Abstract
Charged and neutral silicon clusters comprising Si atoms that are exclusively connected to atoms of the same type serve as models for bulk silicon surfaces. The experimentally known nido-[Si9]4− Zintl cluster is investigated as a building block and allows for a theoretical prediction of novel silicon-rich oligomers and polymers by interconnection of such building units to larger aggregates. The stability and electronic properties of the polymers and , as well as of related oligomers are presented.
1. Introduction
Silicon is the element of choice for fulfilling the desire for novel materials with promising properties. Even though, silicon is an indirect band gap semiconductor resulting in poor efficiency of light emission, the observation of visible photoluminescence from porous silicon or silicon nanoparticles at room temperature reported in the early 90s [1,2] triggered the investigations of low-dimensional silicon quantum structures and had been a subject of extensive investigations due to the potential usage of nano-sized silicon in photonic and optoelectronic devices [3,4].
In recent years, some experimental molecular approaches successfully showed that molecules with low-valent Si atoms can be synthesized. So-called siliconoids [5] are stable unsaturated neutral silicon clusters that show the characteristic structural features of silicon nanoparticles and surfaces in the molecular regime generally realized through the occurrence of one or more unsubstituted Si atoms [5,6,7,8].
Computationally, many novel well-ordered Si allotropes of various dimensionality have been proposed [9,10], but only a few of those were experimentally verified, such as the low-density Si allotropes [11] with clathrate-type structures [12,13,14,15,16,17,18,19]. The focus of the research lies on the description, understanding, and discovery of well-performing photovoltaic materials as well as models for bulk silicon surfaces [20,21]. A few such predicted materials are realizable in laboratory to date also applying physical methods such as ultrafast laser-induced confined microexplosion [22].
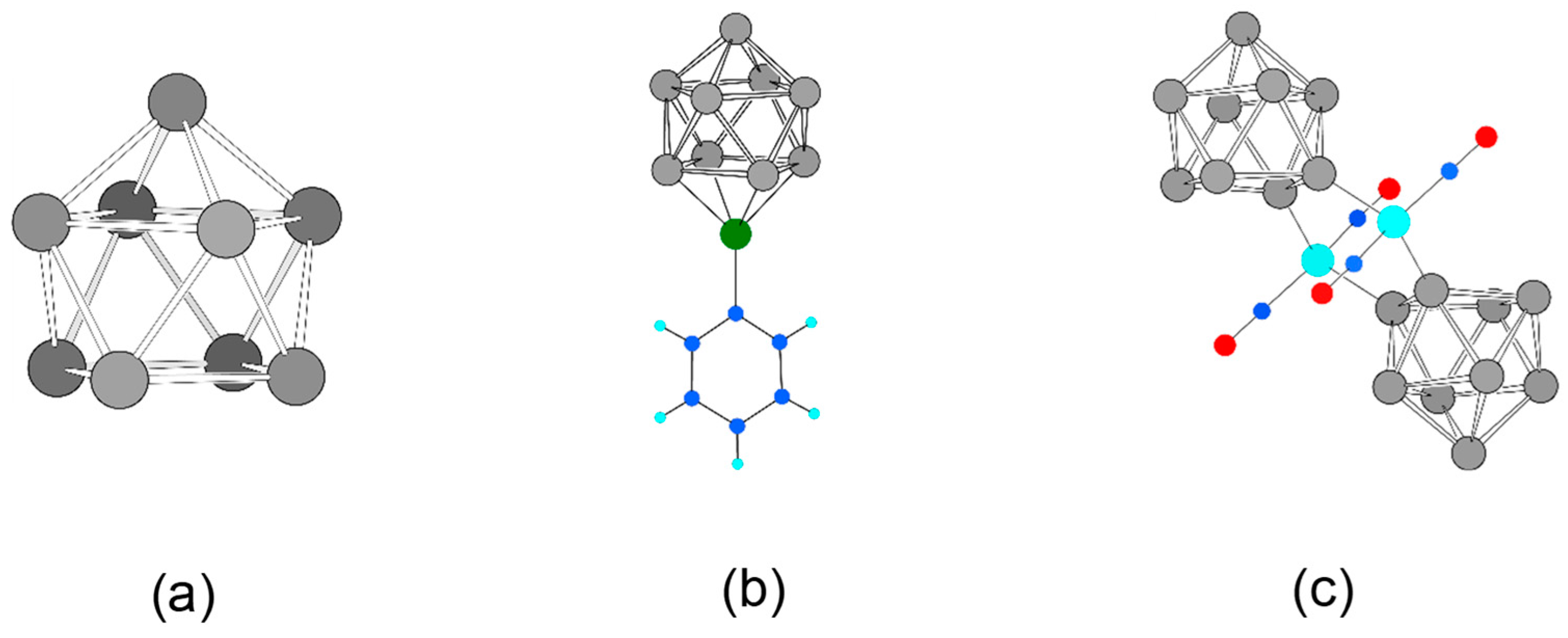
Currently, one of the most investigated two-dimensional materials is silicene [23], the higher homologue of graphene [24]. It is reported as a buckled sheet of sp3/sp2-hybridized Si atoms connected to wrinkled six membered rings, which is stabilized through intrinsic van-der-Waals interactions [25]. Silicene is experimentally accessible only on metal surfaces [26,27,28]. Theoretical investigations on this material are reviewed in several articles [29,30,31]. Another two-dimensional Si modification results from adding ad-atoms to silicene. Si in this MoS2-structure type shows a lower relative energy compared to silicene [32]. In our recent work, we introduced two-dimensional materials, which may overcome surface reconstruction problems by using {Si9} Zintl cages (Figure 1a) that are stable in solution [33].
