A PAlP Pincer Ligand Bearing a 2-Diphenylphosphinophenoxy Backbone
Abstract
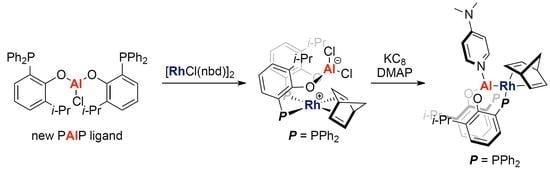
1. Introduction
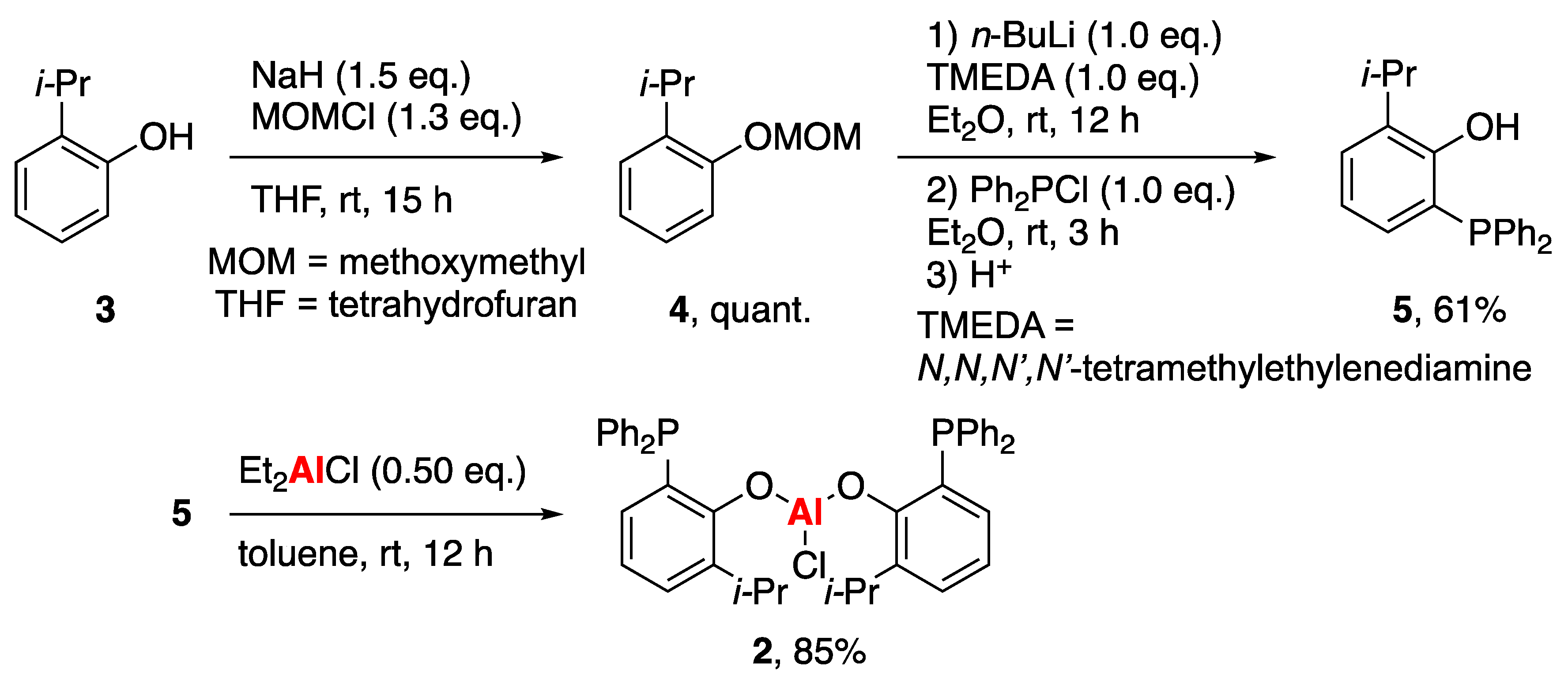
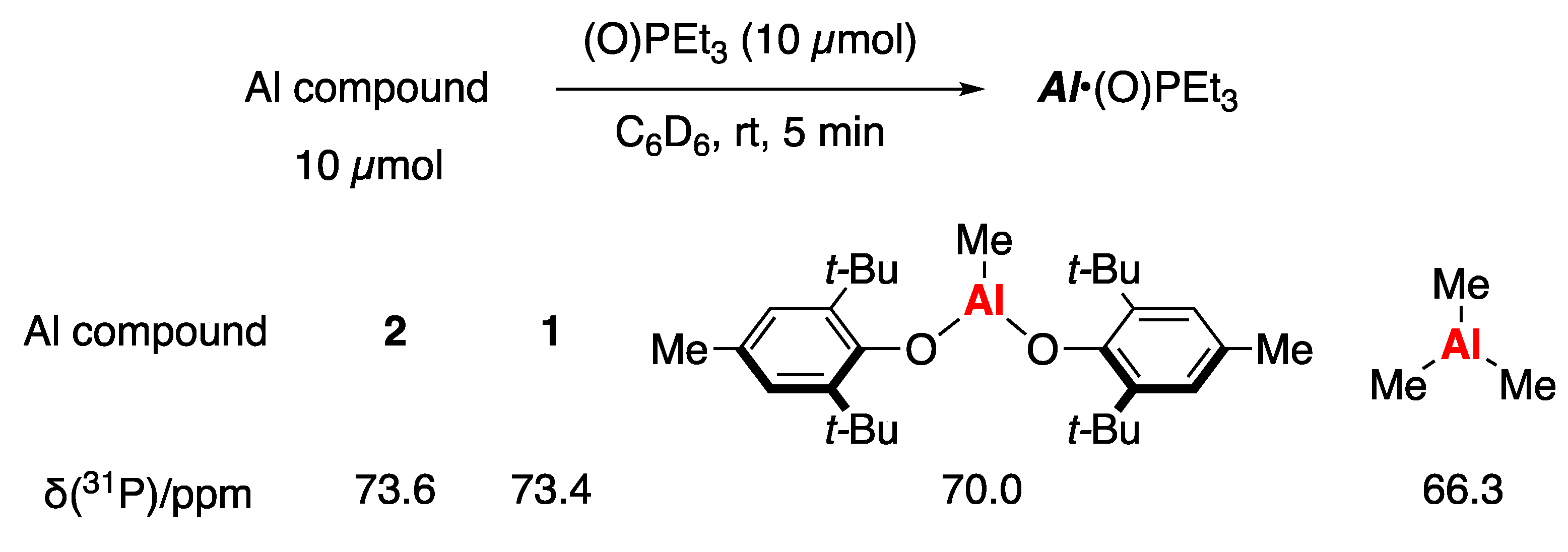
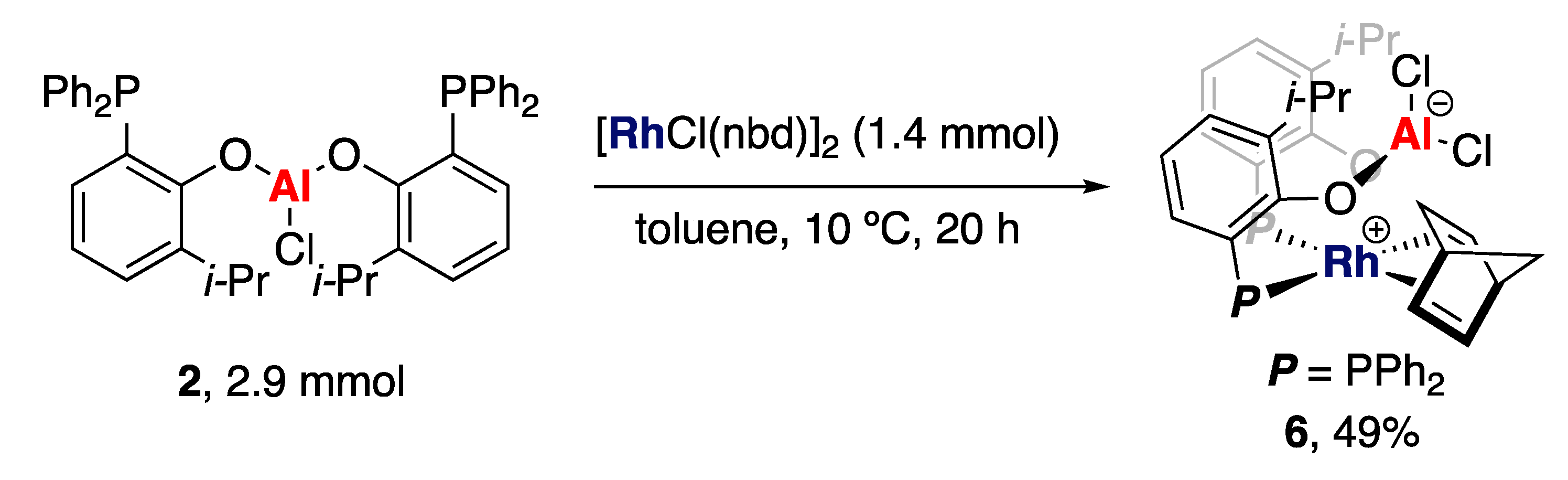
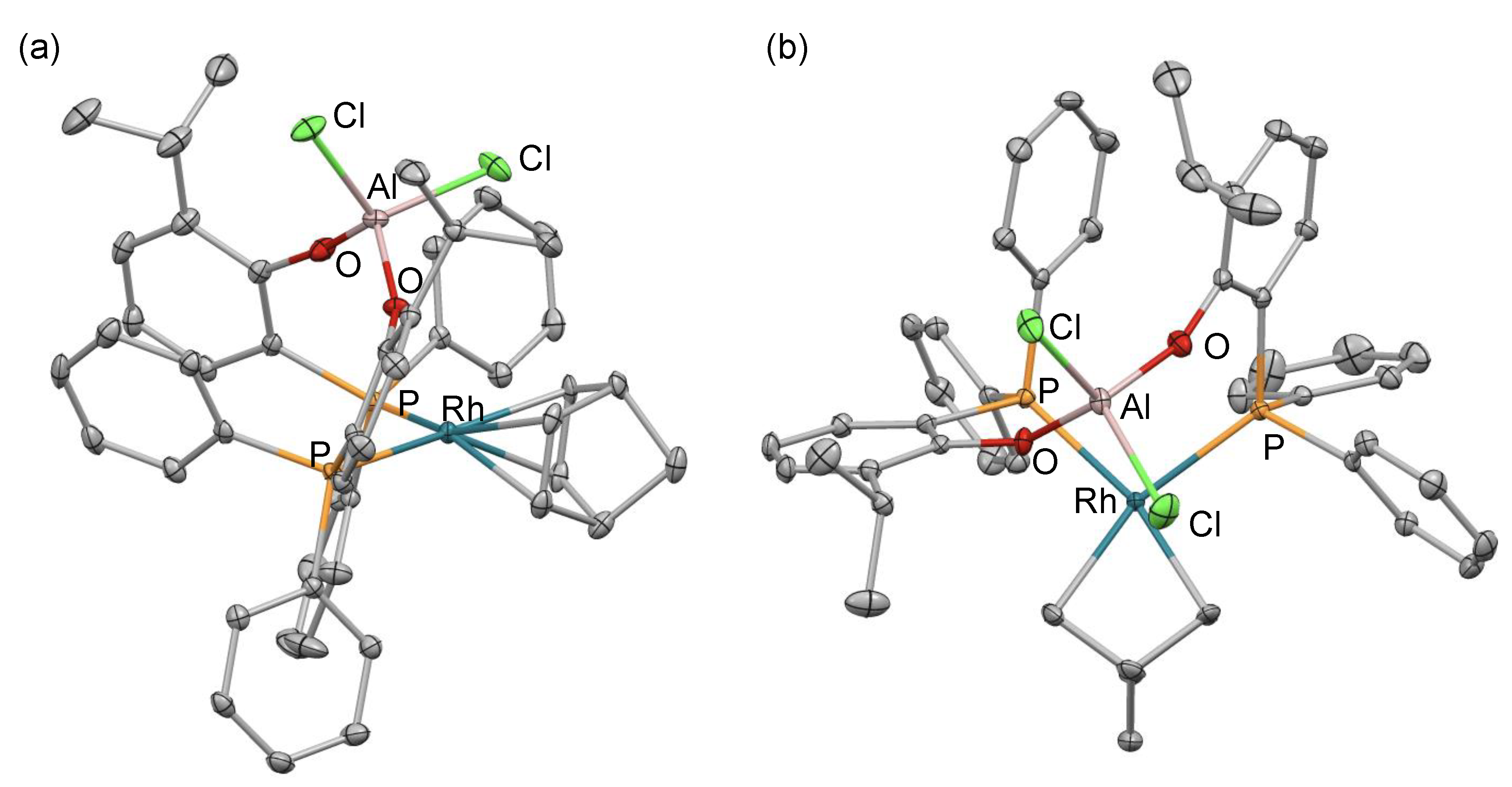
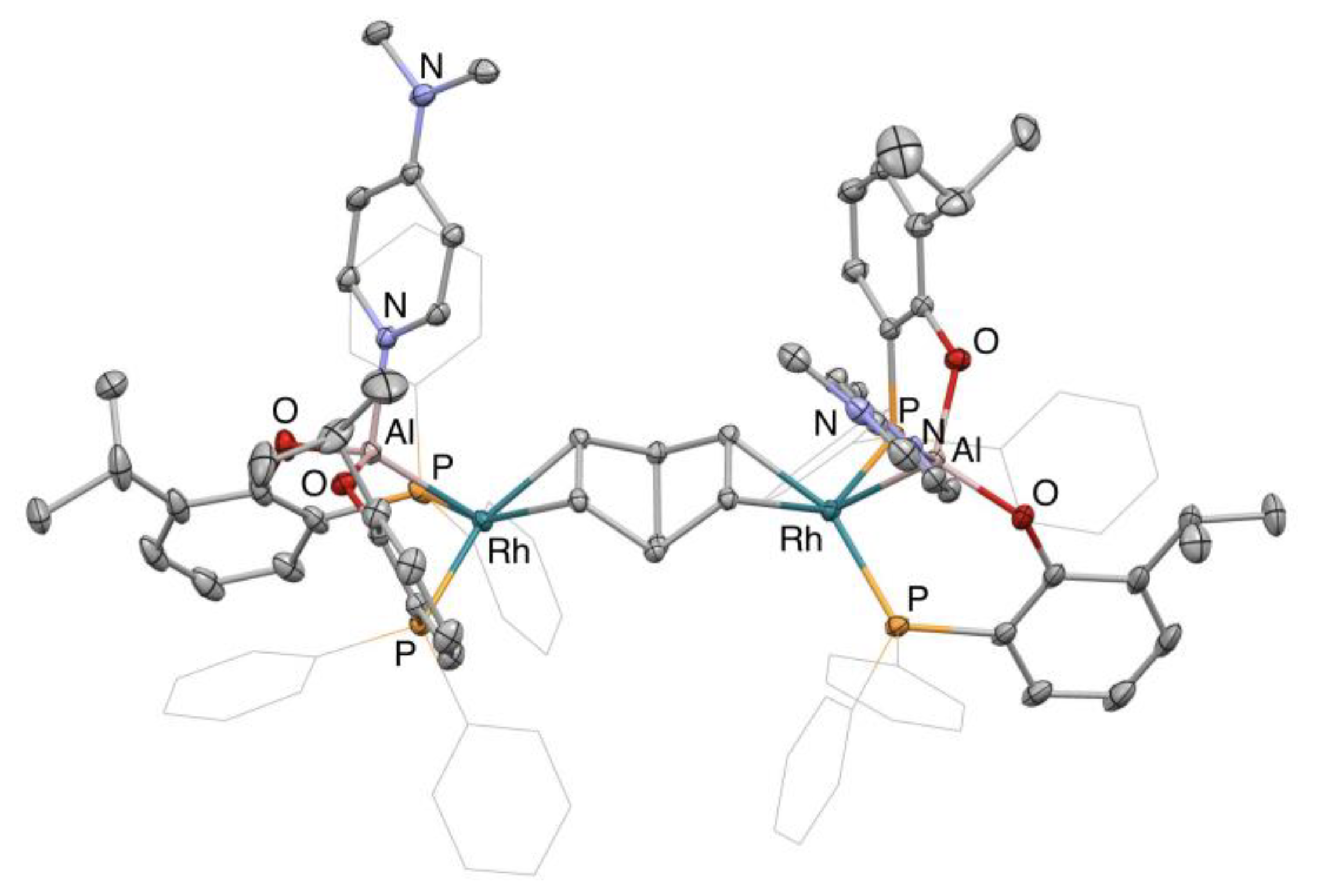
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Apparatus
3.3. Chemicals
3.4. Synthesis of 1-Isopropyl-2-Methoxymethoxybenzene (4)
3.5. Synthesis of 2-Diphenylphosphino-6-Isopropylphenol (5)
3.6. Synthesis of Bis(2-(Diphenylphosphino)-6-Isopropylphenoxy)-Aluminum Chloride (2)
3.7. Synthesis of 6
3.8. Synthesis of 7 and 8
3.9. Evaluation of Lewis Acidity of Aluminum Compounds
3.10. X-Ray Diffraction Study and X-Ray Crystallographic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fontaine, F.G.; Boudreau, J.; Thibault, M.H. Coordination Chemistry of Neutral (Ln)–Z Amphoteric and Ambiphilic Ligands. Eur. J. Inorg. Chem. 2008, 2008, 5439–5454. [Google Scholar] [CrossRef]
- Amgoune, A.; Bourissou, D. σ-Acceptor, Z-type Ligands for Transition Metals. Chem. Commun. 2011, 47, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, G.; Bourissou, D. Complexes of Ambiphilic Ligands: Reactivity and Catalytic Applications. Chem. Soc. Rev. 2016, 45, 1065–1079. [Google Scholar] [CrossRef]
- Yamashita, M. The Organometallic Chemistry of Boron-Containing Pincer Ligands based on Diazaboroles and Carboranes. Bull. Chem. Soc. Jpn. 2016, 89, 269–281. [Google Scholar] [CrossRef]
