Synthesis of Anthraquinones by Iridium-Catalyzed [2 + 2 + 2] Cycloaddition of a 1,2-Bis(propiolyl)benzene Derivative with Alkynes
Abstract
:1. Introduction
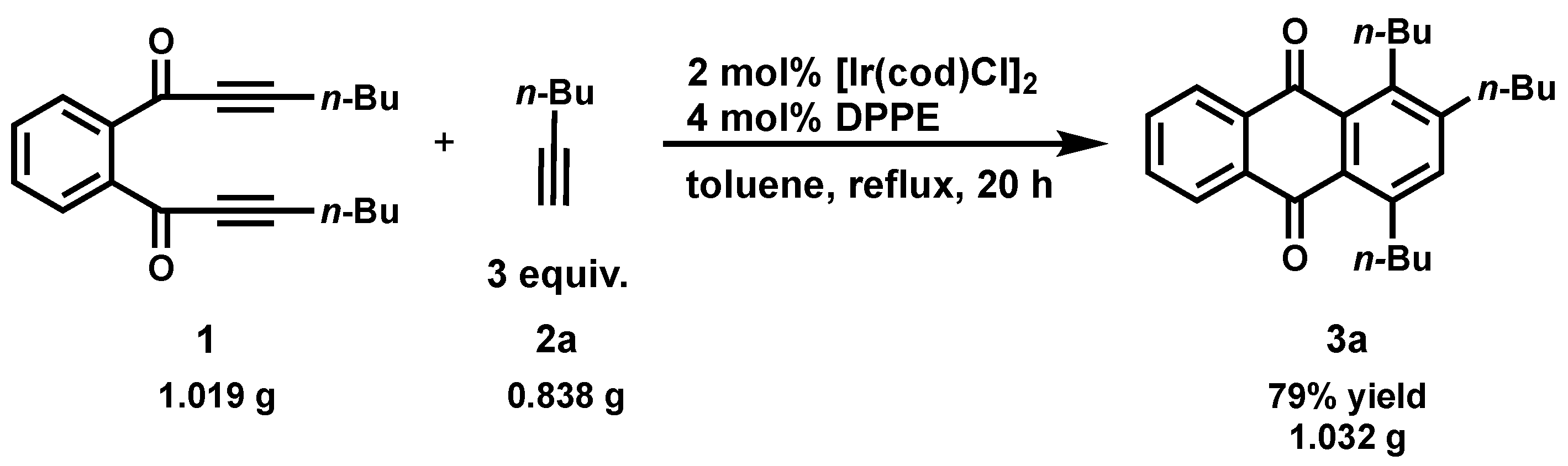
2. Results and Discussion
3. Materials and Methods
3.1. General Methods and Materials
3.2. Preparation of Anthraquinone 1
3.3. Preparation of Fluorenone 4
3.4. General Procedure for the Reaction of 1,2-Bis(propiolyl)benzene Derivative 1 with Alkyne 2
3.5. Characterization of 3a–3l
3.6. Procedure for the Reaction of Diyne 4 with Alkyne 2j
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cichewicz, R.H.; Zhang, Y.; Seeram, N.P.; Nair, M.G. Inhibition of Human Tumor Cell Proliferation by Novel Anthraquinones from Daylilies. Life Sci. 2004, 74, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Gericke, K.M.; Schuberth, I. Synthesis of Highly Functionalized Anthraquinones and Evaluation of Their Antitumor Activity. Eur. J. Org. Chem. 2007, 4563–4577. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Rachtawee, P.; Srichomthong, K.; Srikitikulchai, P.; Kongsaeree, P.; Prabpai, S. Cytotoxic Hydroanthraquinones from the Mangrove-Derived Fungus Paradictyoarthrinium Diffractum BCC 8704. J. Antibiot. 2015, 68, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.-H.; Mack, F.; Debbab, A.; Aly, A.H.; Dicato, M.; Proksch, P.; Diederich, M. Anticancer Effect of Altersolanol A, a Metabolite Produced by the Endophytic Fungus Stemphylium Globuliferum, Mediated by Its Pro-Apoptotic and Anti-Invasive Potential via the Inhibition of NF-κB Activity. Bioorg. Med. Chem. 2013, 21, 3850–3858. [Google Scholar] [CrossRef]
- Shrestha, J.P.; Fosso, M.Y.; Bearss, J.; Chang, C.-W.T. Synthesis and Anticancer Structure Activity Relationship Investigation of Cationic Anthraquinone Analogs. Eur. J. Med. Chem. 2014, 77, 96–102. [Google Scholar] [CrossRef]
- Shrestha, J.P.; Subedi, Y.P.; Chen, L.; Chang, C.-W.T. A Mode of Action Study of Cationic Anthraquinone Analogs: A New Class of Highly Potent Anticancer Agents. Med. Chem. Commun. 2015, 6, 2012–2022. [Google Scholar] [CrossRef]
- Fosso, M.Y.; Chan, K.Y.; Gregory, R.; Chang, C.-W.T. Library Synthesis and Antibacterial Investigation of Cationic Anthraquinone Analogs. ACS Comb. Sci. 2012, 14, 231–235. [Google Scholar] [CrossRef]
- Ondeyka, J.; Buevich, A.V.; Williamson, R.T.; Zink, D.L.; Polishook, J.D.; Occi, J.; Vicente, F.; Basilio, A.; Bills, G.F.; Donald, R.G.K.; et al. Isolation, Structure Elucidation, and Biological Activity of Altersolanol P Using Staphylococcus Aureus Fitness Test Based Genome-Wide Screening. J. Nat. Prod. 2014, 77, 497–502. [Google Scholar] [CrossRef]
- van Gorkom, B.A.P.; de Vries, E.G.E.; Karrenbeld, A.; Kleibeuker, J.H. Review Article: Anthranoid Laxatives and Their Potential Carcinogenic Effects. Aliment. Pharmacol. Ther. 1999, 13, 443–452. [Google Scholar] [CrossRef]
