The Bioinorganic Chemistry of the First Row d-Block Metal Ions—An Introduction
Abstract
:1. Introduction
- Without these elements, a plant cannot complete its life cycle.
- These elements are part of the essential constituents or metabolites of the plant.
- Deficiency of these elements leads to diseases, which can be corrected by their reintroduction.
- “An element is essential when a deficient intake consistently results in impaired function, and supplementation at physiological levels, but not others, restores optimal function” [1].
- “An element is considered essential if it has a defined biochemical function, and its absence results in death or reproductive failure, reversible by dietary supplementation” [35].
- The European Food Safety Authority (EFSA) defines an essential nutrient as “any substance an organism must consume from the diet to support normal health, development, and growth” [36].
- is present in all healthy tissues of living organisms;
- exists in relatively constant concentrations across individuals;
- causes physiological and structural abnormalities in its absence, which are reversible upon reintroduction;
- leads to specific biochemical changes when deficient, correctable by reversing the deficiency.
2. Scandium
3. Titanium
3.1. Biomedical Applications
3.2. Enzyme Inhibition
3.3. Antibiotic Properties
3.4. Role in Agriculture
3.5. Potential Biological Roles
4. Vanadium
4.1. Vanadium in Respiration
4.2. Haloperoxidases
4.3. Vanadium in Marine and Fungal Systems
4.4. Vanadium and Human Biology
5. Chromium
6. Manganese
6.1. Superoxide Dismutase
6.2. Photosystem II
6.3. Examples of Other Mn-Containing Enzymes That Involve a Change in the Metal’s Oxidation State
6.3.1. Ribonucleotide Reductase (RNR)
6.3.2. Manganese Peroxidase
6.3.3. Manganese Lipoxygenase
6.4. Examples of the Non-Redox Bioinorganic Chemistry of Manganese

7. Iron

7.1. The Transport and Storage of O2
7.2. Electron Transport
7.2.1. Cellular Respiration in Mitochondria
7.2.2. Overview of Mitochondrial Respiration
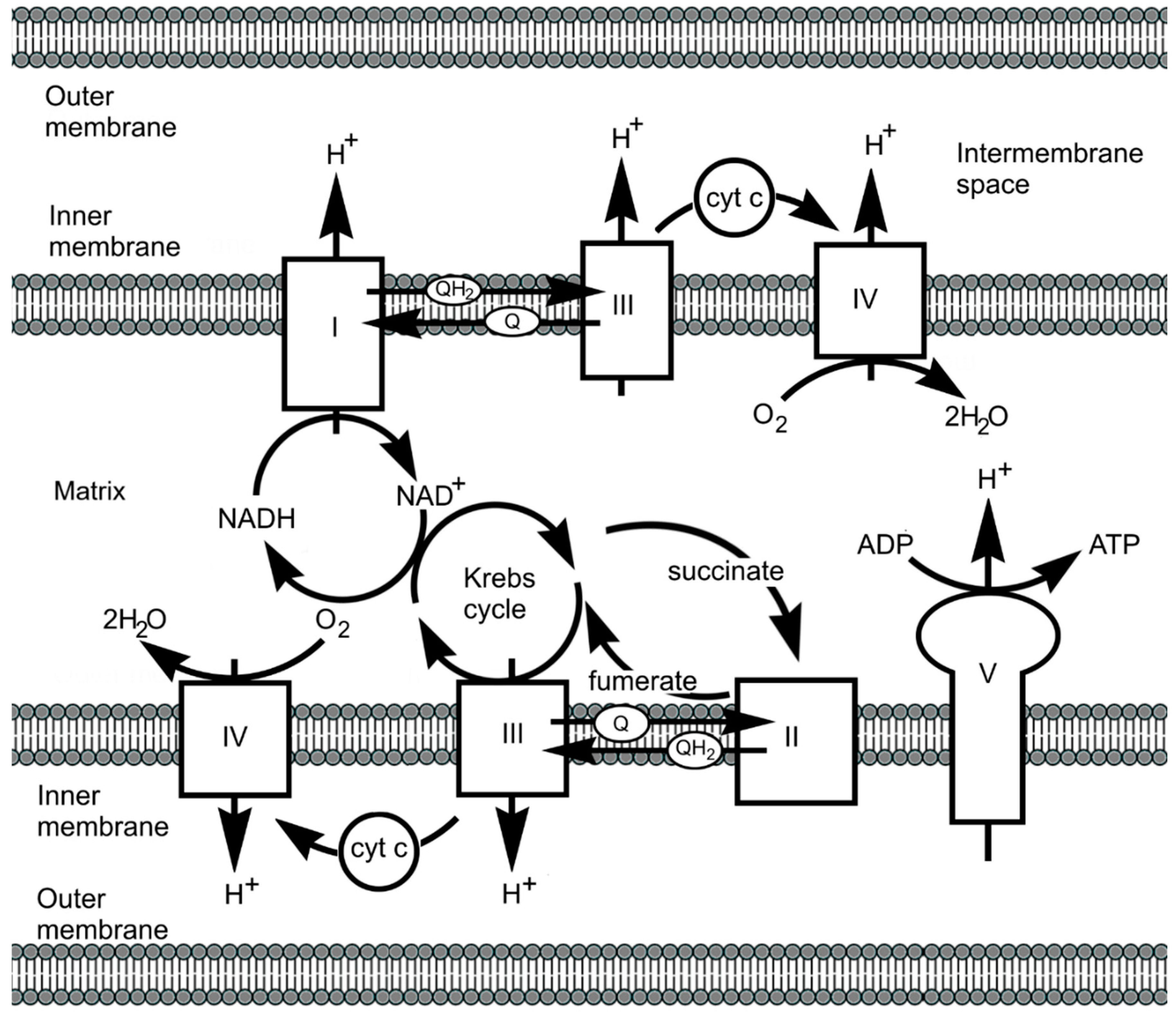
7.2.3. Haems and Electron Transport
7.2.4. Iron–Sulphur Clusters
- Ligand identity and coordination
- ▪
- The type of ligands (e.g., Cys or His) strongly influences E1/2.
- ▪
- Cys ligands, being negatively charged, generally lower E1/2, while harder His ligands, as in Rieske centres, raise E1/2.
- Protein environment and electrostatic effects
- ▪
- The surrounding protein matrix, including electrostatic interactions and hydrogen bonding, fine-tunes the redox potential.
- ▪
- Burial within a hydrophobic environment, proximity to charged residues, and the extent of exposure to solvent significantly impacts E1/2.
- Protonation states and pH dependence
- ▪
- Protonation states of coordinating ligands, especially His, introduce pH-dependent shifts in E1/2.
- ▪
- In Rieske centres, deprotonation of His can lower E1/2 by up to 450 mV.
- Cluster type and delocalisation
- ▪
- The specific iron–sulphur cluster (e.g., [2Fe–2S], [4Fe–4S]) and the accessibility of oxidation states (e.g., valence delocalisation) affect E1/2.
- ▪
- HiPIPs exhibit higher potentials due to cycling between [4Fe–4S]2+/3+, while ferredoxins operate between [4Fe–4S]1+/2+.
7.2.5. From Complex I to Complex IV
7.3. An Example of an Iron Enzyme: The Cytochromes P450
8. Cobalt
8.1. The [MeCbl]-Dependent Enzymes
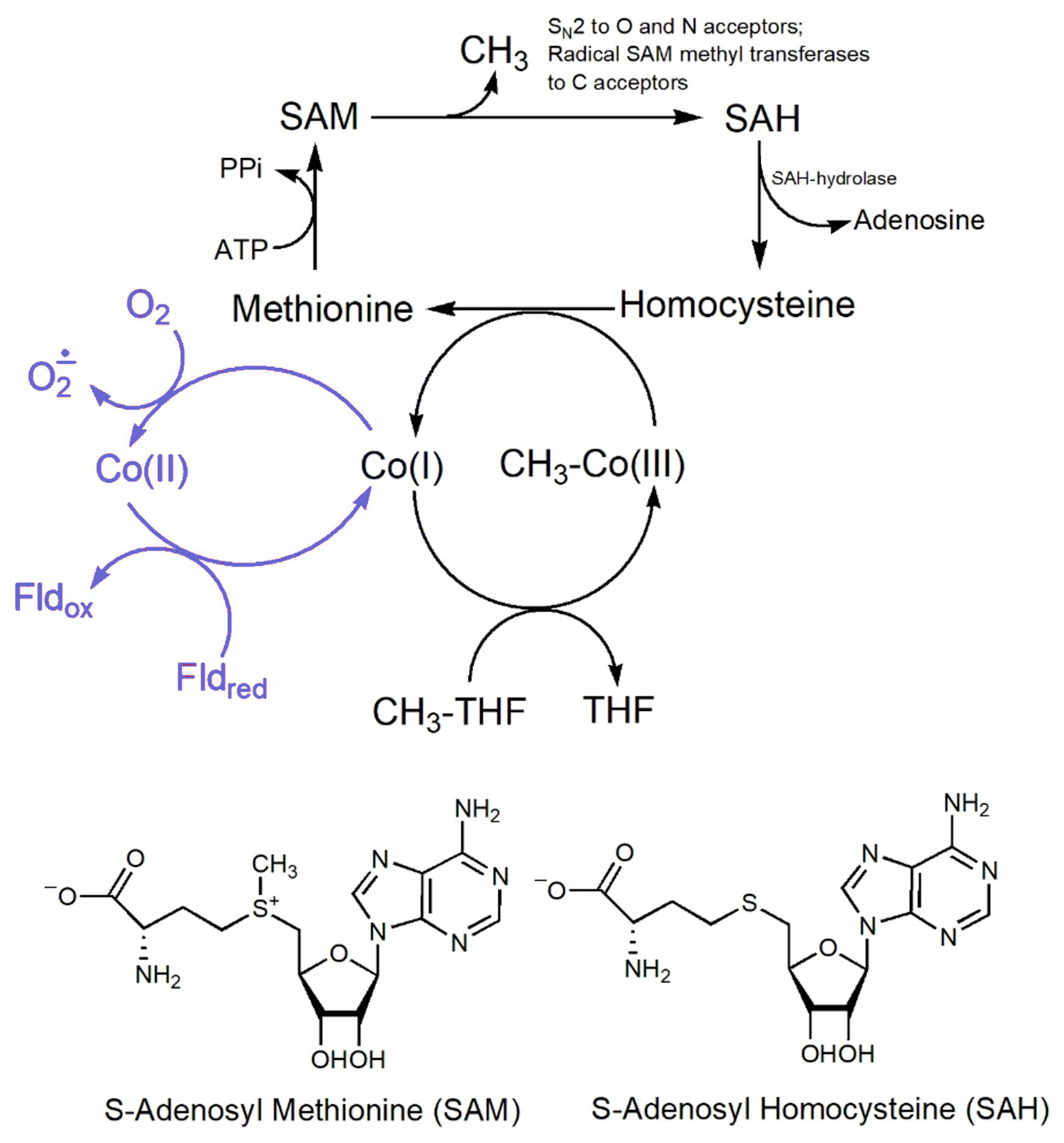
8.2. The [AdoCbl]-Dependent Enzymes

8.3. The Reductive Dehalogenases
8.4. Why Corrin, and Why Cobalt?
9. Nickel
9.1. Urease

9.2. Methyl-Coenzyme M Reductase
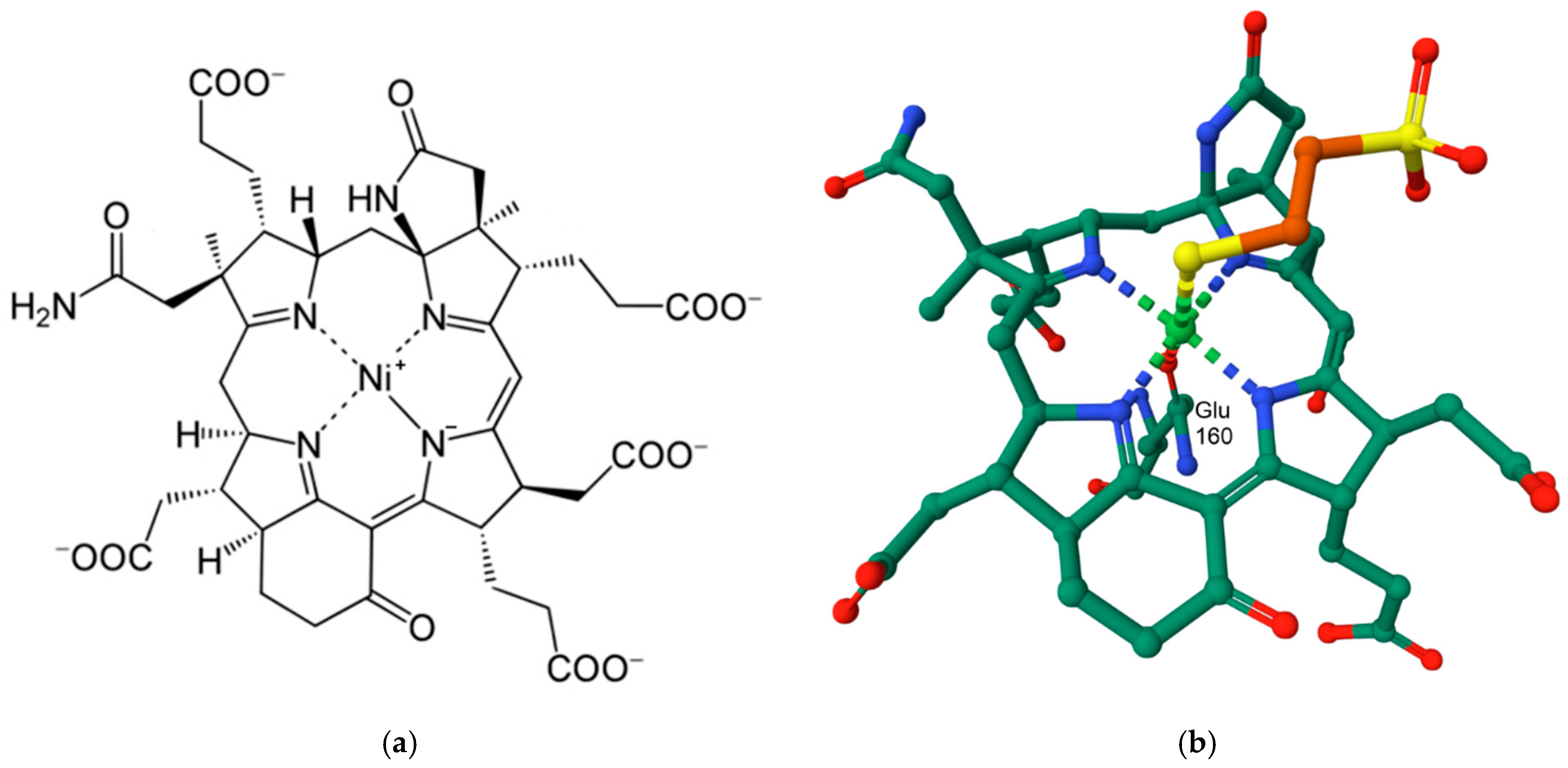
9.3. NiFe Hydrogenases
9.4. Lactate Racemase

10. Copper
10.1. The Coordination Environment of Copper: The Blue Copper Proteins
10.2. The Coordination Environment of Copper: Type II and Type III Centres, and the Multicopper Oxidases (MCOs)
10.3. Some Illustrative Examples of Copper-Based Enzymes
10.3.1. Superoxide Dismutase
10.3.2. Nitrous Oxide Reductase

