Abstract
Nanostructured multilayer anticorrosion coatings offer an effective strategy to mitigate the poor corrosion resistance of aluminum alloys and extend their service life. In this study, four types of Ti/Cr multilayer coatings with varied modulation periods along the growth direction were deposited on 7050 aluminum alloy substrates using direct current magnetron sputtering. The cross-sectional microstructure of the coatings was characterized by scanning electron microscopy (SEM), while their mechanical and corrosion properties were systematically evaluated through nanoindentation and electrochemical measurements. The influence of modulation period distribution on the corrosion resistance of Ti/Cr multilayers was thoroughly investigated. The results show that the average thickness of the Ti/Cr multilayer coatings is 680 nm, the structure is dense, and the coarse columnar crystals are not seen. All Ti/Cr multilayer coatings significantly reduced the corrosion current density of 7050 aluminum alloy by about 10 times compared with that of the substrate, showing good protective effect. Modulation period along the coating growth direction decreases the Ti/Cr multilayer coating surface heterogeneous interface density increases, inhibits the formation of corrosion channels, hindering the penetration of corrosive media, and the other three coatings and aluminum alloy compared to its corrosion surface did not see obvious pore corrosion, showing the most excellent corrosion resistance.
1. Introduction
Aluminum alloys are widely used across various industrial sectors, particularly in critical structural components such as engine housings, transmission casings, and wheels in both commercial and passenger vehicles. Their core advantage lies in significantly reducing structural weight, thus achieving lightweight design objectives [1,2]. Aluminum alloys, with their excellent mechanical properties, lower production costs, and good recyclability, hold a leading position in the manufacturing of various products [3,4]. However, in complex or harsh service environments, the surface mechanical properties and wear resistance of aluminum alloys are relatively poor, and the friction-induced passive films are easily damaged and rapidly corroded, thereby severely weakening their service stability and structural safety [5,6]. Therefore, improving the overall surface performance of aluminum alloys has become a key research direction. In response, researchers have extensively explored various surface modification methods, including anodizing [7], micro-arc oxidation (MAO) [8], surface texturing [9], laser surface cladding [10], electroplating [11], and physical vapor deposition (PVD) [12]. Among these, coating technologies such as electroplating, chemical vapor deposition (CVD), and physical vapor deposition (PVD) have gained widespread application as important surface engineering techniques for enhancing surface performance. Various metal coatings (such as Al [13], Ti [14], and Cr [15]) and ceramic coatings (such as Al2O3 [16], TiN [17], CrN [18]) have been deposited on highly active metal substrates for corrosion and wear protection. These coatings typically exhibit a columnar grain structure, primarily attributed to the limited diffusion of adsorbed atoms at the step edges, leading to high Ehrlich–Schwoebel barriers [19,20]. However, grain boundaries (GBs) in columnar crystals, along with structural defects during their growth process (such as microholes, pinholes, and droplet defects), often serve as crack initiation sites and channels for the penetration of corrosive media, significantly weakening the protective effect of the coatings and drastically shortening the service life of aluminum alloy components.
Compared to the single-layer structure, which often leads to excessive growth and coarsening of columnar crystals, the multilayer structure, due to its interfacial interactions between layers, can effectively refine the coating grains, resulting in superior mechanical properties and corrosion resistance [21,22]. For example, Madhavan [23] found in his study of Cu/Ag multilayer coatings that this structure significantly reduced the wear extent of the material in friction and wear tests. Additionally, the multilayer coating structure can effectively enhance its corrosion resistance by extending the penetration path of corrosive media. Liu et al. [24] prepared single-layer Ti, single-layer TiN, and multilayer Ti/TiN coatings using PVD methods, and their study showed that the multilayer Ti/TiN coating exhibited the best corrosion resistance in corrosive media. Similarly, Zhu et al. [25] found that the Ti/Cr multilayer coating exhibited a higher polarization potential compared to single-layer Ti or Cr coatings, further confirming the effectiveness of the multilayer structure in enhancing the coating’s corrosion resistance. This improvement is mainly attributed to the deflection of micro-crack propagation paths and the passivation effect at the heterogeneous interfaces within the multilayer structure [26,27].
The modulation period is a key structural parameter for regulating the mechanical properties and corrosion behavior of multilayer coatings. Misra et al. [28] systematically studied the mechanical properties of sputter-deposited Cu/Nb multilayer coatings and found that their hardness increased as the modulation period decreased, reaching a maximum value at 2 nm. However, when the modulation period was further reduced, the hardness slightly decreased. This trend can be attributed to the combined effects of the Hall–Petch strengthening mechanism, the constrained layer slip model, and the difficulty of dislocation motion across the interfaces. Similarly, Li et al. [29] observed a similar hardness enhancement phenomenon in Ag/Fe and Ag/Ni multilayer systems, and the study showed that when the modulation period of the coating was reduced from hundreds of nanometers to tens of nanometers, both the hardness and wear resistance of the material were significantly improved. Regarding corrosion behavior, Flores et al. [30] found that although shortening the modulation period of TiN/Ti multilayer coatings might initially exacerbate the local initiation of corrosion, long-term service tests showed that the multilayer structure actually contributed to improving the overall corrosion resistance. Zuo et al. [31] prepared TiN/AlN multilayer coatings using RF reactive magnetron sputtering and found that as the modulation period increased from 2 nm to 12 nm, the coating formed a coherent epitaxial structure, and its hardness and corrosion resistance improved. However, when the modulation period reached 26–36 nm, the coherent interfaces were destroyed, and the alternating stress field weakened, resulting in a significant decrease in hardness, a lower self-corrosion potential, and an increase in self-corrosion current density.
At present, most studies on the corrosion resistance of multilayer coatings have focused on structures with constant modulation periods, while systematic investigations into the role of varying modulation periods along the growth direction remain limited. In this study, a design strategy involving modulation period variation along the coating growth direction was introduced. The microstructural evolution, mechanical properties, and corrosion resistance of Ti/Cr multilayer coatings with different modulation architectures were systematically evaluated. Furthermore, the comprehensive protective efficiency of these coatings on aluminum alloy substrates was thoroughly assessed.
2. Results and Discussions
2.1. Cross-Sectional Morphology Analysis
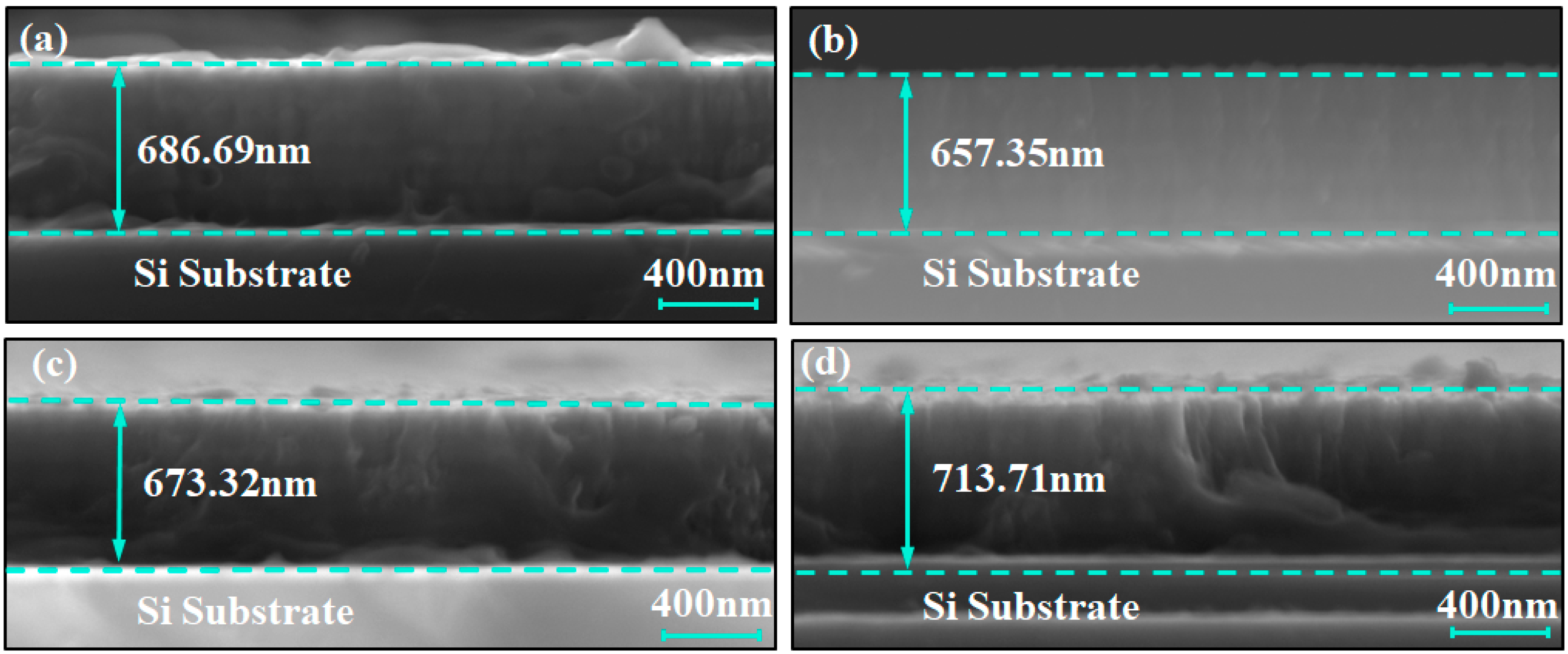
Figure 1 shows the scanning electron microscope (SEM) images of the cross-sections of the coating samples, used to characterize their thickness and structural features. The thickness of the C1, C2, C3, and C4 coatings were 686.69 nm, 657.35 nm, 673.32 nm, and 713.71 nm, respectively, with an average thickness of approximately 680 nm. In addition, no large columnar grains were observed in any of the coatings; only small columnar grains were present. This indicates that the alternating deposition of titanium and chromium sublayers in the multilayer structure has, to a certain extent, inhibited the growth of large columnar grains. However, due to the limited ability of secondary electron imaging to resolve compositional differences, the interfaces of the multilayer structure were difficult to identify directly. To further verify the multilayer structure of the Ti/Cr coatings, backscattered electron imaging (BSE) was employed for structural identification analysis.
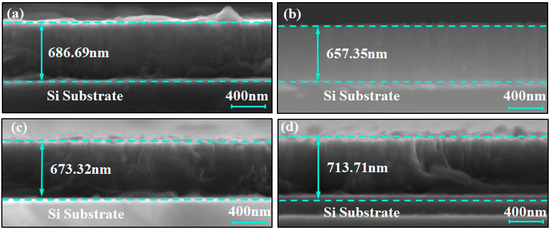
Figure 1.
Cross-sectional secondary electron SEM images of Ti/Cr multilayer coatings with different modulation period distributions along the growth direction: (a) C1; (b) C2; (c) C3; (d) C4.