- Davis, R.H.; Agnew, P.S.; Shapiro, E. Antiarthritic Activity of Anthraquinones Found in Aloe for Podiatric Medicine. J. Am. Podiatr. Med. Assoc. 1986, 76, 61–66. [Google Scholar] [CrossRef]
- Wuthi-udomlert, M.; Kupittayanant, P.; Gritsanapan, W. In Vitro Evaluation of Antifungal Activity of Anthraquinone Derivatives of Senna Alata. J. Health Res. 2010, 24, 117–122. [Google Scholar]
- Seo, E.J.; Ngoc, T.M.; Lee, S.-M.; Kim, Y.S.; Jung, Y.-S. Chrysophanol-8-O-glucoside, an Anthraquinone Derivative in Rhubarb, Has Antiplatelet and Anticoagulant Activities. J. Pharmacol. Sci. 2012, 118, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gan, K.-H.; Teng, C.-H.; Lin, H.-C.; Chen, K.-T.; Chen, Y.-C.; Hsu, M.-F.; Wang, J.-P.; Teng, C.-M.; Lin, C.-N. Antiplatelet Effect and Selective Binding to Cyclooxygenase by Molecular Docking Analysis of 3-Alkylaminopropoxy-9,10-anthraquinone Derivatives. Biol. Pharm. Bull. 2008, 31, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.C.; Verrier, J.D.; Kochanek, P.M. Anthraquinone-2-sulfonic Acid (AQ2S) is a Novel Neurotherapeutic Agent. Cell Death Dis. 2013, 4, 451. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook Beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef]
- Hattori, M.; Seidel, A. Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Wiley: Hoboken, NJ, USA, 2005; Volume 9, pp. 300–349. [Google Scholar]
- Malik, E.M.; Baqi, Y.; Müller, C.E. Syntheses of 2-Substituted 1-Amino-4-bromoanthraquinones (Bromaminic Acid Analogues)–Precursors for Dyes and Drugs. Beilstein J. Org. Chem. 2015, 11, 2326–2333. [Google Scholar] [CrossRef]
- Edward, R. Use of DNA-Specific Anthraquinone Dye to Directly Reveal Cytoplasmic and Nuclear Boundaries in Live and Fixes Cells. Mol. Cells 2009, 27, 391–396. [Google Scholar] [CrossRef]
- Hart, P.W.; Rudie, A.W. Anthraquinone a Review of the Rise and Fall of a Pulping Catalyst. TAPPI J. 2014, 13, 23–31. [Google Scholar] [CrossRef]
- Kampmann, B.; Lian, Y.; Klinkel, K.L.; Vecchi, P.A.; Quiring, H.L.; Soh, C.C.; Sykes, A.G. Luminescence and Structural Comparisons of Strong-Acid Sensor Molecules. 2. J. Org. Chem. 2002, 67, 3878–3883. [Google Scholar] [CrossRef]
- Tandon, P.K.; Gayatri; Sahgal, S.; Srivastava, M.; Singh, S.B. Catalysis by Ir (III), Rh (III) and Pd (II) Metal Ions in the Oxidation of Organic Compounds with H2O2. Appl. Organomet. Chem. 2007, 21, 135–138. [Google Scholar] [CrossRef]
- Charleton, K.D.M.; Prokopchuk, E.M. Coordination Complexes as Catalysts: The Oxidation of Anthracene by Hydrogen Peroxide in the Presence of VO(acac)2. J. Chem. Educ. 2011, 88, 1155–1157. [Google Scholar] [CrossRef]
- Thomson, R. The Chemistry of the Quinoid Compounds, Part I and II; Patai, S., Ed.; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Tietze, L.F.; Güntner, C.; Gericke, K.M.; Schuberth, I.; Bunkoczi, G. Diels–Alder Reaction for the Total Synthesis of the Novel Antibiotic Antitumor Agent Mensacarcin. Eur. J. Org. Chem. 2005, 2459–2467. [Google Scholar] [CrossRef]