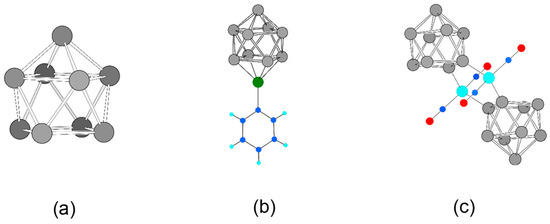
Figure 1.
The building block nido-[Si9]4– and its experimentally accessible complexes from solution. (a) nido-[Si9]4– optimized on a DFT-PBE0/def2-TZVPP/PCM level of theory; (b) closo-[Si9Zn(C6H5)]3− [39]; (c) [{Ni(CO)2}2(μ-Si9]2]8– [40,44]. Si, C, H, Zn, Ni, and O are shown as grey, blue, light blue, green, turquoise, and red spheres.
Our aim is the computational investigation using substructures or molecular building units that are experimentally known, which we call the chemi-inspired attempt [9,10]. We found that Zintl clusters of Group 14 elements, as they occur in neat binary intermetallic solids (Zintl phases) [34], represent ideal candidates for constructing tailor-made materials. They have well-defined structures, which are retained upon solvation, and can thus be functionalized, oligomerized, or even polymerized [35,36]. Best suited for such chemistry is the nine-atomic [E9](2–4)− cluster (E = Si, Ge, Sn, and Pb). For silicon, only a few examples of following-up reactions of Si9 clusters are known whereas the pure clusters are structurally characterized in solids [37] and in salts obtained from solution [38,39,40,41,42]. In all examples, reactions occur via atoms of the open square of the mono-capped square antiprism of the nido-[Si9]4– cluster (Figure 1a); either capped in a η4-coordination with phenylzinc (Figure 1b) [39] as well as Cu-NHC (NHC = N-heterocyclic carbene) [43] or two clusters are bridged by Ni(CO)2 fragments via two neighboring atoms of the open square, each involved in a η1-coordination (Figure 1c) [40,44].
Further, it was shown that such clusters can serve as seed crystals for the synthesis of Si nanoparticles and nanoscale materials [45,46]. In analogy to Ge9 clusters for which covalently bonded Ge9 oligomers and polymers are experimentally known, Si-based materials that contain Si9 units linked via the atoms of the open square are feasible [39,41,44]. The direct linkage has already been discussed and also the stability and properties of two-dimensional Si materials containing sp3-hybrized Si atoms as linkers between Si9 clusters were computationally investigated [33]. In this work, we explore the possibilities of introducing tetrahedrally connecting sp3-Si atoms, which might occur from a formal reaction of the anions with SiCl4 and SiH2Cl2 and nido-[Si9]4− clusters. Thus, we investigate the complexes [Si(Si9)Cl3]3− and [Si(Si9)Me3]3− (Me = –CH3), [Si(Si9)2Cl2]6−, [Si(Si9)3Cl]9−, and [Si(Si9)4]12−. Further, we analyze the hypothetical insertion of nido-[Si9]4− clusters into polysilanes resulting in the neutral polymers and .
2. Computational Details
Neutral polymers containing silane fragments and Si9 clusters as well as the polysilanes themselves were optimized starting from manually drawn structures pre-optimized using UFF [47,48]. The lattice and atomic positions were allowed to relax during optimization within the constraints given by rod group symmetry [49]. All quantum chemical calculations for the polymers were carried out using the CRYSTAL09 program package [50] with a hybrid DFT functional after Perdew, Burke, and Ernzerhof (DFT-PBE0) [51,52]. For silicon, a modified split-valence + polarization (SVP) basis set [53] was applied. The shrinking factor (SHRINK) for generating the Monkhorst-Pack-type grid of k points in the reciprocal space was set to 4, resulting in three k-points in the irreducible Brillouin zone. For the evaluation of the Coulomb and exchange integrals tight tolerance factors (TOLINTEG) of 8, 8, 8, 8, and 16 were chosen. Default optimization convergence thresholds and an extra-large integration grid (XLGRID) for the density-functional part were applied in all calculations. Harmonic vibrational frequencies were calculated numerically to confirm the stationary point on the potential-energy surface as a true minimum.
The investigations on charged complexes containing nido-[Si9]4– as a building block are done with the DFT-PBE0 [51,52] hybrid DFT functional and def2-TVZPP level basis sets for the elements Si, Cl, H, and C using the Gaussian09 program package [54]. For modelling a solvation effect, a solvation model (polarizable continuum model, PCM) with standard settings was applied [55]. For structure optimizations, extremely tight optimization convergence criteria were combined with a large and ultrafine DFT integration grid. The systems were allowed to relax without symmetry restrictions. Harmonic vibrational frequencies were calculated analytically to confirm the stationary point on the potential-energy surface as a true minimum (except for M6).
