Manganese and Iron Catalysts in Alkyd Paints and Coatings
Abstract
:1. Introduction
2. Alkyd Resins
3. Cobalt Soaps
4. Manganese Catalysts
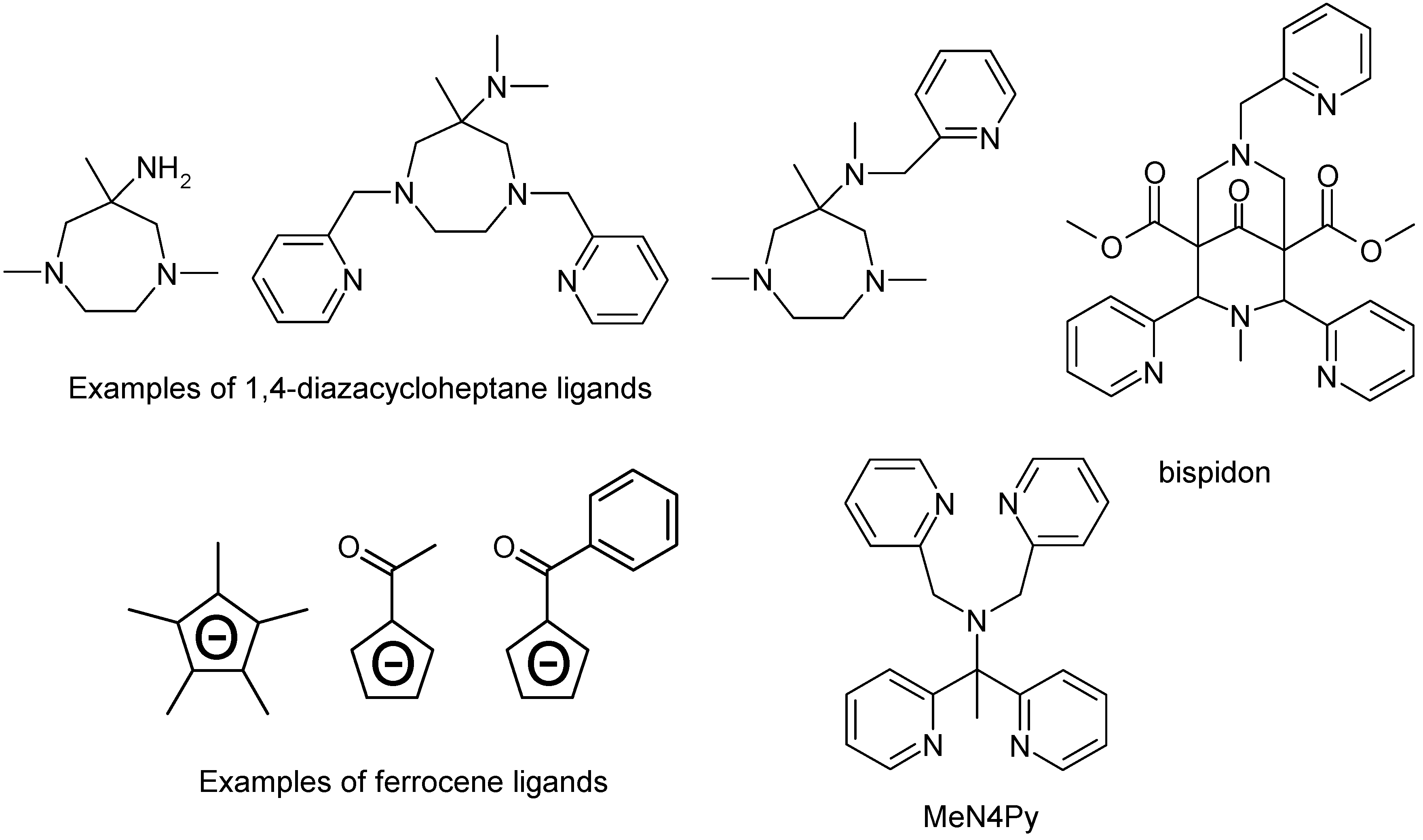
4.1. Manganese Carboxylates
4.2. Manganese Complexes
4.2.1. Manganese Bipyridine Catalysts
4.2.2. Manganese Acetylacetonate Catalysts
4.2.3. Manganese 1,4,7-Triazacyclononane Catalysts
4.2.4. Other Manganese Based Catalysts
5. Iron Catalysts
5.1. Iron Soaps
5.2 Iron Complexes
5.2.1. Iron Bispidon Catalysts
5.2.2. Iron Ferrocene Catalysts
5.2.3. Other Iron-Based Catalysts
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| acac | acetylacetonate |
| bispidon | dimethyl 3-methyl-9-oxo-2,4-di(pyridin-2-yl)-7-(pyridin-2-ylmethyl)-3,7-diazabicyclo[3.3.1]nonane-1,5-dicarboxylate |
| bpy | 2,2′-bipyridine |
| 2-EH | 2-ethylhexanoate |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GC | gas chromatography |
| HMTT | N,N,N′,N″,N′′′,N′′′-hexamethyltriethylenetetraamine |
| HS | high solid |
| Me4DTNE | 1,2-bis(4,7-dimethyl-1,4,7-triazacyclononan-1-yl)-ethane |
| MeN4Py | N,N-bis(pyridin-2-yl-methyl-1,1-bis(pyridin-2-yl)-1-aminoethane |
| Me3TACN | 1,4,7-trimethyl-1,4,7-triazacyclononane |
| MEKO | methylethylketoxime |
| MS | mass spectrometry |
| phen | 1,10-phenanthroline |
| REACH | Registration, Evaluation, Authorisation and Restriction of Chemicals |
| SB | solvent-borne |
| VOC | volatile organic compounds |
| WB | water-borne |
References
- Soucek, M.D.; Khattab, T.; Wu, J. Review of autoxidation and driers. Prog. Org. Coat. 2012, 73, 435–454. [Google Scholar] [CrossRef]
- Hofland, A. Alkyd resins: From down and out to alive and kicking. Prog. Org. Coat. 2012, 73, 274–282. [Google Scholar] [CrossRef]
- Bieleman, J.H. Driers. Chimia 2002, 56, 184–190. [Google Scholar] [CrossRef]
- Van Gorkum, R.; Bouwman, E. The oxidative drying of alkyd paint catalysed by metal complexes. Coord. Chem. Rev. 2005, 249, 1709–1728. [Google Scholar] [CrossRef]
- Greimel, K.J.; Perz, V.; Koren, K.; Feola, R.; Temel, A.; Sohar, C.; Herrero Acero, E.; Klimant, I.; Guebitz, G.M. Banning toxic heavy-metal catalysts from paints: Enzymatic cross-linking of alkyd resins. Green Chem. 2013, 15, 381–388. [Google Scholar] [CrossRef]
- Preininger, O.; Vinklárek, J.; Honzíček, J.; Mikysek, T.; Erben, M. A promising drying activity of environmentally friendly oxovanadium(IV) complexes in air-drying paints. Prog. Org. Coat. 2015, 88, 191–198. [Google Scholar] [CrossRef]