- Amatore, M.; Aubert, C. Recent Advances in Stereoselective [2+2+2] Cycloadditions. Eur. J. Org. Chem. 2015, 265–286. [Google Scholar] [CrossRef]
- Tanaka, K. (Ed.) Transition-Metal-Mediated Aromatic Ring Construction; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Broere, D.L.J.; Ruijter, E. Recent Advances in Transition-Metal-Catalyzed [2+2+2] Cyclo(co)trimerization Reactions. Synthesis 2012, 44, 2639–2672. [Google Scholar] [CrossRef]
- Shibata, Y.; Tanaka, K. Rhodium-Catalyzed [2+2+2] Cycloaddition of Alkynes for the Synthesis of Substituted Benzenes: Catalysts, Reaction Scope, and Synthetic Applications. Synthesis 2012, 44, 323–350. [Google Scholar] [CrossRef]
- Dominguez, G.; Pérez-Castells, J. Recent Advances in [2+2+2] Cycloaddition Reactions. Chem. Soc. Rev. 2011, 40, 3430–3444. [Google Scholar] [CrossRef]
- Agenet, N.; Buisine, O.; Slowinski, F.; Gandon, V.; Aubert, C.; Malacria, M. Cotrimerizations of Acetylenic Compounds. Org. React. 2007, 68, 1–302. [Google Scholar] [CrossRef]
- Wagner, F.; Meier, H. Thermische und Photochemische Umsetzungen von Acetylenen mit Metallcarbonylen–IV: Cycloadditionen von Bis[α-oxo-alkinen] in Gegenwart von Nicikeltetracarbonyl. Tetrahedron 1974, 30, 773–780. [Google Scholar] [CrossRef]
- Müller, E. The Diyne Reaction of 1,4-, 1,5-, 1,6-, and 1,7-Diynes via Transition Metal Complexes to New Compounds. Synlett 1974, 761–774. [Google Scholar] [CrossRef]
- Müeller, E.; Scheller, A.; Winter, W.; Wagner, F.; Meier, H. Diyne Reactions. XXXVII. Thermal and Photochemical Reactions of Acetylenes with Metal Carbonyls. V. Quinone Synthesis from o-Bis(α-oxoalkynes) with Dicarbonylbis(triphenylphosphine) Nickel (0). Chem.-Ztg. 1975, 99, 155–157. [Google Scholar]
- Hillard, R.L., III; Vollhardt, K.P.C. Substituted Benzocyclobutenes, Indans, and Tetralins via Cobalt-Catalyzed Cooligomerization of α, ω-Diynes with Substituted Acetylenes. Formation and Synthetic Utility of Trimethylsilylated Benzocycloalkenes. J. Am. Chem. Soc. 1977, 99, 4058–4069. [Google Scholar] [CrossRef]
- McDonald, F.E.; Zhu, H.Y.H.; Holmquist, C.R. Rhodium-Catalyzed Alkyne Cyclotrimerization Strategies for C-Arylglycoside Synthesis. J. Am. Chem. Soc. 1995, 117, 6605–6606. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hata, K.; Arakawa, T.; Itoh, K. Ru(II)-Catalyzed [2+2+2] Cycloaddition of 1,2-Bis(propiolyl)benzenes with Monoalkynes Leading to Substituted Anthraquinones. Chem. Commun. 2003, 1290–1291. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saigoku, T.; Ohgai, T.; Nishiyama, H.; Itoh, K. Synthesis of C-Arylglycosides via Ru(II)-Catalyzed [2+2+2] Cycloaddition. Chem. Commun. 2004, 2702–2703. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Arakawa, T.; Itoh, K. Synthesis of Naphthoquinone-Fused Cyclobutadiene Ruthenium Complexes. Organometallics 2004, 23, 3610–3614. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saigoku, T.; Nishiyama, H.; Ohgai, T.; Itoh, K. Selective Synthesis of C-Arylglycosides via Cp*RuCl-Catalyzed Partially Intramolecular Cyclotrimerizations of C-alkynylglycosides. Org. Biomol. Chem. 2005, 3, 1768–1775. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hattori, K.; Ishii, J.; Nishiyama, H. Synthesis of Arylboronates via Cp*RuCl-Catalyzed Cycloaddition of Alkynylboronates. Tetrahedron 2006, 62, 4294–4305. [Google Scholar] [CrossRef]
- Tanaka, K.; Suda, T.; Noguchi, K.; Hirano, M. Catalytic [2+2+2] and Thermal [4+2] Cycloaddition of 1,2-Bis(arylpropiolyl)benzenes. J. Org. Chem. 2007, 72, 2243–2246. [Google Scholar] [CrossRef]
- Kezuka, S.; Okado, T.; Niou, E.; Takeuchi, R. Iridium Complex-Catalyzed Reaction of 1,6-Enynes: Cycloaddition and Cycloisomerization. Org. Lett. 2005, 7, 1711–1714. [Google Scholar] [CrossRef]
- Kezuka, S.; Tanaka, S.; Ohe, T.; Nakaya, Y.; Takeuchi, R. Iridium Complex-Catalyzed [2+2+2] Cycloaddition of α, ω-Diynes with Monoynes and Monoenes. J. Org. Chem. 2006, 71, 543–552. [Google Scholar] [CrossRef]
- Onodera, G.; Matsuzawa, M.; Aizawa, T.; Kitahara, T.; Shimizu, Y.; Kezuka, S.; Takeuchi, R. [Ir(cod)Cl]2 /FDPPE-Catalyzed Chemo- and Regioselective Cyclotrimerization of Two Different Terminal Alkynes to Give 1,3,5-Trisubstituted Benzenes. Synlett 2008, 755–758. [Google Scholar] [CrossRef]
- Onodera, G.; Shimizu, Y.; Kimura, J.; Kobayashi, J.; Ebihara, Y.; Kondo, K.; Sakata, K.; Takeuchi, R. Iridium-Catalyzed [2+2+2] Cycloaddition of α, ω -Diynes with Nitriles. J. Am. Chem. Soc. 2012, 134, 10515–10531. [Google Scholar] [CrossRef]
- Onodera, G.; Suto, M.; Takeuchi, R. Iridium-Catalyzed [2+2+2] Cycloaddition of α,ω-Diynes with Isocyanates. J. Org. Chem. 2012, 77, 908–920. [Google Scholar] [CrossRef]
- Hashimoto, T.; Okabe, A.; Mizuno, T.; Izawa, M.; Takeuchi, R. Iridium-Catalyzed [2+2+2] Cycloaddition of α, ω-Diynes with Alkynyl Ketones and Alkynyl Esters. Tetrahedron 2014, 70, 8681–8689. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ishii, S.; Yano, R.; Miura, H.; Sakata, K.; Takeuchi, R. Iridium-Catalyzed [2+2+2] Cycloaddition of α, ω-Diynes with Cyanamides. Adv. Synth. Catal. 2015, 357, 3901–3916. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kato, K.; Yano, R.; Natori, T.; Miura, H.; Takeuchi, R. Iridium-Catalyzed Synthesis of Acylpyridines by [2+2+2] Cycloaddition of Diynes with Acyl Cyanides. J. Org. Chem. 2016, 81, 5393–5400. [Google Scholar] [CrossRef]
- Takeuchi, R.; Fujisawa, S.; Yoshida, Y.; Sagano, J.; Hashimoto, T.; Matsunami, A. Synthesis of Multisubstituted Azatriphenylenes by Iridium-Catalyzed [2+2+2] Cycloaddition of Biaryl-Linked Diynes with Nitriles. J. Org. Chem. 2018, 83, 1852–1860. [Google Scholar] [CrossRef]
- Paneque, M.; Poveda, M.L.; Rendón, N.; Mereiter, K. Isolation of a Stable 1-Iridabicyclo[3.2.0]hepta-1,3,6-triene and Its Reversible Transformation into an Iridacycloheptatriene. J. Am. Chem. Soc. 2004, 126, 1610–1611. [Google Scholar] [CrossRef]
