Cysteine Derivatized 99mTc-Labelled Fatty Acids as β-Oxidation Markers
Abstract
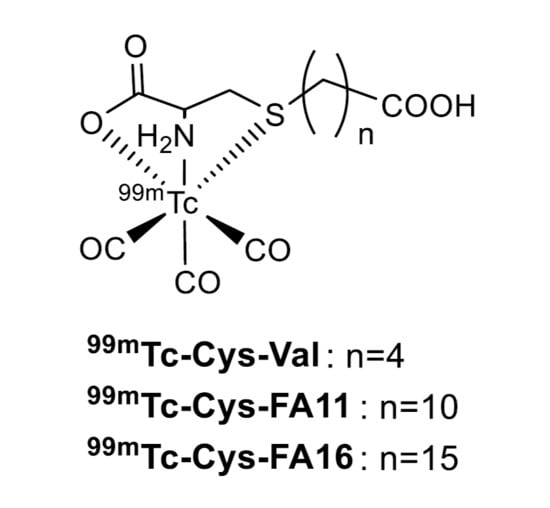
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of 5-((2-Amino-2-Carboxyethyl) Thio) Pentanoic Acid, Cys–Val
3.3. Synthesis of 11-((2-Amino-2-Carboxyethyl) Thio) Undecanoic Acid, Cys–FA11
3.4. Synthesis of 16-((2-Amino-2-Carboxyethyl) Thio) Hexadecanoic Acid, Cys–FA16
3.5. Synthesis of Rhenium Complexes Re–Cys–Val
3.6. Synthesis of 99mTc Complexes
3.7. In Vitro Stability Studies of 99mTc Complexes
3.8. Plasma Stability
3.9. Determination of Distribution Coefficient (Log Do/w)
3.10. Animal Distribution Studies
3.11. Metabolites Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yoshinaga, K.; Tamaki, N. Imaging myocardial metabolism. Curr. Opin. Biotechnol. 2007, 18, 52–59. [Google Scholar] [CrossRef]
- Milger, K.; Herrmann, T.; Becker, C.; Gotthardt, D.; Zickwolf, J.; Ehehalt, R.; Watkins, P.A.; Stremmel, W.; Fullekrug, J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J. Cell Sci. 2006, 119, 4678–4688. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, N.; Morita, K.; Kuge, Y.; Tsukamoto, E. The Role of Fatty Acids in Cardiac Imaging. J. Nucl. Med. 2000, 41, 1525–1534. [Google Scholar]
- Heintz, A.C.; Jung, C.M.; Stehr, S.N.; Mirtschink, P.; Walther, M.; Pietzsch, J.; Bergmann, R.; Pietzsch, H.J.; Spies, H.; Wunderlich, G.; et al. Myocardial uptake and biodistribution of newly designed technetium-labelled fatty acid analogues. Nucl. Med. Commun. 2007, 28, 637–645. [Google Scholar] [CrossRef]
- Eckelman, W.C.; Babich, J.W. Synthesis and validation of fatty acid analogs radiolabeled by nonisotopic substitution. J. Nucl. Cardiol. 2007, 14, S100–S109. [Google Scholar] [CrossRef]
- Herrero, P.; Gropler, R.J. Imaging of myocardial metabolism. J. Nucl. Cardiol. 2005, 12, 345–358. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Wang, S.; Holden, J.E.; Nickles, R.J.; Taylor, M.; Stone, C.K. Synthesis and preliminary evaluation of (18)F-labeled 4-thia palmitate as a PET tracer of myocardial fatty acid oxidation. Nucl. Med. Biol. 2000, 27, 221–231. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Kitapci, M.T.; Wang, S.; Ying, J.; Lopaschuk, G.D. Validation of 18F-fluoro-4-thia-palmitate as a PET probe for myocardial fatty acid oxidation: Effects of hypoxia and composition of exogenous fatty acids. J. Nucl. Med. 2006, 47, 173–181. [Google Scholar] [PubMed]
- Uehara, T.; Uemura, T.; Hirabayashi, S.; Adachi, S.; Odaka, K.; Akizawa, H.; Magata, Y.; Irie, T.; Arano, Y. Technetium-99m-Labeled Long Chain Fatty Acid Analogues Metabolized by β-Oxidation in the Heart. J. Med. Chem. 2007, 50, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Gropler, R.J. Radionuclide Imaging of Myocardial Metabolism. Circ. Cardiovasc. Imaging 2010, 3, 211–222. [Google Scholar] [Green Version]
- DeGrado, T.R.; Holden, J.E.; Ng, C.K.; Raffel, D.M.; Gatley, S.J. Quantitative analysis of myocardial kinetics of 15-p-[iodine-125] iodophenylpentadecanoic acid. J. Nucl. Med. 1989, 30, 1211–1218. [Google Scholar] [PubMed]
