Photocatalysis and Electrocatalysis Properties of a Keggin-Type Inorganic–Organic Hybrid SiW12O40@Ag
Abstract
:1. Introduction
2. Results and Discussion
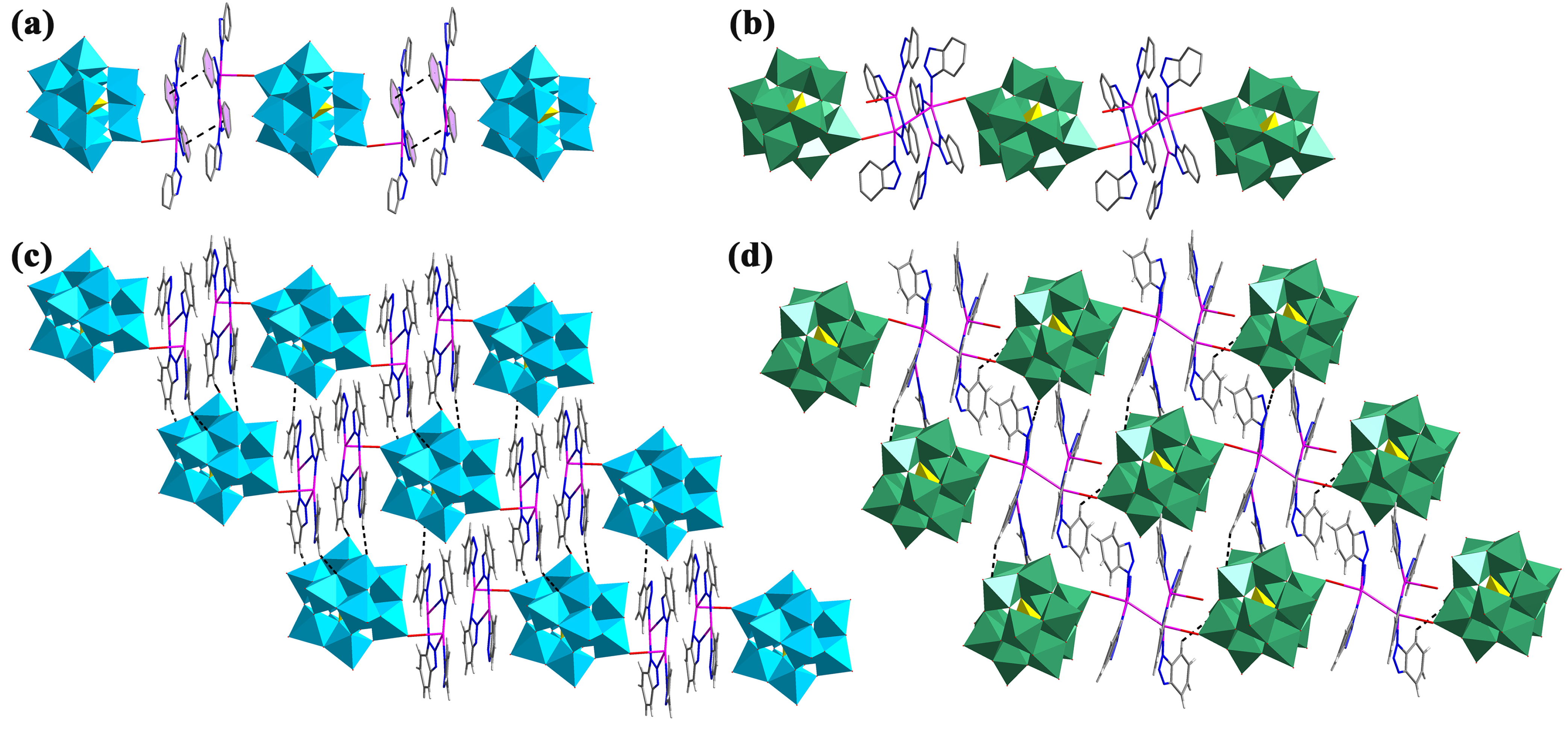
2.1. Crystal Structure Description
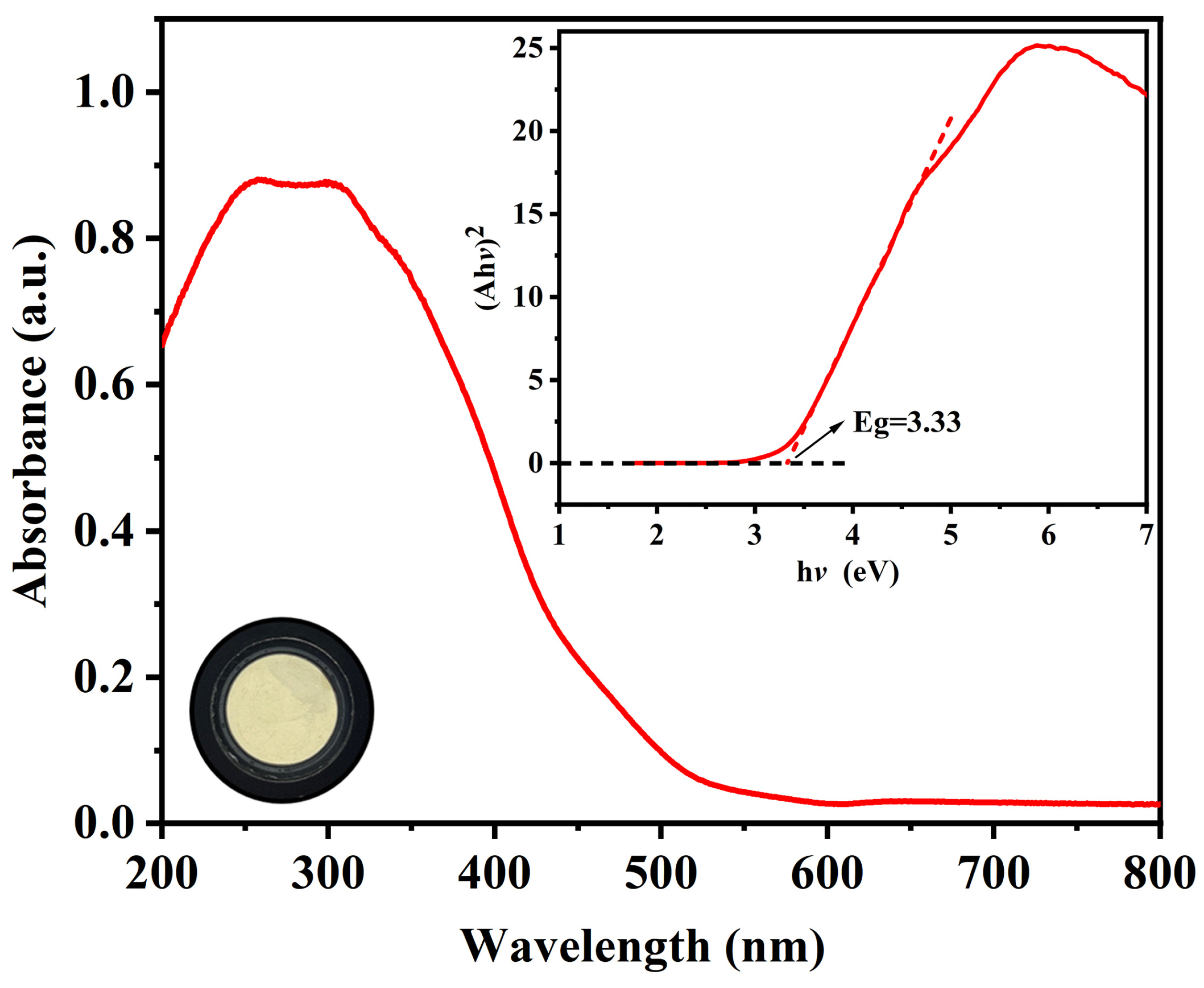
2.2. Spectroscopic and Thermal Analyses
2.3. Photodegradation of MB
2.4. Electrochemical Characteristics
3. Materials and Methods
3.1. Material and Instruments
3.2. Synthesis of {[Ag4(SiW12O40)(HBTA)8][Ag4(SiW12O40)(HBTA)8(H2O)]}n (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Hu, D.; Zhang, Z.; Deng, X. Oxidative Degradation of Methylene Blue via PDS-Based Advanced Oxidation Process Using Natural Pyrite. Int. J. Environ. Res. Public Health 2019, 16, 4773. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Zhu, M.; Fu, D.; Yeap, S.K.; Othman, M.H.D.; Avtar, R.; Ouyang, T. Functionalizing TiO2 with graphene oxide for enhancing photocatalytic degradation of methylene blue (MB) in contaminated wastewater. J. Environ. Manag. 2020, 270, 110871. [Google Scholar] [CrossRef]
- Chen, T.-D.; Wang, J.-H.; Ji, J.-Y.; Zhou, X.; Zhang, S.; Tai, Y.; Zhou, K. Inorganic–organic hybrids based on Keggin-type polyoxometalate@Cu/Ag for degradation/absorption of methylene blue and electrocatalytic property. Vacuum 2025, 232, 113875. [Google Scholar] [CrossRef]
- Qamar, M.A.; Javed, M.; Shahid, S.; Iqbal, S.; Abubshait, S.A.; Abubshait, H.A.; Ramay, S.M.; Mahmood, A.; Ghaithan, H.M. Designing of highly active g-C3N4/Co@ZnO ternary nanocomposites for the disinfection of pathogens and degradation of the organic pollutants from wastewater under visible light. J. Environ. Chem. Eng. 2021, 9, 105534. [Google Scholar] [CrossRef]
- Emmanuel, S.S.; Adesibikan, A.A. Bio-fabricated green silver nano-architecture for degradation of methylene blue water contaminant: A mini-review. Water Environ. Res. 2021, 93, 2873–2882. [Google Scholar] [CrossRef]