- O’Connor, J.M.; Closson, A.; Hiibner, K.; Merwin, R.; Gantzel, P.; Roddick, D.M. Iridacyclopentadiene Reactions with Terminal Alkynes: Tandem Cycloaromatization and Orthometalation. Organometallics 2001, 20, 3710–3717. [Google Scholar] [CrossRef]
- Bianchini, C.; Caulton, K.G.; Johnson, T.J.; Meli, A.; Peruzzini, M.; Vizza, F. Mechanistic Study of Ir (H)2-Assisted Transformations of Ethyne: Cyclotrimerization, Cooligomerization with Ethene, and Reductive Coupling. Organometallics 1995, 14, 933–943. [Google Scholar] [CrossRef]
- Collman, J.P.; Kang, J.W.; Little, W.F.; Sullivan, M.F. Metalocyclopentadiene Complexes of Iridium and Rhodium and Their Role in the Catalytic Cyclotrimerization of Disubstituted Acetylenes. Inorg. Chem. 1968, 7, 1298–1303. [Google Scholar] [CrossRef]
- Perry, P.J.; Read, M.A.; Davies, R.T.; Gowan, S.M.; Reszka, A.P.; Wood, A.A.; Kelland, L.R.; Neidle, S. 2,7-Disubstituted Amidofluorenone Derivatives as Inhibitors of Human Telomerase. J. Med. Chem. 1999, 42, 2679–2684. [Google Scholar] [CrossRef]
- Hu, Q.-F.; Zhou, B.; Huang, J.-M.; Gao, X.-M.; Shu, L.-D.; Yang, G.-Y.; Che, C.-T. Antiviral Phenolic Compounds from Arundina Gramnifolia. J. Nat. Prod. 2013, 76, 292–296. [Google Scholar] [CrossRef]
- Niu, D.-Y.; Han, J.-M.; Kong, W.-S.; Cui, Z.-W.; Hu, Q.-F.; Gao, X.-M. Antiviral Fluorenone Derivatives from Arundina Gramnifolia. Asian J. Chem. 2013, 25, 9514–9516. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, S. Recent Advances of Synthesis of Fluorenone and Fluorene Containing Natural Products. Tetrahedron 2016, 72, 1717–1735. [Google Scholar] [CrossRef]
- Itami, K.; Tonogaki, K.; Nokami, T.; Ohashi, Y.; Yoshida, J. Palladium-Catalyzed Convergent Synthesis and Properties of Conjugated Dendrimers Based on Triarylethene Branching. Angew. Chem. Int. Ed. 2006, 45, 2404–2409. [Google Scholar] [CrossRef]
- Estrada, L.A.; Yarnell, J.E.; Neckers, D.C. Revisiting Fluorenone Photophysics via Dipolar Fluorenone Derivatives. J. Phys. Chem. A 2011, 115, 6366–6375. [Google Scholar] [CrossRef]
- Xia, J.-B.; Zhu, C.; Chen, C. Visible Light-Promoted Metal-Free C-H Activation: Diarylketone-Catalyzed Selective Benzylic Mono- and Difluorination. J. Am. Chem. Soc. 2013, 135, 17494–17500. [Google Scholar] [CrossRef] [Green Version]
- Capodilupo, A.L.; Vergaro, V.; Fabiano, E.; De Giorgi, M.; Baldassarre, F.; Cardone, A.; Maggiore, A.; Maiorano, V.; Sanvitto, D.; Gigli, G.; et al. Design and Synthesis of Fluorenone-Based Dyes: Two-Photon Excited Fluorescent Probes for Imaging of Lysosomes and Mitochondria in Living Cells. J. Mater. Chem. B 2015, 3, 3315–3323. [Google Scholar] [CrossRef]
- Usta, H.; Facchetti, A.; Marks, T.J. Synthesis and Characterization of Electron-Deficient and Highly Soluble (Bis)Indenofluorene Building Blocks for n-Type Semiconducting Polymers. Org. Lett. 2008, 10, 1385–1388. [Google Scholar] [CrossRef]