10.3.3. Tyrosinase

11. Zinc
11.1. Carbonic Anhydrase
11.2. Zinc-Dependent Phosphoesterases

11.3. A Zinc Proteinase: Carboxypeptidase A

11.4. Alkaline Phosphatase
12. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mertz, W. The Essential Trace Elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Interrelations Between Essential Metal Ions and Human Diseases; Springer: Dordrecht, The Netherlands, 2013; Volume 13. [Google Scholar]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chemico-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Williams, R.J.P. Chemical selection of elements by cells. Coord. Chem. Rev. 2001, 216–217, 583–595. [Google Scholar] [CrossRef]
- Sanchez, T.R.; Hu, X.; Zhao, J.; Tran, V.; Loiacono, N.; Go, Y.-M.; Goessler, W.; Cole, S.; Umans, J.; Jones, D.P.; et al. An atlas of metallome and metabolome interactions and associations with incident diabetes in the Strong Heart Family Study. Environ. Intern. 2021, 157, 106810. [Google Scholar] [CrossRef]
- Dupont, C.L.; Butcher, A.; Valas, R.E.; Bourne, P.E.; Caetano-Anollés, G. History of biological metal utilization inferred through phylogenomic analysis of protein structures. Proc. Natl. Acad. Sci. USA 2010, 107, 10567–10572. [Google Scholar] [CrossRef]
- Van Cleave, C.; Crans, D.C. The First-Row Transition Metals in the Periodic Table of Medicine. Inorganics 2019, 7, 111. [Google Scholar] [CrossRef]
- Liang, Y.; Pan, Z.; Zhu, M.; Gao, R.; Wang, Y.; Cheng, Y.; Zhang, N. Exposure to essential and non-essential trace elements and risks of congenital heart defects: A narrative review. Front. Nutr. 2023, 10, 1121826. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Mondal, M.H.; Bhattarai, A.; Saha, B. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Adv. 2022, 12, 9139–9153. [Google Scholar] [CrossRef]
- Nelson, N. Metal ion transporters and homeostasis. EMBO J. 1999, 18, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Ba, L.A.; Doering, M.; Burkholz, T.; Jacob, C. Metal trafficking: From maintaining the metal homeostasis to future drug design. Metallomics 2009, 1, 292–311. [Google Scholar] [CrossRef]
- Palmer, A.E.; Franz, K.J. Introduction to “Cellular Metal Homeostasis and Trafficking”. Chem. Rev. 2009, 109, 4533–4535. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, S.K.; Varma, S.J.; Ralser, M. Metal ion availability and homeostasis as drivers of metabolic evolution and enzyme function. Curr. Opin. Genet. Dev. 2022, 77, 101987. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Terán, I.; Goh, K.G.K.; Ulett, G.C. Resisting death by metal: Metabolism and Cu/Zn homeostasis in bacteria. Emerg. Top. Life Sci. 2024, 8, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, K.; Fang, X.; Zhang, Y.; Miao, R.; Guan, H.; Tian, J. Targeting ion homeostasis in metabolic diseases: Molecular mechanisms and targeted therapies. Pharmacol. Res. 2025, 212, 107579. [Google Scholar] [CrossRef]
- Maret, W. Chromium in Human Health, Metabolic Syndrome, and Diabetes. In Metal Ions in Life Sciences; Carver, P.L., Ed.; W. de Gruyter: Berlin, Germany, 2019; Volume 19, pp. 231–251. [Google Scholar]
- Bansal, S.L.; Asthana, S. Biologically Essential and Non-Essential Elements Causing Toxicity in Environment. J. Environ. Anal. Toxicol. 2018, 8, 1000557. [Google Scholar] [CrossRef]
- Brown, D.H.; Smith, W.E. Metal ions in biological systems. In Enzyme Chemistry: Impact and Applications; Suckling, C.J., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 162–195. [Google Scholar]
- Lippard, S.J.; Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994. [Google Scholar]
- Farrer, B.T.; Pecoraro, V.L. Bioinorganic Chemistry. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 117–139. [Google Scholar]
- Bertini, I.; Gray, H.B.; Stiefel, E.I.; Valentine, J.S. (Eds.) Biological Inorganic Chemistry: Structure and Reactivity; University Science Books: Sausalito, CA, USA, 2007. [Google Scholar]
- Hosmane, N.S. Bioinorganic Chemistry and Applications. In Advanced Inorganic Chemistry; Hosmane, N.S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 225–249. [Google Scholar]
- Crichton, R. (Ed.) Biological Inorganic Chemistry, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Jordan, R.B. Bioinorganic Chemistry. In Principles of Inorganic Chemistry: Basics and Applications; Jordan, R.B., Ed.; Springer International Publishing: Cham, Switzerland, 2024; pp. 823–852. [Google Scholar]
- Williams, R.J.P. The natural selection of the elements. Chem. Br. 1997, 53, 816–829. [Google Scholar]
- Williams, R.J.P.; Frausto da Silva, J.J.R. The Chemistry of Evolution: The Development of our Ecosystem; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Frausto da Silva, J.J.R.; WIlliams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Ghosh, D.; Pecoraro, V.L. Probing metal–protein interactions using a de novo design approach. Curr. Opin. Chem. Biol. 2005, 9, 97–103. [Google Scholar] [CrossRef]
- Belmonte, L.; Mansy, S.S. Metal Catalysts and the Origin of Life. Elements 2016, 12, 413–418. [Google Scholar] [CrossRef]
- Smethurst, D.G.J.; Shcherbik, N. Interchangeable utilization of metals: New perspectives on the impacts of metal ions employed in ancient and extant biomolecules. J. Biol. Chem. 2021, 297, 101374. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Kostenkova, K. Open questions on the biological roles of first-row transition metals. Commun. Chem. 2020, 3, 104. [Google Scholar] [CrossRef]
- Arnon, D.I.; Stout, P.R. The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physiol. 1939, 14, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. New evidence against chromium as an essential trace element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef]
- EFSA: Nutrient. Available online: https://www.efsa.europa.eu/en/glossary/nutrient (accessed on 15 May 2023).
- Underwood, E. Trace Elements in Human and Animal Nutrition, 4th ed.; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Pais, I. The biological importance of titanium. J. Plant Nutr. 1983, 6, 3–131. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; John Wiley & Sons, Inc.: New York, NY, USA, 1972. [Google Scholar]
- Hewitt, E.L. Essential and functional aspects of trace elements. In Chemistry and Agriculture; Royal Chemical Society: London, UK, 1979; pp. 91–127. [Google Scholar]
- Wimmer, M.A.; Abreu, I.; Bell, R.W.; Bienert, M.D.; Brown, P.H.; Dell, B.; Fujiwara, T.; Goldbach, H.E.; Lehto, T.; Mock, H.-P.; et al. Boron: An essential element for vascular plants. New Phytol. 2020, 226, 1232–1237. [Google Scholar] [CrossRef]
- Goldbach, H.E.; Wimmer, M.A. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant Nutr. Soil Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotech. 2022, 13, 58. [Google Scholar] [CrossRef]
- Emsley, J. Unsporting scandium. Nat. Chem. 2014, 6, 1025. [Google Scholar] [CrossRef]
- He, X. Scadium, Biological Effects. In Encyclopedia to Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1882–1884. [Google Scholar]
- Falandysz, J.; Fernandes, A.R. A critical review of the occurrence of scandium and yttrium in mushrooms. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: New York, NY, USA, 2023; Volume 125, pp. 107–141. [Google Scholar]
- Nnomo Assene, A.; Dieme, D.; Jomaa, M.; Côté, J.; Bouchard, M. Toxicokinetic study of scandium oxide in rats. Toxicol. Lett. 2024, 392, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Horovitz, C. (Ed.) Biochemistry of Scandium and Yttrium; Kluwer/Plenum: New York, NY, USA, 2000. [Google Scholar]
- Herath, H.M.T.U.; Silvio, L.D.; Evans, J.R.G. Scandia—A potential biomaterial? J. Mater. Sci. Mater. Med. 2005, 16, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Wang, G.; Okamoto, S.; Ochi, K. The rare earth, scandium, causes antibiotic overproduction in Streptomyces spp. FEMS Microbiol. Lett. 2007, 274, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Shtangeeva, I. Scandium. In Advances in Ecological Sciences: Trace and Ultratrace Elements in Plants and Soil; Shtangeeva, I., Ed.; WIT Press: Southampton, UK, 2005; Volume 20. [Google Scholar]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Li, H.; Sadler, P.J.; Sun, H. Rationalization of the Strength of Metal Binding to Human Serum Transferrin. Eur. J. Biochem. 1996, 242, 387–393. [Google Scholar] [CrossRef]
- Martin, R.B.; Savory, J.; Brown, S.; Bertholf, R.L.; Wills, M.R. Transferrin binding of Al3+ and Fe3+. Clin. Chem. 1987, 33, 405–407. [Google Scholar] [CrossRef]
- Tinoco, A.D.; Valentine, A.M. Ti(IV) Binds to Human Serum Transferrin More Tightly Than Does Fe(III). J. Am. Chem. Soc. 2005, 127, 11218–11219. [Google Scholar] [CrossRef]
- Rosoff, B.; Spencer, H. Binding of rare earths to serum proteins and DNA. Clin. Chim. Acta 1979, 93, 311–319. [Google Scholar] [CrossRef]
- Perkins, D. The interaction of trivalent metal ions with human transferrin. In Protides of the Biological Fluids—14th Colloquium 1966, Bruges; Peeters, H., Ed.; Proteins and Related Subjects; Elsevier: Amsterdam, The Netherlands, 1966; Volume 14. [Google Scholar]
- Shyy, Y.J.; Tsai, T.C.; Tsai, M.D. Metal-nucleotide interactions. 3. Oxygen-17, phosphorus-31, and proton NMR studies on the interaction of scandium(III), lanthanum(III), and lutetium(III) with adenosine 5’-triphosphate. J. Am. Chem. Soc. 1985, 107, 3478–3484. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Lebedev, V.T.; Torok, D.; Melnikov, A.B. Structure of the water salt solutions of DNA with sulfonated scandium diphthalocyanine. J. Struct. Chem. 2007, 48, 740–746. [Google Scholar] [CrossRef]
- Muñoz-Garcia, J.; Mazza, M.; Alliot, C.; Sinquin, C.; Colliec-Jouault, S.; Heymann, D.; Huclier-Markai, S. Antiproliferative Properties of Scandium Exopolysaccharide Complexes on Several Cancer Cell Lines. Mar. Drugs 2021, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.; Palma, G.; Mariconda, A.; Del Vecchio, V.; Iacopetta, D.; Parisi, O.I.; Sinicropi, M.S.; Puoci, F.; Arra, C.; Longo, P.; et al. Synthesis and Antitumor Activity of New Group 3 Metallocene Complexes. Molecules 2017, 22, 526. [Google Scholar] [CrossRef]
- He, X. Scandium and albumin. In Encyclopedia to Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1881–1882. [Google Scholar]
- Lachine, E.E.; Noujaim, A.A.; Ediss, C.; Wieber, L.I. Toxicity, tissue distribution and excretion of 46ScCl3 and 46Sc-EDTA in mice. Int. J. Appl. Rad. Isot. 1976, 27, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Tanida, E.; Usuda, K.; Kono, K.; Kawano, A.; Tsuji, H.; Imanishi, M.; Suzuki, S.; Ohnishi, K.; Yamamoto, K. Urinary scandium as predictor of exposure: Effects of scandium chloride hexahydrate on renal function in rats. Biol. Trace Elem. Res. 2009, 130, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, C.; López-Chaves, C.; Rivas-García, L.; Galindo, P.; Gómez-Aracena, J.; Aranda, P.; Llopis, J. Accumulation of Scandium in Plasma in Patients with Chronic Renal Failure. Sci. World J. 2013, 2013, 782745. [Google Scholar] [CrossRef]
- Kist, A.A.; Zhuk, L.I.; Danilova, E.A.; Makhmudov, E.A. On question of biological role of scandium. In Proceedings of the Abstracts of International Conference on Nuclear Science and Its Application, Samarkand, Uzbekistan, 25–28 September 2012; p. 476. [Google Scholar]
- Barden, J.A.; Curmi, P.M.G.; Dos Remedios, C.G. Crystalline actin tubes: III. The interaction of scandium and yttrium with skeletal muscle actin. Biochim. Biophys Acta 1981, 671, 25–32. [Google Scholar] [CrossRef]
- Gopal, D.; Burke, M. Formation of stable inhibitory complexes of myosin subfragment 1 using fluoroscandium anions. J. Biol. Chem. 1995, 270, 19282–19286. [Google Scholar] [CrossRef]
- Müller, C.; Domnanich, K.A.; Umbricht, C.A.; van der Meulen, N.P. Scandium and terbium radionuclides for radiotheranostics: Current state of development towards clinical application. Brit. J. Radiol. 2018, 91, 20180074. [Google Scholar] [CrossRef]
- Vaughn, B.A.; Koller, A.J.; Boros, E. Aqueous chemistry of the smallest rare earth: Comprehensive characterization of radioactive and non-radioactive scandium complexes for biological applications. In Methods in Enzymology; Cotruvo, J.A., Ed.; Academic Press: New York, NY, USA, 2021; Volume 651, pp. 343–371. [Google Scholar]
- Kilian, K.; Pyrzyńska, K. Scandium Radioisotopes—Toward New Targets and Imaging Modalities. Molecules 2023, 28, 7668. [Google Scholar] [CrossRef]
- Ioannidis, I.; Lefkaritis, G.; Georgiades, S.N.; Pashalidis, I.; Kontoghiorghes, G.J. Towards Clinical Development of Scandium Radioisotope Complexes for Use in Nuclear Medicine: Encouraging Prospects with the Chelator 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic Acid (DOTA) and Its Analogues. Int. J. Molec. Sci. 2024, 25, 5954. [Google Scholar] [CrossRef]
- Vaughn, B.A.; Ahn, S.H.; Aluicio-Sarduy, E.; Devaraj, J.; Olson, A.P.; Engle, J.; Boros, E. Chelation with a twist: A bifunctional chelator to enable room temperature radiolabeling and targeted PET imaging with scandium-44. Chem. Sci. 2020, 11, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Orteca, G.; Sinnes, J.-P.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Piel, M.; Rösch, F.; Ferrari, E.; Asti, M. Gallium-68 and scandium-44 labelled radiotracers based on curcumin structure linked to bifunctional chelators: Synthesis and characterization of potential PET radiotracers. J. Inorg. Biochem. 2020, 204, 110954. [Google Scholar] [CrossRef] [PubMed]
- Zierden, M.R.; Valentine, A.M. Contemplating a role for titanium in organisms. Metallomics 2015, 8, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C. Titanium. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 584–585. [Google Scholar]
- Ipach, I.; Schäfer, R.; Mittag, F.; Leichtle, C.; Wolf, P.; Kluba, T. The development of whole blood titanium levels after instrumented spinal fusion—Is there a correlation between the number of fused segments and titanium levels? BMC Musculoskel. Disord. 2012, 13, 159. [Google Scholar] [CrossRef]
- Williams, D.F. The biological applications of titanium and titanium alloys. In Materials Sciences and Implant Orthopedic Surgery; Kossowsky, R., Kossovsky, N., Eds.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1986; Volume NATO ASI Series; Volume 116, pp. 107–116. [Google Scholar]
- Wilson, W.; Chye Khoon, P. Titanium Alloys in Orthopaedics. In Titanium Alloys; Jan, S., Waldemar, Z., Eds.; IntechOpen: Rijeka, Croatia, 2013; Chapter 1. [Google Scholar]
- Kun, M.; Cuie, W.; Elena, P.I.; Christopher, C.B.; James, W. Sputtered Hydroxyapatite Nanocoatings on Novel Titanium Alloys for Biomedical Applications. In Titanium Alloys; Jan, S., Waldemar, Z., Eds.; IntechOpen: Rijeka, Croatia, 2013; Chapter 1. [Google Scholar]
- Burgos, J.; Hevia, E.; Sanpera, I.; García, V.; de Santos Moreno, M.T.; Mariscal, G.; Barrios, C. Elevated blood metal ion levels in patients undergoing instrumented spinal surgery: A systematic review and meta-analysis. Spine J. 2024, 24, 947–960. [Google Scholar] [CrossRef]
- Asa’ad, F.; Thomsen, P.; Kunrath, M.F. The Role of Titanium Particles and Ions in the Pathogenesis of Peri-Implantitis. J. Bone Metab. 2022, 29, 145–154. [Google Scholar] [CrossRef]
- Abreu, H.; Lallukka, M.; Raineri, D.; Leigheb, M.; Ronga, M.; Cappellano, G.; Spriano, S.; Chiocchetti, A. Evaluation of the immune response of peripheral blood mononuclear cells cultured on Ti6Al4V-ELI polished or etched surfaces. Front. Bioeng. Biotechnol. 2024, 12, 1458091. [Google Scholar] [CrossRef]
- Rabbitt, D.; Villapún, V.M.; Carter, L.N.; Man, K.; Lowther, M.; O’Kelly, P.; Knowles, A.J.; Mottura, A.; Tang, Y.T.; Luerti, L.; et al. Rethinking Biomedical Titanium Alloy Design: A Review of Challenges from Biological and Manufacturing Perspectives. Adv. Healthc. Mater. 2025, 14, 2403129. [Google Scholar] [CrossRef]
- Buettner, K.M.; Valentine, A.M. Bioinorganic Chemistry of Titanium. Chem. Rev. 2012, 112, 1863–1881. [Google Scholar] [CrossRef]
- Köpf-Maier, P.; Martin, R. Subcellular distribution of titanium in the liver after treatment with the antitumor agent titanocene dichloride. Virchows Arch. B 1989, 57, 213–222. [Google Scholar] [CrossRef]
- Meléndez, E. Titanium complexes in cancer treatment. Crit. Rev. Onco./Hemat. 2002, 42, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Rossi, M. Antitumor titanium compounds. Mini Rev. Med. Chem. 2004, 4, 49–60. [Google Scholar] [PubMed]
- Tshuva, E.Y.; Miller, M. Coordination Complexes of Titanium(IV) for Anticancer Therapy. In Metal Ions in Life Sciences; Sigel, A., Sigel, H., Freisinger, E., Sigel, R.K.O., Eds.; Walter de Gruyter GmbH: Berlin, Germany, 2018; Volume 18. [Google Scholar]
- Zhao, T.; Wang, P.; Zhang, X.; Liu, N.; Zhao, W.; Zhang, Y.; Yuan, P.; Li, S.; Yang, M.; Yang, Z.; et al. Anti-tumoral Titanium(IV) Complexes Stabilized with Phenolato Ligands and Structure-Activity Relationship. Curr. Top. Med. Chem. 2023, 23, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, S.; Serrano, R.; Cohen, B.; Martinez-Argudo, I.; Lopez-Sanz, L.; Guadamillas, M.C.; Calero, R.; Ruiz, M.J. Novel Titanocene Y derivative with albumin affinity exhibits improved anticancer activity against platinum resistant cells. J. Inorg. Biochem. 2024, 254, 112520. [Google Scholar] [CrossRef]
- Pedko, A.; Rubanovich, E.; Tshuva, E.Y.; Shurki, A. Hydrolytically Stable and Cytotoxic [ONON]2Ti(IV)-Type Octahedral Complexes. Inorg. Chem. 2022, 61, 17653–17661. [Google Scholar] [CrossRef]
- Vera, J.L.; Román, F.R.; Meléndez, E. Study of titanocene-DNA and molybdenocene-DNA interactions by inductively coupled plasma-atomic emission spectroscopy. Anal. Bioanal. Chem. 2004, 379, 399–403. [Google Scholar] [CrossRef]
- Kumar, N.; Kaushal, R.; Awasthi, P. A Comprehensive Review on the Development of Titanium Complexes as Cytotoxic Agents. Curr. Top. Med. Chem. 2024, 24, 2117–2128. [Google Scholar] [CrossRef]
- Cini, M.; Bradshaw, T.D.; Woodward, S. Using titanium complexes to defeat cancer: The view from the shoulders of titans. Chem. Soc. Rev. 2017, 46, 1040–1051. [Google Scholar] [CrossRef]
- Gao, L.M.; Hernández, R.; Matta, J.; Meléndez, E. Synthesis, Ti(IV) intake by apotransferrin and cytotoxic properties of functionalized titanocene dichlorides. J. Biol. Inorg. Chem. 2007, 12, 959–967. [Google Scholar] [CrossRef]
- Prajapati, A.; Rangra, S.; Patil, R.; Desai, N.; Jyothi, V.G.S.S.; Salave, S.; Amate, P.; Benival, D.; Kommineni, N. Receptor-Targeted Nanomedicine for Cancer Therapy. Receptors 2024, 3, 323–361. [Google Scholar] [CrossRef]
- Hernández, R.; Méndez, J.; Lamboy, J.; Torres, M.; Román, F.R.; Meléndez, E. Titanium(IV) complexes: Cytotoxicity and cellular uptake of titanium(IV) complexes on caco-2 cell line. Toxicol. Vitr. 2010, 24, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Immel, T.A.; Groth, U.; Huhn, T.; Öhlschläger, P. Titanium Salan Complexes Displays Strong Antitumor Properties In Vitro and In Vivo in Mice. PLoS ONE 2011, 6, e17869. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sil, P.C. Mechanistic Insights into Toxicity of Titanium Dioxide Nanoparticles at the Micro- and Macro-levels. Chem. Res. Toxicol. 2024, 37, 1612–1633. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Sriram, K.; Camassa, L.M.A.; Pathak, D.; Bing, H.L.; Mohr, B.; Zienolddiny-Narui, S.; Samulin Erdem, J. Systematic review of mechanistic evidence for TiO2 nanoparticle-induced lung carcinogenicity. Nanotoxicology 2024, 18, 437–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Han, X.; Xu, H.; Zhang, B.; Yu, Q.; Li, M. TiO2 nanoparticles cause cell damage independent of apoptosis and autophagy by impairing the ROS-scavenging system in Pichia pastoris. Chem.-Biol. Interact. 2016, 252, 9–18. [Google Scholar] [CrossRef]
- Pulgarin, C.; Kiwi, J.; Nadtochenko, V. Mechanism of photocatalytic bacterial inactivation on TiO2 films involving cell-wall damage and lysis. Appl. Cat. B Environ. 2012, 128, 179–183. [Google Scholar] [CrossRef]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.-C.; Horvatovich, P.; Gies, J.-P.; Keller, V.; Keller, N.; Andre, P. TiO2 Photocatalysis Damages Lipids and Proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef]
- Bogdan, J.; Pławińska-Czarnak, J.; Zarzyńska, J. Nanoparticles of Titanium and Zinc Oxides as Novel Agents in Tumor Treatment: A Review. Nanoscale Res. Lett. 2017, 12, 225. [Google Scholar] [CrossRef]
- Srivastava, R.; Rahman, Q.; Kashyap, M.; Singh, A.; Jain, G.; Jahan, S.; Lohani, M.; Lantow, M.; Pant, A. Nano-titanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Hum. Exp. Toxicol. 2013, 32, 153–166. [Google Scholar] [CrossRef]
- Deb, V.K.; Jain, U. Ti(3)C(2) (MXene), an advanced carrier system: Role in photothermal, photoacoustic, enhanced drugs delivery and biological activity in cancer therapy. Drug Deliv. Transl. Res. 2024, 14, 3009–3031. [Google Scholar] [CrossRef]
- Li, S.; He, N.; Wu, X.; Chen, F.; Xue, Q.; Li, S.; Zhao, C. Characteristics of Ultrasound-Driven Barium Titanate Nanoparticles and the Mechanism of Action on Solid Tumors. Int. J. Nanomed. 2024, 19, 12769–12791. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, M.; Jiang, L. Advancements in Serine Protease Inhibitors: From Mechanistic Insights to Clinical Applications. Catalysts 2024, 14, 787. [Google Scholar] [CrossRef]
- Paschkowsky, S.; Hsiao, J.M.; Young, J.C.; Munter, L.M. The discovery of proteases and intramembrane proteolysis. Biochem. Cell Biol. 2019, 97, 265–269. [Google Scholar] [CrossRef]
- Duffy, B.; Schwietert, C.; France, A.; Mann, N.; Culbertson, K.; Harmon, B.; McCue, J.P. Transition metals as protease inhibitors. Biol. Trace Elem. Res. 1998, 64, 197–213. [Google Scholar] [CrossRef]
- Ani, A.; Ani, M.; Moshtaghie, A.-A.; Ahmadvand, H. Effect of titanium on lipoprotein lipase activity in vivo and in vitro. J. Trace Elem. Med. Biol. 2010, 24, 95–98. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Lin, L.; Darvesh, A.S.; Sadana, P. Emerging strategies of targeting lipoprotein lipase for metabolic and cardiovascular diseases. Drug Discov. Today 2017, 22, 352–365. [Google Scholar] [CrossRef]
- Shaikh, Z.; Ashiq, U.; Jamal, R.A.; Gul, S.; Mahroof-Tahir, M.; Sultan, S.; Salar, U.; Khan, K.M. Synthesis, characterization, lipoxygease and tyrosinase inhibitor activities of non-cytotoxic titanium(III) and (IV) hydrazide complexes. Bull. Chem. Soc. Ethiop. 2023, 37, 315–333. [Google Scholar] [CrossRef]
- Melchor-Moncada, J.J.; Vasquez-Giraldo, S.; Zuluaga-Vélez, A.; Orozco, L.M.; Veloza, L.A.; Sepúlveda-Arias, J.C. Bioconjugation of Serratiopeptidase with Titanium Oxide Nanoparticles: Improving Stability and Antibacterial Properties. J. Funct. Biomater. 2024, 15, 300. [Google Scholar] [CrossRef]
- Rodríguez, I.; Fernández-Vega, L.; Maser-Figueroa, A.N.; Sang, B.; González-Pagán, P.; Tinoco, A.D. Exploring Titanium(IV) Complexes as Potential Antimicrobial Compounds. Antibiotics 2022, 11, 158. [Google Scholar] [CrossRef]
- Yaghoubi, S.; Schwietert, C.; McCue, J.P. Biological roles of titanium. Biol. Trace Elem. Res. 2000, 78, 205–217. [Google Scholar]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotech. 2016, 14, 73. [Google Scholar] [CrossRef]
- de Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial Effect of Titanium Dioxide Nanoparticles. In Antimicrobial Resistance; Mareș, M., Lim, S.H.E., Lai, K.-S., Cristina, R.-T., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 5. [Google Scholar]
- Tamilselvi, R.; Kalaiarasi, M.; Elumalai, M.; Malarkodi, T.; Venkatesh, A.; Prakash, V. Antimicrobial Activity of Metal Oxide Nanoparticles. Biomed. Pharmacol. J. 2024, 17, 1757–1767. [Google Scholar] [CrossRef]
- Serov, D.A.; Gritsaeva, A.V.; Yanbaev, F.M.; Simakin, A.V.; Gudkov, S.V. Review of Antimicrobial Properties of Titanium Dioxide Nanoparticles. Int. J. Mol. Sci. 2024, 25, 10519. [Google Scholar] [CrossRef]
- Li, W.; Tang, H.; Xiao, A.; Xie, T.; Sun, Y.; Liao, Y. Effects of applying titanium contained trace-element fertilizer to several grain crops in Hunan. Hunan Agric. Sci. 2011, 21, 55–58. [Google Scholar]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Faraz, A.; Faizan, M.; Fariduddin, Q.; Hayat, S. Response of Titanium Nanoparticles to Plant Growth: Agricultural Perspectives. In Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development; Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 101–110. [Google Scholar]
- Chaudhary, I.; Singh, V. Titanium Dioxide Nanoparticles and its Impact on Growth, Biomass and Yield of Agricultural Crops under Environmental Stress: A Review. Res. J. Nanosci. Nanotech. 2020, 10, 1–8. [Google Scholar]
- Silva, S.; Dias, M.C.; Silva, A.M.S. Titanium and Zinc Based Nanomaterials in Agriculture: A Promising Approach to Deal with (A)biotic Stresses? Toxics 2022, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Feizi, H.; Kamali, M.; Jafari, L.; Rezvani Moghaddam, P. Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere 2013, 91, 506–511. [Google Scholar] [CrossRef]
- Yiblet, Y.; Abdu, I.; Belew, B. Comprehensive Literature Review on Metal Nanoparticle for Enhanced Shelf Life of Mango Fruit. Sci. World J. 2024, 2024, 4782328. [Google Scholar] [CrossRef]
- Asztemborska, M.; Jakubiak, M.; Stęborowski, R.; Chajduk, E.; Bystrzejewska-Piotrowska, G. Titanium Dioxide Nanoparticle Circulation in an Aquatic Ecosystem. Water Air Soil Pollut. 2018, 229, 208. [Google Scholar] [CrossRef]
- Shi, W.; Han, Y.; Guo, C.; Su, W.; Zhao, X.; Zha, S.; Wang, Y.; Liu, G. Ocean acidification increases the accumulation of titanium dioxide nanoparticles (nTiO2) in edible bivalve mollusks and poses a potential threat to seafood safety. Sci. Rep. 2019, 9, 3516. [Google Scholar] [CrossRef]
- Valentine, A.M. Exploring a Role for Titanium in Bioinorganic Chemistry. The Chemist 2015, 88, 7–10. [Google Scholar]
- Davis-Gilbert, Z.W.; Tonks, I.A. Titanium redox catalysis: Insights and applications of an earth-abundant base metal. Dalton Trans. 2017, 46, 11522–11528. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. Report: Man survives with titanium heart for 100 days—A world first. Nature 2025, 639, 848–849. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Elsevier: Oxford, UK, 1997. [Google Scholar]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Wever, R.; Renirie, R.; Hasan, Z. Vanadium in Biology. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; Wiley: London, UK, 2011. [Google Scholar]
- Rehder, D. Vanadate-dependent peroxidases in macroalgae: Function, applications, and environmental impact. Oceanography 2014, 2, 121–127. [Google Scholar]
- Costa Pessoa, J.; Garribba, E.; Santos, M.F.A.; Santos-Silva, T. Vanadium and proteins: Uptake, transport, structure, activity and function. Coord. Chem. Rev. 2015, 301–302, 49–86. [Google Scholar] [CrossRef]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Leblanc, C.; Vilter, H.; Fournier, J.-B.; Delage, L.; Potin, P.; Rebuffet, E.; Michel, G.; Solari, P.-L.; Feiters, M.; Czjzek, M. Vanadium haloperoxidases: From the discovery 30 years ago to X-Ray crystallographic and V K-edge absorption spectroscopic studies. Coord. Chem. Rev. 2015, 301–302, 134–146. [Google Scholar] [CrossRef]
- Wever, R.; Krenn, B.E.; Renirie, R. Chapter Six—Marine Vanadium-Dependent Haloperoxidases, Their Isolation, Characterization, and Application. In Methods in Enzymology; Moore, B.S., Ed.; Academic Press: New York, NY, USA, 2018; Volume 605, pp. 141–201. [Google Scholar]
- Campitelli, P.; Crucianelli, M. On the Capability of Oxidovanadium(IV) Derivatives to Act as All-Around Catalytic Promoters Since the Prebiotic World. Molecules 2020, 25, 3073. [Google Scholar] [CrossRef]
- Rehder, D. Is vanadium a more versatile target in the activity of primordial life forms than hitherto anticipated? Org. Biomol. Chem. 2008, 6, 957–964. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Diao, M.; Fu, J.; Xie, M.; Shi, J.; Liu, Z.; Jiang, Y.; Cao, X.; Borthwick, A.G.L. Microbial Community Responses to Vanadium Distributions in Mining Geological Environments and Bioremediation Assessment. J. Geophys. Res. Biogeosci. 2019, 124, 601–615. [Google Scholar] [CrossRef]
- Yan, G.; Sun, X.; Dong, Y.; Gao, W.; Gao, P.; Li, B.; Yan, W.; Zhang, H.; Soleimani, M.; Yan, B.; et al. Vanadate reducing bacteria and archaea may use different mechanisms to reduce vanadate in vanadium contaminated riverine ecosystems as revealed by the combination of DNA-SIP and metagenomic-binning. Water Res. 2022, 226, 119247. [Google Scholar] [CrossRef]
- Lovley, D.R. Microbe Profile: Geobacter metallireducens: A model for novel physiologies of biogeochemical and technological significance. Microbiol. 2022, 168, 001138. [Google Scholar] [CrossRef] [PubMed]
- Yuliani, D.; Morishita, F.; Imamura, T.; Ueki, T. Vanadium Accumulation and Reduction by Vanadium-Accumulating Bacteria Isolated from the Intestinal Contents of Ciona robusta. Mar. Biotech. 2024, 26, 338–350. [Google Scholar] [CrossRef]
- Cockell, C.S.; Santomartino, R.; Finster, K.; Waajen, A.C.; Nicholson, N.; Loudon, C.-M.; Eades, L.J.; Moeller, R.; Rettberg, P.; Fuchs, F.M.; et al. Microbially-Enhanced Vanadium Mining and Bioremediation Under Micro- and Mars Gravity on the International Space Station. Front. Microbiol. 2021, 12, 641387. [Google Scholar] [CrossRef]
- Wever, R.; Hemrika, W. Vanadium Haloperoxidases. In Handbook of Metalloproteins; Messerschmidt, A., Huber, R., Poulas, T., Wieghardt, K., Cygler, M., Bod, W., Eds.; John Wiley & Sons, Ltd.: London, UK, 2006; pp. 1–12. [Google Scholar]
- Antipov, A.N.; Lyalikova, N.N.; Khijniak, T.V.; L’vov, N.P. Molybdenum-free nitrate reductases from vanadate-reducing bacteria. FEBS Lett. 1998, 441, 257–260. [Google Scholar] [CrossRef]
- Rehder, D. Vanadium: Biological, Environmental, and Engineering Aspects. Adv. Chem. Res. 2020, 2, 2001002. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef]
- Santos, M.F.A.; Sciortino, G.; Correia, I.; Fernandes, A.C.P.; Santos-Silva, T.; Pisanu, F.; Garribba, E.; Costa Pessoa, J. Binding of VIVO2+, VIVOL, VIVOL2 and VIVO2L Moieties to Proteins: X-ray/Theoretical Characterization and Biological Implications. Chemistry 2022, 28, e202200105. [Google Scholar] [CrossRef]
- Paolillo, M.; Ferraro, G.; Sahu, G.; Pattanayak, P.D.; Garribba, E.; Halder, S.; Ghosh, R.; Mondal, B.; Chatterjee, P.B.; Dinda, R.; et al. Interaction of VVO2-hydrazonates with lysozyme. J. Inorg. Biochem. 2024, 264, 112787. [Google Scholar] [CrossRef] [PubMed]
- Aissa, T.; Aissaoui-Zid, D.; Moslah, W.; Khamessi, O.; Ksiksi, R.; Oltermann, M.; Ruck, M.; Zid, M.F.; Srairi-Abid, N. Synthesis, physicochemical and pharmacological characterizations of a tetra-[methylimidazolium] dihydrogen decavanadate, inhibiting the IGR39 human melanoma cells development. J. Inorg. Biochem. 2024, 260, 112672. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Krishnan, U.M. Vanadium-Flavonoid Complexes: A Promising Class of Molecules for Therapeutic Applications. J. Med. Chem. 2021, 64, 12435–12452. [Google Scholar] [CrossRef]
- Jia, R.; Huang, X.; Dang, P.; Chen, Q.; Zhong, S.; Fan, F.; Wang, C.; Song, J.; Chorover, J.; Rensing, C. Fe(III) reduction mediates vanadium release and reduction in vanadium contaminated paddy soil under different organic amendments. Environ. Int. 2024, 193, 109073. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Han, X.; Shan, Y.; Li, F.; Shi, L. Biofilm Biology and Engineering of Geobacter and Shewanella spp. for Energy Applications. Front. Bioeng. Biotech. 2021, 9, 786416. [Google Scholar] [CrossRef]
- McMillan, D.G.G.; Marritt, S.J.; Butt, J.N.; Jeuken, L.J.C. Menaquinone-7 Is Specific Cofactor in Tetraheme Quinol Dehydrogenase CymA. J. Biol. Chem. 2012, 287, 14215–14225. [Google Scholar] [CrossRef] [PubMed]
- Portela, P.C.; Silva, M.A.; Teixeira, L.R.; Salgueiro, C.A. A unique aromatic residue modulates the redox range of a periplasmic multiheme cytochrome from Geobacter metallireducens. J. Biol. Chem. 2021, 296, 100711. [Google Scholar] [CrossRef]
- Tielens, A.G.M.; Van Hellemond, J.J. The electron transport chain in anaerobically functioning eukaryotes. Biochim. Biophs. Acta 1998, 1365, 71–78. [Google Scholar] [CrossRef]
- Enomoto, K.; Arikawa, Y.; Muratsubaki, H. Physiological role of soluble fumarate reductase in redox balancing during anaerobiosis in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2002, 215, 103–108. [Google Scholar] [CrossRef]
- Jiang, X.; van Wonderen, J.H.; Butt, J.N.; Edwards, M.J.; Clarke, T.A.; Blumberger, J. Which Multi-Heme Protein Complex Transfers Electrons More Efficiently? Comparing MtrCAB from Shewanella with OmcS from Geobacter. J. Phys. Chem. Lett. 2020, 11, 9421–9425. [Google Scholar] [CrossRef]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim. Biophs. Acta 2011, 1807, 1507–1538. [Google Scholar] [CrossRef] [PubMed]
- Feist, A.M.; Nagarajan, H.; Rotaru, A.-E.; Tremblay, P.-L.; Zhang, T.; Nevin, K.P.; Lovley, D.R.; Zengler, K. Constraint-Based Modeling of Carbon Fixation and the Energetics of Electron Transfer in Geobacter metallireducens. PLoS Comput. Biol. 2014, 10, e1003575. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.; Carter-Franklin, J.N. The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Nat. Prod. Rep. 2004, 21, 180–188. [Google Scholar] [CrossRef]
- Cochereau, B.; Le Strat, Y.; Ji, Q.; Pawtowski, A.; Delage, L.; Weill, A.; Mazéas, L.; Hervé, C.; Burgaud, G.; Gunde-Cimerman, N.; et al. Heterologous Expression and Biochemical Characterization of a New Chloroperoxidase Isolated from the Deep-Sea Hydrothermal Vent Black Yeast Hortaea werneckii UBOCC-A-208029. Mar. Biotechnol. 2023, 25, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Cochereau, B.; Meslet-Cladière, L.; Pouchus, Y.F.; Grovel, O.; Roullier, C. Halogenation in Fungi: What Do We Know and What Remains to Be Discovered? Molecules 2022, 27, 3157. [Google Scholar] [CrossRef]
- Wever, R.; Krenn, B.E.; Renirie, R. Marine Vanadium-Dependent Haloperoxidases, Their Isolation, Characterization, and Application. Methods Enzym. 2018, 605, 141–201. [Google Scholar]
- Winter, J.M.; Moore, B.S. Exploring the Chemistry and Biology of Vanadium-dependent Haloperoxidases. J. Biol. Chem 2009, 284, 18577–18581. [Google Scholar] [CrossRef]
- Gérard, E.F.; Mokkawes, T.; Johannissen, L.O.; Warwicker, J.; Spiess, R.R.; Blanford, C.F.; Hay, S.; Heyes, D.J.; de Visser, S.P. How Is Substrate Halogenation Triggered by the Vanadium Haloperoxidase from Curvularia inaequalis? ACS Catal. 2023, 13, 8247–8261. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, R.; Döbber, J.; Gulder, T. Chemoenzymatic C,C-Bond Forming Cascades by Cryptic Vanadium Haloperoxidase Catalyzed Bromination. Org. Lett. 2025, 27, 159–164. [Google Scholar] [CrossRef]
- Zhao, G.; Dong, H.; Xue, K.; Lou, S.; Qi, R.; Zhang, X.; Cao, Z.; Qin, Q.; Yi, B.; Lei, H.; et al. Nonheme iron catalyst mimics heme-dependent haloperoxidase for efficient bromination and oxidation. Sci. Adv. 2024, 10, eadq0028. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zou, Y.T.; Zeng, Y.Y.; Liu, L.; Chen, B.S. Enantioselectivity in Vanadium-Dependent Haloperoxidases of Different Marine Sources for Sulfide Oxidation to Sulfoxides. Mar. Drugs 2024, 22, 419. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.T.; McKinnie, S.M.K. Regioselective Halogenation of Lavanducyanin by a Site-Selective Vanadium-Dependent Chloroperoxidase. Org. Lett. 2024, 26, 5725–5730. [Google Scholar] [CrossRef]
- Sweeney, D.; Chase, A.B.; Bogdanov, A.; Jensen, P.R. MAR4 Streptomyces: A Unique Resource for Natural Product Discovery. J. Nat. Prod. 2024, 87, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Patton, Z.E.; Shoemaker, C.R.; Bacsa, J.; Biegasiewicz, K.F. N-Halogenation by Vanadium-Dependent Haloperoxidases Enables 1,2,4-Oxadiazole Synthesis. Ange. Chem. Int. Ed. Engl. 2024, 63, e202411387. [Google Scholar] [CrossRef]
- Chen, P.Y.; Adak, S.; Chekan, J.R.; Liscombe, D.K.; Miyanaga, A.; Bernhardt, P.; Diethelm, S.; Fielding, E.N.; George, J.H.; Miles, Z.D.; et al. Structural Basis of Stereospecific Vanadium-Dependent Haloperoxidase Family Enzymes in Napyradiomycin Biosynthesis. Biochemistry 2022, 61, 1844–1852. [Google Scholar] [CrossRef]
- Lazic, J.; Filipovic, V.; Pantelic, L.; Milovanovic, J.; Vojnovic, S.; Nikodinovic-Runic, J. Late-stage diversification of bacterial natural products through biocatalysis. Front. Bioeng. Biotechnol. 2024, 12, 1351583. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Espejo, E. Is there a halo-enzymopathy in Parkinson’s disease? Neurologia 2022, 37, 661–667. [Google Scholar] [CrossRef]
- Radlow, M.; Czjzek, M.; Jeudy, A.; Dabin, J.; Delage, L.; Leblanc, C.; Hartung, J. X-ray Diffraction and Density Functional Theory Provide Insight into Vanadate Binding to Homohexameric Bromoperoxidase II and the Mechanism of Bromide Oxidation. ACS Chem. Biol. 2018, 13, 1243–1259. [Google Scholar] [CrossRef]
- Peters, J.W.; Szilagyi, R.K. Exploring new frontiers of nitrogenase structure and mechanism. Curr. Opin. Chem. Biol. 2006, 10, 101–108. [Google Scholar] [CrossRef]
- Decamps, L.; Rice, D.B.; DeBeer, S. An Fe6C Core in All Nitrogenase Cofactors. Angew. Chem. Int. Ed. Engl. 2022, 61, e202209190. [Google Scholar] [CrossRef]
- Eady, R.R. Structure−Function Relationships of Alternative Nitrogenases. Chem. Rev. 1996, 96, 3013–3030. [Google Scholar] [CrossRef]
- Garcia, A.K.; McShea, H.; Kolaczkowski, B.; Kaçar, B. Reconstructing the evolutionary history of nitrogenases: Evidence for ancestral molybdenum-cofactor utilization. Geobiology 2020, 18, 394–411. [Google Scholar] [CrossRef]
- Miller, R.W.; Eady, R.R. Molybdenum and vanadium nitrogenases of Azotobacter chroococcum. Low temperature favours N2 reduction by vanadium nitrogenase. Biochem. J. 1988, 256, 429–432. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Yang, Z.-Y.; Lukoyanov, D.A.; Harris, D.F.; Dean, D.R.; Raugei, S.; Hoffman, B.M. Reduction of Substrates by Nitrogenases. Chem. Rev. 2020, 120, 5082–5106. [Google Scholar] [CrossRef]
- Sippel, D.; Einsle, O. The structure of vanadium nitrogenase reveals an unusual bridging ligand. Nat. Chem. Biol. 2017, 13, 956–960. [Google Scholar] [CrossRef]
- Howard, J.B.; Rees, D.C. Structural Basis of Biological Nitrogen Fixation. Chem. Rev. 1996, 96, 2965–2982. [Google Scholar] [CrossRef]
- Rutledge, H.L.; Tezcan, F.A. Electron Transfer in Nitrogenase. Chem. Rev. 2020, 120, 5158–5193. [Google Scholar] [CrossRef]
- Van Stappen, C.; Decamps, L.; Cutsail, G.E., III; Bjornsson, R.; Henthorn, J.T.; Birrell, J.A.; DeBeer, S. The Spectroscopy of Nitrogenases. Chem. Rev. 2020, 120, 5005–5081. [Google Scholar] [CrossRef]
- Wahl, I.M.; Sengupta, K.; van Gastel, M.; Decamps, L.; DeBeer, S. Understanding the P-Cluster of Vanadium Nitrogenase: An EPR and XAS Study of the Holo vs. Apo Forms of the Enzyme. ChemBiochem 2024, 26, e202400833. [Google Scholar] [CrossRef]
- Franke, P.; Freiberger, S.; Zhang, L.; Einsle, O. Conformational protection of molybdenum nitrogenase by Shethna protein II. Nature 2025, in press. [Google Scholar] [CrossRef]
- Narehood, S.M.; Cook, B.D.; Srisantitham, S.; Eng, V.H.; Shiau, A.A.; McGuire, K.L.; Britt, R.D.; Herzik, M.A.; Tezcan, F.A. Structural basis for the conformational protection of nitrogenase from O2. Nature 2025, in press. [Google Scholar] [CrossRef]
- Bjornsson, R.; Neese, F.; DeBeer, S. Revisiting the Mössbauer Isomer Shifts of the FeMoco Cluster of Nitrogenase and the Cofactor Charge. Inorg. Chem. 2017, 56, 1470–1477. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M.; Wei, W.-J. The energetics of N2 reduction by vanadium containing nitrogenase. Phys. Chem. Chem. Phys. 2024, 26, 1684–1695. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Jimenez-Vicente, E.; Kallas, H.; Lukoyanov, D.A.; Yang, H.; Martin del Campo, J.S.; Dean, D.R.; Hoffman, B.M.; Seefeldt, L.C. The electronic structure of FeV-cofactor in vanadium-dependent nitrogenase. Chem. Sci. 2021, 12, 6913–6922. [Google Scholar] [CrossRef]
- Burgess, B.K.; Lowe, D.J. Mechanism of Molybdenum Nitrogenase. Chem. Rev. 1996, 96, 2983–3012. [Google Scholar] [CrossRef]
- Lukoyanov, D.; Yang, Z.-Y.; Khadka, N.; Dean, D.R.; Seefeldt, L.C.; Hoffman, B.M. Identification of a Key Catalytic Intermediate Demonstrates That Nitrogenase Is Activated by the Reversible Exchange of N2 for H2. J. Am. Chem. Soc. 2015, 137, 3610–3615. [Google Scholar] [CrossRef]
- Sengupta, K.; Joyce, J.P.; Decamps, L.; Kang, L.; Bjornsson, R.; Rüdiger, O.; DeBeer, S. Investigating the Molybdenum Nitrogenase Mechanistic Cycle Using Spectroelectrochemistry. J. Am. Chem. Soc. 2025, 147, 2099–2144. [Google Scholar] [CrossRef]
- Harwood, C.S. Iron-Only and Vanadium Nitrogenases: Fail-Safe Enzymes or Something More? Ann. Rev. Microbiol. 2020, 74, 247–266. [Google Scholar] [CrossRef]
- Jiang, H.; Ryde, U. H2 formation from the E(2)-E(4) states of nitrogenase. Phys. Chem. Chem. Phys. 2024, 26, 1364–1375. [Google Scholar] [CrossRef]
- Sippel, D.; Rohde, M.; Netzer, J.; Trncik, C.; Gies, J.; Grunau, K.; Djurdjevic, I.; Decamps, L.; Andrade, S.L.A.; Einsle, O. A bound reaction intermediate sheds light on the mechanism of nitrogenase. Science 2018, 359, 1484–1489. [Google Scholar] [CrossRef]
- Benediktsson, B.; Thorhallsson, A.T.; Bjornsson, R. QM/MM calculations reveal a bridging hydroxo group in a vanadium nitrogenase crystal structure. Chem. Commun. 2018, 54, 7310–7313. [Google Scholar] [CrossRef]
- Cao, L.; Caldararu, O.; Ryde, U. Does the crystal structure of vanadium nitrogenase contain a reaction intermediate? Evidence from quantum refinement. J. Biol. Inorg. Chem. 2020, 25, 847–861. [Google Scholar] [CrossRef]
- Lee, C.C.; Hu, Y.; Ribbe, M.W. Vanadium Nitrogenase Reduces CO. Science 2010, 329, 642. [Google Scholar] [CrossRef]
- Rohde, M.; Laun, K.; Zebger, I.; Stripp, S.T.; Einsle, O. Two ligand-binding sites in CO-reducing V nitrogenase reveal a general mechanistic principle. Sci. Adv. 2021, 7, eabg4474. [Google Scholar] [CrossRef]
- Oehlmann, N.N.; Rebelein, J.G. Cover Feature: The Conversion of Carbon Monoxide and Carbon Dioxide by Nitrogenases. Chem. Bio. Chem. 2022, 23, e202100674. [Google Scholar]
- Natzke, J.; Bruno-Bárcena, J.M. Two-Stage Continuous Conversion of Carbon Monoxide to Ethylene by Whole Cells of Azotobacter vinelandii. Appl. Environ. Microbiol. 2020, 86, e00446-20. [Google Scholar] [CrossRef]
- Sickerman, N.S.; Hu, Y.; Ribbe, M.W. Activation of CO2 by Vanadium Nitrogenase. Chem. Asian J. 2017, 12, 1985–1996. [Google Scholar] [CrossRef]
- Rohde, M.; Grunau, K.; Einsle, O. CO Binding to the FeV Cofactor of CO-Reducing Vanadium Nitrogenase at Atomic Resolution. Angew Chem. Int. Edn. 2020, 59, 23626–23630. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Yoshinaga, M.; Kamino, K.; Ueki, T. Vanadium-Binding Ability of Nucleoside Diphosphate Kinase from the Vanadium-Rich Fan Worm, Pseudopotamilla occelata. Zool. Sci. 2016, 33, 266–271. [Google Scholar] [CrossRef]
- Hamada, T.; Asanuma, M.; Ueki, T.; Hayashi, F.; Kobayashi, N.; Yokoyama, S.; Michibata, H.; Hirota, H. Solution Structure of Vanabin2, a Vanadium(IV)-Binding Protein from the Vanadium-Rich Ascidian Ascidia sydneiensis samea. J. Am. Chem. Soc. 2005, 127, 4216–4222. [Google Scholar] [CrossRef]
- da Silva, J.A.L.; Frausto da Silva, J.J.R.; Pombeiro, A.J.L. Amavadin, a vanadium natural complex: Its role and applications. Coord. Chem. Rev. 2013, 257, 2388–2400. [Google Scholar] [CrossRef]
- Bertini, L.; Barbieri, V.; Fantucci, P.; Gioia, L.D.; Zampella, G. DFT characterization of key intermediates in thiols oxidation catalyzed by amavadin. Dalton Trans. 2011, 40, 7704–7712. [Google Scholar] [CrossRef]
- Rehder, D. Vanadium. Its Role for Humans. In Interrelations Between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2013; Volume 13, pp. 139–169. [Google Scholar]
- Kohlmeier, M. Vanadium. In Nutrient Metabolism; Kohlmeier, M., Ed.; Academic Press: London, UK, 2003; pp. 762–766. [Google Scholar]
- Costa Pessoa, J.; Tomaz, I. Transport of Therapeutic Vanadium and Ruthenium Complexes by Blood Plasma Components. Current Med. Chem. 2010, 17, 3701–3738. [Google Scholar] [CrossRef]
- Correia, I.; Jakusch, T.; Cobbinna, E.; Mehtab, S.; Tomaz, I.; Nagy, N.V.; Rockenbauer, A.; Costa Pessoa, J.; Kiss, T. Evaluation of the binding of oxovanadium(iv) to human serum albumin. Dalton Trans. 2012, 41, 6477–6487. [Google Scholar] [CrossRef]
- Goutam Mukherjee, A.; Ramesh Wanjari, U.; Renu, K.; Vellingiri, B.; Valsala Gopalakrishnan, A. Heavy metal and metalloid—Induced reproductive toxicity. Environ. Toxicol. Pharmacol. 2022, 92, 103859. [Google Scholar] [CrossRef]
- Ommati, M.M.; Heidari, R. Amino acids ameliorate heavy metals-induced oxidative stress in male/female reproductive tissue. In Toxicology; Patel, V.B., Preedy, V.R., Eds.; Academic Press: New York, NY, USA, 2021; pp. 371–386. [Google Scholar]
- Aureliano, M.; De Sousa-Coelho, A.L.; Dolan, C.C.; Roess, D.A.; Crans, D.C. Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. Int. J. Mol. Sci. 2023, 24, 5382. [Google Scholar] [CrossRef]
- Vasilaki, A.T.; McMillan, D.C. Lipid Peroxidation. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 2054–2055. [Google Scholar]
- Kehrer, J.P.; Robertson, J.D.; Smith, C.V. Free Radicals and Reactive Oxygen Species. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 277–307. [Google Scholar]
- Hanus-Fajerska, E.; Wiszniewska, A.; Kamińska, I. A Dual Role of Vanadium in Environmental Systems-Beneficial and Detrimental Effects on Terrestrial Plants and Humans. Plants 2021, 10, 1110. [Google Scholar] [CrossRef]
- Domingo, J.L.; Gómez, M. Vanadium compounds for the treatment of human diabetes mellitus: A scientific curiosity? A review of thirty years of research. Food Chem. Toxicol. 2016, 95, 137–141. [Google Scholar] [CrossRef]
- Bailey, N.; Carrington, A.; Lott, K.A.K.; Symons, M.C.R. 55. Structure and reactivity of the oxyanions of transition metals. Part VIII. Acidities and spectra of protonated oxyanions. J. Chem. Soc. 1960, 290–297. [Google Scholar] [CrossRef]
- Shechter, Y.; Karlish, S.J.D. Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl (IV) ions. Nature 1980, 284, 556–558. [Google Scholar] [CrossRef]
- Ramanadham, S.; Mongold, J.J.; Brownsey, R.W.; Cros, G.H.; McNeill, J.H. Oral vanadyl sulfate in treatment of diabetes mellitus in rats. Am. J. Physiol. Heart Circ. Physiol. 1989, 257, H904–H911. [Google Scholar] [CrossRef]
- Sciortino, G.; Maréchal, J.-D.; Garribba, E. Integrated experimental/computational approaches to characterize the systems formed by vanadium with proteins and enzymes. Inorg. Chem. Front. 2021, 8, 1951–1974. [Google Scholar] [CrossRef]
- Crans, D.C.; Henry, L.; Cardiff, G.; Posner, B.I. Developing vanadium as an antidiabetic or anticancer drug: A clinical and historical perspective. Met. Ions Life Sci. 2019, 19, 203–230. [Google Scholar]
- Chromium—Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/ (accessed on 15 May 2023).
- Mertz, W. Chromium occurrence and function in biological systems. Physiol. Rev. 1969, 49, 163–239. [Google Scholar] [CrossRef]
- Lukaski, H.C. Chromium as a supplement. Ann. Rev. Nutr. 1999, 19, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Raman, P.; Elmendorf, J.S. Unifying mechanisms of trivalent chromium in health and disease. In Essential and Toxic Trace Elements and Vitamins in Human Health; Prasad, A.S., Brewer, G.J., Eds.; Academic Press: New York, NY, USA, 2020; pp. 127–139. [Google Scholar]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The Double Face of Metals: The Intriguing Case of Chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Tian, H.; Guo, X.; Wang, X.; He, Z.; Sun, R.; Ge, S.; Zhang, Z. Chromium picolinate supplementation for overweight or obese adults. Cochrane Database Syst. Rev. 2013, 11, CD010063. [Google Scholar]
- Panel, E.; Nda, A. Scientific opinion on dietary reference values for chromium. EFSA J. 2014, 12, 3845. [Google Scholar]
- Vajdi, M.; Khajeh, M.; Safaei, E.; Moeinolsadat, S.; Mousavi, S.; Seyedhosseini-Ghaheh, H.; Abbasalizad-Farhangi, M.; Askari, G. Effects of chromium supplementation on body composition in patients with type 2 diabetes: A dose-response systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2024, 81, 127338. [Google Scholar] [CrossRef]
- Khodavirdipour, A.; Haddadi, F.; Keshavarzi, S. Chromium Supplementation; Negotiation with Diabetes Mellitus, Hyperlipidemia and Depression. J. Diabetes Metab. Disord. 2020, 19, 585–595. [Google Scholar] [CrossRef]
- Trumbo, P.R.; Ellwood, K.C. Chromium Picolinate Intake and Risk of Type 2 Diabetes: An Evidence-Based Review by the United States Food and Drug Administration. Nutr. Rev. 2006, 64, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Ocasio Quinones, G.A. Chromium Deficiency; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Vincent, J.B. Is chromium(III) pharmacologically relevant? An update focused on studies with diabetic rodent models. J. Trace Elem. Med. Biol. 2024, 84, 127453. [Google Scholar] [CrossRef]
- Vincent, J.B. Is chromium(III) supplementation beneficial for dietary rodent models of prediabetes? J. Trace Elem. Med. Biol. 2024, 85, 127482. [Google Scholar] [CrossRef]
- Vincent, J.B.; Love, S. The binding and transport of alternative metals by transferrin. Biochim. Biophys. Acta 2012, 1820, 362–378. [Google Scholar] [CrossRef]
- Petersen, C.M.; Edwards, K.C.; Gilbert, N.C.; Vincent, J.B.; Thompson, M.K. X-ray structure of chromium(III)-containing transferrin: First structure of a physiological Cr(III)-binding protein. J. Inorg. Biochem. 2020, 210, 111101. [Google Scholar] [CrossRef]
- Sun, Y.; Ramirez, J.; Woski, S.A.; Vincent, J.B. The binding of trivalent chromium to low-molecular-weight chromium-binding substance (LMWCr) and the transfer of chromium from transferrin and chromium picolinate to LMWCr. J. Biol. Inorg. Chem. 2000, 5, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Pham, T.H.N.; Lay, P.A. Binding of Chromium(III) to Transferrin Could Be Involved in Detoxification of Dietary Chromium(III) Rather than Transport of an Essential Trace Element. Angew. Chem. Int. Ed. 2016, 55, 8104–8107. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Relationship between Glucose Tolerance Factor and Low-Molecular-Weight Chromium-Binding Substance. J. Nutr. 1994, 124, 117–118. [Google Scholar] [CrossRef]
- Lai, M.-H. Antioxidant Effects and Insulin Resistance Improvement of Chromium Combined with Vitamin C and E Supplementation for Type 2 Diabetes Mellitus. J. Clin. Biochem. Nutr. 2008, 43, 191–198. [Google Scholar] [CrossRef]
- Wallach, S. Clinical and biochemical aspects of chromium deficiency. J. Am. Coll. Nutr. 1985, 4, 107–120. [Google Scholar] [CrossRef]
- Anderson, R.A. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000, 26, 22–27. [Google Scholar]
- Wilbur, S.; Abadin, H.; Fay, M.; Yu, D.; Tencza, B.; Ingerman, L.; Klotzbach, J.; James, S. Toxicological Profile for Chromium. Available online: https://www.ncbi.nlm.nih.gov/books/NBK158855/ (accessed on 24 May 2023).
- Viera, M.; Davis-McGibony, C.M. Isolation and Characterization of Low-Molecular-Weight Chromium-binding Substance (LMWCr) from Chicken Liver. Protein J. 2008, 27, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Toepfer, E.W.; Mertz, W.; Polansky, M.M.; Roginski, E.E.; Wolf, W.R. Preparation of Chromium-Containing Material of Glucose Tolerance Factor Activity from Brewer’s Yeast Extracts and by Synthesis. J. Agric. Food Chem. 1977, 25, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Is the Pharmacological Mode of Action of Chromium(III) as a Second Messenger? Biol. Trace Elem. Res. 2015, 166, 7–12. [Google Scholar] [CrossRef]
- Vincent, J.B. The biochemistry of chromium. J. Nutr. 2000, 130, 715–718. [Google Scholar] [CrossRef]
- Vincent, J.B. Recent advances in the nutritional biochemistry of trivalent chromium. Proc. Nutr. Soc. 2004, 63, 41–47. [Google Scholar] [CrossRef]
- Vincent, J.B. Biochemical Mechanisms. In The Bioinorganic Chemistry of Chromium; Vincent, J.B., Ed.; John Wiley & Sons: Chichester, UK, 2012; pp. 125–167. [Google Scholar]
- Jacquamet, L.; Sun, Y.; Hatfield, J.; Gu, W.; Cramer, S.P.; Crowder, M.W.; Lorigan, G.A.; Vincent, J.B.; Latour, J.-M. Characterization of Chromodulin by X-ray Absorption and Electron Paramagnetic Resonance Spectroscopies and Magnetic Susceptibility Measurements. J. Am. Chem. Soc. 2003, 125, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Elucidating a Biological Role for Chromium at a Molecular. Acc. Chem. Res. 2000, 33, 503–510. [Google Scholar] [CrossRef]
- Clodfelder, B.J.; Emamaullee, J.; Hepburn, D.D.; Chakov, N.E.; Nettles, H.S.; Vincent, J.B. The trail of chromium(III) in vivo from the blood to the urine: The roles of transferrin and chromodulin. J. Biol. Inorg. Chem. 2001, 6, 608–617. [Google Scholar] [CrossRef]
- Hua, Y.; Clark, S.; Ren, J.; Sreejayan, N. Molecular mechanisms of chromium in alleviating insulin resistance. J. Nutr. Biochem. 2012, 23, 313–319. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Hu, F.B. Role of Chromium in Human Health and in Diabetes. Diabetes Care 2004, 27, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, L.; Li, H.; Lai, Y.-T.; Wei, X.; Xu, X.; Cao, Z.; Cao, H.; Wan, Q.; Chang, Y.-Y.; et al. Mitochondrial ATP synthase as a direct molecular target of chromium(III) to ameliorate hyperglycaemia stress. Nat. Commun. 2023, 14, 1738. [Google Scholar] [CrossRef]
- Mattos Pereira, V.; Nair, S. Targeting Mitochondrial ATP-Synthase: Evolving Role of Chromium as a Regulator of Carbohydrate and Fat Metabolism. Biol. Trace Elem. Res. 2024, 202, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, H.; Orhan, C.; Sahin, K. Understanding Cr(III) Action on Mitochondrial ATP Synthase and AMPK Efficacy: Insights from Previous Studies—A Review. Biol. Trace Elem. Res. 2024, 202, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Molec. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Kaikini, A.A.; Kanchan, D.M.; Nerurkar, U.N.; Sathaye, S. Targeting Mitochondrial Dysfunction for the Treatment of Diabetic Complications: Pharmacological Interventions through Natural Products. Pharmacogn. Rev. 2017, 11, 128–135. [Google Scholar]
- Vincent, J.B. What Are the Implications of Cr(III) Serving as an Inhibitor of the Beta Subunit of Mitochondrial ATP Synthase? Biol. Trace Elem. Res. 2024, 202, 1335–1344. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- DesMarias, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Op. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Chen, B.; Xiong, J.; Ding, J.-H.; Yuan, B.-F.; Feng, Y.-Q. Analysis of the Effects of Cr(VI) Exposure on mRNA Modifications. Chem. Res. Toxicol. 2019, 32, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mir, R.A.; Tyagi, A.; Manzar, N.; Kashyap, A.S.; Mushtaq, M.; Raina, A.; Park, S.; Sharma, S.; Mir, Z.A.; et al. Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives. Plants 2023, 12, 1502. [Google Scholar] [CrossRef]
- Suljević, D.; Muhamed, F.; Alijagic, A. Assessing chromium toxicity across aquatic and terrestrial environments: A cross-species review. Drug Chem. Toxicol. 2024, 47, 1312–1324. [Google Scholar] [CrossRef]
- Hassan, M.U.; Lihong, W.; Nawaz, M.; Ali, B.; Tang, H.; Rasheed, A.; Zain, M.; Alqahtani, F.M.; Hashem, M.; Qari, S.H.; et al. Silicon a key player to mitigate chromium toxicity in plants: Mechanisms and future prospective. Plant Physiol. Biochem. 2024, 208, 108529. [Google Scholar] [CrossRef] [PubMed]
- Sazakli, E. Human Health Effects of Oral Exposure to Chromium: A Systematic Review of the Epidemiological Evidence. Int. J. Environ. Res. Public Health 2024, 21, 406. [Google Scholar] [CrossRef]
- Meaza, I.; Williams, A.R.; Wise, S.S.; Lu, H.; Wise, J.P. Carcinogenic Mechanisms of Hexavalent Chromium: From DNA Breaks to Chromosome Instability and Neoplastic Transformation. Curr. Environ. Health Rep. 2024, 11, 484–546. [Google Scholar] [CrossRef]
- Levina, A.; Lay, P.A. Chapter 9—Redox chemistry and biological activities of chromium(III) complexes. In The Nutritional Biochemistry of Chromium (III), 2nd ed.; Vincent, J.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 281–321. [Google Scholar]
- Paul, M.; Pranjaya, P.P.; Thatoi, H. In silico studies on structural, functional, and evolutionary analysis of bacterial chromate reductase family responsible for high chromate bioremediation efficiency. SN Appl. Sci. 2020, 2, 1997. [Google Scholar] [CrossRef]