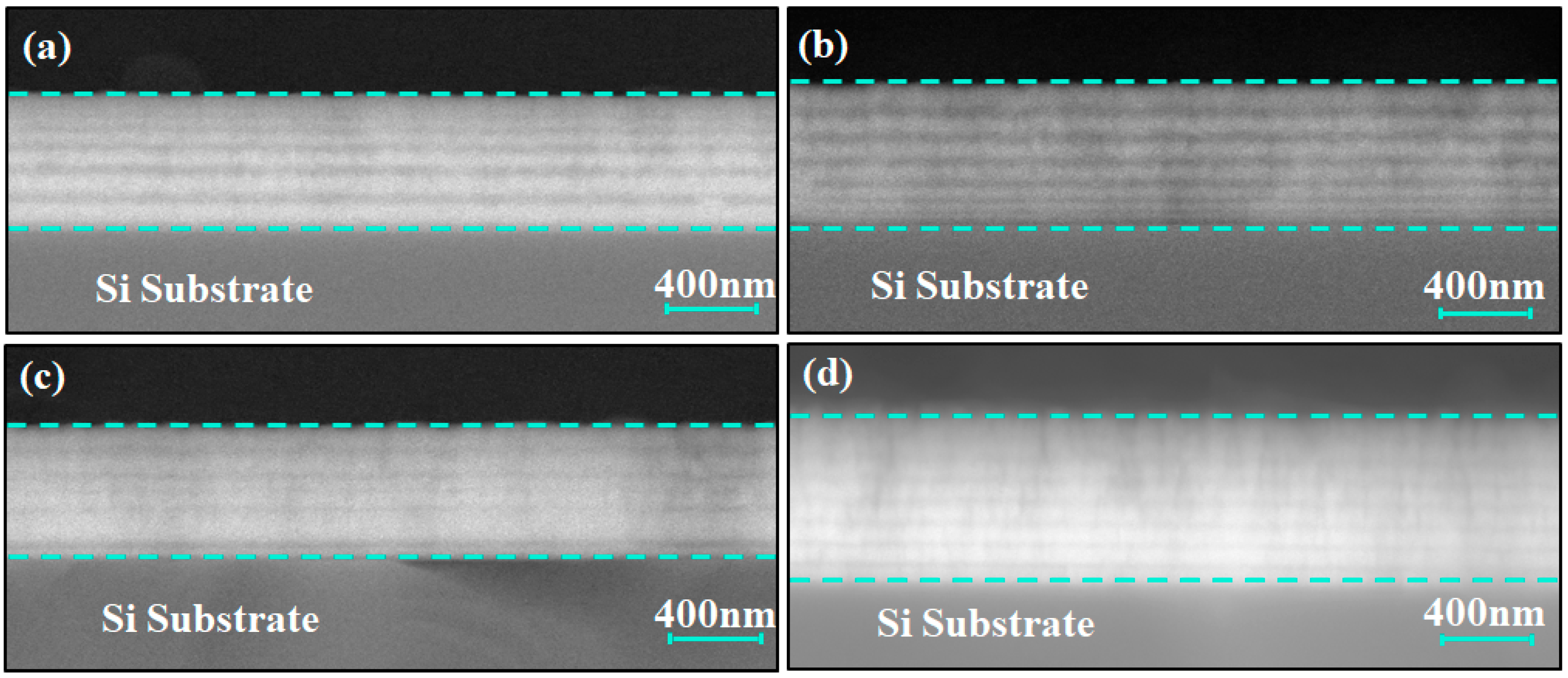
Figure 2 shows the modulation period variation in the Ti/Cr multilayer coating along the growth direction, displaying a distinct periodic pattern. Since Cr has a higher atomic number than Ti, its backscattering electron coefficient is greater, making it appear as brighter regions in the image, while the darker areas correspond to the Ti layers. The image indicates that the Ti and Cr layers alternate with tightly bonded interfaces, and the interfaces are clear without any noticeable structural defects. This result confirms that the deposited multilayer structure is consistent with the intended design.
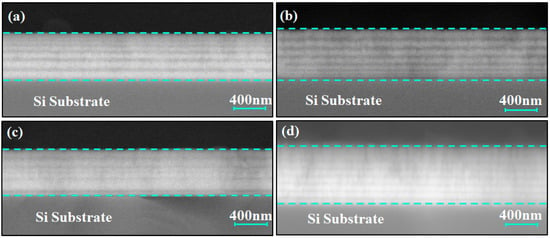
Figure 2.
Cross-sectional backscattered electron (BSE) SEM images of Ti/Cr multilayer coatings with different modulation period distributions along the growth direction: (a) C1; (b) C2; (c) C3; (d) C4.
2.2. Phase Characterization Analysis
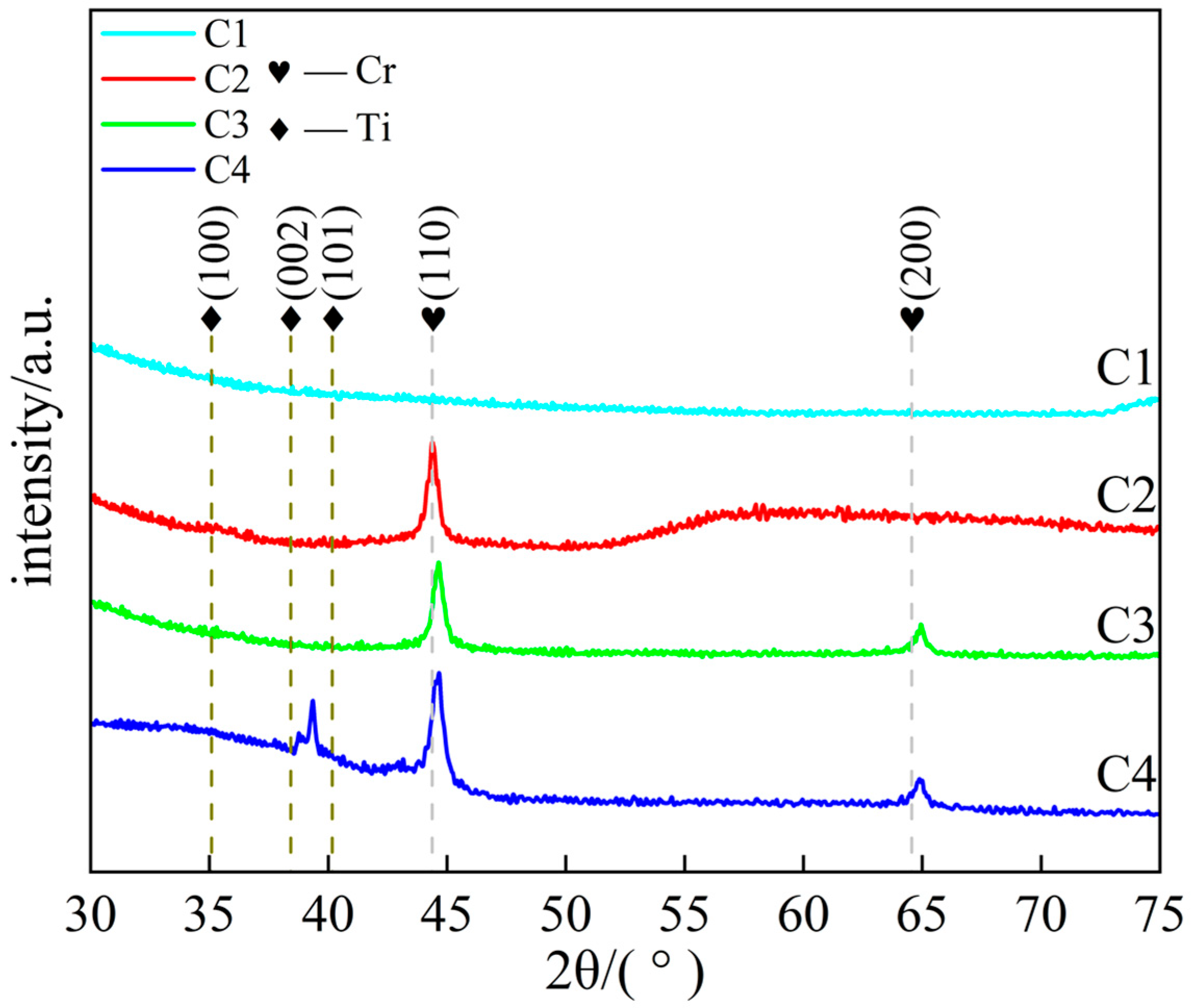
Figure 3 shows the XRD spectra, as the modulation period of the C1 coating decreases with increasing thickness, the intensity of its diffraction peaks significantly weakens and the peak width increases, making it difficult to clearly identify the diffraction peaks, indicating a significant increase in the amorphous degree of the coating. C2, C3, and C4 coatings exhibit distinct diffraction peaks at 2θ ≈ 44.4° and 64.6°, corresponding to the (110) and (200) crystal planes of body-centered cubic Cr, respectively, where the (110) crystal plane exhibits the strongest diffraction intensity due to its lowest surface energy. The internal stress generated by the high-power magnetron sputtering process causes lattice compression in the coating, reducing the interplanar spacing and shifting the Cr diffraction peak toward higher angles [32]. Additionally, a narrow peak at 2θ ≈ 39° was observed in the C4 sample, attributed to the displacement of the Ti diffraction peak due to compressive stress in the Ti layer of the multilayer structure [23,33]. The broad peak at 2θ ≈ 35° originates from the disordered structure formed during the initial deposition stage of the coating [34].
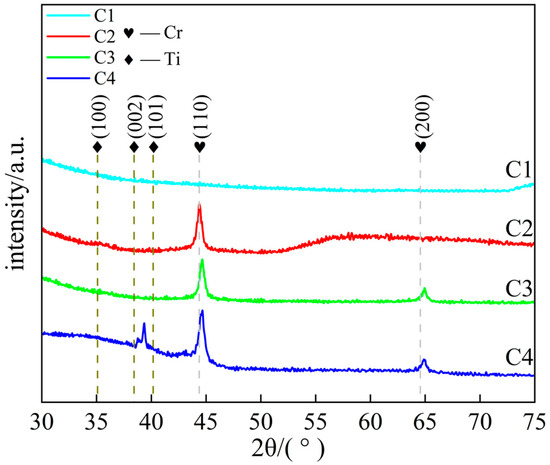
Figure 3.
XRD patterns of Ti/Cr multilayer coatings with different modulation period distributions along the growth direction.