- Pathirana, M.A.; Dissanayake, N.S.L.; Wanasekara, N.D.; Mahltig, B.; Nandasiri, G.K. Chitosan-Graphene Oxide Dip-Coated Polyacrylonitrile-Ethylenediamine Electrospun Nanofiber Membrane for Removal of the Dye Stuffs Methylene Blue and Congo Red. Nanomaterials 2023, 13, 498. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Li, L.; Liu, N.; Zhou, K.; Liang, G.-M.; Tan, Y.-F.; Ji, J.-Y.; Chen, B.-K.; Bi, Y.-F. Carboxylate Ligand-supported Agn (n = 21 and 24) Alkynyl Clusters Induced by Single/Double Carbonate Ions. Chem. Asian J. 2023, 18, e202300041. [Google Scholar] [CrossRef]
- Ali, M.A.; Maafa, I.M.; Qudsieh, I.Y. Photodegradation of Methylene Blue Using a UV/H2O2 Irradiation System. Water 2024, 16, 453. [Google Scholar] [CrossRef]
- Monakhov, K.Y.; Bensch, W.; Kögerler, P. Semimetal-functionalised polyoxovanadates. Chem. Soc. Rev. 2015, 44, 8443–8483. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Duan, X.; Yao, S.; Wang, Z.; Lin, Z.; Li, Y.-G.; Long, L.-S.; Wang, E.-B.; Lin, W. Cation-mediated optical resolution and anticancer activity of chiral polyoxometalates built from entirely achiral building blocks. Chem. Sci. 2016, 7, 4220–4229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, X.; Chi, G.; Shuai, D.; Wang, L.; Chen, B.; Li, J. Research progress on the inhibition of enzymes by polyoxometalates. Inorg. Chem. Front. 2020, 7, 4320–4332. [Google Scholar] [CrossRef]
- Gu, J.; Chen, W.; Shan, G.-G.; Li, G.; Sun, C.; Wang, X.L.; Su, Z. The roles of polyoxometalates in photocatalytic reduction of carbon dioxide. Mater. Today Energy 2021, 21, 100760. [Google Scholar] [CrossRef]
- Ji, T.; Chen, W.; Kang, Z.; Zhang, L. Polyoxometalates for continuous power generation by atmospheric humidity. Nano Res. 2023, 17, 1875–1885. [Google Scholar] [CrossRef]
- Mou, H.-C.; Ying, J.; Tian, A.-X.; Cui, H.-T.; Wang, X.-L. Four Keggin-based compounds constructed by a series of pyridine derivatives: Synthesis, and electrochemical, photocatalytic and fluorescence sensing properties. New J. Chem. 2020, 44, 15122–15130. [Google Scholar] [CrossRef]
- Wang, G.; Chen, T.; Gómez-García, C.J.; Zhang, F.; Zhang, M.; Ma, H.; Pang, H.; Wang, X.; Tan, L. A High-Capacity Negative Electrode for Asymmetric Supercapacitors Based on a PMo12 Coordination Polymer with Novel Water-Assisted Proton Channels. Small 2020, 16, 2001626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bihl, F.; Bonnefont, A.; Boudon, C.; Ruhlmann, L.; Badets, V. Selectivity and efficiency of nitrite electroreduction catalyzed by a series of Keggin polyoxometalates. J. Catal. 2022, 405, 212–223. [Google Scholar] [CrossRef]