- Manzano, E.; Navas, N.; Checa-Moreno, R.L.; Rodriguez-Simón, L.; Capitán-Vallvey, L.F. Preliminary study of UV ageing process of proteinaceuous paint binder by FT-IR and principal component analysis. Talanta 2009, 77, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Payeras, A.M.I.; Fiedler, A.T.; Costas, M.; Kaizer, J.; Stubna, A.; Muenck, E.; Que, L., Jr. Kinetic analysis of the conversion of nonheme (alkylperoxo)iron(III) species to iron(IV) complexes. Inorg. Chem. 2007, 46, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, J.; Costas, M.; Que, L., Jr. A dramatic push effect on the homolysis of FeIII(OOR) intermediates to form non-heme FeIV=O complexes. Angew. Chem. Int. 2003, 42, 3671–3673. [Google Scholar] [CrossRef] [PubMed]
- Coggins, M.K.; Martin-Diaconescu, V.; DeBeer, S.; Kovacs, J.A. Correlation between structural, spectroscopic, and reactivity properties within a series of structurally analogous manganese(III)-alkylperoxo complexes. J. Am. Chem. Soc. 2013, 135, 4260–4272. [Google Scholar] [CrossRef] [PubMed]
- Chavez, F.A.; Rowland, J.M.; Olmstead, M.M.; Mascharak, P.K. Synthesis, structures and reactivities of cobalt(III)-alkylperoxo complexes and their role in stoichiometric and catalytic oxidation of hydrocarbons. J. Am. Chem. Soc. 1998, 120, 9015–9027. [Google Scholar] [CrossRef]
- Clauwaert, E. Cobalt-Based Catalytic Dryer for Polymer Coatings. WO2010076031, 8 July 2010. [Google Scholar]
- Tanase, S.; Hierso, J.-C.; Bouwman, E.; Reedijk, J.; ter Borg, J.; Bieleman, J.H.; Schut, A. New insights in anti-skinning effect of methyl ethyl ketoxime in alkyd paints. New J. Chem. 2003, 27, 854–959. [Google Scholar] [CrossRef]
- Oyman, Z.O.; Ming, W.; van der Linde, R. Oxidation of model compound emulsions for alkyd paints under the influence of cobalt drier. Prog. Org. Coat. 2003, 48, 80–91. [Google Scholar] [CrossRef]
- Muizebelt, W.J.; Hubert, J.C.; Nielen, M.W.F.; Klaasen, R.P.; Zabel, K.H. Crosslink mechanisms of high-solid alkyd resins in the presence of reactive diluents. Prog. Org. Coat. 2000, 40, 121–130. [Google Scholar] [CrossRef]
- Oyman, Z.O.; Ming, W.; van der Linde, R. Oxidation of drying oils containing non-conjugated and conjugated double bonds catalyzed by a cobalt catalyst. Prog. Org. Coat. 2005, 54, 198–204. [Google Scholar] [CrossRef]
- Rondas, F. Manganese-Based Catalytic Dryer for Polymer Coatings. WO2012000934, 5 January 2012. [Google Scholar]
- Warzeska, S.T.; Zonneveld, M.; van Gorkum, R.; Muizebelt, W.J.; Bouwman, E.; Reedijk, J. The influence of bipyridine on the drying of alkyd paints. A model study. Prog. Org. Coat. 2002, 44, 243–248. [Google Scholar] [CrossRef]