- Park, C.H.; Keyhan, M.; Wielinga, B.; Fendorf, S.; Matin, A. Purification to Homogeneity and Characterization of a Novel Pseudomonas putida Chromate Reductase. Appl. Environ. Micro. 2000, 66, 1788–1795. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Buchko, G.W.; Varnum, S.M.; Robinson, H.; Squier, T.C.; Long, P.E. Structure Determination and Functional Analysis of a Chromate Reductase from Gluconacetobacter hansenii. PLoS ONE 2012, 7, e42432. [Google Scholar] [CrossRef]
- Ackerley, D.F.; Gonzalez, C.F.; Keyhan, M.; Blake II, R.; Matin, A. Mechanism of chromate reduction by the Escherichia coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stress during chromate reduction. Environ. Micro. 2004, 6, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Viti, C.; Marchi, E.; Decorosi, F.; Giovannetti, L. Molecular mechanisms of Cr(VI) resistance in bacteria and fungi. FEMS Micro. Rev. 2014, 38, 633–659. [Google Scholar] [CrossRef] [PubMed]
- Alur, A.; John, P.; Xu, D. Effects of hexavalent chromium on mitochondria and their implications in carcinogenesis. J. Env. Sci. Health C 2024, 42, 109–125. [Google Scholar] [CrossRef]
- Gerke, T.L.; Little, B.J. Manganese. In Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth; White, W.M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 864–867. [Google Scholar]
- Čapek, J.; Večerek, B. Why is manganese so valuable to bacterial pathogens? Front. Cell Infect. Micro. 2023, 13, 943390. [Google Scholar] [CrossRef]
- Roth, J.; Ponzoni, S.; Aschner, M. Manganese Homeostasis and Transport. In Matallomics and the Cell; Banci, L., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2013; pp. 169–201. [Google Scholar]
- Soares, M.V.; Quines, C.B.; Ávila, D.S. Manganese. In Essential and Toxic Trace Elements and Vitamins in Human Health; Prasad, A.S., Brewer, G.J., Eds.; Academic Press: London, UK, 2020; pp. 141–152. [Google Scholar]
- Studer, J.M.; Schweer, W.P.; Gabler, N.K.; Ross, J.W. Functions of manganese in reproduction. Anim. Reprod. Sci. 2022, 238, 106924. [Google Scholar] [CrossRef]
- Marques dos Santos, D.; Aschner, M.; Marreilha dos Santos, A.P. Manganese and Neurodegeneration. In Biometals in Neurodegenerative Diseases: Mechanisms and Therapeutics; White, A.R., Aschner, M., Costa, L.G., Bush, A.I., Eds.; Elsevier: London, UK, 2017; pp. 117–151. [Google Scholar]
- Socha, A.L.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 00106. [Google Scholar] [CrossRef] [PubMed]
- Obeng, S.K.; Kulhánek, M.; Balík, J.; Černý, J.; Sedlář, O. Manganese: From Soil to Human Health—A Comprehensive Overview of Its Biological and Environmental Significance. Nutrients 2024, 16, 3455. [Google Scholar] [CrossRef]
- Syiemlieh, I.; Kumar, A.; Kurbah, S.D.; De, A.K.; Lal, R.A. Low-spin manganese(II) and high-spin manganese(III) complexes derived from disalicylaldehyde oxaloyldihydrazone: Synthesis, spectral characterization and electrochemical studies. J. Mol. Str. 2018, 1151, 343–352. [Google Scholar] [CrossRef]
- Saha, A.; Majumdar, P.; Goswami, S. Low-spin manganese(II) and cobalt(III) complexes of N-aryl-2-pyridylazophenylamines: New tridentate N,N,N-donors derived from cobalt mediated aromatic ring amination of 2-(phenylazo)pyridine. Crystal structure of a manganese(II) complex. J. Chem. Soc. Dalton Trans. 2000, 1703–1708. [Google Scholar] [CrossRef]
- Basu, P.; Chakravorty, A. Low-spin tris(quinone oximates) of manganese(II,III). Synthesis, isomerism, and equilibria. Inorg. Chem. 1992, 31, 4980–4986. [Google Scholar] [CrossRef]
- Basumatary, D.; Lal, R.A.; Kumar, A. Synthesis, and characterization of low- and high-spin manganese(II) complexes of polyfunctional adipoyldihydrazone: Effect of coordination of N-donor ligands on stereo-redox chemistry. J. Mol. Str. 2015, 1092, 122–129. [Google Scholar] [CrossRef]
- Baj, J.; Flieger, W.; Barbachowska, A.; Kowalska, B.; Flieger, M.; Forma, A.; Teresiński, G.; Portincasa, P.; Buszewicz, G.; Radzikowska-Büchner, E.; et al. Consequences of Disturbing Manganese Homeostasis. Int. J. Mol. Sci. 2023, 24, 14959. [Google Scholar] [CrossRef]
- Reinert, J.P.; Forbes, L.D. Manganese Toxicity Associated With Total Parenteral Nutrition: A Review. J. Pharm. Technol. 2021, 37, 260–266. [Google Scholar] [CrossRef]
- Wu, Q.; Mu, Q.; Xia, Z.; Min, J.; Wang, F. Manganese homeostasis at the host-pathogen interface and in the host immune system. Semin. Cell Dev. Biol. 2021, 115, 45–53. [Google Scholar] [CrossRef]
- Martins, A.C.; Krum, B.N.; Queirós, L.; Tinkov, A.A.; Skalny, A.V.; Bowman, A.B.; Aschner, M. Manganese in the Diet: Bioaccessibility, Adequate Intake, and Neurotoxicological Effects. J. Agric. Food Chem. 2020, 68, 12893–12903. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Paoliello, M.M.B.; Mazilina, A.N.; Skalny, A.V.; Martins, A.C.; Voskresenskaya, O.N.; Aaseth, J.; Santamaria, A.; Notova, S.V.; Tsatsakis, A.; et al. Molecular Targets of Manganese-Induced Neurotoxicity: A Five-Year Update. Int. J. Mol. Sci. 2021, 22, 4646. [Google Scholar] [CrossRef]
- Soares, A.T.G.; Silva, A.C.; Tinkov, A.A.; Khan, H.; Santamaría, A.; Skalnaya, M.G.; Skalny, A.V.; Tsatsakis, A.; Bowman, A.B.; Aschner, M.; et al. The impact of manganese on neurotransmitter systems. J. Trace Elem. Med. Biol. 2020, 61, 126554. [Google Scholar] [CrossRef]
- Sule, K.; Umbsaar, J.; Prenner, E.J. Mechanisms of Co, Ni, and Mn toxicity: From exposure and homeostasis to their interactions with and impact on lipids and biomembranes. Biochim. Biophys. Acta 2020, 1862, 183250. [Google Scholar] [CrossRef]
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain manganese and the balance between essential roles and neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329. [Google Scholar] [CrossRef]
- McCabe, S.; Limesand, K.; Zhao, N. Recent progress toward understanding the role of ZIP14 in regulating systemic manganese homeostasis. Comput. Struct. Biotechnol. J. 2023, 21, 2332–2338. [Google Scholar] [CrossRef]
- Winslow, J.W.W.; Limesand, K.H.; Zhao, N. The Functions of ZIP8, ZIP14, and ZnT10 in the Regulation of Systemic Manganese Homeostasis. Int. J. Mol. Sci. 2020, 21, 3304. [Google Scholar] [CrossRef]
- Liu, Q.; Barker, S.; Knutson, M.D. Iron and manganese transport in mammalian systems. Biochim. Biophys. Acta 2021, 1868, 118890. [Google Scholar] [CrossRef] [PubMed]
- Mims, M.P.; Prchal, J.T. Divalent metal transporter 1. Hematology 2005, 10, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Kambe, T. Manganese transport in mammals by zinc transporter family proteins, ZNT and ZIP. J. Pharmcol. Sci. 2022, 148, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gunter, T.E.; Gerstner, B.; Gunter, K.K.; Malecki, J.; Gelein, R.; Valentine, W.M.; Aschner, M.; Yule, D.I. Manganese transport via the transferrin mechanism. NeuroToxicology 2013, 34, 118–127. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into Manganese Superoxide Dismutase and Human Diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef]
- Fischer, W.W.; Hemp, J.; Valentine, J.S. How did life survive Earth’s great oxygenation? Curr. Opp. Chem. Biol. 2016, 31, 166–178. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef]
- Koppenol, W.H. The Haber-Weiss cycle—70 years later. Redox Rep. 2001, 6, 229–234. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Hider, R.H. Iron and redox cycling. Do’s and don’ts. Free. Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; Clair, D.K.S. Manganese Superoxide Dismutase: Guardian of the Powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef]
- Steinman, H.M. Bacterial Superoxide Dismutases. In Oxygen Radicals in Biology and Medicine; Simic, M.G., Taylor, K.A., Ward, J.F., von Sonntag, C., Eds.; Springer: Boston, MA, USA, 1988; pp. 641–646. [Google Scholar]
- McCord, J.M. Superoxide dismutase in aging and disease: An overview. In Methods Enzymology; Academic Press: San Diego, CA, USA, 2002; Volume 349, pp. 331–341. [Google Scholar]
- Marques, H.M. Electron transfer in biological systems. J. Biol. Inorg. Chem. 2024, 29, 641–683. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Freudenheim, J.L.; Thompson, P.A.; Bowman, E.; Vena, J.E.; Marshall, J.R.; Graham, S.; Laughlin, R.; Nemoto, T.; Shields, P.G. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999, 59, 602–606. [Google Scholar] [PubMed]
- Valenti, L.; Conte, D.; Piperno, A.; Dongiovanni, P.; Fracanzani, A.L.; Fraquelli, M.; Vergani, A.; Gianni, C.; Carmagnola, L.; Fargion, S. The mitochondrial superoxide dismutase A16V polymorphism in the cardiomyopathy associated with hereditary haemochromatosis. J. Med. Genet. 2004, 41, 946–950. [Google Scholar] [CrossRef]
- Martin, R.C.G.; Ahn, J.; Nowell, S.A.; Hein, D.W.; Doll, M.A.; Martini, B.D.; Ambrosone, C.B. Association between manganese superoxide dismutase promoter gene polymorphism and breast cancer survival. Breast Cancer Res. 2006, 8, R45. [Google Scholar] [CrossRef]
- Hewitt, J.; Morris, J.G. Superoxide dismutase in some obligately anaerobic bacteria. FEBS Lett. 1975, 50, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, G.; Kardinahl, S. Iron superoxide dismutases: Structure and function of an archaic enzyme. Biochem. Soc. Trans. 2003, 31, 1330–1334. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K. Oxidants and Antioxidants|Antioxidants, Enzymatic. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 258–266. [Google Scholar]
- Ryan, K.C.; Johnson, O.E.; Cabelli, D.E.; Brunold, T.C.; Maroney, M.J. Nickel superoxide dismutase: Structural and functional roles of Cys2 and Cys6. J. Biol. Inorg. Chem. 2010, 15, 795–807. [Google Scholar] [CrossRef]
- Perry, J.J.P.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophs. Acta 2010, 1804, 245–262. [Google Scholar] [CrossRef]
- Mendoza, E.R.; Stelmastchuk, L.B.F.; Ferreira, J.R.S.; Garratt, R.C. Crystal Structure Analysis of Superoxide Dismutase from Trichoderma reesei (Protein Databank PDB Code 6DQY). Available online: https://www.rcsb.org/structure/6DQY (accessed on 3 April 2024).
- Lah, M.S.; Dixon, M.M.; Pattridge, K.A.; Stallings, W.C.; Fee, J.A.; Ludwig, M.L. Structure-function in Escherichia coli iron superoxide dismutase: Comparisons with the manganese enzyme from Thermus thermophilus. Biochemistry 1995, 34, 1646–1660. [Google Scholar] [CrossRef]
- Ludwig, M.L.; Metzger, A.L.; Pattridge, K.A.; Stallings, W.C. Manganese superoxide dismutase from Thermus thermophilus: A structural model refined at 1.8 Å resolution. J. Molec. Biol. 1991, 219, 335–358. [Google Scholar] [CrossRef]
- Tierney, D.L.; Fee, J.A.; Ludwig, M.L.; Penner-Hahn, J.E. X-ray Absorption Spectroscopy of the Iron Site in Escherichia coli Fe(III) Superoxide Dismutase. Biochemistry 1995, 34, 1661–1668. [Google Scholar] [CrossRef]
- Whittaker, M.M.; Whittaker, J.W. Low-Temperature Thermochromism Marks a Change in Coordination for the Metal Ion in Manganese Superoxide Dismutase. Biochemistry 1996, 35, 6762–6770. [Google Scholar] [CrossRef]
- Whittaker, M.M.; Whittaker, J.W. A “thermophilic shift” in ligand interactions for Thermus thermophilus manganese superoxide dismutase. J. Biol. Inorg. Chem. 1997, 2, 667–671. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Trickel, S.R.; Borgstahl, G.E.O. Substrate-analog binding aand electrostatic surfaces of human manganese superoxide dismutase. J. Struct. Biol. 2017, 199, 68–75. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Borgstahl, G.E.O. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Lutz, W.E.; Coates, L.; Weiss, K.L.; Borgstahl, G.E.O. Direct detection of coupled proton and electron transfers in human manganese superoxide dismutase. Nat. Commun. 2021, 12, 2079. [Google Scholar] [CrossRef]
- Abreu, I.A.; Cabelli, D.E. Superoxide dismutases—A review of the metal-associated mechanistic variations. Biochim. Biophs. Acta 2010, 1804, 263–274. [Google Scholar] [CrossRef]
- Srnec, M.; Aquilante, F.; Ryde, U.; Rulísek, L. Reaction mechanism of manganese superoxide dismutase studied by combined quantum and molecular mechanical calculations and multiconfigurational methods. J. Phys. Chem. B 2009, 113, 6074–6086. [Google Scholar] [CrossRef]
- Palma, F.R.; He, C.; Danes, J.M.; Paviani, V.; Coelho, D.R.; Gantner, B.N.; Bonini, M.G. Mitochondrial Superoxide Dismutase: What the Established, the Intriguing, and the Novel Reveal About a Key Cellular Redox Switch. Antioxid. Redox Signal. 2020, 32, 701–714. [Google Scholar] [CrossRef]
- Pravda, J. Hydrogen peroxide and disease: Towards a unified system of pathogenesis and therapeutics. Molec. Med. 2020, 26, 41. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Slobodnik, K.; Struble, L.R.; Lutz, W.E.; Coates, L.; Weiss, K.L.; Myles, D.A.A.; Kroll, T.; Borgstahl, G.E.O. Revealing the atomic and electronic mechanism of human manganese superoxide dismutase product inhibition. Nat. Commun. 2024, 15, 5973. [Google Scholar] [CrossRef]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide—Production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015, 13, 39. [Google Scholar] [CrossRef]
- Brudvig, G.W. Structure and Function of Manganese in Photosystem II. In Mechanistic Bioinorganic Chemistry; Thorp, H.H., Pecoraro, V.L., Eds.; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1996; Volume 246, pp. 249–263. [Google Scholar]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese Deficiency in Plants: The Impact on Photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef]
- Narzi, D.; Bovi, D.; De Gaetano, P.; Guidoni, L. Dynamics of the Special Pair of Chlorophylls of Photosystem II. J. Am. Chem. Soc. 2016, 138, 257–264. [Google Scholar] [CrossRef]
- Lokstein, H.; Renger, G.; Götze, J.P. Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions. Molecules 2021, 26, 3378. [Google Scholar] [CrossRef]
- Oliver, N.; Avramov, A.P.; Nürnberg, D.J.; Dau, H.; Burnap, R.L. From manganese oxidation to water oxidation: Assembly and evolution of the water-splitting complex in photosystem II. Photosynth. Res. 2022, 152, 107–133. [Google Scholar] [CrossRef]
- John, C.; Pedraza-González, L.; Betti, E.; Cupellini, L.; Mennucci, B. A Computational Approach to Modeling Excitation Energy Transfer and Quenching in Light-Harvesting Complexes. J. Phys. Chem. B 2024, in press. [Google Scholar] [CrossRef]
- Zhang, M.; Ming, Y.; Wang, H.-B.; Jin, H.-L. Strategies for adaptation to high light in plants. aBIOTECH 2024, 5, 381–393. [Google Scholar] [CrossRef]
- Didaran, F.; Kordrostami, M.; Ghasemi-Soloklui, A.A.; Pashkovskiy, P.; Kreslavski, V.; Kuznetsov, V.; Allakhverdiev, S.I. The mechanisms of photoinhibition and repair in plants under high light conditions and interplay with abiotic stressors. J. Photochem. Photobiol. B 2024, 259, 113004. [Google Scholar] [CrossRef]
- Marulanda Valencia, W.; Pandit, A. Photosystem II Subunit S (PsbS): A Nano Regulator of Plant Photosynthesis. J. Molec. Biol. 2024, 436, 168407. [Google Scholar] [CrossRef]
- Semin, B.; Loktyushkin, A.; Lovyagina, E. Current analysis of cations substitution in the oxygen-evolving complex of photosystem II. Biophys. Rev. 2024, 16, 237–247. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Bryant, D.A.; Macalady, J.L. The role of biology in planetary evolution: Cyanobacterial primary production in low-oxygen Proterozoic oceans. Environ. Microbiol. 2016, 18, 325–340. [Google Scholar] [CrossRef]
- Rexroth, S.; Nowaczyk, M.M.; Rögner, M. Cyanobacterial Photosynthesis: The Light Reactions. In Modern Topics in the Phototrophic Prokaryotes: Metabolism, Bioenergetics, and Omics; Hallenbeck, P.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 163–191. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Mandal, M.; Saito, K.; Ishikita, H. Requirement of Chloride for the Downhill Electron Transfer Pathway from the Water-Splitting Center in Natural Photosynthesis. J. Phys. Chem. B 2022, 126, 123–131. [Google Scholar] [CrossRef]
- Rivalta, I.; Amin, M.; Luber, S.; Vassiliev, S.; Pokhrel, R.; Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N.; Bruce, D.; et al. Structural–Functional Role of Chloride in Photosystem II. Biochemistry 2011, 50, 6312–6315. [Google Scholar] [CrossRef]
- Allgöwer, F.; Pöverlein, M.C.; Rutherford, A.W.; Kaila, V.R.I. Mechanism of proton release during water oxidation in Photosystem II. Proc. Natl. Acad. Sci. USA 2024, 121, e2413396121. [Google Scholar] [CrossRef]
- Hofbauer, W.; Zouni, A.; Bittl, R.; Kern, J.; Orth, P.; Lendzian, F.; Fromme, P.; Witt, H.T.; Lubitz, W. Photosystem II single crystals studied by EPR spectroscopy at 94 GHz: The tyrosine radical Y(D). Proc. Natl. Acad. Sci. USA 2001, 98, 6623–6628. [Google Scholar] [CrossRef]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the Photosynthetic Oxygen-Evolving Center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 2015, 517, 99–103. [Google Scholar] [CrossRef]
- Fatima, S.; Olshansky, L. Conformational control over proton-coupled electron transfer in metalloenzymes. Nat. Rev. Chem. 2024, 8, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Flesher, D.A.; Liu, J.; Wang, J.; Gisriel, C.J.; Yang, K.R.; Batista, V.S.; Debus, R.J.; Brudvig, G.W. Mutation-induced shift of the photosystem II active site reveals insight into conserved water channels. J. Biol. Chem. 2024, 300, 107475. [Google Scholar] [CrossRef]
- Kok, B.; Forbush, B.; McGloin, M. Cooperation of charges in photosynthetic O2 evolution—I. A linear four step mechanism. Photochem. Photobiol. 1970, 11, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Bury, G.; Pushkar, Y. Insights from Ca2+→Sr2+ substitution on the mechanism of O-O bond formation in photosystem II. Photosynth. Res. 2024, 162, 331–351. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Miyagawa, K.; Shoji, M.; Kawakami, T.; Isobe, H.; Yamanaka, S.; Nakajima, T. Theoretical elucidation of the structure, bonding, and reactivity of the CaMn4Ox clusters in the whole Kok cycle for water oxidation embedded in the oxygen evolving center of photosystem II. New molecular and quantum insights into the mechanism of the O–O bond formation. Photosynth. Res. 2024, 162, 291–330. [Google Scholar]
- Mattila, H.; Mishra, S.; Tyystjärvi, T.; Tyystjärvi, E. Singlet oxygen production by photosystem II is caused by misses of the oxygen evolving complex. New Phytol. 2023, 237, 113–125. [Google Scholar] [CrossRef]
- Vinyard, D.J.; Khan, S.; Brudvig, G.W. Photosynthetic water oxidation: Binding and activation of substrate waters for O–O bond formation. Faraday Discuss. 2015, 185, 37–50. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, L. Why nature chose the Mn4CaO5 cluster as water-splitting catalyst in photosystem II: A new hypothesis for the mechanism of O–O bond formation. Dalton Trans. 2018, 47, 14381–14387. [Google Scholar] [CrossRef] [PubMed]
- Yehia, S.; Wang, J.; Brudvig, G.W.; Gunner, M.R.; Brooks, B.R.; Amin, M. An analysis of the structural changes of the oxygen evolving complex of Photosystem II in the S1 and S3 states revealed by serial femtosecond crystallography. Biochim. Biophys. Acta 2025, 1866, 149531. [Google Scholar] [CrossRef]
- Vinyard, D.J.; Ananyev, G.M.; Charles Dismukes, G. Photosystem II: The Reaction Center of Oxygenic Photosynthesis*. Ann. Rev. Biochem. 2013, 82, 577–606. [Google Scholar] [CrossRef]
- Pace, R.J.; Jin, L.; Stranger, R. What spectroscopy reveals concerning the Mn oxidation levels in the oxygen evolving complex of photosystem II: X-ray to near infra-red. Dalton Trans. 2012, 41, 11145–11160. [Google Scholar] [CrossRef]
- Wang, J. Cryo-EM meets crystallography: A model-independent view of the heteronuclear Mn4Ca cluster structure of photosystem II. Proc. Natl. Acad. Sci. USA 2025, 122, e2423012122. [Google Scholar] [CrossRef] [PubMed]
- Cheah, M.H.; Zhang, M.; Shevela, D.; Mamedov, F.; Zouni, A.; Messinger, J. Assessment of the manganese cluster’s oxidation state via photoactivation of photosystem II microcrystals. Proc. Natl. Acad. Sci. USA 2020, 117, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Krewald, V.; Retegan, M.; Cox, N.; Messinger, J.; Lubitz, W.; DeBeer, S.; Neese, F.; Pantazis, D.A. Metal oxidation states in biological water splitting. Chem. Sci. 2015, 6, 1676–1695. [Google Scholar] [CrossRef]
- Mermigki, M.A.; Drosou, M.; Pantazis, D.A. On the nature of high-spin forms in the S(2) state of the oxygen-evolving complex. Chem. Sci. 2025, 16, 4023–4047. [Google Scholar] [CrossRef]
- Li, H.; Nakajima, Y.; Nango, E.; Owada, S.; Yamada, D.; Hashimoto, K.; Luo, F.; Tanaka, R.; Akita, F.; Kato, K.; et al. Oxygen-evolving photosystem II structures during S1–S2–S3 transitions. Nature 2024, 626, 670–677. [Google Scholar] [CrossRef]
- Guo, Y.; Kloo, L.; Sun, L. Quantum Chemical Understanding of the O2 Release Process from Nature’s Water Splitting Cofactor. Angew. Chem. Int. Ed. 2025, 64, e202421383. [Google Scholar] [CrossRef]
- Armstrong, F.A. Why did Nature choose manganese to make oxygen? Phil. Trans. Roy. Soc. B. Biol. Sci. 2008, 363, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Ruskoski, T.B.; Boal, A.K. The periodic table of ribonucleotide reductases. J. Biol. Chem. 2021, 297, 101137. [Google Scholar] [CrossRef]
- Sjöberg, B.M. Ribonucleotide Reductase. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Hofer, A.; Crona, M.; Logan, D.T.; Sjöberg, B.-M. DNA building blocks: Keeping control of manufacture. Crit. Rev. Biochem. Molec. Biol. 2012, 47, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Torrents, E. Ribonucleotide reductases: Essential enzymes for bacterial life. Front. Cell Infect. Micro 2014, 4, 52. [Google Scholar] [CrossRef]
- Grāve, K.; Griese, J.J.; Berggren, G.; Bennett, M.D.; Högbom, M. The Bacillus anthracis class Ib ribonucleotide reductase subunit NrdF intrinsically selects manganese over iron. J. Biol. Inorg. Chem. 2020, 25, 571–582. [Google Scholar] [CrossRef]
- Marques, H.M. The inorganic chemistry of the cobalt corrinoids—An update. J. Inorg. Biochem. 2023, 242, 112154. [Google Scholar]
- Cotruvo, J.A., Jr.; Stich, T.A.; Britt, R.D.; Stubbe, J. Mechanism of Assembly of the Dimanganese-Tyrosyl Radical Cofactor of Class Ib Ribonucleotide Reductase: Enzymatic Generation of Superoxide Is Required for Tyrosine Oxidation via a Mn(III)Mn(IV) Intermediate. J. Am. Chem. Soc. 2013, 135, 4027–4039. [Google Scholar] [CrossRef]
- Yadav, L.R.; Sharma, V.; Shanmugam, M.; Mande, S.C. Structural insights into the initiation of free radical formation in the Class Ib ribonucleotide reductases in Mycobacteria. Curr. Res. Struct. Biol. 2024, 8, 100157. [Google Scholar] [CrossRef]
- John, J.; Aurelius, O.; Srinivas, V.; Saura, P.; Kim, I.-S.; Bhowmick, A.; Simon, P.S.; Dasgupta, M.; Pham, C.; Gul, S.; et al. Redox-controlled reorganization and flavin strain within the ribonucleotide reductase R2b–NrdI complex monitored by serial femtosecond crystallography. eLife 2022, 11, e79226. [Google Scholar] [CrossRef]
- Doyle, L.M.; Bienenmann, R.L.M.; Gericke, R.; Xu, S.; Farquhar, E.R.; Que, L.; McDonald, A.R. Preparation and characterization of MnIIMnIII complexes with relevance to class Ib ribonucleotide reductases. J. Inorg. Biochem. 2024, 257, 112583. [Google Scholar] [CrossRef]
- Doyle, L.; Magherusan, A.; Xu, S.; Murphy, K.; Farquhar, E.R.; Molton, F.; Duboc, C.; Que, L., Jr.; McDonald, A.R. Class Ib Ribonucleotide Reductases: Activation of a Peroxido-MnIIMnIII to Generate a Reactive Oxo-MnIIIMnIV Oxidant. Inorg. Chem. 2024, 63, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Högbom, M.; Stenmark, P.; Voevodskaya, N.; McClarty, G.; Gräslund, A.; Nordlund, P. The Radical Site in Chlamydial Ribonucleotide Reductase Defines a New R2 Subclass. Science 2004, 305, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yun, D.; Saleh, L.; Barr, E.W.; Xing, G.; Hoffart, L.M.; Maslak, M.-A.; Krebs, C.; Bollinger, J.M. A Manganese(IV)/Iron(III) Cofactor in Chlamydia trachomatis Ribonucleotide Reductase. Science 2007, 316, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Kisgeropoulos, E.C.; Gan, Y.J.; Greer, S.M.; Hazel, J.M.; Shafaat, H.S. Pulsed Multifrequency Electron Paramagnetic Resonance Spectroscopy Reveals Key Branch Points for One- vs Two-Electron Reactivity in Mn/Fe Proteins. J. Am. Chem. Soc. 2022, 144, 11991–12006. [Google Scholar] [CrossRef]
- Rozman Grinberg, I.; Berglund, S.; Hasan, M.; Lundin, D.; Ho, F.M.; Magnuson, A.; Logan, D.T.; Sjöberg, B.-M.; Berggren, G. Class Id ribonucleotide reductase utilizes a Mn2(IV,III) cofactor and undergoes large conformational changes on metal loading. J. Biol. Inorg. Chem. 2019, 24, 863–877. [Google Scholar] [CrossRef]
- Sánchez-Ruiz, M.I.; Santillana, E.; Linde, D.; Romero, A.; Martínez, A.T.; Ruiz-Dueñas, F.J. Structure-function characterization of two enzymes from novel subfamilies of manganese peroxidases secreted by the lignocellulose-degrading Agaricales fungi Agrocybe pediades and Cyathus striatus. Biotech. Biofuels Bioprod. 2024, 17, 74. [Google Scholar] [CrossRef]
- Bilal, M.; Zdarta, J.; Jesionowski, T.; Iqbal, H.M.N. Manganese peroxidases as robust biocatalytic tool—An overview of sources, immobilization, and biotechnological applications. Int. J. Biol. Macromolec. 2023, 234, 123531. [Google Scholar] [CrossRef]
- Jofré-Fernández, I.; Matus-Baeza, F.; Merino-Guzmán, C. White-rot fungi scavenge reactive oxygen species, which drives pH-dependent exo-enzymatic mechanisms and promotes CO2 efflux. Front. Microbiol. 2023, 14, 1148750. [Google Scholar] [CrossRef]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase enzymes as green catalysts for bioremediation and biotechnological applications: A review. Sci. Total Environ. 2022, 806, 150500. [Google Scholar] [CrossRef]
- Kumar, V.; Pallavi, P.; Sen, S.K.; Raut, S. Harnessing the potential of white rot fungi and ligninolytic enzymes for efficient textile dye degradation: A comprehensive review. Water Environ. Res. 2024, 96, e10959. [Google Scholar] [CrossRef]
- Liu, X.; Ding, S.; Gao, F.; Wang, Y.; Taherzadeh, M.J.; Wang, Y.; Qin, X.; Wang, X.; Luo, H.; Yao, B.; et al. Exploring the cellulolytic and hemicellulolytic activities of manganese peroxidase for lignocellulose deconstruction. Biotech. Biofuels Bioprod. 2023, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Fan, L.; Yang, Q.; Ning, M.; Zhang, B.; Ren, X. Characterization and mechanism of simultaneous degradation of aflatoxin B1 and zearalenone by an edible fungus of Agrocybe cylindracea GC-Ac2. Front. Microbiol. 2024, 15, 1292824. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, M.; Gold, M.H.; Poulos, T.L. Ultrahigh (0.93Å) resolution structure of manganese peroxidase from Phanerochaete chrysosporium: Implications for the catalytic mechanism. J. Inorg. Biochem. 2010, 104, 683–690. [Google Scholar] [CrossRef]
- Ding, Y.; Cui, K.; Guo, Z.; Cui, M.; Chen, Y. Manganese peroxidase mediated oxidation of sulfamethoxazole: Integrating the computational analysis to reveal the reaction kinetics, mechanistic insights, and oxidation pathway. J. Hazard. Mater. 2021, 415, 125719. [Google Scholar] [CrossRef]
- Hoang, H.G.; Tran, H.T.; Nguyen, M.K.; Nguyen, N.S.H.; Thuy, B.T.P. Investigating the polyethylene degradation mechanism using docking and molecular dynamics simulations. Environ. Sci. Pollut. Res. Int. 2024, 31, 64857–64869. [Google Scholar] [CrossRef]
- Wu, F.; Guo, Z.; Cui, K.; Dong, D.; Yang, X.; Li, J.; Wu, Z.; Li, L.; Dai, Y.; Pan, T. Insights into characteristics of white rot fungus during environmental plastics adhesion and degradation mechanism of plastics. J. Hazard Mater. 2023, 448, 130878. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.; Zhang, L.; Wu, X.; Deng, S.; Yuan, X. Biodegradation of anionic polyacrylamide by manganese peroxidase: Docking, virtual mutation based on affinity, QM/MM calculation and molecular dynamics simulation. Bioprocess Biosyst. Eng. 2022, 45, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Huang, W.; Huang, W.; Jia, R. A mutant R70V/E166A of short manganese peroxidase showing Mn2+-independent dye decolorization. Appl. Microbiol. Biotechnol. 2023, 107, 2303–2319. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar]
- Oliw, E.H. Diversity of the manganese lipoxygenase gene family—A mini-review. Fungal Genet. Biol. 2022, 163, 103746. [Google Scholar] [CrossRef]
- Oliw, E.H. Iron and manganese lipoxygenases of plant pathogenic fungi and their role in biosynthesis of jasmonates. Arch. Biochem. Biophys. 2022, 722, 109169. [Google Scholar] [CrossRef] [PubMed]
- Prigge, S.T.; Boyington, J.C.; Faig, M.; Doctor, K.S.; Gaffney, B.J.; Amzel, L.M. Structure and mechanism of lipoxygenases. Biochimie 1997, 79, 629–636. [Google Scholar] [CrossRef]
- Opalade, A.A.; Grotemeyer, E.N.; Jackson, T.A. Mimicking Elementary Reactions of Manganese Lipoxygenase Using Mn-hydroxo and Mn-alkylperoxo Complexes. Molecules 2021, 26, 7151. [Google Scholar] [CrossRef] [PubMed]
- Wennman, A.; Oliw, E.H.; Karkehabadi, S.; Chen, Y. Crystal Structure of Manganese Lipoxygenase of the Rice Blast Fungus Magnaporthe oryzae. J. Biol. Chem. 2016, 291, 8130–8139. [Google Scholar] [CrossRef] [PubMed]
- Rini, J.M.; Moremen, K.W.; Davis, B.G.; Esko, J.D. Glycosyltransferases and Glycan-Processing Enzymes. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Habor, NY, USA, 2022. [Google Scholar]
- Breton, C.; Šnajdrová, L.; Jeanneau, C.; Koča, J.; Imberty, A. Structures and mechanisms of glycosyltransferases. Glycobiology 2005, 16, 29R–37R. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Boeggeman, E.; Ramasamy, V.; Qasba, P.K. Structure and catalytic cycle of β-1,4-galactosyltransferase. Curr. Opin. Struct. Biol. 2004, 14, 593–600. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Ramasamy, V.; Qasba, P.K. Structural snapshots of beta-1,4-galactosyltransferase-I along the kinetic pathway. J. Mol. Biol. 2006, 357, 1619–1633. [Google Scholar] [CrossRef]
- Kóňa, J. How inverting β-1,4-galactosyltransferase-1 can quench a high charge of the by-product UDP3− in catalysis: A QM/MM study of enzymatic reaction with native and UDP-5′ thio galactose substrates. Org. Biomol. Chem. 2020, 18, 7585–7596. [Google Scholar] [CrossRef]
- Christianson, D.W. Arginase: Structure, Mechanism, and Physiological Role in Male and Female Sexual Arousal. Acc. Chem. Res. 2005, 38, 191–201. [Google Scholar] [CrossRef]
- Ash, D.E.; Cox, J.D.; Christianson, D.W. Arginase: A binuclear manganese metalloenzyme. Met. Ions Biol. Syst. 2000, 37, 407–428. [Google Scholar]
- Li, Z.; Wang, L.; Ren, Y.; Huang, Y.; Liu, W.; Lv, Z.; Qian, L.; Yu, Y.; Xiong, Y. Arginase: Shedding light on the mechanisms and opportunities in cardiovascular diseases. Cell Death Discov. 2022, 8, 413. [Google Scholar] [CrossRef]
- K, D.; Kuramitsu, S.; Yokoyama, S.; Thirumananseri, K.; Ponnuraj, K. Crystal structure analysis and molecular dynamics simulations of arginase from Thermus thermophilus. J. Biomolec. Struct. Dynam. 2023, 41, 6811–6821. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.D.; Cama, E.; Colleluori, D.M.; Pethe, S.; Boucher, J.-L.; Mansuy, D.; Ash, D.E.; Christianson, D.W. Mechanistic and Metabolic Inferences from the Binding of Substrate Analogues and Products to Arginase. Biochemistry 2001, 40, 2689–2701. [Google Scholar] [CrossRef] [PubMed]
- Kanyo, Z.F.; Scolnick, L.R.; Ash, D.E.; Christianson, D.W. Structure of a unique binuclear manganese cluster in arginase. Nature 1996, 383, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Lindley, P.F. Iron in biology: A structural viewpoint. Rep. Prog. Phys. 1996, 59, 867–933. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. (Eds.) The Porphyrin Handbook; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Walker, F.A.; Simonis, U. Iron Porphyrin Chemistry. In Encyclopedia of Inorganic Chemistry; King, R.B., Crabtree, R.H., Lukehart, C.M., Atwood, D.A., Scott, R.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins Containing Cytochrome, Iron–Sulfur, or Copper Redox Centers. Chem. Rev. 2014, 114, 4366–4469. [Google Scholar]
- Crichton, R. Iron Metabolism—From Molecular Mechanisms to Clinical Consequences, 4th, ed.; John Wiley & Sons: Chichester, UK, 2016. [Google Scholar]
- Turilli-Ghisolfi, E.S.; Lualdi, M.; Fasano, M. Ligand-Based Regulation of Dynamics and Reactivity of Hemoproteins. Biomolecules 2023, 13, 683. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reactions with Ligands; North-Holland: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Perutz, M.F.; Fermi, G.; Luisi, B.; Shaanan, B.; Liddington, R.C. Stereochemistry of cooperative mechanisms in hemoglobin. Acc. Chem. Res. 1987, 20, 309–321. [Google Scholar] [CrossRef]
- Collman, J.P.; Fu, L. Synthetic Models for Hemoglobin and Myoglobin. Acc. Chem. Res. 1999, 32, 455–463. [Google Scholar] [CrossRef]
- Olson, J.S. Kinetic mechanisms for O2 binding to myoglobins and hemoglobins. Mol. Asp. Med. 2022, 84, 101024. [Google Scholar] [CrossRef]
- Sil, R.; Chakraborti, A.S. Major heme proteins hemoglobin and myoglobin with respect to their roles in oxidative stress—A brief review. Front. Chem. 2025, 13, 1543455. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.W.; Karbelkar, A.A.; El-Naggar, M.Y. Nature’s conductors: What can microbial multi-heme cytochromes teach us about electron transport and biological energy conversion? Curr. Opin. Chem. Biol. 2018, 47, 7–17. [Google Scholar] [CrossRef]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef]
- Davidson, V.L. What Controls the Rates of Interprotein Electron-Transfer Reactions? Acc. Chem. Res. 2000, 33, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Crowley, P.B.; Ubbink, M. Close Encounters of the Transient Kind: Protein Interactions in the Photosynthetic Redox Chain Investigated by NMR Spectroscopy. Acc. Chem. Res. 2003, 36, 723–730. [Google Scholar] [CrossRef]
- Dell’Acqua, S.; Pauleta, S.R.; Monzani, E.; Pereira, A.S.; Casella, L.; Moura, J.J.G.; Moura, I. Electron Transfer Complex between Nitrous Oxide Reductase and Cytochrome c552 from Pseudomonas nautica: Kinetic, Nuclear Magnetic Resonance, and Docking Studies. Biochemistry 2008, 47, 10852–10862. [Google Scholar] [CrossRef] [PubMed]
- Hille, R. The Mononuclear Molybdenum Enzymes. Chem. Rev. 1996, 96, 2757–2816. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Bonagura, C.A.; Tilley, G.J.; McEvoy, J.P.; Jung, Y.-S.; Armstrong, F.A.; Stout, C.D.; Burgess, B.K. Crystal structures of ferredoxin variants exhibiting large changes in [Fe–S] reduction potential. Nat. Struct. Biol. 2002, 9, 188–192. [Google Scholar] [CrossRef]
- Azzi, A.; Müller, M. Cytochrome c oxidases: Polypeptide composition, role of subunits, and location of active metal centers. Arch. Biochem. Biophys. 1990, 280, 242–251. [Google Scholar] [CrossRef]
- Dawson, J.H. Probing structure-function relations in heme-containing oxygenases and peroxidases. Science 1988, 240, 433–439. [Google Scholar] [CrossRef]
- Esposti, M.D. On the evolution of cytochrome oxidases consuming oxygen. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148304. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Annalora, A.J.; Marcus, C.B.; Jefcoate, C.R.; Sorenson, C.M.; Sheibani, N. Cytochrome P450 1B1: A Key Regulator of Ocular Iron Homeostasis and Oxidative Stress. Cells 2022, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Smulevich, G. The functional role of the key residues in the active site of peroxidases. Biochem. Soc. Trans. 1995, 23, 240–244. [Google Scholar] [CrossRef]
- Isaac, I.S.; Dawson, J.H. Haem iron-containing peroxidases. Essays Biochem. 1999, 34, 51–69. [Google Scholar]
- Battistuzzi, G.; Bellei, M.; Bortolotti, C.A.; Sola, M. Redox properties of heme peroxidases. Arch. Biochem. Biophys. 2010, 500, 21–36. [Google Scholar] [CrossRef]
- Singh, P.K.; Iqbal, N.; Sirohi, H.V.; Bairagya, H.R.; Kaur, P.; Sharma, S.; Singh, T.P. Structural basis of activation of mammalian heme peroxidases. Prog. Biophys. Mol. Biol. 2018, 133, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, A.; Dounce, A.L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol. Rev. 1970, 50, 319–375. [Google Scholar] [CrossRef]
- Bravo, J.; Fita, I.; Gouet, P.; Jouve, H.M.; Melik-Adamyan, W.-L.; Murshudov, G.N. Structure of catalases. Cold Spring Harbor Mongr. Ser. 1997, 34, 407–445. [Google Scholar]
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete identity. Protein Cell 2010, 1, 888–897. [Google Scholar] [CrossRef]
- James, M.O.; Boyle, S.M. Cytochromes P450 in crustacea. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 121, 157–172. [Google Scholar] [CrossRef]
- Vanden Bossche, H.; Koymans, L. Cytochromes P450 in fungi. Mycoses 1998, 41 (Suppl. 1), 32–38. [Google Scholar] [PubMed]
- Pikuleva, I.A.; Waterman, M.R. Cytochromes p450: Roles in diseases. J. Biol. Chem. 2013, 288, 17091–17098. [Google Scholar] [CrossRef]
- Distéfano, A.M.; Setzes, N.; Cascallares, M.; Fiol, D.F.; Zabaleta, E.; Pagnussat, G.C. Roles of cytochromes P450 in plant reproductive development. Int. J. Dev. Biol. 2021, 65, 187–194. [Google Scholar] [CrossRef]
- Mauzerall, D. Porphyrins, Chlorophyll, and Photosynthesis. In Photosynthesis I: Photosynthetic Electron Transport and Photophosphorylation; Trebst, A., Avron, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 117–124. [Google Scholar]
- Boxer, S.G. Model reactions in photosynthesis. Biochim. Biophys. Acta 1983, 726, 265–292. [Google Scholar] [CrossRef]
- Chen, M. Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu. Rev. Biochem. 2014, 83, 317–340. [Google Scholar] [CrossRef]
- Yamazaki, T.; Oyanagi, H.; Fujiwara, T.; Fukumori, Y. Nitrite reductase from the magnetotactic bacterium Magnetospirillum magnetotacticum; A novel cytochrome cd1 with Fe(II):nitrite oxidoreductase activity. Eur. J. Biochem. 1995, 233, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, W.; Liu, D.; Li, Y.; Peng, K.; Lorimer, G.H.; Wang, J. Redox and spectroscopic properties of mammalian nitrite reductase-like hemoproteins. J. Inorg. Biochem. 2022, 237, 111982. [Google Scholar] [CrossRef]
- Murphy, M.J.; Siegel, L.M.; Tove, S.R.; Kamin, H. Siroheme: A New Prosthetic Group Participating in Six-Electron Reduction Reactions Catalyzed by Both Sulfite and Nitrite Reductases. Proc. Natl. Acad. Sci. USA 1974, 71, 612–616. [Google Scholar] [CrossRef]
- Crane, B.R.; Siegel, L.M.; Getzoff, E.D. Structures of the Siroheme- and Fe4S4-Containing Active Center of Sulfite Reductase in Different States of Oxidation: Heme Activation via Reduction-Gated Exogenous Ligand Exchange. Biochemistry 1997, 36, 12101–12119. [Google Scholar] [CrossRef]
- Kappler, U.; Enemark, J.H. Sulfite-oxidizing enzymes. J. Biol. Inorg. Chem. 2015, 20, 253–264. [Google Scholar] [CrossRef]
- Ems, T.; St Lucia, K.; Huecker, M.R. Biochemistry, Iron Absorption. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wang, H.; Shi, H.; Rajan, M.; Canarie, E.R.; Hong, S.; Simoneschi, D.; Pagano, M.; Bush, M.F.; Stoll, S.; Leibold, E.A.; et al. FBXL5 Regulates IRP2 Stability in Iron Homeostasis via an Oxygen-Responsive [2Fe2S] Cluster. Molec. Cell 2020, 78, 31–41.e35. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, L.; Girelli, D.; Vinchi, F.; Cozzolino, M.; Elliott, S.; Mark, P.B.; Valenti, L.; Qian, C.; Guo, Q.; Qian, Z.M.; et al. Iron biology. Nephrol. Dial. Transplant. 2024, 39, 1404–1415. [Google Scholar] [CrossRef]
- Lincoln, S.F. Mechanistic Studies of Metal Aqua Ions: A Semi-Historical Perspective. Helv. Chim. Acta 2005, 88, 523–545. [Google Scholar] [CrossRef]
- Senge, M. Highly Substituted Porphyrins. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2000; Volume 1, pp. 239–347. [Google Scholar]
- Frey, P.A.; Reed, G.H. The Ubiquity of Iron. ACS Chem. Biol. 2012, 7, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Cardona, T. On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol. 2020, 225, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Warke, M.R.; Di Rocco, T.; Zerkle, A.L.; Lepland, A.; Prave, A.R.; Martin, A.P.; Ueno, Y.; Condon, D.J.; Claire, M.W. The Great Oxidation Event preceded a Paleoproterozoic “snowball Earth”. Proc. Natl. Acad. Sci. USA 2020, 117, 13314–13320. [Google Scholar] [CrossRef]
- Kraus, A.; Spät, P.; Timm, S.; Wilson, A.; Schumann, R.; Hagemann, M.; Maček, B.; Hess, W.R. Protein NirP1 regulates nitrite reductase and nitrite excretion in cyanobacteria. Nat. Commun. 2024, 15, 1911. [Google Scholar] [CrossRef]
- Schirrmeister, B.E.; Gugger, M.; Donoghue, P.C.J. Cyanobacteria and the Great Oxidation Event: Evidence from genes and fossils. Palaeontology 2015, 58, 769–785. [Google Scholar] [CrossRef]
- Sweetman, A.K.; Smith, A.J.; de Jonge, D.S.W.; Hahn, T.; Schroedl, P.; Silverstein, M.; Andrade, C.; Edwards, R.L.; Lough, A.J.M.; Woulds, C.; et al. Evidence of dark oxygen production at the abyssal seafloor. Nat. Geosci. 2024, 17, 737–739. [Google Scholar] [CrossRef]
- Schwertmann, U. Solubility and dissolution of iron oxides. Plant Soil 1991, 130, 1–25. [Google Scholar] [CrossRef]
- Hannauer, M.; Barda, Y.; Mislin, G.L.A.; Shanzer, A.; Schalk, I.J. The Ferrichrome Uptake Pathway in Pseudomonas aeruginosa Involves an Iron Release Mechanism with Acylation of the Siderophore and Recycling of the Modified Desferrichrome. J. Bacteriol. 2010, 192, 1212–1220. [Google Scholar] [CrossRef]
- Llamas, M.A.; Sparrius, M.; Kloet, R.; Jiménez, C.R.; Vandenbroucke-Grauls, C.; Bitter, W. The Heterologous Siderophores Ferrioxamine B and Ferrichrome Activate Signaling Pathways in Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Saito, H. Metabolism of Iron Stores. Nagoya J. Med. Sci. 2014, 76, 235–254. [Google Scholar] [PubMed]
- Wallace, D.F. The Regulation of Iron Absorption and Homeostasis. Clin. Biochem. Rev. 2016, 37, 51–62. [Google Scholar]
- Vogt, A.-C.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef]
- Ben Zichri-David, S.; Shkuri, L.; Ast, T. Pulling back the mitochondria’s iron curtain. NPJ Metab. Health Dis. 2025, 3, 6. [Google Scholar] [CrossRef]
- Berndt, C.; Alborzinia, H.; Amen, V.S.; Ayton, S.; Barayeu, U.; Bartelt, A.; Bayir, H.; Bebber, C.M.; Birsoy, K.; Böttcher, J.P.; et al. Ferroptosis in health and disease. Redox Biol. 2024, 75, 103211. [Google Scholar] [CrossRef]
- Glover, H.L.; Schreiner, A.; Dewson, G.; Tait, S.W.G. Mitochondria and cell death. Nat. Cell Biol. 2024, 26, 1434–1446. [Google Scholar] [CrossRef]
- Kraiter, D.C.; Zak, O.; Aisen, P.; Crumbliss, A.L. A Determination of the Reduction Potentials for Diferric and C- and N-Lobe Monoferric Transferrins at Endosomal pH (5.8). Inorg. Chem. 1998, 37, 964–968. [Google Scholar] [CrossRef]
- Dhungana, S.; Taboy, C.H.; Zak, O.; Larvie, M.; Crumbliss, A.L.; Aisen, P. Redox Properties of Human Transferrin Bound to Its Receptor. Biochemistry 2004, 43, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, T.; McNally, J.; Han, T.-H.-L.; Ha-Duong, N.-T.; El-Hage-Chahine, J.-M.; Bou-Abdallah, F. Direct thermodynamic and kinetic measurements of Fe2+ and Zn2+ binding to human serum transferrin. J. Inorg. Biochem. 2014, 136, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Reedy, C.J.; Gibney, B.R. Heme protein assemblies. Chem. Rev. 2004, 104, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Reddi, A.R.; Wang, Z.; Khodaverdian, B.; Hegg, E.L.; Gibney, B.R. Evaluating the Roles of the Heme a Side Chains in Cytochrome c Oxidase Using Designed Heme Proteins. Biochemistry 2006, 45, 12530–12538. [Google Scholar] [CrossRef]
- Perutz, M.F. Regulation of oxygen affinity of hemoglobin: Influence of structure of the globin on the heme iron. Ann. Rev. Biochem. 1979, 48, 327–386. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Ghatge, M.S.; Safo, M.K. Hemoglobin: Structure, Function and Allostery. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and other Body Fluid Proteins; Hoeger, U., Harris, J.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 345–382. [Google Scholar]
- Poole, D.C.; Musch, T.I.; Colburn, T.D. Oxygen flux from capillary to mitochondria: Integration of contemporary discoveries. Eur. J. Appl. Physiol. 2022, 122, 7–28. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Anastasiadi, A.T.; Tzounakas, V.L.; Nemkov, T.; Reisz, J.A.; Kriebardis, A.G.; Zimring, J.C.; Spitalnik, S.L.; Busch, M.P. Red Blood Cell Metabolism In Vivo and In Vitro. Metabolites 2023, 13, 793. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Topunov, A.F. Alternate and Additional Functions of Erythrocyte Hemoglobin. Biochemistry 2018, 83, 1575–1593. [Google Scholar] [CrossRef]
- Feher, J. Oxygen and Carbon Dioxide Transport. In Quantitative Human Physiology, 2nd ed.; Feher, J., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 656–664. [Google Scholar]
- Pauling, L.; Coryell, C.D. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. USA 1936, 22, 210–216. [Google Scholar] [CrossRef]
- Harrington, D.J.; Adachi, K.; Royer, W.E. The high resolution crystal structure of deoxyhemoglobin S11Edited by K. Nagai. J. Molec. Biol. 1997, 272, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Etti, S.; Shanmugam, G.; Karthe, P.; Gunasekaran, K. PDB 3A0G: Crystal Structure Analysis of Guinea Pig Oxyhemoglobin at 2.5 Angstroms Resolution. Available online: https://www.rcsb.org/structure/3A0G (accessed on 18 May 2024).
- Munro, O.Q.; Bradley, J.C.; Hancock, R.D.; Marques, H.M.; Marsicano, F.; Wade, P.W. Molecular Mechanics Study of the Ruffling of Metalloporphyrins. J. Am. Chem. Soc. 1992, 114, 7218–7230. [Google Scholar] [CrossRef]
- Olson, J.S. Lessons Learned from 50 Years of Hemoglobin Research: Unstirred and Cell-Free Layers, Electrostatics, Baseball Gloves, and Molten Globules. Antioxid. Redox Signal. 2020, 32, 228–246. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.J. Nature of the Iron–Oxygen Bond in Oxyhæmoglobin. Nature 1964, 202, 83–84. [Google Scholar] [CrossRef]
- Pauling, L. Nature of the Iron–Oxygen Bond in Oxyhæmoglobin. Nature 1964, 203, 182–183. [Google Scholar] [CrossRef]
- Weiss, J.J. Nature of the Iron–Oxygen Bond in Oxyhæmoglobin. Nature 1964, 203, 183. [Google Scholar] [CrossRef]
- McClure, D.S. Electronic Structure of Transition-Metal Complex Ions. Rad. Res. Suppl. 1960, 2, 218–242. [Google Scholar] [CrossRef]
- Goddard, W.A.; Olafson, B.D. Ozone Model for Bonding of an O2 to Heme in Oxyhemoglobin. Proc. Natl. Acad. Sci. USA 1975, 72, 2335–2339. [Google Scholar] [CrossRef]
- Barlow, C.H.; Maxwell, J.C.; Wallace, W.J.; Caughey, W.S. Elucidation of the mode of binding of oxygen to iron in oxyhemoglobin by infrared spectroscopy. Biochem. Biophys. Res. Commun. 1973, 55, 91–95. [Google Scholar] [CrossRef]
- Maxwell, J.C.; Caughey, W.S. Infrared evidence for similar metal-dioxygen bonding in iron and cobalt oxyhemoglobins. Biochem. Biophys. Res. Commun. 1974, 60, 1309–1314. [Google Scholar] [CrossRef]
- Wittenberg, J.B.; Wittenberg, B.A.; Peisach, J.; Blumberg, W.E. On the State of the Iron and the Nature of the Ligand in Oxyhemoglobin. Proc. Natl. Acad. Sci. USA 1970, 67, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Mildvan, A.S.; Yonetani, T.; Srivastava, T.S. EPR study of 17O nuclear hyperfine interaction in cobalt-oxyhemoglobin: Conformation of bound oxygen. Biochem. Biophys. Res. Commun. 1975, 67, 1005–1012. [Google Scholar] [CrossRef]
- Lang, G.; Marshali, W. Mössbauer effect in some haemoglobin compounds. Proc. Phys. Soc. 1966, 87, 3–34. [Google Scholar] [CrossRef]
- Van Doorslaer, S.; Schweiger, A.; Kräutler, B. A Continuous Wave and Pulse EPR and ENDOR Investigation of Oxygenated Co(II) Corrin Complexes. J. Phys. Chem. B 2001, 105, 7554–7563. [Google Scholar] [CrossRef]
- Schuth, N.; Mebs, S.; Huwald, D.; Wrzolek, P.; Schwalbe, M.; Hemschemeier, A.; Haumann, M. Effective intermediate-spin iron in O2-transporting heme proteins. Proc. Natl. Acad. Sci. USA 2017, 114, 8556–8561. [Google Scholar] [CrossRef]
- Sugawara, Y.; Shikama, K. Autoxidation of Native Oxymyoglobin. Eur. J. Biochem. 1980, 110, 241–246. [Google Scholar] [CrossRef]
- Eaton, W.A.; Henry, E.R.; Hofrichter, J.; Mozzarelli, A. Is cooperative oxygen binding by hemoglobin really understood? Nat. Struct. Biol. 1999, 6, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Fermi, G.; Perutz, M.F.; Shaanan, B.; Fourme, R. The crystal structure of human deoxyhaemoglobin at 1.74 Å resolution. J. Mol. Biol. 1984, 175, 159–174. [Google Scholar] [CrossRef]
- Yi, J.; Thomas, L.M.; Musayev, F.N.; Safo, M.K.; Richter-Addo, G.B. Crystallographic Trapping of Heme Loss Intermediates during the Nitrite-Induced Degradation of Human Hemoglobin. Biochemistry 2011, 50, 8323–8332. [Google Scholar] [CrossRef]
- Perutz, M.F. Stereochemistry of Cooperative Effects in Haemoglobin: Haem–Haem Interaction and the Problem of Allostery. Nature 1970, 228, 726–734. [Google Scholar] [CrossRef]
- Perutz, M.F.; Wilkinson, A.J.; Paoli, M.; Dodson, G.G. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct. 1998, 27, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Karplus, M. A mathematical model for structure-function relations in hemoglobin. J. Molec. Biol. 1972, 72, 163–197. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, C.; Mozzarelli, A.; Rossi, G.L.; Henry, E.R.; Eaton, W.A. Oxygen binding by single crystals of hemoglobin. Biochemistry 1993, 32, 2888–2906. [Google Scholar] [CrossRef]
- Shibayama, N.; Saigo, S. Fixation of the Quaternary Structures of Human Adult Haemoglobin by Encapsulation in Transparent Porous Silica Gels. J. Molec. Biol. 1995, 251, 203–209. [Google Scholar] [CrossRef]
- Yin, Z.; Li, D.; Guo, Q.; Wang, R.; Li, W. Effect of Hb conformational changes on oxygen transport physiology. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2024, 49, 467–475. [Google Scholar]
- D’Alessandro, A.; Xia, Y. Erythrocyte adaptive metabolic reprogramming under physiological and pathological hypoxia. Curr. Opin. Hematol. 2020, 27, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.-S.; Scheiner, S. Electronic structure and bonding in unligated and ligated porphyrins. J. Chem. Phys. 2002, 116, 3635–3645. [Google Scholar] [CrossRef]
- Jensen, K.P.; Ryde, U. How O2 Binds to Heme: Reasons for rapid binding an spin inversion. J. Biol. Chem. 2004, 279, 14561–14569. [Google Scholar] [CrossRef]
- Nakashima, H.; Hasegawa, J.-Y.; Nakatsuji, H. On the reversible O2 binding of the Fe–porphyrin complex. J. Comput. Chem. 2006, 27, 426–433. [Google Scholar] [CrossRef]
- Vacek, J.; Zatloukalova, M.; Kabelac, M. Redox biology and electrochemistry. Towards evaluation of bioactive electron donors and acceptors. Curr. Opin. Electrochem. 2022, 36, 101142. [Google Scholar] [CrossRef]
- Fukuzumi, S. (Ed.) Electron Transfer: Mechanisms and Applications; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2020; pp. i–vii. [Google Scholar]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef]
- Glass, J.B.; Elbon, C.E.; Williams, L.D. Something old, something new, something borrowed, something blue: The anaerobic microbial ancestry of aerobic respiration. Trends Microbiol. 2023, 31, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.L.; Lu, J.; Bai, Y. Mitochondrial Respiratory Complex I: Structure, Function and Implication in Human Diseases. Curr. Med. Chem. 2009, 16, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic type of Mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Berry, B.J.; Trewin, A.J.; Amitrano, A.M.; Kim, M.; Wojtovich, A.P. Use the Protonmotive Force: Mitochondrial Uncoupling and Reactive Oxygen Species. J. Mol. Biol. 2018, 430, 3873–3891. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. The protonmotive Q cycle: A general formulation. FEBS Lett. 1975, 59, 137–139. [Google Scholar] [CrossRef]
- Baldwin, D.A.; Campbell, V.M.; Carleo, L.A.; Marques, H.M.; Pratt, J.M. Reduction of bis(histidine)hemin; model for the proton-coupled reduction of hemoproteins. J. Am. Chem. Soc. 1981, 103, 186–188. [Google Scholar] [CrossRef]
- Baldwin, D.A.; Campbell, V.M.; Marques, H.M.; Pratt, J.M. Protein-free models for the proton-coupled reduction of haemoproteins. FEBS Lett. 1984, 167, 339–342. [Google Scholar] [CrossRef]
- Desbois, A.; Lutz, M. Redox control of proton transfers in membrane b-type cytochromes: An absorption and resonance Raman study on bis(imidazole) and bis(imidazolate) model complexes of iron-protoporphyrin. Eur. Biophys. J. 1992, 20, 321–335. [Google Scholar] [CrossRef]
- Warren, J.J.; Mayer, J.M. Proton-Coupled Electron Transfer Reactions at a Heme-Propionate in an Iron-Protoporphyrin-IX Model Compound. J. Am. Chem. Soc. 2011, 133, 8544–8551. [Google Scholar] [CrossRef]
- Weinberg, D.R.; Gagliardi, C.J.; Hull, J.F.; Murphy, C.F.; Kent, C.A.; Westlake, B.C.; Paul, A.; Ess, D.H.; McCafferty, D.G.; Meyer, T.J. Proton-Coupled Electron Transfer. Chem. Rev. 2012, 112, 4016–4093. [Google Scholar] [CrossRef] [PubMed]
- Tyburski, R.; Liu, T.; Glover, S.D.; Hammarström, L. Proton-Coupled Electron Transfer Guidelines, Fair and Square. J. Am. Chem. Soc. 2021, 143, 560–576. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S. Exploring proton-coupled electron transfer at multiple scales. Nat. Comput. Sci. 2023, 3, 291–300. [Google Scholar] [CrossRef]
- Sazanov, L.A. A giant molecular proton pump: Structure and mechanism of respiratory complex I. Nat. Rev. Mol. Cell Biol. 2015, 16, 375–388. [Google Scholar] [CrossRef]
- Hosseinzadeh, P.; Lu, Y. Design and fine-tuning redox potentials of metalloproteins involved in electron transfer in bioenergetics. Biochim. Biophs. Acta 2016, 1857, 557–581. [Google Scholar] [CrossRef]
- Wenke, B.B.; Spatzal, T.; Rees, D.C. Site-Specific Oxidation State Assignments of the Iron Atoms in the [4Fe:4S]2+/1+/0 States of the Nitrogenase Fe-Protein. Angew. Chem. Int. Ed. 2019, 58, 3894–3897. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.V.; Szilagyi, R.K. Iron–sulfur bond covalency from electronic structure calculations for classical iron–sulfur clusters. J. Comp. Chem. 2014, 35, 540–552. [Google Scholar] [CrossRef]
- Sun, J.; Trumpower, B.L. Superoxide anion generation by the cytochrome bc1 complex. Arch. Biochem. Biophys. 2003, 419, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.H.; Crofts, A.R.; Gennis, R.B. Assignment of the histidine axial ligands to the cytochrome bH and cytochrome bL components of the bc1 complex from Rhodobacter sphaeroides by site-directed mutagenesis. Biochemistry 1991, 30, 6747–6754. [Google Scholar] [CrossRef]
- Fischer, W.W.; Hemp, J.; Johnson, J.E. Evolution of Oxygenic Photosynthesis. Ann. Rev. Earth Planet. Sci. 2016, 44, 647–683. [Google Scholar] [CrossRef]
- Garcia, P.S.; D’Angelo, F.; Ollagnier de Choudens, S.; Dussouchaud, M.; Bouveret, E.; Gribaldo, S.; Barras, F. An early origin of iron–sulfur cluster biosynthesis machineries before Earth oxygenation. Nat. Ecol. Evol. 2022, 6, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.R.; Pettigrew, G.W. Cytochromes c. Evolutionary, Structural and Physicochemical Aspects; Springer-Verlag: Berlin, Germany, 1990. [Google Scholar]
- Johnson, D.C.; Dean, D.R.; Smith, A.D.; Johnson, M.K. Structure, Function, and Formation of Biological Iron Iron-Sulfor Clusters. Ann. Rev. Biochem. 2005, 74, 247–281. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J. Iron–sulfur protein folds, iron–sulfur chemistry, and evolution. J. Biol. Inorg. Chem. 2008, 13, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lill, R. Function and biogenesis of iron–sulphur proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef]
- Garcia, P.S.; Gribaldo, S.; Barras, F. When iron and sulfur met on an anoxic planet and eventually made clusters essential for life. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119730. [Google Scholar] [CrossRef]
- Flint, D.H.; Allen, R.M. Iron−Sulfur Proteins with Nonredox Functions. Chem. Rev. 1996, 96, 2315–2334. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, S.; Chen, X.; Wang, C.; Yang, P.; Qin, S.; Zhao, C.; Fei, F.; Zhao, X.; Tan, P.-H.; et al. Modulation of MagR magnetic properties via iron–sulfur cluster binding. Sci. Rep. 2021, 11, 23941. [Google Scholar] [CrossRef]
- Schmidt, C.L.; Shaw, L. A Comprehensive Phylogenetic Analysis of Rieske and Rieske-Type Iron-Sulfur Proteins. J. Bioenerg. Biomembr. 2001, 33, 9–26. [Google Scholar] [CrossRef]
- Era, I.; Kitagawa, Y.; Yasuda, N.; Kamimura, T.; Amamizu, N.; Sato, H.; Cho, K.; Okumura, M.; Nakano, M. Theoretical Study on Redox Potential Control of Iron-Sulfur Cluster by Hydrogen Bonds: A Possibility of Redox Potential Programming. Molecules 2021, 26, 6129. [Google Scholar] [CrossRef]