The position parameters for all optimized compounds are listed in the Supplementary Materials.
3. Results and Discussion
3.1. Neutral Polymers with SiCl2 or SiH2 Linkers between the Clusters
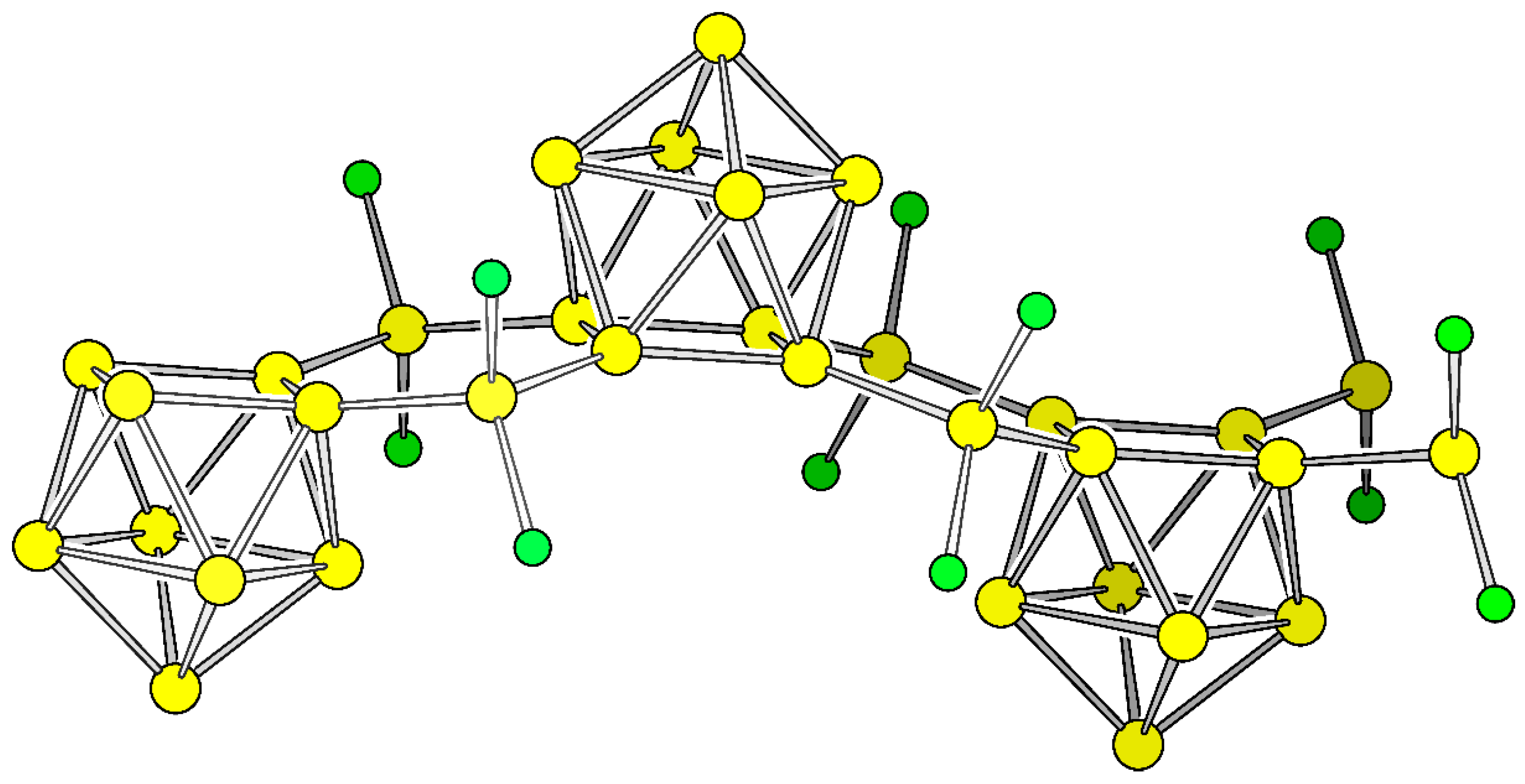
Inspired by the [(Ni(CO)2)2(μ-Si9)2]8− complex [40,44] (Figure 1c) and in continuation of our work on 2D structures containing Si9 clusters [33], we derived two polymers (P1) and (P2) by a formal replacement of Ni(CO)2 with isolobal SiCl2 or SiH2. The polymers can also be regarded as silanes with inserting Si9 units. Pauling electronegativity of Ni and Si are almost identical with a value of χNi = 1.91 and χSi = 1.90, respectively, and the electron withdrawing effect of the CO ligands is considered by choosing ligands whose electronegativity is higher if compared to Si (χCl = 3.16 and χH = 2.20).
