Smart MRI Agents for Detecting Extracellular Events In Vivo: Progress and Challenges
Abstract
:1. Introduction
2. T1 Contrast Agents
2.1. Clinically Available Contrast Agents
2.1.1. Extracellular Fluid Agents
2.1.2. Blood Pool Agents
2.1.3. Hepatobiliary Agents
2.2. Detection of Trace Metal Ions In Vivo
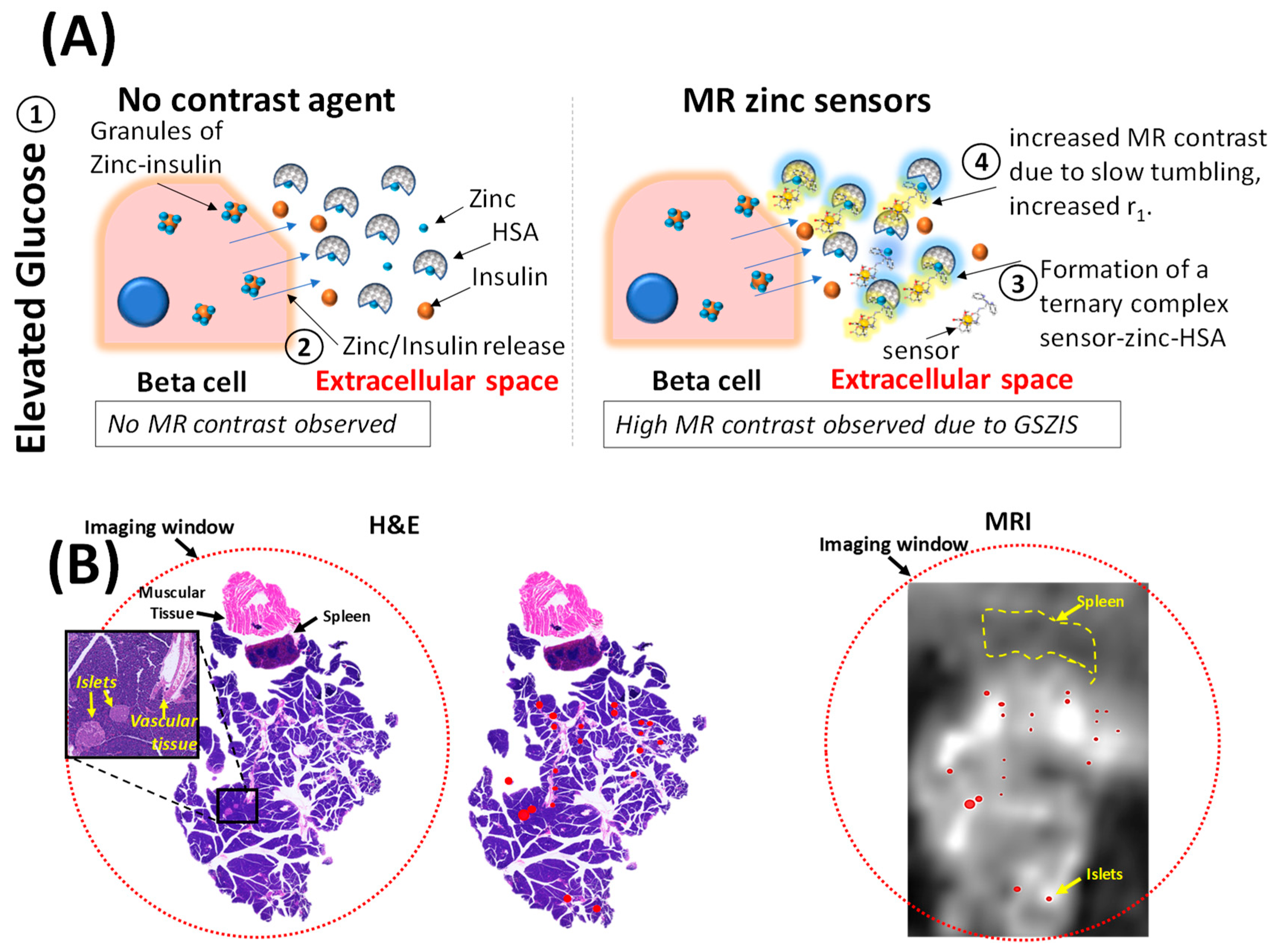
2.2.1. Zinc-Responsive Contrast Agents
2.2.2. Copper-Responsive Contrast Agents
2.2.3. Calcium-Responsive Contrast Agents
2.2.4. Other Metal Ions
2.3. Extracellular pH Contrast Agents Applied In Vivo
2.4. Tissue-Specific Contrast Agents
3. T2 and T2-Exchange Agents In Vivo
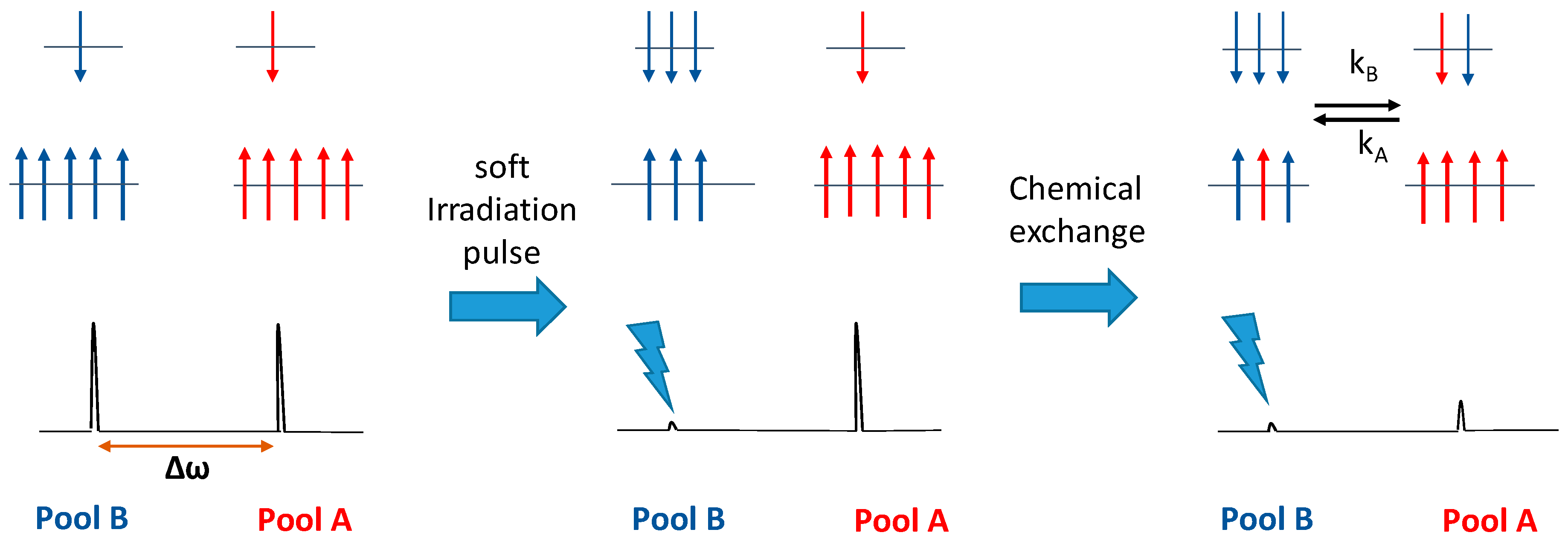
4. CEST Agents
4.1. Mechanism of CEST
4.2. DIACEST Agents (Diamagnetic Molecules with Exchangeable Protons)
4.3. paraCEST Agents (Paramagnetic Molecules with Exchangeable Protons)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolf, G.L. Current status of MR imaging contrast agents: Special report. Radiology 1989, 172, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Gahramanov, S.; Muldoon, L.L.; Varallyay, C.G.; Li, X.; Kraemer, D.F.; Fu, R.; Hamilton, B.E.; Rooney, W.D.; Neuwelt, E.A. Pseudoprogression of Glioblastoma after Chemo- and Radiation Therapy: Diagnosis by Using Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging with Ferumoxytol versus Gadoteridol and Correlation with Survival. Radiology 2013, 266, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.; Powell, D.H.; Tissieres, V.; Merbach, A.E. Water-exchange, electronic relaxation, and rotational dynamics of the MRI contrast agent [Gd(DTPA-BMA)(H2O)] in aqueous solution: A variable pressure, temperature, and magnetic field 17O NMR study. J. Phys. Chem. 1994, 98, 53–59. [Google Scholar] [CrossRef]
- Werner, E.J.; Datta, A.; Jocher, C.J.; Raymond, K.N. High-relaxivity MRI contrast agents: Where coordination chemistry meets medical imaging. Angew. Chem. Int. Ed. Engl. 2008, 47, 8568–8580. [Google Scholar] [CrossRef]
- Sherry, A.D.; Wu, Y. The importance of water exchange rates in the design of responsive agents for MRI. Curr. Opin. Chem. Biol. 2013, 17, 167–174. [Google Scholar] [CrossRef] [PubMed]
- De León-Rodríguez, L.M.; Martins, A.F.; Pinho, M.C.; Rofsky, N.M.; Sherry, A.D. Basic MR relaxation mechanisms and contrast agent design. J. Magn. Reson. Imaging 2015, 42, 545–565. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Martins, A.F.; Preihs, C.; Clavijo Jordan, V.; Chirayil, S.; Zhao, P.; Wu, Y.; Nasr, K.; Kiefer, G.E.; Sherry, A.D. Amplifying the Sensitivity of Zinc(II) Responsive MRI Contrast Agents by Altering Water Exchange Rates. J. Am. Chem. Soc. 2015, 137, 14173–14179. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2018. [Google Scholar] [CrossRef]
- Merbach, A.E.; Helm, L.; Toth, E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; John Wiley & Sons: Chichester, UK, 2013; ISBN 0-471-60778-9. [Google Scholar]
- Lee, S.Y.; Gallagher, D. Assessment methods in human body composition. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 566–572. [Google Scholar] [CrossRef]
- Jordan, M.V.C.; Lo, S.-T.; Chen, S.; Preihs, C.; Chirayil, S.; Zhang, S.; Kapur, P.; Li, W.-H.; Leon-Rodriguez, L.M.D.; Lubag, A.J.M.; et al. Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc. Natl. Acad. Sci. USA 2016, 113, E5464–E5471. [Google Scholar] [CrossRef]
