A Semi-Empirical Method for the Estimation of the Hydration Number of Mn(II)-Complexes
Abstract
:1. Introduction
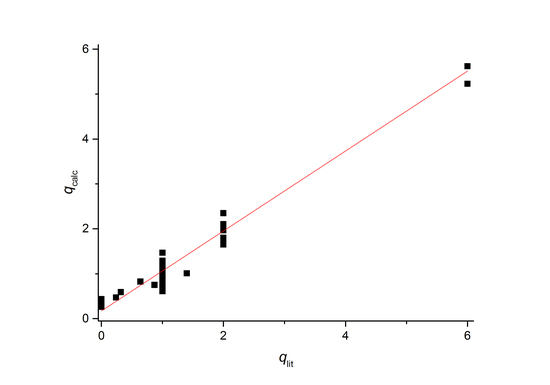
2. Results and Discussion
3. Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Merbach, A.E.; Helm, L.; Tóth, É. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; ISBN 0-471-60778-9. [Google Scholar]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Farrar, C.T.; Frullano, L.; Uppal, R. Influence of molecular parameters and increasing magnetic field strength on relaxivity of gadolinium- and manganese-based T1 contrast agents. Contrast Media Mol. Imaging 2009, 4, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, Z.; Brücher, E.; Uggeri, F.; Maiocchi, A.; Tóth, I.; Andrási, M.; Gáspár, A.; Zékány, L.; Aime, S. The role of equilibrium and kinetic properties in the dissociation of Gd[DTPA-bis(methylamide)] (omniscan) at near to physiological conditions. Chem. Eur. J. 2015, 21, 4789–4799. [Google Scholar] [CrossRef] [PubMed]
- Brücher, E.; Tircsó, G.; Baranyai, Z.; Kovács, Z.; Sherry, A.D. Stability and toxicity of contrast agents. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; Merbach, A.E., Helm, L., Tóth, É., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 157–208. [Google Scholar]
- Kanal, E.; Tweedle, M.F. Residual or retained gadolinium: Practical implications for radiologists and our patients. Radiology 2015, 275, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.I.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Pullicino, R.; Radon, M.; Biswas, S.; Bhojak, M.; Das, K. A review of the current evidence on gadolinium deposition in the brain. Clin. Neuroradiol. 2018, 128, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781498754286. [Google Scholar]
- Alpoim, M.C.; Urbano, A.M.; Geraldes, C.F.G.C.; Peters, J.A. Determination of the number of inner-sphere water-molecules in lanthanide(III) polyaminocarboxylate complexes. J. Chem. Soc. Dalton Trans. 1992, 463–467. [Google Scholar] [CrossRef]
- Djanashvili, K.; Peters, J.A. How to determine the number of inner-sphere water molecules in lanthanide(III) complexes by 17O NMR spectroscopy. A technical note. Contrast Media Mol. Imaging 2007, 2, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Beeby, A.; Clarkson, I.M.; Dickins, R.S.; Faulkner, S.; Parker, D.; Royle, L.; de Sousa, A.S.; Williams, J.A.G.; Woods, M. Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: An improved luminescence method for establishing solution hydration states. J. Chem. Soc. Perkin Trans. 1999, 2, 493–504. [Google Scholar] [CrossRef]
- Esteban-Gómez, D.; Cassino, C.; Botta, M.; Platas-Iglesias, C. 17O and 1H relaxometric and DFT study of hyperfine coupling constants in [Mn(H2O)6]2+. RSC Adv. 2014, 4, 7094–7103. [Google Scholar] [CrossRef]
- Póta, K.; Garda, Z.; Kálmán, F.K.; Barriada Pereira, J.L.; Esteban-Gómez, D.; Platas-Iglesias, C.; Tóth, I.; Brücher, E.; Tircsó, G. Making a next step toward inert Mn2+ complexes of open-chain ligands: The case of the rigid PhDTA ligand. New J. Chem. 2018, 42, 8001–8011. [Google Scholar] [CrossRef]
- Leigh, J.S., Jr. Relaxation times in systems with chemical exchange. Exact solutions. J. Magn. Reson. 1971, 4, 308–311. [Google Scholar] [CrossRef]
- Swift, T.J.; Connick, R.E. NMR (nuclear magnetic resonance)-relaxation mechanisms of O17 in aqueous solutions of paramagnetic cations and the lifetime of water molecules in the first coordination sphere. J. Chem. Phys. 1962, 37, 307–320. [Google Scholar] [CrossRef]
- Solomon, I. Relaxation processes in a system of two spins. Phys. Rev. 1955, 99, 559–565. [Google Scholar] [CrossRef]
- Bloembergen, N. Proton relaxation times in paramagnetic solutions. J. Chem. Phys. 1957, 27, 572–573. [Google Scholar] [CrossRef]
- Bloembergen, N.; Morgan, L.O. Proton relaxation times in paramagnetic solutions. Effects of electron spin relaxation. J. Chem. Phys. 1961, 34, 842–850. [Google Scholar] [CrossRef]
