Transmetalation from Magnesium–NHCs—Convenient Synthesis of Chelating π-Acidic NHC Complexes
Abstract
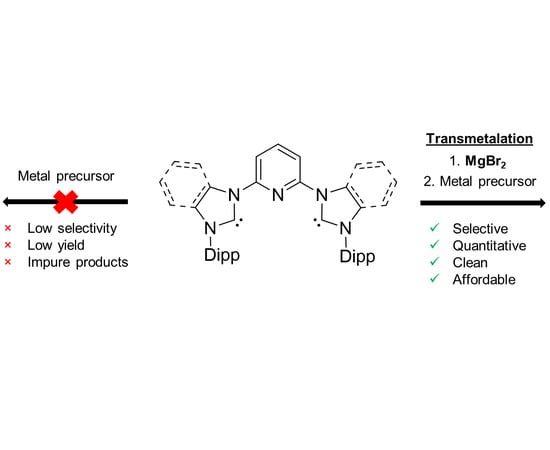
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization of Imidazolinium Salt 1sa
3.3. Synthesis and Characterization of Benzimidazolium Salt 1benz
3.4. Synthesis and Characterization of Free Carbene 2sa
3.5. Synthesis and Characterization of Free Carbene 2benz
3.6. Synthesis and Characterization of Palladium Complex 3sa
3.7. Synthesis and Characterization of Palladium Complex 3benz
3.8. Synthesis and Characterization of Magnesium Complex 4sa
3.9. Synthesis and Characterization of Magnesium Complex 4benz
3.10. Synthesis and Characterization of Iron Complex 5sa
3.11. Effects of Metal Cation on Transmetalation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghadwal, R.S. Carbon-based two electron s-donor ligands beyond classical N-heterocyclic carbenes. Dalton Trans. 2016, 45, 16081–16095. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Munz, D. Pushing Electrons-Which Carbene Ligand for Which Application? Organometallics 2018, 37, 275–289. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Bertrand, G. Carbenes introduction. Chem. Rev. 2009, 109, 3209–3210. [Google Scholar] [CrossRef][Green Version]
- Díez-González, S. N-Heterocyclic Carbenes: From Laboratory to Curiosities to Efficient Synthetic Tools; Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Hahn, F.E. Introduction: Carbene Chemistry. Chem. Rev. 2018, 118, 9455–9456. [Google Scholar] [CrossRef]
- Huynh, H.V. The Organometallic Chemistry of N-Heterocyclic Carbenes; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Nolan, S.P. N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Rovis, T.; Nolan, S.P. Stable Carbenes: From ‘Laboratory Curiosities’ to Catalysis Mainstays. Synlett 2013, 24, 1188–1189. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Köcher, C. N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. Engl. 1997, 36, 2162–2187. [Google Scholar] [CrossRef]
- Melaimi, M.; Soleilhavoup, M.; Bertrand, G. Stable Cyclic Carbenes and Related Species beyond Diaminocarbenes. Angew. Chem. Int. Ed. 2010, 49, 8810–8849. [Google Scholar] [CrossRef]
- Huynh, H.V. Electronic Properties of N-Heterocyclic Carbenes and Their Experimental Determination. Chem. Rev. 2018, 118, 9457–9492. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.J.; Nolan, S.P. Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef] [PubMed]
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Hu, X.; Tang, Y.; Gantzel, P.; Meyer, K. Silver Complexes of a Novel Tripodal N-Heterocyclic Carbene Ligand: Evidence for Significant Metal−Carbene π-Interaction. Organometallics 2003, 22, 612–614. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; Prasang, C.; Donnadieu, B.; Bertrand, G. Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference. Angew. Chem. Int. Ed. 2005, 44, 5705–5709. [Google Scholar] [CrossRef] [PubMed]
- Melaimi, M.; Jazzar, R.; Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)(amino)carbenes (CAACs): Recent Developments. Angew. Chem. Int. Ed. 2017, 56, 10046–10068. [Google Scholar] [CrossRef] [PubMed]
- Paul, U.S.D.; Radius, U. What Wanzlick Did Not Dare To Dream: Cyclic (Alkyl)(amino)carbenes (cAACs) as New Key Players in Transition-Metal Chemistry. Eur. J. Inorg. Chem. 2017, 2017, 3362–3375. [Google Scholar] [CrossRef]
- Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)(Amino)Carbenes (CAACs): Stable Carbenes on the Rise. Acc. Chem. Res. 2015, 48, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Munz, D.; Jazzar, R.; Melaimi, M.; Bertrand, G. Synthesis of Hemilabile Cyclic (Alkyl)(amino)carbenes (CAACs) and Applications in Organometallic Chemistry. J. Am. Chem. Soc. 2016, 138, 7884–7887. [Google Scholar] [CrossRef]
- Munz, D.; Chu, J.; Melaimi, M.; Bertrand, G. NHC-CAAC Heterodimers with Three Stable Oxidation States. Angew. Chem. Int. Ed. 2016, 55, 12886–12890. [Google Scholar] [CrossRef]
- Moerdyk, J.P.; Schilter, D.; Bielawski, C.W. N,N’-Diamidocarbenes: Isolable Divalent Carbons with Bona Fide Carbene Reactivity. Acc. Chem. Res. 2016, 49, 1458–1468. [Google Scholar] [CrossRef]
- Hudnall, T.W.; Tennyson, A.G.; Bielawski, C.W. A Seven-Membered N,N′-Diamidocarbene. Organometallics 2010, 29, 4569–4578. [Google Scholar] [CrossRef]
- Hudnall, T.W.; Bielawski, C.W. An N,N′-Diamidocarbene: Studies in C–H Insertion, Reversible Carbonylation, and Transition-Metal Coordination Chemistry. J. Am. Chem. Soc. 2009, 131, 16039–16041. [Google Scholar] [CrossRef]
- Benhamou, L.; Vujkovic, N.; César, V.; Gornitzka, H.; Lugan, N.; Lavigne, G. Facile Derivatization of a “Chemo-active” NHC Incorporating an Enolate Backbone and Relevant Tuning of Its Electronic Properties. Organometallics 2010, 29, 2616–2630. [Google Scholar] [CrossRef]
- Siemeling, U.; Färber, C.; Bruhn, C.; Leibold, M.; Selent, D.; Baumann, W.; von Hopffgarten, M.; Goedecke, C.; Frenking, G. N-heterocyclic carbenes which readily add ammonia, carbon monoxide and other small molecules. Chem. Sci. 2010, 1, 697–704. [Google Scholar] [CrossRef]
- Yoshida, K.; Yasue, R. Planar-Chiral Ferrocene-Based N-Heterocyclic Carbene Ligands. Chem. Eur. J. 2018, 24, 18575–18586. [Google Scholar] [CrossRef]
- Siemeling, U.; Färber, C.; Leibold, M.; Bruhn, C.; Mücke, P.; Winter, R.F.; Sarkar, B.; von Hopffgarten, M.; Frenking, G. Six-Membered N-Heterocyclic Carbenes with a 1,1′-Ferrocenediyl Backbone: Bulky Ligands with Strong Electron-Donor Capacity and Unusual Non-Innocent Character. Eur. J. Inorg. Chem. 2009, 2009, 4607–4612. [Google Scholar] [CrossRef]
- Khramov, D.M.; Rosen, E.L.; Lynch, V.M.; Bielawski, C.W. Diaminocarbene[3]ferrocenophanes and Their Transition-Metal Complexes. Angew. Chem. Int. Ed. 2008, 47, 2267–2270. [Google Scholar] [CrossRef]
- Hansmann, M.M.; Melaimi, M.; Munz, D.; Bertrand, G. Modular Approach to Kekulé Diradicaloids Derived from Cyclic (Alkyl)(amino)carbenes. J. Am. Chem. Soc. 2018, 140, 2546–2554. [Google Scholar] [CrossRef]
- Messelberger, J.; Grünwald, A.; Pinter, P.; Hansmann, M.M.; Munz, D. Carbene derived diradicaloids—Building blocks for singlet fission? Chem. Sci. 2018, 9, 6107–6117. [Google Scholar] [CrossRef]
- Barry, B.; Soper, G.; Hurmalainen, J.; Mansikkamäki, A.; Robertson, K.N.; McClennan, W.L.; Veinot, A.J.; Roemmele, T.L.; Werner-Zwanziger, U.; Boeré, R.T.; et al. Mono- and Bis-Imidazolidinium Ethynyl Cations and the Reduction of the Latter to give an Extended Bis-1,4-([3]Cumulene)-p-Carbo-Quinoid System. Angew. Chem. Int. Ed. 2018, 57, 749–754. [Google Scholar] [CrossRef]
