Abstract
Crystal violet dye poses significant environmental and human health risks due to its toxicity, persistence, and bioaccumulative nature. It contributes to potential carcinogenicity, cytotoxicity, and systemic toxicity upon human exposure. To address this issue, a novel SrCO3/MgO/CaO/CaCO3 nanocomposite was synthesized using the Pechini sol-gel method, producing AE500 and AE700 at 500 and 700 °C, respectively, for the efficient removal of crystal violet dye from aqueous media. X-ray diffraction (XRD) analysis confirmed the formation of crystalline phases, with average crystallite sizes of 64.53 nm for AE500 and 75.34 nm for AE700. Energy-dispersive X-ray spectroscopy (EDX) revealed elemental compositions with variations in carbon, oxygen, magnesium, calcium, and strontium percentages influenced by synthesis temperature. Field-emission scanning electron microscopy (FE-SEM) showed morphological differences, where AE500 had irregular polyhedral structures, while AE700 exhibited more compact spherical formations, with average grain sizes of 99.98 and 132.23 nm, respectively. High-resolution transmission electron microscopy (HR-TEM) confirmed the structural integrity and nano-scale morphology, showing aggregated irregularly shaped particles in AE500, while AE700 displayed well-defined polyhedral and nearly spherical nanoparticles. The calculated average particle diameters were 21.67 nm for AE500 and 41.19 nm for AE700, demonstrating an increase in particle size with temperature. Adsorption studies demonstrated maximum capacities of 230.41 mg/g for AE500 and 189.39 mg/g for AE700. The adsorption process was exothermic, spontaneous, and physical, following the pseudo-first-order kinetic model and Langmuir isotherm, indicating monolayer adsorption onto a homogenous surface.
1. Introduction
Water contamination by organic dyes is a critical environmental challenge, predominantly driven by industrial activities. Textile, leather, paper, plastics, and pharmaceutical industries discharge large volumes of dye-laden wastewater into natural water bodies [1,2,3]. The non-biodegradable and stable molecular structures of these dyes make them persistent in the environment, posing severe threats to aquatic ecosystems. Inadequate wastewater treatment and improper disposal further exacerbate the problem, leading to the accumulation of these pollutants in rivers, lakes, and groundwater. Additionally, the extensive use of synthetic dyes in domestic applications contributes to their widespread presence in wastewater [4,5]. Organic dyes have significant environmental and human health impacts due to their complex aromatic structures, which make them resistant to degradation. In aquatic ecosystems, dyes reduce light penetration, disrupting photosynthesis and adversely affecting aquatic life [6]. Many dyes exhibit toxicity, mutagenicity, and carcinogenicity, potentially leading to severe ecological imbalances [7]. For humans, exposure to organic dyes, whether through ingestion of contaminated water, dermal contact, or inhalation, can cause respiratory disorders, skin irritation, allergic reactions, and even damage to vital organs. Some dyes also exhibit endocrine-disrupting properties, further elevating their health risks [8]. Crystal violet, a widely used cationic dye, presents significant health and environmental risks. Its extensive application in textile dyeing, biological staining, and as an antifungal agent leads to considerable environmental contamination. Crystal violet dye is highly toxic to aquatic organisms and inhibits microbial activity essential for wastewater treatment. Its persistence in water bodies contributes to bioaccumulation and trophic transfer, further endangering ecosystems [9]. In humans, exposure to crystal violet dye is linked to cytotoxicity, genotoxicity, and potential carcinogenic effects. It can cause dermal and ocular irritation, respiratory distress upon inhalation, and systemic toxicity when ingested, underscoring the urgent need for its effective removal from wastewater [10]. Various techniques have been explored for the removal of organic dyes from wastewater, each with distinct advantages and limitations. Adsorption is a widely used, cost-effective, and efficient technique that employs porous materials to capture dye molecules from aqueous media [11,12]. Coagulation–flocculation is a water treatment process where coagulants neutralize charged particles, allowing them to aggregate into larger flocs, which are then removed through sedimentation or filtration. However, this method has limitations, including the need for precise chemical dosing, high sludge production requiring disposal, and reduced efficiency in removing certain dissolved contaminants. Additionally, the process can be affected by changes in pH, temperature, and the presence of organic matter, requiring careful monitoring and adjustment [13,14]. Membrane filtration includes ultrafiltration [15], nanofiltration [16,17], and reverse osmosis [18,19], offering high removal efficiency but suffering from membrane fouling and high operational costs. Electrodialysis is a membrane-based technique that utilizes an electric field to separate dye molecules but requires substantial energy input [20,21]. Bioremediation exploits microorganisms and enzymes to degrade dyes into less toxic compounds, though it is often slow and depends on environmental conditions [22,23]. Photocatalytic degradation employs semiconductor materials such as TiO2 and ZnO to generate reactive species that degrade dyes under light irradiation, though limited by light penetration and catalyst stability [24,25]. Among the available dye removal methods, adsorption stands out due to its simplicity, cost-effectiveness, and high removal efficiency. Additionally, adsorption allows for easy regeneration and reuse of adsorbents, reducing operational costs. Its adaptability to a wide range of dyes and wastewater conditions makes it one of the most preferred techniques in water treatment applications [26]. Nanomaterials have garnered significant attention in adsorption-based dye removal due to their high surface area, tunable porosity, and active functional groups. Metal oxide nanoparticles, such as Fe3O4 [27], ZnO [28], and CuO [29], exhibit excellent adsorption properties. Their composites, such as ZnO/zeolite [30], CaO/ZrO2/g-C3N4 [31], and chitosan-citrate/ZrO2 [32], further enhance adsorption efficiency by increasing surface reactivity and improving structural stability. Similarly, metal carbonate nanoparticles and their composites like CaCO3 [33], reduced graphene oxide/CaCO3 [34], CaCO3/chitin aerogel [35], chitosan salicylaldehyde/CaO [36], MgO [37], and ZrO2/MnCO3/CdCO3 [38], offer high adsorption capacities due to their unique porous structures and affinity for cationic and anionic dyes. Several adsorbents have been explored for crystal violet dye removal, including surfactant-modified magnetic nanoadsorbent [39], polypyrrole-decorated bentonite magnetic nanocomposite [40], and iron–manganese oxide coated kaolinite [41]. These adsorbents exhibit moderate adsorption capacities; however, novel adsorbents with superior performance are still needed. The present study introduces the facile synthesis of novel SrCO3/MgO/CaO/CaCO3 nanocomposites using the Pechini sol-gel method at 500 °C (AE500) and 700 °C (AE700), for the efficient removal of crystal violet dye from aqueous media. This composite integrates multiple functional components. The incorporation of four functional components within the same composite offers a synergistic effect. The incorporation of different metal oxides and carbonates in the composite structure provides synergistic benefits. For instance, SrCO3 and CaCO3 contribute to surface basicity and adsorption through ion exchange, while MgO and CaO offer high surface reactivity and active sites for dye binding. The combination ensures enhanced adsorption through multiple mechanisms, including electrostatic attraction, surface complexation, and physical trapping. This approach aligns with recent advances in oxide-based nanocomposites, as observed in ZnO-based systems used for sensing and dye degradation, where morphology, charge transfer, and heterostructure play critical roles in performance [42]. Further, the Pechini sol-gel method ensures uniform particle distribution, controlled crystallinity, and improved textural properties, making AE500 and AE700 promising candidates for efficient crystal violet dye removal. Compared to previously reported adsorbents with moderate adsorption capacities, these composites exhibit superior adsorption performance toward crystal violet dye.
2. Results and Discussion
2.1. Characterization
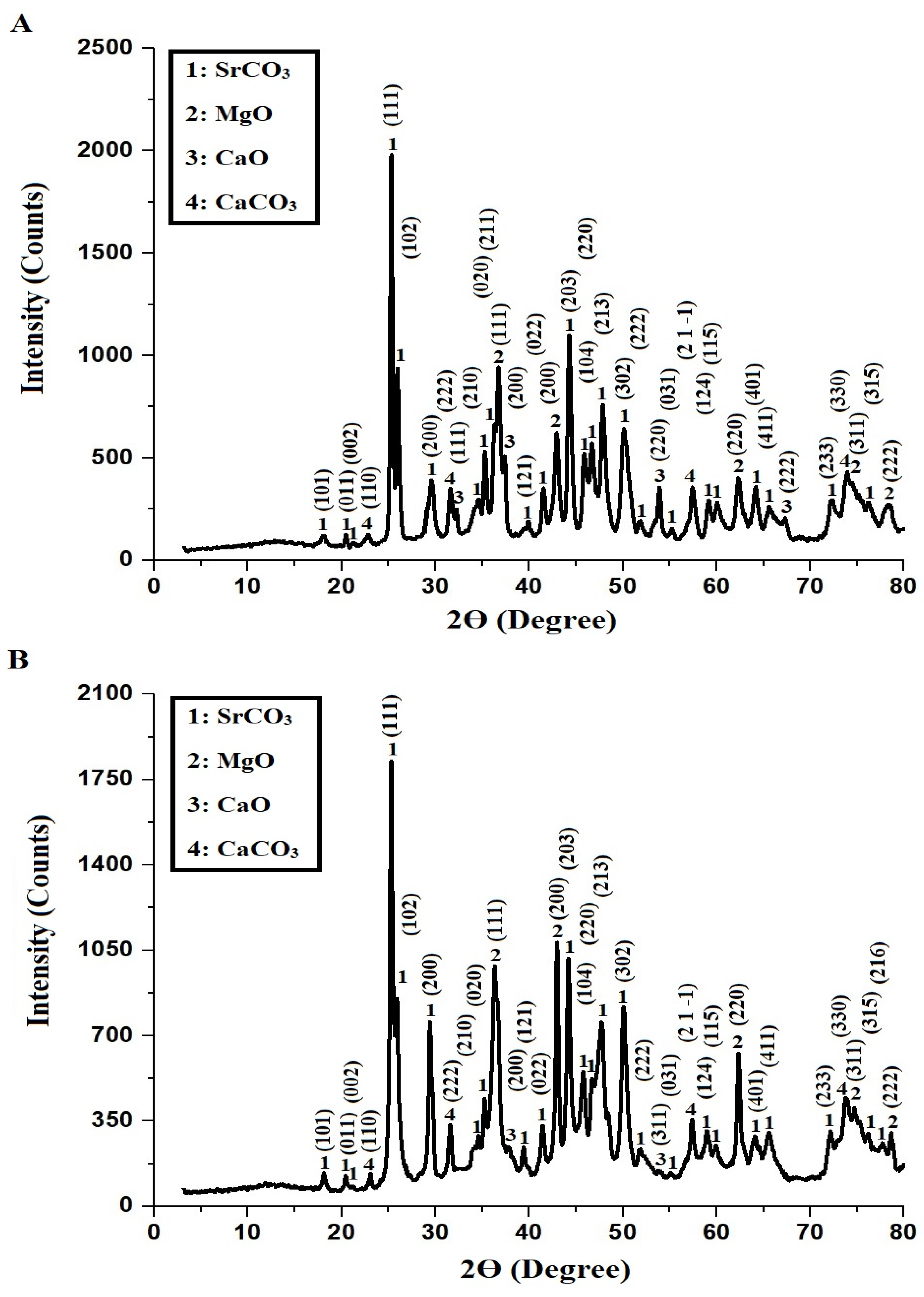
Figure 1A,B present the XRD patterns of AE500 and AE700 nanocomposites, respectively, revealing the presence of multiple crystalline phases, including strontium carbonate (SrCO3), magnesium oxide (MgO), calcium oxide (CaO), and calcium carbonate (CaCO3). The diffraction peaks of SrCO3, identified using JCPDS card No. 01-074-1491, correspond to the orthorhombic crystal system, with characteristic 2θ angles at 18.04°, 20.45°, 21.19°, 25.28°, 25.92°, 29.59°, 34.62°, 35.26°, 36.29°, 39.87°, 41.55°, 44.27°, 45.85°, 46.69°, 47.84°, 50.16°, 51.84°, 55.19°, 59.17°, 60.12°, 65.57°, 72.30°, 76.28°, and 77.64°, corresponding to the Miller indices (101), (011), (002), (111), (102), (200), (210), (020), (211), (121), (022), (203), (104), (220), (213), (302), (222), (031), (124), (115), (411), (233), (315), and (216), respectively. The cubic MgO phase, confirmed by JCPDS card No. 01-078-0430, exhibits diffraction peaks at 36.72°, 42.92°, 62.33°, 74.65°, and 78.27°, corresponding to the Miller indices (111), (200), (220), (311), and (222), respectively. The cubic CaO phase, identified using COD card No. 1000044, presents diffraction peaks at 32.32°, 37.36°, 53.94°, 64.11°, and 67.25°, with corresponding Miller indices (111), (200), (220), (311), and (222), respectively. The rhombohedral CaCO3 phase, confirmed by COD card No. 1010928, exhibits diffraction peaks at 22.96°, 31.57°, 57.40°, and 73.98°, corresponding to the Miller indices (110), (222), (2 1 −1), and (330), respectively. The average crystallite size of the AE500 and AE700 samples is 64.53 nm and 75.34 nm, respectively, indicating an increase in crystallite size with higher synthesis temperatures. This increase can be attributed to enhanced atomic mobility and grain growth at elevated temperatures, which facilitate coalescence of smaller crystallites into larger structures, leading to improved crystallinity and reduced lattice strain.
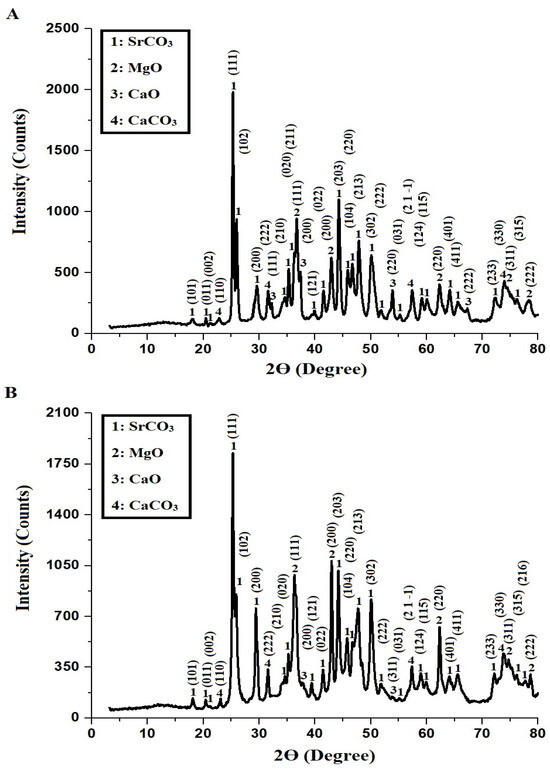
Figure 1.
XRD patterns of (A) AE500 and (B) AE700 nanocomposites.