- Lima, G.E.S.; Nunes, E.V.; Dantes, R.C.; Meneghetti, M.R.; Meneghetti, S.M.P. Systematic investigation of the oxidative polymerization of linseed oil catalysed by Co(II), Mn(II), and Fe(II) complexes with chelating nitrogen ligands. Eur. J. Lipid Sci. Technol. 2015, 117, 229–234. [Google Scholar] [CrossRef]
- Van Gorkum, R.; Bouwman, E. Fast Autoxidation of ethyl linoleate catalyzed by [Mn(acac)3] and bipyridine: A possible drying catalyst for alkyd paints. Inorg. Chem. 2004, 43, 2456–2458. [Google Scholar] [CrossRef] [PubMed]
- Oyman, Z.O.; Ming, W.; van der Linde, R.; Gorkum, R.; Bouwman, E. Effect of [Mn(acac)3] and its combination with 2,2′-bipyridine on the autoxidation and oligomerisation of ethyl linoleate. Polymer 2005, 46, 1731–1738. [Google Scholar] [CrossRef]
- Van Gorkum, R.; Bouwman, E.; Reedijk, J. Drier for Alkyd Based Coating. EP1382648A, 21 January 2004. [Google Scholar]
- Oyman, Z.O.; Ming, W.; Micciche, F.; Oostveen, E.; van Haveren, J.; van der Linde, R. A promising environmentally friendly manganese-based catalyst for alkyd emulsion coatings. Polymer 2004, 45, 7431–7436. [Google Scholar] [CrossRef]
- Oyman, Z.O.; Ming, W.; van der Linde, R.; ter Borg, J.; Schut, A.; Bieleman, J.H. Oxidative drying of alkyd paints catalysed by a dinuclear manganese complex (MnMeTACN). Surf. Coat. Int. B Coat. Trans. 2005, 88, 269–275. [Google Scholar] [CrossRef]
- Gezici-Koç, Ö.; Thomas, C.A.A.M.; Michel, M.E.B.; Erich, S.J.F.; Huinink, H.P.; Flapper, J.; Duivenvoorde, F.L.; van der Ven, L.G.J.; Adan, O.C.G. In-depth study of drying solvent-borne alkyd coatings in presence of Mn- and Fe-based catalysts as cobalt alternatives. Mater. Today Commun. 2016, 7, 22–31. [Google Scholar] [CrossRef]
- Oyman, Z.O.; Ming, W.; van der Linde, R. Catalytic activity of a dinuclear manganese complex (MnMeTACN) on the oxidation of ethyllinoleate. Appl. Catal. A Gen. 2007, 316, 191–196. [Google Scholar] [CrossRef]
- Jansen, J.; Kleuskens, E.C.; van Summeren, R.; Alsters, P.L. Manganese Complex Drier for Coating Compositions. EP2534215B, 18 August 2011. [Google Scholar]
- Jansen, J.; Bergman, F.A.C.; Kleuskens, E.C.; Hage, R. Manganese Salt Complex as Drier for Coating Compositions. WO2011098584, 18 August 2011. [Google Scholar]
- Meijer, M.D.; van Weelde, E.; van Dijk, J.T.M.; Flapper, J. Drier for Auto-Oxidisable Coating Compositions. WO2013092441, 27 July 2013. [Google Scholar]
- Meijer, M.D.; van Weelde, E.; van Dijk, J.T.M.; Flapper, J. Drier for Auto-Oxidisable Coating Compositions. WO2013092442, 27 July 2013. [Google Scholar]
- Meijer, M.D.; Flapper, J. Drier for Auto-Oxidisable COATING compositions. WO2014095670, 26 June 2014. [Google Scholar]
- Hage, R.; de Boer, J.W.; Maaijen, K. Drier for Alkyd-Based Coating. WO2014122433, 14 August 2014. [Google Scholar]