- Zu, Y.; Couture, M.M.J.; Kolling, D.R.J.; Crofts, A.R.; Eltis, L.D.; Fee, J.A.; Hirst, J. Reduction Potentials of Rieske Clusters: Importance of the Coupling between Oxidation State and Histidine Protonation State. Biochemistry 2003, 42, 12400–12408. [Google Scholar] [CrossRef]
- Kuznetsov, A.M.; Zueva, E.M.; Masliy, A.N.; Krishtalik, L.I. Redox potential of the Rieske iron–sulfur protein: Quantum-chemical and electrostatic study. Biochim. Biophys. Acta 2010, 1797, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Fee, J.A.; Hirst, J. Complete Thermodynamic Characterization of Reduction and Protonation of the bc1-type Rieske [2Fe–2S] Center of Thermus thermophilus. J. Am. Chem. Soc. 2001, 123, 9906–9907. [Google Scholar] [CrossRef]
- Danyal, K.; Dean, D.R.; Hoffman, B.M.; Seefeldt, L.C. Electron Transfer within Nitrogenase: Evidence for a Deficit-Spending Mechanism. Biochemistry 2011, 50, 9255–9263. [Google Scholar] [CrossRef]
- Lanzilotta, W.N.; Seefeldt, L.C. Electron Transfer from the Nitrogenase Iron Protein to the [8Fe-(7/8)S] Clusters of the Molybdenum−Iron Protein. Biochemistry 1996, 35, 16770–16776. [Google Scholar] [CrossRef]
- Jeoung, J.-H.; Martins, B.M.; Dobbek, H. Double-Cubane [8Fe9S] Clusters: A Novel Nitrogenase-Related Cofactor in Biology. Chem. Bio. Chem. 2020, 21, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Elghobashi-Meinhardt, N.; Tombolelli, D.; Mroginski, M.A. Electronic and Structural Properties of the Double Cubane Iron-Sulfur Cluster. Catalysts 2021, 11, 245. [Google Scholar] [CrossRef]
- Ullmann, M.G.; Noodleman, L.; Case, D.A. Density functional calculation of pKa values and redox potentials in the bovine Rieske iron-sulfur protein. J. Biol. Inorg. Chem. 2002, 7, 632–639. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron Transfer Reactions in Chemistry: Theory and Experiment. Available online: https://www.nobelprize.org/prizes/chemistry/1992/marcus/lecture/ (accessed on 9 December 2023).
- Gray, H.B.; Winkler, J.R. Electron transfer in proteins. Ann. Rev. Biochem. 1996, 65, 537–561. [Google Scholar] [CrossRef]
- Sánchez, M.; Sabio, L.; Gálvez, N.; Capdevila, M.; Dominguez-Vera, J.M. Iron chemistry at the service of life. IUBMB Life 2017, 69, 382–388. [Google Scholar] [CrossRef]
- Andreini, C.; Rosato, A. Structural Bioinformatics and Deep Learning of Metalloproteins: Recent Advances and Applications. Int. J. Mol. Sci. 2022, 23, 7684. [Google Scholar] [CrossRef]
- Jeong, W.J.; Lee, J.; Eom, H.; Song, W.J. A Specific Guide for Metalloenzyme Designers: Introduction and Evolution of Metal-Coordination Spheres Embedded in Protein Environments. Acc. Chem. Res. 2023, 56, 2416–2425. [Google Scholar] [CrossRef]
- Moore, G.R.; Pettigrew, G.W.; Rogers, N.K. Factors influencing redox potentials of electron transfer proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 4998–4999. [Google Scholar] [CrossRef] [PubMed]
- Perrin, B.S.; Ichiye, T. Characterizing the effects of the protein environment on the reduction potentials of metalloproteins. J. Biol. Inorg. Chem. 2013, 18, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Kono, T.; Shobu, I.; Ishida, M.; Gonda, K.; Hirota, S. Global Structural Flexibility of Metalloproteins Regulates Reactivity of Transition Metal Ion in the Protein Core: An Experimental Study Using Thiol-subtilisin as a Model Protein. Chem. Eur. J. 2018, 24, 2767–2775. [Google Scholar] [CrossRef]
- Lee, Y.-m.; Lim, C. Physical Basis of Structural and Catalytic Zn-Binding Sites in Proteins. J. Mol. Biol. 2008, 379, 545–553. [Google Scholar] [CrossRef]
- Valasatava, Y.; Rosato, A.; Furnham, N.; Thornton, J.M.; Andreini, C. To what extent do structural changes in catalytic metal sites affect enzyme function? J. Inorg. Biochem. 2018, 179, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Zaballa, M.-E.; Abriata, L.A.; Donaire, A.; Vila, A.J. Flexibility of the metal-binding region in apo-cupredoxins. Proc. Natl. Acad. Sci. USA 2012, 109, 9254–9259. [Google Scholar] [CrossRef]
- Ohnishi, T. Iron–sulfur clusters/semiquinones in Complex I. Biochim. Biophys. Acta 1998, 1364, 186–206. [Google Scholar] [CrossRef]
- Carroll, J.; Fearnley, I.M.; Skehel, J.M.; Shannon, R.J.; Hirst, J.; Walker, J.E. Bovine Complex I Is a Complex of 45 Different Subunits. J. Biol. Chem. 2006, 281, 32724–32727. [Google Scholar] [CrossRef]
- Hirst, J.; Carroll, J.; Fearnley, I.M.; Shannon, R.J.; Walker, J.E. The nuclear encoded subunits of complex I from bovine heart mitochondria. Biochim. Biophys. Acta 2003, 1604, 135–150. [Google Scholar] [CrossRef]
- Rees, D.C.; Howard, J.B. The Interface Between the Biological and Inorganic Worlds: Iron-Sulfur Metalloclusters. Science 2003, 300, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Camprubi, E.; Jordan, S.F.; Vasiliadou, R.; Lane, N. Iron catalysis at the origin of life. IUBMB Life 2017, 69, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Haja, D.K.; Schut, G.J.; Wu, C.H.; Meng, X.; Zhao, G.; Li, H.; Adams, M.W.W. Structure of the respiratory MBS complex reveals iron-sulfur cluster catalyzed sulfane sulfur reduction in ancient life. Nat. Commun. 2020, 11, 5953, Erratum in Nat. Commun. 2021, 12, 5831. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Ghosh, K.K.; Chakrabortty, S.; Gulyás, B.; Padmanabhan, P.; Ball, W.B. Mitochondrial Reactive Oxygen Species in Infection and Immunity. Biomolecules 2024, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Selivanov, V.A.; Votyakova, T.V.; Zeak, J.A.; Trucco, M.; Roca, J.; Cascante, M. Bistability of Mitochondrial Respiration Underlies Paradoxical Reactive Oxygen Species Generation Induced by Anoxia. PLoS Comput. Biol. 2009, 5, e1000619. [Google Scholar] [CrossRef]
- Mailloux, R.J. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015, 4, 381–398. [Google Scholar] [CrossRef]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y. Ubiquinol oxidation in the cytochrome bc1 complex: Reaction mechanism and prevention of short-circuiting. Biochim. Biophs. Acta 2005, 1709, 5–34. [Google Scholar] [CrossRef]
- Guzy, R.D.; Schumacker, P.T. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006, 91, 807–819. [Google Scholar] [CrossRef]
- Brzezinski, P.; Moe, A.; Ädelroth, P. Structure and Mechanism of Respiratory III–IV Supercomplexes in Bioenergetic Membranes. Chem. Rev. 2021, 121, 9644–9673. [Google Scholar] [CrossRef]
- Bolisetty, S.; Jaimes, E.A. Mitochondria and Reactive Oxygen Species: Physiology and Pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306–6344. [Google Scholar] [CrossRef] [PubMed]
- Povea-Cabello, S.; Brischigliaro, M.; Fernández-Vizarra, E. Emerging mechanisms in the redox regulation of mitochondrial cytochrome c oxidase assembly and function. Biochem. Soc. Trans. 2024, 52, 873–885. [Google Scholar] [CrossRef]
- Sato, W.; Ishimori, K. Regulation of electron transfer in the terminal step of the respiratory chain. Biochem. Soc. Trans. 2023, 51, 1611–1619. [Google Scholar] [CrossRef]
- Luo, F.; Shinzawa-Itoh, K.; Hagimoto, K.; Shimada, A.; Shimada, S.; Yamashita, E.; Yoshikawa, S.; Tsukihara, T. Structure of bovine cytochrome c oxidase crystallized at a neutral pH using a fluorinated detergent. Acta Cryst. F 2017, 73, 416–422. [Google Scholar] [CrossRef]
- Wikström, M.; Krab, K.; Sharma, V. Oxygen Activation and Energy Conservation by Cytochrome c Oxidase. Chem. Rev. 2018, 118, 2469–2490. [Google Scholar] [CrossRef]
- Rich, P.R. Mitochondrial cytochrome c oxidase: Catalysis, coupling and controversies. Biochem. Soc. Trans. 2017, 45, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Meunier, B.; de Visser, S.P.; Shaik, S. Mechanism of Oxidation Reactions Catalyzed by Cytochrome P450 Enzymes. Chem. Rev. 2004, 104, 3947–3980. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Montellano, P.R. Hydrocarbon Hydroxylation by Cytochrome P450 Enzymes. Chem. Rev. 2010, 110, 932–948. [Google Scholar] [CrossRef]
- Denisov, I.G.; Sligar, S.G. Activation of Molecular Oxygen in Cytochromes P450. In Cytochrome P450: Structure, Mechanism, and Biochemistry; Ortiz de Montellano, P.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 69–109. [Google Scholar]
- Guengerich, F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018, 8, 10964–10976. [Google Scholar] [CrossRef]
- Cook, D.J.; Finnigan, J.D.; Cook, K.; Black, G.W.; Charnock, S.J. Cytochromes P450: History, Classes, Catalytic Mechanism, and Industrial Application. In Advances in Protein Chemistry and Structural Biology; Christov, C.Z., Ed.; Academic Press: New Yrok, NY, USA, 2016; Volume 105, pp. 105–126. [Google Scholar]
- Koroleva, P.I.; Bulko, T.V.; Agafonova, L.E.; Shumyantseva, V.V. Catalytic and Electrocatalytic Mechanisms of Cytochromes P450 in the Development of Biosensors and Bioreactors. Biochemistry 2023, 88, 1645–1657. [Google Scholar]
- Zhou, T.P.; Fan, Y.; Zhang, J.; Wang, B. Mechanistic Perspective on C-N and C-S Bond Construction Catalyzed by Cytochrome P450 Enzymes. ACS Bio. Med. Chem. Au. 2025, 5, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.M.; Shin, W.; Yang, Z.J. Computational Studies of Enzymes for C-F Bond Degradation and Functionalization. Chem. Phys. Chem. 2025, e202401130. [Google Scholar] [CrossRef]
- Leite Dias, S.; D’Auria, J.C. The bitter truth: How insects cope with toxic plant alkaloids. J. Exp. Bot. 2025, 76, 5–15. [Google Scholar] [CrossRef]
- Efimov, I.; Basran, J.; Thackray, S.J.; Handa, S.; Mowat, C.G.; Raven, E.L. Structure and Reaction Mechanism in the Heme Dioxygenases. Biochemistry 2011, 50, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The Molecular Mechanism of the Catalase Reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Aboelnga, M.M. Mechanistic insights into the chemistry of compound I formation in heme peroxidases: Quantum chemical investigations of cytochrome c peroxidase. RSC Adv. 2022, 12, 15543–15554. [Google Scholar] [CrossRef]
- Auclair, K.; Moeenne-Laccoz, P.; Ortiz de Montellano, P.R. Roles of the Proximal Heme Thiolate Ligand in Cytochrome P400cam. J. Am. Chem Soc. 2001, 123, 4877–4885. [Google Scholar] [CrossRef]
- Shaik, S.; Dubey, K.D. The catalytic cycle of cytochrome P450: A fascinating choreography. Trends Chem. 2021, 3, 1027–1044. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Li, C. Engineering Electron Transfer Pathway of Cytochrome P450s. Molecules 2024, 29, 2480. [Google Scholar] [CrossRef]
- Schlichting, I.; Berendzen, J.; Chu, K.; Stock, A.M.; Maves, S.A.; Benson, D.E.; Sweet, R.M.; Ringe, D.; Petsko, G.A.; Sligar, S.G. The catalytic pathway of cytochrome p450cam at atomic resolution. Science 2000, 287, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Bach, R.D.; Dmitrenko, O. The “Somersault” Mechanism for the P-450 Hydroxylation of Hydrocarbons. The Intervention of Transient Inverted Metastable Hydroperoxides. J. Am. Chem. Soc. 2006, 128, 1474–1488. [Google Scholar] [CrossRef]
- Tateishi, Y.; McCarty, K.D.; Martin, M.V.; Yoshimoto, F.K.; Guengerich, F.P. Roles of Ferric Peroxide Anion Intermediates (Fe(3+)O(2) (-), Compound 0) in Cytochrome P450 19A1 Steroid Aromatization and a Cytochrome P450 2B4 Secosteroid Oxidation Model. Angew. Chem. Int. Ed. Engl. 2024, 63, e202406542. [Google Scholar] [CrossRef]
- Tateishi, Y.; McCarty, K.D.; Martin, M.V.; Guengerich, F.P. Oxygen-18 Labeling Defines a Ferric Peroxide (Compound 0) Mechanism in the Oxidative Deformylation of Aldehydes by Cytochrome P450 2B4. ACS Catal. 2024, 14, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- McCarty, K.D.; Tateishi, Y.; Hargrove, T.Y.; Lepesheva, G.I.; Guengerich, F.P. Oxygen-18 Labeling Reveals a Mixed Fe-O Mechanism in the Last Step of Cytochrome P450 51 Sterol 14α-Demethylation. Angew. Chem. Int. Ed. Engl. 2024, 63, e202317711. [Google Scholar] [CrossRef] [PubMed]
- Rittle, J.; Green, M.T. Cytochrome P450 Compound I: Capture, Characterization, and C-H Bond Activation Kinetics. Science 2010, 330, 933–937. [Google Scholar] [CrossRef]
- Han, A.-R.; Jin Jeong, Y.; Kang, Y.; Yoon Lee, J.; Sook Seo, M.; Nam, W. Direct evidence for an iron(iv)-oxo porphyrin π-cation radical as an active oxidant in catalytic oxygenation reactions. Chem. Commun. 2008, 7, 1076–1078. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Hoffman, B.M.; Dean, D.R. Mechanism of Mo-dependent nitrogenase. Annu. Rev. Biochem. 2009, 78, 701–722. [Google Scholar] [CrossRef]
- Pourret, O.; Faucon, M.-P. Cobalt. In Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth; White, W.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–4. [Google Scholar]
- Rollinson, C.L.; Bailar, J.C.; Emeléus, H.J.; Nyholm, R. The Chemistry of Chromium, Molybdenum and Tungsten: Pergamon International Library of Science, Technology, Engineering and Social Studies; Elsevier Science: Oxford, UK, 2015. [Google Scholar]
- Basolo, F.; Pearson, R.G. Mechanisms of Inorganic Reactions: A Study of Metal Complexes in Solution; Wiley: New York, NY, USA, 1958. [Google Scholar]
- Rutenberg, A.C.; Taube, H.J. The Exchange of Water between Co(NH3)5H2O+++ and Solvent. Chem. Phys. 1952, 20, 825–826. [Google Scholar]
- Hunt, J.; Plane, R.A. The Kinetics of the Exchange of Water between Cr(H2O)6+3 and Solvent. J. Am. Chem. Soc. 1954, 76, 5960–5962. [Google Scholar] [CrossRef]
- Fiat, D.; Connick, R.E. Oxygen-17 magnetic resonance studies of ion solvation. The hydration of aluminum (III) and gallium (III) ions. J. Am. Chem. Soc. 1968, 90, 608–615. [Google Scholar] [CrossRef]
- Swift, T.J.; Connick, R.E. NMR-Relaxation Mechanisms of O17 in Aqueous Solutions of Paramagnetic Cations and the Lifetime of Water Molecules in the First Coordination Sphere. J. Chem. Phys. 1962, 37, 307–320. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Prevalence of Cobalt in the Environment and Its Role in Biological Processes. Biology 2023, 12, 1335. [Google Scholar] [CrossRef]
- Kräutler, B.; Jaun, B.M. Vitamin B12 and Cofactor F430. In Fundamentals of Porphyrin Chemistry: A 21st Century Approach; Brothers, P.J., Senge, M.O., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; Volume 1, pp. 777–814. [Google Scholar]
- Kräutler, B. Organometallic B12-derivatives in life processes. In Bioorganometallic Chemistry; Wolfgang, W., Ulf-Peter, A., Eds.; De Gruyter: Berlin, Germany, 2020; pp. 243–284. [Google Scholar]
- Osman, D.; Cooke, A.; Young, T.R.; Deery, E.; Robinson, N.J.; Warren, M.J. The requirement for cobalt in vitamin B12: A paradigm for protein metalation. Biochim. Biophs. Acta 2021, 1868, 118896. [Google Scholar] [CrossRef]
- Mendoza, J.; Purchal, M.; Yamada, K.; Koutmos, M. Structure of full-length cobalamin-dependent methionine synthase and cofactor loading captured in crystallo. Nat. Commun. 2023, 14, 6365. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, Y.; Kamei, Y.; Bito, T.; Arima, J.; Yoneda, K.; Sakuraba, H.; Ohshima, T.; Nakano, Y.; Watanabe, F. Functional and structural characteristics of methylmalonyl-CoA mutase from Pyrococcus horikoshii. Biosci. Biotechnol. Biochem. 2015, 79, 710–717. [Google Scholar] [CrossRef]
- Benjdia, A.; Berteau, O. B12-dependent radical SAM enzymes: Ever expanding structural and mechanistic diversity. Curr. Opin. Structur. Biol. 2023, 83, 102725. [Google Scholar] [CrossRef]
- Fincker, M.; Spormann, A.M. Biochemistry of Catabolic Reductive Dehalogenation. Annu. Rev. Biochem. 2017, 86, 357–386. [Google Scholar] [CrossRef]
- Conrad, K.S.; Jordan, C.D.; Brown, K.L.; Brunold, T.C. Spectroscopic and computational studies of cobalamin species with variable lower axial ligation: Implications for the mechanism of Co-C bond activation by class I cobalamin-dependent isomerases. Inorg. Chem. 2015, 54, 3736–3747. [Google Scholar] [CrossRef]
- Schrapers, P.; Mebs, S.; Goetzl, S.; Hennig, S.E.; Dau, H.; Dobbek, H.; Haumann, M. Axial Ligation and Redox Changes at the Cobalt Ion in Cobalamin Bound to Corrinoid Iron-Sulfur Protein (CoFeSP) or in Solution Characterized by XAS and DFT. PLoS ONE 2016, 11, e0158681. [Google Scholar] [CrossRef]
- Park, K.; Mera, P.E.; Escalante-Semerena, J.C.; Brunold, T.C. Resonance Raman spectroscopic study of the interaction between Co(II)rrinoids and the ATP:corrinoid adenosyltransferase PduO from Lactobacillus reuteri. J. Biol. Inorg. Chem. 2016, 21, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Stracey, N.G.; Costa, F.G.; Escalante-Semerena, J.C.; Brunold, T.C. Spectroscopic Study of the EutT Adenosyltransferase from Listeria monocytogenes: Evidence for the Formation of a Four-Coordinate Cob(II)alamin Intermediate. Biochemistry 2018, 57, 5088–5095. [Google Scholar] [CrossRef] [PubMed]
- Lukinović, V.; Woodward, J.R.; Marrafa, T.C.; Shanmugam, M.; Heyes, D.J.; Hardman, S.J.O.; Scrutton, N.S.; Hay, S.; Fielding, A.J.; Jones, A.R. Photochemical Spin Dynamics of the Vitamin B12 Derivative, Methylcobalamin. J. Phys. Chem. B 2019, 123, 4663–4672. [Google Scholar] [CrossRef]
- Qu, Z.W.; Hansen, A.; Grimme, S. Co-C Bond Dissociation Energies in Cobalamin Derivatives and Dispersion Effects: Anomaly or Just Challenging? J. Chem. Theory. Comput. 2015, 11, 1037–1045. [Google Scholar] [CrossRef]
- Salerno, E.V.; Miller, N.A.; Konar, A.; Li, Y.; Kieninger, C.; Kräutler, B.; Sension, R.J. Ultrafast Excited State Dynamics and Fluorescence from Vitamin B12 and Organometallic [Co]-C≡C-R Cobalamins. J. Phys. Chem. B 2020, 124, 6651–6656. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.A.; Michocki, L.B.; Konar, A.; Alonso-Mori, R.; Deb, A.; Glownia, J.M.; Sofferman, D.L.; Song, S.; Kozlowski, P.M.; Kubarych, K.J.; et al. Ultrafast XANES Monitors Femtosecond Sequential Structural Evolution in Photoexcited Coenzyme B12. J. Phys. Chem. B 2020, 124, 199–209. [Google Scholar] [CrossRef]
- Reckziegel, A.; Kour, M.; Battistella, B.; Mebs, S.; Beuthert, K.; Berger, R.; Werncke, C.G. High-Spin Imido Cobalt Complexes with Imidyl Radical Character. Angew. Chem. Int. Ed. 2021, 60, 15376–15380. [Google Scholar] [CrossRef]
- Toda, M.J.; Lodowski, P.; Mamun, A.A.; Kozlowski, P.M. Electronic and photolytic properties of hydridocobalamin. J. Photochem. Photobiol. B 2021, 224, 112295. [Google Scholar] [CrossRef]
- Lehene, M.; Plesa, D.; Ionescu-Zinca, S.; Iancu, S.D.; Leopold, N.; Makarov, S.V.; Brânzanic, A.M.V.; Silaghi-Dumitrescu, R. Adduct of Aquacobalamin with Hydrogen Peroxide. Inorg. Chem. 2021, 60, 12681–12684. [Google Scholar] [CrossRef]
- Elmendorf, L.D.; Brunold, T.C. Electronic structure studies of free and enzyme-bound B12 species by magnetic circular dichroism and complementary spectroscopic techniques. In Methods in Enzymology; Marsh, E.N.G., Ed.; Academic Press: New York, NY, USA, 2022; Volume 669, pp. 333–365. [Google Scholar]
- Mumcu, T.; Oncuoglu, S.; Hizliates, C.G.; Ertekin, K. Emission-based sensing of cobalt (II) and vitamin B12 via a bis-indole derivative. Luminescence 2024, 39, e4863. [Google Scholar] [CrossRef]
- Elmendorf, L.D.; Brunold, T.C. Vibronic Coupling in Vitamin B12: A Combined Spectroscopic and Computational Study. Inorg. Chem. 2023, 62, 12762–12772. [Google Scholar] [CrossRef]
- Spataru, T. The Miracle of Vitamin B12 Biochemistry. Reactions 2024, 5, 20–76. [Google Scholar] [CrossRef]
- Spataru, T. The complete electronic structure and mechanism of the methionine synthase process as determined by the MCSCF method. J. Organometal. Chem. 2021, 942, 121811. [Google Scholar] [CrossRef]
- Fang, H.; Kang, J.; Zhang, D. Microbial production of vitamin B12: A review and future perspectives. Microb. Cell Fact. 2017, 16, 15. [Google Scholar] [CrossRef]
- Roth, J.R.; Lawrence, J.G.; Bobik, T.A. Cobalamin (coenzymne B12): Synthesis and biological significance. Annu. Rev. Microbiol. 1996, 50, 137–181. [Google Scholar] [CrossRef]
- Martens, J.H.; Barg, H.; Warren, M.; Jahn, D. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 2002, 58, 275–285. [Google Scholar] [CrossRef]
- Scott, A.I.; Roessner, C.A. Biosynthesis of cobalamin (vitamin B12). Biochem. Soc. Trans. 2002, 30, 613–620. [Google Scholar] [CrossRef]
- Mrnjavac, N.; Degli Esposti, M.; Mizrahi, I.; Martin, W.F.; Allen, J.F. Three enzymes governed the rise of O2 on Earth. Biochim. Biophys. Acta 2024, 1865, 149495. [Google Scholar] [CrossRef]
- Mrnjavac, N.; Schwander, L.; Brabender, M.; Martin, W.F. Chemical Antiquity in Metabolism. Acc. Chem. Res. 2024, 57, 2267–2278. [Google Scholar] [CrossRef]
- Dorrell, R.G.; Nef, C.; Altan-Ochir, S.; Bowler, C.; Smith, A.G. Presence of vitamin B12 metabolism in the last common ancestor of land plants. Phil. Trans. Roy. Soc. B 2024, 379, 20230354. [Google Scholar] [CrossRef]
- Modjewski, L.D.; Karavaeva, V.; Mrnjavac, N.; Knopp, M.; Martin, W.F.; Sousa, F.L. Evidence for corrin biosynthesis in the last universal common ancestor. FEBS J. 2025, 292, 827–850. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R. B12 Trafficking in Mammals: A Case for Coenzyme Escort Service. ACS Chem. Biol. 2006, 1, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Gherasim, C.; Padovani, D. The tinker, tailor, soldier in intracellular B12 trafficking. Curr. Opin. Chem. Biol. 2009, 13, 484–491. [Google Scholar] [CrossRef]
- Li, Z.; Gouda, H.; Pillay, S.; Yaw, M.; Ruetz, M.; Banerjee, R. The human B12 trafficking chaperones: CblA, ATR, CblC and CblD. Methods Enzym. 2022, 668, 137–156. [Google Scholar]
- Nijland, M.; Martínez Felices, J.M.; Slotboom, D.J.; Thangaratnarajah, C. Membrane transport of cobalamin. Vitam. Horm. 2022, 119, 121–148. [Google Scholar] [PubMed]
- Calvillo, Á.; Pellicer, T.; Carnicer, M.; Planas, A. Bioprocess Strategies for Vitamin B12 Production by Microbial Fermentation and Its Market Applications. Bioengineering 2022, 9, 365. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, U.; Tiwari, A.; Tiwari, P.; Sahu, J.K.; Sharma, S. Vitamin B12: Strategies for enhanced production, fortified functional food products and health benefits. Process. Biochem. 2023, 127, 44–55. [Google Scholar] [CrossRef]
- Banerjee, R. (Ed.) Chemistry and Biochemistry of B12; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Kräutler, B. Biochemistry of B12-cofactors in human metabolism. Sub-Cell Biochem. 2012, 56, 323–346. [Google Scholar]
- Okamoto, S.; Eltis, L.D. The biological occurrence and trafficking of cobalt. Metallomics 2011, 3, 963–970. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, S. Cobalt proteins. Eur. J. Biochem. 1999, 261, 1–9. [Google Scholar] [CrossRef]
- Harrop, T.C.; Mascharak, P.K. Cobalt-containing Enzymes. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 684–690. [Google Scholar]
- Odaka, M.; Kobayashi, M. Cobalt Proteins, Overview. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 670–678. [Google Scholar]
- Walker, K.W.; Bradshaw, R.A. Methionyl Aminopeptidase Type 1. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: New York, NY, USA, 2013; pp. 1495–1500. [Google Scholar]
- Lee, Y.; Kim, H.; Lee, E.; Hahn, H.; Heo, Y.; Jang, D.M.; Kwak, K.; Kim, H.J.; Kim, H.S. Structural insights into N-terminal methionine cleavage by the human mitochondrial methionine aminopeptidase, MetAP1D. Sci. Rep. 2023, 13, 22326. [Google Scholar] [CrossRef] [PubMed]
- Glueck, D.S. Intramolecular attack on coordinated nitriles: Metallacycle intermediates in catalytic hydration and beyond. Dalton Trans. 2021, 50, 15953–15960. [Google Scholar] [CrossRef]
- Katayama, Y.; Hashimoto, K.; Nakayama, H.; Mino, H.; Nojiri, M.; Ono, T.-a.; Nyunoya, H.; Yohda, M.; Takio, K.; Odaka, M. Thiocyanate Hydrolase Is a Cobalt-Containing Metalloenzyme with a Cysteine-Sulfinic Acid Ligand. J. Am. Chem. Soc. 2006, 128, 728–729. [Google Scholar] [CrossRef] [PubMed]
- Fatima, B.; Javed, M.M. Production, purification and physicochemical characterization of D-xylose/glucose isomerase from Escherichia coli strain BL21. 3 Biotech 2020, 10, 39. [Google Scholar] [CrossRef]
- Hoffman, A.; Hilpert, W.; Dimroth, P. The carboxyltransferase activity of the sodium-ion-translocating methylmalonyl-CoA decarboxylase of Veillonella alcalescens. Eur. J. Biochem. 1989, 179, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.N.G.; Waugh, M.W. Aldehyde Decarbonylases: Enigmatic Enzymes of Hydrocarbon Biosynthesis. ACS Catal. 2013, 3, 2515–2521. [Google Scholar] [CrossRef]
- Broderick, J.B. Iron–Sulfur Clusters in Enzyme Catalysis. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Pergamon: Oxford, UK, 2003; pp. 739–757. [Google Scholar]
- Maity, A.N.; Chen, J.R.; Ke, S.C. Exploring the mechanism of action of lysine 5,6-aminomutase using EPR and ENDOR spectroscopies. Methods Enzym. 2022, 669, 197–228. [Google Scholar]
- Miura, J.; Itoh, N. Determination of Vanadium, Cobalt, Nickel, And Iron In Bromoperoxidases from Pseudomonas Putida and Corallina Pilulifera by High Performance Liquid Chromatography with Spectrophotometric Detection. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 2367–2376. [Google Scholar] [CrossRef]
- Ortiz-Guerrero, J.M.; Polanco, M.C.; Murillo, F.J.; Padmanabhan, S.; Elías-Arnanz, M. Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc. Natl. Acad. Sci. USA 2011, 108, 7565–7570. [Google Scholar] [CrossRef]
- Kennedy, K.J.; Widner, F.J.; Sokolovskaya, O.M.; Innocent, L.V.; Procknow, R.R.; Mok, K.C.; Taga, M.E. Cobalamin Riboswitches Are Broadly Sensitive to Corrinoid Cofactors to Enable an Efficient Gene Regulatory Strategy. mBio 2022, 13, e0112122. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.J.; Mamun, A.A.; Lodowski, P.; Kozlowski, P.M. Why is CarH photolytically active in comparison to other B12-dependent enzymes? J. Photochem. Photobiol. B 2020, 209, 111919. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Pérez-Castaño, R.; Osete-Alcaraz, L.; Polanco, M.C.; Elías-Arnanz, M. Vitamin B12 photoreceptors. Vitam. Horm. 2022, 119, 149–184. [Google Scholar]
- Ghosh, A.P.; Toda, M.J.; Kozlowski, P.M. Photolytic properties of B12-dependent enzymes: A theoretical perspective. Vitam. Horm. 2022, 119, 185–220. [Google Scholar]
- Ghosh, A.P.; Lodowski, P.; Kozlowski, P.M. Aerobic photolysis of methylcobalamin: Unraveling the photoreaction mechanism. Phys. Chem. Chem. Phys. 2022, 24, 6093–6106. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.A.; Kaneshiro, A.K.; Konar, A.; Alonso-Mori, R.; Britz, A.; Deb, A.; Glownia, J.M.; Koralek, J.D.; Mallik, L.; Meadows, J.H.; et al. The Photoactive Excited State of the B(12)-Based Photoreceptor CarH. J. Phys. Chem. B 2020, 124, 10732–10738. [Google Scholar] [CrossRef]
- Kipkorir, T.; Polgar, P.; Barker, D.; D’Halluin, A.; Patel, Z.; Arnvig, K.B. A novel regulatory interplay between atypical B12 riboswitches and uORF translation in Mycobacterium tuberculosis. Nucleic Acids Res. 2024, 52, 7876–7892. [Google Scholar] [CrossRef]
- Degnan, P.H.; Taga, M.E.; Goodman, A.L. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014, 20, 769–778. [Google Scholar] [CrossRef]
- Mok, K.C.; Sokolovskaya, O.M.; Nicolas, A.M.; Hallberg, Z.F.; Deutschbauer, A.; Carlson, H.K.; Taga, M.E. Identification of a Novel Cobamide Remodeling Enzyme in the Beneficial Human Gut Bacterium Akkermansia muciniphila. mBio 2020, 11, e02507. [Google Scholar] [CrossRef]
- Li, H.; Ye, F.; Li, Z.; Peng, X.; Wu, L.; Liu, Q. The response of gut microbiota to arsenic metabolism is involved in arsenic-induced liver injury, which is influenced by the interaction between arsenic and methionine synthase. Environ. Int. 2024, 190, 108824. [Google Scholar] [CrossRef]
- Bannon, C.C.; Mudge, E.M.; Bertrand, E.M. Shedding light on cobalamin photodegradation in the ocean. Limnol. Oceanogr. Lett. 2024, 9, 135–144. [Google Scholar] [CrossRef]
- Lison, D. Cobalt. In Handbook of the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 511–528. [Google Scholar]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Central Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef]
- Danzeisen, R.; Weight, D.; Blakeney, M.; Boyle, D. A tiered approach to investigate the inhalation toxicity of cobalt substances. Introduction: Cobalt’s essential role in nature and technology. Reg. Toxicol. Pharmacol. 2022, 130, 105125. [Google Scholar] [CrossRef]
- Azzini, E.; Raguzzini, A.; Polito, A. A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults. Int. J. Mol. Sci. 2021, 22, 9694. [Google Scholar] [CrossRef] [PubMed]
- Shipton, M.J.; Thachil, J. Vitamin B12 deficiency—A 21st century perspective. Clin. Med. 2015, 15, 145–150. [Google Scholar] [CrossRef]
- Sobczyńska-Malefora, A.; Delvin, E.; McCaddon, A.; Ahmadi, K.R.; Harrington, D.J. Vitamin B12 status in health and disease: A critical review. Diagnosis of deficiency and insufficiency—Clinical and laboratory pitfalls. Crit. Rev. Clinic. Lab. Sci. 2021, 58, 399–429. [Google Scholar] [CrossRef]
- Simonenko, S.Y.; Bogdanova, D.A.; Kuldyushev, N.A. Emerging Roles of Vitamin B12 in Aging and Inflammation. Int. J. Mol. Sci. 2024, 25, 5044. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Coelho, D.; Bossenmeyer-Pourié, C.; Matmat, K.; Arnold, C.; Savladori, A.; Alberto, J.M.; Umoret, R.; Guéant, J.L.; Pourié, G. Cognitive Impairment Is Associated with AMPAR Glutamatergic Dysfunction in a Mouse Model of Neuronal Methionine Synthase Deficiency. Cells 2023, 12, 1267. [Google Scholar] [CrossRef]
- Watson, W.P.; Munter, T.; Golding, B.T. The effect of vitamin B12 on DNA adduction by styrene oxide, a genotoxic xenobiotic. Chem. Biol. Interact. 2023, 382, 110591. [Google Scholar] [CrossRef]
- Kräutler, B. Antivitamins B12—Some Inaugural Milestones. Chem. Eur. J. 2020, 26, 15438–15445. [Google Scholar] [CrossRef]
- Wiedemair, M.; Kieninger, C.; Wurst, K.; Podewitz, M.; Deery, E.; Paxhia, M.D.; Warren, M.J.; Kräutler, B. Solution, Crystal and in Silico Structures of the Organometallic Vitamin B12-Derivative Acetylcobalamin and of its Novel Rhodium-Analogue Acetylrhodibalamin. Helv. Chim. Acta 2023, 106, e202200158. [Google Scholar] [CrossRef]
- Gromova, O.A.; Torshin, I.Y.; Maiorova, L.A.; Koifman, O.I.; Salnikov, D.S. Bioinformatic and chemoneurocytological analysis of the pharmacological properties of vitamin B12 and some of its derivatives. J. Porph. Phthalocyan. 2021, 25, 835–842. [Google Scholar] [CrossRef]
- Salerno, E.V.; Miller, N.A.; Konar, A.; Salchner, R.; Kieninger, C.; Wurst, K.; Spears, K.G.; Kräutler, B.; Sension, R.J. Exceptional Photochemical Stability of the Co–C Bond of Alkynyl Cobalamins, Potential Antivitamins B12 and Core Elements of B12-Based Biological Vectors. Inorg. Chem. 2020, 59, 6422–6431. [Google Scholar] [CrossRef] [PubMed]
- Ruetz, M.; Mascarenhas, R.; Widner, F.; Kieninger, C.; Koutmos, M.; Kräutler, B.; Banerjee, R. A Noble Metal Substitution Leads to B12 Cofactor Mimicry by a Rhodibalamin. Biochemistry 2024, 63, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Billig, E.; Waters, J.H.; Gray, H.B. The Toluenedithiolate and Maleonitriledithiolate Square-Matrix Systems. J. Am. Chem. Soc. 1966, 88, 43–50. [Google Scholar] [CrossRef]
- Röhrscheid, F.; Balch, A.L.; Holm, R.H. Potential Electron Transfer Complexes of the [M-O4] Type: Synthesis and Properties of Complexes Derived from Pyrocatechol and Tetrachloropyrocatechol. Inorg. Chem. 1966, 5, 1542–1551. [Google Scholar] [CrossRef]
- Fleischer, E.B.; Jacobs, S.; Mestichelli, L. The Kinetics of the Reaction of Cobalt (III) and Iron (III) Hematoporphyrin with Cyanide and Thiocyanate. Evidence for a Dissociative Mechanism. J. Am. Chem. Soc. 1968, 90, 2527–2531. [Google Scholar] [CrossRef]
- Kräutler, B.; Arigoni, D.; Golding, B. (Eds.) Vitamin B12 and B12-Proteins; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Toraya, T. Radical catalysis of B12 enzymes: Structure, mechanism, inactivation, and reactivation of diol and glycerol dehydratases. Cell Mol. Life Sci. 2000, 57, 106–127. [Google Scholar] [CrossRef]
- Marsh, E.N.G.; Holloway, D.E. Adenosylcobalamin-dependent enzymes. Subcell Biochem 2000, 35, 351–403. [Google Scholar]
- Marsh, E.N.G. Coenzyme-B12-Dependent Glutamate Mutase. Bioorg. Chem. 2000, 28, 176–189. [Google Scholar] [CrossRef]
- Marsh, E.N.G.; Drennan, C.L. Adenosyl-cobalamin dependent isomerases: New insights into structure and mechanism. Curr. Op. Chem. Biol. 2001, 5, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Frey, P.A. Radical mechanisms of enzymatic catalysis. Ann. Rev. Biochem. 2001, 70, 121–148. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R. Radical peregrinations catalyzed by coenzyme B12-dependent enzymes. Biochemistry 2001, 40, 6191–6198. [Google Scholar] [CrossRef] [PubMed]
- Gruber, K.; Kratky, C. Coenzyme B12 dependent glutamate mutase. Curr. Op. Chem. Biol. 2002, 6, 598–603. [Google Scholar] [CrossRef]
- Brown, K.L. Chemistry and enzymology of vitamin B-12. Chem. Rev. 2005, 105, 2075–2149. [Google Scholar] [CrossRef]
- Heldt, D.; Lawrence, A.D.; Lindenmeyer, M.; Deery, E.; Heathcote, P.; Rigby, S.E.; Warren, M.J. Aerobic synthesis of vitamin B12: Ring contraction and cobalt chelation. Biochem. Soc. Trans. 2005, 33, 815–819. [Google Scholar] [CrossRef]
- Gruber, K.; Puffer, B.; Kräutler, B. Vitamin B12-derivatives—Enzyme cofactors and ligands of proteins and nucleic acids. Chem. Soc. Rev. 2011, 40, 4346–4363. [Google Scholar] [CrossRef]
- Moore, S.J.; Warren, M.J. The anaerobic biosynthesis of vitamin B12. Biochem. Soc. Trans. 2012, 40, 581–586. [Google Scholar] [CrossRef]
- Moore, S.J.; Lawrence, A.D.; Biedendieck, R.; Deery, E.; Frank, S.; Howard, M.J.; Rigby, S.E.J.; Warren, M.J. Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12). Proc. Natl. Acad. Sci. USA 2013, 110, 14906–14911. [Google Scholar] [CrossRef]
- Bridwell-Rabb, J.; Drennan, C.L. Vitamin B12 in the spotlight again. Curr. Opin. Chem. Biol. 2017, 37, 63–70. [Google Scholar] [CrossRef]
- Mattes, T.A.; Deery, E.; Warren, M.J.; Escalante-Semerena, J.C. Cobalamin Biosynthesis and Insertion. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–24. [Google Scholar]
- Monteverde, D.R.; Gómez-Consarnau, L.; Suffridge, C.; Sañudo-Wilhelmy, S.A. Life’s utilization of B vitamins on early Earth. Geobiology 2017, 15, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.M. The Inorganic Chemistry of Vitamin B12; Academic Press: London, UK, 1972. [Google Scholar]
- Giedyk, M.; Gryko, D. Vitamin B12: An efficient cobalt catalyst for sustainable generation of radical species. Chem. Catal. 2022, 2, 1534–1548. [Google Scholar] [CrossRef]
- Shimakoshi, H. Application of bioorganometallic B12 in green organic synthesis. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: New York, NY, USA, 2022; Volume 119, pp. 23–42. [Google Scholar]
- Pleșa, D.; Lehene, M.; Silaghi-Dumitrescu, R. On the reaction of Co(II) cobalamin with hydrogen peroxide. Reac. Kinet. Mech. Catal. 2023, 136, 1791–1799. [Google Scholar] [CrossRef]
- Salnikov, D.S.; Makarov, S.V.; Koifman, O.I. The radical versus ionic mechanisms of reduced cobalamin inactivation by tert-butyl hydroperoxide and hydrogen peroxide in aqueous solution. New J. Chem. 2021, 45, 535–543. [Google Scholar] [CrossRef]
- Lexa, D.; Savéant, J.M. Electrochemistry of vitamin B12. 3. One-electron intermediates in the reduction of methylcobalamin and methylcobinamide. J. Am. Chem. Soc. 1978, 100, 3220–3222. [Google Scholar] [CrossRef]
- Lexa, D.; Savéant, J.M. The electrochemistry of vitamin B12. Acc. Chem. Res. 1983, 16, 235–243. [Google Scholar] [CrossRef]
- Birke, R.L.; Huang, Q.; Spataru, T.; Gosser, D.K. Electroreduction of a Series of Alkylcobalamins: Mechanism of Stepwise Reductive Cleavage of the Co−C Bond. J. Am. Chem. Soc. 2006, 128, 1922–1936. [Google Scholar] [CrossRef]
- Rubinson, K.A.; Parekh, H.V.; Itabashi, E.; Mark, H.B. The Chemistry of [CoICobalamins: Equilibrium Constants and Energies of Formation of Species in Aqueous Solution. Inorg. Chem. 1983, 22, 458–463. [Google Scholar] [CrossRef]
- Martin, B.D.; Finke, R.G. Methylcobalamin’s full- vs half-strenghth cobalt-cobalt bonds and bond dissociation enthalpies: A > 1015 Co-CH3 homolysis rate enhancement following one-antibonding-electron reduction of methylcobalamin. J. Am. Chem. Soc. 1992, 114, 585–592. [Google Scholar] [CrossRef]
- Finke, R.G.; Hay, B.P. Thermolysis of adenosylcobalamin: A product, kinetic, and cobalt-carbon (C5’) bond dissociation energy study. Inorg. Chem. 1984, 23, 3041–3043. [Google Scholar] [CrossRef]
- Finke, R.G. Coenzyme B12-based chemical precedent for Co-C bond homolysis and other key elementary steps. In Vitamin B12 and B12-Proteins; Krautler, B., Arigoni, D., Golding, B.J., Eds.; Wiley-VCH: Weinheim, Germany, 1998; pp. 383–402. [Google Scholar]
- Schrauzer, G.N.; Deutsch, E. Reactions of Cobalt(I) Supernucleophiles: The Alkylations of Vitamin B12s, Cobaloximes(I), and Related Compounds. J. Am. Chem. Soc. 1969, 91, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, R.; Guha, A.; Li, Z.; Ruetz, M.; An, S.; Seravalli, J.; Banerjee, R. Cobalt–Sulfur Coordination Chemistry Drives B12 Loading onto Methionine Synthase. J. Am. Chem. Soc. 2023, 145, 24678–24689. [Google Scholar] [CrossRef]
- Esser, A.J.; Sastre, S.; Dinh, T.J.; Tanner, V.; Wingert, V.; Klotz, K.; Jacobsen, D.W.; Spiekerkoetter, U.; Schilling, O.; Zeida, A.; et al. A Noncatalytic Cysteine Residue Modulates Cobalamin Reactivity in the Human B(12) Processing Enzyme CblC. Biochemistry 2025, 64, 692–709. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Ragsdale, S.W. The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Ann. Rev. Biochem. 2003, 72, 209–247. [Google Scholar] [CrossRef]
- Ludwig, M.L.; Matthews, R.G. Structure-based perspectives on B12-dependent enzymes. Annu. Rev. Biochem. 1997, 66, 269–313. [Google Scholar] [CrossRef]
- Kumar, M.; Hirao, H.; Kozlowski, P.M. Co+–H interaction inspired alternate coordination geometries of biologically important cob(I)alamin: Possible structural and mechanistic consequences for methyltransferases. J. Biol. Inorg. Chem. 2012, 17, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, C.D.; Bernardo-García, N.; Fradale, L.; Grimaldi, S.; Guillot, A.; Brewee, C.; Chavas, L.M.G.; Legrand, P.; Benjdia, A.; Berteau, O. Crystallographic snapshots of a B12-dependent radical SAM methyltransferase. Nature 2022, 602, 336–342. [Google Scholar] [CrossRef]
- Matthews, R.G. Cobalamin-dependent methyltransferases. Acc. Chem. Res. 2001, 34, 681–689. [Google Scholar] [CrossRef]
- Guéant, J.L.; Guéant-Rodriguez, R.M.; Kosgei, V.J.; Coelho, D. Causes and consequences of impaired methionine synthase activity in acquired and inherited disorders of vitamin B12 metabolism. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 133–155. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Gouda, H.; Ruetz, M.; Banerjee, R. Chapter Twelve—Human B12-dependent enzymes: Methionine synthase and Methylmalonyl-CoA mutase. In Methods in Enzymology; Marsh, E.N.G., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 668, pp. 309–326. [Google Scholar]
- Bennett, M.R.; Shepherd, S.A.; Cronin, V.A.; Micklefield, J. Recent advances in methyltransferase biocatalysis. Curr. Opin. Chem. Biol. 2017, 37, 97–106. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, J.; Wang, H.; Wei, Z.; Wang, W.; Hu, J.; Dong, M. B12-Dependent Radical SAM Enzymes Catalyze C-Fluoromethylation via a CH2F-Cobalamin Intermediate. Angew. Chem. Int. Ed. 2025, 64, e202419815. [Google Scholar] [CrossRef] [PubMed]
- Struck, A.-W.; Thompson, M.L.; Wong, L.S.; Micklefield, J. S-Adenosyl-Methionine-Dependent Methyltransferases: Highly Versatile Enzymes in Biocatalysis, Biosynthesis and Other Biotechnological Applications. Chem. Bio. Chem. 2012, 13, 2642–2655. [Google Scholar] [CrossRef]
- Tengg, M.; Stecher, H.; Offner, L.; Plasch, K.; Anderl, F.; Weber, H.; Schwab, H.; Gruber-Khadjawi, M. Methyltransferases: Green Catalysts for Friedel–Crafts Alkylations. Chem. Cat Chem. 2016, 8, 1354–1360. [Google Scholar] [CrossRef]
- Wolthers, K.R.; Scrutton, N.S. Protein interactions in the human methionine synthase-methionine synthase reductase complex and implications for the mechanism of enzyme reactivation. Biochemistry 2007, 46, 6696–6709. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Bayachou, M.; Tejero, J.; Kenney, C.T.; Pearl, N.M.; Im, S.C.; Waskell, L.; Stuehr, D.J. Distinct conformational behaviors of four mammalian dual-flavin reductases (cytochrome P450 reductase, methionine synthase reductase, neuronal nitric oxide synthase, endothelial nitric oxide synthase) determine their unique catalytic profiles. FEBS J. 2014, 281, 5325–5340. [Google Scholar] [CrossRef] [PubMed]
- Brimberry, M.A.; Mathew, L.; Lanzilotta, W. Making and breaking carbon-carbon bonds in class C radical SAM methyltransferases. J. Inorg. Biochem. 2022, 226, 111636. [Google Scholar] [CrossRef]
- Marsh, E.N.G.; Román Meléndez, G.D. Adenosylcobalamin enzymes: Theory and experiment begin to converge. Biochim. Biophs. Acta 2012, 1824, 1154–1164. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Ruetz, M.; McDevitt, L.; Koutmos, M.; Banerjee, R. Mobile loop dynamics in adenosyltransferase control binding and reactivity of coenzyme B12. Proc. Natl. Acad. Sci. USA 2020, 117, 30412–30422. [Google Scholar] [CrossRef]
- Toraya, T.; Tobimatsu, T.; Mori, K.; Yamanishi, M.; Shibata, N. Coenzyme B(12)-dependent eliminases: Diol and glycerol dehydratases and ethanolamine ammonia-lyase. Methods Enzym. 2022, 668, 181–242. [Google Scholar]
- Sintchak, M.D.; Arjara, G.; Kellogg, B.A.; Stubbe, J.; Drennan, C.L. The crystal structure of class II ribonucleotide reductase reveals how an allosterically regulated monomer mimics a dimer. Nat. Struct. Biol. 2002, 9, 293–300. [Google Scholar] [CrossRef]
- Drennan, C.L.; Huang, S.; Drummond, J.T.; Matthews, R.G.; Ludwig, M.L. How a protein binds B12: A 3.0 A X-ray structure of B12-binding domains of methionine synthase. Science 1994, 266, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.B.; Marques, H.M. Fifty years of X-ray crystallography of vitamin B-12 and its derivatives. S. Afr. J. Sci. 2004, 100, 368–380. [Google Scholar]
- Jensen, K.P.; Ryde, U. The axial N-base has minor influence on Co–C bond cleavage in cobalamins. J. Mol. Struct. (THEOCHEM) 2002, 585, 239–255. [Google Scholar] [CrossRef]
- Andruniow, T.; Zgierski, M.Z.; Kozlowski, P.M. Theoretical Determination of the Co-C Bond Energy Dissociation in Cobalamins. J. Am. Chem. Soc. 2001, 123, 2679–2680. [Google Scholar] [CrossRef]
- Dölker, N.; Maseras, F.; Lledós, A. Density functional study on the effect of the trans axial ligand of B12 cofactors on the heterolytic cleavage of the Co-C bond. J. Phys. Chem. B 2003, 107, 306–315. [Google Scholar] [CrossRef]
- Darbyshire, A.L.; Wolthers, K.R. Characterization of a Structurally Distinct ATP-Dependent Reactivating Factor of Adenosylcobalamin-Dependent Lysine 5,6-Aminomutase. Biochemistry 2024, 63, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Banerjee, R. Thermodynamic and Kinetic Characterization of Co−C Bond Homolysis Catalyzed by Coenzyme B12-Dependent Methylmalonyl-CoA Mutase. Biochemistry 2000, 39, 7998–8006. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.H. Radical mechanisms in adenosylcobalamin-dependent enzymes. Curr. Op. Chem. Biol. 2004, 8, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Padmakumar, R.; Banerjee, R. Evidence that cobalt-carbon bond homolysis is coupled to hydrogen atom abstraction from substrate in methylmalonyl-CoA mutase. Biochemistry 1997, 36, 3713–3718. [Google Scholar] [CrossRef]
- Hay, B.P.; Finke, R.G. Thermolysis of the cobalt-carbon bond of adenosylcobalamin. 2. Products, kinetics, and cobalt-carbon bond dissociation energy in aqueous solution. J. Am. Chem. Soc. 1986, 108, 4820–4829. [Google Scholar] [CrossRef]
- Hay, B.P.; Finke, R.G. Thermolysis of the cobalt-carbon bond in adenosylcorrins. 3. Quantification of the axial base effect in adenosylcobalamin by the synthesis and thermolysis of axial base-free adenosylcobinamide. Insights into the energetics of enzyme-assisted cobalt-carbon bond homolysis. J. Am. Chem. Soc. 1987, 109, 8012–8018. [Google Scholar]
- Marzilli, L.G.; Toscano, P.J.; Randaccio, L.; Bresciani-Pahor, N.; Calligaris, M. An unusually long Co-C bond. Molecular structure of trans-Bis(dimethylglyoximato)(isopropyl)(pyridine)cobalt(III). Implications with regard to the conformational trigger mechanism of cobalt-carbon bond cleavage in coenzyme B12. J. Am. Chem. Soc. 1979, 101, 6754–6756. [Google Scholar] [CrossRef]
- Ng, F.T.T.; Rempel, G.L. Ligand effects on transition metal-alkyl bond dissociation energies. J. Am. Chem. Soc. 1982, 104, 621–623. [Google Scholar] [CrossRef]
- Geno, M.K.; Halpern, J. Why does nature not use the porphyrin ligand in vitamin B12? J. Am. Chem. Soc. 1987, 109, 1238–1240. [Google Scholar] [CrossRef]
- Brown, K.L.; Brooks, H.B. Effects of Axial Ligation on the Thermolysis of Benzylcobamides and Neopentylcobamides—Analysis of the Base-on Effect. Inorg. Chem. 1991, 30, 3420–3430. [Google Scholar] [CrossRef]
- Kräutler, B.; Konrat, R.; Stupperich, E.; Färber, G.; Gruber, K.; Kratky, C. Direct Evidence for the Conformational Deformation of the Corrin Ring by the Nucleotide Base in Vitamin B12: Synthesis and Solution Spectroscopic and Crystal Structure Analysis of Co.beta.-Cyanoimidazolylcobamide. Inorg. Chem. 1994, 33, 4128–4139. [Google Scholar]
- Banerjee, R.; Chowdhury, S. Methylmalonyl-CoA mutase. In Chemistry and Biochemistry of B12; Banerjee, R., Ed.; Wiley Interscience: New York, NY, USA, 1999; pp. 707–729. [Google Scholar]
- Sharma, P.K.; Chu, Z.T.; Olsson, M.H.M.; Warshel, A. A New Paradigm for Electrostatic Catalysis of Radical Reactions in Vitamin B12 Enzymes. Proc. Natl. Acad. Sci. USA 2007, 104, 9661–9666. [Google Scholar] [CrossRef]
- Gruber, K.; Csitkovits, V.; Łyskowski, A.; Kratky, C.; Kräutler, B. Structure-Based Demystification of Radical Catalysis by a Coenzyme B12 Dependent Enzyme—Crystallographic Study of Glutamate Mutase with Cofactor Homologues. Angew Chem. Int. Edn. 2022, 61, e202208295. [Google Scholar] [CrossRef]
- Wang, M.; Warncke, K. Entropic Origin of Cobalt−Carbon Bond Cleavage Catalysis in Adenosylcobalamin-Dependent Ethanolamine Ammonia-Lyase. J. Am. Chem. Soc. 2013, 135, 15077–15084. [Google Scholar] [CrossRef]
- Elmendorf, L.D.; Hall, R.L.; Costa, F.G.; Escalante-Semerena, J.C.; Brunold, T.C. Spectroscopic and Computational Insights into the Mechanism of Cofactor Cobalt–Carbon Bond Homolysis by the Adenosylcobalamin-Dependent Enzyme Ethanolamine Ammonia-Lyase. J. Am. Chem. Soc. 2025, 147, 2380–2392. [Google Scholar] [CrossRef]
- Licht, S.S.; Lawrence, C.C.; Stubbe, J. Thermodynamic and Kinetic Studies on Carbon- Cobalt Bond Homolysis by Ribonucleoside Triphosphate Reductase: The Importance of Entropy in Catalysis. Biochemistry 1999, 38, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Toraya, T. Structural Basis for the Activation of the Cobalt-Carbon Bond and Control of the Adenosyl Radical in Coenzyme B12 Catalysis. Chem. Bio. Chem. 2023, 24, e202300021. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W.; Friedrich, P.; Golding, B.T. Hydrogen Bonds Guide the Short-Lived 5′-Deoxyadenosyl Radical to the Place of Action. Angew. Chem. Int. Ed. 2012, 51, 9974–9976. [Google Scholar] [CrossRef]
- Marsh, E.N.G.; Holloway, D.E. Cloning and sequencing of glutamate mutase component S from Clostridium tetanomorphum Homologies with other cobalamin-dependent enzymes. FEBS Lett. 1992, 310, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Ratnatilleke, A.; Vrijbloed, J.W.; Robinson, J.A. Cloning and Sequencing of the Coenzyme B12-binding Domain of Isobutyryl-CoA Mutase from Streptomyces cinnamonensis, Reconstitution of Mutase Activity, and Characterization of the Recombinant Enzyme Produced in Escherichia coli. J. Biol. Chem. 1999, 274, 31679–31685. [Google Scholar] [CrossRef]
- Beatrix, B.; Zelder, O.; Linder, D.; Buckel, W. Cloning, sequencing and expression of the gene encoding the coenzyme B12-dependent 2-methyleneglutarate mutase from Clostridium barkeri in Escherichia coli. Eur. J. Biochem. 1994, 221, 101–109. [Google Scholar] [CrossRef]
- Jensen, K.P.; Ryde, U. How the Co−C bond is Cleaved in Coenzyme B12 Enzymes: A Theoretical Study. J. Am. Chem. Soc. 2005, 127, 9117–9128. [Google Scholar] [CrossRef]
- Payne, K.A.P.; Quezada, C.P.; Fisher, K.; Dunstan, M.S.; Collins, F.A.; Sjuts, H.; Levy, C.; Hay, S.; Rigby, S.E.J.; Leys, D. Reductive dehalogenase structure suggests a mechanism for B12-dependent dehalogenation. Nature 2015, 517, 513–516. [Google Scholar] [CrossRef]
- Miller, E.; Wohlfarth, G.; Diekert, G. Studies on tetrachloroethene respiration in Dehalospirillum multivorans. Arch. Microbiol. 1996, 166, 379–387. [Google Scholar] [CrossRef]
- Holliger, C.; Hahn, D.; Harmsen, H.; Ludwig, W.; Schumacher, W.; Tindall, B.; Vazquez, F.; Weiss, N.; Zehnder, A.J.B. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra-and trichloroethene in an anaerobic respiration. Arch. Microbiol. 1998, 169, 313–321. [Google Scholar] [CrossRef]
- Liao, R.-Z.; Chen, S.-L.; Siegbahn, P.E.M. Which Oxidation State Initiates Dehalogenation in the B12-Dependent Enzyme NpRdhA: CoII, CoI, or Co0? ACS Catal. 2015, 5, 7350–7358. [Google Scholar] [CrossRef]
- Gantzer, C.J.; Wackett, L.P. Reductive dechlorination catalyzed by bacterial transition-metal coenzymes. Environ. Sci. Technol. 1991, 25, 715–722. [Google Scholar] [CrossRef]
- Marques, H.M. Corrins and porphyrins: Two of nature’s pigments of life. J. Coord. Chem. 2024, 77, 1161–1210. [Google Scholar] [CrossRef]
- Scott, A.I. Discovering Nature’s Diverse Pathways to Vitamin B12: A 35-Year Odyssey. J. Org. Chem. 2003, 68, 2529–2539. [Google Scholar] [CrossRef]
- Schubert, T. The organohalide-respiring bacterium Sulfurospirillum multivorans: A natural source for unusual cobamides. World J. Microbiol. Biotechnol. 2017, 33, 93. [Google Scholar] [CrossRef]
- Battersby, A.R. Tetrapyrroles: The pigments of life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef]
- Eschenmoser, A. Robert Robinson Lecture. Post-B12 problems in corrin synthesis. Chem. Soc. Rev. 1976, 5, 377–410. [Google Scholar] [CrossRef]
- Eschenmoser, A. Vitamin B12: Experiments Concerning the Origin of Its Molecular Structure. Angew. Chem. Int. Ed. Engl. 1988, 27, 5–39. [Google Scholar] [CrossRef]
- Jensen, K.P.; Ryde, U. Comparison of the chemical properties of iron and cobalt porphyrins and corrins. Chem. Bio. Chem. 2003, 4, 413–424. [Google Scholar] [CrossRef]
- Jensen, K.P.; Ryde, U. Comparison of chemical properties of iron, cobalt, and nickel porphyrins, corrins, and hydrocorphins. J. Porph. Phthal. 2005, 9, 581–606. [Google Scholar] [CrossRef]
- Li Manni, G.; Alavi, A. Understanding the Mechanism Stabilizing Intermediate Spin States in Fe(II)-Porphyrin. J. Phys. Chem. A 2018, 122, 4935–4947. [Google Scholar] [CrossRef] [PubMed]
- Rovira, C.; Kunc, K.; Hutter, J.; Parrinello, M. Structural and Electronic Properties of Co-corrole, Co-corrin, and Co-porphyrin. Inorg. Chem. 2001, 40, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bieganowski, R.; Friedrich, W. Preparation and some properties of ferribalamin, the Fe(III)-analogue of vitamin B12. FEBS Lett. 1979, 97, 325–326. [Google Scholar]
- Dozza, B.; Rodrigues, B.M.; Tisoco, I.; de Souza, V.B.; Angnes, L.; Iglesias, B.A. Spectroelectrochemistry as a powerful technique for porphyrins/corroles derivatives electro-characterization: Fundamentals and some examples. Microchem. J. 2022, 183, 108041. [Google Scholar] [CrossRef]
- Lexa, D.; Mispelter, J.; Saveant, J.M. Electroreductive alkylation of iron in porphyrin complexes. Electrochemical and spectral characteristics of σ-alkylironporphyrins. J. Am. Chem. Soc. 1981, 103, 6806–6812. [Google Scholar] [CrossRef]
- Battioni, J.-P.; Dupré, D.; Mansuy, D. Synthèse de complexes σ-vinyliques de ferriporphyrines et leur oxydation en N-vinyl-porphyrines: Rétention de la stéréochimie de la double liaison. J. Organomet. Chem. 1987, 328, 173–184. [Google Scholar] [CrossRef]
- Riordan, C.G.; Halpern, J. Kinetics, mechanism and thermodynamics of iron carbon bond dissociation in organoiron porphyrin complexes. Inorg. Chim. Acta 1996, 243, 19–24. [Google Scholar] [CrossRef]
- Arafa, I.M.; Shin, K.; Goff, H.M. Carbon monoxide and carbon dioxide carbon-metal bond insertion chemistry of alkyliron(III) porphyrin complexes. J. Am. Chem. Soc. 1988, 110, 5228–5229. [Google Scholar] [CrossRef]
- Pratt, J.M. Nature’s design and use of catalysts based on Co and the macrocyclic corrin ligand: 4 x 109 years of coordination chemistry. Pure Appl. Chem. 1993, 65, 1513–1520. [Google Scholar] [CrossRef]
- Cao, L.; Caldararu, O.; Ryde, U. Protonation and Reduction of the FeMo Cluster in Nitrogenase Studied by Quantum Mechanics/Molecular Mechanics (QM/MM) Calculations. J. Chem. Theory Comp. 2018, 14, 6653–6678. [Google Scholar] [CrossRef]
- Hu, Y.; Ribbe, M.W. Nitrogenases—A Tale of Carbon Atom(s). Angew. Chem. Int. Ed. 2016, 55, 8216–8226. [Google Scholar] [CrossRef] [PubMed]
- Raugei, S.; Seefeldt, L.C.; Hoffman, B.M. Critical computational analysis illuminates the reductive-elimination mechanism that activates nitrogenase for N2 reduction. Proc. Natl. Acad. Sci. USA 2018, 115, E10521–E10530. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Goenrich, M.; Schick, M.; Hiromoto, T.; Shima, S. Hydrogenases from Methanogenic Archaea, Nickel, a Novel Cofactor, and H2 Storage. Ann. Rev. Biochem. 2010, 79, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Pilak, O.; Vogt, S.; Schick, M.; Stagni, M.S.; Meyer-Klaucke, W.; Warkentin, E.; Thauer, R.K.; Ermler, U. The Crystal Structure of [Fe]-Hydrogenase Reveals the Geometry of the Active Site. Science 2008, 321, 572–575. [Google Scholar] [CrossRef]
- Byer, A.S.; Yang, H.; McDaniel, E.C.; Kathiresan, V.; Impano, S.; Pagnier, A.; Watts, H.; Denler, C.; Vagstad, A.L.; Piel, J.; et al. Paradigm Shift for Radical S-Adenosyl-L-methionine Reactions: The Organometallic Intermediate Ω Is Central to Catalysis. J. Am. Chem. Soc. 2018, 140, 8634–8638. [Google Scholar] [CrossRef] [PubMed]
- Broderick, W.E.; Hoffman, B.M.; Broderick, J.B. Mechanism of Radical Initiation in the Radical S-Adenosyl-l-methionine Superfamily. Acc. Chem. Res. 2018, 51, 2611–2619. [Google Scholar] [CrossRef]
- Dobbek, H.; Svetlitchnyi, V.; Gremer, L.; Huber, R.; Meyer, O. Crystal Structure of a Carbon Monoxide Dehydrogenase Reveals a [Ni–4Fe–5S] Cluster. Science 2001, 293, 1281–1285. [Google Scholar] [CrossRef]
- Jeoung, J.-H.; Dobbek, H. Carbon Dioxide Activation at the Ni,Fe–Cluster of Anaerobic Carbon Monoxide Dehydrogenase. Science 2007, 318, 1461–1464. [Google Scholar] [CrossRef]
- Can, M.; Armstrong, F.A.; Ragsdale, S.W. Structure, Function, and Mechanism of the Nickel Metalloenzymes, CO Dehydrogenase, and Acetyl-CoA Synthase. Chem. Rev. 2014, 114, 4149–4174. [Google Scholar] [CrossRef]
- Duin, E.C. Methyl Coenzyme M Reductase. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1410–1418. [Google Scholar]
- Wongnate, T.; Ragsdale, S.W. The Reaction Mechanism of Methyl-Coenzyme M Reductase. J. Biol. Chem. 2015, 290, 9322–9334. [Google Scholar] [CrossRef]
- Chen, H.; Gan, Q.; Fan, C. Methyl-Coenzyme M Reductase and Its Post-translational Modifications. Front. Microbiol. 2020, 11, 578356. [Google Scholar] [CrossRef] [PubMed]
- Wongnate, T.; Sliwa, D.; Ginovska, B.; Smith, D.; Wolf, M.W.; Lehnert, N.; Raugei, S.; Ragsdale, S.W. The radical mechanism of biological methane synthesis by methyl-coenzyme M reductase. Science 2016, 352, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Boer, J.L.; Mulrooney, S.B.; Hausinger, R.P. Nickel-dependent metalloenzymes. Arch. Biochem. Biophys. 2014, 544, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, R.; Cox, D.L.; Phillips, G.O. Effects of gamma radiation on vitamin B12 systems. J. Chem. Soc. Faraday Trans. 1 1972, 68, 1687–1696. [Google Scholar] [CrossRef]
- Abel, E.W.; Pratt, J.M.; Whelan, E.; Wilkinson, P.J. The mechanism of oxidation of vitamin B12r by oxygen. S. Afr. J. Chem. 1977, 30, 1–12. [Google Scholar]
- Hoffman, B.M.; Petering, D.H. Coboglobins: Oxygen-Carrying Cobalt-Reconstituted Hemoglobin and Myoglobin. Proc. Natl. Acad. Sci. USA 1970, 67, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.C.; Chien, J.C.W. Comparative Biological Chemistry of Cobalt Hemoglobin. J. Biol. Chem. 1973, 248, 5005–5011. [Google Scholar] [CrossRef]
- Stynes, H.C.; Ibers, J.A. Thermodynamics of the reversible oxygenation of amine complexes of cobalt(II) protoporphyrin IX dimethyl ester in a nonaqueous medium. J. Am. Chem. Soc. 1972, 94, 1559–1562. [Google Scholar] [CrossRef]