- Sircoglou, M.; Bouhadir, G.; Saffon, N.; Miqueu, K.; Bourissou, D. A Zwitterionic Gold(I) Complex from an Ambiphilic Diphosphino-Alane Ligand. Organometallics 2008, 27, 1675–1678. [Google Scholar] [CrossRef]
- Sircoglou, M.; Mercy, M.; Saffon, N.; Coppel, Y.; Bouhadir, G.; Maron, L.; Bourissou, D. Gold(I) Complexes of Phosphanyl Gallanes: From Interconverting to Separable Coordination Isomers. Angew. Chem. Int. Ed. 2009, 48, 3454–3457. [Google Scholar] [CrossRef]
- Segawa, Y.; Yamashita, M.; Nozaki, K. Syntheses of PBP Pincer Iridium Complexes: A Supporting Boryl Ligand. J. Am. Chem. Soc. 2009, 131, 9201–9203. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Reuter, M.G.; Stern, C.L.; Ratner, M.A.; Seideman, T.; Mirkin, C.A. Carborane-Based Pincers: Synthesis and Structure of SeBSe and SBS Pd(II) Complexes. J. Am. Chem. Soc. 2009, 131, 9482–9483. [Google Scholar] [CrossRef]
- Harman, W.H.; Peters, J.C. Reversible H2 Addition across a Nickel–Borane Unit as a Promising Strategy for Catalysis. J. Am. Chem. Soc. 2012, 134, 5080–5082. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Segawa, Y.; Yamashita, M.; Nozaki, K. Isolation of a PBP-Pincer Rhodium Complex Stabilized by an Intermolecular C–H σ Coordination as the Fourth Ligand. Angew. Chem. Int. Ed. 2012, 51, 6956–6960. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Hasegawa, M.; Yamashita, M.; Nozaki, K.; Ishida, N.; Murakami, M. Oxidative Addition of a Strained C–C Bond onto Electron-Rich Rhodium(I) at Room Temperature. J. Am. Chem. Soc. 2013, 135, 7142–7145. [Google Scholar] [CrossRef] [PubMed]