- Martins, A.F.; Clavijo Jordan, V.; Bochner, F.; Chirayil, S.; Paranawithana, N.; Zhang, S.; Lo, S.-T.; Wen, X.; Zhao, P.; Neeman, M.; et al. Imaging Insulin Secretion from Mouse Pancreas by MRI Is Improved by Use of a Zinc-Responsive MRI Sensor with Lower Affinity for Zn2+ Ions. J. Am. Chem. Soc. 2018, 140, 17456–17464. [Google Scholar] [CrossRef] [PubMed]
- Daryaei, I.; Pagel, M.D. Double agents and secret agents: The emerging fields of exogenous chemical exchange saturation transfer and T2-exchange magnetic resonance imaging contrast agents for molecular imaging. Res. Rep. Nucl. Med. 2015, 5, 19–32. [Google Scholar]
- Zhang, L.; Martins, A.F.; Zhao, P.; Wu, Y.; Tircsó, G.; Sherry, A.D. Lanthanide-Based T2ex and CEST Complexes Provide Insights into the Design of pH Sensitive MRI Agents. Angew. Chem. Int. Ed. 2017, 56, 16626–16630. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.M.; Aletras, A.H.; Balaban, R.S. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Kovacs, Z.; Green, K.N.; Ratnakar, S.J.; Sherry, A.D. Alternatives to Gadolinium-based MRI Metal Chelates. Chem. Rev. 2010, 110, 2960–3018. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, E.; Sherry, A.D.; Lenkinski, R.E. CEST: From basic principles to applications, challenges and opportunities. J. Magn. Reson. 2013, 229, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Hancu, I.; Dixon, W.T.; Woods, M.; Vinogradov, E.; Sherry, A.D.; Lenkinski, R.E. CEST and PARACEST MR contrast agents. Acta Radiol. 2010, 51, 910–923. [Google Scholar] [CrossRef]
- Fernando, W.S.; Martins, A.F.; Zhao, P.; Wu, Y.; Kiefer, G.E.; Platas-Iglesias, C.; Sherry, A.D. Breaking the Barrier to Slow Water Exchange Rates for Optimal Magnetic Resonance Detection of paraCEST Agents. Inorg. Chem. 2016, 55, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Lux, J.; Sherry, A.D. Advances in gadolinium-based MRI contrast agent designs for monitoring biological processes in vivo. Curr. Opin. Chem. Biol. 2018, 45, 121–130. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Dublin, A.B. Magnetic Resonance Imaging (MRI), Gadolinium. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging 2009, 30, 1259–1267. [Google Scholar] [CrossRef]
- Bellin, M.-F. MR contrast agents, the old and the new. Eur. J. Radiol. 2006, 60, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Mitsumori, L.M.; Bhargava, P.; Essig, M.; Maki, J.H. Magnetic Resonance Imaging Using Gadolinium-Based Contrast Agents. Top. Magn. Reson. Imaging 2014, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Al-Okaili, R.N.; Krejza, J.; Wang, S.; Woo, J.H.; Melhem, E.R. Advanced MR Imaging Techniques in the Diagnosis of Intraaxial Brain Tumors in Adults. RadioGraphics 2006, 26, S173–S189. [Google Scholar] [CrossRef] [PubMed]
- Layne, K.A.; Dargan, P.I.; Archer, J.R.H.; Wood, D.M. Gadolinium deposition and the potential for toxicological sequelae—A literature review of issues surrounding gadolinium-based contrast agents. Br. J. Clin. Pharmacol. 2018, 84, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, T.; Lengsfeld, P.; Schirmer, H.; Hütter, J.; Weinmann, H.-J. Stability of Gadolinium-Based Magnetic Resonance Imaging Contrast Agents in Human Serum at 37 °C. Investig. Radiol. 2008, 43, 817. [Google Scholar] [CrossRef] [PubMed]
- Idée, J.-M.; Port, M.; Robic, C.; Medina, C.; Sabatou, M.; Corot, C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J. Magn. Reson. Imaging 2009, 30, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Ai, T.; Goerner, F.; Hu, X.; Runge, V.M.; Tweedle, M. MRI contrast agents: Basic chemistry and safety. J. Magn. Reson. Imaging 2012, 36, 1060–1071. [Google Scholar] [CrossRef]