- Hsu, G.C.; Spilburg, C.A.; Bull, C.; Hoffman, B.M. Coboglobins: Heterotropic Linkage and the Existence of a Quaternary Structure Change Upon Oxygenation of Cobaltohemoglobin. Proc. Natl. Acad. Sci. USA 1972, 69, 2122–2124. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.A. Reactions of monomeric cobalt-oxygen complexes. I. Thermodynamics of reaction of molecular oxygen with five- and six-coordinate amine complexes of a cobalt porphyrin. J. Am. Chem. Soc. 1973, 95, 1154–1159. [Google Scholar] [CrossRef]
- Wang, M.Y.; Hoffman, B.M.; Hollenberg, P.F. Cobalt-substituted horseradish peroxidase. J. Biol. Chem. 1977, 252, 6268–6275. [Google Scholar] [CrossRef] [PubMed]
- Drago, R.S.; Beugelsdijk, T.; Breese, J.A.; Cannady, J.P. The relationship of thermodynamic data for base adduct formation with cobalt protoporphyrin IX dimethyl ester to the corresponding enthalpies of forming dioxygen adducts with implications to oxygen binding cooperativity. J. Am. Chem. Soc. 1978, 100, 5374–5382. [Google Scholar] [CrossRef]
- Wagner, G.C.; Gunsalus, I.C.; Wang, M.Y.; Hoffman, B.M. Cobalt-substituted cytochrome P-450cam. J. Biol. Chem. 1981, 256, 6266–6273. [Google Scholar] [CrossRef]
- Marden, M.C.; Kiger, L.; Poyart, C.; Rashid, A.K.; Kister, J.; Stetzkowski-Marden, F.; Caron, G.; Haque, M.; Moens, L. Modulation of the oxygen affinity of cobalt-porphyrin by globin. FEBS Lett. 2000, 472, 221–224. [Google Scholar] [CrossRef]
- Brucker, E.A.; Olson, J.S.; Phillips, G.N.; Dou, Y.; Ikeda-Saito, M. High Resolution Crystal Structures of the Deoxy, Oxy, and Aquomet Forms of Cobalt Myoglobin*. J. Biol. Chem. 1996, 271, 25419–25422. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J. Mechanisms of coenzyme B12 dependent rearrangements. Science 1985, 227, 869–875. [Google Scholar] [CrossRef]
- Broderick, J.B.; Broderick, W.E.; Hoffman, B.M. Radical SAM enzymes: Nature’s choice for radical reactions. FEBS Lett. 2023, 597, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L.; Hausinger, R.P. Microbial ureases: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [CrossRef]
- Song, X.; Fiati Kenston, S.S.; Kong, L.; Zhao, J. Molecular mechanisms of nickel induced neurotoxicity and chemoprevention. Toxicology 2017, 392, 47–54. [Google Scholar] [CrossRef]
- Alfano, M.; Cavazza, C. Structure, function, and biosynthesis of nickel-dependent enzymes. Protein Sci. 2020, 29, 1071–1089. [Google Scholar] [CrossRef]
- Macomber, L.; Hausinger, R.P. Mechanisms of nickel toxicity in microorganisms. Metallomics 2011, 3, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Filipovic, D.; Bhattacharya, S.; Cuddapah, S. Epigenetic toxicity of heavy metals—Implications for embryonic stem cells. Environ. Int. 2024, 193, 109084. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Usman, K.; Alsafran, M. Ecological impacts and potential hazards of nickel on soil microbes, plants, and human health. Chemosphere 2024, 357, 142028. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, S.; Jain, A.; Yadav, S.; Dubey, A.; Trivedi, S.P. A review on heavy metal-induced toxicity in fishes: Bioaccumulation, antioxidant defense system, histopathological manifestations, and transcriptional profiling of genes. J. Trace Elem. Med. Biol. 2024, 83, 127377. [Google Scholar] [CrossRef]
- Yu, H.; Li, W.; Liu, X.; Song, Q.; Li, J.; Xu, J. Physiological and molecular bases of the nickel toxicity responses in tomato. Stress Biol. 2024, 4, 25. [Google Scholar] [CrossRef]
- Mao, X.; Ahmad, B.; Hussain, S.; Azeem, F.; Waseem, M.; Alhaj Hamoud, Y.; Shaghaleh, H.; Abeed, A.H.A.; Rizwan, M.; Yong, J.W.H. Microbial assisted alleviation of nickel toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2025, 289, 117669. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Nickel-based Enzyme Systems. J. Biol. Chem. 2009, 284, 18571–18575. [Google Scholar] [CrossRef]
- Campeciño, J.O.; Maroney, M.J. Reinventing the Wheel: The NiSOD Story. In The Biological Chemistry of Nickel; Zamble, D., Rowińska-Żyrek, M., Kozlowski, H., Eds.; The Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Yao, M.; Tu, W.; Chen, X.; Zhan, C.-G. Reaction pathways and free energy profiles for spontaneous hydrolysis of urea and tetramethylurea: Unexpected substituent effects. Org. Biomol. Chem. 2013, 11, 7595–7605. [Google Scholar] [CrossRef]
- Maier, R.J.; Benoit, S.L. Role of Nickel in Microbial Pathogenesis. Inorganics 2019, 7, 80. [Google Scholar] [CrossRef]
- Rutherford, J.C. The Emerging Role of Urease as a General Microbial Virulence Factor. PLoS Pathog. 2014, 10, e1004062. [Google Scholar] [CrossRef]
- de Brito, B.B.; da Silva, F.A.F.; Soares, A.S.; Pereira, V.A.; Santos, M.L.C.; Sampaio, M.M.; Neves, P.H.M.; de Melo, F.F. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 2019, 25, 5578–5589. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.A.; Micus, P.S.; Sunga, S.A.L.; Mazzei, L.; Ciurli, S.; Meloni, G. Metal selectivity and translocation mechanism characterization in proteoliposomes of the transmembrane NiCoT transporter NixA from Helicobacter pylori. Chem. Sci. 2024, 15, 651–665. [Google Scholar] [CrossRef]
- Zhou, C.; Bhinderwala, F.; Lehman, M.K.; Thomas, V.C.; Chaudhari, S.S.; Yamada, K.J.; Foster, K.W.; Powers, R.; Kielian, T.; Fey, P.D. Urease is an essential component of the acid response network of Staphylococcus aureus and is required for a persistent murine kidney infection. PLoS Pathog. 2019, 15, e1007538. [Google Scholar] [CrossRef] [PubMed]
- Clemens, D.L.; Lee, B.Y.; Horwitz, M.A. Purification, characterization, and genetic analysis of Mycobacterium tuberculosis urease, a potentially critical determinant of host-pathogen interaction. J. Bacteriol. 1995, 177, 5644–5652. [Google Scholar] [CrossRef]
- Nim, Y.S.; Fong, I.Y.H.; Deme, J.; Tsang, K.L.; Caesar, J.; Johnson, S.; Pang, L.T.H.; Yuen, N.M.H.; Ng, T.L.C.; Choi, T.; et al. Delivering a toxic metal to the active site of urease. Sci. Adv. 2023, 9, eadf7790. [Google Scholar] [CrossRef]
- Tsang, K.L.; Wong, K.B. Moving nickel along the hydrogenase-urease maturation pathway. Metallomics 2022, 14, mfac003. [Google Scholar] [CrossRef]
- Kumar, S.; Vinella, D.; De Reuse, H. Nickel, an essential virulence determinant of Helicobacter pylori: Transport and trafficking pathways and their targeting by bismuth. Adv. Microb. Physiol. 2022, 80, 1–33. [Google Scholar]
- Masetti, M.; Bertazzo, M.; Recanatini, M.; Ciurli, S.; Musiani, F. Probing the transport of Ni(II) ions through the internal tunnels of the Helicobacter pylori UreDFG multimeric protein complex. J. Inorg. Biochem. 2021, 223, 111554. [Google Scholar] [CrossRef] [PubMed]
- Dixon, N.E.; Gazzola, C.; Blakeley, R.L.; Zerner, B. Jack bean urease (EC 3.5.1.5). Metalloenzyme. Simple biological role for nickel. J. Am. Chem. Soc. 1975, 97, 4131–4133. [Google Scholar] [CrossRef]
- Mazzei, L.; Musiani, F.; Ciurli, S. The structure-based reaction mechanism of urease, a nickel dependent enzyme: Tale of a long debate. J. Biol. Inorg. Chem. 2020, 25, 829–845, Erratum in Commun. Math. Phys. 1976, 46, 206. [Google Scholar] [CrossRef]
- Pearson, M.A.; Michel, L.O.; Hausinger, R.P.; Karplus, P.A. Structures of Cys319 Variants and Acetohydroxamate-Inhibited Klebsiella aerogenes Urease. Biochemistry 1997, 36, 8164–8172. [Google Scholar] [CrossRef] [PubMed]
- Dixon, N.E.; Riddles, P.W.; Gazzola, C.; Blakeley, R.L.; Zerner, B. Jack bean urease (EC 3.5.1.5). V. On the mechanism of action of urease on urea, formamide, acetamide, N-methylurea, and related compounds. Can. J. Biochem. 1980, 58, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Karplus, P.A.; Pearson, M.A.; Hausinger, R.P. 70 Years of Crystalline Urease: What Have We Learned? Acc. Chem. Res. 1997, 30, 330–337. [Google Scholar] [CrossRef]
- Zambelli, B.; Musiani, F.; Benini, S.; Ciurli, S. Chemistry of Ni2+ in Urease: Sensing, Trafficking, and Catalysis. Acc. Chem. Res. 2011, 44, 520–530. [Google Scholar] [CrossRef]
- Martins, C.O.; Sebastiany, L.K.; Lopez-Castillo, A.; Freitas, R.S.; Andrade, L.H.; Toma, H.E.; Netto, C.G.C.M. Urea Decomposition Mechanism by Dinuclear Nickel Complexes. Molecules 2023, 28, 1659. [Google Scholar] [CrossRef]
- Saito, T.; Takano, Y. QM/MM Molecular Dynamics Simulations Revealed Catalytic Mechanism of Urease. J. Phys. Chem. B 2022, 126, 2087–2097. [Google Scholar] [CrossRef]
- Mazzei, L.; Tria, G.; Ciurli, S.; Cianci, M. Exploring the conformational space of the mobile flap in Sporosarcina pasteurii urease by cryo-electron microscopy. Int. J. Biol. Macromol. 2024, 283, 137904. [Google Scholar] [CrossRef]
- Mazzei, L.; Paul, A.; Cianci, M.; Devodier, M.; Mandelli, D.; Carloni, P.; Ciurli, S. Kinetic and structural details of urease inactivation by thiuram disulphides. J. Inorg. Biochem. 2024, 250, 112398. [Google Scholar] [CrossRef]
- Mazzei, L.; Cianci, M.; Ciurli, S. Inhibition of Urease by Hydroquinones: A Structural and Kinetic Study. Chemistry 2022, 28, e202201770. [Google Scholar] [CrossRef]
- Evans, P.N.; Boyd, J.A.; Leu, A.O.; Woodcroft, B.J.; Parks, D.H.; Hugenholtz, P.; Tyson, G.W. An evolving view of methane metabolism in the Archaea. Nat. Rev. Microbiol. 2019, 17, 219–232. [Google Scholar] [CrossRef]
- Timmers, P.H.A.; Welte, C.U.; Koehorst, J.J.; Plugge, C.M.; Jetten, M.S.M.; Stams, A.J.M. Reverse Methanogenesis and Respiration in Methanotrophic Archaea. Archaea 2017, 2017, 1654237. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.F.; Ragsdale, S.W. Activation of Methyl-SCoM Reductase to High Specific Activity after Treatment of Whole Cells with Sodium Sulfide. Biochemistry 1998, 37, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K. Methyl (Alkyl)-Coenzyme M Reductases: Nickel F-430-Containing Enzymes Involved in Anaerobic Methane Formation and in Anaerobic Oxidation of Methane or of Short Chain Alkanes. Biochemistry 2019, 58, 5198–5220. [Google Scholar] [CrossRef] [PubMed]
- Holliger, C.; Pierik, A.J.; Reijerse, E.J.; Hagen, W.R. A spectroelectrochemical study of factor F430 nickel(II/I) from methanogenic bacteria in aqueous solution. J. Am. Chem. Soc. 1993, 115, 5651–5656. [Google Scholar] [CrossRef]
- Grabarse, W.; Mahlert, F.; Shima, S.; Thauer, R.K.; Ermler, U. Comparison of three methyl-coenzyme M reductases from phylogenetically distant organisms: Unusual amino acid modification, conservation and adaptation. J. Molec. Biol. 2000, 303, 329–344. [Google Scholar] [CrossRef]
- Zhou, Y.; Sliwa, D.A.; Ragsdale, S.W. Biochemistry of Methyl-CoM Reductase and Coenzyme F430. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2012; Volume 19, pp. 1–44. [Google Scholar]
- Ohmer, C.J.; Dasgupta, M.; Patwardhan, A.; Bogacz, I.; Kaminsky, C.; Doyle, M.D.; Chen, P.Y.-T.; Keable, S.M.; Makita, H.; Simon, P.S.; et al. XFEL serial crystallography reveals the room temperature structure of methyl-coenzyme M reductase. J. Inorg. Biochem. 2022, 230, 111768. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Oohora, K.; Hayashi, T. Focusing on a nickel hydrocorphinoid in a protein matrix: Methane generation by methyl-coenzyme M reductase with F430 cofactor and its models. Chem. Soc. Rev. 2022, 51, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Sarangi, R.; Ginovska, B.; Raugei, S.; Ragsdale, S.W. Nickel-Sulfonate Mode of Substrate Binding for Forward and Reverse Reactions of Methyl-SCoM Reductase Suggest a Radical Mechanism Involving Long-Range Electron Transfer. J. Am. Chem. Soc. 2021, 143, 5481–5496. [Google Scholar] [CrossRef]
- Wagner, T.; Kahnt, J.; Ermler, U.; Shima, S. Didehydroaspartate Modification in Methyl-Coenzyme M Reductase Catalyzing Methane Formation. Angew. Chem. Int. Ed. 2016, 55, 10630–10633. [Google Scholar] [CrossRef]
- Duin, E.C.; Wagner, T.; Shima, S.; Prakash, D.; Cronin, B.; Yáñez-Ruiz, D.R.; Duval, S.; Rümbeli, R.; Stemmler, R.T.; Thauer, R.K.; et al. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc. Natl. Acad. Sci. USA 2016, 113, 6172–6177. [Google Scholar] [CrossRef]
- Prakash, D.; Wu, Y.; Suh, S.-J.; Duin, E.C. Elucidating the Process of Activation of Methyl-Coenzyme M Reductase. J. Bacteriol. 2014, 196, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Polêto, M.D.; Allen, K.D.; Lemkul, J.A. Structural Dynamics of the Methyl-Coenzyme M Reductase Active Site Are Influenced by Coenzyme F430 Modifications. Biochemistry 2024, 63, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.L.; Vansuch, G.E.; Chica, B.C.; Adams, M.W.W.; Dyer, R.B. Applications of Photogating and Time Resolved Spectroscopy to Mechanistic Studies of Hydrogenases. Acc. Chem. Res. 2017, 50, 2718–2726. [Google Scholar] [CrossRef]
- Tai, H.; Higuchi, Y.; Hirota, S. Comprehensive reaction mechanisms at and near the Ni–Fe active sites of [NiFe] hydrogenases. Dalton Trans. 2018, 47, 4408–4423. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Hirota, S. Mechanism and Application of the Catalytic Reaction of [NiFe] Hydrogenase: Recent Developments. ChemBioChem 2020, 21, 1573–1581. [Google Scholar] [CrossRef]
- Schoelmerich, M.C.; Müller, V. Energy-converting hydrogenases: The link between H(2) metabolism and energy conservation. Cell Mol. Life Sci. 2020, 77, 1461–1481. [Google Scholar] [CrossRef]
- Chenevier, P.; Mugherli, L.; Darbe, S.; Darchy, L.; DiManno, S.; Tran, P.D.; Valentino, F.; Iannello, M.; Volbeda, A.; Cavazza, C.; et al. Hydrogenase enzymes: Application in biofuel cells and inspiration for the design of noble-metal free catalysts for H2 oxidation. Compt. Rend. Chim. 2013, 16, 491–505. [Google Scholar] [CrossRef]
- Ogata, H.; Lubitz, W.; Higuchi, Y. Structure and function of [NiFe] hydrogenases. J. Biochem. 2016, 160, 251–258. [Google Scholar] [CrossRef]
- Kwiatkowski, A.; Caserta, G.; Schulz, A.-C.; Frielingsdorf, S.; Pelmenschikov, V.; Weisser, K.; Belsom, A.; Rappsilber, J.; Sergueev, I.; Limberg, C.; et al. ATP-Triggered Fe(CN)2CO Synthon Transfer from the Maturase HypCD to the Active Site of Apo-[NiFe]-Hydrogenase. J. Am. Chem. Soc. 2024, 146, 30976–30989. [Google Scholar] [CrossRef]
- Schmidt, A.; Kalms, J.; Lorent, C.; Katz, S.; Frielingsdorf, S.; Evans, R.M.; Fritsch, J.; Siebert, E.; Teutloff, C.; Armstrong, F.A.; et al. Stepwise conversion of the Cys6[4Fe–3S] to a Cys4[4Fe–4S] cluster and its impact on the oxygen tolerance of [NiFe]-hydrogenase. Chem. Sci. 2023, 14, 11105–11120. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Nishikawa, K.; Higuchi, Y.; Mao, Z.-w.; Hirota, S. Cysteine SH and Glutamate COOH Contributions to [NiFe] Hydrogenase Proton Transfer Revealed by Highly Sensitive FTIR Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 13285–13290. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Lubitz, W.; Higuchi, Y. [NiFe] hydrogenases: Structural and spectroscopic studies of the reaction mechanism. Dalton Trans. 2009, 37, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Phung, Q.M.; Hallaert, S.D.; Pierloot, K.; Ryde, U. H2 binding to the active site of [NiFe] hydrogenase studied by multiconfigurational and coupled-cluster methods. Phys. Chem. Chem. Phys. 2017, 19, 10590–10601. [Google Scholar] [CrossRef]
- Evans, R.M.; Krahn, N.; Weiss, J.; Vincent, K.A.; Söll, D.; Armstrong, F.A. Replacing a Cysteine Ligand by Selenocysteine in a [NiFe]-Hydrogenase Unlocks Hydrogen Production Activity and Addresses the Role of Concerted Proton-Coupled Electron Transfer in Electrocatalytic Reversibility. J. Am. Chem. Soc. 2024, 146, 16971–16976. [Google Scholar] [CrossRef]
- Tai, H.; Hirota, S.; Stripp, S.T. Proton Transfer Mechanisms in Bimetallic Hydrogenases. Acc. Chem. Res. 2021, 54, 232–241. [Google Scholar] [CrossRef]
- Desguin, B.; Urdiain-Arraiza, J.; Da Costa, M.; Fellner, M.; Hu, J.; Hausinger, R.P.; Desmet, T.; Hols, P.; Soumillion, P. Uncovering a superfamily of nickel-dependent hydroxyacid racemases and epimerases. Sci. Rep. 2020, 10, 18123. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gatreddi, S.; Gupta, S.; Nevarez, J.L.; Rankin, J.A.; Turmo, A.; Hu, J.; Hausinger, R.P. Unveiling the mechanisms and biosynthesis of a novel nickel-pincer enzyme. Biochem. Soc. Trans. 2022, 50, 1187–1196. [Google Scholar] [CrossRef]
- Hausinger, R.P.; Hu, J.; Desguin, B. The nickel-pincer coenzyme of lactate racemase: A case study of uncovering cofactor structure and biosynthesis. Methods Enzym. 2023, 685, 341–371. [Google Scholar]
- Wang, B.; Shaik, S. The Nickel-Pincer Complex in Lactate Racemase Is an Electron Relay and Sink that acts through Proton-Coupled Electron Transfer. Angew. Chem. 2017, 129, 10232–10236. [Google Scholar] [CrossRef]
- Zhang, X.; Chung, L.W. Alternative Mechanistic Strategy for Enzyme Catalysis in a Ni-Dependent Lactate Racemase (LarA): Intermediate Destabilization by the Cofactor. Chem. Eur. J. 2017, 23, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-J.; Chen, S.-L. From NAD+ to Nickel Pincer Complex: A Significant Cofactor Evolution Presented by Lactate Racemase. Chem. Eur. J. 2017, 23, 7545–7557. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Yang, X. A bio-inspired design and computational prediction of scorpion-like SCS nickel pincer complexes for lactate racemization. Chem. Commun. 2017, 53, 11410–11413. [Google Scholar] [CrossRef]
- Rankin, J.A.; Mauban, R.C.; Fellner, M.; Desguin, B.; McCracken, J.; Hu, J.; Varganov, S.A.; Hausinger, R.P. Lactate Racemase Nickel-Pincer Cofactor Operates by a Proton-Coupled Hydride Transfer Mechanism. Biochemistry 2018, 57, 3244–3251. [Google Scholar] [CrossRef]
- Gatreddi, S.; Sui, D.; Hausinger, R.P.; Hu, J. Irreversible inactivation of lactate racemase by sodium borohydride reveals reactivity of the nickel-pincer nucleotide cofactor. ACS Catal. 2023, 13, 1441–1448. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, X. Copper. In Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth; White, W.M., Ed.; Springer: Cham, Switzerland, 2018; pp. 303–305. [Google Scholar]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef] [PubMed]
- Cobine, P.A.; Moore, S.A.; Leary, S.C. Getting out what you put in: Copper in mitochondria and its impacts on human disease. Biochim. Biophs. Acta 2021, 1868, 118867. [Google Scholar] [CrossRef]
- Mydy, L.S.; Chigumba, D.N.; Kersten, R.D. Plant Copper Metalloenzymes As Prospects for New Metabolism Involving Aromatic Compounds. Front. Plant Sci. 2021, 12, 692108. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Johnson, J.E.; Present, T.M.; Valentine, J.S. Iron: Life’s primeval transition metal. Proc. Natl. Acad. Sci. USA 2024, 121, e2318692121. [Google Scholar] [CrossRef]
- Rode, B.M.; Schwendinger, M.G. Copper-catalyzed amino acid condensation in wate—A simple possible way of prebiotic peptide formation. Orig. Life Evol. Biosph. 1990, 20, 401–410. [Google Scholar] [CrossRef]
- Liu, Z.; Mariani, A.; Wu, L.; Ritson, D.; Folli, A.; Murphy, D.; Sutherland, J. Tuning the reactivity of nitriles using Cu(II) catalysis—Potentially prebiotic activation of nucleotides. Chem. Sci. 2018, 9, 7053–7057. [Google Scholar] [CrossRef]
- Fu, X.; Liao, Y.; Aspin, A.; Yang, Z. Effect of Copper Salts on Amide Hydrothermal Formation and Reactivity. ACS Earth Space Chem. 2020, 4, 1596–1603. [Google Scholar] [CrossRef]
- Gaylor, M.O.; Miro, P.; Vlaisavljevich, B.; Kondage, A.A.S.; Barge, L.M.; Omran, A.; Videau, P.; Swenson, V.A.; Leinen, L.J.; Fitch, N.W.; et al. Plausible Emergence and Self Assembly of a Primitive Phospholipid from Reduced Phosphorus on the Primordial Earth. Orig. Life Evol. Biosph. 2021, 51, 185–213. [Google Scholar] [CrossRef]
- Aithal, A.; Dagar, S.; Rajamani, S. Metals in Prebiotic Catalysis: A Possible Evolutionary Pathway for the Emergence of Metalloproteins. ACS Omega 2023, 8, 5197–5208. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Tanwar, L.; Fracassi, A.; Brea, R.J.; Salvador-Castell, M.; Khanal, S.; Sinha, S.K.; Devaraj, N.K. Chemoselective Esterification of Natural and Prebiotic 1,2-Amino Alcohol Amphiphiles in Water. J. Am. Chem. Soc. 2023, 145, 27149–27159. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper Active Sites in Biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar]
- Llases, M.E.; Morgada, M.N.; Vila, A.J. Biochemistry of Copper Site Assembly in Heme-Copper Oxidases: A Theme with Variations. Int. J. Mol. Sci. 2019, 20, 3830. [Google Scholar] [CrossRef]
- Bhadra, M.; Karlin, K.D. Copper Enzymes Involved in Multi-Electron Processes. In Comprehensive Coordination Chemistry III; Constable, E.C., Parkin, G., Que, L., Jr., Eds.; Elsevier: Oxford, UK, 2021; pp. 524–540. [Google Scholar]
- Gao, L.; Zhang, A. Copper-instigated modulatory cell mortality mechanisms and progress in oncological treatment investigations. Front. Immunol. 2023, 14, 1236063. [Google Scholar] [CrossRef]
- Lutsenko, S.; Roy, S.; Tsvetkov, P. Mammalian copper homeostasis: Physiological roles and molecular mechanisms. Physiol. Rev. 2025, 105, 441–491. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Wang, L.; Ji, G.; Dang, Y. Copper homeostasis and cuproptosis in health and disease. Med. Comm. 2024, 5, e724. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular Mechanisms of Plant Responses to Copper: From Deficiency to Excess. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [CrossRef] [PubMed]
- Kosman, D.J. Multicopper oxidases: A workshop on copper coordination chemistry, electron transfer, and metallophysiology. J. Biol. Inorg. Chem. 2010, 15, 15–28. [Google Scholar] [CrossRef]
- Itoh, S. The Role of Copper in Biocatalysis. In Redox-Based Catalytic Chemistry of Transition Metal Complexes; Kojima, T., Ed.; Royal Society of Chemistry: London, UK, 2024; Volume 2, pp. 41–60. [Google Scholar]
- Shim, D.; Han, J. Coordination chemistry of mitochondrial copper metalloenzymes: Exploring implications for copper dyshomeostasis in cell death. BMB Rep. 2023, 56, 575–583. [Google Scholar] [CrossRef]
- Guo, J.; Fisher, O.S. Orchestrating copper binding: Structure and variations on the cupredoxin fold. J. Biol. Inorg. Chem. 2022, 27, 529–540. [Google Scholar] [CrossRef]
- Pérez-Henarejos, S.A.; Alcaraz, L.A.; Donaire, A. Blue Copper Proteins: A rigid machine for efficient electron transfer, a flexible device for metal uptake. Arch. Biochem. Biophys. 2015, 584, 134–148. [Google Scholar] [CrossRef]
- Bím, D.; Alexandrova, A.N. Electrostatic regulation of blue copper sites. Chem. Sci. 2021, 12, 11406–11413. [Google Scholar] [CrossRef]
- Arcos-López, T.; Schuth, N.; Quintanar, L. The Type 1 Blue Copper Site: From Electron Transfer to Biological Function. In Transition Metals and Sulfur—A Strong Relationship for Life; Martha Sosa, T., Peter, K., Eds.; De Gruyter: Berlin, Germany, 2020; pp. 51–90. [Google Scholar]
- Karlin, S.; Zhu, Z.-Y.; Karlin, K.D. The extended environment of mononuclear metal centers in protein structures. Proc. Natl. Acad. Sci. USA 1997, 94, 14225–14230. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 191–248. [Google Scholar]
- Solomon, E.I.; Gorelsky, S.I.; Dey, A. Metal–thiolate bonds in bioinorganic chemistry. J. Comp. Chem. 2006, 27, 1415–1428. [Google Scholar] [CrossRef]
- Solomon, E.I.; Szilagyi, R.K.; DeBeer George, S.; Basumallick, L. Electronic Structures of Metal Sites in Proteins and Models: Contributions to Function in Blue Copper Proteins. Chem. Rev. 2004, 104, 419–458. [Google Scholar] [CrossRef]
- Solomon, E.I.; Jose, A. Spiers Memorial Lecture: Activating metal sites for biological electron transfer. Faraday Discuss. 2022, 234, 9–30. [Google Scholar] [CrossRef]
- Vallee, B.L.; Williams, R.J. Metalloenzymes: The entatic nature of their active sites. Proc. Natl. Acad. Sci. USA 1968, 59, 498–505. [Google Scholar] [CrossRef]
- Williams, R.J.P. Energised (entatic) states of groups and of secondary structures in proteins and metalloproteins. Eur. J. Biochem. 1995, 234, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.B.; Malmstroem, B.G. Long-range electron transfer in multisite metalloproteins. Biochemistry 1989, 28, 7499–7505. [Google Scholar] [CrossRef] [PubMed]
- Comba, P. Coordination compounds in the entatic state. Coord. Chem. Rev. 2000, 200–202, 217–245. [Google Scholar] [CrossRef]
- Comba, P.; Schiek, W. Fit and misfit between ligands and metal ions. Coord. Chem. Rev. 2003, 238–239, 21–29. [Google Scholar] [CrossRef]
- Matyushov, D.V. Reorganization energy of electron transfer. Phys. Chem. Chem. Phys. 2023, 25, 7589–7610. [Google Scholar] [CrossRef]
- Hansen, D.F.; Led, J.J. Determination of the geometric structure of the metal site in a blue copper protein by paramagnetic NMR. Proc. Natl. Acad. Sci. USA 2006, 103, 1738–1743. [Google Scholar] [CrossRef]
- Gray, H.B.; Malmstrom, B.G.; Williams, R.J.P. Copper coordination in blue proteins. J. Biol. Inorg. Chem. 2000, 5, 551–559. [Google Scholar] [CrossRef]
- Kontkanen, O.V.; Biriukov, D.; Futera, Z. Reorganization free energy of copper proteins in solution, in vacuum, and on metal surfaces. J. Chem. Phys. 2022, 156, 175101. [Google Scholar] [CrossRef]
- Bard, A.J. Standard Potentials in Aqueous Solution, 1st ed.; Routeledge: New York, NY, USA, 1985. [Google Scholar]
- Choi, M.; Davidson, V.L. Cupredoxins—A study of how proteins may evolve to use metals for bioenergetic processes. Metallomics 2011, 3, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.J.; Lancaster, K.M.; Richards, J.H.; Gray, H.B. Inner- and outer-sphere metal coordination in blue copper proteins. J. Inorg. Biochem. 2012, 115, 119–126. [Google Scholar] [CrossRef]
- Machczynski, M.C.; Gray, H.B.; Richards, J.H. An outer-sphere hydrogen-bond network constrains copper coordination in blue proteins. J. Inorg. Biochem. 2002, 88, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Mammoser, C.C.; LeMasters, B.E.; Edwards, S.G.; McRae, E.M.; Mullins, M.H.; Wang, Y.; Garcia, N.M.; Edmonds, K.A.; Giedroc, D.P.; Thielges, M.C. The structure of plastocyanin tunes the midpoint potential by restricting axial ligation of the reduced copper ion. Commun. Chem. 2023, 6, 175. [Google Scholar] [CrossRef]
- Lam, Q.; Van Stappen, C.; Lu, Y.; Dikanov, S.A. HYSCORE and QM/MM Studies of Second Sphere Variants of the Type 1 Copper Site in Azurin: Influence of Mutations on the Hyperfine Couplings of Remote Nitrogens. J. Phys. Chem. B 2024, 128, 3350–3359. [Google Scholar] [CrossRef]
- Wang, J.X.; Vilbert, A.C.; Cui, C.; Mirts, E.N.; Williams, L.H.; Kim, W.; Jessie Zhang, Y.; Lu, Y. Increasing Reduction Potentials of Type 1 Copper Center and Catalytic Efficiency of Small Laccase from Streptomyces coelicolor through Secondary Coordination Sphere Mutations. Angew. Chem. Int. Ed. 2023, 62, e202314019. [Google Scholar] [CrossRef]
- Merello Oyarzún, G.; Olivares-Costa, M.; Basile, L.; Pástor, T.P.; Mendoza-Soto, P.; Padilla-Santiago, L.; Mardones, G.A.; Binda, C.; Opazo, J.C. Evolutionary and Functional Analysis of Monoamine Oxidase F: A Novel Member of the Monoamine Oxidase Gene Family. Genome Biol. Evol. 2025, 17, evae280. [Google Scholar] [CrossRef] [PubMed]
- Zaffryar-Eilot, S.; Hasson, P. Lysyl Oxidases: Orchestrators of Cellular Behavior and ECM Remodeling and Homeostasis. Int. J. Mol. Sci. 2022, 23, 11378. [Google Scholar] [CrossRef]
- McGuirl, M.A.; Dooley, D.M. Copper Proteins with Type 2 Sites. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ji, R.; Guan, L.; Hu, Z.; Cheng, Y.; Cai, M.; Zhao, G.; Zang, J. A comprehensive review on hemocyanin from marine products: Structure, functions, its implications for the food industry and beyond. Int. J. Biol. Macromol. 2024, 269, 132041. [Google Scholar] [CrossRef]
- Di Costanzo, L.F. Structural characterization of tyrosinases and an update on human enzymes. In The Enzymes; Supuran, C.T., Ed.; Academic Press: New York, NY, USA, 2024; Volume 56, pp. 55–83. [Google Scholar]
- Pauleta, S.R.; Carepo, M.S.P.; Moura, I. The Tetranuclear Copper-Sulfide Center of Nitrous Oxide Reductase. In Transition Metals and Sulfur—A Strong Relationship for Life; Martha Sosa, T., Peter, K., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2020; pp. 139–164. [Google Scholar]
- Pomowski, A.; Dell’Acqua, S.; Wüst, A.; Pauleta, S.R.; Moura, I.; Einsle, O. Revisiting the metal sites of nitrous oxide reductase in a low-dose structure from Marinobacter nauticus. J. Biol. Inorg. Chem. 2024, 29, 279–290. [Google Scholar] [CrossRef]
- Mellidou, I.; Kanellis, A.K. Revisiting the role of ascorbate oxidase in plant systems. J. Exp. Bot. 2024, 75, 2740–2753. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, T.V.; Sorokin, D.Y.; Hagen, W.R.; Khrenova, M.G.; Muyzer, G.; Rakitina, T.V.; Shabalin, I.G.; Trofimov, A.A.; Tsallagov, S.I.; Popov, V.O. Trinuclear copper biocatalytic center forms an active site of thiocyanate dehydrogenase. Proc. Natl. Acad. Sci. USA 2020, 117, 5280–5290. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, M.; Kim, S.; Wu, M.; Ishida, T.; Momeni, M.H.; Vaaje-Kolstad, G.; Lundberg, D.; Royant, A.; Ståhlberg, J.; Eijsink, V.G.H.; et al. Structural and Electronic Snapshots during the Transition from a Cu(II) to Cu(I) Metal Center of a Lytic Polysaccharide Monooxygenase by X-ray Photoreduction. J. Biol. Chem. 2014, 289, 18782–18792. [Google Scholar] [CrossRef]
- Solomon, E.I.; Augustine, A.J.; Yoon, J. O2 Reduction to H2O by the multicopper oxidases. Dalton Trans. 2008, 30, 3921–3932. [Google Scholar] [CrossRef]
- Gräff, M.; Buchholz, P.C.F.; Le Roes-Hill, M.; Pleiss, J. Multicopper oxidases: Modular structure, sequence space, and evolutionary relationships. Proteins 2020, 88, 1329–1339. [Google Scholar] [CrossRef]
- Sakurai, T.; Kataoka, K. Structure and function of type I copper in multicopper oxidases. Cell Mol. Life Sci. 2007, 64, 2642. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Go, N. Function and molecular evolution of multicopper blue proteins. Cell Mol. Life Sci. 2005, 62, 2050–2066. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Grossmann, J.G.; Eady, R.R.; Hasnain, S.S. Genomic analysis reveals widespread occurrence of new classes of copper nitrite reductases. J. Biol. Inorg. Chem. 2007, 12, 1119–1127. [Google Scholar] [CrossRef]
- Roberts, S.A.; Weichsel, A.; Grass, G.; Thakali, K.; Hazzard, J.T.; Tollin, G.; Rensing, C.; Montfort, W.R. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 2766–2771. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Chong, L.X.; Wedd, A.G.; Xiao, Z. Reaction mechanisms of the multicopper oxidase CueO from Escherichia coli support its functional role as a cuprous oxidase. J. Am. Chem. Soc. 2010, 132, 2005–2015. [Google Scholar] [CrossRef]
- Li, J.; Farrokhnia, M.; Rulíšek, L.; Ryde, U. Catalytic Cycle of Multicopper Oxidases Studied by Combined Quantum- and Molecular-Mechanical Free-Energy Perturbation Methods. J. Phys. Chem. B 2015, 119, 8268–8284. [Google Scholar] [CrossRef] [PubMed]
- Siegbahn, P.E.M. Theoretical Study of O2 Reduction and Water Oxidation in Multicopper Oxidases. J. Phys. Chem. A 2020, 124, 5849–5855. [Google Scholar] [CrossRef]
- Shin, W.; Sundaram, U.M.; Cole, J.L.; Zhang, H.H.; Hedman, B.; Hodgson, K.O.; Solomon, E.I. Chemical and Spectroscopic Definition of the Peroxide-Level Intermediate in the Multicopper Oxidases: Relevance to the Catalytic Mechanism of Dioxygen Reduction to Water. J. Am. Chem. Soc. 1996, 118, 3202–3215. [Google Scholar] [CrossRef]
- Augustine, A.J.; Quintanar, L.; Stoj, C.S.; Kosman, D.J.; Solomon, E.I. Spectroscopic and Kinetic Studies of Perturbed Trinuclear Copper Clusters: The Role of Protons in Reductive Cleavage of the O−O Bond in the Multicopper Oxidase Fet3p. J. Am. Chem. Soc. 2007, 129, 13118–13126. [Google Scholar] [CrossRef]
- Srnec, M.; Ryde, U.; Rulíšek, L. Reductive cleavage of the O–O bond in multicopper oxidases: A QM/MM and QM study. Faraday Discuss. 2011, 148, 41–53. [Google Scholar] [CrossRef]
- Robinett, N.G.; Peterson, R.L.; Culotta, V.C. Eukaryotic copper-only superoxide dismutases (SODs): A new class of SOD enzymes and SOD-like protein domains. J. Biol. Chem. 2018, 293, 4636–4643. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.H.; Kasamatsu, S.; Ihara, H.; Eaton, P. SOD1 is an essential H(2)S detoxifying enzyme. Proc. Natl. Acad. Sci. USA 2023, 120, e2205044120. [Google Scholar] [CrossRef]
- Franco Cairo, J.P.L.; Mandelli, F.; Tramontina, R.; Cannella, D.; Paradisi, A.; Ciano, L.; Ferreira, M.R.; Liberato, M.V.; Brenelli, L.B.; Gonçalves, T.A.; et al. Oxidative cleavage of polysaccharides by a termite-derived superoxide dismutase boosts the degradation of biomass by glycoside hydrolases. Green Chem. 2022, 24, 4845–4858. [Google Scholar] [CrossRef] [PubMed]
- Montllor-Albalate, C.; Kim, H.; Thompson, A.E.; Jonke, A.P.; Torres, M.P.; Reddi, A.R. Sod1 integrates oxygen availability to redox regulate NADPH production and the thiol redoxome. Proc. Natl. Acad. Sci. USA 2022, 119, e2023328119. [Google Scholar] [CrossRef]
- Fielden, E.M.; Roberts, P.B.; Bray, R.C.; Lowe, D.J.; Mautner, G.N.; Rotilio, G.; Calabrese, L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem. J. 1974, 139, 49–60. [Google Scholar] [CrossRef]
- Hough, M.A.; Hasnain, S.S. Crystallographic structures of bovine copper-zinc superoxide dismutase reveal asymmetry in two subunits: Functionally important three and five coordinate copper sites captured in the same crystal. J. Mol. Biol. 1999, 287, 579–592. [Google Scholar] [CrossRef]
- Pantoliano, M.W.; Valentine, J.S.; Burger, A.R.; Lippard, S.J. A pH-dependent superoxide dismutase activity for zinc-free bovine erythrocuprein. Reexamination of the role of zinc in the holoprotein. J. Inorg. Biochem. 1982, 17, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, A.; Chowdhury, S.; Das, B.; Banerjee, A.; Halder, A.; Kumar, A.; Saleem, M.; Naganathan, A.N.; Karmakar, S.; Chattopadhyay, K. The metal cofactor zinc and interacting membranes modulate SOD1 conformation-aggregation landscape in an in vitro ALS model. Elife 2021, 10, e61453. [Google Scholar] [CrossRef]
- Bakavayev, S.; Chetrit, N.; Zvagelsky, T.; Mansour, R.; Vyazmensky, M.; Barak, Z.; Israelson, A.; Engel, S. Cu/Zn-superoxide dismutase and wild-type like fALS SOD1 mutants produce cytotoxic quantities of H2O2 via cysteine-dependent redox short-circuit. Sci. Rep. 2019, 9, 10826. [Google Scholar] [CrossRef]
- Tainer, J.A.; Getzoff, E.D.; Richardson, J.S.; Richardson, D.C. Structure and mechanism of copper, zinc superoxide dismutase. Nature 1983, 306, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Pelmenschikov, V.; Siegbahn, P.E.M. Copper−Zinc Superoxide Dismutase: Theoretical Insights into the Catalytic Mechanism. Inorg. Chem. 2005, 44, 3311–3320. [Google Scholar] [CrossRef] [PubMed]
- Wuebbles, D.J. Nitrous Oxide: No Laughing Matter. Science 2009, 326, 56–57. [Google Scholar] [CrossRef]
- Arat, S.; Bullerjahn, G.S.; Laubenbacher, R. A Network Biology Approach to Denitrification in Pseudomonas aeruginosa. PLoS ONE 2015, 10, e0118235. [Google Scholar] [CrossRef]
- Zumft, W.G.; Matsubara, T. A novel kind of multi-copper protein as terminal oxidoreductase of nitrous oxide respiration in Pseudomonas perfectomarinus. FEBS Lett. 1982, 148, 107–112. [Google Scholar] [CrossRef]
- Brown, K.; Tegoni, M.; Prudêncio, M.; Pereira, A.S.; Besson, S.; Moura, J.J.; Moura, I.; Cambillau, C. A novel type of catalytic copper cluster in nitrous oxide reductase. Nat. Struct. Biol. 2000, 7, 191–195. [Google Scholar] [CrossRef]
- Farrar, J.A.; Thomson, A.J.; Cheesman, M.R.; Dooley, D.M.; Zumft, W.G. A model of the copper centres of nitrous oxide reductase (Pseudomonas stutzeri). FEBS Lett. 1991, 294, 11–15. [Google Scholar] [CrossRef]
- Rathnayaka, S.C.; Mankad, N.P. Coordination chemistry of the CuZ site in nitrous oxide reductase and its synthetic mimics. Coord. Chem. Rev. 2021, 429, 213718. [Google Scholar] [CrossRef] [PubMed]
- Kroneck, P.M.H.; Antholine, W.A.; Riester, J.; Zumft, W.G. The nature of the cupric site in nitrous oxide reductase and of CuA in cytochrome c oxidase. FEBS Lett. 1989, 248, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Morgada, M.N.; Murgida, D.H.; Vila, A.J. Purple Mixed-Valent Copper A. In Transition Metals and Sulfur—A Strong Relationship for Life; De Gruyter: Berlin, Germany, 2020; pp. 91–138. [Google Scholar]
- Zhang, L.; Bill, E.; Kroneck, P.M.H.; Einsle, O. Histidine-Gated Proton-Coupled Electron Transfer to the CuA Site of Nitrous Oxide Reductase. J. Am. Chem. Soc. 2021, 143, 830–838. [Google Scholar] [CrossRef]
- Carreira, C.; Dos Santos, M.M.C.; Pauleta, S.R.; Moura, I. Proton-coupled electron transfer mechanisms of the copper centres of nitrous oxide reductase from Marinobacter hydrocarbonoclasticus—An electrochemical study. Bioelectrochemistry 2020, 133, 107483. [Google Scholar] [CrossRef]
- Johnston, E.M.; Dell’Acqua, S.; Pauleta, S.R.; Moura, I.; Solomon, E.I. Protonation state of the Cu4S2 CuZ site in nitrous oxide reductase: Redox dependence and insight into reactivity. Chem. Sci. 2015, 6, 5670–5679. [Google Scholar] [CrossRef]
- Johnston, E.M.; Dell’Acqua, S.; Ramos, S.; Pauleta, S.R.; Moura, I.; Solomon, E.I. Determination of the Active Form of the Tetranuclear Copper Sulfur Cluster in Nitrous Oxide Reductase. J. Am. Chem. Soc. 2014, 136, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Carreira, C.; Pauleta, S.R.; Moura, I. The catalytic cycle of nitrous oxide reductase—The enzyme that catalyzes the last step of denitrification. J. Inorg. Biochem. 2017, 177, 423–434. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Rodríguez-López, J.N.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Pretzler, M.; Rompel, A. Beyond Phenolics: Alternative Substrates for Type III Copper Enzymes. Chem. Bio. Chem. 2025, 26, e202400982. [Google Scholar] [CrossRef]
- Ivković, Đ.; Andrić, F.; Senćanski, M.; Stević, T.; Krstić Ristivojević, M.; Ristivojević, P. Innovative analytical methodology for skin anti-aging compounds discovery from plant extracts: Integration of High-Performance Thin-Layer Chromatography-in vitro spectrophotometry bioassays with multivariate modeling and molecular docking. J. Chromatogr. A 2024, 1742, 465640. [Google Scholar] [CrossRef] [PubMed]
- Alruhaimi, R.S.; Mahmoud, A.M.; Alnasser, S.M.; Alotaibi, M.F.; Elbagory, I.; El-Bassuony, A.A.; Lamsabhi, A.M.; Kamel, E.M. Integrating Computational Modeling and Experimental Validation to Unveil Tyrosinase Inhibition Mechanisms of Flavonoids from Alhagi graecorum. ACS Omega 2024, 9, 47167–47179. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Ortiz-Ruiz, C.V.; Berna, J.; Tudela, J.; Garcia-Canovas, F. 4-n-butylresorcinol, a depigmenting agent used in cosmetics, reacts with tyrosinase. IUBMB Life 2016, 68, 663–672. [Google Scholar] [CrossRef]
- Lee, S.Y.; Baek, N.; Nam, T.-g. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, G.; Zeng, Q.-H.; Li, Y.; Liu, H.; Wang, J.J.; Zhao, Y. A systematic review of synthetic tyrosinase inhibitors and their structure-activity relationship. Crit. Rev. Food Sci. Nutr. 2022, 62, 4053–4094. [Google Scholar] [CrossRef]
- Min, X.; Su, Z.; Zhou, H.; Kou, X.; Li, D.; Xu, X. Insights into inhibitory action and interaction of bisdemethoxycurcumin on tyrosinase: Spectroscopic and docking analysis. Int. J. Biol. Macromol. 2024, 281, 136655. [Google Scholar] [CrossRef]
- Beaumet, M.; Lazinski, L.M.; Maresca, M.; Haudecoeur, R. Tyrosinase Inhibition and Antimelanogenic Effects of Resorcinol-Containing Compounds. ChemMedChem 2024, 19, e202400314. [Google Scholar] [CrossRef]
- Meena, P.; Das, P.; Rathore, V.; Panda, S.; Popa, C. Snow White’s tale in nephrology: The emerging threat of skin-whitening creams on kidney health. Clin. Kidney J. 2025, 18, sfae358. [Google Scholar] [CrossRef]
- Jujjavarapu, S.E.; Mishra, A. Unravelling the Role of Tyrosine and Tyrosine Hydroxylase in Parkinson’s Disease: Exploring Nanoparticle-based Gene Therapies. CNS Neurol. Disord. Drug Targets 2025, 24, 325–339. [Google Scholar] [CrossRef]
- Panda, S.; Phan, H.; Dunietz, E.M.; Brueggemeyer, M.T.; Hota, P.K.; Siegler, M.A.; Jose, A.; Bhadra, M.; Solomon, E.I.; Karlin, K.D. Intramolecular Phenolic H-Atom Abstraction by a N(3)ArOH Ligand-Supported (μ-η(2):η(2)-Peroxo)dicopper(II) Species Relevant to the Active Site Function of oxy-Tyrosinase. J. Am. Chem. Soc. 2024, 146, 14942–14947. [Google Scholar] [CrossRef]
- Matoba, Y.; Kihara, S.; Bando, N.; Yoshitsu, H.; Sakaguchi, M.; Kayama, K.e.; Yanagisawa, S.; Ogura, T.; Sugiyama, M. Catalytic mechanism of the tyrosinase reaction toward the Tyr98 residue in the caddie protein. PLoS Biol. 2019, 16, e3000077. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, N.; Umakoshi, K.; Ochi, Y.; Nishikawa, Y.; Yanagisawa, S.; Kubo, M.; Kurisu, G.; Itoh, S. Copper–Oxygen Dynamics in the Tyrosinase Mechanism. Angew. Chem. Int. Ed. 2020, 59, 13385–13390. [Google Scholar] [CrossRef] [PubMed]
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A multipurpose trace element. Arch. Toxicol. 2006, 80, 1–9. [Google Scholar] [CrossRef]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Magnusson, K.R.; Sharpton, T.J.; Ho, E. Effects of zinc status on age-related T cell dysfunction and chronic inflammation. BioMetals 2021, 34, 291–301. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. A systematic review of the association between zinc and anxiety. Nutrit. Rev. 2023, 82, 612–621. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Thompson, M.W. Regulation of zinc-dependent enzymes by metal carrier proteins. BioMetals 2022, 35, 187–213. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef]
- Inada, Y.; Mohammed, A.M.; Loeffler, H.H.; Funahashi, S. Water-Exchange Mechanism for Zinc(II), Cadmium(II), and Mercury(II) Ions in Water as Studied by Umbrella-Sampling Molecular-Dynamics Simulations. Helv. Chim. Acta 2005, 88, 461–469. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- McCall, K.A.; Huang, C.-c.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, M.; Zhang, H.; Liu, F.; Pan, Y.; Zhu, J.; Liao, Z.; Chen, X.; Zhang, B. Structures and biological functions of zinc finger proteins and their roles in hepatocellular carcinoma. Biomark. Res. 2022, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Rakhra, G.; Rakhra, G. Zinc finger proteins: Insights into the transcriptional and post transcriptional regulation of immune response. Mol. Biol. Rep. 2021, 48, 5735–5743. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the Zinc-Proteins Encoded in the Human Genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef]
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellularzinc homeostasis. Metallomics 2010, 2, 306–317. [Google Scholar] [CrossRef]
- Maret, W. Zinc and Human Disease. In Interrelations Between Essential Metal Ions and Human Disease; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2013; pp. 389–414. [Google Scholar]
- Maret, W. Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Ong, C.-l.Y.; Walker, M.J.; McEwan, A.G. The Role of Copper and Zinc Toxicity in Innate Immune Defense against Bacterial Pathogens*. J. Biol. Chem. 2015, 290, 18954–18961. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R.R. 12—Zinc: Lewis Acid and Gene Regulator. In Biological Inorganic Chemistry; Crichton, R.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 197–210. [Google Scholar]
- Hu, Y.; Liu, B. Roles of zinc-binding domain of bacterial RNA polymerase in transcription. Trends Biochem. Sci. 2022, 47, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Chanfreau, G.F. Zinc’ing down RNA polymerase I. Transcription 2013, 4, 217–220. [Google Scholar] [CrossRef]
- Markov, D.; Naryshkina, T.; Mustaev, A.; Severinov, K. A zinc-binding site in the largest subunit of DNA-dependent RNA polymerase is involved in enzyme assembly. Genes Dev. 1999, 13, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, W.; Ali, M.A.M.; Schulz, R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. 2021, 288, 7162–7182. [Google Scholar] [CrossRef]
- Tu, C.; Foster, L.; Alvarado, A.; McKenna, R.; Silverman, D.N.; Frost, S.C. Role of zinc in catalytic activity of carbonic anhydrase IX. Arch. Biochem. Biophys. 2012, 521, 90–94. [Google Scholar] [CrossRef]
- Covarrubias, A.S.; Bergfors, T.; Jones, T.A.; Högbom, M. Structural mechanics of the pH-dependent activity of beta-carbonic anhydrase from Mycobacterium tuberculosis. J. Biol. Chem. 2006, 281, 4993–4999. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, C.; Lim, S.W.; Adhikari, A.; Andring, J.T.; McKenna, R.; Ghim, C.-M.; Kim, C.U. Elucidating the role of metal ions in carbonic anhydrase catalysis. Nat. Commun. 2020, 11, 4557. [Google Scholar] [CrossRef]
- Cho, Y.E.; Lomeda, R.A.; Ryu, S.H.; Sohn, H.Y.; Shin, H.I.; Beattie, J.H.; Kwun, I.S. Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats. Nutr. Res. Pract. 2007, 1, 113–119. [Google Scholar] [CrossRef]
- Peretz, A.; Papadopoulos, T.; Willems, D.; Hotimsky, A.; Michiels, N.; Siderova, V.; Bergmann, P.; Neve, J. Zinc supplementation increases bone alkaline phosphatase in healthy men. J. Trace Elem. Med. Biol. 2001, 15, 175–178. [Google Scholar] [CrossRef]
- Holtz, K.M.; Kantrowitz, E.R. The mechanism of the alkaline phosphatase reaction: Insights from NMR, crystallography and site-specific mutagenesis. FEBS Lett. 1999, 462, 7–11. [Google Scholar] [CrossRef]
- Miller, J.; McLachlan, A.D.; Klug, A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985, 4, 1609–1614. [Google Scholar] [CrossRef]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, A.; Sun, L.; Li, W.; Yang, B.; Su, Y.; Ding, R.; Zhang, C.; Liu, H.; Yang, X.; et al. Zinc finger protein Zfp335 controls early T-cell development and survival through β-selection-dependent and -independent mechanisms. eLife 2022, 11, e75508. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, Y.; Miranda, W.F.; Sonnenfeld, M.J. The jing Zn-finger transcription factor is a mediator of cellular differentiation in the Drosophila CNS midline and trachea. Development 2002, 129, 2591–2606. [Google Scholar] [CrossRef] [PubMed]
- Omichinski, J.G.; Clore, G.M.; Appella, E.; Sakaguchi, K.; Gronenborn, A.M. High-resolution three-dimensional structure of a single zinc finger from a human enhancer binding protein in solution. Biochemistry 1990, 29, 9324–9334. [Google Scholar] [CrossRef]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef]
- Wessels, I.; Rolles, B.; Slusarenko, A.J.; Rink, L. Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of Corona Virus Disease 19. Br. J. Nutr. 2022, 127, 214–232. [Google Scholar] [CrossRef]
- Krall, R.F.; Tzounopoulos, T.; Aizenman, E. The Function and Regulation of Zinc in the Brain. Neuroscience 2021, 457, 235–258. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc and Hormones. In Biochemistry of Zinc; Prasad, A.S., Ed.; Springer: Boston, MA, USA, 1993; pp. 93–117. [Google Scholar]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. Review: The role of zinc in the endocrine system. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar]
- Nasiadek, M.; Stragierowicz, J.; Klimczak, M.; Kilanowicz, A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients 2020, 12, 2464. [Google Scholar] [CrossRef] [PubMed]
- Vickram, S.; Rohini, K.; Srinivasan, S.; Nancy Veenakumari, D.; Archana, K.; Anbarasu, K.; Jeyanthi, P.; Thanigaivel, S.; Gulothungan, G.; Rajendiran, N.; et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. Int. J. Mol. Sci. 2021, 22, 2188. [Google Scholar] [CrossRef]
- Knez, M.; Stangoulis, J.C.R. Dietary Zn deficiency, the current situation and potential solutions. Nutr. Res. Rev. 2023, 36, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, W.N. Carboxypeptidase A mechanisms. Proc. Natl. Acad. Sci. USA 1980, 77, 3875–3878. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases--an overview. Curr. Pharm. Des. 2008, 14, 603–614. [Google Scholar] [CrossRef]
- Jonsson, B.H.; Liljas, A. Perspectives on the Classical Enzyme Carbonic Anhydrase and the Search for Inhibitors. Biophys. J. 2020, 119, 1275–1280. [Google Scholar] [CrossRef]
- Jensen, E.L.; Maberly, S.C.; Gontero, B. Insights on the Functions and Ecophysiological Relevance of the Diverse Carbonic Anhydrases in Microalgae. Int. J. Mol. Sci. 2020, 21, 2922. [Google Scholar] [CrossRef]
- Christianson, D.W.; Cox, J.D. Catalysis by metal-activated hydroxide in zinc and manganese metalloenzymes. Annu. Rev. Biochem. 1999, 68, 33–57. [Google Scholar] [CrossRef]
- Mazumdar, P.A.; Kumaran, D.; Swaminathan, S.; Das, A.K. A novel acetate-bound complex of human carbonic anhydrase II. Acta Crystallogr. F 2008, 64, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Lesniewicz, K.; Karlowski, W.M.; Pienkowska, J.R.; Krzywkowski, P.; Poreba, E. The Plant S1-Like Nuclease Family Has Evolved a Highly Diverse Range of Catalytic Capabilities. Plant Cell Physiol. 2013, 54, 1064–1078. [Google Scholar] [CrossRef]
- Byrne, E.M.; Visomirski-Robic, L.; Cheng, Y.W.; Rhee, A.C.; Gott, J.M. RNA Editing in Physarum Mitochondria: Assays and Biochemical Approaches. In Methods in Enzymology; Gott, J.M., Ed.; Academic Press: New York, NY, USA, 2007; Volume 424, pp. 143–172. [Google Scholar]
- Yang, W. Nucleases: Diversity of structure, function and mechanism. Quart. Rev. Biophys. 2011, 44, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Koval, T.; Dohnálek, J. Characteristics and application of S1–P1 nucleases in biotechnology and medicine. Biotech. Adv. 2018, 36, 603–612. [Google Scholar] [CrossRef]
- Kovaľ, T.; Østergaard, L.H.; Lehmbeck, J.; Nørgaard, A.; Lipovová, P.; Dušková, J.; Skálová, T.; Trundová, M.; Kolenko, P.; Fejfarová, K.; et al. Structural and Catalytic Properties of S1 Nuclease from Aspergillus oryzae Responsible for Substrate Recognition, Cleavage, Non–Specificity, and Inhibition. PLoS ONE 2016, 11, e0168832. [Google Scholar] [CrossRef]
- Liao, R.-Z.; Yu, J.-G.; Himo, F. Phosphate Mono- and Diesterase Activities of the Trinuclear Zinc Enzyme Nuclease P1—Insights from Quantum Chemical Calculations. Inorg. Chem. 2010, 49, 6883–6888. [Google Scholar] [CrossRef]
- Stransky, J.; Koval’, T.; Podzimek, T.; Tycova, A.; Lipovova, P.; Matousek, J.; Kolenko, P.; Fejfarova, K.; Duskova, J.; Skalova, T.; et al. Phosphate binding in the active centre of tomato multifunctional nuclease TBN1 and analysis of superhelix formation by the enzyme. Acta Crystallog. F 2015, 71, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Molec. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Quiocho, F.A.; Lipscomb, W.N. Carboxypeptidase A: A protein and an enzyme. Adv. Protein Chem. 1971, 25, 1–78. [Google Scholar]
- Lipscomb, W.N. Relationship of the three dimensional structure of carboxypeptidase A to catalysis: Possible intermediates and pH effects. Tetrahedron 1974, 30, 1725–1732. [Google Scholar] [CrossRef]
- Breslow, R.; Wernick, D.L. Unified picture of mechanisms of catalysis by carboxypeptidase A. Proc. Natl. Acad. Sci. USA 1977, 74, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W.; Lipscomb, W.N. Carboxypeptidase A. Acc. Chem. Res. 1989, 22, 62–69. [Google Scholar] [CrossRef]
- Mock, W.L.; Zhang, J.Z. Mechanistically significant diastereoselection in the sulfoximine inhibition of carboxypeptidase A. J. Biol. Chem. 1991, 266, 6393–6400. [Google Scholar] [CrossRef]
- Lipscomb, W.N.; Sträter, N. Recent Advances in Zinc Enzymology. Chem. Rev. 1996, 96, 2375–2434. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, C.; Xu, D.; Guo, H. Catalysis of Carboxypeptidase A: Promoted-Water versus Nucleophilic Pathways. J. Phys. Chem. B 2010, 114, 9259–9267. [Google Scholar] [CrossRef]
- Simpson, M.C.; Harding, C.J.; Czekster, R.M.; Remmel, L.; Bode, B.E.; Czekster, C.M. Unveiling the Catalytic Mechanism of a Processive Metalloaminopeptidase. Biochemistry 2023, 62, 3188–3205. [Google Scholar] [CrossRef] [PubMed]
- Dembek, G.; Lingens, F. Isolation and characterization of a meta-cleavage product in the degradation of quinaldic acid by Azotobacter sp. FEMS Microbiol. Lett. 1988, 56, 261–264. [Google Scholar] [CrossRef]
- Bobyr, E.; Lassila, J.K.; Wiersma-Koch, H.I.; Fenn, T.D.; Lee, J.J.; Nikolic-Hughes, I.; Hodgson, K.O.; Rees, D.C.; Hedman, B.; Herschlag, D. High-Resolution Analysis of Zn2+ Coordination in the Alkaline Phosphatase Superfamily by EXAFS and X-ray Crystallography. J. Molec. Biol. 2012, 415, 102–117. [Google Scholar] [CrossRef]
- Fernley, H.N. Mammalian alkaline phosphatase. In The Enzymes, 3rd ed.; Boeyer, P.D., Ed.; Acadamic Press: New York, NY, USA, 1971; pp. 417–447. [Google Scholar]
- Coleman, J.E. Structure and Mechanism of Alkaline Phosphatase. Annu. Rev. Biophys. 1992, 21, 441–483. [Google Scholar] [CrossRef]
- Borosky, G.L. Quantum-Mechanical Study on the Catalytic Mechanism of Alkaline Phosphatases. J. Chem. Inf. Model. 2017, 57, 540–549. [Google Scholar] [CrossRef]
- Anderson, R.A.; Bosron, W.F.; Kennedy, F.S.; Vallee, B.L. Role of magnesium in Escherichia coli alkaline phosphatase. Proc. Natl. Acad. Sci. USA 1975, 72, 2989–2993. [Google Scholar] [CrossRef]
- Tibbitts, T.T.; Murphy, J.E.; Kantrowitz, E.R. Kinetic and structural consequences of replacing the aspartate bridge by asparagine in the catalytic metal triad of Escherichia coli alkaline phosphatase. J. Mol. Biol. 1996, 257, 700–715. [Google Scholar] [CrossRef]
- Storozhuk, M.; Cherninskyi, A.; Maximyuk, O.; Isaev, D.; Krishtal, O. Acid-Sensing Ion Channels: Focus on Physiological and Some Pathological Roles in the Brain. Curr. Neuropharmacol. 2021, 19, 1570–1589. [Google Scholar] [CrossRef] [PubMed]
- Ojiakor, O.A.; Rylett, R.J. Modulation of sodium-coupled choline transporter CHT function in health and disease. Neurochem. Int. 2020, 140, 104810. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.J.; Renaud, J.M.; Ørtenblad, N.; Overgaard, K. A century of exercise physiology: Effects of muscle contraction and exercise on skeletal muscle Na(+),K(+)-ATPase, Na(+) and K(+) ions, and on plasma K(+) concentration-historical developments. Eur. J. Appl. Physiol. 2024, 124, 681–751. [Google Scholar] [CrossRef]
- Sommer, B.; Flores-Soto, E.; Gonzalez-Avila, G. Cellular Na+ handling mechanisms involved in airway smooth muscle contraction (Review). Int. J. Mol. Med. 2017, 40, 3–9. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. Ions, the Movement of Water and the Apoptotic Volume Decrease. Front. Cell Dev. Biol. 2020, 8, 611211. [Google Scholar] [CrossRef] [PubMed]
- Orlov, S.N.; Koltsova, S.V.; Kapilevich, L.V.; Dulin, N.O.; Gusakova, S.V. Cation-chloride cotransporters: Regulation, physiological significance, and role in pathogenesis of arterial hypertension. Biochemistry 2014, 79, 1546–1561. [Google Scholar] [CrossRef]
- Pressley, T.A. Structure and function of the Na,K pump: Ten years of molecular biology. Miner. Electrolyte Metab. 1996, 22, 264–271. [Google Scholar]
- Bueno-Orovio, A.; Sánchez, C.; Pueyo, E.; Rodriguez, B. Na/K pump regulation of cardiac repolarization: Insights from a systems biology approach. Pflugers Arch. 2014, 466, 183–193. [Google Scholar] [CrossRef]
- Perrone, M.; Patergnani, S.; Di Mambro, T.; Palumbo, L.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Calcium Homeostasis in the Control of Mitophagy. Antioxid. Redox Signal. 2023, 38, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Ion channels and the regulation of myogenic tone in peripheral arterioles. Curr. Top. Membr. 2020, 85, 19–58. [Google Scholar]
- Hepler, P.K. The role of calcium in cell division. Cell Calcium 1994, 16, 322–330. [Google Scholar] [CrossRef]
- Bachs, O.; Agell, N. Calcium and Calmodulin Fuction in the Cell Nucleus; Springer: Berlin/Heidelberg, Germany, 1995; pp. 1–9. [Google Scholar]
- Schulman, H. Calcium Regulation of Cytosolic Enzymes. In Integrative Aspects of Calcium Signalling; Verkhratsky, A., Toescu, E.C., Eds.; Springer: Boston, MA, USA, 1998; pp. 35–57. [Google Scholar]
- Tokumitsu, H.; Sakagami, H. Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction. Int. J. Mol. Sci. 2022, 23, 11025. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Ecco, G.; Imbeault, M.; Trono, D. KRAB zinc finger proteins. Development 2017, 144, 2719–2729. [Google Scholar] [CrossRef]
- Jen, J.; Wang, Y.C. Zinc finger proteins in cancer progression. J. Biomed. Sci. 2016, 23, 53. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Iyer, A.S.; Shaik, M.R.; Raufman, J.P.; Xie, G. The Roles of Zinc Finger Proteins in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 10249. [Google Scholar] [CrossRef]
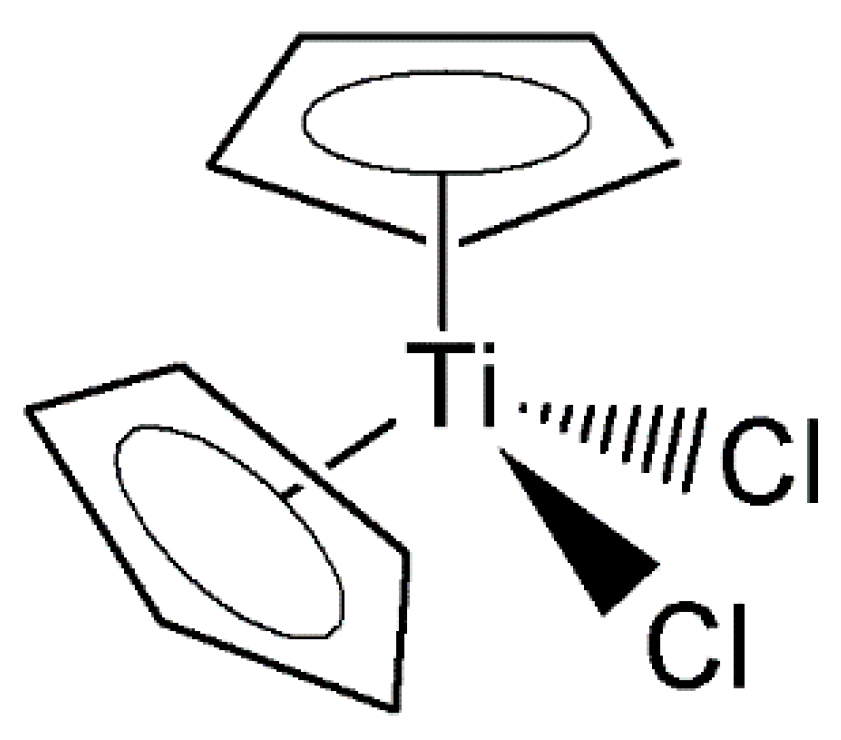