- Sircoglou, M.; Saffon, N.; Miqueu, K.; Bouhadir, G.; Bourissou, D. Activation of M–Cl Bonds with Phosphine–Alanes: Preparation and Characterization of Zwitterionic Gold and Copper Complexes. Organometallics 2013, 32, 6780–6784. [Google Scholar] [CrossRef]
- Lin, T.P.; Peters, J.C. Boryl–Metal Bonds Facilitate Cobalt/Nickel-Catalyzed Olefin Hydrogenation. J. Am. Chem. Soc. 2014, 136, 13672–13683. [Google Scholar] [CrossRef] [PubMed]
- Harman, W.H.; Lin, T.P.; Peters, J.C. A d10 Ni–(H2) Adduct as an Intermediate in H–H Oxidative Addition across a Ni–B Bond. Angew. Chem. Int. Ed. 2014, 53, 1081–1086. [Google Scholar] [CrossRef]
- Cowie, B.E.; Tsao, F.A.; Emslie, D.J.H. Synthesis and Platinum Complexes of an Alane-Appended 1,1’-Bis(phosphino)ferrocene Ligand. Angew. Chem. Int. Ed. 2015, 54, 2165–2169. [Google Scholar] [CrossRef]
- Shih, W.C.; Gu, W.; MacInnis, M.C.; Timpa, S.D.; Bhuvanesh, N.; Zhou, J.; Ozerov, O.V. Facile Insertion of Rh and Ir into a Boron–Phenyl Bond, Leading to Boryl/Bis(phosphine) PBP Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 2086–2089. [Google Scholar] [CrossRef]
- Shih, W.C.; Ozerov, O.V. Selective ortho C–H Activation of Pyridines Directed by Lewis Acidic Boron of PBP Pincer Iridium Complexes. J. Am. Chem. Soc. 2017, 139, 17297–17300. [Google Scholar] [CrossRef]
- Takaya, J.; Iwasawa, N. Synthesis, Structure, and Catalysis of Palladium Complexes Bearing a Group 13 Metalloligand: Remarkable Effect of an Aluminum-Metalloligand in Hydrosilylation of CO2. J. Am. Chem. Soc. 2017, 139, 6074–6077. [Google Scholar] [CrossRef]