- Reske, S.N.; Sauer, W.; Machulla, H.J.; Knust, J.; Winkler, C. Metabolism of 15 (p123I-iodophenyl-) pentadecanoic acid in heart muscle and noncardiac tissues. Eur. J. Nucl. Med. 1985, 10, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Dormehl, I.C.; Hugo, N.; Rossouw, D.; White, A.; Feinendegen, L.E. Planar myocardial imaging in the baboon model with iodine-123-15-(iodophenyl) pentadecanoic acid (IPPA) and iodine-123-15-(iodophenyl)-3-R,S-methylpentadecanoic acid (BMIPP), using time-activity curves for evaluation of metabolism. Nucl. Med. Biol. 1995, 22, 837–847. [Google Scholar] [CrossRef]
- Ambrose, K.R.; Owen, B.A.; Goodman, M.M.; Knapp, F.F.J. Evaluation of the metabolism in rat hearts of two new radioiodinated 3-methyl-branched fatty acid myocardial imaging agents. Eur. J. Nucl. Med. 1987, 12, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.M.; Kirsch, G.; Knapp, F.F.J. Synthesis and evaluation of radioiodinated terminal p-iodophenyl-substituted alpha- and beta-methyl-branched fatty acids. J. Med. Chem. 1984, 27, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, W.C.; Karesh, S.M.; Reba, R.C. New compounds: Fatty acid and long chain hydrocarbon derivatives containing a strong chelating agent. J. Pharm. Sci. 1975, 64, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Karesh, S.M.; Eckelman, W.C.; Reba, R.C. Biological Distribution of Chemical Analogs of Fatty Acids and Long Chain Hydrocarbons Containing a Strong Chelating Agent. J. Pharm. Sci. 1977, 66, 225–228. [Google Scholar] [CrossRef]
- Astheimer, L.; Linse, K.H.; Ramamoorthy, N.; Schwochau, K. Synthesis, Characterization and Evaluation of 99Tc/99mTc DIARS and DMPE Complexes Containing Pentadecanoic Acid. Nucl. Med. Biol. 1987, 14, 545–553. [Google Scholar] [CrossRef]
- Liang, F.H.; Virzi, F.; Hnatowich, D.J. The Use of Diaminodithiol for Labeling Small Molecules with Technetium-99m. Nucl. Med. Biol. 1987, 14, 63–67. [Google Scholar] [CrossRef]
- Bumbalova, A.; Havranek, E.; Harangozo, M.; Krenek, P.; Lubkeova, S. Preparation and biodistribution of 99m Tc-HT18. J. Radioanal. Nucl. Chem. Lett. 1991, 154, 53–59. [Google Scholar] [CrossRef]
- Mach, R.H.; Kung, H.F.; Jungwiwattanaporn, P.; Guo, Y.Z. Synthesis and Biodistribution of a New Class of 99mTc-labeled Fatty Acid Analogs for Myocardial Imaging. Nucl. Med. Biol. 1991, 18, 215–226. [Google Scholar] [CrossRef]
- Jones, G.S., Jr.; Elmaleh, D.R.; Strauss, H.W.; Fischman, A.J. Synthesis and Biodistribution of a New 99mTechnetium Fatty Acid. Nucl. Med. Biol. 1994, 21, 117–123. [Google Scholar] [CrossRef]
- Jones, G.S., Jr.; Elmaleh, D.R.; Strauss, H.W.; Fischman, A.J. 7,10-Bis(2-Mercapto-2-Methyl)Propyl-7,10-Diazapalmitic Acid: A novel, N2S2 Ligand For Technetium-99m. Bioorg. Med. Chem. Lett. 1996, 6, 2399–2404. [Google Scholar] [CrossRef]
