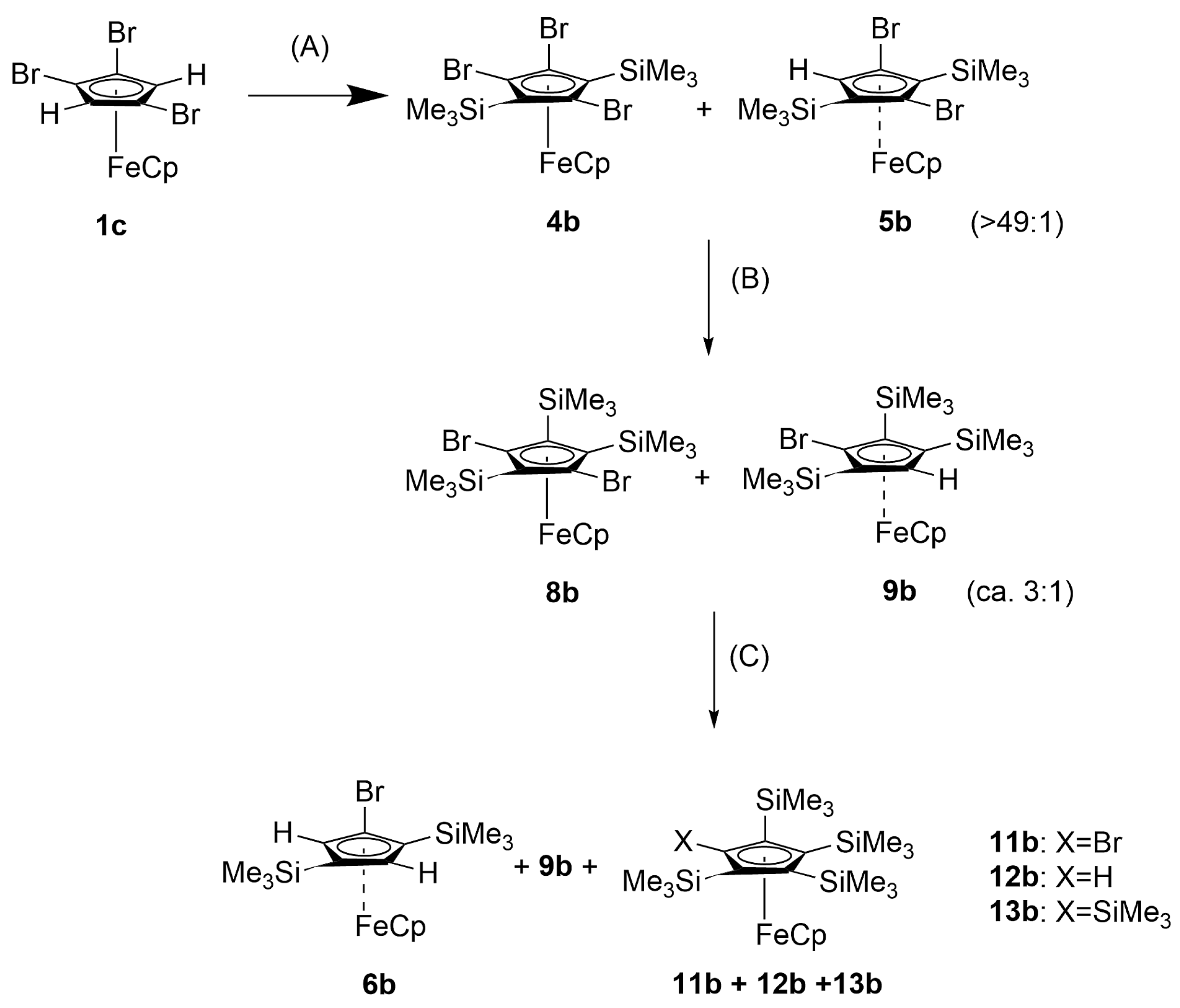
2.1.2. Trimethylsilylferrocenes
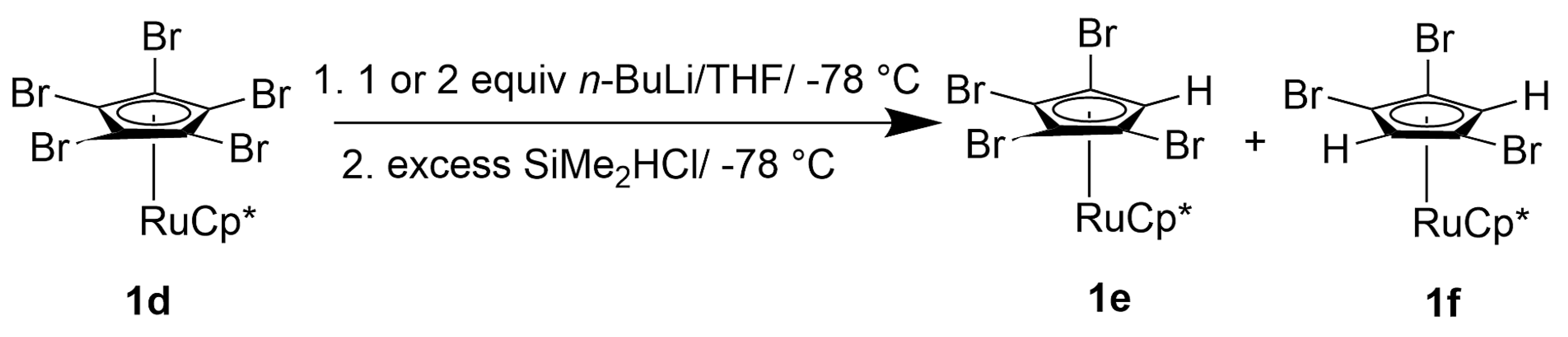
2.1.3. Dimethylsilylruthenocenes
2.2. Crystallography
2.2.1. Pentakis(dimethylsilyl)ferrocene, 13a
2.2.2. 1,2,4-Tribromo-2,5-bis-trimethylsilyl-ferrocene 4b
2.2.3. 1,3-Dibromo-2,4,5-tris-trimethylsilyl-ferrocene 8b
3. Discussion
4. Materials and Methods
4.1. Chemicals and Instrumentation
4.2. Synthesis
4.2.1. 1,2,3,4-Tetrabromo-5-dimethylsilylferrocene, [Fe(C5H5)(C5Br4SiMe2H)], 2a
4.2.2. 1,2,4-Tribromo-3,5-bis(dimethylsilyl)ferrocene, [Fe(C5H5){C5Br3(SiMe2H)2}], 4a
4.2.3. 1,3-Dibromo-2,4,5-tris(dimethylsilyl)ferrocene, [Fe(C5H5){C5Br2(SiMe2H)3}], 8a
4.2.4. 1-Bromo-2,3,4,5-tetrakis(dimethylsilyl)ferrocene, [Fe(C5H5){C5Br(SiMe2H)4}], 11a
4.2.5. 1,2,3,4,5-Pentakis(dimethylsilyl)ferrocene, [Fe(C5H5){C5(SiMe2H)5}], 13a
4.2.6. 1,2,4-Tribromo-3,5-bis(trimethylsilyl)ferrocene, [Fe(C5H5){C5Br3(SiMe3)2}], 4b
4.2.7. 1,3-Dibromo-2,4,5-tris(trimethylsilyl)ferrocene, [Fe(C5H5){C5Br2(SiMe3)3}], 8b
4.2.8. Reaction of 8b with n-BuLi and SiMe3Cl
4.2.9. 1,2,4-Tris(trimethylsilyl)ferrocene, [Fe(C5H5){C5H2(SiMe3)3}] 10b
4.2.10. (1,2,4-Tribromo-2,5-bis(dimethylsilyl)(6,7,8,9,10-pentamethyl)ruthenocene, [Ru(C5Me5){C5Br3(SiMe2H)2}] (4c)
4.2.11. (1,3-Dibromo-2,4,5-tris(dimethylsilyl)(6,7,8,9,10-pentamethyl)ruthenocene, [Ru(C5Me5){C5Br2(SiMe2H)3}] (8c)
4.2.12. Reaction of Crude 8c with Excessive t-BuLi and SiMe2HCl
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winter, C.H.; Zhou, X.-X.; Heeg, M.J. Approaches to Hexane-Soluble Cationic Organometallic Lewis Acids. Synthesis, Structure, and Reactivity of Titanocene Derivatives Containing Polysilylated Cyclopentadienyl Ligands. Inorg. Chem. 1992, 31, 1808–1815. [Google Scholar] [CrossRef]