The energetically optimized polymers P1 and P2 possess nearly ideal C4v symmetric Si9 units in the rod group pmm2 (relevant bond lengths are listed in Table S1, Supplementary Materials). The two bridging Si atoms and two adjacent atoms of each of the two open squares form a planar hexagon which is compressed along the propagation direction of the polymer. The bond angles at the bridging Si atom consequently lie in the range of tetrahedral angles 103.4°–111.0° for both compounds. The torsional angles between these hexagons and the open square of the nido-clusters are 165.8° and 160.3° for P1 and P2, respectively. In order to compare the total energy with respect to α-Si and since the compounds contain also X = H and Cl atoms we include polysilanes with comparable bond situations according to Equation (1):
The resulting values of 7.28 eV and 7.23 eV for X = Cl and H, respectively refer to one formula unit (Si9)(SiX2)2 (X = Cl, H) for P1 and for P2, respectively (Figure 2). Scaled to one Si atom per formula unit these values result in 0.56 eV for both compounds. On a first glance, the values seem high if compared to α-Si, however the polymers must rather be compared to Si nanoparticles than to bulk materials. In this context the values are rather close to the relative energy of the carbon fullerenes of which for example C60 which is 0.48 eV per atom higher in total energy than diamond [56]. The Si bridged clusters are also much lower in energy than the directly connected clusters (0.96 eV) [33]. Energetically, the differences between Si–Cl and Si–H bonds a negligible.
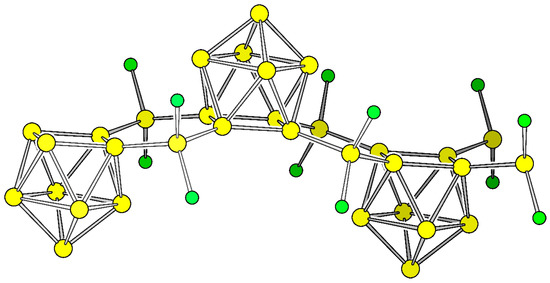
Figure 2.
Cut-out of polymer P1. Si and Cl are shown as yellow and green spheres, respectively.
3.2. Charged Molecules—The Stepwise Ligand Exchange in SiCl4 to [Si17]12−
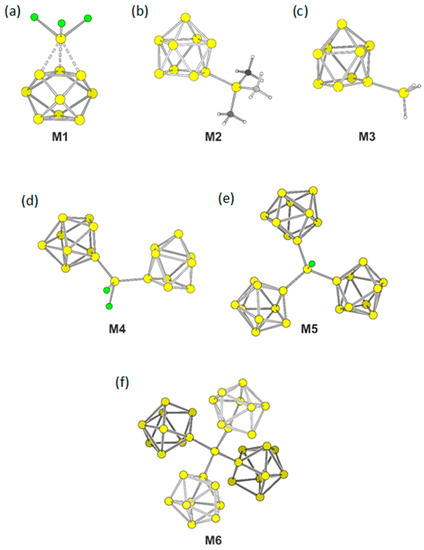
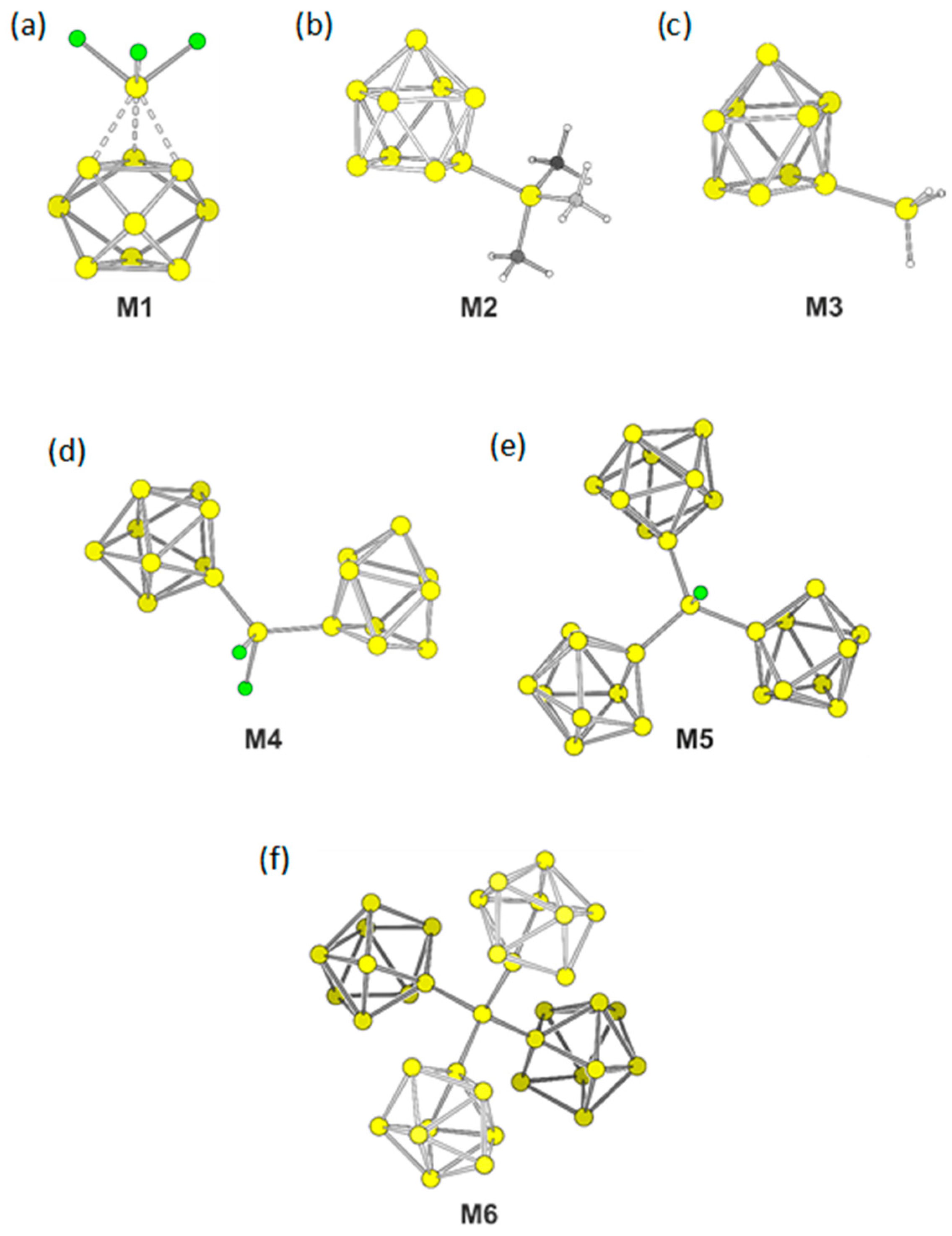
Siliconoid molecules are formed by a stepwise substitution of heteroatomic bonds between ligands bound to a central Si atom with Si terminated groups. We computationally substituted ligands in SiX4 by [Si9]4– for X = Cl, H, and CH3. The resulting molecular ions [Si(Si9)Cl3]3− (M1), [Si(Si9)H3]3– (M2) and [Si(Si9)Me3]3– (M3) differ in the ligands at the sp3-Si atom (Table 1). We started the optimizations for all compounds from a η1-connected isomer with an ideal C4v symmetric nido-[Si9]4– cluster.

Table 1.
Structural analysis of molecular anions (Figure 3). All values are averaged, if more bonds or angles of the same type exist.
As the energetically most favorite structure of M1 we found an arrangement with an almost perfect D3h symmetric trigonal prismatic Si9 cluster with the SiCl3 group symmetrically capping a triangular face of the Si9 cluster. The three distances between the Si atoms of the SiCl3 group and the Si atoms of the clusters are with a value of 2.58 Å identical to the lengths of the triangular face of Si9, consequently forming an almost undistorted tetrahedron. The three Si–Cl distances are 2.20 Å. The shape of Si9 cluster distorts to a tricapped trigonal prism with prism heights of 2.69 Å that are elongated with respect to the triangular faces. The opposing trigonal face, which is not capped, has considerable shorter lengths edges of 2.46 Å and 2.49 Å. Thus the coordination of the SiCl3 group leads to an elongation of edges of the capped triangle.