- Wedeking, P.; Sotak, C.H.; Telser, J.; Kumar, K.; Chang, C.A.; Tweedle, M.F. Quantitative dependence of MR signal intensity on tissue concentration of Gd(HP-DO3A) in the nephrectomized rat. Magn. Reson. Imaging 1992, 10, 97–108. [Google Scholar] [CrossRef]
- Knuttinen, M.-G.; Karow, J.; Mar, W.; Golden, M.; Xie, K.L. Blood Pool Contrast-enhanced Magnetic Resonance Angiography with Correlation to Digital Subtraction Angiography: A Pictorial Review. J. Clin. Imaging Sci. 2014, 4, 63. [Google Scholar]
- Oliveira, I.S.; Hedgire, S.S.; Li, W.; Ganguli, S.; Prabhakar, A.M. Blood pool contrast agents for venous magnetic resonance imaging. Cardiovasc. Diagn. Ther. 2016, 6, 508–518. [Google Scholar] [CrossRef]
- Frydrychowicz, A.; Lubner, M.G.; Brown, J.J.; Merkle, E.M.; Nagle, S.K.; Rofsky, N.M.; Reeder, S.B. Hepatobiliary MR imaging with gadolinium-based contrast agents. J. Magn. Reson. Imaging 2012, 35, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Huh, J.; Woo, D.-C.; Kim, K.W. Use of gadoxetate disodium for functional MRI based on its unique molecular mechanism. BJR 2016, 89, 20150666. [Google Scholar] [CrossRef]
- Haradome, H.; Unno, T.; Morisaka, H.; Toda, Y.; Kwee, T.C.; Kondo, H.; Sano, K.; Ichikawa, T.; Kondo, F.; Sugitani, M.; et al. Gadoxetic acid disodium-enhanced MR imaging of cholangiolocellular carcinoma of the liver: Imaging characteristics and histopathological correlations. Eur. Radiol. 2017, 27, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Cleary, S.; Audet, P.; Balaa, F.; Bhayana, D.; Burak, K.; Chang, S.; Dixon, E.; Haider, M.; Molinari, M.; et al. Consensus Statements from a Multidisciplinary Expert Panel on the Utilization and Application of a Liver-Specific MRI Contrast Agent (Gadoxetic Acid). Am. J. Roentgenol. 2015, 204, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Milon, B.; Feng, P.; Costello, L.C. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front. Biosci. 2005, 10, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; McCormick, N.H.; Velasquez, V.; Lopez, V. Zinc in Specialized Secretory Tissues: Roles in the Pancreas, Prostate, and Mammary Gland. Adv. Nutr. 2011, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lovejoy, K.S.; Jasanoff, A.; Lippard, S.J. Water-soluble porphyrins as a dual-function molecular imaging platform for MRI and fluorescence zinc sensing. Proc. Natl. Acad. Sci. USA 2007, 104, 10780–10785. [Google Scholar] [CrossRef]
- Lee, T.; Zhang, X.; Dhar, S.; Faas, H.; Lippard, S.J.; Jasanoff, A. In Vivo Imaging with a Cell-Permeable Porphyrin-Based MRI Contrast Agent. Chem. Biol. 2010, 17, 665–673. [Google Scholar] [CrossRef]
- De Leon-Rodriguez, L.; Lubag, A.J.M., Jr.; Sherry, A.D. Imaging free zinc levels in vivo—What can be learned? Inorg. Chim. Acta 2012, 393, 12–23. [Google Scholar] [CrossRef]
- Stasiuk, G.J.; Minuzzi, F.; Sae-Heng, M.; Rivas, C.; Juretschke, H.-P.; Piemonti, L.; Allegrini, P.R.; Laurent, D.; Duckworth, A.R.; Beeby, A.; et al. Dual-Modal Magnetic Resonance/Fluorescent Zinc Probes for Pancreatic β-Cell Mass Imaging. Chem. Eur. J. 2015, 21, 5023–5033. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Logothetis, N.K.; Parker, D. Critical In Vitro Evaluation of Responsive MRI Contrast Agents for Calcium and Zinc. Chem. Eur. J. 2011, 17, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.S.; Caillé, F.; Pallier, A.; Morfin, J.-F.; Petoud, S.; Suzenet, F.; Tóth, É. Mechanistic Studies of Gd3+-Based MRI Contrast Agents for Zn2+ Detection: Towards Rational Design. Chem. Eur. J. 2014, 20, 10959–10969. [Google Scholar] [CrossRef] [PubMed]