- Siemeling, U.; Jutzi, P. Trinuclear Homo- and Heterometallic Complexes of a Doubly Bridged Cyclopentadienyl Ligand. Chem. Ber. 1992, 125, 31–35. [Google Scholar] [CrossRef]
- Siddiq, M.A.; Siddiqi, R.A.; Atakan, B.; Roth, N.; Lang, H. Thermal Stability and Sublimation Pressures of Some Ruthenocene Compounds. Materials 2010, 3, 1172–1185. [Google Scholar] [CrossRef]
- Tuchscherer, A.; Georgi, C.; Roth, N.; Schaarschmidt, D.; Rüffer, T.; Waechtler, T.; Schulz, S.E.; Oswald, S.; Gessner, T.; Lang, H. Ruthenocenes and Half-Open Ruthenocenes: Synthesis, Characterization, and Their Use as CVD Precursors for Ruthenium Thin Film Deposition. Eur. J. Inorg. Chem. 2012, 2012, 4867–4876. [Google Scholar] [CrossRef]
- Gaur, R.; Mishra, L.; Siddiqi, M.A.; Atakan, B. Ruthenium complexes as precursors for chemical vapor-deposition (CVD). RSC Adv. 2014, 4, 33785. [Google Scholar] [CrossRef]
- Assim, K.; Jeschke, J.; Jakob, A.; Melzer, M.; Georgi, C.; Schulz, S.E.; Gessner, T.; Lang, H. Manganese half-sandwich complexes as metal-organic chemical vapor deposition precursors for manganese-based thin films. Thin Solid Films 2016, 619, 266–272. [Google Scholar] [CrossRef]
- Okuda, J. Hindering the formation of ferrocenes: Mono(cyclopentadienyl)halo iron complexes [Fe(C5R5)X] containing a sterically bulky cyclopentadienyl ligand. J. Organomet. Chem. 2001, 637–639, 78–792. [Google Scholar] [CrossRef]
- Stammler, H.-G.; Jutzi, P.; Wieland, W.; Neumann, B. (Tributylphosphine-P)[1,2,4-tris(trimethylsilyl)cyclopentadienyl]silver(I). Acta Cryst. 1998, C54, IUC9800064. [Google Scholar] [CrossRef]