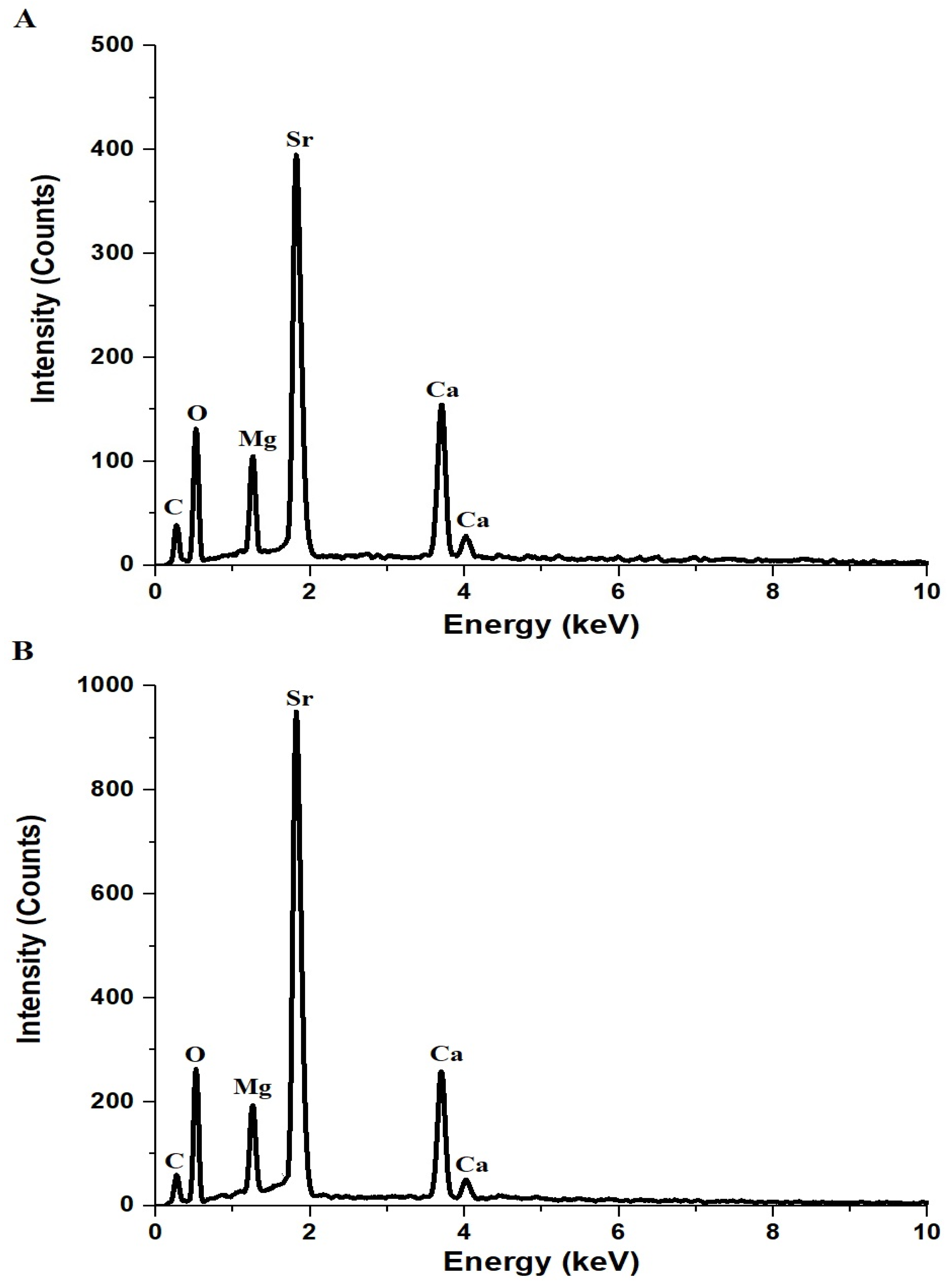
Figure 2A,B presents the EDX spectra of AE500 and AE700 nanocomposites, respectively, confirming the presence of carbon (C), oxygen (O), magnesium (Mg), calcium (Ca), and strontium (Sr) as the primary elemental components. Table 1 provides the atomic percentages of these elements in both samples, revealing variations in elemental composition upon increasing the synthesis temperature. The carbon content in AE500 and AE700 is 19.1% and 16.7%, respectively, which can be attributed to the residual carbonaceous species from the Pechini sol-gel synthesis method that involved tartaric acid and polyethylene glycol 400 as complexing and stabilizing agents. During synthesis, these organic components decompose and volatilize upon heating, with higher temperatures facilitating further carbon removal, explaining the lower carbon content in AE700 compared to AE500. The oxygen content slightly increases from 55.8% in AE500 to 56.8% in AE700, suggesting enhanced oxidation or reduced presence of residual organic species at elevated temperatures. The atomic percentage of magnesium decreases slightly from 5.1% in AE500 to 4.8% in AE700, while calcium exhibits a more pronounced reduction from 8.6% to 7.4%, possibly due to variations in precursor decomposition or phase transformations during heat treatment. In contrast, the strontium content increases from 11.4% to 14.3%, indicating preferential retention or enrichment of Sr-containing phases at higher temperatures. These compositional differences between AE500 and AE700 reflect the thermal decomposition behavior of the precursors and the structural evolution of the nanocomposite, with elevated temperatures promoting crystallite growth, phase stabilization, and a reduction in residual carbonaceous content.
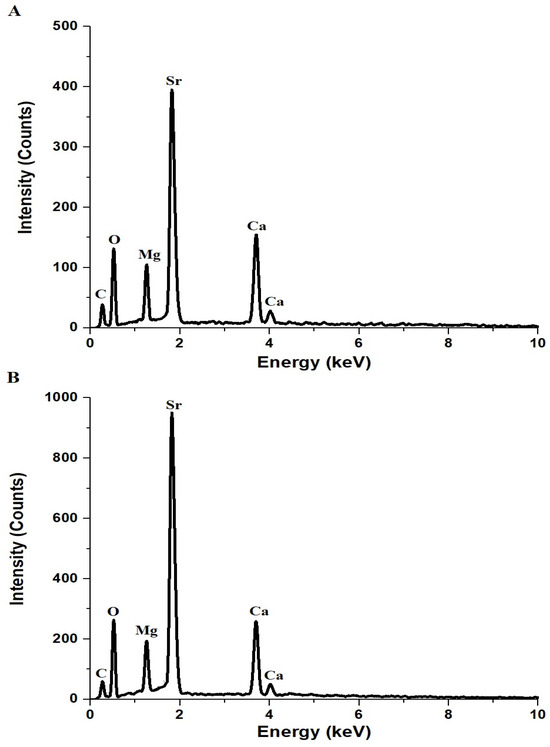
Figure 2.
EDX patterns of (A) AE500 and (B) AE700 nanocomposites.

Table 1.
Atomic percentages of elements in AE500 and AE700 nanocomposites as determined by EDS analysis.
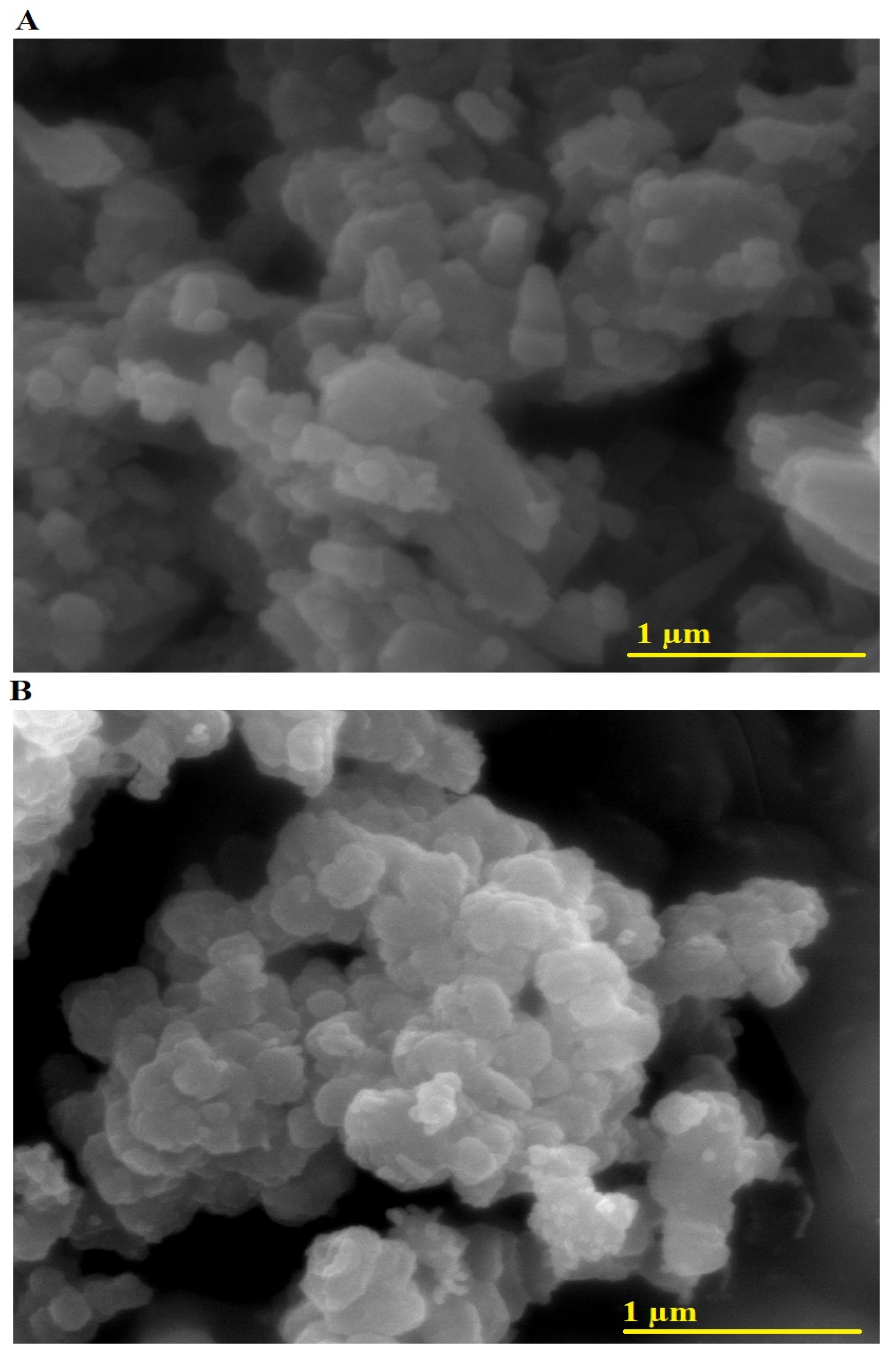
Figure 3A,B presents the FE-SEM images of AE500 and AE700 nanocomposites, respectively, providing insight into their morphological characteristics and grain size distribution. The microstructures reveal agglomerated nanoparticles with distinct morphological features, where AE500 exhibits an irregular and loosely packed structure composed of polyhedral and rod-like particles, while AE700 displays a more compact arrangement with a predominance of nearly spherical and polygonal particles. The observed morphological differences between the two samples can be attributed to the impact of the synthesis temperature on particle growth and agglomeration, with higher temperatures promoting enhanced grain coalescence and structural densification. The average grain size of the AE500 and AE700 nanocomposites, determined from the FE-SEM images, is 99.98 nm and 132.23 nm, respectively, indicating an increase in average grain size with rising synthesis temperature. This increase can be explained by the enhanced atomic diffusion and coarsening effects at elevated temperatures, which facilitate the growth of larger crystallites and the reduction of surface energy.
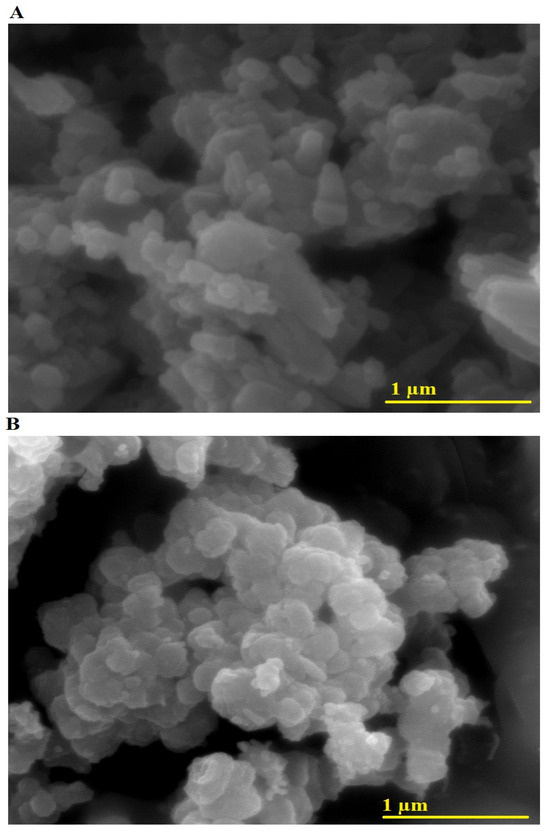
Figure 3.
FE-SEM images of (A) AE500 and (B) AE700 nanocomposites.
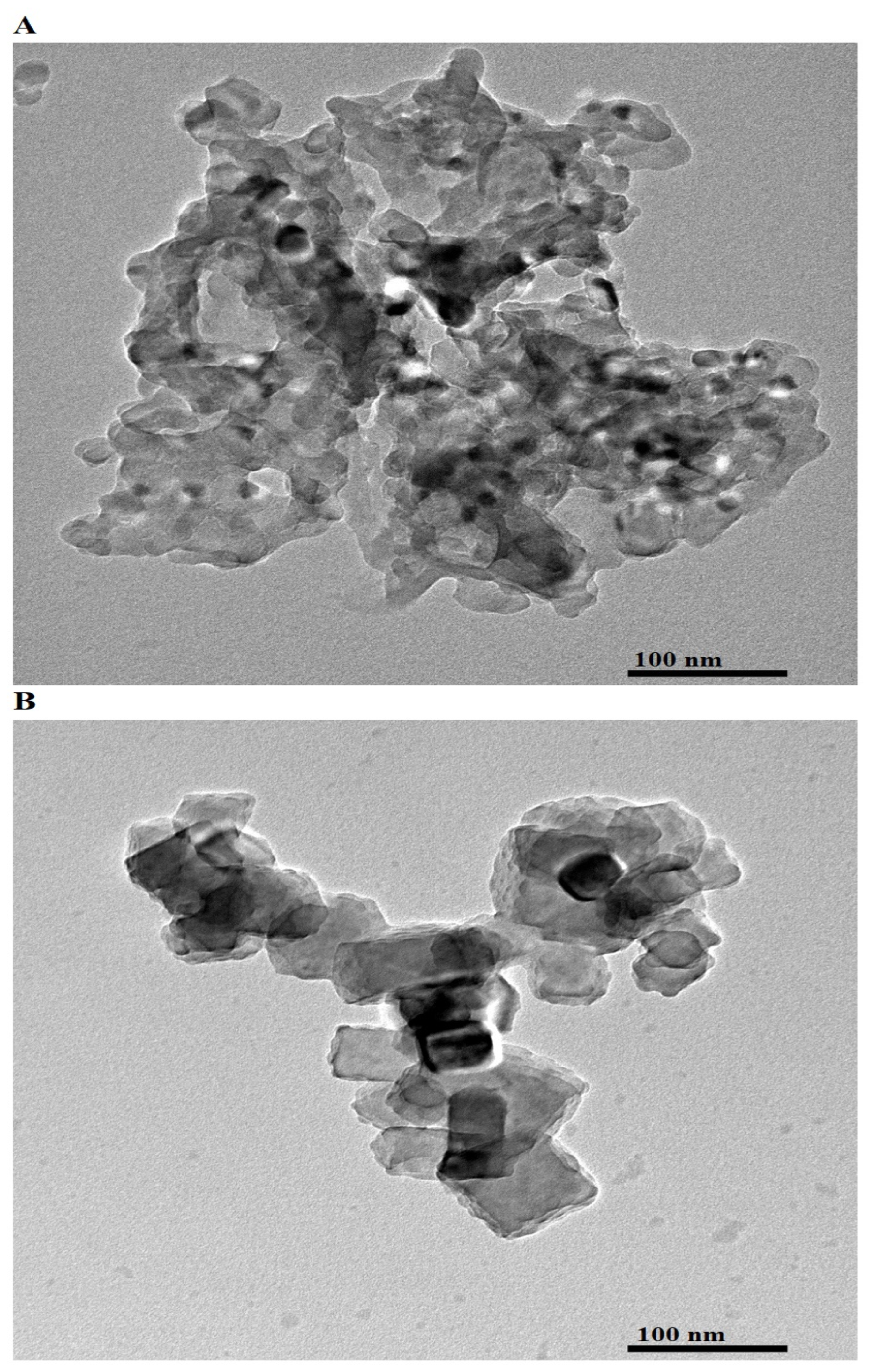
Figure 4A,B presents the HR-TEM images of AE500 and AE700 nanocomposites, respectively, providing detailed insights into their structural and morphological characteristics at the nanoscale. The AE500 sample exhibits an aggregated network of irregularly shaped particles with undefined boundaries, indicating the presence of smaller and loosely connected nanoparticles. In contrast, the AE700 sample shows well-defined polyhedral and nearly spherical particles with enhanced crystallinity and a more compact arrangement. The observed differences between AE500 and AE700 can be attributed to the impact of the higher synthesis temperature, which promotes particle growth, improved crystallinity, and a reduction in structural defects. The increase in average particle size from AE500 to AE700 is further confirmed by the calculated average particle diameters, which are 21.67 nm and 41.19 nm, respectively. This growth can be explained by enhanced atomic diffusion and Ostwald ripening at elevated temperatures, facilitating the coalescence of smaller particles into larger and more thermodynamically stable structures. The morphological transition from irregular and aggregated nanoparticles in AE500 to more uniform and defined polyhedral and spherical shapes in AE700 highlights the role of synthesis temperature in controlling nanoparticle growth and structural evolution.
