Supramolecular Aggregation of a New Substituted Bis(salicylaldiminato)zinc(II) Schiff-Base Complex Derived from trans-1,2-Diaminocyclohexane
Abstract
:1. Introduction
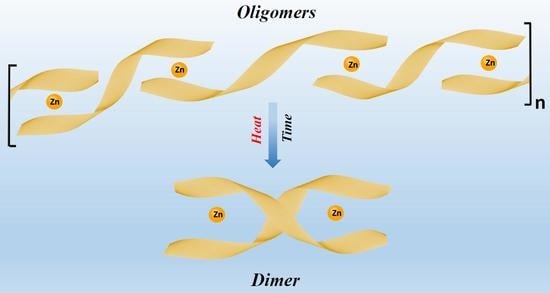
2. Results
3. Discussion
4. Experimental Section
4.1. Materials and General Procedures
4.2. Physical Measurements
4.3. Computational Method
4.4. Syntheses
4.4.1. {N,N-Bis[4-(diethylamino)-2-hydroxybenzylidene]-(1R,2R)-trans-1,2-diaminocyclohexane-diaminato}Zn(II) (R)-2
4.4.2. (R)-2F
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2rd ed.; Wiley: Hoboken, NJ, USA, 2009; ISBN 978-0-470-51233-3. [Google Scholar]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-gelators and their applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.M.-C.; Yam, W.-W. Self-assembly of luminescent alkynylplatinum(II) terpyridyl complexes: Modulation of photophysical properties through aggregation behavior. Acc. Chem. Res. 2011, 44, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Forte, G.; Consiglio, G.; Failla, S.; Di Bella, S. Aggregates of defined stereochemical scaffolds: A study in solution of a zinc(II) Schiff base complex derived from the enantiopure trans-1,2-cyclopentanediamine. Inorg. Chem. 2017, 56, 14206–14213. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. Supramolecular aggregates of defined stereochemical scaffolds: Aggregation/deaggregation in Schiff-base zinc(II) complexes derived from enantiopure trans-1,2-diaminocyclohexane. Inorg. Chem. 2016, 55, 10320–10328. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Oliveri, I.P.; Punzo, F.; Thompson, A.L.; Di Bella, S.; Failla, S. Structure and aggregation properties of a Schiff-base zinc(II) complex derived from cis-1,2-diaminocyclohexane. Dalton Trans. 2015, 44, 13040–13048. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Di Bella, S. An unprecedented structural interconversion in solution of aggregate zinc(II) salen Schiff-base complexes. Inorg. Chem. 2012, 51, 8409–8418. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Di Bella, S. Aggregation properties of bis(salicylaldiminato)zinc(II) Schiff-base complexes and their Lewis acidic character. Dalton Trans. 2012, 41, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Supramolecular aggregation/deaggregation in amphiphilic dipolar Schiff-base zinc(II) complexes. Inorg. Chem. 2010, 49, 5134–5142. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Failla, S.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Controlling the molecular aggregation. An amphiphilic Schiff-base zinc(II) complex as supramolecular fluorescent probe. Dalton Trans. 2009, 10426–10428. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Oliveri, I.P.; Consiglio, G.; Failla, S.; Di Bella, S. On the Lewis acidic character of bis(salicylaldiminato)zinc(II) Schiff-base complexes: A computational and experimental investigation on a series of compounds varying the bridging diimine. Dalton Trans. 2017, 46, 4571–4581. [Google Scholar] [CrossRef] [PubMed]
- Groizard, T.; Kahlal, S.; Dorcet, V.; Roisnel, T.; Bruneau, C.; Halet, J.-F.; Gramage-Doria, R. Nonconventional supramolecular self-assemblies of zinc(II)-salphen building blocks. Eur. J. Inorg. Chem. 2016, 5143–5151. [Google Scholar] [CrossRef]
- Oliveri, I. P.; Failla, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Di Bella, S. Synthesis, characterization, optical absorption/fluorescence spectroscopy, and second-order nonlinear optical properties of aggregate molecular architectures of unsymmetrical Schiff-base zinc(II) complexes. Dalton Trans. 2014, 43, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Failla, S.; Malandrino, G.; Di Bella, S. New molecular architectures by aggregation of tailored zinc(II) Schiff-base complexes. New J. Chem. 2011, 35, 2826–2831. [Google Scholar] [CrossRef]