- Hage, R.; de Boer, J.W.; Maaijen, K. Oxidatively Curable Coating Composition. WO2014122432A, 14 August 2014. [Google Scholar]
- De Boer, J.W.; Maaijen, K.; Hage, R. Composition. WO2015114349A, 6 August 2015. [Google Scholar]
- De Boer, J.W.; Maaijen, K.; Hage, R. Composition. WO2015114352A, 6 August 2015. [Google Scholar]
- Boomgaard, R.E.; Schier, H.; Kirchner, E.J.J.; Klaasen, R.P.; Hartl, F.; van der Leeuw, R.P.C.; Bakkeren, F.J.A.D. Oxidatively Drying Coating Composition. WO2003029371, 10 April 2003. [Google Scholar]
- Srinivasan, K.; Perrier, S.; Kochi, J.K. Dual pathways for manganese catalysis of olefin oxidation with alkyl hydroperoxides. J. Mol. Catal. 1986, 36, 297–317. [Google Scholar] [CrossRef]
- Santhanam, R. Additives for Curable Liquid Compositions. WO2012092034, 5 July 2012. [Google Scholar]
- Hage, R.; de Boer, J.W.; Maaijen, K. Oxidatively Curable Coating Composition. WO2014122434A, 14 August 2014. [Google Scholar]
- Hage, R.; Wesenhagen, P.V. Liquid Hardening. WO2008003652, 10 January 2008. [Google Scholar]
- De Boer, J.W.; Wesenhagen, P.V.; Wenker, E.C.M.; Maaijen, K.; Gol, F.; Gibbs, H.; Hage, R. The quest for cobalt-free alkyd paint driers. Eur. J. Inorg. Chem. 2013, 3581–3591. [Google Scholar] [CrossRef]
- Miccichè, F.; van Haveren, J.; Oostveen, E.; Ming, W.; van der Linde, R. Oxidation and oligomerization of ethyl linoleate under influence of the combination of ascorbic acid 6 palmitate/iron-2-ethylhexanoate. Appl. Catal. A Gen. 2006, 297, 174–181. [Google Scholar] [CrossRef]
- Miccichè, F.; Long, G.J.; Shahin, A.M.; Grandjean, F.; Ming, W.; van Haveren, J.; van der Linde, R. The combination of ascorbic acid 6-palmitate and [Fe3III(μ3-O)]7+ as a catalyst for the oxidation of unsaturated lipids. Inorg. Chim. Acta 2007, 360, 535–545. [Google Scholar] [CrossRef]
- Van Haveren, J.; Oostveen, E.A.; Miccichè, F.; Noordover, B.A.J.; Koning, C.E.; van Benthem, R.A.T.M.; Frissen, A.E.; Weijnen, J.G.J. Resins and additives for powder coatings and alkyd paints, based on renewable sources. J. Coat. Technol. Res. 2007, 4, 177–186. [Google Scholar] [CrossRef]
- Micciche, F. The Combination of Ascorbic Acid Derivatives/Iron Salts as Catalyst for the Oxidative Drying of Alkyd Paints: A Biomimetic Approach. Ph.D. Thesis, Technischie Universiteit Eindhoven, Eindhoven, The Netherlands, 2005. [Google Scholar]
- Hage, R.; Lienke, A. Applications of transition-metal catalysts to textile and wood-pulp bleaching. Angew. Chem. Int. Ed. 2006, 45, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; de Boer, J.W.; Gaulard, F.; Maaijen, K. Manganese and iron bleaching and oxidation catalysts. Adv. Inorg. Chem. 2013, 65, 85–116. [Google Scholar]