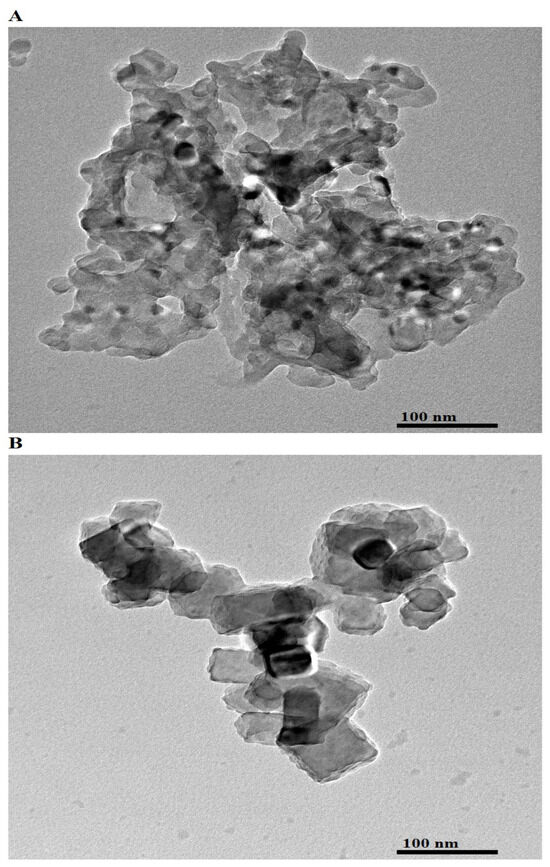
Figure 4.
HR-TEM images of (A) AE500 and (B) AE700 nanocomposites.
2.2. Adsorption of Crystal Violet Dye from Aqueous Media
The dye removal efficiency (% E) and the adsorption capacity (Q) of the nanocomposites were determined using Equations (1) and (2), respectively [43].
where Co (mg/L) and Ce (mg/L) are the initial and equilibrium concentrations of crystal violet dye, respectively. V (L) is the volume of the dye solution, and W (g) is the mass of the adsorbent.
2.2.1. Effect of pH
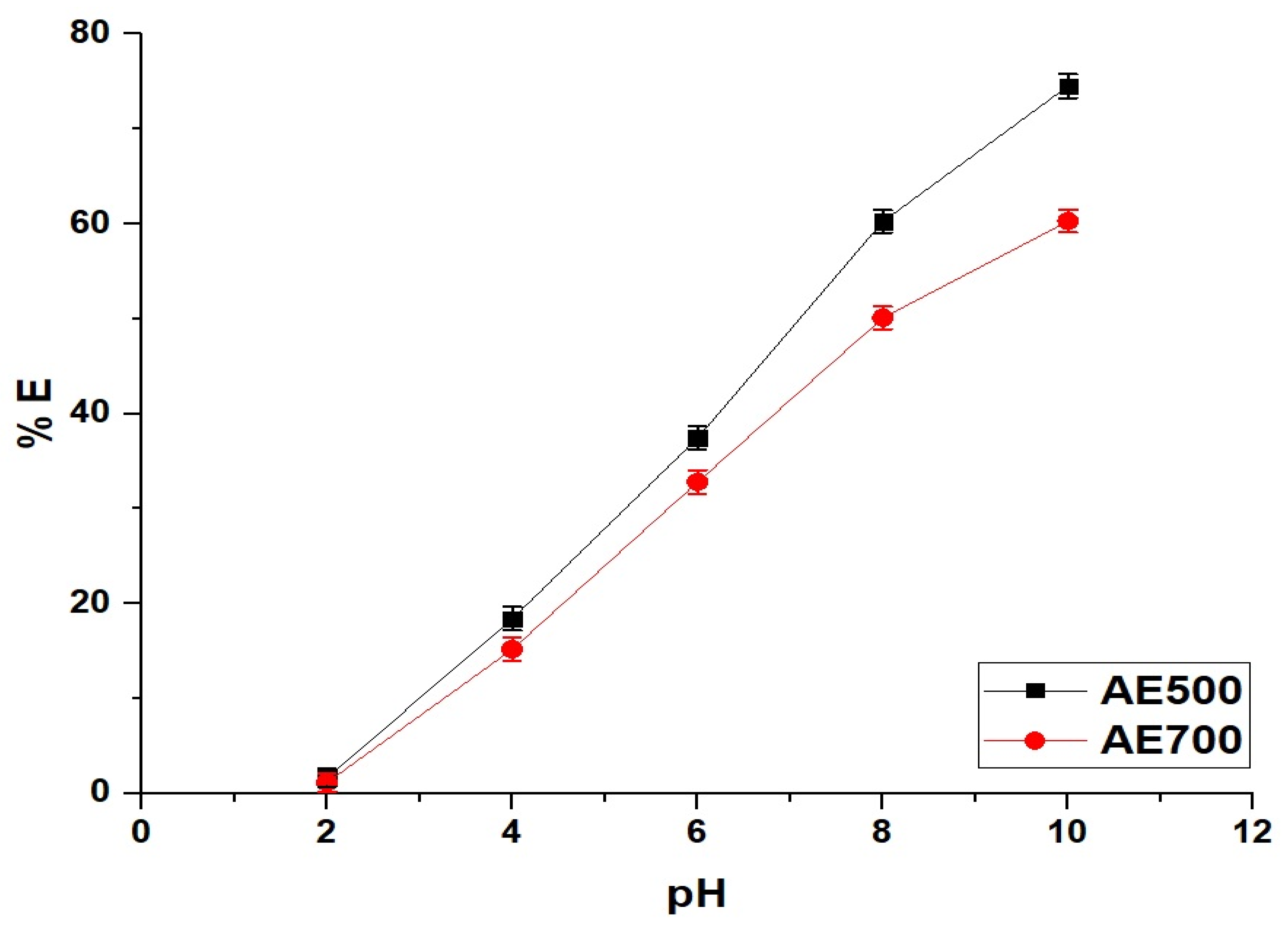
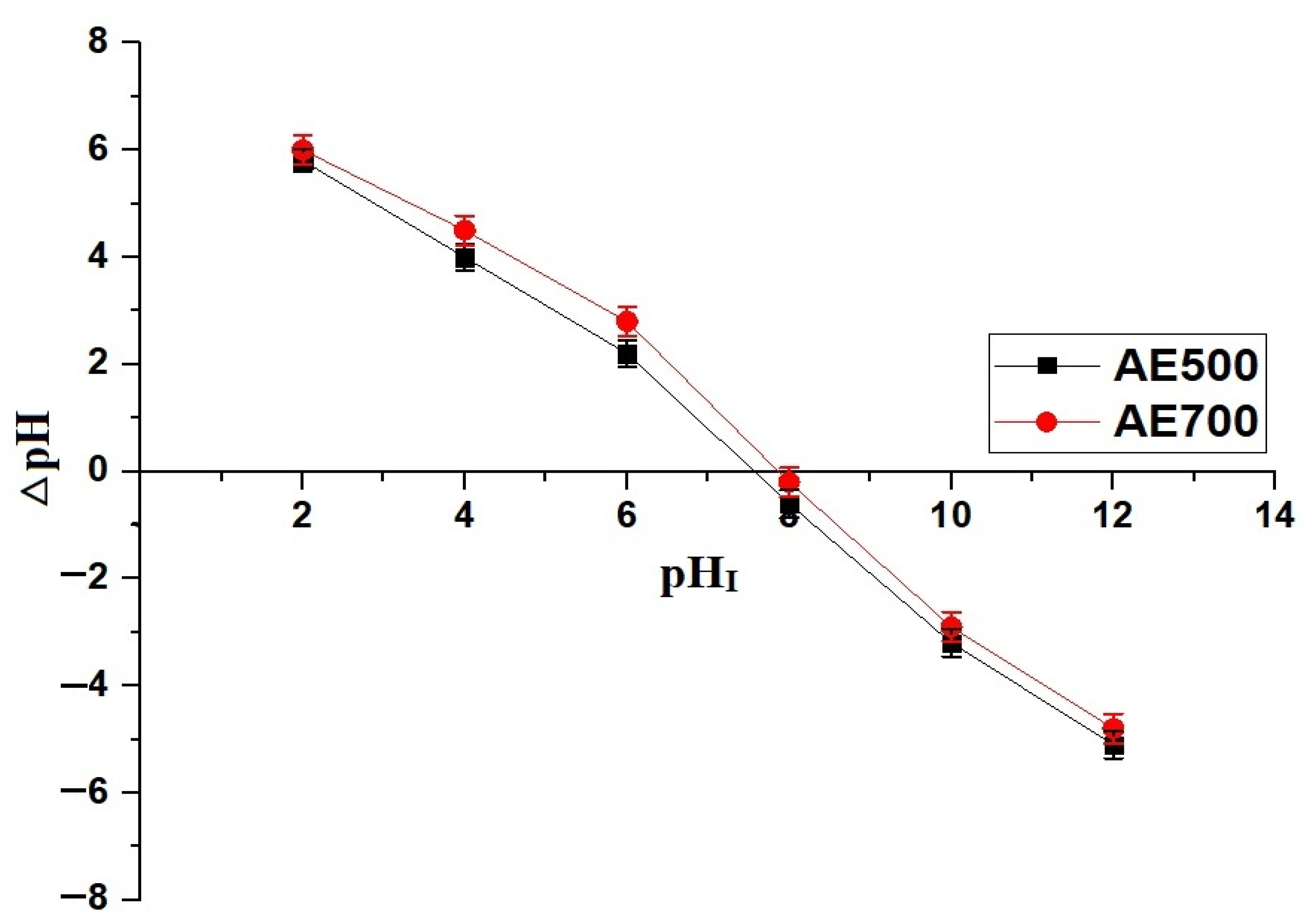
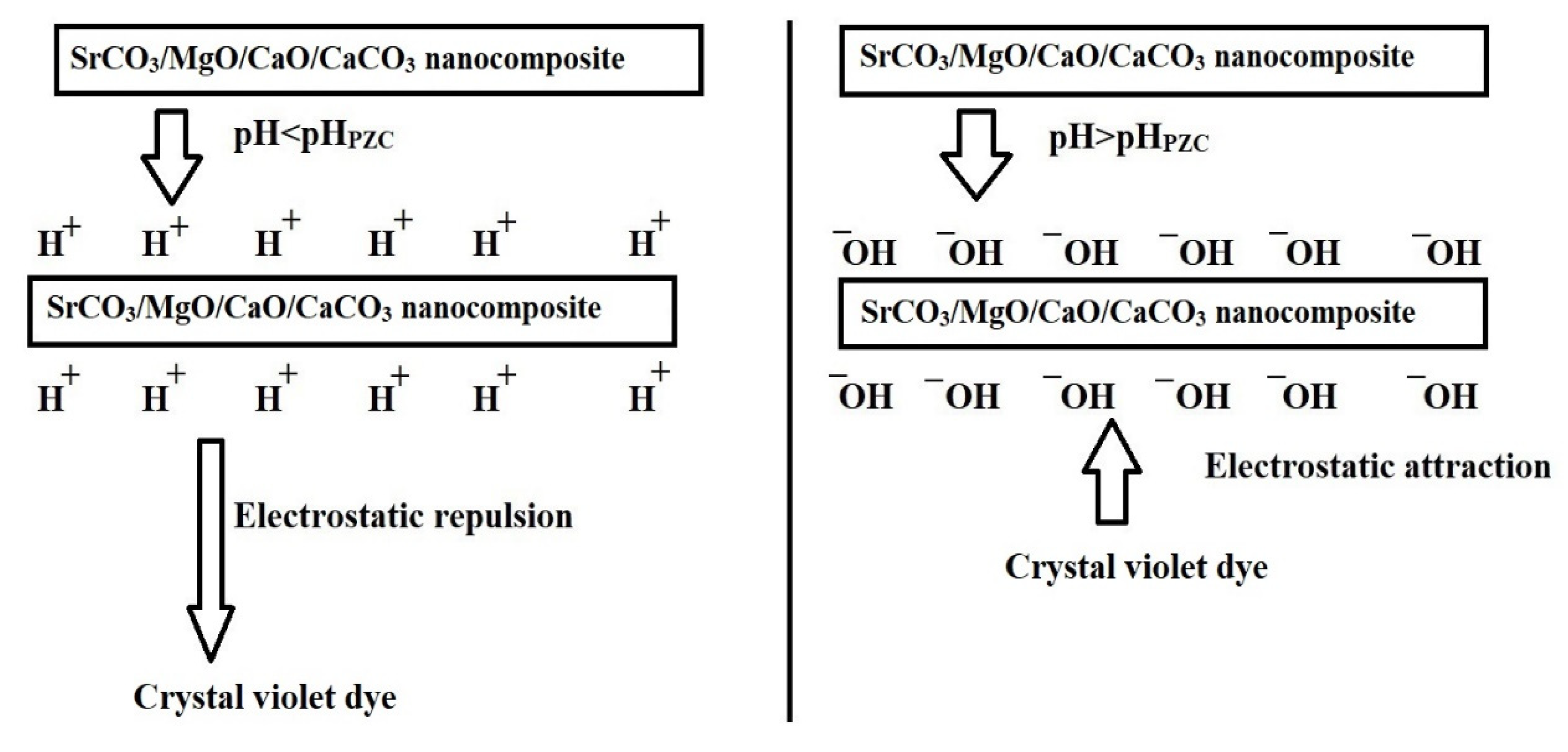
Figure 5 illustrates the effect of pH on the removal efficiency of crystal violet dye using AE500 and AE700 nanocomposites, while Figure 6 presents the point of zero charge (pHPZC) values of both samples. Additionally, Figure 7 provides a schematic representation of their surface charge behavior under different pH conditions. At pH 2, the removal efficiency of crystal violet dye is minimal for both samples, with AE500 exhibiting slightly higher adsorption than AE700. This behavior can be attributed to the highly positive surface charge of both nanocomposites at this pH, as shown in Figure 6, which creates strong electrostatic repulsion with the positively charged crystal violet dye molecules, as depicted in Figure 7. As a result, crystal violet dye uptake is significantly hindered due to the lack of favorable interactions between the adsorbent and adsorbate. Conversely, at pH 10, the removal efficiency increases markedly for both samples, with AE500 showing greater adsorption than AE700. This enhancement can be explained by the fact that at pH values above their respective pHPZC, the surfaces of the nanocomposites acquire a negative charge, as indicated in Figure 6, leading to strong electrostatic attraction with the cationic crystal violet dye, as illustrated in Figure 7. The superior performance of AE500 compared to AE700 can be attributed to its higher surface reactivity and greater availability of active sites, facilitating more efficient dye adsorption. These findings highlight the crucial role of pH in modulating the adsorption behavior of crystal violet dye on the synthesized nanocomposites and demonstrate the significance of surface charge interactions in governing the adsorption process.
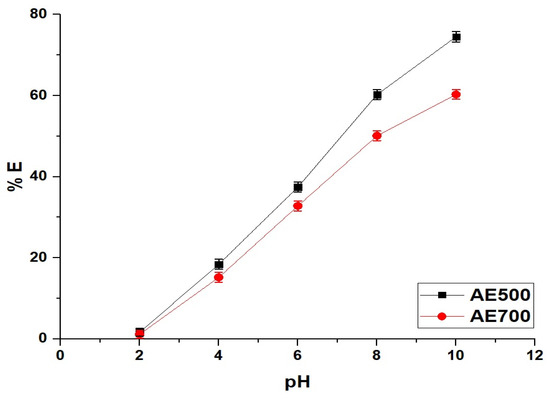
Figure 5.
Influence of pH on the removal efficiency (% E) of crystal violet dye using AE500 and AE700 nanocomposites.
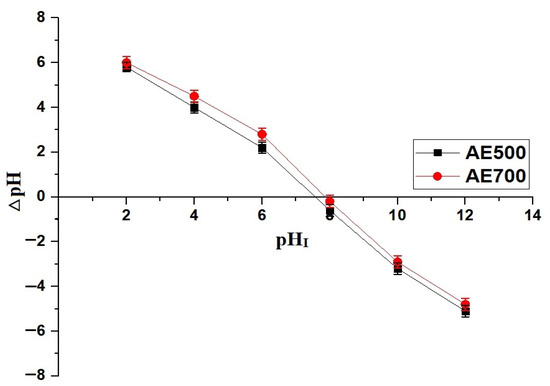
Figure 6.