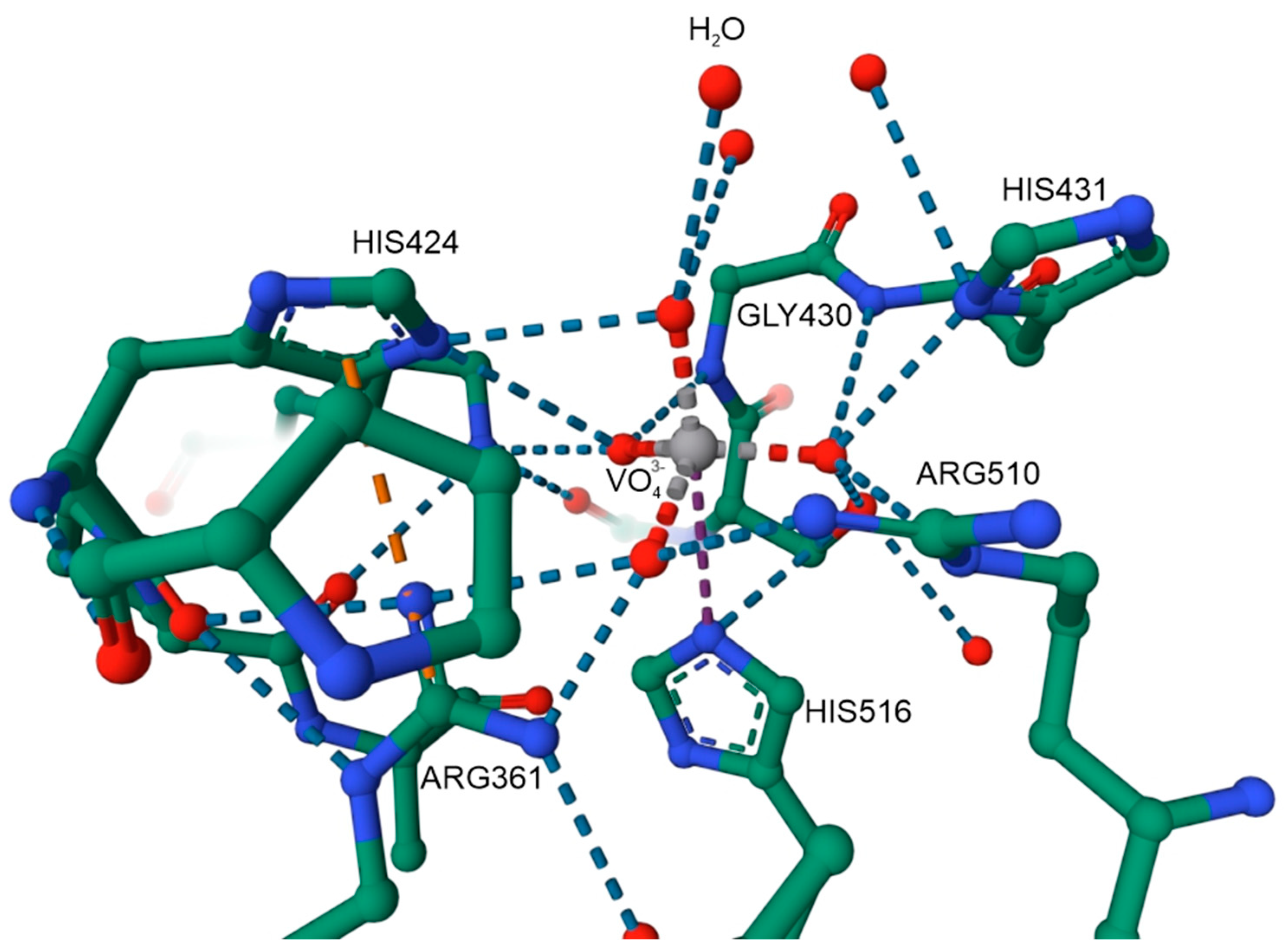

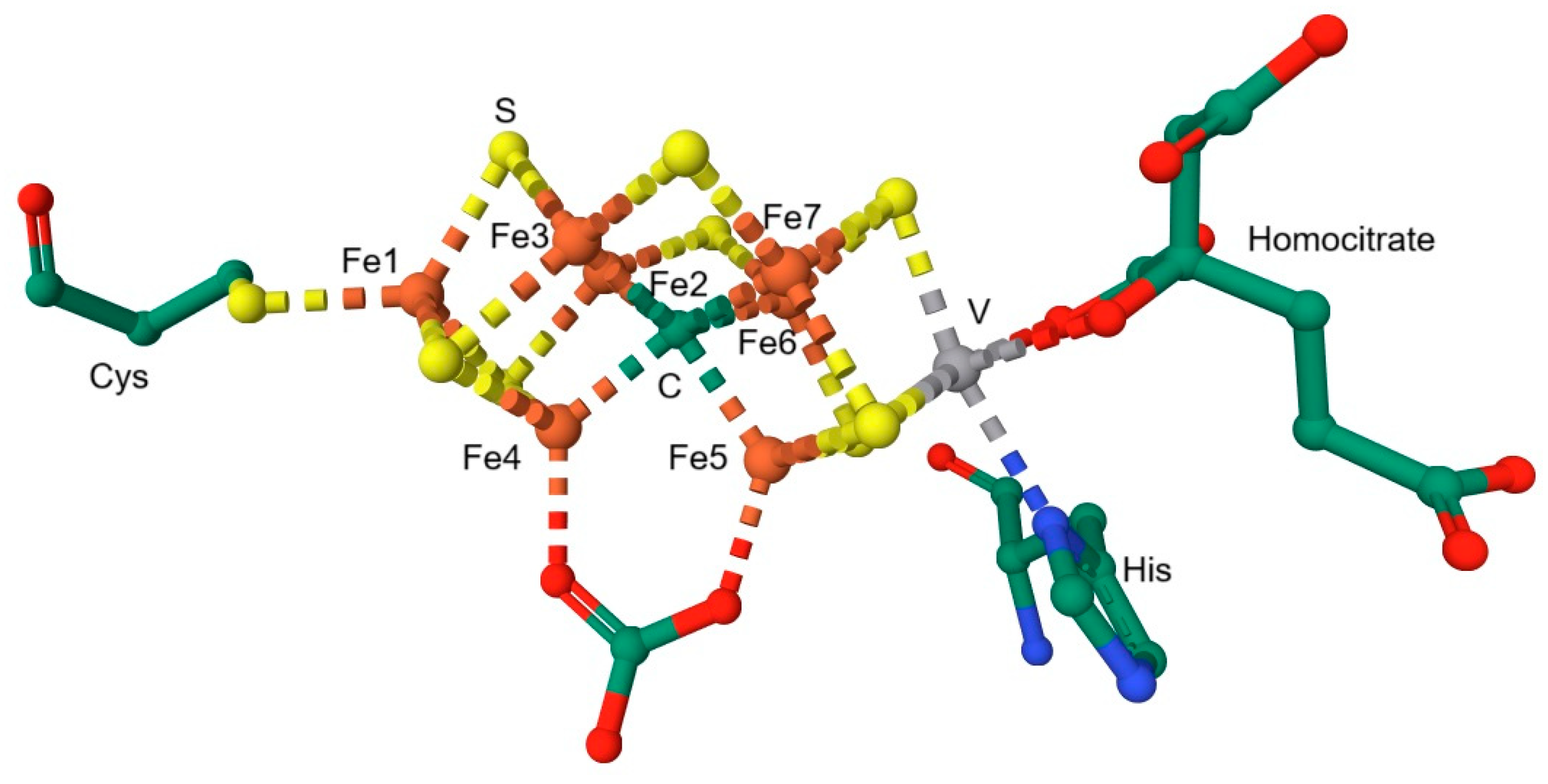

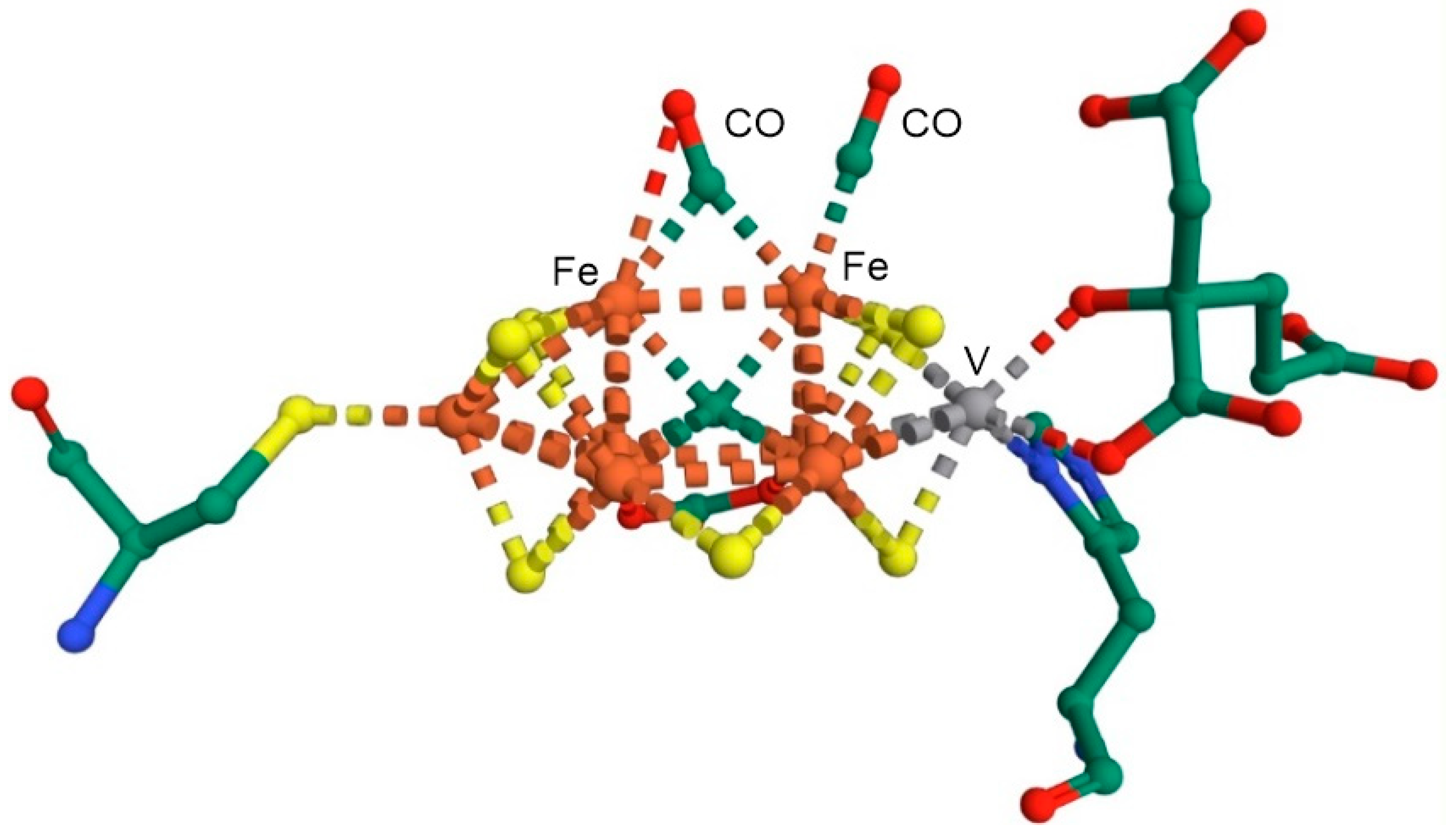
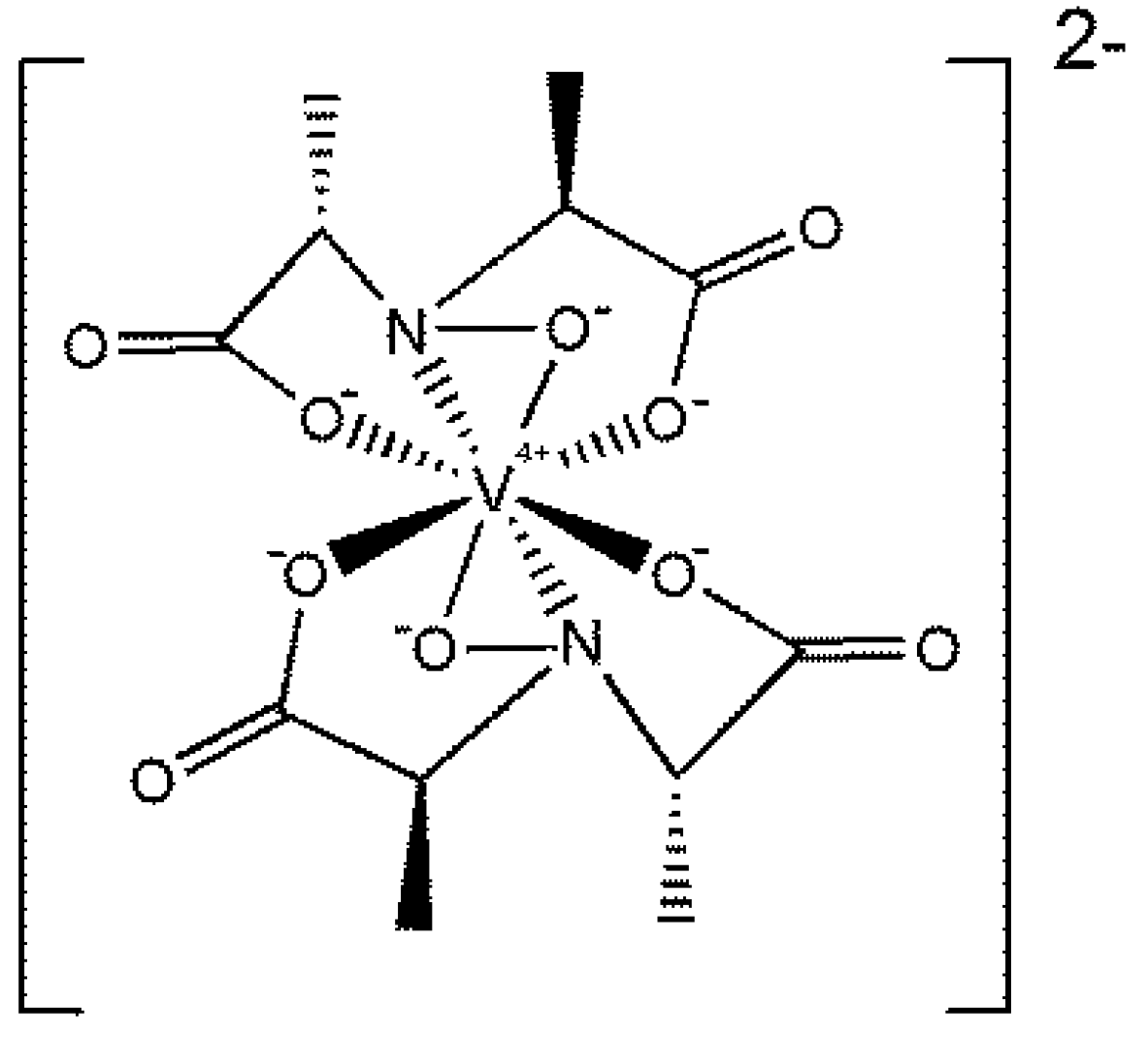
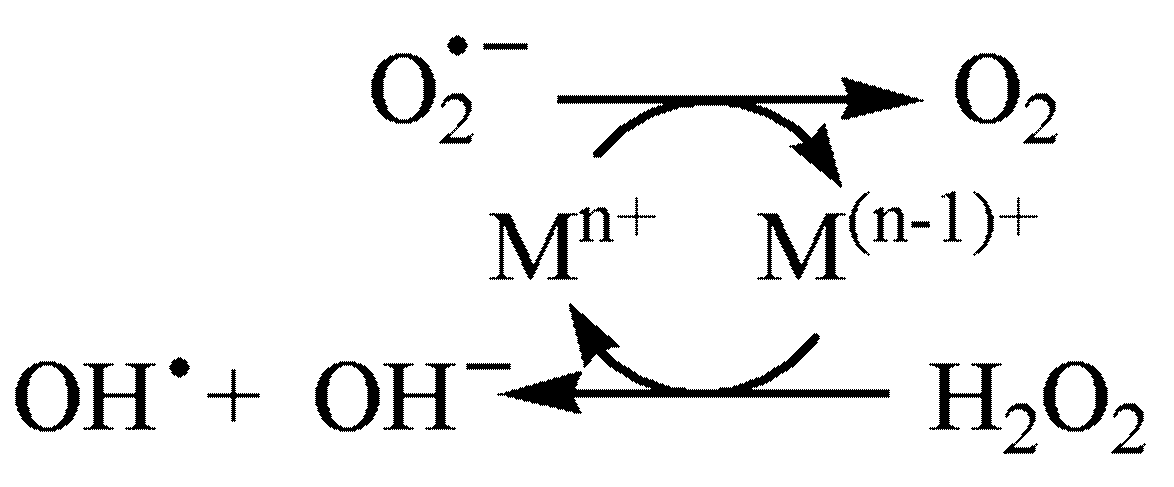
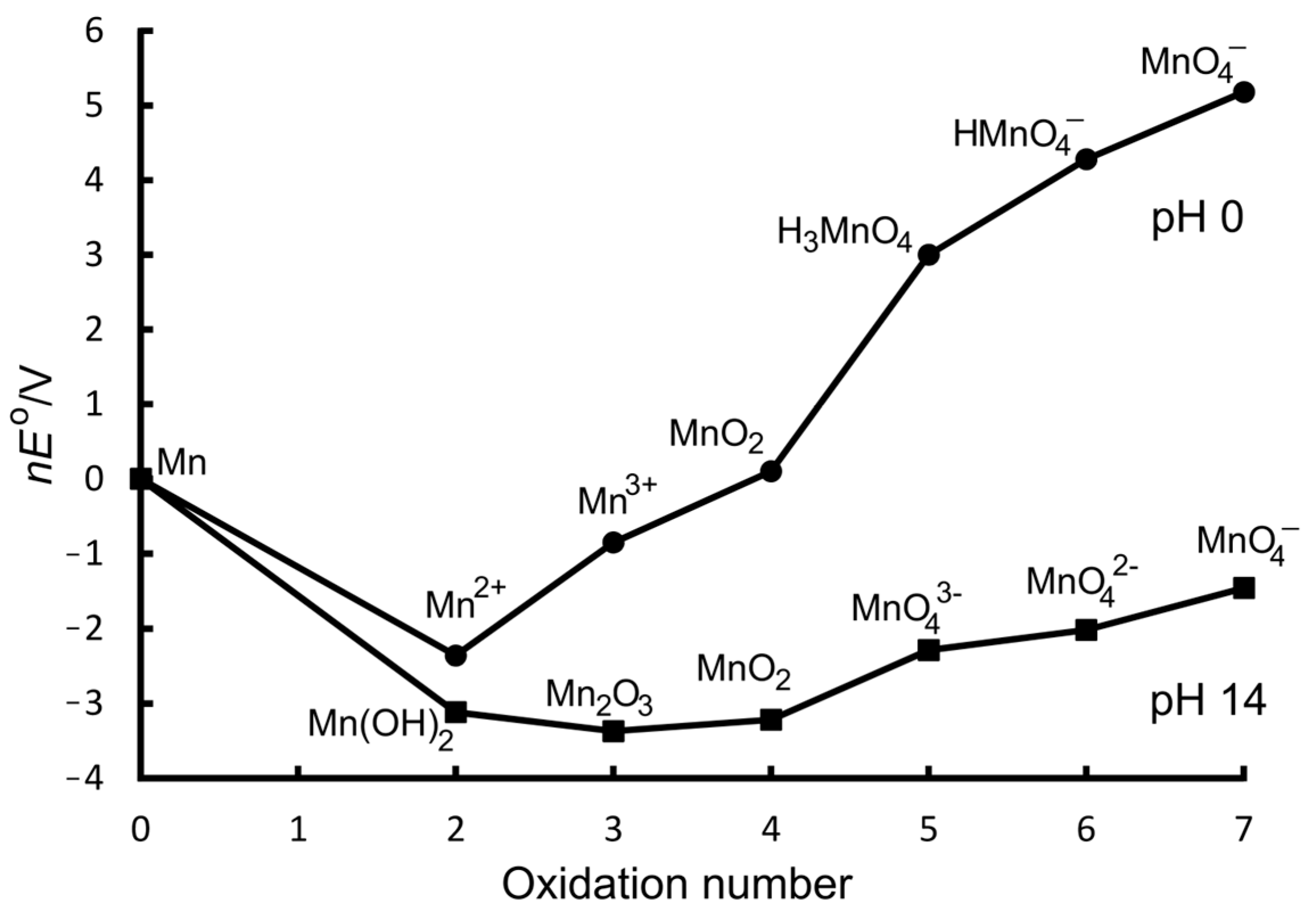
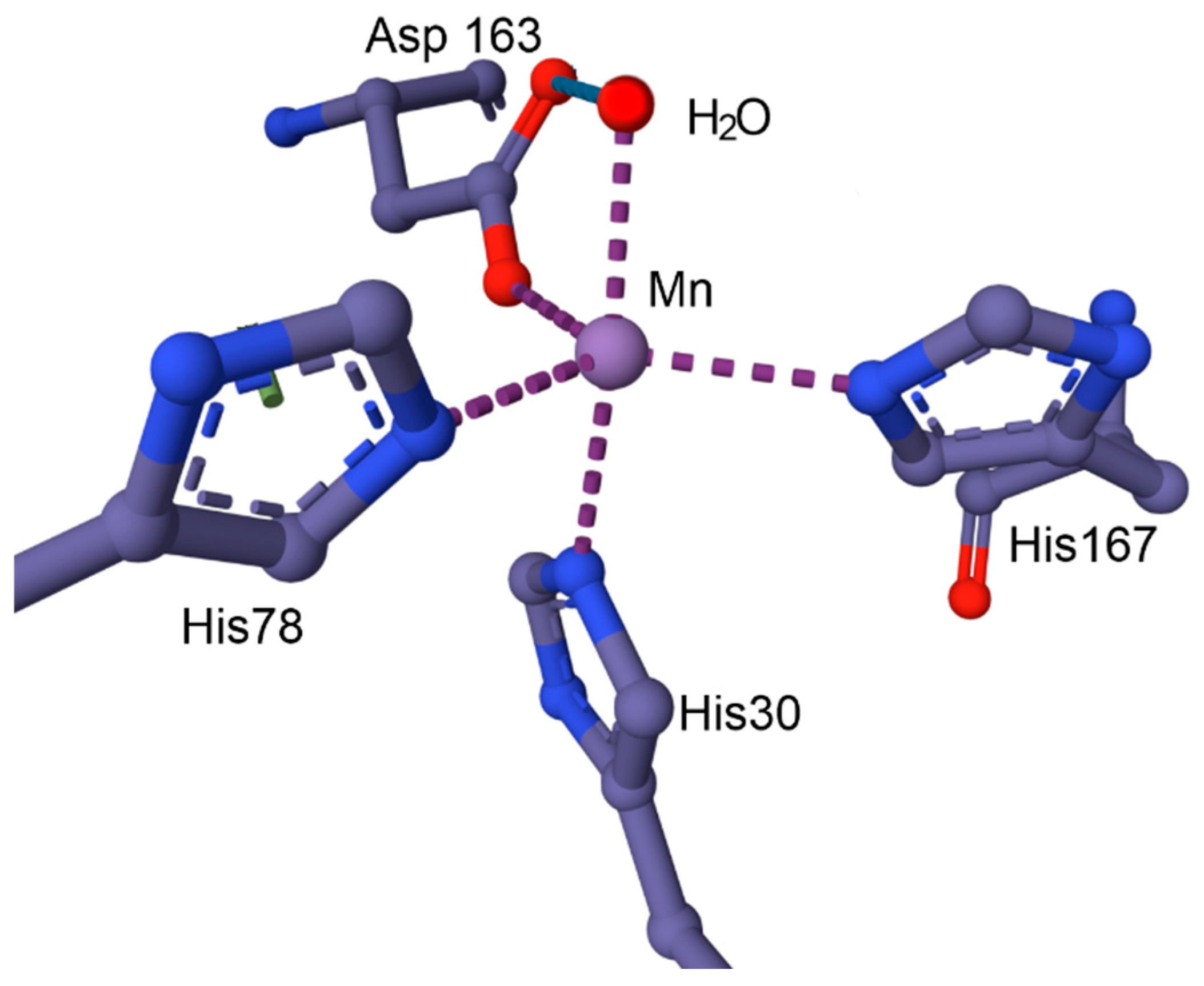
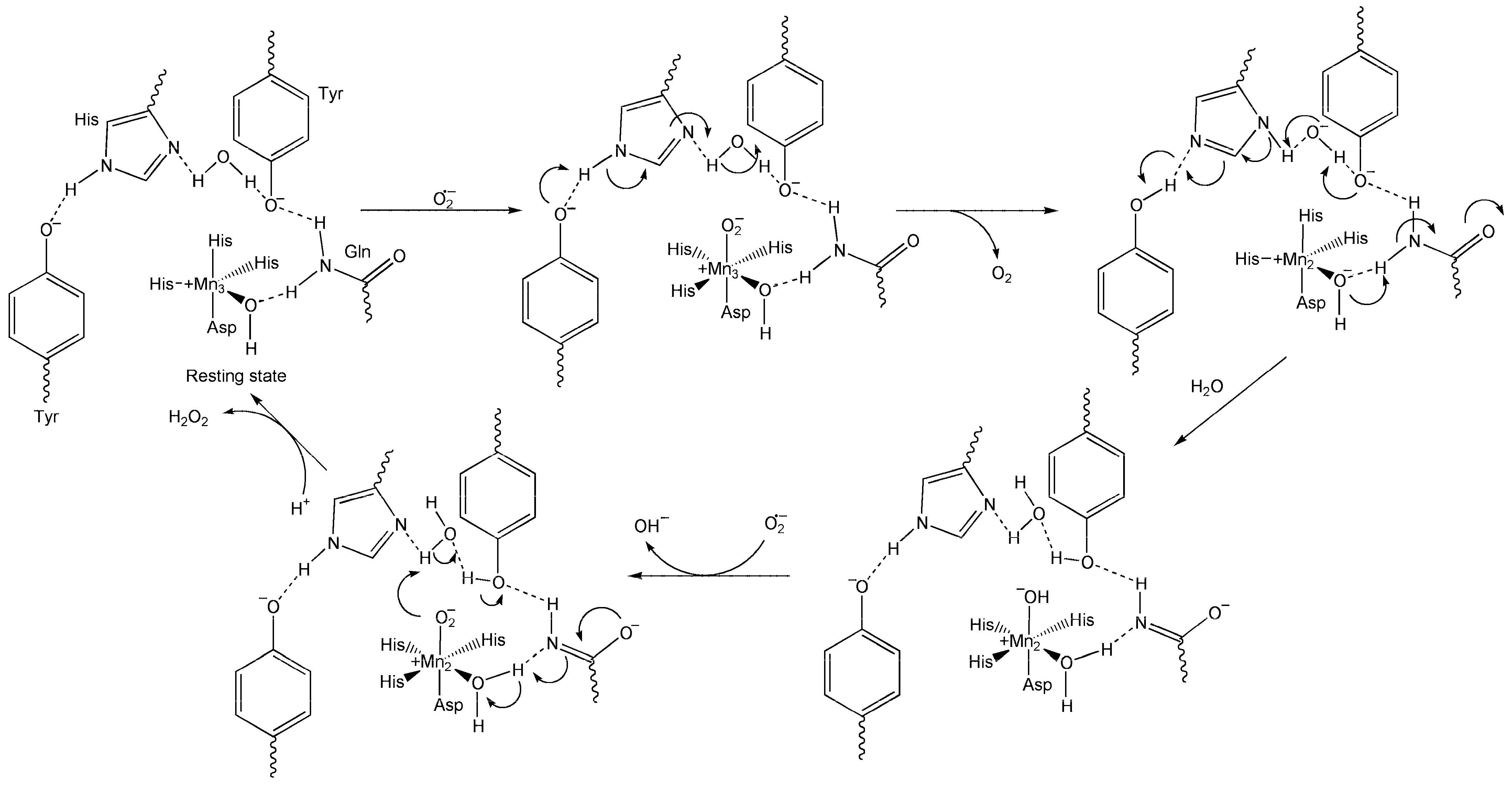
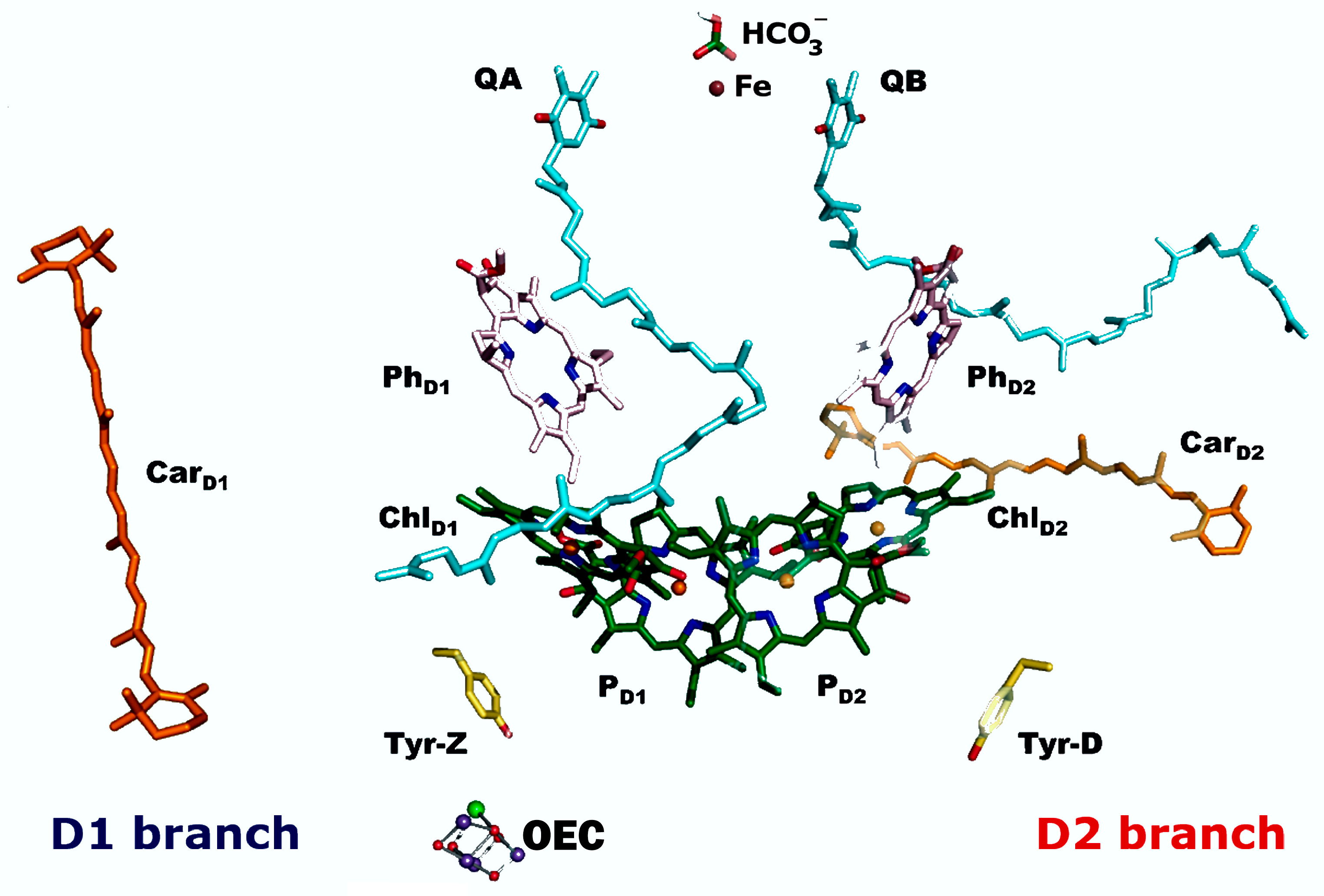
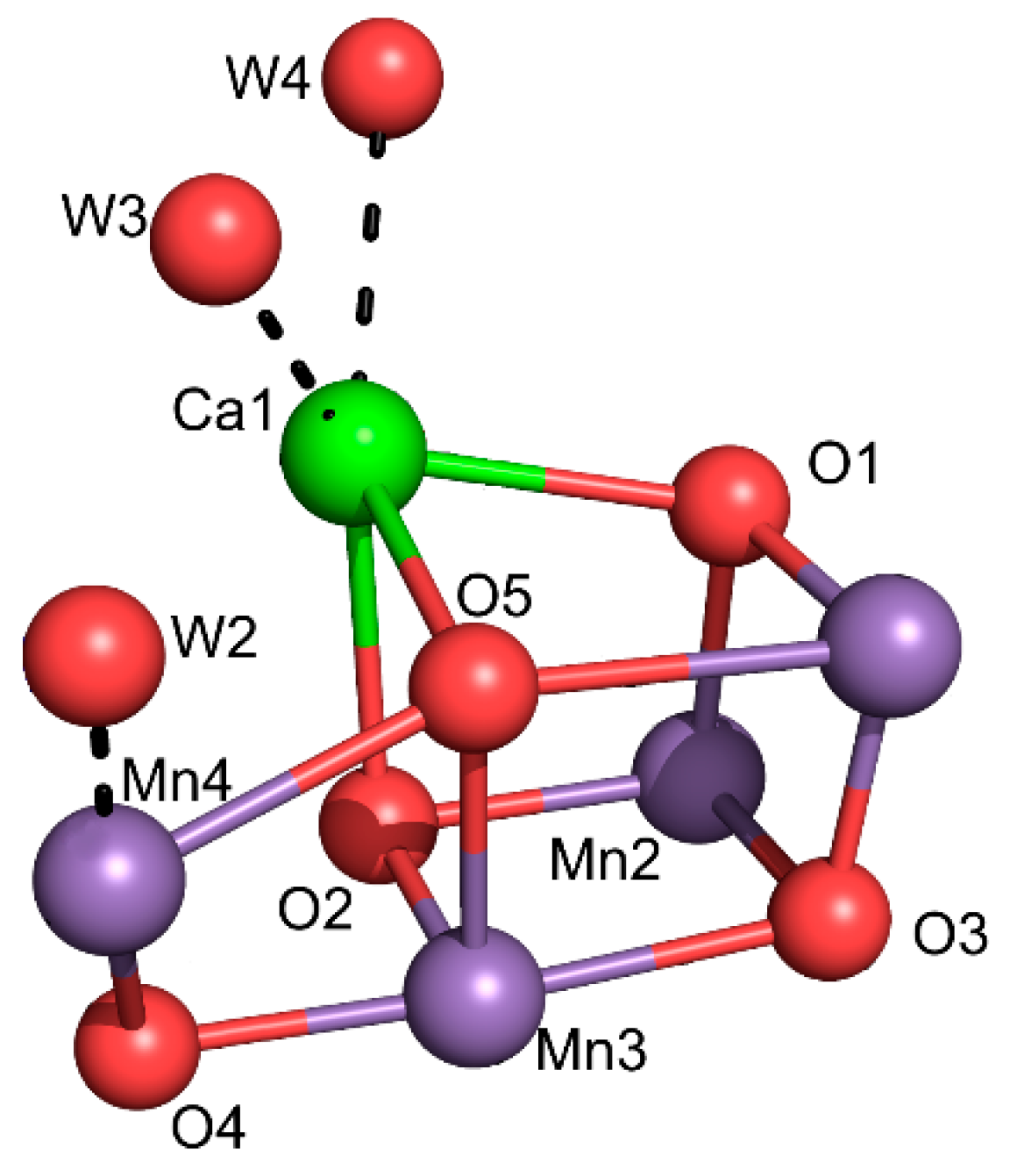