- Bauer, H.; Weismann, J.; Saurenz, D.; Färber, D.; Schär, M.; Gidt, W.; Schädlich, I.; Wolmershäuser, G.; Harder, S.; Sitzmann, H. Chromocene, ferrocene, cobaltocene, and nickelocene derivatives with isopropyl and methyl or trimethylsilyl substituents. J. Organomet. Chem. 2016, 809, 63–73. [Google Scholar] [CrossRef]
- Walter, M.D.; Sofield, C.D.; Booth, C.H.; Andersen, R.A. Spin Equilibria in Mono-meric Manganocenes: Solid-State Magnetic and EXAFS Studies. Organometallics 2009, 28, 2005–2019. [Google Scholar] [CrossRef]
- Harvey, M.J.; Quisenberry, K.T.; Hanusa, T.P.; Young, V.G., Jr. A Homologous Series of Base-Free Organo(alkaline-earth) Metallocenes: Synthesis and Molecular Structures of [1,2,4-(SiMe3)3C5H2]2(Ca, Sr, Ba). Eur. J. Inorg. Chem. 2003, 2023, 3383–3390. [Google Scholar] [CrossRef]
- Corner, S.; Goodwin, C.; Ortu, F.; Evans, P.; Zhang, H.; Gransbury, G.; Whitehead, G.; Mills, D. Synthesis of heteroleptic yttrium and dysprosium 1,2,4-tris(trimethylsilyl)cyclopentadienyl complexes. Aust. J. Chem. 2022, 75, 684–697. [Google Scholar] [CrossRef]
- Ortu, F.; Fowler, J.M.; Burton, M.; Formanuik, A.; Mills, D.P. A structural investigation of heteroleptic lanthanide substituted cyclopentadienyl complexes. New J. Chem. 2015, 39, 7633–7639. [Google Scholar] [CrossRef]
- Wang, S.; Wang, D.; Li, T.; Heng, Y.; Hou, G.; Zi, G.; Walter, M.D. Synthesis, Structure, and Reactivity of the Uranium Bipyridyl Complex [{η5-1,2,4-(Me3Si)3C5H2}2U(bipy)]. Organometallics 2022, 41, 1543–1557. [Google Scholar] [CrossRef]
- Ryan, M.F.; Siedle, A.R.; Burk, M.J.; Richardson, D.E. Parameter Scale for Substituent Effects in Cyclopentadienyl Complexes Based on Gas-Phase Electron-Transfer Equilibrium Studies of Ruthenocene Derivatives. Organometallics 1992, 11, 4231–4237. [Google Scholar] [CrossRef]