Point of zero charge (pHPZC) determination for the AE500 and AE700 nanocomposites.
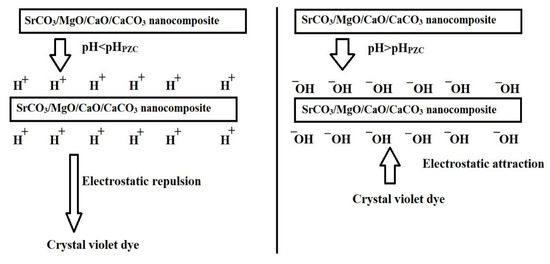
Figure 7.
Schematic representation of the surface charge behavior of synthesized nanocomposites below and above the pHPZC.
2.2.2. Effect of Contact Time
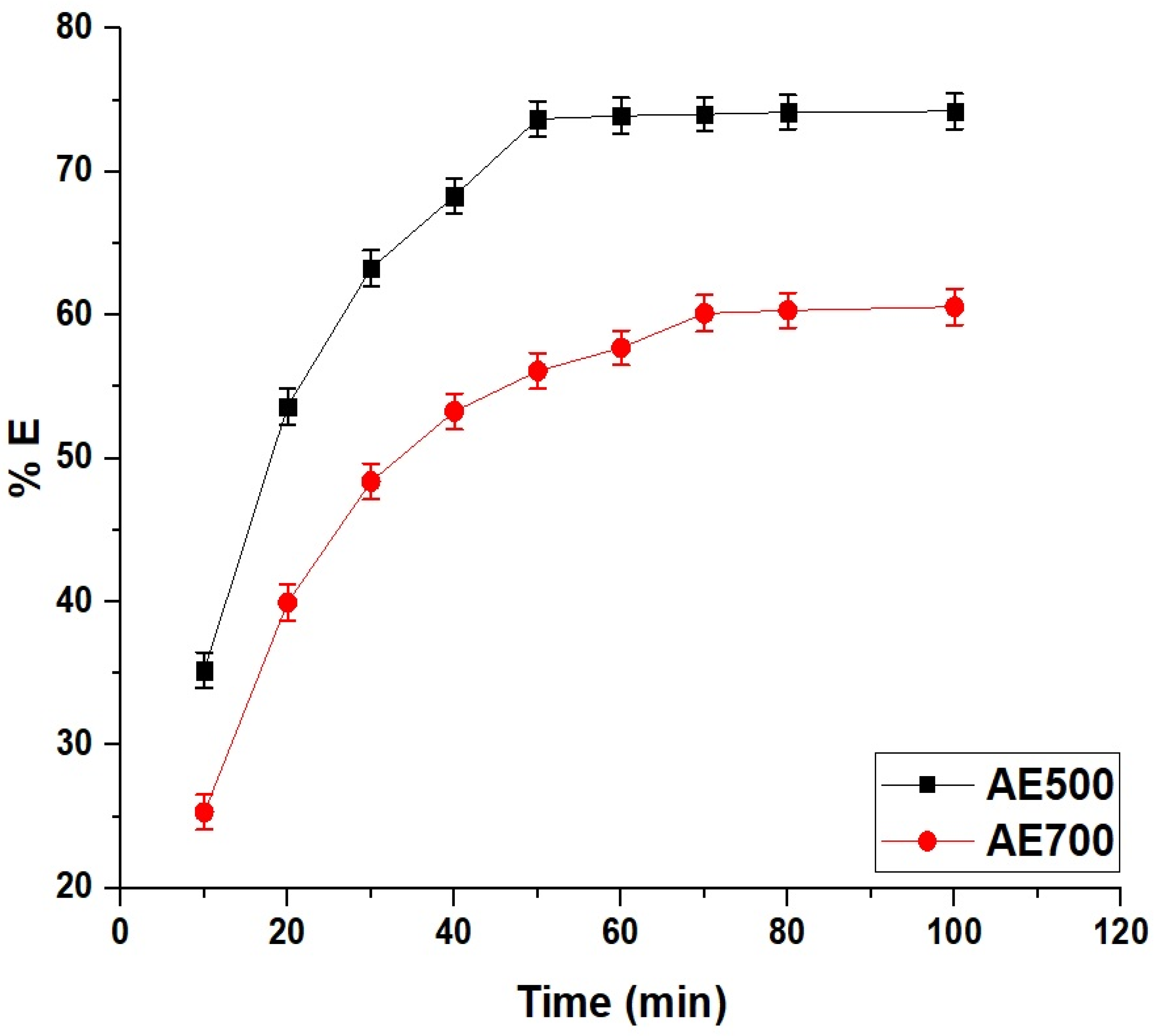
Figure 8 illustrates the time-dependent removal efficiency of crystal violet dye using AE500 and AE700 nanocomposites, demonstrating a rapid increase in dye uptake at the initial stages followed by a gradual approach to equilibrium. The adsorption process is characterized by a fast initial phase due to the availability of abundant active sites on the surface of the nanocomposites, leading to a high diffusion rate of dye molecules onto the adsorbent. Over time, the adsorption percentage decreases as the available active sites become occupied, and equilibrium is reached when no further significant removal occurs. At 10 min, AE500 achieves a removal efficiency of 35.21%, while AE700 reaches 25.31%, indicating the higher adsorption rate of AE500 in the early stages. By 50 min, AE500 reaches its equilibrium with a removal percentage of 73.68%, whereas AE700 continues to adsorb dye molecules until 70 min, reaching a final removal of 60.12%. The lower adsorption efficiency and longer equilibrium time of AE700 compared to AE500 can be attributed to its larger grain size and lower surface reactivity, which reduce the number of available adsorption sites and slow the diffusion of dye molecules. Beyond the equilibrium times, no further significant increase in removal efficiency is observed, confirming the saturation of the adsorbents. These findings highlight the influence of synthesis temperature on adsorption kinetics, where the smaller particle size and higher surface activity of AE500 contribute to its superior adsorption performance and faster equilibrium attainment compared to AE700.
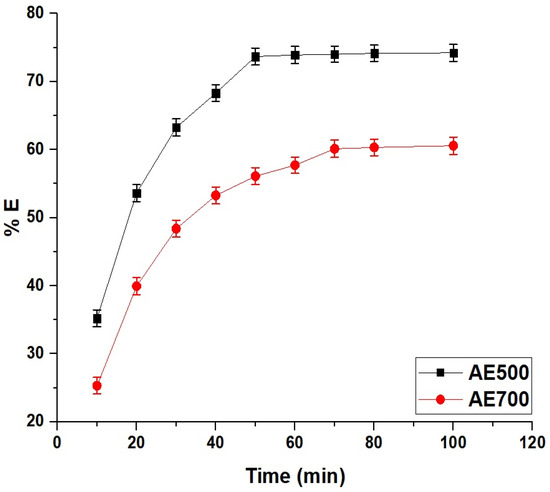
Figure 8.
Time-dependent removal efficiency (% E) of crystal violet dye using AE500 and AE700 nanocomposites.
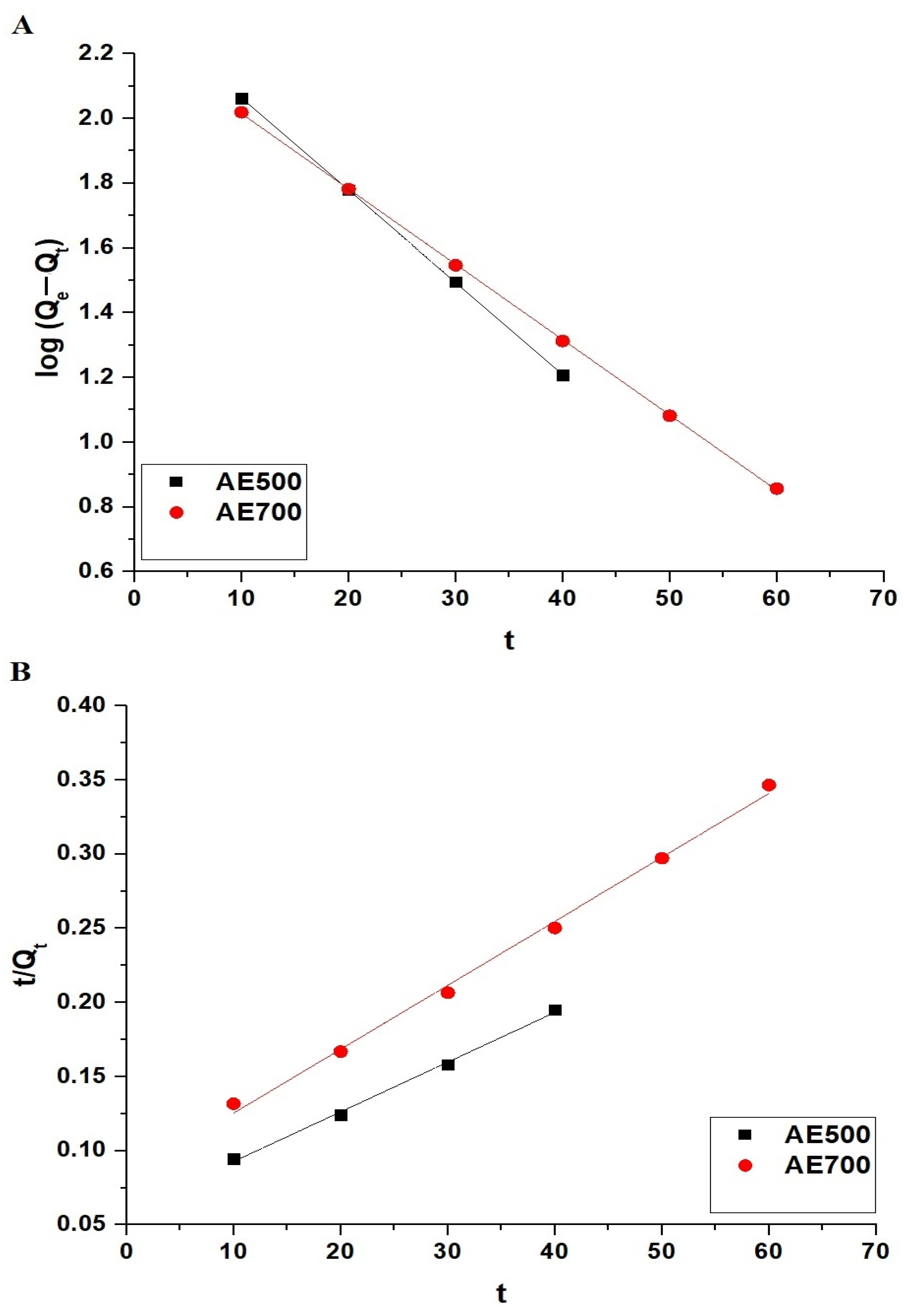
The adsorption kinetics of crystal violet dye onto AE500 and AE700 nanocomposites were analyzed using the pseudo-first-order and pseudo-second-order models, as presented in Figure 9A,B, respectively. The pseudo-first-order model is described by Equation (3) [44].
where Qe (mg/g) represents the amount of crystal violet dye adsorbed at equilibrium, Qt (mg/g) is the amount of crystal violet dye adsorbed at time t, K1 (1/min) is the pseudo-first-order rate constant, and t (min) is the adsorption time.
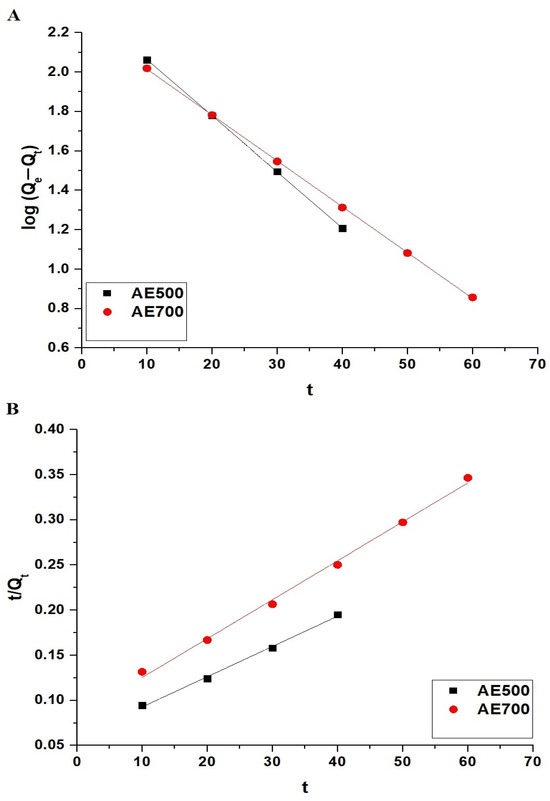
Figure 9.
Kinetic modeling of crystal violet dye adsorption onto AE500 and AE700 nanocomposites: (A) pseudo-first-order model and (B) pseudo-second-order model.
The pseudo-second-order model is expressed by Equation (4) [44].
where K2 (g/mg·min) is the pseudo-second-order rate constant.
The kinetic parameters for both models are summarized in Table 2. The results indicate that the adsorption process follows the pseudo-first-order model for both AE500 and AE700 nanocomposites, as evidenced by the high correlation coefficient (R2 = 0.9999) and the close agreement between the experimentally determined adsorption capacity (QExp) and the theoretical adsorption capacity (Qe) obtained from the pseudo-first-order model. In contrast, the pseudo-second-order model exhibits lower R2 values and a significant discrepancy between QExp and Qe, indicating that it does not accurately describe the adsorption behavior. These findings confirm that the adsorption of crystal violet dye onto AE500 and AE700 nanocomposites is primarily governed by the pseudo-first-order kinetic mechanism.

Table 2.
Kinetic parameters for the adsorption of crystal violet dye onto AE500 and AE700 nanocomposites, based on pseudo-first-order and pseudo-second-order models.