2.3. Mechanical Properties Analysis
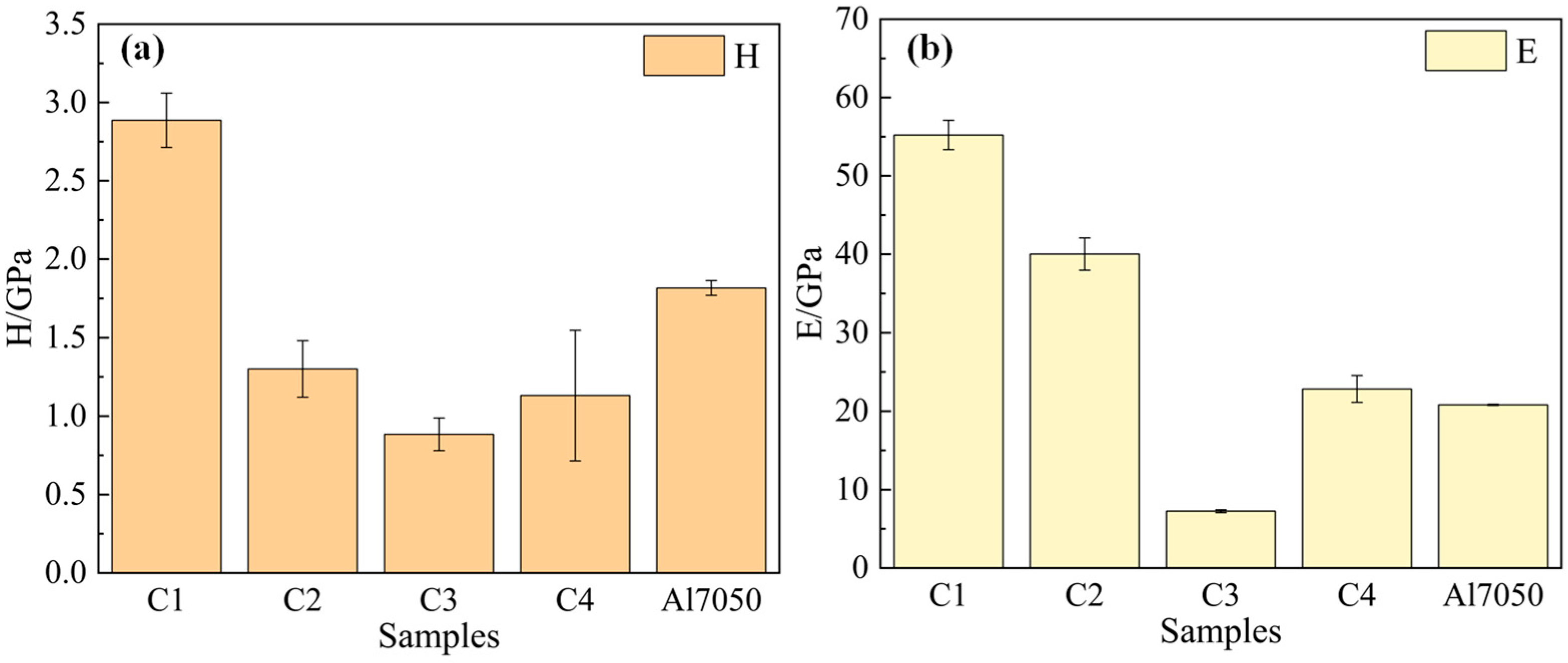
Nanoindentation tests were performed to characterize the hardness (H) and elastic modulus (E) of 7050 aluminum alloy and the four Ti/Cr multilayer coatings. The results are shown in Figure 4. It can be seen that the C1 coating exhibits significantly higher hardness (2.89 GPa) and elastic modulus (55.22 GPa) compared to the 7050 aluminum alloy substrate (1.81 GPa and 20.81 GPa, respectively), whereas the hardness of the C2, C3, and C4 coatings is lower than that of the substrate material. Generally, the hardness of multilayer coatings is influenced by multiple factors, including grain size, lattice structure, and sublayer thickness [35,36]. In the C1 coating, the modulation period gradually decreases along the coating growth direction, leading to a reduction in sublayer thickness. The Constrained Layer Slip (CLS) model accounts for the restricted movement of dislocations at the nanoscale layer interfaces. The optimization of the modulation period and sublayer thickness plays a crucial role in limiting dislocation propagation [29], thereby enhancing the hardness of the C1 coating. In contrast, the C2 coating exhibits an increasing trend in modulation period along the growth direction, leading to thicker sublayers and weakened constraint on dislocations. This results in reduced resistance to plastic deformation and consequently lower hardness. The C3 coating introduces stress mismatch and excess residual stress through alternating modulation periods. This stress accumulation promotes microcrack formation and counteracts dislocation constraint, further deteriorating hardness. Although the C4 coating employs a constant modulation period design, the larger sublayer thickness allows dislocations to easily slip and propagate within the sublayers, weakening the pinning effect of the interface on dislocations. This ultimately results in a hardness lower than that of the C1 coating.
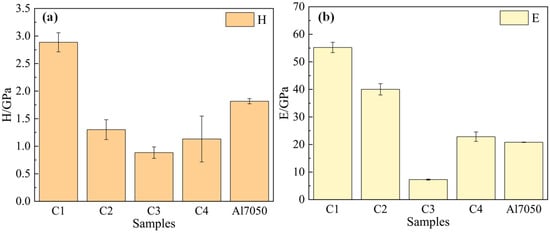
Figure 4.
Hardness (a) and elastic modulus (b) of the substrate and coated samples.
2.4. Corrosion Resistance Analysis
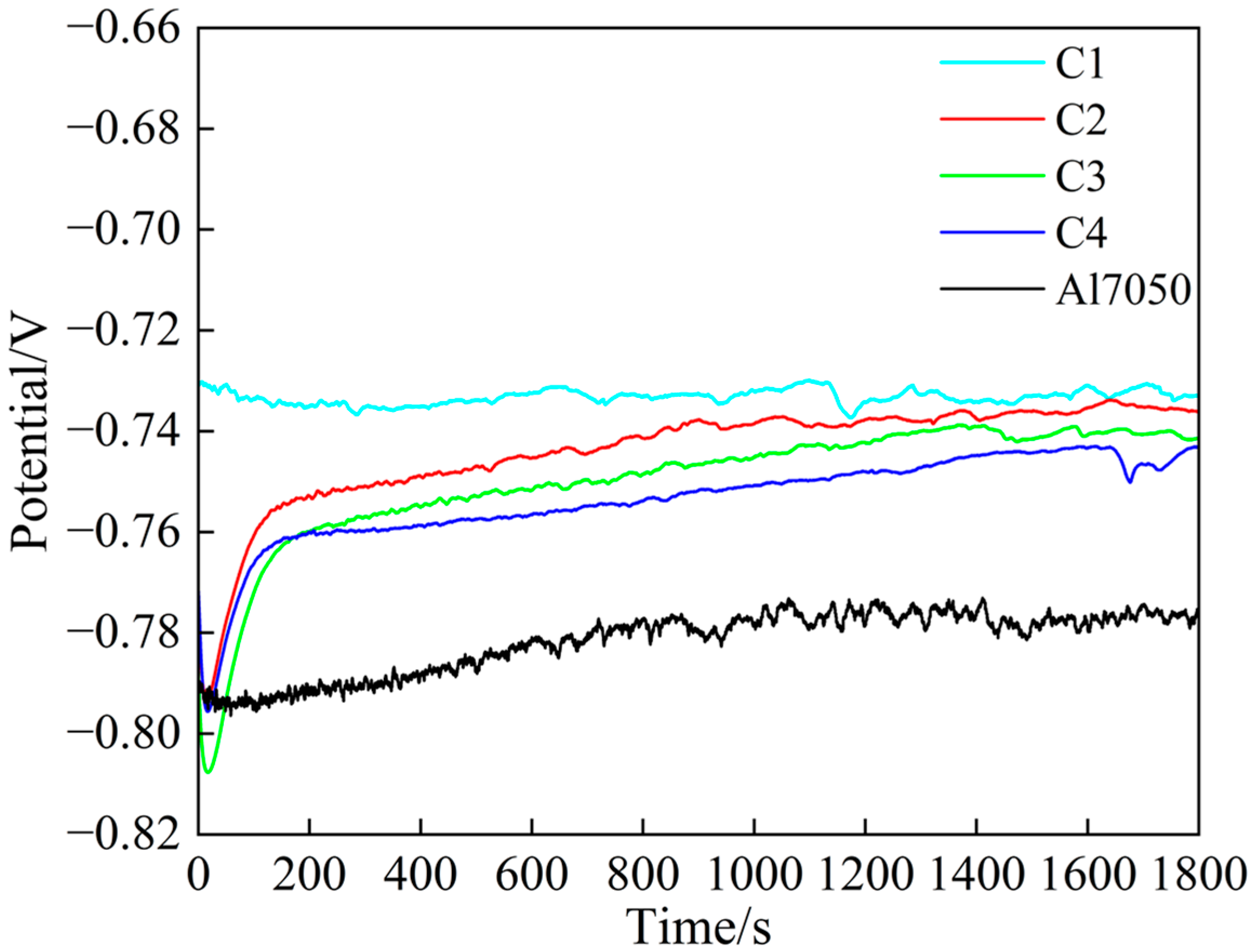
Figure 5 shows the open-circuit potential changes in each sample after being immersed in a 3.5 wt.% NaCl solution for 1800 s. It can be observed that the open-circuit potential of all samples gradually stabilizes over time, indicating that the system has reached a stable electrochemical state, meeting the requirements for subsequent electrochemical testing. Among these, the steady-state potentials of the coated samples are significantly higher than those of the bare A17050 aluminum alloy, indicating that the coating effectively delays the onset of corrosion reactions and exhibits superior passivation capability and corrosion resistance. Notably, the open-circuit potential curve of the C1 sample remained nearly stable throughout the testing process and exhibited the highest steady-state potential, indicating that its surface rapidly formed a dense and stable passivation film upon initial contact with the corrosive medium, effectively preventing the penetration of the corrosive medium [37]. In contrast, the C2, C3, and C4 samples exhibited a noticeable potential increase, indicating that their passivation films had begun to form [38].
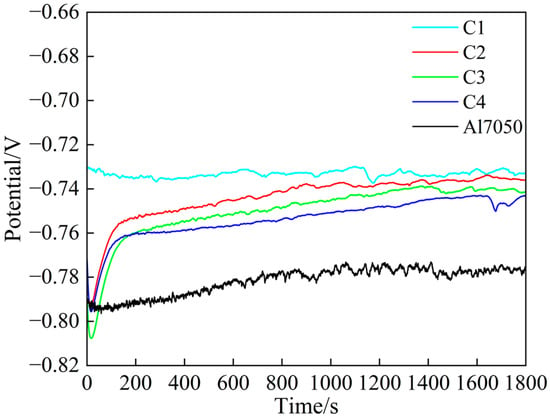
Figure 5.
Open-circuit potential of substrate and coating samples.