- Lubag, A.J.M.; De Leon-Rodriguez, L.M.; Burgess, S.C.; Sherry, A.D. Noninvasive MRI of β-cell function using a Zn2+-responsive contrast agent. Proc. Natl. Acad. Sci. USA 2011, 108, 18400–18405. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.-T.; Martins, A.F.; Jordan, V.C.; Sherry, A.D. Zinc as an Imaging Biomarker of Prostate Cancer. Isr. J. Chem. 2017, 57, 854–861. [Google Scholar] [CrossRef]
- Que, E.L.; New, E.J.; Chang, C.J. A cell-permeable gadolinium contrast agent for magnetic resonance imaging of copper in a Menkes disease model. Chem. Sci. 2012, 3, 1829–1834. [Google Scholar] [CrossRef]
- Que, E.L.; Gianolio, E.; Baker, S.L.; Wong, A.P.; Aime, S.; Chang, C.J. Copper-Responsive Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2009, 131, 8527–8536. [Google Scholar] [CrossRef]
- Que, E.L.; Chang, C.J. A Smart Magnetic Resonance Contrast Agent for Selective Copper Sensing. J. Am. Chem. Soc. 2006, 128, 15942–15943. [Google Scholar] [CrossRef]
- Paranawithana, N.; Martins, A.; Clavijo-Jordan, V.; Zhao, P.; Chirayil, S.; Meloni, G.; Sherry, D. A Responsive MRI Contrast Agent for Detection of Excess copper(II) in the Liver in Vivo. ChemRxiv 2018. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Rigiracciolo, D.C.; Scarpelli, A.; Lappano, R.; Pisano, A.; Santolla, M.F.; Marco, P.D.; Cirillo, F.; Cappello, A.R.; Dolce, V.; Belfiore, A.; et al. Copper activates HIF-1α/GPER/VEGF signalling in cancer cells. Oncotarget 2015, 6, 34158–34177. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Wittung-Stafshede, P. Roles of Copper-Binding Proteins in Breast Cancer. Int. J. Mol. Sci. 2017, 18, 871. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Calcium Control of Neurotransmitter Release. Cold Spring Harb. Perspect. Biol. 2012, 4, a011353. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fraser, S.E.; Meade, T.J. A Calcium-Sensitive Magnetic Resonance Imaging Contrast Agent. J. Am. Chem. Soc. 1999, 121, 1413–1414. [Google Scholar] [CrossRef]
- Dhingra, K.; Fousková, P.; Angelovski, G.; Maier, M.E.; Logothetis, N.K.; Tóth, É. Towards extracellular Ca2+ sensing by MRI: Synthesis and calcium-dependent 1H and 17O relaxation studies of two novel bismacrocyclic Gd3+ complexes. J. Biol. Inorg. Chem. 2008, 13, 35–46. [Google Scholar] [CrossRef]
- Mishra, A.; Fousková, P.; Angelovski, G.; Balogh, E.; Mishra, A.K.; Logothetis, N.K.; Tóth, É. Facile Synthesis and Relaxation Properties of Novel Bispolyazamacrocyclic Gd3+ Complexes: An Attempt towards Calcium-Sensitive MRI Contrast Agents. Inorg. Chem. 2008, 47, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Koretsky, A.P. Manganese ion enhances T1-weighted MRI during brain activation: An approach to direct imaging of brain function. Magn. Reson. Med. 1997, 38, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Moussaron, A.; Vibhute, S.; Bianchi, A.; Gündüz, S.; Kotb, S.; Sancey, L.; Motto-Ros, V.; Rizzitelli, S.; Crémillieux, Y.; Lux, F.; et al. Ultrasmall Nanoplatforms as Calcium-Responsive Contrast Agents for Magnetic Resonance Imaging. Small 2015, 11, 4900–4909. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Bartelle, B.B.; Li, N.; Breton-Provencher, V.; Lee, J.J.; Rodriguez, E.; Melican, J.; Sur, M.; Jasanoff, A. Calcium-dependent molecular fMRI using a magnetic nanosensor. Nat. Nanotechnol. 2018, 13, 473. [Google Scholar] [CrossRef]