Interestingly, no local minimum structure with η1-coordination of the SiCl3 groups were found. In contrast, for [Si(Si9)H3]3– (M2) and [Si(Si9)Me3]3– (M3) the energetically most favored structures remain with η1-coordinated Si9 clusters. For both anions, the Si9 clusters distort to (pseudo-)C2v symmetric cages as an intermediate of the mono-capped square anti prism and the tricapped trigonal prism. With the ratio of diagonal lengths of the open square of d1/d2 = 1.22 is M2 more distorted than M3 (d1/d2 = 1.16). The bond length between the clusters and their ligands are 2.33 Å and 2.35 Å, respectively. The prism heights are 2.63 Å, 2.64 Å, and 3.08 Å for M2 and 2.62 Å, 2.63 Å, and 3.18 Å for M3.
Bridging two clusters by a single SiCl2 fragment, e.g. attaching two clusters to one central Si atom leads to the anion of composition [SiCl2(Si9)2]6– (M4). Both Si9 clusters in M4 form a η1-coordination with distances between the cluster and the bridge atoms of 2.34 Å. The Si–Cl bonds of 2.13 Å and 2.14 Å are shorter than for M1. Both clusters within M4 deform after optimization to (pseudo-)C2v symmetric compounds with d1/d2 = 1.21 and 1.22. (The distortion of C4v symmetric monocapped square anti prism can be characterized by the ratio of the diagonal lengths d1 and d2 of the open square. For an undistorted cage the ratio is equal to 1. Deviation from this ratio leads to cages with C2v symmetry.) The bond angle between the central Si atom and the two bonded Si atoms of the clusters is 121.7° and thus larger than the tetrahedral angel, which is most probably due to the steric demands of the Si9 ligands.
Substitution of another Cl ligand by a third [Si9]4− results in the highly charged cluster [SiCl(Si9)3]9– (M5). In M5 the three (pseudo-)C2v symmetric clusters have diagonal length ratio in the narrow range of d1/d2 = 1.15–1.17 and bond lengths of 2.37 Å between cluster atoms and the central sp3-Si atom for each bond. The distance Si–Cl is 1.18 Å.
A replacement of all Cl ligands by Si9 units results in [Si(Si9)4]12– (M6). Such highly charged clusters are not unrealistic, since a salt containing a [Ge45]12− cluster unit, which is attached to three Au+ ions ([Au3Ge45]9–) had been isolated and structurally characterized [57]. The distances for all four Si–Si bonds at the central Si atom are 2.39 Å. The angles range between 105.1° and 117.3°. The clusters are again distorted towards C2v symmetric cages, but stay close to the C4v symmetric input with ratios of the diagonal lengths of the open square between d1/d2 = 1.11–1.15. A table listening all bond distances and relevant cluster parameters is located in the Supplementary Materials.