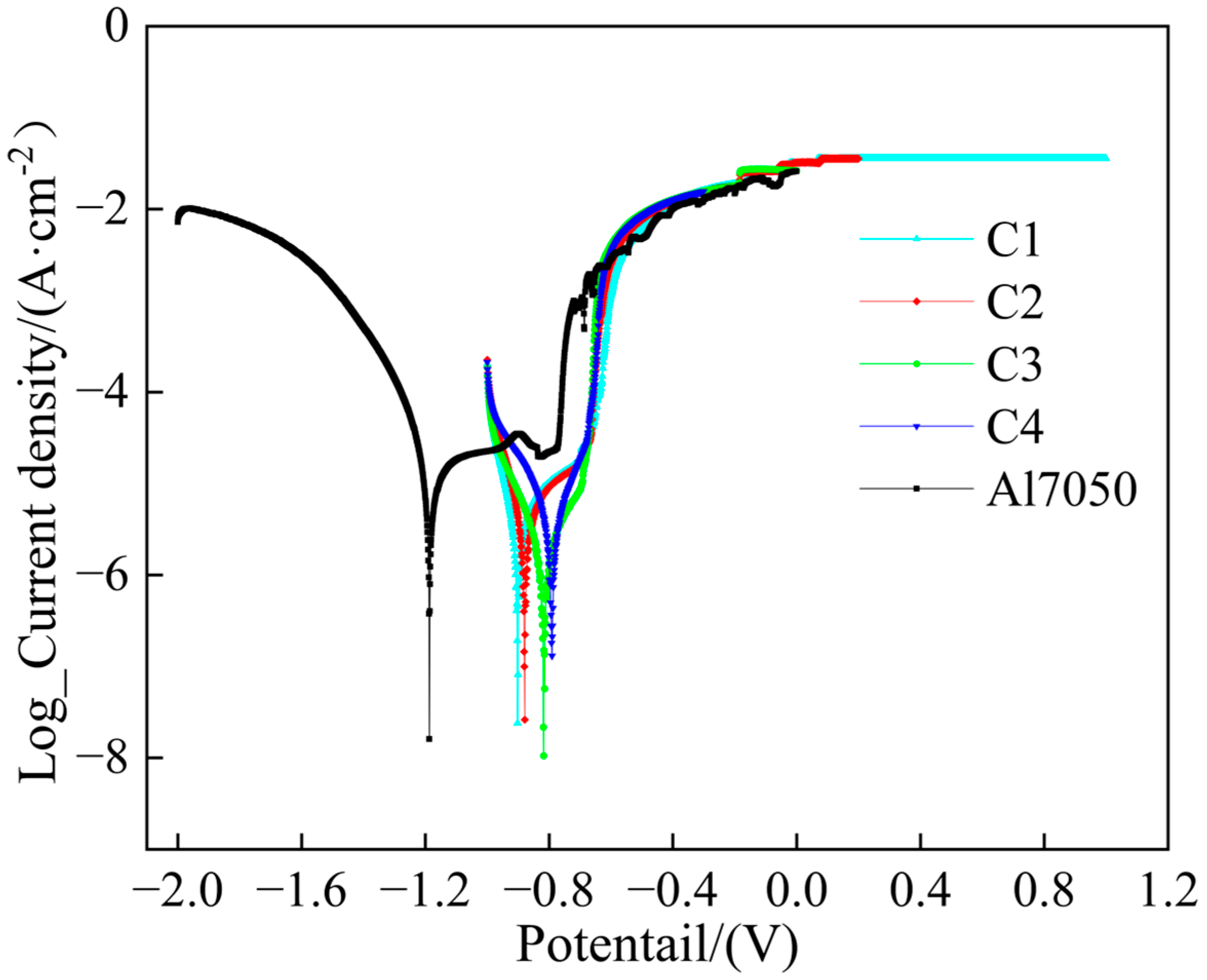
The potentiodynamic polarization tests were conducted on the Ti/Cr multilayer coatings in a 3.5 wt.% NaCl solution, and the results are shown in Figure 6. The 7050 aluminum alloy substrate exhibited pitting corrosion around −0.83 V, where the passive film was broken, whereas none of the four Ti/Cr multilayer coatings showed significant pitting corrosion. This indicates that the multilayer structure effectively enhances the pitting corrosion resistance. The corrosion current density reflects the rate of corrosion, and theoretically, a lower corrosion current density usually suggests higher thermodynamic stability and better corrosion resistance, helping to delay the breakdown and dissolution of the passive film [39,40]. By extrapolating the Tafel curves, the corrosion parameters are listed in Table 1. Compared to the 7050 aluminum alloy bare substrate, the corrosion current densities of all coatings were reduced by about one order of magnitude. This improvement is primarily due to the multiple barrier effects formed by the multilayer coating structure, including a significant reduction in porosity, and an extended penetration path for the corrosive medium, which increases the diffusion energy barrier and effectively hinders the penetration and diffusion of corrosive species [41,42], thus significantly enhancing the protection of the substrate. In the C1 coating, as the modulation period decreased, intrinsic defects such as pores and microcracks were reduced. The coating surface became denser, which decreased the possibility of corrosive media penetrating the coating and reaching the substrate [43]. Furthermore, as the modulation period shortened, the number of heterogeneous interfaces increased, which delayed the formation of a continuous corrosion channel from the coating surface to the substrate. As a result, the corrosion current density in the C1 coating was the lowest among the four coatings, at just 1.19 × 10−6 A/cm2.
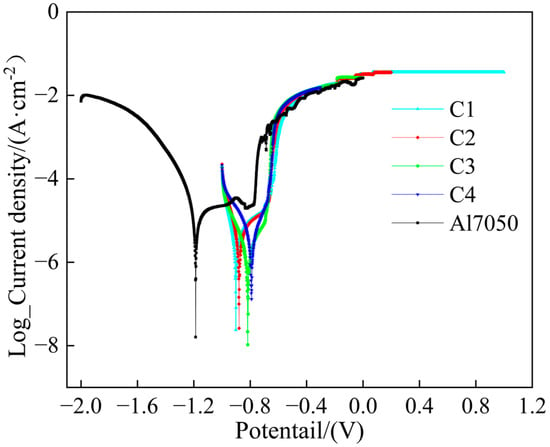
Figure 6.
Potentiodynamic polarization curves of the substrate and coated samples.

Table 1.
Tafel fitting parameters derived from potentiodynamic polarization curves.
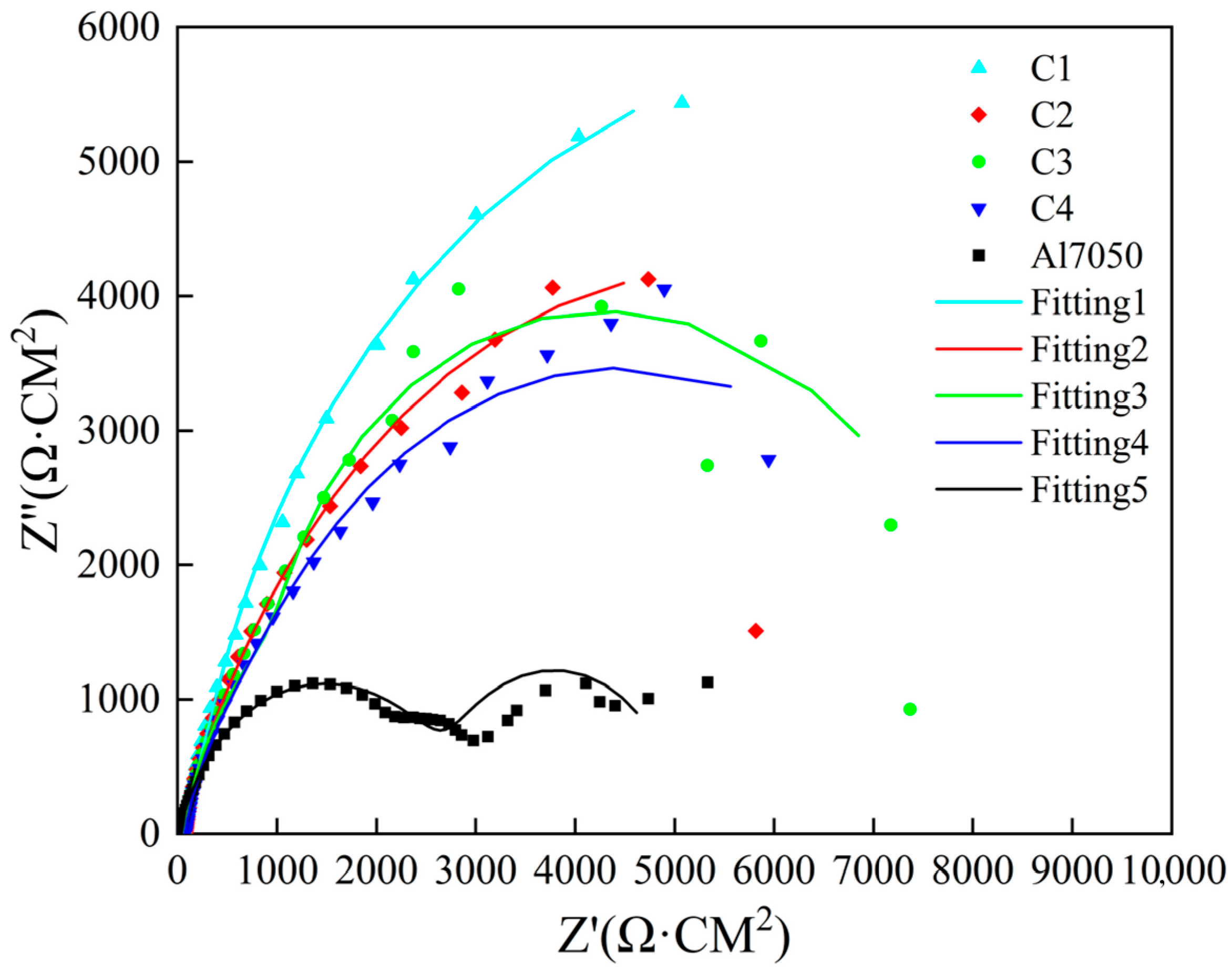
Electrochemical impedance spectroscopy (EIS) was used to evaluate the corrosion resistance of the 7050 aluminum alloy substrate and the samples modified with Ti/Cr multilayer coatings. The results are shown in Figure 7. In the Nyquist plot, the capacitive arc radius in the high-frequency region reflects the charge transfer resistance, which is a key indicator of corrosion resistance. Generally, a larger capacitive arc radius indicates better corrosion resistance of the material [44]. All coating samples presented incomplete semicircular arcs in the Nyquist plot. Compared with untreated 7050 aluminum alloy, samples treated with four different Ti/Cr multilayer coatings exhibited larger capacitive arc radii, indicating increased charge transfer resistance, limited surface reaction kinetics, and improved corrosion resistance. Among the coatings, the C1 coating showed the largest capacitive arc radius, followed by the C3 coating, then the C2 coating, and finally the C4 coating, which had the smallest arc radius. Based on this, it can be concluded that the C1 coating exhibited the best corrosion resistance. It is worth noting that there are no 45° straight segments in the low-frequency region, indicating that no diffusion process has occurred within the multi-layer coating [45].
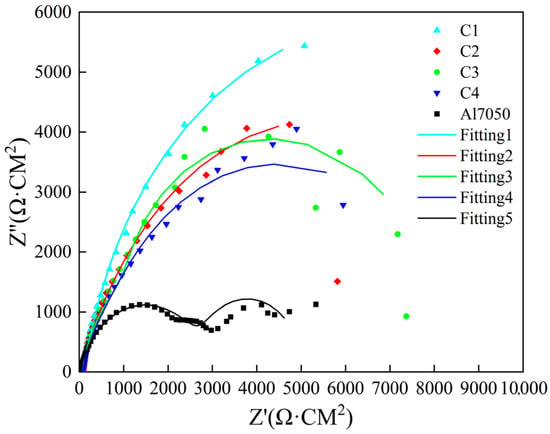
Figure 7.
Nyquist plots of electrochemical impedance spectra for the substrate and coated samples.