- Murmu, G.; Samajdar, S.; Ghosh, S.; Shakeela, K.; Saha, S. Tungsten-based Lindqvist and Keggin type polyoxometalates as efficient photocatalysts for degradation of toxic chemical dyes. Chemosphere 2024, 346, 140576. [Google Scholar] [CrossRef]
- Wang, P.-S.; Wang, Z.-T.; Tan, Y.-Y.; Chen, P.; Zhang, X.-C.; Zhang, L.-N.; Wei, Y.-G.; Ni, L.-B. Structural transformation from Waugh-type to Keggin-type polyoxomolybdate-based crystalline material for photo/electrocatalysis. Rare Met. 2024, 43, 2241–2250. [Google Scholar] [CrossRef]
- Yang, R.; Li, B.; Lai, X.; Yu, X.; Xiao, B.; Hu, S.; Pang, H.; Ma, H.; Wang, X.; Tan, L. An irregular-octagonal-prism-shaped host–guest supramolecular network based on silicotungstate and manganese-complex for light-driven hydrogen evolution. New J. Chem. 2021, 45, 3954–3959. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Liu, G.; Zhang, S.; Zhang, Z.; Wang, X. A new Keggin-type polyoxometallate-based bifunctional catalyst for trace detection and pH-universal photodegradation of phenol. Chin. Chem. Lett. 2024, 35, 109148. [Google Scholar] [CrossRef]
- Cui, H.; Yang, Y.; Bai, X.; Han, X.; Zhang, W.; Lu, Y.; Liu, S. Rare earth inorganic–organic hybrid compounds based on Keggin-type polyoxometalate {SiW12} with fast-responsive photochromism and switchable luminescence properties. J. Rare Earth 2024, 42, 286–292. [Google Scholar] [CrossRef]
- Huang, K.; Huang, L.; Shen, Y.; Hua, Y.; Song, R.; Li, Z.; Zhang, H. Two novel 3D polyoxometalate-based metal–organic frameworks for structure-directed selective adsorption and photodegradation of organic dyes. Inorg. Chem. Commun. 2024, 170, 113350. [Google Scholar] [CrossRef]