- Rottschäfer, D.; Ho, N.K.T.; Neumann, B.; Stammler, H.G.; van Gastel, M.; Andrada, D.M.; Ghadwal, R.S. N-Heterocyclic Carbene Analogues of Thiele and Chichibabin Hydrocarbons. Angew. Chem. Int. Ed. 2018, 57, 5838–5842. [Google Scholar] [CrossRef]
- Ghadwal, R.S.; Rottschafer, D.; Neumann, B.; Stammler, G.; Andrada, D.M. Kekulé Diradicaloids Derived from a Classical N-Heterocyclic Carbene. Chem. Sci. 2018, 9, 4970–4976. [Google Scholar]
- Roy, S.; Mondal, K.C.; Roesky, H.W. Cyclic Alkyl(amino) Carbene Stabilized Complexes with Low Coordinate Metals of Enduring Nature. Acc. Chem. Res. 2016, 49, 357–369. [Google Scholar] [CrossRef]
- Grünwald, A.; Munz, D. How to tame a palladium terminal imido. J. Organomet. Chem. 2018, 864, 26–36. [Google Scholar] [CrossRef]
- Munz, D. How to tame a palladium terminal oxo. Chem. Sci. 2018, 9, 1155–1167. [Google Scholar] [CrossRef]
- Grünwald, A.; Orth, N.; Scheurer, A.; Heinemann, F.W.; Pöthig, A.; Munz, D. An Isolable Terminal Imido Complex of Palladium and Catalytic Implications. Angew. Chem. Int. Ed. 2018, 57, 16228–16232. [Google Scholar] [CrossRef]
- Tulloch, A.A.D.; Danopoulos, A.A.; Tizzard, G.J.; Coles, S.J.; Hursthouse, M.B.; Hay-Motherwell, R.S.; Motherwell, W.B. Chiral 2,6-lutidinyl-biscarbene complexes of palladium. Chem. Commun. 2001, 14, 1270–1271. [Google Scholar] [CrossRef]
- Peris, E.; Mata, J.; Loch, J.A.; Crabtree, R.H. A Pd complex of a tridentate pincer CNC bis-carbene ligand as a robust homogenous Heck catalyst. Chem. Commun. 2001, 2, 201–202. [Google Scholar] [CrossRef]
- Andrew, R.E.; González-Sebastián, L.; Chaplin, A.B. NHC-based pincer ligands: Carbenes with a bite. Dalton Trans. 2016, 45, 1299–1305. [Google Scholar] [CrossRef]
- Poyatos, M.; Mata, J.A.; Peris, E. Complexes with Poly(N-heterocyclic carbene) Ligands: Structural Features and Catalytic Applications. Chem. Rev. 2009, 109, 3677–3707. [Google Scholar] [CrossRef]
- Pugh, D.; Danopoulos, A.A. Metal complexes with ‘pincer’-type ligands incorporating N-heterocyclic carbene functionalities. Coord. Chem. Rev. 2007, 251, 610–641. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Recent homogeneous catalytic applications of chelate and pincer N-heterocyclic carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar] [CrossRef]
- Tu, T.; Malineni, J.; Dötz, K.H. A Novel Pyridine-Bridged Bis-benzimidazolylidene Pincer Palladium Complex: Synthesis and Catalytic Properties. Adv. Synth. Catal. 2008, 350, 1791–1795. [Google Scholar] [CrossRef]
- Chung, L.-H.; Cho, K.-S.; England, J.; Chan, S.-C.; Wieghardt, K.; Wong, C.-Y. Ruthenium(II) and Osmium(II) Complexes Bearing Bipyridine and the N-Heterocyclic Carbene-Based CNC Pincer Ligand: An Experimental and Density Functional Theory Study. Inorg. Chem. 2013, 52, 9885–9896. [Google Scholar] [CrossRef]
- Chen, J.C.C.; Lin, I.J.B. The first dicarbene double helical mercury complex. Dalton Trans. 2000, 6, 839–840. [Google Scholar] [CrossRef]
- MaGee, K.D.M.; Travers, G.; Skelton, B.W.; Massi, M.; Payne, A.D.; Brown, D.H. Synthesis, Solid-state Structures, Solution Behaviour and Catalysis Studies of Nickel Complexes of Bis(benzimidazolin-2-ylidene)pyridine Pincer Ligands. Aust. J. Chem. 2012, 65, 823–833. [Google Scholar] [CrossRef][Green Version]
- Brown, D.H.; Nealon, G.L.; Simpson, P.V.; Skelton, B.W.; Wang, Z. Silver and Palladium Complexes of a Bis(benzimidazolin-2-ylidene)pyridine Pincer Ligand. Organometallics 2009, 28, 1965–1968. [Google Scholar] [CrossRef]