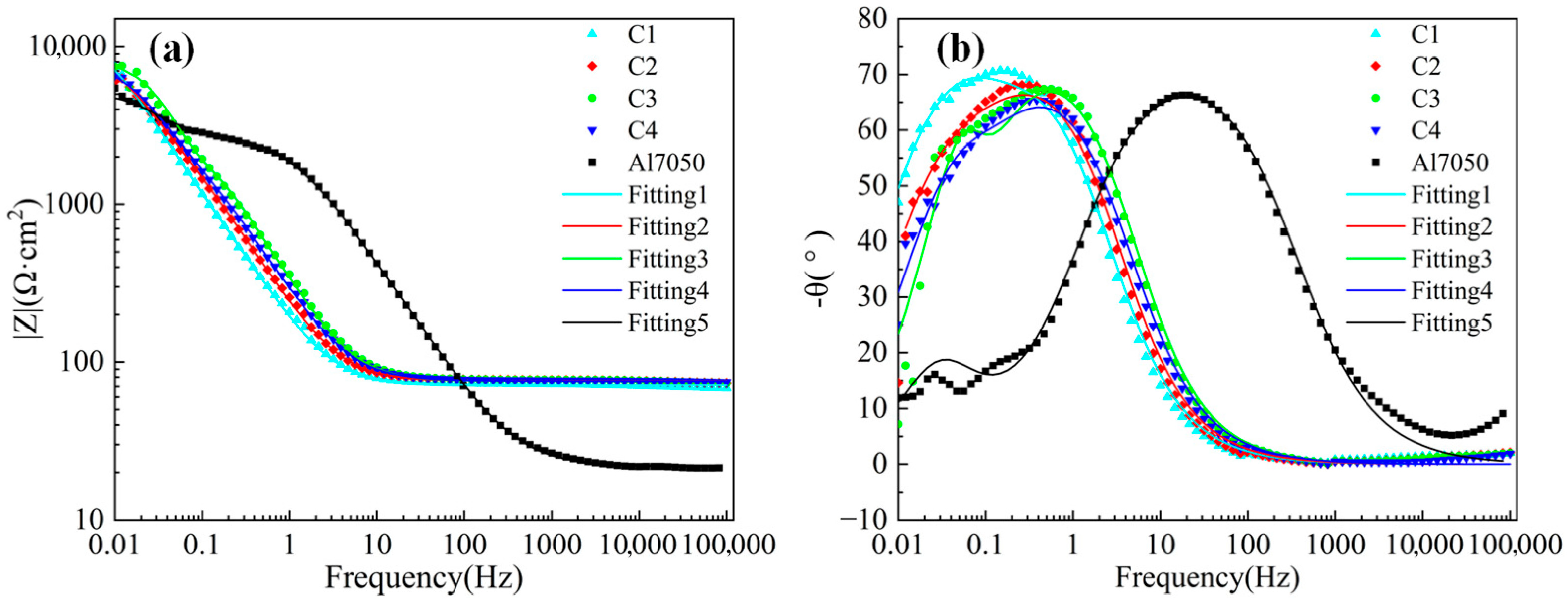
As shown in Figure 8a, the Bode plot curve tends to flatten in the frequency range of 102 to 105 Hz, mainly due to the influence of the solution resistance (Rs) in the artificial seawater medium. Figure 8b shows that in the low-frequency range (0.01–1 Hz), the phase angle peaks of all Ti/Cr multilayer coating samples are higher than those of the bare 7050 aluminum alloy, indicating that the multilayer structure effectively enhances the barrier capability against corrosive media, thereby improving corrosion resistance. Although the phase angle peak values among different coating samples vary insignificantly, their peak frequencies exhibit noticeable shifts. The phase angle peak of the C1 sample appears at a lower frequency range, indicating a slower interfacial reaction process and stronger capacitive behavior of the system, further corroborating its superior corrosion resistance. Conversely, in the mid-to-high frequency range (10–103 Hz), the phase angle curve of the bare substrate exhibits a significant response, with corrosion ions rapidly penetrating the natural oxide film and directly acting on the metal substrate, triggering corrosion reactions [46]. Additionally, the aluminum alloy exhibits a small peak in the low-frequency region, indicating that the passivation film on its surface has developed pitting corrosion [47].
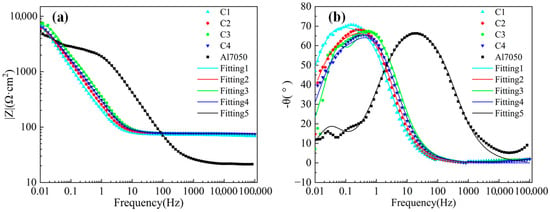
Figure 8.
Bode plots of electrochemical impedance spectra for the substrate and coated samples: (a) Impedance vs. frequency; (b) Phase angle vs. frequency.
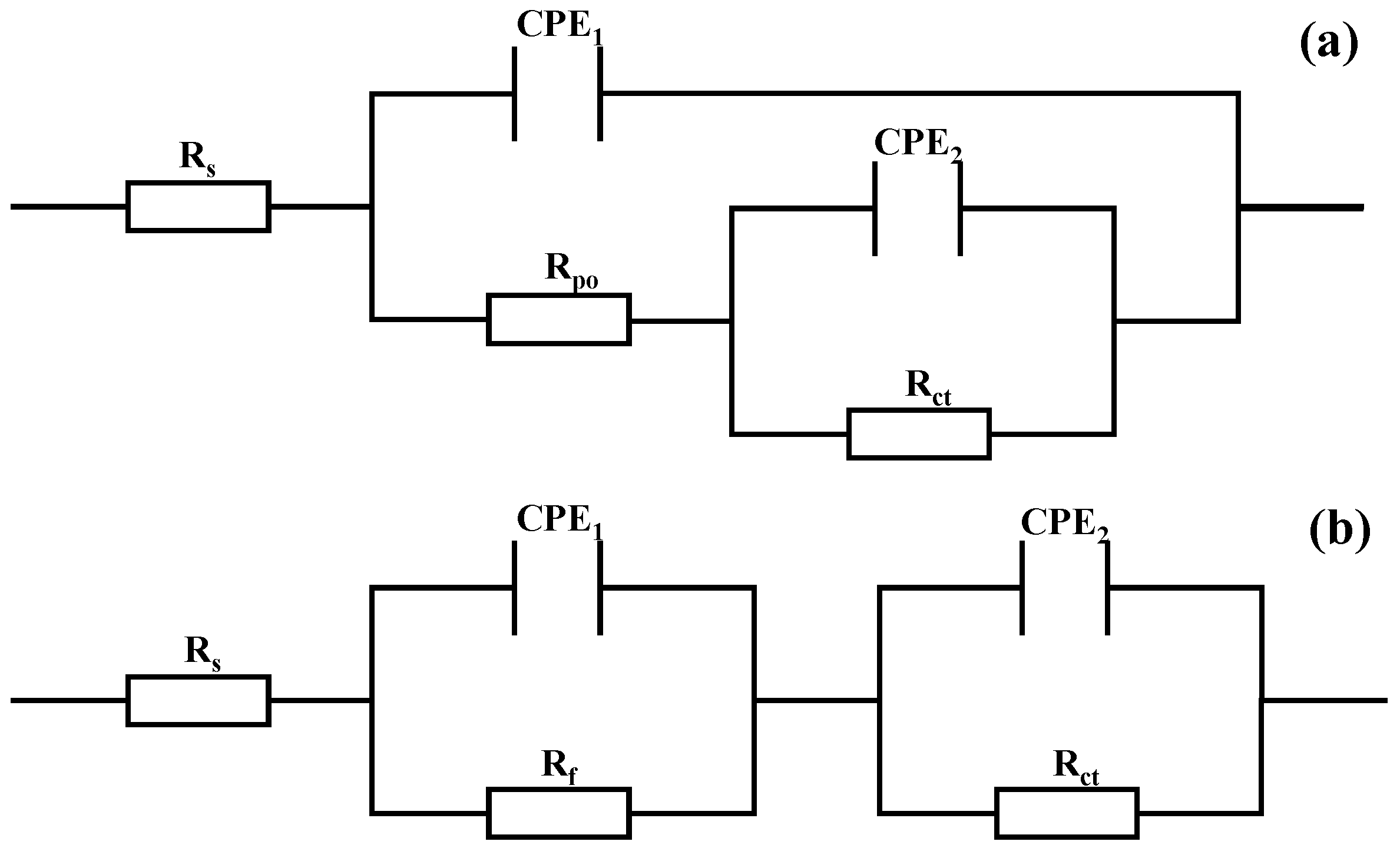
Figure 9a shows the equivalent circuit model used for the coated samples, Rs denotes the solution resistance between the working electrode and the reference electrode, CPE1 represents the capacitance of the coating, Rpo denotes the pore resistance of the coating, CPE2 denotes the double-layer capacitance parallel to Rct, and Rct represents the charge transfer resistance of the test sample, which is an important parameter for evaluating the ease of interfacial Faraday reactions. A higher value indicates greater resistance to charge transfer and stronger corrosion resistance of the system [48]. Figure 9b corresponds to the fitted circuit model for the uncoated 7050 aluminum alloy substrate, where Rs is the solution resistance, Rf represents the resistance layer formed by surface corrosion products (such as Al(OH)3), Rct is the charge transfer resistance at the metal/electrolyte interface, and CPE1 and CPE2 respectively simulate the dielectric response characteristics of the corrosion product layer and the metal interface [49]. The chi-square coefficients reflecting the fitting quality are all in the 10−3 order of magnitude, indicating that the equivalent circuit model has good matching performance. The electrochemical impedance spectroscopy (EIS) fitting results are summarized in Table 2. Combining the data from Table 1 and Table 2, all coatings outperform bare aluminum alloy, further validating the effectiveness of multi-layer coatings in enhancing the corrosion resistance of the substrate. Among them, the C1 coating achieves the maximum values for Rpo (5062 Ω·cm2) and Rct (8742 Ω·cm2), indicating its optimal density and corrosion resistance performance.
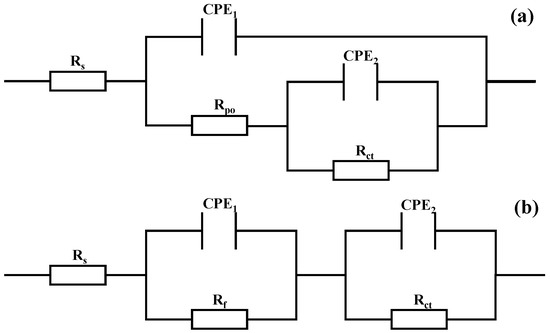
Figure 9.
Equivalent electrical circuit models used for fitting EIS data in 3.5 wt.% NaCl solution: (a) Coated samples; (b) 7050 Al alloy.

Table 2.
EIS fitting results of the substrate and coated samples based on equivalent circuit models.
2.5. Corrosion Morphology Analysis
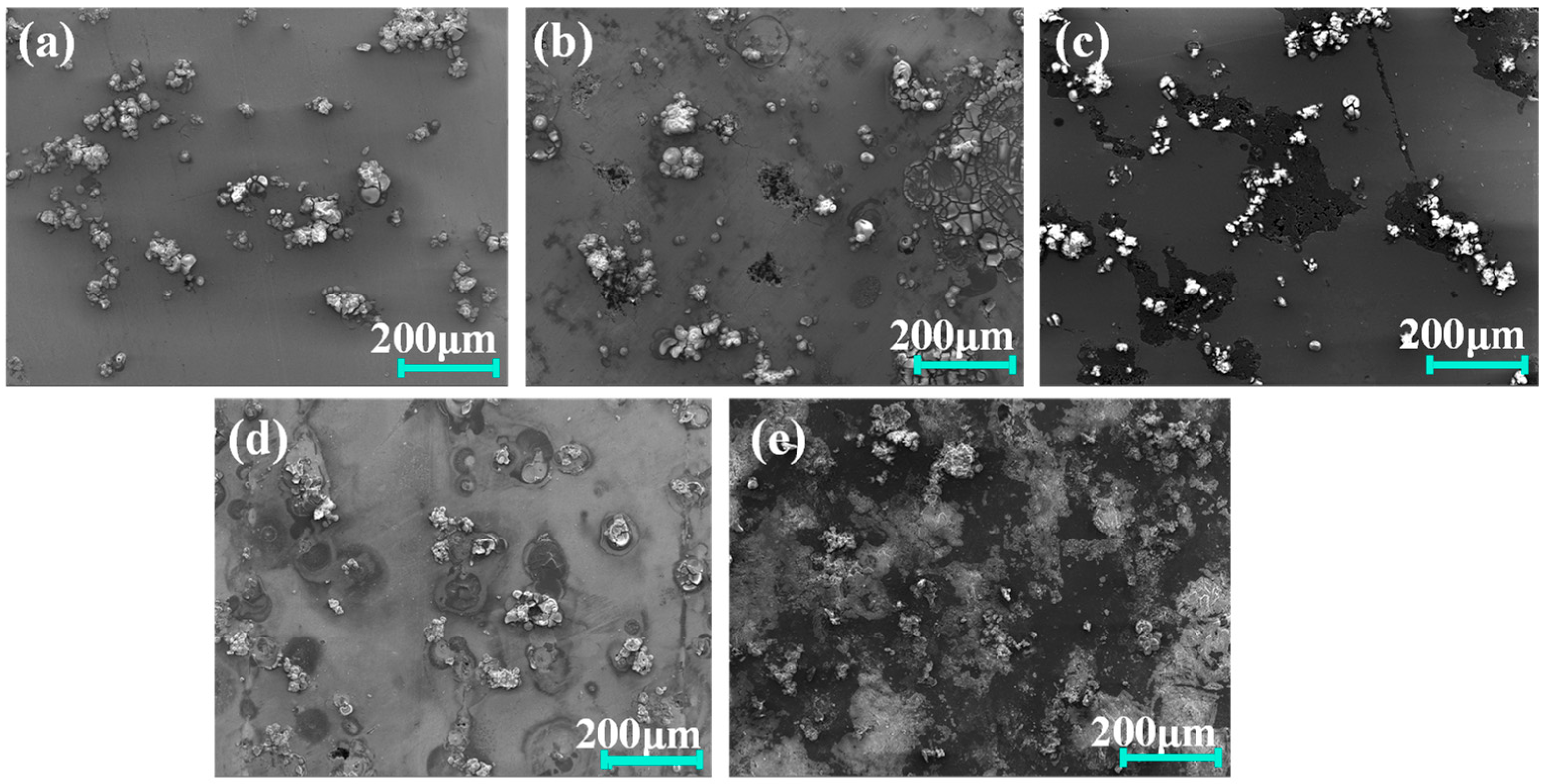
Figure 10 presents the surface morphology changes in Al7050 substrate and Ti/Cr multilayer coatings after electrochemical corrosion tests. The polarization test results indicate severe corrosion on the unprotected 7050 aluminum alloy surface, characterized by extensive accumulation of corrosion products and deep corrosion pits. These rich sediments mainly originate from violent corrosion reactions involving aluminum dissolution and the subsequent formation of aluminum hydroxide and oxide deposits. After corrosion, the surface deposits on the coated samples appear as isolated irregularly shaped particles adhering to the coating surface. These deposits are mainly composed of aluminum-rich corrosion products and oxides, originating from localized corrosion at coating defects or micro-pores. This allows electrolytes to penetrate and react with the aluminum substrate, subsequently depositing corrosion products on the coating surface. The C2, C3, and C4 coatings exhibit more noticeable corrosion marks, with corrosion pits formed due to solution attack. However, the C1 coating shows only mild corrosion traces, with no significant structural damage. Its surface remains smooth and dense, maintaining excellent integrity and demonstrating the best corrosion resistance performance.