- Khajavian, R.; Jodaian, V.; Taghipour, F.; Mague, J.T.; Mirzaei, M. Roles of Organic Fragments in Redirecting Crystal/Molecular Structures of Inorganic–Organic Hybrids Based on Lacunary Keggin-Type Polyoxometalates. Molecules 2021, 26, 5994. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Q.; Yu, X.; Zheng, Y.; Guo, Q.; Fang, C.; Yi, W. Isomeric organic ligands directing octamolybdates-linked Copper(II) functionalized complexes as active catalysts in electrochemistry and desulfurization. J. Mol. Struct. 2025, 1321, 139822. [Google Scholar] [CrossRef]
- Pache, A.; Reinoso, S.; Felices, L.S.; Iturrospe, A.; Lezama, L.; Gutiérrez-Zorrilla, J.M. Single-Crystal to Single-Crystal Reversible Transformations Induced by Thermal Dehydration in Keggin-Type Polyoxometalates Decorated with Copper(II)-Picolinate Complexes: The Structure Directing Role of Guanidinium. Inorganics 2015, 3, 194–218. [Google Scholar] [CrossRef]
- Zhai, Q.-G.; Gao, X.; Li, S.-N.; Jiang, Y.-C.; Hu, M.-C. Solvothermal synthesis, crystal structures and photoluminescence properties of the novel Cd/X/α,ω-bis(benzotriazole)alkane hybrid family (X = Cl, Br and I). CrystEngComm 2011, 13, 1602–1616. [Google Scholar] [CrossRef]
- Cao, Y.; Lv, J.; Yu, K.; Wang, C.-M.; Su, Z.-H.; Wang, L.; Zhou, B.-B. Synthesis and photo-/electro-catalytic properties of Keggin polyoxometalate inorganic–organic hybrid layers based on d10 metal and rigid benzo-diazole/-triazole ligands. New J. Chem. 2017, 41, 12459–12469. [Google Scholar] [CrossRef]
- Shi, C.; Gao, X.-L.; Zhou, K.; Cui, D.-X.; Qu, H.-P.; Ji, J.-Y.; Bi, Y.F. An inorganic–organic hybrid constructed from Keggin polyoxoanions and benzotriazole ligands modulated by two dinuclear silver(I) clusters. Polyhedron 2017, 138, 232–238. [Google Scholar] [CrossRef]
- Loukopoulos, E.; Kostakis, G.E. Recent advances in the coordination chemistry of benzotriazole-based ligands. Coordin. Chem. Rev. 2019, 395, 193–229. [Google Scholar] [CrossRef]
- Song, Z.-J.; Wang, L.-Y.; Kang, N.; Yu, K.; Lv, J.-H.; Zhou, B.-B. 3D host-guest material of {Ag(pz)} modified {BW12O40} with supercapacitor, photocatalytic dye degradation and H2O2 sensing performances. J. Solid State Chem. 2023, 323, 124038. [Google Scholar] [CrossRef]
