Synthesis and Characterisation of Linear and Towards Cyclic Diferrocenes with Alkynyl Spacers
Abstract
:1. Introduction
2. Results
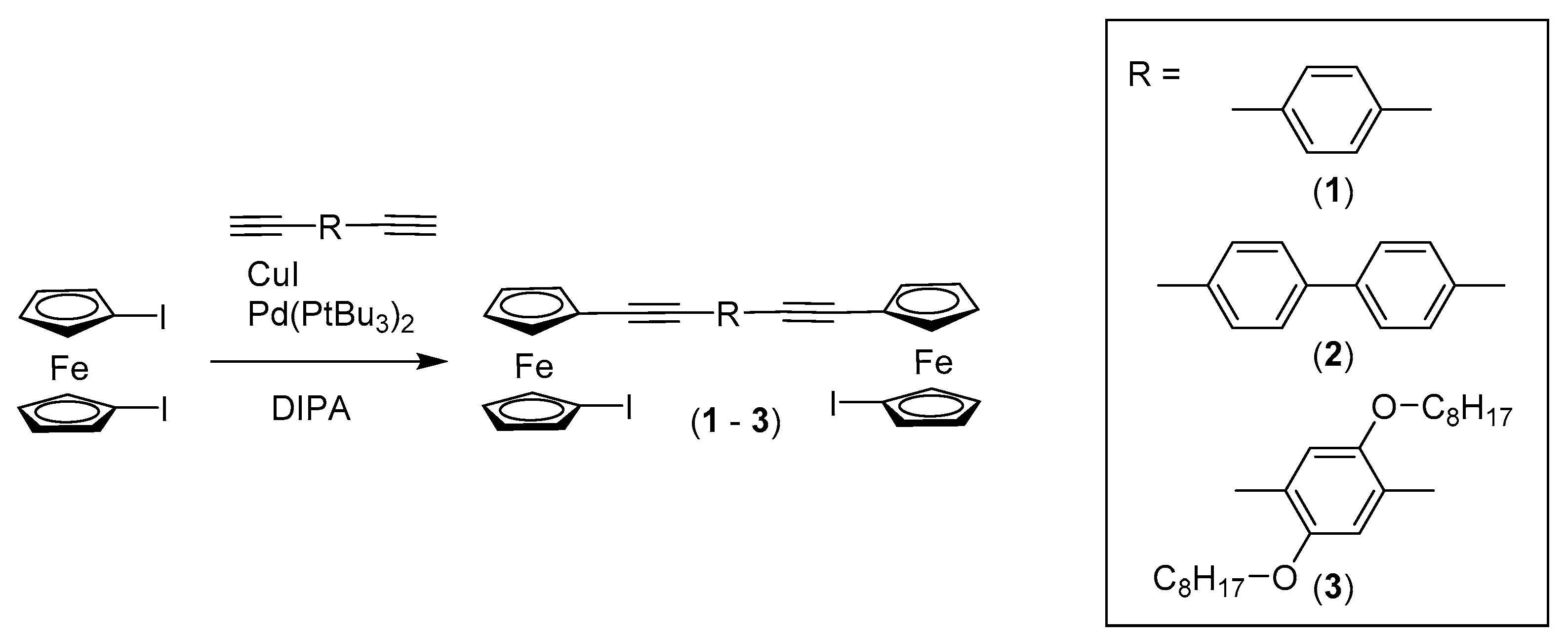
2.1. Synthesis and Characterisation
2.2. X-ray Crystallography
2.3. Electrochemistry
3. Materials and Methods
3.1. Experimental
3.1.1. Synthesis of Compound 1
3.1.2. Synthesis of Compound 2
3.1.3. Synthesis of Compound 3
3.1.4. Synthesis of Compound 4
3.1.5. Synthesis of Compound 5
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 1, 6–29. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Peng, Z.-L.; Hou, R.; Liang, J.-H.; Zheng, J.-F.; Zhou, X.-Y.; Zhou, X.-S.; Jin, S.; Niu, Z.-J.; Mao, B.-W. Enhancing Electron Transport in Molecular Wires by Insertion of a Ferrocene Center. Phys. Chem. Chem. Phys. 2014, 16, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Getty, S.A.; Engtrakul, C.; Wang, L.; Liu, R.; Ke, S.-H.; Baranger, H.-U.; Yang, W.; Fuhrer, M.S.; Sita, L.R. Near-perfect Conduction Through a Ferrocene-based Molecular Wire. Phys. Rev. B Solid State 2005, 71, 241401.1–241401.4. [Google Scholar] [CrossRef]
- Wilson, L.E.; Hassenrück, C.; Winter, R.F.; White, A.J.P.; Albrecht, T.; Long, N.J. Functionalised Biferrocene Systems towards Molecular Electronics. Eur. J. Inorg. Chem. 2017, 2, 496–504. [Google Scholar] [CrossRef]
- Xiao, X.; Brune, D.; He, J.; Lindsay, S.; Gorman, C.B.; Tao, N. Redox-gated electron transport in electrically wired ferrocene molecules. Chem. Phys. 2006, 326, 138–143. [Google Scholar] [CrossRef]
- Sun, R.; Wang, L.; Yu, H.; Abdin, Z.; Chen, Y.; Huang, J.; Tong, R. Molecular Recognition and Sensing Based on Ferrocene Derivatives and Ferrocene-Based Polymers. Organometallics 2014, 33, 4560–4573. [Google Scholar] [CrossRef]
- Diallo, A.K.; Absalon, C.; Ruiz, J.; Astruc, D. Ferrocenyl-Terminated Redox Stars: Synthesis and Electrostatic Effects in Mixed-Valence Stabilization. J. Am. Chem. Soc. 2011, 133, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Valério, C.; Fillaut, J.-L.; Ruiz, J.; Guittard, J.; Blais, J.-C.; Astruc, D. The Dendritic Effect in Molecular Recognition: Ferrocene Dendrimers and their use as Supramolecular Redox Sensors for the Recognition of Small Inorganic Anions. J. Am. Chem. Soc. 1997, 119, 2588–2589. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Ruiz, J. Metallocenyl Dendrimers and their Applications in Molecular Electronics, Sensing, and Catalysis. Acc. Chem. Res. 2008, 41, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Gorman, C.B.; Smith, J.C.; Hager, M.W.; Parkhurst, B.L.; Sierzputowska-Gracz, H.; Haney, C.A. Molecular Structure-property Relationships for Electron-transfer Rate Attenuation in Redox-active Core Dendrimers. J. Am. Chem. Soc. 1999, 121, 9958–9966. [Google Scholar] [CrossRef]
- Inkpen, M.S.; Scheerer, S.; Linseis, M.; White, A.J.P.; Winter, R.F.; Albrecht, T.; Long, N.J. Oligomeric Ferrocene Rings. Nat. Chem. 2016, 8, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, V.; Pleux, L.; Häussinger, D.; Unke, O.T.; Prescimone, A.; Mayor, M. Deltoid versus Rhomboid: Controlling the Shape of Bis-ferrocene Macrocycles by the Bulkiness of the Substituents. Organometallics 2017, 36, 858–866. [Google Scholar] [CrossRef]
- Wilson, L.E.; Hassenrück, C.; Winter, R.F.; White, A.J.P.; Albrecht, T.; Long, N.J. Ferrocene- and Biferrocene-Containing Macrocycles towards Single-Molecule Electronics. Angew. Chem. Int. Ed. 2017, 56, 6838–6842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inkpen, M.S.; White, A.J.P.; Albrecht, T.; Long, N.J. Rapid Sonogashira cross-coupling of iodoferrocenes and the unexpected cyclo-oligomerization of 4-ethynylphenylthioacetate. Chem. Commun. 2013, 49, 5663–5665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, N.J.; Martin, A.J.; Vilar, R.; White, A.J.P.; Williams, D.J. Synthesis, Characterization, and Theoretical Studies of New Alkynylferrocene and -Biferrocene Ligands and their Platinum-containing Dimers and Oligomers. Organometallics 1999, 18, 4261–4269. [Google Scholar] [CrossRef]
- Younus, M.; Long, N.J.; Raithby, P.R.; Lewis, J. Synthetic, Spectroscopic and Electrochemical Characterisation of Mixed-Metal Acetylide Complexes. J. Organomet. Chem. 1998, 570, 55–62. [Google Scholar] [CrossRef]
- Ward, M.D. Metal-metal Interactions in Binuclear Complexes Exhibiting Mixed Valency; Molecular Wires and Switches. Chem. Soc. Rev. 1995, 24, 121–134. [Google Scholar] [CrossRef]