- Ye, F.; Haddad, M.; Michelet, V.; Ratovelomanana-Vidal, V. Access toward Fluorenone Derivatives through Solvent-Free Ruthenium Trichloride Mediated [2+2+2] Cycloadditions. Org. Lett. 2016, 18, 5612–5615. [Google Scholar] [CrossRef]
- Kaiser, R.P.; Hessler, F.; Mosinger, J.; Císařová, I.; Kotora, M. A [2+2+2]-Cyclotrimerization Approach to Selectively Substituted Fluorenes and Fluorenols, and Their Conversion to 9,9’-Spirobifluorenes. Chem. Eur. J. 2015, 21, 13577–13582. [Google Scholar] [CrossRef]
- Crabtree, R.H.; Quirk, J.M.; Felkin, H.; Fillebeen-Khan, T. An Efficient Synthesis of [Ir(cod)Cl]2 and Its Reaction with PMe2Ph to Give FAC-[IrH(PMe2C6H4) (PMe2Ph)3]. Synth. React. Inorg. Met. -Org. Chem. 1982, 12, 407–413. [Google Scholar] [CrossRef]
- Martins, G.M.; Zeni, G.; Back, D.F.; Kaufman, T.S.; Silveira, C.C. Expedient Iodocyclization Approach Toward Polysubstituted 3H-Benzo[e]indoles. Adv. Synth. Catal. 2015, 357, 3255–3261. [Google Scholar] [CrossRef] [Green Version]
- Řezníčková, E.; Tenora, L.; Pospísilová, P.; Galeta, J.; Jorda, R.; Berka, K.; Majer, P.; Potáček, M.; Kryštof, V. ALK5 Kinase Inhibitory Activity and Synthesis of 2,3,4-Substituted 5,5-Dimethyl-5,6-Dihydro-4H-pyrrolo[1,2-b]pyrazoles. Eur. J. Med. Chem. 2017, 127, 632–642. [Google Scholar] [CrossRef]





















| Entry | Ligand (mol %) | Yield of 3a (%) b |
|---|---|---|
| 1 | none | 20 |
| 2 | PPh3 (8) | 64 |
| 3 | DPPE (4) | 83 |
| 4 | DPPP (4) | 40 |
| 5 | DPPB (4) | 45 |
| 6 | DPPF (4) | 22 |

 |  |  |
 |  |  |
 |  |

| Entry | Reaction Conditions | Yield of 3j (%) b |
|---|---|---|
| 1 | Toluene, reflux | 36 |
| 2 | DCE, reflux | 62 |
| 3 | DCM, reflux | 72 |

 |  |  |

| Entry | Ligand (mol %) | Yield of 5j (%) b |
|---|---|---|
| 1 | DPPE (4) | 43 |
| 2 | PPh3 (8) | 45 |
| 3 | BIPHEP (4) | 63 |
| 4 | DPPF (4) | 28 |
| 5 | F-DPPE (4) | 94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawano, T.; Toyoshima, Y.; Takeuchi, R. Synthesis of Anthraquinones by Iridium-Catalyzed [2 + 2 + 2] Cycloaddition of a 1,2-Bis(propiolyl)benzene Derivative with Alkynes. Inorganics 2019, 7, 138. https://doi.org/10.3390/inorganics7110138
Sawano T, Toyoshima Y, Takeuchi R. Synthesis of Anthraquinones by Iridium-Catalyzed [2 + 2 + 2] Cycloaddition of a 1,2-Bis(propiolyl)benzene Derivative with Alkynes. Inorganics. 2019; 7(11):138. https://doi.org/10.3390/inorganics7110138
Chicago/Turabian StyleSawano, Takahiro, Yuko Toyoshima, and Ryo Takeuchi. 2019. "Synthesis of Anthraquinones by Iridium-Catalyzed [2 + 2 + 2] Cycloaddition of a 1,2-Bis(propiolyl)benzene Derivative with Alkynes" Inorganics 7, no. 11: 138. https://doi.org/10.3390/inorganics7110138
APA StyleSawano, T., Toyoshima, Y., & Takeuchi, R. (2019). Synthesis of Anthraquinones by Iridium-Catalyzed [2 + 2 + 2] Cycloaddition of a 1,2-Bis(propiolyl)benzene Derivative with Alkynes. Inorganics, 7(11), 138. https://doi.org/10.3390/inorganics7110138