- Wezenberg, S.J.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Anion-templated formation of supramolecular multinuclear assemblies. Chem. Eur. J. 2009, 15, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Gallant, A.J.; Chong, J.H.; MacLachlan, M.J. Heptametallic bowl-shaped complexes derived from conjugated Schiff-base macrocycles: Synthesis, characterization, and X-ray crystal structures. Inorg. Chem. 2006, 45, 5248–5250. [Google Scholar] [CrossRef] [PubMed]
- Kleij, A.W.; Kuil, M.; Tooke, D.M.; Lutz, M.; Spek, A.L.; Reek, J.N.H. ZnII-salphen complexes as versatile building blocks for the construction of supramolecular box assemblies. Chem. Eur. J. 2005, 11, 4743–4750. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mondal, P.; Prasad, S.K.; Rao, D.S.S.; Bhattacharjee, C.R. Zinc(II)-salphen complexes bearing long alkoxy side arms: Synthesis, solvent dependent aggregation, and spacer group substituent effect on mesomorphism and photophysical property. J. Mol. Liq. 2017, 246, 290–301. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mondal, P.; Prasad, S.K.; Rao, D.S.S.; Bhattacharjee, C.R. Induction of mesomorphism through supramolecular assembly in metal coordination compounds of “salphen”-type Schiff Bases: Photoluminescence and solvatochromism. Eur. J. Inorg. Chem. 2016, 4604–4614. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bhattacharjee, C.R.; Mondal, P.; Prasad, S.K.; Rao, D.S.S. Synthesis and aggregation behaviour of luminescent mesomorphic zinc(II) complexes with ‘salen’ type asymmetric Schiff base ligands. Dalton Trans. 2015, 44, 7477–7488. [Google Scholar] [CrossRef] [PubMed]
- Pucci, D.; Aiello, I.; Bellusci, A.; Crispini, A.; Ghedini, M.; La Deda, M. Coordination induction of nonlinear molecular shape in mesomorphic and luminescent ZnII complexes based on salen-like frameworks. Eur. J. Inorg. Chem. 2009, 4274–4281. [Google Scholar] [CrossRef]
- Piccinno, M.; Angulo-Pachón, C.A.; Ballester, P.; Escuder, B.; Dalla Cort, A. Rational design of a supramolecular gel based on a Zn(II)-salophen bis-dipeptide derivative. RSC Adv. 2016, 6, 57306–57309. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Malandrino, G.; Di Bella, S. Self-assembled nanostructures of amphiphilic Zinc(II) salophen complexes: Role of the solvent on their structure and morphology. Dalton Trans. 2014, 43, 10208–10214. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Failla, S.; Malandrino, G.; Di Bella, S. Controlling the molecular self-assembly into nanofibers of amphiphilic zinc(II) salophen complexes. J. Phys. Chem. C 2013, 117, 15335–15341. [Google Scholar] [CrossRef]
- Hui, J. K.-H.; MacLachlan, M. J. Fibrous aggregates from dinuclear zinc(II) salphen complexes. Dalton Trans. 2010, 39, 7310–7319. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.K.-H.; Yu, Z.; MacLachlan, M.J. Supramolecular assembly of zinc salphen complexes: Access to metal-containing gels and nanofibers. Angew. Chem. Int. Ed. 2007, 46, 7980–7983. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Hao, L.; Chen, C.-Y.; Qiu, Q.-M.; Wang, K.; Song, J.-B.; Li, H. Red-shift in fluorescence emission of D-A type asymmetrical Zn(II) complexes by extending the π–π stacking interaction. RSC Adv. 2017, 7, 20488–20493. [Google Scholar] [CrossRef]
- Minei, P.; Fanizza, E.; Rodríguez, A.M.; Muñoz-García, A.B.; Cimino, P.; Pavone, M.; Pucci, A. Cost-effective solar concentrators based on red fluorescent Zn(II)-salicylaldiminato complex. RSC Adv. 2016, 6, 17474–17482. [Google Scholar] [CrossRef] [Green Version]
- Dumur, F.; Contal, E.; Wantz, G.; Gigmes, D. Photoluminescence of zinc complexes: Easily tunable optical properties by variation of the bridge between the imido groups of Schiff base ligands. Eur. J. Inorg. Chem. 2014, 4186–4198. [Google Scholar] [CrossRef]
- Di Bella, S.; Oliveri, I.P.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D. An unprecedented switching of the second-order nonlinear optical response in aggregate bis(salicylaldiminato)zinc(II) Schiff-base complexes. Dalton Trans. 2012, 41, 7013–7016. [Google Scholar] [CrossRef] [PubMed]