- Saito, T.; Hara, N.; Nakao, Y. Palladium Complexes Bearing Z-type PAlP Pincer Ligands. Chem. Lett. 2017, 46, 1247–1249. [Google Scholar] [CrossRef]
- Hara, N.; Saito, T.; Semba, K.; Kuriakose, N.; Zheng, H.; Sakaki, S.; Nakao, Y. Rhodium Complexes Bearing PAlP Pincer Ligands. J. Am. Chem. Soc. 2018, 140, 7070–7073. [Google Scholar] [CrossRef]
- Saito, N.; Takaya, J.; Iwasawa, N. Stabilized Gallylene in a Pincer-Type Ligand: Synthesis, Structure, and Reactivity of PGaIP-Ir Complexes. Angew. Chem. Int. Ed. 2019, 58, 9998–10002. [Google Scholar] [CrossRef] [PubMed]
- Burlitch, J.M.; Leonowicz, M.E.; Petersen, R.B.; Hughes, R.E. Coordination of Metal Carbonyl Anions to Triphenyaluminum, -gallium, and -indium and the Crystal Structure of Tetraethylammonium Triphenyl((η5-cyclopentadienyl)dicarbonyliron)aluminate (Fe–Al). Inorg. Chem. 1979, 18, 1097–1105. [Google Scholar] [CrossRef]
- Golden, J.T.; Peterson, T.H.; Holland, P.L.; Bergman, R.G.; Andersen, R.A. Adduct Formation and Single and Double Deprotonation of Cp*(PMe3)Ir(H)2 with Main Group Metal Alkyls and Aryls: Synthesis and Structure of Three Novel Ir–Al and Ir–Mg Heterobimetallics. J. Am. Chem. Soc. 1998, 120, 223–224. [Google Scholar] [CrossRef]
- Braunschweig, H.; Gruss, K.; Radacki, K. Interaction between d- and p-Block Metals: Synthesis and Structure of Platinum–Alane Adducts. Angew. Chem. Int. Ed. 2007, 46, 7782–7784. [Google Scholar] [CrossRef]