- Angelovski, G.; Chauvin, T.; Pohmann, R.; Logothetis, N.K.; Tóth, É. Calcium-responsive paramagnetic CEST agents. Bioorg. Med. Chem. 2011, 19, 1097–1105. [Google Scholar] [CrossRef]
- Angelovski, G.; Gottschalk, S.; Milošević, M.; Engelmann, J.; Hagberg, G.E.; Kadjane, P.; Andjus, P.; Logothetis, N.K. Investigation of a Calcium-Responsive Contrast Agent in Cellular Model Systems: Feasibility for Use as a Smart Molecular Probe in Functional MRI. ACS Chem. Neurosci. 2014, 5, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Garello, F.; Vibhute, S.; Gündüz, S.; Logothetis, N.K.; Terreno, E.; Angelovski, G. Innovative Design of Ca-Sensitive Paramagnetic Liposomes Results in an Unprecedented Increase in Longitudinal Relaxivity. Biomacromolecules 2016, 17, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Oukhatar, F.; Même, S.; Même, W.; Szeremeta, F.; Logothetis, N.K.; Angelovski, G.; Tóth, É. MRI Sensing of Neurotransmitters with a Crown Ether Appended Gd3+ Complex. ACS Chem. Neurosci. 2015, 6, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hifumi, H.; Tanimoto, A.; Citterio, D.; Komatsu, H.; Suzuki, K. Novel 15-crown-5 ether or β-diketone incorporated gadolinium complexes for the detection of potassium ions or magnesium and calcium ions. Analyst 2007, 132, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Kubíček, V.; Vitha, T.; Kotek, J.; Hermann, P.; Vander Elst, L.; Muller, R.N.; Lukeš, I.; Peters, J.A. Towards MRI contrast agents responsive to Ca(II) and Mg(II) ions: Metal-induced oligomerization of dota–bisphosphonate conjugates. Contrast Media Mol. Imaging 2010, 5, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Abada, S.; Lecointre, A.; Elhabiri, M.; Esteban-Gómez, D.; Platas-Iglesias, C.; Tallec, G.; Mazzanti, M.; Charbonnière, L.J. Highly relaxing gadolinium based MRI contrast agents responsive to Mg2+ sensing. Chem. Commun. 2012, 48, 4085–4087. [Google Scholar] [CrossRef] [PubMed]
- Raghunand, N.; Howison, C.; Sherry, A.D.; Zhang, S.; Gillies, R.J. Renal and systemic pH imaging by contrast-enhanced MRI. Magn. Reson. Med. 2003, 49, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.V.; Zhang, X.; García-Martín, M.L.; Morse, D.L.; Woods, M.; Sherry, A.D.; Gillies, R.J. Imaging the extracellular pH of tumors by MRI after injection of a single cocktail of T1 and T2 contrast agents. NMR Biomed. 2011, 24, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Kálmán, F.K.; Woods, M.; Caravan, P.; Jurek, P.; Spiller, M.; Tircsó, G.; Király, R.; Brücher, E.; Sherry, A.D. Potentiometric and Relaxometric Properties of a Gadolinium-Based MRI Contrast Agent for Sensing Tissue pH. Inorg. Chem. 2007, 46, 5260–5270. [Google Scholar] [CrossRef] [PubMed]
- Vander Elst, L.; Roch, A.; Gillis, P.; Laurent, S.; Botteman, F.; Bulte, J.W.M.; Muller, R.N. Dy-DTPA derivatives as relaxation agents for very high field MRI: The beneficial effect of slow water exchange on the transverse relaxivities. Magn. Reson. Med. 2002, 47, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.G.; Westmeyer, G.G.; Romero, P.A.; Szablowski, J.O.; Küster, B.; Shah, A.; Otey, C.R.; Langer, R.; Arnold, F.H.; Jasanoff, A. Directed evolution of a magnetic resonance imaging contrast agent for noninvasive imaging of dopamine. Nat. Biotechnol. 2010, 28, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Cai, L.X.; Lelyveld, V.S.; Hai, A.; Jasanoff, A. Molecular-Level Functional Magnetic Resonance Imaging of Dopaminergic Signaling. Science 2014, 344, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.E.; Donnola, S.B.; Jiang, Y.; Batesole, J.; Darrah, R.; Drumm, M.L.; Brady-Kalnay, S.M.; Steinmetz, N.F.; Yu, X.; Griswold, M.A.; et al. Dual Contrast–Magnetic Resonance Fingerprinting (DC–MRF): A Platform for Simultaneous Quantification of Multiple MRI Contrast Agents. Sci. Rep. 2017, 7, 8431. [Google Scholar] [CrossRef] [PubMed]