- Freed, J.H. Dynamic effects of pair correlation functions on spin relaxation by translational diffusion in liquids. II. Finite jumps and independent T1 processes. J. Chem. Phys. 1978, 68, 4034–4037. [Google Scholar] [CrossRef]
- Bertini, I.; Briganti, F.; Xia, Z.; Luchinat, C. Nuclear magnetic relaxation dispersion studies of hexaaquo manganese(II) ions in water-glycerol mixtures. J. Magn. Reson. 1993, 101, 198–201. [Google Scholar] [CrossRef]
- Koenig, S.H.; Brown, R.D.; Studebaker, J. On the interpretation of solvent proton magnetic relaxation data with particular application to the structure of the active site of Mn-carboxypeptidase A. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York, NY, USA, 1971; Volume 36, pp. 551–559. [Google Scholar]
- Balogh, E.; He, Z.; Hsieh, W.; Liu, S.; Tóth, É. Dinuclear complexes formed with the triazacyclononane derivative ENOTA4−: High-pressure 17O NMR evidence of an associative water exchange on [MnII2(ENOTA)(H2O)2]. Inorg. Chem. 2007, 46, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Helm, L. Relaxivity in paramagnetic systems: Theory and mechanism. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 45–64. [Google Scholar] [CrossRef]
- Belorizky, E.; Fries, P.H.; Helm, L.; Kowalewski, J.; Kruk, D.; Sharp, R.R.; Westlund, P.O. Comparison of different methods for calculating the paramagnetic relaxation enhancement of nuclear spins as a function of the magnetic field. J. Chem. Phys. 2008, 128, 052315. [Google Scholar] [CrossRef] [PubMed]
- Troughton, J.S.; Greenfield, M.T.; Greenwood, J.M.; Dumas, S.; Wiethoff, A.J.; Wang, J.; Spiller, M.; McMurry, T.J.; Caravan, P. Synthesis and evaluation of a high relaxivity manganese(II)-based MRI contrast agent. Inorg. Chem. 2004, 43, 6313–6323. [Google Scholar] [CrossRef] [PubMed]
- Belorizky, E.; Fries, P.H. Simple analytical approximation of the longitudinal electronic relaxation rate of Gd(III) complexes in solutions. Phys. Chem. Chem. Phys. 2004, 6, 2341–2351. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C.; Sherry, A.D.; Brown, R.D., III; Koenig, S.H. Magnetic field dependence of solvent proton relaxation rates induced by gadolinium(3+) and manganese(2+) complexes of various polyaza macrocyclic ligands: Implications for NMR imaging. Magn. Reson. Med. 1986, 3, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.H.; Brown, R.D., III. Relaxometry of magnetic resonance imaging contrast agents. Magn. Reson. Annu. 1987, 263–286. [Google Scholar]
- Jackels, S.C.; Durham, M.M.; Newton, J.E.; Henninger, T.C. Aqueous proton NMR relaxation enhancement by manganese(II) macrocyclic complexes: Structure-relaxivity relationships. Inorg. Chem. 1992, 31, 234–239. [Google Scholar] [CrossRef]
- Rolla, G.A.; Platas-Iglesias, C.; Botta, M.; Tei, L.; Helm, L. 1H and 17O NMR relaxometric and computational study on macrocyclic Mn(II) complexes. Inorg. Chem. 2013, 52, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Forgács, A.; Botta, M.; Regueiro-Figueroa, M.; Barriada, J.L.; Esteban-Gómez, D.; de Blas, A.; Rodríguez-Blas, T.; Platas-Iglesias, C. Mono-, bi-, and trinuclear bis-hydrated Mn(2+) complexes as potential MRI contrast agents. Inorg. Chem. 2015, 54, 9576–9587. [Google Scholar] [CrossRef] [PubMed]