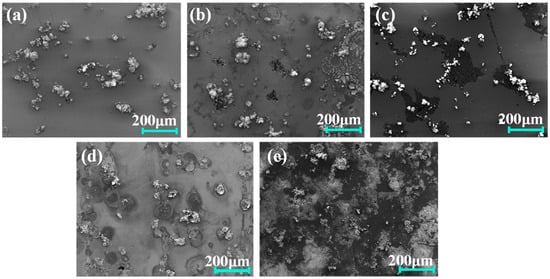
Figure 10.
Secondary electron SEM images of the coating and substrate surfaces after electrochemical testing: (a) C1; (b) C2; (c) C3; (d) C4; (e) 7050 Al alloy.
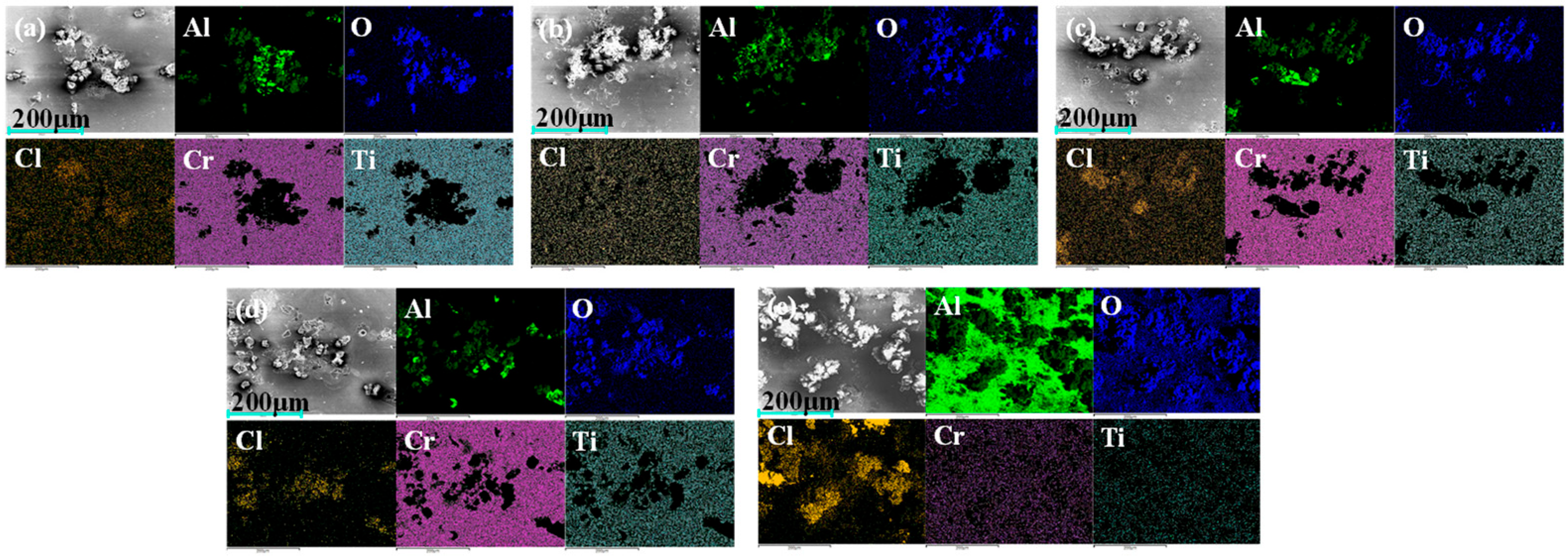
Figure 11 shows the EDS surface scan images of each sample after electrochemical corrosion testing. The results indicate that there are significant differences in elemental distribution between the coated samples and the exposed Al7050 substrate. In the surface scan images of the bare aluminum alloy substrate, oxygen and chlorine elements are widely distributed, indicating that they are prone to forming corrosion products during the corrosion process. In contrast, the C2, C3, and C4 coated samples exhibit varying degrees of corrosion product enrichment, with the concentrated regions of oxygen and aluminum elements gradually expanding, indicating that oxidation and dissolution of the aluminum alloy substrate occurred after localized failure of the coating. In the C1 sample, titanium (Ti) and chromium (Cr) elements have the highest surface coverage, further validating the excellent shielding effect and corrosion resistance of its multi-layer structure.
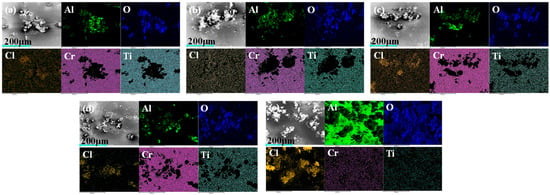
Figure 11.
EDS elemental mapping images of the coating and substrate surfaces after electrochemical testing: (a) C1; (b) C2; (c) C3; (d) C4; (e) Al 7050.
In the Ti/Cr multilayer coatings, the heterogeneous interfaces formed by the multilayer structure can effectively passivate and block the expansion of pitting corrosion within the coating. During the alternating deposition process, these interfaces act as barriers to close the coating pores, suppressing the formation and communication of defects such as pinholes, particles, and microcracks. Although surface defects in the coating are the primary sites of corrosion, the Ti/Cr multilayer coating slows down the penetration rate of the corrosive medium through the interface barrier effect and its multilayer composite structure. Additionally, the Cr element readily forms a stable chromium oxide passivation layer in a corrosive environment [22,50]. These two factors work synergistically to construct a barrier against the penetration of corrosive media, effectively inhibiting the expansion and deepening of surface corrosion pits, and significantly reducing the probability of corrosive media diffusing along defects to the substrate, thereby improving the corrosion resistance of the aluminum alloy.
For the C1 coating, the density of the heterogeneous interfaces gradually increases along the growth direction of the coating. When corrosive media penetrate the surface layer of the coating, they encounter the dense interfaces, which block further diffusion to the substrate [51], resulting in the best corrosion resistance performance during electrochemical testing. For the C2 coating, the interface density decreases along the growth direction. Although the corrosive media face some resistance during their diffusion from the surface to the deeper layers, the presence of microscopic defects like pinholes still provides a pathway for the corrosive media to penetrate. The C3 coating, with its alternating modulation periods, experiences higher residual stress levels, which exacerbate the instability of the internal structure and enlarge the penetration path, making it easier for corrosive media to penetrate [52]. The C4 coating, designed with a uniform modulation period, helps reduce internal stress; however, the larger individual layer thickness results in insufficient interface density to effectively block the penetration of corrosive media. This causes localized corrosion to develop further [53], making pitting corrosion more likely to form and expand.
3. Materials and Experimental
3.1. Substrate Preparation
The substrate samples used were 7050 aluminum alloy disks with dimensions of Ø28 mm × 3 mm. The samples were sequentially polished using SiC sandpapers ranging from #400 to #2000 grit, followed by mechanical polishing with diamond paste. After polishing, the substrates were ultrasonically cleaned in 99.5% acetone for 10 min. It is worth noting that, in order to characterize the microstructure of the coating more efficiently, silicon wafers (P-type, crystal orientation 100, resistivity < 0.02 ohm) were also used for coating preparation, with the same coating deposition conditions as the aluminum substrate.