- Okuda, J.; Herdtweck, E. Synthese und Struktur von 1,1′,2,2′,4,4′-Hexakis(trimethylsilyl)ferrocen (Synthesis and Structure of 1,1‘,2,2‘,4,4‘-Hexakis(trimethylsilyl)ferrocene). Chem. Ber. 1988, 121, 1899–1905. [Google Scholar] [CrossRef]
- Butler, I.R.; Evans, D.M.; Horton, P.N.; Coles, S.J.; Murphy, P.J. 1,1′,2,2′-Tetralithioferrocene and 1,1′,2,2′,3,3′-Hexalithioferrocene: Useful Additions to Ferrocene Precursor Compounds. Organometallics 2021, 40, 600–605. [Google Scholar] [CrossRef]
- Okuda, J.; Albach, R.W.; Herdtweck, E.; Wagner, F.E. Substituent effects in multiply trimethylsilyl-substituted ferrocenes. Molecular Structure of 1,1‘,2,2‘,4,4‘-hexakis(trimethylsilyl)ferrocenium tetrafluoroborate. Polyhedron 1991, 10, 1741–1748. [Google Scholar] [CrossRef]
- Butler, I.R.; Cullen, W.R.; Rettig, S.J. Synthesis of Derivatives of (α-(Dimethylamino)ethyl)ferrocene via Lithiation Reactions and the Structure of 2-(α-(Dimethylamlno)ethyl)-1,1′,3-tris(trimethylsilyl)ferrocene. Organometallics 1986, 5, 1320–1328. [Google Scholar] [CrossRef]
- Sünkel, K.; Stramm, C. Synthesis and molecular structure of C5H2(SiMe3)3-1,2,3]Mn(CO)3, the first complex with a 1,2,3-tris-(trimethylsilyl)cyclopentadienyl ligand. Inorg. Chim. Acta 2000, 298, 33–37. [Google Scholar] [CrossRef]
- Sünkel, K.; Hofmann, J. Systematischer Aufbau von fünffach ringsilylierten Cyc-lopentadienyl-mangan-Komplexen aus [C5Br5]Mn(CO)3. Molekülstruktur von [C5Br3(SiMe3)2]Mn(CO)3. (Systematic Generation of Fivefold Ring-Silylated Cyclopen-tadienyl Manganese Complexes from [C5Br5]Mn(CO)3. Molecular Structure of [C5Br3(SiMe3)2]Mn(CO)3). Chem. Ber. 1993, 126, 1791–1795. [Google Scholar]