- Inkpen, M.S.; Du, S.; Driver, M.; Albrecht, T.; Long, N.J. Oxidative Purification of Halogenated Ferrocenes, Dalton Trans. Dalton Trans 2013, 42, 2813–2816. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Cheng, L.; Luo, J.; Xu, K.; Sun, Q.; Dong, L.; Salhi, F.; Lee, P.P.S.; Chen, J.; Tang, B.Z. Simple Synthesis, Outstanding Thermal Stability, and Tunable Light-emitting and Optical-limiting Properties of Functional Hyperbranched Polyarylenes. Macromolecules 2002, 35, 5349–5351. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, Y.; Cai, R.; Yu, L. Conjugated Liquid-crystalline Polymers—Soluble and Fusible Poly(phenylethylene) by the Heck Coupling Reaction. Macromolecules 1993, 26, 5281–5286. [Google Scholar] [CrossRef]
- SHELXTL v5.1; Bruker AXS: Madison, WI, USA, 1998.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]



| Selected Bond Lengths (Å) | Selected Bond Angles (°) | ||
|---|---|---|---|
| C1–C11 | 1.436(5) | C1–C11–C12 | 178.5(4) |
| C11–C12 | 1.185(5) | C11–C12–C13 | 178.4(4) |
| C12–C13 | 1.441(5) | C11–C5(cent)…C5(cent) | ca. 92.1 |
| C6–I1 | 2.081(4) | I–C5(cent)…C5(cent) | ca. 94.0 |
| Fe1–Fe1 intra | 13.8626(14) | C12–C13–C6H4(cent) | ca. 179.6 |
| Compound | E1/2 (mV) | ∆E (mV) | ipa/ipc |
|---|---|---|---|
| 1 | 0.257 | 0.079 | 0.85 |
| 2 | 0.239 | 0.059 | 0.83 |
| 3 | 0.259 | 0.090 | 0.92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, L.E.; Jian, X.; White, A.J.P.; Long, N.J. Synthesis and Characterisation of Linear and Towards Cyclic Diferrocenes with Alkynyl Spacers. Inorganics 2018, 6, 95. https://doi.org/10.3390/inorganics6030095
Wilson LE, Jian X, White AJP, Long NJ. Synthesis and Characterisation of Linear and Towards Cyclic Diferrocenes with Alkynyl Spacers. Inorganics. 2018; 6(3):95. https://doi.org/10.3390/inorganics6030095
Chicago/Turabian StyleWilson, Lucy E., Xueying Jian, Andrew J. P. White, and Nicholas J. Long. 2018. "Synthesis and Characterisation of Linear and Towards Cyclic Diferrocenes with Alkynyl Spacers" Inorganics 6, no. 3: 95. https://doi.org/10.3390/inorganics6030095
APA StyleWilson, L. E., Jian, X., White, A. J. P., & Long, N. J. (2018). Synthesis and Characterisation of Linear and Towards Cyclic Diferrocenes with Alkynyl Spacers. Inorganics, 6(3), 95. https://doi.org/10.3390/inorganics6030095