- Brown, D.H.; Skelton, B.W. Nickel complexes of a bis(benzimidazolin-2-ylidene)pyridine pincer ligand with four- and five-coordinate geometries. Dalton Trans. 2011, 40, 8849–8858. [Google Scholar] [CrossRef]
- Yu, R.P.; Darmon, J.M.; Semproni, S.P.; Turner, Z.R.; Chirik, P.J. Synthesis of Iron Hydride Complexes Relevant to Hydrogen Isotope Exchange in Pharmaceuticals. Organometallics 2017, 36, 4341–4343. [Google Scholar] [CrossRef]
- Yu, R.P.; Hesk, D.; Rivera, N.; Pelczer, I.; Chirik, P.J. Iron-catalysed tritiation of pharmaceuticals. Nature 2016, 529, 195–199. [Google Scholar]
- Andersen, R.A.; Faegri, K., Jr.; Green, J.C.; Haaland, A.; Lappert, M.F.; Leung, W.P.; Rypdal, K. Synthesis of bis[bis(trimethylsilyl)amido]iron(II). Structure and bonding in M[N(SiMe3)2]2 (M = manganese, iron, cobalt): Two-coordinate transition-metal amides. Inorg. Chem. 1988, 27, 1782–1786. [Google Scholar] [CrossRef]
- Baishya, A.; Baruah, S.; Geetharani, K. Efficient hydroboration of carbonyls by an iron(II) amide catalyst. Dalton Trans. 2018, 47, 9231–9236. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S.; Dagorne, S. Group 1 and 2 and Early Transition Metal Complexes Bearing N-Heterocyclic Carbene Ligands: Coordination Chemistry, Reactivity, and Applications. Chem. Rev. 2014, 114, 8747–8774. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Dias, H.V.R.; Davidson, F.; Harlow, R.L. Carbene adducts of magnesium and zinc. J. Organomet. Chem. 1993, 462, 13–18. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Hill, M.S.; MacDougall, D.J.; Mahon, M.F. A Hydride-Rich Magnesium Cluster. Angew. Chem. Int. Ed. 2009, 48, 4013–4016. [Google Scholar] [CrossRef]
- Baishya, A.; Kumar, L.; Barman, M.K.; Biswal, H.S.; Nembenna, S. N-Heterocyclic Carbene-Carbodiimide (“NHC-CDI”) Adduct or Zwitterionic-Type Neutral Amidinate-Supported Magnesium(II) and Zinc(II) Complexes. Inorg. Chem. 2017, 57, 9535–9546. [Google Scholar] [CrossRef]
- Bantu, B.; Manohar Pawar, G.; Wurst, K.; Decker, U.; Schmidt, A.M.; Buchmeiser, M.R. CO2, Magnesium, Aluminum, and Zinc Adducts of N-Heterocyclic Carbenes as (Latent) Catalysts for Polyurethane Synthesis. Eur. J. Inorg. Chem. 2009, 2009, 1970–1976. [Google Scholar] [CrossRef]
- Ghadwal, R.S.; Rottschäfer, D.; Schürmann, C.J. Expedient Access to Normal- and Abnormal-N-Heterocyclic Carbene (NHC) Magnesium Compounds from Imidazolium Salts. Z. Anorg. Allg. Chem. 2016, 642, 1236–1240. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Klett, J.; Mulvey, R.E.; Robertson, S.D. N-Heterocyclic-Carbene-Induced Monomerization of Sterically Encumbered Dialkylmagnesium and Dialkylmanganese Polymers. Eur. J. Inorg. Chem. 2011, 2011, 4675–4679. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Mulvey, R.E.; Robertson, S.D. N-Heterocyclic carbene stabilized adducts of alkyl magnesium amide, bisalkyl magnesium and Grignard reagents: Trapping oligomeric organo s-block fragments with NHCs. Dalton Trans. 2010, 39, 9091–9099. [Google Scholar] [CrossRef]
- Martínez-Martínez, A.J.; Fuentes, M.Á.; Hernán-Gómez, A.; Hevia, E.; Kennedy, A.R.; Mulvey, R.E.; O’Hara, C.T. Alkali-Metal-Mediated Magnesiations of an N-Heterocyclic Carbene: Normal, Abnormal, and “Paranormal” Reactivity in a Single Tritopic Molecule. Angew. Chem. Int. Ed. 2015, 54, 14075–14079. [Google Scholar] [CrossRef]