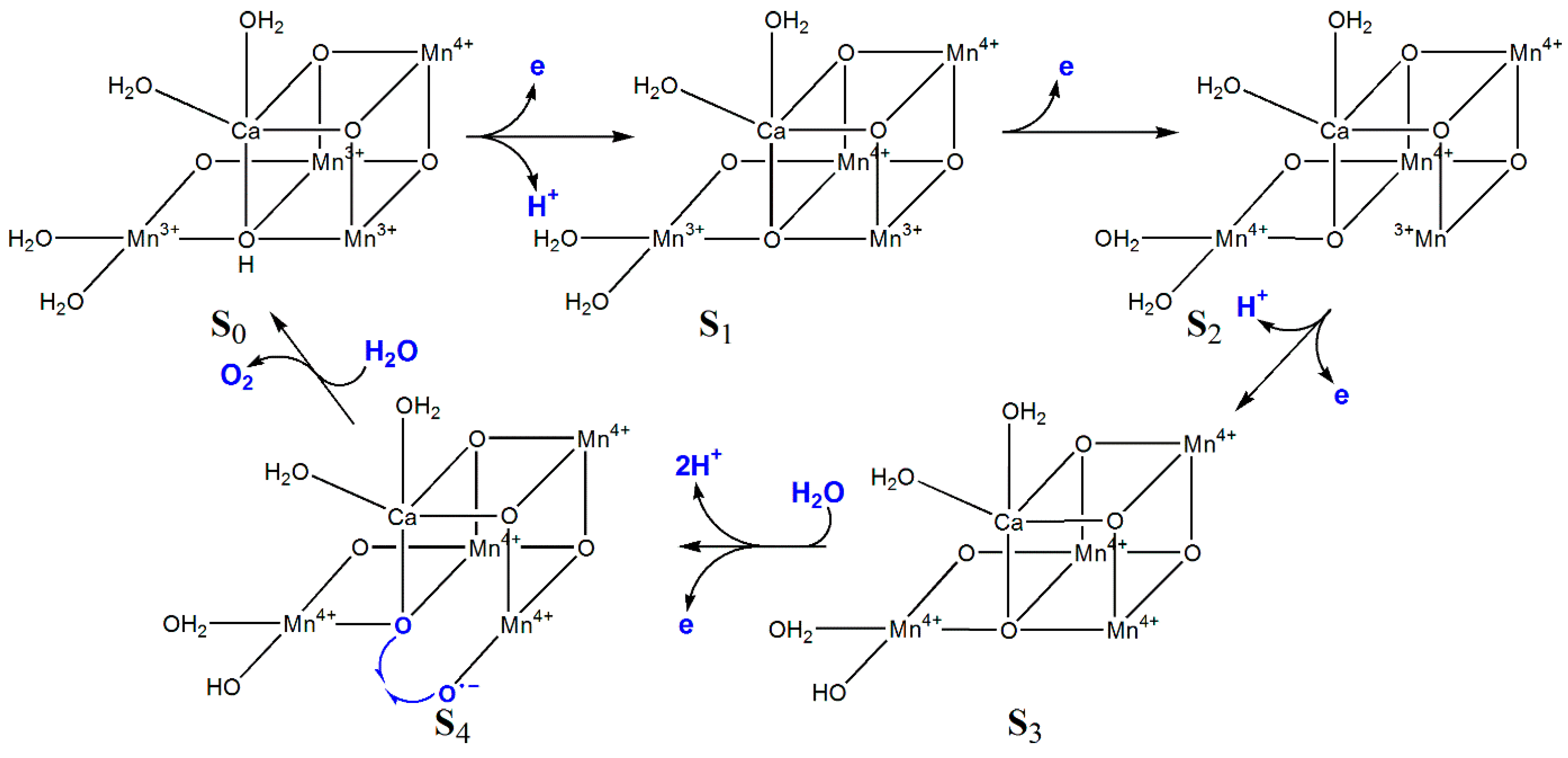

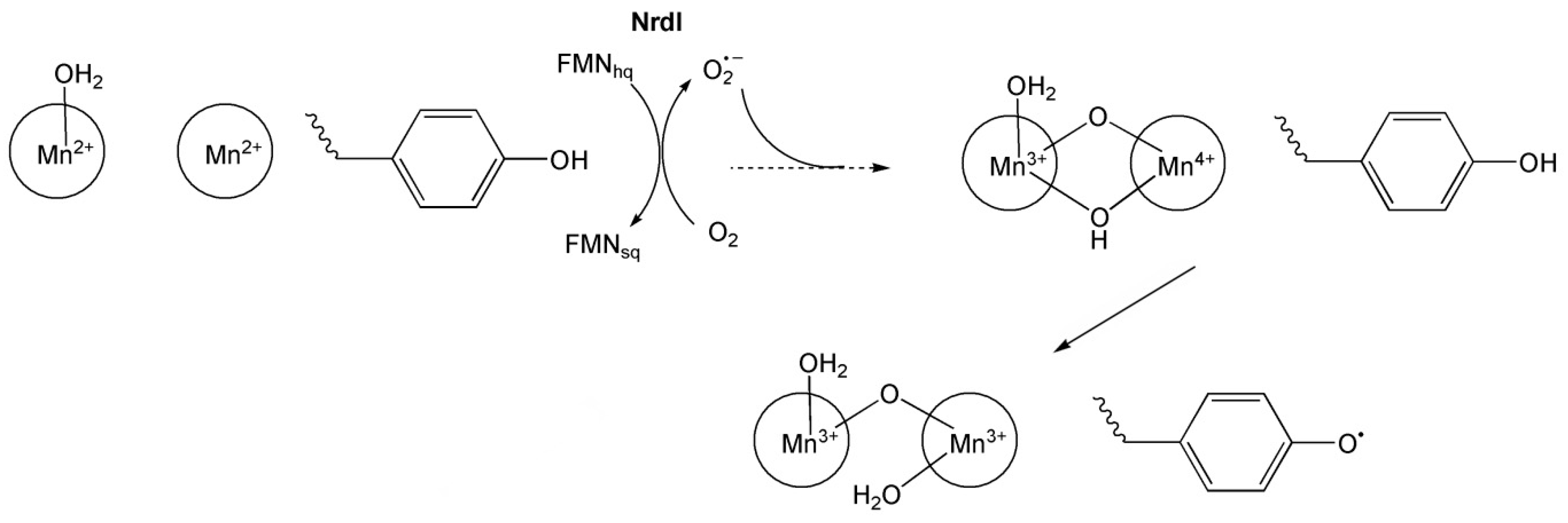
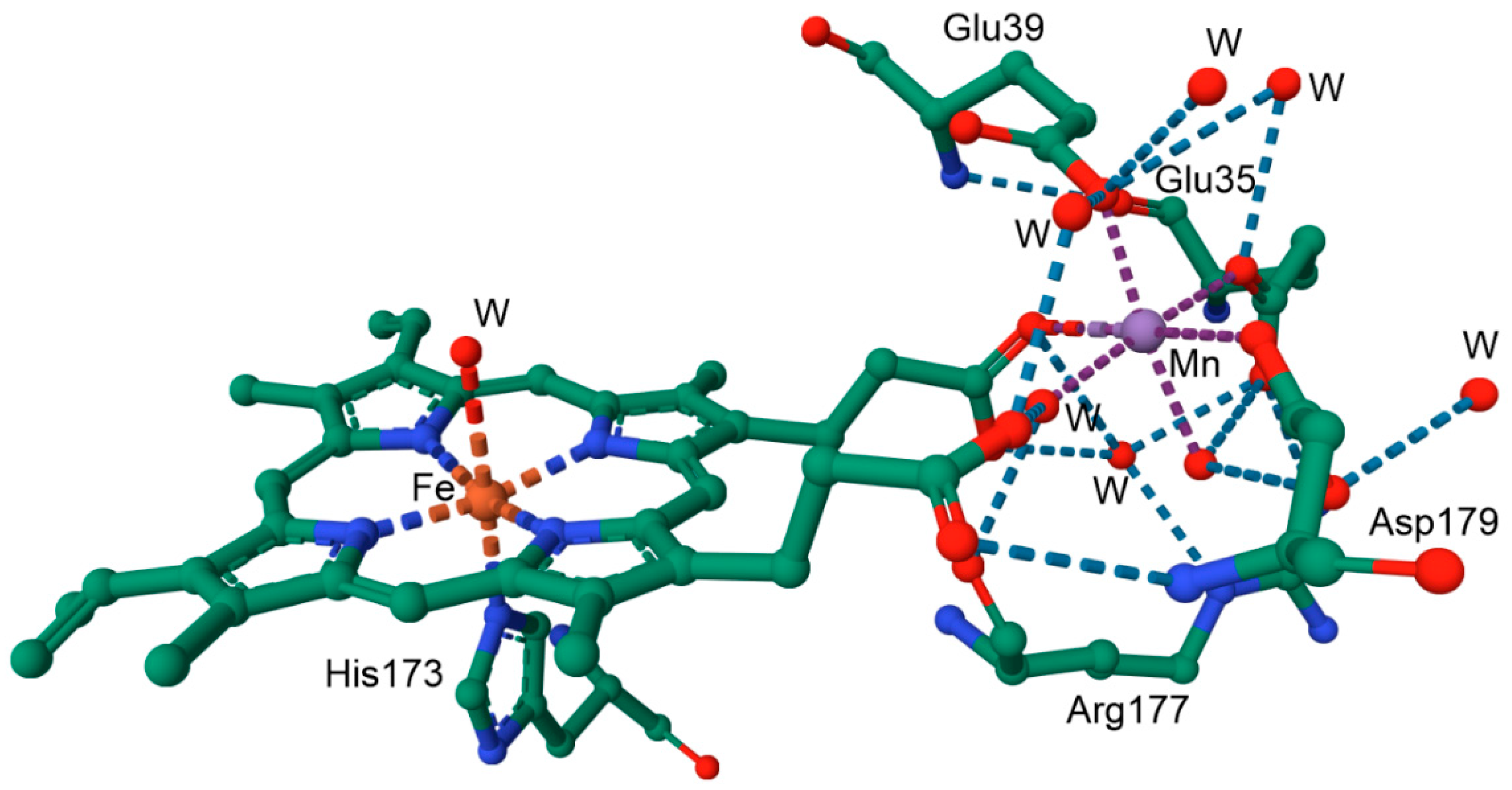
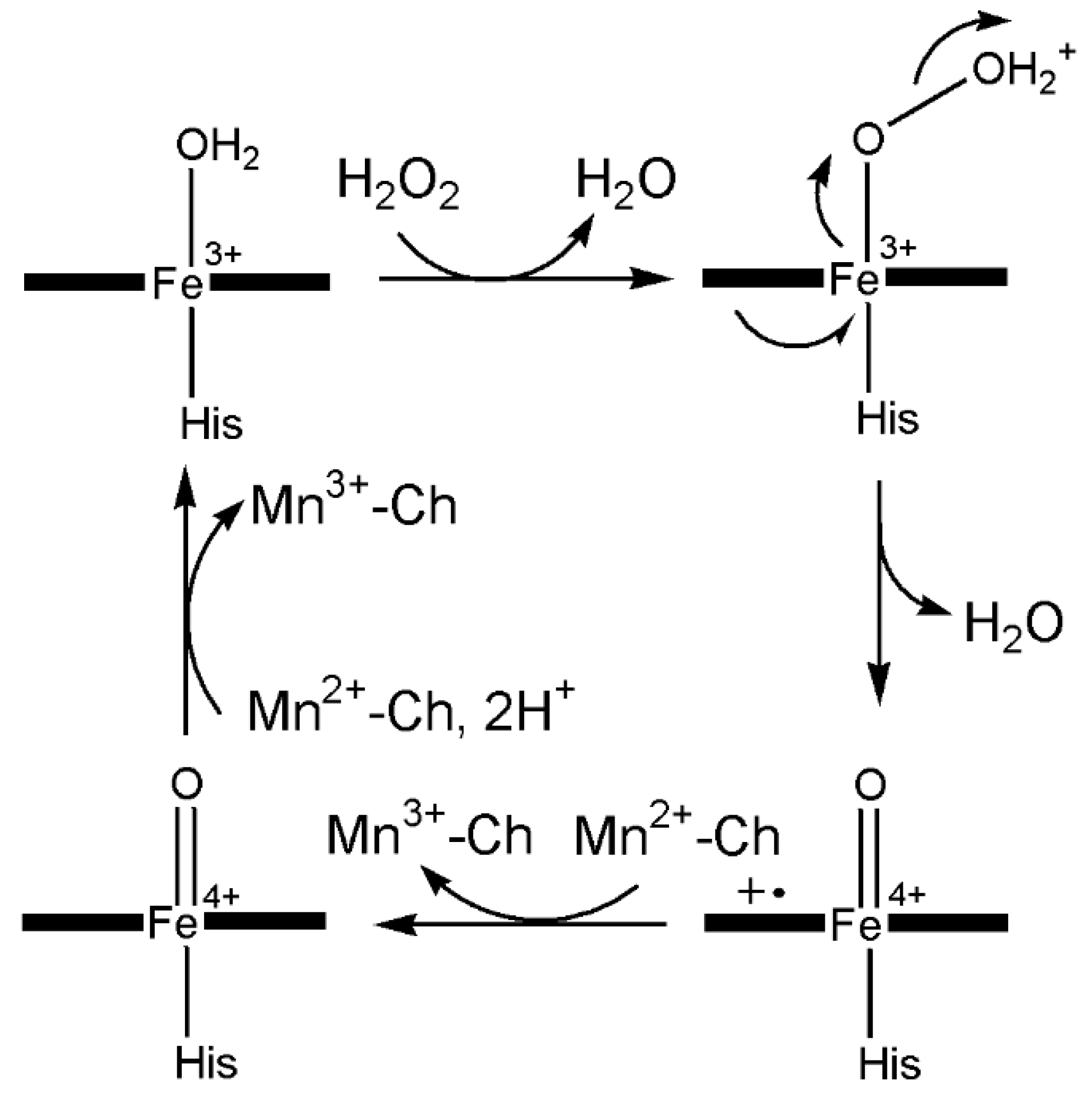



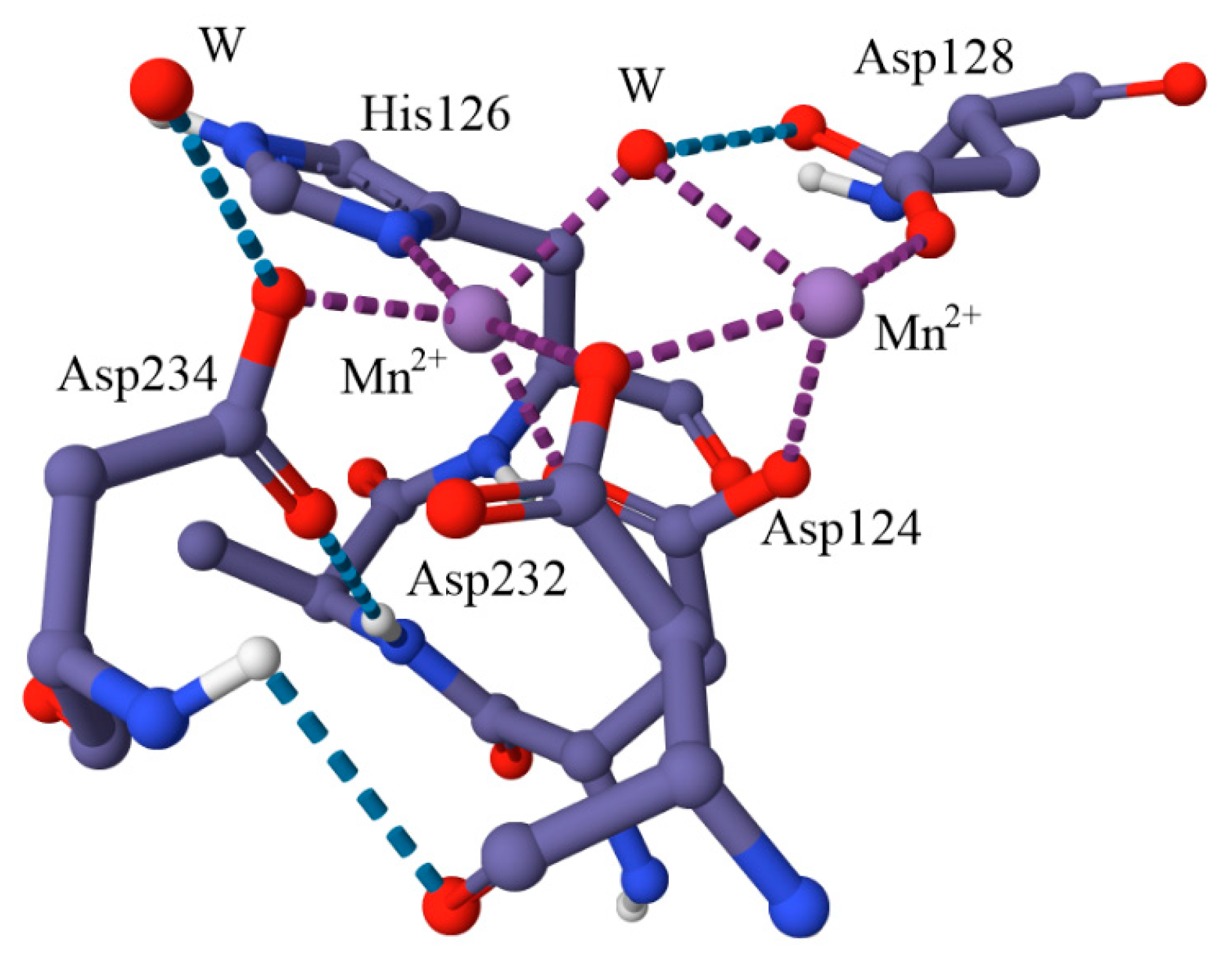
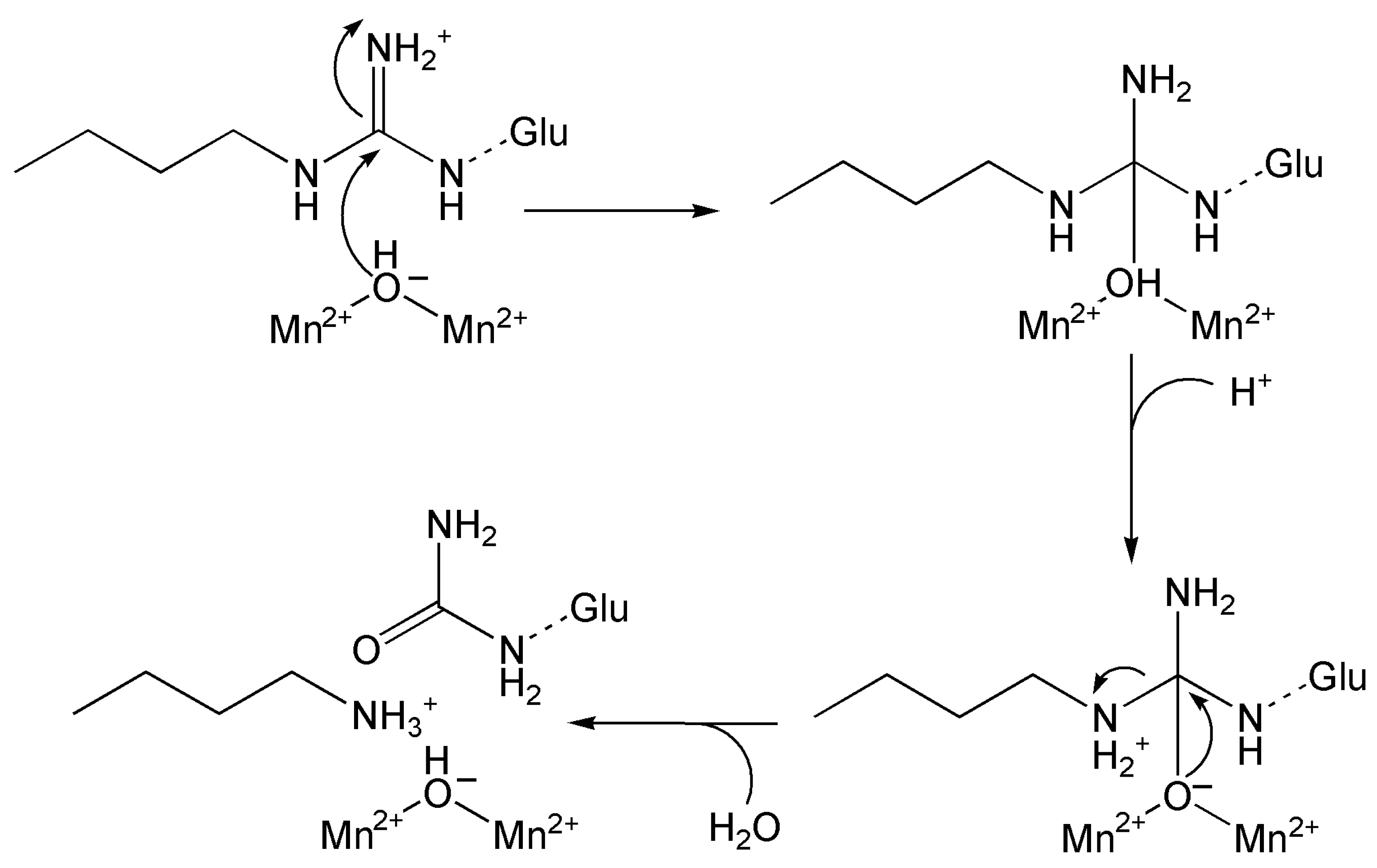
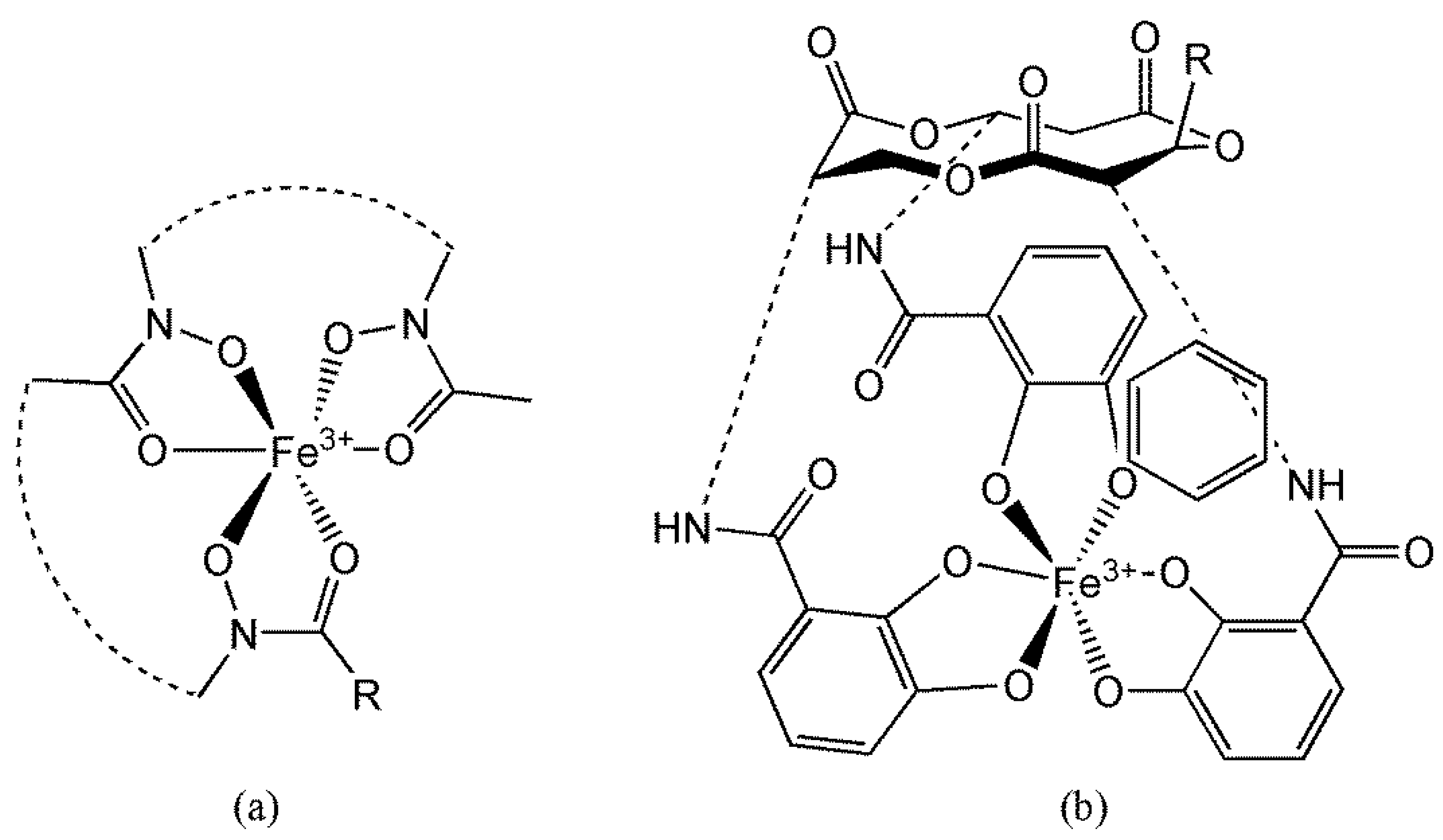
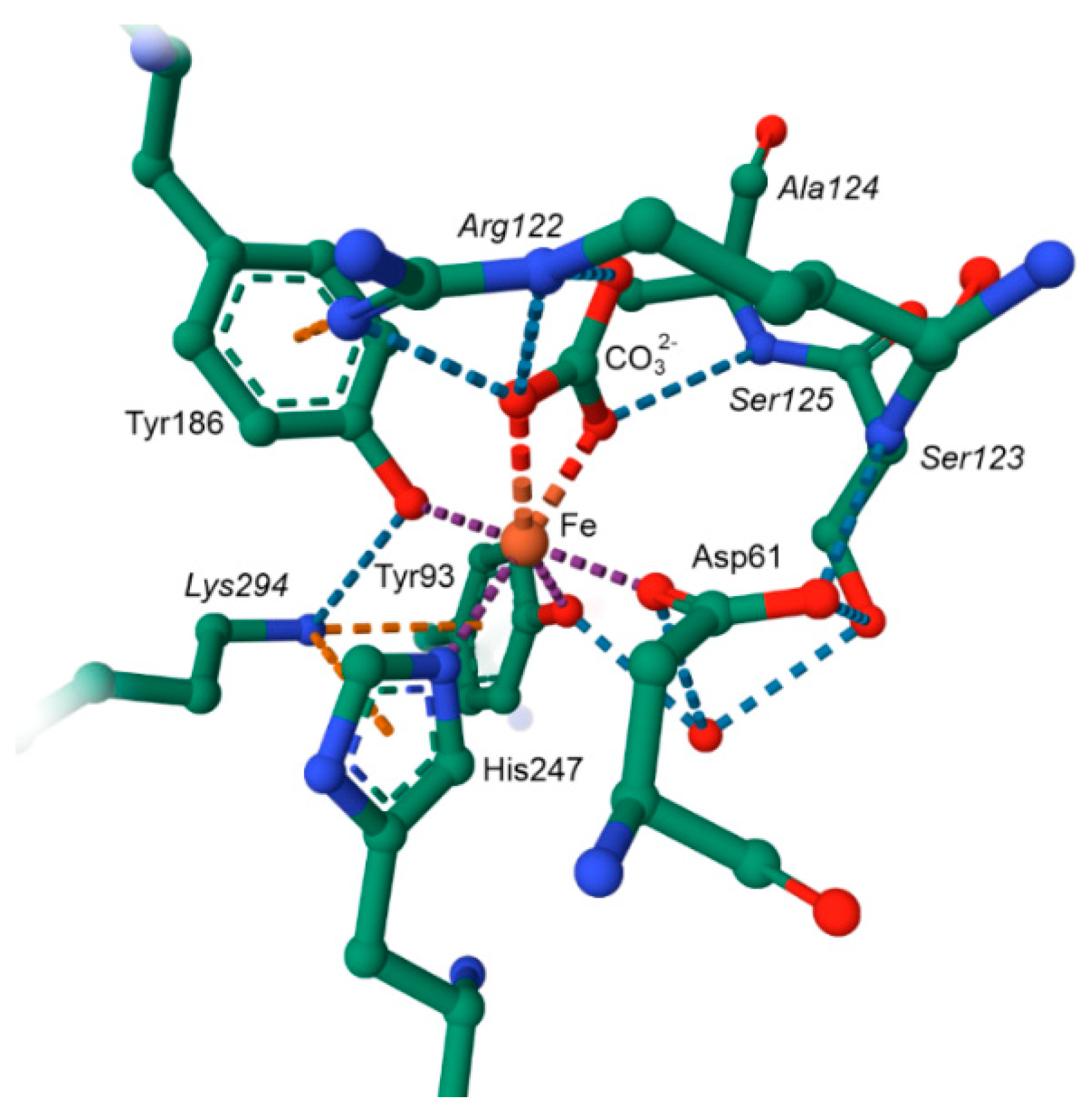
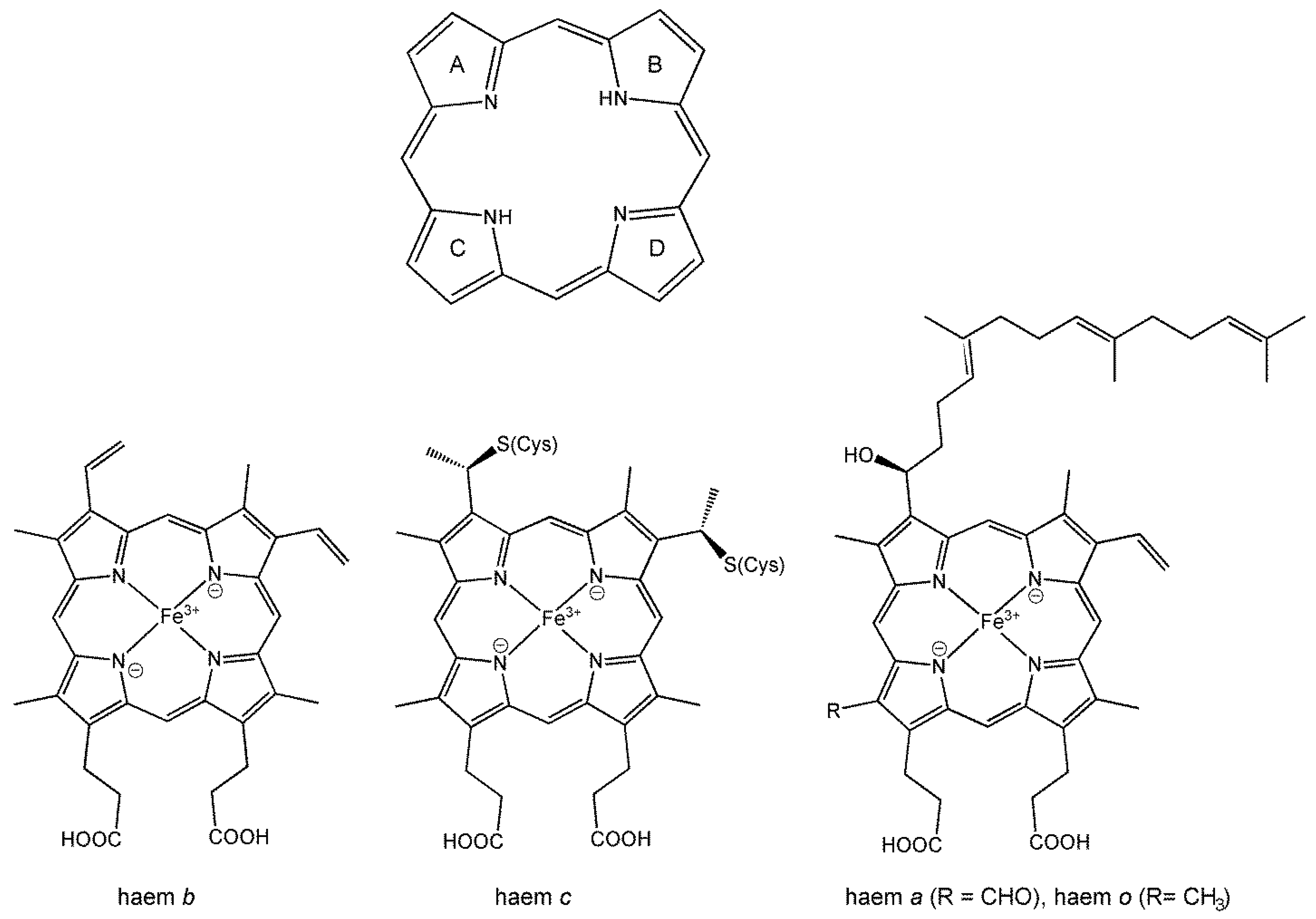
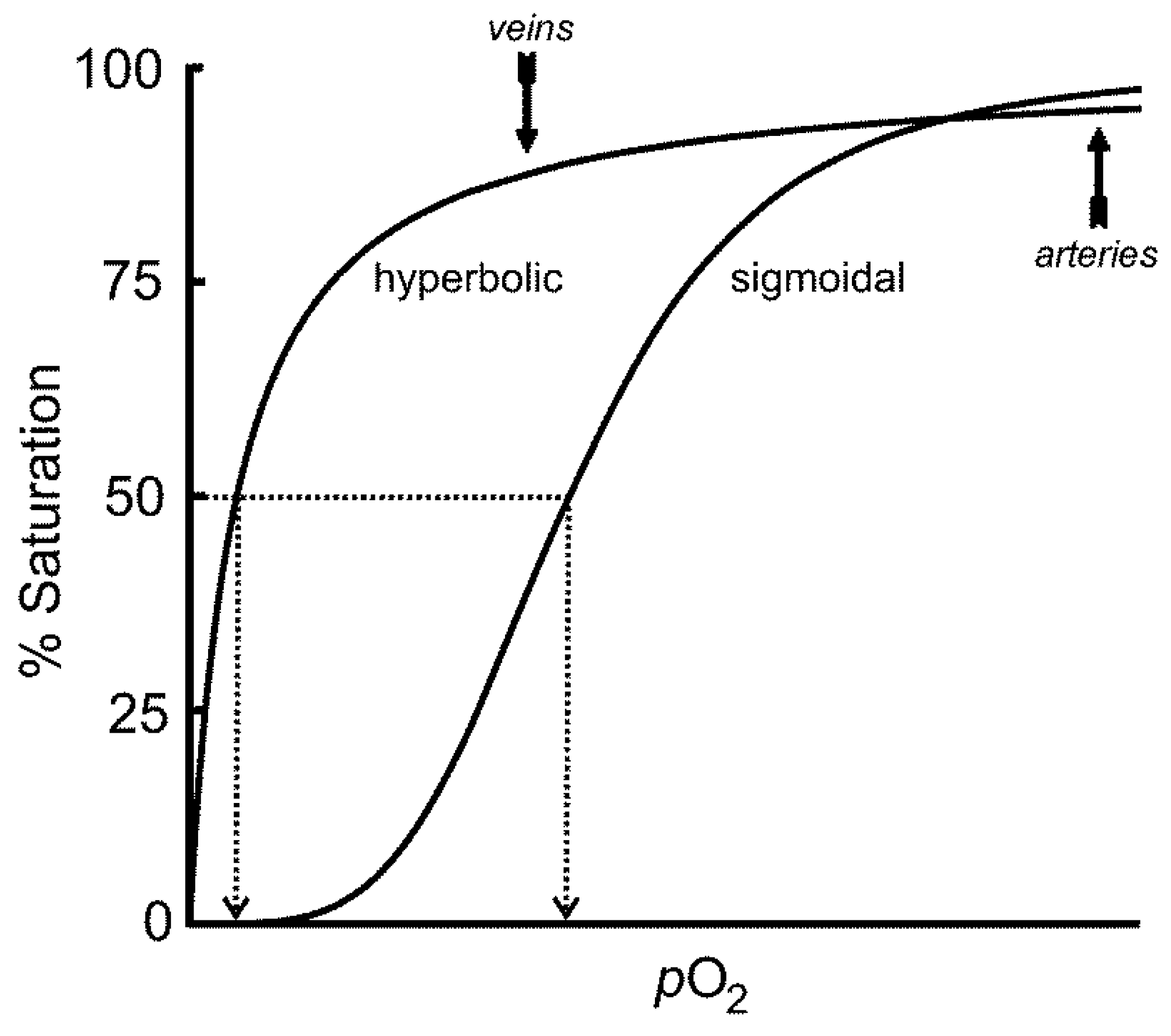

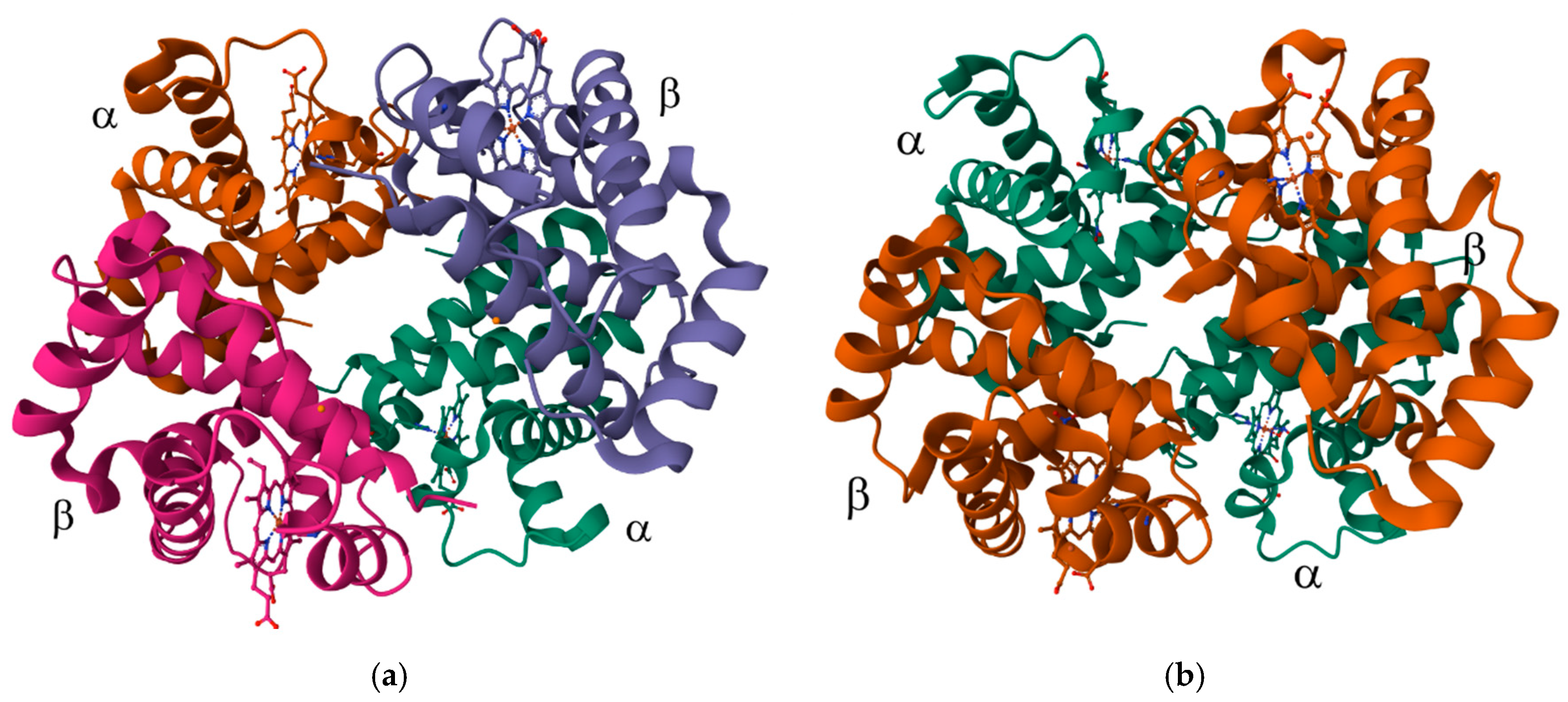

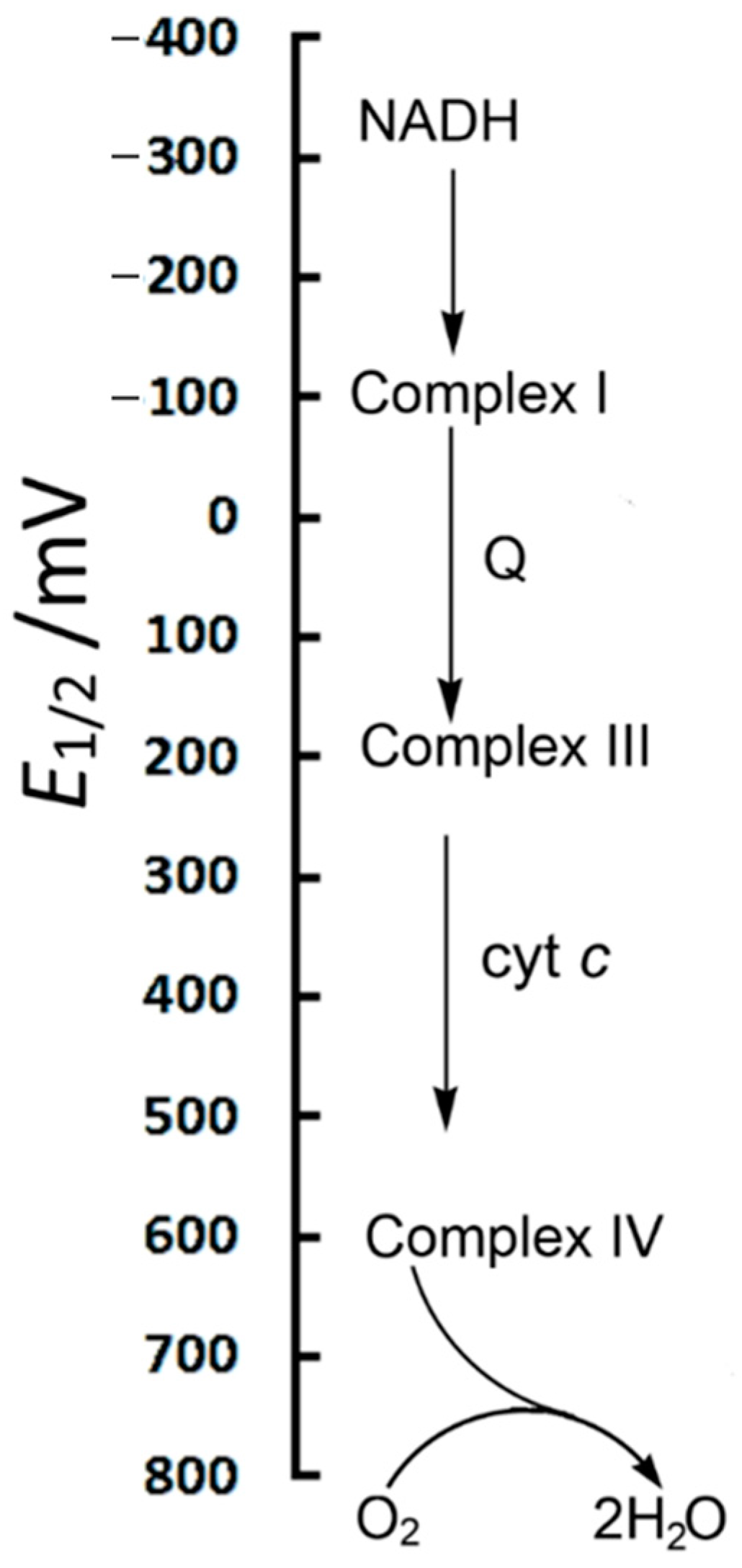
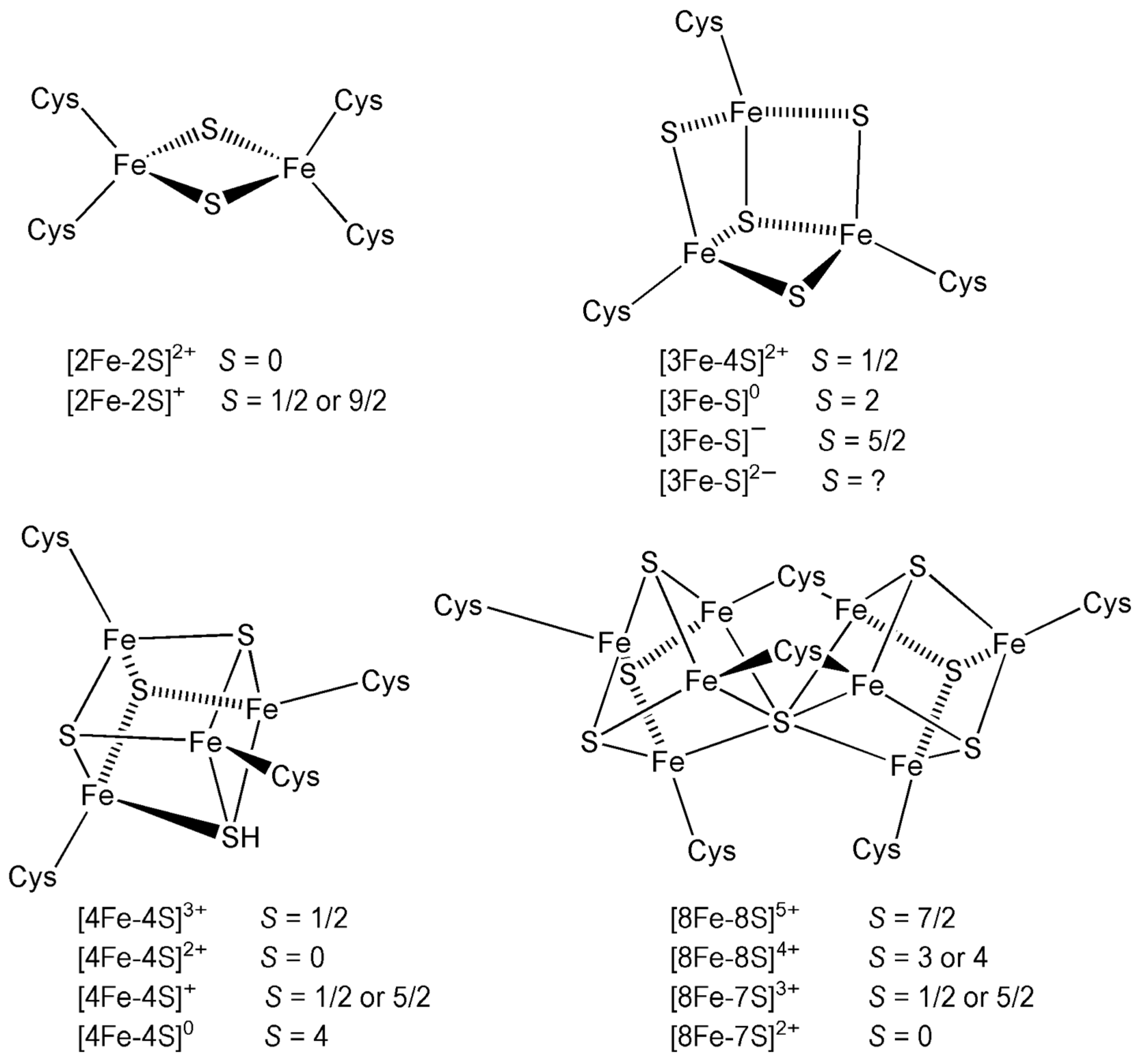
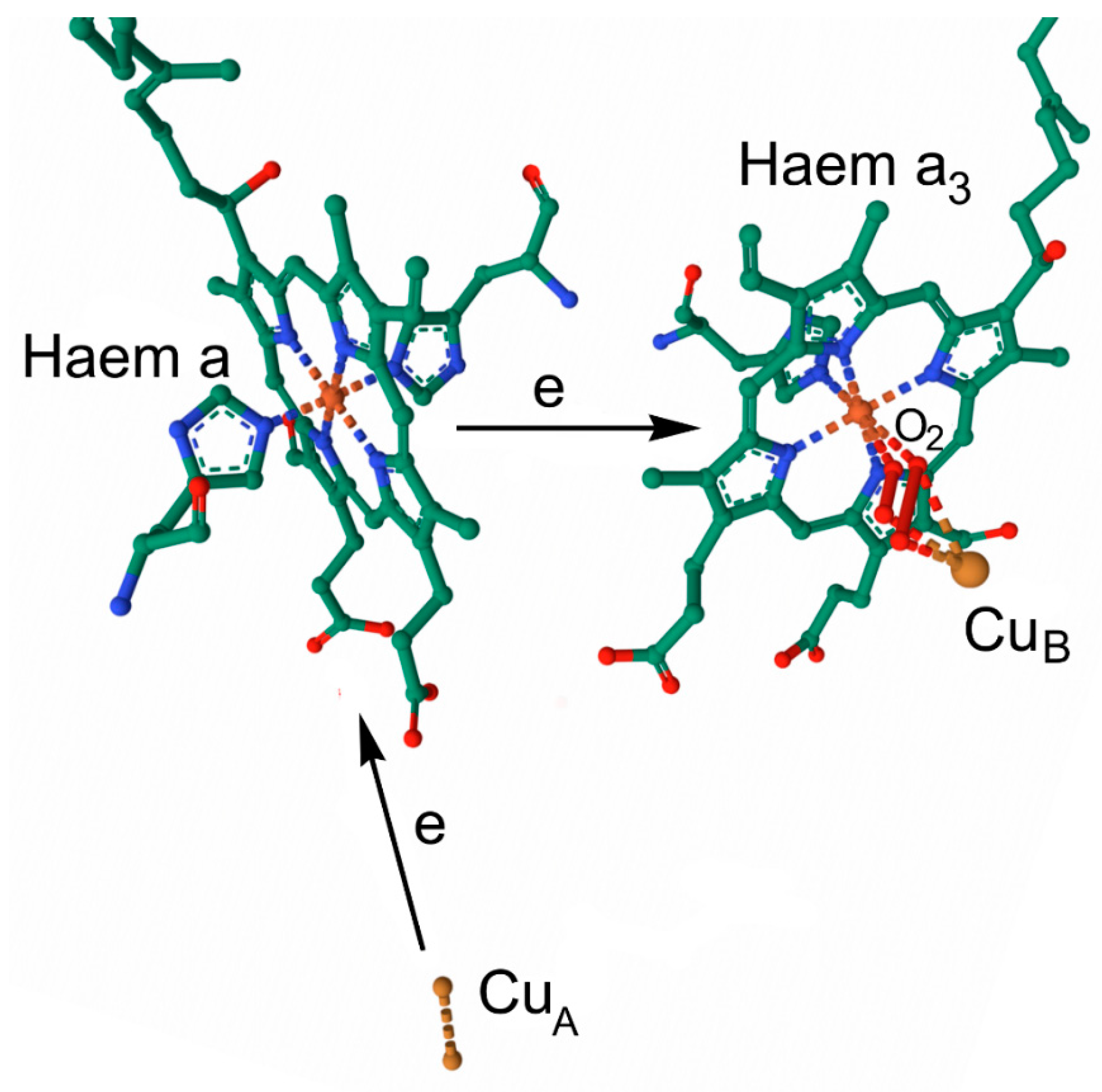
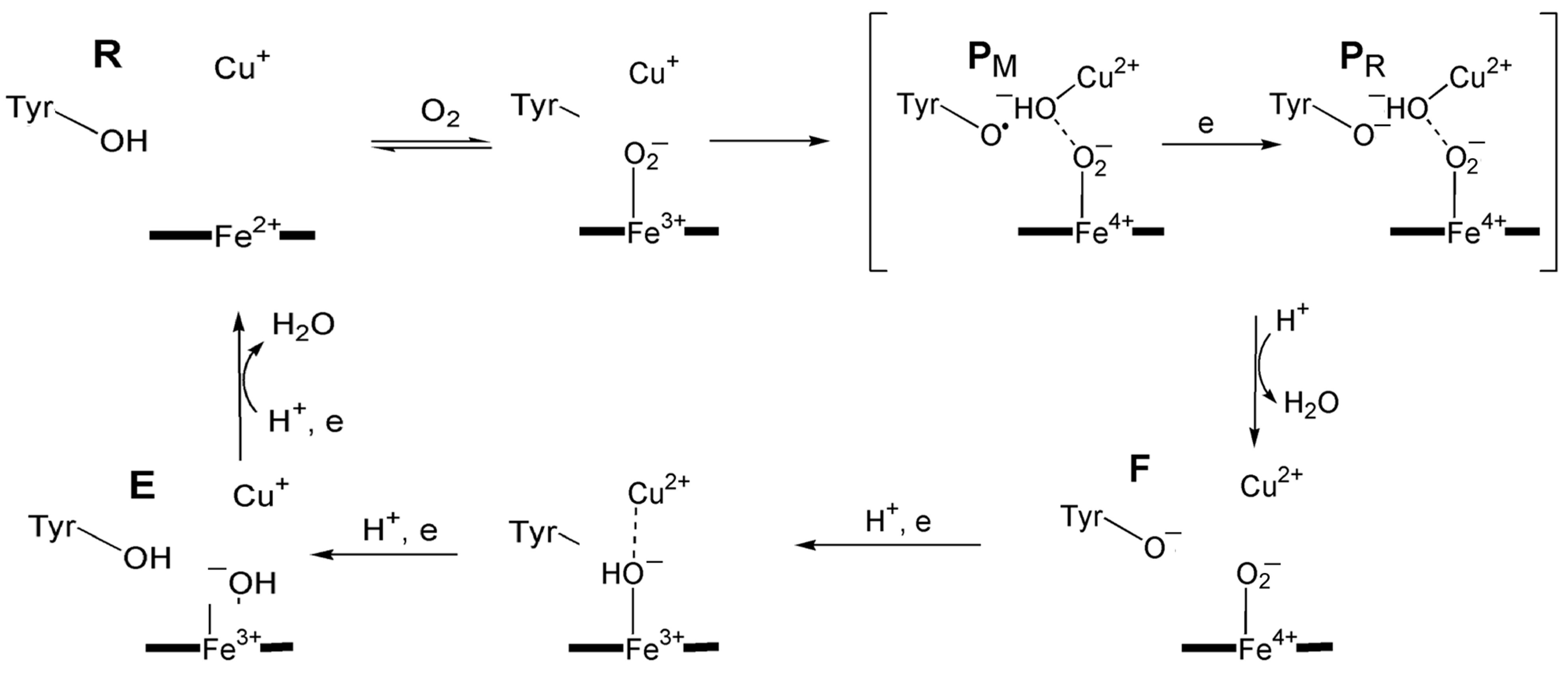
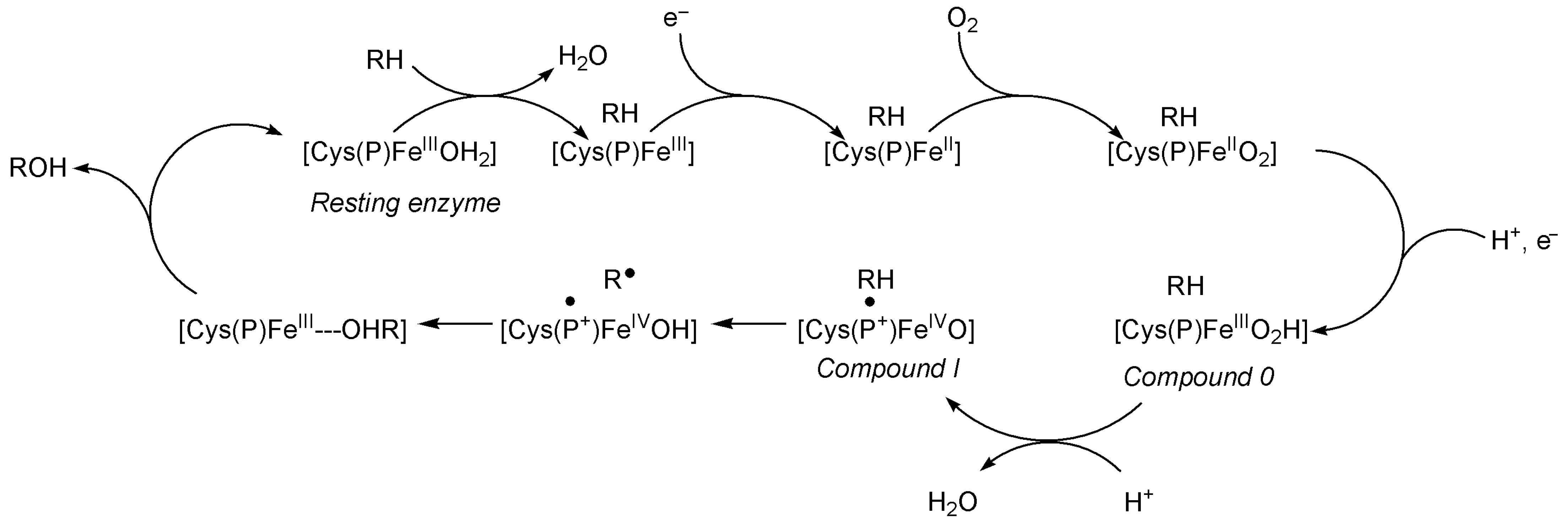