- Yamamura, N.; Magata, Y.; Arano, Y.; Kawaguchi, T.; Ogawa, K.; Konishi, J.; Saji, H. Technetium-99m-Labeled Medium-Chain Fatty Acid Analogues Metabolized by β-Oxidation: Radiopharmaceutical for Assessing Liver Function. Bioconjugate Chem. 1999, 10, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.M.; Kraus, W.; Leibnitz, P.; Pietzsch, H.J.; Kropp, J.; Spies, H. Syntheses and First Crystal Structures of Rhenium Complexes Derived from ω-Functionalized Fatty Acids as Model Compounds of Technetium Tracers for Myocardial Metabolism Imaging. Eur. J. Inorg. Chem. 2002, 2002, 1219–1225. [Google Scholar] [CrossRef]
- Maresca, K.P.; Shoup, T.M.; Femia, F.J.; Burker, M.A.; Fischman, A.; Babich, J.W.; Zubieta, J. Synthesis, characterization, and biodistribution of a Technetium-99m ‘3+1’ fatty acid derivative. The crystal and molecular structures of a series of oxorhenium model complexes. Inorg. Chim. Acta 2002, 338, 149–156. [Google Scholar] [CrossRef]
- Chu, T.; Zhang, Y.; Liu, X.; Wang, Y.; Hu, S.; Wang, X. Synthesis and biodistribution of 99mTc-carbonyltechnetiumlabeled fatty acids. Appl. Rad. Isot. 2004, 60, 845–850. [Google Scholar] [CrossRef]
- Lee, B.C.; Choe, Y.S.; Chi, D.Y.; Paik, J.Y.; Lee, K.H.; Choi, Y.; Kim, B.T. 8-Cyclopentadienyltricarbonyl 99mTc 8-Oxooctanoic Acid: A Novel Radiotracer for Evaluation of Medium Chain Fatty Acid Metabolism in the Liver. Bioconjugate Chem. 2004, 15, 121–127. [Google Scholar] [CrossRef]
- Magata, Y.; Kawaguchi, T.; Ukon, M.; Yamamura, N.; Uehara, T.; Ogawa, K.; Arano, Y.; Temma, T.; Mukai, T.; Tadamura, E.; et al. A Tc-99m-Labeled Long Chain Fatty Acid Derivative for Myocardial Imaging. Bioconjugate Chem. 2004, 15, 389–393. [Google Scholar] [CrossRef]
- Mathur, A.; Mallia, M.B.; Subramanian, S.; Banerjee, S.; Kothari, K.; Sarma, H.D.; Venkatesh, M. Synthesis and bio evaluation of a new fatty acid derivative labelled with technetium-99m. J. Label Compd. Radiopharm. 2006, 49, 1053–1060. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, D.H.; Lee, J.H.; Sung, H.J.; Choe, Y.S.; Chi, D.Y.; Lee, K.H.; Choi, Y.; Kim, B.T. 99mTc(CO)3-15-[N-(Acetyloxy)-2-picolylamino]pentadecanoic Acid: A Potential Radiotracer for Evaluation of Fatty Acid Metabolism. Bioconjugate Chem. 2007, 18, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Walther, M.; Jung, C.M.; Bergmann, R.; Pietzsch, J.; Rode, K.; Fahmy, K.; Mirtschink, P.; Stehr, S.; Heintz, A.; Wunderlich, G. Spies, Synthesis and Biological Evaluation of a New Type of 99mTechnetium-Labeled Fatty Acid for Myocardial Metabolism Imaging. Bioconjugate Chem. 2007, 18, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, Q.; Wang, H.; Wang, H.; Wang, D.; Fang, Y.; Zhang, H. Synthesis and bio-evaluation of Tc-99m-labeled fatty acid derivatives for myocardial metabolism imaging. Appl. Organometal. Chem. 2016, 30, 596–604. [Google Scholar] [CrossRef]
- Tsotakos, T.; Tsoukalas, C.; Patsis, G.; Panagiotopoulou, A.; Nikolić, N.; Janković, D.; Djokić, D.; Raptopoulou, C.P.; Terzis, A.; Papagiannopoulou, D.; et al. Benzimidazole derivatives as NSO ligands for the fac-[M(CO)3]+ (M = Re, 99mTc). Inorg. Chim. Acta 2011, 377, 62–68. [Google Scholar] [CrossRef]