- Soesbe, T.C.; Ratnakar, S.J.; Milne, M.; Zhang, S.; Do, Q.N.; Kovacs, Z.; Sherry, A.D. Maximizing T2-exchange in Dy3+DOTA-(amide)X chelates: Fine-tuning the water molecule exchange rate for enhanced T2 contrast in MRI. Magn. Reson. Med. 2014, 71, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.N.; Xu, J.; Bar-Shir, A.; Qin, Q.; Chan, K.W.Y.; Grgac, K.; Li, W.; McMahon, M.T.; van Zijl, P.C.M. Natural d-glucose as a biodegradable MRI relaxation agent. Magn. Reson. Med. 2014, 72, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.M.; Pagel, M.D.; Cárdenas-Rodríguez, J. Characterization of d-maltose as a T2-exchange contrast agent for dynamic contrast-enhanced MRI. Magn. Reson. Med. 2018, 80, 1158–1164. [Google Scholar] [CrossRef]
- Longo, D.L.; Dastrù, W.; Digilio, G.; Keupp, J.; Langereis, S.; Lanzardo, S.; Prestigio, S.; Steinbach, O.; Terreno, E.; Uggeri, F.; et al. Iopamidol as a responsive MRI-chemical exchange saturation transfer contrast agent for pH mapping of kidneys: In vivo studies in mice at 7 T. Magn. Reson. Med. 2011, 65, 202–211. [Google Scholar] [CrossRef]
- Longo, D.L.; Busato, A.; Lanzardo, S.; Antico, F.; Aime, S. Imaging the pH evolution of an acute kidney injury model by means of iopamidol, a MRI-CEST pH-responsive contrast agent. Magn. Reson. Med. 2013, 70, 859–864. [Google Scholar] [CrossRef]
- Zhou, J.; Payen, J.-F.; Wilson, D.A.; Traystman, R.J.; van Zijl, P.C.M. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef]
- Yang, X.; Song, X.; Ray Banerjee, S.; Li, Y.; Byun, Y.; Liu, G.; Bhujwalla, Z.M.; Pomper, M.G.; McMahon, M.T. Developing imidazoles as CEST MRI pH sensors. Contrast Media Mol. Imaging 2016, 11, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Roalf, D.R.; Nanga, R.P.R.; Rupert, P.E.; Hariharan, H.; Quarmley, M.; Calkins, M.E.; Dress, E.; Prabhakaran, K.; Elliott, M.A.; Moberg, P.J.; et al. Glutamate imaging (GluCEST) reveals lower brain GluCEST contrast in patients on the psychosis spectrum. Mol. Psychiatry 2017, 22, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Bagga, P.; Pickup, S.; Crescenzi, R.; Martinez, D.; Borthakur, A.; D’Aquilla, K.; Singh, A.; Verma, G.; Detre, J.A.; Greenberg, J.; et al. In vivo GluCEST MRI: Reproducibility, background contribution and source of glutamate changes in the MPTP model of Parkinson’s disease. Sci. Rep. 2018, 8, 2883. [Google Scholar] [CrossRef] [PubMed]
- Sinharay, S.; Fernández-Cuervo, G.; Acfalle, J.P.; Pagel, M.D. Detection of Sulfatase Enzyme Activity with a CatalyCEST MRI Contrast Agent. Chem. Eur. J. 2016, 22, 6491–6495. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.R.; Best, M.D.; Wong, C.-H. Sulfatases: Structure, Mechanism, Biological Activity, Inhibition, and Synthetic Utility. Angew. Chem. Int. Ed. 2004, 43, 5736–5763. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yadav, N.N.; Song, X.; Ray Banerjee, S.; Edelman, H.; Minn, I.; van Zijl, P.C.M.; Pomper, M.G.; McMahon, M.T. Tuning Phenols with Intra-Molecular Bond Shifted HYdrogens (IM-SHY) as diaCEST MRI Contrast Agents. Chem. Eur. J. 2014, 20, 15824–15832. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, X.; Zhu, W.; Oskolkov, N.; Zhou, T.; Kim, B.K.; Baig, N.; McMahon, M.T.; Oldfield, E. Chemical Exchange Saturation Transfer (CEST) Agents: Quantum Chemistry and MRI. Chem. Eur. J. 2016, 22, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuervo, G.; Sinharay, S.; Pagel, M.D. A CatalyCEST MRI Contrast Agent that Can Simultaneously Detect Two Enzyme Activities. ChemBioChem 2016, 17, 383–387. [Google Scholar] [CrossRef]