2.2.3. Effect of Temperature
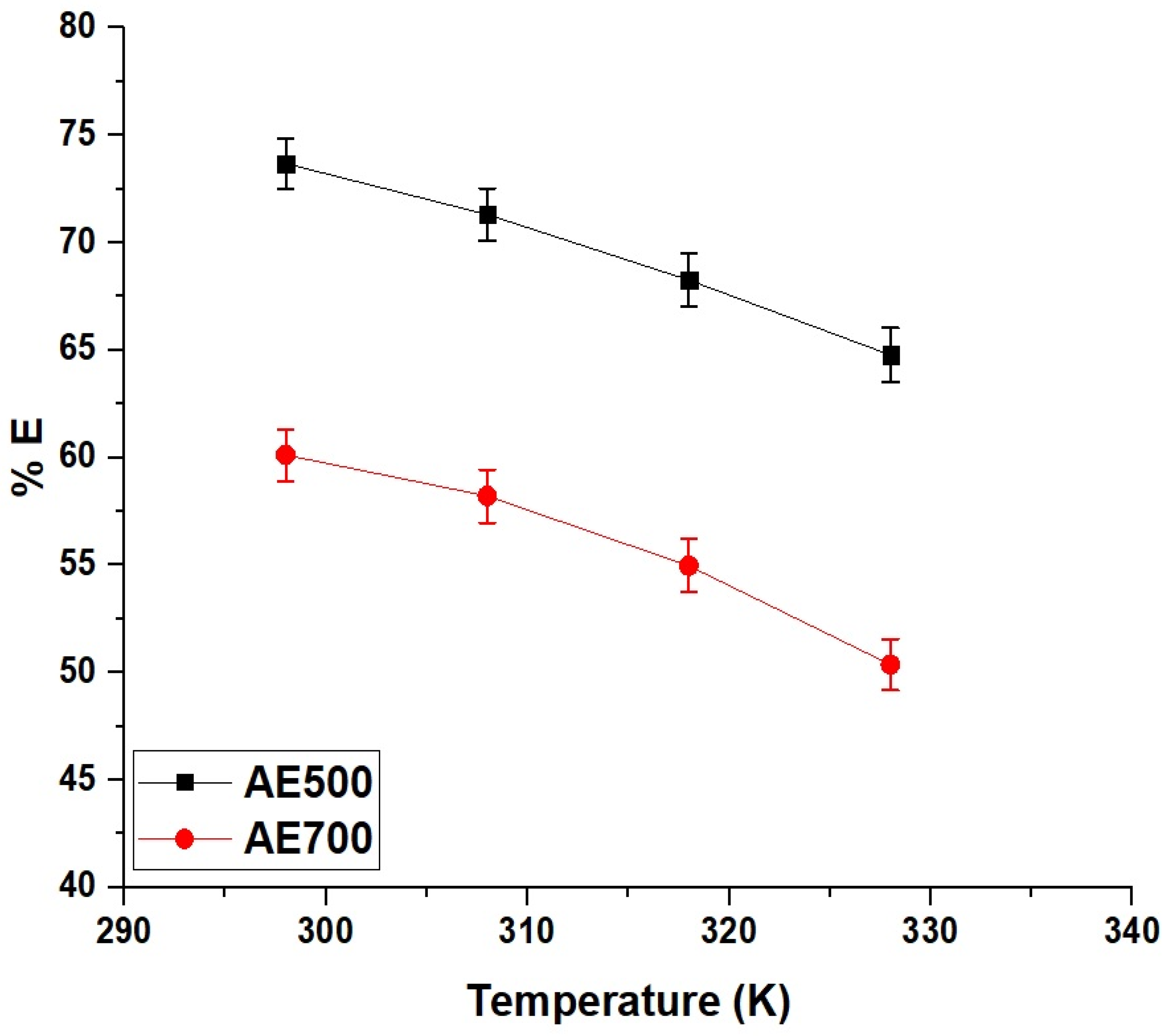
Figure 10 illustrates the effect of temperature on the removal efficiency of crystal violet dye using AE500 and AE700 nanocomposites, demonstrating a decrease in adsorption capacity with increasing temperature. At 298 K, AE500 achieves a removal efficiency of 73.68%, whereas AE700 reaches 60.12%, indicating the superior adsorption performance of AE500 at ambient temperature. As the temperature increases to 328 K, the removal efficiency declines to 64.77% for AE500 and 50.37% for AE700, suggesting a reduction in the affinity between the dye molecules and the adsorbent surface. This decrease in adsorption capacity at higher temperatures can be attributed to the weakening of electrostatic interactions and the enhanced desorption of dye molecules from the surface due to increased kinetic energy. The more pronounced decline in removal efficiency for AE700 compared to AE500 further highlights the lower adsorption capacity of AE700, which can be linked to its larger grain size and reduced surface reactivity. These findings suggest that the adsorption process is exothermic in nature, with stronger dye–adsorbent interactions at lower temperatures, making AE500 a more efficient adsorbent under ambient conditions.
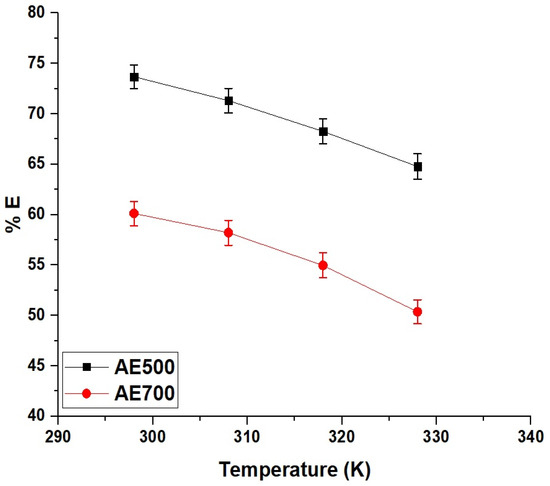
Figure 10.
Effect of temperature on the removal efficiency (% E) of crystal violet dye using AE500 and AE700 nanocomposites.
The thermodynamic parameters for the adsorption of crystal violet dye onto AE500 and AE700 nanocomposites were determined using Equations (5)–(7) [45]. The equilibrium distribution coefficient (Kd, L/g) was calculated using Equation (5).
The Van’t Hoff equation, represented by Equation (6), was employed to determine the enthalpy (ΔH°) and entropy (ΔS°) changes.
where R (8.314 × 10−3 kJ/mol·K) is the universal gas constant and T (K) is the absolute temperature.
The Gibbs free energy change (ΔG°) was calculated using Equation (7).
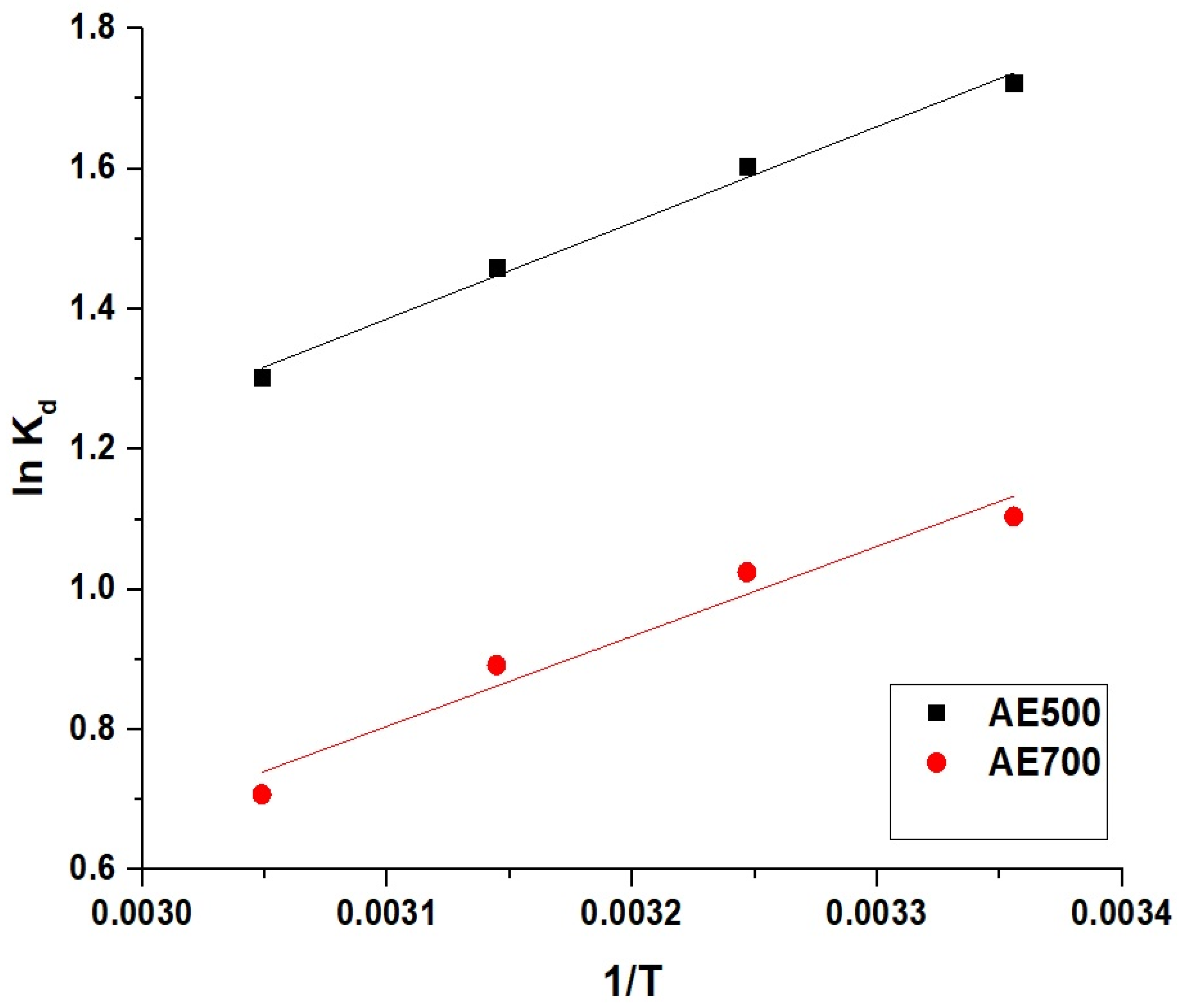
Figure 11 represents the Van’t Hoff plot for the removal of crystal violet dye using AE500 and AE700 nanocomposites. The thermodynamic parameters presented in Table 3 indicate that the adsorption process is physical in nature, as the enthalpy change (ΔH°) is less than 40 kJ/mol. The negative values of ΔH° for both AE500 and AE700 confirm that the adsorption process is exothermic, implying that lower temperatures favor dye uptake. The negative Gibbs free energy values (ΔG°) across all studied temperatures indicate that the adsorption process is spontaneous, with a greater spontaneity at higher temperatures. The positive entropy change (ΔS°) further confirms that the adsorption is thermodynamically feasible, suggesting an increase in the randomness at the solid–liquid interface during dye adsorption. These results collectively demonstrate that the adsorption of crystal violet dye onto AE500 and AE700 is an energetically favorable and spontaneous process, primarily driven by physical interactions.
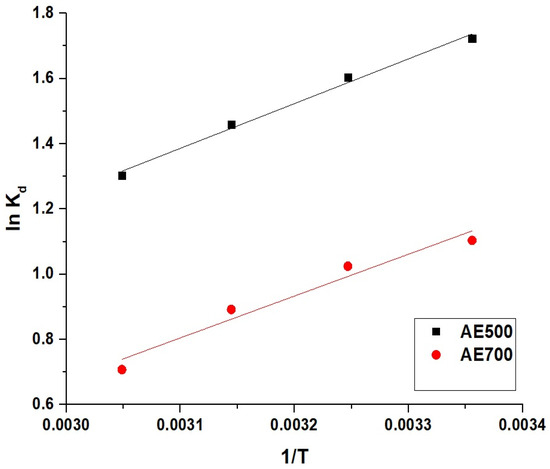
Figure 11.
Van’t Hoff plot for the adsorption of crystal violet dye onto AE500 and AE700 nanocomposites.

Table 3.
Thermodynamic parameters for the uptake of crystal violet dye onto AE500 and AE700 nanocomposites.
2.2.4. Effect of Concentration
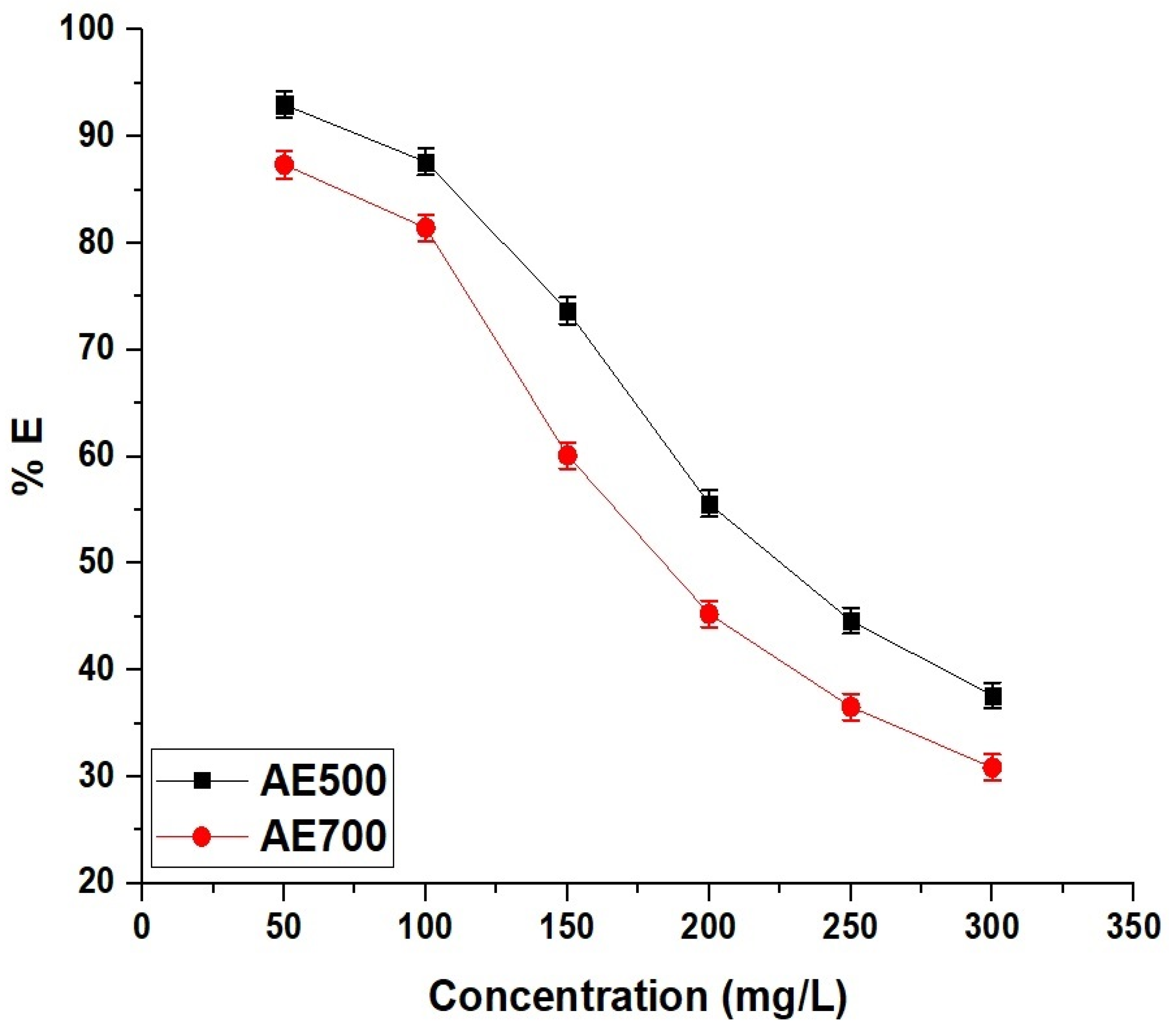
Figure 12 illustrates the effect of initial dye concentration on the removal efficiency of crystal violet dye using AE500 and AE700 nanocomposites, showing a decreasing trend in adsorption efficiency with increasing dye concentration. At a low initial concentration of 50 mg/L, AE500 achieves a removal efficiency of 92.98%, while AE700 reaches 87.36%, indicating the high availability of active adsorption sites at lower dye concentrations. As the concentration increases to 300 mg/L, the removal efficiency decreases to 37.63% for AE500 and 30.87% for AE700, suggesting that the adsorption sites become saturated as more dye molecules compete for the limited active sites on the adsorbent surface. The superior adsorption performance of AE500 compared to AE700 across all concentrations can be attributed to its higher surface reactivity and enhanced dye–adsorbent interactions. These results confirm that the adsorption process is more effective at lower dye concentrations, where a higher proportion of dye molecules can interact with available surface sites, whereas at higher concentrations, saturation effects lead to a decline in removal efficiency.
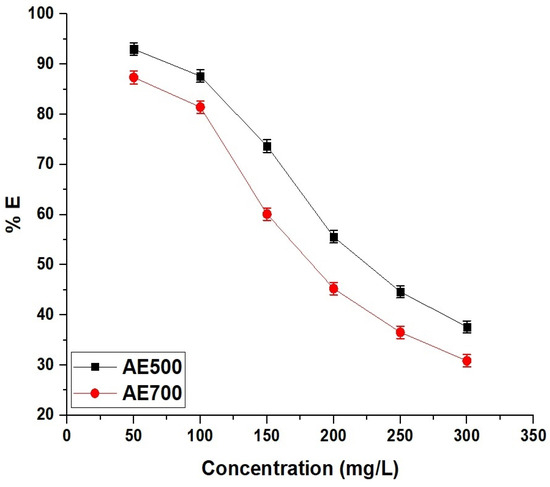
Figure 12.
Effect of initial concentration of dye on the removal efficiency (% E) of crystal violet dye using AE500 and AE700 nanocomposites.