- Cazzola, E.; Benini, E.; Pasquali, M.; Mirtschink, P.; Walther, M.; Pietzsch, H.J.; Uccelli, L.; Boschi, A.; Bolzati, C.; Duatti, A. Labeling of Fatty Acid Ligands with the Strong Electrophilic Metal Fragment [99mTc(N)(PNP)]2+ (PNP=Diphosphane Ligand). Bioconjugate Chem. 2008, 19, 450–460. [Google Scholar] [CrossRef]
- Mirtschink, P.; Stehr, S.N.; Pietzsch, H.J.; Bergmann, R.; Pietzsch, J.; Wunderlich, G.; Heintz, A.C.; Kropp, J.; Spies, H.; Kraus, W.; et al. Modified “4+1” Mixed Ligand Technetium-Labeled Fatty Acids for Myocardial Imaging: Evaluation of Myocardial Uptake and Biodistribution. Bioconjugate Chem. 2008, 19, 97–108. [Google Scholar] [CrossRef]
- Mathur, A.; Subramanian, S.; Mallia, M.B.; Banerjee, S.; Samuel, G.; Sarma, H.D.; Venkatesh, M. Synthesis and bio-evaluation of a new fatty acid derivative for myocardial imaging. Bioorg. Med. Chem. 2008, 16, 7927–7931. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, D.H.; Lee, I.; Choe, Y.S.; Chi, D.Y.; Lee, K.H.; Choi, Y.; Kim, B.T. 16-Cyclopentadienyl Tricarbonyl 99mTc 16-Oxo-hexadecanoic Acid: Synthesis and Evaluation of Fatty Acid Metabolism in Mouse Myocardium. J. Med. Chem. 2008, 51, 3630–3634. [Google Scholar] [CrossRef]
- Mirtschink, P.; Stehr, S.N.; Walther, M.; Pietzsch, J.; Bergmann, R.; Pietzsch, H.J.; Weichsel, J.; Pexa, A.; Dieterich, P.; Wunderlich, G.; et al. Validation of 99mTc-labeled “4+1” fatty acids for myocardial metabolism and flow imaging Part 1: Myocardial extraction and biodistribution. Nucl. Med. Biol. 2009, 36, 833–843. [Google Scholar] [CrossRef]
- Mirtschink, P.; Stehr, S.N.; Walther, M.; Pietzsch, J.; Bergmann, R.; Pietzsch, H.J.; Weichsel, J.; Pexa, A.; Dieterich, P.; Wunderlich, G.; et al. Validation of 99mTc-labeled “4+1” fatty acids for myocardial metabolism and flow imaging Part 2. Subcellular distribution. Nucl. Med. Biol. 2009, 36, 845–852. [Google Scholar] [CrossRef]
- Mathur, A.; Mallia, M.B.; Sarma, H.D.; Banerjee, S.; Venkatesh, M. Evaluation of new positively charged 11- and 12-carbon 99mTc-labeled fatty acid derivatives for myocardial imaging. J. Label. Compd. Radiopharm. 2010, 53, 580–585. [Google Scholar] [CrossRef]
- Mathur, A.; Mallia, M.B.; Sarma, H.D.; Banerjee, S.; Venkatesh, M. Synthesis, radiolabeling and evaluation of a new positively charged 99mTc-labeled fatty acid derivative for myocardial imaging. J. Label. Compd. Radiopharm. 2011, 54, 150–156. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, H.; Wu, X.; Chao, F.; Yu, G.; Zhang, L.; Jiang, H.; Liu, H.; Hou, H.; Zhan, H.; et al. Preliminary studies of a novel cyclopentadienyl tricarbonyl technetium-99m fatty acid derivative for myocardical imaging. J. Label. Compd. Radiopharm. 2013, 56, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, H. Synthesis and biological evaluation of fatty acids conjugates bearing cyclopentadienyl-donors incorporated [99mTc/Re(CO)3]+ for myocardical imaging. Eur. J. Med. Chem. 2014, 72, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Karagiorgou, O.; Patsis, G.; Pelecanou, M.; Raptopoulou, C.P.; Terzis, A.; Siatra-Papastaikoudi, T.; Alberto, R.; Pirmettis, I.; Papadopoulos, M. (S)-(2-(2′-Pyridyl)Ethyl)Cysteamine and (S)-(2-(2′-Pyridyl)Ethyl)-d,l-Homocysteine as Ligands for the “fac-[M(CO)3]+” (M = Re, 99mTc). Core. Inorg. Chem. 2005, 44, 4118–4120. [Google Scholar] [CrossRef] [PubMed]