- Xia, K.; Yatabe, T.; Yamaguchi, K.; Suzuki, K. Multidentate polyoxometalate modification of metal nanoparticles with tunable electronic states. Dalton Trans. 2024, 53, 11088–11093. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Liu, T.; Yang, Y.; Yang, M.; Ying, J.; Tian, A. Three Keggin-Type Polyoxometalate-Based Tetranuclear Cu/Ag Nanostructures with Dual Functions of Supercapacitors and Trace Cr (VI)-Sensitive Detection. ACS Appl. Nano Mater. 2024, 8, 552–561. [Google Scholar] [CrossRef]
- Chupina, A.V.; Shayapov, V.; Novikov, A.S.; Volchek, V.V.; Benassi, E.; Abramov, P.A.; Sokolov, M.N. [{AgL}2Mo8O26]n− complexes: A combined experimental and theoretical study. Dalton Trans. 2020, 49, 1522–1530. [Google Scholar] [CrossRef]
- Komlyagina, V.I.; Romashev, N.F.; Kokovkin, V.V.; Gushchin, A.L.; Benassi, E.; Sokolov, M.N.; Abramov, P.A. Trapping of Ag+ into a Perfect Six-Coordinated Environment: Structural Analysis, Quantum Chemical Calculations and Electrochemistry. Molecules 2022, 27, 6961. [Google Scholar] [CrossRef]
- Abramov, P.A. Study of the structure of Ag(I) solvate complexes by means of polyoxometalates: Crystallization from the AgNO3/(Bu4N)4[β-Mo8O26]/DMF system. review. J. Struct. Chem. 2022, 63, 2068–2082. [Google Scholar] [CrossRef]
- Khan, R.A.; Jaafar, M.H.; Hadi, A.D.; Alsaeedi, H.; Alsalme, A. Luminescent tetranuclear Ag(I)/Cu(I) cubane clusters: Investigating argentophilicity, cuprophilicity, and mixed argento-cuprophilicity. Inorg. Chim. Acta 2025, 578, 122517. [Google Scholar] [CrossRef]
- Ishii, R.; Wada, Y.; Sunada, Y. Silyl- and germyl-bridged neutral square-planar Ag4 clusters with short Ag–Ag distances exhibiting red emission. Chem. Commun. 2025, 61, 4391–4394. [Google Scholar] [CrossRef]
- Shmakova, A.A.; Berezin, A.S.; Abramov, P.A.; Sokolov, M.N. Self-Assembly of Ag+/[PW11NbO40]4– Complexes in Nonaqueous Solutions. Inorg. Chem. 2020, 59, 1853–1862. [Google Scholar] [CrossRef]
- Volchek, V.V.; Berezin, A.S.; Sokolov, M.N.; Abramov, P.A. Stabilization of {Ag20(StBu)10} and {Ag19(StBu)10} Toroidal Complexes in DMSO: HPLC-ICP-AES, PL, and Structural Studies. Inorganics 2022, 10, 225. [Google Scholar] [CrossRef]