- Dalla Cort, A.; De Bernardin, P.; Forte, G.; Mihan, F.Y. Metal-salophen-based receptors for anions. Chem. Soc. Rev. 2010, 39, 3863–3874. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.-Y.; Tang, J.; Zhang, J.-L. Introducing metallosalens to biological studies: The renaissance of traditional coordination complexes. Eur. J. Inorg. Chem. 2017, 5085–5093. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Lewis basicity of relevant monoanions in a non-protogenic organic solvent using a zinc(II) Schiff-base complex as reference Lewis acid. Dalton Trans. 2017, 46, 11608–11614. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, S.; Oliveri, I.P.; Ruffino, F.; Maccarrone, G.; Di Bella, S. Low-cost chemiresistive sensor for volatile amines based on a 2D network of a zinc(II) Schiff-base complex. Appl. Phys. Lett. 2016, 109, 143108. [Google Scholar] [CrossRef]
- Cheng, J.; Gou, F.; Zhang, X.; Shen, G.; Zhou, X.; Xiang, H. A class of multiresponsive colorimetric and fluorescent pH probes via three different reaction mechanisms of salen complexes: A Selective and Accurate pH Measurement. Inorg. Chem. 2016, 55, 9221–9229. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, L.; Bandeira, N.A.G.; Bo, C.; Kleij, A.W. Highly efficient chirality transfer from diamines encapsulated within a self-assembled calixarene-salen host. Chem. Eur. J. 2015, 21, 7144–7150. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, F.; Giannicchi, I.; Acóna, L.; Dalla Cort, A.; Rodríguez, L. Anion selectivity of Zn-salophen receptors: Influence of ligand substituents. Inorg. Chim. Acta 2015, 434, 1–6. [Google Scholar] [CrossRef]
- Kumari, N.; Zelder, F. Detecting biologically relevant phosphates with locked salicylaldehyde probes in water. Chem. Commun. 2015, 51, 17170–17173. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cai, Y.-B.; Jing, J.; Zhang, J.-L. Unravelling the correlation between metal induced aggregation and cellular uptake/subcellular localization of znsalen: an overlooked rule for design of luminescent metal probes. Chem. Sci. 2015, 6, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Malandrino, G.; Di Bella, S. Phase transition and vapochromism in molecular assemblies of a polymorphic zinc(II) Schiff-base complex. Inorg. Chem. 2014, 53, 9771–9777. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ma, X.; Zhang, Y.; Liu, J.; Zhou, X.; Xiang, H. Optical chemosensors based on transmetalation of salen-based Schiff base complexes. Inorg. Chem. 2014, 53, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Brissos, R.; Ramos, D.; Lima, J.C.; Yafteh Mihan, F.; Borràs, M.; de Lapuente, J.; Dalla Cort, A.; Rodríguez, L. Luminescent zinc salophen derivatives: Cytotoxicity assessment and action mechanism studies. New J. Chem. 2013, 37, 1046–1055. [Google Scholar] [CrossRef]
- Jurček, O.; Cametti, M.; Pontini, M.; Kolehmainena, E.; Rissanen, K. A Zinc-salophen/bile-acid conjugate receptor solubilized by CTABr micelles binds phosphate in water. Org. Biomol. Chem. 2013, 11, 4585–4590. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Milione, S.; Maranzana, A.; Grassi, A.; Pellecchia, C. Selective detection of ATP and ADP in aqueous solution by using a fluorescent zinc receptor. Chem. Commun. 2012, 48, 11419–11421. [Google Scholar] [CrossRef] [PubMed]
- Yafteh Mihan, F.; Bartocci, S.; Bruschini, M.; De Bernardin, P.; Forte, G.; Giannicchi, I.; Dalla Cort, A. Ion-pair recognition by metal-salophen and metal-salen complexes. Aust. J. Chem. 2012, 65, 1638–1646. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Maccarrone, G.; Di Bella, S. A Lewis basicity scale in dichloromethane for amines and Common nonprotogenic solvents using a zinc(II) Schiff-base complex as reference Lewis acid. J. Org. Chem. 2011, 76, 8879–8884. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Di Bella, S. Sensitive fluorescent detection and Lewis basicity of aliphatic amines. J. Phys. Chem. A 2011, 115, 14325–14330. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Di Bella, S. Highly sensitive fluorescent probe for detection of alkaloids. Tetrahedron 2011, 67, 9446–9449. [Google Scholar] [CrossRef]