- Tei, L.; Gugliotta, G.; Fekete, M.; Kalman, F.K.; Botta, M. Mn(II) complexes of novel hexadentate AAZTA-like chelators: A solution thermodynamics and relaxometric study. Dalton Trans. 2011, 40, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Rolla, G.A.; Tei, L.; Fekete, M.; Arena, F.; Gianolio, E.; Botta, M. Responsive Mn(II) complexes for potential applications in diagnostic magnetic resonance imaging. Bioorg. Med. Chem. 2011, 19, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Anelli, L.; Botta, M.; Brocchetta, M.; Canton, S.; Fedeli, F.; Gianolio, E.; Terreno, E. Relaxometric evaluation of novel manganese(II) complexes for application as contrast agents in magnetic resonance imaging. J. Biol. Inorg. Chem. 2002, 7, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Artali, R.; Baranyai, Z.; Botta, M.; Giovenzana, G.B.; Maspero, A.; Negri, R.; Palmisano, G.; Sisti, M.; Tollari, S. Solution thermodynamics, computational and relaxometric studies of ditopic DO3A-based Mn(II) complexes. New J. Chem. 2015, 39, 539–547. [Google Scholar] [CrossRef]
- Patinec, V.; Rolla, G.A.; Botta, M.; Tripier, R.; Esteban-Gómez, D.; Platas-Iglesias, C. Hyperfine coupling constants on inner-sphere water molecules of a triazacyclononane-based Mn(II) complex and related systems relevant as mri contrast agents. Inorg. Chem. 2013, 52, 11173–11184. [Google Scholar] [CrossRef] [PubMed]
- Drahoš, B.; Kotek, J.; Císařová, I.; Hermann, P.; Helm, L.; Lukeš, I.; Tóth, É. Mn2+ complexes with 12-membered pyridine based macrocycles bearing carboxylate or phosphonate pendant arm: Crystallographic, thermodynamic, kinetic, redox, and 1H/17O relaxation studies. Inorg. Chem. 2011, 50, 12785–12801. [Google Scholar] [CrossRef] [PubMed]
- Molnár, E.; Camus, N.; Patinec, V.; Rolla, G.A.; Botta, M.; Tircsó, G.; Kálmán, F.K.; Fodor, T.; Tripier, R.; Platas-Iglesias, C. Picolinate-containing macrocyclic Mn2+ complexes as potential MRI contrast agents. Inorg. Chem. 2014, 53, 5136–5149. [Google Scholar] [CrossRef] [PubMed]
- Drahoš, B.; Pniok, M.; Havlíčková, J.; Kotek, J.; Císařová, I.; Hermann, P.; Lukeš, I.; Tóth, É. Mn2+ complexes of 1-oxa-4,7-diazacyclononane based ligands with acetic, phosphonic and phosphinic acid pendant arms: Stability and relaxation studies. Dalton Trans. 2011, 40, 10131–10146. [Google Scholar] [CrossRef] [PubMed]
- Laine, S.; Bonnet, C.S.; Kálmán, F.K.; Garda, Z.; Pallier, A.; Caillé, F.; Suzenet, F.; Tircsó, G.; Tóth, É. Mn2+ complexes of open-chain ligands with a pyridine backbone: Less donor atoms lead to higher kinetic inertness. New J. Chem. 2018, 42, 8012–8020. [Google Scholar] [CrossRef]
- De Sá, A.; Bonnet, C.S.; Geraldes, C.F.G.C.; Tóth, É.; Ferreira, P.M.T.; André, J.P. Thermodynamic stability and relaxation studies of small, triaza-macrocyclic Mn(II) chelates. Dalton Trans. 2013, 42, 4522–4532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forgács, A.; Pujales-Paradela, R.; Regueiro-Figueroa, M.; Valencia, L.; Esteban-Gómez, D.; Botta, M.; Platas-Iglesias, C. Developing the family of picolinate ligands for Mn2+ complexation. Dalton Trans. 2017, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Molnár, E.; Váradi, B.; Garda, Z.; Botár, R.; Kálmán, F.K.; Tóth, É.; Platas-Iglesias, C.; Tóth, I.; Brücher, E.; Tircsó, G. Remarkable differences and similarities between the isomeric Mn(II)-cis- and trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetate complexes. Inorg. Chim. Acta 2018, 472, 254–263. [Google Scholar] [CrossRef]
- Forgács, A.; Tei, L.; Baranyai, Z.; Esteban-Gómez, D.; Platas-Iglesias, C.; Botta, M. Optimising the relaxivities of Mn2+ complexes by targeting human serum albumin (HSA). Dalton Trans. 2017, 8494–8504. [Google Scholar] [CrossRef] [PubMed]
- Vanasschen, C.; Molnár, E.; Tircsó, G.; Kálmán, F.K.; Tóth, É.; Brandt, M.; Coenen, H.H.; Neumaier, B. Novel cdta-based, bifunctional chelators for stable and inert MnII complexation: Synthesis and physicochemical characterization. Inorg. Chem. 2017, 56, 7746–7760. [Google Scholar] [CrossRef] [PubMed]
- Drahoš, B.; Kotek, J.; Hermann, P.; Lukeš, I.; Tóth, É. Mn2+ complexes with pyridine-containing 15-membered macrocycles: Thermodynamic, kinetic, crystallographic, and 1H/17O relaxation studies. Inorg. Chem. 2010, 49, 3224–3238. [Google Scholar] [CrossRef] [PubMed]
- Webplotdigitizer. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 10 August 2018).