- Van Zijl, P.C.M.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef]
- Woods, M.; Woessner, D.E.; Sherry, A.D. Paramagnetic lanthanide complexes as PARACEST agents for medical imaging. Chem. Soc. Rev. 2006, 35, 500–511. [Google Scholar] [CrossRef]
- Hingorani, D.V.; Bernstein, A.S.; Pagel, M.D. A review of responsive MRI contrast agents: 2005–2014. Contrast Media Mol. Imaging 2015, 10, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, X.; Li, Y.; Liu, G.; Ray Banerjee, S.; Pomper, M.G.; McMahon, M.T. Salicylic Acid and Analogues as diaCEST MRI Contrast Agents with Highly Shifted Exchangeable Proton Frequencies. Angew. Chem. Int. Ed. 2013, 52, 8116–8119. [Google Scholar] [CrossRef]
- Cohen, O.; Huang, S.; McMahon, M.T.; Rosen, M.S.; Farrar, C.T. Rapid and quantitative chemical exchange saturation transfer (CEST) imaging with magnetic resonance fingerprinting (MRF). Magn. Reson. Med. 2018, 80, 2449–2463. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, S.; Soesbe, T.C.; Yu, J.; Vinogradov, E.; Lenkinski, R.E.; Sherry, A.D. pH imaging of mouse kidneys in vivo using a frequency-dependent paraCEST agent. Magn. Reson. Med. 2016, 75, 2432–2441. [Google Scholar] [CrossRef] [PubMed]
- Funk, A.M.; Clavijo Jordan, V.; Sherry, A.D.; Ratnakar, S.J.; Kovacs, Z. Oxidative Conversion of a Europium(II)-Based T1 Agent into a Europium(III)-Based paraCEST Agent that can be Detected In Vivo by Magnetic Resonance Imaging. Angew. Chem. 2016, 128, 5108–5111. [Google Scholar] [CrossRef]
- Zhang, L.; Martins, A.F.; Mai, Y.; Zhao, P.; Funk, A.M.; Clavijo Jordan, M.V.; Zhang, S.; Chen, W.; Wu, Y.; Sherry, A.D. Imaging Extracellular Lactate In Vitro and In Vivo Using CEST MRI and a Paramagnetic Shift Reagent. Chem. Eur. J. 2017, 23, 1752–1756. [Google Scholar] [CrossRef]
- Yoo, B.; Sheth, V.R.; Howison, C.M.; Douglas, M.J.K.; Pineda, C.T.; Maine, E.A.; Baker, A.F.; Pagel, M.D. Detection of in vivo enzyme activity with CatalyCEST MRI. Magn. Reson. Med. 2014, 71, 1221–1230. [Google Scholar] [CrossRef]
- Sherry, A.D.; Woods, M. Chemical Exchange Saturation Transfer Contrast Agents for Magnetic Resonance Imaging. Annu. Rev. Biomed. Eng. 2008, 10, 391–411. [Google Scholar] [CrossRef]
- Kumas, C.; Fernando, W.S.; Zhao, P.; Regueiro-Figueroa, M.; Kiefer, G.E.; Martins, A.F.; Platas-Iglesias, C.; Sherry, A.D. Unexpected Changes in the Population of Coordination Isomers for the Lanthanide Ion Complexes of DOTMA–Tetraglycinate. Inorg. Chem. 2016, 55, 9297–9305. [Google Scholar] [CrossRef]
- Zhang, S.; Merritt, M.; Woessner, D.E.; Lenkinski, R.E.; Sherry, A.D. PARACEST Agents: Modulating MRI Contrast via Water Proton Exchange. Acc. Chem. Res. 2003, 36, 783–790. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]










| Agent | Dose | Physiologic Target | Biologic Use | Current Development Status | Ref. |
|---|---|---|---|---|---|
| Mn(III)-(DPA-C2)2-TPPS3 | nM range; direct intracranial injection | Intracellular Zn2+ | Zn2+ signaling in brain tissue | In vivo testing; rat model | [41] |