- Sünkel, K.; Hofmann, J. Synthesis and Molecular structure of Tricarbonyl(tetrakis(trimethylsilyl)cyclopentadienyl)manganese. J. Coord. Chem. 1993, 30, 261–264. [Google Scholar] [CrossRef][Green Version]
- Shimoda, T.; Kawajiri, R.; Isobe, K.; Nakai, H.; Matsuki, Y. Polysilane Production Process. U.S. Patent Application No. US 2011/0158886 A1, 30 June 2011. [Google Scholar]
- Cuenca, T.; Royo, P. Transition metal complexes with functionalized silyl-substituted cyclopentadienyl and related ligands: Synthesis and reactivity. Coord. Chem. Rev. 1999, 193–195, 447–498. [Google Scholar] [CrossRef]
- Sünkel, K.; Hofmann, J. Pentakis(dimethylsilyl)cymantrene. Organometallics 1992, 11, 3923–3925. [Google Scholar]
- Rupf, S.M.; Sievers, R.; Riemann, P.S.; Reimann, M.; Kaupp, M.; Fasting, C.; Malischewski, M. Persilylation of ferrocene: The ultimate discipline in sterically overcrowded metal complexes. Dalton Trans. 2023, 52, 6870–6875. [Google Scholar] [CrossRef]
- Colomer, E.; Corriu, R.J.P.; Pleixats, R. A novel bidentate silicon containing ligand: Cyclopentadienyldimethylsilane. J. Organomet. Chem. 1990, 381, C1–C6. [Google Scholar] [CrossRef]
- Blockhaus, T.; Zott, F.L.; Zehetmaier, P.M.; Klein-Heßling, C.; Sünkel, K. Tricyanated Ferrocenes; Isolation, structure, electrochemistry and DFT calculations. Z. Anorg. Allg. Chem. 2023, 649, e202200277. [Google Scholar] [CrossRef]
- Muratov, D.V.; Romanov, A.S.; Kudinov, A.R. Non-symmetrically substituted ruthenocenes: Synthesis, structures and bond nature. Electronic effects of substituents in ruthenocene. Russ. Chem. Bull. 2014, 63, 2485–2492. [Google Scholar] [CrossRef]