The building unit M6 can be extended by continuation the building scheme with more SiCl4. Successive linking [Si9]4− clusters with SiCl4. under Cl substitution results in a neutral two-dimensional sheet [33].
4. Conclusion
The modelling of polymeric Si9 chains gave interesting insights to the chemistry of this up to now hard to functionalize Si9 cluster. It showed that a replacement of Ni(CO)2 as a ligand by others bridging sp3-Si units with comparable electron withdrawing effects, namely SiH2 and SiCl2, leads to polymers that are energetically in a range for realizable materials. The formation of charged oligomers by the formal substitution of X ligands in SiX4 by [Si9]4− clusters leads to stable anions [SiCl4−x(Si9)x]3x–. For all derivatives, stable anions with covalent Si–Si bonds to the central Si atom form, except for x = 1 and X = Cl. Here, a η3-coordination of the cluster to the Si atom of the SiCl3 group is observed. Whereas the monomeric unit [Si(Si9)4]12– (M6) possesses a relative high charge, further combination with bridging Si atoms leads to a relatively stable two-dimensional neutral Si allotrope.
Supplementary Materials
The following are available online at http://www.mdpi.com/2304-6740/6/1/31/s1, Bond lengths for Si9 clusters within the different compounds; Band Structures and DOSs of Polymers; Position Parameters for Polymers; Vibrational Frequencies for Polymers; Position Parameters for Molecular Compounds; Vibrational Frequencies of Molecular Compounds.
Supplementary File 1Acknowledgments
The authors are grateful to the SolTech (Solar Technologies go Hybrid) program of the Bavarian State Ministry of Education, Science and the Arts for financial support.
Author Contributions
Laura-Alice Jantke and Thomas F. Fässler conceived and designed the structures. Laura-Alice Jantke performed all quantum chemical calculations, Laura-Alice Jantke and Thomas F. Fässler wrote and corrected the paper, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cullis, A.G.; Canham, L.T. Visible light emission due to quantum size effects in highly porous crystalline silicon. Nature 1991, 353, 335–338. [Google Scholar] [CrossRef]
- Canham, L.T. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 1990, 57, 1046–1048. [Google Scholar] [CrossRef]
- Bisi, O.; Ossicini, S.; Pavesi, L. Porous silicon: A quantum sponge structure for silicon based optoelectronics. Surf. Sci. Rep. 2000, 38, 1–126. [Google Scholar] [CrossRef]
- Geppert, T.; Schiling, J.; Wehrspohn, R.; Gösele, U. Silicon-based photonic crystals. In Silicon Photonics; Pavesi, L., Lockwood, D.J., Eds.; Springer: Berlin, Germany, 2004; Volume 94, pp. 295–322. ISBN 978-3-540-21022-1. [Google Scholar]
- Abersfelder, K.; Russell, A.; Rzepa, H.S.; White, A.J.; Haycock, P.R.; Scheschkewitz, D. Contraction and expansion of the silicon scaffold of stable Si6R6 isomers. J. Am. Chem. Soc. 2012, 134, 16008–16016. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Akasaka, N.; Ishida, S. A heavy analogue of the smallest bridgehead alkene stabilized by a base. Nat. Commun. 2014, 5, 5353. [Google Scholar] [CrossRef] [PubMed]
- Abersfelder, K.; White, A.J.; Rzepa, H.S.; Scheschkewitz, D. A tricyclic aromatic isomer of hexasilabenzene. Science 2010, 327, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Scheschkewitz, D. A molecular silicon cluster with a “naked” vertex atom. Angew. Chem. Int. Ed. 2005, 44, 2954–2956. [Google Scholar] [CrossRef] [PubMed]
- Jantke, L.A.; Stegmaier, S.; Karttunen, A.J.; Fässler, T.F. Slicing Diamond—A Guide to Deriving sp3-Si Allotropes. Chem. Eur J. 2017, 23, 2734–2747. [Google Scholar] [CrossRef] [PubMed]
- Jantke, L.A.; Karttunen, A.J.; Fassler, T.F. Slicing Diamond for More sp3-Group 14 Allotropes Ranging from Direct Bandgaps to Poor Metals. ChemPhysChem 2017, 18, 1992–2006. [Google Scholar] [CrossRef] [PubMed]
- Beekman, M.; Wei, K.; Nolas, G.S. Clathrates and beyond: Low-density allotropy in crystalline silicon. Appl. Phys. Rev. 2016, 3, 040804. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Adams, G.B.; Sankey, O.F. Duals of Frank-Kasper structures as C, Si and Ge clathrates: Energetics and structure. Philosoph. Mag. Lett. 1998, 78, 21–28. [Google Scholar] [CrossRef]