- Bauer, J.; Braunschweig, H.; Brenner, P.; Kraft, K.; Radacki, K.; Schwab, K. Late-Transition-Metal Complexes as Tunable Lewis Bases. Chem. Eur. J. 2010, 16, 11985–11992. [Google Scholar] [CrossRef]
- Derrah, E.J.; Sircoglou, M.; Mercy, M.; Ladeira, S.; Bouhadir, G.; Miqueu, K.; Maron, L.; Bourissou, D. Original Transition Metal→Indium Interactions upon Coordination of a Triphosphine-Indane. Organometallics 2011, 30, 657–660. [Google Scholar] [CrossRef]
- Rudd, P.A.; Liu, S.; Gagliardi, L.; Young V.G., Jr.; Lu, C.C. Metal–Alane Adducts with Zero-Valent Nickel, Cobalt, and Iron. J. Am. Chem. Soc. 2011, 133, 20724–20727. [Google Scholar] [CrossRef]
- Devillard, M.; Nicolas, E.; Appelt, C.; Backs, J.; Mallet-Ladeira, S.; Bouhadir, G.; Slootweg, J.C.; Uhl, W.; Bourissou, D. Novel Zwitterionic Complexes Arising from the Coordination of an Ambiphilic Phosphorus–Aluminum Ligand to Gold. Chem. Commun. 2014, 50, 14805–14808. [Google Scholar] [CrossRef]
- Devillard, M.; Nicolas, E.; Ehlers, A.W.; Backs, J.; Mallet-Ladeira, S.; Bouhadir, G.; Slootweg, J.C.; Uhl, W.; Bourissou, D. Dative Au–Al Interactions: Crystallographic Characterization and Computational Analysis. Chem. Eur. J. 2015, 21, 74–79. [Google Scholar] [CrossRef]
- Wang, G.; Xu, L.; Li, P. Double N,B-Type Bidentate Boryl Ligands Enabling a Highly Active Iridium Catalyst for C–H Borylation. J. Am. Chem. Soc. 2015, 137, 8058–8061. [Google Scholar] [CrossRef]