- Pirmettis, I.; Arano, Y.; Tsotakos, T.; Okada, K.; Yamaguchi, A.; Uehara, T.; Morais, M.; Correia, J.D.G.; Santos, I.; Martins, M.; et al. New 99mTc(CO)3 Mannosylated Dextran Bearing S-Derivatized Cysteine Chelator for Sentinel Lymph Node Detection. Mol. Pharm. 2012, 9, 1681–1692. [Google Scholar] [CrossRef]
- Forch, J.; Less, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Schmitz, B.; Reske, S.N.; Machulla, H.J.; Egge, H.; Winkler, C. Cardiac metabolism of omega-(p-iodo-phenyl)-pentadecanoic acid: A gas-liquid chromatographic-mass spectrometric analysis. J. Lipid Res. 1984, 25, 1102–1108. [Google Scholar]
- Kropp, J.; Ambrose, K.R.; Knapp, F.F., Jr.; Nissen, H.P.; Biersack, H.J. Incorporation of radioiodinated IPPA and BMIPP fatty acid analogues into complex lipids from isolated rat hearts. Int. J. Radiat. Appl. Instrum. 1992, 19, 283–288. [Google Scholar] [CrossRef]
- Eisenhut, M.; Lehmann, W.D.; Sutterle, A. Metabolism of 15-(4’-[123I]iodophenyl)pentadecanoic acid ([123I]IPPA) in the rat heart; identification of new metabolites by high pressure liquid chromatography and fast atom bombardment-mass spectrometry. Nucl. Med. Biol. 1993, 20, 747–754. [Google Scholar] [CrossRef]
- Yamamichi, Y.; Kusuoka, H.; Morishita, K.; Shirakami, Y.; Kurami, M.; Okano, K.; Itoh, O.; Nishimura, T. Metabolism of iodine-123-BMIPP in perfused rat hearts. J. Nucl. Med. 1995, 36, 1043–1050. [Google Scholar] [PubMed]
- Alberto, R.; Egli, A.; Abram, U.; Hegetschweiler, K.; Gramlich, V.; Schubiger, P.A. Synthesis and reactivity of [NEt4]2[ReBr3(CO)3]. Formation and structural characterization of the clusters [NEt4][Re3(μ3-OH)(μ-OH)3(CO)9] and [NEt4][Re2(μ-OH)3(CO)6] by alkaline titration. J. Chem. Soc. Dalton Trans. 1994, 2815–2820. [Google Scholar] [CrossRef]
- Alberto, R.; Schibli, R.; Egli, A.; Schubiger, A.P.; Abram, U.; Kaden, T.A. A Novel Organometallic Aqua Complex of Technetium for the Labeling of Biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]− in Aqueous Solution and Its Reaction with a Bifunctional Ligand. J. Am. Chem. Soc. 1998, 120, 7987–7988. [Google Scholar] [CrossRef]
- Jiracek, J.; Collinsova, M.; Rosenberg, I.; Budesinsky, M.; Protivinska, E.; Netusilova, H.; Garrow, T.A. S-Alkylated Homocysteine Derivatives: New Inhibitors of Human Betaine-Homocysteine S-Methyltransferase. J. Med. Chem. 2006, 49, 3982–3989. [Google Scholar] [CrossRef] [Green Version]







| Cys–Val | Re–Cys–Val | Cys–FA11 | Re–Cys–FA11 | Cys–FA16 | Re–Cys–FA16 | |||
|---|---|---|---|---|---|---|---|---|
| H-2 | 2.20 | 2.25 | H-2 | 2.18 | 2.17 | H-2 | 2.28 | 2.27 |
| H-3 | 1.57 | 1.63 | H-3 | 1.47 | 1.48 | H-3 | 1.50 | 1.49 |