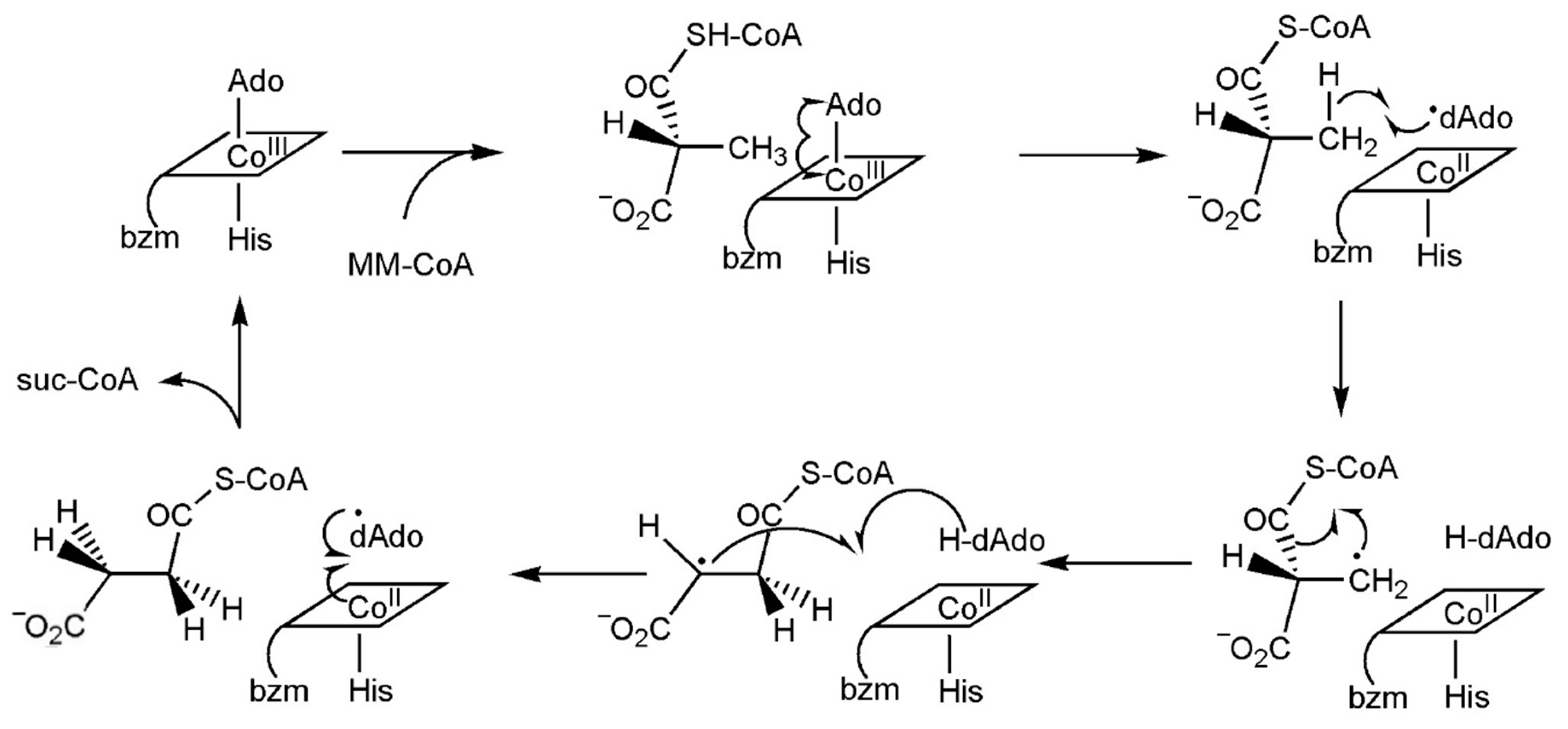

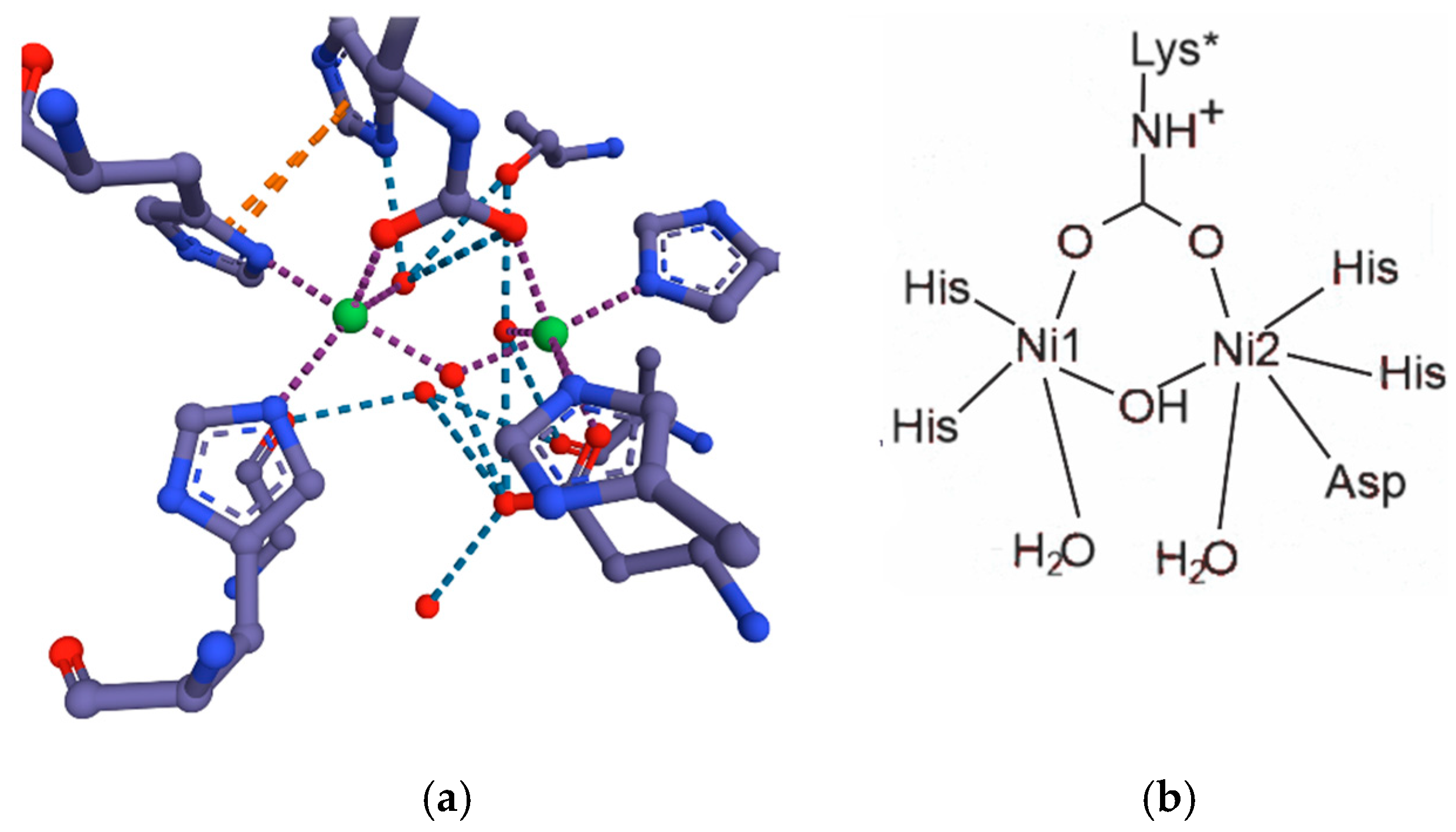

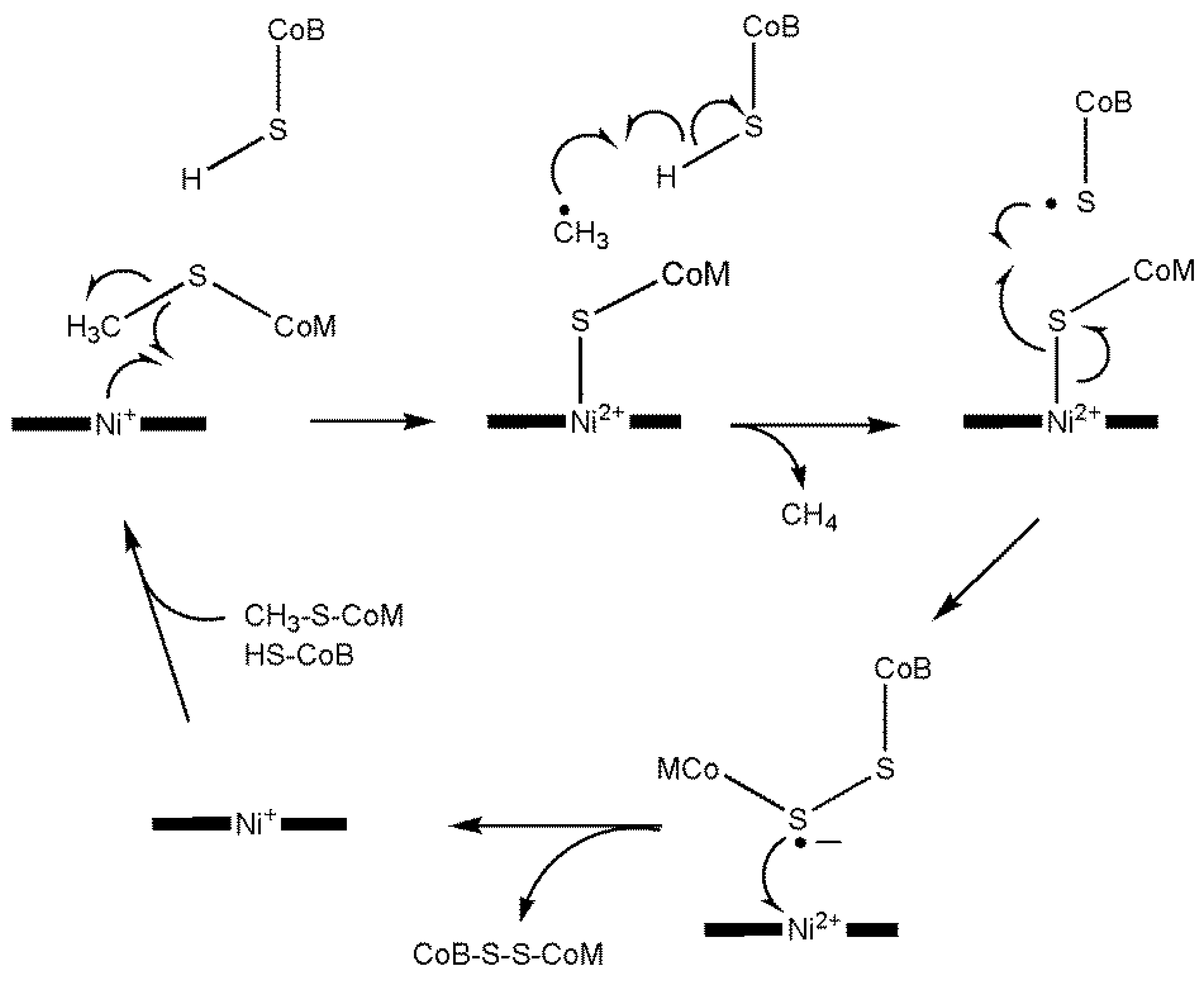
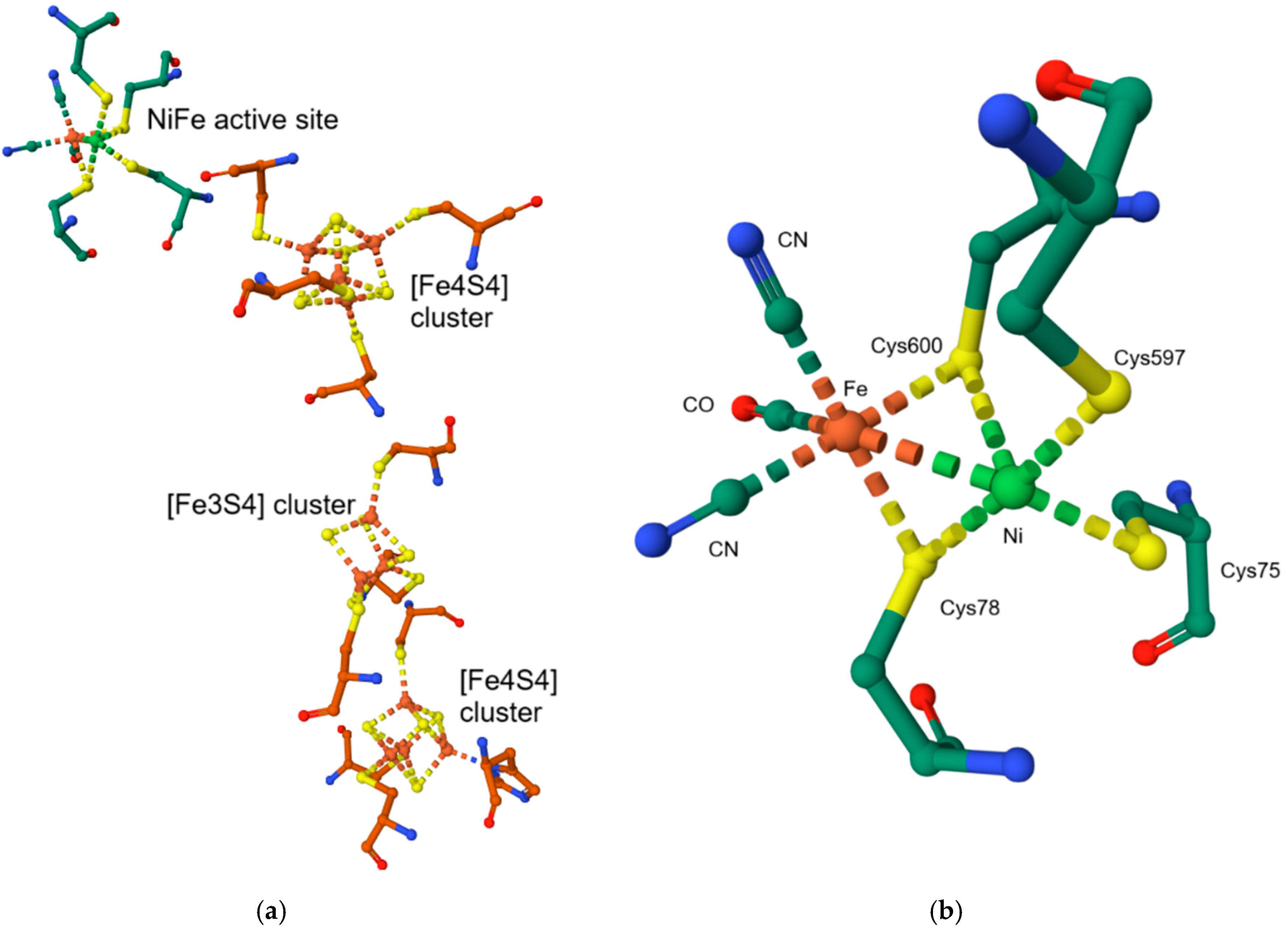
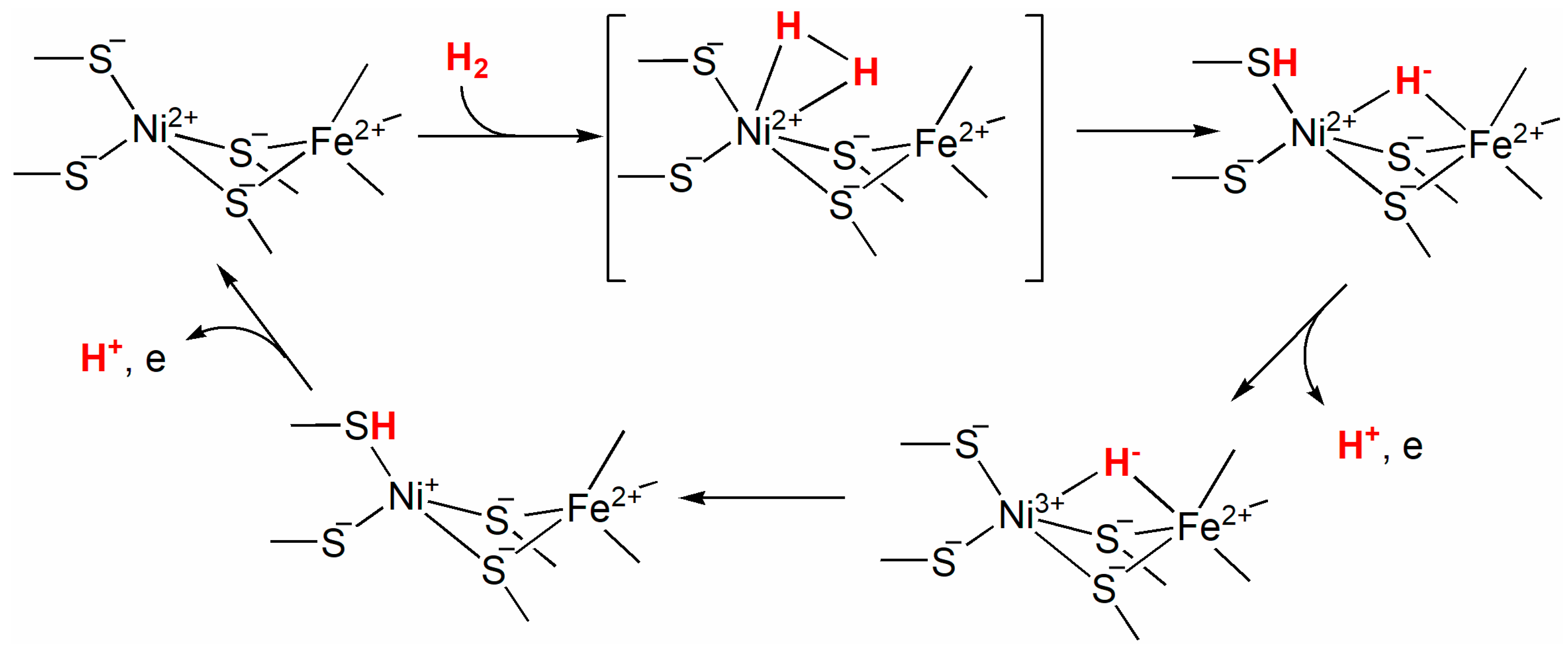

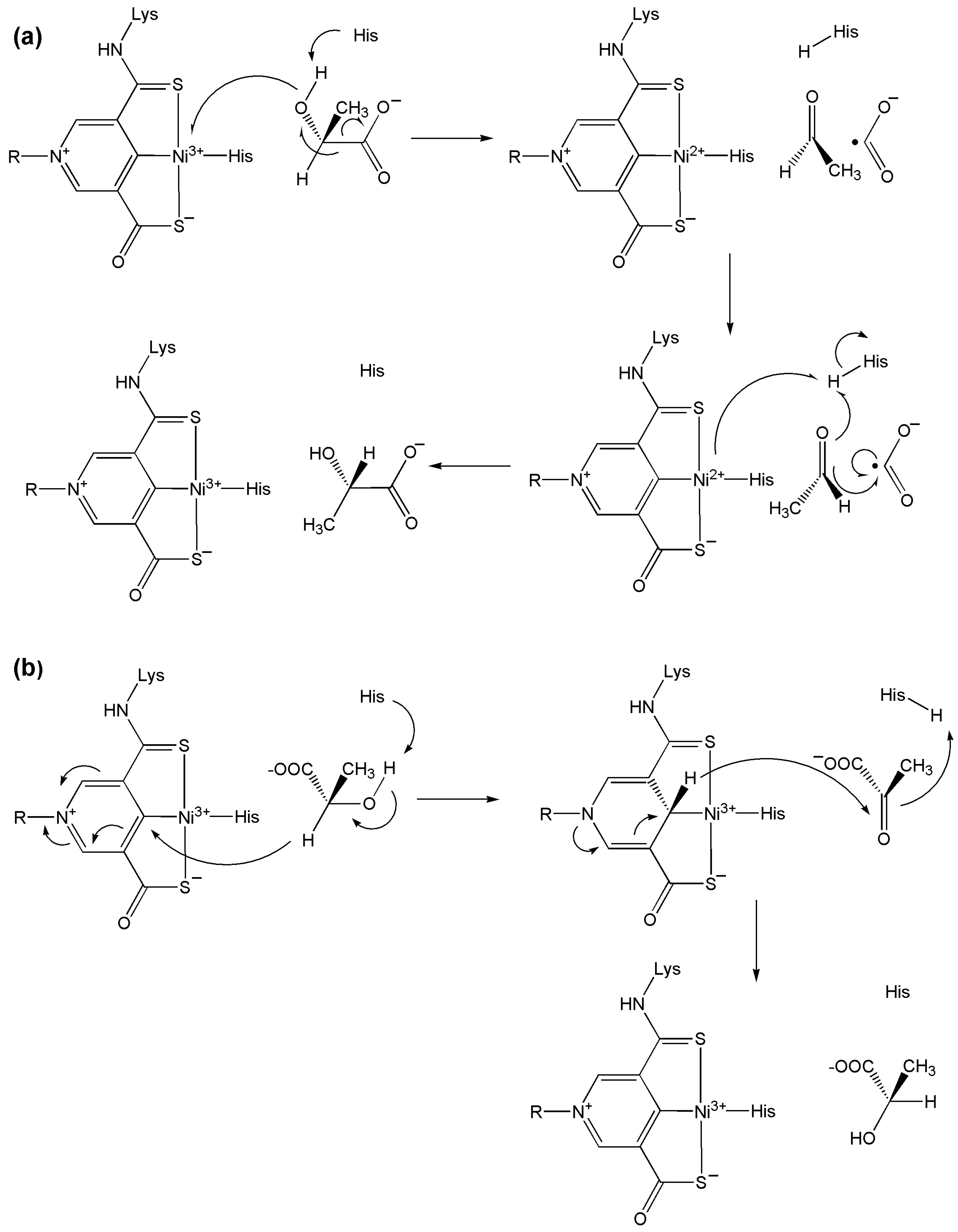
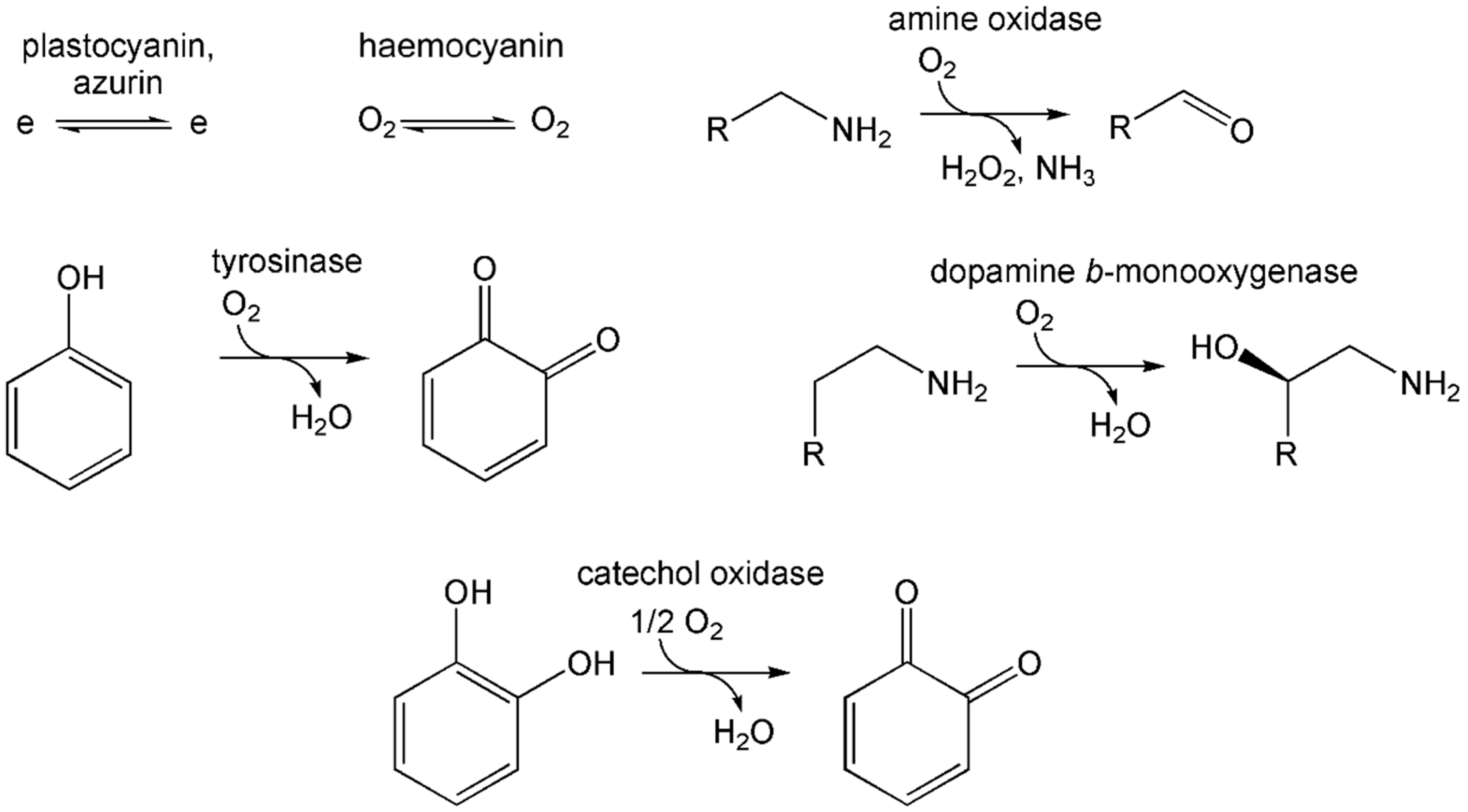
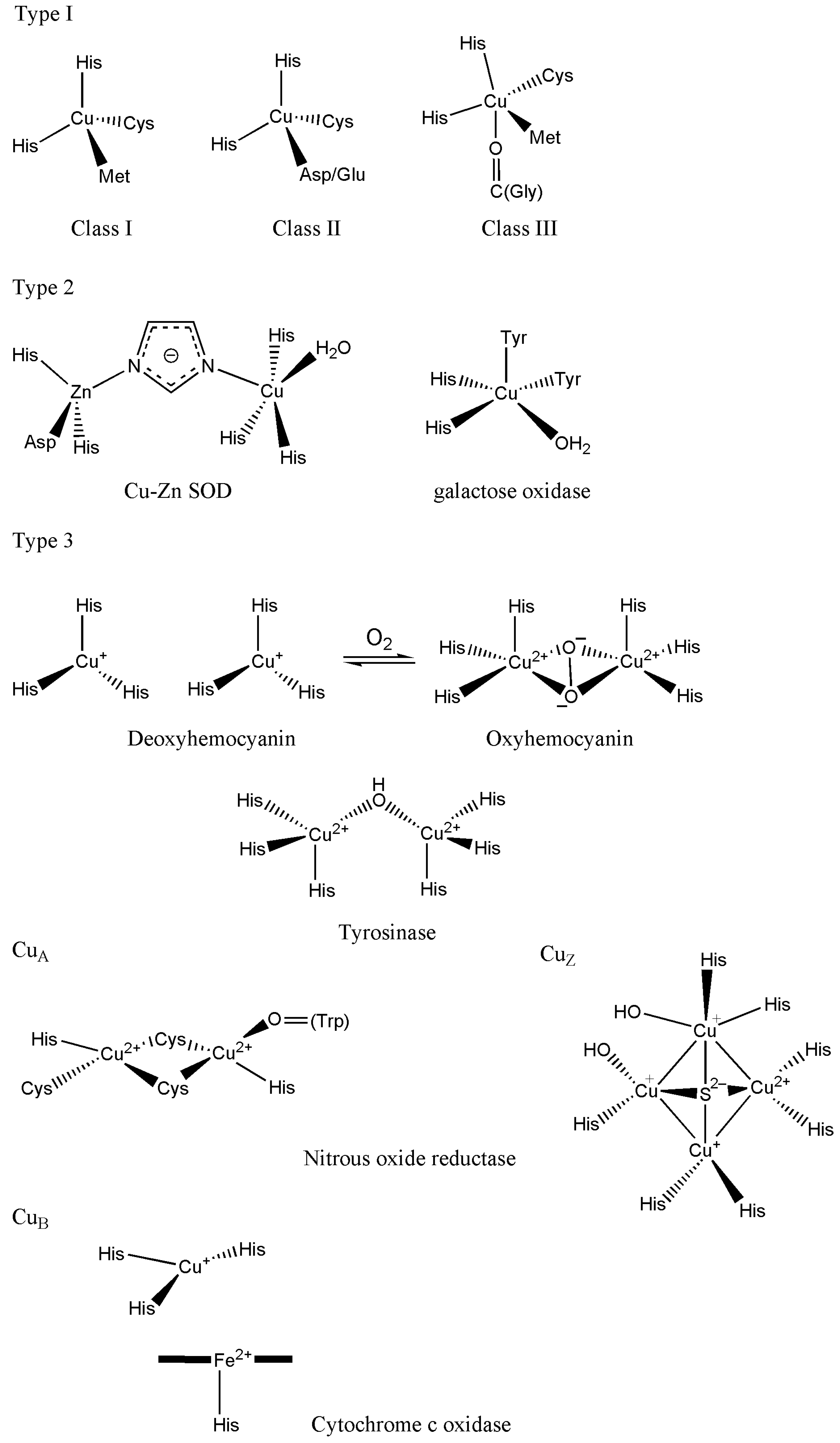
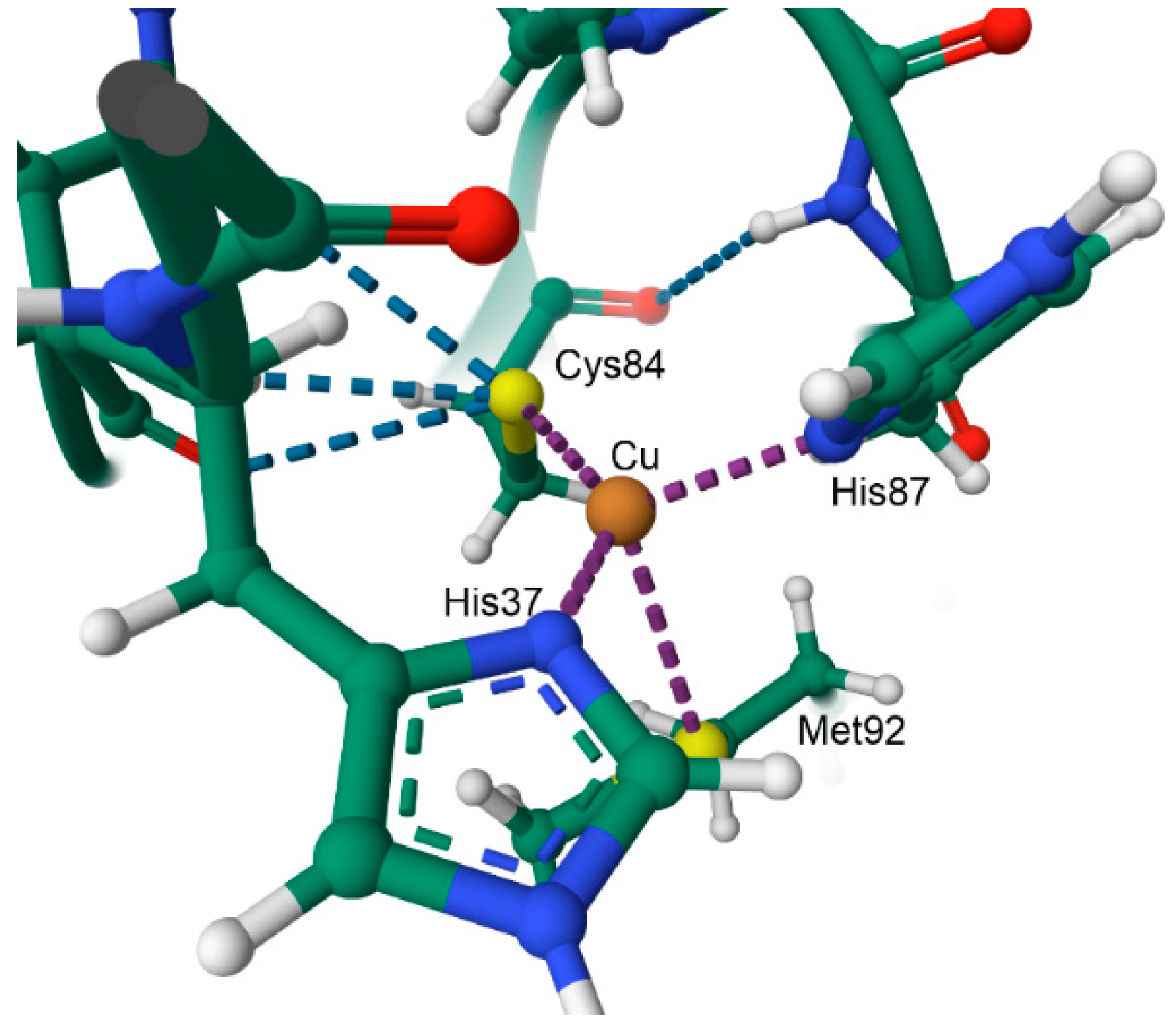
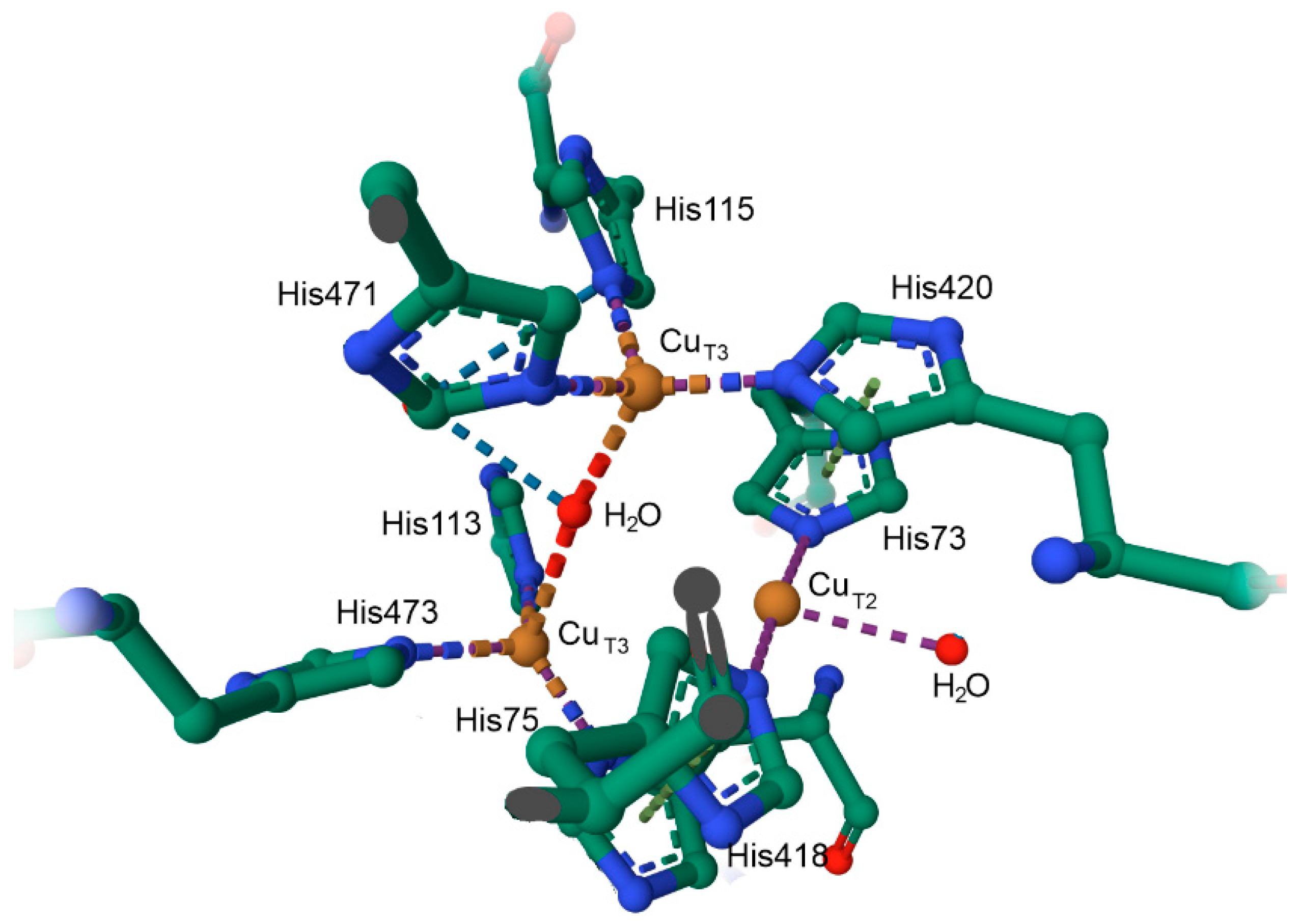


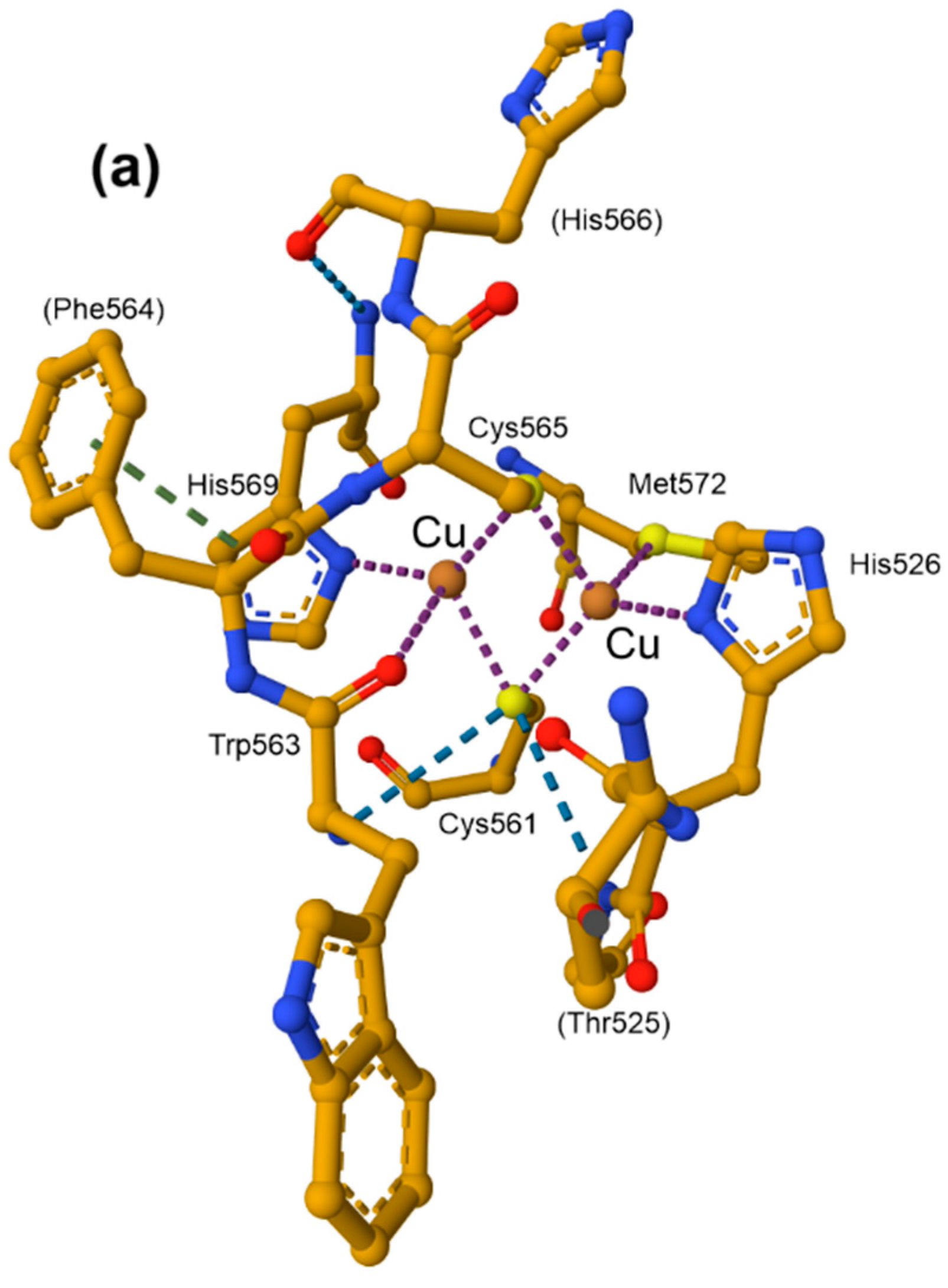

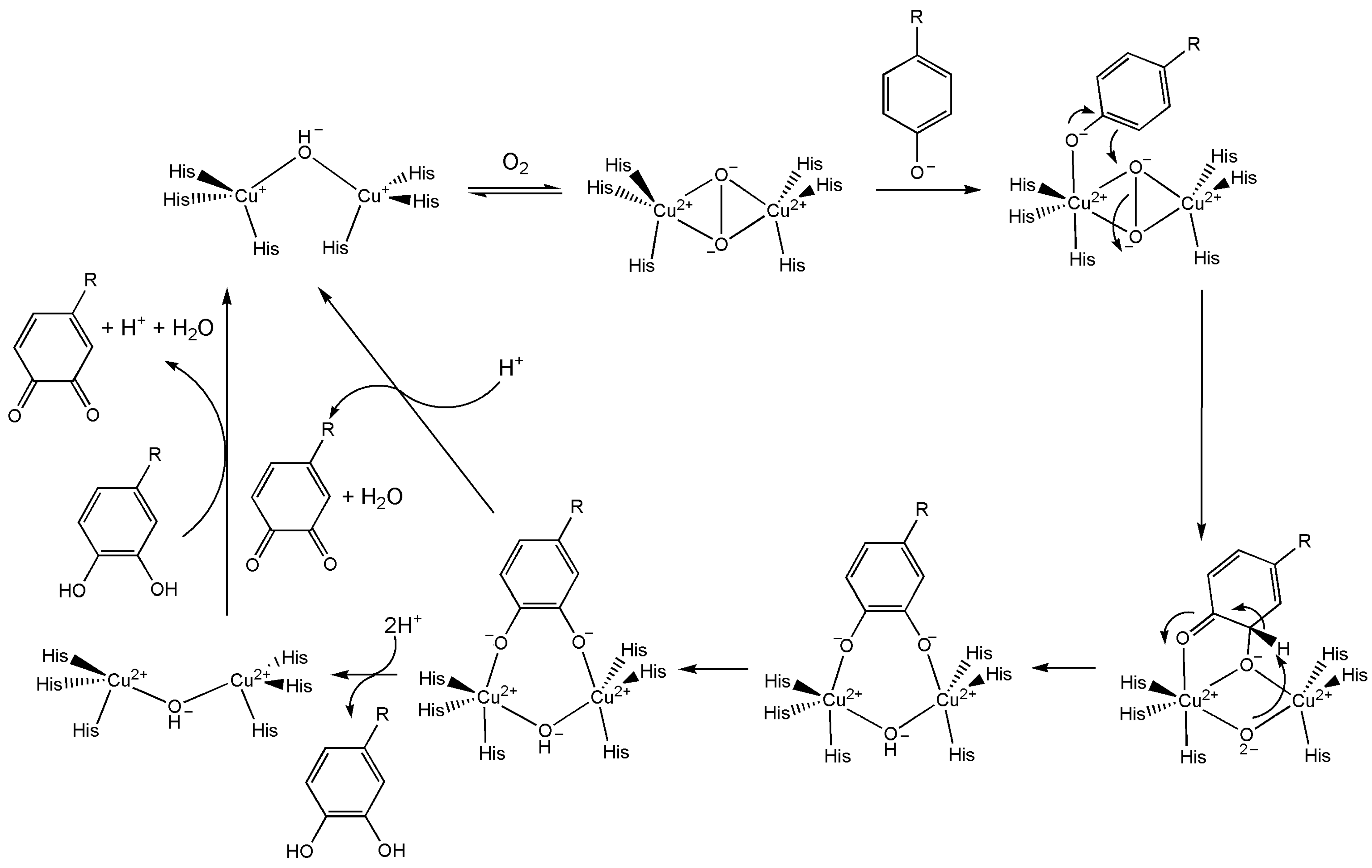
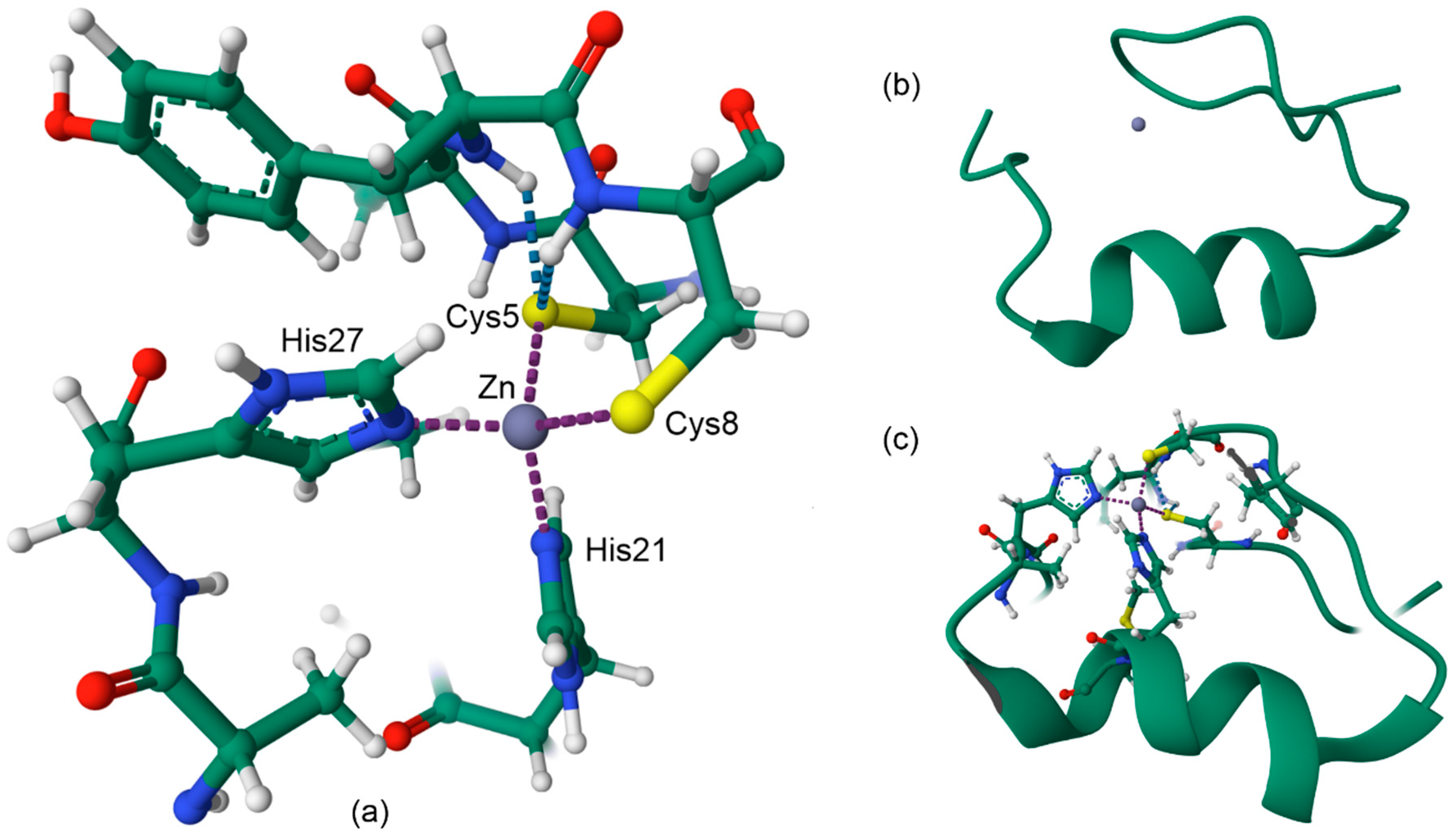

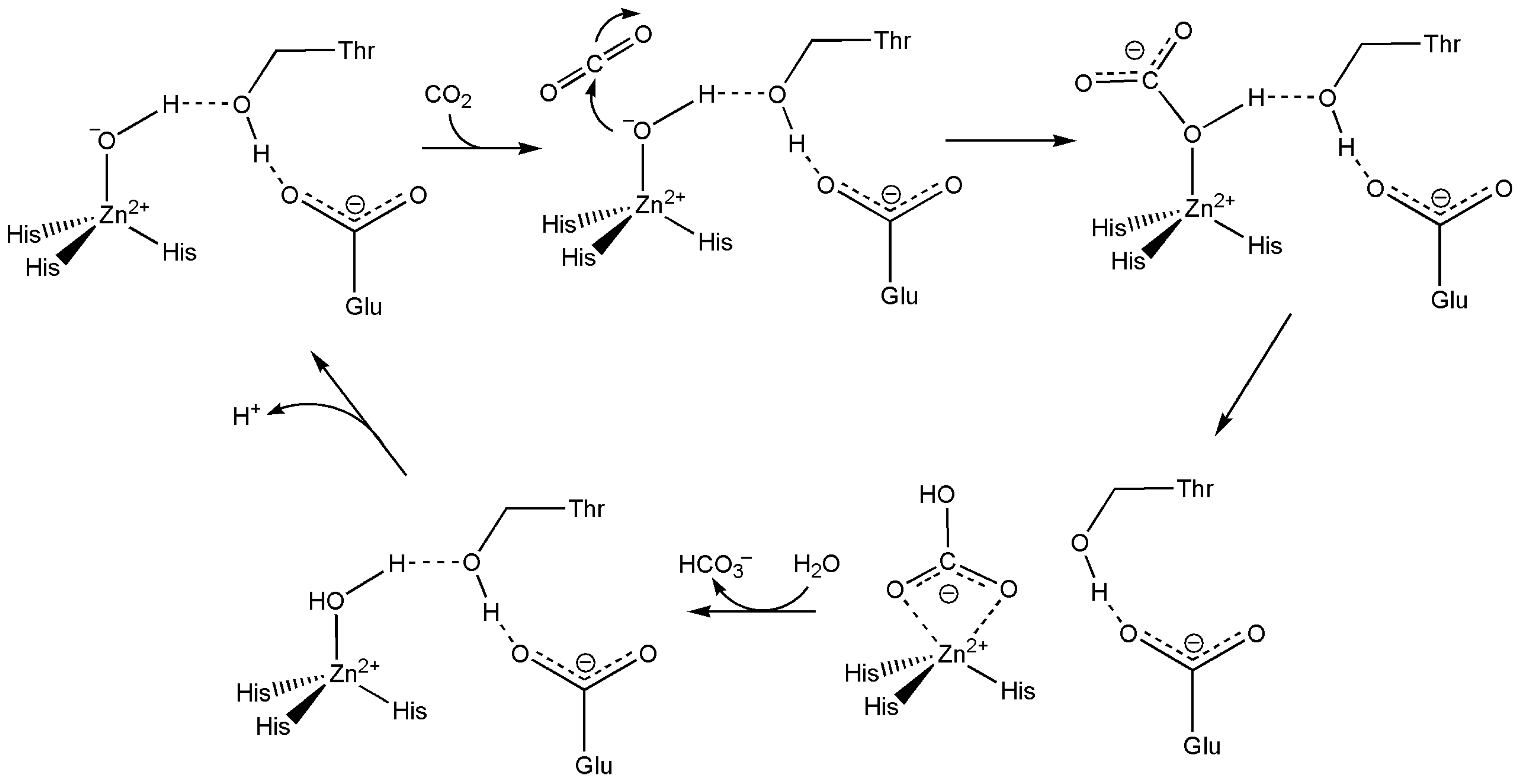



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, H.M. The Bioinorganic Chemistry of the First Row d-Block Metal Ions—An Introduction. Inorganics 2025, 13, 137. https://doi.org/10.3390/inorganics13050137
Marques HM. The Bioinorganic Chemistry of the First Row d-Block Metal Ions—An Introduction. Inorganics. 2025; 13(5):137. https://doi.org/10.3390/inorganics13050137
Chicago/Turabian StyleMarques, Helder M. 2025. "The Bioinorganic Chemistry of the First Row d-Block Metal Ions—An Introduction" Inorganics 13, no. 5: 137. https://doi.org/10.3390/inorganics13050137
APA StyleMarques, H. M. (2025). The Bioinorganic Chemistry of the First Row d-Block Metal Ions—An Introduction. Inorganics, 13(5), 137. https://doi.org/10.3390/inorganics13050137