- Appel, R.; Hage, R.; van der Hoeven, P.C.; Lienke, J.; Smith, R.G. Enhancement of Air Bleaching Catalysts. EP1368450, 27 June 2002. [Google Scholar]
- Appel, R.; Batchelor, S.N.; Grabijn, L.G.; Hage, R.; Jones, S.; Parry, M. Bleaching Composition. WO2005118764, 15 December 2005. [Google Scholar]
- Appel, R.; Hage, R.; Lienke, J. Device Suitable for Analysing Edible Oil Quality. EP1326074, 9 July 2003. [Google Scholar]
- Hage, R.; Gol, F.; Gibbs, H.W.; Maaijen, K. Antiskinning Compositions. WO2012093250, 12 July 2012. [Google Scholar]
- Gibbs, H.W.; Gol, F.; Vrhunec, A.; Stefanec, D.; Pulsar, J. Encapsulated Catalysts. WO2015011430, 29 January 2015. [Google Scholar]
- Pirš, B.; Bogdan, Z.; Skale, S.; Zabret, J.; Godnjavec, J.; Venturini, P. Iron as an alternative drier for high-solid alkyd coatings. J. Coat. Technol. Res. 2015, 12, 965–974. [Google Scholar] [CrossRef]
- Pirš, B.; Bogdan, Z.; Skale, S.; Zabret, J.; Godnjavec, J.; Berce, P.; Venturini, P. The influence of Co/Sr and Fe/Sr driers on film formation of high-solid alkyd coatings. Acta Chim. Slov. 2015, 62, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kalenda, P.; Holeček, J.; Veselý, D.; Erben, M. Influence of methyl groups on ferrocene on rate of drying of oxidizable paints by using model compounds. Prog. Org. Coat. 2006, 56, 111–113. [Google Scholar] [CrossRef]
- Stava, V.; Erben, M.; Vesely, D.; Kalenda, P. Properties of metallocene complexes during the oxidative crosslinking of air drying coatings. J. Phys. Chem. Solids 2007, 68, 799–802. [Google Scholar] [CrossRef]
- Erben, M.; Veselý, D.; Vinklárek, J.; Honzíček, J. Acyl-substituted ferrocenes as driers for solvent-borne alkyd paints. J. Mol. Catal. A Chem. 2012, 353–354, 13–21. [Google Scholar] [CrossRef]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hage, R.; De Boer, J.W.; Maaijen, K. Manganese and Iron Catalysts in Alkyd Paints and Coatings. Inorganics 2016, 4, 11. https://doi.org/10.3390/inorganics4020011
Hage R, De Boer JW, Maaijen K. Manganese and Iron Catalysts in Alkyd Paints and Coatings. Inorganics. 2016; 4(2):11. https://doi.org/10.3390/inorganics4020011
Chicago/Turabian StyleHage, Ronald, Johannes W. De Boer, and Karin Maaijen. 2016. "Manganese and Iron Catalysts in Alkyd Paints and Coatings" Inorganics 4, no. 2: 11. https://doi.org/10.3390/inorganics4020011
APA StyleHage, R., De Boer, J. W., & Maaijen, K. (2016). Manganese and Iron Catalysts in Alkyd Paints and Coatings. Inorganics, 4(2), 11. https://doi.org/10.3390/inorganics4020011