| H-4 | 1.52 | 1.69 | H4, H4a–H4c | 1.24 | 1.25 | H4, H-4a–H-4h | 1.23 | 1.25 |
| H-5 | 2.54 | 2.90 | H-4d | 1.24 | 1.39 | H-4i | 1.23 | 1.36 |
| H-6 | 2.71, 2.97 | 2.72, 2.89 | H-4e | 1.32 | 1.60 | H-4j | 1.29 | 1.63 |
| H-7 | 3.28 | 4.09 | H-4f | 1.49 | 1.50 | H-4k | 1.49 | 1.49 |
| NH2 | not visible | 6.09, 4.91 | H-5 | 2.54 | 2.51 | H-5 | 2.53 | 2.49 |
| OH | not visible | 12.01 | H-6 | 2.95, 2.81 | 2.98, 2.70 | H-6 | 2.98, 2.84 | 2.87, 2.68 |
| H-7 | 3.30 | 4.10 | H-7 | 3.29 | 4.09 | |||
| NH2 | not visible | 6.14, 4.95 | OH | 8.54 | 11.83 | |||
| OH | not visible | 11.82 | NH2 | - | 6.13, 4.95 |
| Cys–Val | Re–Cys–Val | Cys–FA11 | Re–Cys–FA11 | Cys–FA16 | Re–Cys–FA16 | |||
|---|---|---|---|---|---|---|---|---|
| C-1 | 174.92 | 173.96 | C-1 | 174.44 | 174.37 | C-1 | 173.39 | 173.32 |
| C-2 | 33.65 | 32.87 | C-2 | 33.61 | 33.54 | C-2 | 33.50 | 33.18 |
| C-3 | 23.65 | 23.21 | C-3 | 24.40 | 24.35 | C-3 | 24.12 | 24.33 |
| C-4 | 28.28 | 27.07 | C4, C4a–C4d | 28.41–28.79 | 28.23–28.59 | C-4, C-4a–C-4h | 28.92–28.35 | 28.89–28.56 |
| C-5 | 30.54 | 32.04 | C-4e | 28.05 | 27.69 | C-4i | ″ | 28.34 |
| C-6 | 33.33 | 36.22 | C-4f | 28.58 | 28.51 | C-4j | ″ | 27.73 |
| C-7 | 53.58 | 57.62 | C-5 | 30.99 | 31.66 | C-4l | 28.81 | 28.76 |
| C-8 | 168.77 | 177.13 | C-6 | 31.13 | 32.22 | C-5 | 31.15 | 31.59 |
| C≡O | - | 193.36, 195.35 | C-7 | 52.28 | 56.81 | C-6 | 31.16 | 32.30 |
| C-8 | 168.39 | 176.96 | C-7 | 52.25 | 56.98 | |||
| C≡O | - | 193.23, 195.29 | C-8 | 168.58 | 177.16 | |||
| C≡O | - | 195.45, 193.20 |
| 99mTc Complexes | Incubation Time | Protein Binding | Stability of 99mTc Complexes |
|---|---|---|---|
| 99mTc–Cys–FA11 | 15 min | 16.2% ± 1.1% | ≥98% |
| 2 h | 38.2% ± 3% | ≥98% | |
| 99mTc–Cys–FA16 | 15 min | 13.8% ± 0.6% | ≥98% |
| 2 h | 29.2% ± 4.3% | ≥98% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsotakos, T.; Triantis, C.; Kiritsis, C.; Panagiotopoulou, A.; Psimadas, D.; Kyprianidou, P.; Pelecanou, M.; Papadopoulos, M.; Pirmettis, I. Cysteine Derivatized 99mTc-Labelled Fatty Acids as β-Oxidation Markers. Inorganics 2019, 7, 133. https://doi.org/10.3390/inorganics7110133
Tsotakos T, Triantis C, Kiritsis C, Panagiotopoulou A, Psimadas D, Kyprianidou P, Pelecanou M, Papadopoulos M, Pirmettis I. Cysteine Derivatized 99mTc-Labelled Fatty Acids as β-Oxidation Markers. Inorganics. 2019; 7(11):133. https://doi.org/10.3390/inorganics7110133
Chicago/Turabian StyleTsotakos, Theodoros, Charalambos Triantis, Christos Kiritsis, Aggeliki Panagiotopoulou, Dimitrios Psimadas, Patricia Kyprianidou, Maria Pelecanou, Minas Papadopoulos, and Ioannis Pirmettis. 2019. "Cysteine Derivatized 99mTc-Labelled Fatty Acids as β-Oxidation Markers" Inorganics 7, no. 11: 133. https://doi.org/10.3390/inorganics7110133
APA StyleTsotakos, T., Triantis, C., Kiritsis, C., Panagiotopoulou, A., Psimadas, D., Kyprianidou, P., Pelecanou, M., Papadopoulos, M., & Pirmettis, I. (2019). Cysteine Derivatized 99mTc-Labelled Fatty Acids as β-Oxidation Markers. Inorganics, 7(11), 133. https://doi.org/10.3390/inorganics7110133