- Devillard, M.; Declercq, R.; Nicolas, E.; Ehlers, A.W.; Backs, J.; Saffon-Merceron, N.; Bouhadir, G.; Slootweg, J.C.; Uhl, W.; Bourissou, D. A Significant but Constrained Geometry Pt–Al Interaction: Fixation of CO2 and CS2, Activation of H2 and PhCONH2. J. Am. Chem. Soc. 2016, 138, 4917–4926. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, R.C.; Vollmer, M.V.; Xie, J.; Ye, J.; Linehan, J.C.; Burgess, S.A.; Appel, A.M.; Gagliardi, L.; Lu, C.C. A Bimetallic Nickel–Gallium Complex Catalyzes CO2 Hydrogenation via the Intermediacy of an Anionic d10 Nickel Hydride. J. Am. Chem. Soc. 2017, 139, 14244–14250. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, L.; Wang, H.; Ding, Y.S.; Zhou, J.; Mao, S.; Li, P. N,B-Bidentate Boryl Ligand-Supported Iridium Catalyst for Efficient Functional-Group-Directed C–H Borylation. J. Am. Chem. Soc. 2017, 139, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gao, Q.; Xu, S. Chiral Bidentate Boryl Ligand Enabled Iridium-Catalyzed Enantioselective C(sp3)–H Borylation of Cyclopropanes. J. Am. Chem. Soc. 2019, 141, 10599–10604. [Google Scholar] [CrossRef]
- Hicks, J.; Mansikkamäki, A.; Vasko, P.; Goicoechea, J.M.; Aldridge, S. A Nucleophilic Gold Complex. Nat. Chem. 2019, 11, 237–241. [Google Scholar]
- Allred, A.L.; Rochow, E.G. A Scale of Electronegativity Based on Electrostatic Force. J. Inorg. Nucl. Chem. 1958, 5, 264–268. [Google Scholar] [CrossRef]
- Beckett, M.A.; Strickland, G.C.; Holland, J.R.; Varma, K.S. A Convenient n.m.r. Method for the Measurement of Lewis Acidity at Boron Centres: Correlation of Reaction Rates of Lewis Acid Initiated Epoxide Polymerizations with Lewis Acidity. Polymer 1996, 37, 4629–4631. [Google Scholar] [CrossRef]