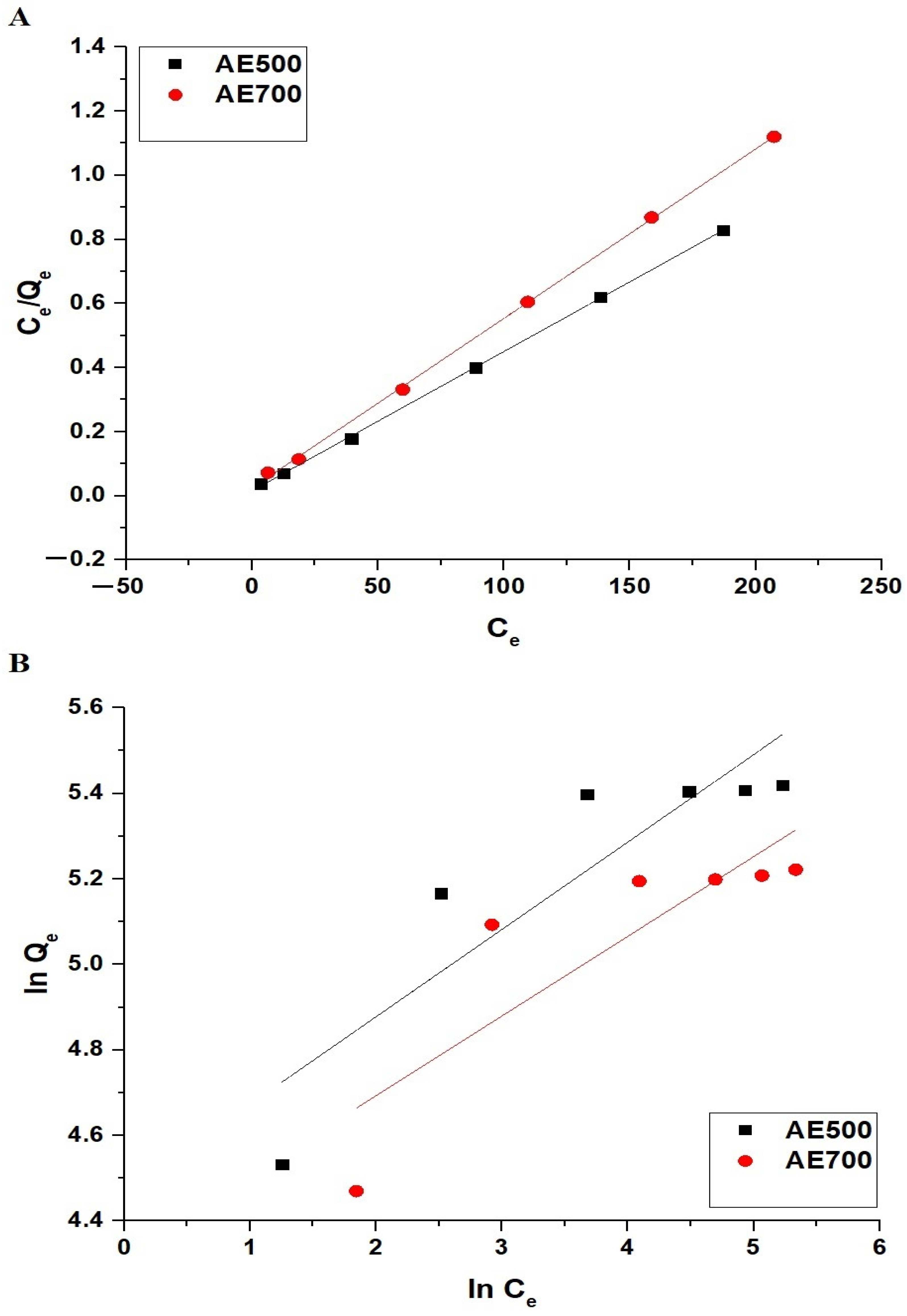
The adsorption behavior of crystal violet dye onto AE500 and AE700 nanocomposites was analyzed using the Langmuir and Freundlich isotherm models, as shown in Figure 13A,B, respectively. The Langmuir isotherm model is expressed by Equation (8) [46].
where Qmax (mg/g) is the maximum monolayer adsorption capacity, and K3 (L/mg) is the Langmuir constant related to the affinity of the adsorbate for the adsorbent surface.
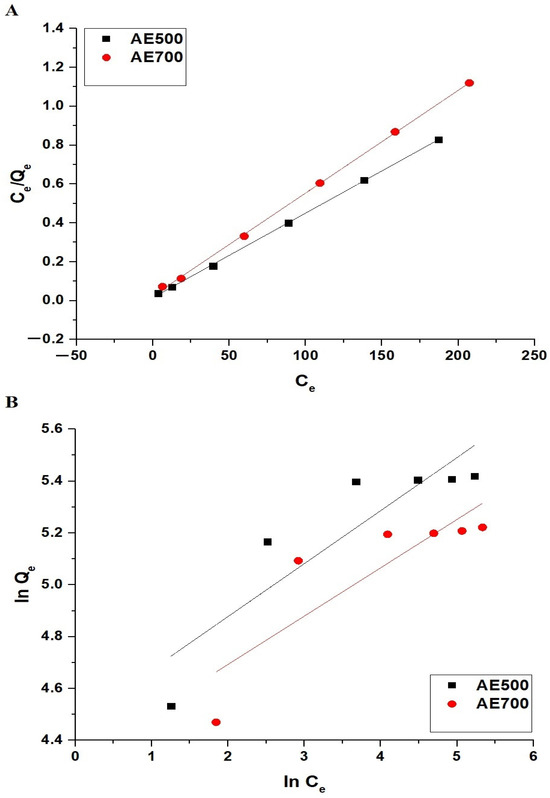
Figure 13.
Adsorption isotherm modeling of crystal violet dye onto AE500 and AE700 nanocomposites: (A) Langmuir isotherm plot and (B) Freundlich isotherm plot.
The Freundlich isotherm model is represented by Equation (9) [46].
where K4 ((mg/g)(L/mg)1/n) is the Freundlich constant indicating adsorption capacity, and n is the Freundlich exponent describing the adsorption intensity.
The empirical relation for estimating Qmax from the Freundlich model is given by Equation (10) [38].
Table 4 indicates that the adsorption process follows the Langmuir model, as evidenced by the high correlation coefficients (R2) of 0.9997 for AE500 and 0.9994 for AE700. The lower R2 values for the Freundlich model, particularly for AE700, suggest that adsorption occurs predominantly as a monolayer on a homogeneous surface rather than as a multilayer adsorption process. The higher Qmax value for AE500 compared to AE700 further confirms the greater adsorption capacity of AE500, which can be attributed to its smaller particle size and higher surface reactivity. These findings indicate that the adsorption of crystal violet dye onto AE500 and AE700 nanocomposites is best described by the Langmuir isotherm, which supports the monolayer adsorption mechanism.

Table 4.
Adsorption isotherm parameters of crystal violet dye onto AE500 and AE700 nanocomposites based on Langmuir and Freundlich models.
Table 5 presents a comparative analysis of the maximum adsorption capacities (Qmax) of various nanoparticle-based adsorbents for the removal of crystal violet dye, highlighting the effectiveness of different materials in dye adsorption applications [39,40,41,47,48,49]. The adsorption capacities vary significantly among the listed adsorbents, with values ranging from 19.75 mg/g for iron–manganese oxide coated kaolinite to 230.41 mg/g for AE500. Among the tested materials, AE500 exhibits the highest adsorption capacity, followed by biochar-based sodium alginate microspheres (223.88 mg/g) and AE700 (189.39 mg/g), demonstrating the superior performance of these adsorbents compared to other nanomaterials. The enhanced adsorption capacity of AE500 and AE700 can be attributed to their unique composition, high surface area, and optimized structural properties, which facilitate more effective dye–adsorbent interactions. The presence of SrCO3, MgO, CaO, and CaCO3 phases in the nanocomposites contributes to their strong affinity for cationic dye through electrostatic attraction and surface complexation mechanisms. Additionally, the nanocomposite structure provides a high density of active sites for dye binding, further enhancing adsorption efficiency. In contrast, adsorbents such as iron–manganese oxide coated kaolinite and polypyrrole-decorated bentonite magnetic nanocomposite exhibit lower adsorption capacities due to their relatively limited surface area and fewer available active sites. The overall comparison in Table 6 confirms that AE500 and AE700 nanocomposites are among the most efficient adsorbents for crystal violet dye removal, making them promising candidates for wastewater treatment applications.

Table 5.
Comparison of maximum uptake capacities (Qmax) for several adsorbents used in the elimination of crystal violet dye.

Table 6.
Experimental parameters for investigating the influences of pH, temperature, contact time, and initial dye concentration on adsorption efficacy of the AE500 and AE700 nanocomposites.
To evaluate the textural properties of the synthesized nanocomposites, N2 adsorption–desorption isotherms were recorded, and the specific surface area was analyzed using the BET method, respectively. The BET surface area of AE500 was found to be 46.42 m2/g with an average pore diameter of 8.86 nm, while AE700 showed a surface area of 38.75 m2/g with an average pore diameter of 7.35 nm. Both samples exhibited type IV isotherms with H3 hysteresis loops (figures omitted for brevity), indicating a mesoporous structure. The higher surface area and pore size of AE500 support its superior adsorption capacity by providing more accessible active sites for dye molecules. The BET results are consistent with the morphological observations and adsorption performance trends.
2.2.5. Effect of Desorption and Reusability
To evaluate the reusability of the synthesized adsorbents, regeneration experiments were carried out by desorbing the crystal violet dye from the dye-loaded AE500 and AE700 nanocomposites. Hydrochloric acid (HCl) was employed as the eluting agent at varying concentrations of 1.0 M, 1.5 M, and 2.0 M. A fixed volume of 50 mL of each HCl solution was used to treat the dye-laden adsorbents under controlled conditions to facilitate the desorption process. The desorption efficiency was assessed by determining the desorption percentage of crystal violet dye (% D), which was calculated using Equation (11).
In this equation, Cd is the concentration of crystal violet dye in the desorbing solution (mg/L), whereas Vd is the volume of the desorbing solution (L).
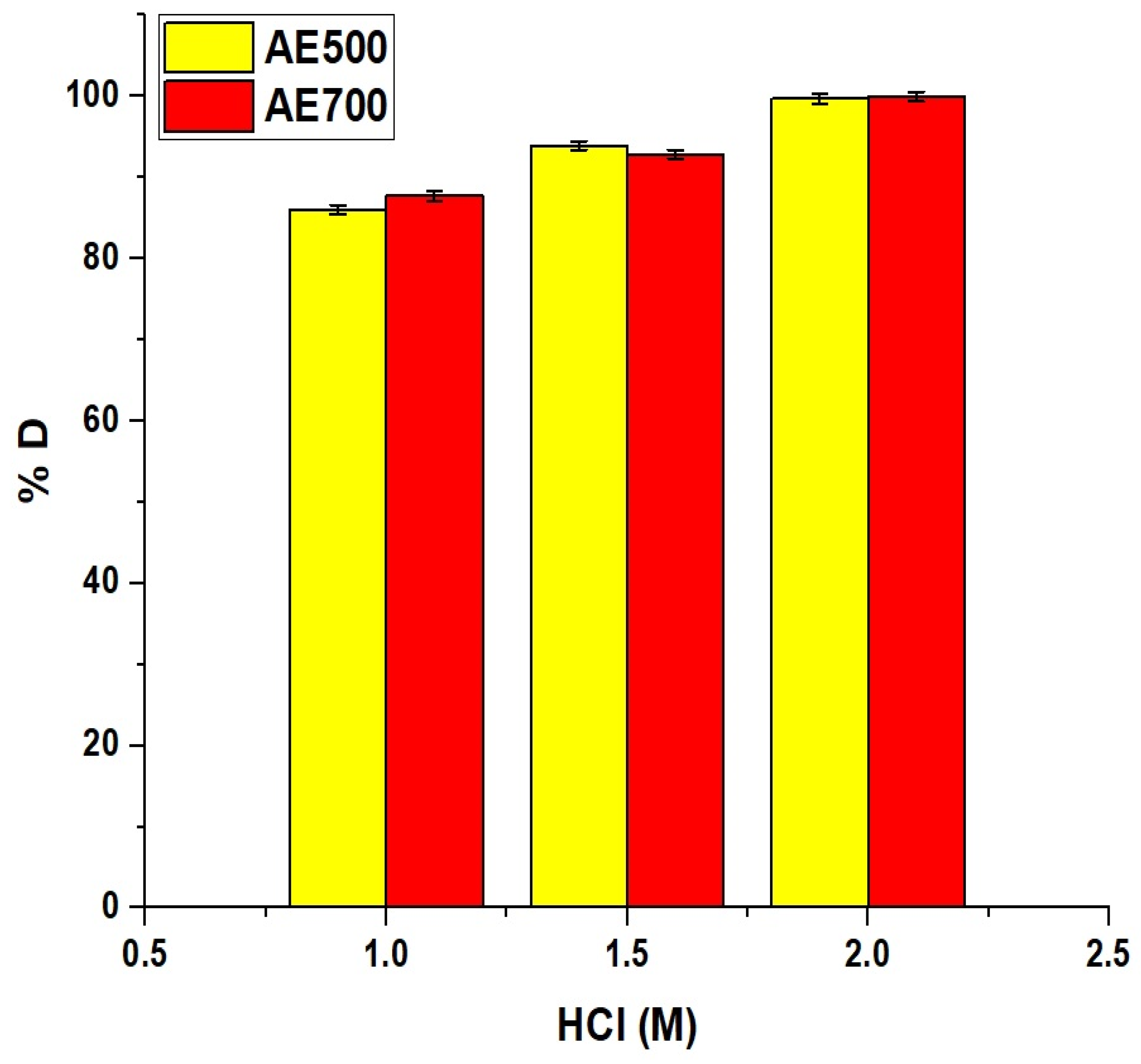
Figure 14 represents the desorption behavior of crystal violet dye from the AE500 and AE700 nanocomposites using HCl as an eluting agent. The desorption percentage increased progressively with increasing acid concentration for both nanocomposites, indicating enhanced dye release at higher acidity. At 1.0 M HCl, the desorption percentage reached 86.03% for AE500 and 87.74% for AE700. When the HCl concentration was increased to 1.5 M, the desorption efficiency improved to 93.93% for AE500 and 92.88% for AE700. At 2.0 M HCl, both samples achieved nearly complete desorption with 99.71% and 99.98% recorded for AE500 and AE700, respectively. This excellent desorption performance can be attributed to the strong protonation effect of HCl, which disrupts electrostatic interactions between the adsorbed dye molecules and the surface of the nanocomposites, thereby facilitating effective dye release and regeneration of the adsorbent surface.
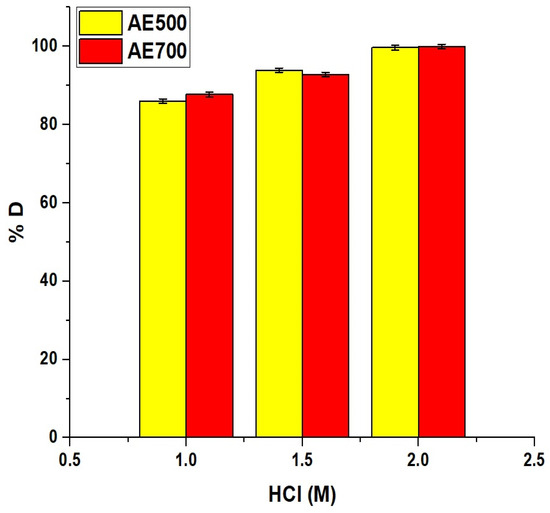
Figure 14.
Desorption efficiency of crystal violet dye from AE500 and AE700 nanocomposites using different concentrations of HCl as the eluting agent.