- Curveexpert Basic, Version 2.1.0. Available online: http://www.curveexpert.net (accessed on 10 August 2018).




| Organic Ligand | qlita | r1b (s−1·mM−1) | FW | qcalcc | τRd (ps) | τS0d (ps) | τMd (ns) | RMnHd (Å) | |AO/ħ| d (106 rad/s) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| DOTA | 0.0 | 2.76 | 455.3 | 0.4 | - | - | - | - | - | [28] |
| DTPA | 0.0 | 2.40 | 390.2 | 0.4 | - | - | - | - | - | [29] |
| DTPA | 0.0 | 2.30 | 390.2 | 0.4 | - | - | - | - | - | [30] |
| 1,7-DO2A | 0.0 | 2.53 | 341.3 | 0.4 | - | 152 | - | - | - | [31] |
| NOTA | 0.0 | 2.30 | 356.2 | 0.4 | - | - | - | - | - | [28] |
| BCPE | 0.0 | 2.23 | 383.3 | 0.4 | - | 87 | - | - | - | [32] |
| DO3A | 0.0 | 2.27 | 398.3 | 0.4 | - | 117 | - | - | - | [31] |
| AAZTA | 0.0 | 2.53 | 412.3 | 0.4 | - | 148 | - | - | - | [33] |
| c-pC-DTPA | 0.0 | 3.02 | 492.4 | 0.4 | - | 161 | - | - | - | [34] |
| t-pC-DTTA | 0.0 | 2.55 | 510.4 | 0.4 | - | 108 | - | - | - | [34] |
| DO3A(BOM)3 | 0.0 | 2.55 | 758.7 | 0.3 | - | 135 | - | - | - | [35] |
| bis-DO3A1 | 0.0 | 2.27 | 837.8 | 0.3 | - | 93 | - | - | - | [36] |
| bis-DO3A2 | 0.0 | 2.48 | 1340.3 | 0.3 | - | 137 | - | - | - | [36] |
| AAZ3MA | 0.2 | 3.04 | 415.3 | 0.5 | 51.0 | 96 | 7.52 | 2.81 | 8.7 | [33] |
| MeAAZ3A | 0.3 | 3.68 | 387.3 | 0.6 | 50.0 | 140 | 7.94 | 2.81 | 7.9 | [33] |
| AAZ3A | 0.6 | 5.05 | 373.3 | 0.8 | 50.0 | 158 | 21.3 | 2.81 | 7.2 | [33] |
| 1,4-DO2A | 0.9 | 4.50 | 359.3 | 0.7 | 46.0 | 74 | 88.2 | 2.83 | 43.0 | [31] |
| DO1A | 1.0 | 4.04 | 302.3 | 0.7 | 22.0 | 88 | 0.168 | 2.83 | 39.4 | [31] |
| MeNO2A | 1.0 | 4.96 | 330.2 | 0.9 | 36.0 | 101 | 1.60 | 2.77 | 46.0 | [37] |
| pyDO1A | 1.0 | 3.54 | 336.3 | 0.6 | 23.0 | 449 | 0.330 | - | - | [38] |
| NOMPA | 1.0 | 6.21 | 336.3 | 1.1 | 51.2 | 129 | 0.361 | 2.77 | −73.3 | [39] |
| EDTA | 1.0 | 5.41 | 361.2 | 0.9 | 56.0 | 81 | 2.12 | 2.83 | 40.5 | [31] |
| EDTA | 1.0 | 5.41 | 361.2 | 0.9 | 57.0 | 81 | 2.12 | 2.83 | 40.5 | [31] |
| EDTA | 1.0 | 5.81 | 361.2 | 1.0 | - | - | - | - | - | [30] |
| EDTA | 1.0 | 5.60 | 361.2 | 0.9 | - | - | - | - | - | [29] |
| pyDO1P | 1.0 | 3.84 | 371.2 | 0.6 | 38.6 | 36 | 0.565 | - | 39.9 | [38] |
| NO2P | 1.0 | 8.03 | 387.1 | 1.3 | 103.0 | 87 | 83.333 | 2.75 | 33.3 | [40] |
| 2,6-diMePyMe3A | 1.0 | 5.23 | 395.2 | 0.8 | 46.0 | 52 | 0.357 | 2.83 | 26.4 | [41] |
| NODAHep | 1.0 | 8.10 g | 414.4 | 1.3 | 84.0 | 37 | 370 | 2.75 | 30.0 | [42] |
| DPAAA | 1.0 | 6.75 | 415.2 | 1.0 | 47.6 | 146 | 7.94 | 2.76 | 31.5 | [43] |