- Krishna, L.; Martinez, A.D.; Baranowski, L.L.; Brawand, N.P.; Koh, C.A.; Stevanovic, V.; Lusk, M.T.; Toberer, E.S.; Tamboli, A.C. Group IV clathrates: Synthesis, optoelectronic properties, and photovoltaic applications. Proceedings 2014, 8981, 898108. [Google Scholar]
- Norouzzadeh, P.; Myles, C.W.; Vashaee, D. Prediction of Giant Thermoelectric Power Factor in Type-VIII Clathrate Si46. Sci. Rep. 2014, 4, 7028. [Google Scholar] [CrossRef] [PubMed]
- Härkönen, V.J.; Karttunen, A.J. Ab initio studies on the lattice thermal conductivity of silicon clathrate frameworks II and VIII. Phys. Rev. B 2016, 93, 024307. [Google Scholar] [CrossRef]
- Ammar, A.; Cros, C.; Pouchard, M.; Jaussaud, N.; Bassat, J.-M.; Villeneuve, G.; Duttine, M.; Ménétrier, M.; Reny, E. On the clathrate form of elemental silicon, Si136: Preparation and characterisation of NaxSi136 (x→0). Solid State Sci. 2004, 6, 393–400. [Google Scholar] [CrossRef]
- Ohashi, F.; Hattori, M.; Ogura, T.; Koketsu, Y.; Himeno, R.; Kume, T.; Ban, T.; Iida, T.; Habuchi, H.; Natsuhara, H.; et al. High-yield synthesis of semiconductive type-II Si clathrates with low Na content. J. Non-Cryst. Solids 2012, 358, 2134–2137. [Google Scholar] [CrossRef]
- Baranowski, L.L.; Krishna, L.; Martinez, A.D.; Raharjo, T.; Stevanovic, V.; Tamboli, A.C.; Toberer, E.S. Synthesis and optical band gaps of alloyed Si–Ge type II clathrates. J. Mater. Chem. C 2014, 2, 3231–3237. [Google Scholar] [CrossRef]
- Krishna, L.; Baranowski, L.L.; Martinez, A.D.; Koh, C.A.; Taylor, P.C.; Tamboli, A.C.; Toberer, E.S. Efficient route to phase selective synthesis of type II silicon clathrates with low sodium occupancy. Cryst. Eng. Comm 2014, 16, 3940–3949. [Google Scholar] [CrossRef]
- Buriak, J.M. Organometallic Chemistry on Silicon and Germanium Surfaces. Chem. Rev. 2002, 102, 1271–1308. [Google Scholar] [CrossRef] [PubMed]
- Wippermann, S.; He, Y.P.; Voros, M.; Galli, G. Novel silicon phases and nanostructures for solar energy conversion. Appl. Phys. Rev. 2016, 3, 040807. [Google Scholar] [CrossRef]
- Rapp, L.; Haberl, B.; Pickard, C.J.; Bradby, J.E.; Gamaly, E.G.; Williams, J.S.; Rode, A.V. Experimental evidence of new tetragonal polymorphs of silicon formed through ultrafast laser-induced confined microexplosion. Nat. Commun. 2015, 6, 7555. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Verri, G.G.; Lew Yan Voon, L.C. Electronic structure of silicon-based nanostructures. Phys. Rev. B 2007, 76, 075131. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Cahangirov, S.; Topsakal, M.; Akturk, E.; Sahin, H.; Ciraci, S. Two- and one-dimensional honeycomb structures of silicon and germanium. Phys. Rev. Lett. 2009, 102, 236804. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Arafune, R.; Kawahara, K.; Tsukahara, N.; Minamitani, E.; Kim, Y.; Takagi, N.; Kawai, M. Structure of Silicene Grown on Ag(111). Appl. Phys. Express 2012, 5, 045802. [Google Scholar] [CrossRef]
- Fleurence, A.; Friedlein, R.; Ozaki, T.; Kawai, H.; Wang, Y.; Yamada-Takamura, Y. Experimental evidence for epitaxial silicene on diboride thin films. Phys. Rev. Lett. 2012, 108, 245501. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, Y.; Zhang, L.; Du, S.; Wu, R.; Li, L.; Zhang, Y.; Li, G.; Zhou, H.; Hofer, W.A.; et al. Buckled Silicene Formation on Ir(111). Nano Lett. 2013, 13, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Xu, X.; Feng, H.; Li, Z.; Wang, X.; Du, Y. Honeycomb silicon: A review of silicene. Sci. Bull. 2015, 60, 1551–1562. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Schreckenbach, G.; Freund, M.S.; Schwingenschlögl, U. Current developments in silicene and germanene. Phys. Status Solidi RRL 2016, 10, 133–142. [Google Scholar] [CrossRef]
- Voon, L.C.L.Y.; Zhu, J.; Schwingenschlögl, U. Silicene: Recent theoretical advances. Appl. Phys. Rev. 2016, 3, 040802. [Google Scholar] [CrossRef]
- Gimbert, F.; Lee, C.-C.; Friedlein, R.; Fleurence, A.; Yamada-Takamura, Y.; Ozaki, T. Diverse forms of bonding in two-dimensional Si allotropes: Nematic orbitals in the MoS2 structure. Phys. Rev. B 2014, 90, 165423. [Google Scholar] [CrossRef]
- Jantke, L.-A.; Karttunen, A.J.; Fässler, T.F. Chemi-inspired silicon allotropes—Experimentally accessible Si9 cages as building block for 1D polymers, 2D sheets, single-walled nanotubes, and nanoparticles. submitted.