3.2. Coating Deposition
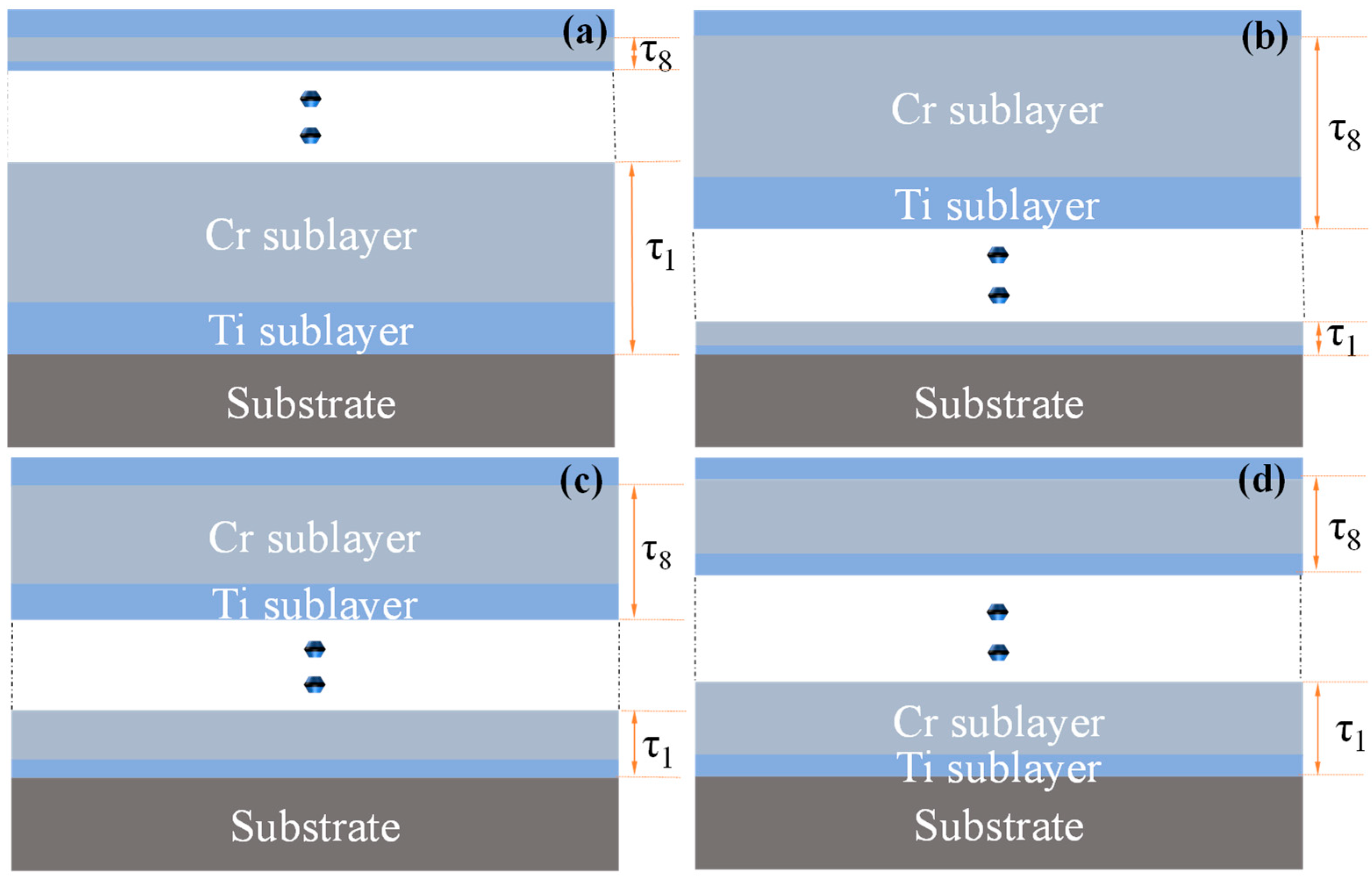
A D.C magnetron sputtering system (MSP-300C, Beijing, China) was used to deposit coatings on the polished aluminum substrates in pure argon gas. The base vacuum of the system was first pumped down to 8 × 10−4 Pa, followed by the introduction of high-purity argon gas to maintain a pressure of 0.3 Pa. The deposition process was carried out at room temperature, with alternating sputtering of high-purity Ti (99.995%, ZhongNuo Advanced Material, Beijing, China) and Cr (99.995%, ZhongNuo Advanced Material, Beijing, China) targets controlled by a shutter system. The power for both the Ti and Cr targets was set to 200 W. Prior to the deposition, each target was cleaned via argon ion sputtering for 10 min. Due to the different sputtering rates of the Ti and Cr targets, four Ti/Cr multilayer coatings with varying modulation periods were prepared by controlling the deposition time of the two targets: C1: Modulation period decreases along the coating growth direction; C2: Modulation period increases along the coating growth direction; C3: Modulation period alternates in size along the coating growth direction; C4: Modulation period remains constant along the coating growth direction. The total deposition time for all four Ti/Cr multilayer coatings was 152 min, with 8 modulation periods (τ1–τ8). The outermost layer was Ti, and the deposition times for each sublayer are shown in Table 3. The structural schematic of the coatings is presented in Figure 12.

Table 3.
Deposition time of individual sublayers in Ti/Cr multilayer coatings with thickness-dependent modulation periods.
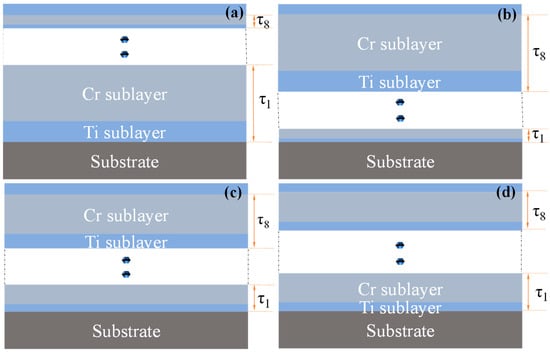
Figure 12.
Schematic diagrams of Ti/Cr multilayer coatings with different modulation period distributions along the growth direction: (a) C1; (b) C2; (c) C3; (d) C4.
3.3. Sample Characterization
The phase composition of the coated silicon wafer samples was examined using XRD analysis with a Cu Kα radiation D/max-2500/PC diffractometer (DMAX-RB, Tokyo, Japan) at a step size of 0.02°, a scanning speed of 5°/min, and a 2θ range of 30° to 75°. The cross-section of the coated silicon wafer samples was observed using a scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectroscopy (EDS) (Hitachi SU5000, Tokyo, Japan) to examine the morphology and thickness of the coating, as well as the distribution of the multi-layer modulation periods.
3.4. Mechanical Property Testing
Nanoindentation tests were carried out on the aluminum alloy substrate and the coatings using a nanoindenter (Bruker Hysitron TS 77, Billerica, MA, USA) with a displacement resolution of 0.2 nm and a load resolution of 75 nN. The maximum indentation depth was controlled at 0.2 μm with a dwell time of 3 s. For each sample, at least five indentations were performed to obtain the average hardness and standard deviation.
3.5. Electrochemical Corrosion Testing
Electrochemical performance of the different coatings was tested using a three-electrode electrochemical workstation (CHI 660D, Shanghai, China). A standard saturated calomel electrode (SCE) was used as the reference electrode (RE), a platinum electrode as the counter electrode (CE), and the coated sample as the working electrode (WE). The exposed area of the working electrode was 1 cm2. The three-electrode setup was immersed in a 3.5 wt% NaCl solution during the tests. The coated aluminum alloy samples were left to stand in a 3.5% wt NaCl solution for half an hour to ensure that the test system was in equilibrium and that the open-circuit potential had stabilized before conducting the EIS test, thereby avoiding misjudgment due to fluctuations in the initial state. EIS tests were then conducted at frequencies ranging from 0.01 Hz to 100 kHz, with an AC amplitude of 10 mV, using Zview 2.0 software to fit the EIS data. Dynamic polarization tests were conducted within a potential scan range of −1 V to +1 V at a scan rate of 0.01 mV/s. Dynamic polarization tests were also performed on the aluminum alloy samples within a potential scan range of −2 V to 0 V at a scan rate of 0.01 mV/s. To ensure the accuracy of the electrochemical test results, each sample was subjected to at least three replicate experiments.
4. Conclusions
In this study, Ti/Cr multilayer coatings were fabricated via magnetron sputtering, with particular emphasis on their microstructural characteristics, interfacial features, and corrosion resistance. All Ti/Cr multilayer coatings with modulation periods along the coating growth direction exhibit a dense morphology. The multilayer structure suppresses the growth of coarse columnar grains, with distinct interlayer interfaces and no obvious cracks or other defects. Ti/Cr multilayer coatings with decreasing modulation periods along the coating growth direction (C1 coating) exhibit significantly enhanced hardness and elastic modulus, reaching 2.89 GPa and 55.22 GPa, respectively, representing approximately 58% and 165% increases compared to 7050 aluminum alloy. Additionally, the Ti/Cr multilayer coating reduces the corrosion current density, which is generally one order of magnitude lower than that of the bare aluminum alloy, indicating that its multilayer structure plays a key role in suppressing the formation of coating defects and enhancing the corrosion resistance of the aluminum alloy substrate. Among the four different coating structures, the C1 coating exhibits a reduced modulation period along the coating growth direction and an increased density of heterogeneous interfaces at the coating surface, effectively blocking the intrusion of corrosive media and demonstrating the best corrosion resistance. Its charge transfer resistance reaches 8742 Ω·cm2, significantly higher than that of the other three coating structures and the substrate, verifying the superiority of the C1 coating’s structure in terms of corrosion resistance.
Author Contributions
Conceptualization, K.C. and T.H.; methodology, K.C.; validation, K.C., T.H. and A.V.; formal analysis, K.C.; investigation, K.C.; resources, T.H.; data curation, K.C., X.D. and Y.D.; writing—original draft preparation, K.C.; writing—review and editing, T.H., Y.D. and J.L.; supervision, T.H., A.V. and C.S.; project administration, T.H. and K.C.; funding acquisition, T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by National Natural Science Foundation of China (Grant No. 52275350), International Cooperation Research Platform Construction Project of Shanghai University of Engineering Science (Grant No. 0301006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhan, H.; Zeng, G.; Wang, Q.; Wang, C.; Wang, P.; Wang, Z.; Xu, Y.; Hess, D.; Crepeau, P.; Wang, J. Unified casting (UniCast) aluminum alloy—A sustainable and low-carbon materials solution for vehicle lightweighting. J. Mater. Sci. Technol. 2023, 154, 251–268. [Google Scholar] [CrossRef]
- Zhang, J.; He, T.; Du, X.; Alexey, V.; Song, M.; Chen, X. Enhanced mechanical properties in 7075 Al alloy fasteners processed by post ECAP-CU aging. Mater. Today Commun. 2025, 45, 112274. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Uggowitzer, P.J.; Roscher, M.; Paolantonio, M.; Liu, C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making sustainable aluminum by recycling scrap: The science of “dirty” alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar] [CrossRef]
- Jia, D.-S.; He, T.; Song, M.; Huo, Y.-M.; Hu, H.-Y. Effects of equal channel angular pressing and further cold upsetting process to the kinetics of precipitation during aging of 7050 aluminum alloy. J. Mater. Res. Technol. 2023, 26, 5126–5140. [Google Scholar] [CrossRef]
- Moffat, A.; Barnes, S.; Mellor, B.; Reed, P. The effect of silicon content on long crack fatigue behaviour of aluminium–silicon piston alloys at elevated temperature. Int. J. Fatigue 2005, 27, 1564–1570. [Google Scholar] [CrossRef]
- Yandouzi, M.; Richer, P.; Jodoin, B. SiC particulate reinforced Al–12Si alloy composite coatings produced by the pulsed gas dynamic spray process: Microstructure and properties. Surf. Coat. Technol. 2009, 203, 3260–3270. [Google Scholar] [CrossRef]
- Mora-Sanchez, H.; del Olmo, R.; Rams, J.; Torres, B.; Mohedano, M.; Matykina, E.; Arrabal, R. Hard Anodizing and Plasma Electrolytic Oxidation of an Additively Manufactured Al-Si alloy. Surf. Coat. Technol. 2021, 420, 127339. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Cai, Z.-B.; Cui, Y.; Liu, J.-H.; Zhu, M.-H. Effect of oxidation time on the impact wear of micro-arc oxidation coating on aluminum alloy. Wear 2019, 426–427, 285–295. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Z.; Shen, Q. Enhancing tribological performance by anodizing micro-textured surfaces with nano-MoS2 coatings prepared on aluminum-silicon alloys. Tribol. Int. 2018, 122, 84–95. [Google Scholar] [CrossRef]
- Bhowmik, A.; Yang, Y.; Zhou, W.; Chew, Y.; Bi, G. On the heterogeneous cooling rates in laser-clad Al-50Si alloy. Surf. Coat. Technol. 2021, 408, 126780. [Google Scholar] [CrossRef]
- Karunakaran, M.; Pugazh Vadivu, M. Magnetic and micro-mechanical behavior of Cu-Ni-P-W-TiO2 hybrid composite electroplating on Al alloy substrate. J. Magn. Magn. Mater. 2019, 475, 359–367. [Google Scholar] [CrossRef]
- Xu, F.; Gong, D. Improved the elevated temperature mechanical properties of Al-Si alloy deposited with Al-Si coating by magnetron sputtering. Vacuum 2018, 150, 1–7. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, P.; Sun, W.; Zhang, W.; Wei, H.; Wang, J.; Li, B.; Yi, X.; Xu, G.; Wu, Y. Effects of bias voltage on coating structures and anticorrosion performances of PA-PVD Al coated NdFeB magnets. J. Rare Earths 2021, 39, 703–711. [Google Scholar] [CrossRef]