- Li, J.-H.; Zhao, Q.; Mao, D.-A.; Zhou, K.; Chen, B.-K.; Bi, Y.-F. Modular assembly of Co4-thiacalix[4]arene units and Keggin-type SiW12O40 for visible-light photothermal conversion. Tungsten 2025, 7, 112–119. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, Q.-G.; Dui, X.J.; Li, S.-N.; Jiang, Y.-C.; Hu, M.-C. Synthesis, crystal structure, and characterizations of a 3-D Cu(I) complex with 1,6-bi(benzotriazole)hexane. J. Coord. Chem. 2010, 63, 214–222. [Google Scholar] [CrossRef]
- Zhu, Q.; An, H.; Xu, T.-Q.; Chen, Y.; Wei, Y.; Sun, H. Polyoxometalates Embedded into Covalent Triazine Frameworks Regulating Charge Transfer for Visible-Light-Driven Synthesis of Functionalized Sulfoxides and Detoxification of Mustard Gas Simulants. ACS Sustain. Chem. Eng. 2024, 12, 1655–1665. [Google Scholar] [CrossRef]
- Yu, J.-Q.; Xue, C.-H.; Zhou, K.; Fang, Y.; Ji, J.-Y.; Chen, B.-K.; Bi, Y.-F. Trapping a [W10O32]6− Decatungstate Anion in an Ag44 Nanowheel. Chem. Asian J. 2022, 17, e202200072. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, W.-X.; Han, K.; Zhou, K.; Kang, L.; Shi, J.; Chen, B.-K.; Bi, Y.-F. Diphosphine modified copper(I)-thiacalixarene supramolecular structure for effective photocurrent response and photodegradation of methylene blue. Polyhedron 2022, 222, 115934. [Google Scholar] [CrossRef]
- Sadek, O.; Touhtouh, S.; Dahbi, A.; Hajjaji, A. Photocatalytic Degradation of Methylene Blue on Multilayer TiO2 Coatings Elaborated by the Sol-gel Spin-Coating Method. Water Air Soil Pollut. 2023, 234, 698. [Google Scholar] [CrossRef]
- Honarmand, M.M.; Mehr, M.E.; Yarahmadi, M.; Siadati, M.H. Efects of diferent surfactants on morphology of TiO2 and Zr-doped TiO2 nanoparticles and their applications in MB dye photocatalytic degradation. SN Appl. Sci. 2019, 1, 505. [Google Scholar] [CrossRef]
- Khan, S.U.; Akhtar, M.; Khan, F.U.; Peng, J.; Hussain, A.; Shi, H.; Du, J.; Yan, G.; Li, Y. Polyoxometalates decorated with metal-organic moieties as new molecular photo- and electro-catalysts. J. Coord. Chem. 2018, 71, 2604–2621. [Google Scholar] [CrossRef]
- Jiang, F.; Kong, L.-Y.; Long, J.-Y.; Fei, B.-L.; Mei, X. An organic-inorganic hybrid based on bicapped Keggin polyoxometalate as a heterogeneous catalyst for MB removal via Fenton-like/photo-Fenton-like degradation or reduction. Solid State Sci. 2024, 149, 107453. [Google Scholar] [CrossRef]