- Fässler, T.F. Zintl phases: Principles and recent developments. In Structure and Bonding; Mingos, D.M.P., Ed.; Springer: Heidelberg, Germany, 2011; Volume 139, ISBN 978-3-642-21150-8. [Google Scholar]
- Scharfe, S.; Kraus, F.; Stegmaier, S.; Schier, A.; Fässler, T.F. Zintl Ions, Cage Compounds, and Intermetalloid Clusters of Group 14 and Group 15 Elements. Angew. Chem. Int. Ed. 2011, 50, 3630–3670. [Google Scholar] [CrossRef] [PubMed]
- Fässler, T.F. Zintl ions: Principles and recent developments. In Structure and Bonding; Mingos, D.M.P., Ed.; Springer: Heidelberg, Germany, 2011; Volume 140, ISBN 978-3-642-21181-2. [Google Scholar]
- Queneau, V.; Todorov, E.; Sevov, S.C. Synthesis and structure of isolated silicon clusters of nine atoms. J. Am. Chem. Soc. 1998, 120, 3263–3264. [Google Scholar] [CrossRef]
- Goicoechea, J.M.; Sevov, S.C. Ligand-free deltahedral clusters of silicon in solution: Synthesis, structure, and electrochemistry of [Si9]2−. Inorganic Chemistry 2005, 44, 2654–2658. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, J.M.; Sevov, S.C. Organozinc derivatives of deltahedral zintl ions: Synthesis and characterization of closo-[E9Zn(C6H5)]3− (E = Si, Ge, Sn, Pb). Organometallics 2006, 25, 4530–4536. [Google Scholar] [CrossRef]
- Joseph, S.; Hamberger, M.; Mutzbauer, F.; Härtl, O.; Meier, M.; Korber, N. Chemistry with Bare Silicon Clusters in Solution: A Transition-Metal Complex of a Polysilicide. Anion Angew. Chem. Int. Ed. 2009, 48, 8770–8772. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Suchentrunk, C.; Kraus, F.; Korber, N. [Si9]4– Anions in Solution—Structures of the Solvates Rb4Si9·4.75NH3 and [Rb(18-crown-6)]Rb3Si9·4NH3, and Chemical Bonding in [Si9]4–. Eur. J. Inorg. Chem. 2009, 2009, 4641–4647. [Google Scholar] [CrossRef]
- Waibel, M.; Kraus, F.; Scharfe, S.; Wahl, B.; Fässler, T.F. [(MesCu)2(η3-Si4)]4–: A Mesitylcopper-Stabilized Tetrasilicide Tetraanion. Angew. Chem. Int. Ed. 2010, 49, 6611–6615. [Google Scholar] [CrossRef] [PubMed]
- Geitner, F.S.; Fässler, T.F. Low Oxidation State Silicon Clusters—Synthesis and Structure of [NHCDippCu(η4-Si9)]2−. Chem. Commun. 2017, 53, 12974–12977. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, S.; Hamberger, M.; Korber, N. The First Chelate-Free Crystal Structure of a Silicide Transition Metal Complex K0.28Rb7.72Si9(Ni(CO)2)2·16NH3. Crystals 2015, 5, 275–282. [Google Scholar] [CrossRef]
- Riley, A.E.; Korlann, S.D.; Richman, E.K.; Tolbert, S.H. Synthesis of semiconducting thin films with nanometer-scale periodicity by solution-phase coassembly of zintl clusters with surfactants. Angew. Chem. Int. Ed. 2005, 45, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Nolan, B.M.; Henneberger, T.; Waibel, M.; Fässler, T.F.; Kauzlarich, S.M. Silicon Nanoparticles by the Oxidation of [Si4]4−- and [Si9]4−- Containing Zintl Phases and Their Corresponding Yield. Inorg. Chem. 2015, 54, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B. Jmol: An open-source Java viewer for chemical structures in 3D; Northfield, MN, USA, 2016. [Google Scholar]
- Rappe, A.M.; Joannopoulos, J.D.; Bash, P.A. A test of the utility of plane-waves for the study of molecules from first principles. J. Am. Chem. Soc. 1992, 114, 6466–6469. [Google Scholar] [CrossRef]
- Dovesi, R.; Saunders, V.R.; Roetti, R.; Orlando, R.; Zicovich-Wilson, C.M.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.J.; et al. CRYSTAL09 User’s Manual; University of Turino: Turino, Italy, 2009. [Google Scholar]
- Dovesi, R.; Orlando, R.; Civalleri, B.; Roetti, C.; Saunders, V.R.; Zicovich-Wilson, C.M. CRYSTAL: A computational tool for the ab initio study of the electronic properties of crystals. Z. Kristallogr. 2005, 220, 571–573. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Karttunen, A.J.; Fässler, T.F.; Linnolahti, M.; Pakkanen, T.A. Structural Principles of Semiconducting Group 14 Clathrate Frameworks. Inorg. Chem. 2011, 50, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
- Karttunen, A.J.; Fässler, T.F.; Linnolahti, M.; Pakkanen, T.A. Two-, One-, and Zero-Dimensional Elemental Nanostructures Based on Ge9-Clusters. ChemPhysChem 2010, 11, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, A.; Hoffmann, S.D.; Kraus, F.; Fässler, T.F. [Au3Ge18]5−—A gold-germanium cluster with remarkable Au–Au interactions. Angew. Chem. Int. Ed. 2007, 46, 1638–1640. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).