- Sünkel, K.; Stramm, C.; Lang, M.; Kempinger, W.; Hofmann, J. Reaction of tetra-bromodiazocyclopentadiene with some halide bridged Rh(I), Ru(II) and Ru(III) complexes. Molecular structure of [C5Br5]Rh(COD), a complex with an η1, η4-coordinated cyclopentadienyl ligand. Inorg. Chim. Acta 1998, 269, 111–116. [Google Scholar] [CrossRef]
- Schuster, I.I.; Weissensteiner, W.; Mislow, K. Dynamic Stereochemistry of Hexakis(dimethylsilyl)benzene. J. Amer. Chem. Soc. 1986, 108, 6661–6663. [Google Scholar] [CrossRef]
- Kahr, B.; Chance, J.M.; Mislow, K. Disorder in the Crystal Structures of Hexakis(dimethylsilyl)benzene and its Tricarbonyl Chromium, Molybdenum and Tungsten Complexes. Mol. Cryst. Liq. Cryst. 1982, 210, 195–214. [Google Scholar] [CrossRef]
- Burkhardt, L.; Mueller, C.; Groß, O.A.; Sun, Y.; Sitzmann, H.; Bauer, M. The Bonding Situation in the Dinuclear Tetra-Hydrido Complex [{5CpFe}2(μ-H)4] Revisited by Hard X-Ray Spectroscopy. Inorg. Chem. 2019, 58, 6609–6618. [Google Scholar] [CrossRef]
- Bunnett, J.F. Base-catalyzed halogen dance, and other reactions of aryl halides. Acc. Chem. Res. 1972, 5, 139. [Google Scholar] [CrossRef]
- Sakhaee, N.; Sakhaee, S.; Mobaraki, A.; Kallou, A.; Sakhaee, M.H. Mechanistic pathways for halogen dance reactions in bromothiophenes: A cascade-like pattern. J. Chem. Sci. 2020, 132, 97–106. [Google Scholar] [CrossRef]
- Inoue, K.; Mori, A.; Okano, K. Ultrafast Halogen Dance Reactions of Bromoarenes Enabled by Catalytic Potassium Hexamethyldisilazide. Chem. Eur. J. 2024, 30, e202400104. [Google Scholar] [CrossRef] [PubMed]
- Korb, M.; Lang, H. Rearrangements and Migrations along the Ferrocene Periphery: On the Way to Planar-Chiral and (Multi)Substitution Patterns. Eur. J. Inorg. Chem. 2022, 2022, e202100946. [Google Scholar] [CrossRef]