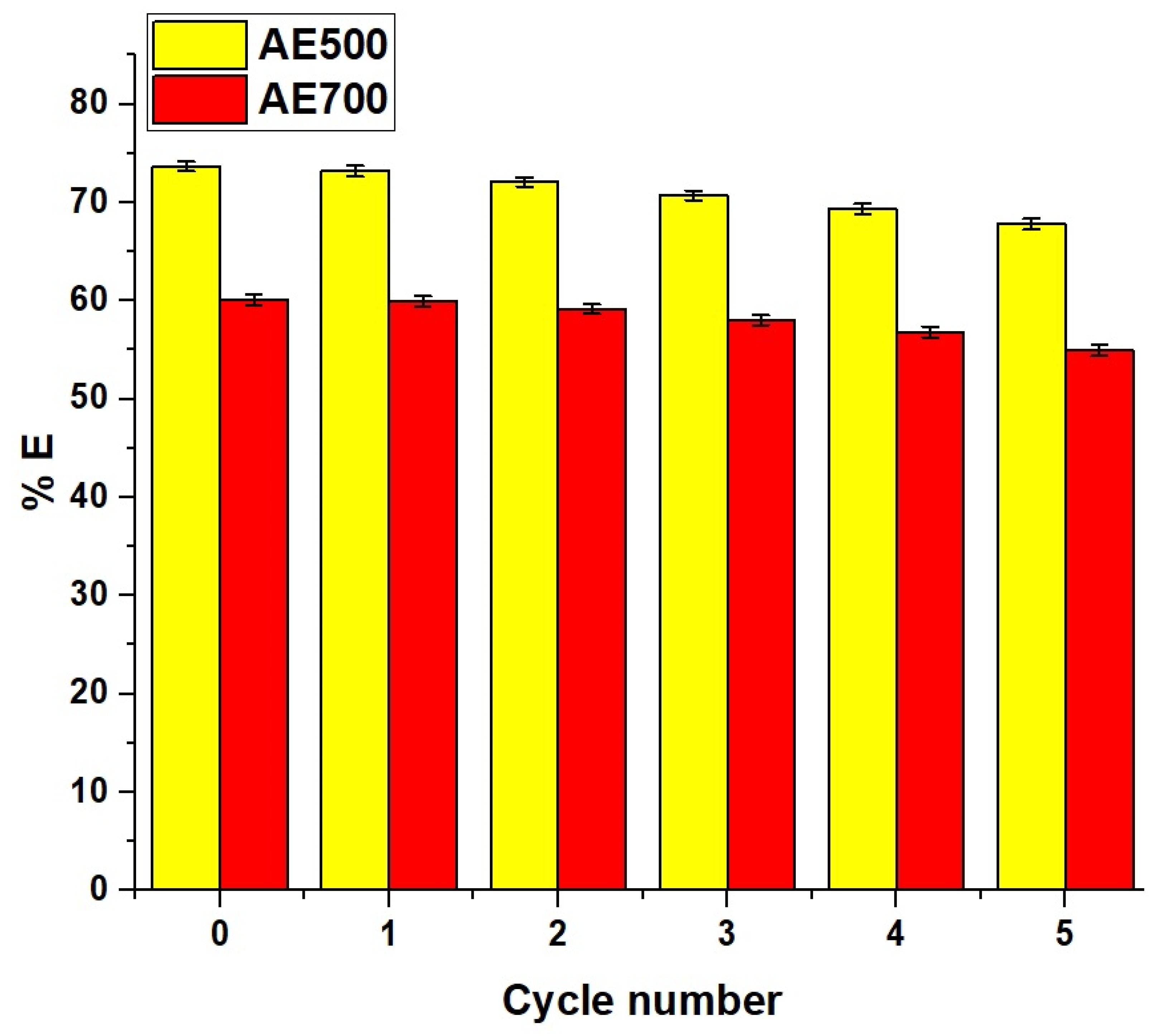
The reusability of the AE500 and AE700 nanocomposites for the removal of crystal violet dye was evaluated over five successive adsorption–desorption cycles under identical experimental conditions. Each cycle was performed using a dye solution with an initial concentration of 150 mg/L and a volume of 100 mL at a constant temperature of 298 K and a fixed pH of 10. An adsorbent dose of 0.05 g was applied in each run to ensure consistency. The contact time required to reach equilibrium was set at 50 min for AE500 and 70 min for AE700 based on their respective adsorption kinetics. After each adsorption cycle, the spent adsorbents were regenerated using hydrochloric acid and reused under the same conditions to assess their stability and efficiency in repeated applications.
Figure 15 represents the reusability performance of AE500 and AE700 nanocomposites. Both materials showed a gradual decline in removal efficiency of crystal violet dye with each regeneration cycle, indicating a slight loss in adsorption capacity over repeated use. Despite this gradual reduction, both nanocomposites retained a considerable portion of their original efficiency, particularly AE500, which demonstrated higher stability and reusability compared to AE700, making it a more reliable candidate for multiple-use applications in crystal violet dye removal.
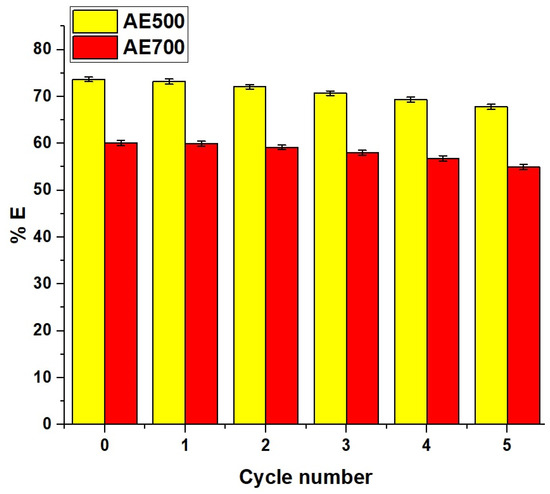
Figure 15.
Reusability performance of AE500 and AE700 nanocomposites for crystal violet dye removal over five consecutive adsorption–desorption cycles.
3. Experimental
3.1. Materials
In this study, all chemicals were of analytical grade and used without further purification. Tartaric acid (C4H6O6), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O), sodium hydroxide (NaOH), polyethylene glycol 400 (H(OCH2CH2)nOH), hydrochloric acid (HCl), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O), potassium chloride (KCl), strontium nitrate (Sr(NO3)2), and crystal violet dye (C25H30ClN3) were all purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Synthesis of SrCO3/MgO/CaO/CaCO3 Nanocomposites
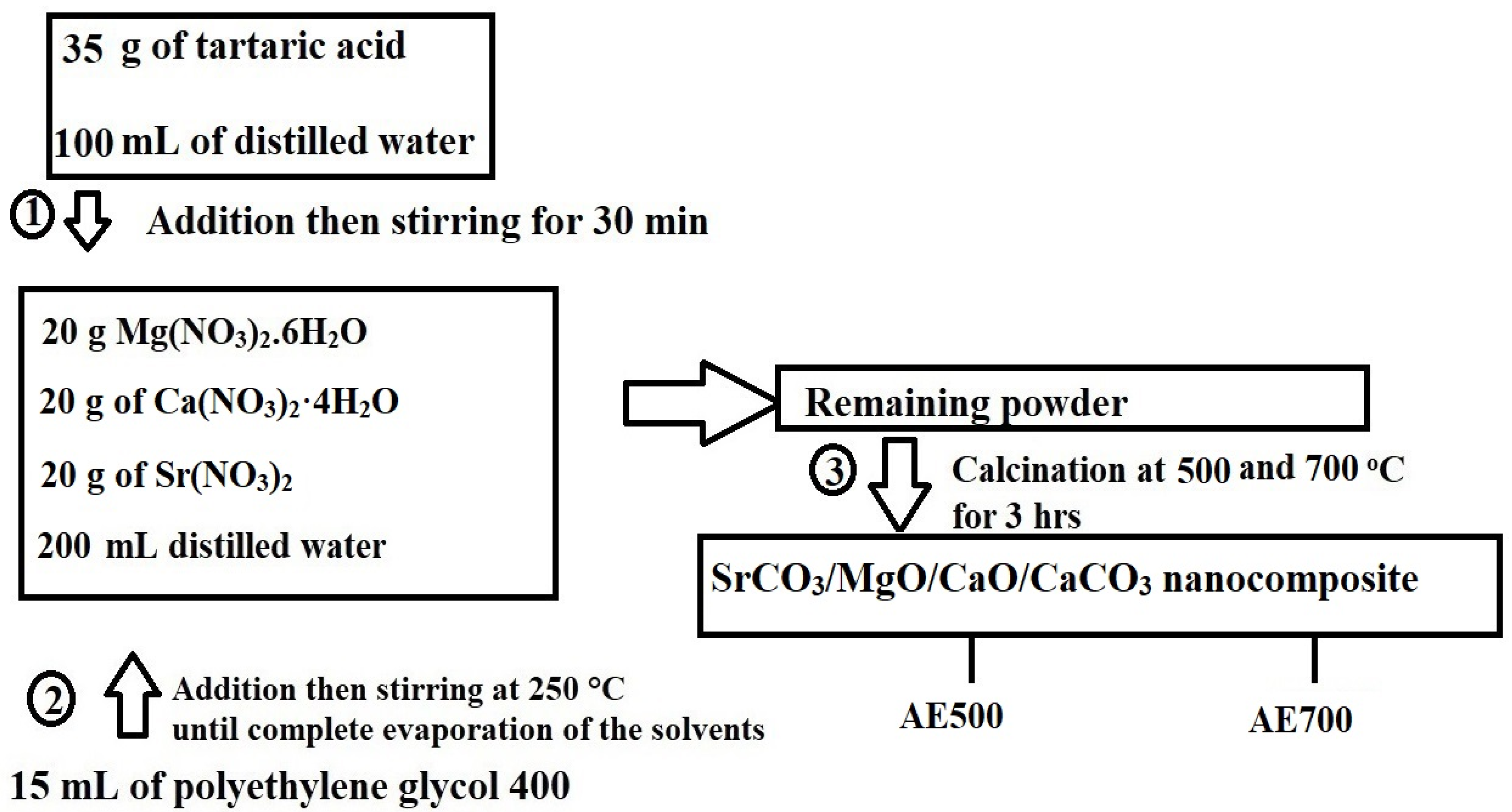
The synthesis of SrCO3/MgO/CaO/CaCO3 nanocomposites was carried out using the Pechini sol-gel method, as illustrated in Figure 16. Initially, 35 g of tartaric acid was dissolved in 100 mL of distilled water under continuous stirring. Separately, 20 g of Mg(NO3)2·6H2O, 20 g of Ca(NO3)2·4H2O, and 20 g of Sr(NO3)2 were dissolved in 200 mL of distilled water. The tartaric acid solution was then gradually added to the nitrate solution with continuous stirring for 30 min. The molar ratio of tartaric acid:Mg(NO3)2·6H2O:Ca(NO3)2·4H2O:Sr(NO3)2 is 15:5:5:6. Subsequently, 15 mL of polyethylene glycol 400 was introduced into the mixture, and the solution was heated at 250 °C until complete evaporation of the solvents (distilled water and polyethylene glycol 400). The resulting precursor powder was subjected to calcination at 500 and 700 °C for 3 h to obtain SrCO3/MgO/CaO/CaCO3 nanocomposites, which were designated as AE500 and AE700, respectively.
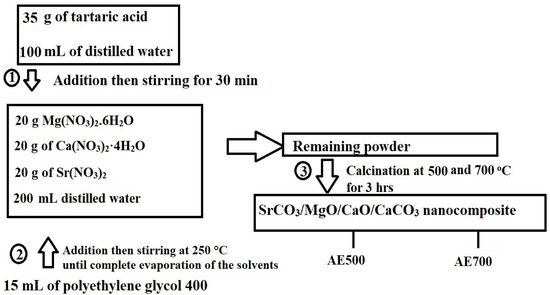
Figure 16.
Schematic representation for the synthesis of SrCO3/MgO/CaO/CaCO3 nanocomposites using the Pechini sol-gel method.
3.3. Instrumentation
The structural characterization of all synthesized samples was conducted using an X-ray diffraction diffractometer (D8 Discover, Bruker, Billerica, MA, USA). The morphological and elemental analyses were performed using a field emission scanning electron microscope equipped with energy dispersive X-ray spectroscopy (FE-SEM/EDX Quanta 250 FEG, Thermo Fisher Scientific, Waltham, MA, USA). Further investigation of the microstructural features was carried out using a high-resolution transmission electron microscope (HR-TEM JEM-2100Plus, JEOL Ltd., Tokyo, Japan). The concentration of crystal violet dye in the solution was determined using a UV-Vis spectrophotometer (Cintra 3030, GBC, Melbourne, Australia).
3.4. Adsorption Procedure of Crystal Violet Dye from Aqueous Solutions
The adsorption efficiency of AE500 and AE700 nanocomposites in removing crystal violet dye was systematically assessed under various experimental conditions, as outlined in Table 6. To examine the effect of pH, adsorption studies were conducted using 0.1 L dye solutions with an initial concentration of 150 mg/L, an adsorbent dose of 50 mg, and a temperature of 298 K. The solution pH was adjusted between 2 and 10 using 0.1 M NaOH or 0.1 M HCl, and the mixtures were stirred for 240 min to reach equilibrium. The impact of contact time on adsorption performance was analyzed by varying the adsorption duration from 10 to 100 min while maintaining a constant solution volume of 0.1 L, an initial dye concentration of 150 mg/L, an adsorbent mass of 50 mg, and a temperature of 298 K. In all experiments, the pH was kept at 10, and the solutions were continuously stirred throughout the process. To explore the influence of temperature, adsorption experiments were carried out at temperatures ranging from 298 to 328 K, using a solution volume of 0.1 L, an initial dye concentration of 150 mg/L, and an adsorbent dosage of 50 mg. The adsorption process was conducted at a pH of 10 for 50 min when using AE500 and for 70 min when using AE700, ensuring sufficient contact time for equilibrium. The effect of the initial dye concentration on adsorption efficiency was investigated by preparing dye solutions with concentrations between 50 and 300 mg/L while keeping all other parameters constant. These experiments were performed at 298 K with a solution volume of 0.1 L, an adsorbent dose of 50 mg, and a pH of 10. The contact times were set at 50 min for AE500 and 70 min for AE700. After the adsorption process, the adsorbents were separated via centrifugation, and the remaining crystal violet dye concentration in the filtrate was measured using a UV-Vis spectrophotometer at 590 nm.
3.5. Point of Zero Charge (pHPZC) of SrCO3/MgO/CaO/CaCO3 Nanocomposites
The point of zero charge (pHPZC) of AE500 and AE700 nanocomposites was determined using the batch equilibrium method with potassium chloride (KCl) as the supporting electrolyte [38]. A series of 50 mL KCl solutions (0.1 M) were prepared, and their initial pH (pHI) was adjusted between 2 and 12 using either 0.1 M HCl or 0.1 M NaOH. Each solution was then transferred into a separate beaker containing 50 mg of the nanocomposite, and the suspensions were continuously stirred at room temperature for 24 hrs to achieve equilibrium. Once equilibrium was established, the final pH (pHF) of each solution was recorded. The pHPZC was determined by plotting the change in pH (ΔpH) against the initial pH (pHI), where ΔpH was calculated using Equation (12) [38].
where ΔpH represents the difference between the final and initial pH values. The pHPZC was identified as the pH at which ΔpH equals zero, indicating the point where the net surface charge of the nanocomposite is neutral.
△pH = pHF − pHI
4. Conclusions
The present study successfully synthesized and characterized SrCO3/MgO/CaO/CaCO3 nanocomposites using the Pechini sol-gel method at 500 °C (AE500) and 700 °C (AE700) for the efficient removal of crystal violet dye from aqueous media. XRD confirmed the formation of multiple crystalline phases, with an increase in crystallite size at higher calcination temperatures. The morphological analysis using FE-SEM and HR-TEM demonstrated significant structural differences between AE500 and AE700, where AE500 exhibited smaller and irregularly shaped particles, while AE700 displayed well-defined polyhedral and nearly spherical morphologies. Elemental composition analysis via EDX revealed variations in carbon, oxygen, magnesium, calcium, and strontium content, which influenced the adsorption performance of the materials. The adsorption studies demonstrated that AE500 exhibited a higher maximum adsorption capacity (230.41 mg/g) compared to AE700 (189.39 mg/g), which was attributed to its smaller particle size, higher surface reactivity, and increased active site availability. Adsorption kinetics followed the pseudo-first-order model, indicating physisorption as the dominant mechanism. The adsorption isotherm analysis confirmed that the Langmuir model best described the adsorption behavior, suggesting monolayer adsorption onto a homogeneous surface. Thermodynamic studies revealed that the adsorption process was exothermic and spontaneous, with a decrease in adsorption capacity at higher temperatures. Overall, the results highlight the potential of SrCO3/MgO/CaO/CaCO3 nanocomposites as efficient adsorbents for crystal violet dye removal, with AE500 demonstrating superior performance. The facile synthesis route, high adsorption capacity, and favorable thermodynamic properties make these nanocomposites promising candidates for wastewater treatment applications.