- Beckett, M.A.; Brassington, D.S.; Coles, S.J.; Hursthouse, M.B. Lewis Acidity of Tris(pentafluorophenyl)borane: Crystal and Molecular Structure of B(C6F5)3·OPEt3. Inorg. Chem. Commun. 2000, 3, 530–533. [Google Scholar] [CrossRef]
- Maruoka, K.; Itoh, T.; Yamamoto, H. Methylaluminum Bis(2,6-di-tert-butyl-4-alkylphenoxide). A New Reagent for Obtaining Unusual Equatorial and Anti-Cram Selectivity in Carbonyl Alkylation. J. Am. Chem. Soc. 1985, 107, 4573–4576. [Google Scholar] [CrossRef]
- Maruoka, K.; Itoh, T.; Sakurai, M.; Nonoshita, K.; Yamamoto, H. Amphiphilic Reactions by Means of Exceptionally Bulky Organoaluminum Reagents. Rational Approach for Obtaining Unusual Equatorial, Anti-Cram, and 1,4 Selectivity in Carbonyl Alkylation. J. Am. Chem. Soc. 1988, 110, 3588–3597. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Wassenaar, J.; Siegler, M.A.; Spek, A.L.; Bruin, B.; Reek, J.N.H.; Vlugt, J.I. Versatile New C3-Symmetric Tripodal Tetraphosphine Ligands; Structural Flexibility to Stabilize CuI and RhI Species and Tune Their Reactivity. Inorg. Chem. 2010, 49, 6495–6508. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.W.; Bennett, M.A.; Wilkinson, G. Norbornadiene–Metal Complexes and Some Related Compounds. J. Chem. Soc. 1959, 3178–3182. [Google Scholar] [CrossRef]