| c-CDTA | 1.0 | 6.12 | 415.3 | 0.9 | 74.0 | 78 | 4.44 | 2.83 | 42.7 | [44] |
| NODAHA | 1.0 | 7.51 g | 429.3 | 1.1 | 80.0 | 32 | 370 | 2.75 | 30.0 | [42] |
| NODABA | 1.0 | 9.80 g | 449.3 | 1.5 | 121.0 | 37 | 769 | 2.75 | 30.0 | [42] |
| t-pC-EDTA | 1.0 | 5.93 | 467.3 | 0.9 | 75.4 | 80 | - | 2.92 | - | [34] |
| EDTA(BOM) | 1.0 | 5.61 e | 495.3 | 0.8 | 83.7 | 87 | 10.7 | 2.90 | - | [35] |
| 1,4-DO2AMBz | 1.0 | 5.67 | 539.6 | 0.8 | 85.0 | 71 | 5.71 | 2.83 | 33.0 | [45] |
| 4-HET-t-CDTA | 1.0 | 6.72 | 570.4 | 0.9 | 104.9 | 56 | 5.68 | 2.83 | 40.0 | [46] |
| 1,4-BzDO2AM | 1.0 | 5.89 | 595.7 | 0.8 | 96.0 | 60 | 3.95 | 2.83 | 31.0 | [45] |
| EDTA(BOM)2 | 1.0 | 7.25 e | 629.5 | 0.9 | 110.8 | 84 | 7.60 | 2.90 | - | [35] |
| ENOTA | 1.0 | 6.46 f | 658.5 | 0.8 | 85.0 | 4317 | 18.2 | 2.75 | 5.2 | [23] |
| NO2A | 1.4 | 5.67 | 317.2 | 1.0 | 22.0 | 160 | 0.840 | 2.75 | 33.3 | [40] |
| 15pyN5 | 2.0 | 9.64 | 340.3 | 1.7 | 28.3 | 8710 | 14.5 | 2.81 | 38.6 | [47] |
| 15pyN3O2 | 2.0 | 11.52 | 342.3 | 2.0 | 40.3 | 7174 | 263 | 2.81 | 38.6 | [47] |
| 15pydieneN5 | 2.0 | 14.20 | 364.3 | 2.3 | - | - | - | - | - | [30] |
| DPAMA | 2.0 | 11.25 | 390.3 | 1.8 | 47.8 | 167 | 3.27 | 2.74 | - | [32] |
| DPAPhA | 2.0 | 11.65 | 452.3 | 1.7 | 81.0 | 87 | 17.9 | 2.78 | 25.0 | [43] |
| mX(DPAMA)2 | 2.0 | 17.65 | 854.6 | 2.1 | 95.8 | 183 | 32.7 | 2.74 | - | [32] |
| mX(DPAMA)3 | 2.0 | 19.72 | 1243.8 | 2.2 | 136.0 | 173 | 32.7 | 2.74 | - | [32] |
| none | 6.0 | 19.52 h | 163.0 | 5.6 | 30.0 | 26042 | 35.5 | 2.83 | 34.6 | [13] |
| none | 6.0 | 20.98 i | 163.0 | 6.0 | - | - | - | - | - | [30] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, J.A.; Geraldes, C.F.G.C. A Semi-Empirical Method for the Estimation of the Hydration Number of Mn(II)-Complexes. Inorganics 2018, 6, 116. https://doi.org/10.3390/inorganics6040116
Peters JA, Geraldes CFGC. A Semi-Empirical Method for the Estimation of the Hydration Number of Mn(II)-Complexes. Inorganics. 2018; 6(4):116. https://doi.org/10.3390/inorganics6040116
Chicago/Turabian StylePeters, Joop A., and Carlos F. G. C. Geraldes. 2018. "A Semi-Empirical Method for the Estimation of the Hydration Number of Mn(II)-Complexes" Inorganics 6, no. 4: 116. https://doi.org/10.3390/inorganics6040116
APA StylePeters, J. A., & Geraldes, C. F. G. C. (2018). A Semi-Empirical Method for the Estimation of the Hydration Number of Mn(II)-Complexes. Inorganics, 6(4), 116. https://doi.org/10.3390/inorganics6040116