Author Contributions
E.A.A. (methodology, funding acquisition, conceptualization, writing—review and editing), M.T.B. (conceptualization, writing—review and editing). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent Advances on the Removal of Dyes from Wastewater Using Various Adsorbents: A Critical Review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Badawi, A.K.; Abd Elkodous, M.; Ali, G.A.M. Recent Advances in Dye and Metal Ion Removal Using Efficient Adsorbents and Novel Nano-Based Materials: An Overview. RSC Adv. 2021, 11, 36528–36553. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Fu, P.; Li, X. Frontiers in Machine Learning Strategies for Dye Removal in Water Treatment. J. Water Process Eng. 2025, 71, 107251. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Ashraf, M.; Ullah, N. A Critical Review on the Removal of Anionic Dyes by Cross-Linked Resin: Recent Progress, Challenges and Future Perspective. Sep. Purif. Technol. 2025, 360, 131111. [Google Scholar] [CrossRef]
- Ahmad, S.; Mujtaba, G.; Zubair, M.; Shah, M.U.H.; Daud, M.; Mu’azu, N.D.; Al-Harthi, M.A. A Review of Performance, Mechanism, and Challenges of Layered Double Hydroxide-Based Biocomposites for the Adsorptive Removal of Dye Contaminants from Water and Wastewater. J. Water Process Eng. 2025, 70, 106837. [Google Scholar] [CrossRef]
- Silina, A.; El Achari, A.; Salaün, F. Metal-Organic Framework Electrospun Nanofibers in Application to Dye Removal from Textile Wastewaters: A Review. J. Environ. Chem. Eng. 2024, 12, 114819. [Google Scholar] [CrossRef]
- Osugi, M.E.; Rajeshwar, K.; Ferraz, E.R.A.; de Oliveira, D.P.; Araújo, Â.R.; Zanoni, M.V.B. Comparison of Oxidation Efficiency of Disperse Dyes by Chemical and Photoelectrocatalytic Chlorination and Removal of Mutagenic Activity. Electrochim. Acta 2009, 54, 2086–2093. [Google Scholar] [CrossRef]
- Park, D.; Nam, S.N.; Jung, B.; Soo Choi, J.; Min Park, C.; Earn Choong, C.; Jang, M.; Cho, K.S.; Jun, B.M.; Yoon, Y. Removal of Selected Contaminants of Dyes and Pharmaceuticals Using MXene-Based Nanoadsorbents: A Review. Sep. Purif. Technol. 2024, 341, 126864. [Google Scholar] [CrossRef]
- Radia, N.D.; Aljeboree, A.M.; Mhammed, A.A.A. Enhanced Removal of Crystal Violet from Aqueous Solution Using Carrageenan Hydrogel Nanocomposite/MWCNTs. Inorg. Chem. Commun. 2024, 167, 112803. [Google Scholar] [CrossRef]
- Brandão, A.T.S.C.; Rosoiu-State, S.; Costa, R.; Enache, L.B.; Mihai, G.V.; Vázquez, J.A.; Valcarcel, J.; Anicai, L.; Enachescu, M.; Pereira, C.M. Unlocking the Power of Amorphous TiO2-Decorated Biocarbon Composite: Enhanced Photocatalytic Performance for Crystal Violet Dye Degradation. J. Water Process Eng. 2025, 71, 107288. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Z.; Zhang, X.; Guo, J.; Yang, Z.; Liu, X.; Han, L. Magnetic Chitosan-Functionalized Bone Char for Efficient Removal of Anionic Dyes: Insights into Adsorption-Enhanced Mechanism. Int. J. Biol. Macromol. 2025, 305, 140941. [Google Scholar] [CrossRef]
- Kishore, K.; Kaur, J.; Saddeek, Y.B.; Sharma, M.; Singh, M.; Thakur, P.; Walia, Y.K.; Lal, M.; Suman, R.; Reddy, A.S.; et al. Efficient Removal of Toxic Dyes from Water Using Mn3O4 Nanoparticles: Synthesis, Characterization, and Adsorption Mechanisms. J. Mol. Struct. 2025, 1333, 141756. [Google Scholar] [CrossRef]
- Muniasamy, S.K.; Alobaid, A.A.; Warad, I.; Ravindiran, G. Removal of Brilliant Green Dye in Aqueous Solution Using Synthetic Coagulation and Flocculation Technique. Desalination Water Treat. 2023, 314, 231–240. [Google Scholar] [CrossRef]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials (Bentonite and Opuntia Ficus Indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Fadeeva, N.P.; Volkova, I.R.; Kharchenko, I.A.; Elsuf’ev, E.V.; Fomenko, E.V.; Akimochkina, G.V.; Afanasova, K.A.; Nemtsev, I.V.; Tarasova, L.S.; Yushkin, A.A.; et al. Development of Composite Ultrafiltration Membrane from Fly Ash Microspheres and Alumina Nanofibers for Efficient Dye Removal from Aqueous Solutions. Ceram. Int. 2024, 50, 52890–52903. [Google Scholar] [CrossRef]
- Rakcho, Y.; Baidou, M.; Naboulsi, A.; Bouazizi, A.; Mouiya, M.; Sehaqui, H.; Abouliatim, Y.; Benhammou, A.; Ouammou, M.; Abourriche, A.; et al. Fabrication of Low-Cost Ceramic Nanofiltration Membrane from Natural Resources for the Removal of Cationic and Anionic Dyes: Experimental and DFT Investigations. Chem. Eng. J. 2025, 505, 159779. [Google Scholar] [CrossRef]
- Lin, T.; Wu, Y.; Chen, H.; Ren, X.; Joshi, R. Graphene Oxide-Polyvinyl Alcohol Nanofiltration Membranes for Efficient Dye Removal in Practical Conditions. J. Membr. Sci. 2025, 719, 123736. [Google Scholar] [CrossRef]
- Cui, T.; Wang, X.; Chen, Y.; Chen, Y.; Fu, B.; Tu, Y. Reverse Osmosis Coupling Multi-Catalytic Ozonation (RO-MCO) in Treating Printing and Dyeing Wastewater and Membrane Concentrate: Removal Performance and Mechanism. Water Resour. Ind. 2023, 30, 100217. [Google Scholar] [CrossRef]
- Al-Bastaki, N. Removal of Methyl Orange Dye and Na2SO4 Salt from Synthetic Waste Water Using Reverse Osmosis. Chem. Eng. Process. Process Intensif. 2004, 43, 1561–1567. [Google Scholar] [CrossRef]
- Leng, Q.; Xu, S.; Wu, X.; Wang, S.; Jin, D.; Wang, P.; Wu, D.; Dong, F. Electrochemical Removal of Synthetic Methyl Orange Dyeing Wastewater by Reverse Electrodialysis Reactor: Experiment and Mineralizing Model. Environ. Res. 2022, 214, 114064. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, X.; Xu, S.; Zhang, Y.; Wang, S. Experimental Investigation on Dyeing Wastewater Treatment and By-Product Hydrogen with a Reverse Electrodialysis Flocculator. Int. J. Hydrogen Energy 2023, 48, 19022–19032. [Google Scholar] [CrossRef]
- Goswami, D.; Mukherjee, J.; Mondal, C.; Bhunia, B. Bioremediation of Azo Dye: A Review on Strategies, Toxicity Assessment, Mechanisms, Bottlenecks and Prospects. Sci. Total Environ. 2024, 954, 176426. [Google Scholar] [CrossRef] [PubMed]
- Pratap Singh, V.; Godara, P.; Srivastava, A. Sustainable Microalgal Bioremediation of Heavy Metals and Dyes from Synthetic Wastewater: Progressing towards United Nations Sustainable Development Goals. Waste Manag. Bull. 2024, 2, 123–135. [Google Scholar] [CrossRef]
- Safaralizadeh, E.; Mahjoub, A.; Janitabardarzi, S. Visible Light-Induced Degradation of Phenolic Contaminants Utilizing Nanoscale TiO2 and ZnO Impregnated with SR 7B (SR) Dye as Advanced Photocatalytic Systems. Ceram. Int. 2024, 51, 1958–1969. [Google Scholar] [CrossRef]
- Amrollahi, R. Comparison of Photocatalytic Activity of Cu/TiO2, Cu/NiO and Cu/ZnO Nanocomposites for the Degradation of Organic Dyes. Appl. Catal. O Open 2024, 195, 207010. [Google Scholar] [CrossRef]
- Mustafa, G.; Munir, R.; Sadia, B.; Younas, F.; Sayed, M.; Muneer, A.; Sardar, M.F.; Albasher, G.; Noreen, S. Synthesis of Polymeric Ferrite Composites (Ni-CoFe2O4/Chitosan, Zn-NiFe2O4/Starch, Co-NiZnFe2O4/Polyaniline, Ni Doped CrZnFe2O4/Alginate, and Cr Doped ZnCoFe2O4/PVA) for the Removal of Reactive Golden Yellow-160 Dye from Wastewater. J. Environ. Chem. Eng. 2024, 12, 112581. [Google Scholar] [CrossRef]
- Iram, M.; Guo, C.; Guan, Y.; Ishfaq, A.; Liu, H. Adsorption and Magnetic Removal of Neutral Red Dye from Aqueous Solution Using Fe3O4 Hollow Nanospheres. J. Hazard. Mater. 2010, 181, 1039–1050. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Wu, F.; Ji, Y. High Adsorption Capability and Selectivity of ZnO Nanoparticles for Dye Removal. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 474–483. [Google Scholar] [CrossRef]
- Arun Viswan, K.K.; Dixit, D.; Bhattacharya, S.; Adhikary, S.; Gangadharan, D. A Sustainable Synthesis of a CuO@C Nanocomposite for the Remediation of Organic Dyes in Water and Its Antibacterial Properties. Nano-Struct. Nano-Objects 2024, 38, 101147. [Google Scholar] [CrossRef]
- Madan, S.; Shaw, R.; Tiwari, S.; Tiwari, S.K. Adsorption Dynamics of Congo Red Dye Removal Using ZnO Functionalized High Silica Zeolitic Particles. Appl. Surf. Sci. 2019, 487, 907–917. [Google Scholar] [CrossRef]
- Aldaghri, O.; El-Badry, B.A.; Ibnaouf, K.H.; Taha, K.K.; Aissa, M.A.B.; Modwi, A. CaO@ZrO2@g-C3N4 Nanosorbent for Superior Malachite Green Dye Selectivity and Adsorption from Contaminated Water. Diam. Relat. Mater. 2024, 144, 110944. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Khan, M.K.A.; Alshahrani, H.; Younes, M.K.; Algburi, S. Newly Developed Polymer Nanocomposite of Chitosan-Citrate/ZrO2 Nanoparticles for Safranin O Dye Adsorption: Physiochemical Properties and Response Surface Methodology. Mater. Chem. Phys. 2024, 324, 129699. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, Z.Z.; Yang, T.Y.; Chatterjee, S.; Cao, M.S.; Peng, H.S. Facile Synthesis of Vaterite CaCO3 Microspheres from Carbon Capture and Solid Waste Utilization towards Microwave Absorption and Dye Wastewater Adsorption. Carbon N. Y. 2024, 226, 119199. [Google Scholar] [CrossRef]
- Nallasamy, P.; Al-Ansari, M.M.; Manikam, P.; Natarajan, S. Agro-Waste Derived RGO/CaCO3 Hybrid Nanostructure as Green Adsorbent for Waste Water Treatment. J. Taiwan Inst. Chem. Eng. 2025, in press. [Google Scholar] [CrossRef]
- Qin, Q.; Li, M.; Lan, P.; Liao, Y.; Sun, S.; Liu, H. Novel CaCO3/Chitin Aerogel: Synthesis and Adsorption Performance toward Congo Red in Aqueous Solutions. Int. J. Biol. Macromol. 2021, 181, 786–792. [Google Scholar] [CrossRef]
- Jawad, A.H.; Maharani, R.A.; Hapiz, A.; ALOthman, Z.A.; Wilson, L.D. A Comparison of Freeze- and Air-Dried Chitosan Salicylaldehyde/Calcium Oxide Biocomposites for Optimized Removal of Acid Red 88 Dye. Int. J. Biol. Macromol. 2025, 292, 139165. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, P.; Kataria, N. Development of Chemically and Green Synthesized MgO Nanoparticles for CR and BG Dye Adsorption from Single and Binary Aqueous System. J. Taiwan Inst. Chem. Eng. 2024, 166, 105566. [Google Scholar] [CrossRef]
- Al-kadhi, N.S.; Abdelrahman, E.A.; Alamro, F.S.; Saad, F.A.; Al-Raimi, D.S. Innovative nanocomposite comprising of ZrO2, MnCO3, and CdCO3 for superior crystal violet dye adsorption: Synthesis, characterization, and regeneration insights. Sci. Rep. 2025, 15, 5525. [Google Scholar] [CrossRef]
- Muthukumaran, C.; Sivakumar, V.M.; Thirumarimurugan, M. Adsorption Isotherms and Kinetic Studies of Crystal Violet Dye Removal from Aqueous Solution Using Surfactant Modified Magnetic Nanoadsorbent. J. Taiwan Inst. Chem. Eng. 2016, 63, 354–362. [Google Scholar] [CrossRef]
- Ahamad, Z.; Nasar, A. Polypyrrole-Decorated Bentonite Magnetic Nanocomposite: A Green Approach for Adsorption of Anionic Methyl Orange and Cationic Crystal Violet Dyes from Contaminated Water. Environ. Res. 2024, 247, 118193. [Google Scholar] [CrossRef]
- Khan, T.A.; Khan, E.A.; Shahjahan. Removal of Basic Dyes from Aqueous Solution by Adsorption onto Binary Iron-Manganese Oxide Coated Kaolinite: Non-Linear Isotherm and Kinetics Modeling. Appl. Clay Sci. 2015, 107, 70–77. [Google Scholar] [CrossRef]
- Marica, I.; Nekvapil, F.; Ștefan, M.; Farcău, C.; Falamaș, A. Zinc Oxide Nanostructures for Fluorescence and Raman Signal Enhancement: A Review. Beilstein J. Nanotechnol. 2022, 13, 472–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Wu, X.; Wang, Y.; Fan, G.; Huang, Y.; Zhang, L. Preparation of Magnetic DTPA-Modified Chitosan Composite Microspheres for Enhanced Adsorption of Pb(II) from Aqueous Solution. Int. J. Biol. Macromol. 2024, 264, 130410. [Google Scholar] [CrossRef]
- Jiang, R.; Shen, T.T.; Zhu, H.Y.; Fu, Y.Q.; Jiang, S.T.; Li, J.B.; Wang, J.L. Magnetic Fe3O4 Embedded Chitosan–Crosslinked-Polyacrylamide Composites with Enhanced Removal of Food Dye: Characterization, Adsorption and Mechanism. Int. J. Biol. Macromol. 2023, 227, 1234–1244. [Google Scholar] [CrossRef]
- Ehab, N.S.A.; Fowzia, A.A.; Fawaz, S.A.; Doaa, A.S. Facile Synthesis of MnCO3/ZrO2/MgCO3 Nanocomposite for High-Efficiency Malachite Green Dye Removal. J. Inorg. Organomet. Polym. Mater. 2025; in press. [Google Scholar] [CrossRef]
- Xinyu, Z.; Tao, F.; Hongwei, Y.; Ming, G.; Nuraje, N. Preparation of Chitosan-like Magnetic Composite Microspheres and Their Adsorption Properties for Cu (II). Mater. Today Proc. 2022, 71, 105–113. [Google Scholar] [CrossRef]
- Tang, L.; Liu, C.; Liu, X.; Zhang, L.; Fan, B.; Wang, B.; Wang, F. Efficient Adsorption of Crystal Violet by Different Temperature Pyrolyzed Biochar-Based Sodium Alginate Microspheres: A Green Solution for Food Industry Dye Removal. Food Chem. X 2025, 26, 102311. [Google Scholar] [CrossRef]
- Azari, A.; Noorisepehr, M.; Dehganifard, E.; Karimyan, K.; Hashemi, S.Y.; Kalhori, E.M.; Norouzi, R.; Agarwal, S.; Gupta, V.K. Experimental Design, Modeling and Mechanism of Cationic Dyes Biosorption on to Magnetic Chitosan-Lutaraldehyde Composite. Int. J. Biol. Macromol. 2019, 131, 633–645. [Google Scholar] [CrossRef]
- Gupta, S.; Prajapati, A.; Kumar, A.; Acharya, S. Synthesis of Silica Aerogel and Its Application for Removal of Crystal Violet Dye by Adsorption. Watershed Ecol. Environ. 2023, 5, 241–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).