- Pangborn, A.B.; Giardello, M.A.; Grubbs, R.H.; Rosen, R.K.; Timmers, F.J. Safe and Convenient Procedure for Solvent Purification. Organometallics 1996, 15, 1518–1520. [Google Scholar] [CrossRef]
- Ihori, Y.; Yamashita, Y.; Ishitani, H.; Kobayashi, S. Chiral Zirconium Catalysts Using Multidentate BINOL Derivatives for Catalytic Enantioselective Mannich-Type Reactions; Ligand Optimization and Approaches to Elucidation of the Catalyst Structure. J. Am. Chem. Soc. 2005, 127, 15528–15535. [Google Scholar] [CrossRef]
- Pflugrath, J.W. Rigaku Corporation, 1999, and CrystalClear Software User’s Guide, Molecular Structure Corporation, 2000. Acta Crystallogr. D 1999, 55, 1718–1725. [Google Scholar] [CrossRef]
- Pflugrath, J.W. The Finer Things in X-ray Diffraction Data Collection. Acta Crystallogr. 1999, 55, 1718–1725. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semba, K.; Fujii, I.; Nakao, Y. A PAlP Pincer Ligand Bearing a 2-Diphenylphosphinophenoxy Backbone. Inorganics 2019, 7, 140. https://doi.org/10.3390/inorganics7120140
Semba K, Fujii I, Nakao Y. A PAlP Pincer Ligand Bearing a 2-Diphenylphosphinophenoxy Backbone. Inorganics. 2019; 7(12):140. https://doi.org/10.3390/inorganics7120140
Chicago/Turabian StyleSemba, Kazuhiko, Ikuya Fujii, and Yoshiaki Nakao. 2019. "A PAlP Pincer Ligand Bearing a 2-Diphenylphosphinophenoxy Backbone" Inorganics 7, no. 12: 140. https://doi.org/10.3390/inorganics7120140
APA StyleSemba, K., Fujii, I., & Nakao, Y. (2019). A PAlP Pincer Ligand Bearing a 2-Diphenylphosphinophenoxy Backbone. Inorganics, 7(12), 140. https://doi.org/10.3390/inorganics7120140