- Naumann, S.; Scholten, P.B.V.; Wilson, J.A.; Dove, A.P. Dual Catalysis for Selective Ring-Opening Polymerization of Lactones: Evolution toward Simplicity. J. Am. Chem. Soc. 2015, 137, 14439–14445. [Google Scholar] [CrossRef]
- Stasch, A. Synthesis of a Dimeric Magnesium(I) Compound by an MgI/MgII Redox Reaction. Angew. Chem. Int. Ed. 2014, 53, 10200–10203. [Google Scholar] [CrossRef]
- Stasch, A.; Sarish, S.P.; Roesky, H.W.; Meindl, K.; Dall’Antonia, F.; Schulz, T.; Stalke, D. Synthesis and Characterization of Alkynyl Complexes of Groups 1 and 2. Chem. Asian J. 2009, 4, 1451–1457. [Google Scholar] [CrossRef]
- Turner, Z.R.; Buffet, J.C. Group 1 and 2 cyclic (alkyl)(amino)carbene complexes. Dalton Trans 2015, 44, 12985–12989. [Google Scholar] [CrossRef]
- Wong, Y.O.; Freeman, L.A.; Agakidou, A.D.; Dickie, D.A.; Webster, C.E.; Gilliard, R.J. Two Carbenes versus One in Magnesium Chemistry: Synthesis of Terminal Dihalide, Dialkyl, and Grignard Reagents. Organometallics 2019, 38, 688–696. [Google Scholar] [CrossRef]
- Arnold, P.L.; Casely, I.J.; Turner, Z.R.; Bellabarba, R.; Tooze, R.B. Magnesium and zinc complexes of functionalised, saturated N-heterocyclic carbene ligands: Carbene lability and functionalisation, and lactide polymerisation catalysis. Dalton Trans. 2009, 35, 7236–7247. [Google Scholar] [CrossRef]
- Arnold, P.L.; Edworthy, I.S.; Carmichael, C.D.; Blake, A.J.; Wilson, C. Magnesium amido N-heterocyclic carbene complexes. Dalton Trans. 2008, 28, 3739–3746. [Google Scholar] [CrossRef]
- Grassi, D.; Dolka, C.; Jackowski, O.; Alexakis, A. Copper-Free Asymmetric Allylic Alkylation with a Grignard Reagent: Design of the Ligand and Mechanistic Studies. Chem. Eur. J. 2013, 19, 1466–1475. [Google Scholar] [CrossRef]
- Liddle, S.T.; Edworthy, I.S.; Arnold, P.L. Anionic tethered N-heterocyclic carbene chemistry. Chem. Soc. Rev. 2007, 36, 1732–1744. [Google Scholar] [CrossRef]
- Nieto, I.; Cervantes-Lee, F.; Smith, J.M. A new synthetic route to bulky “second generation” tris(imidazol-2-ylidene)borate ligands: Synthesis of a four coordinate iron(II) complex. Chem. Commun. 2005, 30, 3811–3813. [Google Scholar] [CrossRef]
- Zhang, D.; Kawaguchi, H. Deprotonation attempts on imidazolium salt tethered by substituted phenol and construction of its magnesium complex by transmetalation. Organometallics 2006, 25, 5506–5509. [Google Scholar] [CrossRef]
- Naumann, S.; Schmidt, F.G.; Frey, W.; Buchmeiser, M.R. Protected N-heterocyclic carbenes as latent pre-catalysts for the polymerization of ε-caprolactone. Polym. Chem. 2013, 4, 4172–4181. [Google Scholar] [CrossRef]
- Armstrong, D.R.; Clegg, W.; Hernan-Gomez, A.; Kennedy, A.R.; Livingstone, Z.; Robertson, S.D.; Russo, L.; Hevia, E. Probing the metallating ability of a polybasic sodium alkylmagnesiate supported by a bulky bis(amido) ligand: Deprotomagnesiation reactions of nitrogen-based aromatic substrates. Dalton Trans. 2014, 43, 4361–4369. [Google Scholar] [CrossRef]
- Baillie, S.E.; Blair, V.L.; Bradley, T.D.; Clegg, W.; Cowan, J.; Harrington, R.W.; Hernán-Gómez, A.; Kennedy, A.R.; Livingstone, Z.; Hevia, E. Isomeric and chemical consequences of the direct magnesiation of 1,3-benzoazoles using β-diketiminate-stabilized magnesium bases. Chem. Sci. 2013, 4, 1895–1905. [Google Scholar] [CrossRef]