- Yumusak, G.; Leyland, A.; Matthews, A. A microabrasion wear study of nitrided α-Ti and β-TiNb PVD metallic thin films, pre-deposited onto titanium alloy substrates. Surf. Coat. Technol. 2022, 442, 128423. [Google Scholar] [CrossRef]
- Martinuzzi, S.M.; Donati, L.; Giurlani, W.; Pizzetti, F.; Galvanetto, E.; Calisi, N.; Innocenti, M.; Caporali, S. A Comparative Research on Corrosion Behavior of Electroplated and Magnetron Sputtered Chromium Coatings. Coatings 2022, 12, 257. [Google Scholar] [CrossRef]
- Yang, K.; Yan, J.; Wang, Q.; Ding, J.; Xu, Q.; Wen, Z.; Zeng, R.; Lu, C.; Fan, T.; Gong, M.; et al. Revealing microstructure and the associated corrosion mechanism of Al/amorphous Al2O3/Al tri-layer coating deposited on depleted uranium by magnetron sputtering. Appl. Surf. Sci. 2024, 659, 159911. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, Y.; Yuan, Z.; Li, J. Structure and tribocorrosion behavior of Ti/TiN multilayer coatings in simulated body fluid by arc ion plating. Surf. Coat. Technol. 2020, 403, 126399. [Google Scholar] [CrossRef]
- Tao, H.; Zhylinski, V.; Vereschaka, A.; Chayeuski, V.; Yuanming, H.; Milovich, F.; Sotova, C.; Seleznev, A.; Salychits, O. Comparison of the Mechanical Properties and Corrosion Resistance of the Cr-CrN, Ti-TiN, Zr-ZrN, and Mo-MoN Coatings. Coatings 2023, 13, 750. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, L. Strongly reduced Ehrlich–Schwoebel barriers at the Cu (111) stepped surface with In and Pb surfactants. Surf. Sci. 2018, 667, 13–16. [Google Scholar] [CrossRef]
- Sbiaai, K.; Boughaleb, Y.; Mazroui, M.; Hajjaji, A.; Kara, A. Energy barriers for diffusion on heterogeneous stepped metal surfaces: Ag/Cu(110). Thin Solid Films 2013, 548, 331–335. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Shi, Q.; Ge, X.; Wang, W. Multilayer Coatings for Tribology: A Mini Review. Nanomaterials 2022, 12, 1338. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, Q.; Yu, Z.; Shi, S.; Liu, Z.; Zhao, Y. Multi-layer structure improves wear and corrosion resistance of chromium. Surf. Coat. Technol. 2025, 508, 132165. [Google Scholar] [CrossRef]
- Madhavan, R.; Bellon, P.; Averback, R.S. Wear Resistance of Cu/Ag Multilayers: A Microscopic Study. ACS Appl. Mater. Interfaces 2018, 10, 15288–15297. [Google Scholar] [CrossRef]
- Liu, T.; Dong, C.; Wu, S.; Tang, K.; Wang, J.; Jia, J. TiN, TiN gradient and Ti/TiN multi-layer protective coatings on Uranium. Surf. Coat. Technol. 2007, 201, 6737–6741. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Liu, T.; Tang, K.; Wei, Q. Interface structure and corrosion resistance of Ti/Cr nanomultilayer film prepared by magnetron sputtering on depleted uranium. ACS Appl. Mater. Interfaces 2013, 5, 6598–6602. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Fu, Q.; Tong, M.; Li, X. Multiple cyclic ablation behaviors of multilayer ZrC-TaC coating with ZrC-SiC interface layer. Corros. Sci. 2022, 200, 110215. [Google Scholar] [CrossRef]
- Yuan, Z.; Han, Y.; Zang, S.; Chen, J.; He, G.; Chai, Y.; Yang, Z.; Fu, Q. Damage evolution behavior of TiN/Ti multilayer coatings under high-speed impact conditions. Surf. Coat. Technol. 2021, 426, 127807. [Google Scholar] [CrossRef]
- Misra, A.; Hirth, J.P.; Hoagland, R.G. Length-scale-dependent deformation mechanisms in incoherent metallic multilayered composites. Acta Mater. 2005, 53, 4817–4824. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Xue, S.; Wang, H.; Zhang, X. Comparison of size dependent strengthening mechanisms in Ag/Fe and Ag/Ni multilayers. Acta Mater. 2016, 114, 154–163. [Google Scholar] [CrossRef]
- Flores, M.; Muhl, S.; Huerta, L.; Andrade, E. The influence of the period size on the corrosion and the wear abrasion resistance of TiN/Ti multilayers. Surf. Coat. Technol. 2005, 200, 1315–1319. [Google Scholar] [CrossRef]
- Zuo, B.; Xu, J.; Lu, G.; Ju, H.; Yu, L. Microstructures, mechanical properties and corrosion resistance of TiN/AlN multilayer films. Ceram. Int. 2022, 48, 11629–11635. [Google Scholar] [CrossRef]
- Shi, W.; Peng, J.; Xu, Z.; Shen, Q.; Wang, C. Effect of Power on Structural and Mechanical Properties of DC Magnetron Sputtered Cr Coatings. Metals 2023, 13, 691. [Google Scholar] [CrossRef]
- Bai, X.M.; Zheng, W.T.; Guo, X.J.; She, H. Microstructure, Interface and Hardness of Ti/TiN Nanolayered Coatings. Key Eng. Mater. 2013, 531, 645–650. [Google Scholar] [CrossRef]
- Debnárová, S.; Souček, P.; Vašina, P.; Zábranský, L.; Buršíková, V.; Mirzaei, S.; Pei, Y.T. The tribological properties of short range ordered W-B-C protective coatings prepared by pulsed magnetron sputtering. Surf. Coat. Technol. 2019, 357, 364–371. [Google Scholar] [CrossRef]
- Tu, Y.; Li, J.; Yuan, Y.; Zhao, J.; Hameed, A.; Yan, C.; Chen, H.; Lan, R.; Cheng, B.; Wang, P.; et al. Thickness modulation influenced mechanical properties of TiN/(CrVTaTiW)N multilayer coatings. Ceram. Int. 2024, 50, 53007–53014. [Google Scholar] [CrossRef]
- Mei, F.; Zhijian, Z.; Yang, Y.; Xiaoliang, L.; Jiangxiong, G.; Tiechui, Y.; Jianguo, L. Microstructure, mechanical, tribological, and oxidizing properties of AlCrSiN/AlCrVN/AlCrNbN multilayer coatings with different modulated thicknesses. Ceram. Int. 2022, 48, 32973–32985. [Google Scholar] [CrossRef]
- Oje, A.M.; Ogwu, A.A.; Oje, A.I. An investigation of the work function and stability of chromium oxide thin films deposited by reactive magnetron sputtering. J. Appl. Electrochem. 2022, 52, 1551–1562. [Google Scholar] [CrossRef]
- Chen, J.; Ding, X.; Wang, J.; Xie, Z.; Wang, S. Corrosion behavior, metal ions release and wear resistance of TiN coating deposited on SLM CoCrMo alloy by magnetron sputtering. J. Alloys Compd. 2024, 1002, 175318. [Google Scholar] [CrossRef]
- Li, J.; He, T.; Du, X.-Y.; Vereschaka, A. Enhancing the corrosion resistance of high-strength Al-Zn-Mg-Cu alloys after equal channel angular pressing by developing retrogression and re-aging strategies. Corros. Sci. 2025, 246, 112736. [Google Scholar] [CrossRef]
- He, T.; Valery, Z.; Vereschaka, A.; Keshin, A.; Huo, Y.; Milovich, F.; Sotova, C.; Seleznev, A. Influence of niobium and hafnium doping on the wear and corrosion resistance of coatings based on ZrN. J. Mater. Res. Technol. 2023, 27, 6386–6399. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, Y.; Wang, C.; Guo, W.; Lu, X.; Sui, Y.; Lan, J. Inhibiting tribocorrosion damage of Cr/CrxN coatings by multi-layer design. Ceram. Int. 2021, 47, 842–850. [Google Scholar] [CrossRef]
- Sun, W.; Lv, Y.; Gao, J.; Gu, G.; Luo, Q.; Feng, Q.; Jia, B.; Ma, F. Enhanced corrosion resistance and electrical conductivity of Nb/NbN multilayer coatings prepared by the combination methods of magnetron sputtering and pulse laser deposition. Int. J. Hydrogen Energy 2025, 127, 160–168. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, M.; Shi, H.; Wang, Y.; Wang, Z.; Cheng, Y.; Liu, M. CrZrN/ZrN multilayer coatings on 316L stainless steel towards anticorrosion application. Thin Solid Films 2022, 755, 139330. [Google Scholar] [CrossRef]
- Li, J.; He, T.; Du, X.-Y.; Vereschaka, A.; Zhang, J.-J. Regulating hardness homogeneity and corrosion resistance of Al-Zn-Mg-Cu alloy via ECAP combined with inter-pass aging. Mater. Charact. 2024, 218, 114489. [Google Scholar] [CrossRef]
- Liu, F.; Cao, H.; Li, H.; Yang, J.; Zhao, N.; Qi, F.; Ouyang, X. Effect of annealing on the microstructure, mechanical and electrochemical properties of CrAlC coatings. Surf. Coat. Technol. 2022, 447, 128800. [Google Scholar] [CrossRef]
- Kroll, R.; Kearns, P.; Usman, B.J.; Zhou, X.; Engelberg, D.L. A novel approach to determine cathodic passivation characteristics and semiconducting properties of pure aluminium 99.5 wt% and aluminium alloy 7075-T6 with an electrochemical pen electrode. Corros. Sci. 2023, 211, 110898. [Google Scholar] [CrossRef]
- Cao, F.H.; Zhang, Z.; Li, J.F.; Cheng, Y.L.; Zhang, J.Q.; Cao, C.N. Exfoliation corrosion of aluminum alloy AA7075 examined by electrochemical impedance spectroscopy. Mater. Corros. 2004, 55, 18–23. [Google Scholar] [CrossRef]
- Yang, J.; Shou, D.; Zhao, N.; Tang, Y.; Cao, H.; Qi, F.; Ouyang, X. Interfacial structure, mechanical properties, and corrosion resistance of Cr/TiSiN nano-multilayer coating by filtered cathode vacuum arc technique. J. Manuf. Process. 2025, 136, 267–281. [Google Scholar] [CrossRef]
- Zhang, J.-J.; He, T.; Du, X.-Y.; Alexer, V.; Song, M.; Chen, X.-L.; Li, J. Effect of pre-heat treatment and subsequent ECAP-CU on microstructure and corrosion behavior of 7075 Al alloy fasteners. J. Cent. South Univ. 2024, 1–21. [Google Scholar] [CrossRef]
- Inman, S.B.; Sur, D.; Han, J.; Ogle, K.; Scully, J.R. Corrosion behavior of a compositionally complex alloy utilizing simultaneous Al, Cr, and Ti passivation. Corros. Sci. 2023, 217, 111138. [Google Scholar] [CrossRef]
- Cai, F.; Zhou, Q.; Chen, J.; Zhang, S. Effect of inserting the Zr layers on the tribo-corrosion behavior of Zr/ZrN multilayer coatings on titanium alloys. Corros. Sci. 2023, 213, 111002. [Google Scholar] [CrossRef]
- Zhao, X.; Munroe, P.; Habibi, D.; Xie, Z. Roles of compressive residual stress in enhancing the corrosion resistance of nano nitride composite coatings on steel. J. Asian Ceram. Soc. 2018, 1, 86–94. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Cai, F.; Yang, Y.; Wang, Q.; Zhang, S. Characterization and corrosion behaviors of TiN/TiAlN multilayer coatings by ion source enhanced hybrid arc ion plating. Surf. Coat. Technol. 2019, 366, 355–365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).