- Tazi, M.; Erb, W.; Halauko, Y.S.; Ivashkevich, O.A.; Matulis, V.E.; Roisnel, T.; Dorcet, V.; Mongin, F. From 2- to 3-Substituted Ferrocene Carboxamides or How to Apply Halogen “Dance” to the Ferrocene Series. Organometallics 2017, 36, 4770–4778. [Google Scholar] [CrossRef]
- Butler, I.R. Sitting Out the Halogen Dance. Room-Temperature Formation of 2,2′-Dilithio-1,1′-dibromoferrocene. TMEDA and Related Lithium Complexes: A Synthetic Route to Multiply Substituted Ferrocenes. Organometallics 2021, 40, 3240–3244. [Google Scholar] [CrossRef]
- Pita-Milleiro, A.; Alferez, M.G.; Moreno, J.J.; Espada, M.F.; Maya, C.; Campos, J. Unveiling the Latent Reactivity of Cp* Ligands (C5Me5-) toward Carbon Nucleophiles on an Iridium Complex. Inorg. Chem. 2023, 62, 5961–5971. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Ebata, K.; Kabuto, C.; Sekiguchi, A. Synthesis, Properties and Molecular Structure of Highly Distorted Hexakis(trimethylsilyl)benzene. J. Amer. Chem. Soc. 1990, 112, 1799–1803. [Google Scholar] [CrossRef]
- Sünkel, K.; Bernhartzeder, S. New high-yield synthesis of monobromoferrocene and simplified procedure for the synthesis of pentabromoferrocene. Molecular structures of 1,2,3-tribromoferrocene and 1,2,3,4,5-pentabromoferrocene. J. Organomet. Chem. 2011, 696, 1536–1540. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT- integrated space group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WINGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]



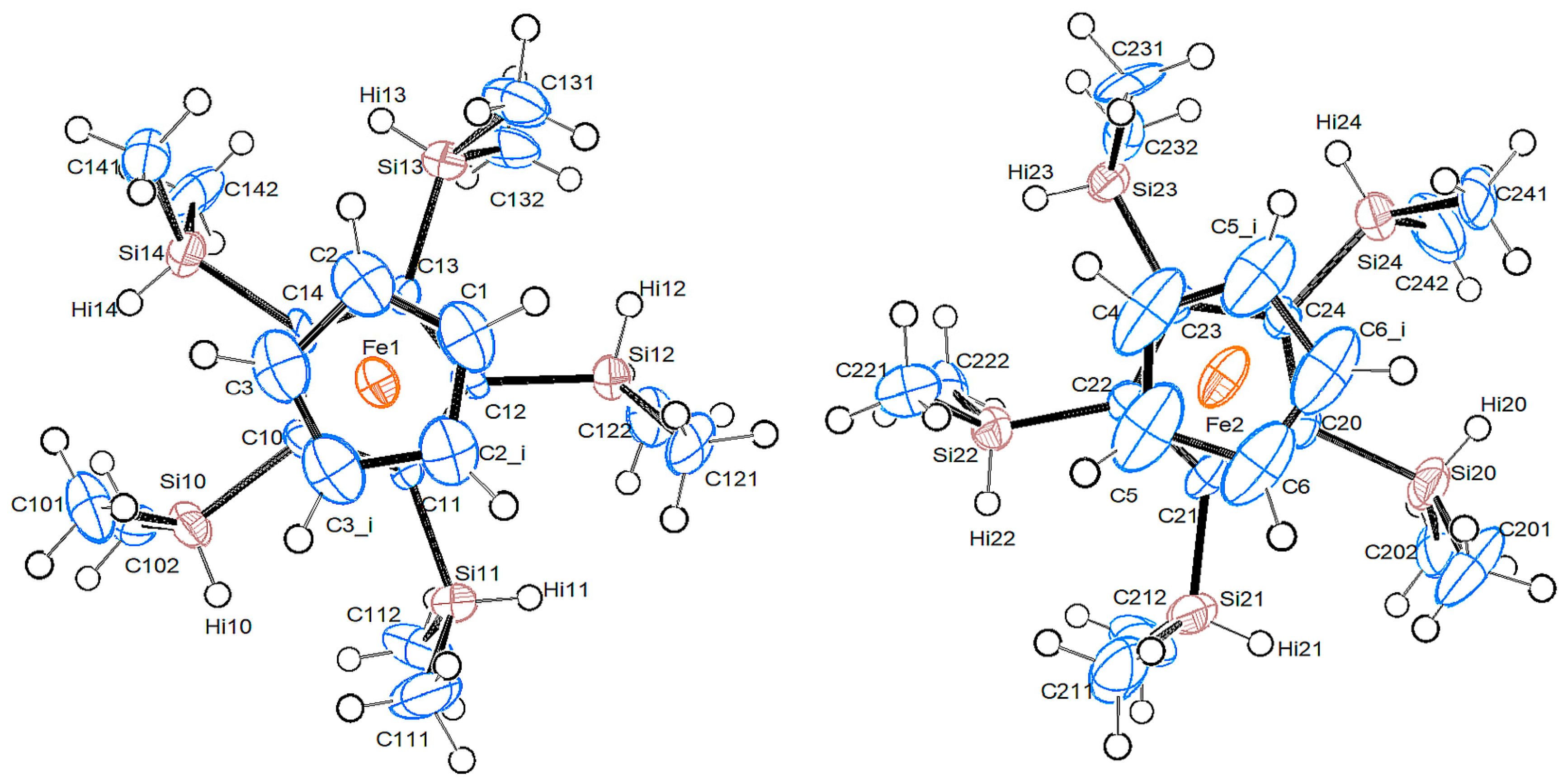

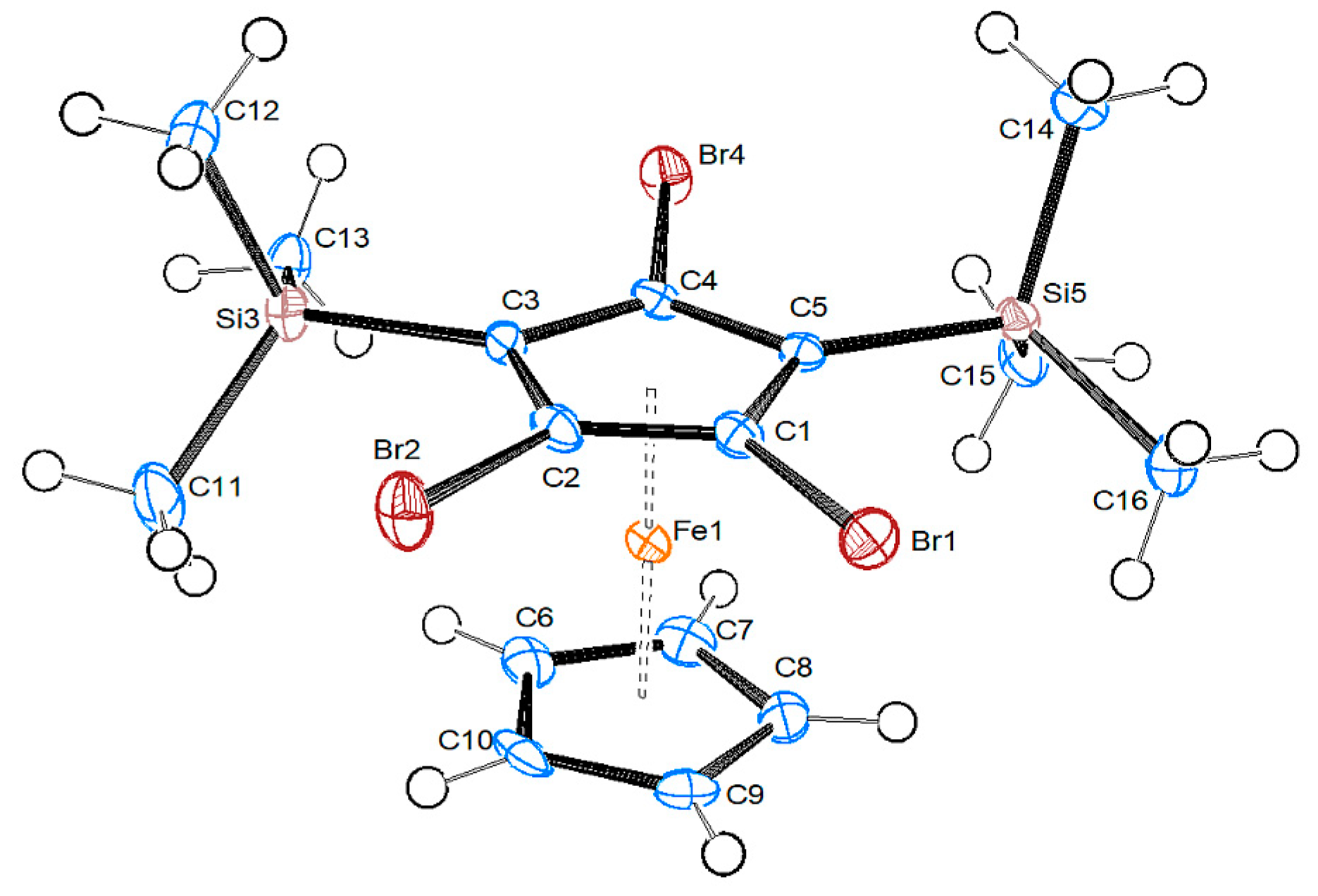
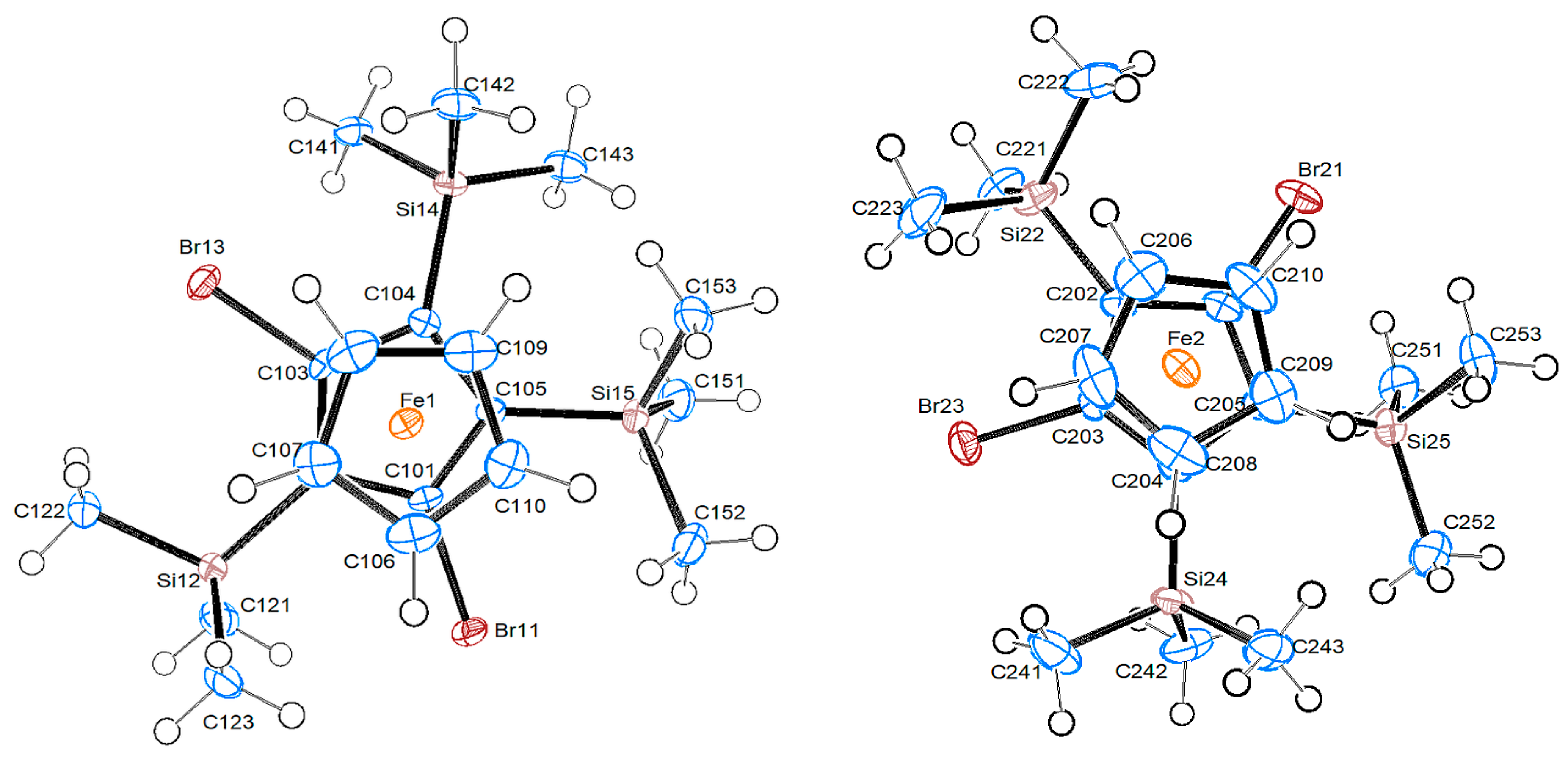

| 4b | “A” | 8b/Mol. A | 8b/Mol. B | |
|---|---|---|---|---|
| C–Br [Å] | 1.889(3)/1.888(3)/1.902(3) | 1.87/1.885(9) | 1.907(3)/1.905(3) | 1.910(3)/1.896(3) |