- Hirsch-Weil, D.; Snead, D.R.; Inagaki, S.; Seo, H.; Abboud, K.A.; Hong, S. In situ generation of novel acyclic diaminocarbene–copper complex. Chem. Commun. 2009, 18, 2475–2477. [Google Scholar] [CrossRef]
- Koch, A.; Krieck, S.; Görls, H.; Westerhausen, M. Alkaline Earth Metal–Carbene Complexes with the Versatile Tridentate 2,6-Bis(3-mesitylimidazol-2-ylidene)pyridine Ligand. Organometallics 2017, 36, 994–1000. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; Cesar, V. Synthetic routes to N-heterocyclic carbene precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Hirano, K.; Biju, A.T.; Glorius, F. Copper-Catalyzed Synthesis of 2-Unsubstituted, N-Substituted Benzimidazoles. J. Org. Chem. 2009, 74, 9570–9572. [Google Scholar] [CrossRef]
- Evrard, D.; Lucas, D.; Mugnier, Y.; Meunier, P.; Hierso, J.C. On the Mechanistic Behavior of Highly Efficient Palladium–Tetraphosphine Catalytic Systems for Cross-Coupling Reactions: First Spectroscopic and Electrochemical Studies of Oxidative Addition on Pd(0)/Multidentate Ferrocenylpolyphosphine Complexes. Organometallics 2008, 27, 2643–2653. [Google Scholar] [CrossRef]
- Drew, D.; Doyle, J.R. Dichloro(η-1,5-Cyclooctadiene)palladium(II). Inorg. Synth. 1990, 28, 348–349. [Google Scholar]
- Westerhausen, M. Synthesis and spectroscopic properties of bis(trimethylsilyl)amides of the alkaline-earth metals magnesium, calcium, strontium, and barium. Inorg. Chem. 1991, 30, 96–101. [Google Scholar] [CrossRef]
- Straub, B.; Bessel, M.; Rominger, F. Modular Trimethylene-Linked Bisimidazol(in)ium Salts. Synthesis 2010, 2010, 1459–1466. [Google Scholar] [CrossRef]
- Hosmane, N.S.; Zhu, D.; McDonald, J.E.; Zhang, H.; Maguire, J.A.; Gray, T.G.; Helfert, S.C. Chemistry of C-Trimethylsilyl-Substituted Heterocarboranes. 23. Synthetic, Spectroscopic, and Structural Investigation on Half- and Full-Sandwich Magnesacarboranes of 2,3- and 2,4-C2B4 Carborane Ligands. Organometallics 1998, 17, 1426–1437. [Google Scholar] [CrossRef]








| Base | Solvent | Crude Product Selectivity |
| LiN(SiMe3)2 | THF | ≈60% |
| LiN(SiMe3)2 | benzene | ≈60% |
| KN(SiMe3)2 | THF | ≈40% |
| KN(SiMe3)2 | benzene | ≈40% |
| MgBr2/KN(SiMe3)2 | THF | ≈80% |
| Mg[N(SiMe3)2]2 | benzene | ≈100% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messelberger, J.; Grünwald, A.; Stegner, P.; Senft, L.; Heinemann, F.W.; Munz, D. Transmetalation from Magnesium–NHCs—Convenient Synthesis of Chelating π-Acidic NHC Complexes. Inorganics 2019, 7, 65. https://doi.org/10.3390/inorganics7050065
Messelberger J, Grünwald A, Stegner P, Senft L, Heinemann FW, Munz D. Transmetalation from Magnesium–NHCs—Convenient Synthesis of Chelating π-Acidic NHC Complexes. Inorganics. 2019; 7(5):65. https://doi.org/10.3390/inorganics7050065
Chicago/Turabian StyleMesselberger, Julian, Annette Grünwald, Philipp Stegner, Laura Senft, Frank W. Heinemann, and Dominik Munz. 2019. "Transmetalation from Magnesium–NHCs—Convenient Synthesis of Chelating π-Acidic NHC Complexes" Inorganics 7, no. 5: 65. https://doi.org/10.3390/inorganics7050065
APA StyleMesselberger, J., Grünwald, A., Stegner, P., Senft, L., Heinemann, F. W., & Munz, D. (2019). Transmetalation from Magnesium–NHCs—Convenient Synthesis of Chelating π-Acidic NHC Complexes. Inorganics, 7(5), 65. https://doi.org/10.3390/inorganics7050065