- Khatua, S.; Choi, S.H.; Lee, J.; Kim, K.; Do, Y.; Churchill, D.G. Aqueous fluorometric and colorimetric sensing of phosphate ions by a fluorescent dinuclear zinc complex. Inorg. Chem. 2009, 48, 2993–2999. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Rodríguez, L.; Lima, J.C.; Pina, F.; Dalla Cort, A.; Pasquini, C.; Schiaffino, L. Specific supramolecular interactions between Zn2+-salophen complexes and biologically relevant anions. Inorg. Chem. 2009, 48, 6229–6235. [Google Scholar] [CrossRef] [PubMed]
- Dalla Cort, A.; Bernardin, P.; Schiaffino, L. A new water soluble Zn-salophen derivative as a receptor for α-aminoacids: Unexpected chiral DISCRIMINATION. Chirality 2009, 21, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Supramolecular adsorption of alkaloids by metallosalphen complexes. Inorg. Chem. 2008, 47, 4256–4263. [Google Scholar] [CrossRef] [PubMed]
- Vladimirova, K.G.; Freidzon, A.Y.; Kotova, O.V.; Vaschenko, A.A.; Lepnev, L.S.; Bagatur’yants, A.A.; Vitukhnovskiy, A.G.; Stepanov, N.F.; Alfimov, M.V. Theoretical study of structure and electronic absorption spectra of some Schiff bases and their zinc complexes. Inorg. Chem. 2009, 48, 11123–11130. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, revision A.02. Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]







| Compound | Species | D × 1010/m2·s−1 | D (solvent) × 1010/m2·s−1 | m (n) 1/Da | m (n) 2/Da |
|---|---|---|---|---|---|
| (R)-2 | 2⋅DMSO-d6 | 2.5 | 7.1(DMSO-d6) | 679 3 | 612.2 |
| (R)-2 | 2A | 1.7 | 26.3(CDCl3) | 14,046(26.6) | 14,256(27) |
| 2B | 1.9 | 26.3(CDCl3) | 11,244(21.3) | 11,088(21) | |
| 2C | 2.2 | 26.3(CDCl3) | 8387(15.9) | 8448(16) | |
| 2D | 5.8 | 26.3(CDCl3) | 1207(2.3) | 1056(2) | |
| 2E | 6.0 | 26.3(CDCl3) | 1128(2.1) | 1056(2) | |
| 2F | 6.2 | 26.3(CDCl3) | 1056(2) | 1056(2) | |
| (R)-2 4 | 2F | 5.9 | 24.8 (CDCl3) | 1017(1.9) | 1056(2) |
| 1C | 6.3 | 24.8 (CDCl3) | 892(2) | 891.7(2) |
| Diamino Bridge | 4-substituent | δ (ppm) | ∆δ (ppm) 1 | Zn(II) Coordination | Aggregate Structure | Ref. |
|---|---|---|---|---|---|---|
| 2,3-diamino-maleonitrile | –OC11H21 | 8.35 | 0.03 | penta | (ZnL)2 | [10] |
| benzene-1,2-diamine | –OC10H20 | 8.47 | 0.39 | penta | (ZnL)2 | [9] |
| ethane-1,2-diamine | –OC16H33 | 7.61 | 0.67 | penta | (ZnL)2 | [8] |
| cis-cyclohexane-1,2-diamine | –OMe | 8.08; 8.33 | 0.22; −0.03 | penta | (ZnL)2 | [7] |
| trans-cyclohexane-1,2-diamine | –OMe | 8.33 | −0.12 | tetra | (ZnL)n 2 | [6] |
| 7.35 | 0.86 | tetra | 1C, Zn2L2 | |||
| trans-cyclohexane-1,2-diamine | –NEt2 | 8.14 | −0.11 | tetra | 2A, (ZnL)n | This work |
| 7.22 | 0.81 | tetra | 2F, Zn2L2 | |||
| trans-cyclopentane-1,2-diamine | –OMe | 7.50 | 0.69 | tetra | Zn2L2 | [5] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. Supramolecular Aggregation of a New Substituted Bis(salicylaldiminato)zinc(II) Schiff-Base Complex Derived from trans-1,2-Diaminocyclohexane. Inorganics 2018, 6, 8. https://doi.org/10.3390/inorganics6010008
Consiglio G, Oliveri IP, Failla S, Di Bella S. Supramolecular Aggregation of a New Substituted Bis(salicylaldiminato)zinc(II) Schiff-Base Complex Derived from trans-1,2-Diaminocyclohexane. Inorganics. 2018; 6(1):8. https://doi.org/10.3390/inorganics6010008
Chicago/Turabian StyleConsiglio, Giuseppe, Ivan Pietro Oliveri, Salvatore Failla, and Santo Di Bella. 2018. "Supramolecular Aggregation of a New Substituted Bis(salicylaldiminato)zinc(II) Schiff-Base Complex Derived from trans-1,2-Diaminocyclohexane" Inorganics 6, no. 1: 8. https://doi.org/10.3390/inorganics6010008
APA StyleConsiglio, G., Oliveri, I. P., Failla, S., & Di Bella, S. (2018). Supramolecular Aggregation of a New Substituted Bis(salicylaldiminato)zinc(II) Schiff-Base Complex Derived from trans-1,2-Diaminocyclohexane. Inorganics, 6(1), 8. https://doi.org/10.3390/inorganics6010008