- Hou, Y.; Pang, H.J.; Gómez-García, C.J.; Ma, H.Y.; Wang, X.M.; Tan, L.C. Polyoxometalate Metal–Organic Frameworks: Keggin Clusters Encapsulated into Silver-Triazole Nanocages and Open Frameworks with Supercapacitor Performance. Inorg. Chem. 2019, 58, 16028–16039. [Google Scholar] [CrossRef]
- Tian, A.; Ni, H.; Ji, X.; Tian, Y.; Liu, G.; Ying, J. Using a flexible bis(pyrazol) ligand to construct four new Keggin-based compounds: Syntheses, structures and properties. RSC Adv. 2017, 7, 5774–5781. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Wang, X.; Xu, N.; Li, X.-H.; Wang, X.-L. Various amide derivatives induced Keggin-type SiW12O404−-based cobalt complexes: Assembly, structure, electrochemical sensing and dye adsorption properties. CrystEngComm 2022, 24, 1195–1202. [Google Scholar] [CrossRef]
- Li, X.J.; Ping, J.F.; Ying, Y.B. Recent developments in carbon nanomaterial-enabled electrochemical sensors for nitrite detection. Trends Anal. Chem. 2019, 113, 1–12. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.-X.; Chen, T.-D.; Gong, X.-J.; Ji, J.-Y.; Zhao, L.-P.; Xie, W.-X.; Zhou, K. Photocatalysis and Electrocatalysis Properties of a Keggin-Type Inorganic–Organic Hybrid SiW12O40@Ag. Inorganics 2025, 13, 132. https://doi.org/10.3390/inorganics13050132
Hu X-X, Chen T-D, Gong X-J, Ji J-Y, Zhao L-P, Xie W-X, Zhou K. Photocatalysis and Electrocatalysis Properties of a Keggin-Type Inorganic–Organic Hybrid SiW12O40@Ag. Inorganics. 2025; 13(5):132. https://doi.org/10.3390/inorganics13050132
Chicago/Turabian StyleHu, Xin-Xin, Tai-Dan Chen, Xiao-Jie Gong, Jiu-Yu Ji, Li-Ping Zhao, Wen-Xuan Xie, and Kun Zhou. 2025. "Photocatalysis and Electrocatalysis Properties of a Keggin-Type Inorganic–Organic Hybrid SiW12O40@Ag" Inorganics 13, no. 5: 132. https://doi.org/10.3390/inorganics13050132
APA StyleHu, X.-X., Chen, T.-D., Gong, X.-J., Ji, J.-Y., Zhao, L.-P., Xie, W.-X., & Zhou, K. (2025). Photocatalysis and Electrocatalysis Properties of a Keggin-Type Inorganic–Organic Hybrid SiW12O40@Ag. Inorganics, 13(5), 132. https://doi.org/10.3390/inorganics13050132