| Ccp–Si | 1.898(3)/1.862(4) | 1.909(9) | 1.894(4)/1.898(3)/1.888(4) | 1.892(4)/1.889(3)/1.905(4) |
| Fe–CTsub | 1.6292(14) | {1.753} | 1.6435(14) | 1.6415(14) |
| Fe–CTC5H5 | 1.6589(15) | – | 1.6682(16) | 1.6686(19) |
| CTsub-Fe-CTC5H5 [°] | 178.52(7) | – | 178.26(9) | 179.51(10) |
| Br-CTsub-CTC5H5-Hcp [°] | 16.3 | – | 19.7 | 5.5 |
| Δ Si—Cp [Å] | 0.064(1)/0.008(1) | −0.092(1)/−0.318(1)/0.196(1) | −0.009(1)/−0.109(1)/0.290(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernhartzeder, S.; Blockhaus, T.; Lang, M.; Sünkel, K. Studies Toward Persilylation of π-Cyclopentadienyl Complexes of Fe and Ru. Molecular Structures of [Fe(C5H5){C5(SiMe2H)5}], [Fe(C5H5){C5Br3(SiMe3)2}] and [Fe(C5H5){C5Br2(SiMe3)3}]. Inorganics 2025, 13, 42. https://doi.org/10.3390/inorganics13020042
Bernhartzeder S, Blockhaus T, Lang M, Sünkel K. Studies Toward Persilylation of π-Cyclopentadienyl Complexes of Fe and Ru. Molecular Structures of [Fe(C5H5){C5(SiMe2H)5}], [Fe(C5H5){C5Br3(SiMe3)2}] and [Fe(C5H5){C5Br2(SiMe3)3}]. Inorganics. 2025; 13(2):42. https://doi.org/10.3390/inorganics13020042
Chicago/Turabian StyleBernhartzeder, Stefanie, Tobias Blockhaus, Markus Lang, and Karlheinz Sünkel. 2025. "Studies Toward Persilylation of π-Cyclopentadienyl Complexes of Fe and Ru. Molecular Structures of [Fe(C5H5){C5(SiMe2H)5}], [Fe(C5H5){C5Br3(SiMe3)2}] and [Fe(C5H5){C5Br2(SiMe3)3}]" Inorganics 13, no. 2: 42. https://doi.org/10.3390/inorganics13020042
APA StyleBernhartzeder, S., Blockhaus, T., Lang, M., & Sünkel, K. (2025). Studies Toward Persilylation of π-Cyclopentadienyl Complexes of Fe and Ru. Molecular Structures of [Fe(C5H5){C5(SiMe2H)5}], [Fe(C5H5){C5Br3(SiMe3)2}] and [Fe(C5H5){C5Br2(SiMe3)3}]. Inorganics, 13(2), 42. https://doi.org/10.3390/inorganics13020042