| GdDOTA-BiPEN derivatives | 0.07 mmol/kg administered intravenously | Extracellular Zn2+ | GSIZS in the pancreas and GSZS in prostate | Extensive in vivo testing; mouse and rat pancreas, and mouse prostate | [7,47] |
| GdDO3A-BPEN | 0.07 mmol/kg administered intravenously | Extracellular Zn2+ | GSIZS in the pancreas | In vivo testing; mouse pancreas | [12] |
| GdDO3A-PEPMA | 0.07 mmol/kg administered intravenously | Extracellular Zn2+ | GSIZS in the pancreas | In vivo testing; mouse pancreas | [12] |
| GdL-1 | 0.10 mmol/kg administered intravenously | Extracellular Cu2+ | Extracellular liver copper stores | In vivo testing; mouse model | [51] |
| MnCl2 | 3.6 μmol/min administered intravenously | Possibly extracellular Ca2+ | Possible Ca2+ signaling in the brain | In vivo testing; rat model | [59] |
| SCA-USRPs | 0.04 mmol/kg administered intravenously | Extracellular Ca2+ | Ca2+ fluctuations in kidneys | In vivo testing; mouse model | [63] |
| MaCaReNas | nM range; direct intracranial injection | Extracellular Ca2+ | Extracellular Ca2+ signaling in the brain | In vivo testing; rat model | [61] |
| GdDOTA-4AMP | 0.2 to 0.4 mmol co-administered intravenously with DDOTA-4AMP or GdDOTP | Extracellular pH | Kidney pH mapping | In vivo testing; mouse model | [69,70] |
| Agent | Dose | Physiologic Target | Biologic Use | Current Development Status | Ref. |
|---|---|---|---|---|---|
| d-glucose | 3.0 mmol/kg administered intravenously | Glucose uptake transporters | Detection of hyperglycolytic cancers | In vivo testing; mouse model | [78] |
| d-maltose | 3.0 mmol/kg administered intravenously | d-maltose uptake | Detection of hyperglycolytic cancers | In vivo testing; mouse model | [79] |
| Agent | Dose | Biological Use | Current Development Status | Ref. |
|---|---|---|---|---|
| Imidazole-4,5-dicarboxamide | 1.0 mmol/kg infused intravenously (sat. power: 5.9 µT) | pH mapping of Kidneys | In vivo testing; mouse model | [83] |
| Eu-DOTA-monoketone-tris(amide) | 0.4 mmol/kg administered intravenously (sat. power: 10 µT) | pH mapping | In vivo testing; mouse model | [96] |
| Eu-DOTA-gly4 complex | 0.05 mmol/kg administered intramuscularly (sat. power: 10 µT) | Redox sensitive agent in vivo | In vivo testing; mouse model | [97] |
| EuDO3A complex | 0.1 mmol/kg administered intravenously (sat. power: 14 µT) | Extracellular lactate | In vivo testing; mouse model | [98] |
| ZGGR-a-amino-(Tm-DOTA) | 0.2 mmol/kg administered intravenously (sat. power: 10 µT) | Urokinase plasminogen activator (uPA) | In vivo testing; cancer mouse model | [99] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrott, D.; Fernando, W.S.; Martins, A.F. Smart MRI Agents for Detecting Extracellular Events In Vivo: Progress and Challenges. Inorganics 2019, 7, 18. https://doi.org/10.3390/inorganics7020018
Parrott D, Fernando WS, Martins AF. Smart MRI Agents for Detecting Extracellular Events In Vivo: Progress and Challenges. Inorganics. 2019; 7(2):18. https://doi.org/10.3390/inorganics7020018
Chicago/Turabian StyleParrott, Daniel, W. Shirangi Fernando, and Andre F. Martins. 2019. "Smart MRI Agents for Detecting Extracellular Events In Vivo: Progress and Challenges" Inorganics 7, no. 2: 18. https://doi.org/10.3390/inorganics7020018
APA StyleParrott, D., Fernando, W. S., & Martins, A. F. (2019). Smart MRI Agents for Detecting Extracellular Events In Vivo: Progress and Challenges. Inorganics, 7(2), 18. https://doi.org/10.3390/inorganics7020018